Investigation of Photorecoordination Kinetics for Complexes of Bis(aza-18-crown-6)-Containing Dienones with Alkali and Alkaline-Earth Metal Cations via Time-Resolved Absorption Spectroscopy: Structure vs. Properties
Abstract
1. Introduction
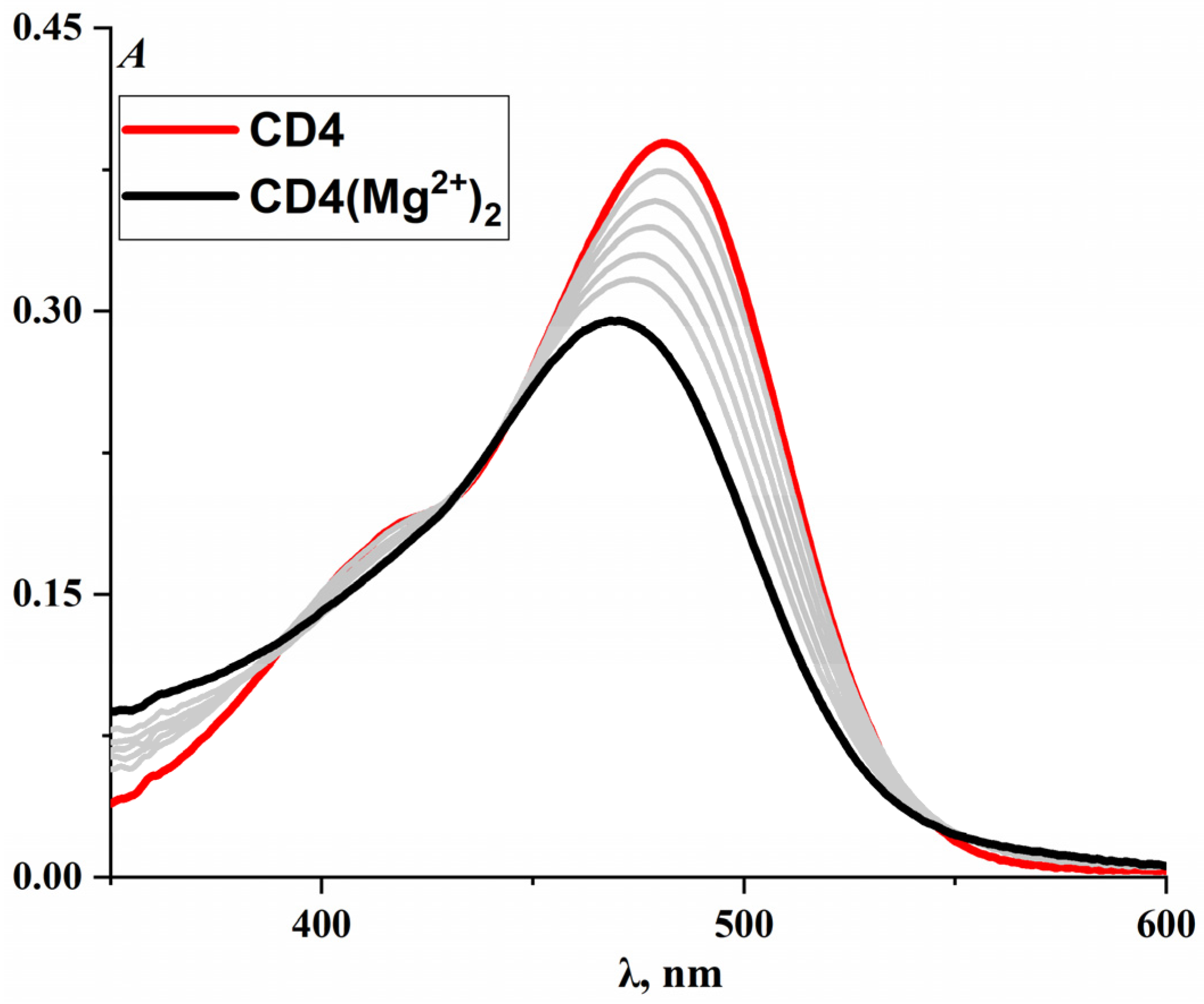
2. Results and Discussion
2.1. Time-Resolved Spectroscopy
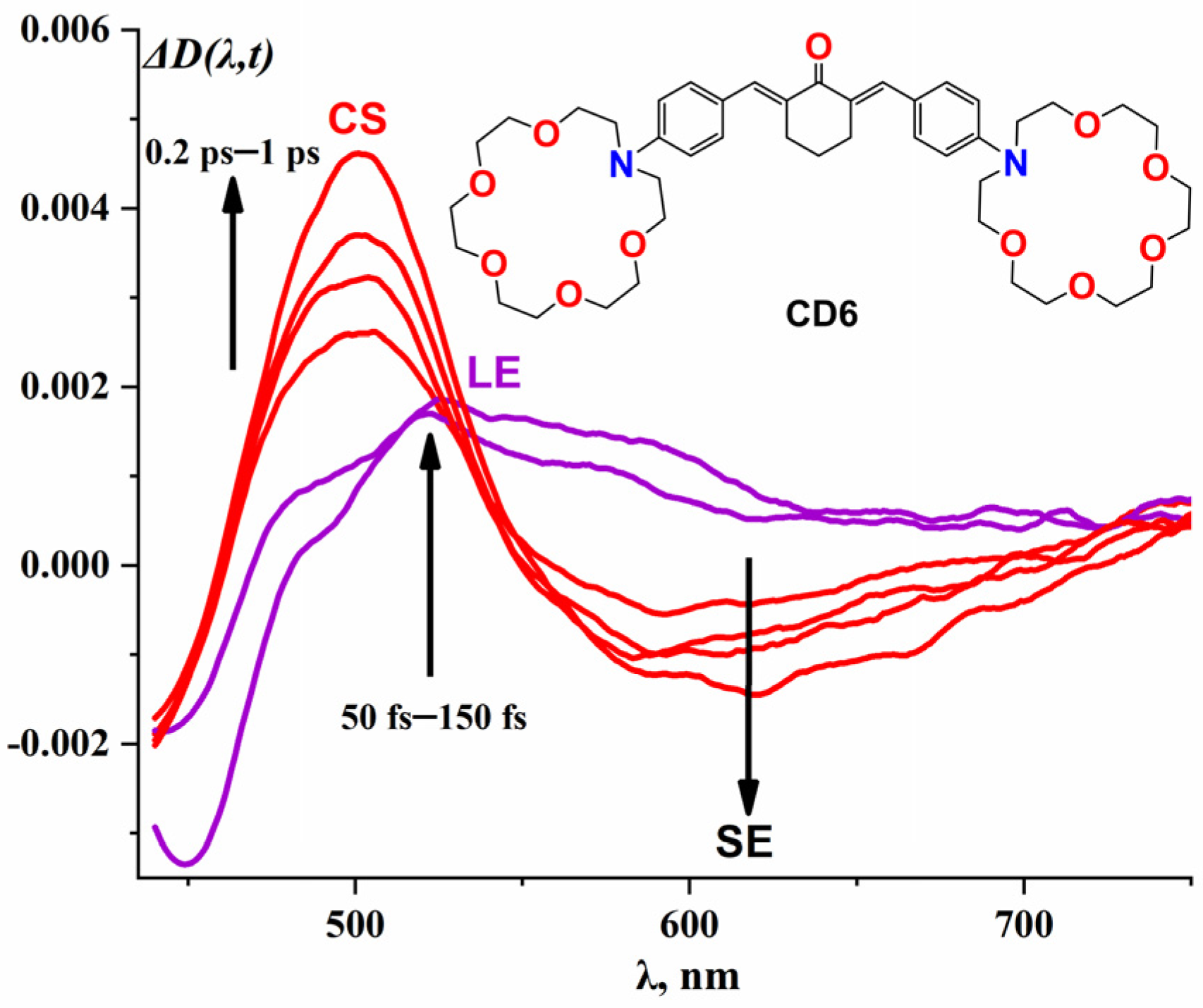
2.1.1. CD6 Dye
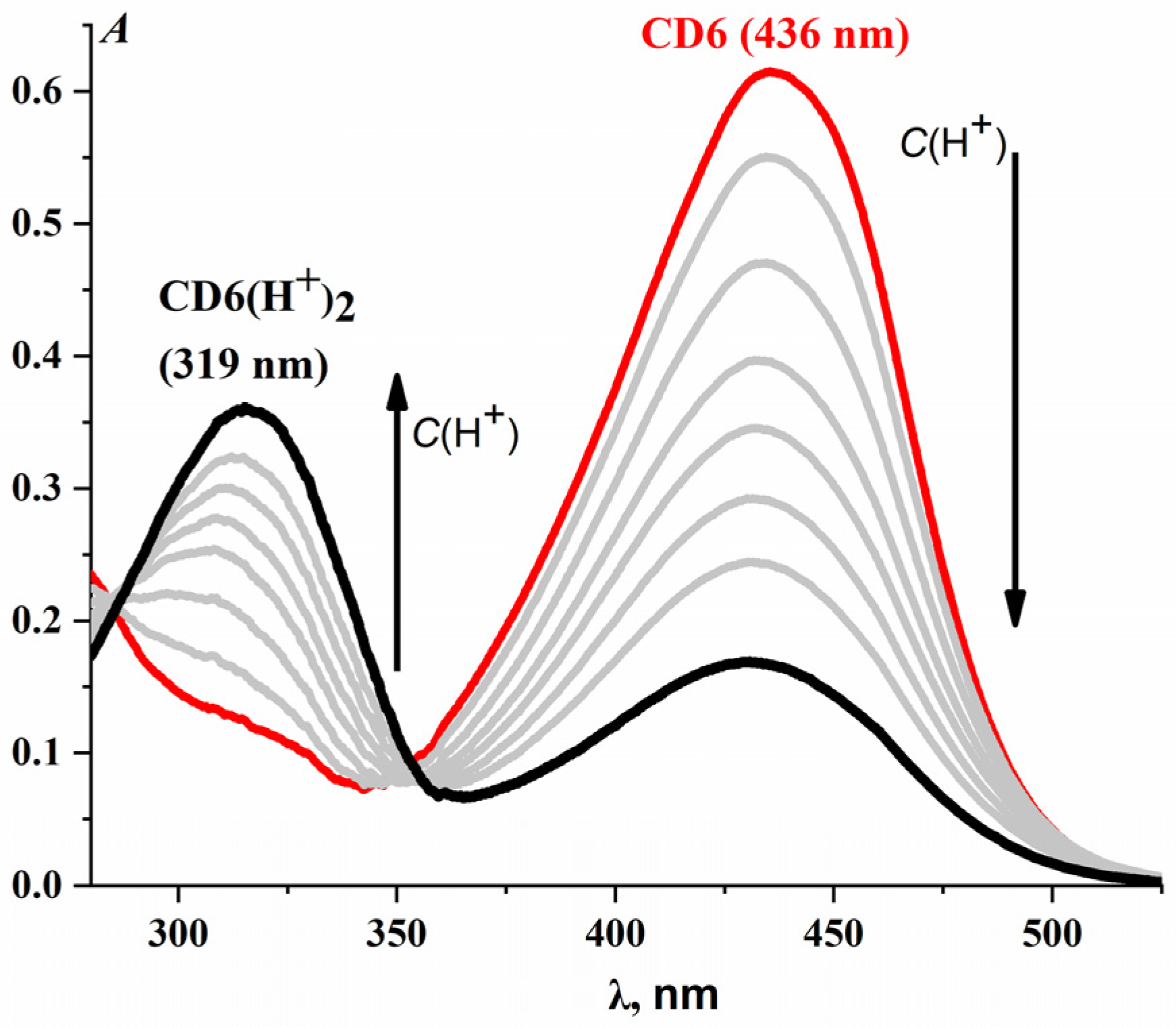
2.1.2. CD6·(H+)2 Complex
2.1.3. CD6·(Mn+)2 Complexes
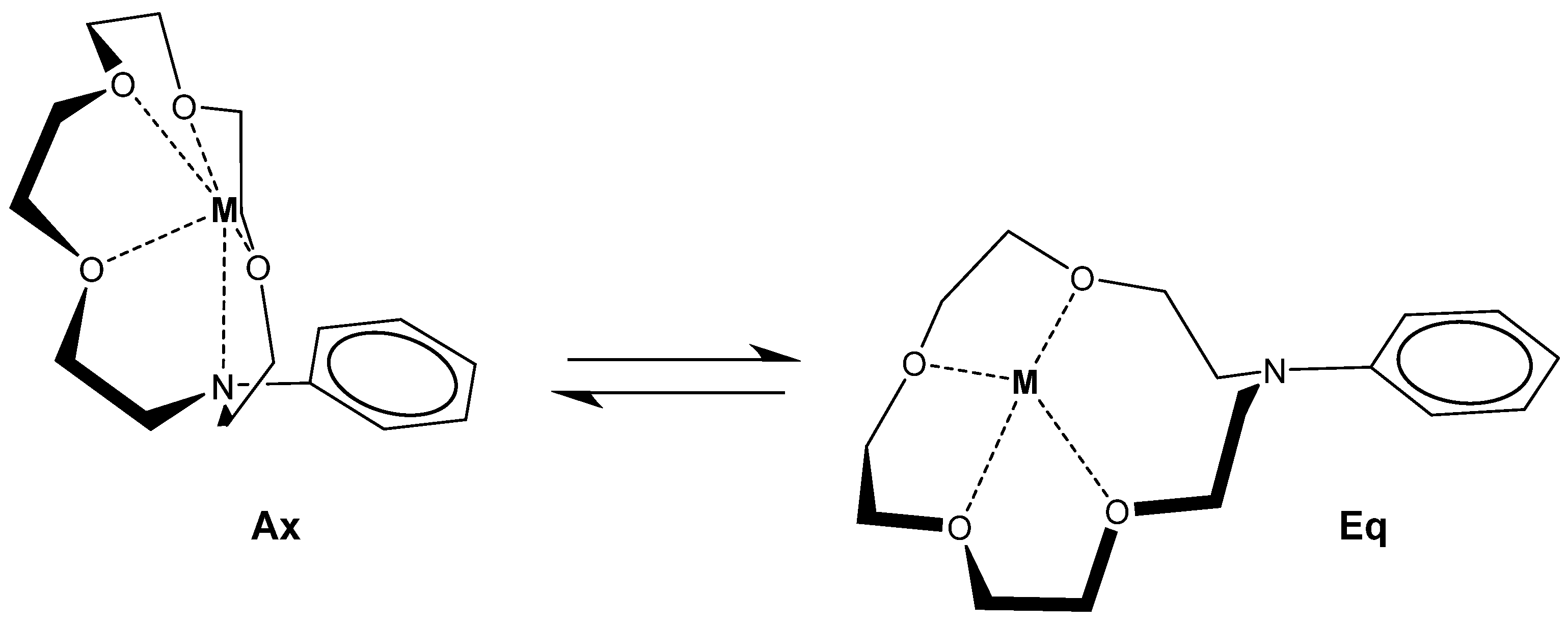
2.1.4. Mn+·CD6·(Mn+)2-Type Complexes
2.2. Quantum-Chemical Calculations
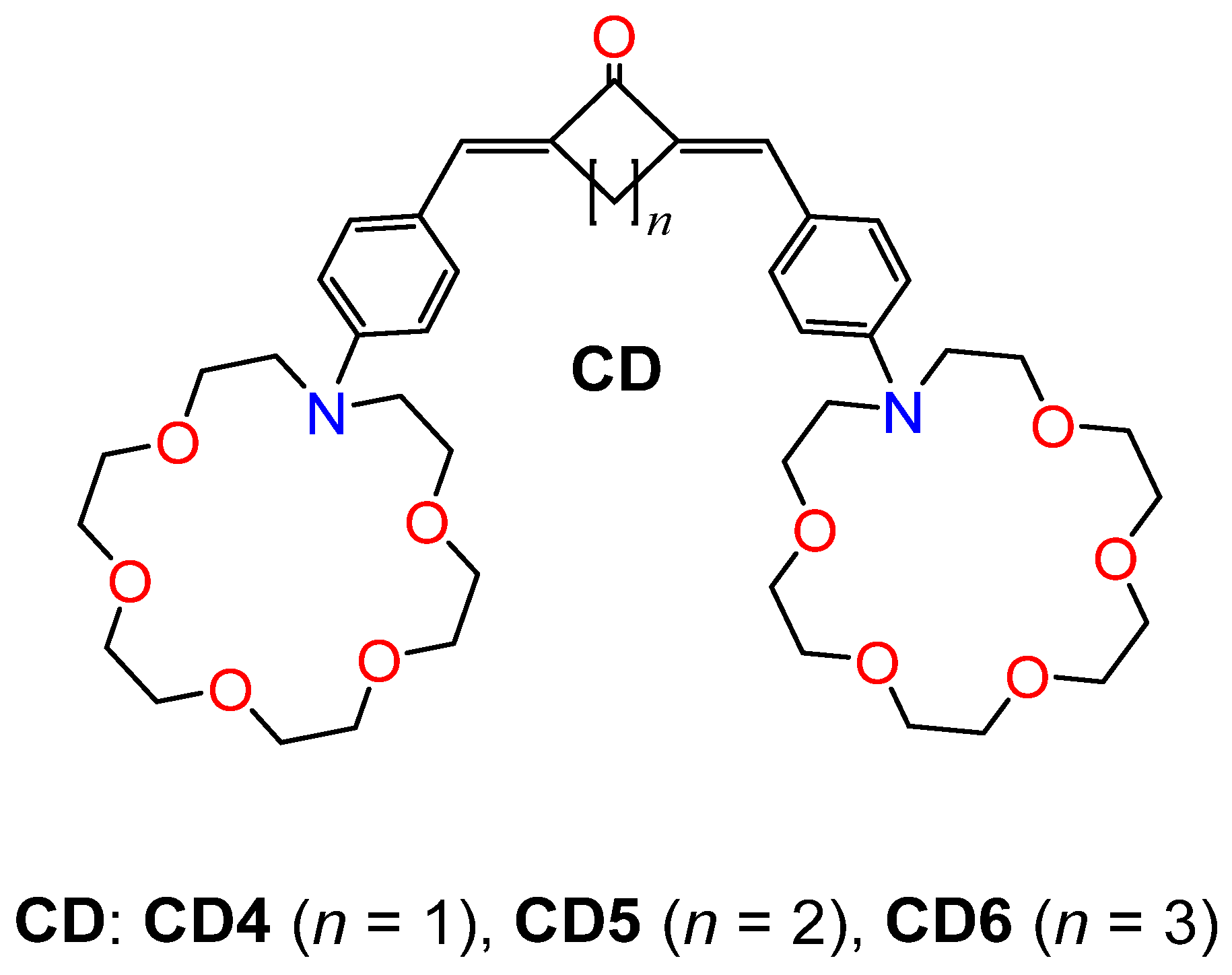
2.3. Structure vs. Properties for CD4–CD6 Dyes
2.4. Thermally Induced Recoordination
3. Materials and Methods
3.1. Materials and Synthesis
3.2. Time-Resolved Absorption Spectra
3.3. TD-DFT Computations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gangadhara; Kishore, K. Novel photocrosslinkable liquid-crystalline polymers: Poly[bis(benzylidene)] esters. Macromolecules 1993, 26, 2995–3003. [Google Scholar] [CrossRef]
- Kannan, P.; Gangadhara; Kishore, K. Novel photocrosslinkable flame retardant polyvanillylidene arylphosphate esters. Polymer 1997, 38, 4349–4355. [Google Scholar] [CrossRef]
- Yakimansky, A.V.; Tenkovtsev, A.V.; Dudkina, M.M.; Voigt-Martin, I.G.; Kolb, U.; Lukoshkin, V.A.; Böhme, F. Studies of structures and properties of polymeric systems containing bis-(hydroxy-arylidene)alkanones as NLO-active chromophores. J. Non-Cryst. Solids 2002, 303, 237–245. [Google Scholar] [CrossRef]
