Aromaticity Tuning in Biaryl Monophosphines and Their Derivatives
Abstract
1. Introduction
2. Results
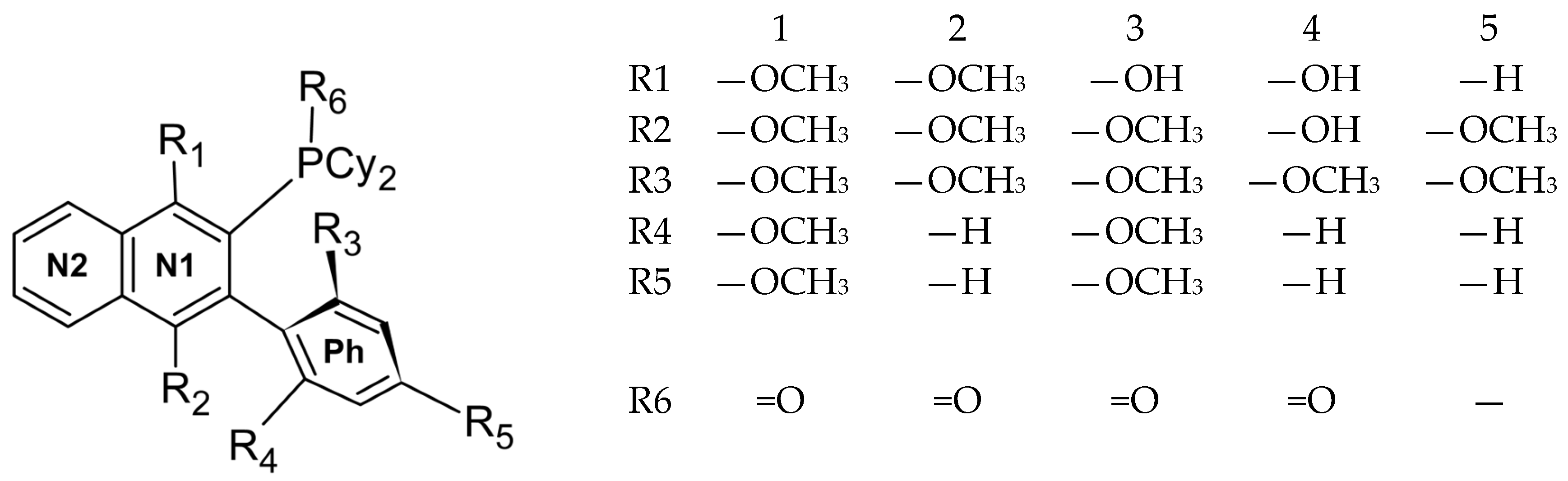
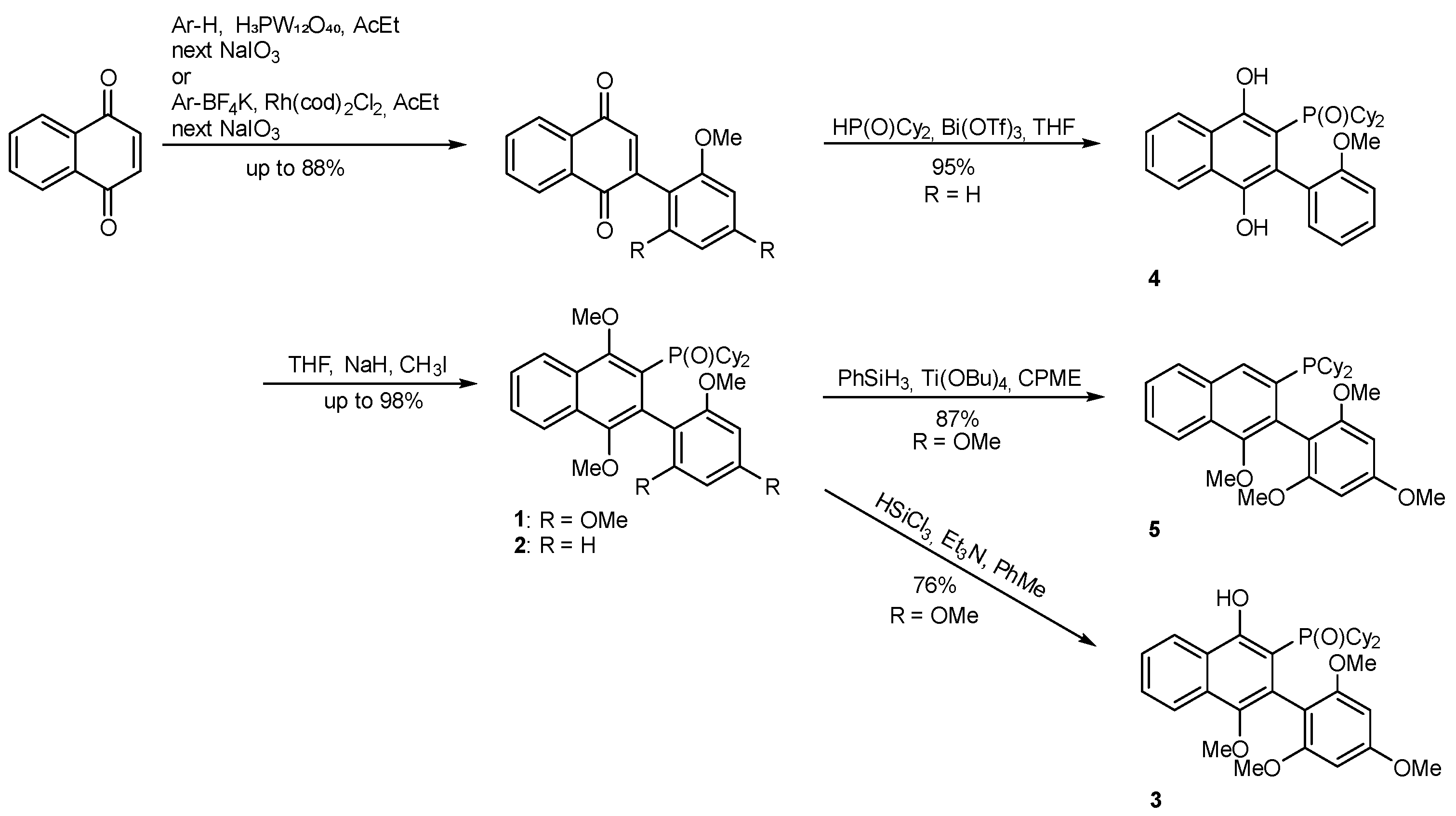
2.1. Synthesis
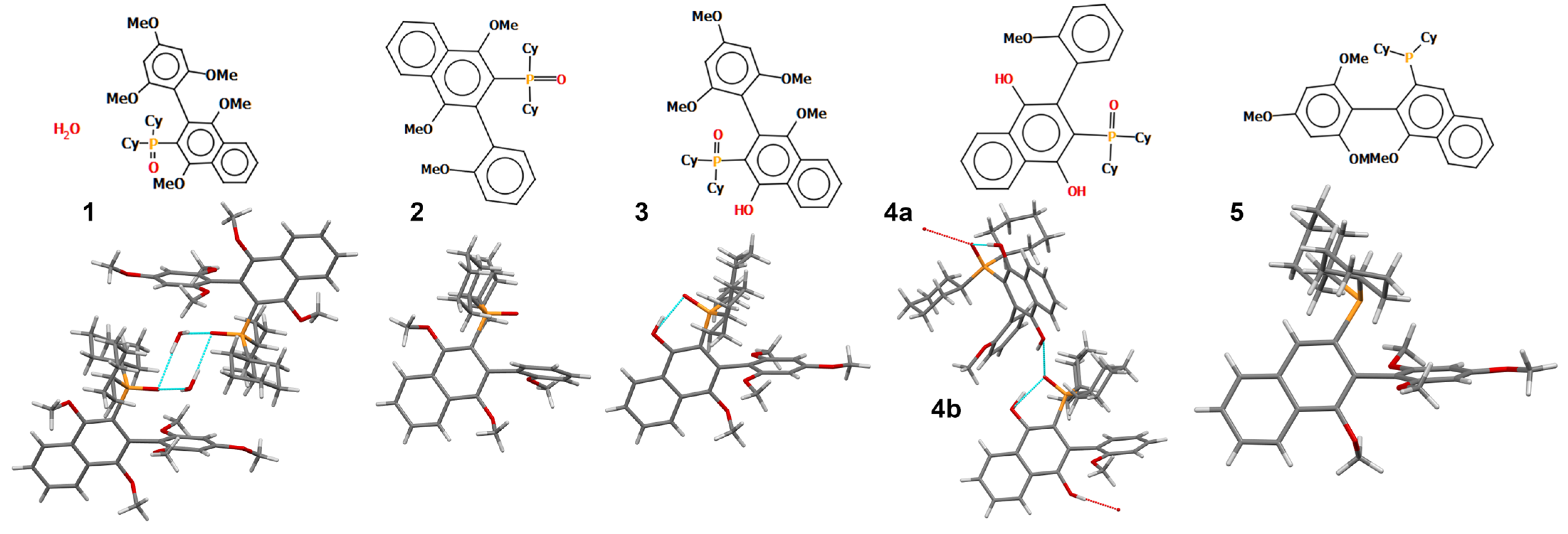
2.2. Crystal Structure
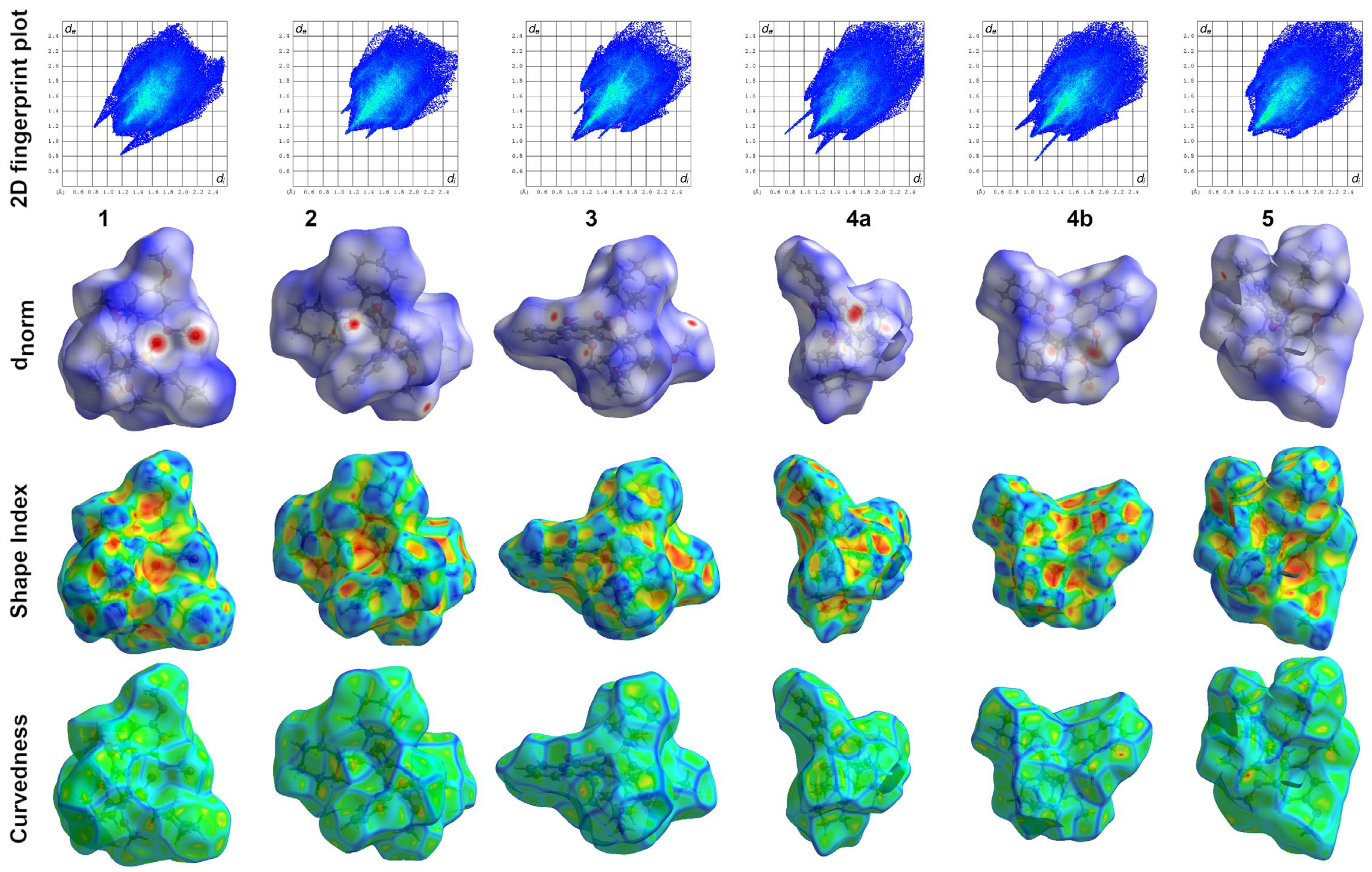
2.3. Hirshfeld Surface Analysis
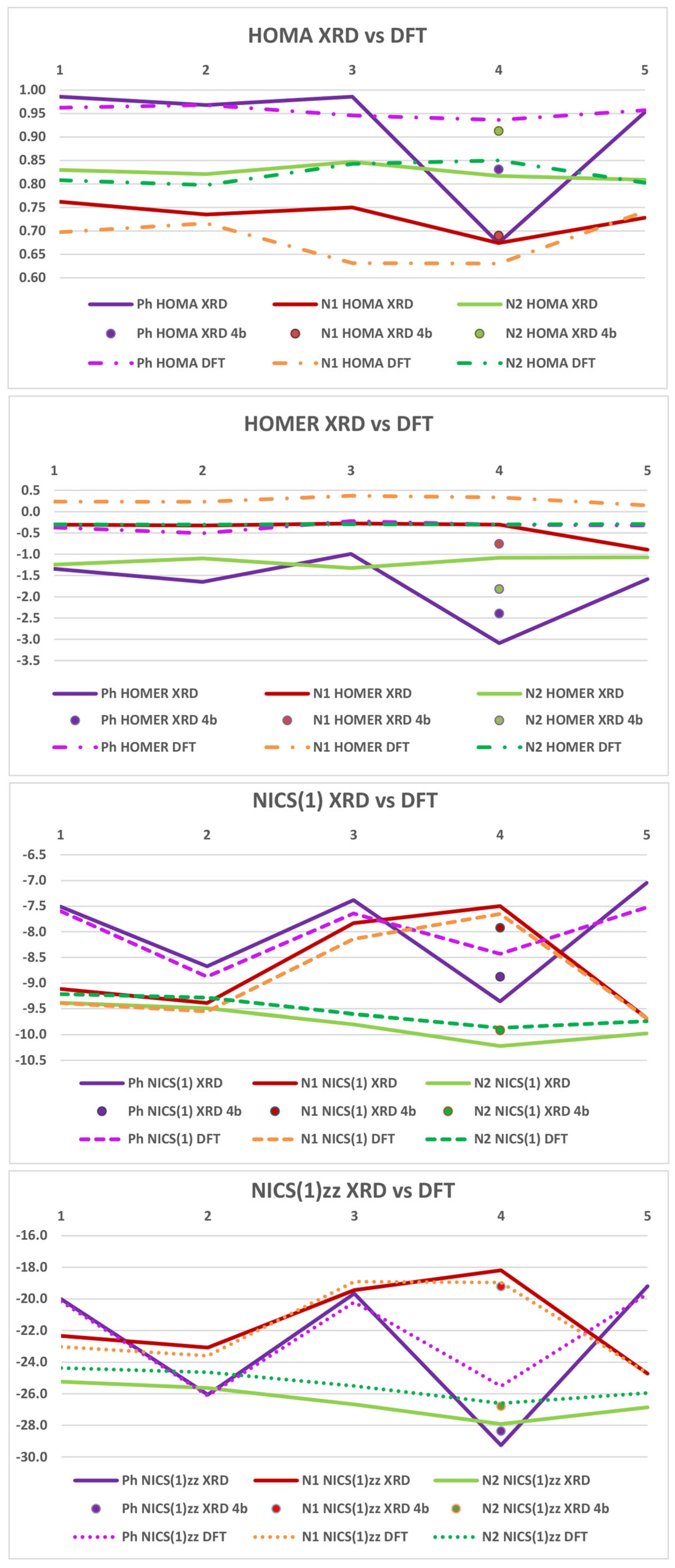
2.4. Aromaticity Evaluation: HOMA, HOMER, and NICS Indices
2.5. HOMA Indices
2.6. HOMER Indices
2.7. Nucleus-Independent Chemical Shift Approach (NICS)
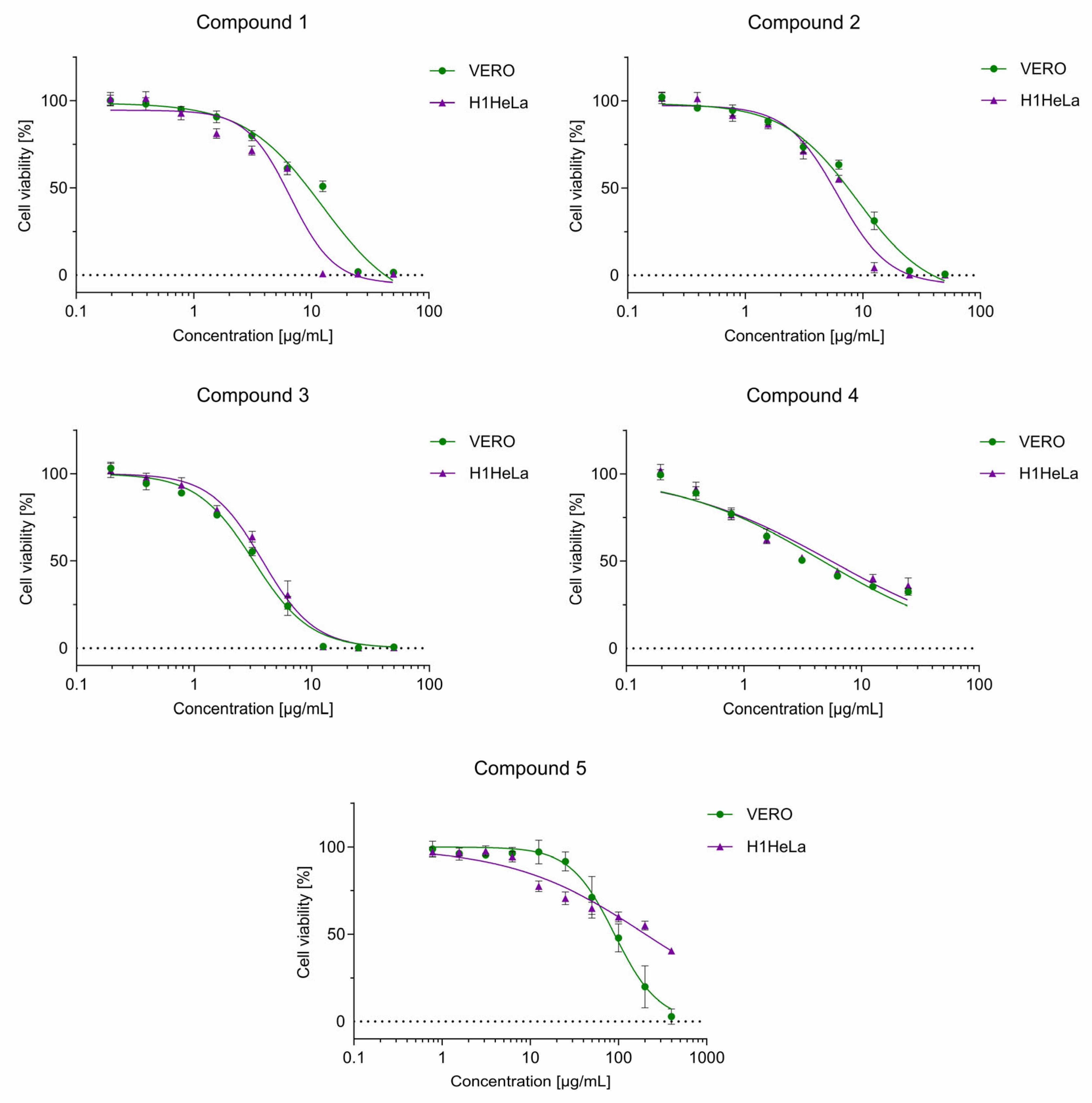
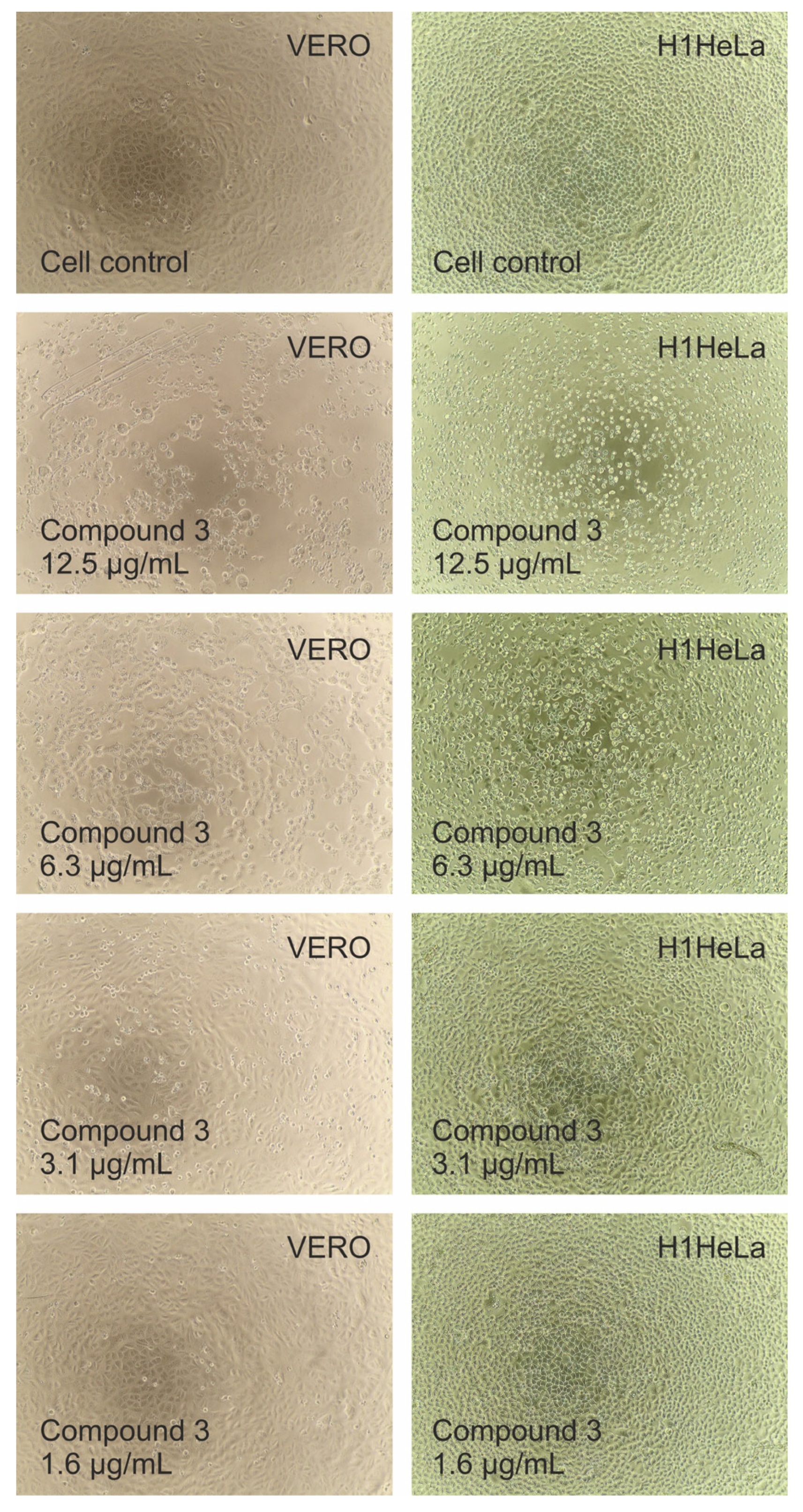
2.8. Cytotoxicity of Studied Compounds
2.9. Antibacterial Activity Screening
3. Materials and Methods
3.1. Compounds
3.2. X-Ray Crystallography
3.3. Hirshfeld Surface Analysis
3.4. HOMA and HOMER Calculations
3.5. Nucleus-Independent Chemical Shift Approach (NICS)
3.6. Cytotoxicity Evaluation
3.7. Antibacterial Activity Screening
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ottosson, H. A Focus on Aromaticity: Fuzzier than Ever Before? Chem. Sci. 2023, 14, 5542–5544. [Google Scholar] [CrossRef] [PubMed]
- Merino, G.; Solà, M.; Fernández, I.; Foroutan-Nejad, C.; Lazzeretti, P.; Frenking, G.; Anderson, H.L.; Sundholm, D.; Cossío, F.P.; Petrukhina, M.A.; et al. Aromaticity: Quo Vadis. Chem. Sci. 2023, 14, 5569–5576. [Google Scholar] [CrossRef]
- Cyrański, M.K.; Krygowski, T.M.; Katritzky, A.R.; Schleyer, P.V.R. To What Extent Can Aromaticity Be Defined Uniquely? J. Org. Chem. 2002, 67, 1333–1338. [Google Scholar] [CrossRef] [PubMed]
- Balaban, A.T.; Oniciu, D.C.; Katritzky, A.R. Aromaticity as a Cornerstone of Heterocyclic Chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef]
- Stanger, A. What Is Aromaticity: A Critique of the Concept of Aromaticity—Can It Really Be Defined? Chem. Commun. 2009, 15, 1939–1947. [Google Scholar] [CrossRef] [PubMed]
- Stanger, A. Singlet Fission and Aromaticity. J. Phys. Chem. A 2022, 126, 8049–8057. [Google Scholar] [CrossRef]
- Von Ragué Schleyer, P.; Manoharan, M.; Wang, Z.X.; Kiran, B.; Jiao, H.; Puchta, R.; Van Eikema Hommes, N.J.R. Dissected Nucleus-Independent Chemical Shift Analysis of π-Aromaticity and Antiaromaticity. Org. Lett. 2001, 3, 2465–2468. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Cyrański, M.K.; Czarnocki, Z.; Häfelinger, G.; Katritzky, A.R. Aromaticity: A Theoretical Concept of Immense Practical Importance. Tetrahedron 2000, 56, 1783–1796. [Google Scholar] [CrossRef]
- Rashid, Z.; Van Lenthe, J.H.; Havenith, R.W.A. Resonance and aromaticity: An ab initio valence bond approach. J. Phys. Chem. A 2012, 116, 4778–4788. [Google Scholar] [CrossRef]
- Pauling, L. The nature of the chemical bond. IV. The energy of single bonds and the relative electronegativity of atoms. J. Am. Chem. Soc. 1932, 54, 3570–3582. [Google Scholar] [CrossRef]
