Recent Developments in Azomethine Ylide-Initiated Double Cycloadditions
Abstract
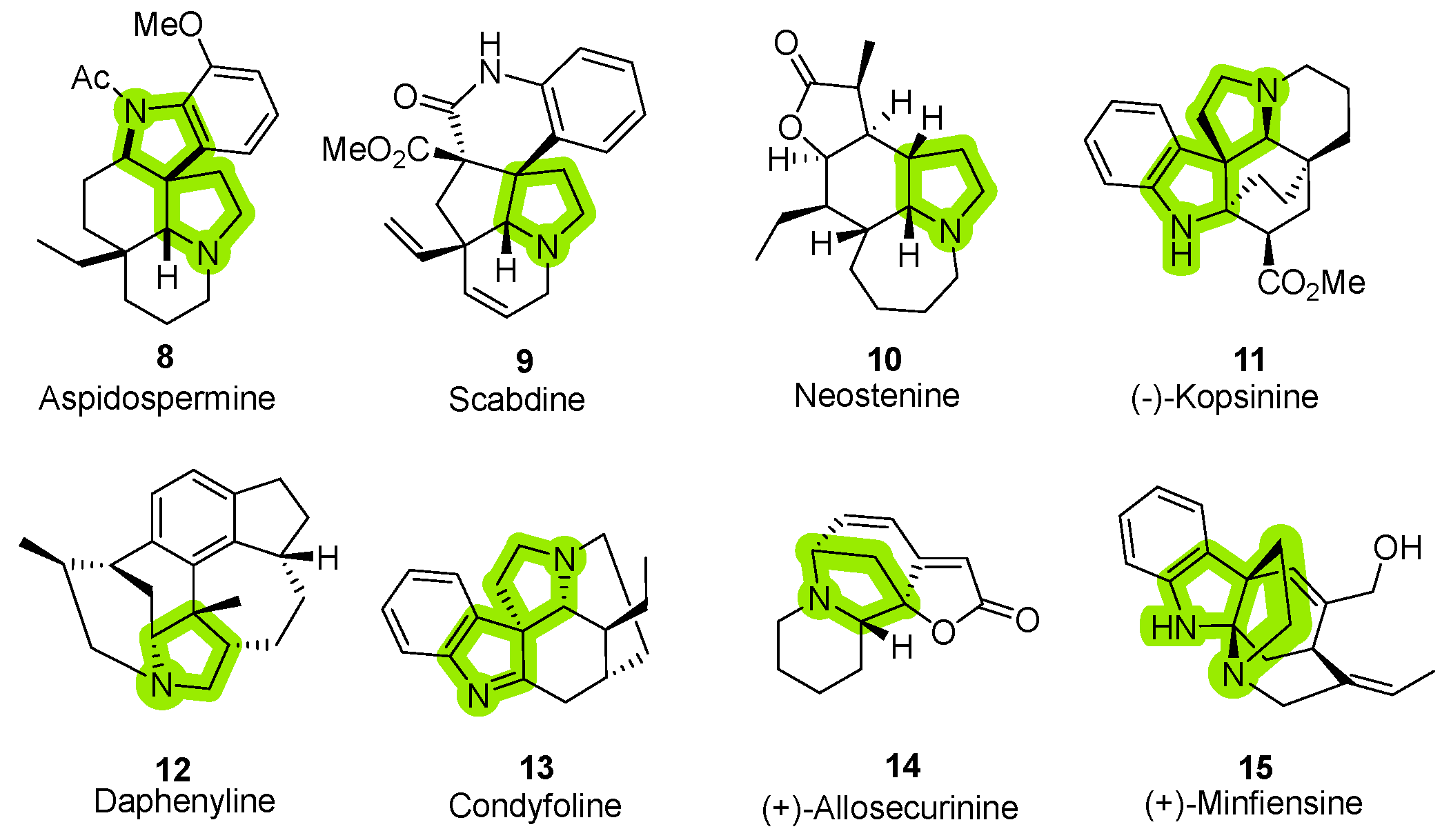
1. Introduction
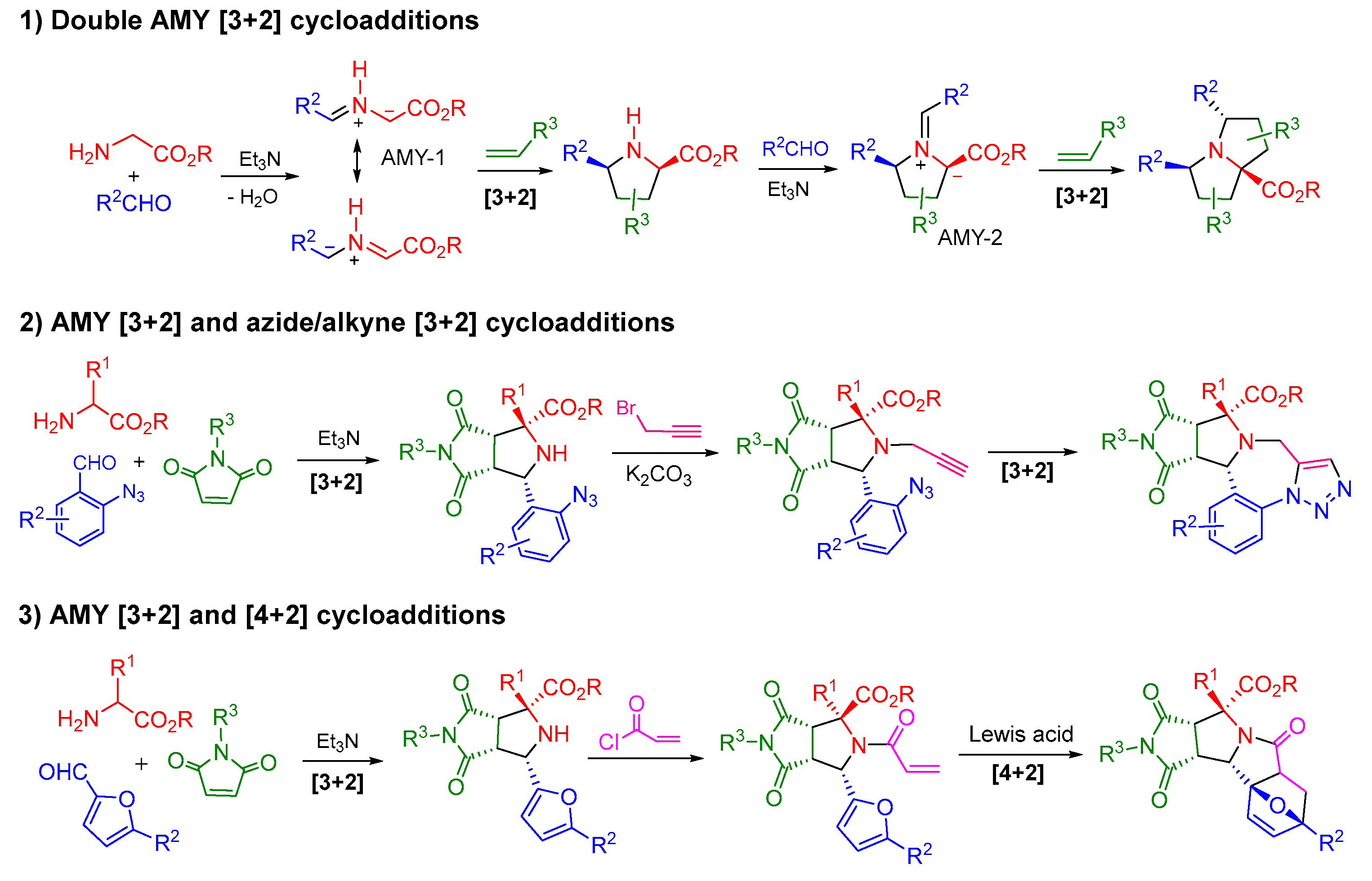
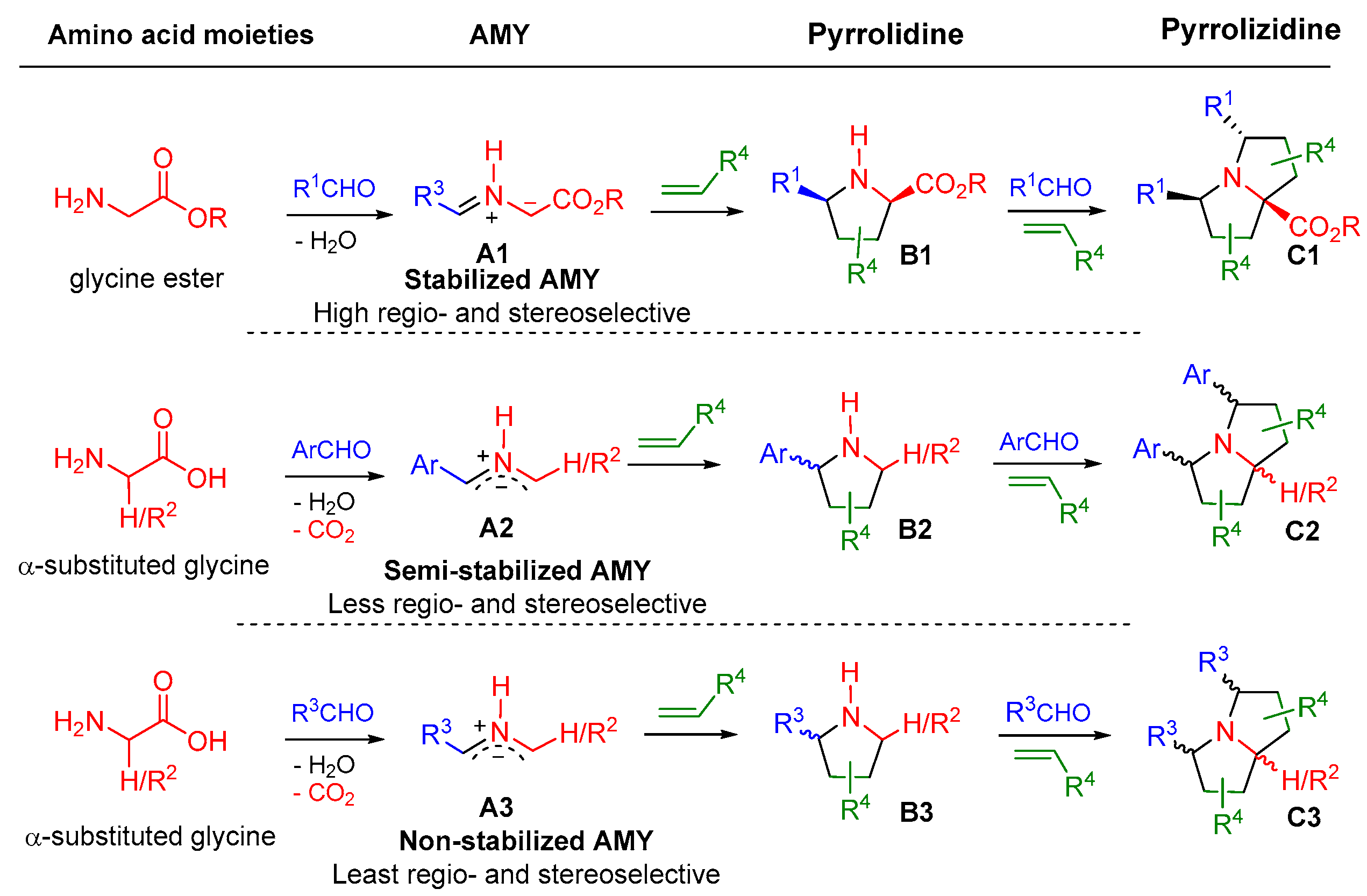
2. Double 1,3-Dipolar Cycloadditions
2.1. Double [3+2] Cycloadditions with Stabilized AMYs
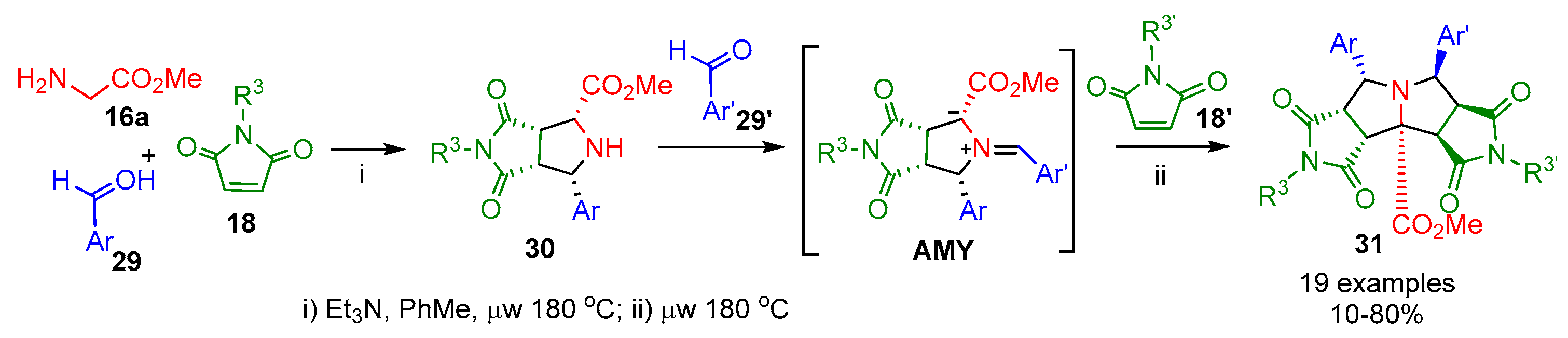
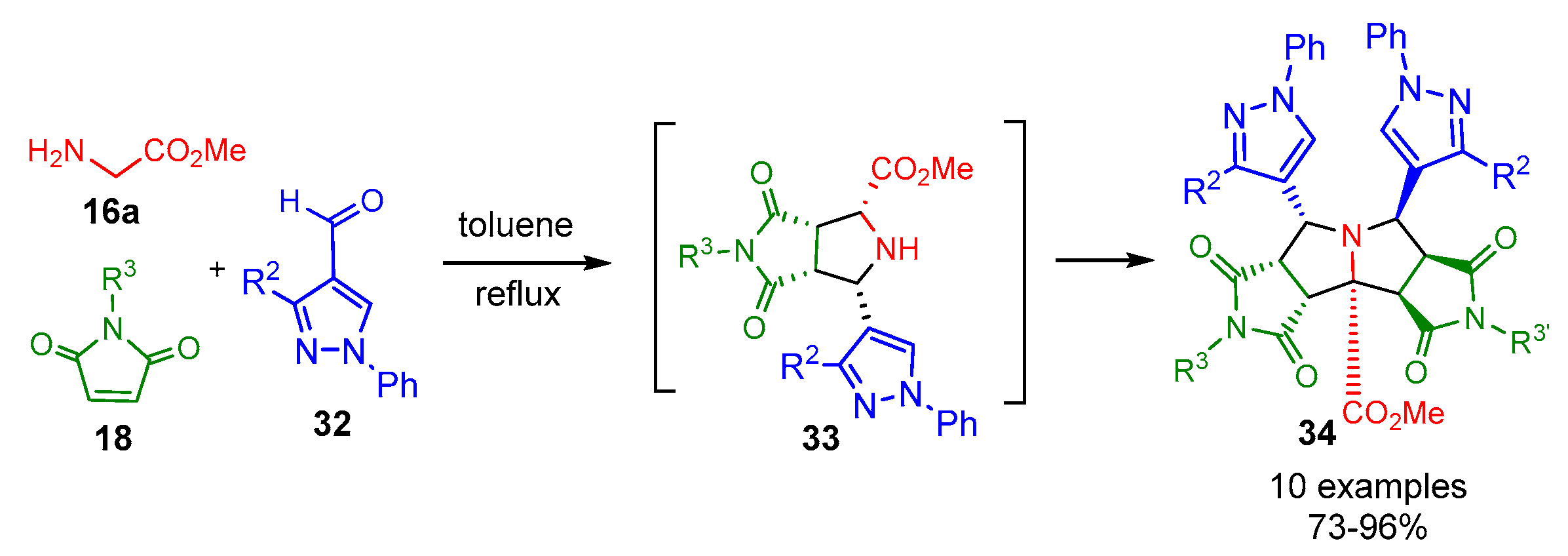
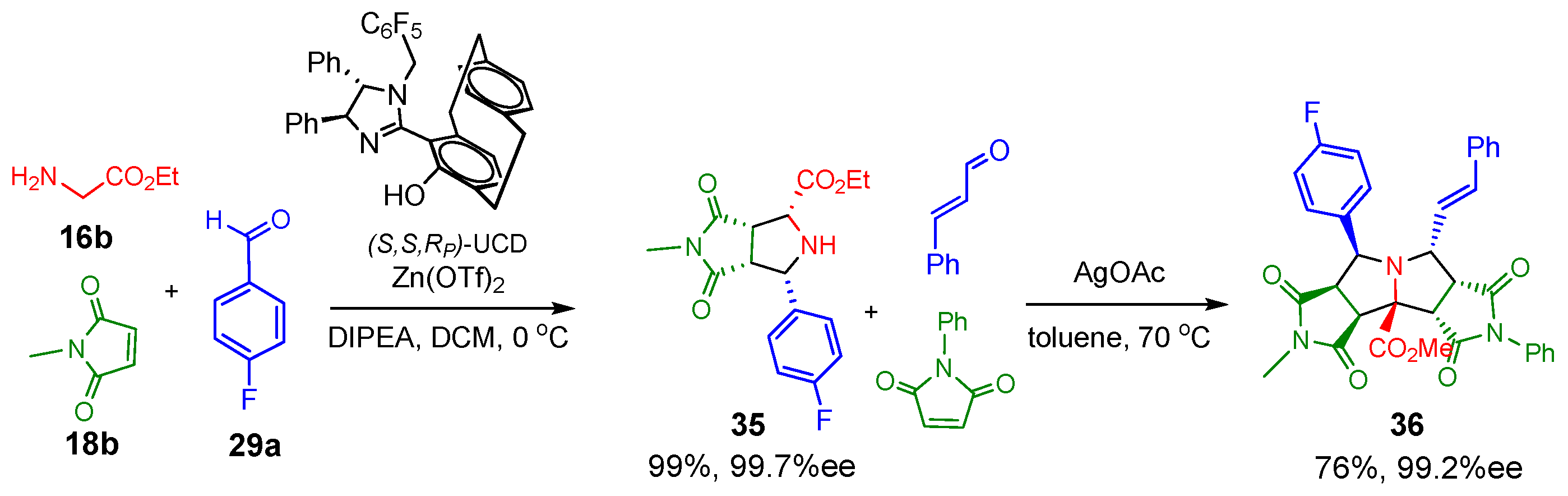
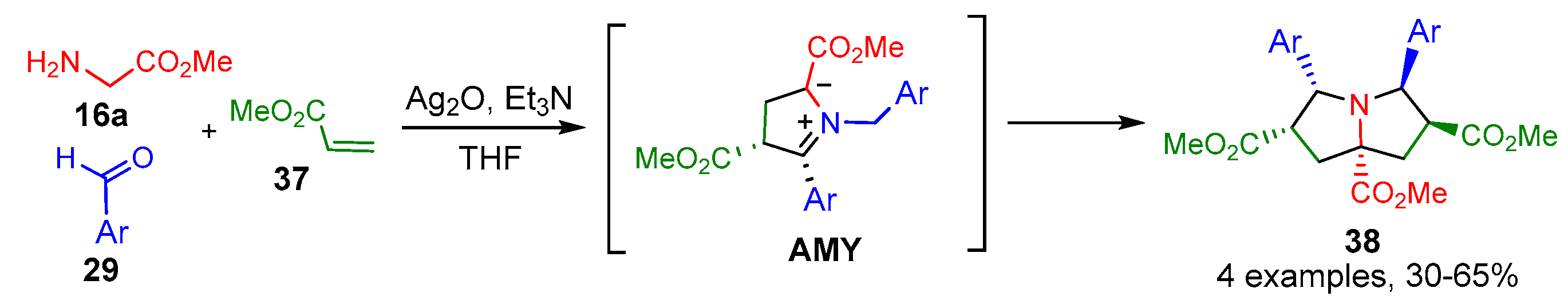
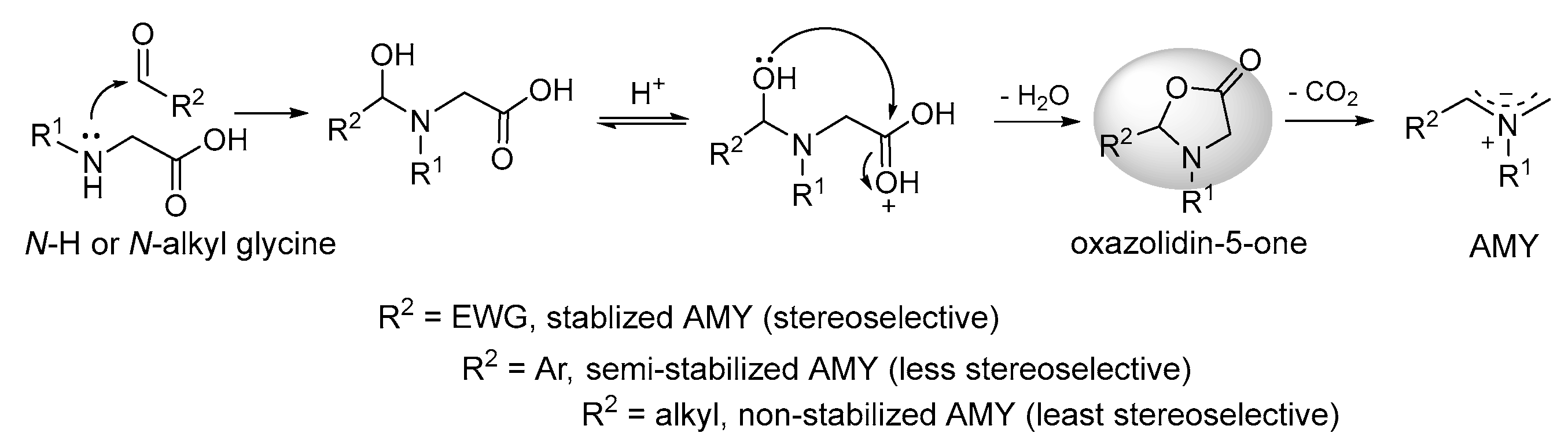
2.1.1. Intermolecular Double [3+2] Cycloadditions
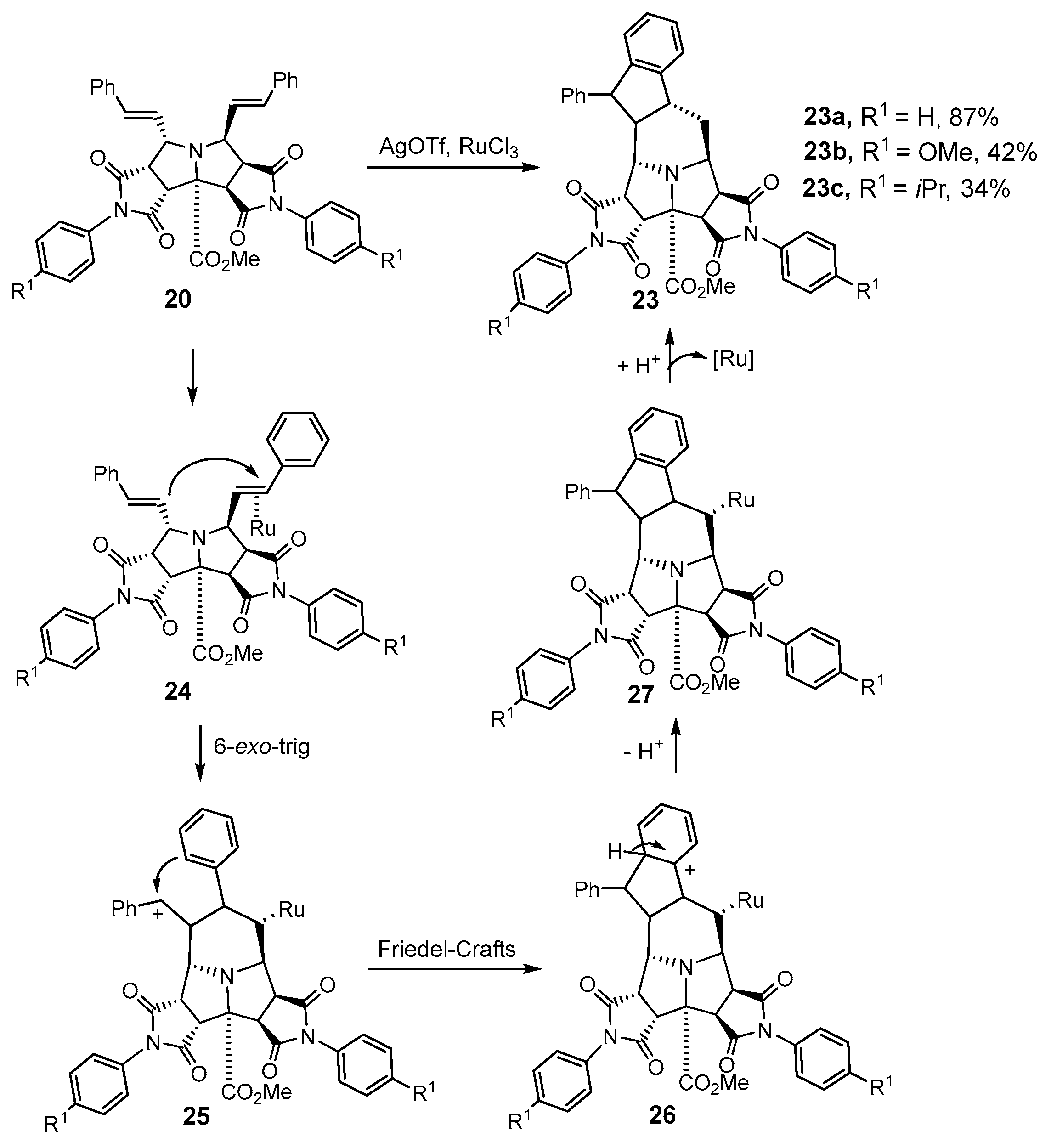
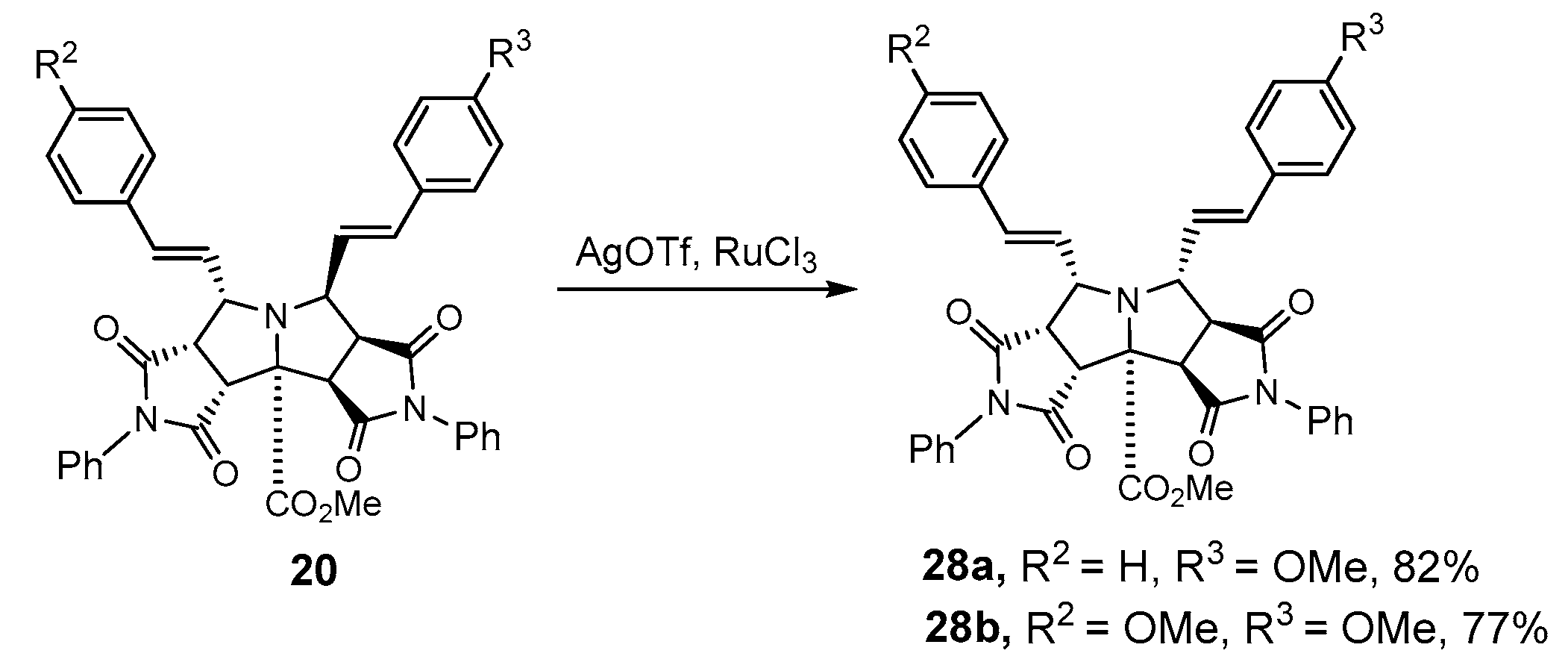
2.1.2. Intramolecular Double [3+2] Cycloadditions
2.1.3. Intermolecular and Intramolecular [3+2] Cycloadditions
2.2. Decarboxylative Double [3+2] Cycloadditions
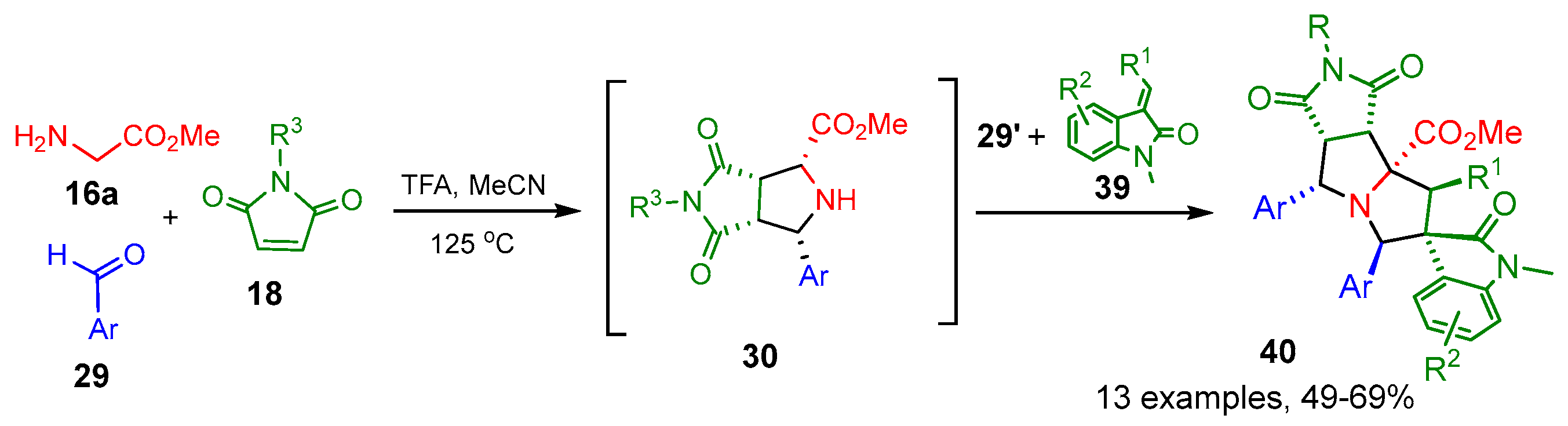
2.2.1. Double [3+2] Cycloadditions with Maleimides
2.2.2. Double [3+2] Cycloadditions with Olefinic Oxindoles
2.2.3. Double [3+2] Cycloadditions with Meta-Dinitrobenzene Derivatives
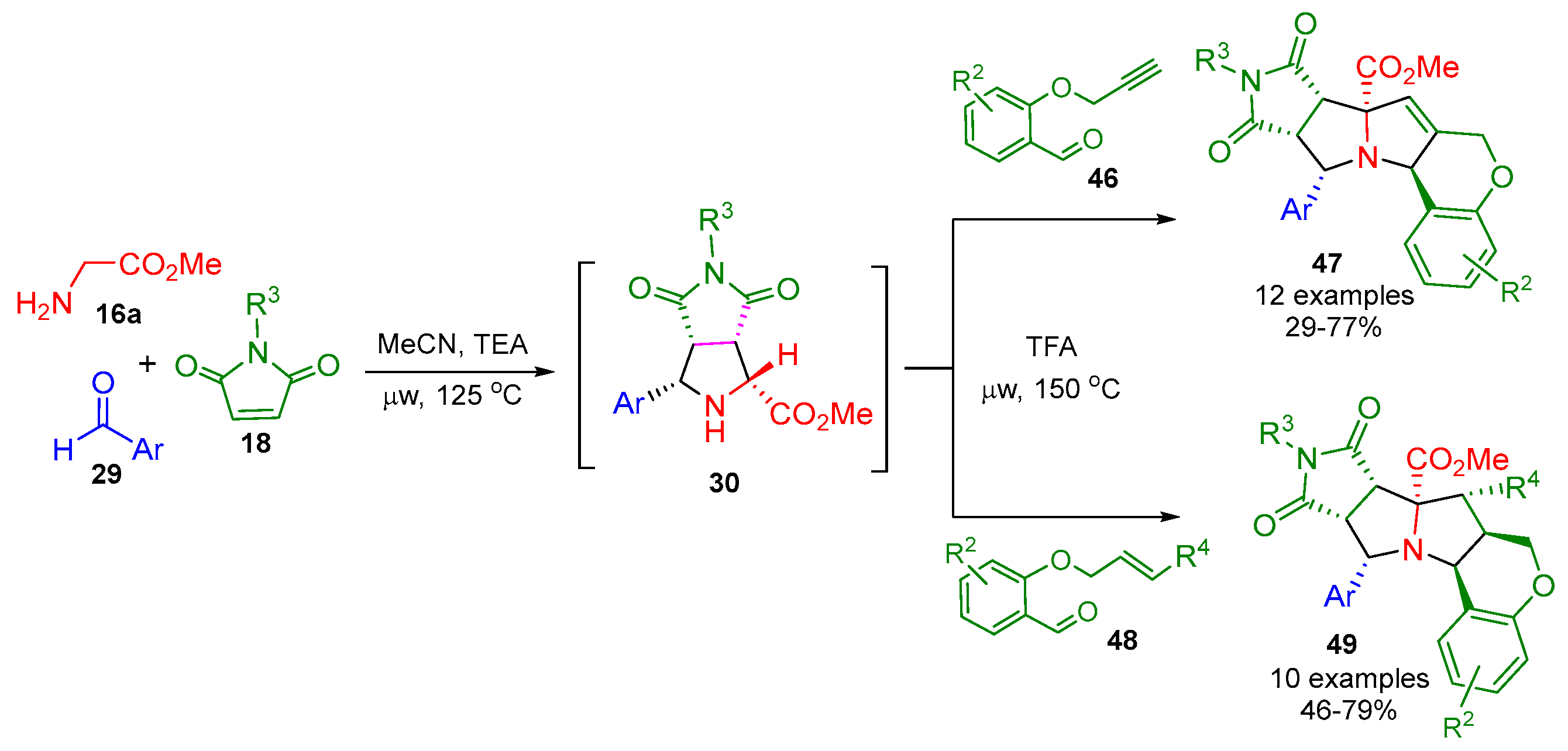
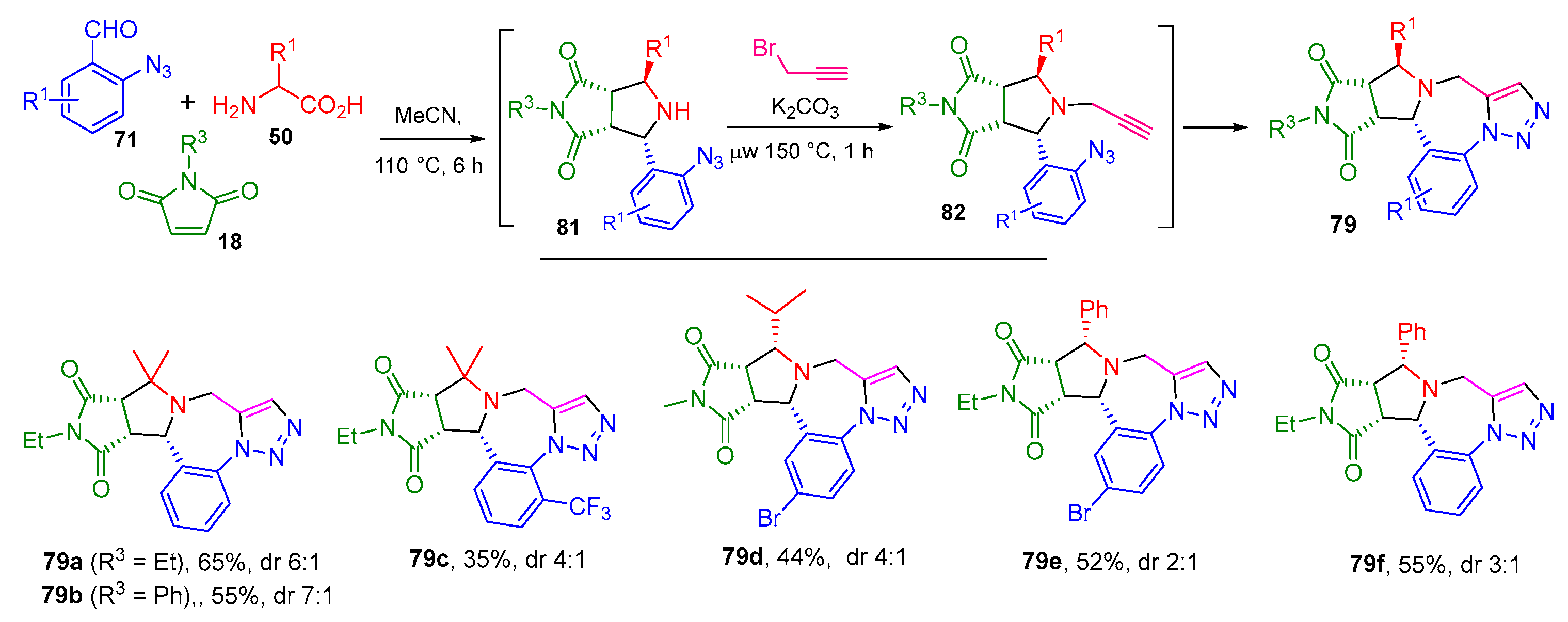
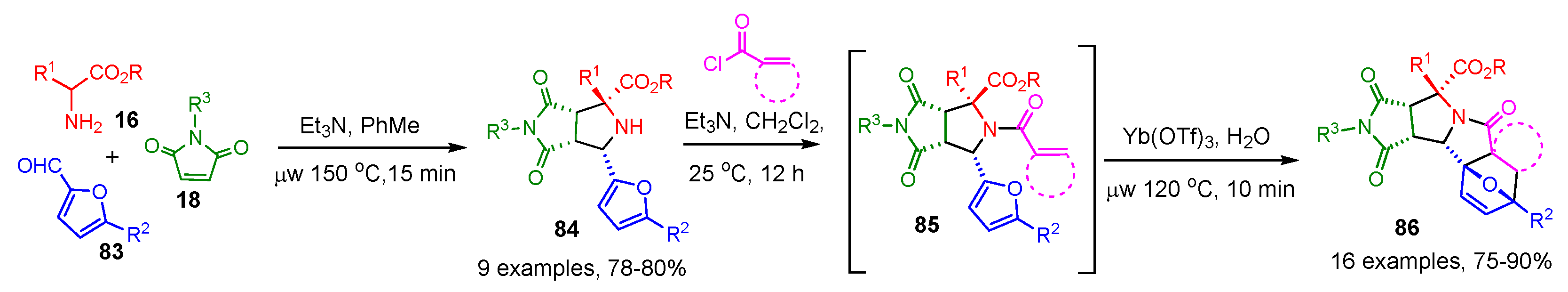
2.3. [3+2] Cycloaddition Followed by a Click Reaction
2.3.1. Stabilized AMY-Based [3+2] Cycloadditions
2.3.2. Semi-Stabilized AMY-Initiated [3+2] Cycloaddition
3. [3+2] Cycloaddition and [4+2] Cycloaddition
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| AMY | Azomethine ylide |
| BzOH | Benzoic acid |
| EWG | Electron-withdrawing group |
| NOESY | Nuclear Overhauser effect spectroscopy |
| PASE | Pot, atom, and step economic |
| TEA | Triethylamine |
| TFA | Trifluoroacetic acid |
| THF | Tetrahydrofuran |
| UCD-Imphanol | Imidazolinyl-[2.2]paracyclophanol |
References
- Panda, S.S.; Aziz, M.N.; Stawinski, J.; Girgis, A.S. Azomethine Ylides—Versatile Synthons for Pyrrolidinyl-Heterocyclic Compounds. Molecules 2023, 28, 668. [Google Scholar] [CrossRef]
- Najera, C.; Sansano, J.M. Azomethine Ylides in Organic Synthesis. Curr. Org. Chem. 2003, 7, 1105–1150. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, X.; Sun, J.; Niu, D.; Chruma, J.J. 2-Azaallyl Anions, 2-Azaallyl Cations, 2-Azaallyl Radicals, and Azomethine Ylides. Chem. Rev. 2018, 118, 10393–10457. [Google Scholar] [CrossRef] [PubMed]