- Cui, J.; Crich, D.; Wink, D.; Lam, M.; Rheingold, A.L.; Case, D.A.; Fu, W.T.; Zhou, Y.; Rao, M.; Olson, A.J.; et al. Design and synthesis of highly constrained factor Xa inhibiors: Amidine-Substituted bis(benzoyl)-1,3-diazepan-2-ones and bis(benzylidene)-bis(gem-dimethyl)cycloketones. Bioorg. Med. Chem. 2003, 11, 3379–3392. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Chen, Q.; Yao, S.; Bai, E.; Fu, W.; Wang, L.; Wang, J.; Du, X.; Wei, T.; Xu, H.; et al. Synthesis and anti-tumor activity of EF24 analogues as IKKβ inhibitors. Eur. J. Med. Chem. 2018, 144, 218–228. [Google Scholar] [CrossRef]
- Cerosimo, U.; Sgorbissa, A.; Foti, C.; Drioli, S.; Angelica, R.; Thomasella, A.; Picco, R.; Semrau, M.S.; Storici, P.; Benedetti, F.; et al. Synthesis, characterization, and optimization for in vivo delivery of a nonselective isopeptidase inhibitor as new antineoplastic agent. J. Med. Chem. 2015, 58, 1691–1704. [Google Scholar] [CrossRef]
- Alatortsev, O.A.; Volchkov, V.V.; Melnikov, M.Y.; Rusalov, M.V.; Fomina, M.V.; Gromov, S.P. Complexation and photoinduced recoordination of bis(aza-18-crown-6)-containing dienones with alkali and alkaline-earth metal cations. Structure-property relationships. Russ. Chem. Bull. 2025, 73, 1578–1589. [Google Scholar]
- Volchkov, V.V.; Khimich, M.N.; Melnikov, M.Y.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Starostin, R.O.; Fomina, M.V.; Freidzon, A.Y.; Gromov, S.P. Femtosecond absorption spectroscopy of bis-aza-18-crown-6-containing dibenzylidenecyclobutanone complexes with alkali and alkali-earth metal cations. Mendeleev Commun. 2023, 33, 769–773. [Google Scholar] [CrossRef]
