Optimization of the Macrocyclic Tetrapeptide [D-Trp]CJ-15,208 to Prevent Stress-Induced Relapse of Cocaine-Seeking Behavior
Abstract
1. Introduction
2. Results
2.1. Design and Synthesis
2.2. Opioid Receptor Affinity and KOR Antagonism In Vitro
2.2.1. Opioid Receptor Affinity
2.2.2. KOR Antagonism
2.3. Metabolic Stability
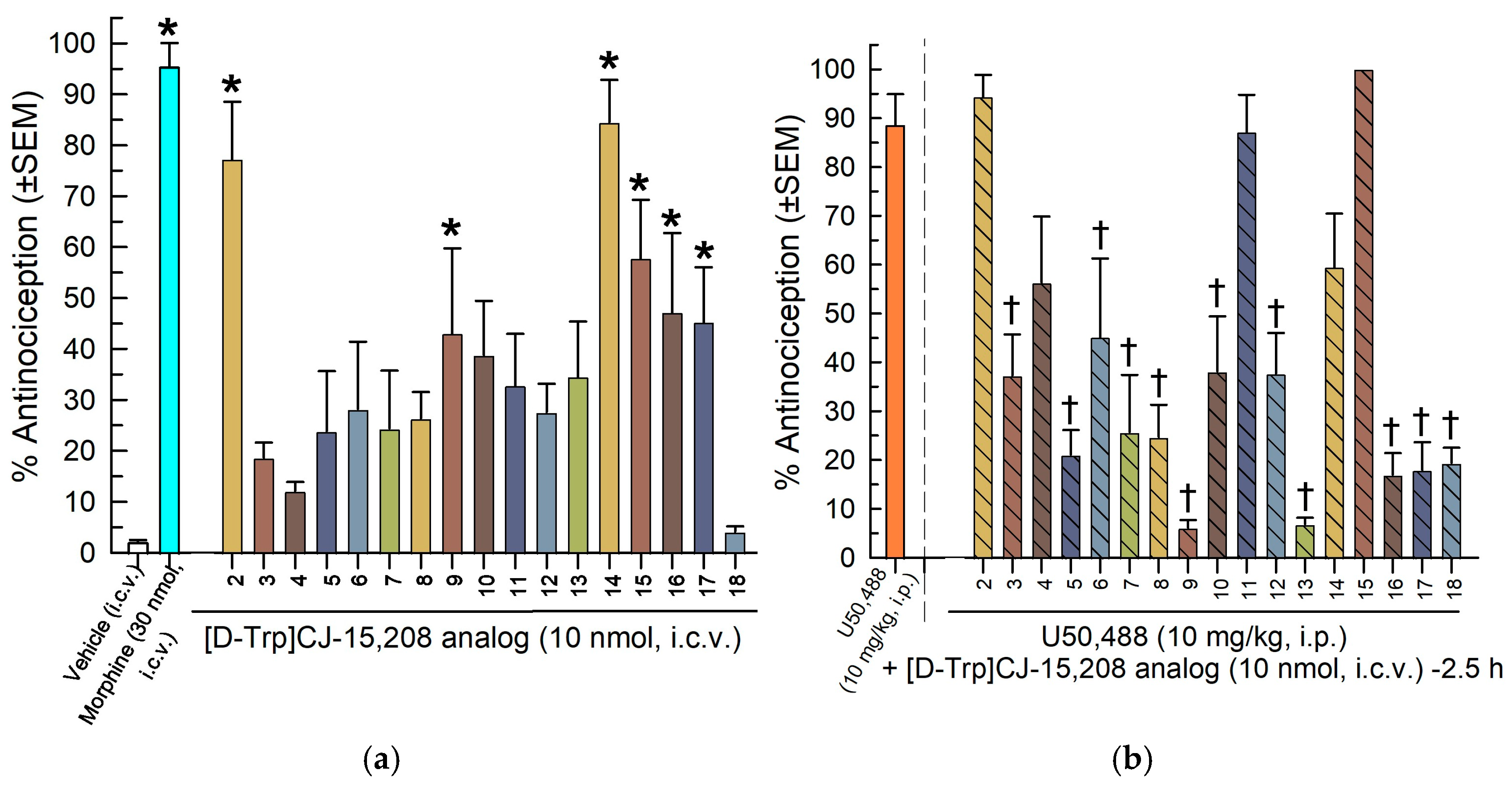
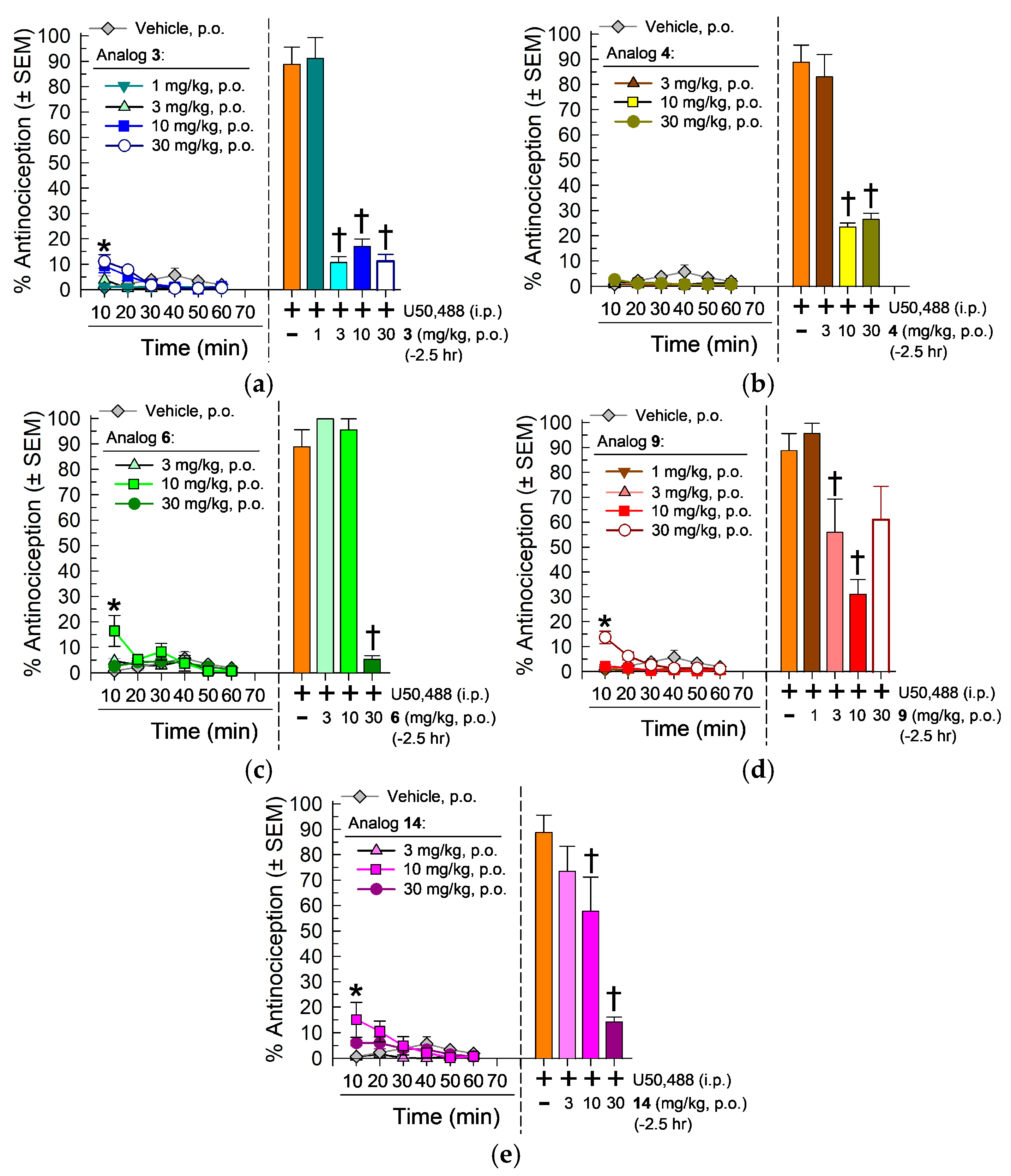
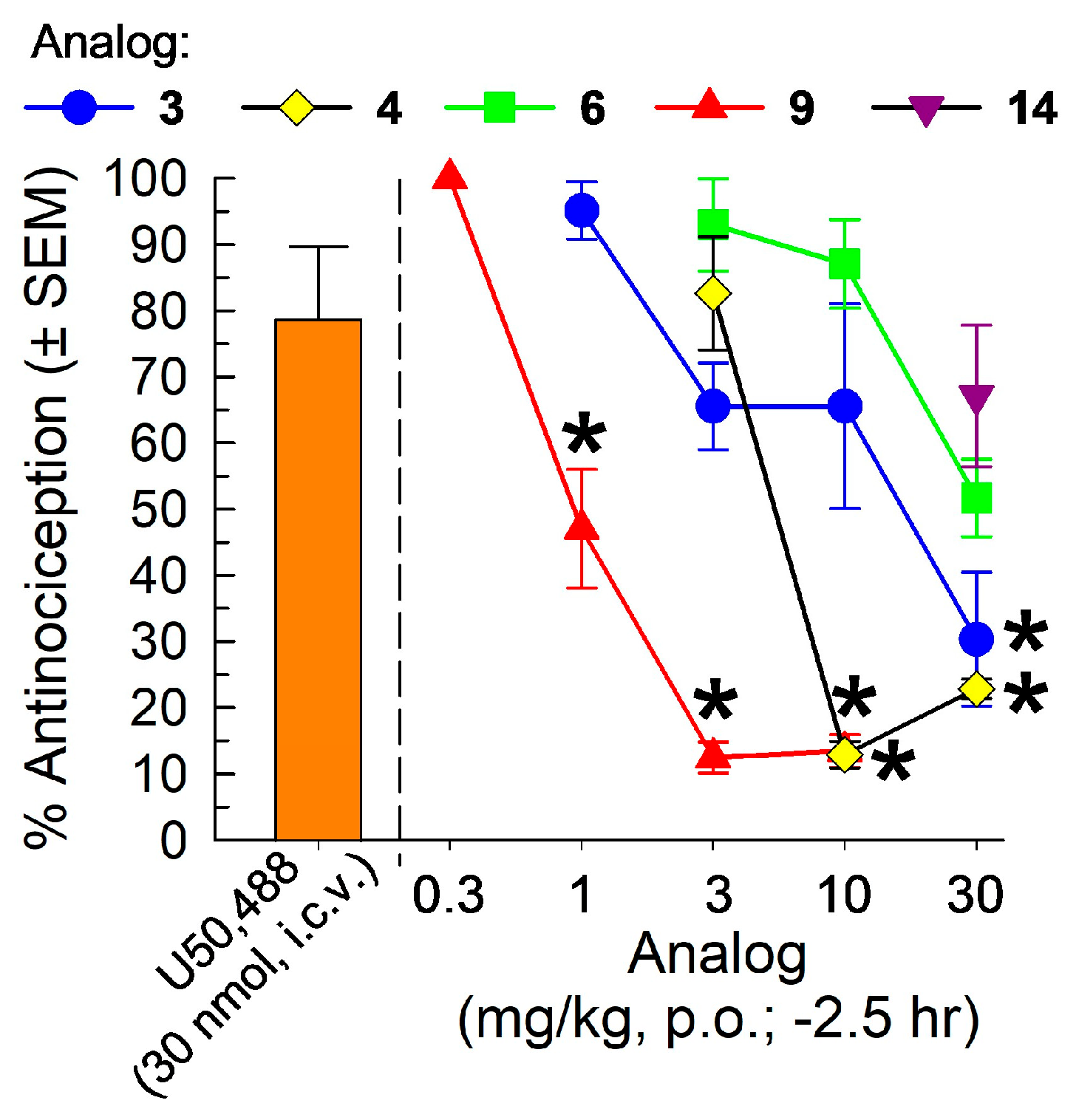
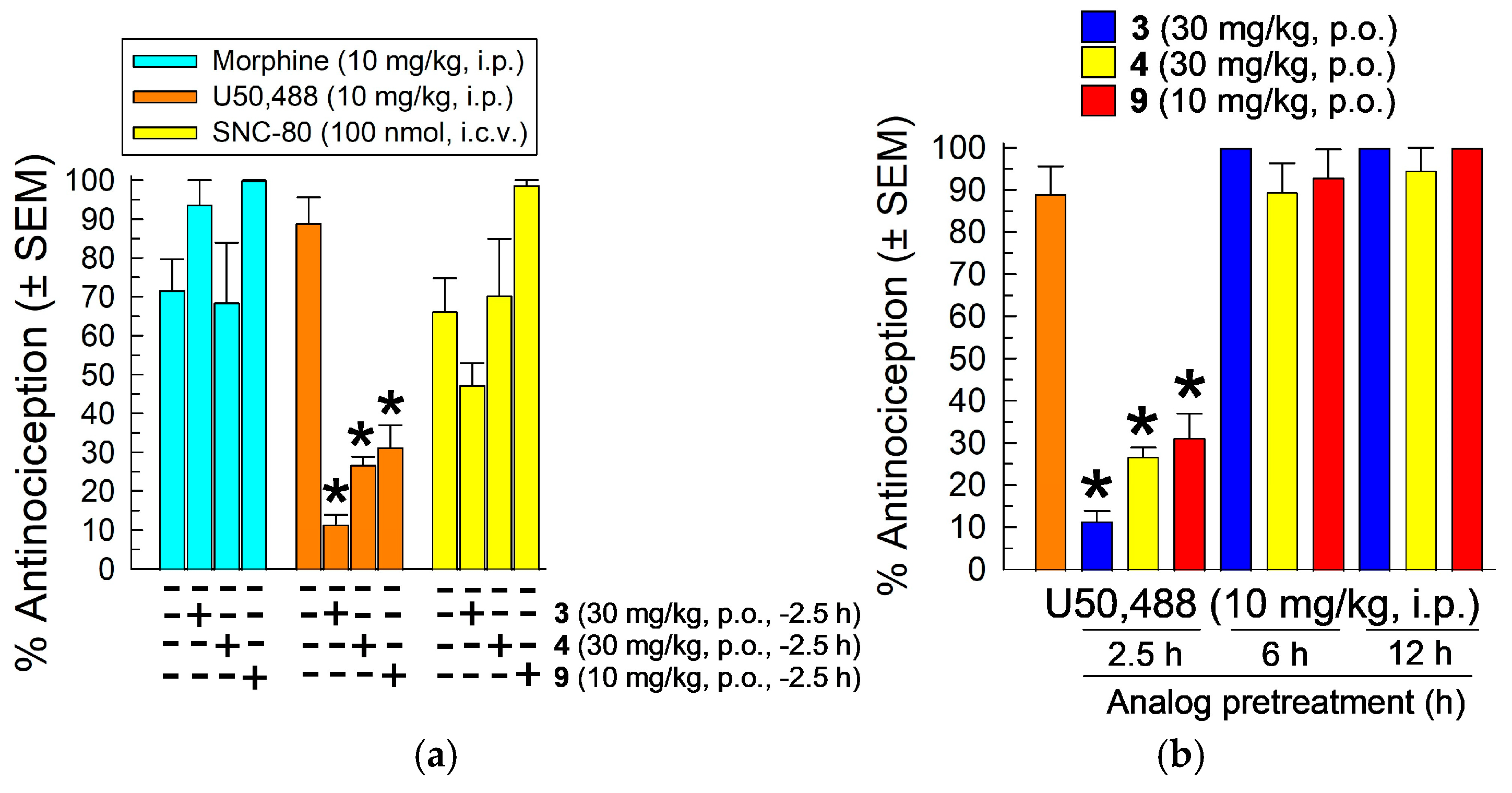
2.4. In Vivo Pharmacological Evaluation
2.4.1. Evaluation in the 55 °C Warm-Water Tail-Withdrawal Assay for Antinociception and KOR Antagonism
Screening of the [D-Trp]- and [D-Phe4]CJ-15,208 Analogs
Characterization of the [D-Trp]- and [D-Phe4]CJ-15,208 Analogs 3, 4, 6, 9 and 14
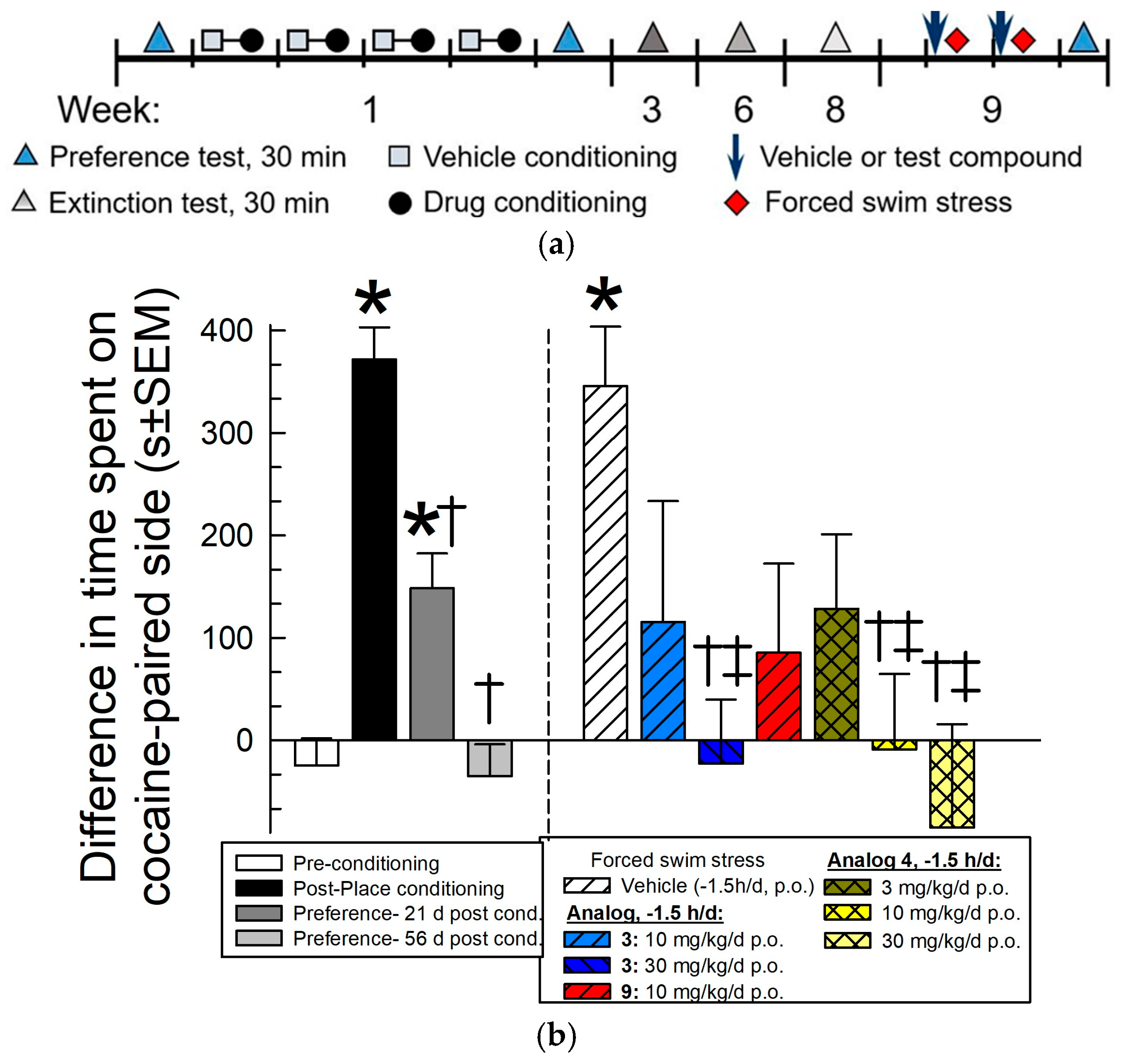
2.4.2. Evaluation of [D-Trp(formamide)]-, 3, [D-Phe)4]-, 4, and [D-Phe(3,5-F2)4]CJ-15,208, 9, for Prevention of Stress-Induced Reinstatement of Extinguished Cocaine-Seeking Behavior in the Conditioned Place Preference (CPP) Assay
3. Discussion
3.1. In Vitro Pharmacological Results
3.2. In Vivo Pharmacological Results
4. Materials and Methods
4.1. Chemicals
4.2. Instruments
4.3. Peptide Synthesis and Purification
