Targeted Chemical Profiling and Dereplication of Australian Plants of the Family Haemodoraceae Using a Combined HPLC-MS and HRLC(ESI)-MS Approach
Abstract
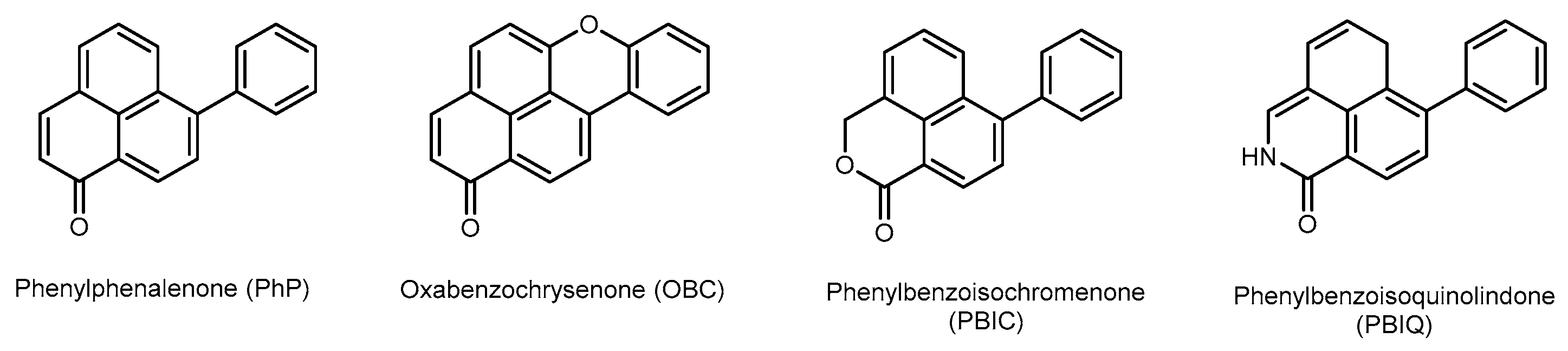
1. Introduction
1.1. Background
1.2. Chemical Profiling Study, Rationale and Methodology
2. Results
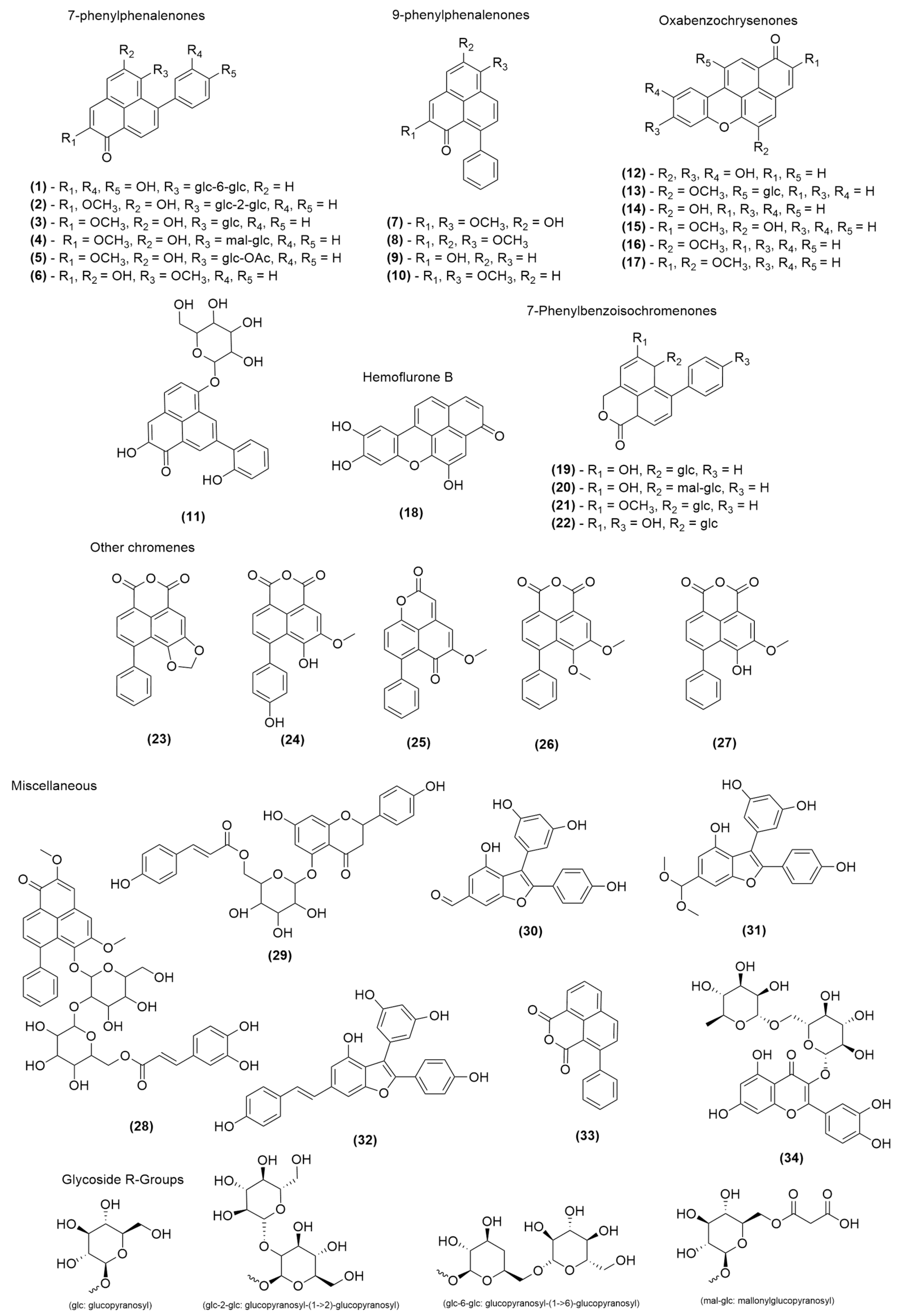
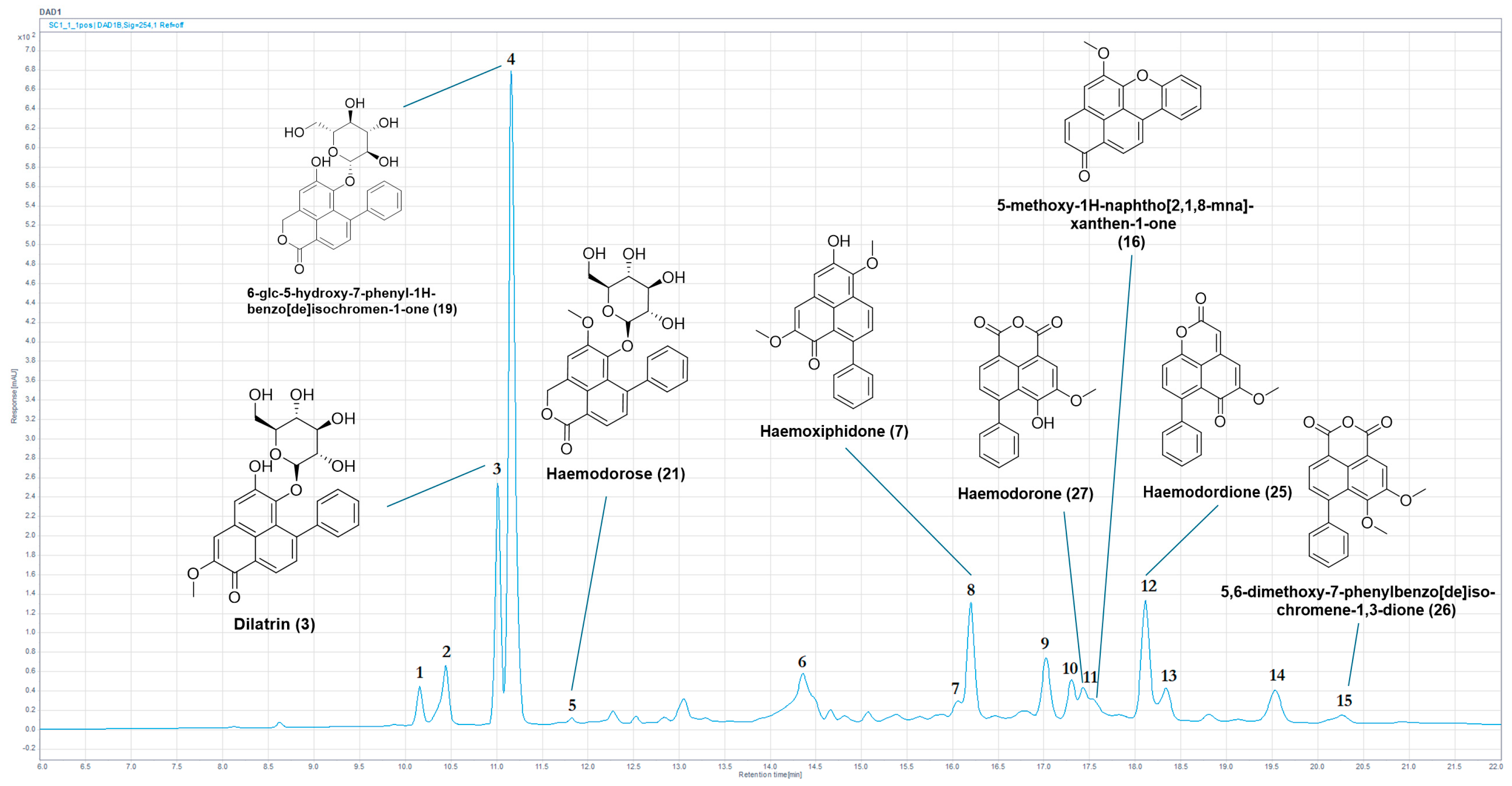
2.1. Chemical Profiling
| Species | Chemical Class(es) Present | Compounds Confirmed |
|---|---|---|
| H. simulans | PhP, OBC, PBIC | (3, 6, 7, 13, 15, 16, 19, 21, 23–27) |
| H. brevisepalum | PhP, OBC, PBIC | (1–3, 7, 11, 13–17, 28) |
| H. spicatum | PhP, OBC, PBIC | (7, 8, 14–16, 27) |
| M. fuliginosa | PhP, OBC, PBIC, benzofurans, other naphthalene derivatives, flavonoid glycosides | (6, 9, 12, 18, 29–34) |
| H. coccineum | PhP, OBC, PBIC | (3–5, 10, 22, 23, 25, 27) |
| H. distichophyllum | PhP, OBC, PBIC, flavonoid glycosides | (3, 4, 13, 15, 16, 19–21, 25, 26, 34) |
| Rt (min) | Compound | Structure Class | Species Present (Material Type) | UV (nm) | m/z | Ref. |
|---|---|---|---|---|---|---|
| 9.41 | (1) | Phenylphenalenone glycoside | H. brevisepalum (stems) | 222, 368, 434 | 644 | [8] |
| 9.42 | (34) | Flavonoid glycoside | M. fuliginosa (all) H. distichophyllum (leaves) | 204, 256, 354 | 610 | [20] |
| 9.88 | (2) | Phenylphenalenone glycoside | H. brevisepalum (bulbs) | 218, 278, 374, 468 | 642 | [8] |
| 10.66 | (28) | Phenylphenalenone glycoside | H. brevisepalum (bulbs) | 220, 278, 334, 470 | 818 | [8] |
| 10.99 | (11) | Phenylphenalenone glycoside | H. brevisepalum (stems) | 248, 288, 458 | 466 | [8] |
| 11.01 | (3) | Phenylphenalenone glycoside | H. simulans (bulbs + stems) H. brevisepalum (bulbs) H. coccineum (bulbs) H. distichophyllum (roots) | 278, 374, 474 | 480 | [8,21,40,41] |
| 11.05 | (12) | Oxabenzochrysenone | M. fuliginosa (all) | 236, 282, 394, 570 | 318 | [20] |
| 11.11 | (4) | Phenylphenalenone glycoside | H. coccineum (bulbs) H. distichophyllum (roots) | 224, 278, 374, 470 | 566 | [21] |
| 11.15 | (19) | Phenylbenzoisochromenone glycoside | H. simulans (bulbs + stems) H. distichophyllum (leaves) | 336, 366 | 454 | [21,40,41] |
| 11.31 | (20) | Phenylbenzoisochromenone glycoside | H. distichophyllum (leaves) | 260, 334, 364 | 540 | [21] |
| 11.41 | (18) | Oxabenzochrysenone | M. fuliginosa (all) | 554 | 318 | [20] |
| 11.65 | (13) | Oxabenzochrysenone glycoside | H. simulans (stems) H. brevisepalum (stems) H. distichophyllum (flowers) | 268, 288, 334, 348, 402, 542 | 478 | [40,41] |
| 11.67 | (5) | Phenylphenalenone glycoside | H. coccineum (bulbs) | 246, 270, 336, 464 | 522 | [21] |
| 11.82 | (21) | Phenylbenzochromenone glycoside | H. simulans (bulbs) H. distichophyllum (leaves) | 254, 346, 394 | 468 | [40,41] |
| 11.99 | (22) | Phenylbenzoisochromenone glycoside | H. coccineum (bulbs) | 228, 254, 326, 384 | 470 | [21] |
| 12.77–13.35 | (29) | Flavonoid glycoside | M. fuliginosa (bulbs) | 236, 316, 370 | 580 | [20] |
| 13.21 | (30) | Benzofuran | M. fuliginosa (bulbs) | 234, 290sh, 322, 364 | 362/408 | [20] |
| 13.21 | (31) | Benzofuran | M. fuliginosa (bulbs) | 234, 290sh, 322, 364 | 362/408 | [20] |
| 14.34 | (32) | Benzofuran | M. fuliginosa (bulbs) | 224, 292, 368 | 452 | [20] |
| 14.66 | (24) | Phenylbenzoisochromenone | H. simulans (bulbs) | 254, 342, 400 | 336 | [40,41] |
