Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight
Abstract
1. Introduction
2. Results and Discussion
2.1. Structural and Chemical Characterization of Ferberite
2.2. Morphological Characterization of Ferberite
2.3. Growth Mechanism of Ferberite Catalyst
2.3.1. Sodium Tungstate Dihydrate Dissolution and Role of Oxalic Acid
2.3.2. Nucleation and Growth Mechanism: Insights from TEM and FTIR Analyses
2.3.3. Proposed Dual-Pathway Growth Mechanism
2.3.4. Influence of Iron Precursor Physical Form on Growth Mechanism and Morphology
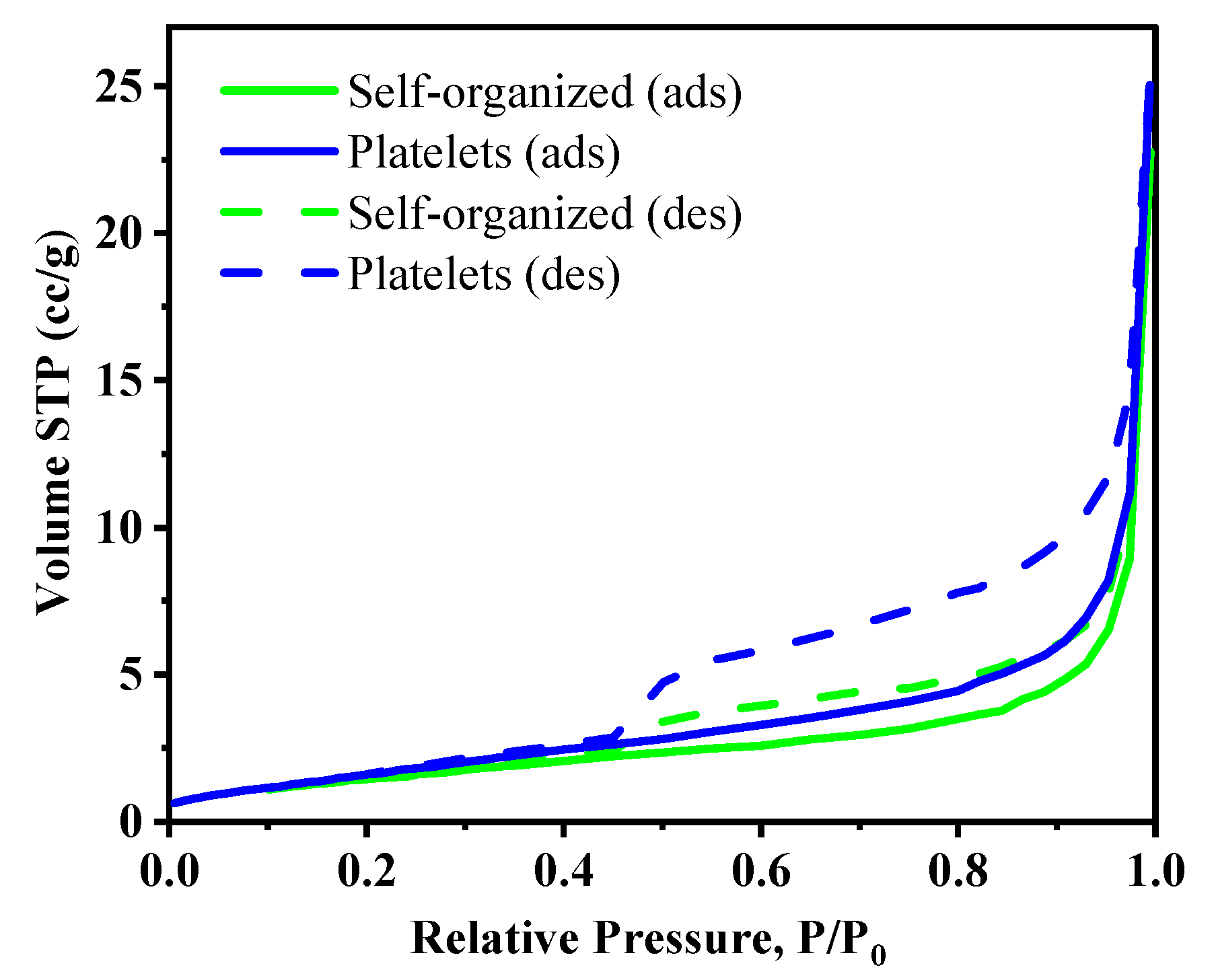
2.4. Textural Properties: Surface Area and Porosity
