The Shift to Bio-Based Auxiliaries in Textile Wet Processing: Recent Advances and Industrial Potential
Abstract
1. Introduction
2. Enzymes
2.1. Desizing
2.2. Scouring
2.3. Bleaching
2.4. Dyeing
2.5. Finishing
2.6. Optimising Enzymatic Treatments
2.6.1. Combined Enzyme Systems and One-Bath Solutions
2.6.2. Immobilised Enzyme Systems
3. Biopolymers
3.1. Pre-Treatment
3.2. Printing
3.3. Finishing
4. Bio-Based Colourants and Functional Agents
4.1. Natural Dyeing
4.1.1. Plant and Agrowaste-Based Colouring Agents
4.1.2. Microbial-Based Colouring Agents
4.2. Functional Finishing
4.2.1. Plant- and Agrowaste-Based Agents
4.2.2. Microbial-Based Agents
5. Conclusions and Future Directions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| AM | Antimicrobial efficacy |
| Aox | Antioxidant activity |
| BOD | Biochemical oxygen demand |
| CJ | Jute |
| CMC | Carboxymethyl cellulose |
| CMG | Carboxymethyl guar gum |
| CNC | Cellulose nanocrystals |
| CO | Cotton |
| COD | Chemical oxygen demand |
| CYT | Cytotoxicity |
| GMO | Genetically modified organisms |
| owf | On weight of fabric |
| K/S | Colour strength |
| MR | Mosquito repellency |
| PA | Polyamide |
| PES | Polyester |
| rPES | Recycled polyester |
| RT | Room temperature |
| SK | Silk |
| TDS | Total dissolved solids |
| UPF | UV protection factor |
| UV | Ultraviolet |
| WO | Wool |
References
- Lara, L.; Cabral, I.; Cunha, J. Ecological Approaches to Textile Dyeing: A Review. Sustainability 2022, 14, 8353. [Google Scholar] [CrossRef]
- Sözen, S.; Dulkadiroglu, H.; Begum Yucel, A.; Insel, G.; Orhon, D. Pollutant Footprint Analysis for Wastewater Management in Textile Dye Houses Processing Different Fabrics. J. Chem. Technol. Biotechnol. 2019, 94, 1330–1340. [Google Scholar] [CrossRef]
- Catarino, M.L.; Sampaio, F.; Gonçalves, A.L. Sustainable Wet Processing Technologies for the Textile Industry: A Comprehensive Review. Sustainability 2025, 17, 3041. [Google Scholar] [CrossRef]
- Ragab, M.; Othman, H.; Hassabo, A. Various Extraction Methods of Different Enzymes and Their Potential Applications in Various Industrial Sector (a Review). Egypt J. Chem. 2022, 65, 495–508. [Google Scholar] [CrossRef]
- Sen, A.; Kapila, R.; Chaudhary, S.; Nigam, A. Biotechnological Applications of Microbial Enzymes to Replace Chemicals in the Textile Industry—A Review. J. Text. Assoc. 2021, 82, 68–72. [Google Scholar]
- Stanescu, M.D. Applications of Enzymes in Processing Cellulosic Textiles—A Review of the Latest Developments. Cellul. Chem. Technol. 2023, 57, 1–15. [Google Scholar] [CrossRef]
- Kabir, S.M.F.; Chakraborty, S.; Hoque, S.M.A.; Mathur, K. Sustainability Assessment of Cotton-Based Textile Wet Processing. Clean Technol. 2019, 1, 232–246. [Google Scholar] [CrossRef]
- Kabir, S.M.M.; Koh, J. Sustainable Textile Processing by Enzyme Applications. In Biodegradation Technology of Organic and Inorganic Pollutants; Mendes, K.F., De Sousa, R., Mielke, K.C., Eds.; IntechOpen: London, UK, 2022. [Google Scholar]
- Hoque, M.T.; Mazumder, N.; Islam, M.T. Enzymatic Wet Processing. In Sustainable Practices in the Textile Industry; Wiley: Hoboken, NJ, USA, 2021; pp. 87–110. [Google Scholar]
- Eyupoglu, S.; Merdan, N. Eco-Friendly Production Methods in Textile Wet Processes. In Sustainable Innovations in Textile Chemical Processes; Springer Nature: Singapore, 2018; pp. 31–65. [Google Scholar]
- Choudhury, R.; Kumar, A. Enzyme Applications in Textile Chemical Processing. In Sustainable Technologies for Fashion and Textiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 91–115. [Google Scholar]
- Kumar, D.; Bhardwaj, R.; Jassal, S.; Goyal, T.; Khullar, A.; Gupta, N. Application of Enzymes for an Eco-Friendly Approach to Textile Processing. Environ. Sci. Pollut. Res. 2021, 30, 71838–71848. [Google Scholar] [CrossRef]
- Tochetto, G.A.; Aragão, A.M.I.; de Oliveira, D.; Immich, A.P.S. Can Enzymatic Processes Transform Textile Processes? A Critical Analysis of the Industrial Application. Process Biochem. 2022, 123, 27–35. [Google Scholar] [CrossRef]
- Zhang, X.; Baek, N.; Xu, J.; Yuan, J.; Fan, X. Differences in the Desizability of Starches and the Mechanism of Inhibiting Desizing. Text. Res. J. 2022, 92, 4789–4798. [Google Scholar] [CrossRef]
- Colombi, B.L.; De Cássia Siqueira Curto Valle, R.; Borges Valle, J.A.; Andreaus, J. Advances in Sustainable Enzymatic Scouring of Cotton Textiles: Evaluation of Different Post-Treatments to Improve Fabric Wettability. Clean. Eng. Technol. 2021, 4, 100160. [Google Scholar] [CrossRef]
- Prajapati, C.D.; Smith, E.; Kane, F.; Shen, J. Laccase-catalysed Coloration of Wool and Nylon. Color. Technol. 2018, 134, 423–439. [Google Scholar] [CrossRef]
- Bai, R.; Yu, Y.; Wang, Q.; Shen, J.; Yuan, J.; Fan, X. Chitosan-templated Bio-coloration of Cotton Fabrics via Laccase-catalyzed Polymerization of Hydroquinone. Eng. Life Sci. 2019, 19, 643–654. [Google Scholar] [CrossRef] [PubMed]
- Atav, R.; Buğdaycı, B.; Bozkurt, Ö.; Yıldız, A.; Güneş, E.; Yakın, İ. Laccase-Catalyzed Enzymatic Dyeing of Cotton Fabrics. Text. Res. J. 2022, 92, 2980–3015. [Google Scholar] [CrossRef]