- Coldham, I.; Hufton, R. Intramolecular Dipolar Cycloaddition Reactions of Azomethine Ylides. Chem. Rev. 2005, 105, 2765–2810. [Google Scholar] [CrossRef] [PubMed]
- Döndas, H.; de Gracia Retamosa, M.; Sansano, J. Current Trends towards the Synthesis of Bioactive Heterocycles and Natural Products using 1,3-Dipolar Cycloadditions (1,3-DC) with Azomethine Ylides. Synthesis 2017, 49, 2819–2851. [Google Scholar] [CrossRef]
- Arrastia, I.; Arrieta, A.; Cossío, F.P. Application of 1,3-Dipolar Reactions between Azomethine Ylides and Alkenes to the Synthesis of Catalysts and Biologically Active Compounds. Eur. J. Org. Chem. 2018, 2018, 5889–5904. [Google Scholar] [CrossRef]
- Pandey, G.; Banerjee, P.; Gadre, S.R. Construction of Enantiopure Pyrrolidine Ring System via Asymmetric [3+2]-Cycloaddition of Azomethine Ylides. Chem. Rev. 2006, 106, 4484–4517. [Google Scholar] [CrossRef]
- Wei, L.; Chang, X.; Wang, C. Catalytic Asymmetric Reactions with N-Metallated Azomethine Ylides. Acc. Chem. Res. 2020, 53, 1084–1100. [Google Scholar] [CrossRef]
- Zhou, T.; Zhang, X.; Zhan, D.; Zhang, W. Glycine-based [3+2] Cycloaddition for the Synthesis of Pyrrolidine-containing Polycyclic Compounds. Molecules 2024, 29, 5726. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, W. PASE Synthesis of Pyrrolidine-Containing Heterocycles Through [3+2] Cycloaddition-Initiated Reactions. Curr. Opin. Green Sustain. Chem. 2018. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Zhang, W. Decarboxylative 1,3-Dipolar Cycloaddition of Amino Acids for the Synthesis of Heterocyclic Compounds. Beilstein J. Org. Chem. 2023, 19, 1677–1693. [Google Scholar] [CrossRef]
- Li, J.; Ye, Y.; Zhang, Y. Cycloaddition/Annulation Strategies for the Construction of Multisubstituted Pyrrolidines and Their Applications in Natural Product Synthesis. Org. Chem. Front. 2018, 5, 864–892. [Google Scholar] [CrossRef]
- Kissane, M.; Maguire, A.R. Asymmetric 1,3-Dipolar Cycloadditions of Acrylamides. Chem. Soc. Rev. 2009, 39, 845–883. [Google Scholar] [CrossRef]
- Nejera, C.; Sansano, J.M. Asymmetric 1,3-Dipolar Cycloadditons of Stabilized Azomethine Ylides with Nitroalkenes. Curr. Top. Med. Chem. 2013, 14, 1271–1282. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Wang, C.-J. Catalytic Asymmetric Construction of Spiropyrrolidines via 1,3-Dipolar Cycloaddition of Azomethine Ylides. Org. Biomol. Chem. 2018, 16, 2591–2601. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.V.; Guiry, P.J. Catalytic Enantioselective [3+2] Cycloaddition of N-Metalated Azomethine Ylides. Chem. Eur. J. 2023, 29, e202300296. [Google Scholar] [CrossRef] [PubMed]