2.5. Optical Properties—UV-Vis Diffuse Reflectance
2.6. Photocatalytic Activity of Ferberite with Self-Organized and Platelet Morphologies
2.6.1. Adsorption Studies
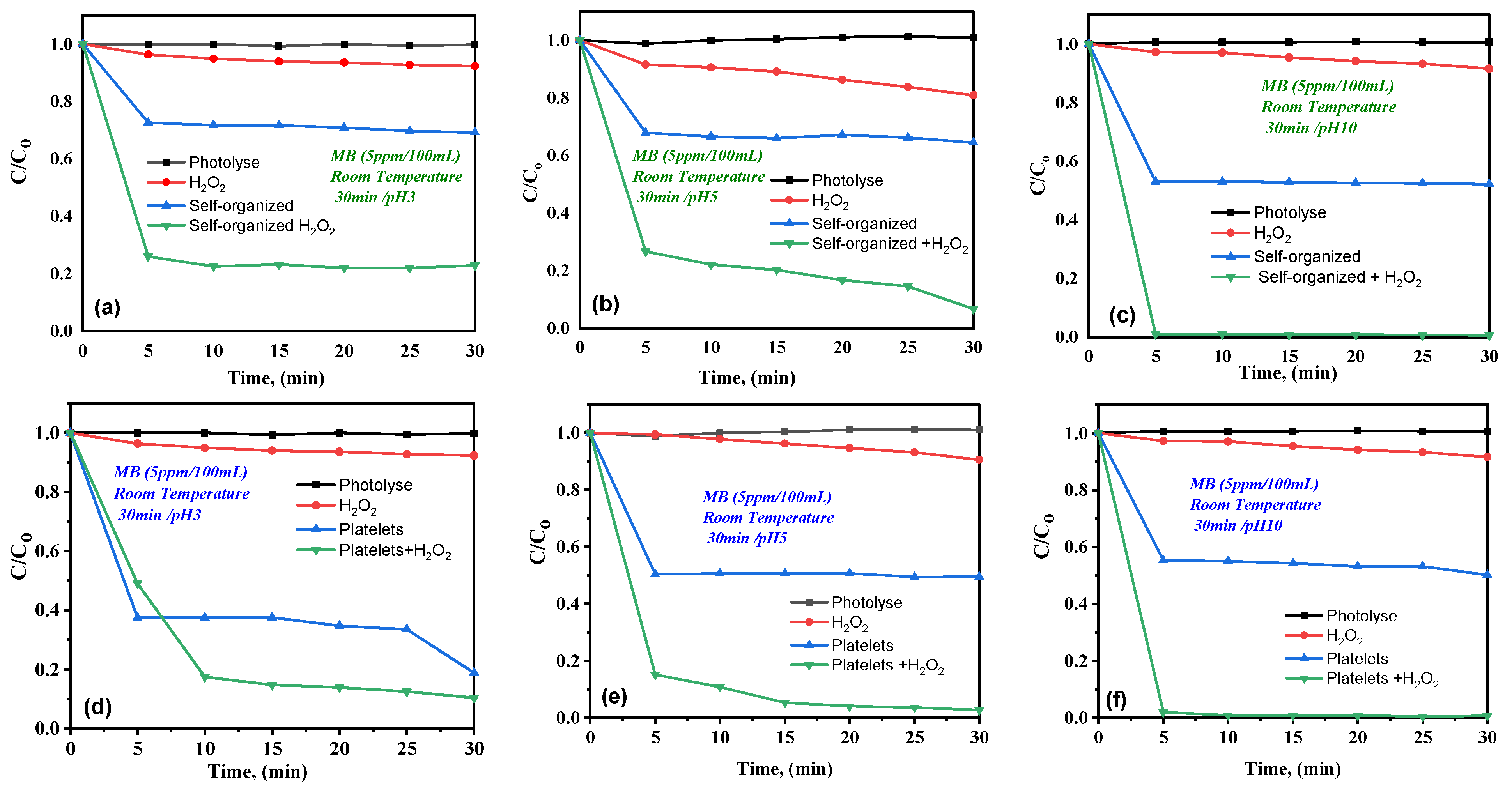
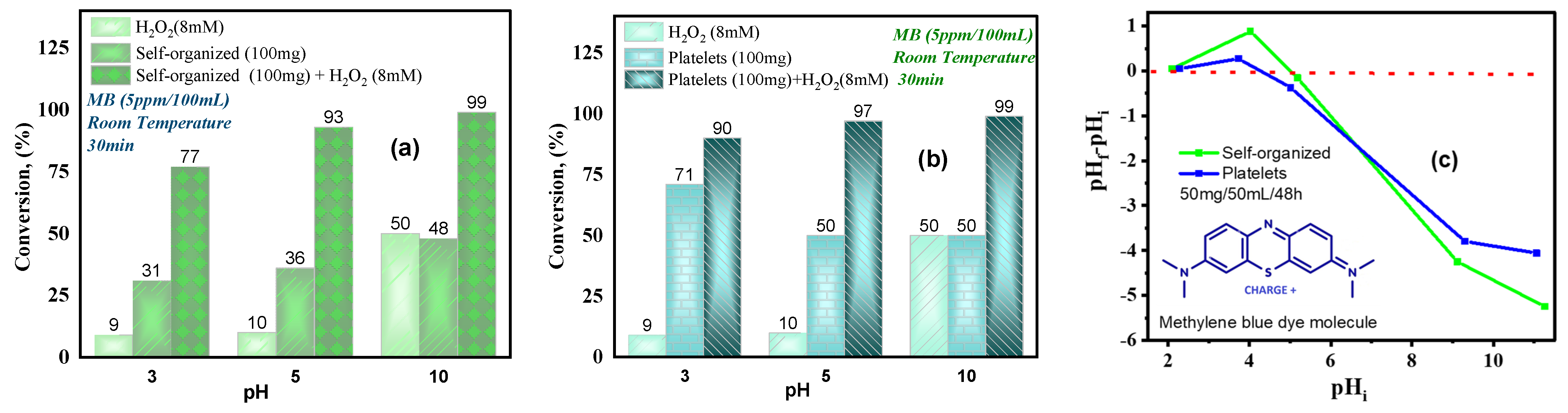
2.6.2. Influence of pH and H2O2 on MB Degradation
- Blank tests under visible light (photolysis, H2O2 alone, FeWO4 alone)
- b.
- Effect of H2O2 addition (photo-Fenton conditions)
- c.
- pH influence and surface charge
- d.
- Morphology effect
- e.
- Influence of H2O2 concentration
- f.
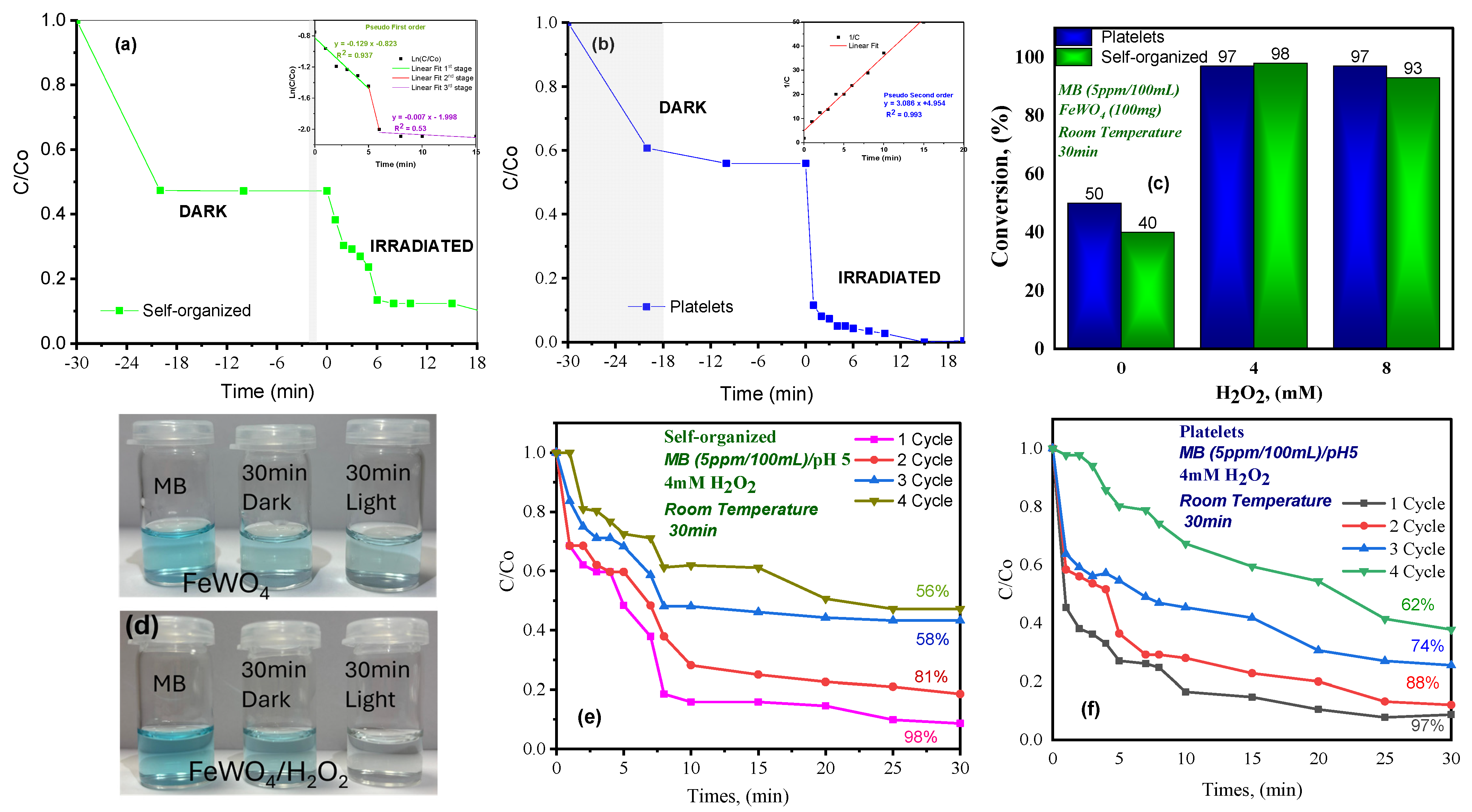
- Kinetic analysis
- g.
- Comparison with literature
- h.
- Recyclability and stability
- i.
