Abstract
Oxazole, a versatile and significant heteroarene, serves as a bridge between synthetic organic chemistry and applications in the medicinal, pharmaceutical, and industrial fields. Polycyclic aromatic compounds with amino groups substituted at the 2-position of an oxazole, such as 2-aminonaphthoxazoles, are expected to be functional probes, but their synthetic methods are extremely limited. Herein, we describe electrochemical reactions of 3-amino-2-naphthol or 3-amino-2-anthracenol and isothiocyanates in DMSO, using a graphite electrode as an anode and a platinum electrode as a cathode in the presence of potassium iodide (KI), which afford N-arylnaphtho- and N-arylanthra[2,3-d]oxazol-2-amines via cyclodesulfurization. This reaction is the first example of synthesis of 2-aminoxazole-based polycyclic compounds using an electrochemical reaction. An examination of the spectroscopic properties of polycyclic oxazoles revealed that the λabs value of the tetracyclic oxazoles was redshifted relative to that of the tricyclic oxazoles. Moreover, synthesized naphthalene/anthracene-fused tricyclic and tetracyclic oxazoles exhibited extended π-conjugated skeletons and fluoresced in the 340–430 nm region in chloroform.
1. Introduction
Oxazoles are important building blocks for the synthesis of functional organic materials and biologically active compounds [1,2,3,4,5]. Among them, N-substituted benzo[d]oxazol-2-amines (2-aminobenzoxazoles) have garnered considerable attention due to their potential applications in biological and pharmaceutical therapies. For example, suvorexant was recently approved as a medication for treating insomnia [6,7]. Furthermore, 2-aminobenzoxazole derivatives have demonstrated significant inhibitory effects on 5-lipoxygenase and p90 ribosomal S6 kinase, as well as strong α-glucosidase inhibition activity [8,9,10]. As a result, numerous synthetic methods for producing 2-aminobenzoxazoles have been developed [11,12,13]. Moreover, 2-aminonaphthoxazole, which has one more fused benzene ring, has also been reported to be a potential probe for detecting γ-hydroxybutyric acid in soft drinks and alcoholic beverages based on color and fluorescence changes [14]. However, only a few methods are known for the synthesis of its parent 2-aminonaphthoxazole [14,15,16,17,18]. For instance, in 2018, synthesis of N-phenylnaphtho[2,3-d]oxazol-2-amine via triphenylbismuth dichloride-promoted cyclodesulfurization of thioureas derived from 3-amino-2-naphthol and phenyl isothiocyanate was reported [15]. Since then, Cu2O/tetrabutylammonium bromide [16] and elemental sulfur/K2CO3 systems [17] have been used as reagents for the oxidative cyclodesulfurization reactions, and artificial hemoglobin-containing cobalt porphyrin [18] has been reported to serve as a catalyst in these reactions. However, these studies were more focused on the synthesis of benzoxazoles than on the synthesis of polycyclic N-arylnaphtho- and anthra[2,3-d]oxazol-2-amines. Moreover, the spectroscopic properties of the synthesized compounds were not investigated despite their potential as functional materials. Electrochemical synthesis attracted attention as an environmentally friendly method [19,20,21]. Bicyclic 2-aminobenzoxazoles have been synthesized through tandem electrochemical reactions from 2-aminophenols and isothiocyanates via thiourea [22]. Inspired by these works, this study aimed to synthesize N-aryloxazol-2-amines fused with naphthalene and anthracene rings, namely N-arylnaphtho- and N-arylanthra[2,3-d]oxazol-2-amines, via electrochemical cyclodesulfurization from 3-amino-2-naphthol or 3-amino-2-anthracenol and isothiocyanates. The optical properties of the synthesized compounds were also investigated.
