Methods for Conjugating Antibodies with Quantum Dots
Abstract
1. Introduction
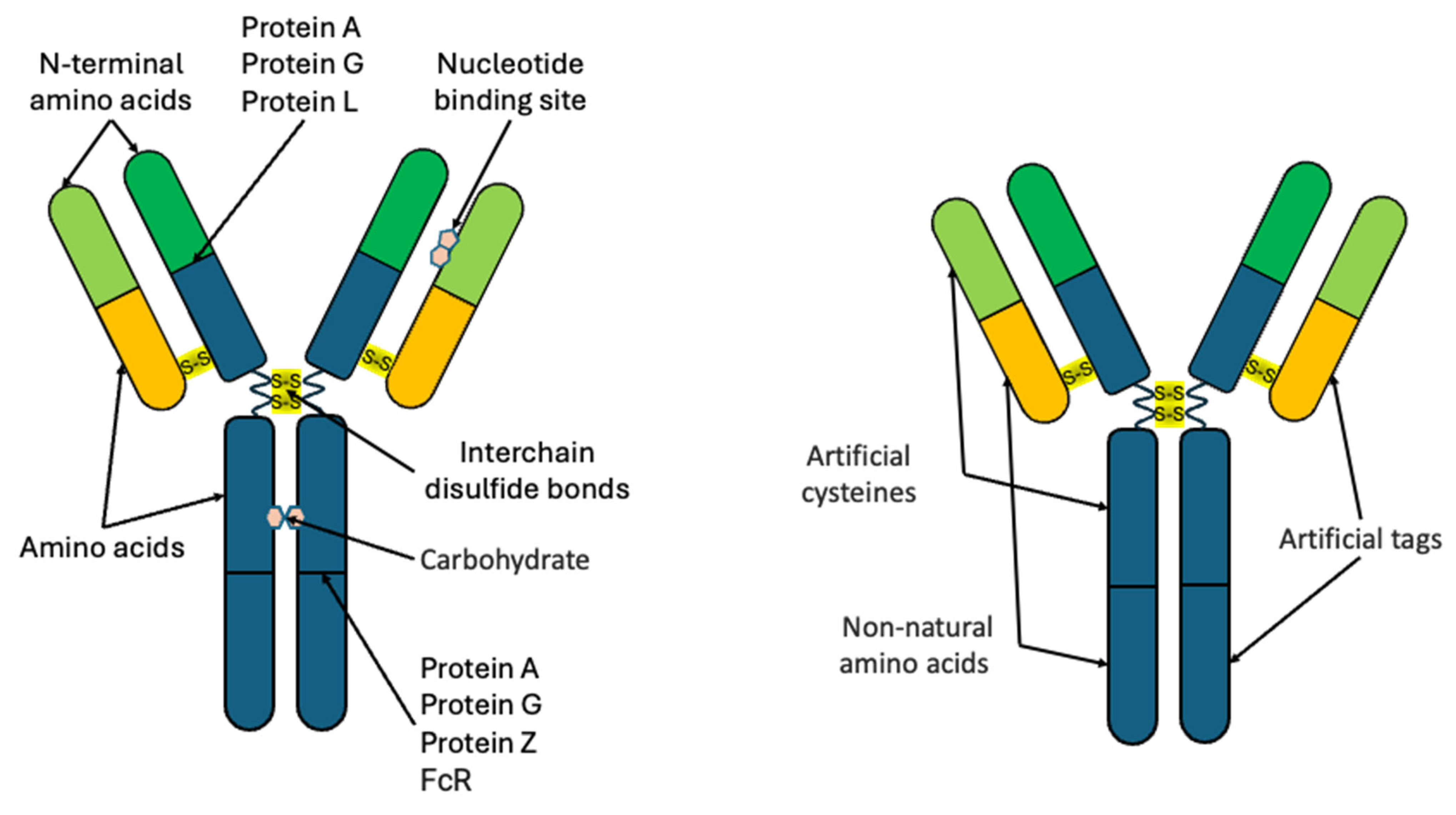
2. Site-Nonspecific Conjugation of Antibodies with Quantum Dots
2.1. Noncovalent Binding Methods
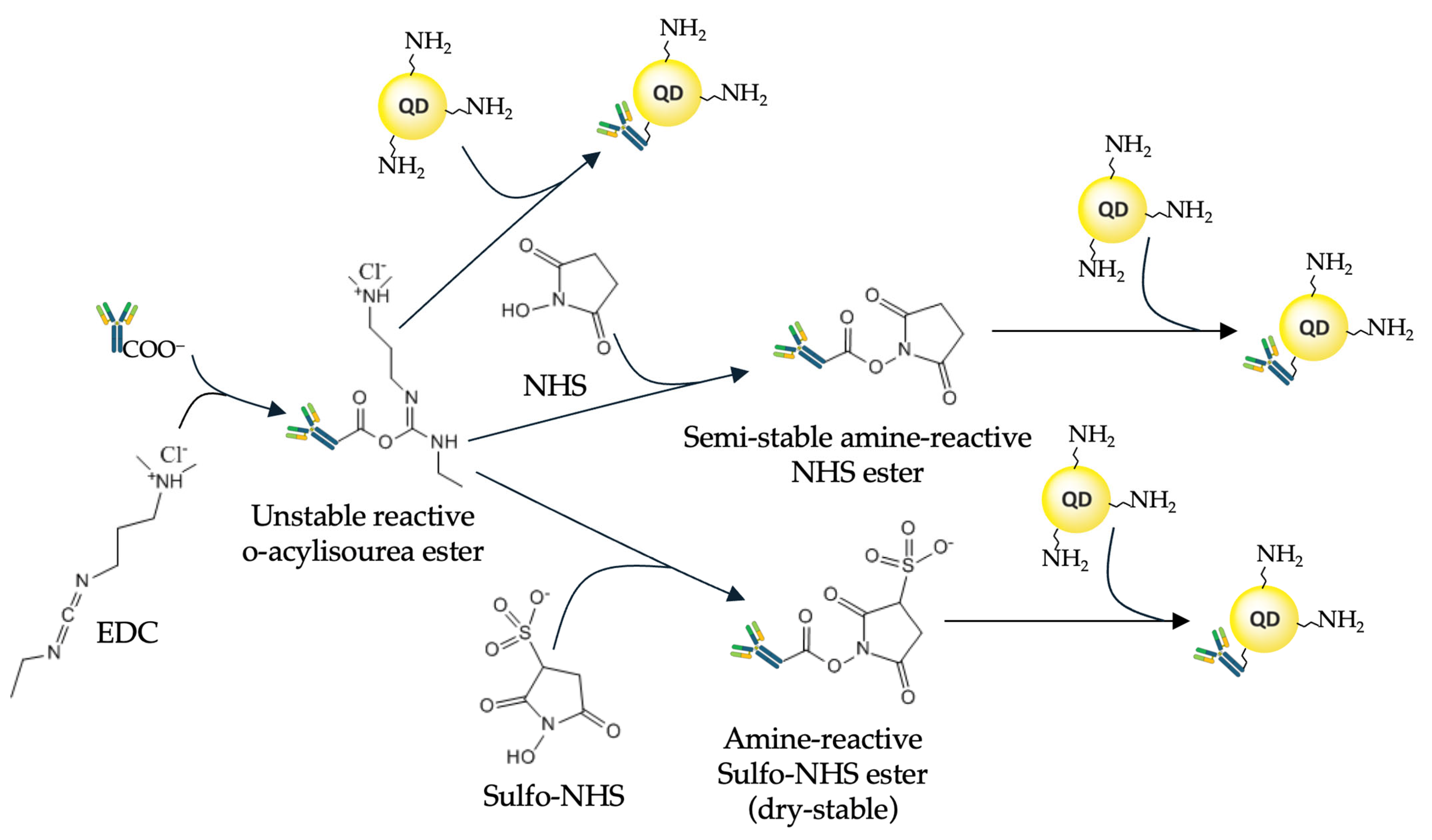
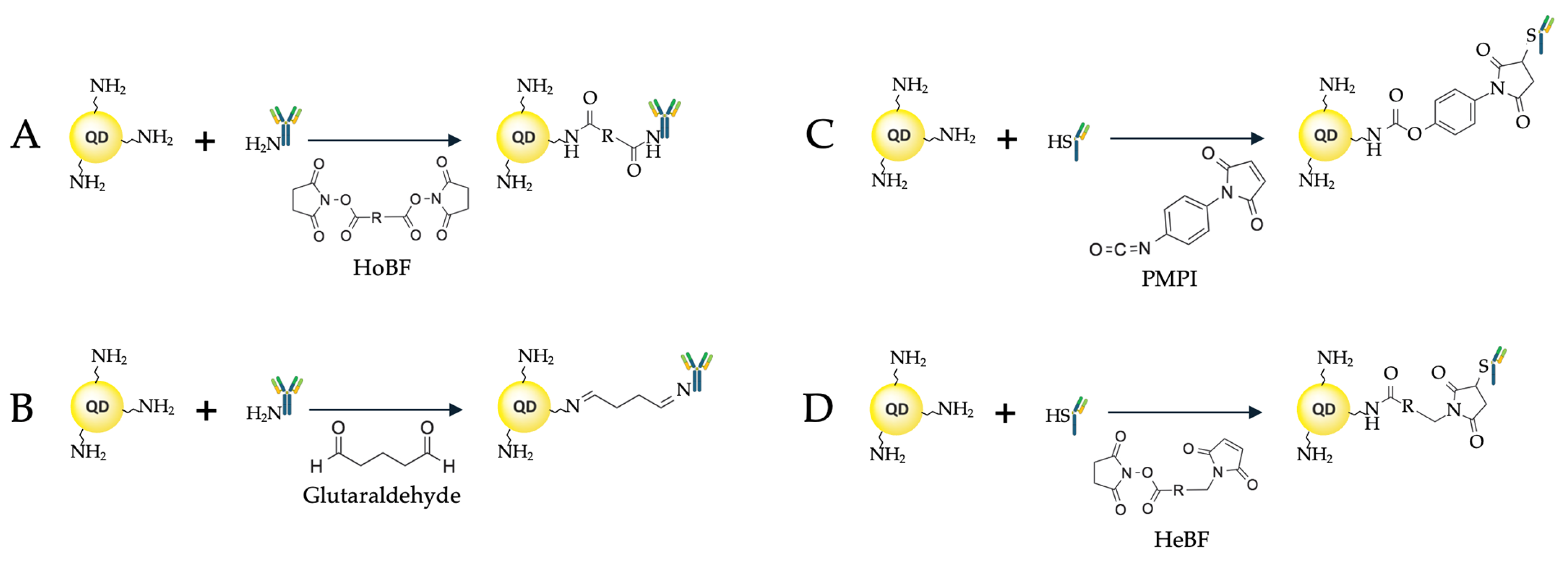
2.2. Covalent Binding Methods
3. Site-Specific Conjugation of Antibodies with Quantum Dots
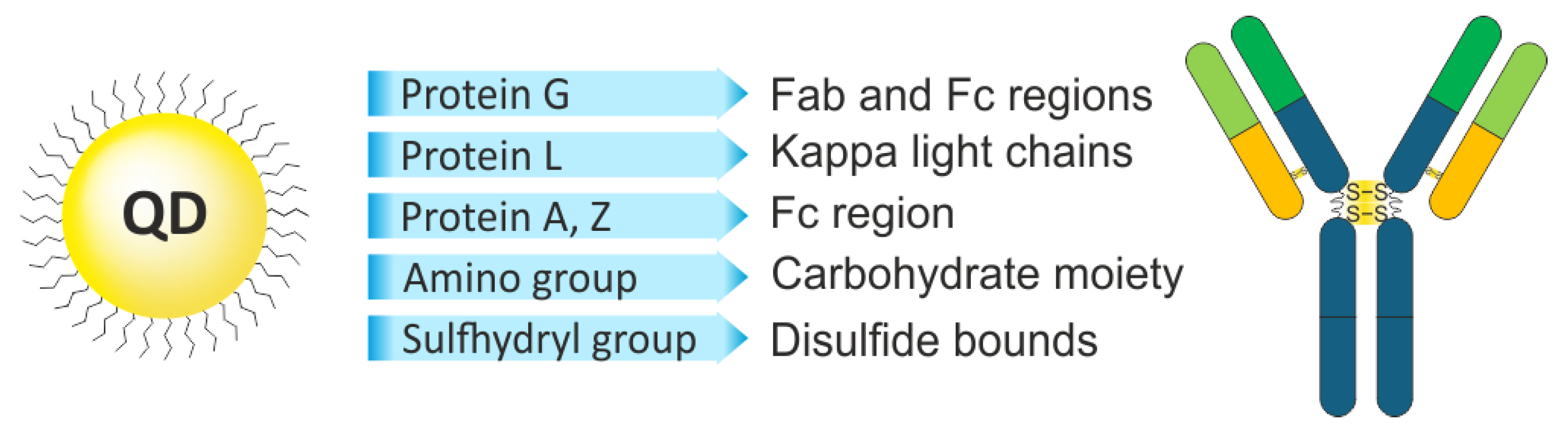
3.1. The Use of Affinity Proteins
3.2. The Use of Carbohydrates
3.3. The Use of Disulfide Bonds
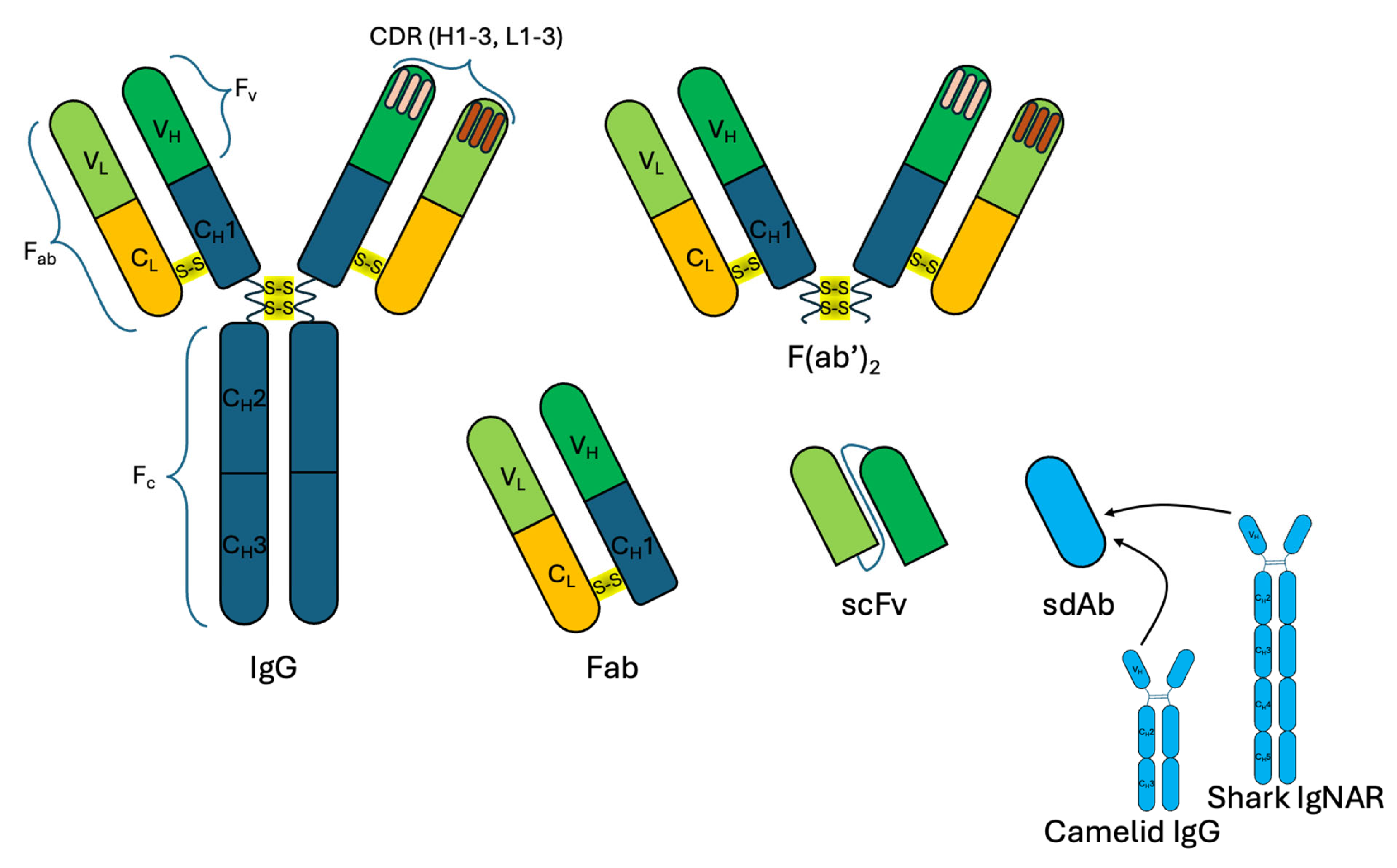
3.4. Conjugation of Antibody Fragments
4. Prospective Conjugation Technologies
4.1. Nucleotide-Binding Sites
4.2. Aptamers
4.3. Enzymatic Conjugation
4.4. Peptide Tags
4.5. Tyrosine Amino Acid Residues
4.6. Non-Natural Amino Acids
5. Conclusions and Prospects
- -
- Methods of QD synthesis, their transfer to the aqueous phase, and functionalization of their surface with organic ligands;
- -
- Methods of preparation and selection of specific Abs for conjugation;
- -
- Comparative data on different adapter molecules and crosslinkers in terms of enhancing QD–Ab conjugation effectiveness;
- -
- Characteristics of the obtained conjugates, including not only the results of testing the conjugate performance in specific techniques but also data on their hydrodynamic diameter, the efficiency of QD–Ab conjugation (number of Abs per QD), changes in the Ab affinity and specificity, as well as the QD optical properties, upon conjugation;
- -
- Data on post-processing of the conjugates for blocking the QD surface and reducing nonspecific binding to biological molecules.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Globus, O.; Sagie, S.; Lavine, N.; Barchana, D.I.; Urban, D. Early Death after a Diagnosis of Metastatic Solid Cancer–Raising Awareness and Identifying Risk Factors from the SEER Database. PLoS ONE 2023, 18, e0281561. [Google Scholar] [CrossRef]
- Manera, V.; Rovini, E.; Wais, P. Editorial: Early Detection of Neurodegenerative Disorders Using Behavioral Markers and New Technologies: New Methods and Perspectives. Front. Aging Neurosci. 2023, 15, 1149886. [Google Scholar] [CrossRef]
- Amemiya, Y.; Nishiura, H. Combined Effect of Early Diagnosis and Treatment on the Case Fatality Risk of COVID-19 in Japan, 2020. Sci. Rep. 2023, 13, 6679. [Google Scholar] [CrossRef] [PubMed]
- Suneja, M.; Beekmann, S.E.; Dhaliwal, G.; Miller, A.C.; Polgreen, P.M. Diagnostic Delays in Infectious Diseases. Diagnosis 2022, 9, 332–339. [Google Scholar] [CrossRef] [PubMed]
- Domsicova, M.; Korcekova, J.; Poturnayova, A.; Breier, A. New Insights into Aptamers: An Alternative to Antibodies in the Detection of Molecular Biomarkers. Int. J. Mol. Sci. 2024, 25, 6833. [Google Scholar] [CrossRef] [PubMed]
- Gosselin, B.; Retout, M.; Jabin, I.; Bruylants, G. Development of a Peptide-Based Lateral Flow Assay for the Detection of the Cancer Biomarker Mdm2. Sens. Diagn. 2024, 3, 248–255. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.; Li, L.; Mao, X.; Shi, M.; Li, K. Capture and Detection of Nucleic Acid Aptamer Target Proteins in Colorectal Cancer. Chem. Eng. J. 2025, 504, 158746. [Google Scholar] [CrossRef]
- Tang, W.; Han, J.; Zhang, W.; Li, H.; Chen, J.; Song, W.; Wang, L. Molecularly Imprinted Polymer Sensors for Biomarker Detection in Cardiovascular Diseases. Analyst 2024, 149, 5617–5637. [Google Scholar] [CrossRef]
- Chávez-Ramírez, A.U.; Vallejo-Becerra, V.; de Dios Galindo-de-la-Rosa, J.; Fernández-Puig, S.; Casanova-Moreno, J.R.; Rohokale, A.; Oza, G.; Valdés-González, A.C. Advancements in Molecularly Imprinted Polymers for Selective Recognition of Cancer Biomarkers. In Molecularly Imprinted Polymers: Path to Artificial Antibodies; Patra, S., Shukla, S.K., Sillanpää, M., Eds.; Springer Nature: Singapore, 2024; pp. 399–442. ISBN 978-981-97-4379-7. [Google Scholar]
- Choi, J.E.; Yun, H.; Jeong, H.-J. Establishment of a Rapid and Convenient Fluoroimmunoassay Platform Using Antibodies Against PDL1 and HER2. Curr. Issues Mol. Biol. 2025, 47, 62. [Google Scholar] [CrossRef]
- Jiao, X.; Zhou, Y.; Zhao, D.; Pang, D.; Wang, C.; Du, H.; Wen, Y.; Zhang, X. An Indirect ELISA-Inspired Dual-Channel Fluorescent Immunoassay Based on MPA-Capped CdTe/ZnS QDs. Anal. Bioanal. Chem. 2019, 411, 5437–5444. [Google Scholar] [CrossRef]
- Ren, Y.; Tian, R.; Wang, T.; Cao, J.; Li, J.; Deng, A. An Extremely Highly Sensitive ELISA in Pg mL−1 Level Based on a Newly Produced Monoclonal Antibody for the Detection of Ochratoxin A in Food Samples. Molecules 2023, 28, 5743. [Google Scholar] [CrossRef]
- Dewulf, J.; Adhikari, K.; Vangestel, C.; Wyngaert, T.V.D.; Elvas, F. Development of Antibody Immuno-PET/SPECT Radiopharmaceuticals for Imaging of Oncological Disorders—An Update. Cancers 2020, 12, 1868. [Google Scholar] [CrossRef] [PubMed]
- Mathiyazhagan, J.; Rajesh, C.; Sagar, S.; Caffrey, T.C.; Huang, Y.; Mohs, A.M.; Swanson, B.J.; Hollingsworth, M.A.; Brooks, C.L.; Radhakrishnan, P. Humanized Anti-MUC16 Antibody-Conjugated Contrast Agents for Magnetic Resonance Imaging of Pancreatic Cancer. Cancers 2025, 17, 957. [Google Scholar] [CrossRef] [PubMed]
- Resch-Genger, U.; Grabolle, M.; Cavaliere-Jaricot, S.; Nitschke, R.; Nann, T. Quantum Dots versus Organic Dyes as Fluorescent Labels. Nat. Methods 2008, 5, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Coons, A.H.; Creech, H.J.; Jones, R.N. Immunological Properties of an Antibody Containing a Fluorescent Group. Exp. Biol. Med. 1941, 47, 200–202. [Google Scholar] [CrossRef]
- Szabó, Á.; Szendi-Szatmári, T.; Ujlaky-Nagy, L.; Rádi, I.; Vereb, G.; Szöllősi, J.; Nagy, P. The Effect of Fluorophore Conjugation on Antibody Affinity and the Photophysical Properties of Dyes. Biophys. J. 2018, 114, 688–700. [Google Scholar] [CrossRef]
