Nickel Thiazoledithiolenes: π-Extended Fused-Ring Metal Dithiolenes as Highly Delocalized π-Electron Systems with Stabilized Frontier Orbitals
Abstract
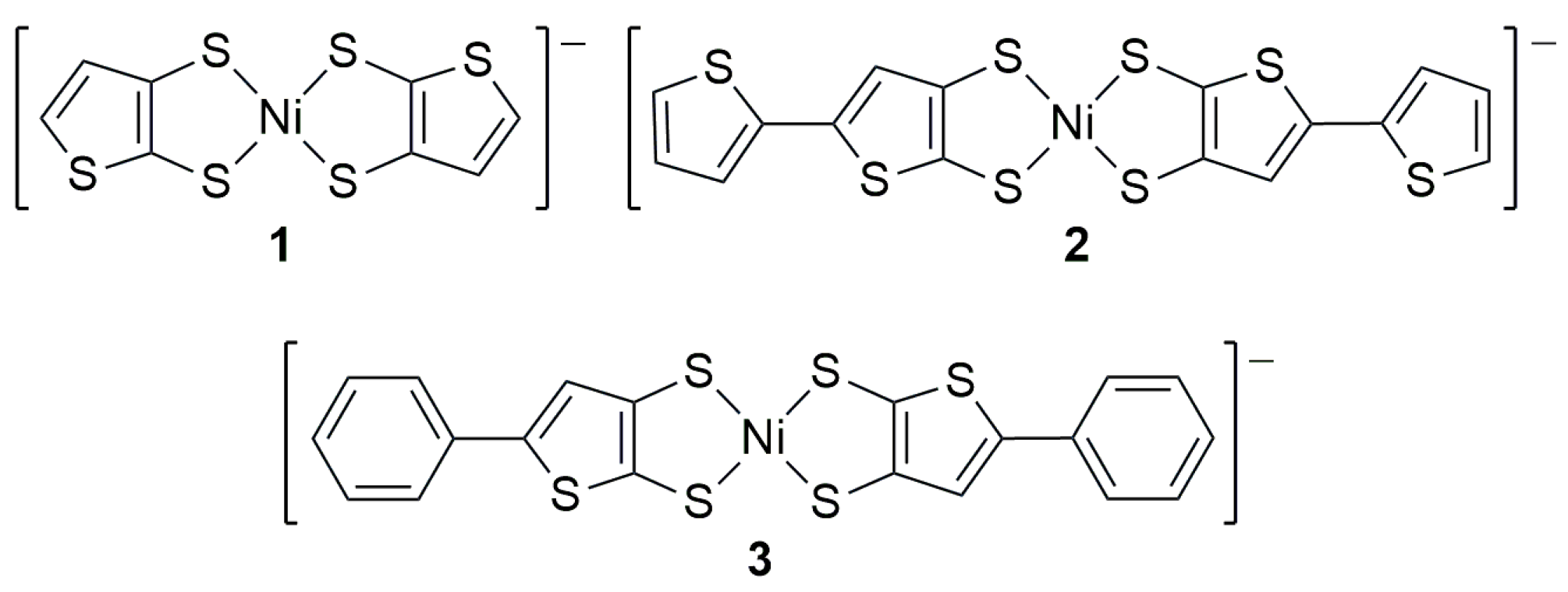
1. Introduction
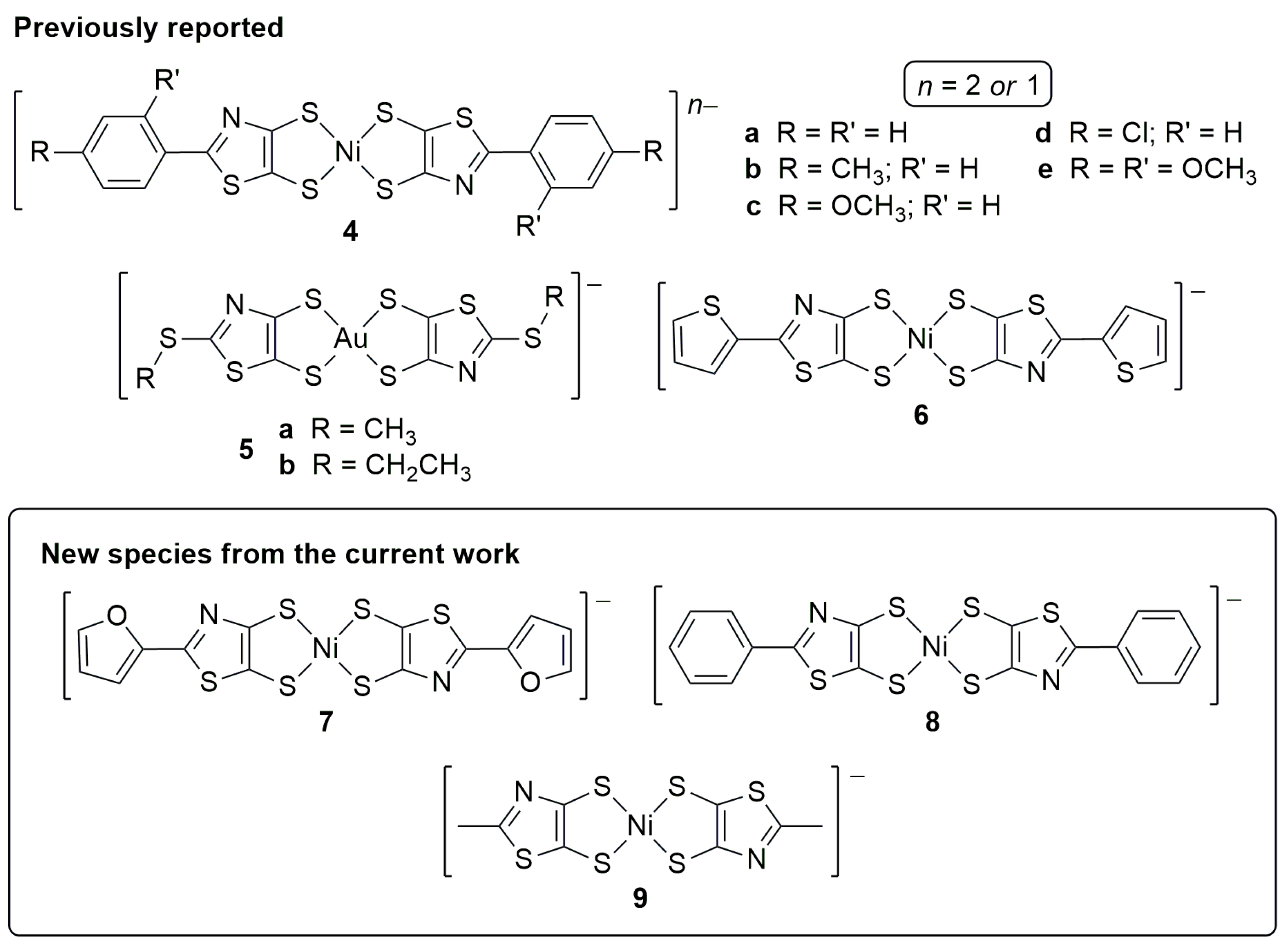
2. Results and Discussion
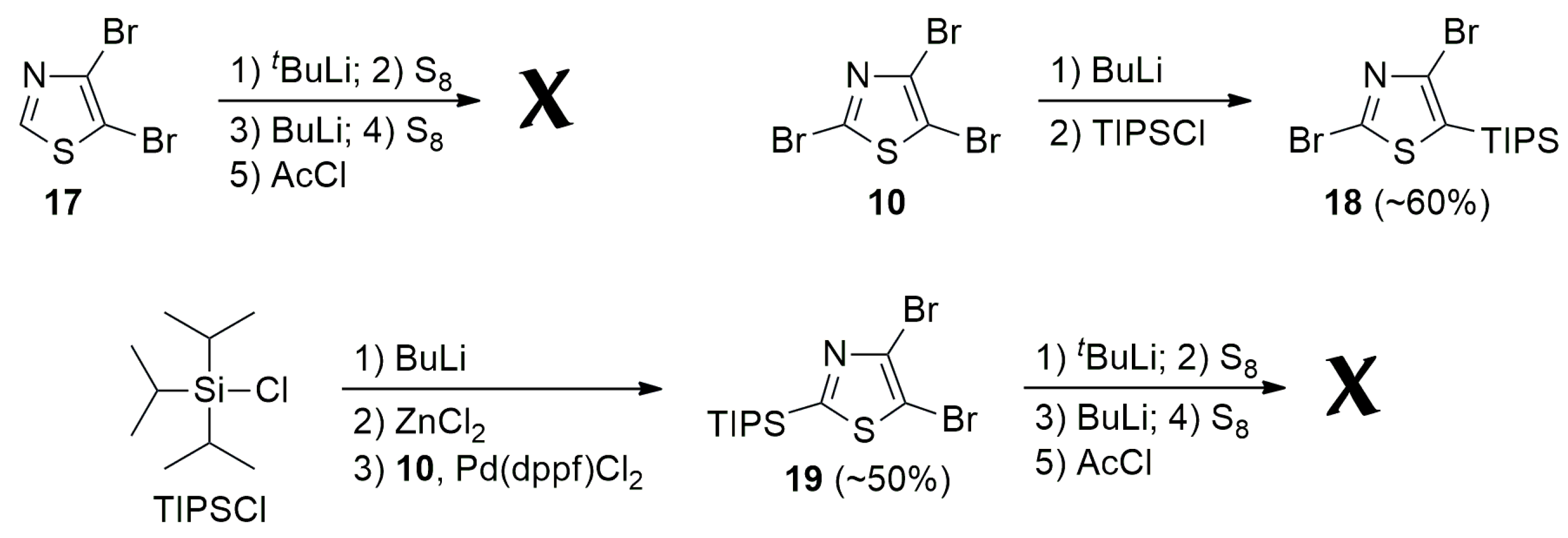
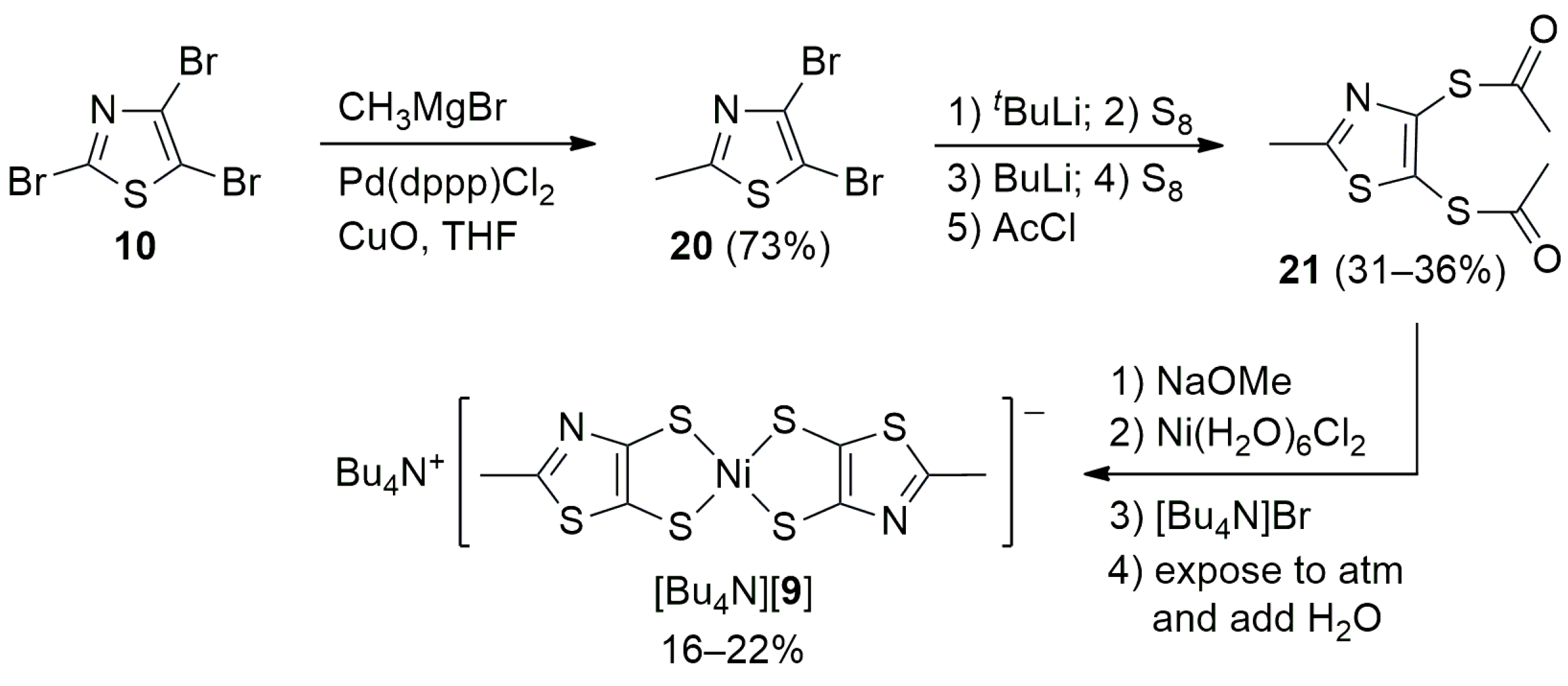
2.1. Synthesis
2.2. X-Ray Crystallography and Structural Analysis
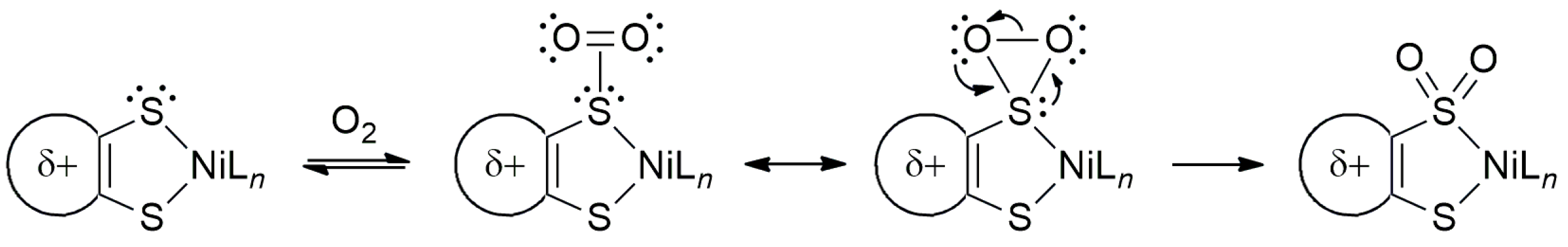
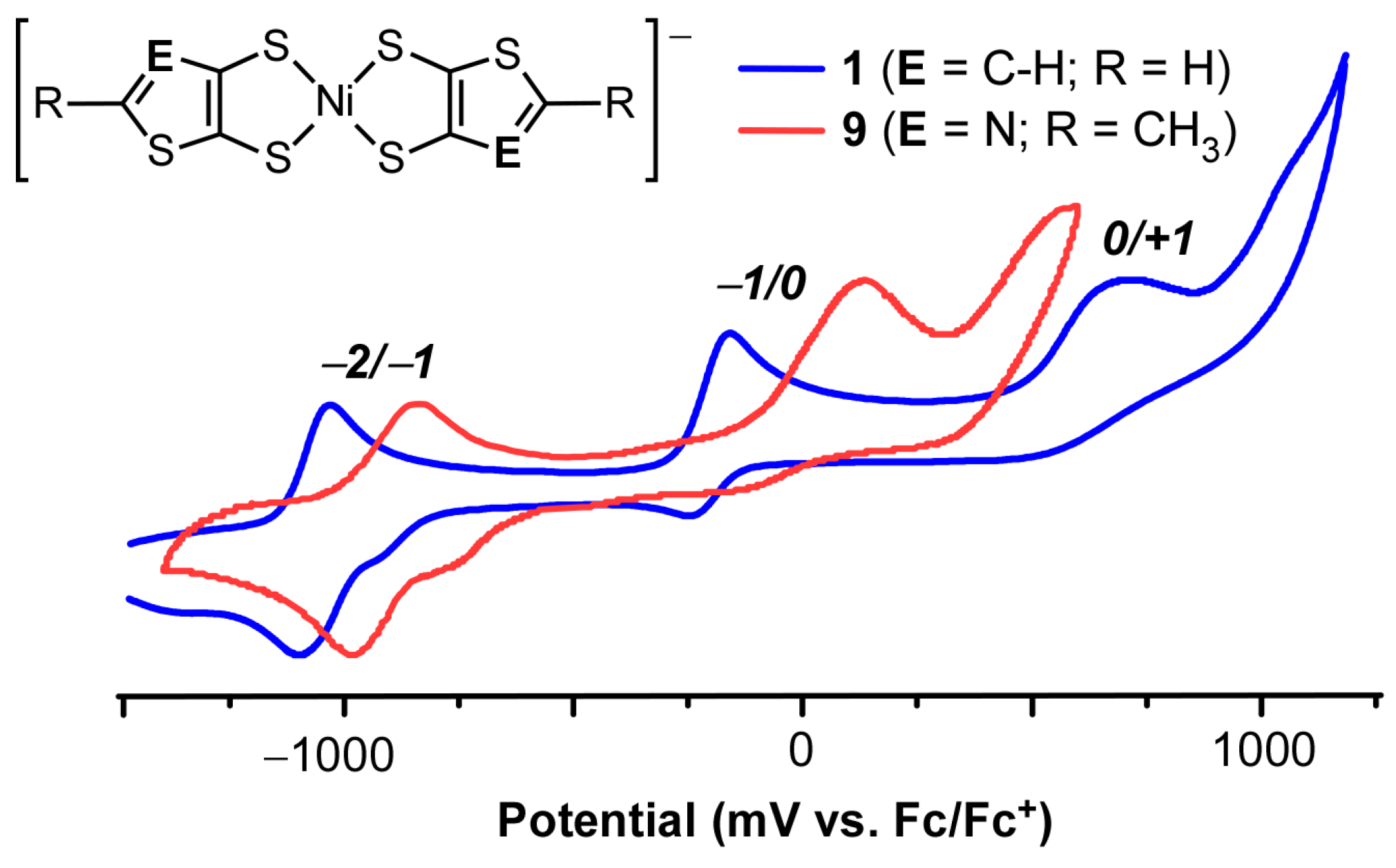
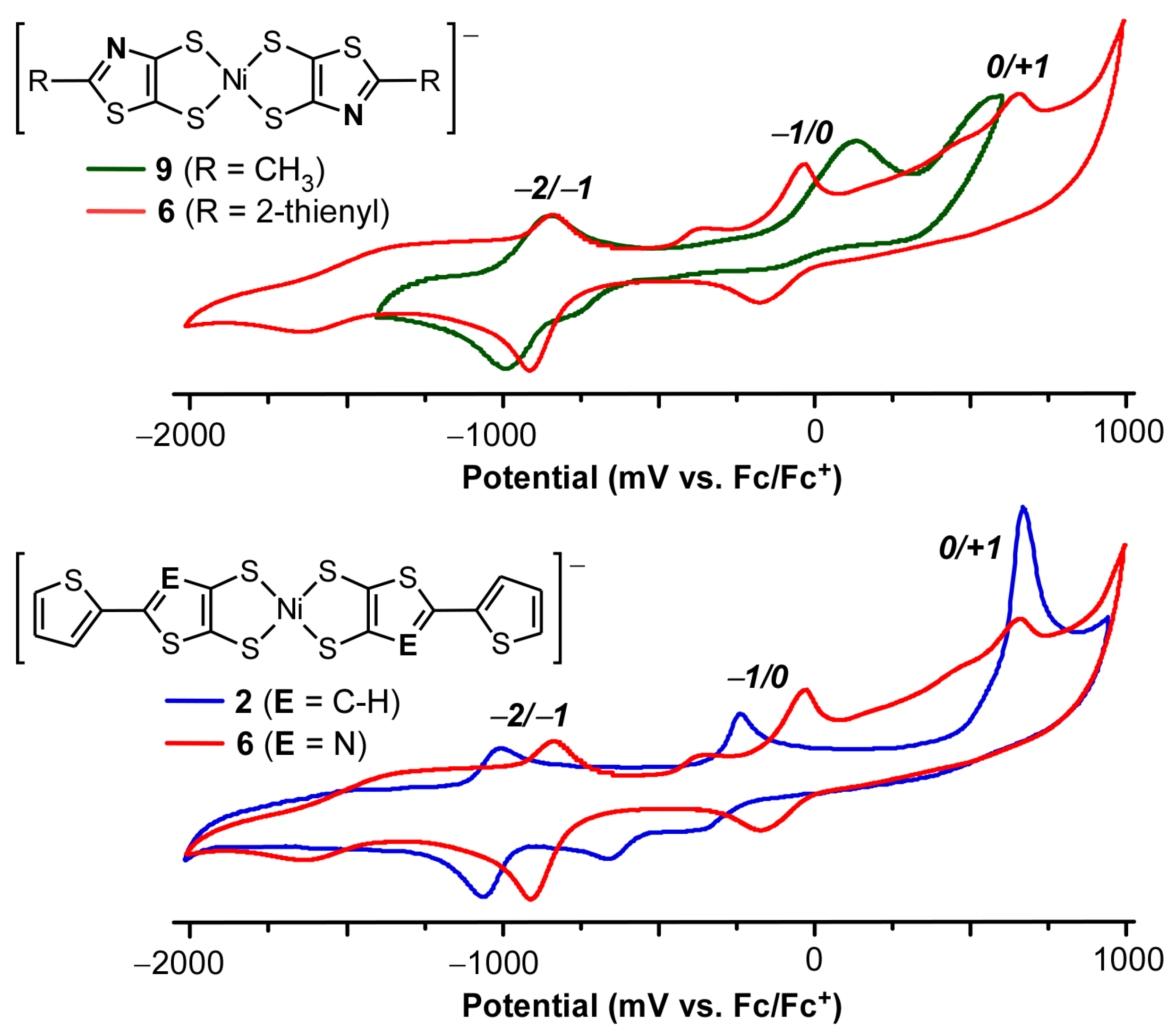
2.3. Electrochemistry
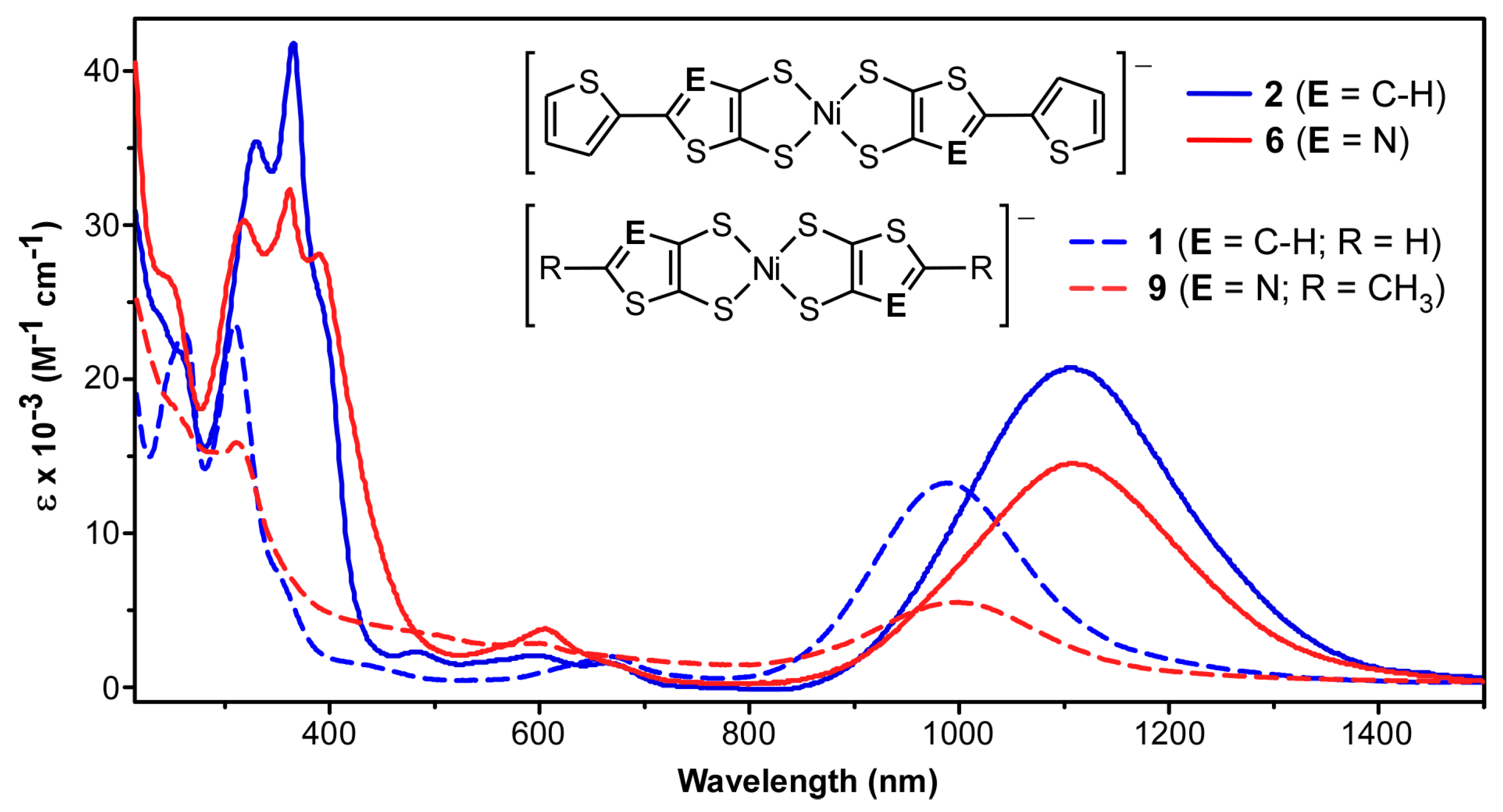
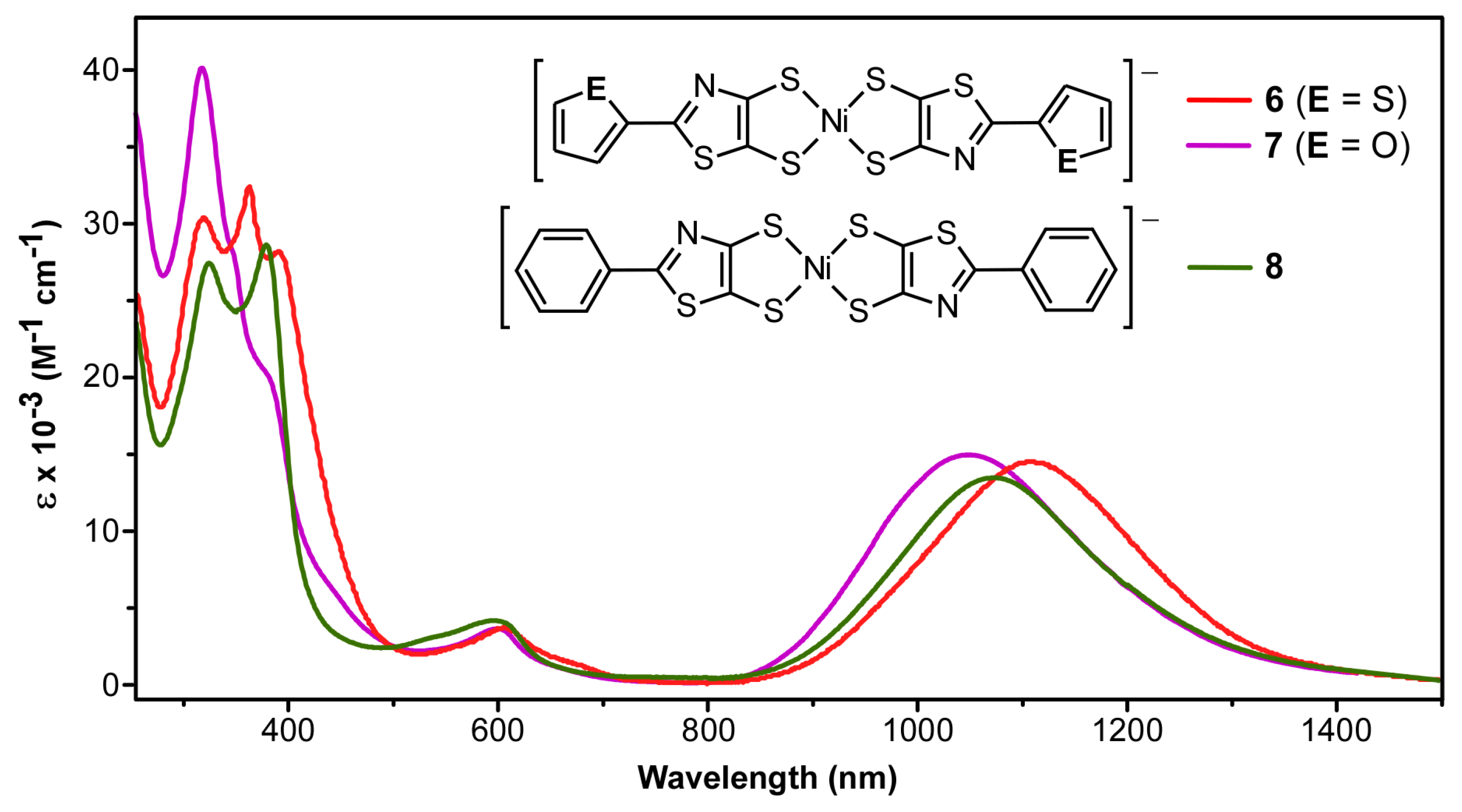
2.4. UV-Vis-NIR Absorption Spectroscopy
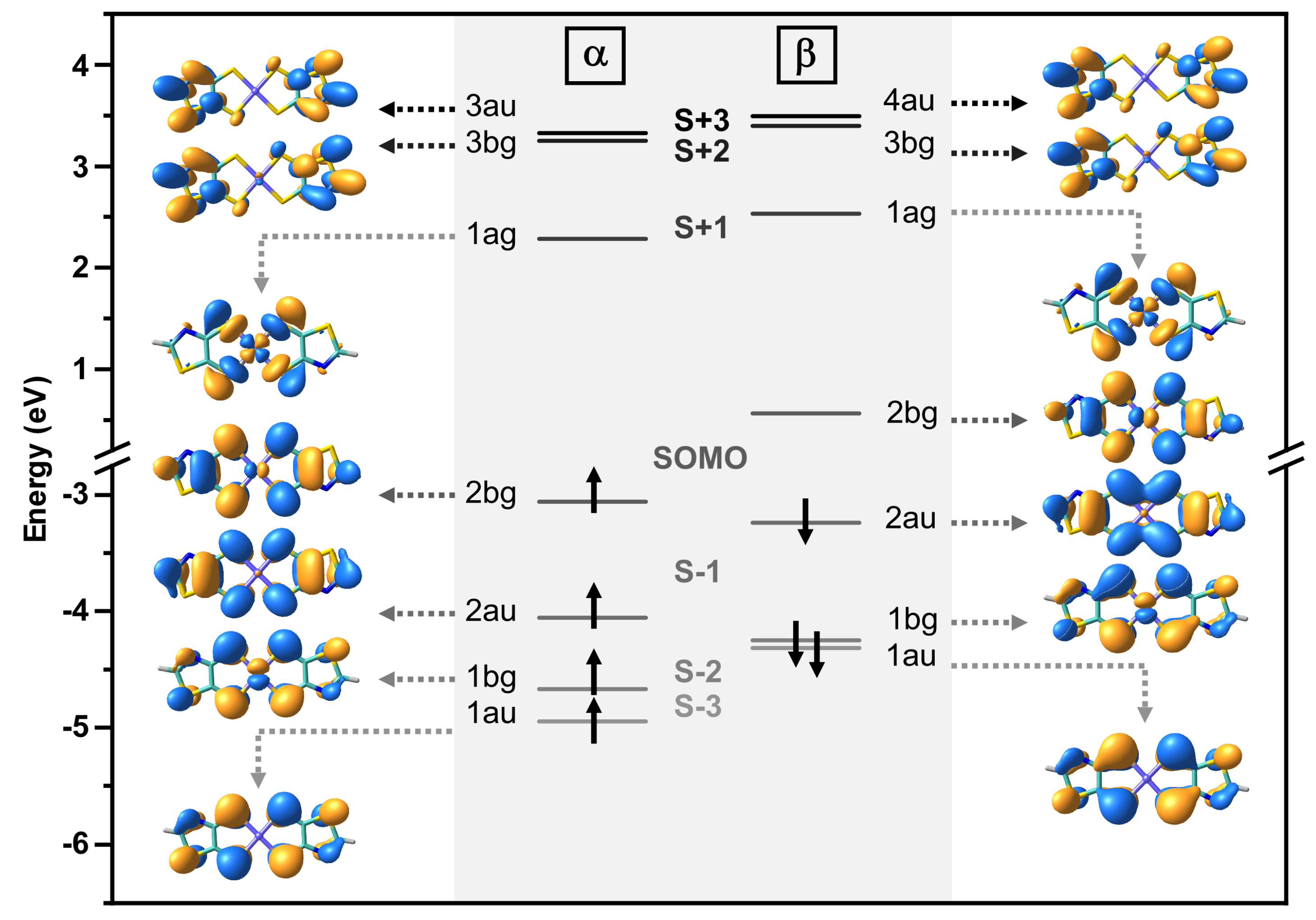
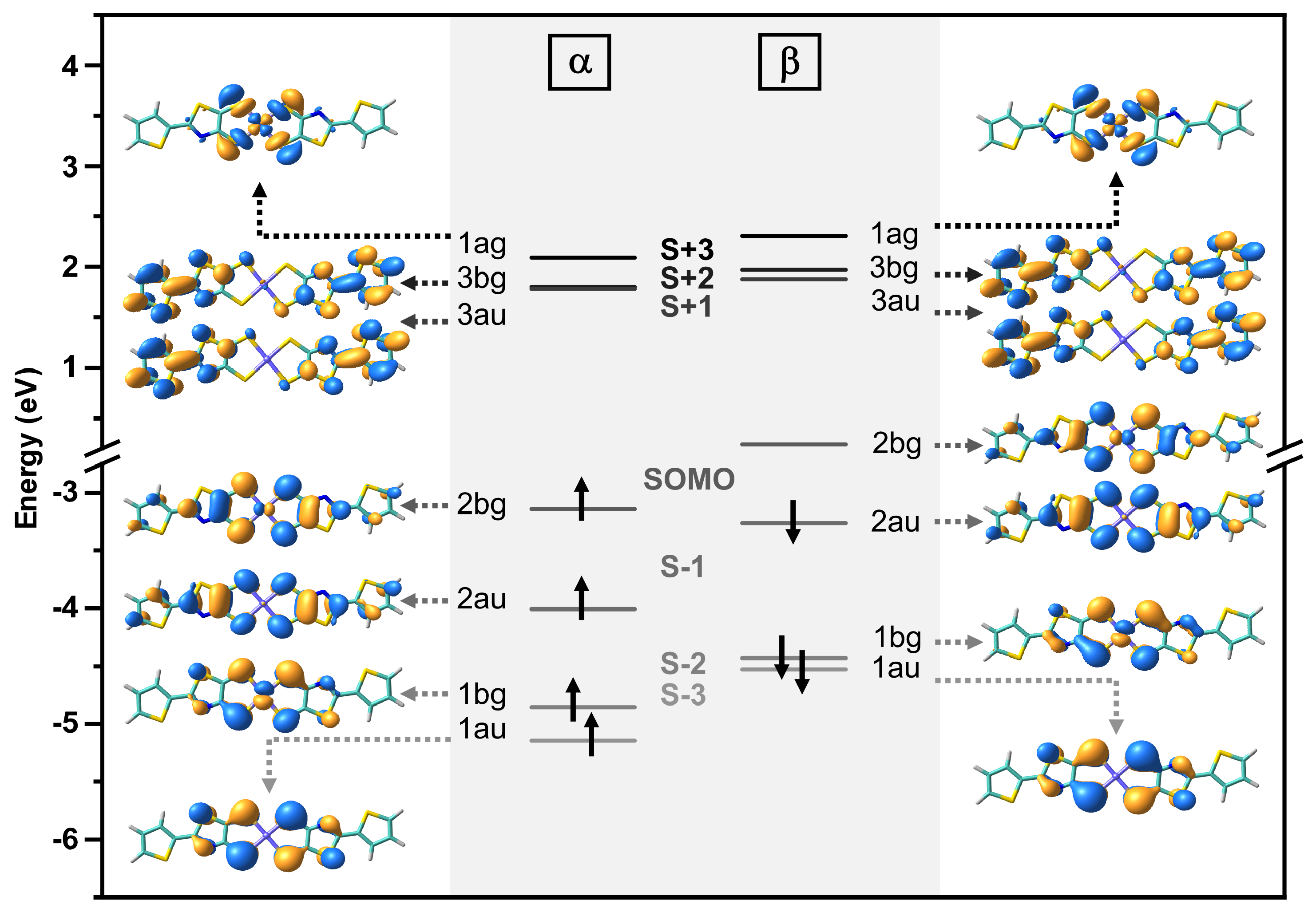
2.5. Theoretical Methods
2.6. Comparison of the Phenyl-Extended Complex 8 with the Previous Work of Kibbel
3. Materials and Methods
3.1. General Procedure for Synthesis of 2-Aryl-4,5-dibromothiazoles
3.1.1. 4,5-Dibromo-2-(2-thienyl)thiazole (11)
3.1.2. 4,5-Dibromo-2-(2-furyl)thiazole (12)
3.1.3. 4,5-Dibromo-2-phenylthiazole (13)
3.2. 4,5-Dibromo-2-methylthiazole (20)
3.3. General Procedure for Synthesis of Acetyl-Protected Ligands
3.3.1. 4,5-Bis(thioacetate)-2-(2-thienyl)thiazole (14)
3.3.2. 4,5-Bis(thioacetate)-2-(2-furyl)thiazole (15)
3.3.3. 4,5-Bis(thioacetate)-2-phenylthiazole (16)
3.3.4. 4,5-Bis(thioacetate)-2-methylthiazole (21)
3.4. General Procedure for Synthesis of Nickel Thiazoledithiolenes
3.4.1. Tetrabutylammonium Bis[2-(2-thienyl)-4,5-thiazoledithiolato]nickelate(1−) ([Bu4N][6])
3.4.2. Tetrabutylammonium Bis[2-(2-furyl)-4,5-thiazoledithiolato]nickelate(1−) ([Bu4N][7])
3.4.3. Tetrabutylammonium Bis(2-phenyl-4,5-thiazoledithiolato)nickelate(1−) ([Bu4N][8])
3.4.4. Tetrabutylammonium Bis(2-methyl-4,5-thiazoledithiolato)nickelate(1−) ([Bu4N][9])
3.5. X-Ray Crystallography
3.6. Theoretical Methodology
3.7. Absorption Spectroscopy
3.8. Electrochemistry
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BuLi | butyllithium |
| CCDC | Cambridge Crystallographic Data Centre |
| CCD | charge-coupled device |
| DFT | density functional theory |
| DMF | dimethylformamide |
| ESI-TOF | electrospray ionization time-of-flight |
| HRMS | high resolution mass spectrometry |
| IVCT | intervalence charge-transfer |
| LMCT | ligand-to-metal charge-transfer |
| MBDT | metal benzenedithiolene |
| MDT | metal dithiolene |
| MTDT | metal thiophenedithiolene |
| MO | molecular orbital |
| NIR | near infra-red |
| NiTzDT | nickel thiazoledithiolene |
| NMP | N-methylpyridinium |
| NMR | nuclear magnetic reasonance |
| f | oscillator strength |
| SCE | saturated calomel electrode |
| SOMO | singly occupied molecular orbital |