- Polak, J.; Wlizło, K.; Pogni, R.; Petricci, E.; Grąz, M.; Szałapata, K.; Osińska-Jaroszuk, M.; Kapral-Piotrowska, J.; Pawlikowska-Pawlęga, B.; Jarosz-Wilkołazka, A. Structure and Bioactive Properties of Novel Textile Dyes Synthesised by Fungal Laccase. Int. J. Mol. Sci. 2020, 21, 2052. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, C.; Gu, M.; Qin, S.; Yin, J.; Wang, X.; Zhang, W. Laccase-Catalyzed the Polymerization of Portulaca Oleracea L. Extract as a Precursor for Ecological Dyeing of Pure Cotton Fabric. Fibers Polym. 2024, 25, 3005–3019. [Google Scholar] [CrossRef]
- Atav, R.; Buğdaycı, B.; Yakın, İ. Laccase-Catalyzed Simultaneous Dye Synthesis and Cotton Dyeing by Using Plant Extracts as Dye Precursor. J. Text. Inst. 2022, 113, 628–636. [Google Scholar] [CrossRef]
- Koksharov, S.A.; Bikbulatova, A.A.; Kornilova, N.L.; Aleeva, S.V.; Lepilova, O.V.; Nikiforova, E.N. Justification of an Approach to Cellulase Application in Enzymatic Softening of Linen Fabrics and Clothing. Text. Res. J. 2022, 92, 4208–4229. [Google Scholar] [CrossRef]
- Li, W.; Zhang, N.; Wang, Q.; Wang, P.; Yu, Y.; Zhou, M. A Sustainable and Effective Bioprocessing Approach for Improving Anti-Felting, Anti-Pilling and Dyeing Properties of Wool Fabric. Fibers Polym. 2021, 22, 3045–3054. [Google Scholar] [CrossRef]
- Popescu, C.; Stanescu, M.D. Eco-Friendly Processing of Wool and Sustainable Valorization of This Natural Bioresource. Sustainability 2024, 16, 4661. [Google Scholar] [CrossRef]
- Zhang, N.; Huang, P.; Wang, P.; Yu, Y.; Zhou, M.; Wang, Q. Combined Cutinase and Keratinolytic Enzyme to Endow Improved Shrink-Resistance to Wool Fabric. Fibers Polym. 2022, 23, 985–992. [Google Scholar] [CrossRef]
- Iglesias, M.S.; Sequeiros, C.; García, S.; Olivera, N.L. Eco-Friendly Anti-Felting Treatment of Wool Top Based on Biosurfactant and Enzymes. J. Clean. Prod. 2019, 220, 846–852. [Google Scholar] [CrossRef]
- Li, R.; Zhu, Q.; Zhou, M.; Xu, B.; Wang, P.; Wang, Q.; Yu, Y. Effective Removal of Scales from Wool Surface through the Combination of Thioglycolic Acid Compounds and Proteases for Anti-Felting Finishing. Int. J. Biol. Macromol. 2025, 306, 141698. [Google Scholar] [CrossRef]
- Li, B.; Li, J.; Shen, Y.; Wu, H.; Sun, Y.; Zhang, P.; Yang, M. Development of Environmentally Friendly Wool Shrink-Proof Finishing Technology Based on L-Cysteine/Protease Treatment Solution System. Int. J. Mol. Sci. 2022, 23, 13553. [Google Scholar] [CrossRef]
- Khan, M.K.R.; Jintun, S. Sustainability Issues of Various Denim Washing Methods. Text. Leather Rev. 2021, 4, 96–110. [Google Scholar] [CrossRef]
- Kakkar, P.; Wadhwa, N. Extremozymes Used in Textile Industry. J. Text. Inst. 2022, 113, 2007–2015. [Google Scholar] [CrossRef]
- Jajpura, L.; Nayak, R. Ultrasound Applications in Textiles and Apparels. In Sustainable Technologies for Fashion and Textiles; Elsevier: Amsterdam, The Netherlands, 2020; pp. 143–161. [Google Scholar]
- Kalia, S.; Bhattacharya, A.; Prajapati, S.K.; Malik, A. Utilization of Starch Effluent from a Textile Industry as a Fungal Growth Supplement for Enhanced α-Amylase Production for Industrial Application. Chemosphere 2021, 279, 130554. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Du, C.; Pensupa, N.; Lin, C.S.K. Optimisation of Fungal Cellulase Production from Textile Waste Using Experimental Design. Process Saf. Environ. Prot. 2018, 118, 133–142. [Google Scholar] [CrossRef]
- Ikbal, M.S.; Tisha, F.A.; Asheque, A.I.; Hasnat, E.; Uddin, M.A. Eco-Friendly Biopolishing of Cotton Fabric through Wasted Sugarcane Bagasse-Derived Enzymes. Heliyon 2024, 10, e26346. [Google Scholar] [CrossRef] [PubMed]
- Dash, A.K.; Sahoo, S.K. Role of Enzymes in Textile Processing. In Bioprospecting of Enzymes in Industry, Healthcare and Sustainable Environment; Springer: Singapore, 2021; pp. 395–410. [Google Scholar]
- Degani, O. Synergism between Cutinase and Pectinase in the Hydrolysis of Cotton Fibers’ Cuticle. Catalysts 2021, 11, 84. [Google Scholar] [CrossRef]
- Singh, A.; Varghese, L.M.; Battan, B.; Patra, A.K.; Mandhan, R.P.; Mahajan, R. Environmental Pollution Reducing Strategy for Scouring of Undegummed Sisal Fibers Using Xylanase and Pectinase Enzymes. Bioprocess Biosyst. Eng. 2021, 44, 607–615. [Google Scholar] [CrossRef]
- Patil, H.; Mudaliar, S.; Athalye, A. Ultrasound-assisted Enzymatic Scouring of Jute Optimised by Response Surface Methodology and Its Natural Dyeing. Color. Technol. 2023, 139, 97–108. [Google Scholar] [CrossRef]
- Islam, M.T.; Huda, S.Z.; Alam, M.S.; Sahariar, M.F. Single-Bath-Single-Stage Enzymatic Treatment of Denim. Results Eng. 2024, 21, 101944. [Google Scholar] [CrossRef]
- El-Fiky, A.F.; Khalil, E.M.; Mowafi, S.; Zaki, R.A.; El-Sayed, H. A Novel Approach towards Removal of Lipid Barrier from Wool Fibers’ Surface Using Thermophilic Lipase. J. Nat. Fibers 2022, 19, 9471–9485. [Google Scholar] [CrossRef]
- El Shehry, A.; Youssef, Y.; Ahmed, N.; Soliman, E.; Hashem, A. Optimization of Enzymatic Treatment and Reactive Dyeing of Viscose Fabric in One-Bath Process. Egypt. J. Chem. 2021, 65, 647–656. [Google Scholar] [CrossRef]
- Benli, H.; Bahtiyari, M.İ. Use of Ultrasound in Biopreparation and Natural Dyeing of Cotton Fabric in a Single Bath. Cellulose 2015, 22, 867–877. [Google Scholar] [CrossRef]
- Sankarraj, N.; Nallathambi, G. Effect of Biopolishing on Structural Degradation and Physical Properties of Cellulose. J. Serbian Chem. Soc. 2017, 82, 567–578. [Google Scholar] [CrossRef]
- Sankarraj, N.; Nallathambi, G. Enzymatic Biopolishing of Cotton Fabric with Free/Immobilized Cellulase. Carbohydr. Polym. 2018, 191, 95–102. [Google Scholar] [CrossRef]
