Lactuca racemosa Willd., Source of Antioxidants with Diverse Chemical Structures
Abstract
1. Introduction
2. Results
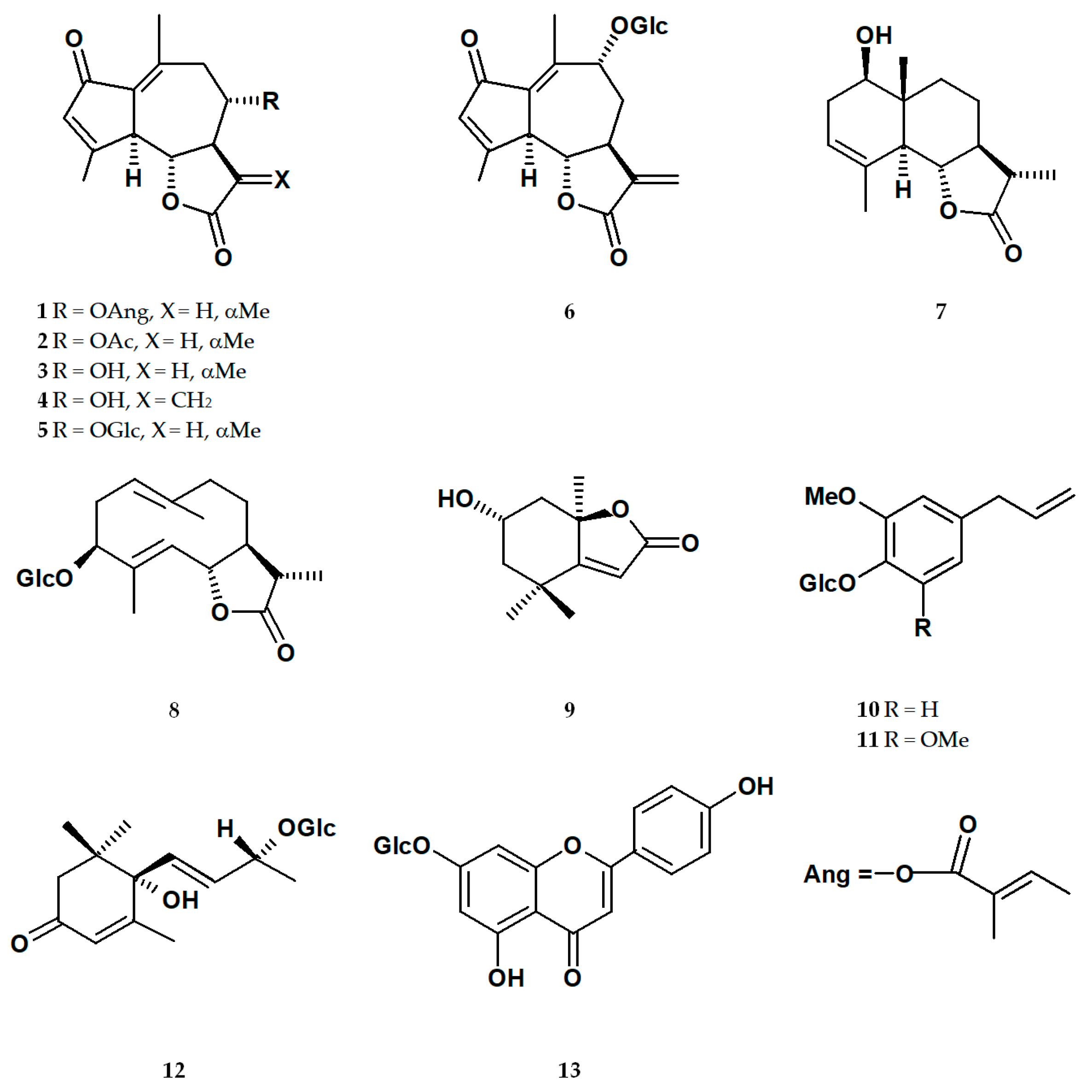
2.1. Isolation of Specialized Metabolites from L. racemosa Extracts
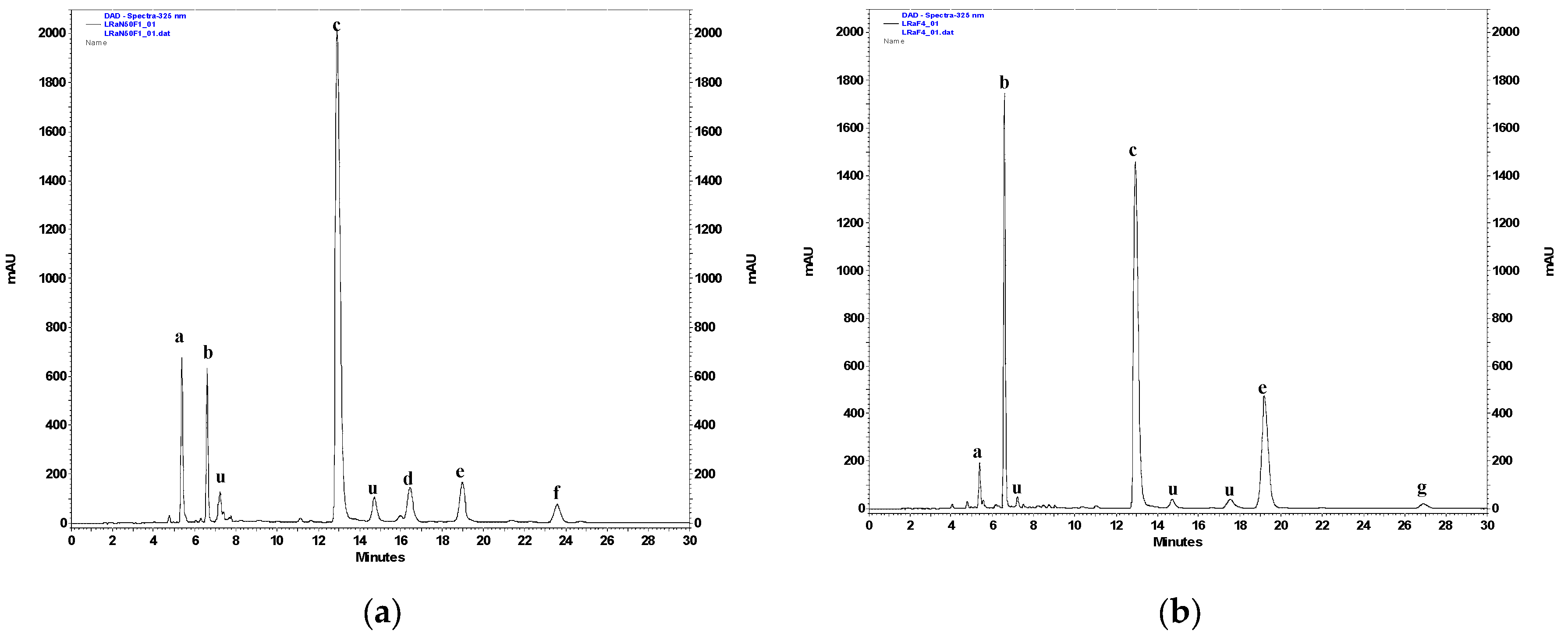
2.2. RP-HPLC-PAD Analysis of Hydroalcoholic Extracts from L. racemosa and Semiquantitative Analysis of Selected Polyphenolic Constituents
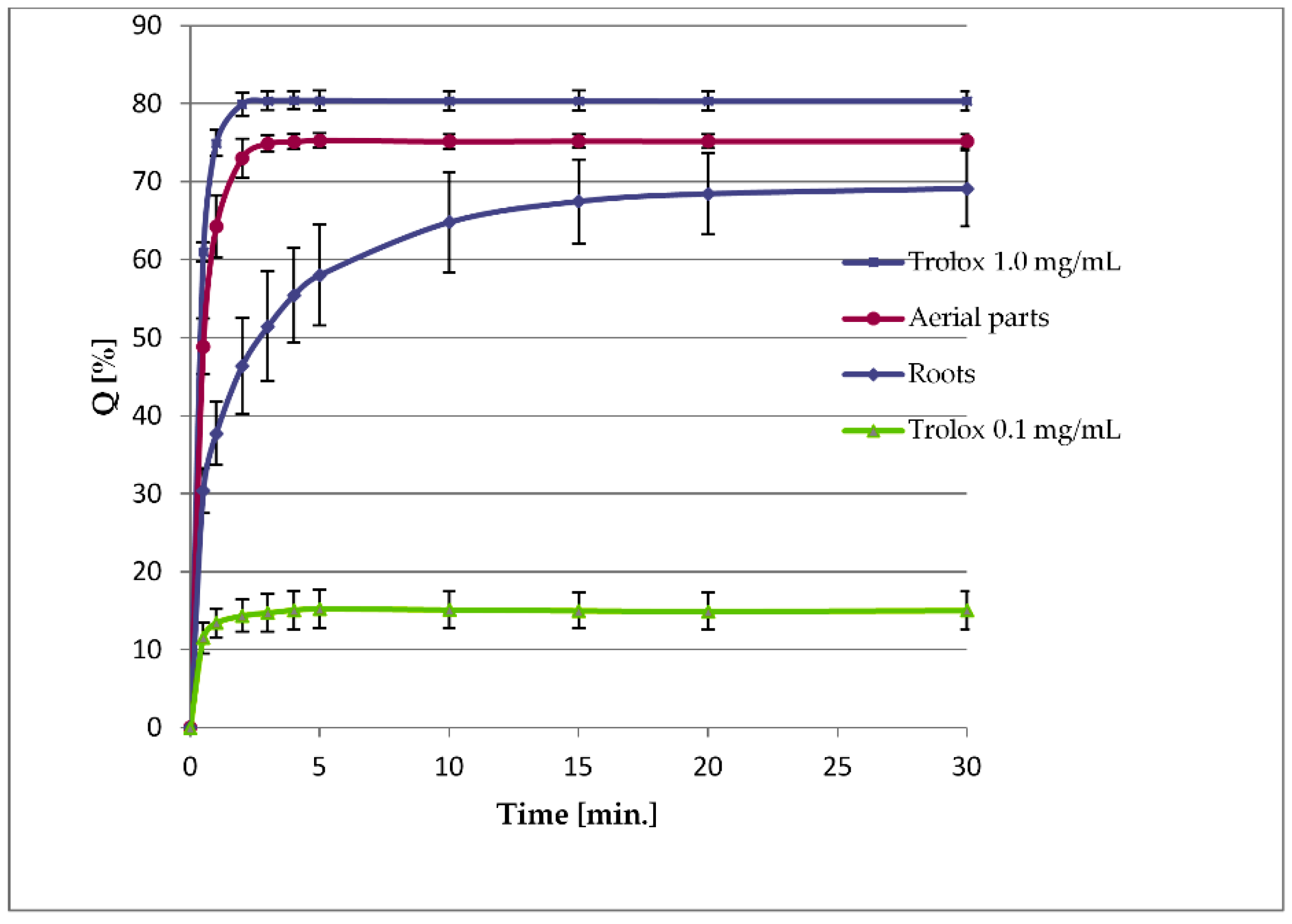
2.3. Measurements of the Reducing Capacity (TPC) and DPPH Radical Scavenging Activity of Extracts from L. racemosa
2.4. Sesquiterpene Lactone Analysis
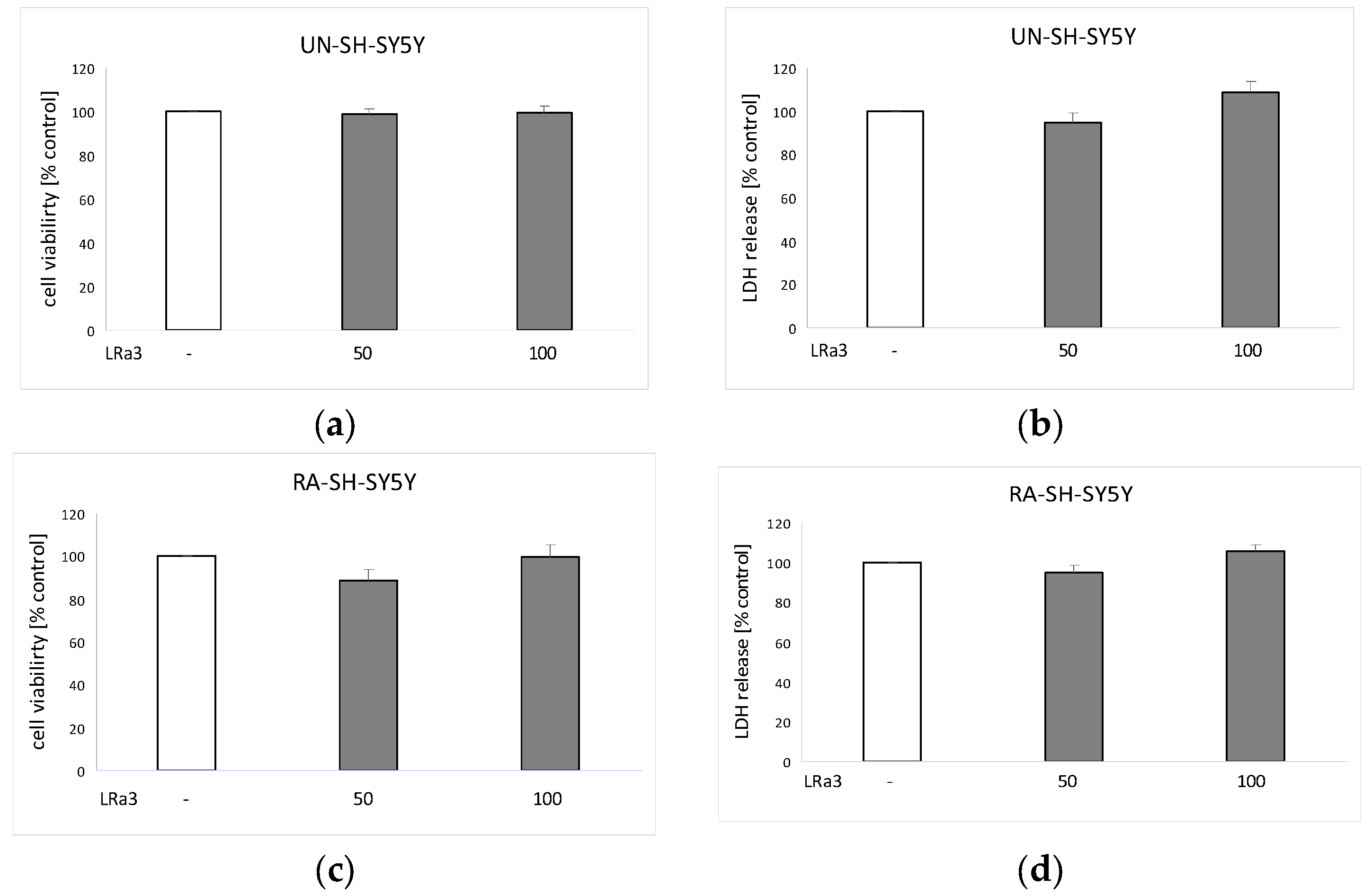