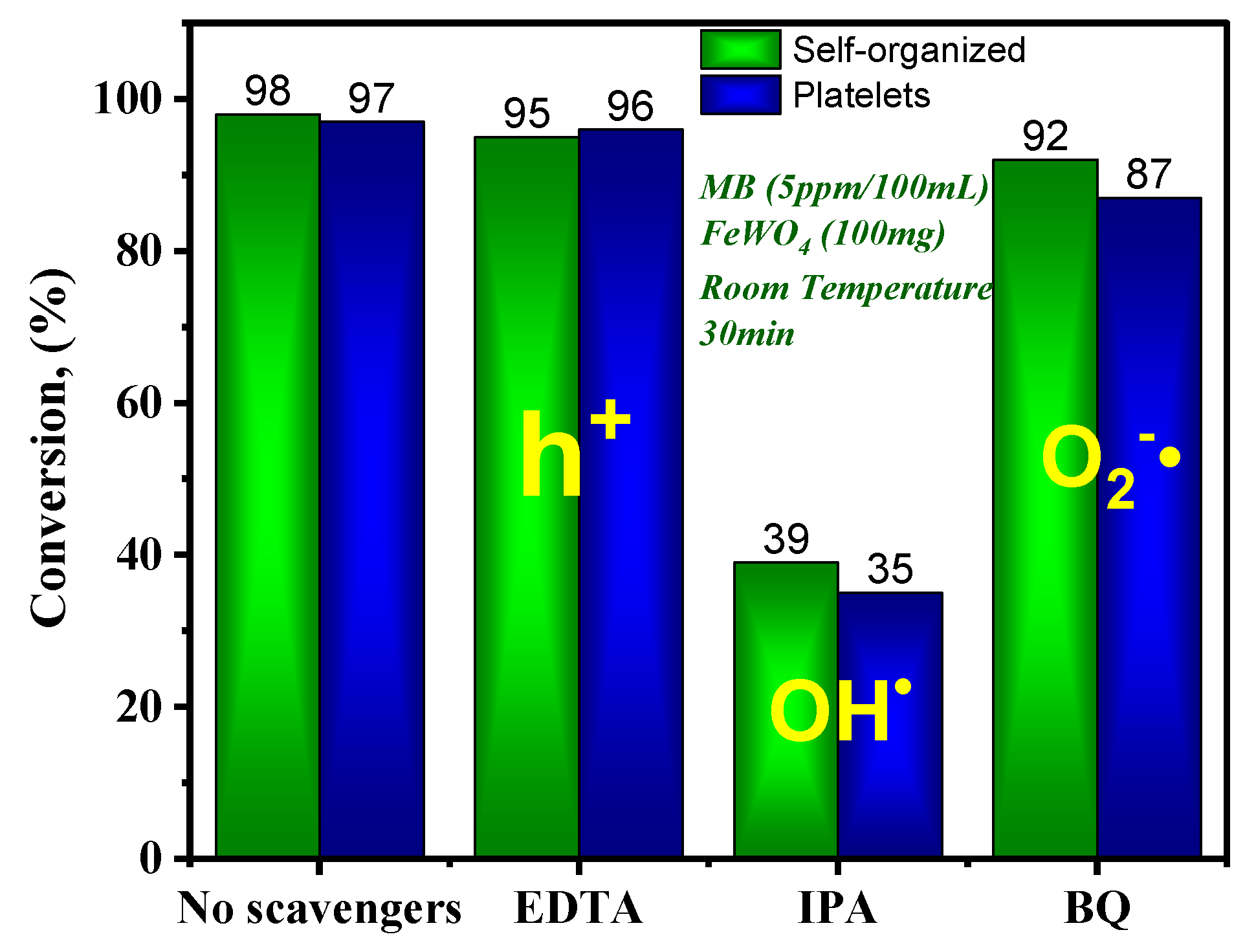
- Radical scavenging experiments
2.7. Photocatalysis Mechanism Based on Photoelectrochemical Investigations
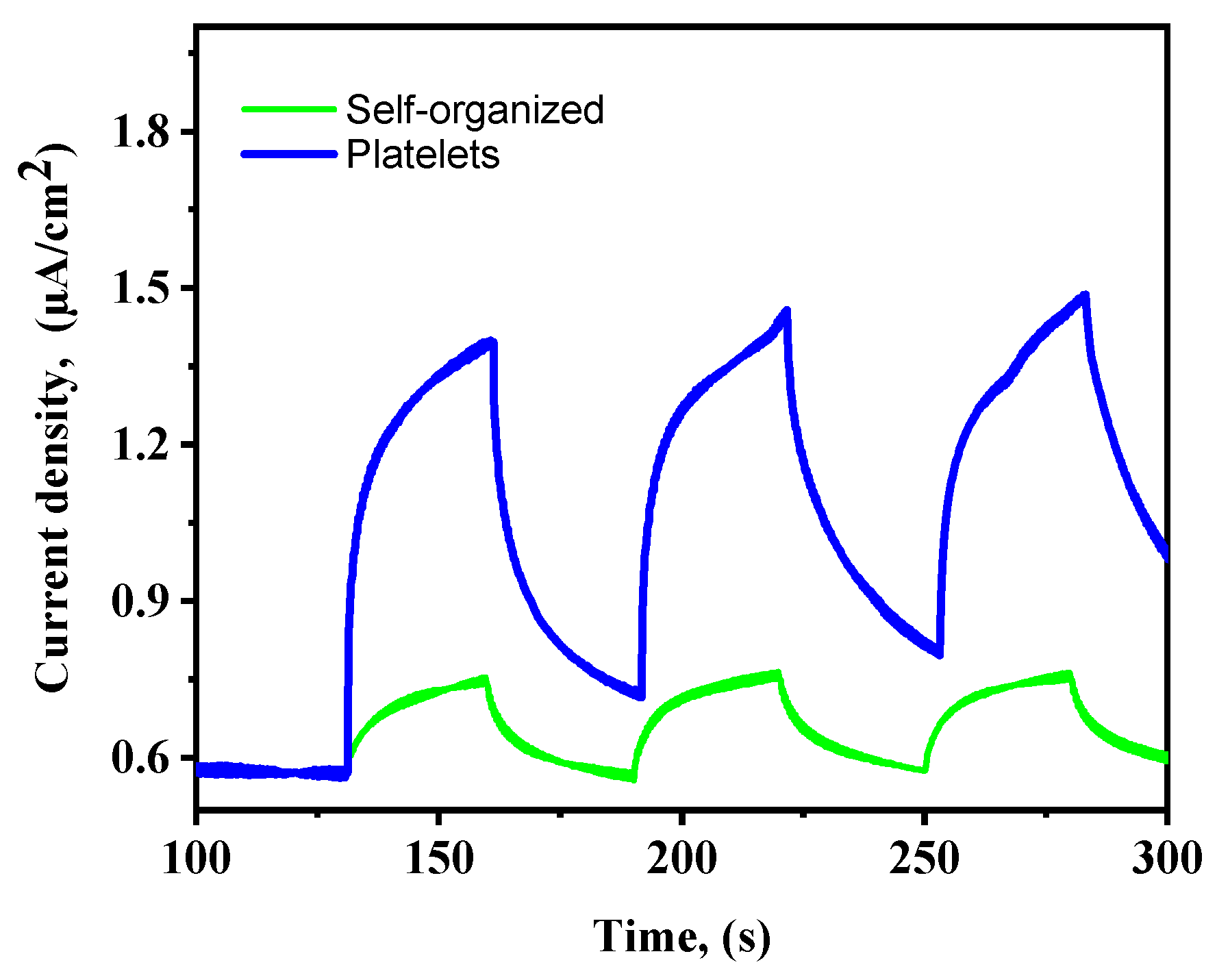
2.7.1. Transient Photocurrent Response
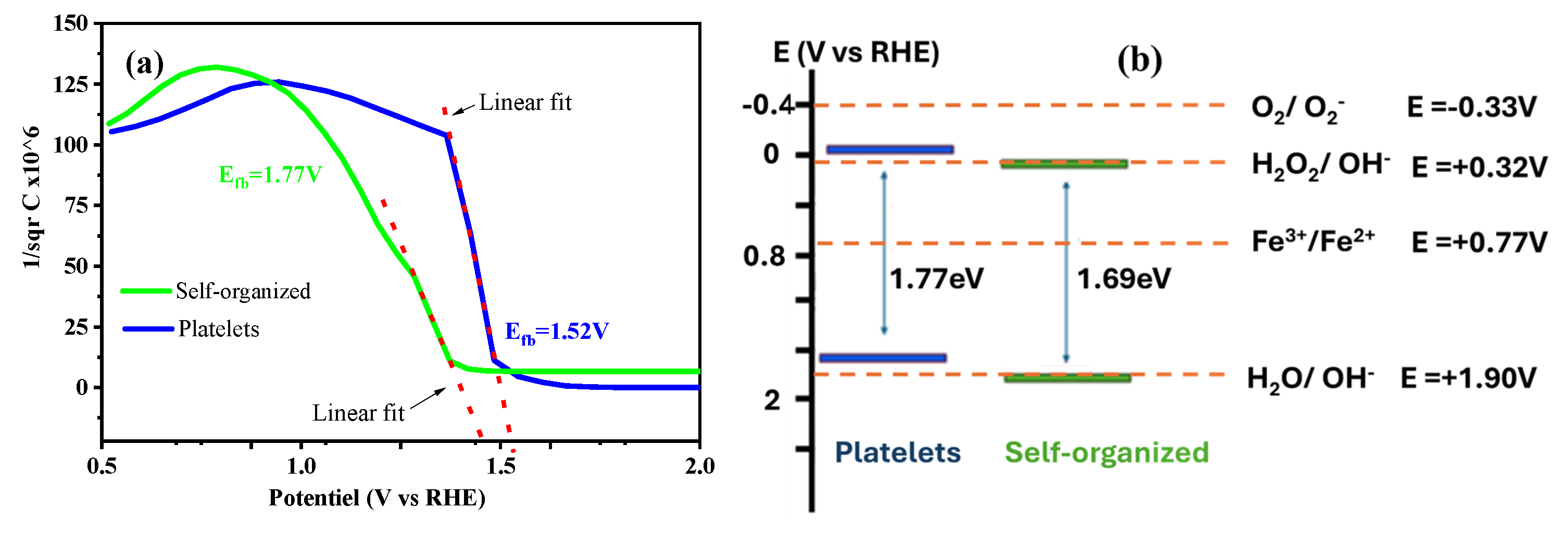
2.7.2. Mott–Schottky Analysis
2.7.3. Photo-Fenton Mechanism and OH• Generation Pathways in FeWO4/H2O2 System
- Reduction of H2O2 by Photogenerated Electrons
- b.
