5-Aminopyrazole Dimerization: Cu-Promoted Switchable Synthesis of Pyrazole-Fused Pyridazines and Pyrazines via Direct Coupling of C-H/N-H, C-H/C-H, and N-H/N-H Bonds
Abstract
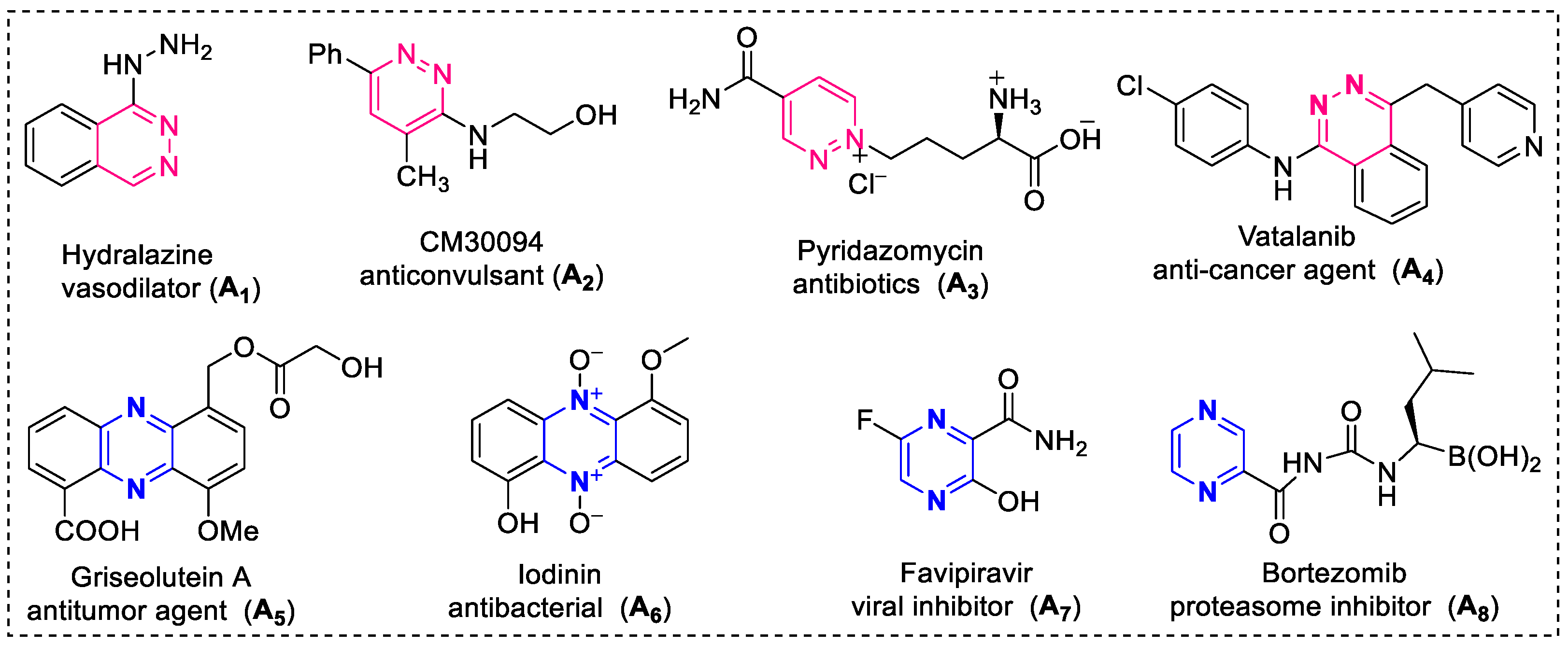
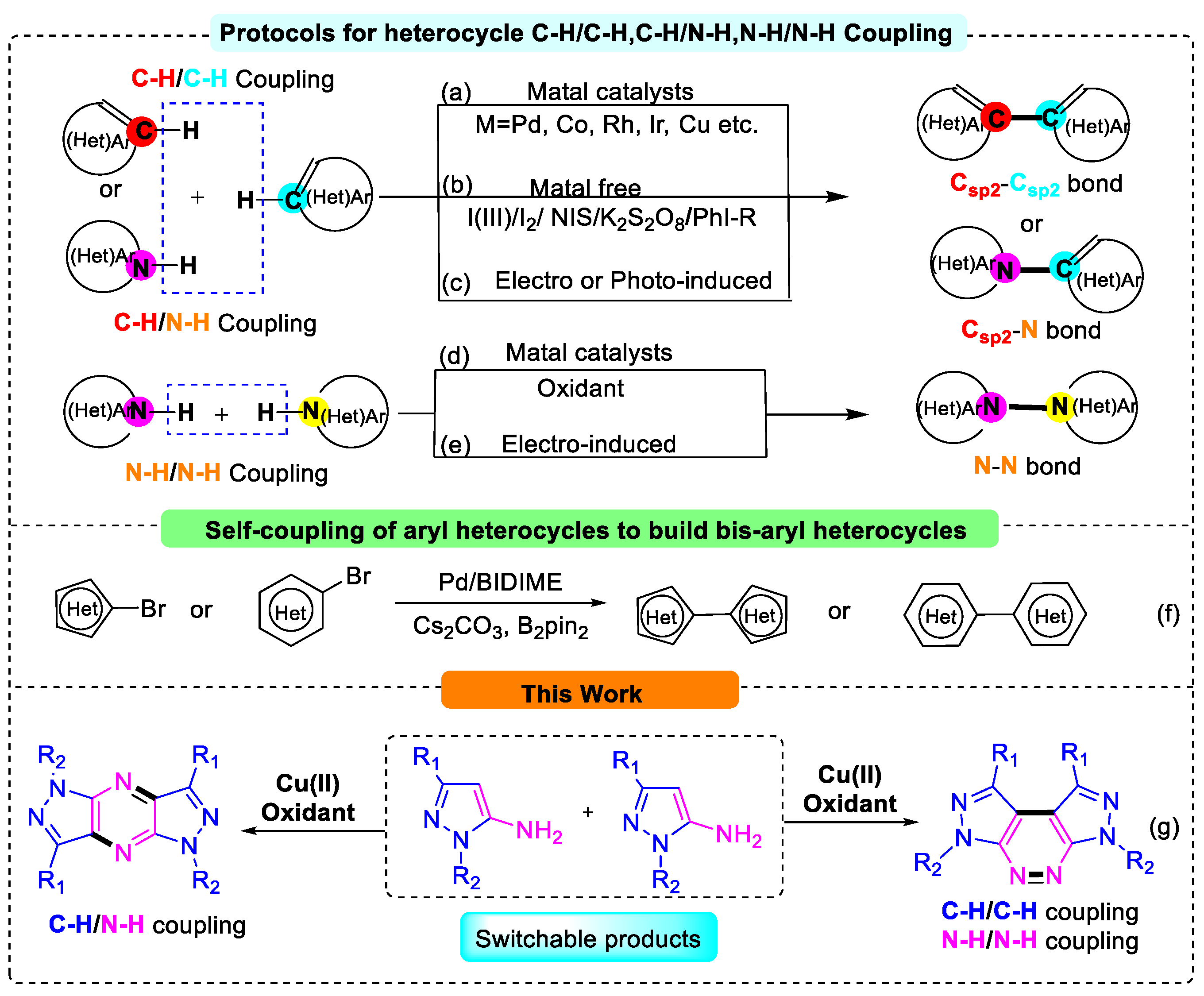
1. Introduction
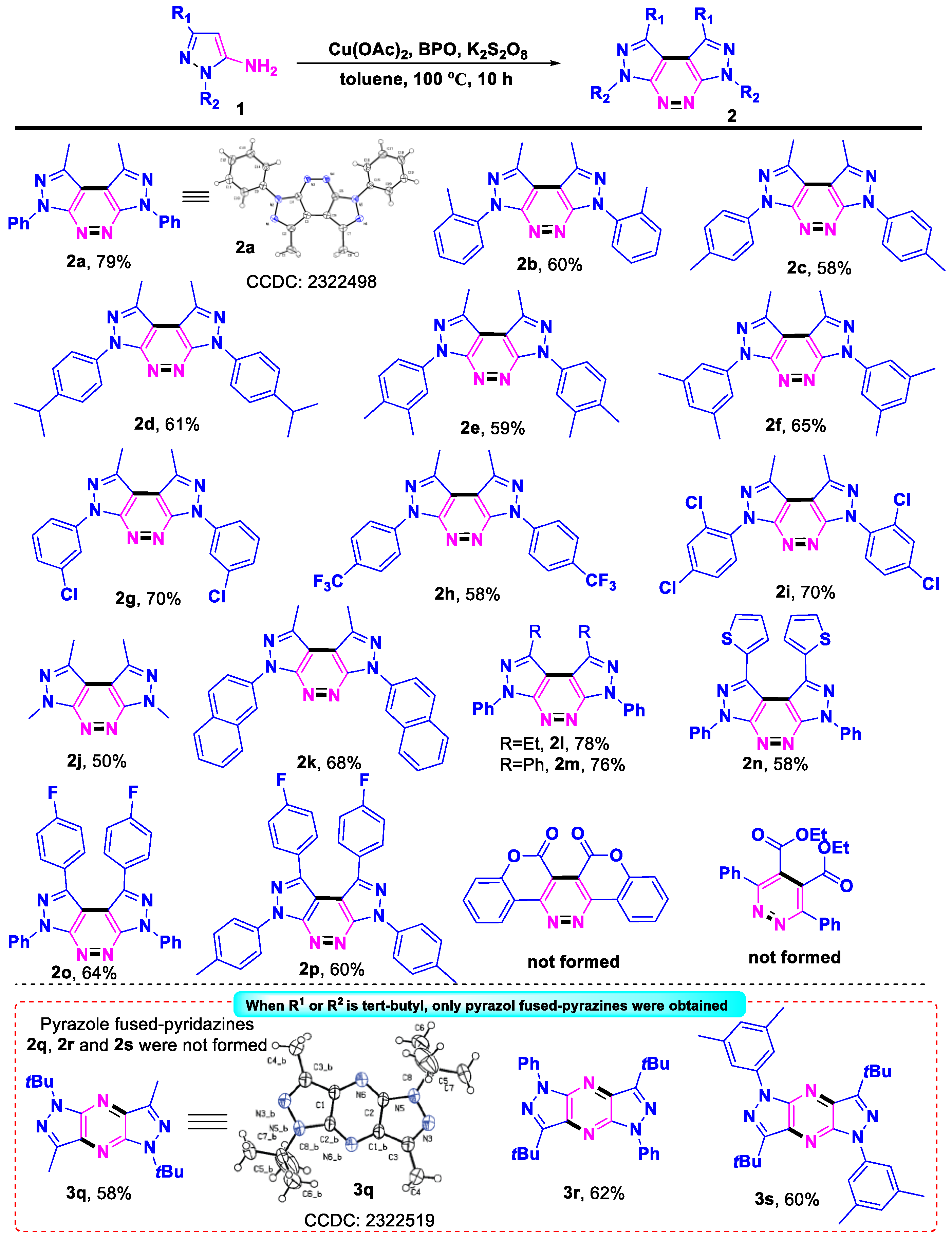
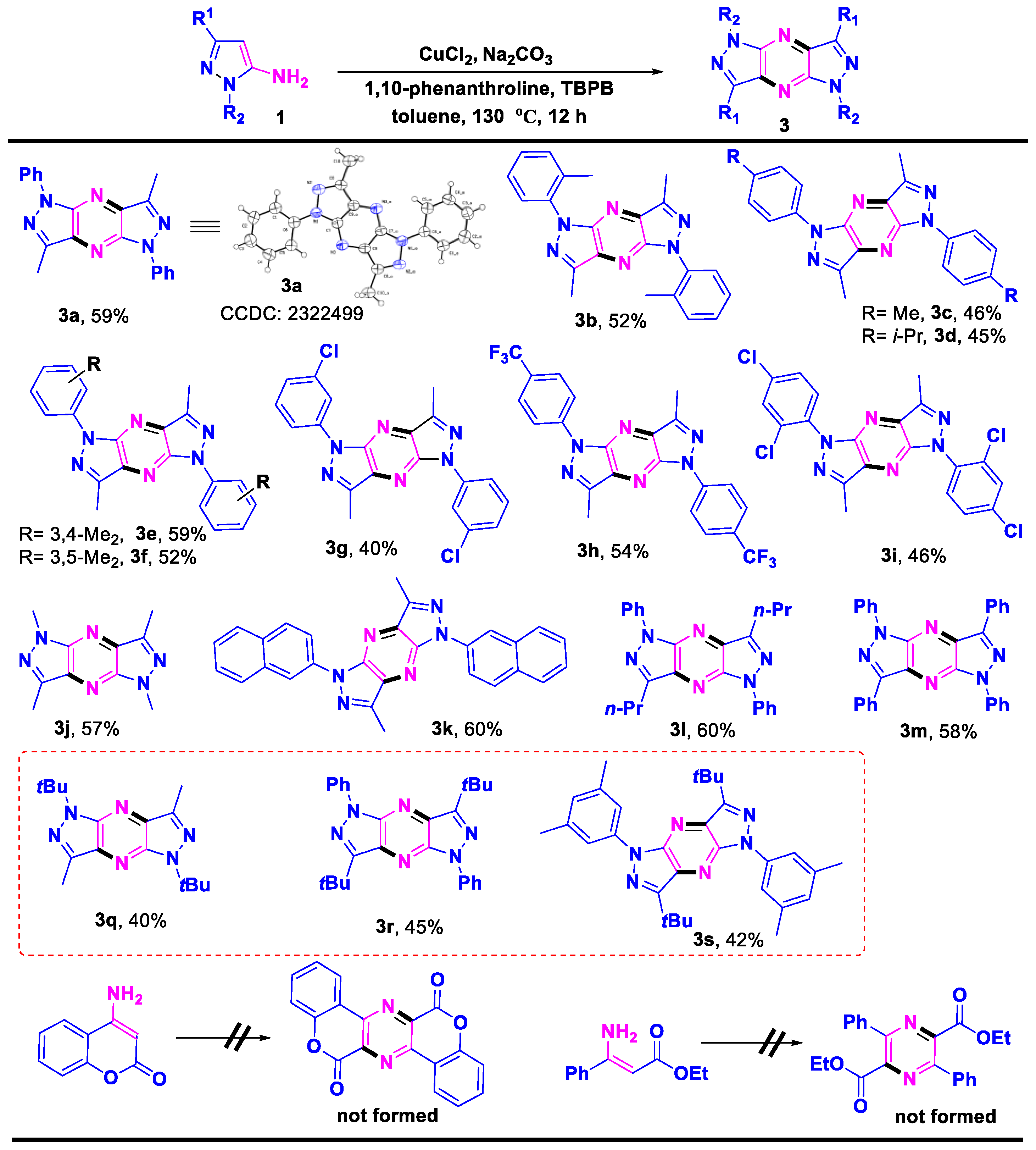
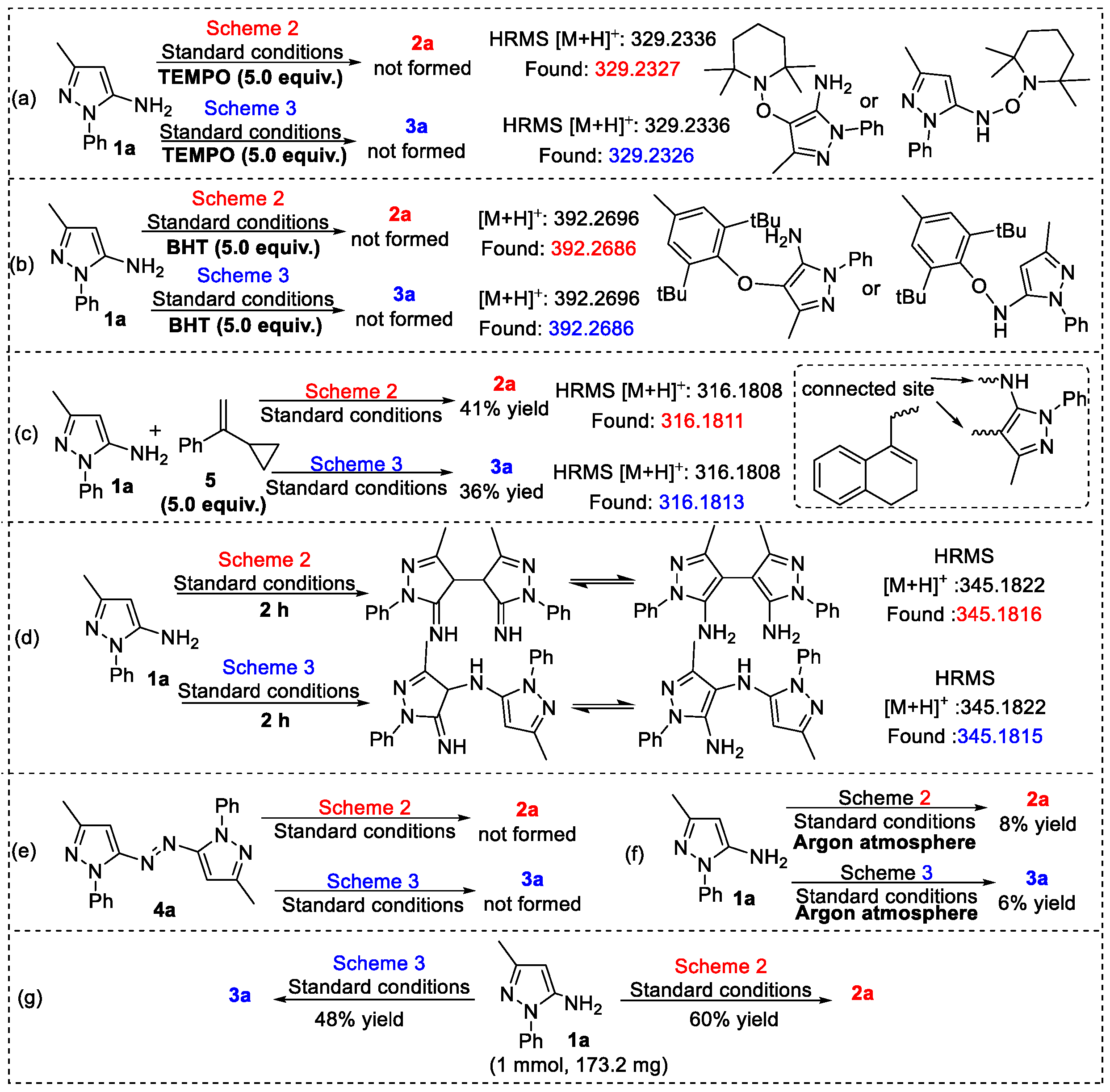
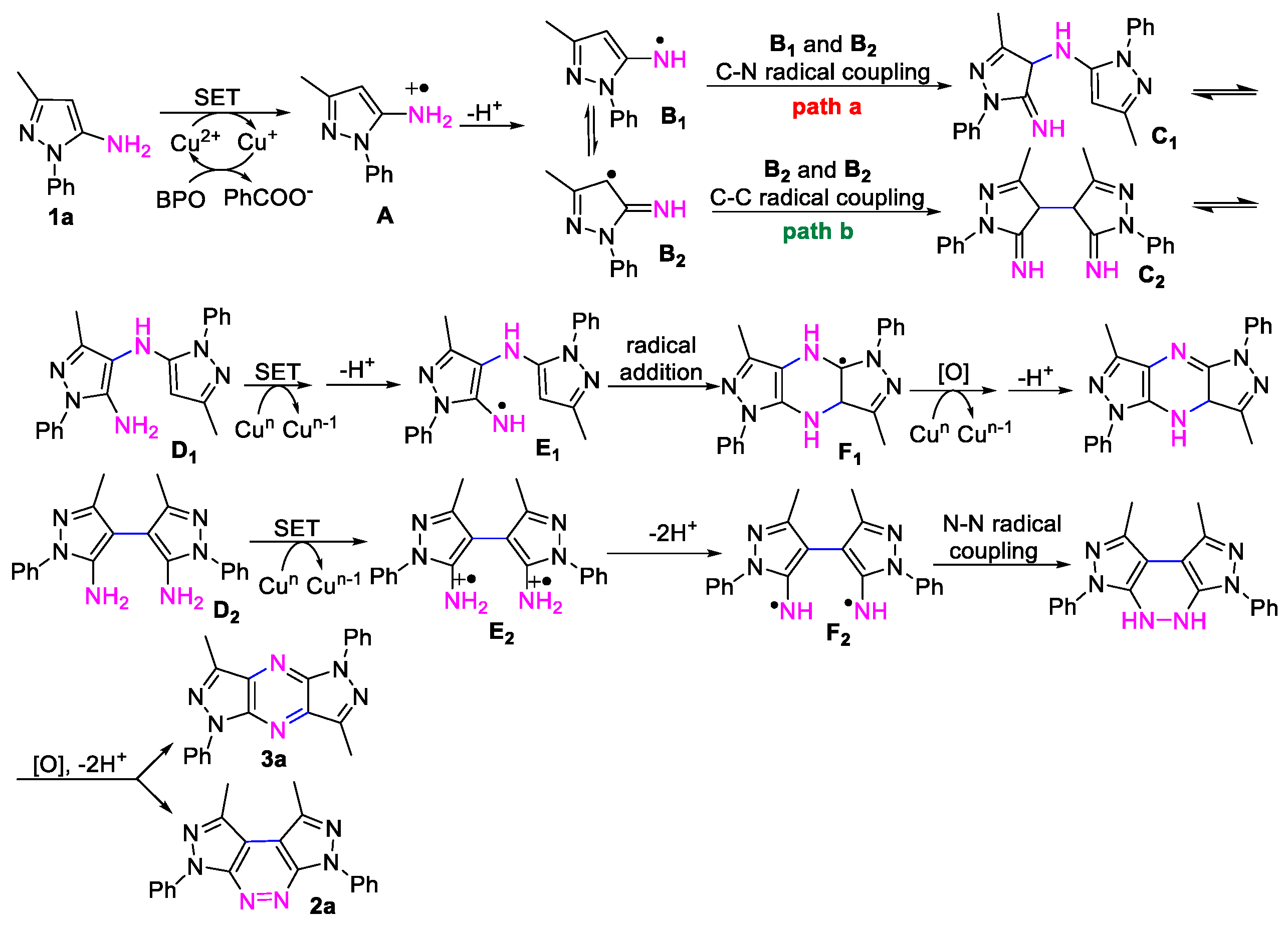
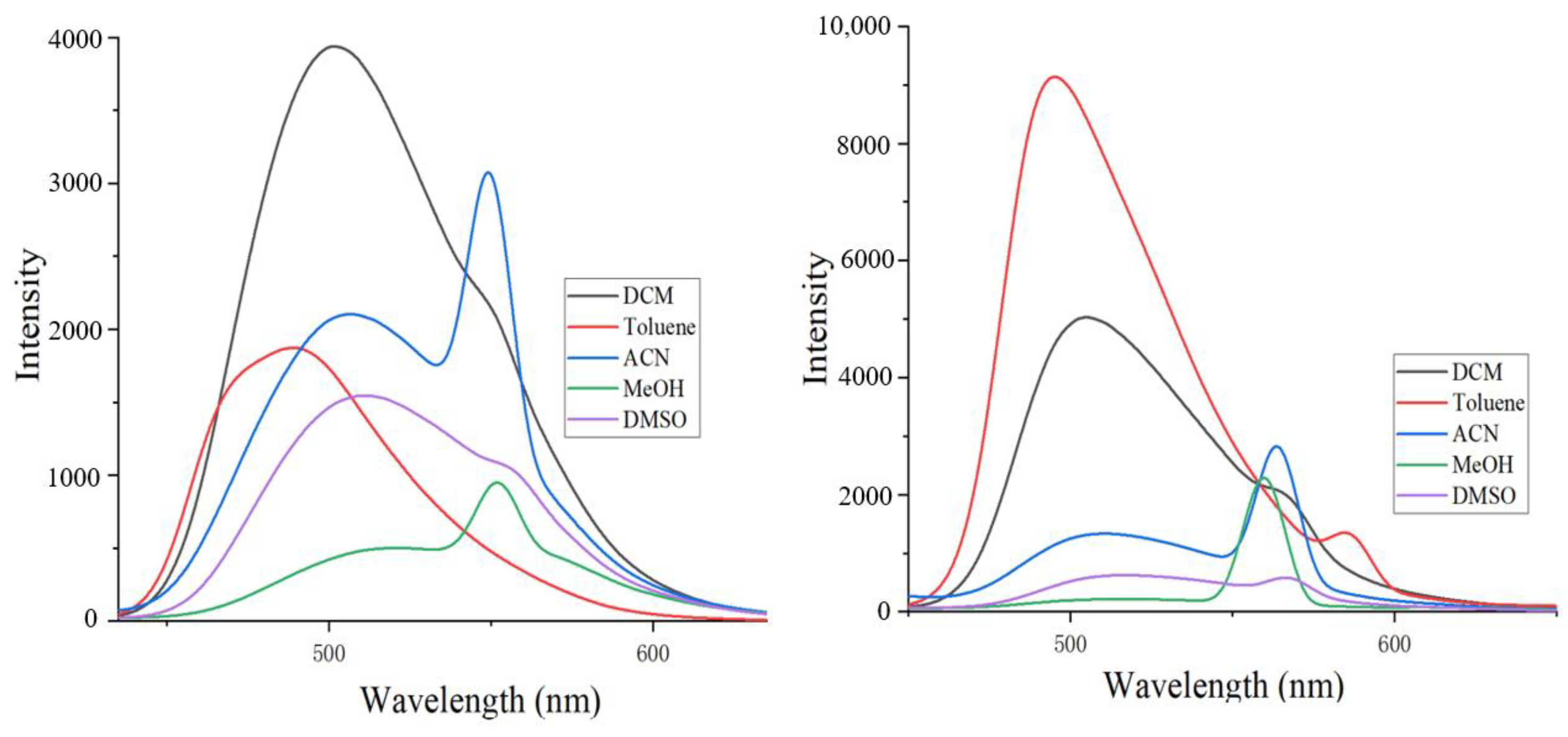
2. Results and Discussion
3. Materials and Methods
3.1. General Information
3.2. Synthetic Procedures
3.3. Characterization of Products
- 1,8-Dimethyl-3,6-diphenyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e]pyridazine (2a), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 27 mg, 70%, yellow solid, m.p.: 242–243 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.46–8.41 (m, 4H), 7.60–7.55 (m, 4H), 7.40–7.35 (m, 2H), 3.01 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 150.6, 140.3, 139.2, 129.2, 126.6, 121.6, 109.7, 15.4. HRMS (ESI): m/z [M + H]+ calcd. for C20H17N6: 341.1509; found: 341.1505.
- 1,8-Dimethyl-3,6-di-o-tolyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e]pyridazine (2b), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 22 mg, 60%, yellow solid, m.p.: 220–221 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.50 (d, J = 7.2 Hz, 2H), 7.43–7.40 (m, 4H), 7.39–7.34 (m, 2H), 3.01 (s, 6H), 2.21 (s, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 151.3, 140.1, 137.2, 135.5, 131.3, 129.2, 127.9, 126.6, 108.1, 18.4, 15.3. HRMS (ESI): m/z [M + H]+ calcd. for C22H21N6: 369.1822; found: 369.1812.
- 1,8-Dimethyl-3,6-di-p-tolyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e]pyridazine (2c), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 21 mg, 58%, yellow solid, m.p.: 272–273 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.27 (d, J = 8.5 Hz, 4H), 7.36 (d, J = 8.5 Hz, 4H), 2.98 (s, 6H), 2.44 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 150.4, 139.9, 136.8, 136.4, 129.8, 121.5, 109.4, 21.1, 15.4. HRMS (ESI): m/z [M + Na]+ calcd. for C22H21N6: 369.1822; found: 369.1816.
