Effect of Thermal Treatment on the Extraction and Antioxidant and Antiglycation Activities of (Poly)phenols from Ribes magellanicum
Abstract
1. Introduction
2. Results
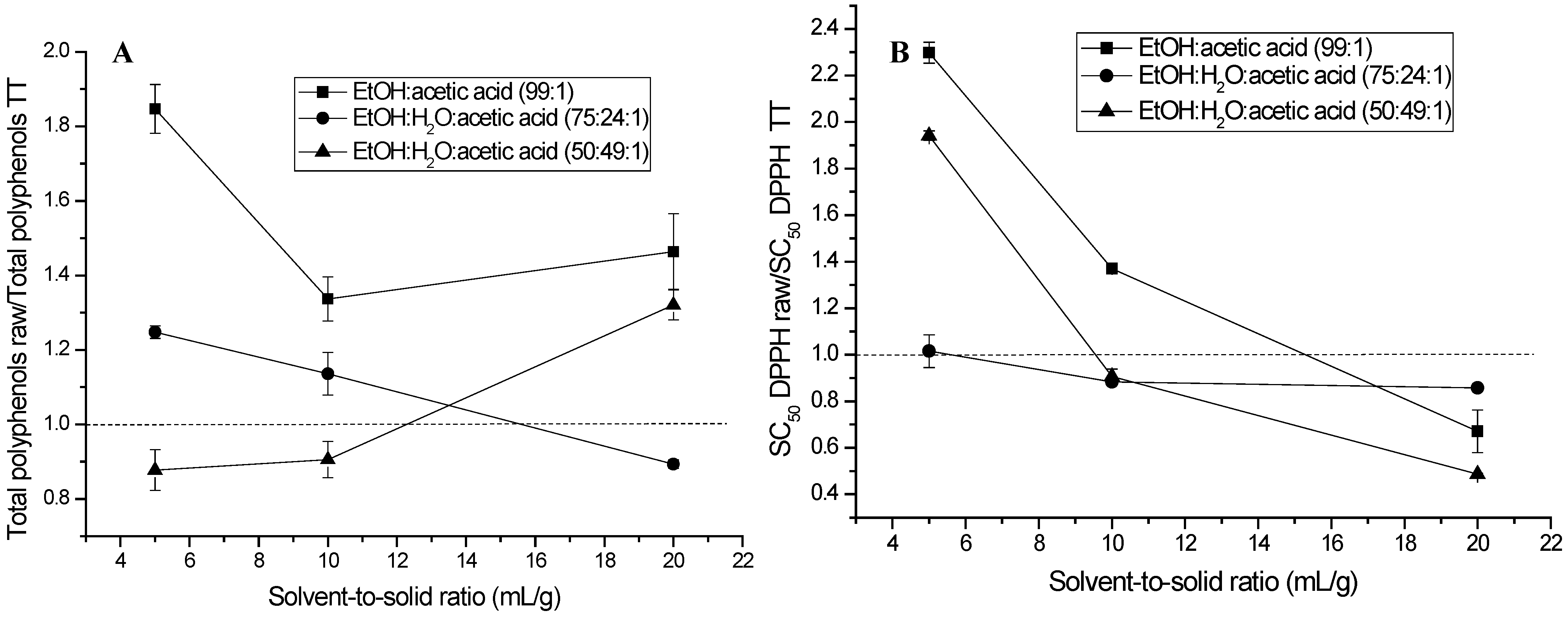
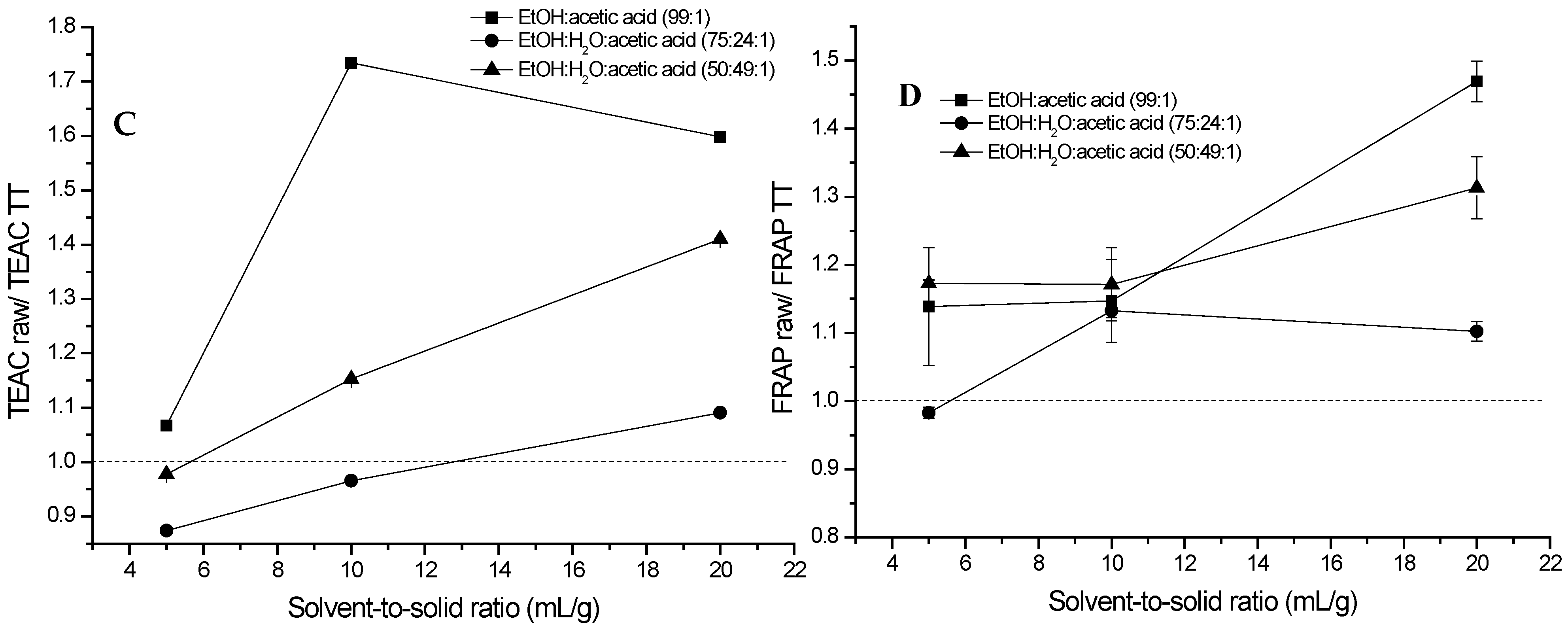
2.1. Extraction of (Poly)phenols from Raw and Thermally Treated Ribes magellanicum Berries and Their Effects on Antioxidant and Antiglycation Activities
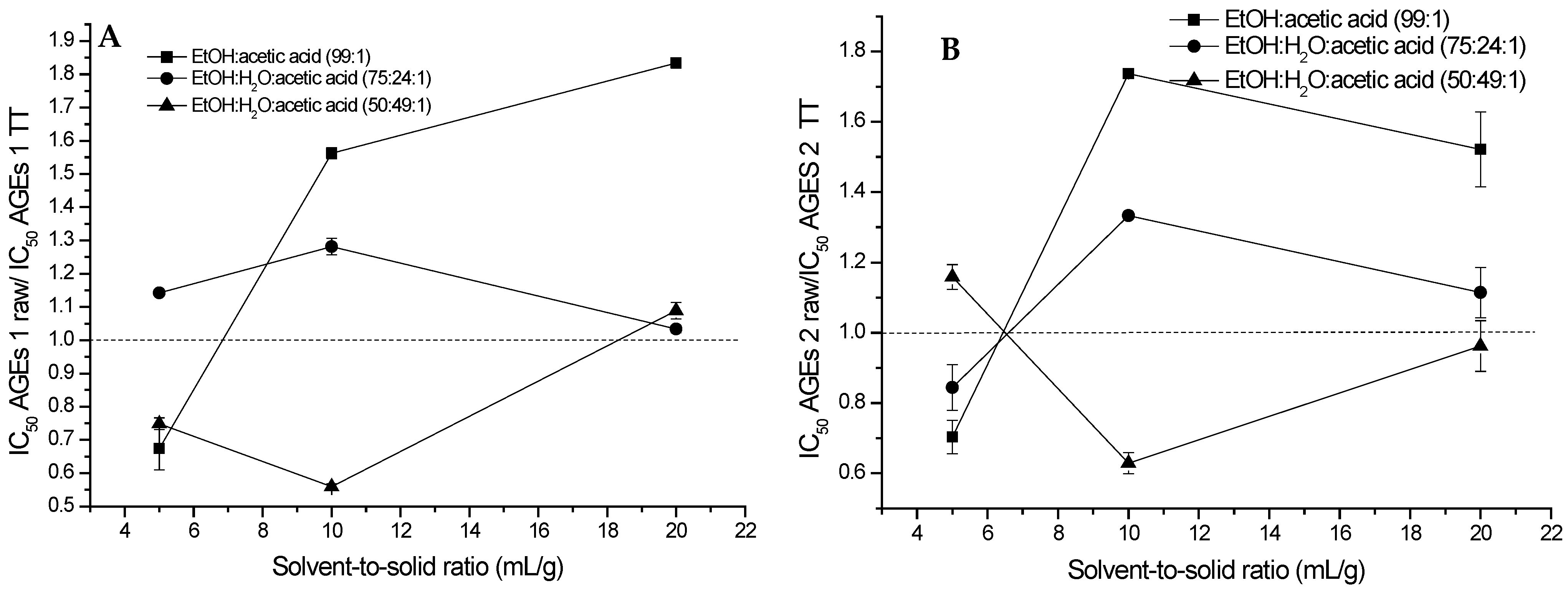
2.2. Extraction of (Poly)phenols from Raw and Thermally Treated Ribes magellanicum and Their Effects on Protein Oxidative Modifications and Advanced Glycation End Products
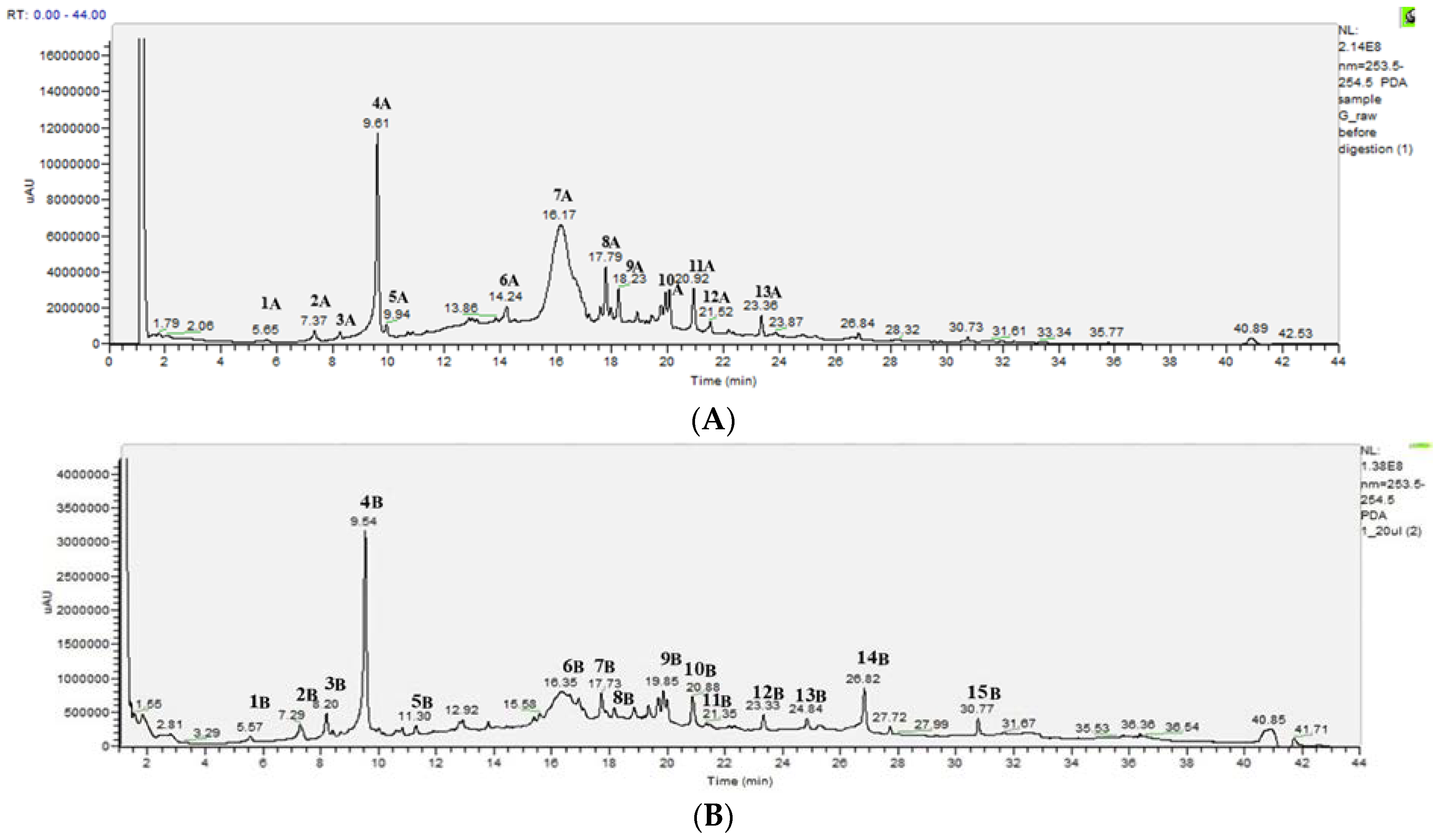
2.3. HPLC-DAD-MS-MS Analysis of the Changes Occurring in PEEs from Raw and Thermally Treated Ribes magellanicum
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Thermal Treatment of Ribes magellanicum
4.3. Polyphenol-Enriched Extract Preparation
4.4. Antioxidant Assays
4.4.1. DPPH Scavenging Assay
4.4.2. TEAC Assay
4.4.3. FRAP Assay
4.5. Determination of Total Phenolic Content (TPC)
4.6. Inhibition of Fluorescent AGEs, Kynurenine and Dityrosine Generation, and Tryptophan Consumption
4.7. Determination of Phenolic Compounds by HPLC-DAD-MS-MS
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lutz, M.; Fuentes, E.; Avila, F.; Alarcon, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef]
- Baye, E.; Kiriakova, V.; Uribarri, J.; Moran, L.J.; de Courten, B. Consumption of diets with low advanced glycation end products improves cardiometabolic parameters: Meta-analysis of randomised controlled trials. Sci. Rep. 2017, 7, 2266. [Google Scholar] [CrossRef] [PubMed]
- Kahleova, H.; Znayenko-Miller, T.; Uribarri, J.; Holubkov, R.; Barnard, N.D. Dietary advanced glycation products and their associations with insulin sensitivity and body weight: A 16-week randomized clinical trial. Obes. Sci. Pract. 2023, 9, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Cai, W.; Tripp, E.; Pyzik, R.; Yee, K.; Goldberg, L.; Tansman, L.; Chen, X.; Mani, V.; Fayad, Z.A.; et al. Oral AGE restriction ameliorates insulin resistance in obese individuals with the metabolic syndrome: A randomised controlled trial. Diabetologia 2016, 59, 2181–2192. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kaur, A.; Thind, S.S.; Singh, B.; Raina, S. Advanced glycation End-products (AGEs): An emerging concern for processed food industries. J. Food Sci. Technol. 2015, 52, 7561–7576. [Google Scholar] [CrossRef]