2. Results and Discussion
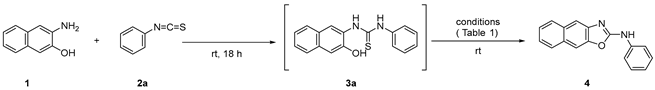
In the electrolytic synthesis of 2-aminobenzoxazoles from 2-aminophenols and isothiocyanates, as reported by Wacharasindhu et al., two reagents, NaI and NaCl, were used as electrolytes and/or reaction mediators with a mixed ethanol–water solvent [22]. Since polycyclic aromatic heterocycles are poorly soluble in water, we attempted to carry out the reaction under simpler electrolysis conditions while considering the solubility of the products. The electrocyclization of thioamide (3a) generated from 3-amino-2-naphthol (1) and phenyl isothiocyanate (2a) in the presence of various electrolytes was carried out to obtain suitable reaction conditions (Table 1). To clarify the effect of the electrolyte, MeCN was used as the solvent with a current of 20 mA using a graphite electrode as the anode [C(+)] and a platinum electrode as the cathode [Pt(−)] (entries 1–5). KI was found to be the best electrolyte for the reaction in terms of the yield of the desired oxazole (4). Solvent screening experiments suggested that the reaction proceeded efficiently in DMSO (87%), MeCN (83%), DMF (79%), and DMA (69%) (entries 4 and 6–8), whereas the reaction did not proceed in EtOH or THF (entries 9 and 10). Next, several combinations of the graphite and platinum electrodes were examined. As evident in Table 1, slightly higher yields were obtained when graphite and platinum were used as the anode and cathode, respectively (entries 4 and 11–13). The yield decreased when the KI concentration was reduced (entry 14). When the applied current was reduced from 20 to 5 mA, the reaction time increased (entry 15). Thus, the best result was obtained when 3a, generated from 1 and 2a, reacted in DMSO in the presence of a KI (0.5 M) electrolyte at a current density of 20 mA using a graphite [C(+)] anode and a platinum [Pt(−)] cathode (entry 8).

Table 1.
Screening of reaction conditions a.
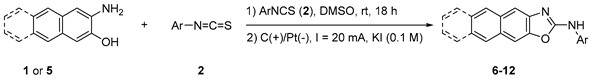
The generality of this protocol was evaluated by examining the reactions of 3-amino-2-naphthol (1) or 3-amino-2-anthracenol (5) with aryl isothiocyanate (2); the results are summarized in Table 2. The reaction of 1 with aryl isothiocyanate (2) gave the corresponding tricyclic naphthoxazoles (6–8) in high yields. When 3-amino-2-anthracenols (5) were used as a substrate instead of 1, the desired tetracyclic anthroxazoles (9–12) were obtained, albeit in low yields. These lower yields can be attributed to the low solubility of the thiourea intermediates (3) derived from 5 and 2.

Table 2.
Synthesis of polycyclic N-aryloxazol-2-amines a,b.
The mechanism of this electrochemical synthesis is unclear at present. However, a plausible mechanism has been proposed (Scheme 1). Iodine (I2) is generated from the iodide (I−) of an electrolyte such as nBuN4I, KI, and NaI at the positive electrode of the electrolytic system [23,24,25]. The thiolic form (A), a tautomer of thiourea derived from aminophenol and isothiocyanate, reacts with iodine to produce B. The electrochemical reaction of 1,3-diphenylthiourea (13) under standard conditions affords carbodiimide (14) in a 62% yield (Scheme 2a), and the reductive elimination of B generates carbodiimide (C), sulfur, and hydroiodic acid (Route A). Finally, C undergoes intramolecular cyclization to give polycyclic oxazole. Another possible route (Route B) could be the intramolecular cyclization of B to give oxazole through dehydrosulfurization via D. Reactions of thiourea (3a) using catalytic or equivalent amounts of iodine, without electrolysis, gave the corresponding product (4) in a 43% yield (Scheme 2b). These results suggest that the addition of iodine alone is less effective and that electrolytic reactions proceed more efficiently using KI, although the reaction mechanism remains unclear.
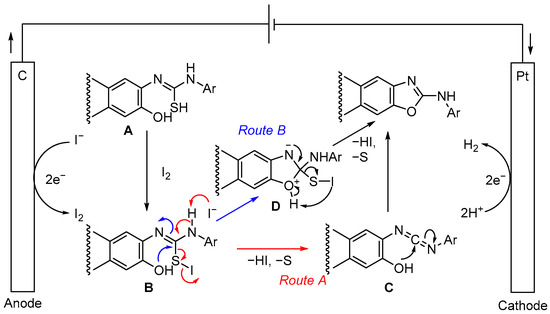
Scheme 1.
Proposed mechanism.
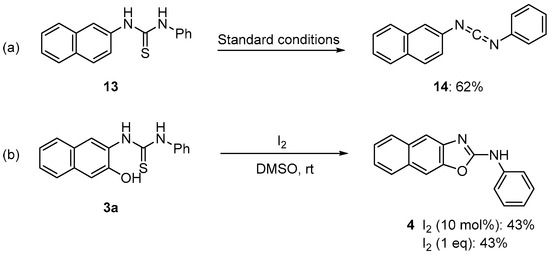
Scheme 2.
Control reactions. (a) Desulfurization of thiourea. (b) Experiments using catalytic or equivalent amounts of iodine.