- Taleb, M.A.; Gomaa, S.K.; Wahba, M.I.; Zaki, R.A.; El-Fiky, A.F.; El-Refai, H.A.; El-Sayed, H. Bioscouring of Wool Fibres Using Immobilized Thermophilic Lipase. Int. J. Biol. Macromol. 2022, 194, 800–810. [Google Scholar] [CrossRef]
- Madhu, A.; Chakraborty, J. Sustainable Approach for Cotton Fabric Pretreatment with Immobilized Enzymes. Fibers Polym. 2022, 23, 993–1007. [Google Scholar] [CrossRef]
- Edo, G.I.; Ndudi, W.; Ali, B.M.; Yousif, E.; Jikah, A.N.; Isoje, E.F.; Igbuku, U.A.; Mafe, A.N.; Opiti, R.A.; Madueke, C.J.; et al. Biopolymers: An Inclusive Review. Hybrid Adv. 2025, 9, 100418. [Google Scholar] [CrossRef]
- Baranwal, J.; Barse, B.; Fais, A.; Delogu, G.L.; Kumar, A. Biopolymer: A Sustainable Material for Food and Medical Applications. Polymer 2022, 14, 983. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Kamrul, H.; Hu, H. Poly(Lactic Acid) Fibers, Yarns and Fabrics: Manufacturing, Properties and Applications. Text. Res. J. 2021, 91, 1641–1669. [Google Scholar] [CrossRef]
- Daget, T.M.; Kassie, B.B.; Tassew, D.F. A Shift from Synthetic to Bio-Based Polymer for Functionalization of Textile Materials: A Review. Int. J. Biol. Macromol. 2025, 306, 141637. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.M.; Mucalo, M.R. Chitosan: A Review of Sources and Preparation Methods. Int J Biol Macromol 2021, 169, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Mahbub, M.A.; Mahmud, M.H.; Ahona, M.J.; Ahmed, T.; Ashraf, S.M.; Sultana, J.A.; Hasan, M.; Islam, M.T. Chitosan as a Cationizing Agent in Pigment Dyeing of Cotton Fabric. Carbohydr. Polym. Technol. Appl. 2024, 7, 100502. [Google Scholar] [CrossRef]
- Rehman, A.; Iqbal, K.; Azam, F.; Safdar, F.; Ashraf, M.; Maqsood, H.S.; Basit, A. To Enhance the Dyeability of Cotton Fiber with the Application of Reactive Dyes by Using Chitosan. J. Text. Inst. 2021, 112, 1208–1212. [Google Scholar] [CrossRef]
- Al-Amir, A.; Gomaa, A.H.; El-Azabawy, R.E.; El-Bayaa, A.A. Valorization Beetroot Waste for Eco-Friendly Extraction of Natural Dye for Textile and Food Applications. Egypt. J. Chem. 2022, 65, 725–736. [Google Scholar] [CrossRef]
- Liu, Q.; Zhang, Y.; Ma, Z.; Wang, Y.; Tian, Y.; Xu, H.; Hou, X. Improving the Dyeing Properties of Peanut Skin Extracts to Flax Fabrics by Chitosan Pretreatment. J. Nat. Fibers 2023, 20, 2229516. [Google Scholar] [CrossRef]
- Hong, K.H. Preparation and Properties of Cotton Fabrics Dyed by Aronia (Aronia melanocarpa) Extract and Chitosan. Fash. Text. 2023, 10, 14. [Google Scholar] [CrossRef]
- Rahman, M.M.; Kim, M.; Youm, K.; Kumar, S.; Koh, J.; Hong, K.H. Sustainable One-Bath Natural Dyeing of Cotton Fabric Using Turmeric Root Extract and Chitosan Biomordant. J. Clean. Prod. 2023, 382, 135303. [Google Scholar] [CrossRef]
- Verma, M.; Gahlot, N.; Singh, S.S.J.; Rose, N.M. UV Protection and Antibacterial Treatment of Cellulosic Fibre (Cotton) Using Chitosan and Onion Skin Dye. Carbohydr. Polym. 2021, 257, 117612. [Google Scholar] [CrossRef]
- Eser, C.O.; Yavas, A. Effect of Silk Sericin Pre-Treatment on Dyeability of Woollen Fabric. Ind. Textila 2021, 72, 203–209. [Google Scholar] [CrossRef]
- Ahmed, M.; Sukumar, N.; Yusuf, A.; Awol, Y. Cationisation of Cotton with Natural Source Based Gelatin for Salt-Free Reactive Dyeing of Cationised Cotton. J. Nat. Fibers 2022, 19, 15353–15366. [Google Scholar] [CrossRef]
- Majeed, H.; Iftikhar, T. Ecofriendly Reactive Printing of Cellulosic Fabric with Sustainable Novel Techniques. Cellulose 2024, 31, 7067–7081. [Google Scholar] [CrossRef]
- Ebrahim, S.A.; Othman, H.A.; Mosaad, M.M.; Hassabo, A.G. Eco-Friendly Natural Thickener (Pectin) Extracted from Fruit Peels for Valuable Utilization in Textile Printing as a Thickening Agent. Textiles 2023, 3, 26–49. [Google Scholar] [CrossRef]
- Hassabo, A.; Othman, H.; Ebrahim, S. Natural Thickener in Textile Printing (A Mini Review). J. Text. Color. Polym. Sci. 2021, 18, 55–64. [Google Scholar] [CrossRef]
- Khajeh Mehrizi, M.; Jokar, M.; Sadeghyan, T.; Azizi, M.E.; Rahmani, M.R. Modified Guar Gum: An Alternative Source for Printing of Cotton Fabric with Reactive Dye. Color. Technol. 2023, 139, 385–394. [Google Scholar] [CrossRef]
- Zhang, M.; Qiao, X.; Liu, X.; Fang, K.; Gong, J.; Lu, X.; Gao, W.; Si, J.; Sun, F. Environmental Urea-Free Pre-treatment Process to Form New Surface on Hemp for Enhancing the Inkjet Printing Performance. Prog. Org. Coat. 2023, 174, 107317. [Google Scholar] [CrossRef]
- Hassabo, A.; Mohamed, N.; Abd El-Salam, N.; Gouda, N.; Othman, H. Application of Modified Xanthan as Thickener in the Printing of Natural and Synthetic Fabrics. J. Text. Color. Polym. Sci. 2023, 20, 41–56. [Google Scholar] [CrossRef]
- Mongkholrattanasit, R.; Klaichoi, C.; Rungruangkitkrai, N.; Vuthiganond, N.; Nakpathom, M. Eco-Printing on Cotton Fabric with Natural Indigo Dye Using Wild Taro Corms as a New Thickening Agent. J. Nat. Fibers 2022, 19, 5435–5450. [Google Scholar] [CrossRef]
- Vastrad, J.V.; Kotur, R.S. Mango Kernel Starch: A Bio-Thickener for Natural Printing on Fabric. Pharma Innov. J. 2021, 10, 722–726. [Google Scholar]
- Zhu, F.; Chen, L.; Feng, Q. Waste Gelatin Based Layer by Layer Assembly for Sustainable Solution to Cotton Fabrics Flame Retardancy. Prog. Org. Coat. 2022, 163, 106688. [Google Scholar] [CrossRef]