- Ávila, F.; Cruz, N.; Alarcon-Espósito, J.; Nina, N.; Paillan, H.; Márquez, K.; Fuentealba, D.; Burgos-Edwards, A.; Theoduloz, C.; Vejar-Vivar, C.; et al. Inhibition of advanced glycation end products and protein oxidation by leaf extracts and phenolics from Chilean bean landraces. J. Funct. Foods 2022, 98, 105270. [Google Scholar] [CrossRef]
- Liu, H.; Liu, H.; Wang, W.; Khoo, C.; Taylor, J.; Gu, L. Cranberry phytochemicals inhibit glycation of human hemoglobin and serum albumin by scavenging reactive carbonyls. Food Funct. 2011, 2, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Totlani, V.M.; Peterson, D.G. Epicatechin Carbonyl-Trapping Reactions in Aqueous Maillard Systems: Identification and Structural Elucidation. J. Agric. Food Chem. 2006, 54, 7311–7318. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.P.; O’Donnell, C.; Tiwari, B.K. Effect of thermal processing on anthocyanin stability in foods; mechanisms and kinetics of degradation. Trends Food Sci. Technol. 2010, 21, 3–11. [Google Scholar] [CrossRef]
- Chamorro, S.; Goñi, I.; Viveros, A.; Hervert-Hernández, D.; Brenes, A. Changes in polyphenolic content and antioxidant activity after thermal treatments of grape seed extract and grape pomace. Eur. Food Res. Technol. 2012, 234, 147–155. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef]
- Dewanto, V.; Wu, X.; Liu, R.H. Processed Sweet Corn Has Higher Antioxidant Activity. J. Agric. Food Chem. 2002, 50, 4959–4964. [Google Scholar] [CrossRef]
- Patras, A.; Brunton, N.; Da Pieve, S.; Butler, F.; Downey, G. Effect of thermal and high pressure processing on antioxidant activity and instrumental colour of tomato and carrot purées. Innov. Food Sci. Emerg. Technol. 2009, 10, 16–22. [Google Scholar] [CrossRef]
- Xu, B.; Chang, S.K. Total phenolics, phenolic acids, isoflavones, and anthocyanins and antioxidant properties of yellow and black soybeans as affected by thermal processing. J. Agric. Food Chem. 2008, 56, 7165–7175. [Google Scholar] [CrossRef] [PubMed]
- Gancel, A.-L.; Feneuil, A.; Acosta, O.; Pérez, A.M.; Vaillant, F. Impact of industrial processing and storage on major polyphenols and the antioxidant capacity of tropical highland blackberry (Rubus adenotrichus). Food Res. Int. 2011, 44, 2243–2251. [Google Scholar] [CrossRef]