- Ley, C.; Lacombat, F.; Plaza, P.; Martin, M.M.; Leray, I.; Valeur, B. Femtosecond to subnanosecond multistep calcium photoejection from a crown ether-linked merocyanine. ChemPhysChem 2009, 10, 276–281. [Google Scholar] [CrossRef]
- Plaza, P.; Leray, I.; Changenet-Barret, P.; Martin, M.M.; Valeur, B. Reversible bulk photorelease of strontium ion from a crown-linked merocyanine. Chem. Phys. Chem. 2002, 3, 668–674. [Google Scholar] [CrossRef]
- Lewis, J.D.; Perutz, R.N.; Moore, J.N. Light-controlled ion switching: Direct observation of the complete nanosecond release and microsecond recapture cycle of an azacrown-substituted [(bpy)Re(CO)3L]+ complex. J. Phys. Chem. 2004, 108, 9037–9047. [Google Scholar] [CrossRef]
- Thieulloy, L.D.; Barois, C.; Mongin, C.; Leray, I.; Guerrin, C.; Aloise, S.; Buntinx, A.; Perrier, J. Is it possible to “simply” predict the photoejection of a cation? Example of azacrown-substituted [(bpy)Re(CO)]. J. Photochem. Photobiol. A Chem. 2022, 426, 113714–113725. [Google Scholar] [CrossRef]
- Douhal, A.; Roshal, A.D.; Organero, J.A. Stepwise interactions, sodium ion photoejection and proton transfer inhibition in a crown-ether and proton-transfer dye. Chem. Phys. Lett. 2003, 381, 519–525. [Google Scholar] [CrossRef]