| tBuLi | tert-butyllithium |
| Bu4N | tetrabutylammonium |
| THF | tetrahydrofuran |
| TDDFT | time dependent density functional theory |
| TIPS | triisopropylsilyl |
| UV | ultraviolet |
References
- Eisenberg, R.; Gray, H.B. Noninnocence in Metal Complexes: A Dithiolene Dawn. Inorg. Chem. 2011, 50, 9741–9751. [Google Scholar] [CrossRef]
- Tiefel, E.I.; Karlin, K.D. Dithiolene Chemistry: Synthesis Properties, and Applications; Tiefel, E.I., Karlin, K.D., Eds.; Progress in Inorganic Chemistry; John Wiley & Sons: Hoboken, NJ, USA, 2004; Volume 52. [Google Scholar]
- Cassoux, P. Molecular (super)conductors derived from bisdithiolate metal complexes. Coord. Chem. Rev. 1999, 185–186, 213–232. [Google Scholar] [CrossRef]
- Robertson, N.; Cronin, L. Metal bis-1,2-dithiolene complexes in conducting or magnetic crystalline assemblies. Coord. Chem. Rev. 2002, 227, 93–127. [Google Scholar] [CrossRef]
- Kato, R. Conducting Metal Dithiolene Complexes: Structural and Electronic Properties. Chem. Rev. 2004, 104, 5319–5346. [Google Scholar] [CrossRef]
- Dalgleish, S.; Robertson, N. Electropolymerisable dithiolene complexes. Coord. Chem. Rev. 2010, 254, 1549–1558. [Google Scholar] [CrossRef]
- Belo, D.; Almeida, M. Transition metal complexes based on thiophene-dithiolene ligands. Coord. Chem. Rev. 2010, 254, 1479–1492. [Google Scholar] [CrossRef]
- Papavassiliou, G.C.; Anyfantis, G.C.; Mousdis, G.A. Neutral Metal 1,2-Dithiolenes: Preparations, Properties and Possible Applications of Unsymmetrical in Comparison to the Symmetrical. Crystals 2012, 2, 762–811. [Google Scholar] [CrossRef]
- Rasmussen, S.C.; Amb, C.M. Synthesis and Structural Characterization of Thiophene-Functionalized Metal Dithiolenes. In Chemical Crystallography; Connelly, B.L., Ed.; NOVA Publishers: Hauppauge, NY, USA, 2010; pp. 69–101. [Google Scholar]
- Garreau-de Bonneval, B.; Moineau-Chane Ching, K.I.; Alary, F.; Bui, T.-T.; Valadea, L. Neutral d8 metal bis-dithiolene complexes: Synthesis, electronic properties and applications. Coord. Chem. Rev. 2010, 254, 1457–1467. [Google Scholar] [CrossRef]
- Castillo, O.; Delgado, E.; Gómez-García, C.J.; Hernández, D.; Hernández, E.; Martín, A.; Martínez, J.I.; Zamora, F. Group 10 Metal Benzene-1,2-dithiolate Derivatives in the Synthesis of Coordination Polymers Containing Potassium Countercations. Inorg. Chem. 2017, 56, 11810–11818. [Google Scholar] [CrossRef]
- Shi, W.; Wu, G.; Yong, X.; Deng, T.; Wang, J.-S.; Zheng, J.-C.; Xu, J.; Sullivan, M.B.; Yang, S.-W. Orbital-Engineering-Based Screening of π-Conjugated d8 Transition-Metal Coordination Polymers for High-Performance n-Type Thermoelectric Applications. ACS Appl. Mater. Interfaces 2018, 10, 35306–35315. [Google Scholar] [CrossRef]