- Schleyer, P.V.R.; Jiao, H. What is aromaticity? Pure Appl. Chem. 1996, 68, 209–218. [Google Scholar] [CrossRef]
- Chen, Z.; Wannere, C.S.; Corminboeuf, C.; Puchta, R.; Von, P.; von Ragué Schleyer, P. Nucleus-Independent Chemical Shifts (NICS) as an Aromaticity Criterion. Chem. Rev. 2005, 105, 3842–3888. [Google Scholar] [CrossRef]
- Geuenich, D.; Hess, K.; Köhler, F.; Herges, R. Anisotropy of the Induced Current Density (ACID), a General Method to Quantify and Visualize Electronic Delocalization. Chem. Rev. 2005, 105, 3758–3772. [Google Scholar] [CrossRef] [PubMed]
- Klod, S.; Kleinpeter, E. Ab Initio Calculation of the Anisotropy Effect of Multiple Bonds and the Ring Current Effect of Arenes—Application in Conformational and Configurational Analysis. J. Chem. Soc. Perkin Trans. 2 2001, 1, 1893–1898. [Google Scholar] [CrossRef]
- Fliegl, H.; Taubert, S.; Lehtonen, O.; Sundholm, D. The Gauge Including Magnetically Induced Current Method. Phys. Chem. Chem. Phys. 2011, 13, 20500–20518. [Google Scholar] [CrossRef]
- Kruszewski, J.; Krygowski, T.M. Definition of Aromaticity Basing on the Harmonic Oscillator Model. Tetrahedron Lett. 1972, 13, 3839–3842. [Google Scholar] [CrossRef]
- Dobrowolski, J.C.; Ostrowski, S. The Form of the HOMA Geometric Aromaticity Index Is Universal and Allows the Design of Electronic and Magnetic Descriptors. J. Org. Chem. 2025, 90, 8674–8686. [Google Scholar] [CrossRef]
- Arpa, E.M.; Durbeej, B. HOMER: A Reparameterization of the Harmonic Oscillator Model of Aromaticity (HOMA) for Excited States. Phys. Chem. Chem. Phys. 2023, 25, 16763–16771. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kertesz, M. Bond Length Alternation and Energy Band Gap of Polyyne. J. Phys. Chem. 2006, 110, 9771–9774. [Google Scholar] [CrossRef]
- Bird, C.W. A New Aromaticity Index and Its Application to Five-Membered Ring Heterocycles. Tetrahedron 1985, 41, 1409–1414. [Google Scholar] [CrossRef]
- Güell, M.; Matito, E.; Luis, J.M.; Poater, J.; Solà, M. Analysis of Electron Delocalization in Aromatic Systems: Individual Molecular Orbital Contributions to Para-Delocalization Indexes (PDI). J. Phys. Chem. A 2006, 110, 11569–11574. [Google Scholar] [CrossRef]
- Poater, J.; Fradera, X.; Duran, M.; Solà, M. The Delocalization Index as an Electronic Aromaticity Criterion: Application to a Series of Planar Polycyclic Aromatic Hydrocarbons. Chem. A Eur. J. 2003, 9, 400–406. [Google Scholar] [CrossRef]
- Matito, E.; Duran, M.; Solà, M. The Aromatic Fluctuation Index (FLU): A New Aromaticity Index Based on Electron Delocalization. J. Chem. Phys. 2005, 122, 014109. [Google Scholar] [CrossRef] [PubMed]
