DPAGT1—Perspective as an Anticancer Drug Target
Abstract
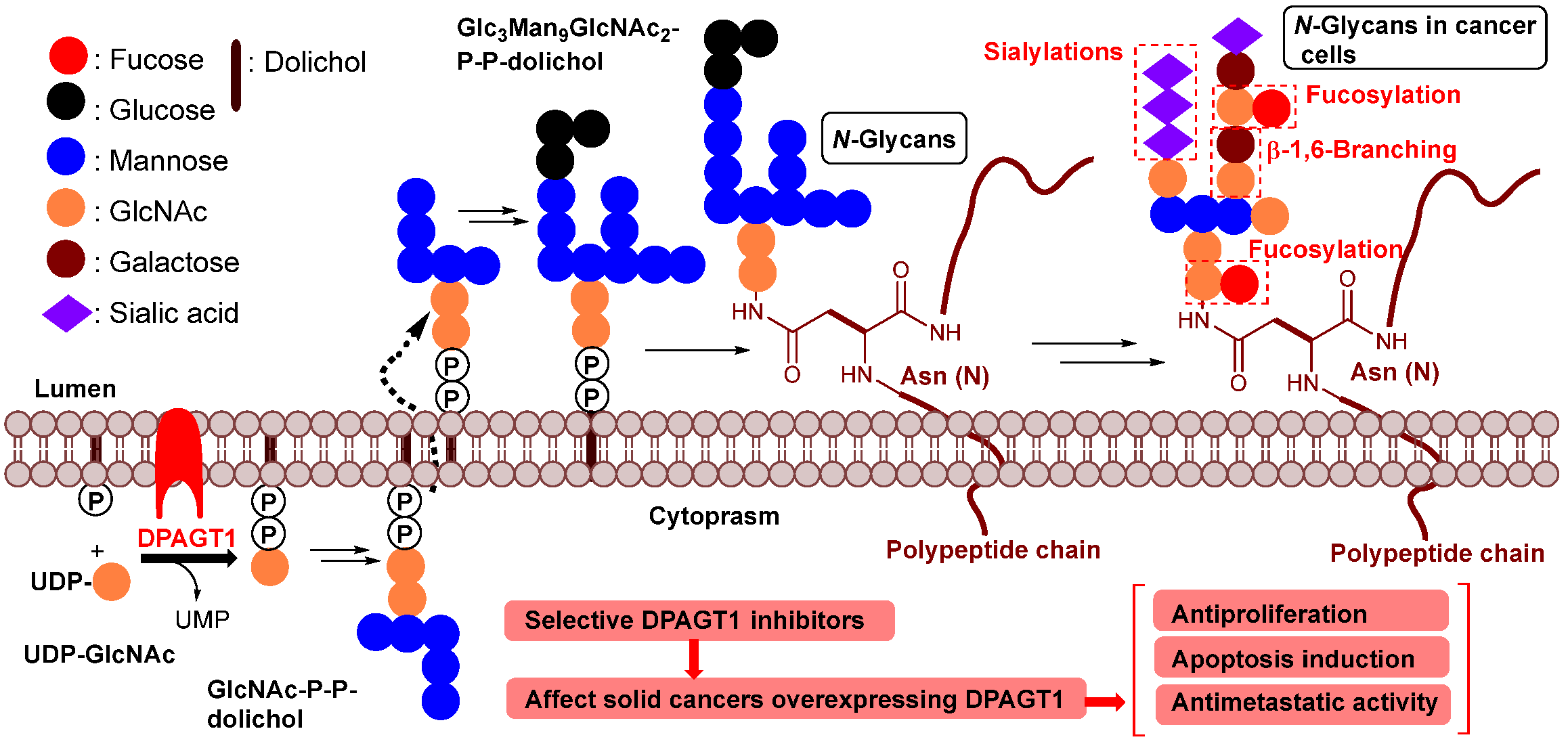
1. Introduction
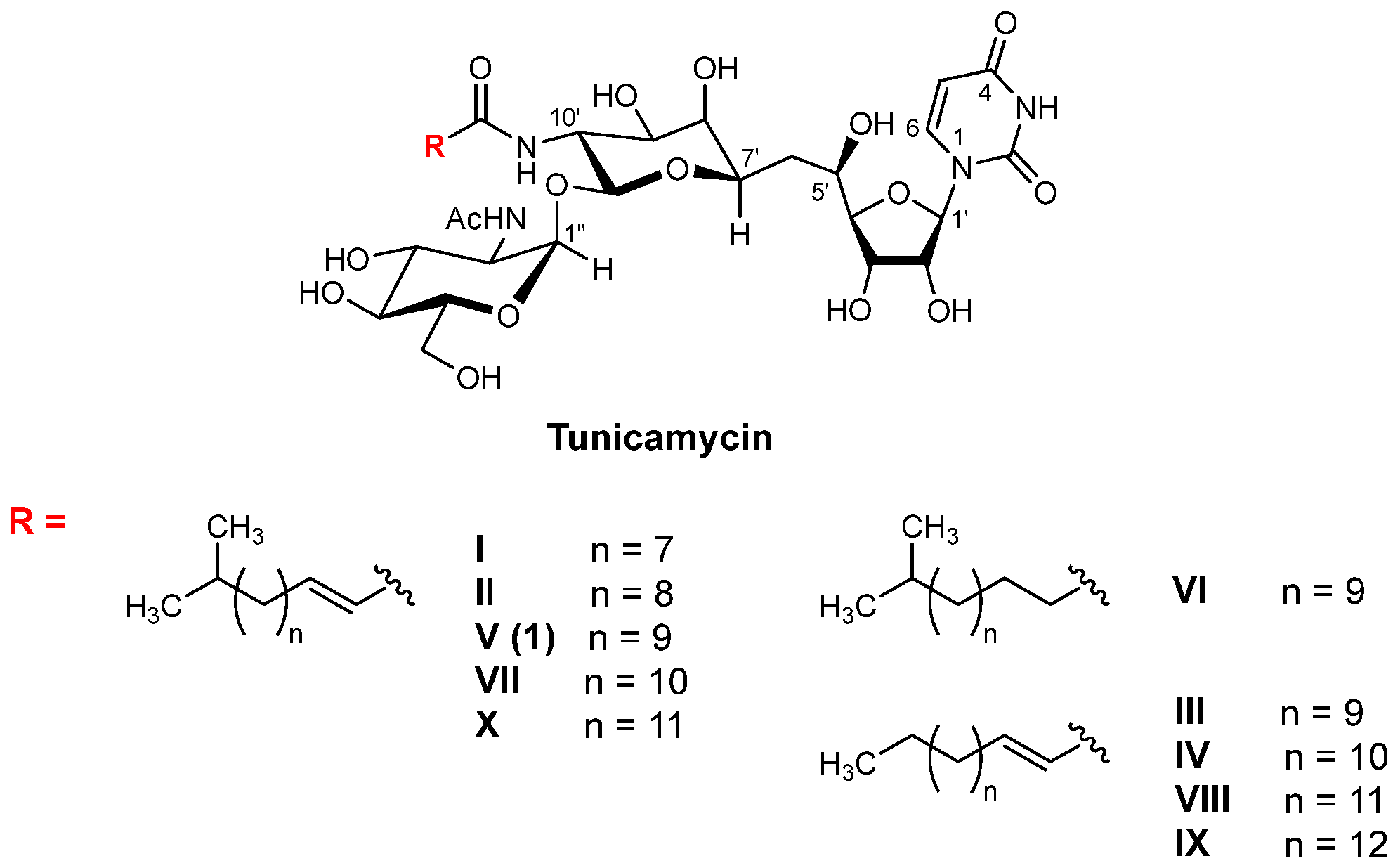
2. DPAGT1 and Its Inhibitor Molecules
3. Discovery of a New DPAGT1 Inhibitor of Natural Product
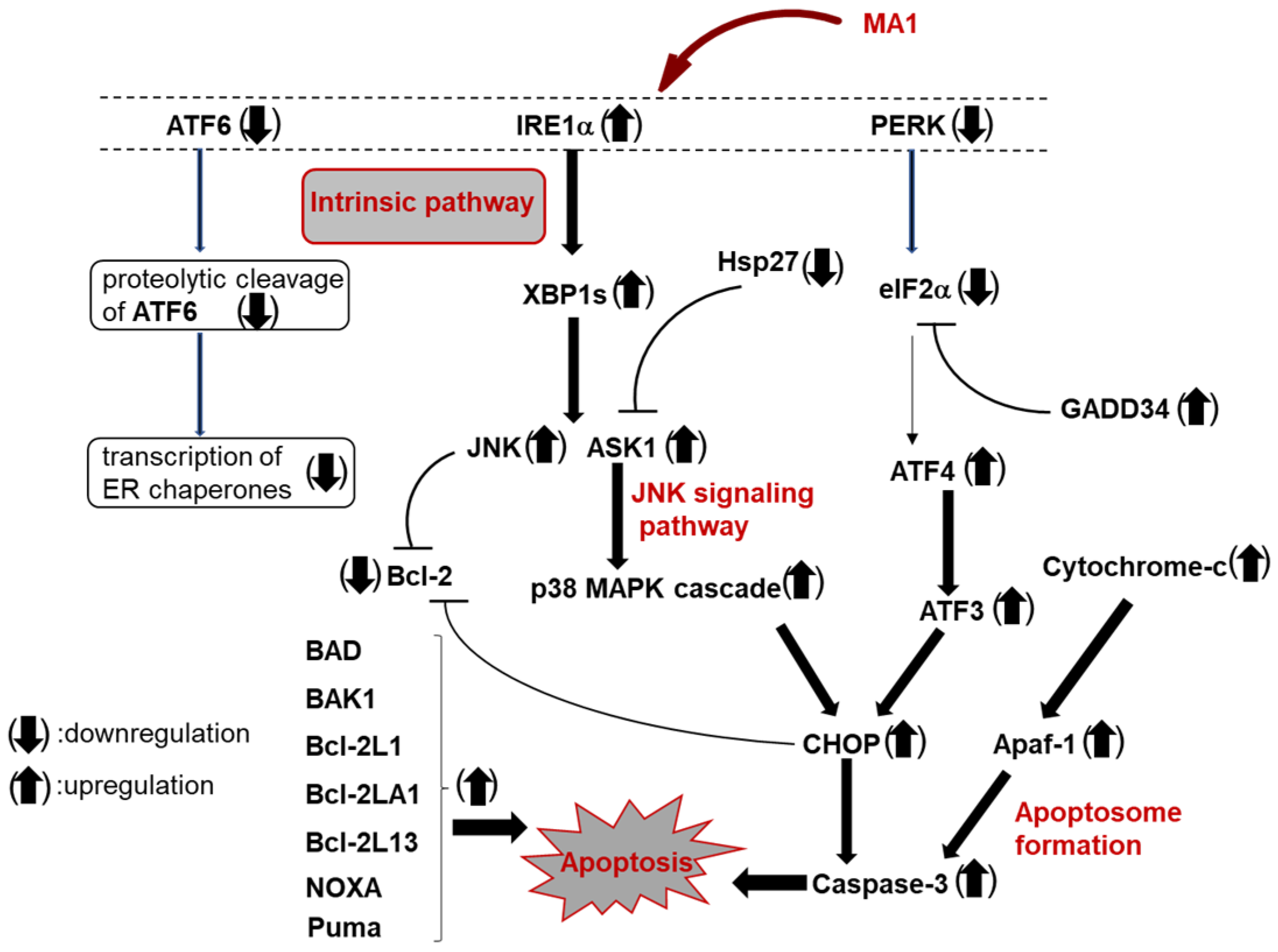
4. Muraymycin A1 Induces G2/M Cell Cycle Arrest and Apoptosis
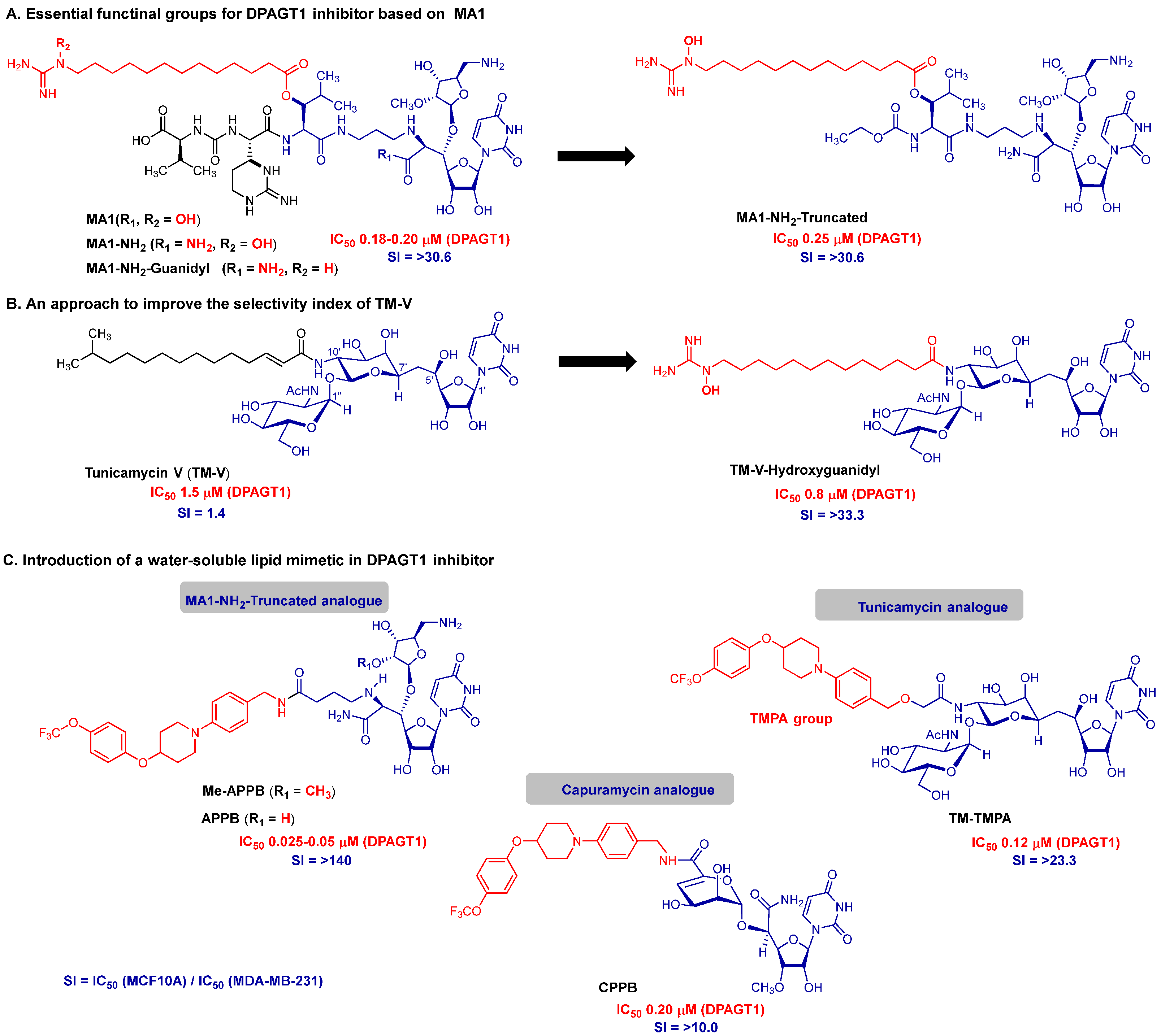
5. Discovery of Novel DPAGT1 Inhibitors Inspired by Muraymycin A1
6. Proof-of-Pharmacological Concept
7. Advancing DPAGT1 Inhibitors Toward Basic to Translational Studies
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPAGT1 | Dolichyl-phosphate N-acetylglucosamine-phosphotransferase 1 |
| ER | Endoplasmic reticulum |
| TM-V | Tunicamycin V |
| LD | Linear dichroism |
| UDP-GlcNAc | Uridine diphosphate N-acetylglucosamine |
| Asn | Asparagine |
| MraY | Phospho-MurNAc-pentapeptide translocase |
| WecA | N-Acetylglucosamine-1-phosphate transferase |
| C. albicans | Candida albicans |
| C. neoformans | Cryptococcus neoformans |
| C. gattii | Cryptococcus gattii |
| A. fumigatus | Aspergillus fumigatus |
| MDA-MB-231 | Human triple negative breast cancer |
| MDA-MB-453 | Human triple negative breast cancer |
| MDA-MB-468 | Human triple negative breast cancer |
| SkBr3 | HER2-overexpressing human breast cancer |
| BT-474 | HER2+ human ductal breast carcinoma |
| HCC38 | Triple-negative breast cancer |
| MCF7 | A human breast adeno-carcinoma cell (ER+) |
| HCT116 | Human colon cancer. |
| HT-29 | Human colorectal adenocarcinoma |
| HepG2 | Human hepatoma cell |
| Caco-2 | Human colorectal adenocarcinoma cell |
| HL-60 | Human leukemia cell |
| L1210 | Mouse lymphocytic leukemia cell |
| MCF10A | Human mammary epithelial cell |
| HPNE | Human pancreatic ductal cell |
| Vero | African green monkey kidney cell. |
| MA1 | Muraymycin A1 |
| IC50 | Half-maximal inhibitory concentration |
| MIC | Minimum inhibitory concentration |
| PERK | Protein kinase R (PKR)-like endoplasmic reticulum kinase |
| IRE1 | Inositol-requiring enzyme 1 |
| ATF6 | Activating transcription factor 6 |
| UPR | Unfolded protein response |
| 7-AAD | 7-Aminoactinomycin D |
| TNFR1 | Tumor necrosis factor receptor 1 |
| IL | Interleukin |
| CL | Cardiolipin |
| PS | Phosphatidylserine |
| BiP | Binding immunoglobulin protein |
| GRP78 | 78 kDa glucose-regulated protein |
| CHOP | C/EBP homologous protein (DNA damage-inducible transcript 3) |
| BCL-2 | B-cell lymphoma 2 protein |
| APPB | Aminouridyl phenoxypiperidinbenzylbutanamide, a DPAGT1 inhibitor |
| Me-APPM | A methylated analog of APPB |
| CPPB | Capuramycin phenoxypiperidinbenzimide, a DPAGT1 inhibitor |