- Oxidation of OH− by Photogenerated Holes
- c.
- Oxidation of H2O2 by Photogenerated Holes (Equation (13))
- d.
- Classical Fenton Reaction via Fe2+/Fe3+ Cycling
3. Experimental Section
3.1. Ferberite Hydrothermally Synthesis
3.2. Structural, Morphological, Chemical, Textural Properties and Optical Investigation
3.3. Photoelectrochemical (PEC) Measurements
3.4. Photocatalytic Action Evaluation
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- How Is Fast Fashion Degrading Our Water Resources? | Bosaq. Available online: https://bosaq.com/water-and-fashion-industry/ (accessed on 7 July 2025).
- Niinimäki, K.; Peters, G.; Dahlbo, H.; Perry, P.; Rissanen, T.; Gwilt, A. The Environmental Price of Fast Fashion. Nat. Rev. Earth Environ. 2020, 1, 189–200. [Google Scholar] [CrossRef]
- Bailey, K.; Basu, A.; Sharma, S. The Environmental Impacts of Fast Fashion on Water Quality: A Systematic Review. Water 2022, 14, 1073. [Google Scholar] [CrossRef]
- Chavan, R.B. Thirsty Textile and Fashion Industry PART I: Water Distribution on Earth and Virtual Water, Water Footprint Concepts. Latest Trends Text. Fash. Des. 2018, 2, 1–22. [Google Scholar] [CrossRef]
- Ahmed, S.; Rasul, M.G.; Brown, R.; Hashib, M.A. Influence of Parameters on the Heterogeneous Photocatalytic Degradation of Pesticides and Phenolic Contaminants in Wastewater: A Short Review. J. Environ. Manag. 2011, 92, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Saeed, K.; Ali, N.; Khan, I.; Zhang, B.; Sadiq, M. Heterogeneous Photodegradation of Industrial Dyes: An Insight to Different Mechanisms and Rate Affecting Parameters. J. Environ. Chem. Eng. 2020, 8, 104364. [Google Scholar] [CrossRef]
- Chengli, Z.; Ronghua, M.; Qi, W.; Mingrui, Y.; Rui, C.; Xiaonan, Z. Photocatalytic Degradation of Organic Pollutants in Wastewater by Heteropolyacids: A Review. J. Coord. Chem. 2021, 74, 1751–1764. [Google Scholar] [CrossRef]
- Antonopoulou, M. Homogeneous and Heterogeneous Photocatalysis for the Treatment of Pharmaceutical Industry Wastewaters: A Review. Toxics 2022, 10, 539. [Google Scholar] [CrossRef]
- Van Thuan, D.; Ngo, H.L.; Thi, H.P.; Chu, T.T.H. Photodegradation of Hazardous Organic Pollutants Using Titanium Oxides -Based Photocatalytic: A Review. Environ. Res. 2023, 229, 116000. [Google Scholar] [CrossRef]
- Masuleh, M.T.; Hasheminiasari, M.; Ashiri, R. Enhanced Photocatalytic Efficiency of Eco-Friendly Synthesized ZnO for Rapid Full Degradation of Methylene Blue Dye. Mater. Adv. 2025, 6, 2611–2621. [Google Scholar] [CrossRef]
- Zong, M.; Song, D.; Zhang, X.; Huang, X.; Lu, X.; Rosso, K.M. Facet-Dependent Photodegradation of Methylene Blue by Hematite Nanoplates in Visible Light. Environ. Sci. Technol. 2021, 55, 677–688. [Google Scholar] [CrossRef]
- Wang, T.; Nie, C.; Ao, Z.; Wang, S.; An, T. Recent Progress in G-C3N4 Quantum Dots: Synthesis, Properties and Applications in Photocatalytic Degradation of Organic Pollutants. J. Mater. Chem. A 2020, 8, 485–502. [Google Scholar] [CrossRef]
- Zhang, Y.; Yu, H.; Zhai, R.; Zhang, J.; Gao, C.; Qi, K.; Yang, L.; Ma, Q. Recent Progress in Photocatalytic Degradation of Water Pollution by Bismuth Tungstate. Molecules 2023, 28, 8011. [Google Scholar] [CrossRef] [PubMed]
- Alam, U.; Verma, N. Direct Z-Scheme-Based Novel Cobalt Nickel Tungstate/Graphitic Carbon Nitride Composite: Enhanced Photocatalytic Degradation of Organic Pollutants and Oxidation of Benzyl Alcohol. Colloids Surf. Physicochem. Eng. Asp. 2021, 630, 127606. [Google Scholar] [CrossRef]