- Méndez-Lagunas, L.; Rodríguez-Ramírez, J.; Cruz-Gracida, M.; Sandoval-Torres, S.; Barriada-Bernal, G. Convective drying kinetics of strawberry (Fragaria ananassa): Effects on antioxidant activity, anthocyanins and total phenolic content. Food Chem. 2017, 230, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Carle, R.; Stintzing, F.C. Thermal degradation of anthocyanins and its impact on color and in vitro antioxidant capacity. Mol. Nutr. Food Res. 2007, 51, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, K.; Ah-Hen, K.S.; Vega-Gálvez, A.; Vásquez, V.; Quispe-Fuentes, I.; Rojas, P.; Lemus-Mondaca, R. Changes in bioactive components and antioxidant capacity of maqui, Aristotelia chilensis [Mol] Stuntz, berries during drying. LWT Food Sci. Technol. 2016, 65, 537–542. [Google Scholar] [CrossRef]
- Speisky, H.; López-Alarcón, C.; Gómez, M.; Fuentes, J.; Sandoval-Acuña, C. First Web-Based Database on Total Phenolics and Oxygen Radical Absorbance Capacity (ORAC) of Fruits Produced and Consumed within the South Andes Region of South America. J. Agric. Food Chem. 2012, 60, 8851–8859. [Google Scholar] [CrossRef]
- Harris, C.S.; Cuerrier, A.; Lamont, E.; Haddad, P.S.; Arnason, J.T.; Bennett, S.A.; Johns, T. Investigating wild berries as a dietary approach to reducing the formation of advanced glycation endproducts: Chemical correlates of in vitro antiglycation activity. Plant Foods Hum. Nutr. 2014, 69, 71–77. [Google Scholar] [CrossRef]
- Schmeda-Hirschmann, G.; Jiménez-Aspee, F.; Theoduloz, C.; Ladio, A. Patagonian berries as native food and medicine. J. Ethnopharmacol. 2019, 241, 111979. [Google Scholar] [CrossRef]
- Simirgiotis, M.J.; Schmeda-Hirschmann, G. Determination of phenolic composition and antioxidant activity in fruits, rhizomes and leaves of the white strawberry (Fragaria chiloensis spp. chiloensis form chiloensis) using HPLC-DAD–ESI-MS and free radical quenching techniques. J. Food Compos. Anal. 2010, 23, 545–553. [Google Scholar] [CrossRef]
- Ávila, F.; Ravello, N.; Manriquez, C.; Jiménez-Aspee, F.; Schmeda-Hirschmann, G.; Theoduloz, C. Antiglycating Effect of Phenolics from the Chilean Currant Ribes cucullatum under Thermal Treatment. Antioxidants 2021, 10, 665. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aspee, F.; Theoduloz, C.; Vieira, M.N.; Rodríguez-Werner, M.A.; Schmalfuss, E.; Winterhalter, P.; Schmeda-Hirschmann, G. Phenolics from the Patagonian currants Ribes spp.: Isolation, characterization and cytoprotective effect in human AGS cells. J. Funct. Foods 2016, 26, 11–26. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Thomas-Valdés, S.; Schulz, A.; Ladio, A.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and phenolic profiles of the wild currant Ribes magellanicum from Chilean and Argentinean Patagonia. Food Sci. Nutr. 2016, 4, 595–610. [Google Scholar] [CrossRef] [PubMed]