The optical properties of tricyclic and tetracyclic oxazole derivatives 4 and 6–12 were evaluated based on their UV–Vis absorption and fluorescence spectra in chloroform (Figure 1 and Figure 2 and Table 3). Tricyclic oxazoles 4 and 6–8 exhibited an absorption band with a maximum (λabs) at around 330 nm. The absorption maxima were largely unaffected by the substituents at the 4-position of the N-phenyl ring. Tetracyclic oxazoles 9–12 displayed three bands (λabs) ranging from 350 to 400 nm, which were largely unaffected by the substituents at the 4-position of the N-phenyl ring. It is evident that the λabs value of the tetracyclic oxazoles was redshifted by ~70 nm relative to that of the tricyclic oxazoles, as presented in Table 3. Compounds 4 and 6–8 exhibited fluorescence maxima (λem) at 339–357 nm. The 4-methoxyphenyl derivative (6) exhibited negligible fluorescence, while the 4-trifluoromethyl derivative (8) showed stronger fluorescence (ΦF = 32%) than compounds 4 and 7. Furthermore, the λem of 4 and 6–8 was redshifted from 339 to 357 nm with an increase in the electron-donating ability of the substituents at the 4-position on the N-phenyl ring. Tetracyclic oxazoles 9–12 exhibited two fluorescence emission bands at around 410 and 430 nm, with ΦF values of 14–27%. The λem values of tetracyclic oxazoles 9–12 were not affected by the electronic property of the substituent on the N-phenyl ring.
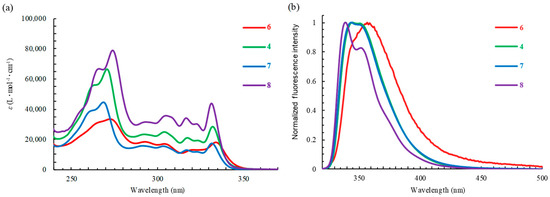
Figure 1.
The (a) absorption and (b) fluorescence spectra of N-arylnaphtho[2,3-d]oxazol-2-amines 4 and 6–8 in CHCl3. The excitation wavelength was 310 nm.
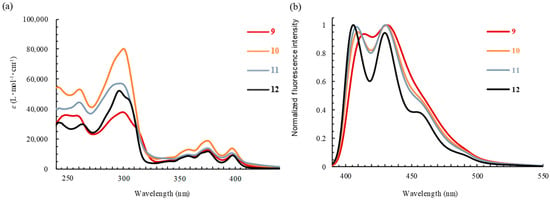
Figure 2.
The (a) absorption and (b) fluorescence spectra of N-arylanthra[2,3-d]oxazol-2-amines 9–12 in CHCl3. The excitation wavelength was 380 nm.

Table 3.
Absorption (C = 1.30 × 10−5–2.98 × 10−5 M) and fluorescence (C = 1.51 × 10−6–4.73 × 10−6 M) data for 4 and 6–12 in CHCl3 at room temperature.
3. Materials and Methods
3.1. General Information
Unless otherwise stated, all reagents and solvents were purchased from commercial suppliers and used without further purification. 1H NMR (DMSO: δ 2.48 ppm as an internal standard), 13C NMR (DMSO-d6: δ 39.5 ppm as an internal standard), and 19F NMR (trifluoromethylbenzene: δ −64.0 ppm as an external standard) spectra were recorded on a JEOL ECZ-400S (400 MHz, 100 MHz, and 376 MHz) spectrometer (JEOL Ltd., Tokyo, Japan) in DMSO-d6. Melting points were measured on a Yanagimoto micro melting point hot stage apparatus (Yanaco Technical Science Co., Ltd., Tokyo, Japan) and were not corrected. GC-MS (EI) spectra were recorded on an Agilent 5977E Diff-SST MSD-230V spectrometer (Agilent Technologies Japan, Ltd., Tokyo, Japan). HRMS (ESI) were measured on an Agilent 6230 TOF mass spectrometer (Agilent Technologies Japan, Ltd., Tokyo, Japan). IR spectra were recorded on an FTIR-8400S system from Shimadzu (SHIMADZU Corp., Kyoto, Japan) and are reported using the frequency of absorption (cm−1). UV–Vis spectra were recorded at room temperature on a HITACHI U-2800A spectrophotometer (Hitachi High-Tech Corporation, Tokyo, Japan, C = 1.30 × 10−5–2.98 × 10−5 M in CHCl3), and fluorescence spectra were recorded on a JASCO FP-8300 luminescence spectrometer (JASCO Corporation, Tokyo, Japan, C = 1.51 × 10−6–4.73 × 10−6 M in CHCl3). Only selected IR absorbencies are reported. All chromatographic separations were accomplished with Silica Gel 60N (Kanto Chemical Co., Inc., Tokyo, Japan). Thin-layer chromatography (TLC) was performed with Macherey-Nagel Sil G25 UV254 pre-coated TLC plates. 3-Amino-2-naphthol (1) and isothiocyanates (2) were purchased from TCI Fine Chemicals, Japan. Aminophenol (5) [27] was prepared according to the reported procedure. All electrochemical reactions were carried out in an IKA ElectraSyn 2.0 using ElectraSyn 2.0 undivided cells (a 5 mL vial) equipped with standard IKA ElectraSyn 2.0 electrodes (IKA Japan K.K., Osaka, Japan).
