[(2-Dimesitylboryl)phenyl]ethynyl-Substituted [2.2]Paracyclophane Exhibiting Circularly Polarized Luminescence in Both Solution and Solid-State
Abstract
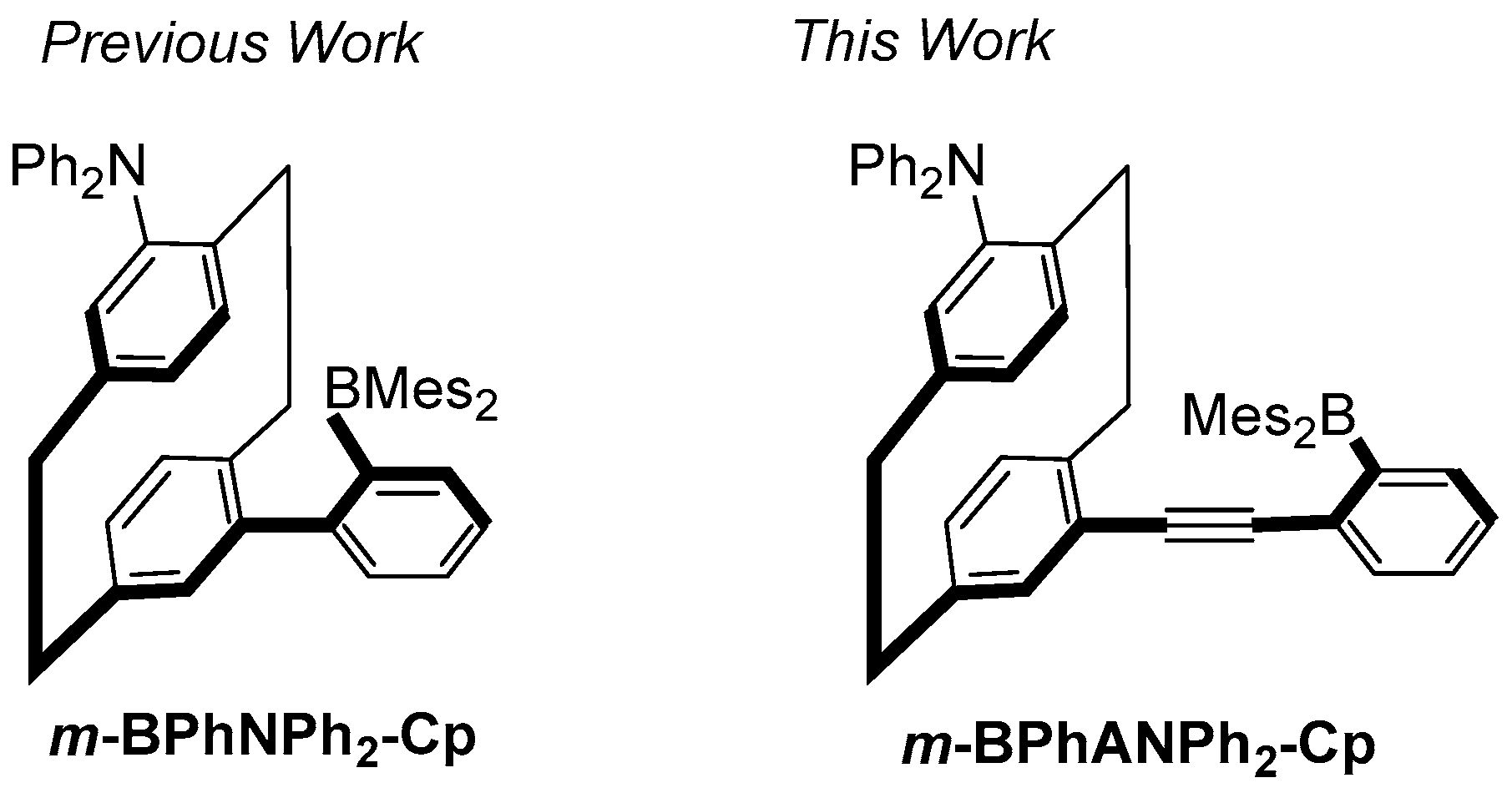