| TM-TMPA | A DPAGT1 inhibitor of tunicamycin analog |
| LD50 | The median lethal dose |
| IV | Intravenous administration |
| KPC-1 | Mouse pancreatic ductal adenocarcinoma |
| Her2 | Human epidermal growth factor receptor 2 |
| mTORC1 | Mammalian target of rapamycin complex 1 |
| ADAM10 | Metalloproteinase domain-containing protein 10 |
| UPR | The unfolded protein response |
| Akt | Protein kinase B |
| NF-kB | Nuclear factor kappa-light-chain-enhancer of activated B |
| Wnt | A proto-oncogene Wnt-1 protein |
| β-catenin | A protein encoded by the CTNNB1 gene |
| EGF | Epidermal growth factor |
| PD-L1 | Programmed death-ligand 1 |
| PI3K | Phosphoinositide 3-kinase |
| PTX3 | Pentraxin-related protein, TNF-inducible gene 14 protein |
| NSCLC | Non-small cell lung cancer |
| WYC | A retinoic acid receptor agonist |
| 5-FU | 5-Fluorouracil |
| BT474 Clone 5 | An epithelial-like cell line clone of ductal carcinoma breast cancer |
References
- Shivatare, S.S.; Vidya, S.; Shivatare, V.S.; Wong, C.-H. Glycoconjugates: Synthesis, Functional Studies, and Therapeutic Developments. Chem. Rev. 2022, 122, 15603–15671. [Google Scholar] [CrossRef]
- Kurosu, M. Structure-based drug discovery by targeting N-glycan biosynthesis, dolichyl-phosphate N-acetylglucosaminephosphotransferase. Future Med. Chem. 2019, 11, 927–933. [Google Scholar] [CrossRef]
- Timur, T.L.; Samatov, R.; Tonevitsky, A.G.; Schumacher, U. Importance of altered glycoprotein-bound N- and O-glycans for epithelial-to-mesenchymal transition and adhesion of cancer cells. Carbohydr. Res. 2014, 389, 39–45. [Google Scholar] [CrossRef]
- Newsom-Davis, T.E.; Wang, D.; Steinman, L.; Chen, P.F.T.; Wang, L.X.; Simon, A.K.; Screaton, G.R. Enhanced immune recognition of cryptic glycan markers in human tumors. Cancer Res. 2009, 69, 2018–2025. [Google Scholar] [CrossRef]
- Vallejo-Ruiz, V.; Reyes-Leyva, J. Glycobiology of Cancer. In Pathogens Associated with the Development of Cancer in Humans; Velázquez-Márquez, N., Paredes-Juárez, G.A., Vallejo-Ruiz, V., Eds.; Springer: Cham, Switzerland, 2024. [Google Scholar] [CrossRef]
- Haga, Y.; Ueda, K. Glycosylation in cancer: Its application as a biomarker and recent advances of analytical techniques. Glycoconj. J. 2022, 39, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Pinho, S.; Reis, C. Glycosylation in cancer: Mechanisms and clinical implications. Nat. Rev. Cancer 2015, 15, 540–555. [Google Scholar] [CrossRef]
- Dong, Y.Y.; Wang, H.; Pike, A.C.W.; Cochrane, S.A.; Hamedzadeh, S.; Wyszyński, F.J.; Bushell, S.R.; Royer, S.F.; Widdick, D.A.; Sajid, A.; et al. Structures of DPAGT1 explain glycosylation disease mechanisms and advance TB antibiotic design. Cell 2018, 175, 1045–1058. [Google Scholar] [CrossRef]
