Novel Isolongifolenone-Based Caprolactam Derivatives as Potential Anticancer Agents via the p53/mTOR/Autophagy Pathway
Abstract
1. Introduction
2. Results and Discussion
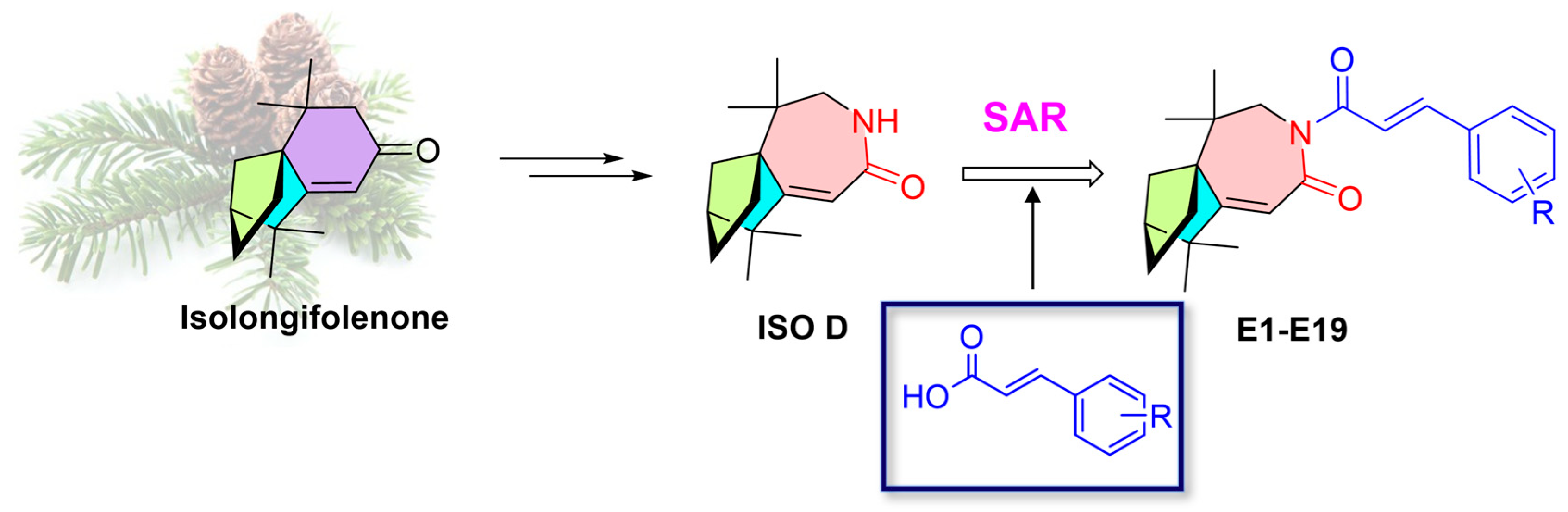
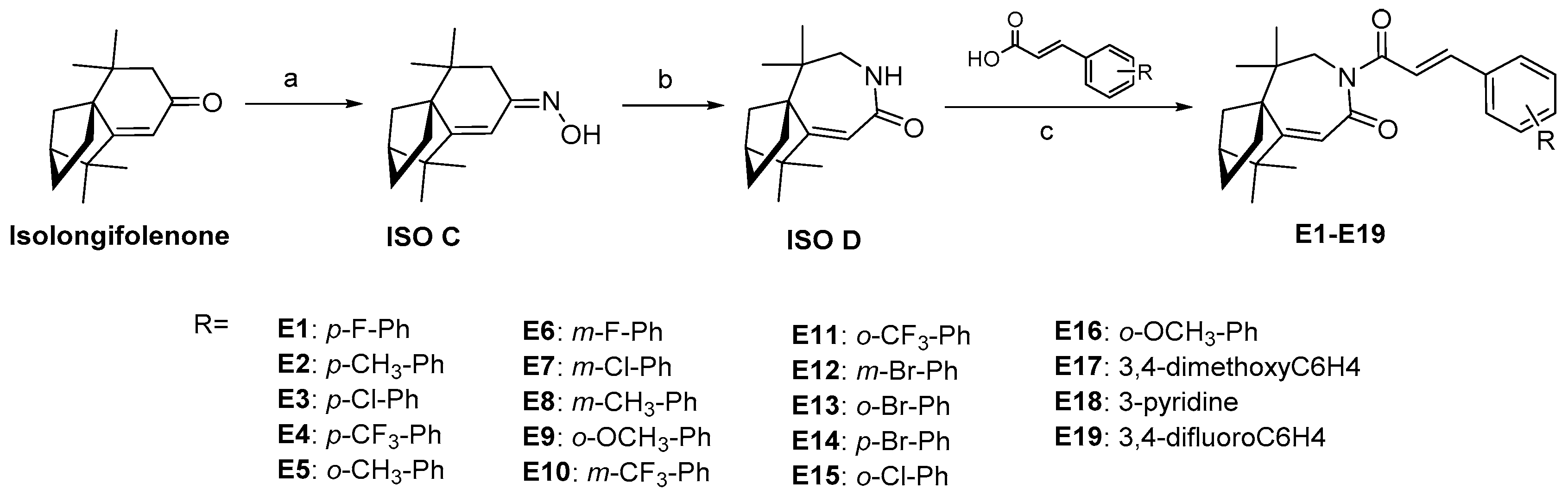
2.1. Design and Synthesis
2.2. In Vitro Antiproliferative Activity of Isolongifolenone Bicyclocaprolactam Derivatives and Structure-Activity Relationship Studies
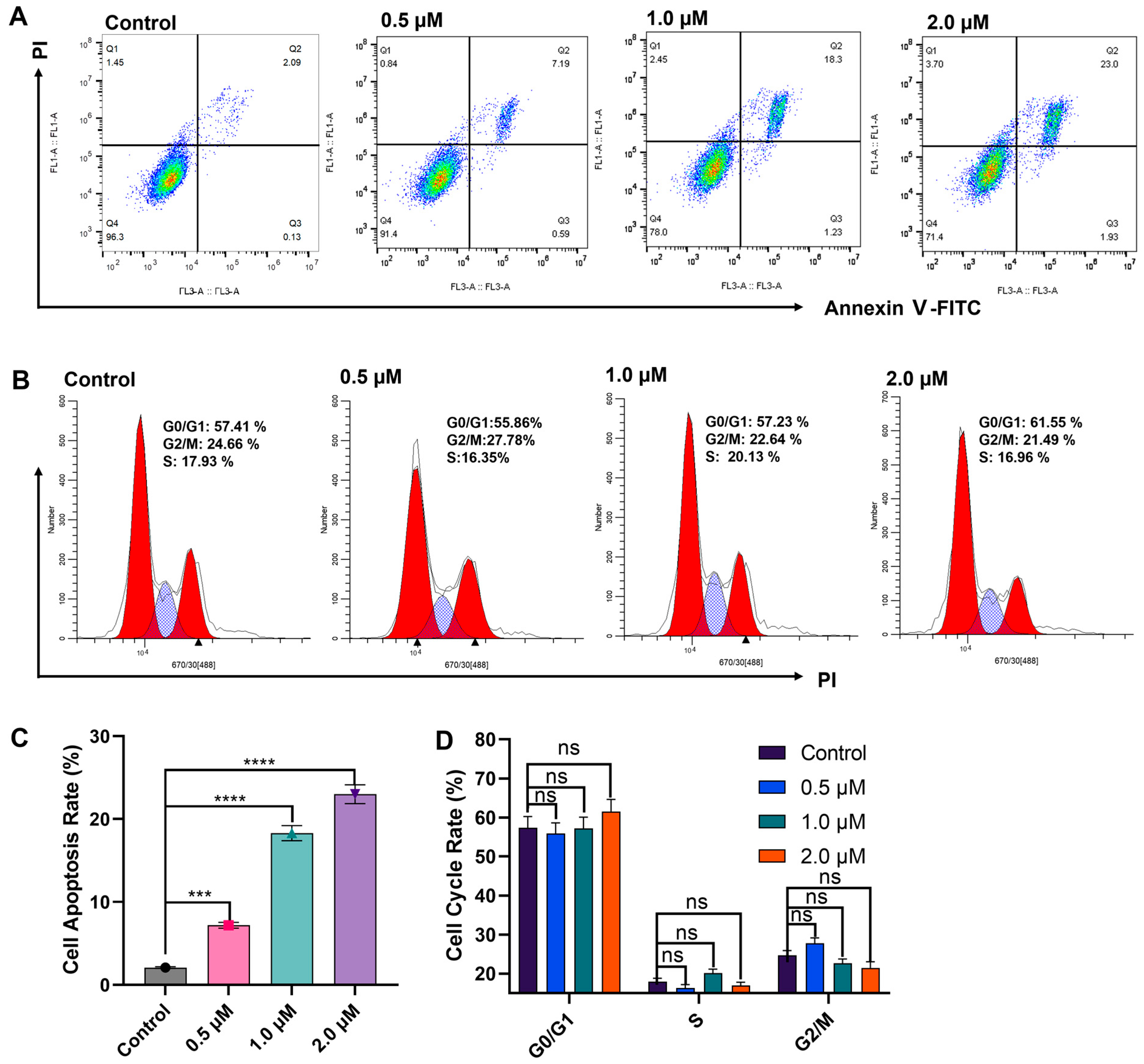
2.3. E10 Induced Cell Apoptosis and Cell Cycle Arrest
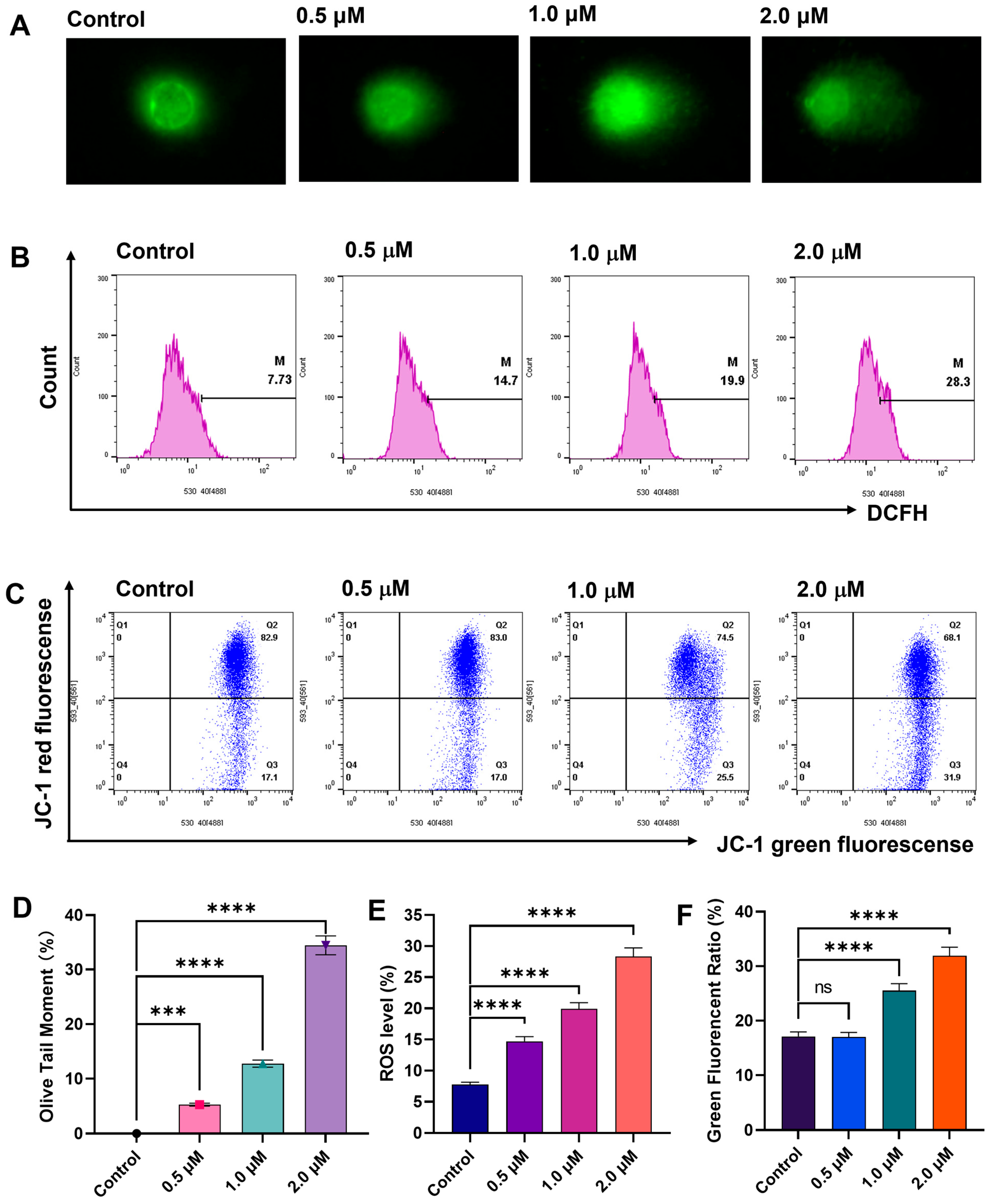
2.4. DNA Damage Studies of Compound E10
2.5. E10 Induced ROS Generation and Loss of MMP
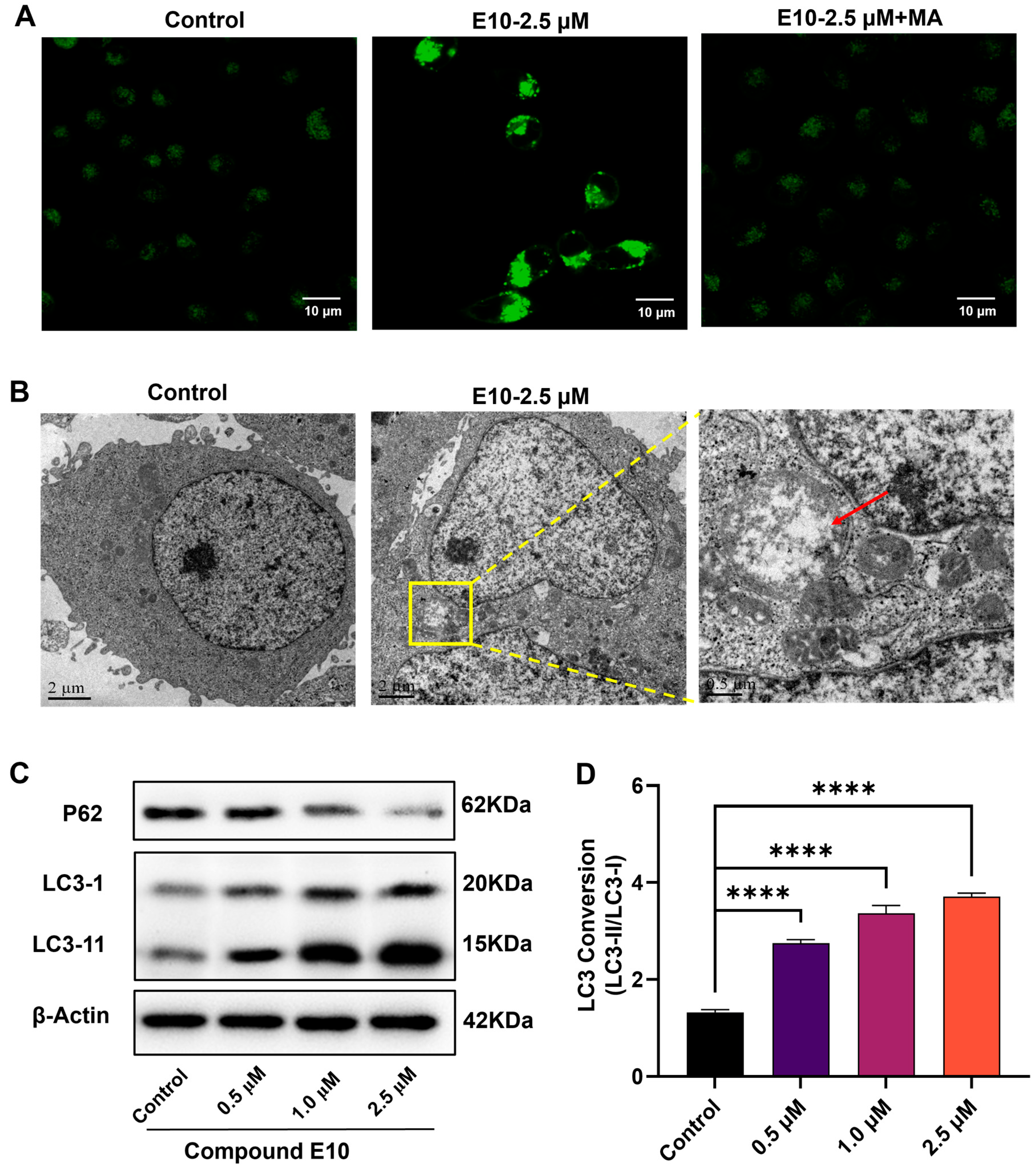
2.6. E10 Induced Autophagy in MCF-7 Cells
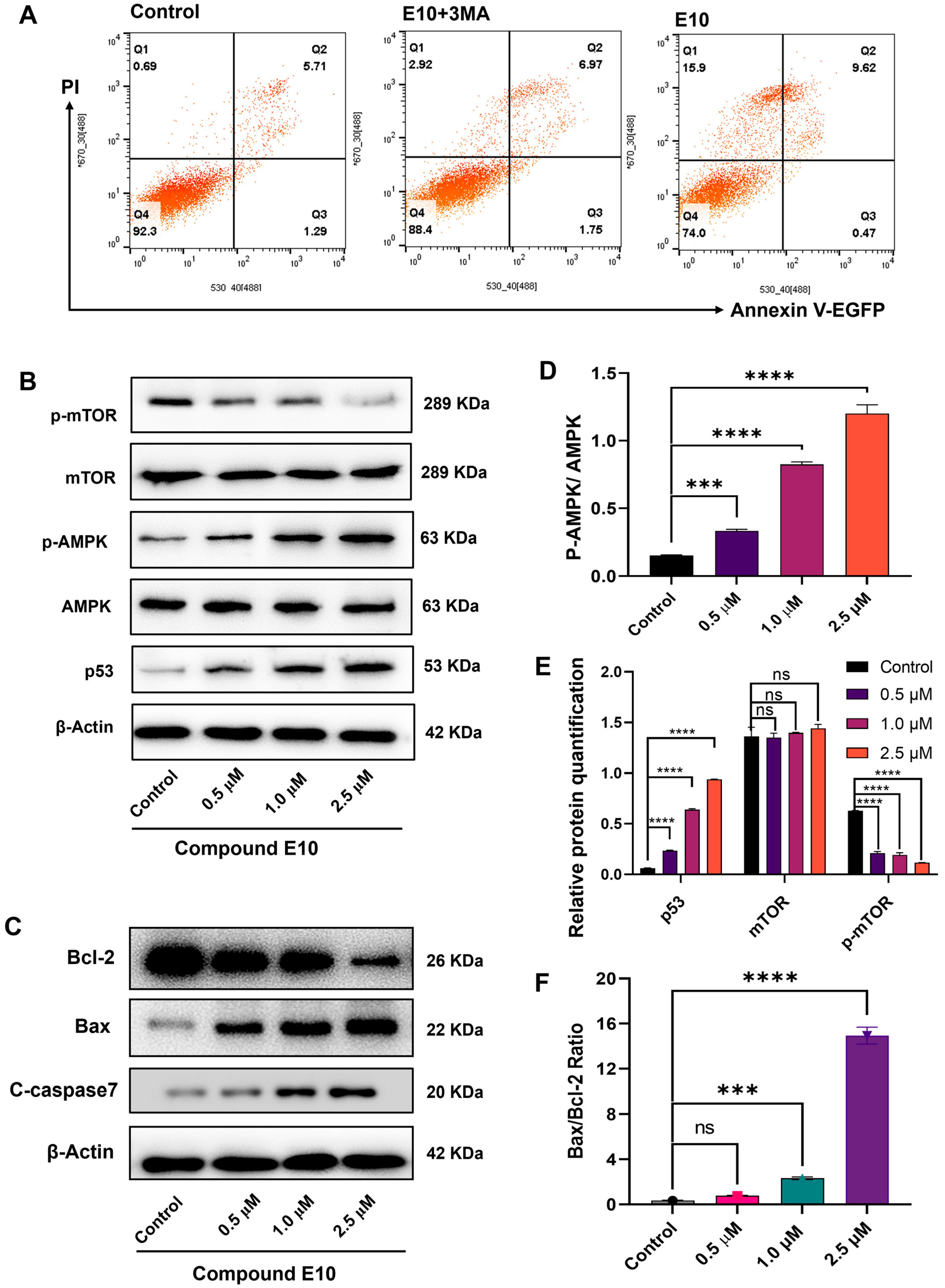
2.7. E10 Induced Cell Death via the AMPK-mTOR Pathway
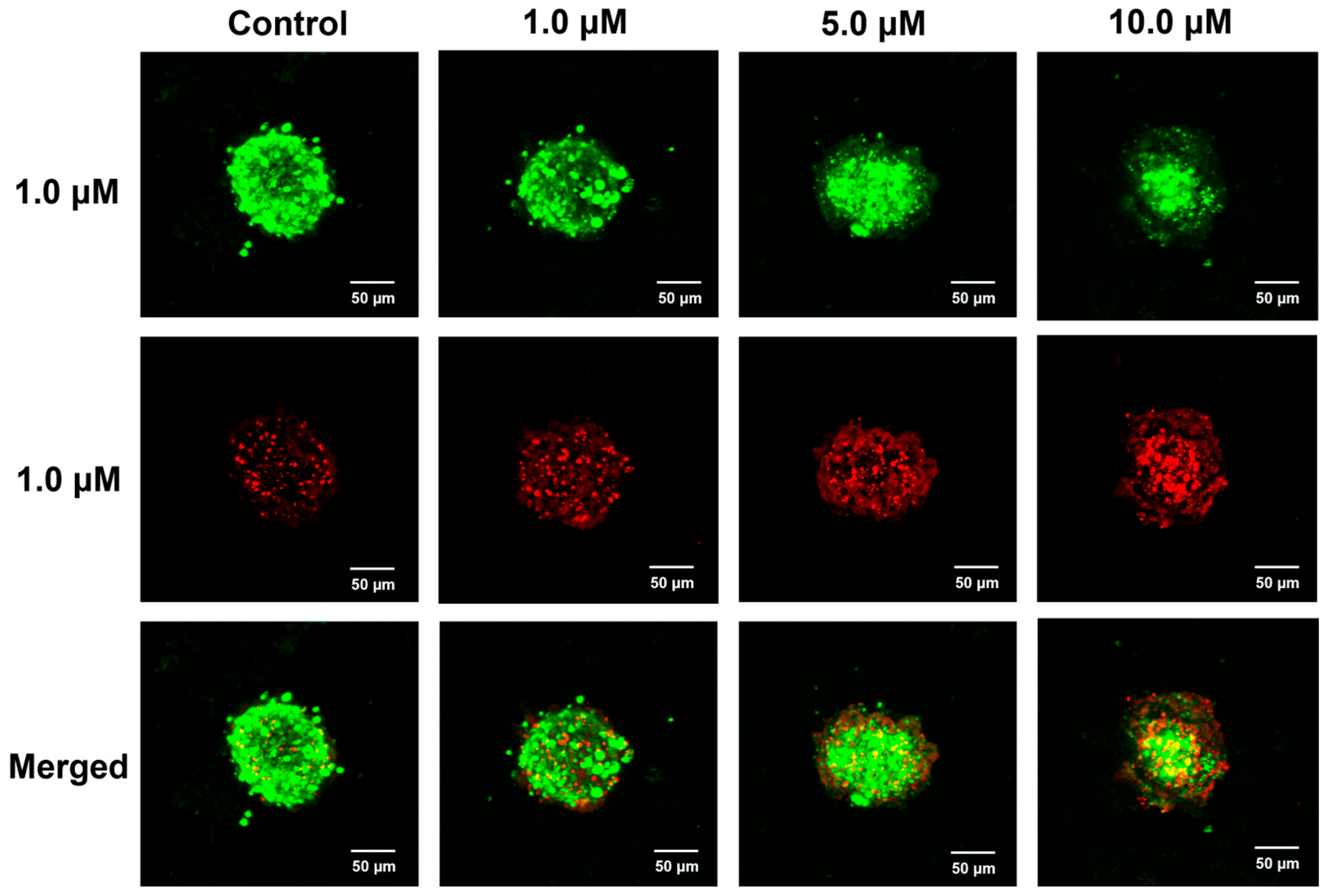
2.8. Antiproliferative Activity of E10 in 3D Spheroid Tumor Model
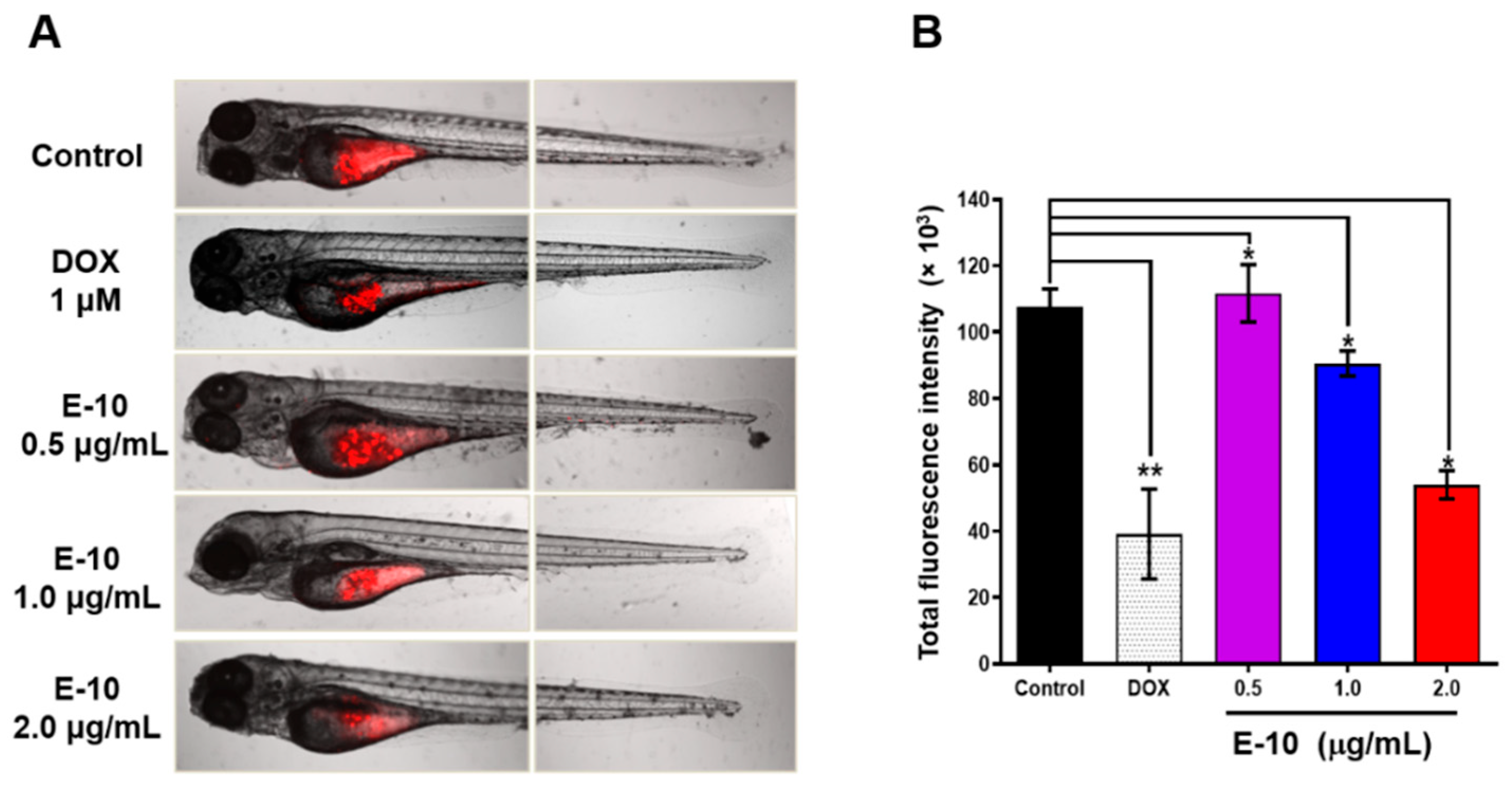
2.9. Compound E10 Suppresses Tumor Growth In Vivo
3. Conclusions
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for the Synthesis of Compound ISO C
4.1.2. General Procedure for the Synthesis of Compound ISO D
4.1.3. General Procedure for the Synthesis of Derivatives E1–E19
4.2. Cell Culture and Cell Cytotoxicity Assay
Cell Culture and CCK-8 Assay
4.3. Anticancer Mechanism Studies
4.3.1. Cell Apoptosis Assay
4.3.2. Cell Cycle Assay
4.3.3. Monodansylcadaverine (MDC) Staining Assay
4.3.4. Intracellular ROS Assay
4.3.5. MMP Assay
4.3.6. Comet Assay
4.3.7. Western Blotting Analysis
4.3.8. Autophagy Assay Using Transmission Electron Microscopy
4.4. Three-Dimensional Cell Culture
4.5. Anticancer Activity in Zebrafish Models