1. Introduction
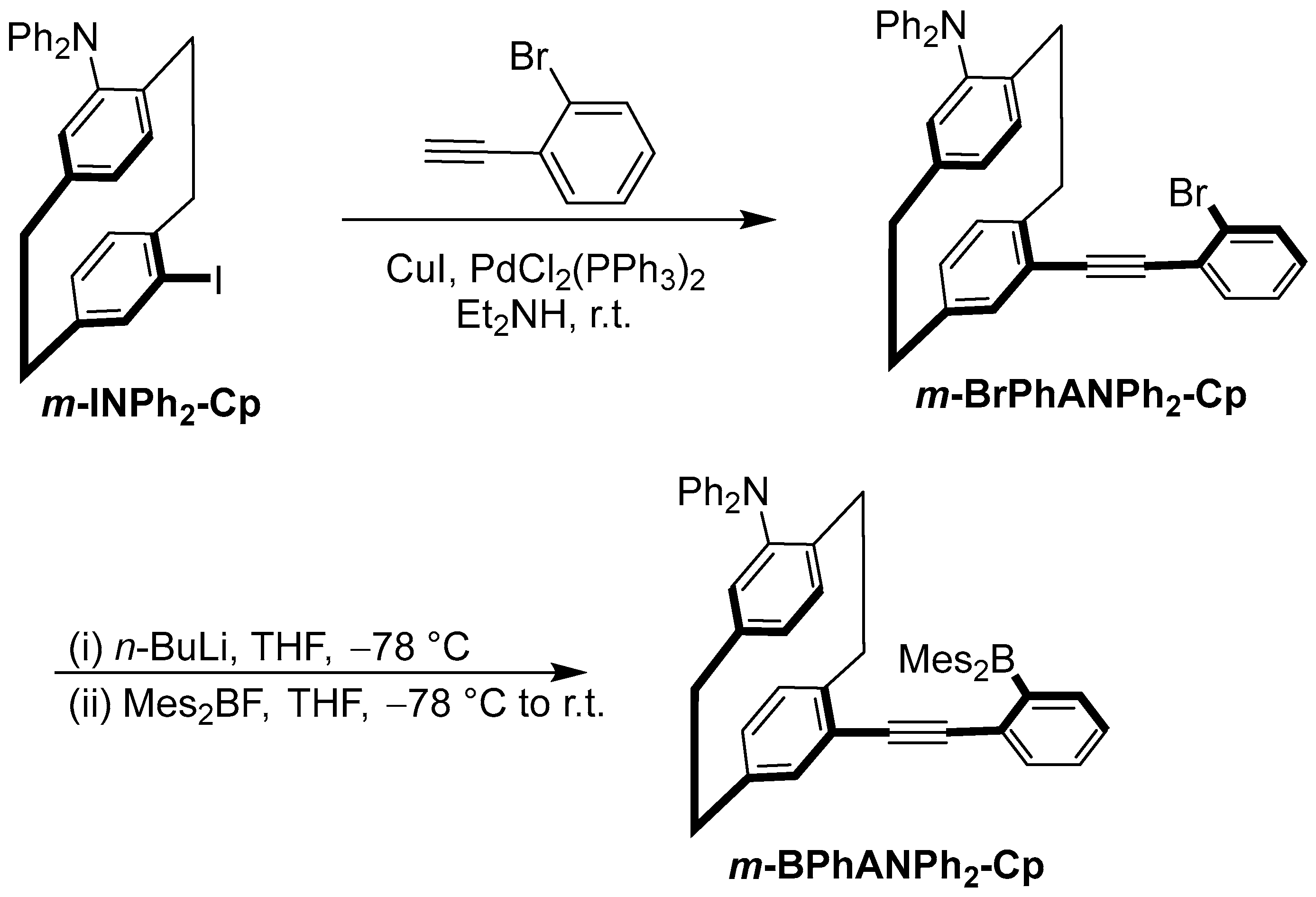
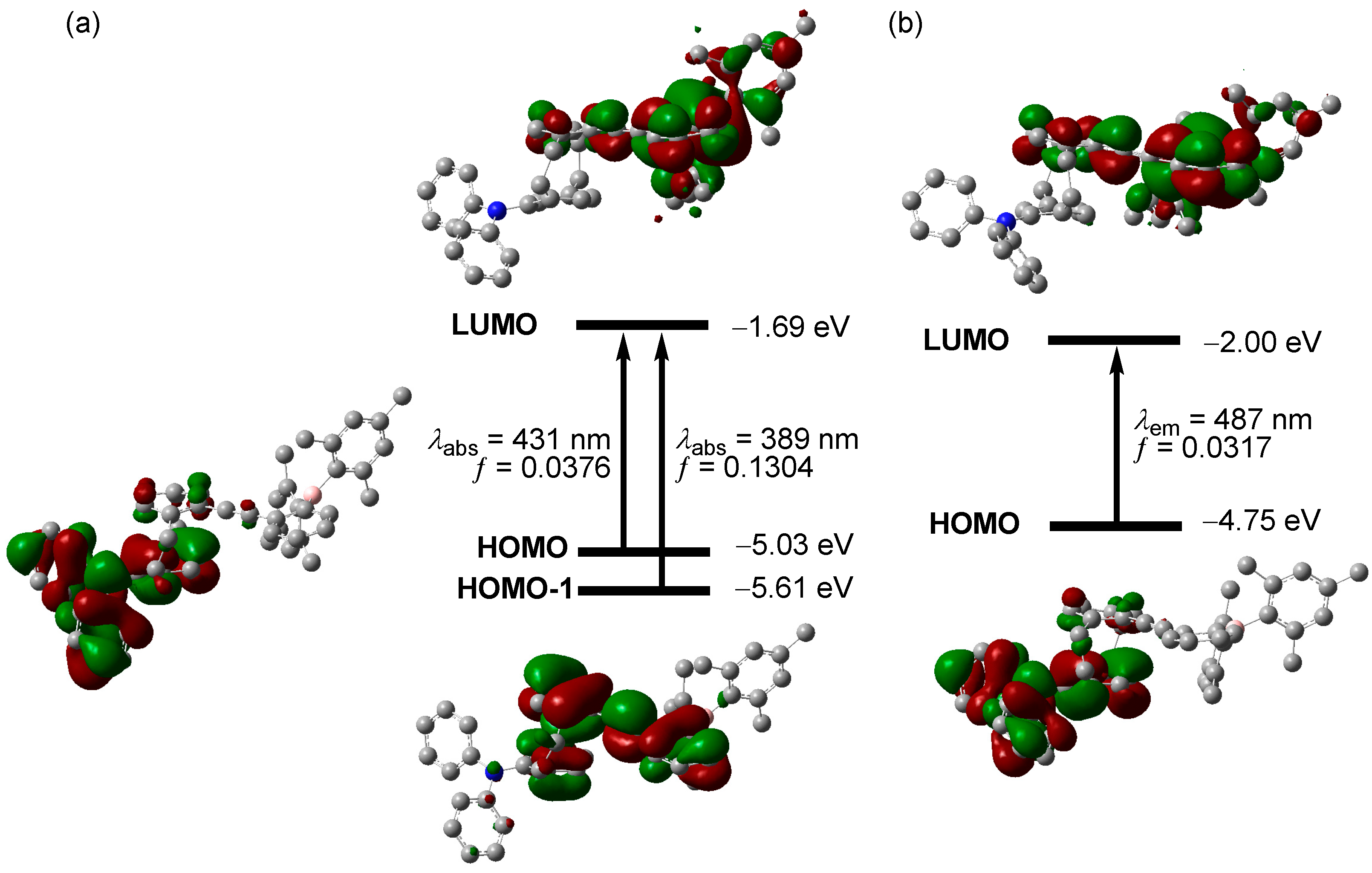
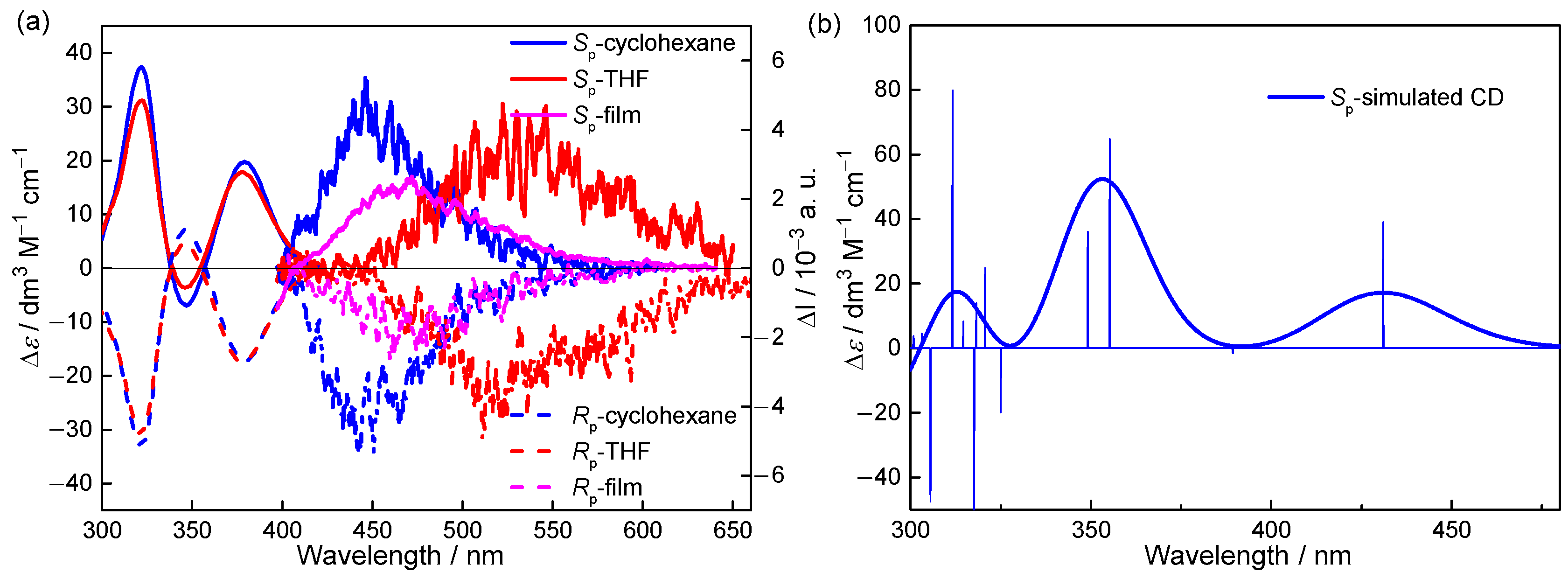
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Reagents and Instruments
4.2. Computational Methods
4.3. Synthetic Methods
4.4. Chiral SFC Separation Data
- Instrument: Waters UPC2 analytical SFC (SFC-H).