- Chan, W.C.W.; Nie, S. Quantum Dot Bioconjugates for Ultrasensitive Nonisotopic Detection. Science 1998, 281, 2016–2018. [Google Scholar] [CrossRef]
- Sokolov, P.; Nifontova, G.; Samokhvalov, P.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Nontoxic Fluorescent Nanoprobes for Multiplexed Detection and 3D Imaging of Tumor Markers in Breast Cancer. Pharmaceutics 2023, 15, 946. [Google Scholar] [CrossRef]
- Xu, L.; Yang, F.; Dias, A.C.P.; Zhang, X. Development of Quantum Dot-Linked Immunosorbent Assay (QLISA) and ELISA for the Detection of Sunset Yellow in Foods and Beverages. Food Chem. 2022, 385, 132648. [Google Scholar] [CrossRef]
- Buranda, T.; Wu, Y.; Sklar, L.A. Quantum Dots for Quantitative Flow Cytometry. In Flow Cytometry Protocols; Hawley, T.S., Hawley, R.G., Eds.; Humana Press: Totowa, NJ, USA, 2011; pp. 67–84. ISBN 978-1-61737-950-5. [Google Scholar]
- Sokolov, P.; Evsegneeva, I.; Karaulov, A.; Sukhanova, A.; Nabiev, I. Allergen Microarrays and New Physical Approaches to More Sensitive and Specific Detection of Allergen-Specific Antibodies. Biosensors 2024, 14, 353. [Google Scholar] [CrossRef]
- Ayadi, N.; Lafont, F.; Charlier, C.; Benhelli-Mokrani, H.; Sokolov, P.; Sukhanova, A.; Fleury, F.; Nabiev, I. Comparative Advantages and Limitations of Quantum Dots in Protein Array Applications. In Quantum Dots: Applications in Biology; Fontes, A., Santos, B.S., Eds.; Springer: New York, NY, USA, 2020; pp. 259–273. ISBN 978-1-0716-0463-2. [Google Scholar]
- Jessy Mercy, D.; Girigoswami, K.; Girigoswami, A. A Mini Review on Biosensor Advancements-Emphasis on Quantum Dots. Results Chem. 2024, 7, 101271. [Google Scholar] [CrossRef]
- Sokolov, P.; Samokhvalov, P.; Sukhanova, A.; Nabiev, I. Biosensors Based on Inorganic Composite Fluorescent Hydrogels. Nanomaterials 2023, 13, 1748. [Google Scholar] [CrossRef]
- Nikolaev, V.V.; Lepekhina, T.B.; Alliluev, A.S.; Bidram, E.; Sokolov, P.M.; Nabiev, I.R.; Kistenev, Y.V. Quantum Dot-Based Nanosensors for In Vitro Detection of Mycobacterium Tuberculosis. Nanomaterials 2024, 14, 1553. [Google Scholar] [CrossRef]
- Sukhanova, A.; Ramos-Gomes, F.; Chames, P.; Sokolov, P.; Baty, D.; Alves, F.; Nabiev, I. Multiphoton Deep-Tissue Imaging of Micrometastases and Disseminated Cancer Cells Using Conjugates of Quantum Dots and Single-Domain Antibodies. In Multiplexed Imaging: Methods and Protocols; Zamir, E., Ed.; Springer: New York, NY, USA, 2021; pp. 105–123. ISBN 978-1-0716-1593-5. [Google Scholar]
- Wang, L.-W.; Peng, C.-W.; Chen, C.; Li, Y. Quantum Dots-Based Tissue and in Vivo Imaging in Breast Cancer Researches: Current Status and Future Perspectives. Breast Cancer Res. Treat. 2015, 151, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Francis, J.E.; Mason, D.; Lévy, R. Evaluation of Quantum Dot Conjugated Antibodies for Immunofluorescent Labelling of Cellular Targets. Beilstein J. Nanotechnol. 2017, 8, 1238–1249. [Google Scholar] [CrossRef] [PubMed]
- Tomita, M.; Tsumoto, K. Hybridoma Technologies for Antibody Production. Immunotherapy 2011, 3, 371–380. [Google Scholar] [CrossRef] [PubMed]
- Leenaars, M.; Hendriksen, C.F.M. Critical Steps in the Production of Polyclonal and Monoclonal Antibodies: Evaluation and Recommendations. ILAR J. 2005, 46, 269–279. [Google Scholar] [CrossRef]
- Conroy, P.J.; Hearty, S.; Leonard, P.; O’Kennedy, R.J. Antibody Production, Design and Use for Biosensor-Based Applications. Semin. Cell Dev. Biol. 2009, 20, 10–26. [Google Scholar] [CrossRef]
- Kinman, A.W.L.; Pompano, R.R. Optimization of Enzymatic Antibody Fragmentation for Yield, Efficiency, and Binding Affinity. Bioconjug. Chem. 2019, 30, 800–807. [Google Scholar] [CrossRef]
- Ahmad, Z.A.; Yeap, S.K.; Ali, A.M.; Ho, W.Y.; Alitheen, N.B.M.; Hamid, M. scFv Antibody: Principles and Clinical Application. Clin. Dev. Immunol. 2012, 2012, 980250. [Google Scholar] [CrossRef]
- Holliger, P.; Prospero, T.; Winter, G. “Diabodies”: Small Bivalent and Bispecific Antibody Fragments. Proc. Natl. Acad. Sci. USA 1993, 90, 6444–6448. [Google Scholar] [CrossRef] [PubMed]
- Natale, V.; Stadlmayr, G.; Benedetti, F.; Stadlbauer, K.; Rüker, F.; Wozniak-Knopp, G. Trispecific Antibodies Produced from mAb2 Pairs by Controlled Fab-Arm Exchange. Biol. Chem. 2022, 403, 509–523. [Google Scholar] [CrossRef] [PubMed]
- Harmsen, M.M.; De Haard, H.J. Properties, Production, and Applications of Camelid Single-Domain Antibody Fragments. Appl. Microbiol. Biotechnol. 2007, 77, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, K.; Dolezal, O.; Parisi, K.; Angerosa, J.; Dogovski, C.; Barraclough, M.; Sanalla, A.; Casey, J.; González, I.; Perugini, M.; et al. Shark Variable New Antigen Receptor (VNAR) Single Domain Antibody Fragments: Stability and Diagnostic Applications. Antibodies 2013, 2, 66–81. [Google Scholar] [CrossRef]
- Ryu, J.; Kim, E.-J.; Kim, J.-K.; Park, T.H.; Kim, B.-G.; Jeong, H.-J. Development of a CHO Cell Line for Stable Production of Recombinant Antibodies against Human MMP9. BMC Biotechnol. 2022, 22, 8. [Google Scholar] [CrossRef]
- Kim, J.-K.; Lim, G.-M.; Kim, E.-J.; Kim, W.; Lee, C.-S.; Kim, B.-G.; Jeong, H.-J. Generation of Recombinant Antibodies in HEK293F Cells for the Detection of Staphylococcus aureus. ACS Omega 2022, 7, 9690–9700. [Google Scholar] [CrossRef]
- Goncalves, A.M. Pichia Pastoris: A Recombinant Microfactory for Antibodies and Human Membrane Proteins. J. Microbiol. Biotechnol. 2013, 23, 587–601. [Google Scholar] [CrossRef]