4.6. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Weiderpass, E.; Soerjomataram, I. The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer 2021, 127, 3029–3030. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Hijazi, M.A.; Gessner, A.; El-Najjar, N. Repurposing of Chronically Used Drugs in Cancer Therapy: A Chance to Grasp. Cancers 2023, 15, 3199. [Google Scholar] [CrossRef]
- Clardy, J.; Walsh, C. Lessons from natural molecules. Nature 2004, 432, 829–837. [Google Scholar] [CrossRef]
- Butler, M.S. Natural products to drugs: Natural product-derived compounds in clinical trials. Nat. Prod. Rep. 2008, 25, 475–516. [Google Scholar] [CrossRef] [PubMed]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; International Natural Product Sciences Taskforce; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rinkel, J.; Goldfuss, B.; Dickschat, J.S.; Tiefenbacher, K. Sesquiterpene Cyclisations Catalysed inside the Resorcinarene Capsule and Application in the Short Synthesis of Isolongifolene and Isolongifolenone. Nat. Catal. 2018, 1, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari, T.; Kafil, H.S.; Asnaashari, S.; Farajnia, S.; Delazar, A.; Baek, S.C.; Hamishehkar, H.; Kim, K.H. Chemical Composition and Antimicrobial Activity of Essential Oils from the Aerial Parts of Pinus eldarica Grown in Northwestern Iran. Molecules 2019, 24, 3203. [Google Scholar] [CrossRef]
- Li, J.; Li, J.; Fang, H.; Yang, H.; Wu, T.; Shi, X.; Pang, C. Isolongifolene alleviates liver ischemia/reperfusion injury by regulating AMPK-PGC1α signaling pathway-mediated inflammation, apoptosis, and oxidative stress. Int. Immunopharmacol. 2022, 113 Pt A, 109185. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Musharraf, S.G.; Khan, M.T.H.; Abdelrahman, D.; Parvez, M.; Shaheen, F.; Atta-ur-Rahman. Microbial transformation of isolongifolen-4-one. Helv. Chim. 2003, 86, 3450–3460. [Google Scholar] [CrossRef]