- Szczepanik, D.W.; Andrzejak, M.; Dyduch, K.; Żak, E.; Makowski, M.; Mazur, G.; Mrozek, J. A Uniform Approach to the Description of Multicenter Bonding. Phys. Chem. Chem. Phys. 2014, 16, 20514–20523. [Google Scholar] [CrossRef] [PubMed]
- Schmider, H.L.; Becke, A.D. Chemical Content of the Kinetic Energy Density. J. Mol. Struct. THEOCHEM 2000, 527, 51–61. [Google Scholar] [CrossRef]
- Giambiagi, M.; De Giambiagi, M.S.; dos Santos Silva, C.D.; De Figueiredo, A.P. Multicenter bond indices as a measure of aromaticity. Phys. Chem. Chem. Phys. 2000, 2, 3381–3392. [Google Scholar] [CrossRef]
- Bultinck, P.; Ponec, R.; Van Damme, S. Multicenter bond indices as a new measure of aromaticity in polycy-clic aromatic hydrocarbons. J. Phys. Org. Chem. 2005, 18, 706–718. [Google Scholar] [CrossRef]
- Becke, A.D.; Edgecombe, K.E. A simple measure of electron localization in atomic and molecular systems. J. Chem. Phys. 1990, 92, 5397–5403. [Google Scholar] [CrossRef]
- Barbon, S.M.; Staroverov, V.N.; Gilroy, J.B. Effect of Extended π Conjugation on the Spectroscopic and Electrochemical Properties of Boron Difluoride Formazanate Complexes. J. Org. Chem. 2015, 80, 5226–5235. [Google Scholar] [CrossRef]
- Woller, T.; Geerlings, P.; De Proft, F.; Champagne, B.t.; Alonso, M. Aromaticity as a Guiding Concept for Spectroscopic Features and Nonlinear Optical Properties of Porphyrinoids. Molecules 2018, 23, 1333. [Google Scholar] [CrossRef]
- Setiawan, D.; Kraka, E.; Cremer, D. Quantitative Assessment of Aromaticity and Antiaromaticity Utilizing Vibrational Spectroscopy. J. Org. Chem. 2016, 81, 9669–9686. [Google Scholar] [CrossRef]
- Tran Ngoc, T.; Grabicki, N.; Irran, E.; Dumele, O.; Teichert, J.F. Photoswitching Neutral Homoaromatic Hydrocarbons. Nat. Chem. 2023, 15, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Alonso, M.; Herradón, B. Substituent Effects on the Aromaticity of Carbocyclic Five-Membered Rings. Phys. Chem. Chem. Phys. 2010, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Wieczorkiewicz, P.A.; Zborowski, K.K.; Krygowski, T.M.; Szatylowicz, H. Substituent Effect versus Aromaticity─A Curious Case of Fulvene Derivatives. J. Org. Chem. 2023, 88, 14775–14780. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Ejsmont, K.; Stepień, B.T.; Cyrański, M.K.; Poater, J.; Solà, M. Relation between the Substituent Effect and Aromaticity. J. Org. Chem. 2004, 69, 6634–6640. [Google Scholar] [CrossRef]
- Szatylowicz, H.; Jezuita, A.; Krygowski, T.M. On the Relations between Aromaticity and Substituent Effect. Struct. Chem. 2019, 30, 1529–1548. [Google Scholar] [CrossRef]
- Maksić, Z.B.; Kovačević, B.; Lesar, A. Protonation of Archetypal Aromatic and Antiaromatic Systems—G2 Studies of Benzene and Cyclobutadiene. Chem. Phys. 2000, 253, 59–71. [Google Scholar] [CrossRef]