- Robinson, M.-P.; Ke, N.; Lobstein, J.; Peterson, C.; Szkodny, A.; Mansell, T.J.; Tuckey, C.; Riggs, P.D.; Colussi, P.A.; Noren, C.J.; et al. Efficient Expression of Full-Length Antibodies in the Cytoplasm of Engineered Bacteria. Nat. Commun. 2015, 6, 8072. [Google Scholar] [CrossRef]
- Kunert, R.; Reinhart, D. Advances in Recombinant Antibody Manufacturing. Appl. Microbiol. Biotechnol. 2016, 100, 3451–3461. [Google Scholar] [CrossRef]
- Tyther, R.; Jenkins, N. Quality Issues Arising from Post-Translational Modification of Recombinant Antibodies. In Antibody Expression and Production; Al-Rubeai, M., Ed.; Springer: Dordrecht, The Netherlands, 2011; pp. 293–303. ISBN 978-94-007-1257-7. [Google Scholar]
- Szymanski, C.J.; Yi, H.; Liu, J.L.; Wright, E.R.; Payne, C.K. Imaging Intracellular Quantum Dots: Fluorescence Microscopy and Transmission Electron Microscopy. In NanoBiotechnology Protocols; Rosenthal, S.J., Wright, D.W., Eds.; Humana Press: Totowa, NJ, USA, 2013; pp. 21–33. ISBN 978-1-62703-468-5. [Google Scholar]
- Sun, J.; Liu, F.; Yu, W.; Jiang, Q.; Hu, J.; Liu, Y.; Wang, F.; Liu, X. Highly Sensitive Glutathione Assay and Intracellular Imaging with Functionalized Semiconductor Quantum Dots. Nanoscale 2019, 11, 5014–5020. [Google Scholar] [CrossRef]
- Foubert, A.; Beloglazova, N.V.; Rajkovic, A.; Sas, B.; Madder, A.; Goryacheva, I.Y.; De Saeger, S. Bioconjugation of Quantum Dots: Review & Impact on Future Application. TrAC Trends Anal. Chem. 2016, 83, 31–48. [Google Scholar] [CrossRef]
- Yemets, A.; Plokhovska, S.; Pushkarova, N.; Blume, Y. Quantum Dot-Antibody Conjugates for Immunofluorescence Studies of Biomolecules and Subcellular Structures. J. Fluoresc. 2022, 32, 1713–1723. [Google Scholar] [CrossRef]
- Kang, M.S.; Kong, T.W.S.; Khoo, J.Y.X.; Loh, T.-P. Recent Developments in Chemical Conjugation Strategies Targeting Native Amino Acids in Proteins and Their Applications in Antibody–Drug Conjugates. Chem. Sci. 2021, 12, 13613–13647. [Google Scholar] [CrossRef]
- Priya, L.; Mehta, S.; Gevariya, D.; Sharma, R.; Panjwani, D.; Patel, S.; Ahlawat, P.; Dharamsi, A.; Patel, A. Quantum Dot-Based Bio-Conjugates as an Emerging Bioimaging Tool forCancer Theranostic—A Review. Curr. Drug Targets 2024, 25, 241–260. [Google Scholar] [CrossRef]
- Bilan, R.; Nabiev, I.; Sukhanova, A. Quantum Dot-Based Nanotools for Bioimaging, Diagnostics, and Drug Delivery. ChemBioChem 2016, 17, 2103–2114. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Shim, Y.; Myong Song, J. Quantum Dot as Probe for Disease Diagnosis and Monitoring. Biotechnol. J. 2016, 11, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Torcello-Gómez, A.; Santander-Ortega, M.J.; Peula-García, J.M.; Maldonado-Valderrama, J.; Gálvez-Ruiz, M.J.; Ortega-Vinuesa, J.L.; Martín-Rodríguez, A. Adsorption of Antibody onto Pluronic F68-Covered Nanoparticles: Link with Surface Properties. Soft Matter 2011, 7, 8450. [Google Scholar] [CrossRef]
- Lidke, D.S.; Nagy, P.; Jovin, T.M.; Arndt-Jovin, D.J. Biotin-Ligand Complexes With Streptavidin Quantum Dots for In Vivo Cell Labeling of Membrane Receptors. In Quantum Dots: Applications in Biology; Bruchez, M.P., Hotz, C.Z., Eds.; Humana Press: Totowa, NJ, USA, 2007; pp. 69–79. ISBN 978-1-59745-369-1. [Google Scholar]
- Tran, L.; Park, S. Highly Sensitive Detection of Dengue Biomarker Using Streptavidin-Conjugated Quantum Dots. Sci. Rep. 2021, 11, 15196. [Google Scholar] [CrossRef]
- Goldman, E.R.; Balighian, E.D.; Mattoussi, H.; Kuno, M.K.; Mauro, J.M.; Tran, P.T.; Anderson, G.P. Avidin: A Natural Bridge for Quantum Dot-Antibody Conjugates. J. Am. Chem. Soc. 2002, 124, 6378–6382. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, H.L.; Liu, N.; Sai, N.; Liu, X.; Liu, Z.; Gao, Z.; Ning, B.A. A Fluoroimmunoassay Based on Quantum Dot−Streptavidin Conjugate for the Detection of Chlorpyrifos. J. Agric. Food Chem. 2010, 58, 8895–8903. [Google Scholar] [CrossRef]
- Pathak, S.; Davidson, M.C.; Silva, G.A. Characterization of the Functional Binding Properties of Antibody Conjugated Quantum Dots. Nano Lett. 2007, 7, 1839–1845. [Google Scholar] [CrossRef]
- Jaiswal, J.K.; Simon, S.M. Potentials and Pitfalls of Fluorescent Quantum Dots for Biological Imaging. Trends Cell Biol. 2004, 14, 497–504. [Google Scholar] [CrossRef]
- Mueller, B.M.; Wrasidlo, W.A.; Reisfeld, R.A. Determination of the Number of e -Amino Groups Available for Conjugation of Effector Molecules to Monoclonal Antibodies. Hybridoma 1988, 7, 453–456. [Google Scholar] [CrossRef]
- Wang, L.; Amphlett, G.; Blättler, W.A.; Lambert, J.M.; Zhang, W. Structural Characterization of the Maytansinoid–Monoclonal Antibody Immunoconjugate, huN901–DM1, by Mass Spectrometry. Protein Sci. 2005, 14, 2436–2446. [Google Scholar] [CrossRef] [PubMed]
- East, D.A.; Todd, M.; Bruce, I.J. Quantum Dot–Antibody Conjugates via Carbodiimide-Mediated Coupling for Cellular Imaging. In Quantum Dots: Applications in Biology; Fontes, A., Santos, B.S., Eds.; Springer: New York, NY, USA, 2014; pp. 67–83. ISBN 978-1-4939-1280-3. [Google Scholar]
- Liang, C.; Huang, H.; Chen, W.; Liu, H.; Zhou, J.; Chen, Y.; Zhu, X.; Liu, E.; Wang, A. Quantum Dot-Based Fluorescence-Linked Immunosorbent Assay for High-Throughput Detection of Apramycin Residues in Animal-Derived Foods. Food Control 2026, 181, 111709. [Google Scholar] [CrossRef]
- Lv, Y.; Fan, J.; Zhao, M.; Wu, R.; Li, L.S. Recent Advances in Quantum Dot-Based Fluorescence-Linked Immunosorbent Assays. Nanoscale 2023, 15, 5560–5578. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, Y.; Hu, Y.; Jiang, N.; Patel, R.P.; Yetisen, A.K.; Cordeiro, M.F. Fluorescent Quantum Dots Based Lateral Flow Assay for Rapid Quantitative Detection of Ciliary Neurotrophic Factor in Glaucoma. Adv. Mater. Technol. 2024, 9, 2400238. [Google Scholar] [CrossRef]
