Optimal Extraction of Antioxidants, Flavonoids, and Phenolic Acids from the Leaves of Apocynum venetum L. by Response Surface Methodology with Integrated Chemical Profiles and Bioactivity Evaluation
Abstract
1. Introduction
2. Results and Discussion
2.1. Phytochemical Profile of the Leaves of A. venetum L. by UHPLC-QTOF-MS
2.2. Model Adequacy
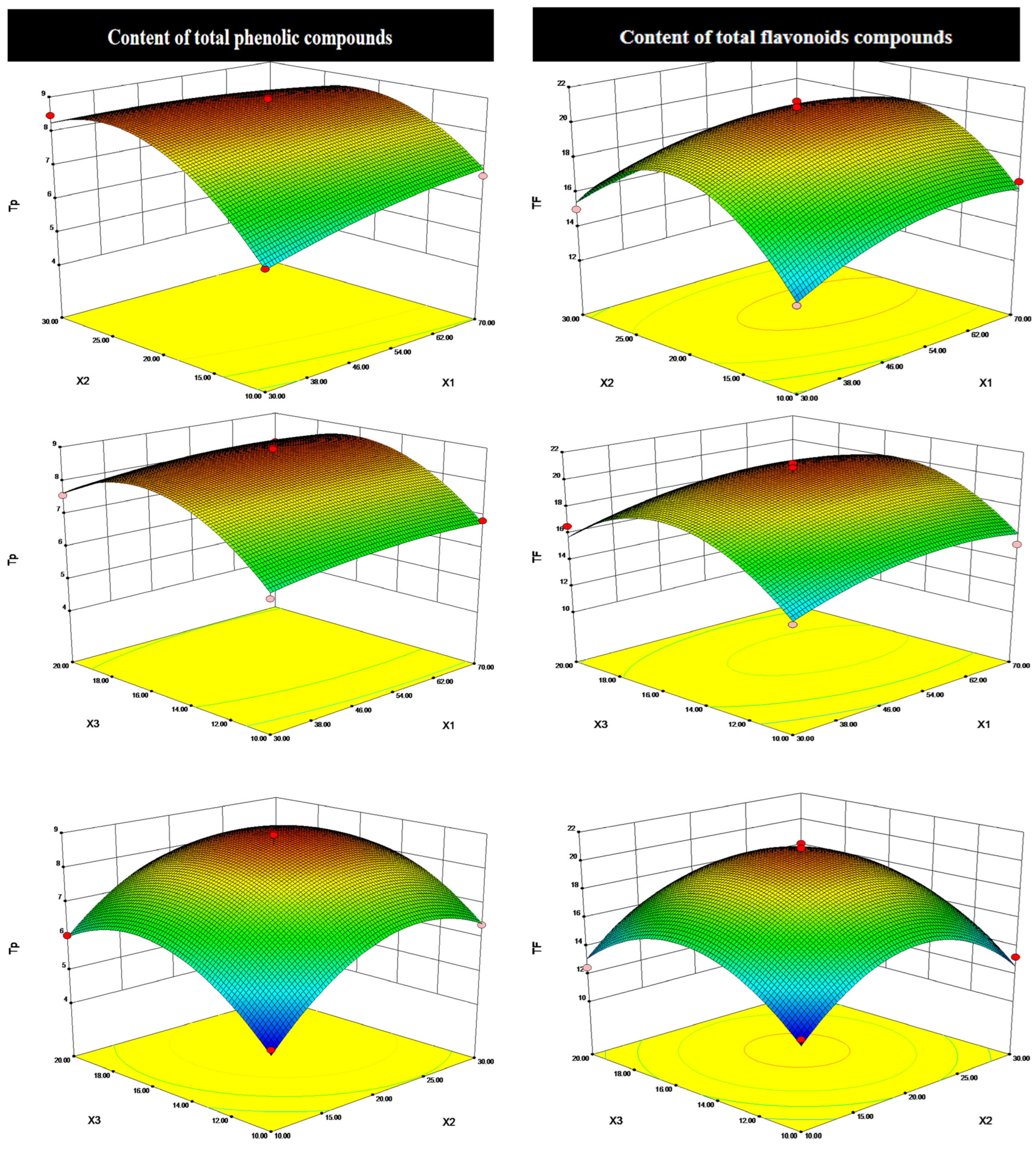
2.3. Optimization and Validation of the Extraction Conditions
2.4. Comparison of Present Method with Other Reported Methods
2.5. Contribution of Phenolic Acids and Flavonoids to Antioxidant Activity
3. Materials and Methods
3.1. Materials, Chemicals and Reagents
3.2. Preparation of Standard Solution
3.3. Preparation of Samples Solution
3.3.1. Conventional Refluxing Extraction
3.3.2. Ultrasound Extraction
3.4. Optimization of Processing Method by Box–Behnken Design (BBD)
3.5. Qualitative and Quantitative Analysis of Phytochemicals by UHPLC-DAD-QTOF-MS
3.6. Semi-Quantitative Analysis
3.7. Determination of Antioxidant Activities
3.7.1. ABTS Assay
3.7.2. DPPH Assay
3.8. Verification of the Model
3.9. Correlations Between Levels of the Major Chemical Constituents and Their Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abubakar, A.S.; Ahmad, B.; Ahmad, N.; Liu, L.L.; Liu, B.; Qu, Y.T.; Chen, J.K.; Chen, P.; Zhao, H.H.; Chen, J.; et al. Physicochemical evaluation, structural characterization, in vitro and in vivo bioactivities of water-soluble polysaccharides from Luobuma (Apocynum L.) tea. Food Chem. 2024, 460, 140453. [Google Scholar] [CrossRef]
- Xie, W.L.; Li, F.J.; Ding, X.R.; Xu, Z.C.; Cui, Y.Y.; Fu, X.J.; Xu, K. Ethnomedical uses, phytochemistry and pharmacology of Apocynum venetum L. J. Ethnopharmacol. 2025, 337, 118967. [Google Scholar] [CrossRef]
- Lv, J.; Xu, X.X.; Gong, J.X.; Wang, Z.H.; Shi, D.W.; Dai, L.J. Extraction of polyphenols from Apocynum venetum leaves using customized deep eutectic solvents: Process optimization and antioxidant evaluation. Process Biochem. 2024, 147, 305–317. [Google Scholar] [CrossRef]
- Li, C.; Tan, F.; Yang, J.J.; Yang, Y.; Gou, Y.T.; Li, S.T.; Zhao, X. Antioxidant Effects of Apocynum venetum Tea Extracts on d-Galactose-Induced Aging Model in Mice. Antioxidants 2019, 8, 381. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, S.; Ma, J.L.; Chen, C.; Huang, P.; Ji, J.H.; Wu, D.; Ren, L.Q. Apocynum venetum leaf extract alleviated doxorubicin-induced cardiotoxicity through the AKT/Bcl-2 signaling pathway. Phytomedicine 2022, 94, 153815. [Google Scholar] [CrossRef]