- 3,6-Bis(4-isopropylphenyl)-1,8-dimethyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2d), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 26 mg, 61%, yellow solid, m.p.: 227–228 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.29 (d, J = 8.5 Hz, 4H), 7.42 (d, J = 8.5 Hz, 4H), 3.05–3.00 (m, 2H), 2.99 (s, 6H), 1.33 (s, 6H), 1.31 (s, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 150.5, 147.5, 140.0, 137.0, 127.2, 121.7, 109.4, 33.8, 24.0, 15.4. HRMS (ESI): m/z [M + H]+ calcd. for C26H29N6: 425.2448; found: 425.2440.
- 3,6-Bis(3,4-dimethylphenyl)-1,8-dimethyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2e), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 23 mg, 59%, yellow solid, m.p.: 269–270 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.10 (d, J = 6.4 Hz, 4H), 7.31 (d, J = 8.8 Hz, 2H), 2.98 (s, 6H), 2.40 (s, 6H), 2.35 (s, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 150.5, 139.8, 137.6, 137.0, 135.2, 130.2, 122.8, 119.3, 109.3, 20.1, 19.4, 15.4. HRMS (ESI): m/z [M + H]+ calcd. for C24H25N6: 397.2135; found: 397.2125.
- 3,6-Bis(3,5-dimethylphenyl)-1,8-dimethyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2f), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 25 mg, 63%, yellow solid, m.p.: 272–273 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.99 (s, 4H), 7.02 (s, 2H), 2.99 (s, 6H), 2.46 (s, 12H). 13C NMR (101 MHz, CDCl3) δ (ppm) 150.5, 140.0, 139.0, 128.5, 119.6, 109.4, 29.7, 21.5, 15.4. HRMS (ESI): m/z [M + H]+ calcd. for C24H25N6: 397.2135; found: 397.2134.