- Solihat, N.N.; Purwanti, T.; Husna, N.; Oktaviani, M.; Zulfiana, D.; Fatriasari, W.; Nawawi, D.S. Capability Lignin from Acacia Crassicarpa Black Liquor as an Environmentally Benign Antibacterial Agent to Produce Antibacterial and Hydrophobic Textiles. Bioresour. Technol. 2024, 413, 131409. [Google Scholar] [CrossRef]
- Mosaad, R.M.; Alhalafi, M.H.; Emam, E.-A.M.; Ibrahim, M.A.; Ibrahim, H. Enhancement of Antimicrobial and Dyeing Properties of Cellulosic Fabrics via Chitosan Nanoparticles. Polymers 2022, 14, 4211. [Google Scholar] [CrossRef]
- Mostafa, K.; Ameen, H.; Morsy, M.; el-ebiassy, A.; El-Sanabary, A.; Adel, M.; Salah, A. Production of High-Performance Textiles via Pioneering Strengthening Approach Using Starch Nanoparticles. J. Ind. Text. 2020, 50, 278–292. [Google Scholar] [CrossRef]
- Juikar, S.J.; Nadanathangam, V. Microbial Production of Nanolignin from Cotton Stalks and Its Application onto Cotton and Linen Fabrics for Multifunctional Properties. Waste Biomass Valorization 2020, 11, 6073–6083. [Google Scholar] [CrossRef]
- Shukla, A.; Sharma, V.; Basak, S.; Ali, S.W. Sodium Lignin Sulfonate: A Bio-Macromolecule for Making Fire Retardant Cotton Fabric. Cellulose 2019, 26, 8191–8208. [Google Scholar] [CrossRef]
- Faheem, S.; Baheti, V.; Tunak, M.; Wiener, J.; Militky, J. Comparative Performance of Flame Retardancy, Physiological Comfort, and Durability of Cotton Textiles Treated with Alkaline and Acidic Casein Suspension. J. Ind. Text. 2019, 48, 969–991. [Google Scholar] [CrossRef]
- Daget, T.M.; Kassie, B.B.; Abate, M.T.; Teshome, M.F.; Arega, M.M.; Atalie, D. Eco-Friendly Flame-Retardant Finishing of Polyester Fabrics with Diammonium Hydrogen Phosphate, Gelatin, and Silica Gel for Cultural Heritage Preservation. J. Sol-Gel Sci. Technol. 2025, 114, 1095–1108. [Google Scholar] [CrossRef]
- Fang, Y.; Wu, J.; Chang, S.; Wu, L. Flame Retardant and Anti-Dripping Polyethylene Terephthalate Fabric Based on Bio-Based Phytic Acid/Gelatine Coating. Surf. Eng. 2023, 39, 49–55. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Multifunctional Linen Fabric Obtained through Finishing with Chitosan-Gelatin Microcapsules Loaded with Cinnamon Oil. J. Nat. Fibers 2022, 19, 4780–4790. [Google Scholar] [CrossRef]
- Singh, N.; Sheikh, J. Novel Chitosan-Gelatin Microcapsules Containing Rosemary Essential Oil for the Preparation of Bioactive and Protective Linen. Ind. Crops Prod. 2022, 178, 114549. [Google Scholar] [CrossRef]
- Zerin, I.; Farzana, N.; Sayem, A.S.M.; Anang, D.M.; Haider, J. Potentials of Natural Dyes for Textile Applications. In Encyclopedia of Renewable and Sustainable Materials; Elsevier: Amsterdam, The Netherlands, 2020; pp. 873–883. [Google Scholar]
- Datta, S.; Uddin, M.; Afreen, K.; Akter, S.; Bandyopadhyay, A. Assessment of Antimicrobial Effectiveness of Natural Dyed Fabrics. Bangladesh J. Sci. Ind. Res. 2013, 48, 179–184. [Google Scholar] [CrossRef]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of Synthetic Dyes from Textile Industries, Discharge Impacts and Treatment Methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Mansour, R.; Ben Ali, H. Investigating the Use of Chitosan: Toward Improving the Dyeability of Cotton Fabrics Dyed with Roselle (Hibiscus sabdariffa L.). J. Nat. Fibers 2021, 18, 1007–1016. [Google Scholar] [CrossRef]
- Ayele, M.; Tesfaye, T.; Alemu, D.; Limeneh, M.; Sithole, B. Natural Dyeing of Cotton Fabric with Extracts from Mango Tree: A Step towards Sustainable Dyeing. Sustain. Chem. Pharm. 2020, 17, 100293. [Google Scholar] [CrossRef]
- Repon, M.R.; Dev, B.; Rahman, M.A.; Jurkonienė, S.; Haji, A.; Alim, M.A.; Kumpikaitė, E. Textile Dyeing Using Natural Mordants and Dyes: A Review. Environ. Chem. Lett. 2024, 22, 1473–1520. [Google Scholar] [CrossRef]
- Iqbal, S.; Ansari, T.N. Extraction and Application of Natural Dyes. In Sustainable Practices in the Textile Industry; Wiley: Hoboken, NJ, USA, 2021; pp. 1–40. [Google Scholar]
- Hosseinnezhad, M.; Gharanjig, K.; Imani, H.; Razani, N. Green Dyeing of Wool Yarns with Yellow and Black Myrobalan Extract as Bio-Mordant with Natural Dyes. J. Nat. Fibers 2022, 19, 3893–3915. [Google Scholar] [CrossRef]
- Hossain, S.; Jalil, M.A.; Kamal, S.M.B.; Kader, A. A natural dye extracted from leaves of Mimusops elengi Linn and its dyeing properties on cotton and silk fabrics. J. Text. Inst. 2020, 122, 455–461. [Google Scholar] [CrossRef]
- Manicketh, T.J.; Francis, M.S.; Joseph, G. Extraction of Polygenetic Natural Dyes from Cordyline fruticosa and Mussaenda erythrophylla for Textile Substrates. Nat. Prod. Res. 2022, 36, 4040–4044. [Google Scholar] [CrossRef]
- Benli, H.; Bahtiyari, M.İ. An Approach for the Valorization of Bio-Waste Pistachio Shells (Pistacia vera L.): Dyeing of Cellulose-Based Fabrics. J. Clean. Prod. 2024, 445, 141213. [Google Scholar] [CrossRef]
- Ozuzu, S.A.; Fidelis, G.D.; Toshmatov, Z.O.; Kuchkarova, N.N.; Ochekwu, B.E.; Shao, H. Characterizing Dyeing Constituents from Walnut Green Husks: Utilization of a Harmful Agricultural by-Product as Natural Dye to Alleviate Its Environmental Pollution. Ind. Crops Prod. 2025, 223, 120172. [Google Scholar] [CrossRef]
- Yang, R.; Li, J.; Cheng, G.; Inta, A.; Yang, L. Textiles Dyeing with Pomegranate (Punica granatum) Peel Extract Using Natural Mordant. J. Nat. Fibers 2023, 20, 2282056. [Google Scholar] [CrossRef]