- Burgos-Edwards, A.; Jiménez-Aspee, F.; Thomas-Valdés, S.; Schmeda-Hirschmann, G.; Theoduloz, C. Qualitative and quantitative changes in polyphenol composition and bioactivity of Ribes magellanicum and R. punctatum after in vitro gastrointestinal digestion. Food Chem. 2017, 237, 1073–1082. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.N.; Prabhakar, S. Techniques and modeling of polyphenol extraction from food: A review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Laczkó-Zöld, E.; Komlósi, A.; Ülkei, T.; Fogarasi, E.; Croitoru, M.; Fülöp, I.; Domokos, E.; Ştefănescu, R.; Varga, E. Extractability of polyphenols from black currant, red currant and gooseberry and their antioxidant activity. Acta Biol. Hung. 2018, 69, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Stampar, F.; Veberic, R.; Jakopic, J. Anthocyanins profile, total phenolics and antioxidant activity of black currant ethanolic extracts as influenced by genotype and ethanol concentration. Food Chem. 2013, 141, 961–966. [Google Scholar] [CrossRef]
- Stoica, R.; Senin, R.M.; Ion, R.-M. Ethanol Concentration Effect on the Extraction of Phenolic Compounds from Ribes nigrum. Assessed by Spectrophotometric and HPLC-DAD Methods. Rev. Chim. 2013, 64, 620–624. [Google Scholar]
- Kajdžanoska, M.; Petreska, J.; Stefova, M. Comparison of Different Extraction Solvent Mixtures for Characterization of Phenolic Compounds in Strawberries. J. Agric. Food Chem. 2011, 59, 5272–5278. [Google Scholar] [CrossRef]
- Cruz, N.; Basoalto-Cubillos, A.; Márquez, K.; Nina, N.; Vallejos-Almirall, A.; Armijo, F.; Schmeda-Hirschmann, G.; Ávila, F. Thermal treatment under oxidative conditions increases the antioxidant and antiglycation activity of Chilean Tórtola beans (Phaseolus vulgaris). Food Chem. 2024, 463 Pt 1, 141085. [Google Scholar] [CrossRef]
- Tan, H.; Cui, B.; Zheng, K.; Gao, N.; An, X.; Zhang, Y.; Cheng, Z.; Nie, Y.; Zhu, J.; Wang, L.; et al. Novel inhibitory effect of black chokeberry (Aronia melanocarpa) from selected eight berries extracts on advanced glycation end-products formation and corresponding mechanism study. Food Chem. X 2024, 21, 101032. [Google Scholar] [CrossRef] [PubMed]