- Feng, Y.L.; Jiang, C.; Yang, F.; Chen, Z.X.; Li, Z. Apocynum venetum leaf extract protects against H2O2-induced oxidative stress by increasing autophagy in PC12 cells. Biomed. Rep. 2020, 13, 6. [Google Scholar] [CrossRef]
- Fu, H.M.; Yin, C.L.; Shen, Z.Y.; Yang, M.H. Flavonoids from the leaves of Apocynum venetum and their anti-inflammatory activity. J. Chem. Res. 2022, 46, 17475198211073871. [Google Scholar] [CrossRef]
- Xiang, T.; Wu, L.J.; Isah, M.B.; Chen, C.; Zhang, X.Y. Apocynum venetum, a medicinal, economical and ecological plant: A review update. PeerJ 2023, 11, e14966. [Google Scholar] [CrossRef]
- Kim, D.; Choi, G.; Kim, S.; Kim, J.; Oh, D.; Jin, T.; Yang, J.W.; Cho, S. Sleep-promoting effects and potential mechanisms of Apocynum venetum leaf extract (VENETRON®): Effects of acute and chronic treatment on the sleep-wake profiles in mice. J. Ethnopharmacol. 2025, 350, 120008. [Google Scholar] [CrossRef]
- Chinese Pharmacopeia Commission. Pharmacopeia of the People’s Republic of China, 2025th ed.; China Medical Science Publisher: Beijing, China, 2025; pp. 228–229. [Google Scholar]
- Shi, Q.M.; Zhang, K.; Wu, Q.M.; Yin, C.H. Effect of detection instrument and digestion methods on the detection of trace elements of Apocynum venetum. J. Food Saf. Qual. 2020, 11, 926–931. [Google Scholar] [CrossRef]
- Zhang, D.M.; Li, Y.G.; Ma, W.X.; Zhu, Y.Q.; Dai, N.F. Research on the extraction of flavonoids from Apocynum venetum L. leaves by ultrasonic-assisted aqueous two-phase method and their antioxidant properties. Food Sci. Technol. 2025, 50, 254–262. [Google Scholar] [CrossRef]
- Shao, D.Y.; Gao, G.; Abubakar, A.S.; Hazaisi, H.; Chen, P.; Chen, J.K.; Chen, K.M.; Wang, X.F.; Wang, Y.; Chen, Y.; et al. Total Flavonoids Extracts of Apocynum L. from the Ili River Valley Region at Different Harvesting Periods and Bioactivity Analysis. Molecules 2022, 27, 7343. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Liu, Q.Y.; Fan, K.J.; Mei, H. Study on enrichment and purification technology of total flavonoids from lotus leaves and Apocynum venetum L. Cereals Oils 2022, 38, 1–3+11. [Google Scholar] [CrossRef]
- Sun, S.W.; Zhao, Y.W.; Wang, L.Y.; Tan, Y.Z.; Shi, Y.N.; Sedjoah, R.-C.A.-A.; Shao, Y.T.; Li, L.X.; Wang, M.X.; Wan, J.S.; et al. Ultrasound-assisted extraction of bound phenolic compounds from the residue of Apocynum venetum tea and their antioxidant activities. Food Biosci. 2022, 47, 101646. [Google Scholar] [CrossRef]