- Ahmad Najib, M.; Selvam, K.; Khalid, M.F.; Ozsoz, M.; Aziah, I. Quantum Dot-Based Lateral Flow Immunoassay as Point-of-Care Testing for Infectious Diseases: A Narrative Review of Its Principle and Performance. Diagnostics 2022, 12, 2158. [Google Scholar] [CrossRef]
- Xu, W.; Xiong, Y.; Lai, W.; Xu, Y.; Li, C.; Xie, M. A Homogeneous Immunosensor for AFB1 Detection Based on FRET between Different-Sized Quantum Dots. Biosens. Bioelectron. 2014, 56, 144–150. [Google Scholar] [CrossRef]
- Grazon, C.; Chern, M.; Lally, P.; Baer, R.C.; Fan, A.; Lecommandoux, S.; Klapperich, C.; Dennis, A.M.; Galagan, J.E.; Grinstaff, M.W. The Quantum Dot vs. Organic Dye Conundrum for Ratiometric FRET-Based Biosensors: Which One Would You Chose? Chem. Sci. 2022, 13, 6715–6731. [Google Scholar] [CrossRef]
- Shamirian, A.; Ghai, A.; Snee, P. QD-Based FRET Probes at a Glance. Sensors 2015, 15, 13028–13051. [Google Scholar] [CrossRef]
- Han, H.-S.; Niemeyer, E.; Huang, Y.; Kamoun, W.S.; Martin, J.D.; Bhaumik, J.; Chen, Y.; Roberge, S.; Cui, J.; Martin, M.R.; et al. Quantum Dot/Antibody Conjugates for in Vivo Cytometric Imaging in Mice. Proc. Natl. Acad. Sci. USA 2015, 112, 1350–1355. [Google Scholar] [CrossRef]
- Popov, A.; Lisyte, V.; Kausaite-Minkstimiene, A.; Bernotiene, E.; Ramanaviciene, A. Experimental Evaluation of Quantum Dots and Antibodies Conjugation by Surface Plasmon Resonance Spectroscopy. Int. J. Mol. Sci. 2022, 23, 12626. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.L.; Liu, C.-H.; Kumari, M.; Wu, W.-C.; Wang, C.-C. Biocompatible Quantum Dot-Antibody Conjugate for Cell Imaging, Targeting and Fluorometric Immunoassay: Crosslinking, Characterization and Applications. RSC Adv. 2019, 9, 32791–32803. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.; Oliveira, P.; Xu, A.; Rodrigues, E.; Guerreiro, S.G.; Castro, R.C.; Ribeiro, D.S.M.; Santos, J.L.M.; Piloto, A.M.L. Optical Immunosensor Panel Using Quantum Dot-Antibody Conjugates for Highly Sensitive Detection of Carbohydrate Antigen 19–9 (CA19-9). Anal. Chim. Acta 2025, 1333, 343399. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.-M.; Bao, R.-M.; Yu, C.-M.; Lv, Y.-N.; Zhang, W.-F.; Tang, J.-B. Fc-Specific Biotinylation of Antibody Using an Engineered Photoactivatable Z–Biotin and Its Biosensing Application. Anal. Chim. Acta 2017, 949, 76–82. [Google Scholar] [CrossRef]
- Gao, X.-Y.; Xu, C.-M.; Zhang, X.-K.; Li, M.-R.; Gong, X.-M.; Yang, H.-M.; Tang, J.-B. Development of Fc-Specific Multi-Biotinylated Antibodies via Photoreactive Tandem AviTag Repeats for the Ultrasensitive Determination of Ochratoxin A. Food Control 2022, 132, 108525. [Google Scholar] [CrossRef]
- Dvorakova, V.; Cadkova, M.; Datinska, V.; Kleparnik, K.; Foret, F.; Bilkova, Z.; Korecka, L. An Advanced Conjugation Strategy for the Preparation of Quantum Dot-Antibody Immunoprobes. Anal. Methods 2017, 9, 1991–1997. [Google Scholar] [CrossRef]
- Jin, T.; Tiwari, D.K.; Tanaka, S.; Inouye, Y.; Yoshizawa, K.; Watanabe, T.M. Antibody–ProteinA Conjugated Quantum Dots for Multiplexed Imaging of Surface Receptors in Living Cells. Mol. Biosyst. 2010, 6, 2325. [Google Scholar] [CrossRef]
- Kobayashi, H.; Hama, Y.; Koyama, Y.; Barrett, T.; Regino, C.A.S.; Urano, Y.; Choyke, P.L. Simultaneous Multicolor Imaging of Five Different Lymphatic Basins Using Quantum Dots. Nano Lett. 2007, 7, 1711–1716. [Google Scholar] [CrossRef]
- Oda, M. Evidence of Allosteric Conformational Changes in the Antibody Constant Region upon Antigen Binding. Int. Immunol. 2003, 15, 417–426. [Google Scholar] [CrossRef]
- Nilsson, J.; Nilsson, P.; Williams, Y.; Pettersson, L.; Uhlén, M.; Nygren, P. Competitive Elution of Protein A Fusion Proteins Allows Specific Recovery Under Mild Conditions. Eur. J. Biochem. 1994, 224, 103–108. [Google Scholar] [CrossRef]
- Makride, S.C.; Gasbarro, C.; Bello, J.M. Bioconjugation of Quantum Dot Luminescent Probes for Western Blot Analysis. BioTechniques 2005, 39, 501–506. [Google Scholar] [CrossRef]
- Jansson, B.; Uhlén, M.; Nygren, P.-Å. All Individual Domains of Staphylococcal Protein A Show Fab Binding. FEMS Immunol. Med. Microbiol. 2006, 20, 69–78. [Google Scholar] [CrossRef]
- Linhult, M.; Binz, H.K.; Uhlén, M.; Hober, S. Mutational Analysis of the Interaction between Albumin-binding Domain from Streptococcal Protein G and Human Serum Albumin. Protein Sci. 2002, 11, 206–213. [Google Scholar] [CrossRef]
- Tsuboi, S.; Sasaki, A.; Sakata, T.; Yasuda, H.; Jin, T. Immunoglobulin Binding (B1) Domain Mediated Antibody Conjugation to Quantum Dots for in Vitro and in Vivo Molecular Imaging. Chem. Commun. 2017, 53, 9450–9453. [Google Scholar] [CrossRef] [PubMed]
- Goldman, E.R.; Anderson, G.P.; Tran, P.T.; Mattoussi, H.; Charles, P.T.; Mauro, J.M. Conjugation of Luminescent Quantum Dots with Antibodies Using an Engineered Adaptor Protein To Provide New Reagents for Fluoroimmunoassays. Anal. Chem. 2002, 74, 841–847. [Google Scholar] [CrossRef] [PubMed]
- Åkerström, B.; Björck, L. Protein L: An Immunoglobulin Light Chain-Binding Bacterial Protein. J. Biol. Chem. 1989, 264, 19740–19746. [Google Scholar] [CrossRef] [PubMed]
- Lao, U.L.; Mulchandani, A.; Chen, W. Simple Conjugation and Purification of Quantum Dot−Antibody Complexes Using a Thermally Responsive Elastin-Protein L Scaffold As Immunofluorescent Agents. J. Am. Chem. Soc. 2006, 128, 14756–14757. [Google Scholar] [CrossRef]
- Li, N.K.; Quiroz, F.G.; Hall, C.K.; Chilkoti, A.; Yingling, Y.G. Molecular Description of the LCST Behavior of an Elastin-Like Polypeptide. Biomacromolecules 2014, 15, 3522–3530. [Google Scholar] [CrossRef]