- Atala, E.; Fuentes, J.; Wehrhahn, M.J.; Speisky, H. Quercetin and related flavonoids conserve their antioxidant properties despite undergoing chemical or enzymatic oxidation. Food Chem. 2017, 234, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Xue, M.; Shi, H.; Zhang, J.; Liu, Q.Q.; Guan, J.; Zhang, J.Y.; Ma, Q. Stability and Degradation of Caffeoylquinic Acids under Different Storage Conditions Studied by High-Performance Liquid Chromatography with Photo Diode Array Detection and High-Performance Liquid Chromatography with Electrospray Ionization Collision-Induced Dissociation Tandem Mass Spectrometry. Molecules 2016, 21, 948. [Google Scholar] [CrossRef] [PubMed]
- Sadilova, E.; Stintzing, F.C.; Carle, R. Thermal Degradation of Acylated and Nonacylated Anthocyanins. J. Food Sci. 2006, 71, C504–C512. [Google Scholar] [CrossRef]
- Loypimai, P.; Moongngarm, A.; Chottanom, P. Thermal and pH degradation kinetics of anthocyanins in natural food colorant prepared from black rice bran. J. Food Sci. Technol. 2016, 53, 461–470. [Google Scholar] [CrossRef]
- Adams, J.B. Thermal degradation of anthocyanins with particular reference to the 3-glycosides of cyanidin. I. In acidified aqueous solution at 100 °C. J. Sci. Food Agric. 1973, 24, 747–762. [Google Scholar] [CrossRef]
- Lopes, P.; Richard, T.; Saucier, C.; Teissedre, P.L.; Monti, J.P.; Glories, Y. Anthocyanone A: A quinone methide derivative resulting from malvidin 3-O-glucoside degradation. J. Agric. Food Chem. 2007, 55, 2698–2704. [Google Scholar] [CrossRef]
- Kamiya, H.; Yanase, E.; Nakatsuka, S. Novel oxidation products of cyanidin 3-O-glucoside with 2,2′-azobis-(2,4-dimethyl)valeronitrile and evaluation of anthocyanin content and its oxidation in black rice. Food Chem. 2014, 155, 221–226. [Google Scholar] [CrossRef]
- Fuentes, J.; Atala, E.; Pastene, E.; Carrasco-Pozo, C.; Speisky, H. Quercetin Oxidation Paradoxically Enhances its Antioxidant and Cytoprotective Properties. J. Agric. Food Chem. 2017, 65, 11002–11010. [Google Scholar] [CrossRef]
- de Ferrars, R.M.; Czank, C.; Zhang, Q.; Botting, N.P.; Kroon, P.A.; Cassidy, A.; Kay, C.D. The pharmacokinetics of anthocyanins and their metabolites in humans. Br. J. Pharmacol. 2014, 171, 3268–3282. [Google Scholar] [CrossRef]
- Buchner, N.; Krumbein, A.; Rohn, S.; Kroh, L.W. Effect of thermal processing on the flavonols rutin and quercetin. Rapid Commun. Mass Spectrom. 2006, 20, 3229–3235. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, F.; Hang, T.; Dramou, P. Degradation study of rutin as template from magnetic composite molecularly imprinted polymer supernatant samples by liquid chromatography-mass spectrometry. J. Chromatogr. A 2022, 1673, 463199. [Google Scholar] [CrossRef] [PubMed]