- Yoo, J.; Mashalidis, E.H.; Kuk, A.C.Y.; Yamamoto, K.; Kaeser, B.; Ichikawa, S.; Lee, S.Y. GlcNAc-1-P-transferase-tunicamycin complex structure reveals basis for inhibition of N-glycosylation. Nat. Struct. Mol. Biol. 2018, 25, 217–224. [Google Scholar] [CrossRef]
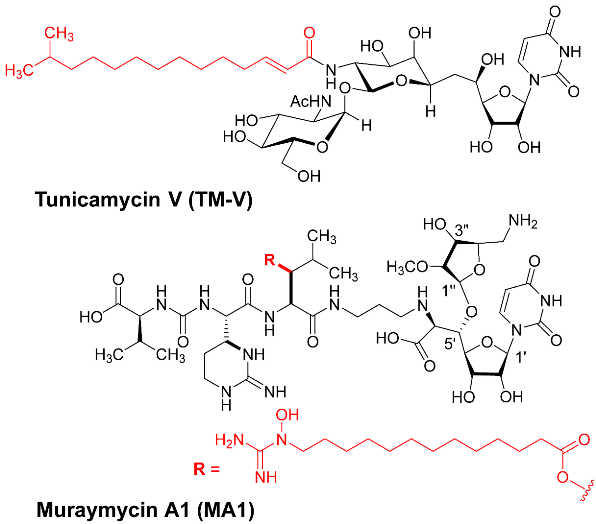
- Mitachi, K.; Mingle, D.; Effah, W.; Sánchez-Ruiz, A.; Hevener, K.E.; Narayanan, R.; Clemons, W.M.; Sarabia, F.; Kurosu, M. Concise synthesis of tunicamycin V and discovery of a cytostatic DPAGT1 inhibitor. Angew. Chem. Int. Ed. 2022, 61, e202203225. [Google Scholar] [CrossRef]
- Li, H.; You, L.; Tian, Y.; Guo, J.; Fang, X.; Zhou, C.; Shi, L.; Su, Y.-Q. DPAGT1-Mediated Protein N-Glycosylation Is Indispensable for Oocyte and Follicle Development in Mice. Adv. Sci. 2020, 7, 2000531. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; He, Z.; Deng, J.; Zhang, Z.; Liu, L.; Ye, W.; Liu, S. Tunicamycin induces ER stress and inhibits tumorigenesis of head and neck cancer cells by inhibiting N-glycosylation. Am. J. Transl. Res. 2020, 12, 541–550. [Google Scholar] [PubMed] [PubMed Central]
- Abhari, B.A.; McCarthy, N.; Le Berre, M.; Kilcoyne, M.; Joshi, L.; Agostinis, P.; Fulda, S. Smac mimetic suppresses tunicamycin-induced apoptosis via resolution of ER stress. Cell Death Dis. 2019, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Chen, S.; Liu, H.; Zhang, Z.; Ni, Z.; Chen, J.; Yang, Z.; Nie, Y.; Fan, D. Tunicamycin specifically aggravates ER stress and overcomes chemoresistance in multidrug-resistant gastric cancer cells by inhibiting N-glycosylation. J. Exp. Clin. Cancer Res. 2018, 37, 272. [Google Scholar] [CrossRef]
- Huang, S.H.; Wang, D.I.; Zhang, S.H.; Huang, X.I.; Wang, D.; Ijaz, M.; Shi, Y. Tunicamycin potentiates paclitaxel-induced apoptosis through inhibition of PI3K/AKT and MAPK pathways in breast cancer. Cancer Chemother. Pharmacol. 2017, 80, 685–696. [Google Scholar] [CrossRef]
- Nami, B.; Donmez, H.; Kocak, N. Tunicamycin-induced endoplasmic reticulum stress reduces in vitro subpopulation and invasion of CD44+/CD24-phenotype breast cancer stem. Exp. Toxicol. Pathol. 2016, 68, 419–426. [Google Scholar] [CrossRef]
- Hou, H.; Sun, H.; Lu, P.; Ge, C.H.; Zhang, L.; Li, H.; Zhao, F.; Tian, H.; Zhang, L.; Chen, T.; et al. Tunicamycin potentiates cisplatin anticancer efficacy through the DPAGT1/Akt/ABCG2 pathway in mouse xenograft models of human hepatocellular carcinoma. Mol. Cancer Ther. 2013, 12, 2874–2884. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, A.; Arima, K.; Tamura, G. Tunicamycin, a new antibiotic. I. Isolation and characterization of tunicamycin. J. Antibiot. 1971, 24, 215–223. [Google Scholar] [CrossRef]