- Mao, L.J.; Xie, X.Y.; Gao, Y.; Lai, P.X.; Ma, Q.L. Chemical Composition, Antibacterial and Antioxidant Activities of Essential oil from Leonurus pseudomacranthus Kitag. Nat. Prod. 2019, 13, 91–95. [Google Scholar] [CrossRef]
- Muthyala, R.S.; Sheng, S.; Carlson, K.E.; Katzenellenbogen, B.S.; Katzenellenbogen, J.A. Bridged bicyclic cores containing a 1,1-diarylethylene motif are high-affinity subtype-selective ligands for the estrogen receptor. J. Med. Chem. 2003, 46, 1589–1602. [Google Scholar] [CrossRef]
- Okitsu, T.; Sato, K.; Iwatsuka, K.; Sawada, N.; Nakagawa, K.; Okano, T.; Yamada, S.; Kakuta, H.; Wada, A. Replacement of the hydrophobic part of 9-cis-retinoic acid with cyclic terpenoid moiety results in RXR-selective agonistic activity. Bioorg. Med. Chem. 2011, 19, 2939–2949. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Zhao, L.; Yang, W.; Pan, L.; Bai, Y.; Wang, Y.; Li, Y. P129, a pyrazole ring-containing isolongifolanone-derivate: Synthesis and investigation of anti-glioma action mechanism. Discov. Oncol. 2024, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shi, W.; Wu, C.; Wan, L.; Zhao, Y.; Zhang, C.; Gu, W.; Wang, S. Pyrazole ring-containing isolongifolanone derivatives as potential CDK2 inhibitors: Evaluation of anticancer activity and investigation of action mechanism. Biomed. Pharmacother. 2021, 139, 111663. [Google Scholar] [CrossRef]
- Ma, C.; Wang, Y.; Dong, F.; Wang, Z.; Zhao, Y.; Shan, Y.; Gu, W.; Wang, S. Synthesis and antitumor activity of isolongifoleno[7,8-d]thiazolo[3,2-a]pyrimidine derivatives via enhancing ROS level. Chem. Biol. Drug Des. 2019, 94, 1457–1466. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Wu, C.; Wang, Y.; Huang, Z.; Zhang, Q.; Dong, F.; Gu, W.; Shan, Y.; Wang, S. Synthesis and Antitumor Activity of Novel Isolongifolic-Alkyl Dihydropyrimidinethione Derivatives. Chin. J. Org. Chem. 2019, 39, 821–829. [Google Scholar] [CrossRef]