4.4. In Vitro Pharmacological Evaluation
4.4.1. Opioid Receptor Binding Assays
4.4.2. KOR cAMP Assay Using GloSensor-22F
Plasmid DNA Cloning
Production of GloSensor-22F KOR CHO Cell Stable Lines (KOR-CHO-GLO)
Performing GloSensor cAMP Assay
Data Analysis—Agonist Mode
Data Analysis—Antagonist Mode
4.5. Metabolic Stability in Liver Microsomes
4.6. In Vivo Pharmacological Evaluation
4.6.1. Animals
4.6.2. Peptide Solutions for In Vivo Pharmacological Testing
4.6.3. Injection Techniques
4.6.4. Antinociceptive Testing
4.6.5. Place Conditioning to Determine Conditioned Place Preference or Aversion
4.6.6. Extinction Testing
4.6.7. Reinstatement Testing
4.7. Statistical Analysis
5. Conclusions
6. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| cAMP | Cyclic adenosine monophosphate |
| CHO | Chinese hamster ovary |
| CI | Confidence interval |
| CJ-15,208 | cyclo[Phe-D-Pro-Phe-Trp] |
| CPP | Conditioned place preference |
| CUD | Cocaine use disorder |
| [D-Phe4]CJ-15,208 | cyclo[Phe-D-Pro-Phe-D-Phe] |
| [D-Trp]CJ-15,208 | cyclo[Phe-D-Pro-Phe-D-Trp] |
| DAMGO | [D-Ala2,N-MPhe4,glyol]enkephalin |
| D-Bta | D-benzothienylalanine |
| DCM | Dichloromethane |