- Takatsuki, A.; Tamura, G. Tunicamycin, a new antibiotic. II. Biological properties of the antiviral activity of tunicamycin. J. Antibiot. 1971, 24, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Takatsuki, A.; Tamura, G. Tunicamycin, a new antibiotic. III. Reversal of the antiviral activity of tunicamycin by aminosugars and their derivatives. J. Antibiot. 1971, 24, 232–238. [Google Scholar] [PubMed]
- Mitachi, K.; Kurosu, M. Development of novel DPAGT1 inhibitors based on tunicamycin V and its homologous structures. J. Synth. Org. Chem. 2023, 81, 220–234. [Google Scholar] [CrossRef]
- Wang, A.; Yan, S.; Liu, J.; Chen, X.; Hu, M.; Du, X.; Jiang, W.; Pan, Z.; Fan, L.; Su, G. Endoplasmic reticulum stress-related super enhancer promotes epithelial-mesenchymal transformation in hepatocellular carcinoma through CREB5 mediated activation of TNC. Cell Death Dis. 2025, 16, 73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.Y.; Du, Z.X.; Liu, B.Q.; Gao, Y.Y.; Meng, X.; Guan, Y.; Deng, W.W.; Wang, H.Q. Tunicamycin enhances TRAIL-induced apoptosis by inhibition of cyclin D1 and the subsequent downregulation of survivin. Exp. Mol. Med. 2009, 41, 362–369. [Google Scholar] [CrossRef]
- Yoshida, T.; Sakai, T. Tunicamycin. In Encyclopedia of Cancer; Schwab, M., Ed.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Lim, E.J.; Heo, J.; Kim, Y.H. Tunicamycin promotes apoptosis in leukemia cells through ROS generation and downregulation of survivin expression. Apoptosis 2015, 20, 1087–1098. [Google Scholar] [CrossRef] [PubMed]
- Bravo-Sagua, R.; López-Crisosto, C.; Parra, V. Rodriguez-Peña, M.; Rothermel, B.A.; Quest, A.F.G.; Lavandero, S. mTORC1 inhibitor rapamycin and ER stressor tunicamycin induce differential patterns of ER-mitochondria coupling. Sci. Rep. 2016, 6, 36394. [Google Scholar] [CrossRef] [PubMed]
- Kishino, A.; Hayashi, K.; Maeda, M.; Jike, T.; Hidai, C.; Nomura, Y.; Oshima, T. Caspase-8 Regulates Endoplasmic Reticulum Stress-Induced Necroptosis Independent of the Apoptosis Pathway in Auditory Cells. Int. J. Mol. Sci. 2019, 20, 5896. [Google Scholar] [CrossRef]
- Nita-Lazar, M.; Noonan, V.; Rebustini, I.; Walker, J.; Menko, A.S.; Kukuruzinska, M.A. Overexpression of DPAGT1 leads to aberrant N-glycosylation of E-cadherin and cellular discohesion in oral cancer. Cancer Res. 2009, 69, 5673–5680. [Google Scholar] [CrossRef]
- The Human Protein Atlas. Available online: https://www.proteinatlas.org/ENSG00000172269-DPAGT1/cancer (accessed on 27 September 2025).