- Bilska-Markowska, M.; Cytlak, T.; Kaźmierczak, M. Trifluoromethylated lactams: Promising small molecules in the search for effective drugs. Chem. Commun. 2025, 61, 785–802. [Google Scholar] [CrossRef]
- Fu, D.J.; Zhang, Y.F.; Chang, A.Q.; Li, J. β-Lactams as promising anticancer agents: Molecular hybrids, structure activity relationships and potential targets. Eur. J. Med. Chem. 2020, 201, 112510. [Google Scholar] [CrossRef]
- Zhang, X.; Jia, Y. Recent Advances in β-lactam Derivatives as Potential Anticancer Agents. Curr. Top. Med. Chem. 2020, 20, 1468–1480. [Google Scholar] [CrossRef]
- Lima, L.M.; Silva, B.N.M.D.; Barbosa, G.; Barreiro, E.J. β-lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef]
- Fratoni, A.J.; Nicolau, D.P.; Kuti, J.L. A guide to therapeutic drug monitoring of β-lactam antibiotics. Pharmacotherapy 2021, 41, 220–233. [Google Scholar] [CrossRef]
- Xie, D.; Zhong, S.; Xia, Y. Asymmetric Synthesis of Seven-Membered Lactams: Recent Advances and Future Perspectives. Eur. J. Org. Chem. 2024, 27, e202400687. [Google Scholar] [CrossRef]
- Yue, X.; Li, Y.; Liu, M.; Sang, D.; Huang, Z.; Chen, F. Biocatalytic dynamic reductive kinetic resolution of aryl α-chloro β-keto esters: Divergent, stereocontrolled synthesis of diltiazem, clentiazem, and siratiazem. Chem. Commun. 2022, 58, 9010–9013. [Google Scholar] [CrossRef]