| DIEA | N,N-Diisopropylethylamine |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DOR | Delta opioid receptor |
| DPDPE | cyclo[D-Pen2,D-Pen5]enkephalin |
| FSS | Forced swim stress |
| HATU | 2-(1H-7-Azabenzotriazol-1-yl)-1,1,3,3-tetramethyluronium hexafluorophosphate |
| HOBt | hydroxybenzotriazole |
| i.c.v. | intracerebroventricular |
| i.p. | intraperitoneal |
| ITR | Inverted terminal repeat |
| KOR | Kappa opioid receptor |
| MOR | Mu opioid receptor |
| ND | Not determined |
| p.o. | Per os |
| RM | Repeated measures |
| PyBOP | Benzotriazole-1yloxy-tris-pyrrolidinophophonium hexafluorphosphate |
| SAR | Structure–activity relationships |
| SEM | Standard error of the mean |
| SNC-80 | (+)-4-[(αR)-α-((2S,5R)-4-Allyl-2,5-dimethyl-1-piperazinyl)-3-methoxybenzyl]-N,N-diethylbenzamide |
| TFA | Trifluoroacetic acid |
| U50,488 | (±)-trans-3,4-Dichloro-N-methyl-N-[2-(1-pyrrolidinyl)cyclohexyl]benzeneacetamide |
| UHPLC | Ultrahigh performance liquid chromatography |
| WWTW | Warm-water tail withdrawal |
Appendix A
Appendix A.1
| HLM | MLM | Solubility 2 | |||
|---|---|---|---|---|---|
| Analog 3 | t1/2 (min) | CLint 4 | t1/2 (min) | CLint 4 | (µM) |
| 1 | 5.5 | 127 | 2.6 | 269 | 23 |
| 2, D-Trp(Me) | 2.4 | 286 | 3.3 | 211 | 12 |