- 3,6-Bis(3-chlorophenyl)-1,8-dimethyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2g), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 29 mg, 70%, yellow solid, m.p.: 251–252 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.50–8.44 (m, 4H), 7.50 (t, J = 8.0 Hz, 2H), 7.34 (ddd, J = 8.1, 2.0, 1.0 Hz, 2H), 2.99 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 150.7, 141.0, 140.1, 135.1, 130.3, 126.6, 121.3, 119.2, 110.1, 15.4. HRMS (ESI): m/z [M + H]+ calcd. for C20H15Cl2N6: 409.0730; found: 409.0723.
- 1,8-Dimethyl-3,6-bis(4-(trifluoromethyl)phenyl)-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2h), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 28 mg, 58%, white solid, m.p.: 252–253 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.70 (d, J = 8.5 Hz, 4H), 7.83 (d, J = 8.5 Hz, 4H), 3.01 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 150.9, 141.8, 141.5, 126.5 (q, JCF = 3.7 Hz), 120.8, 110.5, 15.5. 19F NMR (470 MHz, CDCl3) δ (ppm) −62.13. HRMS (ESI): m/z [M + H]+ calcd. for C22H15F6N6: 477.1257; found: 477.1249.
- 3,6-Bis(2,4-dichlorophenyl)-1,8-dimethyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2i), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 33 mg, 70%, yellow solid, m.p.: 199–200 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.65 (d, J = 2.5 Hz, 2H), 7.59 (d, J = 8.5 Hz, 2H), 7.45 (dd, J = 8.5, 2.0 Hz, 2H), 3.01 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 151.4, 141.5, 135.8, 134.4, 133.0, 130.6, 130.5, 127.9, 108.8, 15.4. HRMS (ESI): m/z [M + H]+ calcd. for C20H13Cl4N6: 476.9950; found: 476.9943.
- 1,3,6,8-Tetramethyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2j), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 11 mg, 50%, yellow solid, m.p.: 106–107 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 4.39 (s, 6H), 2.84 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 150.6, 138.0, 107.4, 35.1, 14.9. HRMS (ESI): m/z [M + H]+ calcd. for C10H13N6: 217.1196; found: 217.1188.