- Dirany, N.; Arab, M.; Leroux, C.; Villain, S.; Madigou, V.; Gavarri, J.R. Effect of WO3 Nanoparticles Morphology on the Catalytic Properties. Mater. Today Proc. 2016, 3, 230–234. [Google Scholar] [CrossRef]
- El Aouni, A.; El Ouardi, M.; Arab, M.; Saadi, M.; Haspel, H.; Kónya, Z.; Ben Ali, A.; Jada, A.; BaQais, A.; Ait Ahsaine, H. Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation. Materials 2024, 17, 1029. [Google Scholar] [CrossRef]
- Dirany, N.; Hallaoui, A.; Valmalette, J.C.; Arab, M. Effect of morphology and temperature treatment control on the photocatalytic and photoluminescence properties of SrWO4 crystals. Photochem Photobiol Sci. 2020, 19, 235–250. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Xue, Q.; Liu, Y.; Liang, F.; Li, W.; Liu, T.; Zhuang, C.; Zhao, Z.; Li, S. Manipulating interfacial charge redistribution in Mn0.5Cd0.5S/N-rich C3N5 S-scheme heterojunction for high-performance photocatalytic removal of emerging contaminants. J. Mater. Sci. Technol. 2026, 243, 265–274. [Google Scholar] [CrossRef]
- Chen, W.; Yan, R.; Chen, G.; Chen, M.; Huang, G.; Liu, X. Hydrothermal route to synthesize helical CdS@ZnIn2S4 core-shell heterostructures with enhanced photocatalytic hydrogeneration activity. Ceram. Int. 2019, 45, 1803–1811. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, S.; Yan, R.; Song, C.; Jin, Y.; Huang, G.; Huang, J. Enhanced photocatalytic properties of Mn doped CdS catalysts by decomposition of complex precursors. Opt. Mater. 2020, 109, 110324. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, K.; Chan, L.; Yan, R.; Zhang, J.; Zhang, M.; Wang, L.; Chen, W.; Huang, G. Construction of S-scheme MIL-101(Fe)/Bi2MoO6 heterostructures for enhanced catalytic activities towards tetracycline hydrochloride photodegradation and nitrogen photofixation. Sol. Energy. 2023, 264, 112042. [Google Scholar] [CrossRef]
- Qian, J.; Shen, L.; Wang, Y.; Li, L.; Zhang, Y. Photo-Fenton Catalytic and Photocatalytic Performance of FeWO4 Nanorods Prepared at Different pH. Mater. Lett. 2023, 334, 133705. [Google Scholar] [CrossRef]
- Mahendran, N.; Udayakumar, S.; Praveen, K. PH-Controlled Photocatalytic Abatement of RhB by an FeWO4/BiPO4 p–n Heterojunction under Visible Light Irradiation. New J. Chem. 2019, 43, 17241–17250. [Google Scholar] [CrossRef]
- Abdelbasir, S.M.; Elseman, A.M.; Harraz, F.A.; Ahmed, Y.M.Z.; El-Sheikh, S.M.; Rashad, M.M. Superior UV-Light Photocatalysts of Nano-Crystalline (Ni or Co) FeWO4: Structure, Optical Characterization and Synthesis by a Microemulsion Method. New J. Chem. 2021, 45, 3150–3159. [Google Scholar] [CrossRef]
- Narendhran, S.; Shakila, P.B.; Manikandan, M.; Vinoth, V.; Rajiv, P. Spectroscopic Investigation on Photocatalytic Degradation of Methyl Orange Using Fe2O3/WO3/FeWO4 Nanomaterials. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2020, 232, 118164. [Google Scholar] [CrossRef]
- Jansi Rani, B.; Ravi, G.; Yuvakkumar, R.; Praveenkumar, M.; Ravichandran, S.; Muthu Mareeswaran, P.; Hong, S.I. Bi2WO6 and FeWO4 Nanocatalysts for the Electrochemical Water Oxidation Process. ACS Omega 2019, 4, 5241–5253. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, X.; Lu, Y.; Zhang, X.; Kuang, S.; Hou, W. Monodisperse Spindle-like FeWO4 Nanoparticles: Controlled Hydrothermal Synthesis and Enhanced Optical Properties. J. Solid State Chem. 2012, 196, 550–556. [Google Scholar] [CrossRef]
- Almeida, M.A.P.; Cavalcante, L.S.; Morilla-Santos, C.; Filho, P.N.L.; Beltrán, A.; Andrés, J.; Gracia, L.; Longo, E. Electronic Structure and Magnetic Properties of FeWO4 Nanocrystals Synthesized by the Microwave-Hydrothermal Method. Mater. Charact. 2012, 73, 124–129. [Google Scholar] [CrossRef]