3.2. Synthesis of N-Arylnaphtho- and Anthra[2,3-d]oxazol-2-amines
In a 5.0 mL ElectraSyn vial, a mixture of aminophenol (1 or 5: 0.4 mmol) and arylisothiocyanate (2: 0.4 mmol) in DMSO (5.0 mL) was stirred well for 18 h. After complete conversion of the reaction, KI (83.0 mg, 0.5 mmol, and 0.1 M) was added to the reaction mixture. The vial was sealed with an ElectraSyn vial cap fitted with graphite SK-50 as an anode and platinum foil as a cathode. Constant-current electrolysis was performed at 20 mA for 2–4 h. The reaction mixture was diluted with H2O (20 mL) and AcOEt (20 mL), and the aqueous phase was extracted with AcOEt (3 × 30 mL). The combined organic phase was washed with brine (20 mL) and dried over MgSO4. Evaporation of the solvent provided the crude product. The crude product was then purified by column chromatography on silica gel (copies of 1H and 13C NMR see Supplementary Materials).
3.3. Characterization Data
3.3.1. N-Phenylnaphtho[2,3-d]oxazol-2-amine (4) [15]
Yellow prisms (90.4 mg, 87%), m.p.: 198–200 °C (from CH2Cl2-Hexane), Rf = 0.45 (Hexane/AcOEt = 8:2). 1H NMR (400 MHz, DMSO-d6): δ 10.9 (brs, 1H, NH), 7.93–7.90 (m, 3H, Ar-H), 7.84 (s, 1H, Ar-H), 7.79 (d, J = 8.0 Hz, 2H, Ar-H), 7.42–7.37 (m, 4H, Ar-H), and 7.05 (t, J = 7.6 Hz, 1H, Ar-H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 159.3 (C), 146.9 (C), 142.9 (C), 138.4 (C), 131.3 (C), 129.6 (C), 129.0 (CH), 127.6 (CH), 127.5 (CH), 124.4 (CH), 124.0 (CH), 122.6 (CH), 118.0 (CH), 112.3 (CH), and 104.5 (CH) ppm. HRMS: m/z [M + H]+ calculated for C17H13N2O: 261.1022. Found: 261.1019. IR (KBr): ν 3019 (NH) cm−1.
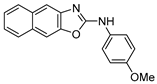
3.3.2. N-(4-Methoxyphenyl)naphtho[2,3-d]oxazol-2-amine (6) [16]
Colorless needles (104 mg, 90%), m.p.: 190–191 °C (from CH2Cl2), Rf = 0.40 (Hexane/AcOEt = 8:2). 1H NMR (400 MHz, DMSO-d6): δ 10.6 (brs, 1H, NH), 7.92–7.88 (m, 3H, Ar-H), 7.78 (s, 1H, Ar-H), 7.68 (td, J = 9.2, 2.9 Hz, 2H, Ar-H), 7.41–7.35 (m, 2H, Ar-H), 6.96 (td, J = 9.2, 2.9 Hz, 2H, Ar-H), and 3.73 (s, 3H, OCH3) ppm. 13C NMR (100 MHz, DMSO-d6): δ 160.2 (C), 155.5 (C), 147.7 (C), 143.7 (C), 132.1 (C), 131.9 (C), 130.0 (C), 128.2 (CH), 128.0 (CH), 124.9 (CH), 124.5 (CH), 120.2 (CH), 114.9 (CH), 112.5 (CH), 104.9 (CH), and 55.9 (CH3) ppm. HRMS: m/z [M + H]+ calculated for C18H15N2O2: 291.1128. Found: 291.1118. IR (KBr): ν 3018 (NH) cm−1.