- Lednev, I.K.; Ye, T.-Q.; Hester, R.E.; Moore, J.N. Photocontrol of cation complexation with a benzothiazolium styryl azacrown ether dye: Spectroscopic studies on picoseconds and kiloseconds time scale. J. Phys. Chem. 1997, 101, 4966–4972. [Google Scholar] [CrossRef]
- Lednev, I.K.; Hester, R.E.; Moore, J.N. Direct observation of photocontrolled ion release: A nanosecond time-resolved spectroscopic study of a benzothiazolium styryl azacrown ether dye complexed with barium. J. Phys. Chem. 1997, 101, 7371–7378. [Google Scholar] [CrossRef]
- Freidzon, A.Y.; Bagatur’yants, A.A.; Gromov, S.P.; Alfimov, M.V. Recoordination of a metal ion in the cavity of a crown compound: A theoretical study. 1. Conformers of arylazacrown ethers and their complexes with Ca2+ cation. Russ. Chem. Bull. 2003, 52, 2646–2655. [Google Scholar] [CrossRef]
- Baroncini, M.; Canton, M.; Casimiro, L.; Corra, S.; Groppi, J.; Rosa, M.; Silvi, S.; Credi, A. Photoactive molecular-based devices, machines and materials: Recent advances. Eur. J. Inorg. Chem. 2018, 42, 4589–4603. [Google Scholar]
- Ceroni, P.; Credi, A.; Venturi, M. Light to investigate (read) and operate (write) molecular devices and machines. Chem. Soc. Rev. 2014, 43, 4068–4083. [Google Scholar] [CrossRef]
- Budyka, M.F. Molecular switches and logic gates for information processing, the bottom-up strategy: From silicon to carbon, from molecules to supermolecules. Russ. Chem. Rev. 2017, 86, 181–210. [Google Scholar] [CrossRef]
- Mako, L.T.; Racicot, J.M.; Levine, M. Supramolecular luminescent sensors. Chem. Rev. 2019, 119, 322–477. [Google Scholar] [CrossRef]