- Zhu, Q.; Chen, S.; Chen, D.; Lin, L.; Xiao, K.; Zhao, L.; Solà, M.; Zhu, J. The Application of Aromaticity and Antiaromaticity to Reaction Mechanisms. Fundam. Res. 2023, 3, 926–938. [Google Scholar] [CrossRef]
- Solà, M.; Boldyrev, A.I.; Cyranski, M.K.; Krygowski, T.M.; Merino, G. Aromaticity and Antiaromaticity: Concepts and Applications; John Wiley & Sons, Ltd.: New York, NY, USA, 2022. [Google Scholar] [CrossRef]
- Krygowski, T.M.; Stȩpień, B.T. Sigma- and Pi-Electron Delocalization: Focus on Substituent Effects. Chem. Rev. 2005, 105, 3482–3512. [Google Scholar] [CrossRef]
- Ajami, D.; Oeckler, O.; Simon, A.; Herges, R. Synthesis of a Möbius Aromatic Hydrocarbon. Nature 2003, 426, 819–821. [Google Scholar] [CrossRef]
- Boldyrev, A.I.; Wang, L.S. All-Metal Aromaticity and Antiaromaticity. Chem. Rev. 2005, 105, 3716–3757. [Google Scholar] [CrossRef]
- Zubarev, D.Y.; Averkiev, B.B.; Zhai, H.J.; Wang, L.S.; Boldyrev, A.I. Aromaticity and Antiaromaticity in Transition-Metal Systems. Phys. Chem. Chem. Phys. 2008, 10, 257–267. [Google Scholar] [CrossRef] [PubMed]
- Gershoni-Poranne, R.; Stanger, A. Magnetic Criteria of Aromaticity. Chem. Soc. Rev. 2015, 44, 6597–6615. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, G.M.A.; Dos Santos, H.F. Theoretical Study of the Solvent Effect on the Aromaticity of Benzene: A NICS Analysis. J. Mol. Model. 2014, 20, 2152. [Google Scholar] [CrossRef]
- Han, Z.; Yu, X.; Sang, Y.; Xu, Y.; Zhao, A.; Lu, X. Aromaticity-Enhanced PH-Responsive Electrochemiluminescence of Cyclopentadienols. Anal. Chem. 2022, 94, 6036–6043. [Google Scholar] [CrossRef] [PubMed]
- Baird, N.C. Quantum Organic Photochemistry. II. Resonance and Aromaticity in the Lowest 3.Pi.Pi.* State of Cyclic Hydrocarbons. J. Am. Chem. Soc. 1972, 94, 4941–4948. [Google Scholar] [CrossRef]
- Rosenberg, M.; Dahlstrand, C.; Kilså, K.; Ottosson, H. Excited State Aromaticity and Antiaromaticity: Opportunities for Photophysical and Photochemical Rationalizations. Chem. Rev. 2014, 114, 5379–5425. [Google Scholar] [CrossRef]
- Barder, T.E.; Walker, S.D.; Martinelli, J.R.; Buchwald, S.L. Catalysts for Suzuki-Miyaura Coupling Processes: Scope and Studies of the Effect of Ligand Structure. J. Am. Chem. Soc. 2005, 127, 4685–4696. [Google Scholar] [CrossRef]
- Doherty, S.; Knight, J.G.; Smyth, C.H.; Jorgenson, G.A. Electron-Rich, Bicyclic Biaryl-like KITPHOS Monophosphines via [4+2] Cycloaddition between 1-Alkynylphosphine Oxides and Anthracene: Highly Efficient Ligands for Palladium-Catalysed C-N and C-C Bond Formation. Adv. Synth. Catal. 2008, 350, 1801–1806. [Google Scholar] [CrossRef]