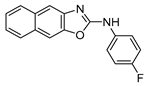
3.3.3. N-(4-Fluorophenyl)naphtho[2,3-d]oxazol-2-amine (7)
Colorless prisms (95.6 mg, 86%), m.p.: 250–252 °C (from CH2Cl2-Hexane), Rf = 0.4 (Hexane/AcOEt = 9:1). 1H NMR (400 MHz, DMSO-d6): δ 10.9 (brs, 1H, NH), 7.93–7.89 (m, 3H, Ar-H), 7.82–7.78 (m, 3H, Ar-H), 7.42–7.36 (m, 2H, Ar-H), and 7.23 (tt, J = 9.2, 3.6 Hz, 2H, Ar-H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 159.9 (C), 158.3 (d, 1JC,F = 237 Hz, C), 147.6 (C), 143.4 (C), 135.4 (C), 131.9 (C), 130.1 (C), 128.2 (CH), 128.0 (CH), 125.0 (CH), 124.6 (CH), 120.2 (d, 3JC,F = 7.7 Hz, CH), 116.2 (d, 2JC,F = 22.0 Hz, CH), 112.9 (CH), and 105.1 (CH) ppm. 19F NMR (376 MHz, DMSO-d6): δ −120.4 ppm. HRMS: m/z [M + H]+ calculated for C17H12FN2O: 279.0928. Found: 279.0923. IR (KBr): ν 3057 (NH) cm−1.
3.3.4. N-[4-(Trifluoromethyl)phenyl]naphtho[2,3-d]oxazol-2-amine (8)
Colorless prisms (116 mg, 89%), m.p.: 201–202 °C (from CH2Cl2-Hexane), Rf = 0.43 (Hexane/AcOEt = 8:2). 1H NMR (400 MHz, DMSO-d6): δ 11.3 (brs, 1H, NH), 8.00–7.90 (m, 6H, Ar-H), 7.76 (d, J = 9.2 Hz, 2H, Ar-H), and 7.45–7.39 (m, 2H, Ar-H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 159.3 (C), 147.4 (C), 142.9 (C), 142.6 (C), 131.8 (C), 130.4 (C), 128.3 (CH), 128.2 (CH), 127.0 (q, 3JC,F = 3.8 Hz, CH), 125.4 (q, 1JC,F = 270 Hz, C), 125.1 (CH), 124.9 (CH), 123.0 (q, 2JC,F = 31.6 Hz, C), 118.4 (CH), 113.5 (CH), and 105.4 (CH) ppm. 19F NMR (376 MHz, DMSO-d6): δ −60.0 ppm. HRMS: m/z [M + H]+ calculated for C18H12F3N2O: 329.0896. Found: 329.0888. IR (KBr): ν 3018 (NH) cm−1.
3.3.5. N-(4-Methoxyphenyl)anthra[2,3-d]oxazol-2-amine (9)
Yellow powder (23.1 mg, 17%), m.p. > 300 °C (from THF-Hexane), Rf = 0.40 (Hexane/AcOEt = 7:3). 1H NMR (400 MHz, DMSO-d6): δ 10.8 (brs, 1H, NH), 8.52 (d, J = 7.9 Hz, 2H, Ar-H), 7.99 (t, J = 4.6 Hz, 3H, Ar-H), 7.88 (s, 1H, Ar-H), 7.69 (d, J = 8.6 Hz, 2H, Ar-H), 7.43–7.39 (m, 2H, Ar-H), 6.98 (d, J = 9.1 Hz, 2H, Ar-H), and 3.74 (s, 3H, OCH3) ppm. 13C NMR (100 MHz, DMSO-d6): δ 160.5 (C), 155.7 (C), 148.3 (C), 144.6 (C), 137.9 (C), 131.8 (C), 131.7 (C), 130.9 (CH), 130.5 (C), 129.2 (C), 128.14 (CH), 128.05 (CH), 126.1 (CH), 125.5 (CH), 125.3 (CH), 120.4 (CH), 114.9 (CH), 111.2 (CH), 103.8 (CH), and 55.9 (CH3) ppm. HRMS: m/z [M + H]+ calculated for C22H17N2O2: 341.1285. Found: 341.1275. IR (KBr): ν 2955 (NH) cm−1.
