Study of Ultrasound-Assisted Low-Pressure Closed Acid Digestion Method for Trace Element Determination in Rock Samples by Inductively Coupled Plasma Mass Spectrometry
Abstract
1. Introduction
2. Results and Discussion
2.1. Extraction Property of Ultrasound-Assisted Low-Pressure Closed Digestion Method
2.2. Pressure Relief Study of the Proposed Ultrasound-Assisted Low-Pressure Closed Digestion Method
2.3. A Comparison of the Digestion Efficiency to Traditional Low-Pressure Closed Digestion Method
2.4. Trace Element Quantification Accuracy of the Proposed Method Based on HNO3–HF–Mannitol
3. Materials and Methods
3.1. Apparatus for Ultrasonication
3.2. ICP-MS Instrumentation
3.3. Chemicals and Reagent Preparation
3.4. Silicate Standard Materials
3.5. Digestion Method Description
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, C.T. Geochemical classification of elements. In Encyclopedia of Geochemistry, Encyclopedia of Earth Sciences Series; White, W.M., Ed.; Springer: Cham, Switzerland, 2018; pp. 545–549. [Google Scholar]
- Aurisicchio, C.; Conte, A.M.; Medeghini, L.; Ottolini, L.; De Vito, C. Major and trace element geochemistry of emerald from several deposits: Implications for genetic models and classification schemes. Ore Geol. Rev. 2018, 94, 351–366. [Google Scholar] [CrossRef]
- Wood, B.J.; Blundy, J.D. Trace element partitioning under crustal and uppermost mantle conditions: The influences of ionic radius, cation charge, pressure, and temperature. Treatise Geochem. 2003, 2, 395–424. [Google Scholar]
- Albarède, F. Geochemistry: An Introduction; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Misra, K.C. Introduction to Geochemistry: Principles and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- White, W.M. Geochemistry; John Wiley & Sons: Hoboken, NJ, USA, 2020. [Google Scholar]
- Hanson, G.N. The application of trace elements to the petrogenesis of igneous rocks of granitic composition. Earth Planet. Sci. Lett. 1978, 38, 26–43. [Google Scholar] [CrossRef]
- O’Sullivan, G.; Chew, D.; Kenny, G.; Henrichs, I.; Mulligan, D. The trace element composition of apatite and its application to detrital provenance studies. Earth Sci. Rev. 2020, 201, 103044. [Google Scholar] [CrossRef]
- Rottier, B.; Casanova, V. Trace element composition of quartz from porphyry systems: A tracer of the mineralizing fluid evolution. Miner. Depos. 2021, 56, 843–862. [Google Scholar] [CrossRef]
- Sciuba, M.; Beaudoin, G.; Grzela, D.; Makvandi, S. Trace element composition of scheelite in orogenic gold deposits. Miner. Depos. 2020, 55, 1149–1172. [Google Scholar] [CrossRef]