- Column: Chiralpak AD-3 50 × 4.6mm I.D., 3 µm.
- Mobile phase: A for CO2 and B for ethanol (0.05%DEA).
- Gradient: B 5-40%.
- Flow rate: 2.5 mL/min.
- Back pressure: 100 bar.
- Column temperature: 35 °C.
- Instrument: WATERS150 preparative SFC(SFC-26).
- Instrument: WATERS150 preparative SFC(SFC-26).
- Column: ChiralPak AD, 250 × 30mm I.D., 10 µm.
- Mobile phase: A for CO2 and B for ethanol.
- Gradient: B 25%.
- Flow rate: 120 mL/min.
- Back pressure: 100 bar.
- Column temperature: 38 °C.
- Cycle time: ~2.5 min.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, M.J.; Guo, Q.; Li, Z.Y.; Zhou, Y.J.; Zhao, S.S.; Tong, Z.; Wang, Y.X.; Li, G.G.; Jin, S.; Zhu, M.Z.; et al. Processable circularly polarized luminescence material enables flexible stereoscopic 3D imaging. Sci. Adv. 2023, 9, eadi9944. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Tian, G.K.; Li, D.; Jiang, Z.H.; Quan, Y.W.; Cheng, Y.X. Color-Tunable White Circularly Polarized Electroluminescence Triggered Using Chiral Co-Assembly-Sensitized Strategy. Laser Photonics Rev. 2024, 18, 2400223. [Google Scholar] [CrossRef]
- Zhong, X.S.; Yuan, L.; Liao, X.J.; Hu, J.J.; Xing, S.; Song, S.Q.; Xi, J.Q.; Zheng, Y.X. Circularly Polarized Organic Light-Emitting Diodes Based on Chiral Hole Transport Enantiomers. Adv. Mater. 2024, 36, 2311857. [Google Scholar] [CrossRef]
- Ai, Y.Y.; Fei, Y.X.; Shu, Z.; Zhu, Y.H.; Liu, J.Q.; Li, Y.G. Visible-light-controlled ternary chiroptical switches with high-performance circularly polarized luminescence for advanced optical information storage and anti-counterfeiting materials. Chem. Eng. J. 2022, 450, 138390. [Google Scholar] [CrossRef]
- Yao, K.; Li, Y.; Shen, Y.H.; Quan, Y.W.; Cheng, Y.X. A photosensitive-type CPL response controlled by intermolecular dynamic FRET and chiral transfer in ternary chiral emissive nematic liquid crystals. J. Mater. Chem. C 2021, 9, 12590–12595. [Google Scholar] [CrossRef]
- Cerdan, L.; Moreno, F.; Johnson, M.; Muller, G.; de la Moya, S.; García-Moreno, I. Circularly polarized laser emission in optically active organic dye solutions. Phys. Chem. Chem. Phys. 2017, 19, 22088–22093. [Google Scholar] [CrossRef] [PubMed]
- Jiménez, J.; Cerdán, L.; Moreno, F.; Maroto, B.L.; García-Moreno, I.; Lunkley, J.L.; Muller, G.; de la Moya, S. Chiral Organic Dyes Endowed with Circularly Polarized Laser Emission. J. Phys. Chem. C 2017, 121, 5287–5292. [Google Scholar] [CrossRef]
- Imai, Y.; Nakano, Y.; Kawai, T.; Yuasa, J. A Smart Sensing Method for Object Identification Using Circularly Polarized Luminescence from Coordination-Driven Self-Assembly. Angew. Chem. Int. Ed. 2018, 57, 8973–8978. [Google Scholar] [CrossRef]
- Song, F.Y.; Wei, G.; Jiang, X.X.; Li, F.; Zhu, C.J.; Cheng, Y.X. Chiral sensing for induced circularly polarized luminescence using an Eu (III)-containing polymer and D- or L-proline. Chem. Commun. 2013, 49, 5772–5774. [Google Scholar] [CrossRef]