- Adrio, J.; Carretero, J.C. Recent Advances in the Catalytic Asymmetric 1,3-Dipolar Cycloaddition of Azomethine Ylides. Chem. Commun. 2014, 50, 12434–12446. [Google Scholar] [CrossRef]
- Narayan, R.; Potowski, M.; Jia, Z.-J.; Antonchick, A.P.; Waldmann, H. Catalytic Enantioselective 1,3-Dipolar Cycloadditions of Azomethine Ylides for Biology-Oriented Synthesis. Acc. Chem. Res. 2014, 47, 1296–1310. [Google Scholar] [CrossRef]
- Bakthadoss, M.; Mushaf, M.; Agarwal, V.; Reddy, T.T.; Sharada, D.S. Azomethine Ylide Cycloaddition of 1,3-Dienyl Esters: Highly Regio- and Diastereoselective Synthesis of Functionalized Pyrrolidinochromenes. Org. Biomol. Chem. 2022, 20, 778–782. [Google Scholar] [CrossRef]
- Antonchick, A.P.; Gerding-Reimers, C.; Catarinella, M.; Schürmann, M.; Preut, H.; Ziegler, S.; Rauh, D.; Waldmann, H. Highly Enantioselective Synthesis and Cellular Evaluation of Spirooxindoles Inspired by Natural Products. Nat. Chem. 2009, 2, 735–740. [Google Scholar] [CrossRef]
- Assen, A.H.; Adil, K.; Belmabkhout, Y. Lab-Scale Insights into Green Metal–Organic Framework Sorbents for Gas Separation or Purification. Curr. Opin. Green Sustain. Chem. 2024, 49, 100948. [Google Scholar] [CrossRef]
- Mishra, M.; Sharma, M.; Dubey, R.; Kumari, P.; Ranjan, V.; Pandey, J. Green Synthesis Interventions of Pharmaceutical Industries for Sustainable Development. Curr. Res. Green Sustain. Chem. 2021, 4, 100174. [Google Scholar] [CrossRef]
- Wang, W.; Brown, M.K. Photosensitized [4+2]- and [2+2]-Cycloaddition Reactions of N-Sulfonylimines. Angew. Chem. Int. Ed. 2023, 62, e202305622. [Google Scholar] [CrossRef]
- Gouault, N.; Roydor, M.; Renault, J.; Banik, S.; Reddy, B.V.S.; Lalli, C. One-Pot Double Cyclizations Involving an Aza-Prins Process. Eur. J. Org. Chem. 2024, 27, e202400617. [Google Scholar] [CrossRef]
- Kalita, S.J.; Mecadon, H.; Deka, D.C. Pot, Atom and Step Economic (PASE) Synthesis of 5-Monoalkylbarbiturates through Domino Aldol-Michael Reaction. Tetrahedron Lett. 2015, 56, 731–734. [Google Scholar] [CrossRef]