- Al-Rooqi, M.M.; Sadiq, A.; Obaid, R.J.; Ashraf, Z.; Nazir, Y.; Jassas, R.S.; Naeem, N.; Alsharif, M.A.; Shah, S.W.A.; Moussa, Z.; et al. Evaluation of 2,3-Dihydro-1,5-benzothiazepine Derivatives as Potential Tyrosinase Inhibitors: In Vitro and In Silico Studies. ACS Omega 2023, 8, 17195–17208. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Tian, E.; Liu, Z.; Zhou, C.; Yang, P.; Tian, K.; Liao, W.; Li, J.; Ren, C. Small molecule angiotensin converting enzyme inhibitors: A medicinal chemistry perspective. Front. Pharmacol. 2022, 13, 968104. [Google Scholar] [CrossRef] [PubMed]
- Piska, K.; Gunia-Krzyżak, A.; Koczurkiewicz, P.; Wójcik-Pszczoła, K.; Pękala, E. Piperlongumine (piplartine) as a lead compound for anticancer agents—Synthesis and properties of analogues: A mini-review. Eur. J. Med. Chem. 2018, 156, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.S.; Sahoo, S.K. Piperlongumine and its derivatives against cancer: A recent update and future prospective. Arch. Pharm. 2024, 357, e2300768. [Google Scholar] [CrossRef]
- Díaz-Brochero, C.; Valderrama-Rios, M.C.; Nocua-Báez, L.C.; Cortés, J.A. First-generation cephalosporins for the treatment of complicated upper urinary tract infection in adults: A systematic literature review. Int. J. Infect. Dis. 2022, 116, 403–410. [Google Scholar] [CrossRef]
- Kurz, T.; Terman, A.; Brunk, U.T. Autophagy, ageing and apoptosis: The role of oxidative stress and lysosomal iron. Arch. Biochem. Biophys. 2007, 462, 220–230. [Google Scholar] [CrossRef]
- Jacobson, J.; Duchen, M.R. Mitochondrial oxidative stress and cell death in astrocytes-requirement for stored Ca2+ and sustained opening of the permeability transition pore. J. Cell Sci. 2002, 115 Pt 6, 1175–1188. [Google Scholar] [CrossRef]
- Mizushima, N.; Yoshimori, T.; Levine, B. Methods in mammalian autophagy research. Cell 2010, 140, 313–326. [Google Scholar] [CrossRef]