- Ragazzon, G.; Baroncini, M.; Silvi, S.; Venturi, M.; Credi, A. Light-powered, artificial molecular pumps: A minimalistic approach. Beilstein J. Nanotechnol. 2015, 6, 2096–2104. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. 2005. Available online: https://webbook.nist.gov/chemistry/ (accessed on 1 March 2025).
- Gromov, S.P.; Dmitrieva, S.N.; Vedernikov, A.I.; Kurchavov, N.A.; Kuz’mina, L.G.; Sazonov, S.K.; Strelenko, Y.A.; Alfimov, M.V.; Howard, J.A.K.; Ushakov, E.N. Synthesis, structure, and characterization of chromo(fluoro)ionophores with cation-triggered emission based on N-methylazacrown-ether styryl dyes. J. Org. Chem. 2013, 78, 9834–9847. [Google Scholar] [CrossRef] [PubMed]
- Fomina, M.V.; Freidzon, A.Y.; Kuz’mina, L.G.; Moiseeva, A.A.; Starostin, R.O.; Kurchavov, N.A.; Nuriev, V.N.; Gromov, S.P. Synthesis, structure and photochemistry of dibenzylidenecyclobutanones. Molecules 2022, 27, 7602. [Google Scholar] [CrossRef] [PubMed]
- Shelaev, I.V.; Gostev, F.E.; Vygodina, T.V.; Lepeshkevich, S.V.; Dzhagarov, B.M. Femtoseconds absorption spectroscopy of reduced and oxidized forms of cytochrome c oxidase: Excited states and relaxation processes in heme a and a3 centers. Opt. Spectrosc. 2019, 127, 756–762. [Google Scholar] [CrossRef]
- Demas, J.N.; Crosby, G.A. Measurement of photoluminescence quantum yields. J. Phys. Chem. 1971, 75, 991–1024. [Google Scholar] [CrossRef]
- Granovsky, A.A. Firefly 8.2, Build 10203. 2017. Available online: http://classic.chem.msu.su/gran/firefly/index.html/ (accessed on 1 February 2025).