- Feng, Y.; Tian, N.; Li, Y.; Jia, C.; Li, X.; Wang, L.; Cui, X. Construction of Fused Polyheterocycles through Sequential [4 + 2] and [3 + 2] Cycloadditions. Org. Lett. 2017, 19, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Bastrakov, M.A.; Starosotnikov, A.M.; Kachala, V.V.; Fedyanin, I.V.; Shevelev, S.A. Facile Dearomatization of Nitroquinolines through [3+2] and [4+2] Cycloaddition Reactions. Asian J. Org. Chem. 2015, 4, 146–153. [Google Scholar] [CrossRef]
- Shang, X.; Liu, K.; Zhang, Z.; Xu, X.; Li, P.; Li, W. Direct Access to Spirobiisoxazoline via the Double 1,3-Dipolar Cycloaddition of Nitrile Oxide with Allenoate. Org. Biomol. Chem. 2018, 16, 895–898. [Google Scholar] [CrossRef]
- Lautens, M.; Jang, A. Palladium-Catalyzed Formal [5+4] and [3+2] Cycloaddition. Synfacts 2017, 13, 0382. [Google Scholar] [CrossRef]
- Paul, A.; Kim, J.H.; Daniel, S.D.; Seidel, D. Diversification of Unprotected Alicyclic Amines by C−H Bond Functionalization: Decarboxylative Alkylation of Transient Imines. Angew. Chem. Int. Ed. 2021, 60, 1625–1628. [Google Scholar] [CrossRef]
- Zhang, B.; Ruan, J.; Seidel, D.; Chen, W. Palladium-Catalyzed Arylation of Endocyclic 1-Azaallyl Anions: Concise Synthesis of Unprotected Enantioenriched Cis-2,3-Diarylpiperidines. Angew. Chem. Int. Ed. 2023, 62, e202307638. [Google Scholar] [CrossRef] [PubMed]
- Seidel, D. The Azomethine Ylide Route to Amine C–H Functionalization: Redox-Versions of Classic Reactions and a Pathway to New Transformations. Acc. Chem. Res. 2015, 48, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Paul, A.; Ghiviriga, I.; Seidel, D. A-C–H Bond Functionalization of Unprotected Alicyclic Amines: Lewis-Acid-Promoted Addition of Enolates to Transient Imines. Org. Lett. 2021, 23, 797–801. [Google Scholar] [CrossRef]
- Paul, A.; Seidel, D. A-Functionalization of Cyclic Secondary Amines: Lewis Acid Promoted Addition of Organometallics to Transient Imines. J. Am. Chem. Soc. 2019, 141, 8778–8782. [Google Scholar] [CrossRef]
- Korotaev, V.Y.; Zimnitskiy, N.S.; Barkov, A.Y.; Kutyashev, I.B.; Sosnovskikh, V.Y. Stabilized Azomethine Ylides Derived from Indeno[1,2-b]Quinoxalinones in [3+2] Cycloaddition Reactions with Electrophilic Alkenes. Chem. Heterocycl. Compd. 2018, 54, 905–922. [Google Scholar] [CrossRef]
- Cui, P.; Xu, L.; Shi, Z.; Gan, L. Synthesis of Decahydropyrrolo[2,1,5-Cd]Indolizine through Consecutive [2 + 3] Cycloadditions and 6-Exo-Trig Cyclization. J. Org. Chem. 2011, 76, 4210–4212. [Google Scholar] [CrossRef]