- 1,8-Dimethyl-3,6-di(naphthalen-2-yl)-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2k), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 30 mg, 68%, yellow solid, m.p.: 242–243 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.96 (d, J = 2.4 Hz, 2H), 8.59 (dd, J = 9.2, 2.4 Hz, 2H), 8.05–7.99 (m, 4H), 7.90 (d, J = 8.0 Hz, 2H), 7.57–7.49 (m, 4H), 3.01 (s, 6H). 13C NMR (126 MHz, CDCl3) δ 150.8, 140.5, 136.8, 133.6, 131.9, 129.2, 128.4, 127.7, 126.7, 126.0, 120.4, 119.0, 109.8, 15.5. HRMS (ESI): m/z [M + H]+ calcd. for C28H21N6: 441.1822; found: 441.1815.
- 1,8-Diethyl-3,6-diphenyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2l), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 29 mg, 78%, yellow solid, m.p.: 246–247 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.45 (d, J = 7.5 Hz, 4H), 7.57 (t, J = 7.5 Hz, 4H), 7.37 (t, J = 7.5 Hz, 2H), 3.37 (q, J = 7.5 Hz, 4H), 1.58 (d, J = 7.5 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ 150.5, 145.6, 139.3, 129.2, 126.5, 121.6, 108.9, 22.8, 13.4. HRMS (ESI): m/z [M + H]+ calcd. for C22H21N6: 369.1822; found: 369.1817.
- 1,3,6,8-Tetraphenyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2m), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 35 mg, 76%, orange solid, m.p.: 217–218 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.54–8.50 (m, 4H), 7.64–7.59 (m, 4H), 7.43 (t, J = 7.6 Hz, 2H), 7.37 (d, J = 6.8 Hz, 4H), 7.21 (t, J = 7.6 Hz, 2H), 7.03 (t, J = 8.0 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ (ppm) 151.1, 144.7, 139.1, 132.3, 129.3, 128.6, 127.8, 127.2, 122.3, 108.2. HRMS (ESI): m/z [M + H]+ calcd. for C30H21N6: 465.1822; found: 465.1813.
- 3,6-Diphenyl-1,8-di(thiophen-2-yl)-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2n), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 28 mg, 58%, orange solid, m.p.: 253–354 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.46 (d, J = 8.0 Hz, 4H), 7.61 (t, J = 8.0 Hz, 4H), 7.44 (t, J = 7.5 Hz, 2H), 7.32 (d, J = 5.0 Hz, 2H), 6.82 (d, J = 3.5 Hz, 2H), 6.80–6.78 (m, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 150.8, 138.8, 138.6, 133.0, 129.3, 129.0, 127.5, 127.4, 126.8, 122.5, 108.4. HRMS (ESI): m/z [M + H]+ calcd. for C26H17N6S2: 477.0951; found: 477.0945.
- 1,8-Bis(4-fluorophenyl)-3,6-diphenyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2o), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 32 mg, 64%, orange solid, m.p.: 254–255 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.51–8.47 (m, 4H), 7.64–7.59 (m, 4H), 7.47–7.42 (m, 2H), 7.37–7.32 (m, 4H), 6.84–6.77 (m, 4H). 13C NMR (126 MHz, CDCl3) δ (ppm) 164.6, 162.1, 151.0, 143.5, 139.0, 130.4 (d, JCF = 8.3 Hz), 128.4, 127.4, 122.3, 115.0, 114.9 (d, JCF = 21.9 Hz), 108.0. 19F NMR (470 MHz, CDCl3) δ (ppm) −112.51. HRMS (ESI): m/z [M + H]+ calcd. for C30H19F2N6: 501.1634; found: 501.1626.
- 1,8-Bis(4-fluorophenyl)-3,6-di-p-tolyl-3,6-dihydrodipyrazolo [3,4-c:4′,3′-e] pyridazine (2p), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 5:1), 32 mg, 60%, orange solid, m.p.: 253–254 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.33 (d, J = 8.5 Hz, 4H), 7.40 (d, J = 8.5 Hz, 4H), 7.33 (dd, J = 8.5, 5.5 Hz, 4H), 6.79 (t, J = 8.5 Hz, 4H), 2.47 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 164.3, 162.3, 150.8, 143.1, 137.3, 136.6, 130.4 (d, JCF = 8.3 Hz), 128.5 (d, JCF = 3.2 Hz), 122.2, 115.0, 114.8 (d, JCF = 22.0 Hz), 107.8, 21.1. 19F NMR (470 MHz, CDCl3) δ (ppm) −112.67. HRMS (ESI): m/z [M + H]+ calcd. for C32H23F2N6: 529.1947; found: 529.1939.
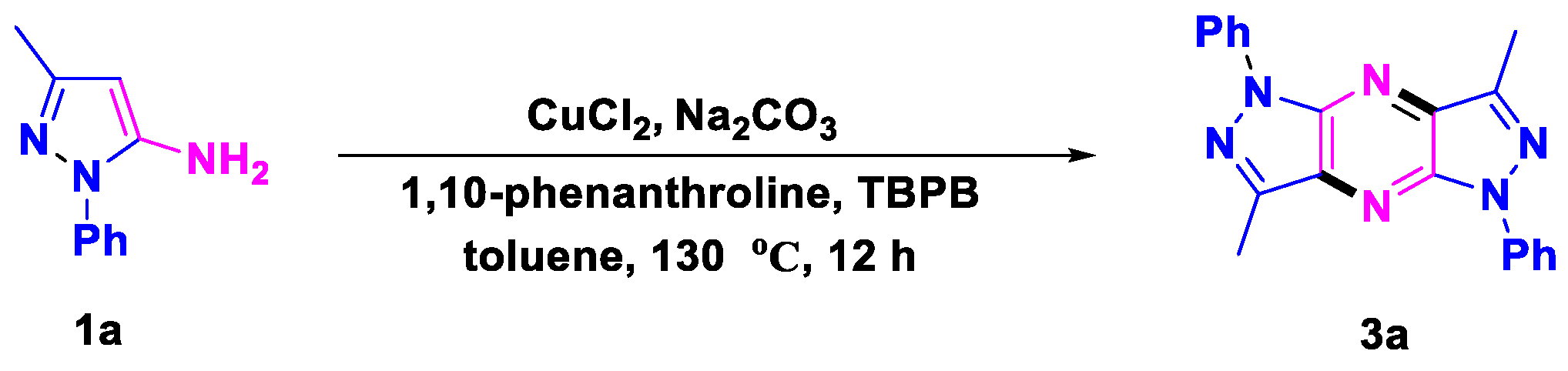
- 3,7-Dimethyl-1,5-diphenyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3a), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 20 mg,59%, orange solid, m.p.: 276–277 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.40 (d, J = 8.0 Hz, 4H), 7.57 (t, J = 8.0 Hz, 4H), 7.32 (t, J = 7.5 Hz, 2H), 2.84 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 143.1, 142.3, 139.5, 134.1, 129.2, 125.6, 119.8, 11.6. HRMS (ESI): m/z [M + H]+ calcd. for C20H17N6: 341.1509; found: 341.1501.
- 3,7-Dimethyl-1,5-di-o-tolyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3b), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 19 mg, 52%, yellow solid, m.p.: 234–235 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.50–7.47 (m, 2H), 7.44 (td, J = 7.1, 6.6, 2.1 Hz, 4H), 7.41–7.36 (m, 2H), 2.74 (s, 6H), 2.28 (s, 6H). 13C NMR (101 MHz, CDCl3) δ (ppm) 143.3, 142.6, 137.3, 135.5, 133.3, 131.4, 128.7, 127.7, 126.7, 18.7, 11.7. HRMS (ESI): m/z [M + H]+ calcd. for C22H21N6: 369.1822; found: 369.1816.
- 3,7-Dimethyl-1,5-di-p-tolyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3c), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 17 mg, 46%, yellow solid, m.p.: 273–274 °C. Due to the low solubility of the product, 0.6 mL of CDCl3/TFA (v:v = 20:1) was used to measure the NMR spectrum. 1H NMR ((400 MHz, CDCl3/TFA = 20:1(v:v)) δ (ppm) 7.71 (d, J = 8.4 Hz, 4H), 7.43 (d, J = 8.0 Hz, 4H), 2.87 (s, 6H), 2.48 (s, 6H). 13C NMR (101 MHz, CDCl3/TFA = 20:1(v:v)) δ (ppm) 162.3 (q, JCF = 43.8 Hz, CO), 143.40, 142.11, 139.87, 133.84, 133.62, 130.53, 124.03, 114.3 (q, JCF = 285.6 Hz, CF3), 20.95, 10.76. HRMS (ESI): m/z [M + H]+ calcd. for C17H14NO5: 369.1822; found: 369.1813.
- 1,5-Bis(4-isopropylphenyl)-3,7-dimethyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3d), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 19 mg, 45%, white solid, m.p.: 236–237 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.26 (d, J = 8.6 Hz, 4H), 7.42 (d, J = 8.6 Hz, 4H), 3.00 (m, 2H), 2.82 (s, 6H), 1.33 (d, J = 6.9 Hz, 12H). 13C NMR (101 MHz, CDCl3) δ 146.3, 142.6, 142.1, 137.3, 133.9, 127.2, 120.0, 33.8, 24.1, 11.6. HRMS (ESI): m/z [M + H]+ calcd. for C26H29N6: 425.2448; found: 425.2441.
- 1,5-Bis(3,4-dimethylphenyl)-3,7-dimethyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3e), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 23 mg, 59%, yellow solid, m.p.: 251–252 °C. Due to the low solubility of the product, 0.6 mL of CDCl3/TFA (v:v = 20:1) was used to measure the NMR spectrum. 1H NMR (500 MHz, CDCl3/TFA = 20:1(v:v)) δ (ppm) 7.55 (d, J = 8.8 Hz, 1H), 7.37 (d, J = 8.4 Hz, 1H), 2.87 (s, 1H), 2.38 (s, 3H). 13C NMR (126 MHz, CDCl3/TFA = 20:1(v:v)) δ (ppm) 162.2 (q, JCF = 44.1 Hz, CO), 143.1, 142.0, 138.8, 138.5, 133.7, 130.9, 125.1, 121.5, 114.3 (q, JCF = 285.4 Hz, CF3), 19.7, 19.4, 10.8. HRMS (ESI): m/z [M + H]+ calcd. for C24H25N6: 397.2135; found: 397.2125.