- Tan, Z.J.; Yi, Y.J.; Wang, H.Y.; Zhou, W.L.; Wang, C.Y. Extraction, Preconcentration and Isolation of Flavonoids from Apocynum venetum L. Leaves Using Ionic Liquid-Based Ultrasonic-Assisted Extraction Coupled with an Aqueous Biphasic System. Molecules 2016, 21, 262. [Google Scholar] [CrossRef]
- Lin, X.; Wang, Y.Z.; Liu, X.J.; Huang, S.Y.; Zeng, Q. ILs-based microwave-assisted extraction coupled with aqueous two-phase for the extraction of useful compounds from Chinese medicine. Analyst 2012, 137, 4076–4085. [Google Scholar] [CrossRef]
- Wang, H.Z.; Zhang, C.; Liu, C. Optimization of Extraction Process of Total Flavones from Apocynum venetum L. with Microwave-Method by Orthogonal Experiment. J. Jilin Inst. Chem. Technol. 2013, 30, 22–25. [Google Scholar] [CrossRef]
- Ran, M.L.; Li, X.F.; Luo, L.J.; Yu, L.; Luo, D.; Li, P.; Li, P.P. Optimization of Extraction Technology of Total Flavone in Apocynum venetum by Central Composite Design-Response Surface Methodology. J. Chengdu Univ. TCM 2014, 37, 4–7. [Google Scholar] [CrossRef]
- Bai, M.; Li, L.Z.; Wang, Y.N.; Wang, X.L. The Optimization of Flash-type Extractional Process of Total Flavonoids in Apocynum venetum Leaves by Orthogonal Experiment. J. Anhui Agric. Sci. 2013, 41, 7761–7762, 7767. [Google Scholar] [CrossRef]
- Wu, D.Q.; Yang, X.J.; An, H.G.; Lin, M.; Ren, X.F. Optimization of extraction technology of flavonoids from Apocynun venetum leaves by response surface methodology. Sci. Technol. Food Ind. 2011, 32, 327–329. [Google Scholar] [CrossRef]
- Yunusjan, T.; Turghun, M. Extraction of Polyphenols from Apocynum venetum L. Leaves and Antioxidant Activity. Lishizhen Med. Mater. Med. Res. 2012, 23, 818–820. [Google Scholar] [CrossRef]
- Liu, D.; Wang, S.Y.; Bao, Y.L.; Zheng, L.H.; Wang, G.N.; Sun, Y.; Yang, X.G.; Liu, L. Extraction, purification and structural characterization of polysaccharides from Apocynum venetum L. roots with anti-inflammatory activity. Process Biochem. 2022, 121, 100–112. [Google Scholar] [CrossRef]
- Peng, J.; Abdulla, R.; Li, Y.; Liu, X.Y.; He, F.; Xin, X.L.; Aisa, H.A. Potential anti-diabetic components of Apocynum venetum L. flowers: Optimization, chemical characterization and quality evaluation. J. Food Compos. Anal. 2023, 115, 104930. [Google Scholar] [CrossRef]
- Jing, C.L.; Dong, X.F.; Tong, J.M. Optimization of Ultrasonic-Assisted Extraction of Flavonoid Compounds and Antioxidants from Alfalfa Using Response Surface Method. Molecules 2015, 20, 15550–15571. [Google Scholar] [CrossRef]