- Cui, P.; Xu, L.; Cheng, H.; Gan, L. Synthesis of Decahydropyrrolo[2,1,5-Cd]Indolizine Derivatives through RuCl3/AgOTf Induced Alkene–Alkene and Alkene–Arene Double Cycloisomerizations. Tetrahedron 2012, 68, 152–158. [Google Scholar] [CrossRef]
- Lu, Q.; Song, G.; Jasinski, J.P.; Keeley, A.C.; Zhang, W. One-Pot Double [3 + 2] Cycloaddition for Diastereoselective Synthesis of Tetracyclic Pyrrolidine Compounds. Green Chem. 2012, 14, 3010–3012. [Google Scholar] [CrossRef]
- Quiroga, J.; Gálvez, J.; Abonia, R.; Insuasty, B.; Ortíz, A.; Cobo, J.; Nogueras, M. Highly Efficient and Diastereoselective Synthesis of New Pyrazolylpyrrolizine and Pyrazolylpyrrolidine Derivates by a Three-Component Domino Process. Molecules 2014, 19, 4284–4300. [Google Scholar] [CrossRef]
- Kumar, S.V.; Guiry, P.J. Zinc-Catalyzed Enantioselective [3+2] Cycloaddition of Azomethine Ylides Using Planar Chiral [2.2]Paracyclophane-Imidazoline N,O-ligands. Angew. Chem. Int. Ed. 2022, 61, e202205516. [Google Scholar] [CrossRef]
- Egorov, V.A.; Khasanova, L.S.; Gimalova, F.A.; Lobov, A.N.; Miftakhov, M.S. Straightforward Synthesis of Pyrrolizidines. Mendeleev Commun. 2017, 27, 163–165. [Google Scholar] [CrossRef]
- Zhang, X.; Ma, X.; Qiu, W.; Awad, J.; Zhang, W. Double [3 + 2] Cycloadditions for Diastereoselective Synthesis of Spirooxindole Pyrrolizidines. Green Process. Synth. 2022, 11, 1128–1135. [Google Scholar] [CrossRef]
- Zhang, W.; Lu, Y.; Geib, S. Synthesis of Fluorous and Nonfluorous Polycyclic Systems by One-Pot, Double Intramolecular 1,3-Dipolar Cycloaddition of Azomethine Ylides. Org. Lett. 2005, 7, 2269–2272. [Google Scholar] [CrossRef] [PubMed]
- Matsugi, M.; Curran, D.P. Reverse Fluorous Solid-Phase Extraction: A New Technique for Rapid Separation of Fluorous Compounds. Org. Lett. 2004, 6, 2717–2720. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Luo, Z.; Curran, D.P. Separation of “Light Fluorous” Reagents and Catalysts by Fluorous Solid-Phase Extraction: Synthesis and Study of a Family of Triarylphosphines Bearing Linear and Branched Fluorous Tags. J. Org. Chem. 2000, 65, 8866–8873. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, W.; Ma, X.; Evans, J.; Kaur, M.; Jasinski, J.P.; Zhang, W. One-Pot Double [3 + 2] Cycloadditions for Diastereoselective Synthesis of Pyrrolidine-Based Polycyclic Systems. J. Org. Chem. 2018, 83, 13536–13542. [Google Scholar] [CrossRef]