- Haji, A.; Shahmoradi Ghaheh, F.; Indrie, L. Pomegranate Fallen Leaves as a Source of Natural Dye for Mordant-free Dyeing of Wool. Color. Technol. 2023, 139, 165–170. [Google Scholar] [CrossRef]
- Popescu, V.; Blaga, A.C.; Pruneanu, M.; Cristian, I.N.; Pîslaru, M.; Popescu, A.; Rotaru, V.; Crețescu, I.; Cașcaval, D. Green Chemistry in the Extraction of Natural Dyes from Colored Food Waste, for Dyeing Protein Textile Materials. Polymers 2021, 13, 3867. [Google Scholar] [CrossRef]
- Fonseca, F.D.; Symochko, L.; Pinheiro, M.N.C. Grape Pomace (Vitis vinifera L.) Waste Valorization: Assessing Its Potential as a Sustainable Natural Dye for Textiles Applications. Sustainability 2024, 16, 3167. [Google Scholar] [CrossRef]
- Islam, M.R.; Khan, A.N.N.; Mahmud, R.U.; Haque, S.M.N.; Khan, M.M.I. Sustainable Dyeing of Jute-Cotton Union Fabrics with Onion Skin (Allium CEPA) Dye Using Banana Peel (Musa) and Guava Leaves (Psidium guajava) Extract as Biomordants. Pigment. Resin Technol. 2024, 53, 369–375. [Google Scholar] [CrossRef]
- Baig, U.; Khatri, A.; Ali, S.; Sanbhal, N.; Ishaque, F.; Junejo, N. Ultrasound-Assisted Dyeing of Cotton Fabric with Natural Dye Extracted from Marigold Flower. J. Text. Inst. 2021, 112, 801–808. [Google Scholar] [CrossRef]
- Bueno, A.M.; Hoffmann, T.G.; Krebs de Souza, C.; de Carvalho, L.F.; Bertoli, S.L.; Barcellos, I.O.; Gonçalves, M.J. Optimal Process Conditions to Recycled Polyester Dyeing Using Natural Annatto Dye. J. Clean. Prod. 2022, 370, 133497. [Google Scholar] [CrossRef]
- Atav, R.; Namırtı, O. An Ecofriendly Dyeing Method for Polyester Fibers: To Bring Traditional Natural Dyeing into Industrial Production. Fibers Polym. 2023, 24, 2027–2038. [Google Scholar] [CrossRef]
- Rehman, F.U.; Adeel, S.; Haddar, W.; Bibi, R.; Azeem, M.; Mia, R.; Ahmed, B. Microwave-Assisted Exploration of Yellow Natural Dyes for Nylon Fabric. Sustainability 2022, 14, 5599. [Google Scholar] [CrossRef]
- Sadannavar, M.K.; Dong, X.; Manj, R.Z.A.; Shafiq, F.; Irfan, M.; Hatamvand, M.; Zhao, T. Extraction of Natural Dye from Broccoli (Brassica oleracea) and Evaluation of Its Antimicrobial, Ultraviolet and Dyeing Properties on Cotton Fabrics. Cellulose 2024, 31, 9503–9522. [Google Scholar] [CrossRef]
- Kıcık, H.; Gökbulut, Ç. Investigation of the Dyeability of Cotton Fabrics with Bacterial Colorants. Tekst. Mühendis 2023, 30, 231–235. [Google Scholar] [CrossRef]
- Mazotto, A.M.; de Ramos Silva, J.; de Brito, L.A.A.; Rocha, N.U.; de Souza Soares, A. How Can Microbiology Help to Improve Sustainability in the Fashion Industry? Environ. Technol. Innov. 2021, 23, 101760. [Google Scholar] [CrossRef]
- Janković, V.; Marković, D.; Nikodinovic-Runic, J.; Radetić, M.; Ilic-Tomic, T. Eco-Friendly Dyeing of Polyamide and Polyamide-Elastane Knits with Living Bacterial Cultures of Two Streptomyces sp. Strains. World J. Microbiol. Biotechnol. 2023, 39, 32. [Google Scholar] [CrossRef]
- Kramar, A.; Kostic, M.M. Bacterial Secondary Metabolites as Biopigments for Textile Dyeing. Textiles 2022, 2, 252–264. [Google Scholar] [CrossRef]
- de Oliveira Barreto, J.V.; Casanova, L.M.; Junior, A.N.; Reis-Mansur, M.C.P.P.; Vermelho, A.B. Microbial Pigments: Major Groups and Industrial Applications. Microorganisms 2023, 11, 2920. [Google Scholar] [CrossRef]
- Hartvigsen, M.L.G.; Reea, V.E. Bacterial Colouring: Using Multi-Disciplinary Methods for Ecofriendly Textile Design. J. Int. Colour Assoc. 2022, 30, 10–23. [Google Scholar]
- Aman Mohammadi, M.; Ahangari, H.; Mousazadeh, S.; Hosseini, S.M.; Dufossé, L. Microbial Pigments as an Alternative to Synthetic Dyes and Food Additives: A Brief Review of Recent Studies. Bioprocess Biosyst. Eng. 2022, 45, 1–12. [Google Scholar] [CrossRef]
- Van Den Bergen, J.; Parker, G. Textile Processing Guide: Pre-Treatment, Colouration and Finishing; Fashion For Good: Amsterdam, The Netherlands, 2022; pp. 1–36. [Google Scholar]
- Chadni, Z.; Rahaman, M.H.; Jerin, I.; Hoque, K.M.F.; Reza, M.A. Extraction and Optimisation of Red Pigment Production as Secondary Metabolites from Talaromyces verruculosus and Its Potential Use in Textile Industries. Mycology 2017, 8, 48–57. [Google Scholar] [CrossRef]
- Sengupta, S.; Bhowal, J. Characterization of a Blue-Green Pigment Extracted from Pseudomonas aeruginosa and Its Application in Textile and Paper Dyeing. Environ. Sci. Pollut. Res. 2022, 30, 30343–30357. [Google Scholar] [CrossRef] [PubMed]
- Kalebek, N.A. Fastness and Antibacterial Properties of Polypropylene Surgical Face Masks Dyed with Coffee Grounds. J. Text. Inst. 2022, 113, 1309–1315. [Google Scholar] [CrossRef]
- Fang, J.; Meng, C.; Zhang, G. Agricultural Waste of Ipomoea batatas Leaves as a Source of Natural Dye for Green Coloration and Bio-Functional Finishing for Textile Fabrics. Ind. Crops Prod. 2022, 177, 114440. [Google Scholar] [CrossRef]
- Chakraborty, L.; Pandit, P.; Roy Maulik, S. Acacia auriculiformis—A Natural Dye Used for Simultaneous Coloration and Functional Finishing on Textiles. J. Clean. Prod. 2020, 245, 118921. [Google Scholar] [CrossRef]
- Khan, A.; Hussain, M.T.; Jiang, H.; Gul, S. Development of Functional Wool Fabric by Treatment with Aqueous and Alkaline Extracts of Cinnamomum camphora Plant Leaves. J. Nat. Fibers 2020, 17, 472–481. [Google Scholar] [CrossRef]