- Suami, T.; Sasai, H.; Matsuno, K.; Suzuki, N.; Fukuda, Y.; Sakanaka, O. Synthetic approach toward antibiotic tunicamycins—VI total synthesis of tunicamycins. Tetrahedron Lett. 1984, 25, 4533–4536. [Google Scholar] [CrossRef]
- Suami, T.; Sasai, H.; Matsuno, K.; Suzuki, N. Total synthesis of tunicamycin. Carbohydr. Res. 1985, 143, 85–96. [Google Scholar] [CrossRef]
- Myers, A.G.; Gin, D.Y.; Rogers, D.H. Synthetic Studies of the Tunicamycin Antibiotics. Preparation of (+)-Tunicaminyluracil, (+)-Tunicamycin-V, and 5′-epi-Tunicamycin-V. J. Am. Chem. Soc. 1994, 116, 4697–4718. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yakushiji, F.; Matsumuru, T.; Ichikawa, S. Total Synthesis of Tunicamycin V. Org. Lett. 2018, 20, 256–259. [Google Scholar] [CrossRef]
- Li, J.; Yu, B. A modular approach to the total synthesis of tunicamycins. Angew. Chem. Int. Ed. 2015, 54, 6618–6621. [Google Scholar] [CrossRef]
- Mitachi, K.; Yun, H.G.; Kurosu, S.M.; Eslamimehr, S.; Lemieux, M.R.; Klaić, L.; Clemons, W.M.; Kurosu, M. Novel FR-900493 analogs that inhibit outgrowth of Clostridium difficile spores. ACS Omega 2018, 3, 1726–1739. [Google Scholar] [CrossRef]
- Mitachi, K.; Kurosu, S.M.; Eslamimehr, S.; Lemieux, M.R.; Ishizaki, Y.; Clemons, W.M.; Kurosu, M. Semi-synthesis of an anticancer DPAGT1 Inhibitor from a muraymycin biosynthetic intermediate. Org. Lett. 2019, 21, 876–879. [Google Scholar] [CrossRef]
- Lin, Y.; Lubman, D.M. The role of N-glycosylation in cancer. Acta Pharm. Sin. B 2024, 14, 1098–1110. [Google Scholar] [CrossRef] [PubMed]
- AlDoughaim, M.; AlSuhebany, N.; AlZahrani, M.; AlQahtani, T.; AlGhamdi, S.; Badreldin, H.; AlAlshaykh, H. Cancer Biomarkers and Precision Oncology: A Review of Recent Trends and Innovations. Clin. Med. Insights Oncol. 2024, 18, 11795549241298541. [Google Scholar] [CrossRef]
- Li, Q.; Xie, Y.; Wong, M.; Barboza, M.; Lebrilla, C.B. Comprehensive structural glycomic characterization of the glycocalyxes of cells and tissues. Nat. Protoc. 2020, 15, 2668–2704. [Google Scholar] [CrossRef]
- Thomas, D.; Rathinavel, A.K.; Radhakrishnan, P. Altered glycosylation in cancer: A promising target for biomarkers and therapeutics. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188464. [Google Scholar] [CrossRef] [PubMed]
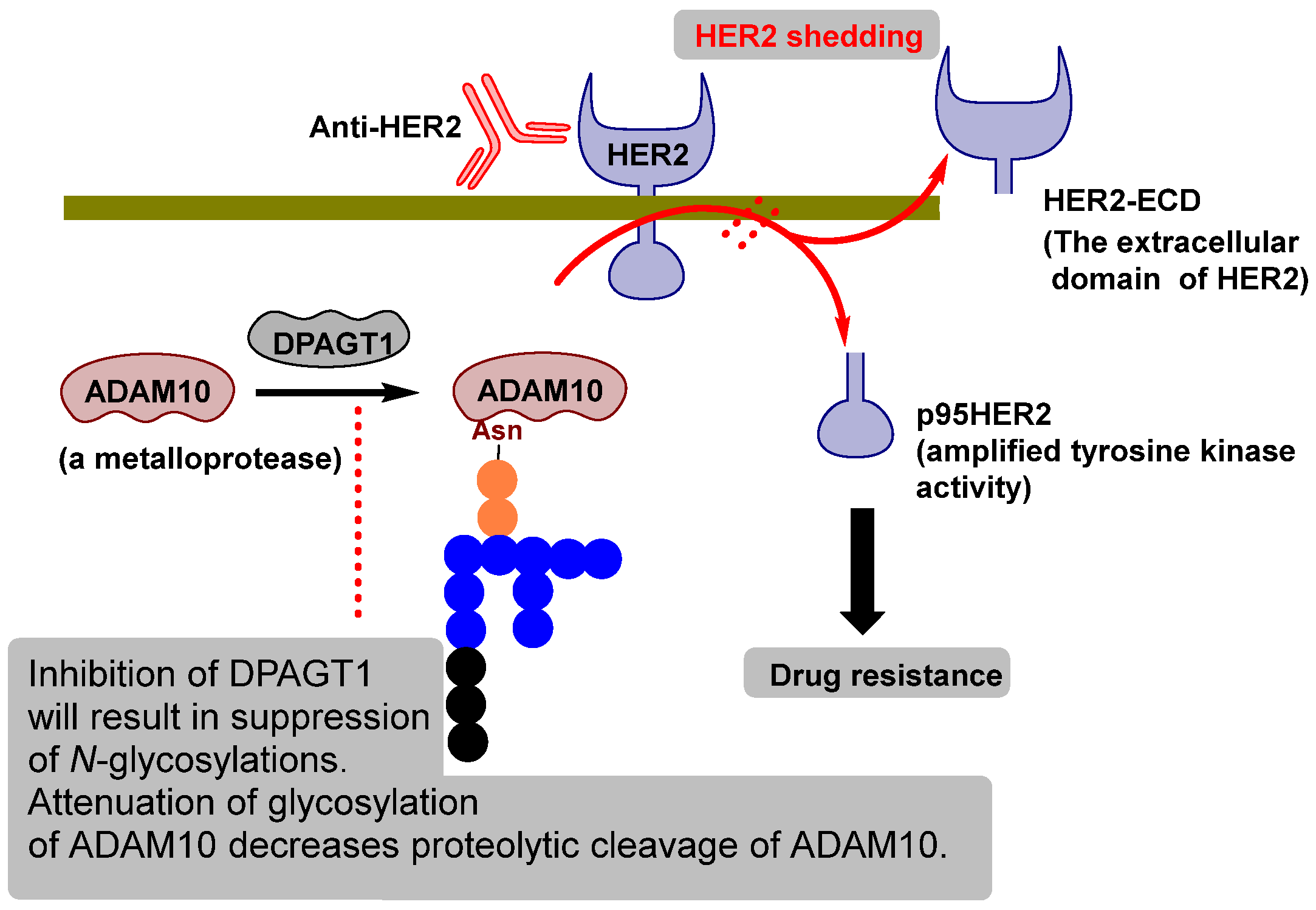
- Yang, M.; Li, Y.; Kong, L.; Huang, S.; He, L.; Liu, P.; Mo, S.; Lu, X.; Lin, X.; Xiao, Y.; et al. Inhibition of DPAGT1 suppresses HER2 shedding and trastuzumab resistance in human breast cancer. J. Clin. Investig. 2023, 133, e164428. [Google Scholar] [CrossRef] [PubMed]
- Patel, B.; Ryan, P.; Makwana, V.; Zunk, M.; Rudrawar, S.; Grant, G. Caprazamycins: Promising lead structures acting on a novel antibacterial target MraY. Eur. J. Med. Chem. 2019, 171, 462. [Google Scholar] [CrossRef]
- Mitachi, K.; Aleiwi, B.A.; Schneider, C.M.; Siricilla, S.; Kurosu, M. Stereocontrolled total synthesis of muraymycin D1 having a dual mode of action against Mycobacterium tuberculosis. J. Am. Chem. Soc. 2016, 138, 12975–12980. [Google Scholar] [CrossRef]
- Ubukata, M.; Kimura, K.; Isono, K.; Nelson, C.C.; Gregson, J.M.; McCloskey, J.A. Structure elucidation of liposidomycins, a class of complex lipid nucleoside antibiotics. J. Org. Chem. 1992, 57, 6392–6403. [Google Scholar] [CrossRef]
- Kurosu, M.; Li, K.; Crick, D.C. Concise Synthesis of Capuramycin. Org. Lett. 2009, 11, 2393–2396. [Google Scholar] [CrossRef]
- Thomson, J.M.; Lamont, I.L. Nucleoside Analogues as Antibacterial Agents. Front. Microbiol. 2019, 22, 952. [Google Scholar] [CrossRef]
- Serpi, M.; Ferrari, V.; Pertusati, F. Nucleoside Derived Antibiotics to Fight Microbial Drug Resistance: New Utilities for an Established Class of Drugs? J. Med. Chem. 2016, 59, 10343–10382. [Google Scholar] [CrossRef]
- Bugg, T.D.H.; Lloyd, A.J.; Roper, D.I. Phospho-MurNAc-pentapeptide translocase (MraY) as a target for antibacterial agents and antibacterial proteins. Infect. Dis. Drug Targets 2006, 6, 85–106. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.A.; Barbieri, L.R.; Carter, G.T.; Lenoy, E.; Lotvin, J.; Petersen, P.J.; Siegel, M.M.; Singh, G.; Williamson, R.T. Structures of the muraymycins, novel peptidoglycan biosynthesis inhibitors. J. Am. Chem. Soc. 2002, 124, 10260–10261. [Google Scholar] [CrossRef] [PubMed]