- Edirs, S.; Turak, A.; Numonov, S.; Xin, X.L.; Aisa, H.A. Optimization of Extraction Process for Antidiabetic and Antioxidant Activities of Kursi Wufarikun Ziyabit Using Response Surface Methodology and Quantitative Analysis of Main Components. Evid. Based Complement. Alternat. Med. 2017, 2017, 6761719. [Google Scholar] [CrossRef]
- Li, R.F.; Zhu, H.; Du, W.J.; Li, G.P.; Zhao, Y.Z.; Song, K.H.; Qiu, J.; Liu, J.J.; Fang, S. Effect of ultrasound-assisted extraction combined with enzymatic pretreatment on bioactive compounds, antioxidant capacity and flavor characteristics of grape pulp extracts. Ultrason. Sonochem. 2025, 121, 107572. [Google Scholar] [CrossRef]
- Parveen, S.; Bukhari, N.; Nazir, M.; Qureshi, W.A.; Yaqoob, A.; Shahid, M. Phytochemical analysis, in-vitro biological activities and Pearson correlation of total polyphenolic content with antioxidant activities of Ziziphus mauritiana fruit pulp and seed during different ripening stages. S. Afr. J. Bot. 2023, 157, 346–354. [Google Scholar] [CrossRef]
- Li, F.; Xiang, T.J.; Jiang, L.; Cheng, Y.; Song, G.S.; Wang, D.L.; Yuan, T.L.; Li, L.; Chen, F.; Luo, Z.S.; et al. New insights into ultrasound-assisted noncovalent nanocomplexes of β-lactoglobulin and neochlorogenic acid/cryptochlorogenic acid and its potential application for curcumin loading. Food Res. Int. 2025, 199, 115384. [Google Scholar] [CrossRef]
- Rüfer, C.E.; Maul, R.; Donauer, E.; Fabian, E.J.; Kulling, S.E. In vitro and in vivo metabolism of the soy isoflavone glycitein. Mol. Nutr. Food Res. 2007, 51, 813–823. [Google Scholar] [CrossRef]
- Ossowski, S.; Rybak, K.; Pobiega, K.; Sękul, J.; Domżalska, Z.; Gregorek, K.; Gramza-Michałowska, A.; Janiszewska-Turak, E. Antioxidant Activity and Microbial Quality of Freeze-Dried, Lactic Acid Fermented Peach Products. Molecules 2025, 30, 2360. [Google Scholar] [CrossRef]
- Yang, N.; Chen, P.; Li, W.H.; Feng, C.P.; Wang, X.W.; Wang, Q.L. Synthesis of Nano-Selenium from Bombyx batryticatus Polypeptide and Exploring Its Antioxidant and Skin Whitening Ability. Molecules 2025, 30, 1153. [Google Scholar] [CrossRef]
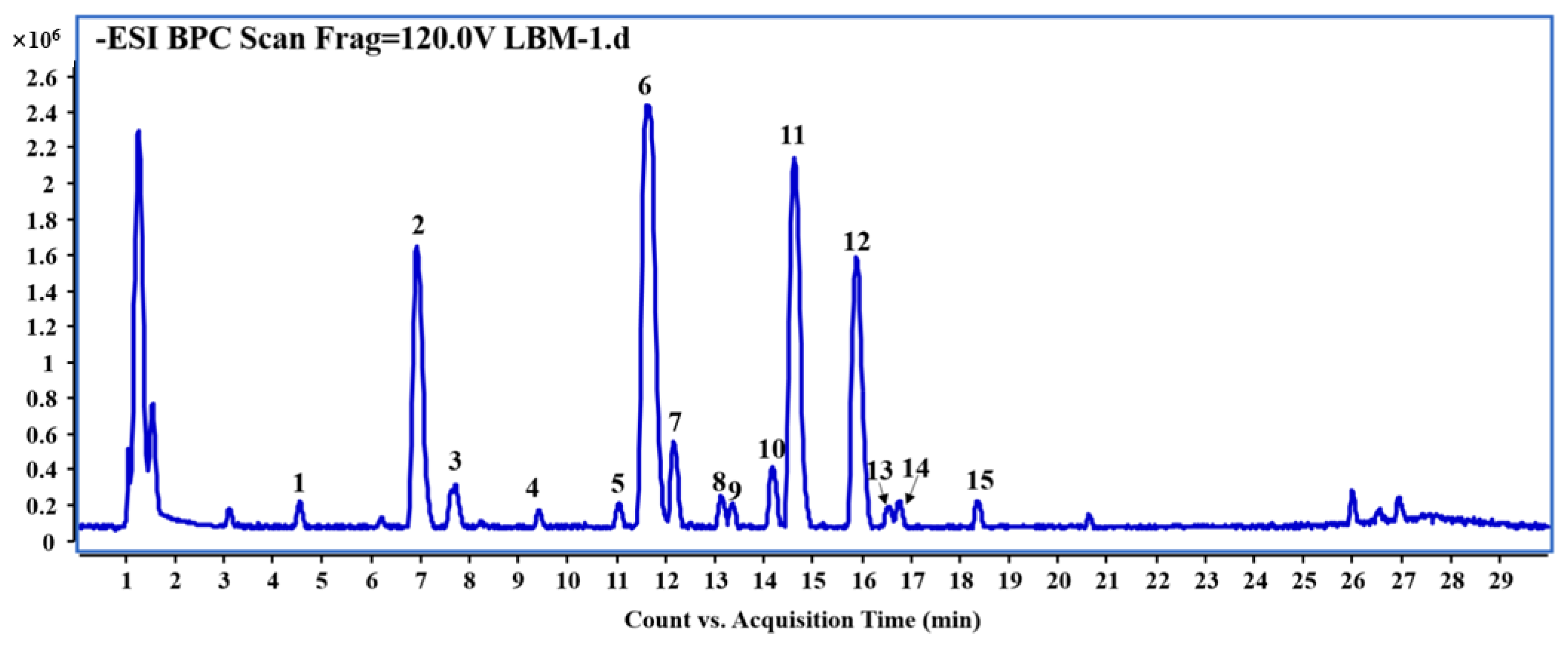


| Peak | tR | Identification | Formula | Quasi-Molecular | Observed | Calculated | ppm | Fragement Ions |
|---|---|---|---|---|---|---|---|---|
| No. | (min) | Ion | Mass (Da) | Mass (Da) | ||||
| 1 | 4.56 | Neochlorogenic acid (NCA) | C16H18O9 | [M−H]− | 353.0889 | 353.0878 | 3.12 | 191.0595 [M−H-C9H6O3]− 179.0384 [M−H-C7H10O5]− |
| 2 | 6.98 | Chlorogenic acid (CA) | C16H18O9 | [M−H]− | 353.0893 | 353.0878 | 4.25 | 191.0606 [M−H-C9H6O3]− |