- Wang, S.; Hu, D.P.; Guan, X.Y.; Cai, S.L.; Shi, G.; Shuai, Z.G.; Zhang, J.; Peng, Q.; Wan, X.H. Brightening up Circularly Polarized Luminescence of Monosubstituted Polyacetylene by Conformation Control: Mechanism, Switching, and Sensing. Angew. Chem. Int. Ed. 2021, 60, 21918–21926. [Google Scholar] [CrossRef] [PubMed]
- Heffern, M.C.; Matosziuk, L.M.; Meade, T.J. Lanthanide Probes for Bioresponsive Imaging. Chem. Rev. 2014, 114, 4496–4539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Dai, L.X.; Webster, A.M.; Chan, W.T.K.; Mackenzie, L.E.; Pal, R.; Cobb, S.L.; Law, G.L. Unusual Magnetic Field Responsive Circularly Polarized Luminescence Probes with Highly Emissive Chiral Europium (III) Complexes. Angew. Chem. Int. Ed. 2021, 60, 1004–1010. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sato, M.; Ishiguro, S.; Saito, T.; Morishita, Y.; Sato, I.; Nishino, H.; Inoue, Y.; Soai, K. Enantioselective synthesis of near enantiopure compound by asymmetric autocatalysis triggered by asymmetric photolysis with circularly polarized light. J. Am. Chem. Soc. 2005, 127, 3274–3275. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, Y.; Ren, X.; Hu, J.; Yu, H.; Liu, J.; Huang, H.; Han, J. Chiral Polar Bifunctional Polyimide Enantiomers for Asymmetric Photo- and Piezo-catalysis. Angew. Chem. Int. Ed. 2024. [Google Scholar] [CrossRef]
- Arrico, L.; Di Bari, L.; Zinna, F. Quantifying the Overall Efficiency of Circularly Polarized Emitters. Chem. Eur. J. 2021, 27, 2920–2934. [Google Scholar] [CrossRef]
- Hasegawa, M.; Hasegawa, C.; Nagaya, Y.; Tsubaki, K.; Mazaki, Y. Multiply Twisted Chiral Macrocycles Clamped by Tethered Binaphthyls Exhibiting High Circularly Polarized Luminescence Brightness. Chem. Eur. J. 2022, 28, e202202218. [Google Scholar] [CrossRef] [PubMed]
- Hananel, U.; Schwartz, G.; Paiss, G.; Arrico, L.; Zinna, F.; Di Bari, L.; Cheshnovsky, O.; Markovich, G. Time-resolved circularly polarized luminescence of Eu3+-based systems. Chirality 2021, 33, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Willis, O.G.; Petri, F.; De Rosa, D.F.; Mandoli, A.; Pal, R.; Zinna, F.; Di Bari, L. Two-Photon Circularly Polarized Luminescence of Chiral Eu Complexes. J. Am. Chem. Soc. 2023, 145, 25170–25176. [Google Scholar] [CrossRef]
- Huang, Z.Z.; Hu, Y.; Jin, X.; Zhao, Y.H.; Su, J.H.; Ma, X. Light-Responsive Circularly Polarized Luminescence Polymers with INHIBIT Logic Function. Adv. Opt. Mater. 2021, 9, 2100135. [Google Scholar] [CrossRef]
- Yan, H.W.; He, Y.L.; Wang, D.; Han, T.; Tang, B.Z. Aggregation-induced emission polymer systems with circularly polarized luminescence. Aggregate 2023, 4, e331. [Google Scholar] [CrossRef]
- Chen, N.B.; Yan, B. Recent Theoretical and Experimental Progress in Circularly Polarized Luminescence of Small Organic Molecules. Molecules 2018, 23, 3376. [Google Scholar] [CrossRef]
- Li, M.; Lin, W.B.; Fang, L.; Chen, C.F. Recent Progress on Circularly Polarized Luminescence of Chiral Organic Small Molecules. Acta Chim. Sin. 2017, 75, 1150–1163. [Google Scholar] [CrossRef]