- Liu, C.; Lü, H.; Yu, C.; Wu, X.; Wang, P. Hydrothermal-Assisted Microemulsion Synthesis of FeWO4 Nanorods and Their Superior Visible-Light- Driven Photocatalytic Activity. Mater. Lett. 2019, 257, 126707. [Google Scholar] [CrossRef]
- Irfan, M.; Tahir, N.; Zahid, M.; Noreen, S.; Yaseen, M.; Shahbaz, M.; Mustafa, G.; Shakoor, R.A.; Shahid, I. The Fabrication of Halogen-Doped FeWO4 Heterostructure Anchored over Graphene Oxide Nanosheets for the Sunlight-Driven Photocatalytic Degradation of Methylene Blue Dye. Molecules 2023, 28, 7022. [Google Scholar] [CrossRef]
- Dadigala, R.; Bandi, R.; Gangapuram, B.R.; Guttena, V. Construction of in Situ Self-Assembled FeWO4/g-C3N4 Nanosheet Heterostructured Z-Scheme Photocatalysts for Enhanced Photocatalytic Degradation of Rhodamine B and Tetracycline. Nanoscale Adv. 2019, 1, 322–333. [Google Scholar] [CrossRef]
- Shi, X.; Wang, L.; Zuh, A.A.; Jia, Y.; Ding, F.; Cheng, H.; Wang, Q. Photo-Fenton Reaction for the Degradation of Tetracycline Hydrochloride Using a FeWO4/BiOCl Nanocomposite. J. Alloys Compd. 2022, 903, 163889. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y.; Li, S.; Wang, X.; Huang, F.; Xie, A.; Shen, Y. Controlled Synthesis, Growth Mechanism and Optical Properties of FeWO4 Hierarchical Microstructures. CrystEngComm 2011, 13, 5744–5750. [Google Scholar] [CrossRef]
- He, D.; Liu, X.; Li, X.; Lyu, P.; Chen, J.; Rao, Z. Regulating the Polysulfide Redox Kinetics for High-Performance Lithium-Sulfur Batteries through Highly Sulfiphilic FeWO4 Nanorods. Chem. Eng. J. 2021, 419, 129509. [Google Scholar] [CrossRef]
- Yu, F.; Cao, L.; Huang, J.; Wu, J. Effects of pH on the Microstructures and Optical Property of FeWO4 Nanocrystallites Prepared via Hydrothermal Method. Ceram. Int. 2013, 39, 4133–4138. [Google Scholar] [CrossRef]
- Parasuraman, B.; Kandasamy, B.; Murugan, I.; Alsalhi, M.S.; Asemi, N.; Thangavelu, P.; Perumal, S. Designing the Heterostructured FeWO4/FeS2 Nanocomposites for an Enhanced Photocatalytic Organic Dye Degradation. Chemosphere 2023, 334, 138979. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Liu, Y.; Chen, P. Room-Temperature Multiferroic Properties of Single-Crystalline FeWO4 Nanowires. Scr. Mater. 2014, 89, 17–20. [Google Scholar] [CrossRef]
- 38. Variation of Unit Cell Parameters in Wolfranite Series. Available online: https://www.jstage.jst.go.jp/article/minerj1953/2/6/2_6_375/_article (accessed on 8 July 2025).
- Kovács, T.N.; Pokol, G.; Gáber, F.; Nagy, D.; Igricz, T.; Lukács, I.E.; Fogarassy, Z.; Balázsi, K.; Szilágyi, I.M. Preparation of Iron Tungstate (FeWO4) Nanosheets by Hydrothermal Method. Mater. Res. Bull. 2017, 95, 563–569. [Google Scholar] [CrossRef]
- Li, L.; Zhao, J.; Wang, Y.; Li, Y.; Ma, D.; Zhao, Y.; Hou, S.; Hao, X. Oxalic Acid Mediated Synthesis of WO3·H2O Nanoplates and Self-Assembled Nanoflowers under Mild Conditions. J. Solid State Chem. 2011, 184, 1661–1665. [Google Scholar] [CrossRef]
- Patil, V.B.; Adhyapak, P.V.; Suryavanshi, S.S.; Mulla, I.S. Oxalic Acid Induced Hydrothermal Synthesis of Single Crystalline Tungsten Oxide Nanorods. J. Alloys Compd. 2014, 590, 283–288. [Google Scholar] [CrossRef]
- Lassner, E.; Schubert, W.-D.; Lüderitz, E.; Wolf, H.U. Tungsten, Tungsten Alloys, and Tungsten Compounds. In Ullmann’s Encyclopedia of Industrial Chemistry; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2000; ISBN 978-3-527-30673-2. [Google Scholar]
- Fang, Z.; Jiao, S.; Wang, B.; Yin, W.; Liu, S.; Gao, R.; Liu, Z.; Pang, G.; Feng, S. Synthesis of Reduced Cubic Phase WO3−x Nanosheet by Direct Reduction of H2WO4·H2O. Mater. Today Energy 2017, 6, 146–153. [Google Scholar] [CrossRef]