- Lamark, T.; Johansen, T. Mechanisms of Selective Autophagy. Annu. Rev. Cell Dev. Biol. 2021, 37, 143–169. [Google Scholar] [CrossRef]
- Saxton, R.A.; Sabatini, D.M. mTOR Signaling in Growth, Metabolism, and Disease. Cell 2017, 168, 960–976. [Google Scholar] [CrossRef]
- Niemann, A.; Baltes, J.; Elsässer, H.P. Fluorescence properties and staining behavior of monodansylpentane, a structural homologue of the lysosomotropic agent monodansylcadaverine. J. Histochem. Cytochem. 2001, 49, 177–185. [Google Scholar] [CrossRef]
- Wu, Y.T.; Tan, H.L.; Shui, G.; Bauvy, C.; Huang, Q.; Wenk, M.R.; Ong, C.N.; Codogno, P.; Shen, H.M. Dual role of 3-methyladenine in modulation of autophagy via different temporal patterns of inhibition on class I and III phosphoinositide 3-kinase. J. Biol. Chem. 2010, 285, 10850–10861. [Google Scholar] [CrossRef] [PubMed]
- García-Pérez, B.E.; Pérez-Torres, C.; Baltierra-Uribe, S.L.; Castillo-Cruz, J.; Castrejón-Jiménez, N.S. Autophagy as a Target for Non-Immune Intrinsic Functions of Programmed Cell Death-Ligand 1 in Cancer. Int. J. Mol. Sci. 2023, 24, 15016. [Google Scholar] [CrossRef]
- Yuan, H.X.; Xiong, Y.; Guan, K.L. Nutrient sensing, metabolism, and cell growth control. Mol. Cell 2013, 49, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [PubMed]
- Kruiswijk, F.; Labuschagne, C.F.; Vousden, K.H. p53 in survival, death and metabolic health: A lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 2015, 16, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Moll, U.M.; Wolff, S.; Speidel, D.; Deppert, W. Transcription-independent pro-apoptotic functions of p53. Curr. Opin. Cell Biol. 2005, 17, 631–636. [Google Scholar] [CrossRef]
