Iron-Doped Molybdenum Sulfide Nanoflowers on Graphene for High-Performance Supercapacitors
Abstract
1. Introduction
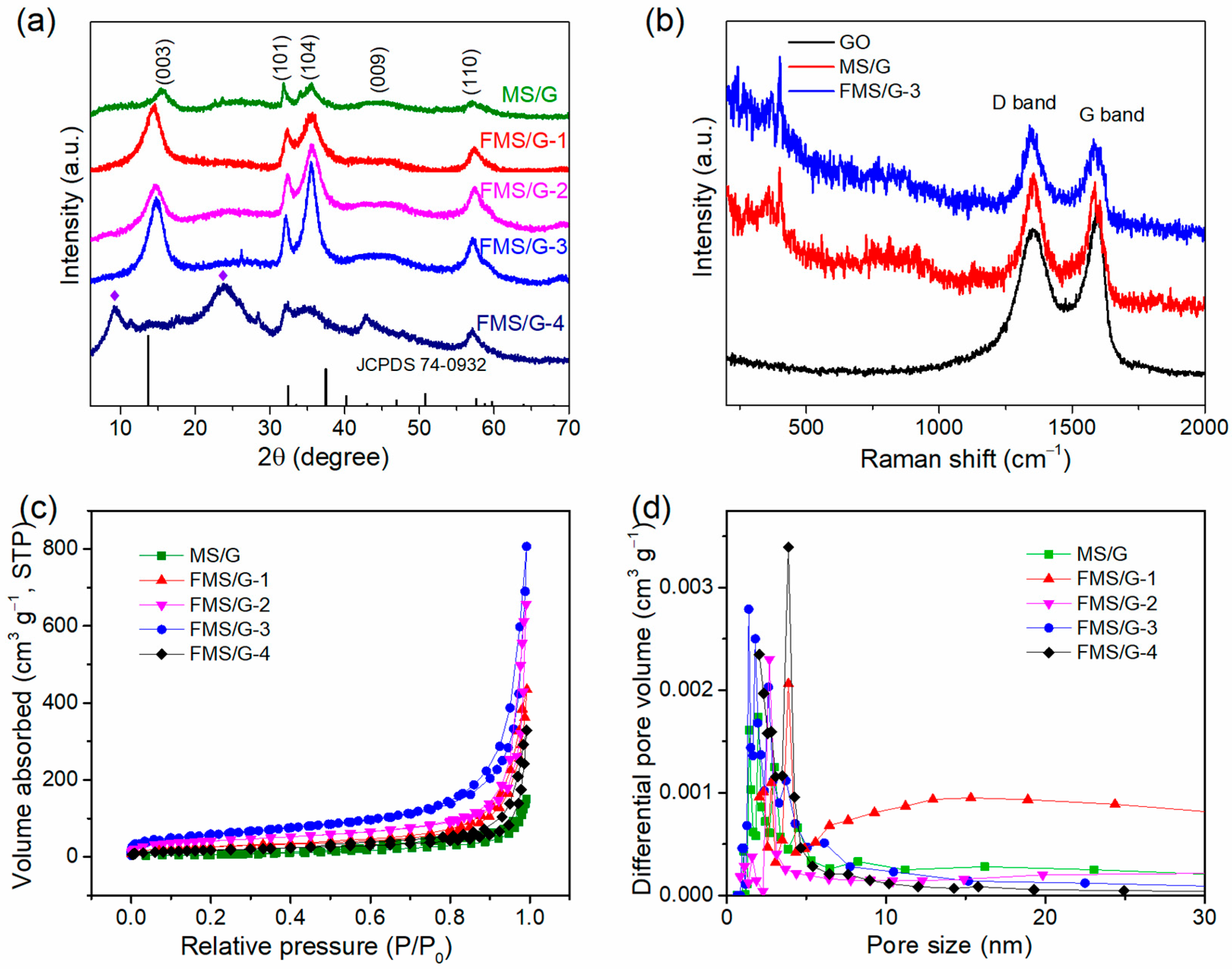
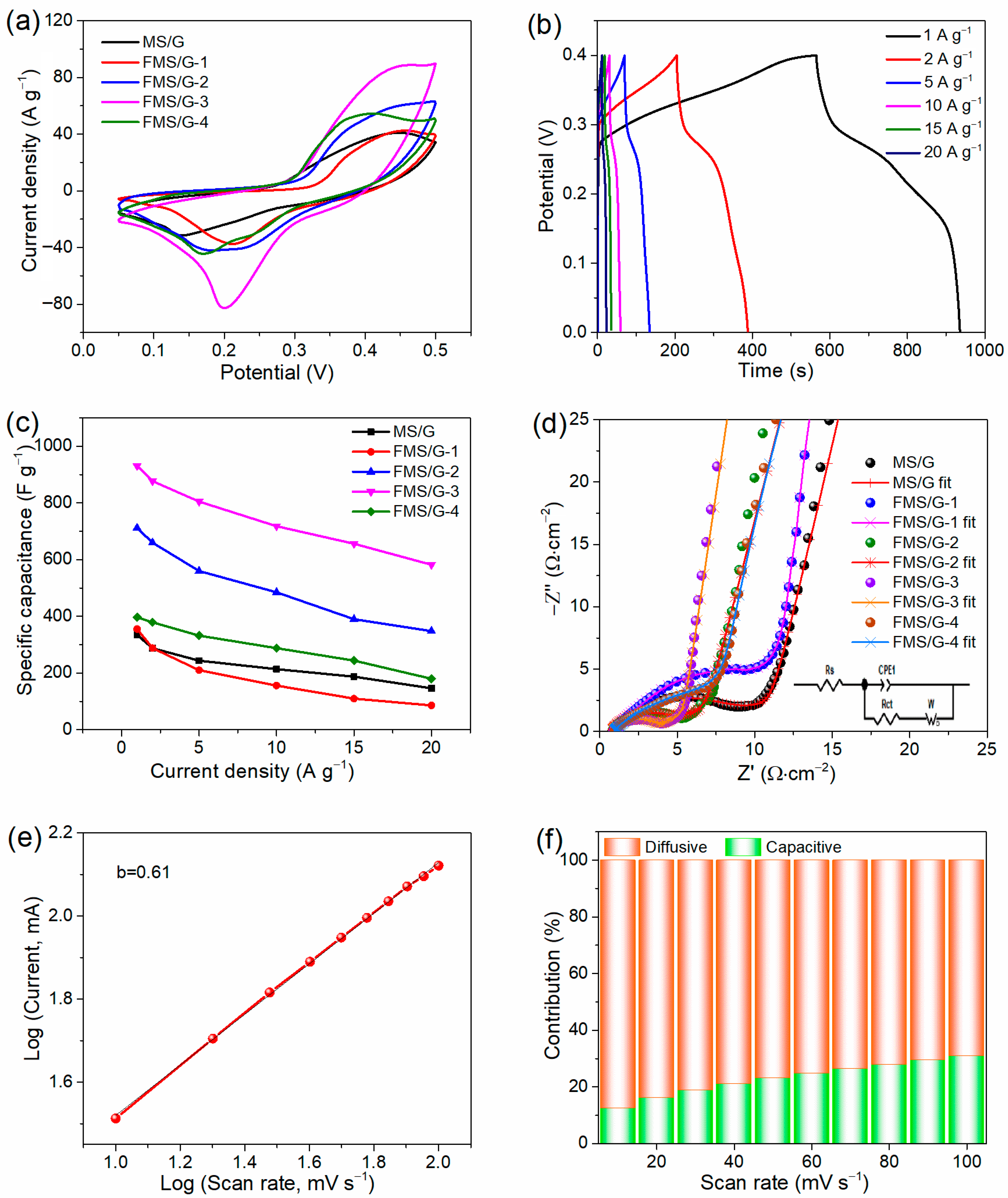
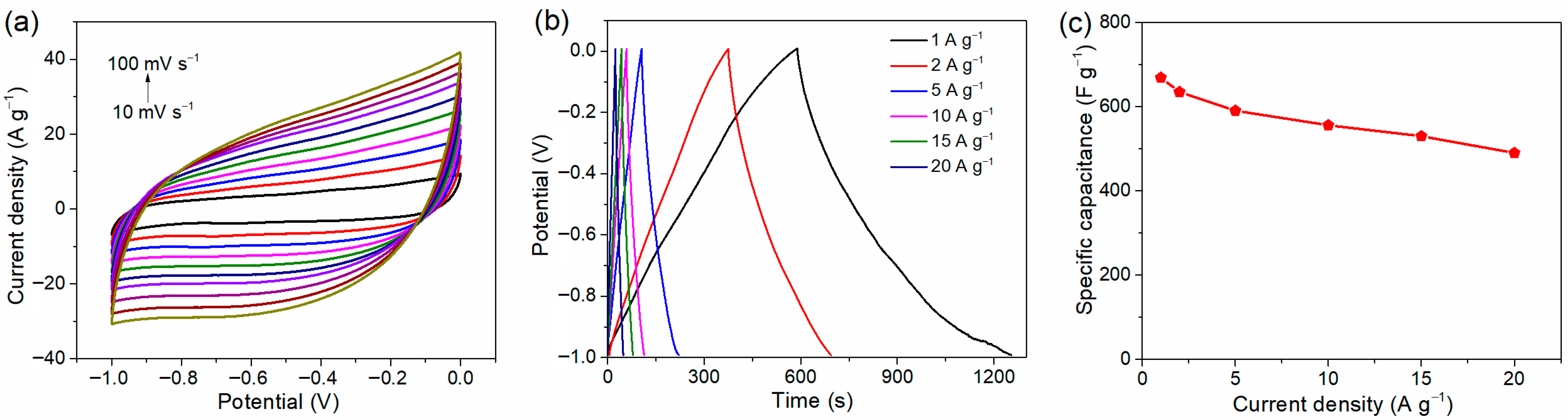
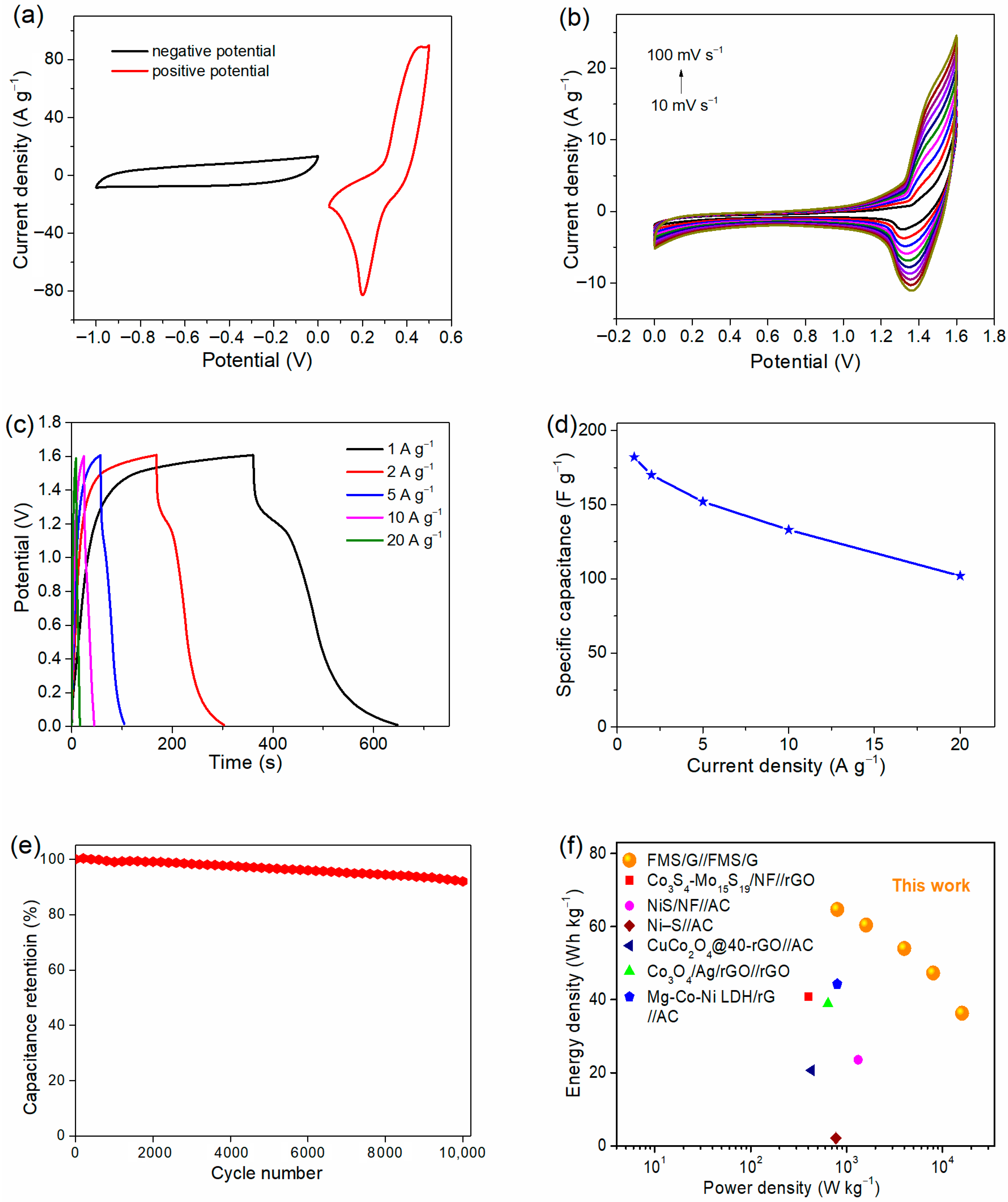
2. Results and Discussion
3. Experimental
3.1. Materials
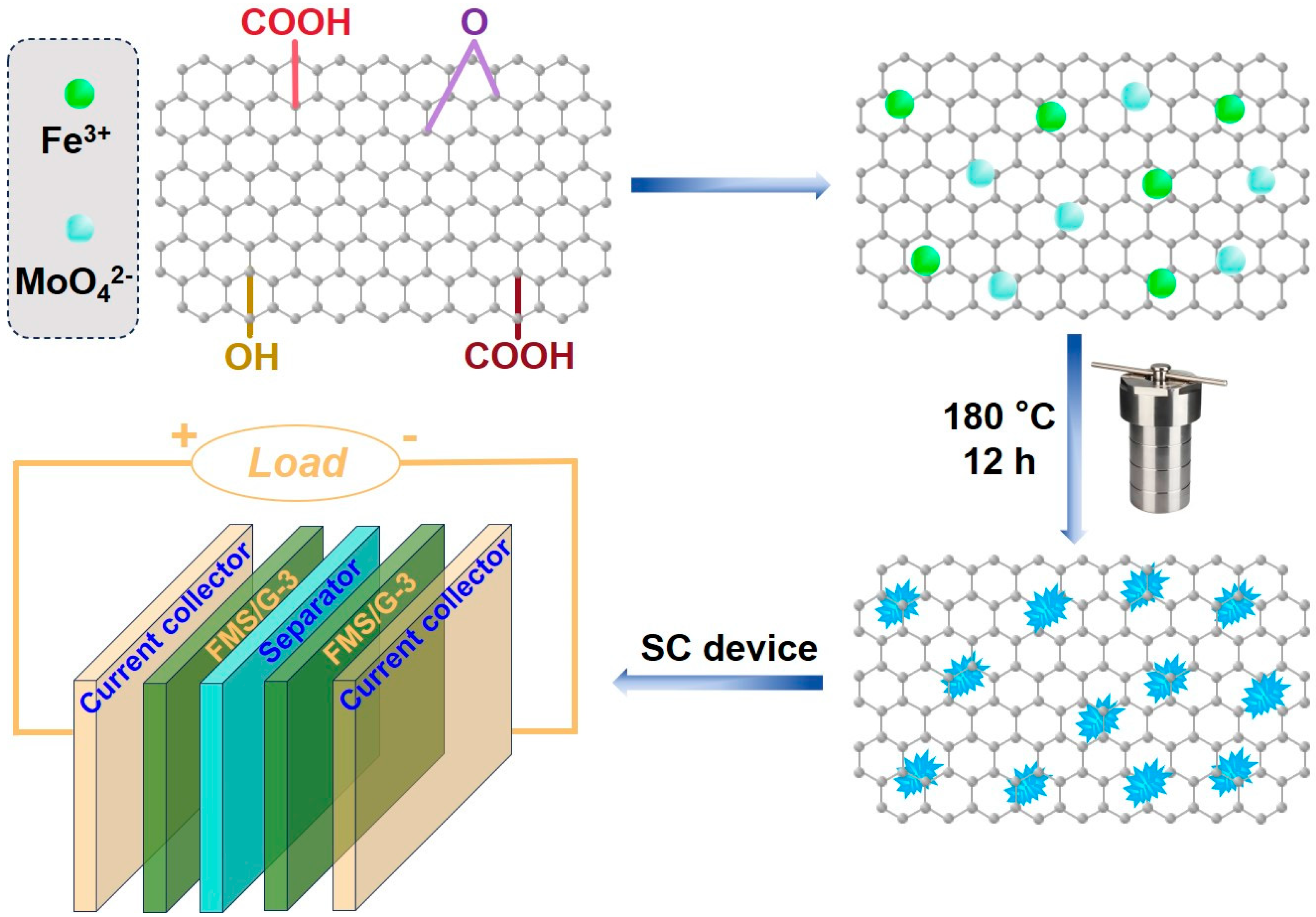
3.2. Synthesis of FMS/G Composite
3.3. Material Characterization
3.4. Electrochemical Analysis
3.5. Fabrication of the Supercapacitor Device
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banerjee, S.; Mordina, B.; Sinha, P.; Kar, K.K. Recent advancement of supercapacitors: A current era of supercapacitor devices through the development of electrical double layer, pseudo and their hybrid supercapacitor electrodes. J. Energy Storage 2024, 108, 115075. [Google Scholar] [CrossRef]
- Shuja, A.; Khan, H.R.; Murtaza, I.; Ashraf, S.; Abid, Y.; Farid, F.; Sajid, F. Supercapacitors for energy storage applications: Materials, devices and future directions: A comprehensive review. J. Alloys Compd. 2024, 1009, 176924. [Google Scholar] [CrossRef]
- Saghafizadeh, M.A.; Zardkhoshoui, A.M.; Davarani, S.S.H. Hybrid supercapacitors with MnTe@ZnMnTe hollow octahedrons encapsulated with reduced graphene oxide for high-performance hybrid supercapacitors. Chem. Eng. J. 2025, 508, 160817. [Google Scholar] [CrossRef]
- Jayakumar, S.; Chinnappan Santhosh, P.; Ramakrishna, S.; Radhamani, A.V. 2D (Ti3C2Tx) MXene: A comprehensive review of advancements in synthesis protocols, applications in supercapacitors, sustainability targets and future prospects. J. Energy Storage 2024, 97, 112741. [Google Scholar] [CrossRef]
- Li, W.; Zhang, W.; Xu, Y.; Wang, G.; Sui, W.; Xu, T.; Yuan, Z.; Si, C. Heteroatom-doped lignin derived carbon materials with improved electrochemical performance for advanced supercapacitors. Chem. Eng. J. 2024, 497, 154829. [Google Scholar] [CrossRef]
- Saha, P.; Nath, N.C.D.; Islam, M.M.; Abdul Aziz, M.; Saleh Ahammad, A.J. Recent progress of high-energy density supercapacitors based on nanostructured nickel oxides. Electrochim. Acta 2024, 504, 144892. [Google Scholar] [CrossRef]
- Zhang, A.; Zhang, Q.; Huang, J.; Fu, H.; Zong, H.; Guo, H. NiMnCo-LDH in-situ derived from ZIF-67@ZnO as self-supporting electrode for asymmetric supercapacitor device. Chem. Eng. J. 2024, 487, 150587. [Google Scholar] [CrossRef]