2.5. Biosafety Assessment of Deacetylmatricarin in UN-SH-SY5Y and RA-SH-SY5Y Cells
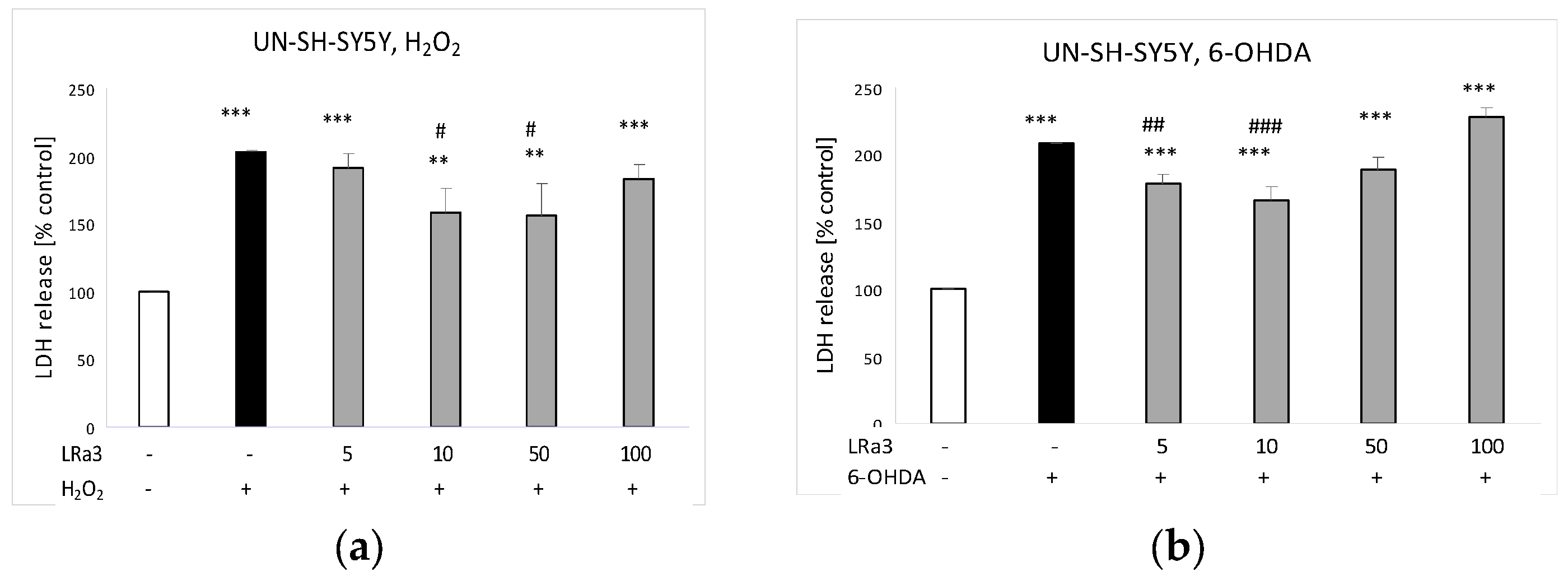
2.6. The Effect of Deacetylmatricarin on H2O2- and 6-OHDA-Induced Cell Damage in UN-SH-SY5Y Cells
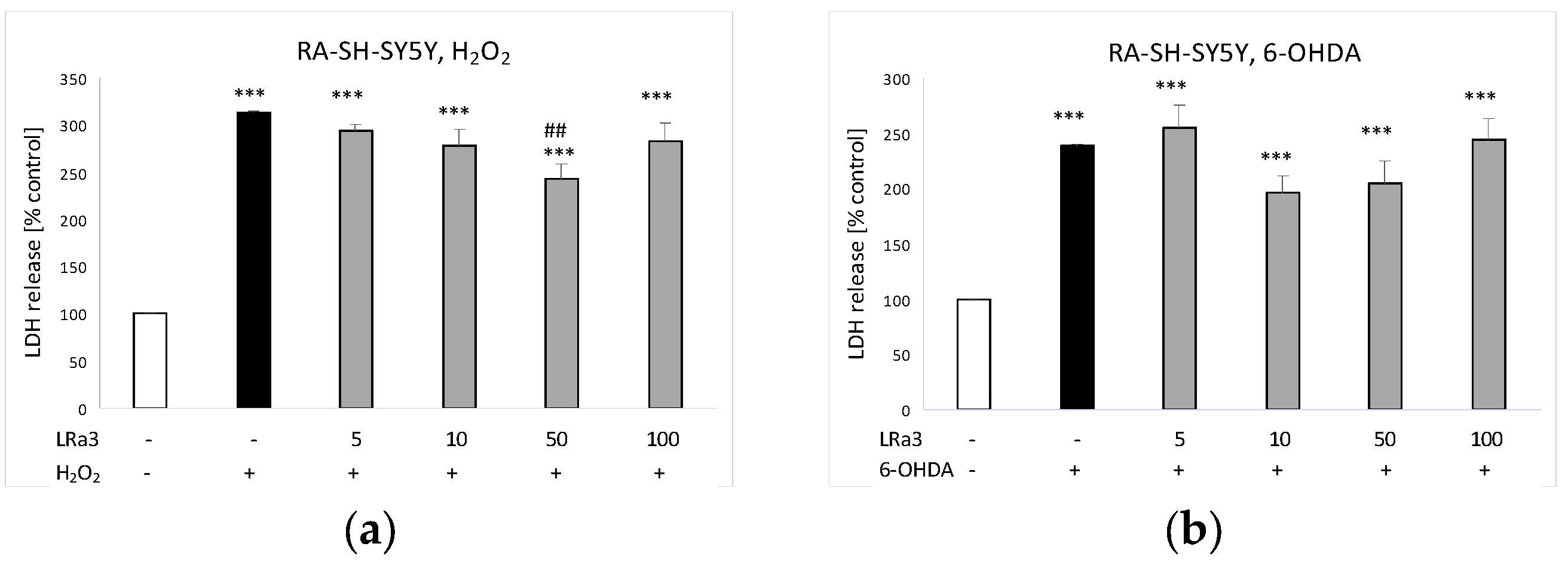
2.7. The Effect of Deacetylmatricarin on H2O2- and 6-OHDA-Induced Cell Damage in RA-SH-SY5Y Cells
3. Discussion
4. Materials and Methods
4.1. Chemicals and Solvents
4.2. General Experimental Procedures
4.3. Plant Material
4.4. Extraction and Isolation
4.5. Identification of Major Phenolic Constituents from Roots and Aerial Parts of L. racemosa and Estimation of Their Contents
4.6. Measurement of the Reducing Capacity of the Plant Material (TPC)