| 3, D-Trp(COH) | 2.8 | 244 | 1.1 | 607 | 4.6 |
| [D-Phe(X)4]CJ-15,208: | |||||
| 4, X = H | 4.2 | 165 | 1.6 | 447 | 24 |
| 5, X = o-F | 1.9 | 373 | 1.6 | 430 | 15 |
| 6, X = m-F | 1.8 | 376 | 2.1 | 331 | 26 |
| 7, X = p-F | 2.3 | 296 | 3.4 | 202 | 25 |
| 8, X = 3,4-F2 | 2.2 | 309 | 1.1 | 629 | 9.5 |
| 9, X = 3,5-F2 | 2.9 | 237 | 1.2 | 600 | 8.6 |
| 10, X = 2,4,5-F3 | 1.5 | 462 | 1.0 | 699 | 7.4 |
| 11, X = 3,4,5-F2 | 1.6 | 447 | 2.9 | 238 | 4.8 |
| 14, X = m-Me | 4.1 | 170 | 1.5 | 467 | 19 |
| 15, X = p-Me | 5.4 | 129 | 2.9 | 236 | 24 |
| 16, X = p-NO2 | 6.7 | 103 | 4.4 | 159 | 6.7 |
| UHPLC Syst. 1 2,3 | UHPLC Syst. 2 4 | ESI (m/z 5) | ||
|---|---|---|---|---|
| Analog 2 | t1/2 (min) | t1/2 (min) | Observed | Calc |
| 2 | 8.05 2 | 16.13 | 614.9 | 614.3 |
| 3 | 7.33 2 | 14.94 | 628.9 | 628.3 |
| 4 | 5.71 6 | 12.4 6 | 539.1 7, 561.3 | 539.1 7, 561.3 |
| 5 | 9.89 3 | 14.61 | 579.4 | 579.3 |
| 6 | 7.76 2 | 15.19 | 579.9 | 579.3 |
| 7 | 7.67 2 | 15.27 | 598.0 | 579.3 |
| 8 | 10.12 3 | 15.49 | 575.2 7, 597.6 | 575.2 7, 597.2 |
| 9 | 10.25 3 | 15.71 | 597.6 | 597.2 |
| 10 | 10.42 3 | 16.13 | 593.2 7, 615.6 | 593.2 7, 615.2 |
| 11 | 10.59 3 | 16.68 | 593.2 7, 615.6 | 593.1 7, 615.2 |
| 12 | 11.04 3 | 17.58 | 629.4 7, 651.3 | 629.2 7, 651.2 |