- McDonald, L.A.; Barbieri, L.R.; Carter, G.T.; Kruppa, G.; Feng, X.; Lotvin, J.A.; Siegel, M.M. FTMS structure elucidation of natural products: Application to muraymycin antibiotics using ESI multi-CHEF SORI-CID FTMSn, the top-down/bottom-up approach, and HPLC ESI capillary-skimmer CID FTMS. Anal. Chem. 2003, 75, 2730–2739. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, X.; Koppermann, S.; Thorson, J.S.; Ducho, C.; Van Lanen, S.G. Antibacterial Muraymycins from Mutant Strains of Streptomyces sp. NRRL 30471. J. Nat. Prod. 2018, 81, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Mitachi, K.; Cheng-Sánchez, I.; Sánchez-Ruiz, A.; Sarabia, F.; Kurosu, M. Total Synthesis of Muraymycin A1: A Protecting Group Strategy for Nucleoside Antibiotic Synthesis. Org. Lett. 2025, 27, 9448–9453. [Google Scholar] [CrossRef]
- Mitachi, K.; Daria, D.; Kirsh, J.M.; Clemons, W.M., Jr.; Kurosu, M. Syntheses and New Insights of Muraymycin A1 and Its Analogs as DPAGT1 Inhibitors. J. Med. Chem. 2025. manuscript submitted for publication. [Google Scholar]
- Moon, H.; Thak, E.J.; Choi, Y.; Kim, S.; Park, J.; Lee, N.; Shin, S.; Jeon, H.; Winarto, J.; Choi, S.; et al. Integrative glycomic analysis reveals the crucial role of protein glycosylation in fungal pathogenesis. PLoS Pathog. 2025, 21, e1013325. [Google Scholar] [CrossRef]
- Hyde, L.F.; Kong, Y.; Zhao, L.; Rao, S.R.; Wang, J.; Stone, L.; Njaa, A.; Collin, G.B.; Krebs, M.P.; Chang, B.; et al. A Dpagt1 Missense Variant Causes Degenerative Retinopathy without Myasthenic Syndrome in Mice. Int. J. Mol. Sci. 2022, 23, 12005. [Google Scholar] [CrossRef] [PubMed]
- Mitachi, K.; Kansal, R.; Hevener, K.; Gillman, C.; Yun, H.-G.; Hussain, M.; Miranda-Carboni, G.; Glazer, E.; Clemons, W.M.; Kurosu, M. DPAGT1 Inhibitors of capuramycin analogues and their antimigratory activities of solid tumors. J. Med. Chem. 2020, 63, 10855–10878. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C.; Zhang, K.; Kaufman, R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020, 21, 421–438. [Google Scholar] [CrossRef]
- Malhotra, J.D.; Kaufman, R.J. The endoplasmic reticulum and the unfolded protein response. Semin. Cell Dev. Biol. 2007, 18, 716–731. [Google Scholar] [CrossRef]
- Seres, M.; Pavlikova, L.; Bohacova, V.; Kyca, T.; Borovska, I.; Lakatos, B.; Breier, A.; Sulova, Z. Overexpression of GRP78/BiP in P-glycoprotein-positive L1210 cells is responsible for altered response of cells to tunicamycin as a stressor of the endoplasmic reticulum. Cells 2020, 9, 890. [Google Scholar] [CrossRef] [PubMed]
- Ettlinger, C.; Schindler, J.; Lehle, L. Cell-cycle arrest of plant suspension cultures by tunicamycin. Planta 1986, 168, 101–105. [Google Scholar] [CrossRef]
- Savage, K.E.; Baur, P.S. Effect of tunicamycin, an inhibitor of protein glycosylation, on division of tumor cells in vitro. J. Cell Sci. 1983, 64, 295–306. [Google Scholar] [CrossRef]
- Nishikawa, Y.; Yamamoto, Y.; Kaji, K.; Mitsui, H. Reversible G1 arrest of a human Burkitt lymphoma cell line(Raji) induced by tunicamycin. Biochem. Biophys. Res. Commun. 1980, 97, 1296–1303. [Google Scholar] [CrossRef]
- Martinez, J.A.; Torres-Negron, I.; Amigo, L.A.; Roldan, R.A.; Mendez, A.; Banerjee, D.K. Tunicamycin inhibits capillary endothelial cell proliferation by inducing apoptosis. Targeting dolichol-pathway for generation of new anti-angiogenic therapeutics. Adv. Exp. Med. Biol. 2000, 476, 197–208. [Google Scholar]
- Jiang, C.C.; Chen, L.H.; Gillespie, S.; Kiejda, K.A.; Mhaidat, N.; Wang, Y.F.; Thorne, R.; Zhang, X.D.; Hersey, P. Tunicamycin sensitizes human melanoma cells to tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by up-regulation of TRAIL-R2 via the unfolded protein response. Cancer Res. 2007, 67, 5880–5888. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, T.; Yoshida, T.; Nakata, S.; Horinaka, M.; Wakada, M.; Mizutani, Y.; Miki, T.; Sakai, T. Tunicamycin Enhances Tumor Necrosis Factor-Related Apoptosis-Inducing Ligand-Induced Apoptosis in Human Prostate Cancer Cells. Cancer Res. 2005, 65, 6364–6370. [Google Scholar] [CrossRef]
- Leaver, D.D.; Schneider, K.M.; Rand, M.J.; Anderson, R.M.; Gage, P.W.; Malbon, R. The neurotoxicity of tunicamycin. Toxicology 1988, 49, 179–187. [Google Scholar] [CrossRef]
- Finnie, J.W. Effect of tunicamycin on hepatocytes in vitro. J. Compar. Pathol. 2001, 125, 318–321. [Google Scholar] [CrossRef]