| 14.85 | (14) | Oxabenzochrysenone | H. brevisepalum (stems) H. spicatum (stems) | 234, 312, 520 | 286 | [8] |
| 16.22 | (7) | Phenylphenalenone | H. simulans (bulbs) H. brevisepalum (stems) H. spicatum (bulbs) | 276, 372, 460 | 332 | [40,41] |
| 16.47 | (6) | Phenylphenalenone | M. fuliginosa (bulbs) | 250, 280, 374, 476 | 318 | [20] |
| 16.57 | (15) | Oxabenzochrysenone | H. simulans (bulbs + stems) H. brevisepalum (stems) H. spicatum (stems) H. distichophyllum (flowers) | 236, 320, 388, 412, 540 | 316 | [8,19,40,41] |
| 16.75 | (23) | Phenylbenzoisochromenone | H. simulans (bulbs) | 254, 327, 368, 421 | 318 | [21,40,41] |
| 17.44 | (27) | Phenylbenzoisochromenone | H. simulans (bulbs + stems) H. spicatum (bulbs) H. coccineum (bulbs) | 256, 344, 396 | 320 | [19,21,40,41] |
| 17.55 | (16) | Oxabenzochrysenone | H. simulans (bulbs + stems) H. spicatum (bulbs) H. brevisepalum (stems) H. distichophyllum (flowers) | 244, 344, 378, 522 | 300 | [19,40,41] |
| 17.64 | (17) | Oxabenzochrysenone | H. brevisepalum (bulbs) | 238, 320, 364, 384, 536 | 330 | [8] |
| 17.86 | (33) | Naphthalic anhydride | M. fuliginosa (bulbs) | 246, 344 | 274 | [20] |
| 18.13 | (25) | Phenylbenzoisochromenone | H. simulans (bulbs) H. coccineum (bulbs) H. distichophyllum (roots) | 266, 340, 384 | 304 | [19,40,41] |
| 19.72 | (8) | Phenylphenalenone | H. spicatum (bulbs) | 238, 276, 372, 462 | 346 | [19] |
| 19.97 | (9) | Phenylphenalenone | M. fuliginosa (bulbs) | 242, 260, 350, 366, 416 | 272 | [20] |
| 20.16 | (10) | Phenylphenalenone | H. coccineum (bulbs) | 272, 370, 436 | 316 | [19,21] |
| 20.29 | (26) | Phenylbenzoisochromenone | H. simulans (bulbs) H. distichophyllum (roots/bulbs) | 258, 346, 394 | 334 | [40,41] |
2.2. Anti-Microbial and Anthelmintic Activity
3. Discussion
3.1. Haemodorum Simulans
3.2. Haemodorum Coccineum
3.3. Haemodorum Distichophyllum
4. Materials and Methods
4.1. Plant Material
4.2. Extraction
4.3. Chemical Profiling
4.4. High-Resolution Liquid Chromatography–Electrospray Mass Spectrometry (HRLC(ESI)-MS)
4.5. Nematode Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Norman, E.O.; Lever, J.; Brkljača, R.; Urban, S. Distribution, biosynthesis, and biological activity of phenylphenalenone-type compounds derived from the family of plants, Haemodoraceae. Nat. Prod. Rep. 2019, 36, 753–768. [Google Scholar] [CrossRef]
- Opitz, S.; Hoelscher, D.; Oldham, N.J.; Bartram, S.; Schneider, B. Phenylphenalenone-related compounds: Chemotaxonomic markers of the Haemodoraceae from Xiphidium caeruleum. J. Nat. Prod. 2002, 65, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Opitz, S.; Schneider, B. Organ-specific analysis of phenylphenalenone-related compounds in Xiphidium caeruleum. Phytochemistry 2002, 61, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Cooke, R.G.; Thomas, R.L. Coloring matters of Australian plants. XVIII. Constituents of Anigozanthos rufus. Aust. J. Chem. 1975, 28, 1053. [Google Scholar] [CrossRef]
- Cooke, R.; Dagley, I. Colouring Matters of Australian Plants. XXI. Naphthoxanthenones in the Haemodoraceae. Aust. J. Chem. 1979, 32, 1841–1847. [Google Scholar] [CrossRef]
- Bazan, A.C.; Edwards, J.M. Pigments of Lachnanthes tinctoria Ell. Part 6. Phenalenone pigments of the flowers of Lachnanthes tinctoria. Phytochemistry 1976, 15, 1413. [Google Scholar] [CrossRef]