| 13 | 8.13 2 | 16.57 | 576.0 | 575.3 |
| 14 | 8.19 2 | 16.57 | 576.0 | 575.3 |
| 15 | 8.14 2 | 16.53 | 576.0 | 575.3 |
| 16 | 9.62 3 | 14.01 | 606.3 | 606.2 |
| 17 | 5.07 3 | 6.69 | 554.3 7, 576.8 | 554.3 7, 576.3 |
| 18 | 9.41 3 | 13.83 | 569.5 7, 591.5 | 569.3 7, 591.3 |
References
- Estrallado, N. National Statistics on Relapse Rates for Various Addictions. Available online: https://www.addictiongroup.org/resources/relapse-rates-statistics/ (accessed on 15 May 2025).
- Kreek, M.J.; LaForge, K.S.; Butelman, E. Pharmacotherapy of addictions. Nat. Rev. Drug Discov. 2002, 1, 710–726. [Google Scholar] [CrossRef] [PubMed]
- Sinha, R.; Catapano, D.; O’Malley, S. Stress-induced craving and stress response in cocaine dependent individuals. Psychopharmacology 1999, 142, 343–351. [Google Scholar] [CrossRef]
- Sinha, R.; Garcia, M.; Paliwal, P.; Kreek, M.J.; Rounsaville, B.J. Stress-induced cocaine craving and hypothalamic-pituitary-adrenal responses are predictive of cocaine relapse outcomes. Arch. Gen. Psychiatry 2006, 63, 324–331. [Google Scholar] [CrossRef]