- Santoro, L.; Putzolu, F.; Mondillo, N.; Boni, M.; Herrington, R. Trace element geochemistry of iron-(oxy)-hydroxides in Ni (Co)-laterites: Review, new data and implications for ore forming processes. Ore Geol. Rev. 2022, 140, 104501. [Google Scholar] [CrossRef]
- Ghosh, U.; Upadhyay, D.; Mishra, B.; Abhinay, K. In-situ trace element and Li-isotope study of zinnwaldite from the Degana tungsten deposit, India: Implications for hydrothermal tungsten mineralization. Chem. Geol. 2023, 632, 121550. [Google Scholar] [CrossRef]
- Jarvis, I. Sample preparation for ICP-MS. In Handbook of Inductively Coupled Plasma-Mass Spectrometry; Jarvis, K.E., Gray, A.L., Houk, R.S., Eds.; Blackie: London, UK, 1992; pp. 172–224. [Google Scholar]
- Totland, M.; Jarvis, I.; Jarvis, K.E. An assessment of dissolution techniques for the analysis of geological samples by plasma spectrometry. Chem. Geol. 1992, 95, 35–62. [Google Scholar] [CrossRef]
- Beauchemin, D. Inductively coupled plasma mass spectrometry. Anal. Chem. 2010, 82, 4786–4810. [Google Scholar] [CrossRef]
- Chao, T.T.; Sanzolone, R.F. Decomposition techniques. J. Geochem. Explor. 1992, 44, 65–106. [Google Scholar] [CrossRef]
- Yu, Z.; Robinson, P.; McGoldrick, P. An evaluation of methods for the chemical decomposition of geological materials for trace element determination using ICP-MS. Geostand. Newsl. 2001, 25, 199–217. [Google Scholar] [CrossRef]
- Balaram, V.; Subramanyam, K.S.V. Sample preparation for geochemical analysis: Strategies and significance. Adv. Sample Prep. 2022, 1, 100010. [Google Scholar] [CrossRef]
- Bayon, G.; Barrat, J.A.; Etoubleau, J.; Benoit, M.; Bollinger, C.; Révillon, S. Determination of rare earth elements, Sc, Y, Zr, Ba, Hf and Th in geological samples by ICP-MS after Tm addition and alkaline fusion. Geostand. Geoanal. Res. 2009, 33, 51–62. [Google Scholar]
- Mnculwane, H.T. Rare earth elements determination by inductively coupled plasma mass spectrometry after alkaline fusion preparation. Analytica 2022, 3, 135–143. [Google Scholar] [CrossRef]
- Panteeva, S.V.; Gladkochoub, D.P.; Donskaya, T.V.; Markova, V.V.; Sandimirova, G.P. Determination of 24 trace elements in felsic rocks by inductively coupled plasma mass spectrometry after lithium metaborate fusion. Spectrochim. Acta B 2003, 58, 341–350. [Google Scholar] [CrossRef]
- Zivkovic, Z.; Danyushevsky, L.; Halley, S.; Barker, S.; Baker, M. Comparison of lithium borate fusion and four-acid digestions for the determination of whole-rock chemistry–implications for lithogeochemistry and mineral exploration. Geochem. Explor. Environ. Anal. 2023, 23, 2022–2054. [Google Scholar] [CrossRef]
- Matusiewicz, H. Wet Digestion Methods. Sample Prep. Trace Elem. Anal. 2003, 41, 193–233. [Google Scholar]