- Chen, Y.; Paetz, C.; Menezes, R.C.; Schneider, B. Phenylbenzoisoquinolindione alkaloids accumulate in stamens of Xiphidium caeruleum Aubl. flowers. Phytochemistry 2016, 128, 95–101. [Google Scholar] [CrossRef]
- Norman, E.O.; Hombsch, S.; Lever, J.; Brkljaca, R.; White, J.; Gasser, R.B.; Taki, A.C.; Urban, S. Phytochemical Profiling and Biological Testing of the Constituents of the Australian Plant Haemodorum brevisepalum. J. Nat. Prod. 2021, 84, 2832–2844. [Google Scholar] [CrossRef]
- Song, X.; Ku, C.-F.; Si, T.-X.; Jaiswal, Y.S.; Williams, L.L.; Lu, D.-Y.; Huang, J.-J.; He, Z.-D.; Wang, M.-Z. Synthesis and Biological Activities Assessment of 4-, 6-, and 9-Phenylphenalenone Derivatives. ChemistrySelect 2022, 7, e202203793. [Google Scholar] [CrossRef]
- Song, X.; Wei, P.; Si, T.-X.; Lu, D.-Y.; Qiu, H.-L.; Huang, J.-J.; He, Z.-D.; Wang, M.-Z. Synthesis and biological activities of some new antimicrobial and cytotoxic 5-/7-/8-phenylphenalenone derivatives. Phytochem. Lett. 2023, 55, 169–174. [Google Scholar] [CrossRef]
- Lazzaro, A.; Corominas, M.; Martí, C.; Flors, C.; Izquierdo, L.R.; Grillo, T.A.; Luis, J.G.; Nonell, S. Light- and singlet oxygen-mediated antifungal activity of phenylphenalenone phytoalexins. Photochem. Photobiol. Sci. 2004, 3, 706–710. [Google Scholar] [CrossRef] [PubMed]
- Bucher, G.; Bresoli-Obach, R.; Brosa, C.; Flors, C.; Luis, J.G.; Grillo, T.A.; Nonell, S. β-Phenyl quenching of 9-phenylphenalenones: A novel photocyclisation reaction with biological implications. Phys. Chem. Chem. Phys. 2014, 16, 18813–18820. [Google Scholar] [CrossRef] [PubMed]
- Luis, J.G.; Quinones, W.; Echeverri, F.; Grillo, T.A.; Kishi, M.P.; Garcia-Garcia, F.; Torres, F.; Cardona, G. Musanolones: Four 9-phenylphenalenones from rhizomes of Musa acuminata. Phytochemistry 1996, 41, 753. [Google Scholar] [CrossRef]
- Luis, J.G. Phenylphenalenone-type phytoalexins and phytoanticipins from susceptible and resistant cultivars of Musa species. Its potential for engineering resistance to fungi and nematodes into banana. Acta Hortic. 1998, 490, 425–431. [Google Scholar] [CrossRef]
- Hoss, R.; Helbig, J.; Bochow, H. Function of Host and Fungal Metabolites in Resistance Response of Banana and Plantain in the Black Sigatoka Disease Pathosystem (Musa spp. –Mycosphaerella fijiensis). J. Phytopathol. 2000, 148, 387–394. [Google Scholar] [CrossRef]
- Quinones, W.; Escobar, G.; Echeverri, F.; Torres, F.; Rosero, Y.; Arango, V.; Cardona, G.; Gallego, A. Synthesis and antifungal activity of Musa phytoalexins and structural analogs. Molecules 2000, 5, 974–980. [Google Scholar] [CrossRef]
- Kamo, T. Phenylphenalenones and their biosynthetic pathway. Shinshu Daigaku Nogakubu Kiyo 2004, 40, 1–6. [Google Scholar]
- Echeverri, F.; Torres, F.; Quinones, W.; Escobar, G.; Archbold, R. Phenylphenalenone phytoalexins, will they be a new type of fungicide? Phytochem. Rev. 2012, 11, 1–12. [Google Scholar] [CrossRef]
- Brkljača, R.; Urban, S. HPLC-NMR and HPLC-MS Profiling and Bioassay-Guided Identification of Secondary Metabolites from the Australian Plant Haemodorum spicatum. J. Nat. Prod. 2015, 78, 1486–1494. [Google Scholar] [CrossRef]