3.3.6. N-Phenylanthra[2,3-d]oxazol-2-amine (10) [15]
Yellow powder (44.7 mg, 36%), m.p.: 278–281 °C (from AcOEt-Hexane), Rf = 0.37 (Hexane/AcOEt = 8:2). 1H NMR (400 MHz, DMSO-d6): δ 10.9 (brs, 1H, NH), 8.56 (d, J = 4.4 Hz, 2H, Ar-H), 8.03–8.00 (m, 3H, Ar-H), 7.95 (s, 1H, Ar-H), 7.81 (d, J = 7.2 Hz, 2H, Ar-H), 7.45–7.38 (m, 4H, Ar-H), and 7.08 (t, J = 7.6 Hz, 1H, Ar-H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 159.7 (C), 147.5 (C), 143.7 (C), 138.2 (C), 130.3 (C), 130.2 (C), 129.9 (C), 129.0 (CH), 128.7 (CH), 127.6 (CH), 127.5 (CH), 125.5 (CH), 125.1 (CH), 124.9 (CH), 124.7 (CH), 122.8 (CH), 118.2 (CH), 111.0 (CH), and 103.3 (CH) ppm. HRMS: m/z [M + H]+ calculated for C21H15N2O: 311.1179. Found: 311.1168. IR (KBr): ν 2997 (NH) cm−1.
3.3.7. N-(4-Fluorophenyl)anthra[2,3-d]oxazol-2-amine (11)
Yellow prisms (61.7 mg, 47%), m.p.: 285–286 °C (from AcOEt-Hexane), Rf = 0.25 (Hexane/AcOEt = 8:2). 1H NMR (400 MHz, DMSO-d6): δ 11.0 (brs, 1H, NH), 8.55 (d, J = 4.8 Hz, 2H, Ar-H), 8.03–8.00 (m, 3H, Ar-H), 7.94 (s, 1H, Ar-H), 7.84–7.81 (m, 2H, Ar-H), 7.46–7.41 (m, 2H, Ar-H), and 7.26 (t, J = 8.0 Hz, Ar-H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 160.3 (C), 158.4 (d, 1JC,F = 237 Hz, C), 148.1 (C), 144.2 (C), 135.2 (C), 130.9 (C), 130.8 (C), 130.5 (C), 129.2 (C), 128.13 (CH), 128.11 (CH), 126.1 (C), 125.7 (C), 125.5 (CH), 125.3 (CH), 120.3 (d, 3JC,F = 7.7 Hz, CH), 116.3 (d, 2JC,F = 23.0 Hz, CH), 111.6 (CH), and 103.9 (CH) ppm. 19F NMR (376 MHz, DMSO-d6): δ -120.1 ppm. HRMS: m/z [M+] calculated for C21H14FN2O: 329.1085. Found: 329.1076. IR (KBr): ν 2928 (NH) cm−1.
3.3.8. N-[4-(Trifluoromethyl)phenyl]anthra[2,3-d]oxazol-2-amine (12)
Yellow powder (80.1 mg, 53%), m.p. > 300 °C (from THF-Hexane), Rf = 0.35 (Hexane/AcOEt = 8:2). 1H NMR (400 MHz, DMSO-d6): δ 11.4 (brs, 1H, NH), 8.57 (s, 2H, Ar-H), 8.08 (s, 1H, Ar-H), 8.03–8.00 (m, 5H, Ar-H), 7.77 (d, J = 8.2 Hz, 2H, Ar-H), and 7.44–7.42 (m, 2H, Ar-H) ppm. 13C NMR (100 MHz, DMSO-d6): δ 159.8 (C), 147.9 (C), 143.8 (C), 142.4 (C), 130.9 (C), 130.73 (C), 130.69 (C), 129.4 (C), 128.1 (q, 3JC,F = 4.8 Hz, CH), 127.0 (q, 4JC,F = 3.8 Hz, CH), 126.2 (CH), 125.9 (CH), 125.7 (CH), 125.5 (CH), 124.9 (q, 1JC,F = 273 Hz, C), 123.3 (q, 2JC,F = 31.3 Hz, C), 118.7 × 2 (CH), 112.4 (CH), and 104.3 (CH) ppm. 19F NMR (376 MHz, DMSO-d6): δ −60.0 ppm. HRMS: m/z [M + H]+ calculated for C22H14F3N2O: 379.1053. Found: 379.1047. IR (KBr): ν 3059 (NH) cm−1.
4. Conclusions
In conclusion, we have demonstrated a simple method for the synthesis of polycyclic benzene-fused oxazoles using electrochemical cyclodesulfurization reactions. These are environmentally friendly reactions that use commercially available and inexpensive KI as an electrolytic mediator. Oxazoles 4 and 6–12 exhibited fluorescence in the 340–430 nm region in chloroform. Detailed mechanistic investigations of these electrochemical reactions, structural characterization, and elucidation of the physical properties of the polycyclic oxazoles are currently underway in our laboratory.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30020319/s1.