- 1,5-Bis(3,5-dimethylphenyl)-3,7-dimethyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3f), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 21 mg, 52%, yellow solid, m.p.: 254–255 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 7.98 (s, 4H), 6.97 (s, 2H), 2.83 (s, 6H), 2.47 (s, 12H). 13C NMR (101 MHz, CDCl3) δ (ppm) 153.3, 142.7, 142.2, 139.3, 139.0, 134.0, 127.4, 117.9, 21.6, 11.6. HRMS (ESI): m/z [M + H]+ calcd. for C24H25N6: 397.2135; found: 397.2123.
- Methyl 6-chloro-9-oxo-3,3a-dihydro-9H-chromeno [3,2-c]isoxazole-3-carboxylate (3g), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 16 mg, 40%, yellow solid, m.p.: 238–239 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.47 (s, 2H), 8.39 (d, J = 8.5 Hz, 2H), 7.49 (t, J = 8.0 Hz, 2H), 7.28 (d, J = 10.0 Hz, 2H), 2.84 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 143.8, 142.3, 140.4, 135.0, 134.2, 130.3, 125.5, 119.5, 117.3, 11.6. HRMS (ESI): m/z [M + H]+ calcd. for C20H15Cl2N6: 397.2135; found: 397.2130.
- 3,7-Dimethyl-1,5-bis(4-(trifluoromethyl)phenyl)-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3h), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 26 mg, 54%, yellow solid, m.p.: 252–253 °C. Due to the low solubility of the product, 0.6 mL of CDCl3/TFA (v:v = 20:1) was used to measure the NMR spectrum. 1H NMR (500 MHz, CDCl3/TFA = 20:1 (v:v)) δ (ppm) 8.34 (d, J = 8.0 Hz, 4H), 7.88 (d, J = 8.5 Hz, 4H), 2.90 (s, 6H). 13C NMR (126 MHz, CDCl3/TFA = 20:1 (v:v)) δ (ppm) 162.2 (q, JCF = 44.0 Hz, CO), 145.1, 142.6, 140.6, 134.5, 126.9 (q, JCF = 3.7 Hz), 121.2, 114.2(q, JCF = 284.8 Hz, CF3), 11.2. 19F NMR (470 MHz, CDCl3) δ (ppm)-62.13. HRMS (ESI): m/z [M + H]+ calcd. for C22H15F6N6: 477.1257; found: 477.1249.
- 1,5-Bis(2,4-dichlorophenyl)-3,7-dimethyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3i), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 22 mg, 46%, light yellow solid, m.p.: 256–257 °C. Due to the low solubility of the product, 0.6 mL of CDCl3/TFA (v:v = 20:1) was used to measure the NMR spectrum. 1H NMR (500 MHz, CDCl3/TFA = 20:1 (v:v)) δ (ppm) 7.69 (d, J = 2.5 Hz, 2H), 7.55 (d, J = 8.0 Hz, 2H), 7.51 (dd, J = 8.5, 2.5 Hz, 2H), 2.80 (s, 6H). 13C NMR (126 MHz, CDCl3/TFA = 20:1 (v:v)) δ (ppm) 161.7 (q, JCF = 43.5Hz, CO), 144.5, 143.1, 137.4, 133.8, 133.4, 132.3, 130.8, 130.8, 128.4, 114.2 (q, JCF = 284.8Hz, CF3). HRMS (ESI): m/z [M + H]+ calcd. for C20H13Cl4N6: 476.9950; found: 476.9945.
- 1,3,5,7-Tetramethyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3j), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 12 mg, 57%, yellow solid, m.p.: 216–217 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 4.16 (s, 6H), 2.72 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 142.9, 140.1, 132.6, 34.1, 11.5. HRMS (ESI): m/z [M + H]+ calcd. for C10H13N6: 217.1196; found: 217.1190.
- 3,7-Dimethyl-1,5-di(naphthalen-2-yl)-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3k), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 26 mg, 60%, yellow solid, m.p.: 252–253 °C. Due to the low solubility of the product, 0.6 mL of CDCl3/TFA (v:v = 20:1) was used to measure the NMR spectrum. 1H NMR (500 MHz, CDCl3/TFA = 20:1 (v:v)) δ 8.45 (s, 2H), 8.10 (d, J = 1.5 Hz, 4H), 7.97 (ddd, J = 12.0, 7.6, 2.0 Hz, 4H), 7.61 (m, 4H), 2.93 (s, 6H). 13C NMR (126 MHz, CDCl3/TFA = 20:1 (v:v)) δ 162.1 (q, JCF = 42.8 Hz, CO), 144.01, 142.41, 134.29, 134.13, 133.45, 132.68, 129.99, 128.27, 128.01, 127.45, 127.09, 121.35, 120.98, 114.2 (q, JCF = 284.8 Hz, CF3), 11.01. HRMS (ESI): m/z [M + H]+ calcd. for C28H21N6: 441.1822; found: 441.1816.
- 1,5-Diphenyl-3,7-dipropyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3l), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 24 mg, 60%, yellow solid, m.p.: 228–229 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.42 (d, J = 7.4 Hz, 4H), 7.59–7.55 (m, 4H), 7.31 (t, J = 7.5 Hz, 2H), 3.23 (t, J = 7.5 Hz, 4H), 2.07 (m, 4H), 1.12 (t, J = 7.5 Hz, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 146.8, 142.3, 139.6, 133.8, 129.2, 125.4, 119.7, 28.5, 21.6, 14.1. HRMS (ESI): m/z [M + H]+ calcd. for C24H25N6: 397.2135; found: 397.2127.
- 1,3,5,7-Tetraphenyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3m), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 27 mg, 58%, orange solid, m.p.: 275–276 °C. Due to the low solubility of the product, 0.6 mL of CDCl3/TFA (v:v = 20:1) was used to measure the NMR spectrum. 1H NMR (400 MHz, CDCl3/TFA = 20:1 (v:v)) δ (ppm) 8.45 (d, J = 6.4 Hz, 4H), 8.17 (d, J = 7.6 Hz, 4H), 7.71–7.51 (m, 12H). 13C NMR (101 MHz, CDCl3/TFA = 20:1 (v:v)) δ (ppm) 162.7 (q, JCF = 43.4 Hz, CO), 158.1, 137.3, 130.8, 129.9, 129.5, 128.6, 128.1, 123.2, 114.4 (q, JCF = 285.8 Hz, CF3). HRMS (ESI): m/z [M + H]+ calcd. for C30H21N6: 465.1822; found: 465.1816.
- 1,5-Di-tert-butyl-3,7-dimethyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3q), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 12 mg, 40%, yellow solid, m.p.: 228–229 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 2.68 (s, 6H), 1.85 (s, 18H). 13C NMR (101 MHz, CDCl3) δ (ppm) 142.3, 138.8, 132.7, 59.9, 29.1, 11.3. HRMS (ESI): m/z [M + H]+ calcd. for C16H25N6: 301.2135; found: 301.2134.
- 3,7-Di-tert-butyl-1,5-diphenyl-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3r), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 19 mg, 45%,yellow solid, m.p.: 252–253 °C. 1H NMR (400 MHz, CDCl3) δ (ppm) 8.49–8.44 (m, 4H), 7.59–7.54 (m, 4H), 7.29 (m, 2H), 1.72 (s, 18H). 13C NMR (101 MHz, CDCl3) δ (ppm) 153.1, 142.0, 139.9, 132.4, 129.1, 125.1, 119.4, 34.3, 29.2. HRMS (ESI): m/z [M + H]+ calcd. for C26H29N6: 425.2448; found: 425.2441.
- 3,7-Di-tert-butyl-1,5-bis(3,5-dimethylphenyl)-1,5-dihydrodipyrazolo [3,4-b:3′,4′-e] pyrazine (3s), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 10:1), 20 mg, 42%, yellow solid, m.p.: 262–263 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 8.10 (s, 4H), 6.94 (s, 2H), 2.47 (s, 12H), 1.72 (s, 18H). 13C NMR (126 MHz, CDCl3) δ (ppm) 152.8, 141.9, 139.7, 138.8, 132.2, 126.8, 117.3, 34.2, 29.1, 21.7. HRMS (ESI): m/z [M + H]+ calcd. for C30H37N6: 481.3074; found: 481.3070.
- (E)-1,2-bis(3-methyl-1-phenyl-1H-pyrazol-5-yl)diazene (4a), (silica gel: 200–300 mesh, solvent system: petroleum ether/ethyl acetate = 8:1), 14 mg, 40%, orange solid, m.p.: 198–199 °C. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.70 (d, J = 7.5 Hz, 4H), 7.50 (t, J = 7.5 Hz, 4H), 7.40 (t, J = 7.5 Hz, 2H), 6.40 (s, 2H), 2.39 (s, 6H). 13C NMR (126 MHz, CDCl3) δ (ppm) 154.3, 150.0, 139.0, 128.8, 127.5, 124.8, 94.6, 14.0. HRMS (ESI): m/z [M + H]+ calcd. for C20H19N6: 343.1666; found: 343.1663.
- (1-Cyclopropylvinyl)benzene (5), (silica gel: 200–300 mesh, solvent system: petroleum ether), 20 mg, 70%, white solid. 1H NMR (500 MHz, CDCl3) δ (ppm) 7.58 (d, J = 6.0 Hz, 2H), 7.31 (t, J = 7.0 Hz, 2H), 7.26 (d, J = 7.5 Hz, 1H), 5.26 (s, 1H), 4.92 (s, 1H), 1.63 (m, 1H), 0.83–0.79 (m, 2H), 0.58 (dd, J = 5.5, 2.2 Hz, 2H). 13C NMR (126 MHz, CDCl3) δ (ppm) 149.3, 141.6, 128.1, 127.4, 126.1, 109.0, 15.6, 6.6. HRMS (ESI): m/z [M + H]+ calcd. for C11H13: 145.1012; found: 145.1008.