- Neese, F.; Wennmohs, F.; Becker, U.; Riplinger, C. The ORCA quantum chemistry program package. J. Chem. Phys. 2020, 152, 224108. [Google Scholar] [CrossRef]
- Kohn, W.; Becke, A.D.; Parr, R.G. Density functional theory of electronic structure. J. Phys. Chem. 1996, 100, 12974–12980. [Google Scholar] [CrossRef]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elber, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matgunaga, N.; Nguyen, K.A.; Su, S.J.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347. [Google Scholar] [CrossRef]
- Marenich, A.V.; Cramer, C.J.; Trular, D.G. Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions. Phys. Chem. B 2009, 18, 6378–6396. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Vosko, S.H.; Wilk, L.; Nusair, M. Accurate spin-dependent electron liquid correlation energies for local spin density calculations: A critical analysis. Can. J. Phys. 1980, 58, 1200–1211. [Google Scholar] [CrossRef]

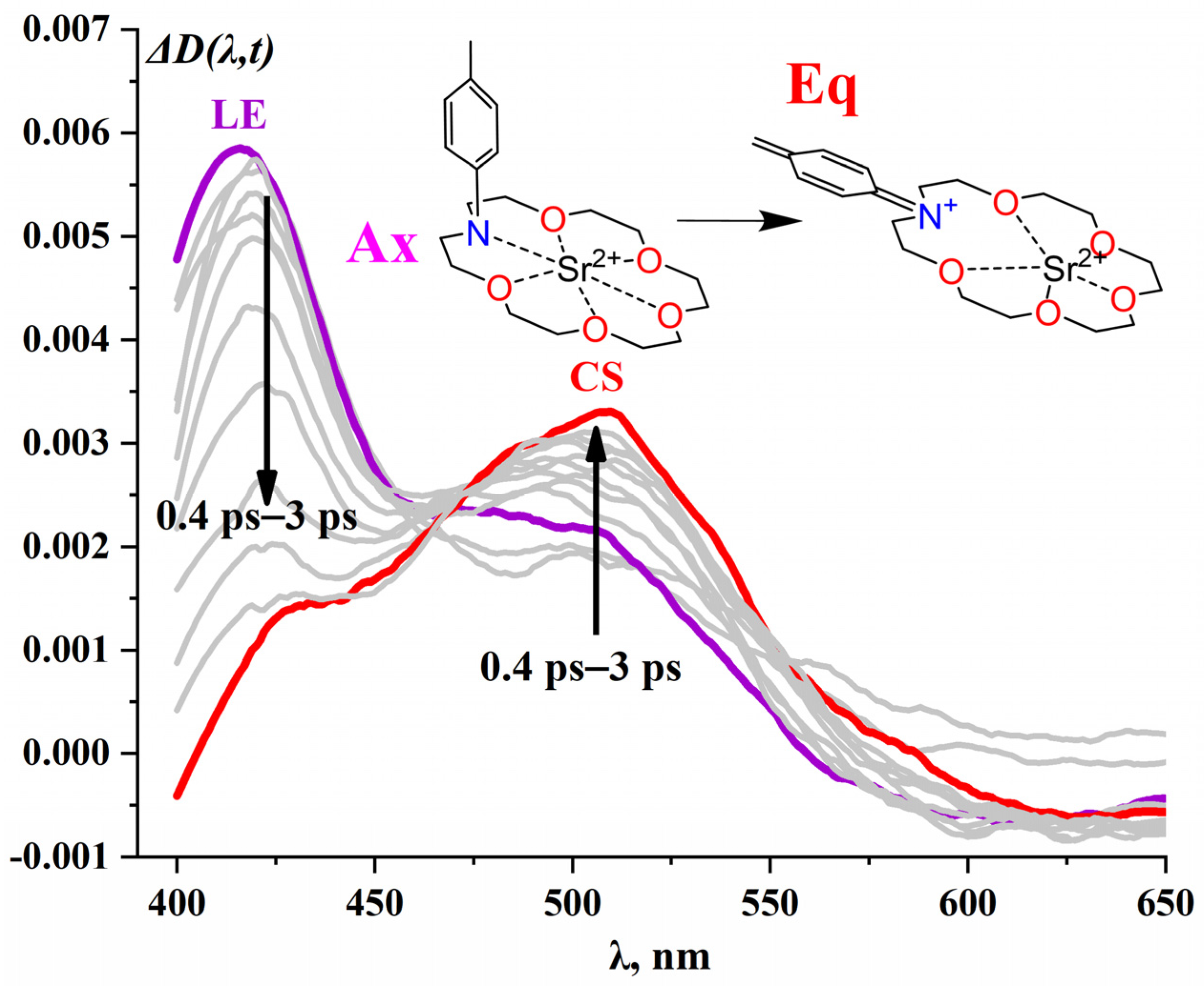
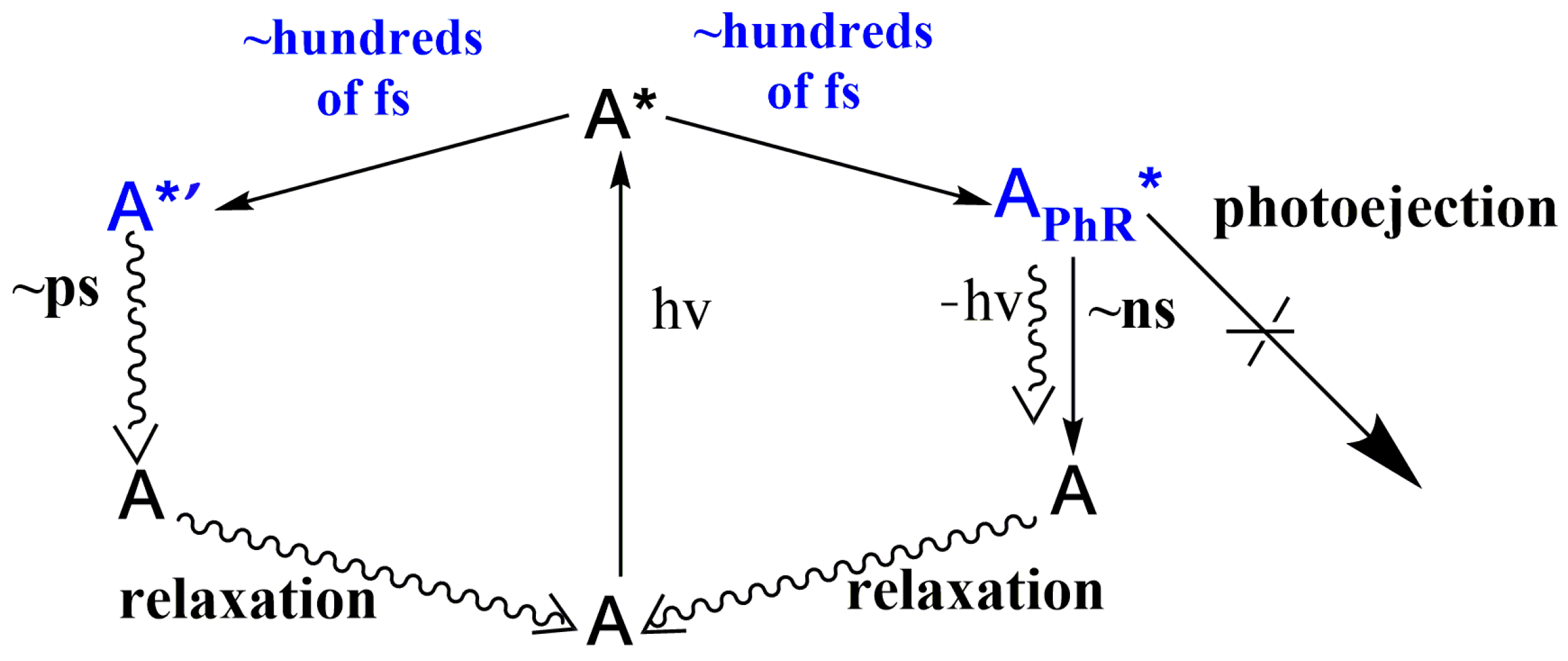

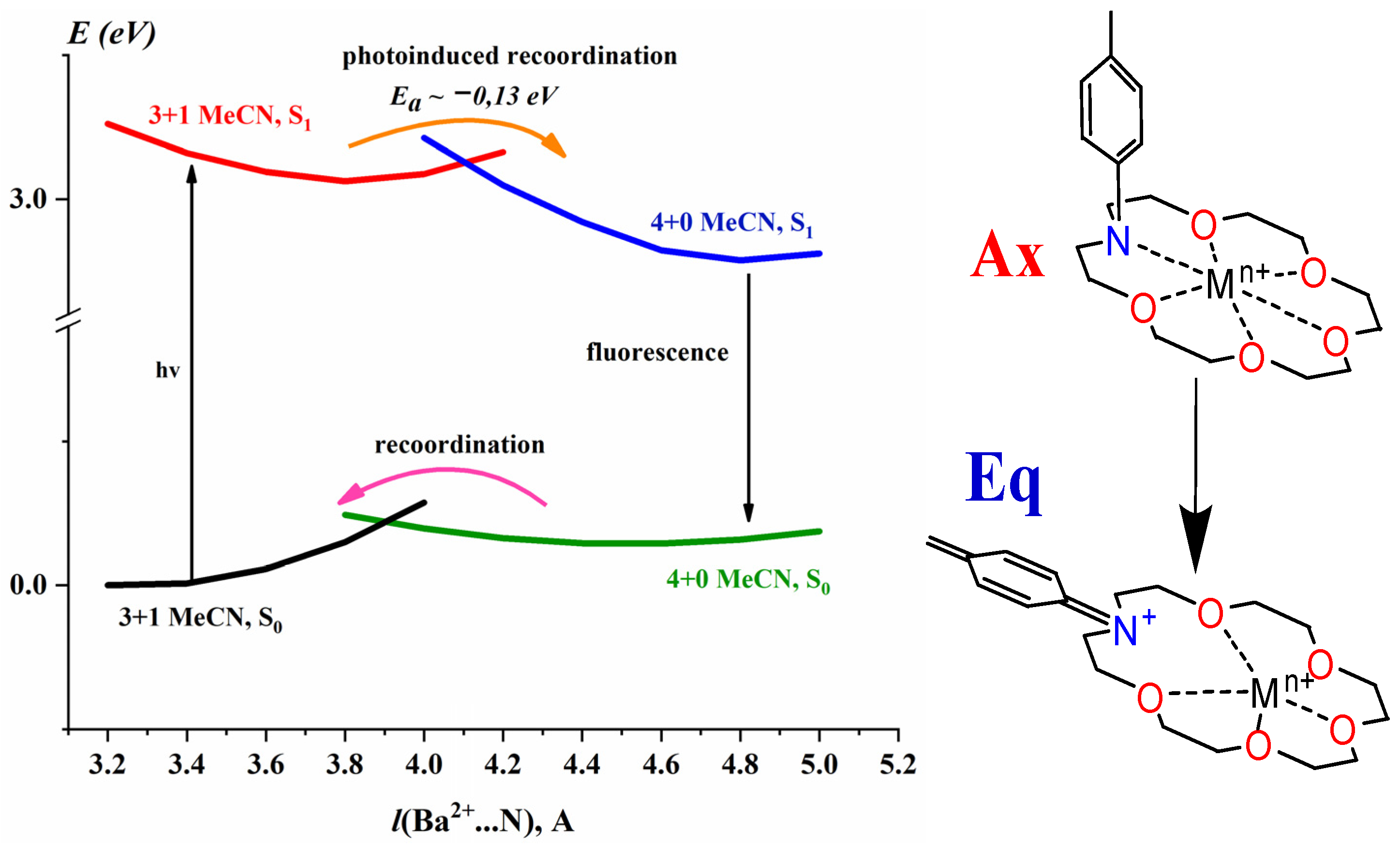
| System | λTAmax, nm | t1, fs | t2, ps | t3, ns | lgKs1:2 b | λSEmax, nm | φf c | kf × 10−8, c−1 | kd × 10−9, c−1 |
|---|---|---|---|---|---|---|---|---|---|
| CD6 | 500 | 236 ± 4 | — | 0.167 ± 0.001 | — | 630 | ~0.03 | 1.79 | 5.79 |
| CD6·(Sr2+)2 a | 416 | 124 ± 3 | 2.71 ± 0.07 | — | 5.29 | ~600 | ~0.01 | 0.80 | 7.92 |
| 500 | 875 ± 25 | — | 0.125 ± 0.004 | ||||||
| CD6·(Ba2+)2 | 417 | 111 ± 2 | 2.94 ± 0.07 | — | 5.57 | ~600 | <0.01 | — d | — d |
| 500 | 934 ± 97 e | 27 ± 7 | — | ||||||
| CD6·(Ca2+)2 | 450 | 102 ± 6 f | 1 ± 0.1 f | — | 4.29 | ~600 | ~0.02 | 0.41 | 1.74 |
| 500 | 443 ± 10 f | — | 0.562 ± 0.029 | ||||||
| CD6·(K+)2 | — g | — | — | — | 2.72 | ~620 | ~0.02 | 1.20 | 6.20 |