- Zhang, Y.; Huang, Y.; Li, R.; Wang, H.; Zhang, Z.; Lin, H.; Li, Z. Site-Specific Labeling of Antibodies with Quantum Dots Could Promote to Retain the Antigen Binding Capacity of Antibodies. Food Chem. 2023, 413, 135655. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, M.D.; Turner, B.L.; Rivera, K.R.; Menegatti, S.; Daniele, M. Quantum Dot Enabled Lateral Flow Immunoassay for Detection of Cardiac Biomarker NT-proBNP. Sens. Bio-Sens. Res. 2018, 21, 46–53. [Google Scholar] [CrossRef]
- Bilan, R.; Brazhnik, K.; Chames, P.; Baty, D.; Nabiev, I.; Sukhanova, A. Oriented Conjugates of Single-Domain Antibodies and Fluorescent Quantum Dots for Highly Sensitive Detection of Tumor-Associated Biomarkers in Cells and Tissues. Phys. Procedia 2015, 73, 228–234. [Google Scholar] [CrossRef]
- Brazhnik, K.; Nabiev, I.; Sukhanova, A. Advanced Procedure for Oriented Conjugation of Full-Size Antibodies with Quantum Dots. In Quantum Dots: Applications in Biology; Fontes, A., Santos, B.S., Eds.; Springer: New York, NY, USA, 2014; pp. 55–66. ISBN 978-1-4939-1280-3. [Google Scholar]
- Brazhnik, K.; Nabiev, I.; Sukhanova, A. Oriented Conjugation of Single-Domain Antibodies and Quantum Dots. In Quantum Dots: Applications in Biology; Fontes, A., Santos, B.S., Eds.; Springer: New York, NY, USA, 2014; pp. 129–140. ISBN 978-1-4939-1280-3. [Google Scholar]
- Zhang, B.; Yu, J.; Liu, C.; Wang, J.; Han, H.; Zhang, P.; Shi, D. Improving Detection Sensitivity by Oriented Bioconjugation of Antibodies to Quantum Dots with a Flexible Spacer Arm for Immunoassay. RSC Adv. 2016, 6, 50119–50127. [Google Scholar] [CrossRef]
- Huh, J.H.; White, A.J.; Brych, S.R.; Franey, H.; Matsumura, M. The Identification of Free Cysteine Residues Within Antibodies a Potential Role for Free Cysteine Residues in Covalent Aggregation Because of Agitation Stress. J. Pharm. Sci. 2013, 102, 1701–1711. [Google Scholar] [CrossRef]
- Hwang, D.; Nilchan, N.; Park, H.; Roy, R.N.; Roush, W.R.; Rader, C. Sculpting a Uniquely Reactive Cysteine Residue for Site-Specific Antibody Conjugation. Bioconjug. Chem. 2022, 33, 1192–1200. [Google Scholar] [CrossRef]
- Anderson, G.P.; Glaven, R.H.; Algar, W.R.; Susumu, K.; Stewart, M.H.; Medintz, I.L.; Goldman, E.R. Single Domain Antibody–Quantum Dot Conjugates for Ricin Detection by Both Fluoroimmunoassay and Surface Plasmon Resonance. Anal. Chim. Acta 2013, 786, 132–138. [Google Scholar] [CrossRef]
- Sukhanova, A.; Even-Desrumeaux, K.; Millot, J.-M.; Chames, P.; Baty, D.; Artemyev, M.; Oleinikov, V.; Cohen, J.; Nabiev, I. Oriented Conjugates of Monoclonal and Single-Domain Antibodies with Quantum Dots for Flow Cytometry and Immunohistochemistry Diagnostic Applications. Prog. Biomed. Opt. Imaging—Proc. SPIE 2012, 8232, 18. [Google Scholar] [CrossRef]
- Wang, W.; Hou, X.; Yang, X.; Liu, A.; Tang, Z.; Mo, F.; Yin, S.; Lu, X. Highly Sensitive Detection of CTLA-4-positive T-cell Subgroups Based on Nanobody and Fluorescent Carbon Quantum Dots. Oncol. Lett. 2019, 18, 109–116. [Google Scholar] [CrossRef]
- Wegner, K.D.; Lindén, S.; Jin, Z.; Jennings, T.L.; Khoulati, R.E.; Van Bergen En Henegouwen, P.M.P.; Hildebrandt, N. Nanobodies and Nanocrystals: Highly Sensitive Quantum Dot-Based Homogeneous FRET Immunoassay for Serum-Based EGFR Detection. Small 2014, 10, 734–740. [Google Scholar] [CrossRef]
- Gonzalez-Sapienza, G.; Rossotti, M.A.; Tabares-da Rosa, S. Single-Domain Antibodies As Versatile Affinity Reagents for Analytical and Diagnostic Applications. Front. Immunol. 2017, 8, 977. [Google Scholar] [CrossRef]
- Pedroso, C.C.S.; Mann, V.R.; Zuberbühler, K.; Bohn, M.-F.; Yu, J.; Altoe, V.; Craik, C.S.; Cohen, B.E. Immunotargeting of Nanocrystals by SpyCatcher Conjugation of Engineered Antibodies. ACS Nano 2021, 15, 18374–18384. [Google Scholar] [CrossRef]
- Zakeri, B.; Fierer, J.O.; Celik, E.; Chittock, E.C.; Schwarz-Linek, U.; Moy, V.T.; Howarth, M. Peptide Tag Forming a Rapid Covalent Bond to a Protein, through Engineering a Bacterial Adhesin. Proc. Natl. Acad. Sci. USA 2012, 109, E690–E697. [Google Scholar] [CrossRef]
- Xu, W.; Liu, L.; Brown, N.J.; Christian, S.; Hornby, D. Quantum Dot-Conjugated Anti-GRP78 scFv Inhibits Cancer Growth in Mice. Molecules 2012, 17, 796–808. [Google Scholar] [CrossRef] [PubMed]
- Kolossov, V.L.; Kanakaraju, K.; Sarkar, S.; Arogundade, O.H.; Kuo, C.-W.; Mara, N.R.; Smith, A.M. Quantum Dot-Fab′ Conjugates as Compact Immunolabels for Microtubule Imaging and Cell Classification. ACS Nano 2024, 18, 15084–15095. [Google Scholar] [CrossRef] [PubMed]
- Umakoshi, T.; Udaka, H.; Uchihashi, T.; Ando, T.; Suzuki, M.; Fukuda, T. Quantum-Dot Antibody Conjugation Visualized at the Single-Molecule Scale with High-Speed Atomic Force Microscopy. Colloids Surf. B Biointerfaces 2018, 167, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Andris, S.; Seidel, J.; Hubbuch, J. Kinetic Reaction Modeling for Antibody-Drug Conjugate Process Development. J. Biotechnol. 2019, 306, 71–80. [Google Scholar] [CrossRef]
- Weggen, J.T.; González, P.; Hui, K.; Bean, R.; Wendeler, M.; Hubbuch, J. Kinetic Modeling of the Antibody Disulfide Bond Reduction Reaction With Integrated Prediction of the Drug Load Profile for Cysteine-Conjugated ADCs. Biotechnol. Bioeng. 2025, 122, 579–593. [Google Scholar] [CrossRef]
- Noriega, H.A.; Wang, X.S. AI-Driven Innovation in Antibody-Drug Conjugate Design. Front. Drug Discov. 2025, 5, 1628789. [Google Scholar] [CrossRef]
- Mustafaoglu, N.; Kiziltepe, T.; Bilgicer, B. Site-Specific Conjugation of an Antibody on a Gold Nanoparticle Surface for One-Step Diagnosis of Prostate Specific Antigen with Dynamic Light Scattering. Nanoscale 2017, 9, 8684–8694. [Google Scholar] [CrossRef]
- Wang, W.; He, Y.; He, S.; Deng, L.; Wang, H.; Cao, Z.; Feng, Z.; Xiong, B.; Yin, Y. A Brief Review of Aptamer-Based Biosensors in Recent Years. Biosensors 2025, 15, 120. [Google Scholar] [CrossRef]