- Martinez, D.; Slifstein, M.; Matuskey, D.; Nabulsi, N.; Zheng, M.Q.; Lin, S.F.; Ropchan, J.; Urban, N.; Grassetti, A.; Chang, D.; et al. Kappa-opioid receptors, dynorphin, and cocaine addiction: A positron emission tomography study. Neuropsychopharmacology 2019, 44, 1720–1727. [Google Scholar] [CrossRef]
- Carey, A.N.; Borozny, K.; Aldrich, J.V.; McLaughlin, J.P. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur. J. Pharmacol. 2007, 569, 84–89. [Google Scholar] [CrossRef]
- Aldrich, J.V.; Patkar, K.A.; McLaughlin, J.P. Zyklophin, a systemically active selective kappa opioid receptor peptide antagonist with short duration of action. Proc. Natl. Acad. Sci. USA 2009, 106, 18396–18401. [Google Scholar] [CrossRef]
- Ross, N.C.; Reilley, K.J.; Murray, T.F.; Aldrich, J.V.; McLaughlin, J.P. Novel opioid cyclic tetrapeptides: Trp isomers of CJ-15,208 exhibit distinct opioid receptor agonism and short-acting kappa opioid receptor antagonism. Br. J. Pharmacol. 2012, 165, 1097–1108. [Google Scholar] [CrossRef]
- Eans, S.O.; Ganno, M.L.; Reilley, K.J.; Patkar, K.A.; Senadheera, S.N.; Aldrich, J.V.; McLaughlin, J.P. The macrocyclic tetrapeptide [D-Trp]CJ-15,208 produces short acting κ opioid receptor antagonism in the CNS after oral administration. Br. J. Pharmacol. 2013, 169, 426–436. [Google Scholar] [CrossRef]