- Mansour, R.; Dhouib, S.; Sakli, F. UV Protection and Dyeing Properties of Wool Fabrics Dyed with Aqueous Extracts of Madder Roots, Chamomiles, Pomegranate Peels, and Apple Tree Branches Barks. J. Nat. Fibers 2022, 19, 610–620. [Google Scholar] [CrossRef]
- Jabar, J.M.; Ogunsade, A.F.; Odusote, Y.A.; Yılmaz, M. Utilization of Nigerian Mango (Mangifera indica L.) Leaves Dye Extract for Silk Fabric Coloration: Influence of Extraction Technique, Mordant and Mordanting Type on the Fabric Color Attributes. Ind. Crops Prod. 2023, 193, 116235. [Google Scholar] [CrossRef]
- Maqbool, M.; Ali, S.; Hussain, M.T.; Khan, A.; Majeed, S. Comparison of Dyeing and Functionalization Potential of Some Selected Plant Extracts Applied on Cotton Fabric. J. Nat. Fibers 2021, 18, 42–50. [Google Scholar] [CrossRef]
- Rahman, M.M.; Koh, J.; Hong, K.H. Coloration and Multi-Functionalization of Cotton Fabrics Using Different Combinations of Aqueous Natural Plant Extracts of Onion Peel, Turmeric Root, and Pomegranate Rind. Ind. Crops Prod. 2022, 188, 115562. [Google Scholar] [CrossRef]
- Zhou, Q.; Rather, L.J.; Ali, A.; Wang, W.; Zhang, Y.; Rizwanul Haque, Q.M.; Li, Q. Environmental Friendly Bioactive Finishing of Wool Textiles Using the Tannin-Rich Extracts of Chinese Tallow (Sapium sebiferum L.) Waste/Fallen Leaves. Dye. Pigment. 2020, 176, 108230. [Google Scholar] [CrossRef]
- Nazir, F.; Siddique, A.; Nazir, A.; Javed, S.; Hussain, T.; Abid, S. Eco-friendly Dyeing of Cotton Using Waste-derived Natural Dyes and Mordants. Color. Technol. 2022, 138, 684–692. [Google Scholar] [CrossRef]
- Grujić, D.; Savić, A.; Topalić-Trivunović, L.; Bizjak, M.; Velemir, A.; Milanović, J. Ultrasonic-Assisted Antimicrobial Functionalization of Cotton Knitwear with Plant Extracts. J. Text. Inst. 2023, 114, 1206–1217. [Google Scholar] [CrossRef]
- Tambi, S.; Mangal, A.; Singh, N.; Sheikh, J. Cleaner Production of Dyed and Functional Polyester Using Natural Dyes Vis-a-Vis Exploration of Secondary Shades. Prog. Color Color. Coat. 2021, 14, 121–128. [Google Scholar]
- Sadeghi-Kiakhani, M.; Tehrani-Bagha, A.R.; Safapour, S.; Eshaghloo-Galugahi, S.; Etezad, S.M. Ultrasound-Assisted Extraction of Natural Dyes from Hawthorn Fruits for Dyeing Polyamide Fabric and Study Its Fastness, Antimicrobial, and Antioxidant Properties. Environ. Dev. Sustain. 2021, 23, 9163–9180. [Google Scholar] [CrossRef]
- Bouaziz, A.; Dridi, D.; Gargoubi, S.; Chelbi, S.; Boudokhane, C.; Kenani, A.; Aroui, S. Analysis of the Coloring and Antibacterial Effects of Natural Dye: Pomegranate Peel. Coatings 2021, 11, 1277. [Google Scholar] [CrossRef]
- Grifoni, D.; Roscigno, G.; Falco, E.D.; Vece, A.; Camilli, F.; Sabatini, F.; Fibbi, L.; Zipoli, G. Evaluation of Dyeing and UV Protective Properties on Hemp Fabric of Aqueous Extracts from Vegetal Matrices of Different Origin. Fibers Polym. 2020, 21, 1750–1759. [Google Scholar] [CrossRef]
- Venil, C.K.; Dufossé, L.; Velmurugan, P.; Malathi, M.; Lakshmanaperumalsamy, P. Extraction and Application of Pigment from Serratia marcescens SB08, an Insect Enteric Gut Bacterium, for Textile Dyeing. Textiles 2021, 1, 21–36. [Google Scholar] [CrossRef]
- Darwesh, O.M.; Marzoog, A.; Matter, I.A.; Okla, M.K.; El-Tayeb, M.A.; Aufy, M.; Dawoud, T.M.; Abdel-Maksoud, M.A. Natural Dyes Developed by Microbial-Nanosilver to Produce Antimicrobial and Anticancer Textiles. Microb. Cell Factories 2024, 23, 189. [Google Scholar] [CrossRef]
- Madhu, C.R.; Agrawal, B.J. Sustainable Environment Friendly Approach for Psilocybe zapotecorum. Sustain. Agri Food Environ. Res.-Discontin. 2024, 12, 719–3726. [Google Scholar]
- Ren, Y.; Gong, J.; Fu, R.; Li, Z.; Li, Q.; Zhang, J.; Yu, Z.; Cheng, X. Dyeing and Antibacterial Properties of Cotton Dyed with Prodigiosins Nanomicelles Produced by Microbial Fermentation. Dye. Pigment. 2017, 138, 147–153. [Google Scholar] [CrossRef]
- Ren, Y.; Fu, R.; Fang, K.; Xie, R.; Hao, L.; Chen, W.; Shi, Z. Clean Dyeing of Acrylic Fabric by Sustainable Red Bacterial Pigment Based on Nano-Suspension System. J. Clean. Prod. 2021, 281, 125295. [Google Scholar] [CrossRef]
- Govindaraj, C.; Ugamoorthi, R.; Ramarethinam, S. Isolation of Pseudomonas aeruginosa for Bacterial Pigment Production and Its Application on Synthetic Knitted Fabric. Indian J. Fibre Text. Res. 2021, 46, 168–173. [Google Scholar] [CrossRef]
- Wang, B.; Li, Z.; Wang, Y.; Zhang, B.; Lv, C.; Bi, X.; Zhao, T. Eco-Friendly Dyeing of Cotton Fabrics with Microbial Pigments: Anionic Modification for Superior Color, Antibacterial, Hydrophobic and UV Protection Properties. Ind. Crops Prod. 2025, 223, 120276. [Google Scholar] [CrossRef]
- Bisht, G.; Srivastava, S.; Kulshreshtha, R.; Sourirajan, A.; Baumler, D.J.; Dev, K. Applications of Red Pigments from Psychrophilic Rhodonellum psychrophilum GL8 in Health, Food and Antimicrobial Finishes on Textiles. Process Biochem. 2020, 94, 15–29. [Google Scholar] [CrossRef]
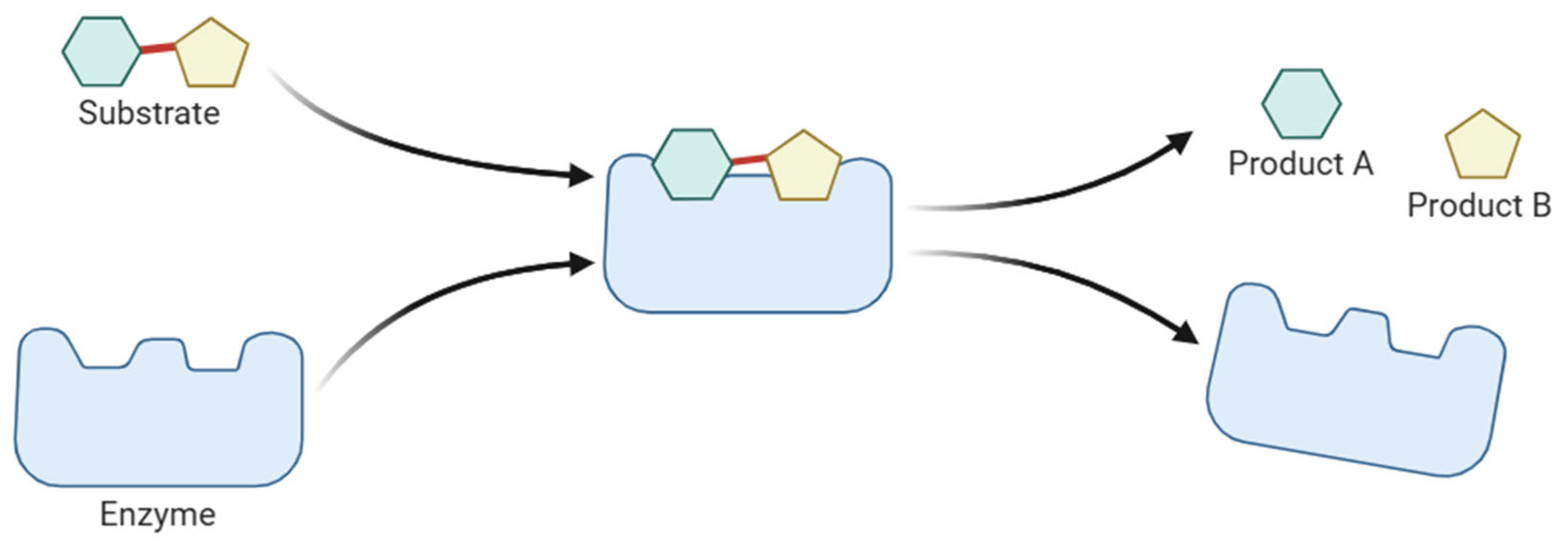
| Classification | Enzyme | Wet Processing Step | Function |
|---|---|---|---|
| Hydrolases | Amylase | Desizing | Removal of starch-based sizes |
| Lipase | Desizing and scouring | Removal of fats and oils | |
| Pectinase | Scouring | Removal of non-cellulosic contents (e.g., pectin, waxes, protein, ashes and seed husk fragments) | |
| Cutinase | Scouring | Removal of cutin and waxes | |
| Protease | Scouring and finishing | Degumming of silk; modification of wool and silk | |