4.7. DPPH Radical Scavenging Assay
4.8. Measurement of the Matricarin Derivatives Content
4.9. SH-SY5Y Cell Culture
4.10. Cell Treatment
4.11. Cell Viability Assay
4.12. Cytotoxicity Assay (LDH Release Assay)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WFO. Lactuca racemosa Willd. 2024. Available online: http://www.worldfloraonline.org/taxon/wfo-0000005317 (accessed on 11 September 2024).
- Compositae Working Group (CWG). Global Compositae Database. Lactuca racemosa Willd. 2024. Available online: https://www.compositae.org/gcd/aphia.php?p=taxdetails&id=1103863 (accessed on 11 September 2024).
- IPNI. International Plant Names Index. Lactuca racemosa Willd.; The Royal Botanic Gardens, Kew, Harvard University Herbaria & Libraries and Australian National Herbarium. 2024. Available online: https://www.ipni.org/n/228203-1 (accessed on 11 September 2024).
- WFO. Cicerbita racemosa Beauverd. 2024. Available online: https://wfoplantlist.org/taxon/wfo-0000135671-2023-06?page=1 (accessed on 11 September 2024).
- POWO—Plants of the World Online. Facilitated by the Royal Botanic Gardens, Kew. Cicerbita racemosa (Willd.) Beauverd. 2024. Available online: https://powo.science.kew.org/taxon/urn:lsid:ipni.org:names:194484-1 (accessed on 11 September 2024).
- Güzel, M.E.; Coşkunçelebi, K.; Kilian, N.; Makbul, S.; Gültepe, M. Phylogeny and systematics of the Lactucinae (Asteraceae) focusing on their SW Asian centre of diversity. Plant Syst. Evol. 2021, 307, 7. [Google Scholar] [CrossRef]
- Chu, R.; Xu, X.; Lu, Z.; Ma, Y.; Cheng, H.; Zhu, S.; Bakker, F.T.; Schranz, M.E.; Wei, Z. Plastome-based phylogeny and biogeography of Lactuca L. (Asteraceae) support revised lettuce gene pool categories. Front. Plant Sci. 2022, 13, 978417. [Google Scholar] [CrossRef] [PubMed]
- Saraç, D.U.; Özkan, Z.C.; Akbulut, S. Ethnobotanic features of Rize/Turkey province. Biol. Divers. Conserv. 2013, 6, 57–66. Available online: https://dergipark.org.tr/en/download/article-file/1191743 (accessed on 26 September 2024).