- Marshall, K.; Painter, G.; Lotito, K.; Noto, A.; Chang, P. Transition Metal Dithiolene Near-IR Dyes and Their Applications in Liquid Crystal Devices. Mol. Cryst. Liq. Cryst. 2006, 454, 449–481. [Google Scholar] [CrossRef]
- Nishijo, J.; Iwama, K.; Enomoto, M. Unexpected Strong Antiferromagnetic Interaction in [CuCyclam][Ni(1,2-benzenedithiolate)2]2. Eur. J. Inorg. Chem. 2015, 2015, 348–354. [Google Scholar] [CrossRef]
- Schlindwein, S.H.; Bader, K.; Sibold, C.; Frey, W.; Neugebauer, P.; Orlita, M.; van Slageren, J.; Gudat, D. New Selective Synthesis of Dithiaboroles as a Viable Pathway to Functionalized Benzenedithiolenes and Their Complexes. Inorg. Chem. 2016, 55, 6186–6194. [Google Scholar] [CrossRef]
- Nakamura, Y.; Matsumoto, T.; Sakazume, Y.; Murata, J.; Chang, H.-C. Tuning the Mesomorphism and Redox Response of Anionic-Ligand-Based Mixed-Valent Nickel(II) Complexes by Alkyl-Substituted Quaternary Ammonium Cations. Chem. Eur. J. 2018, 24, 7398–7409. [Google Scholar] [CrossRef]
- Castillo, O.; Delgado, E.; Hernández, E.; Pérez, M.; Zamora, F. Structural Factors Governing the Formation of Extended Structures in Group 10 and 12 Metal-Dithiolenes. Cryst. Growth Des. 2020, 20, 4573–4584. [Google Scholar] [CrossRef]
- Du, J.; Cheng, B.; Yuan, H.; Tao, Y.; Chen, Y.; Ming, M.; Han, Z.; Eisenberg, R. Molecular Nickel Thiolate Complexes for Electrochemical Reduction of CO2 to C1–3 Hydrocarbons. Angew. Chem. Int. Ed. 2023, 62, e202211804. [Google Scholar] [CrossRef]
- Alves, H.; Neves, A.I.S.; Gouveia, W.; Silva, R.A.L.; Belo, D. Conducting films based on single-component molecular metals. Chem. Commun. 2015, 51, 13117–13119. [Google Scholar] [CrossRef]
- Higashino, T.; Jeannin, O.; Kawamoto, T.; Lorcy, D.; Mori, T.; Fourmigué, M. A Single-Component Conductor Based on a Radical Gold Dithiolene Complex with Alkyl-Substituted Thiophene-2,3-dithiolate Ligand. Inorg. Chem. 2015, 54, 9908–9913. [Google Scholar] [CrossRef]
- Perepichka, I.F.; Perepichka, D.F. Handbook of Thiophene-Based Materials; Perepichka, I.F., Perepichka, D.F., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Mishra, A.; Ma, C.-Q.; Bäuerle, P. Functional Oligothiophenes: Molecular Design for Multidimensional Nanoarchitectures and Their Applications. Chem. Rev. 2009, 109, 1141–1276. [Google Scholar] [CrossRef]
- Amb, C.M.; Rasmussen, S.C. Synthesis and Characterization of a New π-Extended Nickel Dithiolene Complex for Molecular Materials. Synth. Met. 2009, 159, 2390–2393. [Google Scholar] [CrossRef]
- Amb, C.M.; Heth, C.L.; Evenson, S.J.; Pokhodnya, K.I.; Rasmussen, S.C. Thiophene-fused Nickel Dithiolenes: A Synthetic Scaffold for Highly Delocalized π-electron Systems. Inorg. Chem. 2016, 55, 10978–10989. [Google Scholar] [CrossRef] [PubMed]