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Bondžić, A.M.; Lazarević-Pašti, T.D.; Bondžić, B.P.; Čolović, M.B.; Jadranin, M.B.; Vasić, V.M. Investigation of reaction between quercetin and Au(iii) in acidic media: Mechanism and identification of reaction products. New J. Chem. 2013, 37, 901–908. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Jiménez-Aspee, F.; Theoduloz, C.; Ávila, F.; Thomas-Valdés, S.; Mardones, C.; von Baer, D.; Schmeda-Hirschmann, G. The Chilean wild raspberry (Rubus geoides Sm.) increases intracellular GSH content and protects against H2O2 and methylglyoxal-induced damage in AGS cells. Food Chem. 2016, 194, 908–919. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Aspee, F.; Quispe, C.; Soriano, M.d.P.C.; Fuentes Gonzalez, J.; Hüneke, E.; Theoduloz, C.; Schmeda-Hirschmann, G. Antioxidant activity and characterization of constituents in copao fruits (Eulychnia acida Phil., Cactaceae) by HPLC–DAD–MS/MSn. Food Res. Int. 2014, 62, 286–298. [Google Scholar] [CrossRef]
- Márquez, K.; Márquez, N.; Ávila, F.; Cruz, N.; Burgos-Edwards, A.; Pardo, X.; Carrasco, B. Oleuropein-Enriched Extract From Olive Mill Leaves by Homogenizer-Assisted Extraction and Its Antioxidant and Antiglycating Activities. Front Nutr. 2022, 9, 895070. [Google Scholar] [CrossRef] [PubMed]
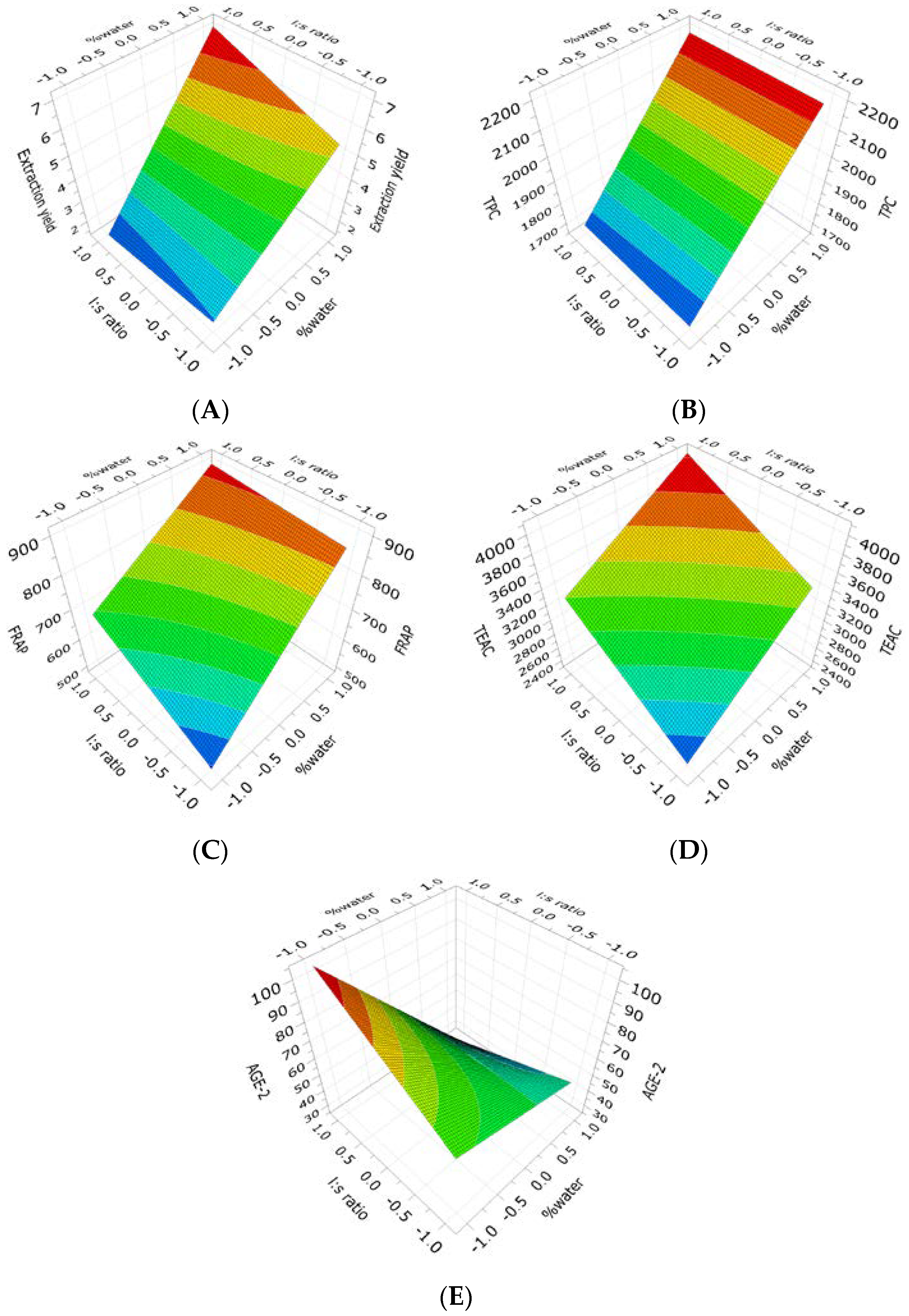
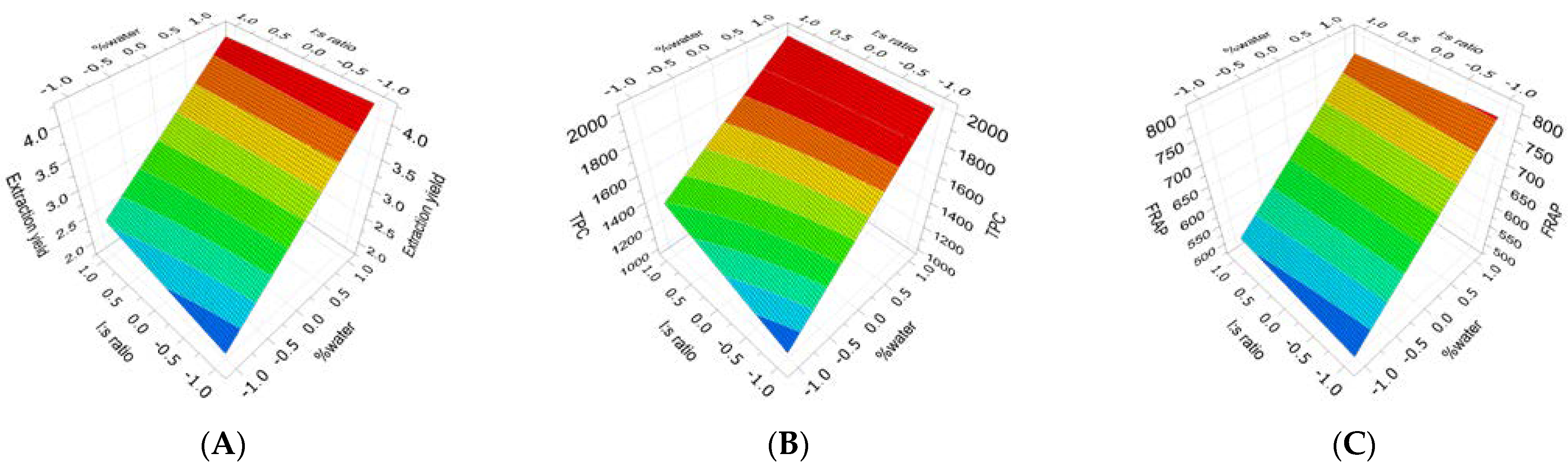


| Sample | Response | PLS Model | Coefficients |
|---|---|---|---|
| Raw Ribes | Yield | R2 = 0.887; Q2 = 0.598 RSD = 0.714 Reproducibility = 0.98 | 3.95 Cte. + 1.96 × X1 + 0.23 × X2 (ns) + 0.40 × [X1 × X2] (Equation (1)) |
| Raw Ribes | TPC | R2 = 0.809; Q2 = 0.447 RSD = 111 Reproducibility = 0.93 | 1938 Cte. + 214 × X1 + 7.2 × X2 (ns) − 2.6 × [X1 × X2] (ns) (Equation (2)) |
| Raw Ribes | FRAP | R2 = 0.767; Q2 = 0.318 RSD = 81 Reproducibility = 0.93 | 737 Cte. + 126 × X1 + 48.9 × X2 − 28.3 × [X1 × X2] (ns) (Equation (3)) |
| Raw Ribes | TEAC | R2 = 0.840; Q2 = 0.453 RSD = 239 Reproducibility = 0.98 | 3295 Cte. + 400 × X1+ 346 × X2 − 46.8 × [X1 × X2] (ns) (Equation (4)) |
| Raw Ribes | AGE-2 | R2 = 0.654; Q2 = 0.225 RSD = 18 Reproducibility = 0.98 | 59.6 Cte. − 20.5 × X1 + 4.62 × X2 (ns) − 11.1 × [X1 × X2] (Equation (5)) |
| TT Ribes | Yield | R2 = 0.554; Q2 = 0.306 RSD = 0.744 Reproducibility = 0.97 | 3.20 Cte. + 0.87 × X1 + 0.101 × X2 (ns) − 0.112 × [X1 × X2] (ns) (Equation (6)) |
| TT Ribes | TPC | R2 = 0.647; Q2 = 0.329 RSD = 282 Reproducibility = 0.99 | 1614 Cte. + 344 × X1 + 81.6 × X2 (ns) − 68.1 × [X1 × X2] (ns) (Equation (7)) |
| TT Ribes | FRAP | R2 = 0.581; Q2 = 0.281 RSD = 104 Reproducibility = 0.94 | 639 Cte. + 121 × X1 + 5.77 × X2 (ns) − 10.9 × [X1 × X2] (ns) (Equation (8)) |