- Sun, M.; Xu, N.; Cao, Y.W.; Yao, J.N.; Wang, E.G. Nanocrystalline Tungsten Oxide Thin Film: Preparation, Microstructure, and Photochromic Behavior. J. Mater. Res. 2000, 15, 927–933. [Google Scholar] [CrossRef]
- Nayak, A.K.; Lee, S.; Choi, Y.I.; Yoon, H.J.; Sohn, Y.; Pradhan, D. Crystal Phase and Size-Controlled Synthesis of Tungsten Trioxide Hydrate Nanoplates at Room Temperature: Enhanced Cr(VI) Photoreduction and Methylene Blue Adsorption Properties. ACS Sustain. Chem. Eng. 2017, 5, 2741–2750. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmett, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The Determination of Pore Volume and Area Distributions in Porous Substances. I. Computations from Nitrogen Isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Sun, D.; Iqbal, N.; Liao, W.; Lu, Y.; He, X.; Wang, K.; Ma, B.; Zhu, Y.; Sun, K.; Sun, Z.; et al. Efficient Degradation of MB Dye by 1D FeWO4 Nanomaterials through the Synergistic Effect of Piezo-Fenton Catalysis. Ceram. Int. 2022, 48, 25465–25473. [Google Scholar] [CrossRef]
- Ali, M.A.; Maafa, I.M.; Qudsieh, I.Y. Photodegradation of Methylene Blue Using a UV/H2O2 Irradiation System. Water 2024, 16, 453. [Google Scholar] [CrossRef]
- Diaz-Anichtchenko, D.; Aviles-Coronado, J.E.; López-Moreno, S.; Turnbull, R.; Manjón, F.J.; Popescu, C.; Errandonea, D. Electronic, Vibrational, and Structural Properties of the Natural Mineral Ferberite (FeWO4): A High-Pressure Study. Inorg. Chem. 2024, 63, 6898–6908. [Google Scholar] [CrossRef]
- Bera, S.; Rawal, S.B.; Kim, H.J.; Lee, W.I. Novel Coupled Structures of FeWO4/TiO2 and FeWO4/TiO2/CdS Designed for Highly Efficient Visible-Light Photocatalysis. ACS Appl. Mater. Interfaces 2014, 6, 9654–9663. [Google Scholar] [CrossRef]
- Wang, H.; Wang, C.; Cui, X.; Qin, L.; Ding, R.; Wang, L.; Liu, Z.; Zheng, Z.; Lv, B. Design and Facile One-Step Synthesis of FeWO4/Fe2O3 Di-Modified WO3 with Super High Photocatalytic Activity toward Degradation of Quasi-Phenothiazine Dyes. Appl. Catal. B Environ. 2018, 221, 169–178. [Google Scholar] [CrossRef]
- Ojha, D.P.; Karki, H.P.; Song, J.H.; Kim, H.J. Decoration of G-C3N4 with Hydrothermally Synthesized FeWO4 Nanorods as the High-Performance Supercapacitors. Chem. Phys. Lett. 2018, 712, 83–88. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Zhang, X.; Dong, X.; Ma, C.; Zhang, X.; Ma, H.; Xue, M. Construction of G-C3N4 and FeWO4 Z-Scheme Photocatalyst: Effect of Contact Ways on the Photocatalytic Performance. RSC Adv. 2018, 8, 18419–18426. [Google Scholar] [CrossRef]
- Lau, A.; Goh, C.Y.; Guo, Y.; Alsultan, A.G.; Hin, T.-Y.Y.; Nurhadi, M.; Lai, S.Y. Visible-Light Degradation of Methylene Blue Using Energy-Efficient Carbon-Doped TiO2: Kinetic Study and Mechanism. Bull. Chem. React. Eng. Catal. 2025, 20, 177–192. [Google Scholar] [CrossRef]
- Mohammed, W.; Matalkeh, M.; Soubaihi, R.M.A.; Elzatahry, A.; Saoud, K.M. Visible Light Photocatalytic Degradation of Methylene Blue Dye and Pharmaceutical Wastes over Ternary NiO/Ag/TiO2 Heterojunction. ACS Omega 2023, 8, 40063. [Google Scholar] [CrossRef]
- Albiss, B.; Abu-Dalo, M. Photocatalytic Degradation of Methylene Blue Using Zinc Oxide Nanorods Grown on Activated Carbon Fibers. Sustainability 2021, 13, 4729. [Google Scholar] [CrossRef]
- Mahlaule-Glory, L.M.; Mapetla, S.; Makofane, A.; Mathipa, M.M.; Hintsho-Mbita, N.C. Biosynthesis of Iron Oxide Nanoparticles for the Degradation of Methylene Blue Dye, Sulfisoxazole Antibiotic and Removal of Bacteria from Real Water. Heliyon 2022, 8, e10536. [Google Scholar] [CrossRef] [PubMed]