- Tang, W.; Capacci, A.G.; Wei, X.; Li, W.; White, A.; Patel, N.D.; Savoie, J.; Gao, J.J.; Rodriguez, S.; Qu, B.; et al. A General and Special Catalyst for Suzuki-Miyaura Coupling Processes. Angew. Chemie Int. Ed. 2010, 49, 5879–5883. [Google Scholar] [CrossRef]
- Walker, S.D.; Barder, T.E.; Martinelli, J.R.; Buchwald, S.L. A Rationally Designed Universal Catalyst for Suzuki-Miyaura Coupling Processes. Angew. Chemie Int. Ed. 2004, 43, 1871–1876. [Google Scholar] [CrossRef]
- Deangelis, A.J.; Gildner, P.G.; Chow, R.; Colacot, T.J. Generating Active L-Pd(0) via Neutral or Cationic π-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphosphines: Synthetic, Mechanistic, and Structure-Activity Studies in Challenging Cross-Coupling Reactions. J. Org. Chem. 2015, 80, 6794–6813. [Google Scholar] [CrossRef]
- Biscoe, M.R.; Fors, B.P.; Buchwald, S.L. A New Class of Easily Activated Palladium Precatalysts for Facile C-N Cross-Coupling Reactions and the Low Temperature Oxidative Addition of Aryl Chlorides. J. Am. Chem. Soc. 2008, 130, 6686–6687. [Google Scholar] [CrossRef]
- Singh, C.; Rathod, J.; Jha, V.; Panossian, A.; Kumar, P.; Leroux, F.R. Modular Synthesis of Biaryl-Substituted Phosphine Ligands: Application in Microwave-Assisted Palladium-Catalyzed C-N Cross-Coupling Reactions. European J. Org. Chem. 2015, 2015, 6515–6525. [Google Scholar] [CrossRef]
- Dooleweerdt, K.; Fors, B.P.; Buchwald, S.L. Pd-Catalyzed Cross-Coupling Reactions of Amides and Aryl Mesylates. Org. Lett. 2010, 12, 2350–2353. [Google Scholar] [CrossRef]
- Coffey, S.B.; Bernhardson, D.J.; Wright, S.W. Synthesis and Characterization of an IsopropylBippyPhos Precatalyst. Tetrahedron 2022, 104, 132597. [Google Scholar] [CrossRef]
- Kapłon, K.; Frynas, S.; Mirosław, B.; Lipkowski, J.; Demchuk, O.M. An Efficient Asymmetric Cross-Coupling Reaction in Aqueous Media Mediated by Chiral Chelating Mono Phosphane Atropisomeric Biaryl Ligand. Catalysts 2023, 13, 353. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Yoruk, B.; Blackburn, T.; Snieckus, V. A Mixed Naphthyl-Phenyl Phosphine Ligand Motif for Suzuki, Heck, and Hydrodehalogenation Reactions. Synlett 2006, 2006, 2908–2913. [Google Scholar] [CrossRef]
- Ikawa, T.; Barder, T.E.; Biscoe, M.R.; Buchwald, S.L. Pd-Catalyzed Amidations of Aryl Chlorides Using Monodentate Biaryl Phosphine Ligands: A Kinetic, Computational, and Synthetic Investigation. J. Am. Chem. Soc. 2007, 129, 13001–13007. [Google Scholar] [CrossRef] [PubMed]
- Pratap, R.; Parrish, D.; Gunda, P.; Venkataraman, D.; Lakshman, M.K. Influence of Biaryl Phosphine Structure on C-N and C-C Bond Formation. J. Am. Chem. Soc. 2009, 131, 12240–12249. [Google Scholar] [CrossRef]