- Kaltalioğlu, K.; Karaköse, M.; Şahın, H.; Bektaş, E.; İnan Bektaş, K. Determination of antioxidant and antimicrobial activities, and phenolics compounds by RP-HPLC-DAD of some medicinal plants from Gümüşhane (Turkey). GÜFBED/GUSTIJ 2019, 9, 362–372. [Google Scholar] [CrossRef]
- Zidorn, C. Bioprospecting of plant natural products in Schleswig-Holstein (Germany) I: Chemodiversity of the Cichorieae tribe (Asteraceae) in Schleswig-Holstein. Phytochem. Rev. 2019, 18, 1223–1253. [Google Scholar] [CrossRef]
- Yang, X.; Gil, M.I.; Yang, Q.; Tomás-Barberán, F.A. Bioactive compounds in lettuce: Highlighting the benefits to human health and impacts of preharvest and postharvest practices. Compr. Rev. Food Sci. Food Saf. 2022, 21, 4–45. [Google Scholar] [CrossRef]
- Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Phytochemistry 2008, 69, 2270–2296. [Google Scholar] [CrossRef]
- Shulha, O.; Zidorn, C. Sesquiterpene lactones and their precursors as chemosystematic markers in the tribe Cichorieae of the Asteraceae revisited: An update (2008–2017). Phytochemistry 2019, 163, 149–177. [Google Scholar] [CrossRef]
- Sareedenchai, V.; Zidorn, C. Flavonoids as chemosystematic markers in the tribe Cichorieae of the Asteraceae. Biochem. Syst. Ecol. 2010, 38, 935–957. [Google Scholar] [CrossRef]
- Stojakowska, A.; Michalska, K.; Malarz, J.; Beharav, A.; Kisiel, W. Root tubers of Lactuca tuberosa as a source of antioxidant phenolic compounds and new furofuran lignans. Food Chem. 2013, 138, 1250–1255. [Google Scholar] [CrossRef]
- Stojakowska, A.; Michalska, K.; Kłeczek, N.; Malarz, J.; Beharav, A. Phenolics and terpenoids from a wild edible plant Lactuca orientalis (Boiss.) Boiss.: A preliminary study. J. Food Compos. Anal. 2018, 69, 20–24. [Google Scholar] [CrossRef]
- Malarz, J.; Michalska, K.; Stojakowska, A. Stem Lettuce and Its Metabolites: Does the variety make any difference? Foods 2021, 10, 59. [Google Scholar] [CrossRef]
- Michalska, K.; Malarz, J.; Paul, W.; Stojakowska, A. Natural products from Tolpis barbata (L.) Gaertn. (Asteraceae, Cichorieae). Biochem. Syst. Ecol. 2019, 86, 103922. [Google Scholar] [CrossRef]
- Beharav, A.; Stojakowska, A.; Ben-David, R.; Malarz, J.; Michalska, K.; Kisiel, W. Variation of sesquiterpene lactone contents in Lactuca georgica natural populations from Armenia. Gen. Resour. Crop. Evol. 2015, 62, 431–441. [Google Scholar] [CrossRef]
- Kisiel, W.; Michalska, K. Matricarin-type guaianolides from Taraxacum bessarabicum and their chemotaxonomic significance. Biochem. Syst. Ecol. 2006, 34, 356–359. [Google Scholar] [CrossRef]
- Michalska, K.; Szneler, E.; Kisiel, W. Sesquiterpene lactones from Lactuca canadensis and their chemotaxonomic significance. Phytochemistry 2013, 90, 90–94. [Google Scholar] [CrossRef]
- Walle, T.; Browning, A.M.; Steed, L.L.; Reed, S.G.; Walle, U.K. Flavonoid glucosides are hydrolyzed and thus activated in the oral cavity in humans. J. Nutr. 2005, 135, 48–52. [Google Scholar] [CrossRef]
- Seto, M.; Miyase, T.; Umehara, K.; Ueno, A.; Hirano, Y.; Otani, N. Sesquiterpene lactones from Cichorium endivia L. and C. intybus L. and cytotoxic activity. Chem. Pharm. Bull. 1988, 36, 2423–2429. [Google Scholar] [CrossRef]
- Stojanović, M.; Savić, S.; Delcourt, A.; Hilbert, J.-L.; Hance, P.; Dragišić Maksimović, J.; Maksimović, V. Phenolics and sesquiterpene lactones profile of red and green lettuce: Combined effect of cultivar, microbiological fertiliser, and season. Plants 2023, 12, 2616. [Google Scholar] [CrossRef]
- Llorach, R.; Martínez-Sánchez, A.; Tomás-Barberán, F.A.; Gil, M.I.; Ferreres, F. Characterisation of polyphenols and antioxidant properties of five lettuce varieties and escarole. Food Chem. 2008, 108, 1028–1038. [Google Scholar] [CrossRef]