- Chen, W.; Yan, L.; Song, Z.; Li, X. NiMn2O4/CoS nanostructure electrode material for flexible asymmetric supercapacitors. Electrochim. Acta 2024, 491, 144329. [Google Scholar] [CrossRef]
- Chen, Z.; Xue, R.; Fan, B.; Wang, Y.; Tian, W.; Pei, L.; Jin, Y.; Guo, Z.; Sun, Z.; Ren, F.; et al. Bimetallic nickel-cobalt sulfide grown on graphene foam for high-performance asymmetric supercapacitor. J. Alloys Compd. 2024, 1007, 176483. [Google Scholar] [CrossRef]
- Wesley, R.J.; Vasanth, S.; Durairaj, A.; Justinabraham, R.; Viswanathan, C.; Obadiah, A.; Vasanthkumar, S. Nickel-molybdenum bimetallic sulfide decorated biomass derived carbon support for high performance asymmetric supercapacitor application. J. Energy Storage 2024, 91, 112092. [Google Scholar] [CrossRef]
- Su, Q.; Wang, S.; Feng, M.; Du, G.; Xu, B. Direct studies on the lithium-storage mechanism of molybdenum disulfide. Sci. Rep. 2017, 7, 7275. [Google Scholar] [CrossRef]
- Veeramalai, C.P.; Li, F.; Liu, Y.; Xu, Z.; Guo, T.; Kim, T.W. Enhanced field emission properties of molybdenum disulphide few layer nanosheets synthesized by hydrothermal method. Appl. Surf. Sci. 2016, 389, 1017–1022. [Google Scholar] [CrossRef]
- Tsyganov, A.; Vikulova, M.; Zotov, I.; Korotaev, E.; Plugin, I.; Sysoev, V.; Kirilenko, D.; Rabchinskii, M.; Asoyan, A.; Gorokhovsky, A.; et al. Application of W1.33CTz MXenes obtained by hydrothermal etching as an additive to enhance the electrochemical energy storage properties of binder-free Ti3C2Tx MXene films. Dalton Trans. 2025, 54, 8547–8558. [Google Scholar] [CrossRef]
- Yang, L.; Mukhopadhyay, A.; Jiao, Y.; Hamel, J.; Benamara, M.; Xing, Y.; Zhu, H. Aligned and stable metallic MoS2 on plasma-treated mass transfer channels for the hydrogen evolution reaction. J. Mater. Chem A 2017, 5, 25359–25367. [Google Scholar] [CrossRef]
- Wang, Y.; Zhen, M.; Liu, H.; Wang, C. Interlayer-expanded MoS2 /graphene composites as anode materials for high-performance lithium-ion batteries. J. Solid. State. Electr. 2018, 22, 3069–3076. [Google Scholar] [CrossRef]
- Liu, C.; Bai, Y.; Zhao, Y.; Yao, H.; Pang, H. MoS2/graphene composites: Fabrication and electrochemical energy storage. Energy Storage Mater. 2020, 33, 470–502. [Google Scholar] [CrossRef]
- Rahman, R.; Samanta, D.; Pathak, A.; Nath, T.K. Tuning of structural and optical properties with enhanced catalytic activity in chemically synthesized Co-doped MoS2 nanosheets. RSC Adv. 2021, 11, 1303. [Google Scholar] [CrossRef]