- Li, X.N.; Xie, Y.J.; Li, Z. The Progress of Circularly Polarized Luminescence in Chiral Purely Organic Materials. Adv. Photonics Res. 2021, 2, 2000136. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, C.H. Chiral Triarylborane-based Small Organic Molecules for Circularly Polarized Luminescence. Chem. Rec. 2022, 22, e202100199. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.L.; Li, M.; Lu, H.Y.; Chen, C.F. Advances in Helicene Derivatives with Circularly Polarized Luminescence. Chem. Commun. 2019, 55, 13793–13803. [Google Scholar] [CrossRef]
- Takaishi, K.; Hinoide, S.; Matsumoto, T.; Ema, T. Axially Chiral peri-Xanthenoxanthenes as a Circularly Polarized Luminophore. J. Am. Chem. Soc. 2019, 141, 11852–11857. [Google Scholar] [CrossRef]
- Ito, S.; Ikeda, K.; Nakanishi, S.; Imai, Y.; Asami, M. Concentration-Dependent Circularly Polarized Luminescence (CPL) of Chiral N, N’-Dipyrenyldiamines: Sign-Inverted CPL Switching between Monomer and Excimer Regions under Retention of the Monomer Emission for Photoluminescence. Chem. Commun. 2017, 53, 6323–6326. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.J.; Pu, D.; Yuan, L.; Tong, J.; Xing, S.; Tu, Z.L.; Zuo, J.L.; Zheng, W.H.; Zheng, Y.X. Planar Chiral Multiple Resonance Thermally Activated Delayed Fluorescence Materials for Efficient Circularly Polarized Electroluminescence. Angew. Chem. Int. Ed. 2023, 62, e202217045. [Google Scholar] [CrossRef]
- Liang, Y.H.; Xu, C.; Zhang, H.Q.; Wu, S.Y.; Li, J.A.; Yang, Y.F.; Mao, Z.; Luo, S.L.; Liu, C.; Shi, G.; et al. Color-Tunable Dual-Mode Organic Afterglow from Classical Aggregation-Caused Quenching Compounds for White-Light-Manipulated Anti-Counterfeiting. Angew. Chem. Int. Ed. 2023, 62, e202217616. [Google Scholar] [CrossRef]
- Wang, J.W.; Liu, Z.D.; Yang, S.M.; Lin, Y.Z.; Lin, Z.H.; Ling, Q.D. Large Changes in Fluorescent Color and Intensity of Symmetrically Substituted Arylmaleimides Caused by Subtle Structure Modifications. Chem. Eur. J. 2018, 24, 322–326. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.C.; Zhao, W.L.; Tan, K.K.; Li, M.; Chen, C.F. B, N-Embedded Hetero [9]helicene Toward Highly Efficient Circularly Polarized Electroluminescence. Angew. Chem. Int. Ed. 2024, 63, e202401835. [Google Scholar] [CrossRef] [PubMed]
- Takaishi, K.; Iwachido, K.; Takehana, R.; Uchiyama, M.; Ema, T. Evolving Fluorophores into Circularly Polarized Luminophores with a Chiral Naphthalene Tetramer: Proposal of Excimer Chirality Rule for Circularly Polarized Luminescence. J. Am. Chem. Soc. 2019, 141, 6185–6190. [Google Scholar] [CrossRef] [PubMed]
- Dhbaibi, K.; Favereau, L.; Srebro-Hooper, M.; Quinton, C.; Vanthuyne, N.; Arrico, L.; Roisnel, T.; Jamoussi, B.; Poriel, C.; Cabanetos, C.; et al. Modulation of circularly polarized luminescence through excited-state symmetry breaking and interbranched exciton coupling in helical push-pull organic systems. Chem. Sci. 2020, 11, 567–576. [Google Scholar] [CrossRef]
- Zhao, Z.H.; Liang, X.; He, M.X.; Zhang, M.Y.; Zhao, C.H. Triarylborane-Based [5]Helicenes with Full-Color Circularly Polarized Luminescence. Org. Lett. 2019, 21, 9569–9573. [Google Scholar] [CrossRef] [PubMed]