- Soxpollard, N.; Strauss, S.; Jungmann, R.; MacPherson, I.S. Selection of Antibody-Binding Covalent Aptamers. Commun. Chem. 2024, 7, 174. [Google Scholar] [CrossRef]
- Jeger, S.; Zimmermann, K.; Blanc, A.; Grünberg, J.; Honer, M.; Hunziker, P.; Struthers, H.; Schibli, R. Site-Specific and Stoichiometric Modification of Antibodies by Bacterial Transglutaminase. Angew. Chem. Int. Ed. 2010, 49, 9995–9997. [Google Scholar] [CrossRef]
- Strop, P.; Liu, S.-H.; Dorywalska, M.; Delaria, K.; Dushin, R.G.; Tran, T.-T.; Ho, W.-H.; Farias, S.; Casas, M.G.; Abdiche, Y.; et al. Location Matters: Site of Conjugation Modulates Stability and Pharmacokinetics of Antibody Drug Conjugates. Chem. Biol. 2013, 20, 161–167. [Google Scholar] [CrossRef]
- Ramakrishnan, B.; Boeggeman, E.; Manzoni, M.; Zhu, Z.; Loomis, K.; Puri, A.; Dimitrov, D.S.; Qasba, P.K. Multiple Site-Specific in Vitro Labeling of Single-Chain Antibody. Bioconjug. Chem. 2009, 20, 1383–1389. [Google Scholar] [CrossRef] [PubMed]
- Möhlmann, S.; Mahlert, C.; Greven, S.; Scholz, P.; Harrenga, A. In Vitro Sortagging of an Antibody Fab Fragment: Overcoming Unproductive Reactions of Sortase with Water and Lysine Side Chains. ChemBioChem 2011, 12, 1774–1780. [Google Scholar] [CrossRef] [PubMed]
- Petershans, A.; Wedlich, D.; Fruk, L. Bioconjugation of CdSe/ZnS Nanoparticles with SNAP Tagged Proteins. Chem. Commun. 2011, 47, 10671. [Google Scholar] [CrossRef]
- Ohyanagi, T.; Shima, T.; Okada, Y.; Tsukasaki, Y.; Komatsuzaki, A.; Tsuboi, S.; Jin, T. Compact and Stable SNAP Ligand-Conjugated Quantum Dots as a Fluorescent Probe for Single-Molecule Imaging of Dynein Motor Protein. Chem. Commun. 2015, 51, 14836–14839. [Google Scholar] [CrossRef]
- Fellouse, F.A.; Wiesmann, C.; Sidhu, S.S. Synthetic Antibodies from a Four-Amino-Acid Code: A Dominant Role for Tyrosine in Antigen Recognition. Proc. Natl. Acad. Sci. USA 2004, 101, 12467–12472. [Google Scholar] [CrossRef]
- Ban, H.; Nagano, M.; Gavrilyuk, J.; Hakamata, W.; Inokuma, T.; Barbas, C.F. Facile and Stabile Linkages through Tyrosine: Bioconjugation Strategies with the Tyrosine-Click Reaction. Bioconjug. Chem. 2013, 24, 520–532. [Google Scholar] [CrossRef]
- Griebenow, N.; Greven, S.; Lobell, M.; Dilmaç, A.M.; Bräse, S. A Study on the Trastuzumab Conjugation at Tyrosine Using Diazonium Salts. RSC Adv. 2015, 5, 103506–103511. [Google Scholar] [CrossRef]
- Szijj, P.A.; Kostadinova, K.A.; Spears, R.J.; Chudasama, V. Tyrosine Bioconjugation—An Emergent Alternative. Org. Biomol. Chem. 2020, 18, 9018–9028. [Google Scholar] [CrossRef] [PubMed]
- Hallam, T.J.; Smider, V.V. Unnatural Amino Acids in Novel Antibody Conjugates. Future Med. Chem. 2014, 6, 1309–1324. [Google Scholar] [CrossRef] [PubMed]
- Hutchins, B.M.; Kazane, S.A.; Staflin, K.; Forsyth, J.S.; Felding-Habermann, B.; Schultz, P.G.; Smider, V.V. Site-Specific Coupling and Sterically Controlled Formation of Multimeric Antibody Fab Fragments with Unnatural Amino Acids. J. Mol. Biol. 2011, 406, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Cimaglia, F.; Aliverti, A.; Chiesa, M.; Poltronieri, P.; De Lorenzis, E.; Santino, A.; Sechi, L.A. Quantum Dots Nanoparticle-Based Lateral Flow Assay for Rapid Detection of Mycobacterium Species Using Anti-FprA Antibodies. Nanotechnol. Dev. 2012, 2, 5. [Google Scholar] [CrossRef]
- Kalvaityte, U.; Bagdonas, E.; Kirdaite, G.; Kausaite-Minkstimiene, A.; Uzieliene, I.; Ramanaviciene, A.; Popov, A.; Butkiene, G.; Karabanovas, V.; Denkovskij, J.; et al. Development of a Sensitive Quantum Dot-Linked Immunoassay for the Multiplex Detection of Biochemical Markers in a Microvolumeric Format. Int. J. Nanomedicine 2025, 20, 1717–1729. [Google Scholar] [CrossRef]
- Di Nardo, F.; Anfossi, L.; Giovannoli, C.; Passini, C.; Goftman, V.V.; Goryacheva, I.Y.; Baggiani, C. A Fluorescent Immunochromatographic Strip Test Using Quantum Dots for Fumonisins Detection. Talanta 2016, 150, 463–468. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, X.; Qian, W.; Yang, Q.; Qi, Y.; Chen, Y.; Wang, A. Quantum Dots-based Fluorescence Immunoassay for Detection of Tiamulin in Pork. J. Food Saf. 2021, 41, e12930. [Google Scholar] [CrossRef]

| QD Type | QD Surface Ligand | Conjugation Principle | Comments | Tested Application | Ref. |
|---|---|---|---|---|---|
| Site-specific QD–Ab conjugation | |||||
| CdSe/ZnS; CdSeTe/CdS | GSH | Immunoglobulin-binding domain of protein G (GB1) was expressed with 6 His tags at its N-terminus (HisGB1). HisGB1 directly binds to QDs via complexation between histidine tags and Zn2+ or Cd2+ ions at the QD surface because of the high affinity of His to these ions. | Since the GB1 domain recognizes the Fc region of IgG, its antigen-binding activity is not affected by Ab-conjugation reactions. | Fluorescent microscopy and in vivo fluorescence imaging | [84] |
| CdSe/ZnS | DHLA | QDs capped with negatively charged DHLA interact with a mixture of PG-zb and MBP-zb via the positively charged zb peptide tag. | PG interacts with the Fc region of the anti-SEB or anti-TNT Ab. The amount of Abs bound to the QD surface can be controlled by varying the ratio of PG-zb and MBP-zb in the mixture. MBP can be used to purify conjugates. | Fluorescent immunosorbent assay; continuous-flow immunoassay | [85] |
| CdSe/ZnS | Carboxyl compound | The tripartite fusion protein (His–ELP–PL) consists of an N-terminal His tag used for QD conjugation via strong metal-affinity coordination with Zn2+ on the QD surface, the ELP midblock of 78 repeating VPGVG units for stimulus-responsive purification, and the C-terminal PL. | PL has a high affinity for Ig κ-light chains. ELP tag can undergo a reversible phase transition from water-soluble forms into aggregates and can be used for purification of QDs IgGs conjugates. | Western blot analysis; microarrays | [87] |