- Charapale, M.R.; Shembade, U.V.; Ahir, S.A.; Kothavale, V.P.; Jadhav, N.T.; Sankpal, V.G.; Waifalkarf, P.P.; Moholkar, A.V.; Dongale, T.D.; Masti, S.A. Enhancing capacitive performance of MoS2 through Fe doping: Synthesis, characterization, and electrochemical evaluation for supercapacitor applications. Surf. Interfaces 2024, 52, 104814. [Google Scholar] [CrossRef]
- Guo, M.; Liu, X.; Du, J.; Cao, Y.; Li, X.; Zhang, Y. Synthesis, analysis and characterization of Mo-doped Fe3O4 nanoparticles decorated on rGO as an anode for high-performance supercapacitors. J. Mater. Sci. Mater. Electron. 2024, 35, 1496. [Google Scholar] [CrossRef]
- Guo, M.; Du, J.; Liu, X.; Cao, Y.; Li, X.; Wu, K.; Li, Z. Cobalt-doped vanadium sulfide nanorods anchored on graphene for high-performance supercapacitors. ACS Appl. Nano Mater. 2024, 7, 15469–15477. [Google Scholar] [CrossRef]
- Kumbhar, M.B.; Patil, V.V.; Chandak, V.S.; Shaikh, S.B.; Chitare, Y.M.; Gunjakar, J.L.; Kulal, P.M. Exploring copper-doped nickel oxide as a superior cathode electrode material for flexible hybrid solid-state supercapacitor device. J. Ind. Eng. Chem. 2024, 147, 422–435. [Google Scholar] [CrossRef]
- Jimoh, M.F.; Carson, G.S.; Anderson, M.B.; El-Kady, M.F.; Kaner, R.B. Direct fabrication of 3D electrodes based on graphene and conducting polymers for supercapacitor applications. Adv. Funct. Mater. 2025, 35, 2405569. [Google Scholar] [CrossRef]
- Ryu, C.; Do, H.M.; In, J.B. Enhanced performance of densified laser-induced graphene supercapacitor electrodes in dimpled polyimide. Appl. Surf. Sci. 2024, 643, 158696. [Google Scholar] [CrossRef]
- Dighe, P.S.; Redekar, R.S.; Tarwal, N.L.; Sarawade, P.B. Unveiling the performance of nickel cobalt-layered double hydroxide/reduced graphene oxide composite for high performance aqueous supercapacitor. J. Power Sources 2025, 634, 236474. [Google Scholar] [CrossRef]
- Xu, X.; Liu, W.; Chen, Y.; Wang, S.; Wang, X.; Jiang, H.; Ma, S.; Yuan, F.; Zhang, Q. n-n heterogeneous MoS2/SnO2 nanotubes and the excellent triethylamine (TEA) sensing performances. Mater. Lett. 2022, 311, 131522. [Google Scholar] [CrossRef]
- Umar, A.; Ahmed, F.; Ullah, N.; Ansari, S.A.; Hussain, S.; Ibrahim, A.A.; Qasem, H.; Kumar, S.A.; Alhamami, M.A.; Almehbad, N.; et al. Exploring the potential of reduced graphene oxide/polyaniline (rGO@PANI) nanocomposites for high-performance supercapacitor application. Electrochim. Acta 2024, 479, 143743. [Google Scholar] [CrossRef]