| TT Ribes | NFK | R2 = 0.595; Q2 = 0.561 RSD = 6.95 Reproducibility = 0.99 | 29.3 Cte. + 8.28 × X1 + 1.02 × X2 (ns) − 1.10 × [X1 × X2] (ns) (Equation (9)) |
| TT Ribes | DiTyr | R2 = 0.589; Q2 = 0.540 RSD = 8.02 Reproducibility = 0.99 | 29.9 Cte. + 8.89 × X1 + 0.99 × X2 (ns) − 1.12 × [X1 × X2] (ns) (Equation (10)) |
| TT Ribes | AGE-1 | R2 = 0.590; Q2 = 0.552 RSD = 6.80 Reproducibility = 0.98 | 29.0 Cte. + 7.84 × X1 + 1.11 × X2 (ns) − 1.12 × [X1 × X2] (ns) (Equation (11)) |
| Peak | Retention Time (min) | λmax (nm) | F. Weight (g/mol) | Ion Detection Mode (+ or −) | Detected Mass (Fragmentation) (m/z) | Tentative Identification |
|---|---|---|---|---|---|---|
| 1A | 5.65 | 322, 214 | 354 | − | 353 (191, 179, 135) | Caffeoyl quinic acid |
| 2A | 7.37 | 320, 260 | 354 | − | 353 (191, 179, 135) | Caffeoyl quinic acid |
| 3A | 8.27 | 320, 260 | 354 | − | 191, 179, 135 | 3-Caffeoyl quinic acid |
| 4A | 9.61 | 324, 290 (sh), 240, 222 (sh) | 354 180 | + − | 355 (285, 268.98) 377 (355) 179 (135) | 3-Caffeoyl quinic acid 3-Caffeoyl quinic acid + Na+ Caffeic acid |
| 5A | 9.95 | 325, 2920 (sh), 242 | 342 | − | 341 (161, 133) | Caffeoyl hexoside |
| 6A | 14.24 | 340, 280, 240 | 368 | + | 369 (207, 185) | Feruloylquinic acid |
| 7A | 16.17 | 520, 280, 240 | 448 449 | − + | 447 (285, 255) 450 (287) | Kaempferol hexoside Cyanidin glucoside |
| 8A | 17.79 | 354, 266, 244 | 448 | + | 460 (286) | Kaempferol derivate |
| 9A | 18.23 | 354, 254 | 464 154 | − − | 463 (301, 271, 255) 153 (125, 107, 83) | Quercetin hexoside Phloroglucynaldehyde |
| 10A | 20.05 | 354, 246 | 464 | + | 466 (303) | Quercetine hexoside |
| 11A | 20.92 | 350, 266, 246 | 449 | − | 447 (285) | Kaempferol derivate |
| 12A | 21.52 | − | 447 (285) | Kaempferol derivate | ||
| 13A | 23.36 | − | 447 (285) | Kaempferol derivate | ||
| 1B | 5.57 | 322, 306 (sh), 220 | 354 | − | 353 (328, 229, 191, 179, 135) | Caffeoyl quinic acid derivative |
| 2B | 7.29 | 320, 260 314, 398, 240 | 354 154 | − − | 353 (191, 179, 135) 153 (109) | Caffeoyl quinic acid Protocatechuic acid |
| 3B | 8.2 | 320, 240 | 336 | − | 335 (161, 133) | Dehydrated 3-caffeoyl quinic acid |
| 4B | 9.54 | 324, 290 (sh), 240, 220 (sh) | 354 | − | 353 (191, 179, 135, 134) 375 (353) | 3-Caffeoyl quinic acid |
| 5B | 11.30 | 310, 236 | 350 | − | 349 (328, 187, 178, 164) | Caffeoyl quinic acid oxidized derivative |
| 6B | 16.35 | 520, 280, 240 | 594 | − | 593 (447, 343, 285) 595 (449, 355, 287) | Cyanidin hexoside rahmnoside |