Author Contributions
Conceptualization, Y.M. and S.Y.; methodology, Y.M. and S.Y.; validation, A.M. and Y.M.; formal analysis, M.K. and M.M.; investigation, A.M. and Y.M.; writing—original draft preparation, Y.M. and S.Y.; writing—review and editing, Y.M., M.M. and S.Y.; supervision, S.Y.; project administration, S.Y.; funding acquisition, M.K., Y.M., M.M. and S.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by a research grant from the Institute of Pharmaceutical Life Sciences, Aichi Gakuin University, and a Nagai Memorial Research Scholarship from the Pharmaceutical Society of Japan (M.K.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from the authors upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Yan, X.; Wen, J.; Zhou, L.; Fan, L.; Wang, X.; Xu, Z. Current scenario of 1,3-oxazole derivatives for anticancer activity. Curr. Top. Med. Chem. 2020, 20, 1916–1937. [Google Scholar] [CrossRef] [PubMed]
- Demmer, C.S.; Bunch, L. Benzoxazoles and oxazolopyridines in medicinal chemistry studies. Eur. J. Med. Chem. 2015, 97, 778–785. [Google Scholar] [CrossRef]
- Kaur, A.; Wakode, S.; Pathak, D.P. Benzoxazole: The molecule of diverse pharmacological importance. Int. J. Pharm. Pharm. Sci. 2015, 7, 16–23. [Google Scholar]
- Singh, S.; Veeraswamy, G.; Bhattarai, D.; Goo, J.; Lee, K.; Choi, Y. Recent advances in the development of pharmacologically active compounds that contain a benzoxazole scaffold. Asian J. Org. Chem. 2015, 4, 1338–1361. [Google Scholar] [CrossRef]
- Oliveira, E.; Santos, H.M. An overview on sensing materials depending on the electromagnetic spectra region applied. Dye. Pigment. 2016, 135, 3–25. [Google Scholar] [CrossRef]
- Flick, A.C.; Ding, H.X.; Leverett, C.A.; Kyne, R.E., Jr.; Liu, K.K.C.; Fink, S.J.; O’Donnell, C.J. Synthetic approaches to the 2014 new drugs. Bioorg. Med. Chem. 2016, 24, 1937–1980. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Handu, S.S.; Mediratta, P.K. Suvorexant: The first orexin receptor antagonist to treat insomnia. J. Pharmacol. Pharmacother. 2015, 6, 118–121. [Google Scholar] [CrossRef]
- Song, H.; Oh, S.R.; Lee, H.K.; Han, G.; Kim, J.H.; Chang, H.W.; Doh, K.E.; Rhee, H.K.; Choo, H.Y.P. Synthesis and evaluation of benzoxazole derivatives as 5-lipoxygenase inhibitors. Bioorg. Med. Chem. 2010, 18, 7580–7585. [Google Scholar] [CrossRef] [PubMed]
- Costales, A.; Mathur, M.; Ramurthy, S.; Lan, J.; Subramanian, S.; Jain, R.; Atallah, G.; Setti, L.; Lindvall, M.; Appleton, B.A.; et al. 2-Amino-7-substituted benzoxazole analogs as potent RSK2 inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 1592–1596. [Google Scholar] [CrossRef]
- Wang, G.; Peng, Z.; Wang, J.; Li, J.; Li, X. Synthesis, biological evaluation and molecular docking study of N-arylbenzo[d]oxazol-2-amines as potential α-glucosidase inhibitors. Bioorg. Med. Chem. 2016, 24, 5374–5379. [Google Scholar] [CrossRef]
- Kadagathur, M.; Shaikh, A.S.; Jadhav, G.S.; Sigalapalli, D.K.; Shankaraiah, N.; Tangellamudi, N.D. Cyclodesulfurization: An enabling protocol for synthesis of various heterocycles. ChemstrySelect 2021, 6, 2621–2640. [Google Scholar] [CrossRef]
- Gu, Y.; Li, Y.D.; Ge, Y.; Huang, J.L.; Xu, H.J.; Hu, Y. Hypervalent iodine mediated synthesis of 2-aminobenzazoles and 2-aminobenzothiazoles. Asian J. Org. Chem. 2024, 13, e202400076. [Google Scholar] [CrossRef]
- Kant, K.; Patel, C.K.; Banerjee, S.; Naik, P.; Padhi, A.; Sharma, V.; Singh, V.; Almeer, R.; Keremane, K.S.; Atta, A.K.; et al. HFIP-mediated cyclodesulfurization approach for the synthesis of 2-aminobenzoxazole and 2-aminobenzothiazole derivatives. Asian J. Org. Chem. 2024, 13, e202400223. [Google Scholar] [CrossRef]