- Banks, R.E. Isolation of flourine by Moissan: Setting the scene. J. Fluor. Chem. 1986, 33, 3–26. [Google Scholar] [CrossRef]
- Makishima, A. Thermal Ionization Mass Spectrometry (TIMS): Silicate Digestion, Separation, and Measurement; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Tan, X.J.; Wang, Z.M. General high-pressure closed acidic decomposition method of rock samples for trace element determination using inductively coupled plasma mass spectrometry. J. Anal. Chem. 2020, 75, 1295–1303. [Google Scholar]
- Liu, Y.H.; Guo, S.; Li, W.J.; Xue, D.S.; Li, C.F.; Wang, B. Rapid and complete digestion of refractory geological samples using ultrafine powder for accurate analyses of trace elements. Anal. Chem. 2024, 96, 6523–6527. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Luo, L.; Guo, W.; Jin, L.L.; Chen, B.; Hu, S.H. Accurate determination of trace cadmium in geological reference materials by closed vessel acid digestion ETAAS. At. Spectrosc. 2015, 36, 141–145. [Google Scholar] [CrossRef]
- Navarro, M.S.; Andrade, S.; Ulbrich, H.; Gomes, C.B.; Girardi, V.A.V. The direct determination of rare earth elements in basaltic and related rocks using ICP-MS: Testing the efficiency of microwave oven sample decomposition procedures. Geostand. Geoanal. Res. 2008, 32, 167–180. [Google Scholar] [CrossRef]
- Hu, Z.C.; Qi, L. Sample Digestion Methods. In Treatise on Geochemistry; Holland, H.D., Turekian, K.K., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2014; Volume 15, pp. 87–109. [Google Scholar]
- Gao, J.J.; Liu, J.H.; Li, X.G.; Yan, Q.S.; Wang, X.J.; Wang, H.M. The determination of 52 elements in marine geological samples by an inductively coupled plasma optical emission spectrometry and an inductively coupled plasma mass spectrometry with a high-pressure closed digestion method. Acta Oceanol. Sin. 2017, 36, 109–117. [Google Scholar] [CrossRef]
- Qi, L.; Hu, J.; Gregoire, D.C. Determination of trace elements in granites by inductively coupled plasma mass spectrometry. Talanta 2000, 51, 507–513. [Google Scholar]
- Bendicho, C.; De La Calle, I.; Pena, F.; Costas, M.; Cabaleiro, N.; Lavilla, I. Ultrasound-assisted pretreatment of solid samples in the context of green analytical chemistry. Trends Anal. Chem. 2012, 31, 50–60. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. Trends Analyt. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chemat, F.; Rombaut, N.; Sicaire, A.G.; Meullemiestre, A.; Fabiano-Tixier, A.S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications: A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef]