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Singh, A.-K.; Singh, H.; Vijayan, V.; Kumar, D.; Naik, J.; Thareja, S.; Yadav, J.-P.; Pathak, P.; Grishina, M.; et al. Nitrogen Containing Heterocycles as Anticancer Agents: A Medicinal Chemistry Perspective. Pharmaceuticals 2023, 16, 299. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, W.; Shen, L.; Qiu, N.; Hu, M.; Liu, Y.; Liu, Q.; Huang, L. Vasodilator Hydralazine Promotes Nanoparticle Penetration in Advanced Desmoplastic Tumors. ACS Nano 2019, 13, 1751–1763. [Google Scholar] [CrossRef]
- Komendantova, A.-S.; Fakhrutdinov, A.-N.; Menchikov, L.-G.; Sukhorukov, A.-Y.; Zavarzin, I.-V.; Volkova, Y.-A. Cyclization of β-Chlorovinyl Thiohydrazones into Pyridazines: A Mechanistic Study. Eur. J. Org. Chem. 2019, 2019, 527–536. [Google Scholar] [CrossRef]
- Grote, R.; Chen, Y.; Zeeck, A.; Antibiotics, J. Metrabolic Products of Microorganisms. J. Antibiot. 1988, 41, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Scott, E.-N.; Meinhardt, G.; Jacques, C.; Laurent, D.; Thomas, A.-L. Vatalanib: The clinical development of a tyrosine kinase inhibitor of angiogenesis in solid tumours. Expert Opin. Investig. Drugs 2007, 16, 367–379. [Google Scholar] [CrossRef]
- Cao, J.; Miao, Q.; Miao, S.; Bi, L.; Zhang, S.; Yang, Q.; Zhou, X.; Zhang, M.; Xie, Y.; Zhang, J.; et al. Tetramethylpyrazine (TMP) exerts antitumor effects by inducing apoptosis and autophagy in hepatocellular carcinoma. Int. Immunopharmacol. 2015, 26, 212–220. [Google Scholar] [CrossRef]
- De Wang, X.; Li, T.; Li, Y.; Yuan, W.-H.; Zhao, Y.-Q. 2-Pyrazine-PPD, a novel dammarane derivative, showed anticancer activity by reactive oxygen species-mediate apoptosis and endoplasmic reticulum stress in gastric cancer cells. Eur. J. Pharmacol. 2020, 881, 173211. [Google Scholar] [CrossRef]
- Łagocka, R.; Dziedziejko, V.; Kłos, P.; Pawlik, A. Favipiravir in Therapy of Viral Infections. J. Clin. Med. 2021, 10, 273. [Google Scholar] [CrossRef]
- Takeo, F.; Hiroyoshi, D.; Kazuhiro, W.; Hideo, I.; Masakazu, M.; Minoru, N.; Nobuyoshi, S. Antitumor effects and drug interactions of the proteasome inhibitor bortezomib (PS341) in gastric cancer cells. Anti-Cancer Drugs 2007, 18, 677–686. [Google Scholar] [CrossRef]
- Henry, M.; Mostafa, M.; Sutherland, A. Recent Advances in Transition-Metal-Catalyzed, Directed Aryl C–H/N–H Cross-Coupling Reactions. Synthesis 2017, 49, 4586–4598. [Google Scholar] [CrossRef]
- Bansal, S.; Shabade, A.-B.; Punji, B. Advances in C(sp2) − H/C(sp2) − H Oxidative Coupling of (Hetero)arenes Using 3d Transition Metal Catalysts. Adv. Synth. Catal. 2021, 363, 1998–2022. [Google Scholar] [CrossRef]
- Giri, R.; Shi, B.-F.; Engle, K.M.; Maugel, N.; Yu, J.-Q. Transition metal-catalyzed C–H activation reactions: Diastereoselectivity and enantioselectivity. Chem. Soc. Rev. 2009, 38, 3242–3272. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.-F.; Zeng, L.; Zhu, Y.-P.; Li, J.-H. Copper-catalyzed photoredox 1,4-amidocyanation of 1,3-enynes with N-amidopyridin-1-ium salts and TMSCN: Facile access to α-amido allenyl nitriles. Chin. Chem. Lett. 2024, 35, 109685. [Google Scholar] [CrossRef]
- Han, X.-L.; Lin, P.-P.; Li, Q.-J. Recent advances of allenes in the first-row transition metals catalyzed C-H activation reactions. Chin. Chem. Lett. 2019, 30, 1495–1502. [Google Scholar] [CrossRef]
- Yi, R.-N.; He, W.-M. Photoinduced bismuth vanadate-catalyzed C(sp2)-H functionalization. Chin. Chem. Lett. 2025, 35, 109253. [Google Scholar] [CrossRef]
- Gambouz, K.; Abbouchi, A.-E.; Nassiri, S.; Suzenet, F.; Bousmina, M.; Akssira, M.; Guillaumet, G.; El Kazzouli, S. Palladium-Catalyzed Oxidative Arylation of 1H-Indazoles with Arenes. Eur. J. Org. Chem. 2020, 48, 7435–7439. [Google Scholar] [CrossRef]
- Chen, C.; Chen, X.; Zhao, H.; Jiang, H.; Zhang, M. Direct Access to Nitrogen Bi-heteroarenes via Iridium-Catalyzed Hydrogen-Evolution Cross-Coupling Reaction. Org. Lett. 2017, 19, 3390–3393. [Google Scholar] [CrossRef]
- Cao, L.; Zhao, H.; Guan, R.; Jiang, H.; Dixneuf, P.-H.; Zhang, M. Practical iridium-catalyzed direct α-arylation of N-heteroarenes with (hetero)arylboronic acids by H2O-mediated H2 evolution. Nat. Commun. 2021, 12, 4206. [Google Scholar] [CrossRef]
- Zhao, S.; Yuan, J.; Li, Y.-C.; Shi, B.-F. Copper-catalyzed oxidative C–H/C–H cross-coupling of benzamides and thiophenes. Chem. Commun. 2015, 51, 12823–12826. [Google Scholar] [CrossRef]
- Zhao, H.; Li, X.; Guan, R.; Jiang, H.; Zhang, M. Synthesis of Diverse Functionalized Quinoxalines by Oxidative Tandem Dual C−H Amination of Tetrahydroquinoxalines with Amines. Chem. Eur. J. 2019, 25, 15858–15862. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Fu, Y.; Huang, J.; Qin, J.; Zuo, H.; Wu, Y.; Zhong, F. Enantioselective Oxidative Phenol-Indole [3+2] Coupling Enabled by Biomimetic Mn(III)/Brønsted Acid Relay Catalysis. ACS Catal. 2019, 9, 7285–7291. [Google Scholar] [CrossRef]
- Zhang, L.-B.; Zhu, M.-H.; Du, W.-B.; Ni, S.-F.; Wen, L.-R.; Li, M. Silver-promoted regioselective [4+2] annulation reaction of indoles with alkenes to construct dihydropyrimidoindolone scaffolds. Chem. Commun. 2019, 55, 14383–14386. [Google Scholar] [CrossRef]
- Tian, S.-H.; Luo, T.; Zhu, Y.-P.; Wan, J.-P. Recent advances in the diversification of chromones and flavones by direct C-H bond activation or functionalization. Chin. Chem. Lett. 2020, 31, 3073–3082. [Google Scholar] [CrossRef]
- Varaksin, M.; Moseev, T.; Chupakhin, O.; Charushin, V.; Trofimov, B. Metal-free C–H functionalization of 2H-imidazole 1-oxides with pyrrolyl fragments in the design of novel azaheterocyclic ensembles. Org. Biomol. Chem. 2017, 15, 8280–8284. [Google Scholar] [CrossRef]
- Badigenchala, S.; Sekar, G. NIS Mediated Cross-Coupling of C(sp2)–H and N–H Bonds: A Transition-Metal-Free Approach toward Indolo[1,2-a]quinazolinones. J. Org. Chem. 2017, 82, 7657–7665. [Google Scholar] [CrossRef] [PubMed]
- Shakoor, S.-M.-A.; Mandal, S.-K.; Sakhuja, R. An Articulate Oxidative Transition-Metal-Free Homocoupling of Imidazo Heterocycles through C(sp2)–C(sp2) Bond Formation. Eur. J. Org. Chem. 2017, 2017, 2596–2602. [Google Scholar] [CrossRef]
- Dai, M.; Zhang, Y.; Zhang, X.; Wang, R.; Wei, W.; Zhang, Z.; Liang, T. Iodine-Mediated C2,3–H Aminoheteroarylation of Indoles. J. Org. Chem. 2023, 88, 15106–15117. [Google Scholar] [CrossRef]
- Li, Y.-X.; Ji, K.-G.; Wang, H.-X.; Ali, S.; Liang, Y.-M. Iodine-Induced Regioselective C−C and C−N Bonds Formation of N-Protected Indoles. J. Org. Chem. 2011, 76, 744–747. [Google Scholar] [CrossRef]
- Wu, Y.-C.; Jiang, S.-S.; Song, R.-J.; Li, J.-H. A metal- and oxidizing-reagent-free anodic para-selective amination of anilines with phenothiazines. Chem. Commun. 2019, 55, 4371–4374. [Google Scholar] [CrossRef]
- Wang, Y.; Tian, B.; Ding, M.; Shi, Z. Electrochemical Cross-Dehydrogenative Coupling between Phenols and β-Dicarbonyl Compounds: Facile Construction of Benzofurans. Chem. Eur. J. 2020, 26, 4297–4303. [Google Scholar] [CrossRef] [PubMed]
- Feng, P.; Ma, G.; Chen, X.; Wu, X.; Lin, L.; Liu, P.; Chen, T. Electrooxidative and Regioselective C−H Azolation of Phenol and Aniline Derivatives. Angew. Chem. Int. Ed. 2019, 58, 8400–8404. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.-J.; Li, Y.; Ouyang, X.-H.; Li, J.-H.; He, D.-L. Electrochemical dehydrogenative cross-coupling of two anilines: Facile synthesis of unsymmetrical biaryls. Chem. Commun. 2020, 56, 2707–2710. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.-Y.; Hu, J.-L.; Song, Y.-X.; Jia, G.-K.; Lin, Y.-W.; He, J.-Y.; Cao, Z.; He, W.-M. Visible-Light-Initiated Cross-Dehydrogenative Coupling of Quinoxalin-2(1H)-ones and Simple Amides with Air as an Oxidant. ACS Sustain. Chem. Eng. 2019, 7, 19993–19999. [Google Scholar] [CrossRef]
- Qin, J.-H.; Luo, M.-J.; An, D.-L.; Li, J.-H. Electrochemical 1,2-Diarylation of Alkenes Enabled by Direct Dual C–H Functionalizations of Electron-Rich Aromatic Hydrocarbons. Angew. Chem. Int. Ed. 2021, 60, 1861–1868. [Google Scholar] [CrossRef]
- Yu, Y.; Yuan, Y.; Liu, H.; He, M.; Yang, M.; Liu, P.; Yu, B.; Dong, X.; Lei, A. Electrochemical oxidative C–H/N–H cross-coupling for C–N bond formation with hydrogen evolution. Chem. Commun. 2019, 55, 1809–1812. [Google Scholar] [CrossRef]
- Liu, K.; Wu, J.; Deng, Y.; Song, C.; Song, W.; Lei, A. Electrochemical C−H/N−H Oxidative Cross Coupling of Imidazopyridines with Diarylamines to Synthesize Triarylamine Derivatives. ChemElectroChem 2019, 6, 4173–4176. [Google Scholar] [CrossRef]
- Song, C.; Liu, K.; Jiang, X.; Dong, X.; Weng, Y.; Chiang, C.-W.; Lei, A. Electrooxidation Enables Selective Dehydrogenative [4+2] Annulation between Indole Derivatives. Angew. Chem. Int. Ed. 2020, 59, 7193–7197. [Google Scholar] [CrossRef]
- Jiang, B.; Ning, Y.; Fan, W.; Tu, S.-J.; Li, G. Oxidative Dehydrogenative Couplings of Pyrazol-5-amines Selectively Forming Azopyrroles. J. Org. Chem. 2014, 79, 4018–4024. [Google Scholar] [CrossRef]
- Ryan, M.C.; Martinelli, J.R.; Stahl, S.-S. Cu-Catalyzed Aerobic Oxidative N–N Coupling of Carbazoles and Diarylamines Including Selective Cross-Coupling. J. Am. Chem. Soc. 2018, 140, 9074–9077. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.-W.; Xu, H.-C.; Wang, L. Electrochemical dehydrogenative N–H/N–H coupling reactions. Curr. Opin. Electrochem. 2022, 34, 100988. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Zhang, C.; Zhang, C.; Ma, H.; Guo, Z.; Liu, N.; Xu, M.; Ma, H.; Qiu, J. Green electrosynthesis of 3,3′-diamino-4,4′-azofurazan energetic materials coupled with energy-efficient hydrogen production over Pt-based catalysts. Nat. Commun. 2023, 14, 8146. [Google Scholar] [CrossRef] [PubMed]
- Lv, S.; Han, X.; Wang, J.-Y.; Zhou, M.; Wu, Y.; Ma, L.; Niu, L.; Gao, W.; Zhou, J.; Hu, W.; et al. Tunable Electrochemical C−N versus N−N Bond Formation of Nitrogen-Centered Radicals Enabled by Dehydrogenative Dearomatization: Biological Applications. Angew. Chem. Int. Ed. 2020, 59, 11583–11590. [Google Scholar] [CrossRef]
- Luo, B.-K.; Dong, W.-F.; Ma, Q.-J.; Yang, H.; Tang, W.-J. Synthesis of Biheteroaryls by Pd-Catalyzed Homocoupling of Heteroaryl Bromides. Org. Lett. 2024, 26, 8736–8740. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, G.-K.; Deb, M.-L.; Baruah, P.-K. Copper-catalysed dehydrogenative self-coupling/cyclization of 5-aminopyrazoles: Synthesis and photophysical study of pyridazines. Chem. Commun. 2023, 59, 9642–9645. [Google Scholar] [CrossRef]
- CCDC 2322498, 2322499, 2322519 Contain the Supplementary Crystallographic Data for this Paper. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 29 October 2024).