- Gon, M.; Sawada, R.; Morisaki, Y.; Chujo, Y. Enhancement and Controlling the Signal of Circularly Polarized Luminescence Based on a Planar Chiral Tetrasubstituted [2.2] Paracyclophane Framework in Aggregation System. Macromolecules 2017, 50, 1790–1802. [Google Scholar] [CrossRef]
- Morisaki, Y.; Gon, M.; Sasamori, T.; Tokitoh, N.; Chujo, Y. Planar Chiral Tetrasubstituted [2.2] Paracyclophane: Optical Resolution and Functionalization. J. Am. Chem. Soc. 2014, 136, 3350–3353. [Google Scholar] [CrossRef]
- Teng, J.M.; Zhang, D.W.; Chen, C.F. Recent Progress in Circularly Polarized Luminescence of [2.2] Paracyclophane Derivatives. ChemPhotoChem 2021, 6, e202100228. [Google Scholar] [CrossRef]
- Xu, D.; Hua, X.; Liu, C.; Luo, J.; Zheng, W.; Cheng, Y. Induced Red Circularly Polarized Luminescence Emission Promoted by Intermolecular Förster Resonance Energy Transfer through a Cholesteric Liquid Crystal Medium. ACS Appl. Mater. Interfaces 2023, 15, 25783–25790. [Google Scholar] [CrossRef]
- Xu, D.; Zheng, W.H. Synthesis and Chiroptical Properties of Planar Chiral Azahelicenes Based on [2.2] Paracyclophane. Org. Lett. 2021, 23, 8612–8616. [Google Scholar] [CrossRef]
- Li, S.Y.; Sun, Z.B.; Zhao, C.H. Charge-Transfer Emitting Triarylborane π-Electron Systems. Inorg. Chem. 2017, 56, 8705–8717. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.H.; Wakamiya, A.; Inukai, Y.; Yamaguchi, S. Highly emissive organic solids containing 2,5-diboryl-1,4-phenylene unit. J. Am. Chem. Soc. 2006, 128, 15934–15935. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Y.; Li, Z.Y.; Lu, B.; Wang, Y.; Ma, Y.D.; Zhao, C.H. Solid-State Emissive Triarylborane-Based [2.2] Paracyclophanes Displaying Circularly Polarized Luminescence and Thermally Activated Delayed Fluorescence. Org. Lett. 2018, 20, 6868–6871. [Google Scholar] [CrossRef]
- Zhang, M.Y.; Liang, X.; Ni, D.N.; Liu, D.H.; Peng, Q.; Zhao, C.H. 2-(Dimesitylboryl) phenyl-Substituted [2.2] Paracyclophanes Featuring Intense and Sign-Invertible Circularly Polarized Luminescence. Org. Lett. 2021, 23, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.F.; Wang, M.; Zhao, C.H. The Solid-State Multi-Color Fluorescence Switching from a [2.2] Paracyclophane-Based Triarylborane. Chem. Eur. J. 2024, 30, e202402287. [Google Scholar] [CrossRef] [PubMed]
- Entwistle, C.D.; Marder, T.B. Boron Chemistry Lights the Way: Optical Properties of Molecular and Polymeric Systems. Angew. Chem. Int. Ed. 2002, 41, 2927–2931. [Google Scholar] [CrossRef]
- Ji, L.; Griesbeck, S.; Marder, T.B. Recent developments in and perspectives on three-coordinate boron materials: A bright future. Chem. Sci. 2017, 8, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Wakamiya, A.; Yamaguchi, S. Designs of Functional π-Electron Materials based on the Characteristic Features of Boron. Bull. Chem. Soc. Jpn. 2015, 88, 1357–1377. [Google Scholar] [CrossRef]
- Ren, Y.; Jäkle, F. Merging thiophene with boron: New building blocks for conjugated materials. Dalton Trans. 2016, 45, 13996–14007. [Google Scholar] [CrossRef]