- Fowler, P.W.; Anstöter, C.S. Tuning (Anti)Aromaticity: Variations on the [8]-Circulene Framework. ChemPhysChem 2024, 25, e202300791. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, L.G.F.; Chagas, J.C.V.; Ferrão, L.F.A.; Aquino, A.J.A.; Nieman, R.; Lischka, H.; Machado, F.B.C. Tuning Aromaticity, Stability and Radicaloid Character of Periacenes by Chemical BN Doping. J. Comput. Chem. 2025, 46, e70039. [Google Scholar] [CrossRef] [PubMed]
- Plasser, F. Exploitation of Baird Aromaticity and Clar’s Rule for Tuning the Triplet Energies of Polycyclic Aromatic Hydrocarbons. Chem. 2021, 3, 532–549. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Justyniak, I.; Miroslaw, B.; Jasinski, R. 2-Methoxynaphthylnaphthoquinone and Its Solvate: Synthesis and Structure-Properties Relationship. J. Phys. Org. Chem. 2014, 27, 66–73. [Google Scholar] [CrossRef]
- Dudek, W.M.; Ostrowski, S.; Dobrowolski, J.C. On Aromaticity of the Aromatic α-Amino Acids and Tuning of the NICS Indices to Find the Aromaticity Order. J. Phys. Chem. A 2022, 126, 3433–3444. [Google Scholar] [CrossRef]
- Milner, P.J.; Maimone, T.J.; Su, M.; Chen, J.; Müller, P.; Buchwald, S.L. Investigating the Dearomative Rearrangement of Biaryl Phosphine-Ligated Pd(II) Complexes. J. Am. Chem. Soc. 2012, 134, 19922–19934. [Google Scholar] [CrossRef]
- Miroslaw, B.; Dybala, I.; Jasiński, R.; Demchuk, O.M. A Single Biaryl Monophosphine Ligand Motif—The Multiverse of Coordination Modes. Inorganics 2023, 11, 399. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Kapłon, K.; Mazur, L.; Strzelecka, D.; Pietrusiewicz, K.M. Readily Available Catalysts for Demanding Suzuki–Miyaura Couplings under Mild Conditions. Tetrahedron 2016, 72, 6668–6677. [Google Scholar] [CrossRef]
- Allman, T.; Goel, R.G. The Basicity of Phosphines. Can. J. Chem. 1982, 60, 716–722. [Google Scholar] [CrossRef]
- Demchuk, O.M.; Kielar, K.; Pietrusiewicz, K.M. Rational Design of Novel Ligands for Environmentally Benign Cross-Coupling Reactions. Pure Appl. Chem. 2011, 83, 633–644. [Google Scholar] [CrossRef]
- Krygowski, T.M. Crystallographic Studies of Inter- and Intramolecular Interactions Reflected in Aromatic Character of Pi.-Electron Systems. J. Chem. Inf. Model. 1993, 33, 70–78. [Google Scholar] [CrossRef]
- Wang, Z. py.Aroma: An Intuitive Graphical User Interface for Diverse Aromaticity Analyses. Chemistry 2024, 6, 1692–1703. [Google Scholar] [CrossRef]
- Tao, J.; Perdew, J.P.; Staroverov, V.N.; Scuseria, G.E. Climbing the Density Functional Ladder: Nonempirical Meta–Generalized Gradient Approximation Designed for Molecules and Solids. Phys. Rev. Lett. 2003, 91, 146401. [Google Scholar] [CrossRef]
- Schleyer, P.v.R.; Jiao, H.; Hommes, N.J.R.v.E.; Malkin, V.G.; Malkina, O.L. An Evaluation of the Aromaticity of Inorganic Rings: Refined Evidence from Magnetic Properties. J. Am. Chem. Soc. 1997, 119, 12669–12670. [Google Scholar] [CrossRef]