- Aldrich, J.V.; Senadheera, S.N.; Ross, N.C.; Reilley, K.J.; Ganno, M.L.; Eans, S.O.; Murray, T.F.; McLaughlin, J.P. Alanine analogues of [D-Trp]CJ-15,208: Novel opioid activity profiles and prevention of drug- and stress-induced reintatement of cocaine-seeking behaviour. Br. J. Pharmacol. 2014, 171, 3212–3222. [Google Scholar] [CrossRef] [PubMed]
- Ferracane, M.J.; Brice-Tutt, A.; Coleman, J.; Simpson, G.; Wilson, L.; Eans, S.O.; Stacy, H.; Murray, T.F.; McLaughlin, J.P.; Aldrich, J.V. Design, Synthesis, and Characterization of the Macrocyclic Tetrapeptide cyclo[Pro-Sar-Phe-D-Phe]: A Mixed Opioid Receptor Agonist-Antagonist Following Oral Administration. ACS Chem. Neurosci. 2020, 11, 1324–1336. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Hirai, H.; Kim, Y.-J.; Kojima, Y.; Matsunaga, Y.; Nishida, H.; Sakakibara, T.; Suga, O.; Sujaku, T.; Kojima, N. CJ-15,208, a novel kappa opioid receptor antagonist, from a fungus, Ctenomyces serratus ATCC15502. J. Antibiot. 2002, 55, 847–854. [Google Scholar] [CrossRef]
- Ross, N.C.; Kulkarni, S.S.; McLaughlin, J.P.; Aldrich, J.V. Synthesis of CJ-15,208, a novel κ-opioid receptor antagonist. Tetrahedron Lett. 2010, 51, 5020–5023. [Google Scholar] [CrossRef]