- Brkljača, R.; White, J.M.; Urban, S. Phytochemical Investigation of the Constituents Derived from the Australian Plant Macropidia fuliginosa. J. Nat. Prod. 2015, 78, 1600–1608. [Google Scholar] [CrossRef]
- Carpinelli de Jesus, M.; Church, T.; Wapling, J.A.; Collins, R.; Leach, G.J.; Leach, D.; De Voss, J.J.; Blanchfield, J.T. Differentiating Dyes: A Spectroscopic Investigation into the Composition of Scarlet Bloodroot (Haemodorum coccineum R.Br.) Rhizome. Molecules 2023, 28, 7422. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Paetz, C.; Menezes, R.C.; Schneider, B. Cultured roots of Xiphidium caeruleum: Phenylphenalenones and their biosynthetic and extractant-dependent conversion. Phytochemistry 2017, 133, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Holscher, D.; Schneider, B. HPLC-NMR analysis of phenylphenalenones and a stilbene from Anigozanthos flavidus. Phytochemistry 1998, 50, 155–161. [Google Scholar] [CrossRef]
- Edwards, J.M.; Weiss, U. Pigments of Lachnanthes tinctoria. V. Phenalenone pigments of the root system of Lachnanthes tinctoria. Phytochemistry 1974, 13, 1597. [Google Scholar] [CrossRef]
- Hoelscher, D.; Schneider, B. The biosynthesis of 8-phenylphenalenones from Eichhornia crassipes involves a putative aryl migration step. Phytochemistry 2005, 66, 59–64. [Google Scholar] [CrossRef]
- Opitz, S.; Otalvaro, F.; Echeverri, F.; Quinones, W.; Schneider, B. Isomeric oxabenzochrysenones from Musa acuminata and Wachendorfia thyrsiflora. Nat. Prod. Lett. 2002, 16, 335–338. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Sun, Q.-Y.; Yang, F.-M.; Gu, W.; Yang, J.; Niu, H.-M.; Wang, Y.-H.; Long, C.-L. Diarylheptanoids and phenylphenalenones from Musa itinerans fruits. Phytochemistry 2014, 103, 171–177. [Google Scholar] [CrossRef]
- Holscher, D.; Schneider, B. Phenylphenalenones from root cultures of Anigozanthos preissii. Phytochemistry 1997, 45, 87–91. [Google Scholar] [CrossRef]
- Holscher, D.; Schneider, B. Phenylphenalenones from Ensete ventricosum. Phytochemistry 1998, 49, 2155–2157. [Google Scholar] [CrossRef]
- Munde, T.; Brand, S.; Hidalgo, W.; Maddula, R.K.; Svatos, A.; Schneider, B. Biosynthesis of tetraoxygenated phenylphenalenones in Wachendorfia thyrsiflora. Phytochemistry 2013, 91, 165–176. [Google Scholar] [CrossRef]
- Schneider, B.; Paetz, C.; Hoelscher, D.; Opitz, S. HPLC-NMR for tissue-specific analysis of phenylphenalenone-related compounds in Xiphidium caeruleum (Haemodoraceae). Magn. Reson. Chem. 2005, 43, 724–728. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.-B.; He, J.; Li, X.-Y.; Wu, X.-D.; Deng, X.; Xu, G.; Peng, L.-Y.; Zhao, Y.; Li, Y.; Gong, X.; et al. Chemical constituents from the aerial parts of Musella lasiocarpa. Nat. Prod. Bioprospect. 2011, 1, 41–47. [Google Scholar] [CrossRef]
- Fang, J.-J.; Paetz, C.; Hoelscher, D.; Munde, T.; Schneider, B. Phenylphenalenones and related natural products from Wachendorfia thyrsiflora L. Phytochem. Lett. 2011, 4, 203–208. [Google Scholar] [CrossRef]
- Ocampos, F.M.M.; Paetz, C.; Antar, G.M.; Menezes, R.C.; Miguel, O.G.; Schneider, B. Phytochemical profile of Schiekia orinocensis (Haemodoraceae). Phytochem. Lett. 2017, 21, 139–145. [Google Scholar] [CrossRef]
- Fang, J.; Kai, M.; Schneider, B. Phytochemical profile of aerial parts and roots of Wachendorfia thyrsiflora L. studied by LC-DAD-SPE-NMR. Phytochemistry 2012, 81, 144–152. [Google Scholar] [CrossRef]