- Vollmer, W.; Blanot, D.; De Pedro, M.A. Peptidoglycan Structure and Architecture. FEMS Microbiol. Rev. 2008, 32, 149–167. [Google Scholar] [CrossRef]
- Koebnik, R.; Locher, K.P.; Van Gelder, P. Structure and Function of Bacterial Outer Membrane Proteins: Barrels in a Nutshell. Mol. Microbiol. 2000, 37, 239–253. [Google Scholar] [CrossRef] [PubMed]
- Kleanthous, C.; Armitage, J.P. The Bacterial Cell Envelope. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20150019. [Google Scholar] [CrossRef] [PubMed]
- Janowski, M.; Janowska, S.; Andrzejczuk, S.; Kosikowska, U.; Jasiński, R.; Mirosław, B.; Feldo, M.; Wujec, M.; Demchuk, O.M. New 4-(Morpholin-4-Yl)-3-Nitrobenzhydrazide Based Scaffold: Synthesis, Structural Insights, and Biological Evaluation. Molecules 2025, 30, 3343. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spackman, P.R.; Turner, M.J.; McKinnon, J.J.; Wolff, S.K.; Grimwood, D.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer: A Program for Hirshfeld Surface Analysis, Visualization and Quantitative Analysis of Molecular Crystals. J. Appl. Crystallogr. 2021, 54, 1006–1011. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09, Revision A.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA Quantum Chemistry Program Package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced Basis Sets of Split Valence, Triple Zeta Valence and Quadruple Zeta Valence Quality for H to Rn: Design and Assessment of Accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef] [PubMed]
- Weigend, F. Accurate Coulomb-Fitting Basis Sets for H to Rn. Phys. Chem. Chem. Phys. 2006, 8, 1057–1065. [Google Scholar] [CrossRef] [PubMed]
- Łaska, G.; Sieniawska, E.; Świątek, Ł.; Czapiński, J.; Rivero-Müller, A.; Kiercul, S.; Tekwani, B.L.; Pasco, D.S.; Balachandran, P. Evaluating the Impact of Xanthoparmelia conspersa Extracts on Signaling in HeLa Cells and Exploring Their Diverse Biological Activities. Sci. Rep. 2024, 14, 28531. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing (EUCAST). Break-Point Tables for Interpretation of MICs and Zone Diameters. Version 15.0.2025. Available online: https://www.eucast.org/ (accessed on 1 May 2025).
| Modulation Method | Effect on Aromaticity | Ref. |
|---|---|---|
| Substituent effects | π-Density is modulated by electron-donating (lowering) or -withdrawing groups (increasing of aromaticity). | [33,34,35,36] |
| Protonation/ deprotonation | Changes π-electron count, affects Hückel/Möbius character. | [37,38] |
| Redox modulation | Alters number of π-electrons. | [12] |
| Structural constraints | Strain, ring size or planarity influence electron delocalization. | [39] |
| Heteroatom incorporation | Heteroatoms modulate π-electron count, delocalization, and aromatic stabilization via lone pair donation or withdrawal. | [40] |