- Tsuge, O.; Kanemasa, S.; Hatada, A.; Matsuda, K. Synthetic Versatility of N-(Silylmethyl)imines: Water-Induced Generation of N-Protonated Azomethine Ylides of Nonstabilized Type and Fluoride-Induced Generation of 2-Azaallyl Anions. Bull. Chem. Soc. Jpn. 1986, 59, 2537–2545. [Google Scholar] [CrossRef]
- Vedejs, E.; West, F.G. Ylides by the Desilylation of α-Silyl Onium Salts. Chem. Rev. 1986, 86, 941–955. [Google Scholar] [CrossRef]
- Pearson, W.H.; Walters, M.A.; Oswell, K.D. Intramolecular 2-Azaallyl Anion Cycloadditions. Application to the Synthesis of Fused Bicyclic Pyrrolidines. J. Am. Chem. Soc. 1986, 108, 2769–2771. [Google Scholar] [CrossRef]
- Tsuge, O.; Kanemasa, S.; Yamada, T.; Matsuda, K. Regioselective Cycloadditions of N-Protonated Azomethine Ylides and 2-Azaallyl Anions Generated from N-(Silylmethyl) Thioimidates, Synthetic Equivalents of Nonstabilized Nitrile Ylides. J. Org. Chem. 1987, 52, 2523–2530. [Google Scholar] [CrossRef]
- Ardill, H.; Grigg, R.; Sridharan, V.; Surendrakumar, S. X=Y-ZH Systems as Potential 1,3-Dipoles: Part 19. Intramolecular Cycloadditions of Non-stabilised Azomethine Ylides Generated via the Decarboxylative Route from α- Amino Acids. Tetrahedron 1988, 44, 4953–4966. [Google Scholar] [CrossRef]
- Kanemasa, S.; Sakamoto, K.; Tsuge, O. Nonstabilized Azomethine Ylides Generated by Decarboxylative Condensation of α-Amino Acids. Structural Variation, Reactivity, and Stereoselectivity. Bull. Chem. Soc. Jpn. 1989, 62, 1960–1968. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, W.; Evans, J.; Kaur, M.; Jasinski, J.P.; Zhang, W. Double 1,3-Dipolar Cycloadditions of Two Nonstabilized Azomethine Ylides for Polycyclic Pyrrolidines. Org. Lett. 2019, 21, 2176–2179. [Google Scholar] [CrossRef]
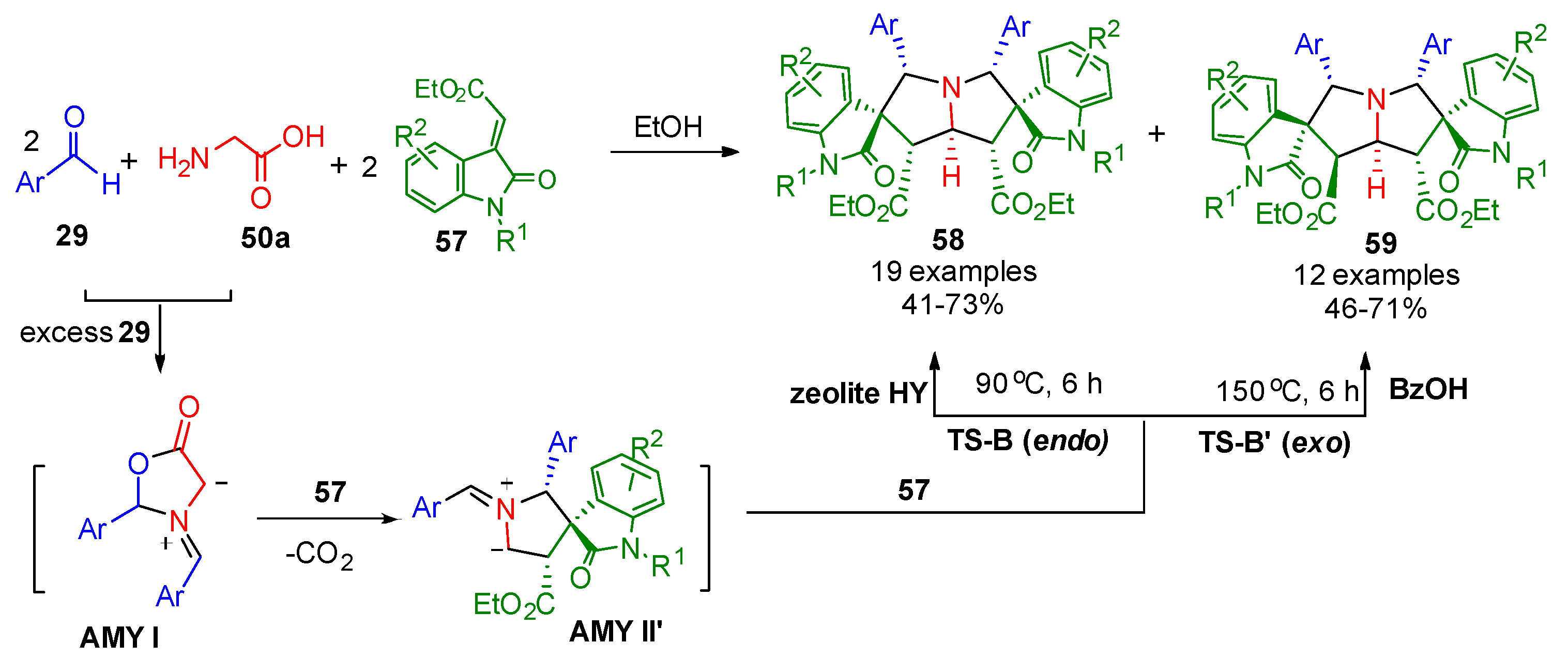
- Zhan, D.; Yang, G.; Zhou, T.; Nallapati, S.; Zhang, X. Decarboxylation-Driven Double Annulations: Innovative Multi-Component Reaction Pathways. Molecules 2025, 30, 1594. [Google Scholar] [CrossRef]
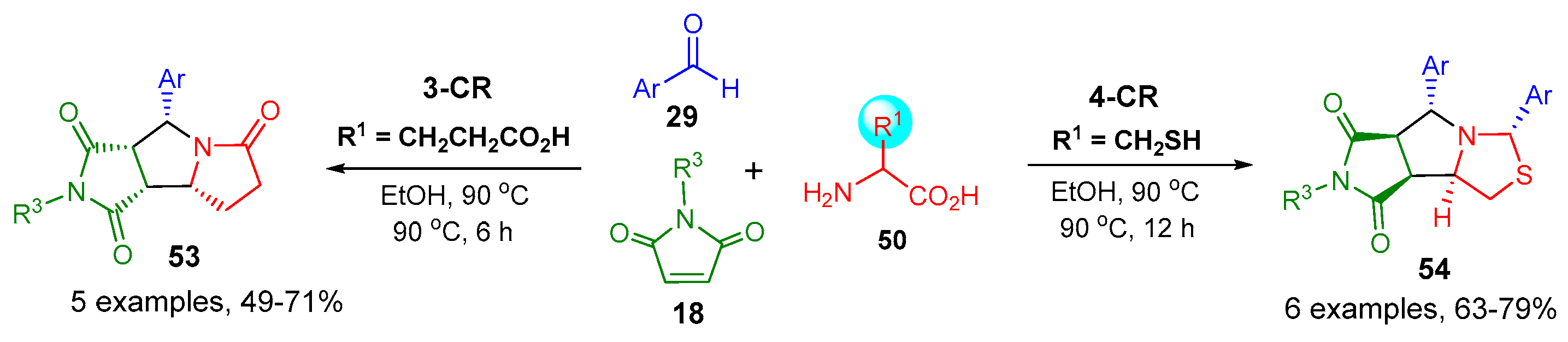
- Ma, X.; Qiu, W.; Liu, L.; Zhang, X.; Awad, J.; Evans, J.; Zhang, W. Synthesis of Tetrahydropyrrolothiazoles through One-Pot and Four-Component N,S-Acetalation and Decarboxylative [3+2] Cycloaddition. Green Synth. Catal. 2021, 2, 74–77. [Google Scholar] [CrossRef]