- Chen, Y.; Paetz, C.; Schneider, B. Organ-specific distribution and non-enzymatic conversions indicate a metabolic network of phenylphenalenones in Xiphidium caeruleum. Phytochemistry 2019, 159, 30–38. [Google Scholar] [CrossRef]
- Opitz, S.; Schnitzler, J.P.; Hause, B.; Schneider, B. Histochemical analysis of phenylphenalenone-related compounds in Xiphidium caeruleum (haemodoraceae). Planta 2003, 216, 881–889. [Google Scholar] [CrossRef]
- Morrison, G.A.; Laundon, B.; Brooks, J.S. Naturally occurring compounds related to phenalenone. I. Synthesis of lachnanthocarpone. J. Chem. Soc. C 1971, 36–40. [Google Scholar] [CrossRef]
- Bick, I.; Blackman, A. Haemodorin; A phenalenone pigment. Aust. J. Chem. 1973, 26, 1377–1380. [Google Scholar] [CrossRef]
- Dias, D.A.; Goble, D.J.; Silva, C.A.; Urban, S. Phenylphenalenones from the Australian Plant Haemodorum simplex. J. Nat. Prod. 2009, 72, 1075–1080. [Google Scholar] [CrossRef]
- Urban, S.; Brkljaca, R.; White, J.M.; Timmers, M.A. Phenylphenalenones and oxabenzochrysenones from the Australian plant Haemodorum simulans. Phytochemistry 2013, 95, 351–359. [Google Scholar] [CrossRef]
- Luque-Ortega, J.R.; Martínez, S.; Saugar, J.M.; Izquierdo, L.R.; Abad, T.; Luis, J.G.; Piñero, J.; Valladares, B.; Rivas, L. Fungus-Elicited Metabolites from Plants as an Enriched Source for New Leishmanicidal Agents: Antifungal Phenyl-Phenalenone Phytoalexins from the Banana Plant (Musa acuminata) Target Mitochondria of Leishmania donovani Promastigotes. Antimicrob. Agents Chemother. 2004, 48, 1534–1540. [Google Scholar] [CrossRef]
- Gutiérrez, D.; Flores, N.; Abad-Grillo, T.; McNaughton-Smith, G. Evaluation of substituted phenalenone analogues as antiplasmodial agents. Exp. Parasitol. 2013, 135, 456–458. [Google Scholar] [CrossRef]
- Fang, J.; Hoelscher, D.; Schneider, B. Co-occurrence of phenylphenalenones and flavonoids in Xiphidium caeruleum Aubl. flowers. Phytochemistry 2012, 82, 143–148. [Google Scholar] [CrossRef]
- Reddy, P.; Rochfort, S.; Read, E.; Deseo, M.; Jaehne, E.; Van Den Buuse, M.; Guthridge, K.; Combs, M.; Spangenberg, G.; Quinn, J. Tremorgenic effects and functional metabolomics analysis of lolitrem B and its biosynthetic intermediates. Sci. Rep. 2019, 9, 9364. [Google Scholar] [CrossRef]
- Taki, A.C.; Byrne, J.J.; Wang, T.; Sleebs, B.E.; Nguyen, N.; Hall, R.S.; Korhonen, P.K.; Chang, B.C.H.; Jackson, P.; Abdul, J.; et al. High-throughput phenotypic assay to screen for anthelmintic activity on Haemonchus contortus. Pharmaceuticals 2021, 14, 616. [Google Scholar] [CrossRef]
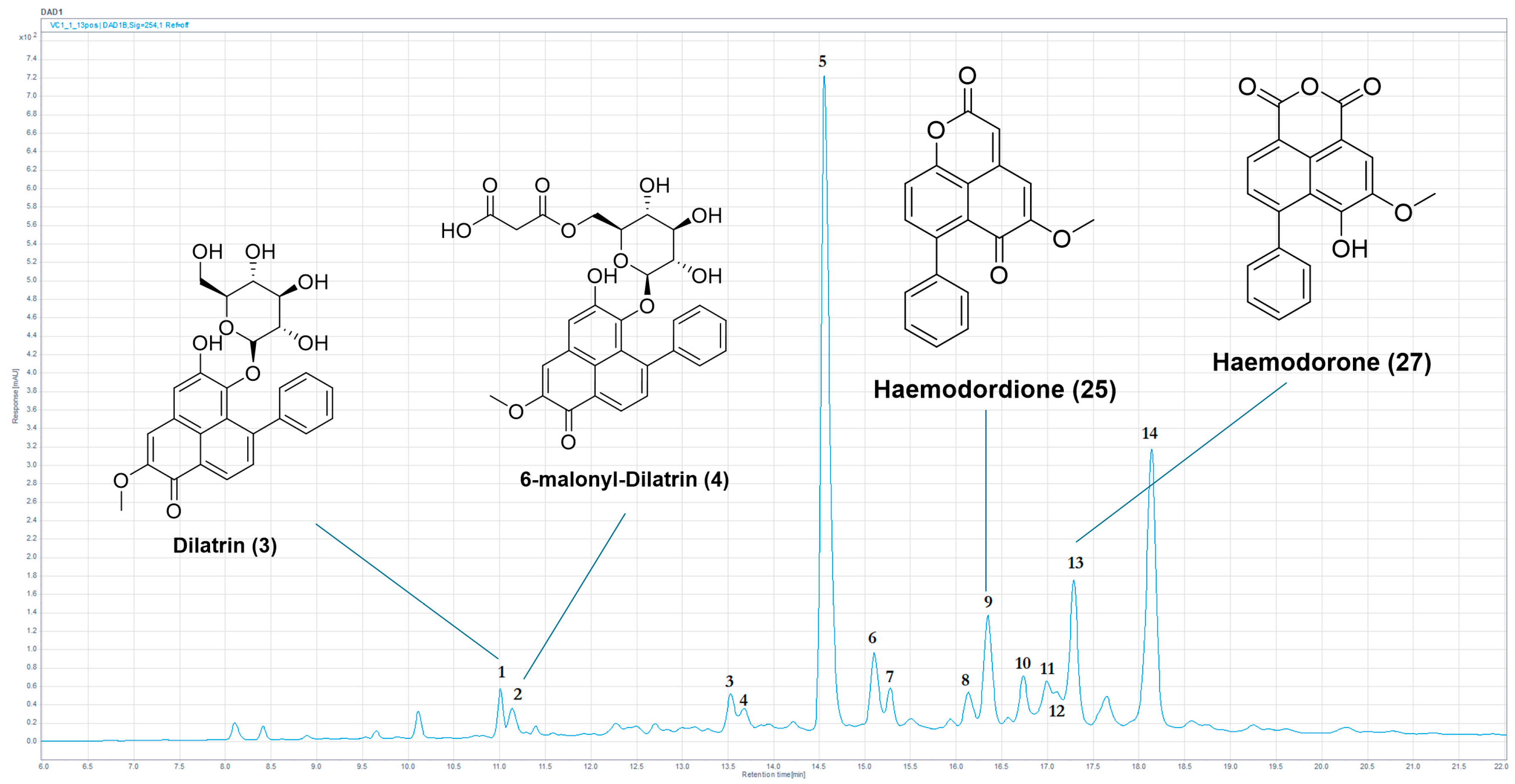
| Species | Material Type (Source) | Motility Reduction, 168 h (%) | Abnormal Phenotype (%) |
|---|---|---|---|
| H. simulans | Bulbs (2005_01a) | 64.0 ± 13.2 = Partial | Skinny (10) |
| Stems (2005_01b) | 7.9 ± 18.4 | Skinny (10) | |
| Bulbs (2007_01a) | 45.8 ± 11.8 | Skinny (30) | |
| Stems (2007_01b) | 57.5 ± 5.5 = Partial | Skinny (10) | |
| Bulbs (2010_17a) | 39.8 ± 8.9 | Skinny (30) | |
| Stems (2010_17b) | 73.7 ± 10.6 = Active * | Skinny (50) | |
| H. brevisepalum | Bulbs (2010_19a) | −1.5 ± 7.3 | Skinny (3) |
| Stems (2010_19b) | 34.7 ± 7.5 | Skinny (17) | |
| H. spicatum | Bulbs (2010_20a) | 12.0 ± 7.5 | - |