- Jiang, J.; Zhou, W.; Li, W.; Huang, Z.; Zhang, M.; Jin, J.; Xie, J. Construction of electron-interactive CoMoO4@CoP core–shell structure on boron-doped graphene aerogel as strongly interface coupled hybrid electrodes for high flexible supercapacitor. Chem. Eng. J. 2024, 496, 154123. [Google Scholar] [CrossRef]
- Zhao, Y.; Hu, H.; Wang, F.; Yan, Y.; Ye, K.; Zeng, W.; Tang, K.; Huang, A.; Cai, S.; Lan, L.; et al. Dual-basic carbonate template-assisted construction of graphene-like porous carbon nanosheets from waste biomass for enhanced supercapacitor performance. J. Power Sources 2024, 629, 236016. [Google Scholar] [CrossRef]
- Najafi, M.; Ehsani, A.; Nabatian, M.; Hamza, Z.; Neekzad, N. Advanced supercapacitor electrodes: Synthesis and electrochemical characterization of graphene oxide-bismuth metal-organic framework composites for superior performance. Electrochim. Acta 2024, 498, 144636. [Google Scholar] [CrossRef]
- Li, Q.; Wei, Q.; Zuo, W.; Huang, L.; Luo, W.; An, Q.; Pelenovich, V.O.; Mai, L.; Zhang, Q. Greigite Fe3S4 as a new anode material for high-performance sodium-ion batteries. Chem. Sci. 2017, 8, 160–164. [Google Scholar] [CrossRef]
- Ravichandran, V.; Nardekar, S.S.; Kesavan, D.; Das, J.P.; Elumalai, V.; Kim, S.-J. High-performance redox-active organic molecule grafted graphene based on-chip micro-supercapacitor towards self-powered environmental monitoring station. Chem. Eng. J. 2024, 482, 148822. [Google Scholar] [CrossRef]
- Cheng, X.; Tian, X.; Liao, S.; Wang, Q.; Wei, Q. Wet spinning for high-performance fiber supercapacitor based on Fe-doped MnO2 and graphene. Carbon 2024, 230, 119572. [Google Scholar] [CrossRef]
- Guo, X.; Hou, Y.; Ren, R.; Chen, J. Temperature-dependent crystallization of MoS2 nanoflakes on graphene nanosheets for electrocatalysis. Nanoscale. Res. Lett. 2017, 12, 479. [Google Scholar] [CrossRef]
- Zhang, J.; Xie, Y.-L. Hierarchical porous hollow of carbon spheres with high surface area for high performance supercapacitor electrode materials. Sci. Rep. 2025, 15, 15125. [Google Scholar] [CrossRef]
- Li, P.; Xuan, Y.; Jiang, B.; S Zhang, H.; Xia, C. Hollow La0.6Sr0.4Ni0.2Fe0.75Mo0.05O3-δ electrodes with exsolved FeNi3 in quasi-symmetrical solid oxide electrolysis cells for direct CO2 electrolysis. Electrochem. Commun. 2021, 134, 107188. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, B.; Chen, Y. Iron phosphides supported on three-dimensional iron foam as an efficient electrocatalyst for water splitting reactions. J. Mater. Sci. 2019, 54, 1–12. [Google Scholar] [CrossRef]