| Ring size and fusion | Ring size affects planarity and π-delocalization; fusion alters local versus global aromaticity. | [41] |
| Metal coordination | Metal centers alter electron density, affecting aromaticity. | [42,43] |
| Solvent/pH effects | Environmental factors influence tautomerism or charge states. | [44,45,46] |
| Excited states (Baird’s rule) | Reverses aromatic or antiaromatic behavior in triplet states. | [47,48] |
| Compound | CC50 (µg/mL) * | |
|---|---|---|
| VERO | H1HeLa | |
| 1 | 9.82 ± 0.59 | 6.78 ± 0.27 |
| 2 | 7.78 ± 0.44 | 5.92 ± 0.40 |
| 3 | 3.11 ± 0.14 | 3.83 ± 0.33 |
| 4 | 4.70 ± 0.41 | 5.53 ± 0.12 |
| 5 | 89.88 ± 4.11 | 200.85 ± 13.08 |
| 1 | 2 | 3 | 4 | 5 | Amox | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Bacterial Strain | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| S. aureus ATCC 29213 | 15.63–31.25 | >1000 | 7.82–15.63 | >1000 | 62.5–125 | >1000 | 250 | >1000 | 125–250 | >1000 | 0.98 | 0.98 |
| S. epidermidis ATCC 12228 | 15.63–31.25 | 250 | 3.91–7.89 | >1000 | 31.25 | >1000 | 500 | >1000 | 31.25–62.5 | 1000 | 31.25 | 31.25 |
| M. luteus ATCC 10240 | 1.95–3.91 | 125 | 1.95 | >1000 | 0.98 | >1000 | 15.63–31.25 | >1000 | 1.95–3.51 | 125 | 31.25 | 31.25 |
| E. coli ATCC 25922 | 500–1000 | nd | >1000 | nd | 250–500 | >1000 | 500–1000 | nd | >1000 | nd | 3.91 | 7.81 |
| K. pneumoniae ATCC 13883 | >1000 | nd | >1000 | nd | 500 | nd | 500 | nd | >1000 | nd | 250 | 250 |
| P. mirabilis ATCC 12453 | >1000 | nd | >1000 | nd | >1000 | nd | 1000 | nd | >1000 | nd | 0.49 | 0.49 |
| A. baumannii ATCC 19606 | 1000/1000 | nd | 500/500 | nd | >1000 | nd | 1000 | nd | 1000 | nd | 125 | 125 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miroslaw, B.; Rejmak, P.; Dybala, I.; Kosikowska, U.; Andrzejczuk, S.; Świątek, Ł.; Salwa, K.; Demchuk, O.M. Aromaticity Tuning in Biaryl Monophosphines and Their Derivatives. Molecules 2025, 30, 4018. https://doi.org/10.3390/molecules30194018
Miroslaw B, Rejmak P, Dybala I, Kosikowska U, Andrzejczuk S, Świątek Ł, Salwa K, Demchuk OM. Aromaticity Tuning in Biaryl Monophosphines and Their Derivatives. Molecules. 2025; 30(19):4018. https://doi.org/10.3390/molecules30194018
Chicago/Turabian StyleMiroslaw, Barbara, Pawel Rejmak, Izabela Dybala, Urszula Kosikowska, Sylwia Andrzejczuk, Łukasz Świątek, Kinga Salwa, and Oleg M. Demchuk. 2025. "Aromaticity Tuning in Biaryl Monophosphines and Their Derivatives" Molecules 30, no. 19: 4018. https://doi.org/10.3390/molecules30194018
APA StyleMiroslaw, B., Rejmak, P., Dybala, I., Kosikowska, U., Andrzejczuk, S., Świątek, Ł., Salwa, K., & Demchuk, O. M. (2025). Aromaticity Tuning in Biaryl Monophosphines and Their Derivatives. Molecules, 30(19), 4018. https://doi.org/10.3390/molecules30194018