| Stems (2010_20b) | 9.5 ± 22.7 | - | |
| M. fuliginosa | Bulbs (2011_01a) | 84.1 ± 6.6 = Active * | nca |
| Stems (2011_01b) | 14.1 ± 4.8 | nca | |
| Bulbs (2011_02a) | 64.2 ± 8.4 = Partial | nca | |
| Stems (2011_02b) | 42.6 ± 6.0 | Skinny (10) | |
| Bulbs (2012_01a) | 40.6 ± 13.9 | nca | |
| Stems (2012_01b) | 18.7 ± 6.7 | nca | |
| Flowers (2012_05a) | 63.4 ± 9.1 | nca | |
| Stems/leaves (2012_05b) | 31.4 ± 5.9 | Skinny (7) | |
| Stems (2012_05c) | 31.0 ± 17.0 | Skinny (30) | |
| Flowers (2013_02) | 55.0 ± 8.0 = Partial | nca | |
| H. coccineum | Leaves/stems (2021_17a) | 18.4 ± 15.0 | Skinny (10) |
| Roots (2021_17b) | 50 ± 12.2 = Partial | Skinny (50) | |
| Leaves/bulbs (2022_08) | 40.9 ± 17.0 = Partial | Skinny (50) | |
| Bulbs (2023_01a) | 44.3 ± 17.1 | nca | |
| Stems (2023_01b) | 10.0 ± 8.7 | Skinny (10) | |
| H. distichophyllum | Leaves (2021_18a) | 62.1 ± 1.9 = Partial | Skinny (50) |
| Flowers/seeds (2021_18b) | 12.0 ± 15.0 | Skinny (20) | |
| Roots (2021_18c) | 93.3 ± 4.1 = Active * | Skinny (70) | |
| Leaves (2022_07a) | 52.8 ± 3.4 = Partial | Skinny (40) | |
| Roots/bulbs (2022_07b) | 45.3 ± 9.9 | Skinny (80) | |
| Negative control | DMSO | −15.1 ± 11.8 | - |
| Positive controls | Moxidectin | 92.8 ± 3.6 = Active * | - |
| Monepantel | 100.0 ± 2.5 = Active * | Coiled (100) |
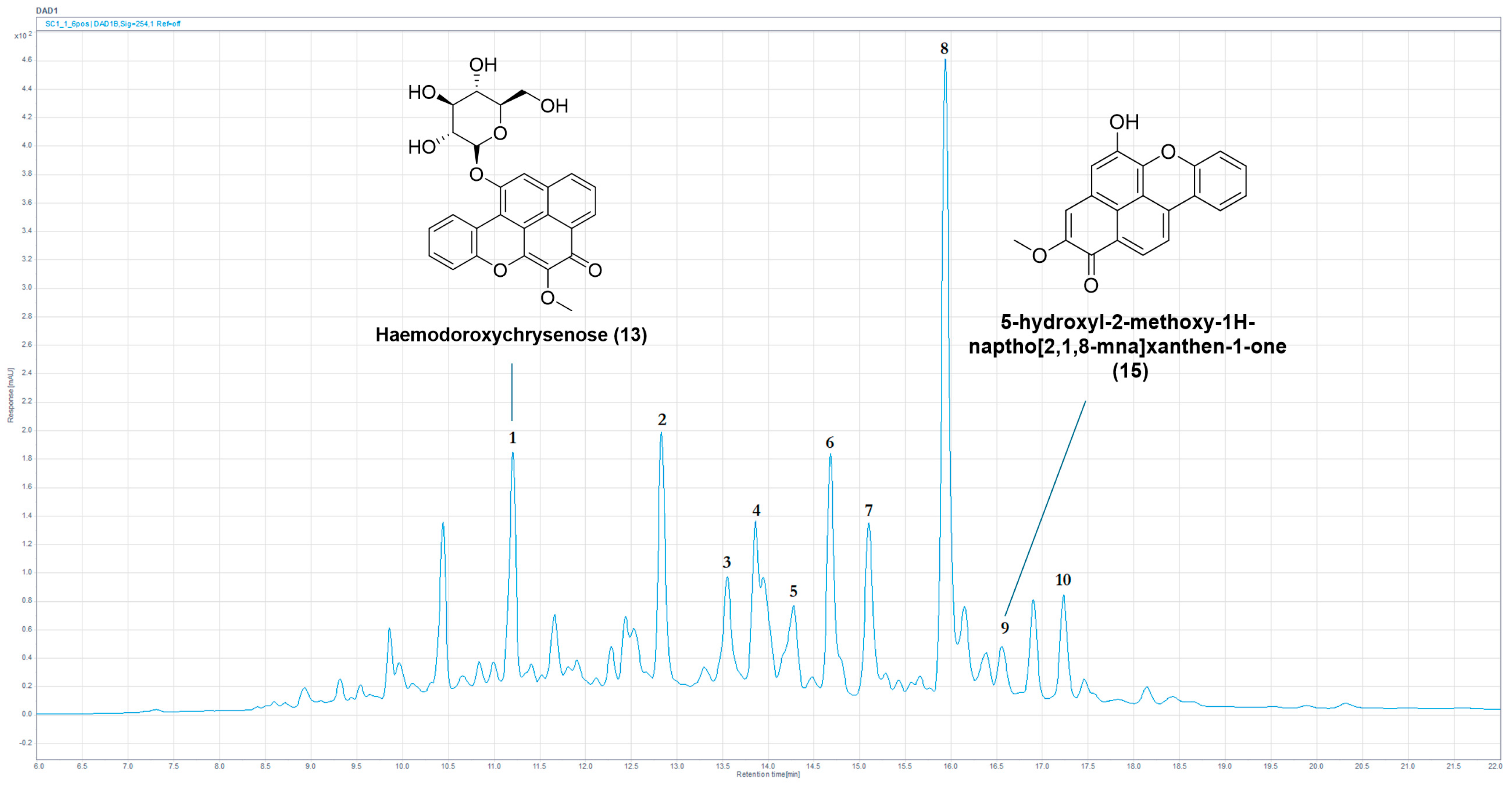
| Peak | Tr (min) | UV (nm) | (m/z) | HRLC(ESI)-MS ([M+H]+) | Compound Identification |
|---|---|---|---|---|---|
| 1 | 10.15 | 240, 322, 436 | 460 | 461.1812 | New mass |
| 2 | 10.35 | 240, 324, 442 | 446/470 | 447.1656 | New mass or new isomer |
| 3 | 11.01 | 278, 374, 472 | 480 | 481.1485 * | (3) |
| 4 | 11.15 | 214, 260, 336, 366 | 454 | 455.1328 * | (19) |
| 5 | 11.83 | 254, 348, 380 | 468 | 469.1490 * | (21) |
| 6 | 14.36 | 234, 320, 432 | 389 | 390.1330 | Potential PBIQ |
| 7 | 16.06 | 250, 350, 394 | 420 | 421.1275 | New mass |
| 8 | 16.21 | 276, 372, 460 | 332 | 333.1116 * | (7) |
| 9 | 17.03 | 268, 340, 434 | 332 | 333.1115 | Potential isomer of (7) |
| 10 | 17.31 | 266, 346, 410 | 320 | 321.1119 | PBIC |
| 11 | 17.44 | 256, 344, 396 | 320 | 321.0756 * | (27) |
| 11a | 17.54 | 234, 322, 522 | 300 | 301.0857 * | (16) |
| 12 | 18.12 | 266, 340, 384 | 304/350 | 305.0804 */351.1223 | (25)/New mass |
| 13 | 18.33 | 266, 346, 416 | 350 | 351.1224 | New mass |
| 14 | 19.53 | 242, 262, 298, 366, 456 | 316 | 317.1167 | PhP |
| 15 | 20.28 | 258, 344, 394 | 334 | 335.0912 * | (26) |
| Peak | Tr (min) | UV (nm) | (m/z) | HRLC(ESI)-MS ([M+H]+) | Compound Identification |
|---|---|---|---|---|---|
| 1 | 11.67 | 238, 268, 332, 404, 548 | 478 | 479.1329 * | (13) |
| 2 | 12.84 | 230, 306, 342, 496 | 336 | 337.0339 | New mass (HRLC(ESI)-MS) |
| 3 | 13.56 | 252, 346, 420 | 322 | 323.0548 | New isomer—potentially of (24) |
| 4 | 13.86 | 268, 338, 388 | 308 | Not detected | Potential new compound/isomer |
| 5 | 14.29 | 246, 484 | 350 | 351.0495 | New mass |
| 6 | 14.69 | 260, 330, 348, 416 | 336 | 337.0703 | Possible derivative of (27) |
| 7 | 15.10 | 264, 338, 386, 550 | 302/322 | 303.0648/323.0909 | OBC |
| 8 | 15.94 | 222, 268, 346, 412 | 306 | 307.0596 | PhP/PBIC |
| 9 | 16.57 | 242, 270, 372, 412, 540 | 316 | 317.0805 * | (15) |
| 10 | 17.23 | 266, 346, 410 | 320 | 321.0755 | Potential isomer of (27) |