- Uzelac, E.J.; McCausland, C.B.; Rasmussen, S.C. Pyrrolo[2,3-d:5,4-d′]bisthiazoles: Alternate Synthetic Routes and a Comparative Study to Analogous Fused-ring Bithiophenes. J. Org. Chem. 2018, 83, 664–671. [Google Scholar] [CrossRef]
- Uzelac, E.J.; Badía-Domínguez, I.; Gilman, S.J.; Delgado, M.C.R.; Rasmussen, S.C. Molecular Tuning in Diaryl-capped Pyrrolo[2,3-d:5,4-d′]bisthiazoles: Effects of Terminal Aryl Unit and Comparison to Dithieno[3,2-b:2′,3′-d]pyrrole Analogues. Molecules 2022, 27, 6638. [Google Scholar] [CrossRef]
- Kibbel, H.U.; Jeroschewski, P.; Rudolf, G. Nickelkomplexe von 2-Arylthiazoldithiolaten. Z. Chem. 1988, 28, 446–447. [Google Scholar] [CrossRef]
- Filatre-Furcate, A.; Auban-Senzier, P.; Fourmigué, M.; Roisnel, T.; Dorceta, V.; Lorcy, D. Gold dithiolene complexes: Easy access to 2-alkylthio-thiazoledithiolate complexes. Dalton Trans. 2015, 44, 15683–15689. [Google Scholar] [CrossRef]
- Filatre-Furcate, A.; Roisnel, T.; Lorcy, D. Chemical transformation of dithiolene ligands in heteroleptic and homoleptic complexes (M = Ti, Zn, Au). J. Organomet. Chem. 2016, 819, 182–188. [Google Scholar] [CrossRef]
- Uzelac, E.J.; Rasmussen, S.C. Thiophene-Extended Nickel Thiazoledithiolene: π-Extended Fused-ring Metal Dithiolenes with Stabilized Frontier Orbitals. Eur. J. Inorg. Chem. 2017, 2017, 3878–3883. [Google Scholar] [CrossRef]
- Amb, C.M.; Rasmussen, S.C. Sterics vs. Electronics: Regioselective Cross-coupling of Polybrominated Thiophenes. Eur. J. Org. Chem. 2008, 2008, 801–804. [Google Scholar] [CrossRef]
- Uzelac, E.J.; Rasmussen, S.C. Synthesis of Brominated Thiazoles via Sequential Bromination-Debromination Methods. J. Org. Chem. 2017, 82, 5947–5951. [Google Scholar] [CrossRef]
- Schnürch, M.; Spina, M.; Khan, A.F.; Mihovilovic, M.D.; Stanetty, P. Halogen dance reactions—A review. Chem. Soc. Rev. 2007, 36, 1046–1057. [Google Scholar] [CrossRef]
- Hronec, M.; Malik, L.; Stasko, A. Reaction of bis-(1,2-diphenylethylene-1,2-dithiolato)nickel with cumene hydroperoxide. J. Mol. Catal. 1985, 30, 251–258. [Google Scholar] [CrossRef]
- Sugimoto, K.; Kuroda-Sowa, T.; Maekawa, M.; Munakata, M. Unsymmetrical Oxygenation Products of [Pd(mnt)2]2−: Syntheses and Crystal Structures of (Bu4N)2[Pd(mnt){O2S2C2(CN)2}] and One-Dimensional Coordination Polymer (Bu4N)2[AgPd(mnt){O2S2C2(CN)2}]2 (mnt = 1,2-Dicyano-1,2-enthylenedithiolato). Bull. Chem. Soc. Jpn. 2000, 73, 391–394. [Google Scholar] [CrossRef]
- Grapperhaus, C.A.; Mullins, C.S.; Kozlowski, P.M.; Mashuta, M.S. Synthesis and Oxygenation of a Nickel(II) and Zinc(II) Dithiolate: An Experimental and Theoretical Comparison. Inorg. Chem. 2004, 43, 2859–2866. [Google Scholar] [CrossRef] [PubMed]