- Seidi, S.; Yamini, Y. Analytical sonochemistry; developments, applications, and hyphenations of ultrasound in sample preparation and analytical techniques. Cent. Eur. J. Chem. 2012, 10, 938–976. [Google Scholar] [CrossRef]
- Mason, T.J.; Lorimer, J.P. Applied Sonochemistry: The Uses of Power Ultrasound in Chemistry and Processing; Wiley-VCH: Weinheim, Germany, 2002. [Google Scholar]
- Priego-Capote, F.; de Castro, L. Ultrasound-assisted digestion: A useful alternative in sample preparation. J. Biochem. Biophys. Methods 2007, 70, 299–310. [Google Scholar] [CrossRef]
- Bendicho, C.; Lavilla, I. Ultrasound-assisted metal extractions. In Reference Module in Chemistry, Molecular Sciences and Chemical Engineering; Reedijk, J., Ed.; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ilander, A.; Väisänen, A. An ultrasound-assisted digestion method for the determination of toxic element concentrations in ash samples by inductively coupled plasma optical emission spectrometry. Anal. Chim. Acta 2007, 602, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Ilander, A.; Väisänen, A. The determination of trace element concentrations in fly ash samples using ultrasound-assisted digestion followed with inductively coupled plasma optical emission spectrometry. Ultrason. Sonochem. 2009, 16, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Zuma, M.C.; Nomngongo, P.N.; Mketo, N. Simultaneous determination of REEs in coal samples using the combination of microwave-assisted ashing and ultrasound-assisted extraction methods followed by ICP-OES analysis. Minerals 2021, 11, 1103. [Google Scholar] [CrossRef]
- Özkan, M.H.; Özkan, A.; Gürkan, R.; Akçay, M. Determination of sodium and potassium in certified rock samples by AES after ultrasonic leaching. J. Serb. Chem. Soc. 2006, 71, 949–955. [Google Scholar] [CrossRef]
- Pumure, I.; Renton, J.J.; Smart, R.B. Accelerated aqueous leaching of selenium and arsenic from coal associated rock samples with selenium speciation using ultrasound extraction. Environ. Geol. 2009, 56, 985–991. [Google Scholar] [CrossRef]
- Welna, M.; Borkowska-Burnecka, J.; Popko, M. Ultrasound-and microwave-assisted extractions followed by hydride generation inductively coupled plasma optical emission spectrometry for lead determination in geological samples. Talanta 2015, 144, 953–959. [Google Scholar] [CrossRef]
- Verni, E.R.; Londonio, A.; Bazán, C.; Strasser, E.; Perino, E.; Gil, R.A. REE profiling in basic volcanic rocks after ultrasonic sample treatment and ICPMS analysis with oxide ion formation in ICP enriched with O2. Microchem. J. 2017, 130, 14–20. [Google Scholar] [CrossRef]