- Zhang, X.; Qiu, W.; Murray, S.A.; Zhan, D.; Evans, J.; Jasinski, J.P.; Wang, X.; Zhang, W. Pseudo-Five-Component Reaction for Diastereoselective Synthesis of Butterfly Shaped Bispiro[Oxindole-Pyrrolidine]s. J. Org. Chem. 2021, 86, 17395–17403. [Google Scholar] [CrossRef] [PubMed]
- Zhan, D.; Yao, B.; Huan, C.; Lu, J.; Ji, Y.; Zhang, X. A Step-Economical and Diastereoselective Synthesis of Dispirooxindole-Pyrrolizines Bearing Seven Stereocenters. Tetrahedron Lett. 2023, 126, 154638. [Google Scholar] [CrossRef]
- Bastrakov, M.A.; Starosotnikov, A.M.; Pechenkin, S.Y.; Kachala, V.V.; Glukhov, I.V.; Shevelev, S.A. Double 1,3-dipolar Cycloaddition of N-methyl Azomethine Ylide to Meta-Dinitrobenzene Annelated with Nitrogen Aromatic Heterocycles. J. Heterocycl. Chem. 2010, 47, 893–896. [Google Scholar] [CrossRef]
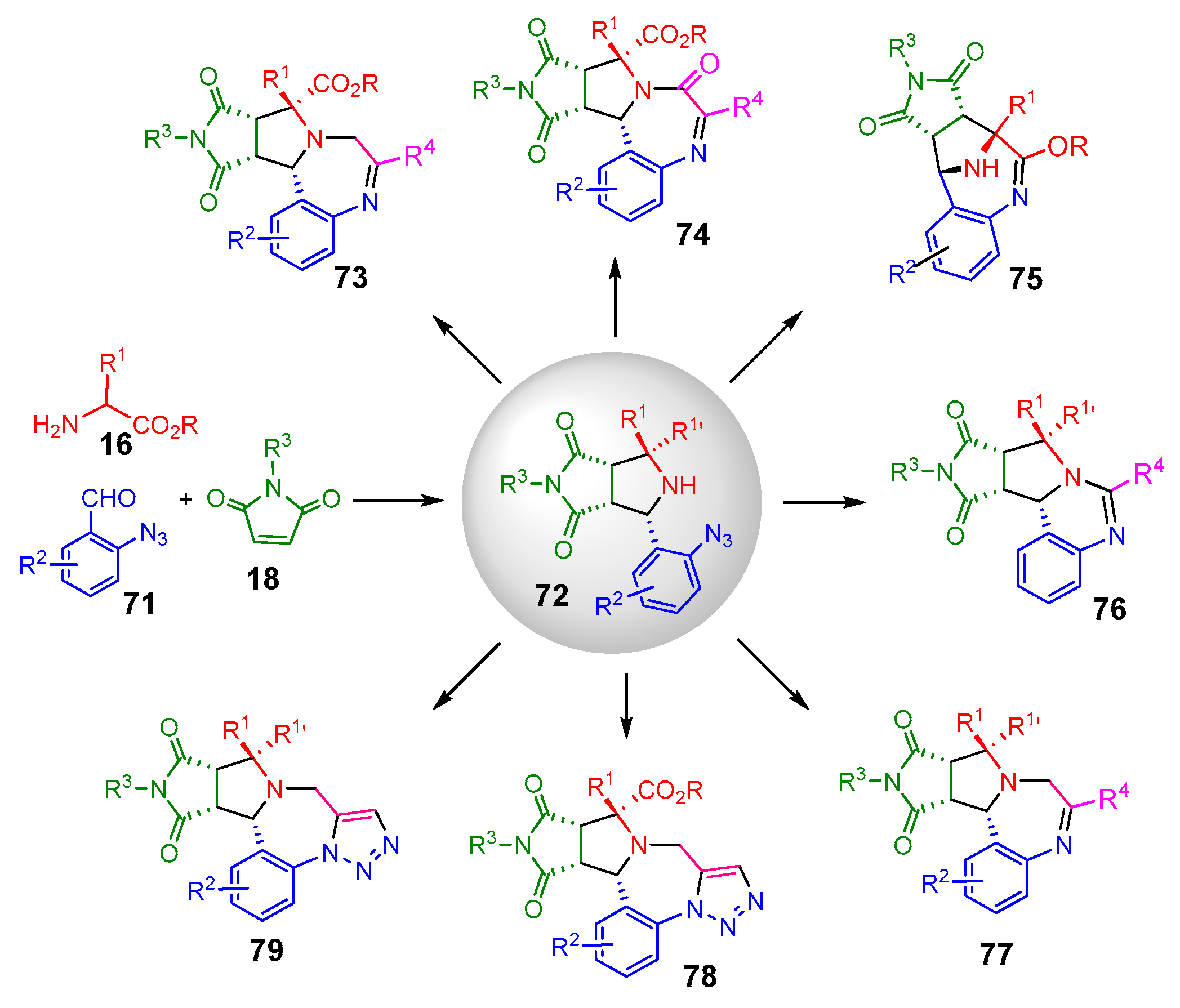
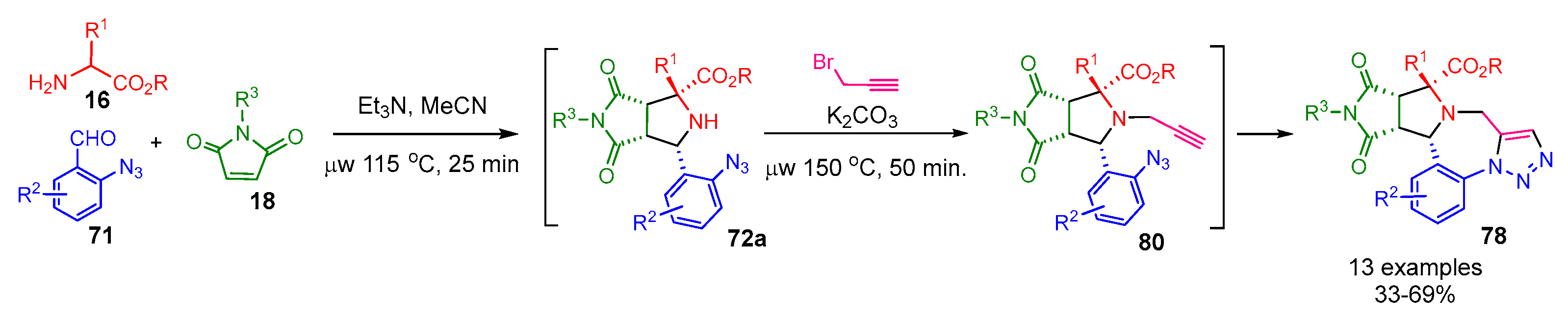
- Zhang, X.; Zhi, S.; Wang, W.; Liu, S.; Jasinski, J.P.; Zhang, W. A Pot-Economical and Diastereoselective Synthesis Involving Catalyst-Free Click Reaction for Fused-Triazolobenzodiazepines. Green Chem. 2016, 18, 2642–2646. [Google Scholar] [CrossRef]
- Ma, X.; Meng, S.; Zhang, X.; Zhang, Q.; Yan, S.; Zhang, Y.; Zhang, W. Synthesis of Pyrrolidinedione-Fused Hexahydropyrrolo[2,1-a]Isoquinolines via Three-Component [3 + 2] Cycloaddition Followed by One-Pot N-Allylation and Intramolecular Heck Reactions. Beilstein J. Org. Chem. 2020, 16, 1225–1233. [Google Scholar] [CrossRef]
- Takao, K.; Munakata, R.; Tadano, K. Recent Advances in Natural Product Synthesis by Using Intramolecular Diels−Alder Reactions. Chem. Rev. 2005, 105, 4779–4807. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R. Recent Developments in Catalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reaction. Chem. Rev. 2013, 113, 5515–5546. [Google Scholar] [CrossRef] [PubMed]
- Briou, B.; Améduri, B.; Boutevin, B. Trends in the Diels–Alder Reaction in Polymer Chemistry. Chem. Soc. Rev. 2021, 50, 11055–11097. [Google Scholar] [CrossRef] [PubMed]
- Tandem Diels–Alder [4+2] Cycloadditions and Intramolecular [3+2] Cross-Cycloadditions of Dienylcyclopropane 1,1-Diesters. Synlett 2014, 25, 2260–2264. [CrossRef]
- Shevelev, S.A.; Starosotnikov, A.M. Pericyclic [4+2] and [3+2] Cycloaddition Reactions of Nitroarenes in Heterocyclic Synthesis. Chem. Heterocycl. Compd. 2013, 49, 92–115. [Google Scholar] [CrossRef]