| Xylanase | Scouring and bleaching | Removal of residual hemicellulose | |
| Cellulase | Finishing | Removal of cellulosic fibres on the textile’s surface (fibrils) | |
| Oxidoreductases | Catalase | Bleaching | Decomposition of residual H2O2 after bleaching |
| Laccase | Bleaching and dyeing | Removal of indigo from denim fabrics and oxidation of dye precursors | |
| Peroxidase | Bleaching | Oxidation of dyes that are not bond covalently and removal of residual H2O2 | |
| Glucose oxidase | Bleaching | Generation of H2O2 |
| Classification | Origin | Biopolymer | Structure | Wet Processing Step | Function |
|---|---|---|---|---|---|
| Polysaccharides | Animal | Chitosan | 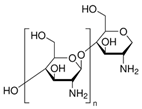 | Pre-treatment and finishing | Dye uptake improvement Antimicrobial Flame retardancy Microencapsulation |
| Plant | Alginate | 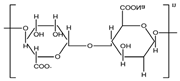 | Printing | Thickener | |
| Plant | Starch |  | Printing and finishing | Thickener | |
| Plant | Natural gums | 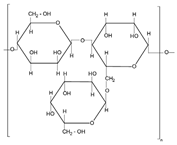 | Printing | Thickener Swelling agent | |
| Polyphenolics (not true polysaccharides) | Plant | Lignin | 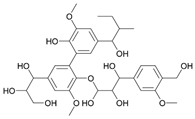 | Pre-treatment and finishing | Antibacterial UV protection Antioxidant Flame retardancy |
| Proteins | Animal | Sericin | 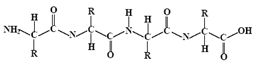 | Pre-treatment | Dye uptake improvement |
| Animal | Casein | 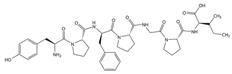 | Finishing | Flame retardancy | |
| Animal | Gelatine | 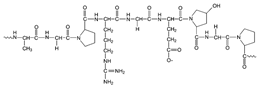 | Pre-treatment and finishing | Dye uptake improvement Flame retardancy Microencapsulation |
| Dye Source | Colour | Mordant/ Application | Substrate | Colour Fastness | Ref |
|---|---|---|---|---|---|
| Plant-Based Dyes | |||||
| Mimusops elengi Linn leaves | N.a. | Aloe vera (pre-, meta- and post-mordanting) | Cotton (CO) Silk (SK) | CO/No mordant: W 5; R 4-5 CO/Aloe vera: W 5; R 5 SK/No mordant: W 5; R 5 SK/Aloe vera: W 5; R 5 | [88] |
| Mussaenda erythrophylla red sepals | Peach | Lemon juice (pre-mordanting) | Silk (SK) | W 4; R 3; P 2-3; L 3-4 | [89] |
| Madder | Reddish Brown | Yellow myrobalan (YM) Black myrobalan (BM) (pre- and a meta-mordanting) | Wool (WO) | 20% madder/20% YM: W 3; R 4-5; L 4-5 10% madder/20% BM: W 4; R 4-5; L 3 10% reseda/10% YM: W 3-4; R 4-5; L 4 10% reseda/10% BM: W 3-4; R 4-5; L 3 | [87] |
| Turmeric root | Yellow | Chitosan (meta-mordanting: added at the beginning or after reaching dyeing temperature) | Cotton (CO) | W 4-5 | [57] |
| St. John’s wort Onion peel | Light brown Orange | Laccase-assisted colouration | Cotton (CO) | St. John’s: W 4; R 4; L 3 Onion: W 4-5; R 4; L 3 | [21] |
| Agrowaste-Based Dyes | |||||
| Pistachio soft and hard shells | Light brown | Tannic acid (TA) Acorn powder (AP, pre- and meta-mordanting) | Cotton (CO) | Soft shells/TA: W 4-5; R 4-5; P 5; L 3 Hard shells/TA: W 5; R 4-5; P 5; L 3-4 Soft shells/AP: W 5; R 4-5; P 5; L 3 Hard shells/AP: W 5; R 4-5; P 5; L 3 | [90] |
| Walnut green husks | Brown Orange Grey | No mordant No salt | Cotton (CO) | W 2; R 3-4 | [91] |
| Onion peel | Brown | Banana peel Guava leaves (pre-, meta- and post-mordanting) | Jute/Cotton (CJ/CO) | Banana: W 3-4 Guava: W 3 | [96] |
| Pomegranate peel | Grey Brown | Chinese quince fruit (pre-, meta- and post-mordanting) | Silk (SK) | W 3-4; R 4-5; P 4 | [92] |
| Pomegranate fallen leaves | N.a. | No mordant | Wool (WO) | W 4-5; R 4; L 6-7 | [93] |
| Beetroot waste | N.a. | Chitosan (pre-mordanting) | Cotton (CO) Wool (WO) Silk (SK) | CO: W 4; P 4-5; L 4-5 WO: W 4-5; P 5; L 5 SK: W 5; P 5; L 5 | [54] |
| Beetroot peel | Red | Acetic acid (pre-mordanting) | Wool (WO) | W 5; R 4-5; L 5 | [94] |
| Grape pomace | Brown | Cationisation (CO) No mordant (WO) | Cotton (CO) Wool (WO) | N.a. | [95] |
| Marigold flower | Yellow | Ultrasound-assisted | Cotton (CO) | W 3; R 4-5; L 7 | [97] |
| Annato | Orange | No mordant | Recycled polyester (rPES) | W 5; R 5 | [98] |
| Turmeric Madder Indigo Henna Catechu (Powder, Pd, and Liquid, Lq, forms) | Yellow Red Blue Green Brown | No mordant | Polyester (PES) | Turmeric Pd (5%): W 5; R 4-5; L 1 Turmeric Lq (5%): W 4; R 3-4; L 1-2 Madder Pd (10%): W 5; R 3-4; L 3 Madder Lq (10%): W 4-5; R 2; L 3 Indigo Pd (3%): W 4-5; R 3-4; L 3 Indigo Lq (3%): W 5; R 4; L 3-4 Henna Pd (5%): W 5; R 3-4; L 2 Henna Lq (5%): W 5; R 2-3; L 2 Catechu Pd (2.5%): W 5; R 3; L 1-2 Catechu Lq (2.5%): W 4; R 2; L 3-4 | [99] |
| Saffron powder | Yellow | Pomegranate Turmeric (pre- and post-mordanting) | Polyamide (PA) | Pomegranate: W 4-5; P 4-5; L 5 Turmeric: W 4; P 4-5; L 4-5 | [100] |
| Start-Up | Microorganism | Colours | Substrate |
|---|---|---|---|
| Colorifix | GMO | Diverse | Natural and Synthetic |
| Pili | GMO | Indigo | Cellulosic, proteic and blended fibres |
| Huue | GMO | Indigo | Cellulosic, proteic and blended fibres |
| Vienna Textile Lab | Bacteria and Fungi | Diverse | Natural and Synthetic |
| KBCols Sciences | Bacteria and Fungi | Diverse | Natural and Synthetic |
| Dye Source | Mordant/ Application | Substrate | Colour Fastness | Functionalities | Ref |
|---|---|---|---|---|---|
| Sweet potato | No mordant No salt | Silk (SK) Wool (WO) Cotton (CO) Polyester (PES) Polyamide (PA) | N.a. | AM: >80% after 30 washing cycles (E. coli and S. aureus) UPF: WO, Excellent after 1 month; PA, PES, Very good after 1 month | [113] |