| 7B | 17.73 | 350, 268 (sh), 240 | 447 (285) | Kaempferol hexoside | ||
| 8B | 18.2 | 344, 266 | 620 154 | − − | 619 (317) 153 (125, 107, 83) | Petunidin derivative Phloroglucynaldehyde |
| 9B | 19.85 | 352, 266 (sh), 240 | 610 | − | 609 (301, 271, 255, 243,151) | Rutin |
| 10B | 20.88 | 348, 266, 244 | 464 432 | − − | 463 (382, 343, 301, 254) 431 (269) | Quercetin hexoside Apigenin hexoside |
| 11B | 21.35 | 594 | − | 593 (363, 336, 284-284, 255) 561 (460, 350) 784 (460, 360, 294) 789 (478, 407, 350, 241) 696 (460, 246) 617 (460, 331) 460 (301) | Kaempferol 3-o-rutinoside Quercetin hexoside | |
| 12B | 23.33 | 348, 268, 242 | 490 | − | 489 (285) | Kaempferol hexoside monoacetate |
| 13B | 24.84 | 348, 268, 242 | 464 | − | 447 (301) | Quercetin hexoside |
| 14B | 26.82 | 344, 268, 242 | 432 | − | 431 (285, 133) | Kaempferol rhamnoside |
| 15B | 30.77 | 276, 234 | 348 | − | 347 (303, 233) | Quercetin oxidative derivate |
| Experiment | Factorial DOE | Description of Variables | ||
|---|---|---|---|---|
| Number | % Water | l:s Ratio | X1 % Water | X2 l:s Ratio |
| 1 | −1 | −1 | 0 | 5 mL/g |
| 2 | −1 | 0 | 0 | 10 mL/g |
| 3 | −1 | 1 | 0 | 20 mL/g |
| 4 | 0 | −1 | 24 | 5 mL/g |
| 5 | 0 | 0 | 24 | 10 mL/g |
| 6 | 0 | 1 | 24 | 20 mL/g |
| 7 | 1 | −1 | 49 | 5 mL/g |
| 8 | 1 | 0 | 49 | 10 mL/g |
| 9 | 1 | 1 | 49 | 20 mL/g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ávila, F.; Martinez, N.; Mora, N.; Márquez, K.; Jiménez-Aspee, F. Effect of Thermal Treatment on the Extraction and Antioxidant and Antiglycation Activities of (Poly)phenols from Ribes magellanicum. Molecules 2025, 30, 318. https://doi.org/10.3390/molecules30020318
Ávila F, Martinez N, Mora N, Márquez K, Jiménez-Aspee F. Effect of Thermal Treatment on the Extraction and Antioxidant and Antiglycation Activities of (Poly)phenols from Ribes magellanicum. Molecules. 2025; 30(2):318. https://doi.org/10.3390/molecules30020318
Chicago/Turabian StyleÁvila, Felipe, Natalia Martinez, Nicolás Mora, Katherine Márquez, and Felipe Jiménez-Aspee. 2025. "Effect of Thermal Treatment on the Extraction and Antioxidant and Antiglycation Activities of (Poly)phenols from Ribes magellanicum" Molecules 30, no. 2: 318. https://doi.org/10.3390/molecules30020318
APA StyleÁvila, F., Martinez, N., Mora, N., Márquez, K., & Jiménez-Aspee, F. (2025). Effect of Thermal Treatment on the Extraction and Antioxidant and Antiglycation Activities of (Poly)phenols from Ribes magellanicum. Molecules, 30(2), 318. https://doi.org/10.3390/molecules30020318