- Mellerup, S.K.; Wang, S. Boron-based stimuli responsive materials. Chem. Soc. Rev. 2019, 48, 3537–3549. [Google Scholar] [CrossRef]
- Fu, G.L.; Zhang, H.Y.; Yan, Y.Q.; Zhao, C.H. p-Quaterphenyls Laterally Substituted with a Dimesitylboryl Group: A Promising Class of Solid-State Blue Emitters. J. Org. Chem. 2012, 77, 1983–1990. [Google Scholar] [CrossRef] [PubMed]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2019. [Google Scholar]
- Bruhn, T.; Schaumlöffel, A.; Hemberger, Y.; Bringmann, G. SpecDis: Quantifying the Comparison of Calculated and Experimental Electronic Circular Dichroism Spectra. Chirality 2013, 25, 243–249. [Google Scholar] [CrossRef] [PubMed]
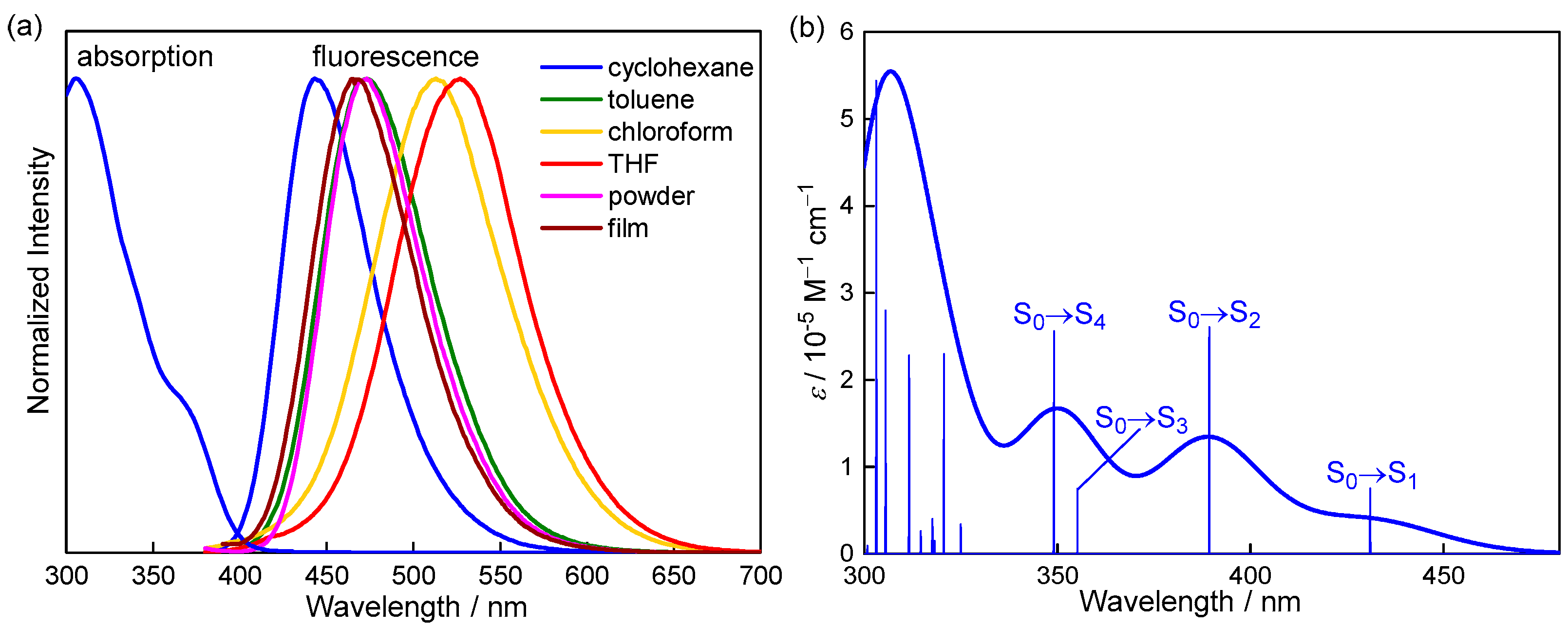
| λabs/nm (ε/104 M−1 cm−1) | λem/nm | ∆ν/103 cm−1 | ΦF | τ/ns | kr/107 s−1 | knr/107 s−1 | |
|---|---|---|---|---|---|---|---|
| cyclohexane | 305 (3.19), 373 (0.91) | 443 | 4.23 | 0.37 1 | 12 | 3.08 | 5.25 |
| toluene | 306 (3.34), 369 (1.00) | 473 | 5.96 | 0.28 1 | − | − | − |
| chloroform | 305 (3.17), 372 (0.85) | 513 | 7.39 | 0.16 1 | − | − | − |
| THF | 305 (4.28), 368 (1.38) | 527 | 8.20 | 0.15 1 | − | − | − |
| film | − | 465 | − | 0.64 2 | 15 | 4.27 | 2.40 |
| powder | − | 473 | − | 0.64 2 | 14 | 4.57 | 2.57 |
| Rp-Isomer | Sp-Isomer | |||||
|---|---|---|---|---|---|---|
| gabs/10−3 (λ/nm) | glum/10−3 (λ/nm) | BCPL/ M−1 cm−1 | gabs/10−3 (λ/nm) | glum/10−3 (λ/nm) | BCPL/ M−1 cm−1 | |
| cyclohexane | −7.27 (380) | −9.13 (454) | 53.7 | +7.57 (379) | +9.62 (456) | 56.7 |
| THF | −6.03 (378) | −7.23 (531) | 23.1 | +6.51 (378) | +8.29 (536) | 26.6 |
| film | − | −4.32 (466) | − | − | +5.40 (466) | − |
| Fraction | Retention Time/min | Area | Area% | ee | |
|---|---|---|---|---|---|
| rac-form | Rp-isomer | 2.427 | 2,190,497 | 50.6 | |
| Sp-isomer | 3.185 | 2,141,703 | 49.4 | ||
| Rp-isomer | Rp-isomer | 2.479 | 7,241,036 | 100 | >99% |
| Sp-isomer | − | − | − | − | |
| Sp-isomer | Rp-isomer | 2.476 | 6226 | 0.08% | − |
| Sp-isomer | 3.224 | 7,993,768 | 99.92% | >99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, L.; Zhang, M.; Zhao, C. [(2-Dimesitylboryl)phenyl]ethynyl-Substituted [2.2]Paracyclophane Exhibiting Circularly Polarized Luminescence in Both Solution and Solid-State. Molecules 2025, 30, 390. https://doi.org/10.3390/molecules30020390
Guo L, Zhang M, Zhao C. [(2-Dimesitylboryl)phenyl]ethynyl-Substituted [2.2]Paracyclophane Exhibiting Circularly Polarized Luminescence in Both Solution and Solid-State. Molecules. 2025; 30(2):390. https://doi.org/10.3390/molecules30020390
Chicago/Turabian StyleGuo, Lianfeng, Mengyuan Zhang, and Cuihua Zhao. 2025. "[(2-Dimesitylboryl)phenyl]ethynyl-Substituted [2.2]Paracyclophane Exhibiting Circularly Polarized Luminescence in Both Solution and Solid-State" Molecules 30, no. 2: 390. https://doi.org/10.3390/molecules30020390
APA StyleGuo, L., Zhang, M., & Zhao, C. (2025). [(2-Dimesitylboryl)phenyl]ethynyl-Substituted [2.2]Paracyclophane Exhibiting Circularly Polarized Luminescence in Both Solution and Solid-State. Molecules, 30(2), 390. https://doi.org/10.3390/molecules30020390