| [2M−H]− | 707.1812 | 707.1829 | 2.40 | 179.0376 [M−H-C7H10O5]− | ||||
| 3 | 7.70 | Cryptochlorogenic acid (CCA) | C16H18O9 | [M−H]− | 353.0890 | 353.0878 | 3.40 | 191.0594 [M−H-C9H6O3]− |
| 179.0378 [M−H-C7H10O5]− | ||||||||
| 4 | 9.40 | (−)-Epicatechin (EC) | C15H14O6 | [M−H]− | 289.0737 | 289.0718 | 6.43 | 245.0847 [M−H-CO2]− |
| 5 | 11.04 | Rutin-hexoside (RH) | C33H40O21 | [M−H]− | 771.1969 | 771.1989 | −2.59 | 609.1435 [M−H-C6H10O5]− |
| 301.0355 [M−H-C6H10O5 -C12H20O9]− | ||||||||
| 6 | 11.64 | Quercetin 3-O-β-D-glucosyl-(1→2)-β-D- glucoside (Baimaside, BM) | C27H30O17 | [M−H]− | 625.1416 | 625.1410 | 0.96 | 463.0865 [M−H-C6H10O5]− |
| 301.0351 [M−H-2×C6H10O5]− | ||||||||
| 7 | 12.18 | Apocynoside I (AI) | C19H30O8 | [M+Cl]− | 421.1630 | 421.1635 | −1.19 | 223.1368 [M−H-C6H10O5]− |
| [M+HCOO]− | 431.1919 | 431.1923 | −0.93 | 205.1264 [M−H-C6H10O5 -H2O]− | ||||
| 8 | 13.14 | Quercetin 3-O-(6‴-O-malonyl)-β-D- glucosyl- (1→2)-β-D-glucoside (Malonated baimaside, MBM) | C30H32O20 | [M−H]− | 711.1389 | 711.1414 | −3.52 | 625.1395 [M−H-C3H2O3]− |
| 463.0868 [M−H-C3H2O3 -C6H10O5]− | ||||||||
| 301.0346 [M−H-C3H2O3 -2×C6H10O5]− -2×C6H10O5]− | ||||||||
| 9 | 13.34 | Rutin (Ru) | C27H30O16 | [M−H]− | 609.1436 | 609.1461 | −4.10 | 301.0347 [M−H-C12H20O9]− |
| [M+Cl]− | 645.1207 | 645.1228 | −3.26 | 177.9897 [M−H-C19H27O11]− | ||||
| 10 | 14.20 | Hyperoside (Hyp) | C21H20O12 | [M−H]− | 463.0866 | 463.0882 | −3.46 | 301.0369 [M−H-C6H10O5]− |
| 11 | 14.63 | Isoquercetin (Ique) | C21H20O12 | [M−H]− | 463.0883 | 463.0882 | 0.22 | 301.0371 [M−H-C6H10O5]− |
| 12 | 15.89 | Quercetin-3-O-(6″-O-acetyl)-galactoside (Acetylated hyperoside, AHyp) | C23H22O13 | [M−H]− | 505.0979 | 505.0988 | −1.78 | 463.0866 [M−H-C2H2O]− |
| 301.0370 [M−H-C2H2O -C6H10O5]− | ||||||||
| 13 | 16.52 | Quercetin-3-O-(6″-O-acetyl)-glucoside (Acetylated isoquercetin, AI) | C23H22O13 | [M−H]− | 505.0969 | 505.0988 | −3.76 | 463.0866 [M−H-C2H2O]− |
| 301.0365 [M−H-C2H2O -C6H10O5]− | ||||||||
| 14 | 16.78 | Astragalin | C21H20O11 | [M−H]− | 447.0927 | 447.0933 | −3.76 | 285.0420 [M−H-C6H10O5]− |
| 15 | 18.38 | Kaempferol-3-O-(6″-O-acetyl)-glucoside (Acetylated astragalin) | C23H22O12 | [M−H]− | 489.1022 | 489.1038 | −3.27 | 447.0918 [M−H-C2H2O]− |
| 285.0427 [M−H-C2H2O-C6H10O5]− |
| Run | Independent | Phenolic Acids (mg/g) | Flavonoids (mg/g) | IC50 (mg/mL) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variables | ||||||||||||||||
| X1 | X2 | X3 | 1 | 2 | 3 | Tp | 6 | 9 | 10 | 11 | 12 | 14 | TF | ABTS | DPPH | |