- Li, B.; Jiang, L.; Li, X.; Ran, P.; Zuo, P.; Wang, A.; Qu, L.; Zhao, Y.; Cheng, Z.; Lu, Y. Preparation of monolayer MoS2 quantum dots using temporally shaped femtosecond laser ablation of bulk MoS2 targets in water. Sci. Rep. 2025, 7, 11182. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Du, J.; Liu, X.; Liu, W.; Zhao, M.; Wang, J.; Li, X. Rational fabrication of nickel vanadium sulfide encapsulated on graphene as an advanced electrode for high-performance supercapacitors. Molecules 2024, 29, 3642. [Google Scholar] [CrossRef] [PubMed]
- Johra, F.T.; Jung, W.-G. Hydrothermally reduced graphene oxide as a supercapacitor. Appl. Surf. Sci. 2015, 357, 1911–1914. [Google Scholar] [CrossRef]
- Sekhar, M.C.; Reddy, B.P.; Kuchi, C.; Basha, C.K.; Al-Zahrani, F.A.M.; Mangiri, R. Enhanced solar-driven photocatalytic hydrogen production, dye degradation, and supercapacitor functionality using MoS2–TiO2 nanocomposite. Ceram. Int. 2024, 50, 38679–38687. [Google Scholar] [CrossRef]
- Salagean, C.A.; Cotet, L.C.; Baia, M.; Fort, C.I.; Turdean, G.L.; Barbu-Tudoran, L.; Lazar, M.D.; Baia, L. Influence of precursors on physical characteristics of MoS2 and their correlation with potential electrochemical applications. Materials 2025, 18, 2111. [Google Scholar] [CrossRef]
- Lakshmi-Narayana, A.; Attarzadeh, N.; Shutthanandan, V.; Ramana, C.V. High-performance NiCo2O4/graphene quantum dots for asymmetric and symmetric supercapacitors with enhanced energy efficiency. Adv. Funct. Mater. 2024, 34, 2316379. [Google Scholar] [CrossRef]
- Nayak, S.; Kittur, A.A.; Nayak, S.; Murgunde, B. Binderless nano marigold flower like structure of nickel sulfide electrode for sustainable supercapacitor energy storage applications. J. Energy Storage 2023, 62, 106963. [Google Scholar] [CrossRef]
- Moradi, M.; Zolfaghari, S.; Pooriraj, M.; Babamoradi, M.; Hajati, S. One-step synthesis of Zn-doped nickel sulfide/graphene derived from Ni-MOF for supercapacitor application. Mater. Chem. Phys. 2025, 329, 130068. [Google Scholar] [CrossRef]
- Vinothkumar, V.; Naveenkumar, P.; Oh, D.E.; Maniyazagan, M.; Yang, H.W.; Bong, S.; Kim, S.J.; Kim, T.H. Nickel-mixed chromium sulfide nanoparticle synthesis, characterization, and supercapacitor applications. Vacuum 2024, 225, 113234. [Google Scholar] [CrossRef]