| CdSe/ZnS | Amino compound | A photoactivated probe (Bpa) with a Cys handle (an adapter) is integrated into the Z domain of protein A covalently coupled to the Fc region of the Ab using ultraviolet irradiation. This construct is conjugated to QDs via its free Cys sulfhydryl group and amino groups on QD surface in the presence of maleimide. | The method yields site-specific covalent QD–Ab conjugates that have a higher antigen affinity than randomly oriented conjugates. | Immunofluorescence; lateral flow immunoassay | [89] |
| CdSe/CdZnS | Gluthathione | Protein A coupled to QD surface by a carbodiimide chemistry technique is used for non-covalent conjugation with the Fc fragment of full-length Abs. | The hydrodynamic size of protein A–modified QDs is smaller than 10 nm. These QDs are monodisperse and do not aggregate in PBS over two months. | Multiplexed confocal fluorescence microscopy | [77] |
| CdSe/ZnS | Streptavidin | Biotin coupled with the divalent ZZ domain based on the B domain of protein A is used as a linker for conjugation of a streptavidin-coated QD and the Fc domain. | The ZZ peptide does not bind the Fab region of Ig, in contrast to all individual domains of protein A, which offers the advantage of a more uniform binding to antibodies (via the Fc region), leaving the Fab domain free to bind its target. | Fluorescent Western blot | [81] |
| CdS/ZnS | Amino compound | Oxidized glycosylated Fc regions of Abs were used for binding with amino-functionalized QDs, and the resultant conjugate was stabilized with sodium cyanoborohydride. | Site-specific conjugation increased the detection limit by a factor of 8 and enhanced the fluorescent signal compared with conjugation using carbodiimide chemistry. | Lateral flow assay | [90] |
| CdSe/ZnSe/ZnS | GSH | Succinimidyl valerate–PEG–maleimide (SMPEG) was used for obtaining maleimide-functionalized QDs subsequently conjugated with sulfhydryl groups of Cys in the hinge region of reduced Abs. | The number of Abs conjugated on the surface of one QD by SMPEG-mediated coupling was 2.13 ± 0.5, versus 0.76 ± 0.2 in the case of the site-nonspecific EDC-mediated coupling. | Fluorescent microscopy; dot blot assay | [94] |
| Qdot® 585 (CdSe/ZnS) | Amino-modified polyethylene glycol | Affinity-purified goat anti-mouse Abs were partially reduced with DTT or 2-MEA and conjugated with SMCC-activated QDs via sulfhydryl groups of the Ab hinge region. | This method allows site-specific conjugation of full-length Abs with QDs without the use of affinity proteins. | Dot blot analysis; flow cytometry | [92] |
| Qdot® 605 (CdSe/ZnS) | Carboxyl compound | Carboxyl-modified QDs functionalized with maleimide are conjugated, via the reduced Ab –SH groups, with Ab fragments obtained by splitting full-length Abs with tris(2-carboxyetheyl)phosphine hydrochloride. | The QD–Ab conjugates are imaged by high-speed atomic force microscopy. The number of attached Abs varied from 2 to 7 per QD (4.3 ± 1.5 on average). | Fluorescence microscopy | [106] |
| CdSe/ZnS | PEG derivatives containing terminal hydroxyl | QDs containing hydroxyl group on their surface were conjugated to the sdAbs containing cysteine residue in C-terminus using the PMPI crosslinker | Hydrodynamic diameter of QD-sdAb conjugate was about 12 nm and the contained four molecules of sdAbs on the surface of one QD | Flow cytometry | [91] |
| CdSe/ZnS | DHLA | sdAb with three repeats of 6 histidine residues separated by short spacer sequences at its C-terminus is expressed and coordinated to zinc ions on the QD surface. | Site-specifically conjugated sdAb–His–QDs provide a 4-fold higher signal in SPR than site-non-specifically conjugated sdAb–biotin–streptavidin–QDs. | Fluorescent immunoassay; SPR | [97] |
| CdSe/ZnS | SH-, NH2-, or OH-modified PEG | sdAb with an additional Cys at its C-terminus is expressed. | Ultrasmall conjugates. The hydrodynamic diameter of QDs functionalized with PEG derivatives is 8.8 nm; that of sdQD–Ab conjugates is 11.9 nm. | Flow cytometry; fluorescence immunohistochemistry | [98] |
| CdSe/CdS | PAOA | An scFv Ab fragment with the SpyTag peptide at the C-terminus interacts with a SpyCatcher-coated QD. | SpyCatcher proteins spontaneously form covalent isopeptide bonds with SpyTag peptides, thereby forming conjugates of engineered Abs and QDs with controlled stability, orientation, and stoichiometry. | Fluorescence microscopy | [102] |
| Qdot® 625 (CdSe/ZnS) | Polymer | Qdot® 625 activated with SMCC covalently bind with DTT-reduced scFv fragments. | The scFv–QD conjugates not only serve as detection tags, but also have tumoricidal activity, inhibiting xenograft breast tumor growth in vivo. | Fluorescence immunohistochemistry; Western blot analysis | [104] |
| HgxCd1−xSe/CdzZn1−zS | Polymer P(IM-N3) | A full-length Ab is reacted with DBCO–sulfo-N-hydroxysuccinimidyl ester to obtain DBCO bound to the Ab via amide bonds. The Fab′ fragment is conjugated at the hinge region with DBCO–maleimide. The conjugates are formed by covalent click reactions between the azides and cyclooctyne-functionalized Abs. | Conjugates of QDs with full-length Abs have a hydrodynamic diameter of about 22 nm, and conjugates with Fab fragments are only 12.3 nm in size, which is only slightly larger than the size of bare QDs (7.5 nm). It has been shown that smaller bioaffinity agents and site-specific orientation of Abs/Ab fragments on QDs are required to enhance penetration into biospecimens and minimize nonspecific staining. | Fluorescence Immunohistochemistry and microscopy | [105] |