| 495 | 181 ± 4 | — | 0.158 ± 0.002 | ||||||
| CD6·(H+)2 | 408 | 191 ± 5 | 2.21 ± 0.05 | — d | — d | — d | — d | — d | — d |
| System | λexc, nm | λTAmax, nm | t1, fs | t3, ns | lgKs1:3 a | λSE, nm | q/d, 1/Å b |
|---|---|---|---|---|---|---|---|
| Na+·CD6·(Na+)2 | 440 | - c | - | - | ~0 | 610 | ~0.14 |
| 500 | 454 ± 8 | 0.217 ± 0.002 | |||||
| Li+·CD6·(Li+)2 | 515 | - c | - | - | ~0 | 625 | ~0.58 |
| 535 | 500 ± 5 | 0.153 ± 0.001 | |||||
| Mg2+·CD6·(Mg2+)2 | 515 | - c | - | - | ~0.32 | ~630 | ~0.91 |
| - | 191 ± 7 | 0.088 ± 0.001 d |
| System | λamax, nm | λTAmax, nm | λSEmax, nm | t1, fs | t3, ns | φf a | kf × 10−8, s−1 | kd × 10−9, s−1 |
|---|---|---|---|---|---|---|---|---|
| CD4 | 475 | 523 | 596 | ~430 | 1.33 | ~0.20 | 1.50 | 0.60 |
| CD5 | 466 | 511 | 605 | ~340 | 0.77 | ~0.13 | 1.70 | 1.13 |
| CD6 | 436 | 500 | 630 | ~236 | 0.17 | ~0.03 | 1.79 | 5.79 |
| System | lgKs1:2 | ∆λa1;2, nm | λfmax, nm | λTAmax, nm | λSEmax, nm | t1, fs | t3, ns | φf | kf × 10−8, s−1 | kd × 10−9, s−1 |
|---|---|---|---|---|---|---|---|---|---|---|
| CD4·(Ba2+)2 | 3.68 | 104 | 612 | 504 | ~598 | ~520 | ~1.55 | 0.169 | 1.09 | 0.50 |
| CD4·(Ca2+)2 | 4.05 | 33 | 604 | 496 | ~598 | ~230 | ~1.53 | 0.151 | 0.99 | 0.56 |
| CD4·(K+)2 | 1.77 | 30 | 609 | ~502 | ~605 | ~250 | ~0.98 | 0.085 | 0.87 | 0.93 |
| System | ∆λa1:3, nm | λTAmax, nm | t1, fs | t3, ns | lgKs1:3 | λSEmax, nm |
|---|---|---|---|---|---|---|
| Mg2+·CD4·(Mg2+)2 | −52 | 497 | ~130 | 0.82 | 2.1 | 644 |
| Mg2+·CD5·(Mg2+)2 | −59 | 490 | ~140 | 0.47 | 1.9 | 607 |