- Al-Abawi, B.T.; Parveen, N.; Ansari, S.A. Controllable synthesis of sphere-shaped interconnected interlinked binder-free nickel sulfide@nickel foam for high-performance supercapacitor applications. Sci. Rep. 2022, 12, 14413. [Google Scholar] [CrossRef] [PubMed]
- Balu, R.; Panneerselvam, A.; Rajabathar, J.R.; Devendrapandi, G.; Subburaj, S.; Anand, S.; Veerasamy, U.S.; Palani, S. Synergistic effect of Echinops flower-like Copper sulfide@Cadmium sulfide heterostructure for high-performance all-solid-state. J. Energy Storage 2023, 72, 108447. [Google Scholar] [CrossRef]
- Kuo, T.R.; Lin, K.H.; Chen, M.W.; Yougbare, S.; Lin, L.Y.; Wu, Y.F. Tailing copper cobalt sulfide particle-decorated tube-like structure as efficient active material of battery supercapacitor hybrid. J. Energy Storage 2023, 67, 107564. [Google Scholar] [CrossRef]
- Hussain, S.; Vikraman, D.; Sarfraz, M.; Faizan, M.; Patil, S.A.; Batoo, K.M.; Nam, K.W.; Kim, H.S.; Jung, J. Design of XS2 (X = W or Mo)-decorated VS2 hybrid nano-architectures with abundant active edge sites for high-rate asymmetric supercapacitors and hydrogen evolution reactions. Small 2023, 19, 2205881. [Google Scholar] [CrossRef]
- Mohamed, S.G.; Morad, M.M.; Siddiqui, M.R.; Mahmud, A.A.; Zoubi, W.A. Improving the electrochemical performance of zinc sulfides by iron doping toward supercapacitor applications. Adv. Mater. Interfaces 2024, 11, 2300790. [Google Scholar] [CrossRef]
- Azimov, F.; Lee, J.; Park, S.; Jung, H.M. Fabrication of assembled FeS2 nanosheet and application for high-performance supercapacitor electrodes. ACS Appl. Mater. Interfaces 2023, 15, 26967–26976. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.Y.; Liu, L.Y.; He, J.H.; Zhou, Y.; Wu, S.B.; Zheng, M.X.; Demir, M.; Ma, P.P. Preparation and electrochemical properties of bimetallic carbide Fe3Mo3C/Mo2C@carbon nanotubes as negative electrode material for supercapacitor. J. Energy Storage 2023, 72, 108656. [Google Scholar] [CrossRef]
- Wu, S.; Hu, R.; Zhou, Y.; He, J.; Demir, M.; Ma, P. Phase modulation and electrochemical behavior of Fe3Mo3C/Mo2C composite nanofibers as supercapacitor negative electrodes. ACS Appl. Energy Mater. 2024, 7, 9827–9838. [Google Scholar] [CrossRef]
- Jabeen, N.; Hussain, A.; Elsaeedy, H.I.; Rahman, A.U.; Tarique, M. Unique hierarchical architecture of SnO2 hexagonal interconnected nanolayered arrays as negative electrode for high performance asymmetric supercapacitors. Mater. Chem. Phys. 2023, 303, 127796. [Google Scholar] [CrossRef]