| 1 | 50 | 30 | 20 | 1.851 | 4.732 | 0.551 | 7.132 | 6.393 | 0.174 | 0.051 | 3.491 | 3.163 | 0.162 | 13.421 | 0.040 | 0.450 |
| ±0.021 | ±0.081 | ±0.012 | ±0.112 | ±0.172 | ±0.006 | ±0.002 | ±0.040 | ±0.036 | ±0.005 | ±0.171 | ±0.002 | ±0.018 | ||||
| 2 | 50 | 30 | 10 | 1.421 | 4.561 | 0.402 | 6.381 | 6.402 | 0.153 | 0.043 | 3.880 | 2.642 | 0.141 | 13.252 | 0.079 | 0.471 |
| ±0.032 | ±0.054 | ±0.015 | ±0.109 | ±0.068 | ±0.003 | ±0.002 | ±0.042 | ±0.040 | ±0.006 | ±0.162 | ±0.003 | ±0.021 | ||||
| 3 | 50 | 20 | 15 | 1.873 | 6.352 | 0.773 | 8.992 | 9.991 | 0.172 | 0.031 | 6.792 | 4.030 | 0.201 | 21.212 | 0.035 | 0.213 |
| ±0.029 | ±0.067 | ±0.020 | ±0.098 | ±0.110 | ±0.004 | ±0.001 | ±0.062 | ±0.043 | ±0.005 | ±0.218 | ±0.001 | ±0.008 | ||||
| 4 | 30 | 10 | 15 | 1.916 | 3.353 | 0.478 | 5.753 | 6.332 | 0.123 | 0.021 | 4.558 | 2.421 | 0.070 | 13.520 | 0.067 | 0.477 |
| ±0.042 | ±0.058 | ±0.021 | ±0.061 | ±0.081 | ±0.005 | ±0.001 | ±0.053 | ±0.036 | ±0.003 | ±0.180 | ±0.003 | ±0.014 | ||||
| 5 | 50 | 10 | 20 | 1.393 | 4.144 | 0.501 | 6.028 | 6.158 | 0.149 | 0.039 | 3.443 | 2.532 | 0.161 | 12.475 | 0.075 | 0.488 |
| ±0.026 | ±0.047 | ±0.012 | ±0.072 | ±0.065 | ±0.004 | ±0.001 | ±0.049 | ±0.044 | ±0.004 | ±0.152 | ±0.003 | ±0.016 | ||||
| 6 | 30 | 20 | 10 | 1.422 | 4.276 | 0.491 | 6.187 | 7.958 | 0.132 | 0.022 | 3.340 | 2.258 | 0.103 | 13.812 | 0.044 | 0.372 |
| ±0.029 | ±0.041 | ±0.017 | ±0.059 | ±0.119 | ±0.004 | ±0.001 | ±0.056 | ±0.029 | ±0.004 | ±0.165 | ±0.001 | ±0.016 | ||||
| 7 | 70 | 20 | 10 | 1.854 | 4.402 | 0.592 | 6.843 | 7.191 | 0.131 | 0.041 | 4.801 | 2.880 | 0.181 | 15.220 | 0.046 | 0.387 |
| ±0.030 | ±0.043 | ±0.017 | ±0.102 | ±0.132 | ±0.003 | ±0.002 | ±0.071 | ±0.038 | ±0.007 | ±0.180 | ±0.002 | ±0.017 | ||||
| 8 | 70 | 20 | 20 | 2.047 | 5.263 | 0.738 | 8.052 | 8.082 | 0.193 | 0.049 | 4.722 | 3.822 | 0.242 | 17.101 | 0.041 | 0.272 |
| ±0.031 | ±0.062 | ±0.026 | ±0.074 | ±0.090 | ±0.004 | ±0.001 | ±0.101 | ±0.044 | ±0.011 | ±0.203 | ±0.002 | ±0.010 | ||||
| 9 | 50 | 20 | 15 | 1.803 | 5.791 | 0.751 | 8.344 | 9.801 | 0.152 | 0.031 | 6.972 | 3.751 | 0.193 | 20.894 | 0.038 | 0.223 |
| ±0.024 | ±0.066 | ±0.019 | ±0.092 | ±0.110 | ±0.003 | ±0.001 | ±0.071 | ±0.039 | ±0.005 | ±0.218 | ±0.001 | ±0.007 | ||||
| 10 | 50 | 20 | 15 | 1.991 | 5.573 | 0.833 | 8.393 | 9.326 | 0.139 | 0.042 | 6.639 | 3.993 | 0.220 | 20.364 | 0.043 | 0.203 |
| ±0.031 | ±0.059 | ±0.021 | ±0.088 | ±0.096 | ±0.003 | ±0.001 | ±0.067 | ±0.041 | ±0.006 | ±0.199 | ±0.002 | ±0.009 | ||||
| 11 | 70 | 10 | 15 | 1.824 | 4.284 | 0.642 | 6.741 | 7.993 | 0.181 | 0.043 | 5.013 | 3.232 | 0.210 | 16.663 | 0.051 | 0.327 |