- Hameed, M.K.; Umar, W.; Razzaq, A.; Wei, S.; Niu, Q.; Huang, D.; Chang, L. Quantification of total polyphenols, antioxidants, anthocyanins and secondary metabolites by UPLC VION IMS QTOF MS/MS analysis in green and red lettuce cultivars. Sci. Hortic. 2023, 315, 111994. [Google Scholar] [CrossRef]
- Abu-Reidah, I.M.; Contreras, M.M.; Arráez-Romána, D.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Reversed-phase ultra-high-performance liquid chromatography coupled to electrospray ionization-quadrupole-time-of-flight mass spectrometry as a powerful tool for metabolic profiling of vegetables: Lactuca sativa as an example of its application. J. Chromatogr. A 2013, 1313, 212–227. [Google Scholar] [CrossRef]
- Viacava, G.E.; Roura, S.I.; López-Márquez, D.M.; Berrueta, L.A.; Gallo, B.; Alonso-Salces, R.M. Polyphenolic profile of butterhead lettuce cultivar by ultrahigh performance liquid chromatography coupled online to UV–visible spectrophotometry and quadrupole time-of-flight mass spectrometry. Food Chem. 2018, 260, 239–273. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.F.; Braidy, N.; Gortzi, O.; Sobarzo-Sanchez, E.; Daglia, M.; Skalicka-Woźniak, K.; Nabavi, S.M. Luteolin as an anti-inflammatory and neuroprotective agent: A brief review. Brain Res. Bull. 2015, 119, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Jayawickreme, D.K.; Ekwosi, C.; Anand, A.; Andres-Mach, M.; Wlaź, P.; Socała, K. Luteolin for neurodegenerative diseases: A review. Pharmacol. Rep. 2024, 76, 644–664. [Google Scholar] [CrossRef]
- Wang, N.; Li, R.; Feng, B.; Cheng, Y.; Guo, Y.; Qian, H. Chicoric acid prevents neuroinflammation and neurodegeneration in a mouse Parkinson’s disease model: Immune response and transcriptome profile of the spleen and colon. Int. J. Mol. Sci. 2022, 23, 2031. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Shen, C.; Xiao, Y.; Wang, Y.; Liu, Z.; Liu, X. Chicoric acid supplementation prevents systemic inflammation-induced memory impairment and amyloidogenesis via inhibition of NF-κB. FASEB J. 2017, 31, 1494–1507. [Google Scholar] [CrossRef]
- Althagafi, H.A. Neuroprotective role of chlorogenic acid against hippocampal neuroinflammation, oxidative stress, and apoptosis following acute seizures induced by pentylenetetrazole. Metab. Brain. Dis. 2024, 39, 1307–1321. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Alcazar Magaña, A.; Kamimura, N.; Soumyanath, A.; Stevens, J.F.; Maier, C.S. Caffeoylquinic acids: Chemistry, biosynthesis, occurrence, analytical challenges, and bioactivity. Plant J. 2021, 107, 1299–1319. [Google Scholar] [CrossRef]
- Zheng, Y.; Ji, S.; Li, X.; Feng, Q. Active ingredients and molecular targets of Taraxacum mongolicum against hepatocellular carcinoma: Network pharmacology, molecular docking, and molecular dynamics simulation analysis. PeerJ 2022, 10, e13737. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Choi, E.J.; Yoo, G.S.; Kim, K.-M.; Ryu, S.Y. Desacetylmatricarin, an anti-allergic component from Taraxacum platycarpum. Planta Med. 1998, 64, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Plutno, A.B.; Sham’yanov, I.D.; Aizikov, M.I.; Prokhorova, M.R.; Galyust’yan, G.G.; Kurmukov, A.G. Modification of the sesquiterpene lactones leukomisin and austricin biological activities of some of their derivatives. Chem. Nat. Compd. 1995, 31, 579–583. [Google Scholar] [CrossRef]
- Li, H.; Li, J.; Liu, M.; Xie, R.; Zang, Y.; Li, J.; Aisa, H.A. Guaianolide sesquiterpene lactones from Achillea millefolium L. Phytochemistry 2021, 186, 112733. [Google Scholar] [CrossRef]