- Jabeen, N.; Hassan, N.U.; Bokhari, A.; Khan, M.F.; Eldin, S.M.; Arifeen, W.U.; Hussain, A.; Bahajjaj, A.A.A. High performance δ-Bi2O3 nanosheets transformed Bi2S3 nanoflakes interconnected nanosheets as negative electrode for supercapacitor applications. Fuel 2023, 347, 128392. [Google Scholar] [CrossRef]
- Zhu, W.; Yan, X.; Huang, X.; Wu, S.; Chen, H.; Pan, J.; Li, T.; Shahnavaz, Z. Three-dimensional carbon-based endogenous-exogenous MoO2 composites as high-performance negative electrode in asymmetric supercapacitors and efficient electrocatalyst for oxygen evolution reaction. Ceram. Int. 2023, 49, 5646–5656.s. [Google Scholar] [CrossRef]
- Wang, Y.; Lu, W.; Wang, L.; Li, Y.; Wu, H.; Zhu, X.; Zhang, C.; Wang, K. Vanadate-based Fe-MOFs as promising negative electrode for hybrid supercapacitor device. Nanotechnology 2024, 35, 205402. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, F.; Jing, R.; Gu, S.; Zhang, Q.; Li, Z.; Zhu, Y.; Xiao, Z.; Wang, L. The precise building units modulation of iron-bismuth sulfide triple-level hierarchical structure for enhanced supercapacitor performance. J. Power Sources 2024, 597, 234128. [Google Scholar] [CrossRef]
- Saha, S.; Kandasamy, M.; Potphode, D.; Sharma, C.S.; Chakraborty, B.; Hameed, N.; Salim, N. Zeolitic imidazolate framework derived stellate shaped cobalt-molybdenum hybrid sulfide microflower for enhanced supercapacitor properties. J. Energy Storage 2024, 99, 113294. [Google Scholar] [CrossRef]
- Semerci, F.; Egin, H. Step-wise synthesis of mesoporous CuCo2O4@reduced graphene oxide composites for supercapacitor applications. Electroanal. Chem. 2024, 970, 118550. [Google Scholar] [CrossRef]
- Kalia, S.; Choudhary, D.; Shrivastav, M.; Bala, R.; Singh, R.K.; Khan, M.S.; Dhiman, R. Synergistic effects of Ag nanoparticles in the rGO and Co3O4 based electrode materials for asymmetric supercapacitors. Electrochim. Acta 2024, 491, 144337. [Google Scholar] [CrossRef]
- Yao, Y.; Yu, Y.; Wan, L.; Du, C.; Zhang, Y.; Chen, J.; Xie, M. Structurally-stable Mg-Co-Ni LDH grown on reduced graphene by ball-milling and ion-exchange for highly-stable asymmetric supercapacitor. J. Colloid Interface Sci. 2023, 649, 519–527. [Google Scholar] [CrossRef] [PubMed]

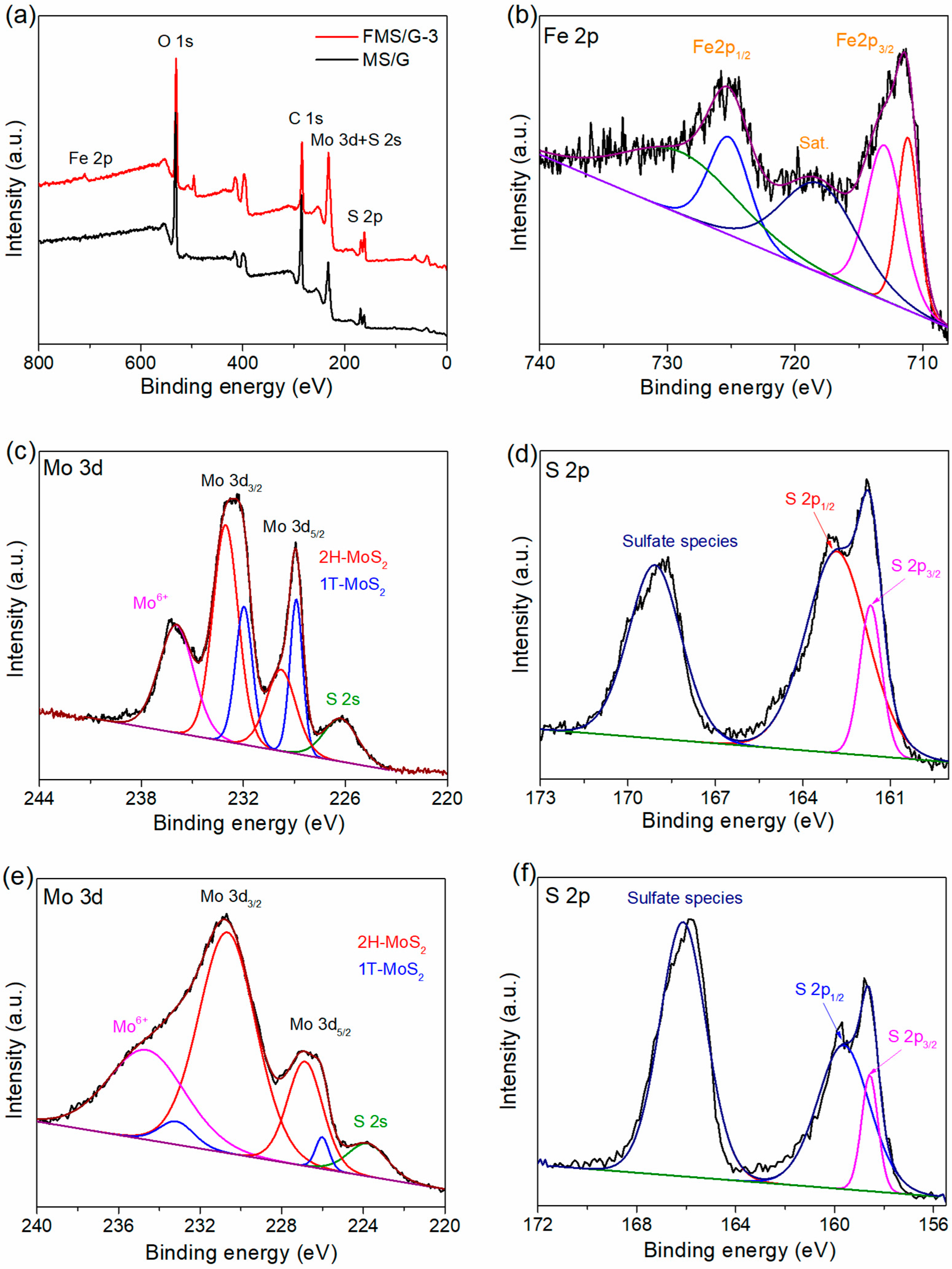
| Samples | Mo 3d3/2 (eV) | Mo 3d5/2 (eV) | ||
|---|---|---|---|---|
| 2H | 1T | 2H | 1T | |
| MS/G | 233.05 | 230.7 | 226.8 | 226.0 |
| FMS/G-1 | 232.7 | 231.4 | 229.2 | 228.2 |
| FMS/G-2 | 232.9 | 231.7 | 229.8 | 228.6 |