- Moulder, D.E.; Hatoum, D.; Tay, E.; Lin, Y.; McGowan, E.M. The Roles of p53 in Mitochondrial Dynamics and Cancer Metabolism: The Pendulum between Survival and Death in Breast Cancer? Cancers 2018, 10, 189. [Google Scholar] [CrossRef] [PubMed]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef]
- Brown, H.K.; Schiavone, K.; Tazzyman, S.; Heymann, D.; Chico, T.J. Zebrafish xenograft models of cancer and metastasis for drug discovery. Expert Opin. Drug Discov. 2017, 12, 379–389. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freema, J.L.; Gamse, J.T.; Iturria, I. Use of zebrafish in drug discovery toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Dryer, Y.; Berghause, J.; Creswell, K.; Glasgow, E.; Brelidze, T. Comparison of tumor growth assessment using GFP fluorescence and DiI labeling in a zebrafish xenograft model. Cancer Biol. Ther. 2023, 24, 2234140. [Google Scholar] [CrossRef]
- Astell, K.R.; Sieger, D. Zebrafish In Vivo Models of Cancer and Metastasis. Cold Spring Harb. Perspect. Med. 2020, 10, a037077. [Google Scholar] [CrossRef]

| Compd. | R | IC50 * (µM) | |||
|---|---|---|---|---|---|
| MCF-7 | HepG2 | A549 | LO2 | ||
| E1 | 4-F-C6H4 | 9.18 ± 0.11 | >20 | 11.73 ± 0.16 | >20 |
| E2 | 4-CH3-C6H4 | 1.29 ± 0.04 | 6.60 ± 0.07 | 3.18 ± 0.16 | >20 |
| E3 | 4-Cl-C6H4 | 18.97 ± 0.13 | 7.09 ± 0.33 | 16.93 ± 0.27 | >20 |
| E4 | 4-CF3-C6H4 | 16.74 ± 0.16 | >20 | 14.98 ± 0.10 | >20 |
| E5 | 2-CH3-C6H4 | 2.86 ± 0.12 | 1.86 ± 0.09 | 3.89 ± 0.44 | >20 |
| E6 | 3-F-C6H4 | 5.32 ± 0.11 | 5.96 ± 0.05 | 5.34 ± 0.16 | >20 |
| E7 | 3-Cl-C6H4 | 2.67 ± 0.11 | 6.67 ± 0.06 | 2.28 ± 0.10 | >20 |
| E8 | 3-CH3-C6H4 | 1.38 ± 0.12 | 1.62 ± 0.10 | 3.71 ± 0.08 | >20 |
| E9 | 2-OCH3-C6H4 | 2.22 ± 0.07 | 2.17 ± 0.24 | 2.13 ± 0.16 | >20 |
| E10 | 3-CF3-C6H4 | 0.32 ± 0.05 | 1.36 ± 0.18 | 1.39 ± 0.32 | 14.41± 0.04 |