- Wang, Y.-F.; Zhu, X.; Chiba, S. Copper-Catalyzed Aerobic [3+2]-Annulation of N-Alkenyl Amidines. J. Am. Chem. Soc. 2012, 134, 3679–3682. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-F.; Chen, H.; Zhu, X.; Chiba, S. Copper-Catalyzed Aerobic Aliphatic C–H Oxygenation Directed by an Amidine Moiety. J. Am. Chem. Soc. 2012, 134, 11980–11983. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Zhang, F.-L.; Chiba, S. Oxidative Radical Skeletal Rearrangement Induced by Molecular Oxygen: Synthesis of Quinazolinones. Org. Lett. 2013, 15, 2842–2845. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Lv, D.; Yu, F.; Chiou, M.-F.; Li, Y.; Bao, H. Regioselective Three-Component Synthesis of Vicinal Diamines via 1,2-Diamination of Styrenes. Org. Lett. 2021, 23, 3184–3189. [Google Scholar] [CrossRef]
- Yan, M.; Zhu, L.; Zhang, X.; Yin, S.-F.; Kambe, N.; Qiu, R. Nickel-Catalyzed N, N-Diarylation of 8-Aminoquinoline with Large Steric Aryl Bromides and Fluorescence of Products. Org. Lett. 2021, 23, 2514–2520. [Google Scholar] [CrossRef]
- Yang, T.; Cao, X.; Zhang, X.-X.; Ou, Y.; Au, C.-T.; Yin, S.-F.; Qiu, R. Iodine-Catalyzed Synthesis of N, N′-Chelate Organoboron Aminoquinolate. J. Org. Chem. 2020, 85, 12430–12443. [Google Scholar] [CrossRef]

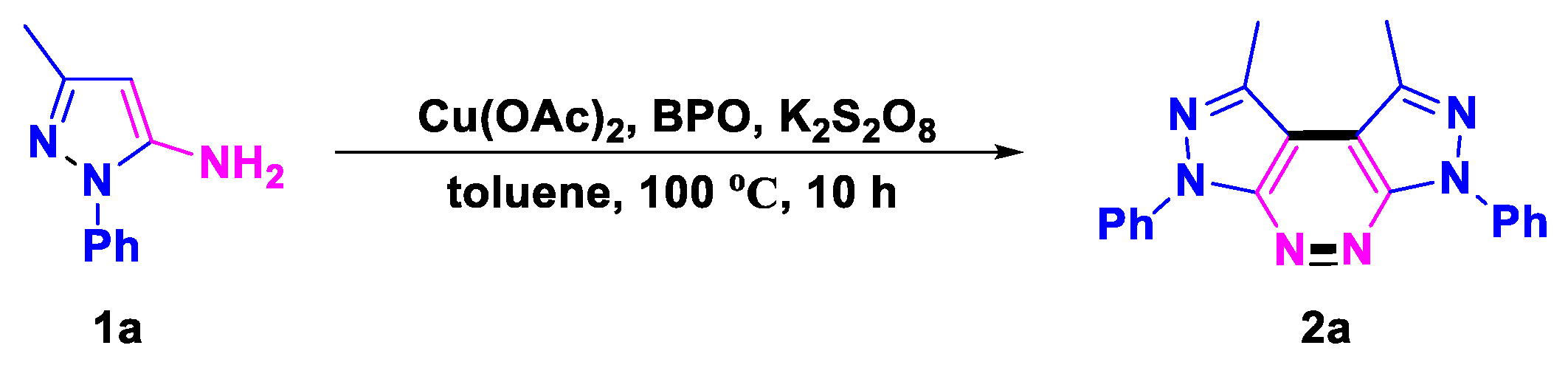
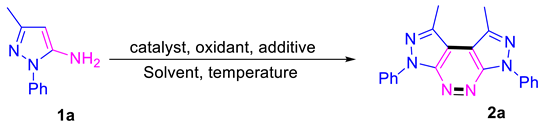 | ||||||
| Entry | Catalysts (Equiv.) | Oxidants (Equiv.) | Additives (Equiv.) | Temp (℃) | Solvent | Yield (%) |
| 1 | Cu(OAc)2 (3.0) | 100 | Toluene | 50 | ||
| 2 | Cu(OAc)2 (3.0) | BPO (0.5) | 100 | Toluene | 58 | |
| 3 | Cu(OAc)2 (3.0) | TBHP (0.5) | 100 | Toluene | 52 | |
| 4 | Cu(OAc)2 (3.0) | DDQ (0.5) | 100 | Toluene | 42 | |
| 5 | Cu(OAc)2 (3.0) | TBPB (0.5) | 100 | Toluene | 38 | |
| 6 | CuCl2(3.0) | BPO (0.5) | 100 | Toluene | 46 | |
| 7 | CuBr (3.0) | BPO (0.5) | 100 | Toluene | 40 | |
| 8 | CuI (3.0) | BPO (0.5) | 100 | Toluene | 38 | |
| 9 | CuCl (3.0) | BPO (0.5) | 100 | Toluene | 42 | |
| 10 | Cu(OAc)2.H2O (3.0) | BPO (0.5) | 100 | Toluene | 48 | |
| 11 | Zn (OAc)2 (3.0) | BPO (0.5) | 100 | Toluene | 36 | |
| 12 | FeCl3(3.0) | BPO (0.5) | 100 | Toluene | 12 | |
| 13 | Cu(OAc)2 (3.0) | BPO (0.5) | 110 | Toluene | 55 | |
| 14 | Cu(OAc)2 (3.0) | BPO (0.5) | 120 | Toluene | 53 | |
| 15 | Cu(OAc)2 (3.0) | BPO (0.5) | 90 | Toluene | 51 | |
| 16 | Cu(OAc)2 (3.0) | BPO (0.5) | 80 | Toluene | 44 | |
| 17 | Cu(OAc)2 (2.0) | BPO (0.5) | 100 | Toluene | 56 | |
| 18 | Cu(OAc)2 (1.0) | BPO (0.5) | 100 | Toluene | 48 | |
| 19 | Cu(OAc)2 (3.0) | BPO (0.25) | 100 | Toluene | 55 | |
| 20 | Cu(OAc)2 (3.0) | BPO (0.75) | 100 | Toluene | 53 | |
| 21 | Cu(OAc)2 (3.0) | BPO (0.5) | 100 | DMSO | 50 | |
| 22 | Cu(OAc)2 (3.0) | BPO (0.5) | 100 | Dioxane | 40 | |
| 23 | Cu(OAc)2 (3.0) | BPO (0.5) | 100 | DEC | 42 | |
| 24 | Cu(OAc)2 (3.0) | BPO (0.5) | 100 | MeCN | 40 | |
| 25 | Cu(OAc)2 (3.0) | BPO (0.5) | 100 | DMF | 56 | |
| 26 | Cu(OAc)2 (3.0) | BPO (0.5) | Na2CO3 (2.5) | 100 | Toluene | 54 |
| 27 | Cu(OAc)2 (3.0) | BPO (0.5) | Cs2CO3 (2.5) | 100 | Toluene | 41 |
| 28 | Cu(OAc)2 (3.0) | BPO (0.5) | K2S2O8 (2.5) | 100 | Toluene | 79 |
| 29 | Cu(OAc)2 (3.0) | BPO (0.5) | K2S2O8 (1.5) | 100 | Toluene | 60 |
| 30 | Cu(OAc)2 (3.0) | BPO (0.5) | K2S2O8 (3.5) | 100 | Toluene | 70 |
 | |||||||
| Entry | Catalysts (Equiv.) | Oxidants (Equiv.) | Additives (Equiv.) | Ligands (Equiv.) | Temp (℃) | Solvent | Yield (%) |
| 1 | Cu(OAc)2 (20%) | 130 | Toluene | 36 | |||
| 2 | Cu(OAc)2 (20%) | BPO (0.5) | 130 | Toluene | 38 | ||
| 3 | Cu(OAc)2 (20%) | TBHP (0.5) | 130 | Toluene | 37 | ||
| 4 | Cu(OAc)2 (20%) | DDQ (0.5) | 130 | Toluene | 32 | ||
| 5 | Cu(OAc)2 (20%) | TBPB (0.5) | 130 | Toluene | 41 | ||
| 6 | CuCl2 (20%) | TBPB (0.5) | 130 | Toluene | 47 | ||
| 7 | CuBr (20%) | TBPB (0.5) | 130 | Toluene | 40 | ||
| 8 | CuI (20%) | TBPB (0.5) | 130 | Toluene | 38 | ||
| 9 | CuCl (20%) | TBPB (0.5) | 130 | Toluene | 41 | ||
| 10 | Cu(OAc)2.H2O(20%) | TBPB (0.5) | 130 | Toluene | 43 | ||
| 11 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | Toluene | 51 | |
| 12 | CuCl2 (20%) | TBPB (0.5) | Cs2CO3 (2.5) | 130 | Toluene | 34 | |
| 13 | CuCl2 (20%) | TBPB (0.5) | tBuONa (2.5) | 130 | Toluene | 36 | |