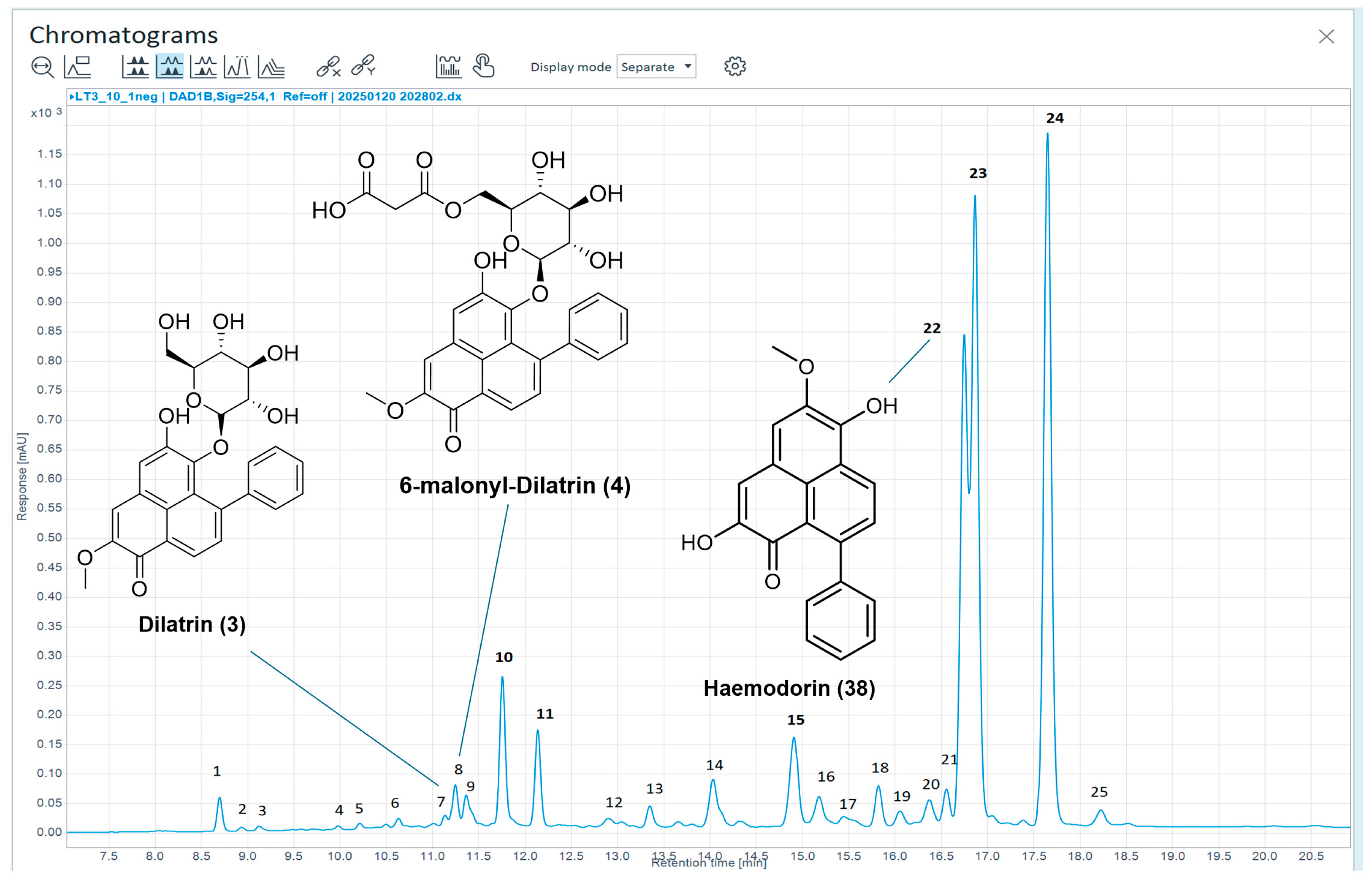
| Peak | Tr (min) | UV (nm) | m/z | HRLC(ESI)-MS [M+H]+ | Compound Identification |
|---|---|---|---|---|---|
| 1 | 8.69 | 200, 224, 328 | 594 | Not detected | New mass—unlikely to be PhP-type |
| 2 | 8.94 | 218, 328 | 386 | 387.1278 | New mass—unlikely to be PhP-type |
| 3 | 9.13 | 218, 328 | 386 | 387.1277 | New mass—unlikely to be PhP-type |
| 4 | 9.99 | 218, 326 | 662 | Not detected | New mass—unlikely to be PhP-type |
| 5 | 10.21 | 220, 330 | 754 | Not detected | New mass—unlikely to be PhP-type |
| 6 | 10.63 | 220, 328, 380sh | 556 | 557.1281 | Potential isomer of a known PBIC glycoside |
| 7 | 11.15 | 220, 280, 374, 474 | 480 | 481.1487 * | (3) |
| 8 | 11.25 | 218, 278, 374, 468 | 566 | 567.1486 * | (4) |
| 9 | 11.37 | 218, 286, 346, 404 | 462 | 463.1603 | New mass |
| 10 | 11.75 | 216, 332 | 676 | 677.1848 | New mass—unlikely to be PhP-type |
| 11 | 12.14 | 222, 254, 326, 384 | 556 | 557.1280 | Potential isomer of a known PBIC glycoside |
| 12 | 12.90 | 222, 324, 384 | 512 | 513.1357 | New mass—similar UV to peak 11 |
| 13 | 13.35 | 222, 268, 326, 388 | 338 | 339.0856 | New mass—PBIC |
| 14 | 14.04 | 224, 398 | 306 | 307.0596 | PBIC |
| 15 | 14.91 | 222, 256, 274, 330, 394 | 324 | 325.0701 | New mass—PBIC |
| 16 | 15.18 | 222, 282, 378 | 322 | 323.0545 | No matching UVs for mass |
| 17 | - | - | - | - | Below limit of detection for UV and MS |
| 18 | 15.82 | 222, 268, 338, 386 | 322 | 323.0908 * | PBIC (potentially (36)) |
| 19 | 16.06 | 224, 286, 348, 406 | 391 | 392.1120 | Potential PBIQ (37) |
| 20 | 16.37 | 224, 296, 372, 422 | 332 | 333.1114 | PhP |
| 21 | 16.56 | 224, 268, 338, 386 | 336 | 337.1062 | PBIC |
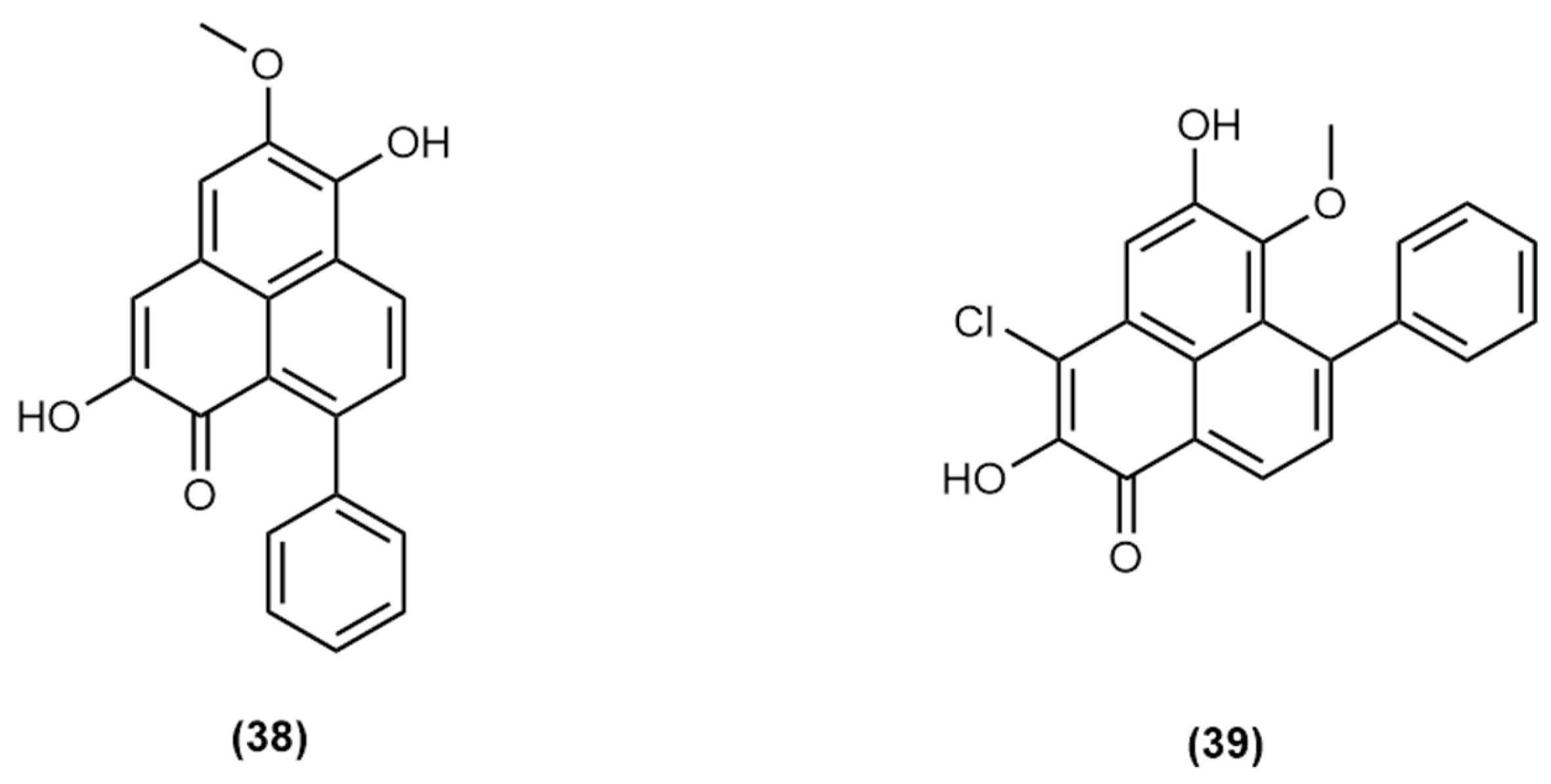
| 22 | 16.75 | 214, 278, 374, 514 | 318 | 319.0956 * | (38) |
| 23 | 16.86 | 220, 256, 278, 332, 400 | 338 | 339.0856 | New mass—PBIC/PhP |
| 24 | 17.65 | 218, 252, 282, 332, 386 | 352 | 353.1013 | PBIC—potentially new compound |
| 25 | 18.23 | 226, 338, 414 | 336 | 337.0702 | PBIC/PhP |
| Chemical Class | No. of Compounds | Compounds Confirmed |
|---|---|---|
| Phenylphenalenone | 2 | (3)(4) |
| Oxabenzochrysenone | 3 | (13)(15)(16) |
| Phenylbenzoisochromenone | 5 | (19–21)(25)(26) |
| Other (flavonoid) | 1 | (34) |
| Peak | Tr (min) | UV (nm) | (m/z) | HRLC(ESI)-MS ([M+H]+) | Compound Identification |
|---|---|---|---|---|---|
| 1 | 11.01 | 230, 278, 374, 474 | 480 | 481.1483 * | (3) |
| 2 | 11.12 | 234, 276, 374, 470 | 566 | 567.1485 * | (4) |
| 3 | 13.53 | 260, 336, 384 | 350 | 351.0858 | New mass—PBIC |
| 4 | 13.68 | 268, 332 | 564/896 | - | None |
| 5 | 14.56 | 238, 320, 366, 384, 544 | 316 | 317.0799 | OBC |
| 6 | 15.11 | 262, 322, 386, 550 | 322 | 323.0908 | PBIC/OBC |
| 7 | 15.28 | 244, 296, 340sh, 380sh, 460sh | 302 | 303.1011 | PhP |
| 8 | 16.14 | 244, 292, 438 | 316 | 317.0806 | PhP |
| 9 | 16.35 | 244, 388 | 304 | 305.0804 * | (25) |
| 10 | 16.74 | 256, 374 | 336 | 337.1066 | PBIC |
| 11 | 16.99 | 242, 268, 312, 342, 436 | 288/334/446 | 335.0908/289.0490 | PhP |
| 12 | 17.06 | 246, 270, 446, 512, 550, 590 | 334/408/716 | 335.0909 | OBC |
| 13 | 17.29 | 266, 340, 386 | 320 | 321.0755 | (27) |
| 14 | 18.14 | 266, 340, 384 | 350 | 351.1218 | New mass—PBIC similar UV chromophores to (27) |