| Mg2+·CD6·(Mg2+)2 | −49 | – | 191 ± 7 | 0.088 | ~0.3 | 630 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alatortsev, O.A.; Volchkov, V.V.; Khimich, M.N.; Sorokin, I.D.; Melnikov, M.Y.; Gostev, F.E.; Shelaev, I.V.; Nadtochenko, V.A.; Fomina, M.V.; Gromov, S.P. Investigation of Photorecoordination Kinetics for Complexes of Bis(aza-18-crown-6)-Containing Dienones with Alkali and Alkaline-Earth Metal Cations via Time-Resolved Absorption Spectroscopy: Structure vs. Properties. Molecules 2025, 30, 4005. https://doi.org/10.3390/molecules30194005
Alatortsev OA, Volchkov VV, Khimich MN, Sorokin ID, Melnikov MY, Gostev FE, Shelaev IV, Nadtochenko VA, Fomina MV, Gromov SP. Investigation of Photorecoordination Kinetics for Complexes of Bis(aza-18-crown-6)-Containing Dienones with Alkali and Alkaline-Earth Metal Cations via Time-Resolved Absorption Spectroscopy: Structure vs. Properties. Molecules. 2025; 30(19):4005. https://doi.org/10.3390/molecules30194005
Chicago/Turabian StyleAlatortsev, Oleg A., Valeriy V. Volchkov, Mikhail N. Khimich, Ivan D. Sorokin, Mikhail Ya. Melnikov, Fedor E. Gostev, Ivan V. Shelaev, Victor A. Nadtochenko, Marina V. Fomina, and Sergey P. Gromov. 2025. "Investigation of Photorecoordination Kinetics for Complexes of Bis(aza-18-crown-6)-Containing Dienones with Alkali and Alkaline-Earth Metal Cations via Time-Resolved Absorption Spectroscopy: Structure vs. Properties" Molecules 30, no. 19: 4005. https://doi.org/10.3390/molecules30194005
APA StyleAlatortsev, O. A., Volchkov, V. V., Khimich, M. N., Sorokin, I. D., Melnikov, M. Y., Gostev, F. E., Shelaev, I. V., Nadtochenko, V. A., Fomina, M. V., & Gromov, S. P. (2025). Investigation of Photorecoordination Kinetics for Complexes of Bis(aza-18-crown-6)-Containing Dienones with Alkali and Alkaline-Earth Metal Cations via Time-Resolved Absorption Spectroscopy: Structure vs. Properties. Molecules, 30(19), 4005. https://doi.org/10.3390/molecules30194005