- Diehl, L.O.; Gatiboni, T.L.; Mello, P.A.; Muller, E.I.; Duarte, F.A.; Flores, E.M.M. Ultrasound-assisted extraction of rare-earth elements from carbonatite rocks. Ultrason. Sonochem. 2018, 40, 24–29. [Google Scholar] [CrossRef]
- Gatiboni, T.L.; Iop, G.D.; Diehl, L.O.; Flores, E.M.M.; Muller, E.I.; Mello, P.A. An ultrasound-assisted sample preparation method of carbonatite rock for determination of rare earth elements by inductively coupled plasma mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8732. [Google Scholar] [CrossRef]
- Benali, K.; Kounbach, S.; Boulif, R.; Benhida, R.; Khaless, K. Development of a simple and fast ultrasound-assisted extraction method for iodine determination in phosphate rock and phosphogypsum by-product using titrimetric method. Int. J. Environ. Anal. Chem. 2024, 104, 3742–3758. [Google Scholar] [CrossRef]
- Al-Merey, R.; Al-Masri, M.S.; Bozou, R. Cold ultrasonic acid extraction of copper, lead and zinc from soil samples. Anal. Chim. Acta 2002, 452, 143–148. [Google Scholar] [CrossRef]
- Collasiol, A.; Pozebon, D.; Maia, S.M. Ultrasound assisted mercury extraction from soil and sediment. Anal. Chim. Acta 2004, 518, 157–164. [Google Scholar] [CrossRef]
- Da Silva Medeiros, D.C.C.; Piechontcoski, F.; da Rocha Watanabe, E.R.L.; Chaves, E.S.; Inglez, S.D. Fast and effective simultaneous determination of metals in soil samples by ultrasound-assisted extraction and flame atomic absorption spectrometry: Assessment of trace elements contamination in agricultural and native forest soils from Paraná-Brazil. Environ. Monit. Assess. 2020, 192, 111. [Google Scholar] [CrossRef]
- Pinto, F.G.; Junior, R.E.; Saint’Pierre, T.D. Sample preparation for determination of rare earth elements in geological samples by ICP-MS: A critical review. Anal. Lett. 2012, 45, 1537–1556. [Google Scholar] [CrossRef]
- Pedrotti, M.F.; Enders, M.S.P.; Pereira, L.S.F.; Meskoc, M.F.; Floresb, E.M.M.; Bizzi, C.A. Intensification of ultrasonic-assisted crude oil demulsification based on acoustic field distribution data. Ultrason. Sonochem. 2018, 40, 53–59. [Google Scholar] [CrossRef]
- Jochum, K.P.; Nohl, U.; Herwig, K.; Lammel, E.; Stoll, B.; Hofmann, A.W. GeoReM: A new geochemical database for reference materials and isotopic standards. Geostand. Geoanal. Res. 2005, 29, 333–338. [Google Scholar] [CrossRef]
- Xie, X.J.; Yan, M.C.; Li, L.Z.; Shen, H.J. Usable values for Chinese standard reference samples of stream sediments, soils, and rocks: GSD 9-12, GSS 1-8 and GSR 1-6. Geostand. Newsl. 1985, 9, 277–280. [Google Scholar]
- Xie, X.J.; Yan, M.C.; Wang, C.S.; Li, L.Z. Geochemical standard reference samples GSD 9-12, GSS 1-8 and GSR 1-6. Geostand. Newsl. 1989, 13, 83–179. [Google Scholar] [CrossRef]