| Species | No. of Compounds Identified * | No. of Compounds Reported | % of Compounds Identified |
|---|---|---|---|
| H. simulans | 10 | 10 | 100% |
| H. brevisepalum | 11 | 30 | 37% |
| H. spicatum | 5 | 11 | 45% |
| M. fuliginosa | 10 | 13 | 77% |
| 64% (average % of compounds identified) |
| Species | Voucher Codes | Collection Location/s | Identified/Collected by |
|---|---|---|---|
| Haemodorum simulans | 2005_01, 2007_01, 2010_17 | Arrowsmith River region, Eneabba, Western Australia | Mr Allan Tinker, 2005–2010 (Plant licence: SW008335) |
| Haemodorum brevisepalum | 2010_19 | Arrowsmith River region, Eneabba, Western Australia | Mr Allan Tinker, 2010 (Plant licence: SW008335) |
| Haemodorum spicatum | 2010_20 | Arrowsmith River region, Eneabba, Western Australia | Mr Allan Tinker, 2010 (Plant licence: SW008335) |
| Macropidia fuliginosa | 2011_01, 2011_02, 2012_01, 2012_05, 2013_02 | 2011_01, 2012_01, 2012_05—Kuranga Native Australian Nursery, Mt Evelyn, Victoria 2011_02, 2013_02—Arrowsmith River region, Eneabba, Western Australia | 2011_01, 2012_01, 2012_05—Dr Robert Brkljača (purchased), 2011–12 2011_02, 2013_02—Mr Allan Tinker, 2011–2013 (Plant licence: SW008335) |
| Haemodorum coccineum | 2021_17, 2022_08, 2023_01 | 2021_17, 2022_08—Cranbourne Botanical Gardens, Cranbourne, Victoria 2023_01—Territory Native Plants, Darwin, Northern Territory | 2021_17, 2022_08—Ms Bronwyn Swartz, Ms Deepika Dugan, Ms Mandy Thomson, 2021–2022 (MTA—Royal Botantical Gardens Victoria) 2023_01—Ms Amber Wildi, (purchased) 2023 |
| Haemodorum distichophyllum | 2021_18, 2022_07 | Cranbourne Botanical Gardens, Cranbourne, Victoria | 2021_18, 2022_07—Mrs Bronwyn Swartz, Ms Deepika Dugan, Ms Mandy Thomson, 2021–2022 (MTA—Royal Botanical Gardens Victoria) |
| Time (min) | %H2O | %CH3CN |
|---|---|---|
| 0–2 | 90 | 10 |
| 14–24 | 25 | 75 |
| 26–30 | 0 | 100 |
| 32–40 | 90 | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thompson, L.; Chow, V.; Chen, S.; Reddy, P.; Brkljača, R.; Rix, C.; Byrne, J.J.; Taki, A.C.; Gasser, R.B.; Urban, S. Targeted Chemical Profiling and Dereplication of Australian Plants of the Family Haemodoraceae Using a Combined HPLC-MS and HRLC(ESI)-MS Approach. Molecules 2025, 30, 4044. https://doi.org/10.3390/molecules30204044
Thompson L, Chow V, Chen S, Reddy P, Brkljača R, Rix C, Byrne JJ, Taki AC, Gasser RB, Urban S. Targeted Chemical Profiling and Dereplication of Australian Plants of the Family Haemodoraceae Using a Combined HPLC-MS and HRLC(ESI)-MS Approach. Molecules. 2025; 30(20):4044. https://doi.org/10.3390/molecules30204044
Chicago/Turabian StyleThompson, Liam, Valerie Chow, Shan Chen, Priyanka Reddy, Robert Brkljača, Colin Rix, Joseph J. Byrne, Aya C. Taki, Robin B. Gasser, and Sylvia Urban. 2025. "Targeted Chemical Profiling and Dereplication of Australian Plants of the Family Haemodoraceae Using a Combined HPLC-MS and HRLC(ESI)-MS Approach" Molecules 30, no. 20: 4044. https://doi.org/10.3390/molecules30204044
APA StyleThompson, L., Chow, V., Chen, S., Reddy, P., Brkljača, R., Rix, C., Byrne, J. J., Taki, A. C., Gasser, R. B., & Urban, S. (2025). Targeted Chemical Profiling and Dereplication of Australian Plants of the Family Haemodoraceae Using a Combined HPLC-MS and HRLC(ESI)-MS Approach. Molecules, 30(20), 4044. https://doi.org/10.3390/molecules30204044