- Schrauzer, G.N.; Zhang, C.; Chadha, R. The cis-Bis(cis-2-mercaptostilbene-1-sulfinato)nickel(II) Dianion: A Product of the Irreversible Oxidation of the Bis(cis-stilbene-1,2-dithiolato)nickeI(II) Dianion. Inorg. Chem. 1990, 29, 4104–4107. [Google Scholar] [CrossRef]
- Bolligarla, R.; Das, S.K. Sulfur Oxygenation of [Ni(btdt)2]2– by Aerial Oxidation under Ambient Conditions—Syntheses, Crystal Structures, and Properties of [Bu4N]2[Ni(btdt)2] and [Bu4N]2[Ni(btdtO2)2]·H2O ({btdt}2– = 2,1,3-Benzenethiadiazole-5,6-dithiolate). Eur. J. Inorg. Chem. 2012, 2012, 2933–2939. [Google Scholar] [CrossRef]
- Shen, K.; Fu, Y.; Li, J.N.; Liu, L.; Guo, Q. What are the pKa values of C–H bonds in aromatic heterocyclic compounds in DMSO? Tetrahedron 2007, 63, 1568–1576. [Google Scholar] [CrossRef]
- Mo, H.; Radke, K.R.; Ogawa, K.; Heth, C.L.; Erpelding, B.T.; Rasmussen, S.C. Solution and solid-state properties of highly fluorescent dithieno[3,2-b:2’,3’-d]pyrrole-based oligothiophenes. Phys. Chem. Chem. Phys. 2010, 12, 14585–14595. [Google Scholar] [CrossRef]
- Gronowitz, S.; Bjork, P.; Malm, J.; Hornfeldt, A.-B. The effect of some additives on the Stille Pd0-catalyzed cross-coupling reaction. J. Organomet. Chem. 1993, 460, 127–129. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Pozharskii, A.F. Handbook of Heterocyclic Chemistry, 2nd ed.; Pergamon: New York, NY, USA, 2000; pp. 68, 101, 115. [Google Scholar]
- Rasmussen, S.C.; Gilman, S.J.; Wilcox, W.D. Conjugated Polymers: Synthesis & Design; ACS in Focus; American Chemical Society: Washington, DC, USA, 2023. [Google Scholar]
- Mascal, M. Statistical analysis of C–H···N hydrogen bonds in the solid state: There are real precedents. Chem. Commun. 1998, 303–304. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical Considerations for Determining Absolute Frontier Orbital Energy Levels of Conjugated Polymers for Solar Cell Applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef]
- Ray, K.; Weyhermuller, T.; Neese, F.; Wieghardt, K. Electronic Structure of Square Planar Bis(benzene-1,2-dithiolato)metal Complexes [M(L)2]z (z = 2-, 1-, 0; M = Ni, Pd, Pt, Cu, Au): An Experimental, Density Functional, and Correlated ab Initio Study. Inorg. Chem. 2005, 44, 5345–5360. [Google Scholar] [CrossRef]
- Petrenko, T.; Ray, K.; Wieghardt, K.E.; Neese, F. Vibrational Markers for the Open-Shell Character of Transition Metal Bis-dithiolenes: An Infrared, Resonance Raman, and Quantum Chemical Study. J. Am. Chem. Soc. 2006, 128, 4422–4436. [Google Scholar] [CrossRef] [PubMed]
- D’Alessandro, D.M.; Keene, F.R. Current trends and future challenges in the experimental, theoretical and computational analysis of intervalence charge transfer (IVCT) transitions. Chem. Soc. Rev. 2006, 35, 424–440. [Google Scholar] [CrossRef] [PubMed]