| 14 | CuCl2 (20%) | TBPB (0.5) | KI (2.5) | 130 | Toluene | 29 | |
| 15 | CuCl2 (20%) | TBPB (0.5) | K2S2O8 (2.5) | 130 | Toluene | 38 | |
| 16 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | Toluene | 48 | |
| 17 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | DMF | 35 | |
| 18 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | DMSO | 32 | |
| 19 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | NMP | 24 | |
| 20 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | Dioxane | 26 | |
| 21 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | DEC | 24 | |
| 22 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 130 | DMAC | 21 | |
| 23 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | Bipy (0.3) | 130 | Toluene | 49 |
| 24 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 1,10-Phen (0.3) | 130 | Toluene | 59 |
| 25 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 1,10-Phen (0.3) | 120 | Toluene | 46 |
| 26 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 1,10-Phen (0.3) | 100 | Toluene | 38 |
| 27 | CuCl2 (20%) | TBPB (0.5) | Na2CO3 (2.5) | 1,10-Phen (0.3) | 80 | Toluene | 29 |
| 28 | CuCl2 (40%) | TBPB (0.5) | Na2CO3 (2.5) | 1,10-Phen (0.3) | 130 | Toluene | 50 |
| 29 | CuCl2 (10%) | TBPB (0.5) | Na2CO3 (2.5) | 1,10-Phen (0.3) | 130 | Toluene | 35 |
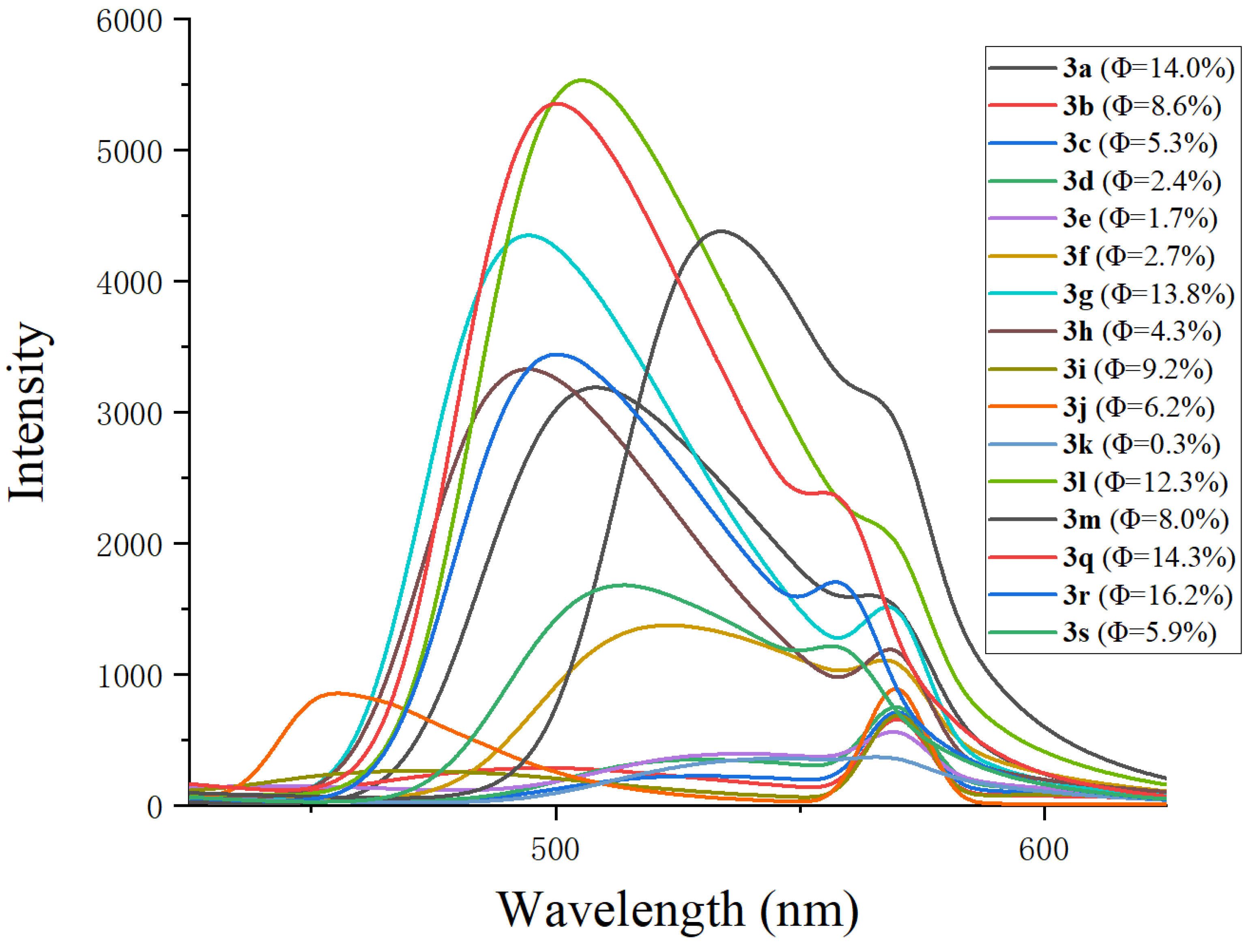
| Compd. | λmax a (nm) | λem b (nm) | Stokes Shift (nm) | ΦF c (%) |
|---|---|---|---|---|
| 2a | 279 | 502 | 223 | 3.0 |
| 2b | 266 | 298 | 32 | 5.2 |
| 2c | 272 | 295 | 23 | 21.8 |
| 2d | 273 | 300 | 27 | 10.3 |
| 2e | 238 | 304 | 66 | 2.2 |
| 2f | 274 | 296 | 22 | 9.0 |
| 2g | 309 | 492 | 183 | 7.4 |
| 2h | 312 | 478 | 166 | 7.6 |
| 2i | 271 | 294 | 23 | 11.6 |
| 2j | 273 | 300 | 27 | 9.1 |
| 2k | 273 | 293 | 20 | 2.0 |
| 2l | 271 | 294 | 23 | 12.6 |
| 2m | 273 | 511 | 238 | 8.0 |
| 2n | 272 | 296 | 24 | 7.5 |
| 2o | 277 | 510 | 233 | 5.8 |
| 2p | 274 | 298 | 24 | 2.0 |
| Compd. | λmax a (nm) | λem b (nm) | Stokes Shift (nm) | ΦF c (%) |
|---|---|---|---|---|
| 3a | 284 | 505 | 221 | 14.0 |
| 3b | 304 | 501 | 197 | 8.6 |
| 3c | 284 | 523 | 239 | 5.3 |
| 3d | 286 | 530 | 244 | 2.4 |
| 3e | 285 | 530 | 245 | 1.7 |
| 3f | 285 | 522 | 237 | 2.7 |
| 3g | 285 | 495 | 210 | 13.8 |
| 3h | 284 | 496 | 212 | 4.3 |
| 3i | 287 | 474 | 187 | 9.2 |
| 3j | 274 | 302 | 28 | 6.2 |
| 3k | 287 | 544 | 257 | 0.3 |
| 3l | 284 | 503 | 219 | 12.3 |
| 3m | 289 | 534 | 245 | 8.0 |
| 3q | 284 | 499 | 215 | 14.3 |
| 3r | 283 | 500 | 217 | 16.2 |
| 3s | 285 | 512 | 227 | 5.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chai, Y.-X.; Ren, J.-J.; Li, Y.-M.; Bai, Y.-C.; Zhang, Q.-Q.; Zhao, Y.-Z.; Yang, X.; Zhang, X.-H.; Zhang, X.-S.; Wu, A.-X.; et al. 5-Aminopyrazole Dimerization: Cu-Promoted Switchable Synthesis of Pyrazole-Fused Pyridazines and Pyrazines via Direct Coupling of C-H/N-H, C-H/C-H, and N-H/N-H Bonds. Molecules 2025, 30, 381. https://doi.org/10.3390/molecules30020381
Chai Y-X, Ren J-J, Li Y-M, Bai Y-C, Zhang Q-Q, Zhao Y-Z, Yang X, Zhang X-H, Zhang X-S, Wu A-X, et al. 5-Aminopyrazole Dimerization: Cu-Promoted Switchable Synthesis of Pyrazole-Fused Pyridazines and Pyrazines via Direct Coupling of C-H/N-H, C-H/C-H, and N-H/N-H Bonds. Molecules. 2025; 30(2):381. https://doi.org/10.3390/molecules30020381
Chicago/Turabian StyleChai, Yi-Xin, Jun-Jie Ren, Yi-Ming Li, Yi-Cheng Bai, Qing-Qing Zhang, Yi-Zhen Zhao, Xue Yang, Xiao-Han Zhang, Xin-Shuang Zhang, An-Xin Wu, and et al. 2025. "5-Aminopyrazole Dimerization: Cu-Promoted Switchable Synthesis of Pyrazole-Fused Pyridazines and Pyrazines via Direct Coupling of C-H/N-H, C-H/C-H, and N-H/N-H Bonds" Molecules 30, no. 2: 381. https://doi.org/10.3390/molecules30020381
APA StyleChai, Y.-X., Ren, J.-J., Li, Y.-M., Bai, Y.-C., Zhang, Q.-Q., Zhao, Y.-Z., Yang, X., Zhang, X.-H., Zhang, X.-S., Wu, A.-X., Zhu, Y.-P., & Sun, Y.-Y. (2025). 5-Aminopyrazole Dimerization: Cu-Promoted Switchable Synthesis of Pyrazole-Fused Pyridazines and Pyrazines via Direct Coupling of C-H/N-H, C-H/C-H, and N-H/N-H Bonds. Molecules, 30(2), 381. https://doi.org/10.3390/molecules30020381