- Jantas, D.; Malarz, J.; Le, T.N.; Stojakowska, A. Neuroprotective properties of kempferol derivatives from Maesa membranacea against oxidative stress-induced cell damage: An association with cathepsin D inhibition and PI3K/Akt activation. Int. J. Mol. Sci. 2021, 22, 10363. [Google Scholar] [CrossRef]
- Jantas, D.; Chwastek, J.; Malarz, J.; Stojakowska, A.; Lasoń, W. Neuroprotective effects of methyl caffeate against hydrogen peroxide-induced cell damage: Involvement of caspase 3 and cathepsin D inhibition. Biomolecules 2020, 10, 1530. [Google Scholar] [CrossRef]
- Lee, K.Y.; Hwang, L.; Jeong, E.J.; Kim, S.H.; Kim, Y.C.; Sung, S.H. Effect of neuroprotective flavonoids of Agrimonia eupatoria on glutamate-induced oxidative injury to HT22 hippocampal cells. Biosci. Biotechnol. Biochem. 2010, 74, 1704–1706. [Google Scholar] [CrossRef]
- Park, H.-J.; Kim, H.-N.; Kim, C.Y.; Seo, M.-D.; Baek, S.-H. Synergistic protection by isoquercitrin and quercetin against glutamate-induced oxidative cell death in HT22 cells via activating Nrf2 and HO-1 signaling pathway: Neuroprotective principles and mechanisms of Dendropanax morbifera leaves. Antioxidants 2021, 10, 554. [Google Scholar] [CrossRef]
- Magalingam, K.B.; Radhakrishnan, A.; Haleagrahara, N. Protective effects of flavonol isoquercitrin, against 6-hydroxydopamine (6-OHDA)-induced toxicity in PC12 cells. BMC Res. Notes 2014, 7, 49. [Google Scholar] [CrossRef]
- Cheung, Y.T.; Lau, W.K.; Yu, M.S.; Lai, C.S.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Glasl, S.; Mucaji, P.; Werner, I.; Presser, A.; Jurenitsch, J. Sesquiterpenes and flavonoid aglycones from a Hungarian taxon of the Achillea millefolium group. Z. Naturforsch. 2002, 57, 976–982. [Google Scholar] [CrossRef]
- Daniewski, W.M.; Skibicki, P.; Gumułka, M.; Drożdż, B.; Grabarczyk, H.; Błoszczyk, E. Sesquiterpene lactones constituents of Reichardia tingitana L. Roth. and their antifeedant activity. Acta Soc. Bot. Pol. 1988, 57, 539–545. [Google Scholar] [CrossRef]
| Lactuca racemosa | Phenolic Constituents (% Dry Weight) 1 | |||||
|---|---|---|---|---|---|---|
| CTA | 5-CQA | DCTA | 3,5-DCQA | 4,5-DCQA | Luteolin 7-O-β-glucoside | |
| Aerial parts | 0.39 ± 0.04 | 0.23 ± 0.03 | 3.00 ± 0.16 | 0.28 ± 0.09 | nd | 0.45 ± 0.04 |
| Roots | 0.06 ± 0.01 | 0.28 ± 0.08 | 1.18 ± 0.08 | 0.45 ± 0.08 | 0.020 ± 0.004 | nd |
| Lactuca racemosa | Sesquiterpene Lactones (% Dry Weight) 1 | ||
|---|---|---|---|
| Matricarin (2) | Deacetylmatricarin (3) | Deacetylmatricarin 8-O-β-glucopyranoside (5) | |
| Aerial parts | 0.010 ± 0.008 | 0.046 ± 0.020 | 0.326 ± 0.162 |
| Roots | 0.033 ± 0.011 | 0.007 ± 0.002 | 1.260 ± 0.572 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalska, K.; Jantas, D.; Malarz, J.; Jakubowska, K.; Paul, W.; Stojakowska, A. Lactuca racemosa Willd., Source of Antioxidants with Diverse Chemical Structures. Molecules 2024, 29, 5975. https://doi.org/10.3390/molecules29245975
Michalska K, Jantas D, Malarz J, Jakubowska K, Paul W, Stojakowska A. Lactuca racemosa Willd., Source of Antioxidants with Diverse Chemical Structures. Molecules. 2024; 29(24):5975. https://doi.org/10.3390/molecules29245975
Chicago/Turabian StyleMichalska, Klaudia, Danuta Jantas, Janusz Malarz, Klaudia Jakubowska, Wojciech Paul, and Anna Stojakowska. 2024. "Lactuca racemosa Willd., Source of Antioxidants with Diverse Chemical Structures" Molecules 29, no. 24: 5975. https://doi.org/10.3390/molecules29245975
APA StyleMichalska, K., Jantas, D., Malarz, J., Jakubowska, K., Paul, W., & Stojakowska, A. (2024). Lactuca racemosa Willd., Source of Antioxidants with Diverse Chemical Structures. Molecules, 29(24), 5975. https://doi.org/10.3390/molecules29245975