- Rodríguez-Nuévalos, S.; Costero, A.M.; Arroyo, P.; Sáez, J.A.; Parra, M.; Sancenón, F.; Martínez-Máñez, R. Protection against chemical submission: Naked-eye detection of γ-hydroxybutyric acid (GHB) in soft drinks and alcoholic beverages. Chem. Commun. 2020, 56, 12600–12603. [Google Scholar] [CrossRef] [PubMed]
- Murata, Y.; Matsumoto, N.; Miyata, M.; Kitamura, Y.; Kakusawa, N.; Matsumura, M.; Yasuike, S. One-pot reaction for the synthesis of N-substituted 2-aminobenzoxazoles using triphenylbismuth dichloride as cyclodesulfurization reagent. J. Organomet. Chem. 2018, 859, 18–23. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, L.; Dong, Y.; Yang, J.; Wu, Y. A Cu2O/TBAB-promoted approach to synthesize heteroaromatic 2-amines via one-pot cyclization of aryl isothiocyanates with ortho-substituted amines in water. Org. Biomol. Chem. 2020, 18, 7425–7430. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.T.; Huynh, T.N.; Nguyen, P.C.; Phan, N.T.S.; Nguyen, T.T. Synthesis of 2-aminobenzoxazoles from elemental sulfur mediated cyclization of 2-aminophenols and aryl isothiocyanates. Tetrahedron Lett. 2023, 122, 154510. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zhao, N.; Su, J.; Wang, C.; Wang, C.; Li, Z.; Wang, L. Environment-friendly and efficient synthesis of 2-aminobenzo-xazoles and 2-aminobenzothiazoles catalyzed by Vitreoscilla hemoglobin incorporating a cobalt porphyrin cofactor. Green Chem. 2021, 23, 8047–8052. [Google Scholar] [CrossRef]
- Listratova, A.V.; Sbei, N.; Voskressensky, L.G. Catalytic electrosynthesis of N,O-heterocycles—Recent advances. Eur. J. Org. Chem. 2020, 2020, 2012–2027. [Google Scholar] [CrossRef]
- Sbei, N.; Listratova, A.V.; Titov, A.A.; Voskressensky, L.G. Recent advances in electrochemistry for the synthesis of N-heterocycles. Synthesis 2019, 51, 2455–2473. [Google Scholar] [CrossRef]
- Jiang, Y.; Xu, K.; Zeng, C. Use of electrochemistry in the synthesis of heterocyclic structures. Chem. Rev. 2018, 118, 4485–4540. [Google Scholar] [CrossRef]
- Huynh, T.N.T.; Tankam, T.; Koguchi, S.; Rerkrachaneekorn, T.; Sukwattanasinitt, M.; Wacharasindhu, S. Electrochemical NaI/NaCl-mediated one-pot synthesis of 2-aminobenzoxazoles in aqueous media via tandem addition–cyclization. Green Chem. 2021, 23, 5189–5194. [Google Scholar] [CrossRef]
- Liu, K.; Song, C.; Lei, A. Recent advances in iodine mediated electrochemical oxidative cross-coupling. Org. Biomol. Chem. 2018, 16, 2375–2387. [Google Scholar] [CrossRef]
- Bentlety, C.L.; Bond, A.M.; Hollenkamp, A.F.; Mahon, P.J.; Zhang, J. Electrochemistry of iodide, iodine, and iodine monochloride in chloride containing nonhaloaluminate ionic liquids. Anal. Chem. 2016, 88, 1915–1921. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, C.; Jiang, H.; Sun, L. Convenient synthesis of selenyl-indoles via iodide ion-catalyzed electrochemical C–H selenation. Chem. Commun. 2018, 54, 8781–8784. [Google Scholar] [CrossRef] [PubMed]
- Eaton, D.F. Reference materials for fluorescence measurement. Pure Appl. Chem. 1988, 60, 1107–1114. [Google Scholar] [CrossRef]
- Partes, C.; Yildirim, C.; Schuster, S.; Kind, M.; Bats, J.W.; Zharnikov, M.; Terfort, A. Self-assembled monolayers of pseudo-C2v-Symmetric, low-band-gap areneoxazolethiolates on gold surfaces. Langmuir 2016, 32, 11474–11484. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).