- Josse, P.; Chavez, P.; Dindault, C.; Dalinot, C.; McAfee, S.; Dabos-Seignon, S.; Tondelier, D.; Welch, G.; Blanchard, P.; Leclerc, N.; et al. Thiophene vs thiazole: Effect of the π-connector on the properties of phthalimide end-capped diketopyrrolopyrrole based molecular acceptors for organic photovoltaics. Dye. Pigm. 2017, 137, 576–583. [Google Scholar] [CrossRef]
- Bronstein, H.; Collado-Fregoso, E.; Hadipour, A.; Soon, Y.W.; Huang, Z.; Dimitrov, S.D.; Ashraf, R.S.; Rand, B.P.; Watkins, S.E.; Tuladhar, P.S.; et al. Thieno[3,2-b]thiophene-diketopyrrolopyrrole Containing Polymers for Inverted Solar Cells Devices with High Short Circuit Currents. Adv. Funct. Mater. 2013, 23, 5647–5654. [Google Scholar] [CrossRef]
- Soto-Rojo, R.; Baldenebro-López, J.; Glossman-Mitnik, D. Theoretical Study of the π-Bridge Influence with Different Units of Thiophene and Thiazole in Coumarin Dye-Sensitized Solar Cells. Int. J. Photoenergy 2016, 2016, 6479649. [Google Scholar] [CrossRef]
- Braude, E.A. Studies in light absorption. Part IX. The relation between absorption intensities and molecular dimensions, and its application to the electronic spectra of polyenes and polycyclic benzenoid hydrocarbons. J. Chem. Soc. 1950, 379–384. [Google Scholar] [CrossRef]
- Gidron, O.; Dadvand, A.; Shenynin, Y.; Bendikov, M.; Perepichka, D.F. Towards “green” electronic materials. α-Oligofurans as semiconductors. Chem. Commun. 2011, 47, 1976–1978. [Google Scholar] [CrossRef]
- Li, R.; Lv, X.; Shi, D.; Cheng, Y.; Zhang, G.; Wang, P. Dye-Sensitized Solar Cells Based on Organic Sensitizers with Different Conjugated Linkers: Furan, Bifuran, Thiophene, Bithiophene, Selenophene, and Biselenophene. J. Phys. Chem. C 2009, 113, 7469–7479. [Google Scholar] [CrossRef]
- Lee, S.; Lee, H.; Han, A.; Lee, J.; Oh, J.; Yang, C. High-Performance Furan-Containing Conjugated Polymer for Environmentally Benign Solution Processing. ACS Appl. Mater. Interfaces 2017, 9, 15652–15661. [Google Scholar] [CrossRef]
- Szilagyi, R.K.; Lim, B.S.; Glaser, T.; Holm, R.H.; Hedman, B.; Hodgson, K.O.; Solomon, E.I. Description of the Ground State Wave Functions of Ni Dithiolenes Using Sulfur K-edge X-ray Absorption Spectroscopy. J. Am. Chem. Soc. 2003, 125, 9158–9169. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.02; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Grimme, S.; Stephan, E.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Runge, E.; Gross, E.K.U. Density-Functional Theory for Time-Dependent Systems. Phys. Rev. Lett. 1984, 52, 997–1000. [Google Scholar] [CrossRef]