| ±0.022 | ±0.054 | ±0.021 | ±0.068 | ±0.113 | ±0.006 | ±0.002 | ±0.094 | ±0.022 | ±0.008 | ±0.170 | ±0.002 | ±0.012 | ||||
| 12 | 30 | 20 | 20 | 2.488 | 4.296 | 0.782 | 7.569 | 8.451 | 0.092 | 0.041 | 5.152 | 2.701 | 0.151 | 16.578 | 0.044 | 0.352 |
| ±0.037 | ±0.051 | ±0.023 | ±0.125 | ±0.082 | ±0.004 | ±0.002 | ±0.106 | ±0.031 | ±0.006 | ±0.150 | ±0.002 | ±0.010 | ||||
| 13 | 50 | 20 | 15 | 1.923 | 5.922 | 0.779 | 8.622 | 8.968 | 0.174 | 0.041 | 6.144 | 3.962 | 0.211 | 19.486 | 0.037 | 0.231 |
| ±0.030 | ±0.062 | ±0.020 | ±0.086 | ±0.101 | ±0.004 | ±0.001 | ±0.065 | ±0.041 | ±0.006 | ±0.196 | ±0.001 | ±0.008 | ||||
| 14 | 50 | 10 | 10 | 1.384 | 2.841 | 0.381 | 4.601 | 6.052 | 0.153 | 0.040 | 3.170 | 2.674 | 0.130 | 12.212 | 0.062 | 0.513 |
| ±0.021 | ±0.031 | ±0.013 | ±0.052 | ±0.116 | ±0.005 | ±0.002 | ±0.031 | ±0.040 | ±0.005 | ±0.163 | ±0.002 | ±0.011 | ||||
| 15 | 50 | 20 | 15 | 2.147 | 5.982 | 0.818 | 8.952 | 9.843 | 0.172 | 0.042 | 6.061 | 4.030 | 0.203 | 20.342 | 0.035 | 0.253 |
| ±0.032 | ±0.055 | ±0.021 | ±0.090 | ±0.101 | ±0.004 | ±0.001 | ±0.061 | ±0.041 | ±0.007 | ±0.175 | ±0.001 | ±0.011 | ||||
| 16 | 30 | 30 | 15 | 2.581 | 5.091 | 0.802 | 8.473 | 8.301 | 0.091 | 0.031 | 3.429 | 3.082 | 0.121 | 15.050 | 0.058 | 0.358 |
| ±0.041 | ±0.059 | ±0.026 | ±0.131 | ±0.096 | ±0.004 | ±0.001 | ±0.044 | ±0.068 | ±0.003 | ±0.162 | ±0.002 | ±0.012 | ||||
| 17 | 70 | 30 | 15 | 2.282 | 4.660 | 0.731 | 7.670 | 7.314 | 0.208 | 0.053 | 4.422 | 3.911 | 0.251 | 16.151 | 0.060 | 0.310 |
| ±0.033 | ±0.057 | ±0.011 | ±0.114 | ±0.101 | ±0.004 | ±0.002 | ±0.050 | ±0.069 | ±0.008 | ±0.170 | ±0.002 | ±0.009 | ||||
| Predicted | 64.09 | 20.42 | 15.82 | 2.082 | 5.852 | 0.824 | 8.751 | 9.418 | 0.181 | 0.051 | 6.383 | 4.140 | 0.238 | 20.411 | 0.036 | 0.200 |
| Experimental | 64 | 20 | 16 | 2.152 | 5.968 | 0.811 | 8.932 | 9.390 | 0.172 | 0.042 | 6.560 | 4.161 | 0.241 | 20.530 | 0.034 | 0.203 |
| ±0.031 | ±0.061 | ±0.012 | ±0.091 | ±0.103 | ±0.003 | ±0.001 | ±0.069 | ±0.042 | ±0.005 | ±0.198 | ±0.001 | ±0.009 | ||||
| Reflux | 50 | 30 | 100 | 2.283 | 4.941 | 0.314 | 7.531 | 6.441 | 0.133 | 0.023 | 5.162 | 3.252 | 0.132 | 15.132 | 0.049 | 0.363 |
| ±0.048 | ±0.092 | ±0.011 | ±0.170 | ±0.121 | ±0.004 | ±0.001 | ±0.098 | ±0.051 | ±0.004 | ±0.260 | ±0.002 | ±0.017 | ||||
| Vc | 0.005 | 0.041 | ||||||||||||||
| ±0.000 | ±0.002 | |||||||||||||||
| Factor | Coefficient (β) | |||
|---|---|---|---|---|
| Tp | TF | ABTS | DPPH | |
| Intercept | 8.66 | 20.46 | 0.037 | 0.22 |