- Zhang, W.; Hu, Z.C.; Liu, Y.S.; Chen, L.; Chen, H.H.; Li, M.; Zhao, L.S.; Hu, S.H.; Gao, S. Reassessment of HF/HNO3 decomposition capability in the high-pressure digestion of felsic rocks for multi-element determination by ICP-MS. Geostand. Geoanal. Res. 2012, 36, 271–289. [Google Scholar] [CrossRef]
- Tan, X.J.; Zhou, R.L.; Feng, Y.G.; Liang, T. In-depth method investigation for determination of boron in silicate samples using an improved boron–mannitol complex digestion method by inductively coupled plasma mass spectrometry. Molecules 2023, 28, 441. [Google Scholar] [CrossRef]
- Tan, X.J.; Feng, Y.G.; Zhou, R.L.; Wang, D.H.; Liang, T.; Wang, Y. Accurate boron determination in tourmaline by inductively coupled plasma mass spectrometry: An insight into the boron–mannitol complex-based wet acid digestion method. Molecules 2024, 29, 2701. [Google Scholar] [CrossRef]
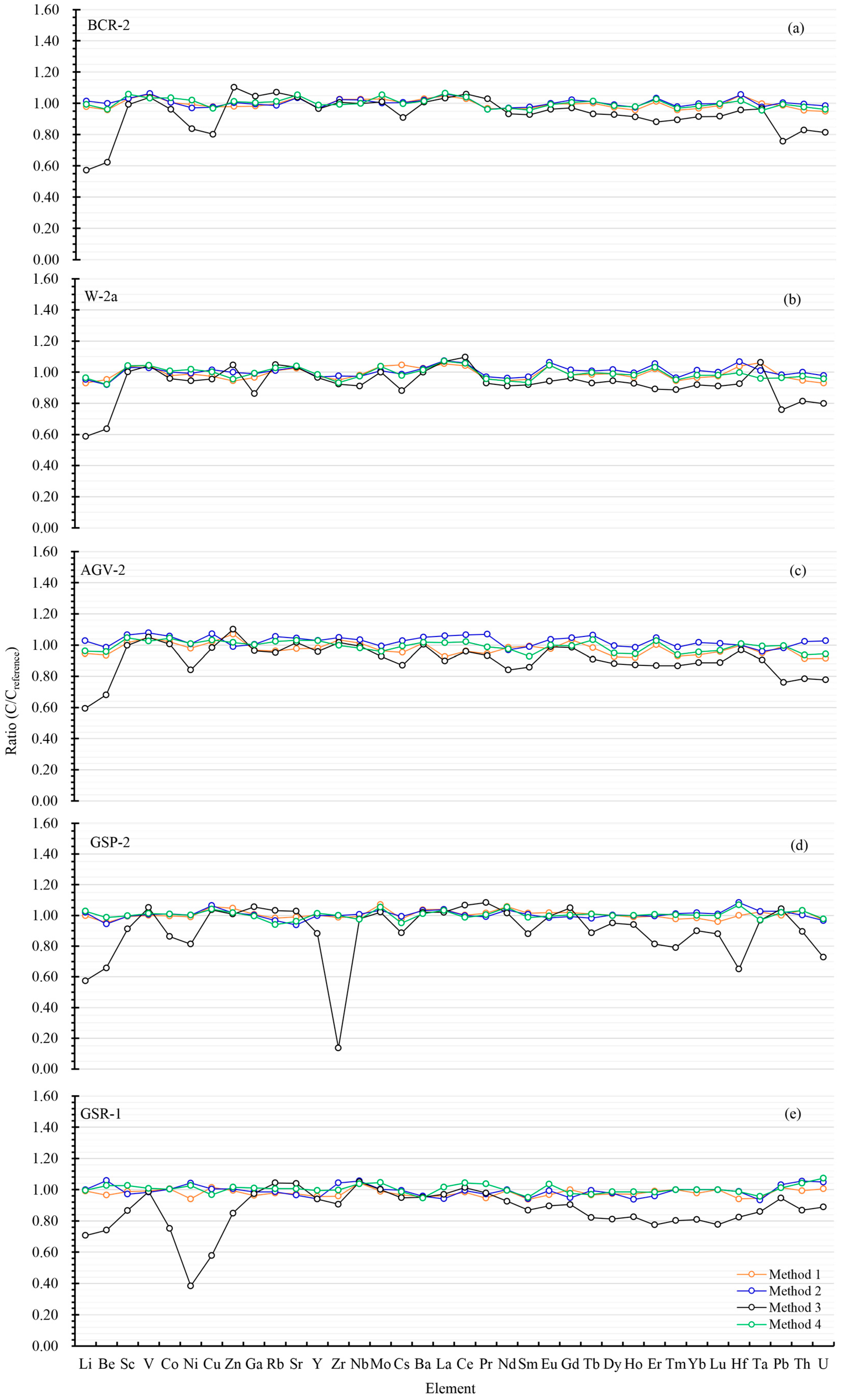
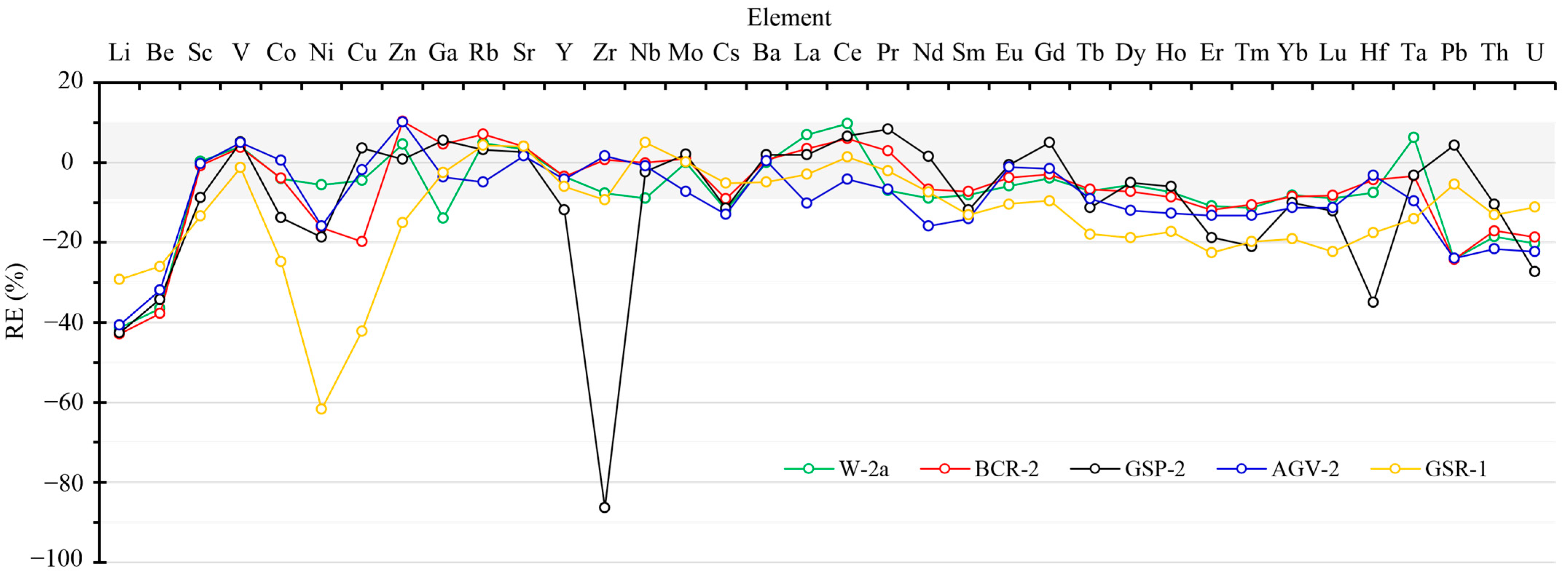
| Sample | BCR-2 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Element | Method 1 | Method 2 | Method 3 | Method 4 | Reference μg/g | ||||
| Content μg/g | RE 2 % | Content μg/g | RE % | Content μg/g | RE % | Content μg/g | RE % | ||
| Li | 8.92 ± 0.11 | −2.25 | 9.27 ± 0.05 | 1.51 | 5.21 ± 0.15 | −42.90 | 9.06 ± 0.10 | −0.75 | 9.13 ± 0.22 |
| Be | 2.08 ± 0.01 | −4.20 | 2.17 ± 0.13 | −0.14 | 1.35 ± 0.08 | −37.70 | 2.08 ± 0.10 | −3.95 | 2.17 ± 0.1 |
| Sc | 34.53 ± 0.14 | 3.00 | 34.60 ± 0.26 | 3.19 | 33.27 ± 0.23 | −0.78 | 35.47 ± 0.39 | 5.79 | 33.53 ± 0.4 |
| V | 441.9 ± 1.7 | 5.83 | 443.8 ± 2.5 | 6.27 | 433.4 ± 1.4 | 3.79 | 431.9 ± 4.9 | 3.41 | 417.6 ± 4.5 |
| Co | 37.55 ± 0.31 | 0.58 | 37.58 ± 0.15 | 0.66 | 35.86 ± 0.17 | −3.94 | 38.61 ± 0.39 | 3.43 | 37.33 ± 0.38 |
| Ni | 12.48 ± 0.22 | −0.74 | 12.20 ± 0.29 | −2.93 | 10.51 ± 0.58 | −16.36 | 12.82 ± 0.15 | 2.03 | 12.57 ± 0.37 |
| Cu | 19.23 ± 0.30 | −2.19 | 19.19 ± 0.27 | −2.38 | 15.78 ± 0.20 | −19.76 | 19.01 ± 0.37 | −3.29 | 19.66 ± 0.72 |
| Zn | 126.9 ± 0.78 | −1.97 | 130.1 ± 0.75 | 0.43 | 142.9 ± 0.17 | 10.34 | 131.0 ± 1.63 | 1.19 | 129.5 ± 1.8 |
| Ga | 21.68 ± 0.52 | −1.78 | 21.96 ± 0.18 | −0.51 | 23.08 ± 0.18 | 4.58 | 22.17 ± 0.21 | 0.45 | 22.07 ± 0.19 |
| Rb | 45.82 ± 0.21 | −0.43 | 45.39 ± 0.35 | −1.37 | 49.28 ± 0.11 | 7.09 | 46.57 ± 0.45 | 1.19 | 46.02 ± 0.56 |
| Sr | 350.6 ± 3.2 | 3.91 | 349.5 ± 3.6 | 3.60 | 350.9 ± 1.6 | 3.99 | 355.7 ± 5.3 | 5.5 | 337.4 ± 6.7 |
| Y | 34.97 ± 0.26 | −3.04 | 35.01 ± 0.39 | −2.97 | 34.81 ± 0.20 | −3.48 | 35.69 ± 0.38 | −1.05 | 36.07 ± 0.37 |
| Zr | 191.5 ± 1.62 | 2.67 | 191.1 ± 1.21 | 2.46 | 187.7 ± 1.12 | 0.63 | 185.1 ± 1.49 | −0.75 | 186.5 ± 1.5 |