| Acacia auriculiformis bark | No mordant | Wool (WO) Silk (SK) Cotton (CO) | WO, SK: W 4-5; R 4-5; L 4-5 CO: W 4; R 4-5; L 3-4 | AM: >90% (E. coli and S. aureus) UPF: WO, Excellent; SK, CO, Good | [114] |
| Cinnamomum camphora leaves | No mordant | Wool (WO) | W 4-5; L 3 | AM: NaOH extract, >90% (E. coli and S. aureus), >85% (C. albicans); water extract, 65–80% (E. coli, S. aureus and C. albicans) UPF: Excellent | [115] |
| Pomegranate peel Chamomile Apple tree bark Madder roots | No mordant | Wool (WO) | Pomegranate: W 5; R 4; L 7 Chamomile: W 5; R 4; L 4 Apple: W 5; R 4-5; L 3-4 Madder: W 5; R 3-4; L 2-3 | UPF: Excellent | [116] |
| Chinese tallow leaves | Chlorophyll extract (pre-mordanting) | Wool (WO) | No mordant: W 4-5; R 4 Chlorophyll: W 5; R 4-5 | AM: No mordant, >95% (B. subtilis), >90%, (P. aeruginosa); Chlorophyll, >95% (B. subtilis), 70% (P. aeruginosa) UPF: Very good Aox: No mordant, 70%; chlorophyll, 50% | [120] |
| Mango leaves | Mucuna pruriens L. leaves Justicia carnea L. leaves (pre- and post-mordanting) | Silk (SK) | Mucuna/No microwave: W 5; R 5; L 7 Mucuna/Microwave: W 5; R 5; L 7-8 Justicia/No microwave: W 5; R 5; L 7-8 Justicia/Microwave: W 5; R 5; L 8 | UPF: Excellent after 3 exposure periods Aox: >80% after 3 washing cycles | [117] |
| Henna leaves Turmeric rhizome Pomegranate peel Onion peel Marigold flowers Eucalyptus bark Acacia bark | No mordant | Cotton (CO) | Henna: W 4; R 4-5; L 5 Turmeric: W 3; R 4-5; L 5-6 Pomegranate: W 3; R 4-5; L 4 Onion: W 4; R 4-5; L 5 Marigold: W 3; R 4-5; L 4 Eucalyptus W 4; R 4-5; L 4 Acacia: W 4; R 4-5; L 4 | AM: No activity (E. coli and S. aureus) UPF: Marigold, Pomegranate, Eucalyptus, Excellent; Henna, Acacia, Onion, Very good; Turmeric, Insufficient MR: Turmeric, Onion, Marigold, >50% | [118] |
| Onion peel | Chitosan (pre-mordanting) | Cotton (CO) | N.a. | AM: >95% (E. coli and S. aureus); >80% after 20 washing cycles UPF: Excellent after 20 washing cycles | [58] |
| Pomegranate rind Onion peel Turmeric root | No mordant | Cotton (CO) | Pomegranate: W 4-5; R 4-5 Onion: W 4-5; R 4-5 Turmeric: W 3-4; R 4 | AM: Pomegranate, 99.9% (S. aureus and K. pneumoniae); Onion and Turmeric, 99.9% (S. aureus) UPF: Pomegranate, Very good; Onion, Good; turmeric, Insufficient Aox: Pomegranate, Onion, >90% | [119] |
| Sugarcane bagasse Wheat bran Rice husk | Seed coats tamarind (pre-, meta- and post-mordanting) | Cotton (CO) | Sugarcane/Pre-mordanting: W 4; L 2-3 Sugarcane/Post-mordanting: W 4; L 4 Wheat/Pre-mordanting: W 4; L 2-3 Wheat/Post-mordanting: W 3-4; L 4 Rice/Pre- mordanting: W 4; L 2-3 Rice/Post-mordanting: W 3-4; L 4 | UPF: Rice/Pre-mordanting, Very good; Sugarcane/Pre-mordanting, Wheat/Pre-mordanting, Good; Post-mordanting, Insufficient | [121] |
| Achillea millefolium L. | Ultrasound-assisted | Cotton (CO) | N.a. | AM: Contact inhibition (E. coli and S. aureus) | [122] |
| Pomegranate peel | No mordant | Polyester (PES) | W 4; R 4; L 5 | AM: 90% (S. aureus) UPF: Excellent Aox: 93.97% | [123] |
| Hawthorn fruits | Ultrasound-assisted | Polyamide (PA) | W 5; R 4; P 4-5; L 7 | AM: 50% owf, 60% (E. coli) 47% (S. aureus); 100% owf, 91% (E. coli) 81% (S. aureus) Aox: 50% owf, 80%; 100% owf, >85% | [124] |
| Pomegranate peel | Gallnut (meta-mordanting) | Polyamide (PA) | W 3-4; R 4-5; L 2-3 | AM: 60% (E. coli), 50% (S. aureus) | [125] |
| Microorganism /Pigment | Colour | Substrate | Colour Fastness | Functionalities | Ref |
|---|---|---|---|---|---|
| Serratia marcescens | Red | Acrylic (PAC) | W 5; R 5; P 5 | N.a. | [131] |
| Pseudomonas aeruginosa | N.a. | Polyester (PES) | W 4-5; P 4-5 | N.a. | [132] |
| Streptomyces sp. strains | N.a. | Polyamide (PA) Polyamide/Elastane (PA/EA) | N.a. | AM: S. epidermidis and C. albicans CYT: HaCaT cells | [104] |
| Prodigiosin (from Serratia marcescens) | Red | Cotton (CO) | W 5; R 4; L 2 | AM: 96.9% (S. aureus) UPF: Good | [133] |
| Serratia marcescens | Red | Cotton (CO) Silk (SK) | CO: W 4-5; R 4-5; L 3 SK: W 4-5; R 4-5; L 4 | AM: B. subtilis, E. coli, K. pneumoniae, Proteus vulgaris, P. aeruginosa | [127] |
| Rhodonellum psychrophilum | Red | Cotton (CO) Viscose (CV) Silk (SK) | N.a. | AM: E. coli, S. aureus, C. albicans, S. cerevisiae Aox: 83.6% | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Catarino, M.L.; Sampaio, F.; Pacheco, L.; Gonçalves, A.L. The Shift to Bio-Based Auxiliaries in Textile Wet Processing: Recent Advances and Industrial Potential. Molecules 2025, 30, 4016. https://doi.org/10.3390/molecules30194016
Catarino ML, Sampaio F, Pacheco L, Gonçalves AL. The Shift to Bio-Based Auxiliaries in Textile Wet Processing: Recent Advances and Industrial Potential. Molecules. 2025; 30(19):4016. https://doi.org/10.3390/molecules30194016
Chicago/Turabian StyleCatarino, Maria L., Filipa Sampaio, Luísa Pacheco, and Ana L. Gonçalves. 2025. "The Shift to Bio-Based Auxiliaries in Textile Wet Processing: Recent Advances and Industrial Potential" Molecules 30, no. 19: 4016. https://doi.org/10.3390/molecules30194016
APA StyleCatarino, M. L., Sampaio, F., Pacheco, L., & Gonçalves, A. L. (2025). The Shift to Bio-Based Auxiliaries in Textile Wet Processing: Recent Advances and Industrial Potential. Molecules, 30(19), 4016. https://doi.org/10.3390/molecules30194016