- Zhao, H.; Sun, L.; Wang, L.; Xu, Z.; Zhou, F.; Su, J.; Jin, J.; Yang, Y.; Hu, Y.; Zha, X. N-glycosylation at Asn residues 554 and 566 of E-cadherin affects cell cycle progression through extracellular signal-regulated protein kinase signaling pathway. Acta Biochim. Biophys. Sin. 2008, 40, 140–148. [Google Scholar] [CrossRef]
- Coker-Gurkan, A.; Can, E.; Sahin, S.; Obakan-Yerlikaya, P.; Arisan, E.-D. Atiprimod triggered apoptotic cell death via acting on PERK/eIF2α/ATF4/CHOP and STAT3/NF- κB axis in MDA-MB-231 and MDA-MB-468 breast cancer cells. Mol. Biol. Rep. 2021, 48, 5233–5247. [Google Scholar] [CrossRef]
- Tian, R.-D.; Chen, Y.-Q.; He, Y.-H.; Tang, Y.-J.; Chen, G.-M.; Yang, F.-W.; Li, Y.; Huang, W.-G.; Chen, H.; Liu, X.; et al. Phosphorylation of eIF2α mitigates endoplasmic reticulum stress and hepatocyte necroptosis in acute liver injury. Ann. Hepatol. 2020, 19, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Qiang, X.; Zechmann, B.; Reitz, M.U.; Kogel, K.-H.; Schaefer, P. The mutualistic fungus Piriformospora indica colonizes Arabidopsis roots by inducing an endoplasmic reticulum stress-triggered caspase-dependent cell death. Plant Cell 2012, 24, 794–809. [Google Scholar] [CrossRef] [PubMed]
- Oliver, B.L.; Cronin, C.G.; Zhang-Benoit, Y.; Goldring, M.B.; Tanzer, M.L. Divergent stress responses to IL-1β, nitric oxide, and tunicamycin by chondrocytes. J. Cell. Physiol. 2005, 204, 45–50. [Google Scholar] [CrossRef]
- Adolph, T.E.; Grabherr, F.; Mayr, L.; Grander, C.; Enrich, B.; Moschen, A.R.; Tilg, H. Weight loss induced by bariatric surgery restricts hepatic GDF15 Expression. J. Obes. 2018, 7108075. [Google Scholar] [CrossRef]
- Wang, C.; Youle, R.J. The role of mitochondria in apoptosis. Annu. Rev. Genet. 2009, 43, 95–118. [Google Scholar] [CrossRef]
- Emaus, K.J.; Fogo, G.M.; Wider, J.M.; Sanderson, T.H. The role of cardiolipin in mitochondrial dynamics and quality control in neuronal ischemia/reperfusion injury. Cell Death Dis. 2025, 16, 494. [Google Scholar] [CrossRef]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease. Mol. Pharmacol. Asp. Cells 2019, 8, 728. [Google Scholar] [CrossRef]
- Gomes, M.T.; Palasiewicz, K.; Gadiyar, V.; Lahey, K.; Calianese, D.; Birge, R.B.; Ucker, D.S. Phosphatidylserine externalization by apoptotic cells is dispensable for specific recognition leading to innate apoptotic immune responses. J. Biol. Chem. 2022, 298, 102034. [Google Scholar] [CrossRef]
- Lee, S.H.; Meng, X.W.; Flatten, K.S.; Loegering, D.A.; Kaufmann, S.H. Phosphatidylserine exposure during apoptosis reflects bidirectional trafficking between plasma membrane and cytoplasm. Cell Death Differ. 2013, 20, 64–76. [Google Scholar] [CrossRef]
- Wang, M.; Wey, S.; Zhang, Y.; Ye, R.; Lee, A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009, 11, 2307–2316. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ni, M.; Lee, B.; Barron, E.; Hinton, D.R.; Lee, A.S. The unfolded protein response regulator GRP78/BiP is required for endoplasmic reticulum integrity and stress-induced autophagy in mammalian cells. Cell Death Differ. 2008, 15, 1460–1471. [Google Scholar] [CrossRef] [PubMed]
- Oyadomari, S.; Mori, M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004, 11, 381–389. [Google Scholar] [CrossRef]
- Engel, T.; Henshall, D.C. Apoptosis, Bcl-2 family proteins and caspases: The ABCs of seizure-damage and epileptogenesis? Int. J. Physiol. Pathophysiol. Pharmacol. 2009, 1, 97–115. [Google Scholar] [PubMed] [PubMed Central]
- Kirsch, D.G.; Doseff, A.; Chau, B.N.; Kastan, M.B.; Lazebnik, Y.A.; Hardwick, J.M. Caspase-3-dependent Cleavage of Bcl-2 Promotes Release of Cytochrome c. J. Biol. Chem. 1999, 274, 21155–21161. [Google Scholar] [CrossRef] [PubMed]
- Hou, H.; Ge, C.; Sun, H.; Li, H.; Li, J.; Tian, H. Tunicamycin inhibits cell proliferation and migration in hepatocellular carcinoma through suppression of CD44s and the ERK1/2 pathway. Cancer Sci. 2018, 109, 1088–1100. [Google Scholar] [CrossRef]
- Vogel, P.; Ellis, Z.L.; Carlin, J.J. Effect of microsomal enzyme inducers and inhibitors on the toxicity of tunicamycin and corynetoxin, the causal agent of annual ryegrass toxicity. Vet. Hum. Toxicol. 1986, 28, 530–532. [Google Scholar] [PubMed]
- Available online: https://bioshopcanada.com/Content/Docs/msds/TUN301.pdf (accessed on 27 September 2025).