| Nb | 12.76 ± 0.05 | 2.60 | 12.72 ± 0.12 | 2.21 | 12.43 ± 0.06 | −0.08 | 12.43 ± 0.13 | −0.11 | 12.44 ± 0.2 |
| Mo | 257.6 ± 7.1 | 2.78 | 251.1 ± 6.9 | 0.21 | 253.1 ± 13.5 | 1.01 | 263.9 ± 10.8 | 5.33 | 250.6 ± 6.7 |
| Cs | 1.17 ± 0.01 | 0.47 | 1.17 ± 0.01 | 0.61 | 1.05 ± 0.01 | −9.09 | 1.16 ± 0.01 | −0.25 | 1.16 ± 0.02 |
| Ba | 704.4 ± 6.9 | 2.99 | 697.8 ± 5.9 | 2.03 | 688.8 ± 2.8 | 0.71 | 694.0 ± 4.8 | 1.47 | 683.9 ± 4.7 |
| La | 26.24 ± 0.15 | 4.64 | 26.58 ± 0.23 | 5.97 | 25.94 ± 0.21 | 3.42 | 26.71 ± 0.24 | 6.49 | 25.08 ± 0.16 |
| Ce | 54.65 ± 0.36 | 2.88 | 55.17 ± 0.06 | 3.86 | 56.27 ± 0.19 | 5.93 | 55.17 ± 0.17 | 3.85 | 53.12 ± 0.33 |
| Pr | 6.60 ± 0.15 | −3.31 | 6.57 ± 0.03 | −3.75 | 7.02 ± 0.02 | 2.89 | 6.56 ± 0.04 | −3.94 | 6.827 ± 0.04 |
| Nd | 27.29 ± 0.15 | −3.44 | 27.43 ± 0.19 | −2.93 | 26.37 ± 0.11 | −6.69 | 27.38 ± 0.31 | −3.12 | 28.26 ± 0.37 |
| Sm | 6.34 ± 0.06 | −3.19 | 6.39 ± 0.02 | −2.38 | 6.08 ± 0.04 | −7.19 | 6.27 ± 0.06 | −4.28 | 6.547 ± 0.02 |
| Eu | 1.98 ± 0.02 | −0.29 | 1.99 ± 0.01 | −0.16 | 1.91 ± 0.01 | −3.74 | 1.98 ± 0.01 | −0.69 | 1.989 ± 0.05 |
| Gd | 6.79 ± 0.09 | −0.28 | 6.96 ± 0.05 | 2.18 | 6.61 ± 0.04 | −2.91 | 6.86 ± 0.05 | 0.68 | 6.811 ± 0.08 |
| Tb | 1.08 ± 0.01 | 0.11 | 1.09 ± 0.02 | 1.14 | 1.00 ± 0.01 | −6.69 | 1.09 ± 0.01 | 1.32 | 1.077 ± 0.03 |
| Dy | 6.26 ± 0.05 | −2.58 | 6.37 ± 0.03 | −0.90 | 5.96 ± 0.05 | −7.29 | 6.33 ± 0.07 | −1.52 | 6.424 ± 0.06 |
| Ho | 1.26 ± 0.01 | −4.32 | 1.28 ± 0.01 | −2.54 | 1.20 ± 0.01 | −8.62 | 1.28 ± 0.01 | −2.24 | 1.313 ± 0.01 |
| Er | 3.71 ± 0.03 | 1.21 | 3.79 ± 0.01 | 3.37 | 3.23 ± 0.03 | −11.92 | 3.77 ± 0.04 | 2.70 | 3.67 ± 0.04 |
| Tm | 0.51 ± 0.01 | −4.27 | 0.52 ± 0.01 | −2.20 | 0.48 ± 0.01 | −10.52 | 0.52 ± 0.01 | −3.12 | 0.5341 ± 0.01 |
| Yb | 3.28 ± 0.04 | −3.31 | 3.38 ± 0.03 | −0.29 | 3.10 ± 0.02 | −8.52 | 3.33 ± 0.02 | −1.76 | 3.392 ± 0.04 |
| Lu | 0.50 ± 0.01 | −1.44 | 0.50 ± 0.04 | −0.19 | 0.46 ± 0.01 | −8.28 | 0.50 ± 0.02 | −0.26 | 0.5049 ± 0.01 |
| Hf | 5.24 ± 0.06 | 5.45 | 5.25 ± 0.03 | 5.59 | 4.76 ± 0.05 | −4.35 | 5.06 ± 0.05 | 1.71 | 4.972 ± 0.03 |
| Ta | 0.78 ± 0.01 | −0.21 | 0.77 ± 0.01 | −2.29 | 0.76 ± 0.01 | −3.41 | 0.75 ± 0.01 | −4.49 | 0.785 ± 0.02 |
| Pb | 10.44 ± 0.05 | −1.38 | 10.63 ± 0.33 | 0.42 | 8.02 ± 0.30 | −24.28 | 10.53 ± 0.17 | −0.58 | 10.59 ± 0.17 |
| Th | 5.57 ± 0.06 | −4.48 | 5.80 ± 0.05 | −0.44 | 4.83 ± 0.03 | −17.06 | 5.69 ± 0.05 | −2.32 | 5.828 ± 0.05 |
| U | 1.60 ± 0.02 | −5.04 | 1.66 ± 0.01 | −1.52 | 1.37 ± 0.01 | −18.65 | 1.62 ± 0.01 | −3.91 | 1.683 ± 0.02 |
| Instrument Parameter | Operating Condition | Instrument Parameter | Operating Condition |
|---|---|---|---|
| Spray chamber | Scott chamber at 2 °C | Extract 1, V | 0 |