- Heinze, H.H.; Görling, A.; Rösch, N. Efficient method for calculating molecular excitation energies by time-dependent density-functional theory. J. Chem. Phys. 2000, 113, 2088–2099. [Google Scholar] [CrossRef]
- Chemcraft—Graphical Software for Visualization of Quantum Chemistry Computations. Available online: https://www.chemcraftprog.com (accessed on 5 October 2025).
- Lee, C.; Yang, W.; Parr, R. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Turro, N.J. Modern Molecular Photochemistry; University Science Books: Sausalito, CA, USA, 1991; pp. 86–90. [Google Scholar]
- Larson, R.C.; Iwamoto, R.T.; Adams, R.N. Reference electrodes for voltammetry in acetonitrile. Anal. Chim. Acta 1961, 25, 371–374. [Google Scholar] [CrossRef]




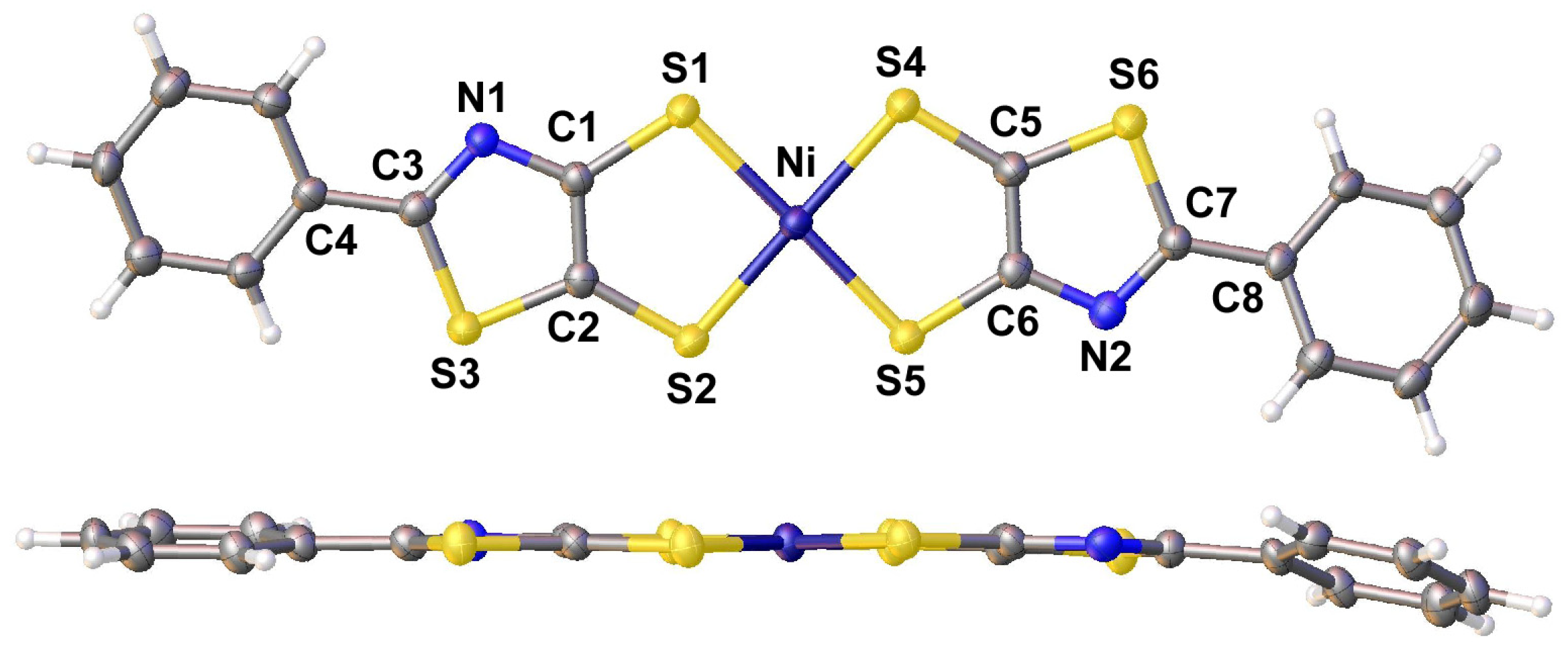
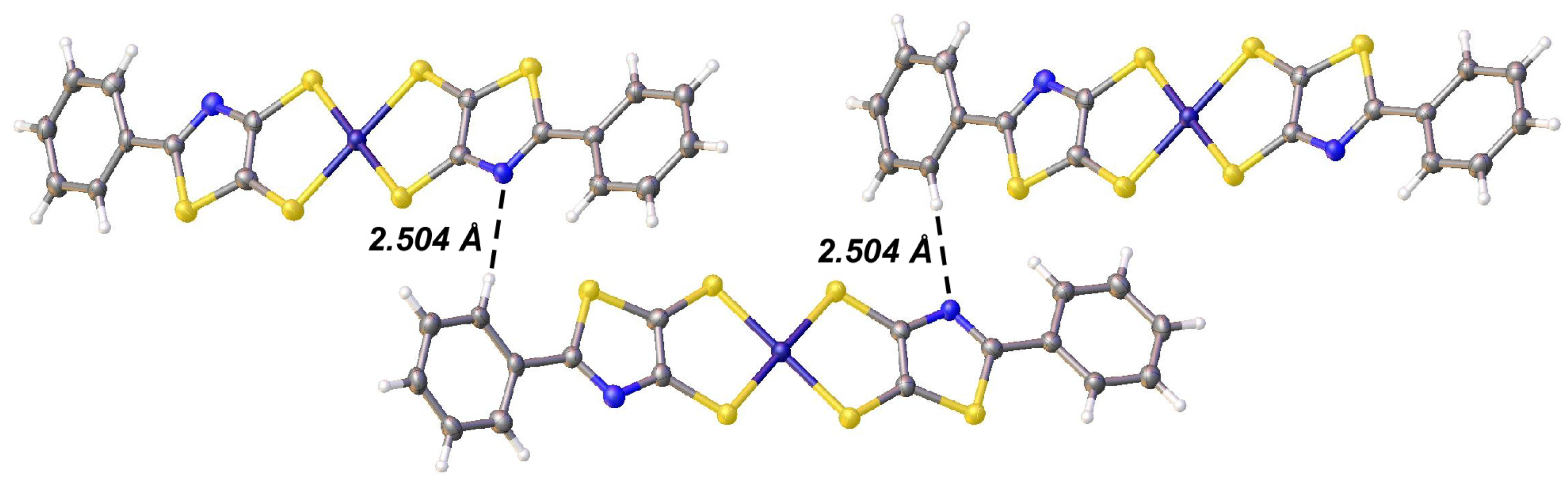
| Parameter | Thiazole 1 | [Bu4N][6] 2 | [Bu4N][8] | [NMP][2] 3 | [NMP][3] 4 |
|---|---|---|---|---|---|
| Ni-S1 | 2.1762(9) | 2.172(1) | 2.175(2) | 2.174(3) | |
| Ni-S2 | 2.1656(9) | 2.170(2) | 2.170(2) | 2.176(3) | |
| S1-C1 | 1.733(3) | 1.739(6) | 1.708(6) | 1.732(9) | |
| S2-C2 | 1.717(3) | 1.712(5) | 1.733(7) | 1.731(10) | |
| C1-C2 | 1.367 | 1.376(4) | 1.371(7) | 1.396(8) | 1.442(12) |
| C2-S3 | 1.713 | 1.728(3) | 1.732(6) | 1.748(6) | 1.725(8) |
| C1-N1 | 1.372 | 1.378(4) | 1.373(7) | ||
| N1-C3 | 1.304 | 1.310(4) | 1.306(7) | ||
| S2-C3 | 1.724 | 1.754(3) | 1.752(5) | 1.746(6) | 1.739(8) |
| C3-C4 | 1.441(4) | 1.469(8) | 1.472(8) | 1.51(1) |
| Compound | E1/22−/1− (V) 1 | ΔE (mV) | E1/20/1− (V) 1 | ΔE (mV) | Ep0/n+ (V) 1 |
|---|---|---|---|---|---|
| [Bu4N][9] | −0.92 | 150 | 0.01 | 250 | 0.58 |
| [Bu4N][6] | −0.88 | 80 | −0.10 | 120 | 0.67 |
| [Bu4N][7] | −0.87 | 180 | −0.05 | 250 | 0.85 |
| [Bu4N][8] | −0.90 | 100 | −0.18 | 200 | 0.65 |
| Compound | λmax (nm) | ε (M−1 cm−1) | f | Assignment |
|---|---|---|---|---|
| [Bu4N][9] | 1014 | 5000 | 0.05 | IVCT |
| 295 | 16,000 | 0.10 | π → π* | |
| [Bu4N][6] | 1108 | 14,600 | 0.14 | IVCT |
| 610 | 3800 | 0.06 | LMCT | |
| 365 | 32,500 | 0.60 | π → π* | |
| [Bu4N][7] | 1051 | 15,000 | 0.15 | IVCT |
| 605 | 3600 | 0.06 | LMCT | |
| 316 | 40,000 | 0.62 | π → π* | |
| [Bu4N][8] | 1072 | 13,500 | 0.12 | IVCT |
| 600 | 4100 | 0.06 | LMCT | |
| 377 | 38,800 | 0.52 | π → π* |
| Compound | λmaxcalc (nm) | fcalc | Description | Assignment |
|---|---|---|---|---|
| NiTzDT | 1082 | 0.14 | SOMO-1β → SOMOβ (97%) | IVCT |
| 612 | 0.02 | SOMO-3β → SOMOβ (96%) | LMCT | |
| 297 | 0.09 | SOMOα → SOMO+3α (17%) | MLCT | |
| SOMO-3β → SOMO+2β (15%) | π → π* | |||
| SOMO-1β → SOMO+2β (14%) | π → π* | |||
| SOMO-2β → SOMO+3β (13%) | MLCT | |||
| 270 | 0.36 | SOMO-4α → SOMO+1α (16%) | π → π* | |
| SOMO-5β → SOMO+1β (25%) | π → π* | |||
| SOMO-7β → SOMO+1β (15%) | π → π* | |||