- Yang, H.; Khalil, R.A. ADAM and ADAMTS disintegrin and metalloproteinases as major factors and molecular targets in vascular malfunction and disease. Adv. Pharmacol. 2022, 94, 255–363. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, B.N.; Vanderkerken, M.; Hammad, H. The emerging role of ADAM metalloproteinases in immunity. Nat. Rev. Immunol. 2018, 18, 745–758. [Google Scholar] [CrossRef]
- Arora, S.; Scott, A.M.; Janes, P.W. ADAM Proteases in Cancer: Biological Roles, Therapeutic Challenges, and Emerging Opportunities. Cancers 2025, 17, 1703. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.; Nagini, S. ADAM and ADAMTS Family of Metalloproteinases: Role in Cancer Progression and Acquisition of Hallmarks. In Proteases in Human Diseases; Chakraborti, S., Chakraborti, T., Dhalla, N., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Feldinger, K.; Generali, D.; Kramer-Marek, G.; Gijsen, M.; Ng, T.B.; Wong, J.H.; Strina, C.; Cappelletti, M.; Andreis, D.; Li, J.L.; et al. ADAM10 mediates trastuzumab resistance and is correlated with survival in HER2 positive breast cancer. Oncotarget 2014, 5, 6633–6646. [Google Scholar] [CrossRef]
- Sebastian, W.; Lisa, S.; Paul, S. The metalloproteinase ADAM10: A useful therapeutic target? Biochim. Biophys. Acta—Mol. Cell Res. 2017, 1864, 2071–2081. [Google Scholar] [CrossRef]
- Liu, P.C.C.; Liu, X.; Li, Y.; Covington, M.; Wynn, R.; Huber, R.; Burn, T.C. Identification of ADAM10 as a major source of HER2 ectodomain sheddase activity in HER2 overexpressing breast cancer cells. Cancer Biol. Ther. 2006, 5, 657–664. [Google Scholar] [CrossRef]
- Guha, P.; Kaptan, E.; Gade, P.; Kalvakolanu, D.V.; Ahmed, H. Tunicamycin induced endoplasmic reticulum stress promotes apoptosis of prostate cancer cells by activating mTORC1. Oncotarget 2017, 8, 68191–68207. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Li, H.; Huang, S.; Zhang, S.; Wang, F.; Shi, Y. Tunicamycin enhances the antitumor activity of trastuzumab on breast cancer in vitro and in vivo. Oncotarget 2015, 6, 38912–38925. [Google Scholar] [CrossRef]
- Lee, H.H.; Wang, Y.N.; Xia, W.; Chen, C.H.; Rau, K.M.; Ye, L.; Wei, Y.; Chou, C.K.; Wang, S.C.; Yan, M.; et al. Removal of N-Linked Glycosylation Enhances PD-L1 Detection and Predicts Anti-PD-1/PD-L1 Therapeutic Efficacy. Cancer Cell 2019, 36, 168–178.e4. [Google Scholar] [CrossRef] [PubMed]
- You, Z.; He, L.; Yan, N. Tunicamycin-Induced Endoplasmic Reticulum Stress Promotes Breast Cancer Cell MDA-MB-231 Apoptosis through Inhibiting Wnt/β-Catenin Signaling Pathway. J. Healthc. Eng. 2021, 2021, 6394514. [Google Scholar] [CrossRef] [PubMed]
- Raiter, A.; Yerushalmi, R.; Hardy, B. Pharmacological induction of cell surface GRP78 contributes to apoptosis in triple negative breast cancer cells. Oncotarget 2014, 5, 11452–11463. [Google Scholar] [CrossRef]
- Zhong, J.T.; Yu, J.; Wang, H.J.; Shi, Y.; Zhao, T.S.; He, B.X.; Qiao, B.; Feng, Z.W. Effects of endoplasmic reticulum stress on the autophagy, apoptosis, and chemotherapy resistance of human breast cancer cells by regulating the PI3K/AKT/mTOR signaling pathway. Tumor Biol. 2017, 39, 1010428317697562. [Google Scholar] [CrossRef]
- Salatino, M.; Girotti, M.R.; Rabinovich, G.A. Glycans Pave the Way for Immunotherapy in Triple-Negative Breast Cancer. Cancer Cell 2018, 33, 155–157. [Google Scholar] [CrossRef]
- Ahmmed, B.; Khan, M.N.; Nisar, M.A.; Kampo, S.; Zheng, Q.; Li, Y.; Yan, Q. Tunicamycin enhances the suppressive effects of cisplatin on lung cancer growth through PTX3 glycosylation via AKT/NF-κB signaling pathway. Int. J. Oncol. 2019, 54, 431–442. [Google Scholar] [CrossRef]
- Ling, Y.H.; Li, T.; Perez-Soler, R.; Haigentz, M., Jr. Activation of ER stress and inhibition of EGFR N-glycosylation by tunicamycin enhances susceptibility of human non-small cell lung cancer cells to erlotinib. Cancer Chemother. Pharmacol. 2009, 64, 539–548. [Google Scholar] [CrossRef]
- Banerjee, S.; Ansari, A.A.; Upadhyay, S.P.; Mettman, D.J.; Hibdon, J.R.; Quadir, M.; Ghosh, P.; Kambhampati, A.; Banerjee, S.K. Benefits and Pitfalls of a Glycosylation Inhibitor Tunicamycin in the Therapeutic Implication of Cancers. Cells 2024, 13, 395. [Google Scholar] [CrossRef] [PubMed]

 | |||
|---|---|---|---|
| Target | IC50 or MIC | TM-V | MA1 |
| Enzyme | DPAGT1 (IC50) | 1.5 μM | 0.18 μM |
| Fungus | C. albicans 18M (MIC) | <0.24 μg/mL | >35 μg/mL |
| C. neoformans NIH9hi90 (MIC) | 0.24 μg/mL | >35 μg/mL | |
| C. gatti C17 (MIC) | <0.24 μg/mL | >35 μg/mL | |
| A. fumigaus ASFU-2263 (MIC) | 0.96 μg/mL | >35 μg/mL | |
| DPAGT1-high expression cancer cells (Breast cancers) | MDA-MB-231 (IC50) | 0.17 μM (48 h) | 0.98 μM (96 h) |
| MDA-MB-453 (IC50) | 0.16 μM (48 h) | 0.85 μM (96 h) | |
| MDA-MB-468 (IC50) | 0.25 μM (48 h) | 0.85 μM (96 h) | |
| SkBr3 (IC50) | 0.11 μM (48 h) | 0.56 μM (96 h) | |
| BT-474 (IC50) | 0.15 μM (48 h) | 0.85 μM (96 h) | |
| HCC38 (IC50) | 0.35 μM (48 h) | 0.95 μM (96 h) | |
| MCF7 (IC50) | 0.54 μM (48 h) | 0.95 μM (96 h) | |
| DPAGT1-low expression cancer cells | HCT116 (IC50) | 0.50 μM (48 h) | >35 μM (96 h) |
| HT-29 (IC50) | 0.45 μM (48 h) | >35 μM (96 h) | |
| HepG2 (IC50) | 0.98 μM (48 h) | >35 μM (96 h) | |
| Caco-2 (IC50) | 0.55 μM (48 h) | >35 μM (96 h) | |
| HL-60 (IC50) | 0.45 μM (48 h) | >35 μM (96 h) | |
| L1210 (IC50) | 0.35 μM (48 h) | >35 μM (96 h) | |
| Normal (healthy) cells | MCF10A (IC50) | 0.25 μM (48 h) | >35 μM (96 h) |
| HPNE (IC50) | 0.15 μM (48 h) | >35 μM (96 h) | |
| Vero (IC50) | 0.25 μM (48 h) | >35 μM (96 h) | |
| TM-V | MA1 | |
|---|---|---|
| DPAGT1 (IC50) | 1.5 μM | 0.18 μM |
| Cell cycle arrest | G0/G1 | G2/M |
| Cell death | Apoptosis (+) (non-apoptotic cell death processes) | Apoptosis (+++) |
| Cytotoxicity rate | 24−48 h (at 0.10−1.0 μM) | 72−96 h (at 0.50−0.98 μM) |
| Selectivity Index (SI) | 0.5−2.3 | >30 |
| Migration inhibition | + (0.4−1.0 μM) | +++ (0.4−1.0 μM) |
| Cancer Type | Mechanisms of Action(s) | Synergies |
|---|---|---|
| Prostate cancer [94] |
| Docetaxel |
| Breast cancer (Her2+) [41,95,96] |
| Anti-Her2 |
| Breast cancer (TNBC) [97,98,99,100] |
| Doxorubcin Anti-Her2 |
| Lung (NSCLC) and Liver cancer [101,102] |
| Erlotinib Cisplatin |
| Pancreatic cancer [103] |
| Paclitaxel Gemcitabine 5-FU |
| Pancreatic cancer [56] |
| Paclitaxel |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurosu, M.; Mitachi, K. DPAGT1—Perspective as an Anticancer Drug Target. Molecules 2025, 30, 4049. https://doi.org/10.3390/molecules30204049
Kurosu M, Mitachi K. DPAGT1—Perspective as an Anticancer Drug Target. Molecules. 2025; 30(20):4049. https://doi.org/10.3390/molecules30204049
Chicago/Turabian StyleKurosu, Michio, and Katsuhiko Mitachi. 2025. "DPAGT1—Perspective as an Anticancer Drug Target" Molecules 30, no. 20: 4049. https://doi.org/10.3390/molecules30204049
APA StyleKurosu, M., & Mitachi, K. (2025). DPAGT1—Perspective as an Anticancer Drug Target. Molecules, 30(20), 4049. https://doi.org/10.3390/molecules30204049