| Site-nonspecific QD–Ab conjugation | |||||
| Qdot® 655 (CdSe/ZnS) | Streptavidin directly bound to the polymer shell | Streptavidin on the QD surface binds biotinylated Abs | The size of streptavidin-functionalized QDs is 14.7 ± 3.7 nm; that of QD–Ab conjugates is 29.6 ± 11.8 nm, as estimated by transmission electron microscopy. | Fluorescence quenching upon binding the analyte | [55] |
| CdTe | 3-Mercaptopropionic acid | Sterptavidin is bound to the QD surface by carbodiimide chemistry methods and then conjugated with biotinylated Abs. | The use of QD–Ab conjugates in immunosorbent assay increases the sensitivity of detection of chlorpyrifos 5.5-fold compared to the conventional ELISA using HRP–Ab conjugates. | Fluorescence-linked immunosorbent assay | [57] |
| Carbon | Carboxyl compound | Amino groups of sdAbs are conjugated with carboxylic group on the QD surface by carbodiimide chemistry methods. | The small allows the sdQD–Ab conjugates diffuse more efficiently into solid tumors. The conjugates are nontoxic for normal human cells and for 4 weeks after injection. | Flow cytometry, immunofluorescent staining | [99] |
| CdSe/ZnS | DHLA | An avidin-functionalized QD is conjugated with randomly biotinylated Abs. | Functionalization of the QD surface with a mixture of avidin and MBP-leucine zipper interaction domain allows controlling the amount of avidin on the QD surface and, hence, the amount of conjugated Abs per QD. | Sandwich immunoassay | [56] |
| CdSe/ZnS | Streptavidin | An streptavidin-functionalized QD is conjugated with randomly biotinylated Abs. | The LoD of specific mycobacterial flavoprotein reductase is 12.5 pg/μL. | Fluorescent lateral flow immunoassay | [125] |
| Qdot® 655 (CdSe/ZnS) | Streptavidin | An streptavidin-functionalized QD is conjugated with randomly biotinylated Abs. | The LoD of cartilage oligomeric matrix protein is 3.125 nM and LoD of human growth hormone—5 nM. | 96-well plate and microvolome slide fluorescence-linked immunesorbent assay | [126] |
| eFluor 490 (CdSe/ZnS) | Carboxyl compound | Carbodiimide-mediated conjugation of full-length Abs with QDs. | A protocol for carbodiimide-mediated QD–Ab conjugation and conjugate optimization is used. | Immunofluorescence imaging; membrane sandwich immunoassay | [62] |
| CdSe/CdS/ZnS | Polymer coated QDs with carboxyl groups | Carbodiimide-mediated conjugation of full-length Abs. | The LoD of fumonisin B1 is 2.8 μg/L. | Fluorescent lateral flow immunoassay | [127] |
| CdSe/ZnS | Commercial QDs with carboxyl groups | Carbodiimide-mediated conjugation of full-length Abs. | The LoD of ciliary neurotrophic factor is 6.45 pg/mL. | Fluorescent lateral flow immunoassay | [65] |
| ZnCdSe/ZnS | Commercial QDs with carboxyl groups | Carbodiimide-mediated conjugation of full-length Abs. | The LoD of tiamulin—0.309 pg/mL. | Indirect competitive fluorescence-linked immunosorbent assay | [128] |
| ZnCdSe/ZnS | Commercial QDs with carboxyl groups | Carbodiimide-mediated conjugation of full-length Abs. | The LoD of apramycin is 0.38 ng/mL. | Fluorescence-linked immunosorbent assay | [63] |
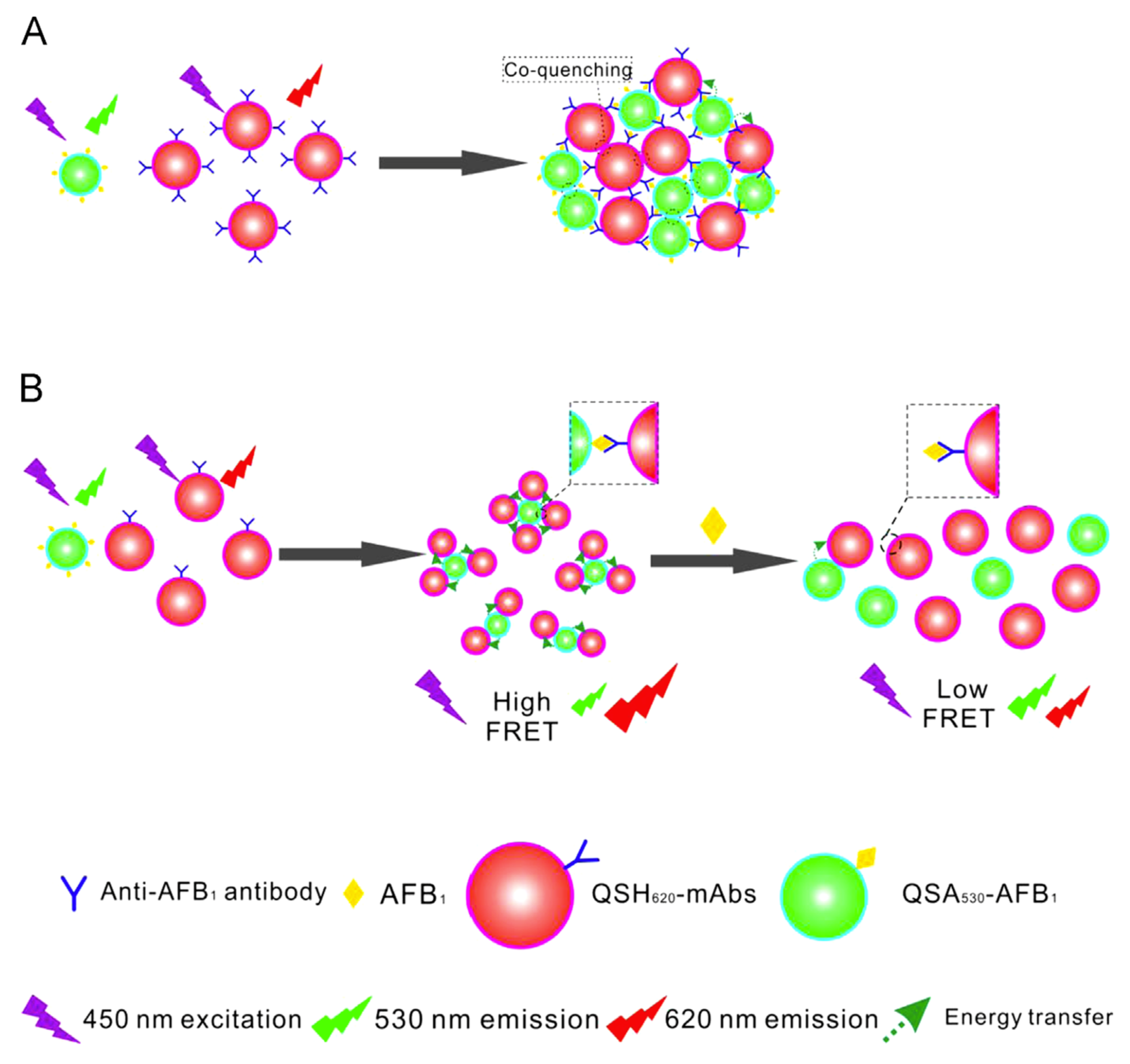
| QSH620 | Carboxylic acid-modified QDs with emission at 620 nm | Carbodiimide-mediated conjugation of full-length Abs. | The LoD of Aflatoxin B1 is 0.04 ng/mL. | FRET based immunosensor | [67] |
| CdSe/CdS; CdSe/CdZn0.3Zn0.7S; InAs/Cd0.2Zn0.8S | NBPILs | Tetrazine-modified Abs interact with NBPILs-functionalized QDs via tetrazine–norbornene cycloaddition. | The hydrodynamic diameter of the conjugate is 15–17 nm (that of free Abs is 12 nm); the quantum yield of the conjugated QDs is as high as ~80%. | Flow cytometry; in vivo microscopy; multiplexed single-cell imaging in vivo | [70] |
| CdSe/ZnS | Carboxyl compound | The aldehyde groups of Abs oxidized by sodium periodate are conjugated with the amino groups of BSA-modified QDs. | BSA acts as a QD-stabilizing agent and protect the Ab structure upon conjugation. Addition of BSA reduces nonspecific binding of the conjugates. | Confocal microscopy; fluorometric immunoassay | [72] |
| CdTe | 3-Mercaptopropionic acid | QDs are coated with BSA via the carbonyldiimidazole linker. Anti-CA19-9 Abs are conjugated to BSA via aldehyde groups. | The CA19-9 detection limit is about 1.66 10−4 U mL−1, which is significantly below the cutoff for early pancreatic cancer risk. | CA19-9 immunosensor based on QD fluorescence quenching | [73] |
| Qdot® 565 (CdSe/ZnS) | Carboxyl compound | Carbodiimide conjugation of full-length Abs. | Conjugation of QDs with anti-ApoE Abs bound with ApoE-modified magnetic particles protecting the Ab binding sites. | Capillary electrophoresis–laser induced fluorescence | [76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolov, P.; Knysh, A.; Kriukova, I.; Samokhvalov, P.; Kistenev, Y.V. Methods for Conjugating Antibodies with Quantum Dots. Molecules 2025, 30, 3999. https://doi.org/10.3390/molecules30193999
Sokolov P, Knysh A, Kriukova I, Samokhvalov P, Kistenev YV. Methods for Conjugating Antibodies with Quantum Dots. Molecules. 2025; 30(19):3999. https://doi.org/10.3390/molecules30193999
Chicago/Turabian StyleSokolov, Pavel, Alexander Knysh, Irina Kriukova, Pavel Samokhvalov, and Yury V. Kistenev. 2025. "Methods for Conjugating Antibodies with Quantum Dots" Molecules 30, no. 19: 3999. https://doi.org/10.3390/molecules30193999
APA StyleSokolov, P., Knysh, A., Kriukova, I., Samokhvalov, P., & Kistenev, Y. V. (2025). Methods for Conjugating Antibodies with Quantum Dots. Molecules, 30(19), 3999. https://doi.org/10.3390/molecules30193999