| FMS/G-3 | 233.1 | 231.9 | 229.8 | 228.8 |
| FMS/G-4 | 233.5 | 232.2 | 230.4 | |
| Materials | Specific Capacitance | Electrolyte | Stability | Ref. |
|---|---|---|---|---|
| NiS | 613 F g−1 at 1.1 A g−1 | 3 M KOH | 97% (5000 cycles) | [43] |
| Zn-NiS/G | 442.66 F g−1 at 1 A g−1 | 6 M KOH | - | [44] |
| Ni-Cr2S3 | 187.53 F g−1 at 0.5 A g−1 | 3 M KOH | 93.31% (5000 cycles) | [45] |
| SS-NiS@3DNF | 470 F g−1 at 1 A g−1 | 2 M KOH | 88% (6700 cycles) | [46] |
| CuS/CdS | 543.6 F g−1 at 1 A g−1 | 3 M KOH | 89.06% (10,000 cycles) | [47] |
| CuCo2S4 | 831.7 F g−1 at 20 mV s−1 | 3 M KOH | 78.8% (10,000 cycles) | [48] |
| WS2@VS2 | 615 F g−1 at 2.5 A g−1 | 1 M KOH | 98.85% (5000 cycles) | [49] |
| Zn0.7Fe0.3S | 700 F g−1 at 0.5 A g−1 | 6 M KOH | 80% (2000 cycles) | [50] |
| FMS/G | 931 F·g−1 at 1 A·g−1 | 3 M KOH | 90.5% (10,000 cycles) | This work |
| Materials | Specific Capacitance | Electrolyte | Ref. |
|---|---|---|---|
| NSA-FeS2/polyaniline | 976 F g−1 at 0.5 A g−1 | 1 M Na2SO3 | [51] |
| Fe3Mo3C/Mo2C@CNTs | 202.3 F g−1 at 1 A g−1 | 1 M KOH | [52] |
| Mo2C/Fe3Mo3C | 354.9 F g−1 at 1 A g−1 | 1 M KOH | [53] |
| SnO2 | 325 F g−1 at 1 A g−1 | 2 M Na2SO4 | [54] |
| Bi2S3 | 565 F g−1 at 1 A g−1 | 2 M Na2SO4 | [55] |
| MoO2 | 411 F g−1 at 1 A g−1 | 1 M Na2SO4 | [56] |
| GO/Fe-VO4-BIPY | 190 F g−1 at 0.5 A g−1 | 6 M KOH | [57] |
| (Fe/Bi)2S3 | 610 F g−1 at 1 A g−1 | 6 M KOH | [58] |
| FMS/G | 669 F·g−1 at 1 A·g−1 | 3 M KOH | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Zhao, M.; Li, S.; Cheng, S.; Zuo, Y.; Wang, K.; Guo, M. Iron-Doped Molybdenum Sulfide Nanoflowers on Graphene for High-Performance Supercapacitors. Molecules 2025, 30, 4045. https://doi.org/10.3390/molecules30204045
Li X, Zhao M, Li S, Cheng S, Zuo Y, Wang K, Guo M. Iron-Doped Molybdenum Sulfide Nanoflowers on Graphene for High-Performance Supercapacitors. Molecules. 2025; 30(20):4045. https://doi.org/10.3390/molecules30204045
Chicago/Turabian StyleLi, Xuyang, Mingjian Zhao, Shuyi Li, Shiyuan Cheng, Yiting Zuo, Kaixuan Wang, and Meng Guo. 2025. "Iron-Doped Molybdenum Sulfide Nanoflowers on Graphene for High-Performance Supercapacitors" Molecules 30, no. 20: 4045. https://doi.org/10.3390/molecules30204045
APA StyleLi, X., Zhao, M., Li, S., Cheng, S., Zuo, Y., Wang, K., & Guo, M. (2025). Iron-Doped Molybdenum Sulfide Nanoflowers on Graphene for High-Performance Supercapacitors. Molecules, 30(20), 4045. https://doi.org/10.3390/molecules30204045