| 6 | 1203 | 0.28 | SOMO-1β → SOMOβ (96%) | IVCT |
| 616 | 0.02 | SOMO-3β → SOMOβ (91%) | LMCT | |
| 590 | 0.06 | SOMOα → SOMO+1α (36%) | MLCT | |
| SOMO-1α → SOMO+2α (21%) | π → π* | |||
| SOMO-1β → SOMO+2β (17%) | π → π* | |||
| 389 | 0.64 | SOMOα → SOMO+1α (32%) | MLCT | |
| SOMO-1β → SOMO+2β (35%) | π → π* | |||
| 283 | 0.31 | SOMO-2α → SOMO+1α (28%) | MLCT | |
| SOMO-3α → SOMO+2α (22%) | π → π* | |||
| 253 | 0.21 | SOMO-4α → SOMO+3α (24%) | π → π* | |
| SOMO-5β → SOMO+3β (31%) | π → π* | |||
| 7 | 1341 | 0.26 | SOMO-1β → SOMOβ (96%) | IVCT |
| 631 | 0.02 | SOMO-3β → SOMOβ (89%) | LMCT | |
| 590 | 0.06 | SOMOα → SOMO+1α (35%) | MLCT | |
| SOMO-1α → SOMO+2α (22%) | π → π* | |||
| SOMO-1β → SOMO+2β (19%) | π → π* | |||
| 382 | 0.71 | SOMOα → SOMO+1α (32%) | MLCT | |
| SOMO-1β → SOMO+2β (36%) | π → π* | |||
| 302 | 0.45 | SOMO-8β → SOMOβ (52%) | LMCT | |
| 257 | 0.19 | SOMO-4α → SOMO+3α (20%) | π → π* | |
| SOMO-5β → SOMO+3β (25%) | π → π* | |||
| SOMO-7β → SOMO+3β (20%) | MLCT | |||
| 8 | 1184 | 0.26 | SOMO-1β → SOMOβ (96%) | IVCT |
| 618 | 0.02 | SOMO-3β → SOMOβ (93%) | LMCT | |
| 551 | 0.06 | SOMOα → SOMO+1α (37%) | MLCT | |
| SOMO-1α → SOMO+2α (21%) | π → π* | |||
| SOMO-1β → SOMO+2β (18%) | π → π* | |||
| 375 | 0.71 | SOMOα → SOMO+1α (31%) | MLCT | |
| SOMO-1β → SOMO+2β (42%) | π → π* | |||
| 278 | 0.45 | SOMO-2α → SOMO+1α (19%) | MLCT | |
| 252 | 0.19 | SOMO-3α → SOMO+2α (13%) | π → π* | |
| SOMO-4α → SOMO+3α (23%) | π → π* | |||
| SOMO-5β → SOMO+3β (29%) | π → π* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uzelac, E.J.; Sánchez-Rincón, J.; Ruiz Delgado, M.C.; Rasmussen, S.C. Nickel Thiazoledithiolenes: π-Extended Fused-Ring Metal Dithiolenes as Highly Delocalized π-Electron Systems with Stabilized Frontier Orbitals. Molecules 2025, 30, 3998. https://doi.org/10.3390/molecules30193998
Uzelac EJ, Sánchez-Rincón J, Ruiz Delgado MC, Rasmussen SC. Nickel Thiazoledithiolenes: π-Extended Fused-Ring Metal Dithiolenes as Highly Delocalized π-Electron Systems with Stabilized Frontier Orbitals. Molecules. 2025; 30(19):3998. https://doi.org/10.3390/molecules30193998
Chicago/Turabian StyleUzelac, Eric J., Juan Sánchez-Rincón, M. Carmen Ruiz Delgado, and Seth C. Rasmussen. 2025. "Nickel Thiazoledithiolenes: π-Extended Fused-Ring Metal Dithiolenes as Highly Delocalized π-Electron Systems with Stabilized Frontier Orbitals" Molecules 30, no. 19: 3998. https://doi.org/10.3390/molecules30193998
APA StyleUzelac, E. J., Sánchez-Rincón, J., Ruiz Delgado, M. C., & Rasmussen, S. C. (2025). Nickel Thiazoledithiolenes: π-Extended Fused-Ring Metal Dithiolenes as Highly Delocalized π-Electron Systems with Stabilized Frontier Orbitals. Molecules, 30(19), 3998. https://doi.org/10.3390/molecules30193998