- Villela, L.F.; Zidan, M.; Barriault, L. Photoactivated Formal [3 + 2]/[4+2] Cycloaddition of N-Aryl Cyclopropyl and Cyclobutylamines. Eur. J. Org. Chem. 2024, 27, e202400558. [Google Scholar] [CrossRef]
- Chauhan, A.N.S.; Vini, V.; Kumar, A.; Erande, R.D. Synthesis of Indol-3-Yl-Benzofurans and Carbazoles via Cu(OTf) 2 -Catalyzed [3 + 2] and [4 + 2] Cycloaddition. Org. Biomol. Chem. 2024, 22, 6690–6694. [Google Scholar] [CrossRef]
- Lu, Q.; Huang, X.; Song, G.; Sun, C.-M.; Jasinski, J.P.; Keeley, A.C.; Zhang, W. Sequential [3 + 2] and [4 + 2] Cycloadditions for Stereoselective Synthesis of a Novel Polyheterocyclic Scaffold. ACS Comb. Sci. 2013, 15, 350–355. [Google Scholar] [CrossRef]








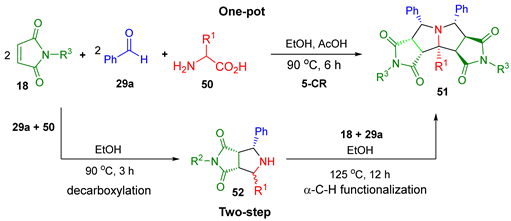 | |||||
|---|---|---|---|---|---|
| Amino Acids 50 | R1 | R2 | Product | One-Pot Yield (dr) 3 | Two-Step Yield (dr) 4 |
| glycine 1 | H | Et | 51a | 91% (>9:1) | 48.5% (3:1) |
| alanine | Me | Et | 51b | 88% (>8:1) | -- |
| butyrine | Et | Et | 51c | 88% (>8:1) | -- |
| norvaline | Pr | Et | 51d | 75% (>8:1) | -- |
| serine | CH2OH | Me | 51e | 83% (>8:1) | -- |
| leucine | iBu | Me | 51f | 53% (>8:1) | -- |
| aspartic acid 2 | CH2CO2H | Et | 51g | 66% (~4:1) | -- |
| valine | iPr | Et | 51h | -- | -- |
| phenylglycine | Ph | Et | 51i | -- | -- |
| glutamic acid | CH2CH2CO2H | Et | 51j | -- | -- |
| cysteine | CH2SH | Et | 51k | -- | -- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, T.; Zhang, X.; Sheng, Y.J.; Zhan, D.; Zhang, W. Recent Developments in Azomethine Ylide-Initiated Double Cycloadditions. Molecules 2025, 30, 4019. https://doi.org/10.3390/molecules30194019
Zhou T, Zhang X, Sheng YJ, Zhan D, Zhang W. Recent Developments in Azomethine Ylide-Initiated Double Cycloadditions. Molecules. 2025; 30(19):4019. https://doi.org/10.3390/molecules30194019
Chicago/Turabian StyleZhou, Tieli, Xiaofeng Zhang, Yan Jan Sheng, Desheng Zhan, and Wei Zhang. 2025. "Recent Developments in Azomethine Ylide-Initiated Double Cycloadditions" Molecules 30, no. 19: 4019. https://doi.org/10.3390/molecules30194019
APA StyleZhou, T., Zhang, X., Sheng, Y. J., Zhan, D., & Zhang, W. (2025). Recent Developments in Azomethine Ylide-Initiated Double Cycloadditions. Molecules, 30(19), 4019. https://doi.org/10.3390/molecules30194019