| E11 | 2-CF3-C6H4 | 5.63 ± 0.30 | 4.53 ± 0.12 | 18.30 ± 0.21 | >20 |
| E12 | 3-Br-C6H4 | 1.26 ± 0.19 | 4.89 ± 0.52 | 3.55 ± 0.04 | 11.26 ± 0.06 |
| E13 | 2-Br-C6H4 | 2.12 ± 0.04 | 2.67 ± 0.16 | 4.64 ± 0.06 | >20 |
| E14 | 4-Br-C6H4 | 1.27 ± 0.04 | 2.38 ± 0.16 | 3.21 ± 0.16 | >20 |
| E15 | 2-Cl-C6H4 | 1.58 ± 0.16 | 2.09 ± 0.16 | 3.47 ± 0.16 | >20 |
| E16 | 2-OCH3-C6H4 | 2.67 ± 0.18 | 2.44 ± 0.16 | 5.28 ± 0.10 | >20 |
| E17 | 3,4-OCH3-C6H4 | 2.16 ± 0.03 | 2.56 ± 0.16 | 5.28 ± 0.08 | >20 |
| E18 | 3-pyridine | >20 | >20 | >20 | >20 |
| E19 | 3,4-F-C6H4 | 17.07 ± 0.06 | 18.43 ± 0.16 | 18.90 ± 0.16 | >20 |
| PL | − | 2.79 ± 0.43 | 3.46 ± 0.25 | 5.83 ± 0.26 | − |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, M.; Han, J.; Zhao, Y.; Xiong, B.; Li, P.; Wang, S. Novel Isolongifolenone-Based Caprolactam Derivatives as Potential Anticancer Agents via the p53/mTOR/Autophagy Pathway. Molecules 2025, 30, 4013. https://doi.org/10.3390/molecules30194013
Wang Y, Hu M, Han J, Zhao Y, Xiong B, Li P, Wang S. Novel Isolongifolenone-Based Caprolactam Derivatives as Potential Anticancer Agents via the p53/mTOR/Autophagy Pathway. Molecules. 2025; 30(19):4013. https://doi.org/10.3390/molecules30194013
Chicago/Turabian StyleWang, Yunyun, Min Hu, Jiale Han, Yuxun Zhao, Biao Xiong, Peihai Li, and Shifa Wang. 2025. "Novel Isolongifolenone-Based Caprolactam Derivatives as Potential Anticancer Agents via the p53/mTOR/Autophagy Pathway" Molecules 30, no. 19: 4013. https://doi.org/10.3390/molecules30194013
APA StyleWang, Y., Hu, M., Han, J., Zhao, Y., Xiong, B., Li, P., & Wang, S. (2025). Novel Isolongifolenone-Based Caprolactam Derivatives as Potential Anticancer Agents via the p53/mTOR/Autophagy Pathway. Molecules, 30(19), 4013. https://doi.org/10.3390/molecules30194013