| RF power, W | 1550 | Extract 2 *, V | −190 |
| Plasma gas, L/min Ar | 15.0 | Omega bias *, V | −95 |
| Auxiliary gas, L/min Ar | 1.0 | Omega lens *, V | 9.1 |
| Nebulizer gas, L/min Ar | 1.05 | Discriminators *, mV | 3.8 |
| Sample/skimmer cone, mm | Nickle, 1.0/0.45 | Analog HV *, V | 2221 |
| Sampling depth *, mm | 9.0 | Pulse HV *, V | 1258 |
| Dwell time, s | 0.3 | Detector mode | Dual |
| Readings/replicate | 5 | Scan mode | Peak jumping |
| Procedure | Method | |||
|---|---|---|---|---|
| Method 1 | Method 2 | Method 3 | Method 4 | |
| Pressure relief | Add 0.5 mL of HNO3 and 0.3 mL of HF into 50 mg of sample, then dry at 140 °C | – | – | – |
| Ultrasound-assisted decomposition | Add 0.5 mL of HNO3 and 0.2 mL of HF, then place in an ultrasonic bath for 4 h | Add 1.0 mL of HNO3 and 0.5 mL of HF into 50 mg of sample, then place in an ultrasonic bath for 4 h | – | Add 30 μL of HNO3, 0.6 mL of HF, and 50 μL of 2% mannitol into 50 mg of sample, then place in an ultrasonic bath for 4 h |
| Low-pressure hotplate digestion | Flux 12 h at 140 °C | Flux 12 h at 140 °C | Add 1.0 mL of HNO3 and 0.5 mL HF into 50 mg of sample, then flux 12 h at 140 °C | Flux 12 h at 140 °C |
| Excess HF removal | When incipient dry was obtained, add 1 mL of HNO3 and heat to dry again | |||
| Sample redissolution | Add 2.0 mL of 40% HNO3 (wt.) and flux over 4 h | |||
| Solution for ICP-MS | Age overnight, and dilute to 50 g using 2% HNO3 (v/v) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, X.; Ren, Y.; Liang, T.; Wang, D. Study of Ultrasound-Assisted Low-Pressure Closed Acid Digestion Method for Trace Element Determination in Rock Samples by Inductively Coupled Plasma Mass Spectrometry. Molecules 2025, 30, 342. https://doi.org/10.3390/molecules30020342
Tan X, Ren Y, Liang T, Wang D. Study of Ultrasound-Assisted Low-Pressure Closed Acid Digestion Method for Trace Element Determination in Rock Samples by Inductively Coupled Plasma Mass Spectrometry. Molecules. 2025; 30(2):342. https://doi.org/10.3390/molecules30020342
Chicago/Turabian StyleTan, Xijuan, Yunxiu Ren, Ting Liang, and Denghong Wang. 2025. "Study of Ultrasound-Assisted Low-Pressure Closed Acid Digestion Method for Trace Element Determination in Rock Samples by Inductively Coupled Plasma Mass Spectrometry" Molecules 30, no. 2: 342. https://doi.org/10.3390/molecules30020342
APA StyleTan, X., Ren, Y., Liang, T., & Wang, D. (2025). Study of Ultrasound-Assisted Low-Pressure Closed Acid Digestion Method for Trace Element Determination in Rock Samples by Inductively Coupled Plasma Mass Spectrometry. Molecules, 30(2), 342. https://doi.org/10.3390/molecules30020342