| Linear | ||||
| X1 | 2.56 | 6.66 * | 1.75 | 12.00 * |
| X2 | 63.54 *** | 1.69 | 1.12 | 8.09 * |
| X3 | 33.90 ** | 4.76 | 4.86 | 5.68 * |
| Quadratic | ||||
| X12 | 1.79 | 7.81 * | 1.47 × 10−3 | 0.11 |
| X22 | 86.73 *** | 97.51 *** | 123.14 *** | 113.01 *** |
| X32 | 85.71 *** | 80.64 *** | 10.82 * | 79.74 *** |
| Interaction | ||||
| X1X2 | 9.70 * | 1.45 | 5.04 | 3.61 |
| X1X3 | 0.088 | 0.31 | 0.39 | 3.13 |
| X2X3 | 1.38 | 6.53 × 10−3 | 32.93 ** | 5.55 × 10−3 |
| R2 | 0.9771 | 0.9689 | 0.9635 | 0.9714 |
| Adj.R2 | 0.9477 | 0.9288 | 0.9166 | 0.9347 |
| F value (model) | 33.20 *** | 24.19 ** | 20.54 ** | 26.43 ** |
| F value (Lack of Fit) | 0.76 | 2.36 | 1.98 | 3.30 |
| Phenolic Acids | Flavonoids | ABTS | DPPH | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (1) | (2) | (3) | TP | (6) | (9) | (10) | (11) | (12) | (14) | TF | |||
| ABTS | −0.352 | −0.672 ** | −0.693 ** | −0.686 ** | −0.804 ** | −0.118 | −0.048 | −0.671 ** | −0.629 ** | −0.452 | −0.771 ** | 1 | 0.793 ** |
| DPPH | −0.448 | −0.863 ** | −0.879 ** | −0.879 ** | −0.933 ** | −0.280 | −0.117 | −0.850 ** | −0.860 ** | −0.704 ** | −0.956 ** | 0.793 ** | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qin, R.; Song, J.; Wang, Q.; Guan, Y.; Lv, C. Optimal Extraction of Antioxidants, Flavonoids, and Phenolic Acids from the Leaves of Apocynum venetum L. by Response Surface Methodology with Integrated Chemical Profiles and Bioactivity Evaluation. Molecules 2025, 30, 4006. https://doi.org/10.3390/molecules30194006
Qin R, Song J, Wang Q, Guan Y, Lv C. Optimal Extraction of Antioxidants, Flavonoids, and Phenolic Acids from the Leaves of Apocynum venetum L. by Response Surface Methodology with Integrated Chemical Profiles and Bioactivity Evaluation. Molecules. 2025; 30(19):4006. https://doi.org/10.3390/molecules30194006
Chicago/Turabian StyleQin, Rulan, Jinhang Song, Qiang Wang, Yingli Guan, and Chongning Lv. 2025. "Optimal Extraction of Antioxidants, Flavonoids, and Phenolic Acids from the Leaves of Apocynum venetum L. by Response Surface Methodology with Integrated Chemical Profiles and Bioactivity Evaluation" Molecules 30, no. 19: 4006. https://doi.org/10.3390/molecules30194006
APA StyleQin, R., Song, J., Wang, Q., Guan, Y., & Lv, C. (2025). Optimal Extraction of Antioxidants, Flavonoids, and Phenolic Acids from the Leaves of Apocynum venetum L. by Response Surface Methodology with Integrated Chemical Profiles and Bioactivity Evaluation. Molecules, 30(19), 4006. https://doi.org/10.3390/molecules30194006