- Lin, R.; Chen, H.; Cui, T.; Zhang, Z.; Zhou, Q.; Nan, L.; Cheong, W.-C.; Schröck, L.; Ramm, V.; Ding, Q.; et al. Optimization of P-Type Cu2O Nanocube Photocatalysts Based on Electronic Effects. ACS Catal. 2023, 13, 11352–11361. [Google Scholar] [CrossRef]
- Ferrari, M.; Lutterotti, L. Method for the Simultaneous Determination of Anisotropic Residual Stresses and Texture by X-ray Diffraction. J. Appl. Phys. 1994, 76, 7246–7255. [Google Scholar] [CrossRef]
- Kalaycıoğlu, Z.; Uysal, B.Ö.; Pekcan, Ö.; Erim, F.B. Efficient Photocatalytic Degradation of Methylene Blue Dye from Aqueous Solution with Cerium Oxide Nanoparticles and Graphene Oxide-Doped Polyacrylamide. ACS Omega 2023, 8, 13004–13015. [Google Scholar] [CrossRef]
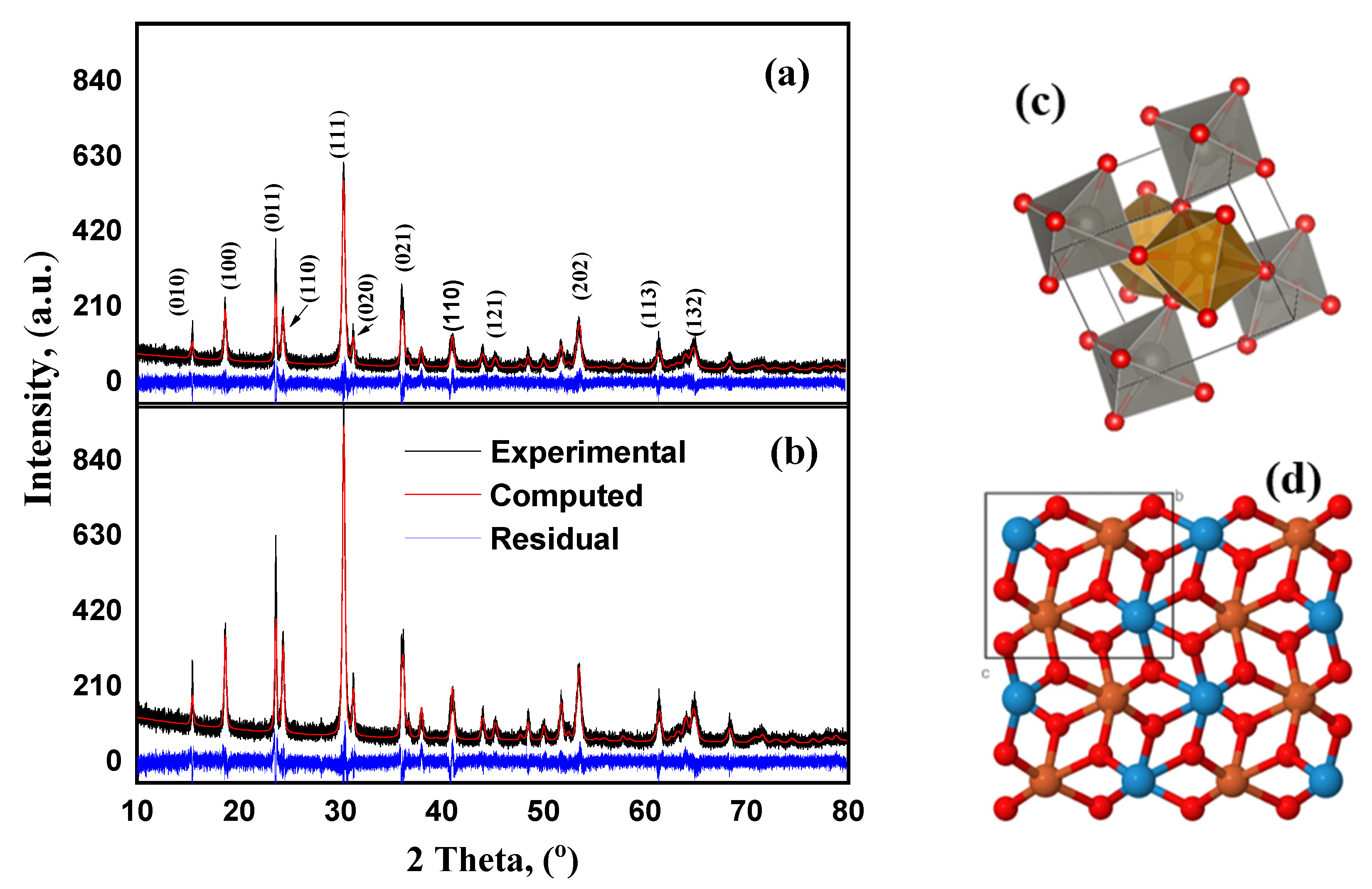

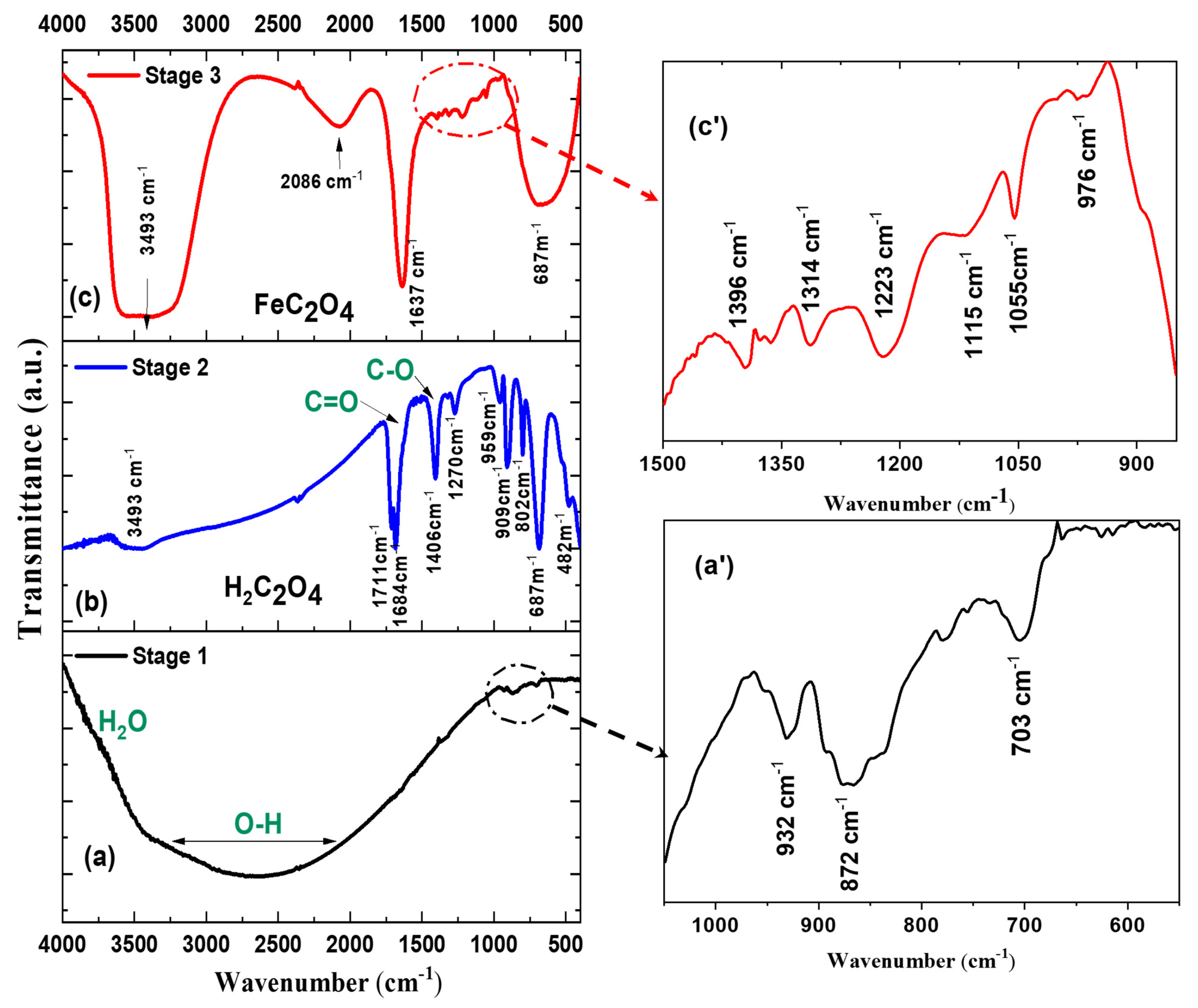
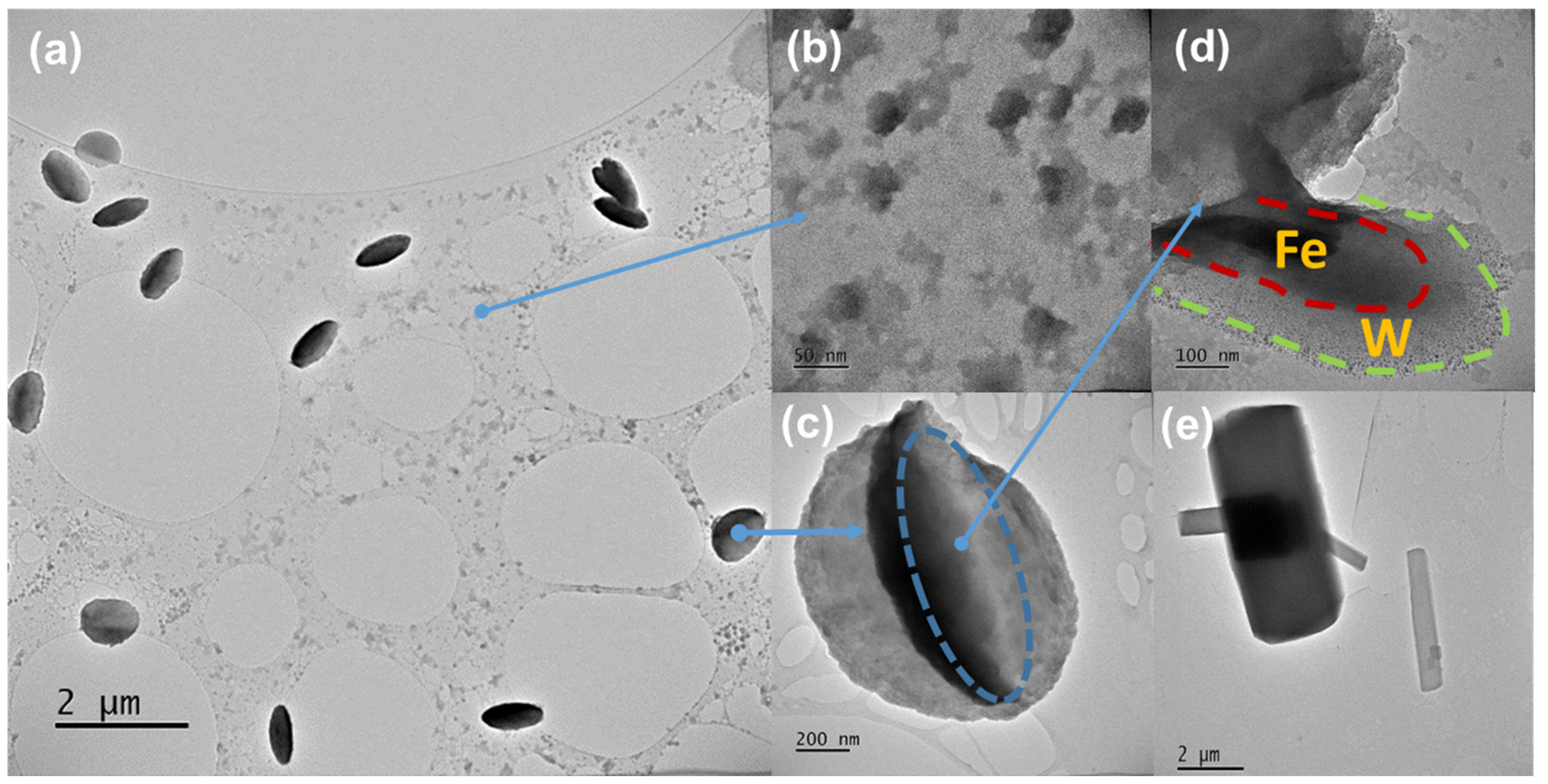
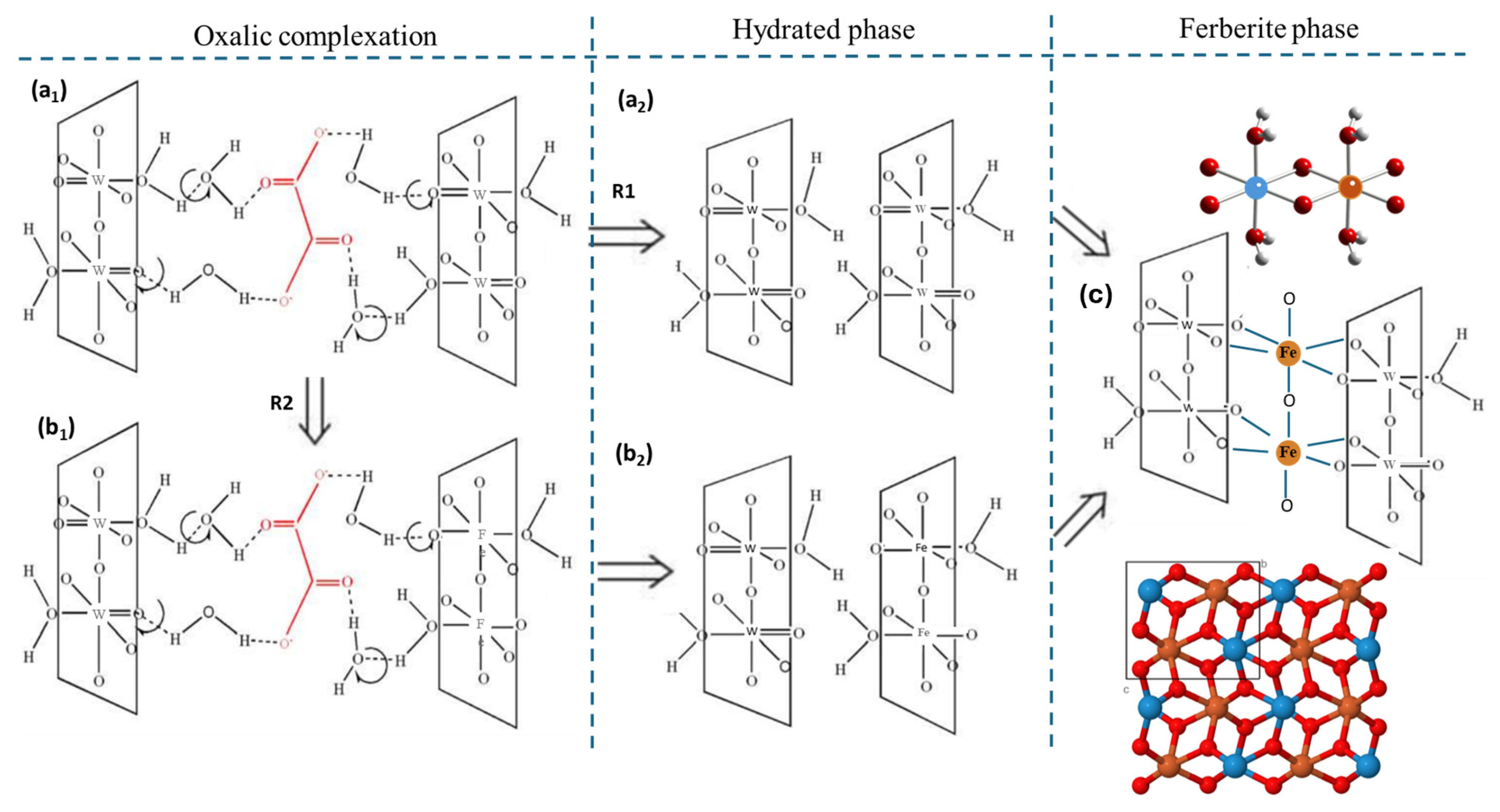

| Compound FeWO4 | Self-Organized | Platelets |
|---|---|---|
| Space group | P2/c:1 | |
| Cell parameters (Å) | a = 4.733 | a = 4.731 |
| b = 5.710 | b = 5.708 | |
| c = 4.977 | c = 4.976 | |
| Angle (degrees) | β = 90.334 | β = 90.292 |
| Density (g/cm3) | 7.49 | 7.50 |
| Crystal size (nm) | 67.78 | 87.28 |
| Reliability factors | ||
| Rwp (%) | 14.9 | 12.8 |
| Rexp (%) | 12.9 | 10.5 |
| Rwp/Rexp(S) | 1.16 | 1.22 |
| Synthesis Method | Morphology/ Composite | Volume (mL) | Mass Masse (mg) | Irradiation | [H2O2] (mM) | pH | Time (min) | MB Removal (%) | Reference |
|---|---|---|---|---|---|---|---|---|---|
| Hydrothermal | Platelets/ FeWO4 | 100 | 100 | Simulated sunlight | 4 | 5 | 30 | 94–100 | This work |
| Hydrothermal | Self-organized /FeWO4 | 100 | 100 | Simulated sunlight | 4 | 5 | 30 | 92–99 | This work |
| Hydrothermal | Nanorods/FeWO4 | 300 | 300 | Visible light | 66 | 5 | 60 | ~90 | [22] |
| Microemulsion/ Hydrothermal | Nanorods/FeWO4 | 100 | 25 | Visible light | – | 5 | 120 | 75 | [29] |
| Microemulsion | Ni–FeWO4 | - | 100 | UV light | – | 3 | 60 | 98 | [24] |
| Co-precipitation-assisted hydrothermal | Nanosheets/FeWO4–GO (I-doped) | 100 | 15–40 | Sunlight | – | 7 | 25 | 95 | [30] |
| Hydrothermal | Nanofibers/ FeWO4 | 50 | 100 | Visible light + ultrasound | 2 | 7 | 14 | 99.5 | [49] |
| Hydrothermal | NI */(30wt%-TiO2-250) | 500 | 100 | UV light | 5 | 10 | 120 | 89.53 | [56] |
| Coprecipitation | Spherical/ (NiO/Ag/TiO2) | 80 | 2/4/8 | UV light | – | NI | 60 | 93.15 | [57] |
| Precipitation/ Hydrothemal | Nanorods/ZnO-NR/ACF | 100 | 50 | UV light | – | 6.7 | 120 | 99 | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gomes dos Santos, A.; Elaadssi, Y.; Chevallier, V.; Leroux, C.; Lopes-Moriyama, A.L.; Arab, M. Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight. Molecules 2025, 30, 4026. https://doi.org/10.3390/molecules30194026
Gomes dos Santos A, Elaadssi Y, Chevallier V, Leroux C, Lopes-Moriyama AL, Arab M. Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight. Molecules. 2025; 30(19):4026. https://doi.org/10.3390/molecules30194026
Chicago/Turabian StyleGomes dos Santos, Andarair, Yassine Elaadssi, Virginie Chevallier, Christine Leroux, Andre Luis Lopes-Moriyama, and Madjid Arab. 2025. "Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight" Molecules 30, no. 19: 4026. https://doi.org/10.3390/molecules30194026
APA StyleGomes dos Santos, A., Elaadssi, Y., Chevallier, V., Leroux, C., Lopes-Moriyama, A. L., & Arab, M. (2025). Insights of Nanostructured Ferberite as Photocatalyst, Growth Mechanism and Photodegradation Under H2O2-Assisted Sunlight. Molecules, 30(19), 4026. https://doi.org/10.3390/molecules30194026