- Scherrer, K.H.; Eans, S.O.; Medina, J.M.; Senadheera, S.N.; Khaliq, T.; Murray, T.F.; McLaughlin, J.P.; Aldrich, J.V. Tryptophan Substitution in CJ-15,208 (cyclo[Phe-D-Pro-Phe-Trp]) Introduces delta-Opioid Receptor Antagonism, Preventing Antinociceptive Tolerance and Stress-Induced Reinstatement of Extinguished Cocaine-Conditioned Place Preference. Pharmaceuticals 2023, 16, 1218. [Google Scholar] [CrossRef]
- Demyttenaere, K. Aticaprant, a kappa opioid receptor antagonist, and the recovered ‘interest and pleasure’ in the concept of major depressive disorder. Eur. Arch. Psychiatry Clin. Neurosci. 2024. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Mathew, S.J.; Cutler, A.J.; Visitacion, N.C.; Gold, M.; Yuan, J.; Aurora, B. Navacaprant, a Novel and Highly Selective Kappa Opioid Receptor Antagonist, in Adults With Major Depressive Disorder: A Randomized, Double-Blind Phase 2 Clinical Trial. J. Clin. Psychopharmacol. 2025, 45, 267–276. [Google Scholar] [CrossRef]
- Brice-Tutt, A.C.; Senadheera, S.N.; Ganno, M.L.; Eans, S.O.; Khaliq, T.; Murray, T.F.; McLaughlin, J.P.; Aldrich, J.V. Phenylalanine stereoisomers of CJ-15,208 and [D-Trp]CJ-15,208 exhibit distinctly different opioid activity profiles. Molecules 2020, 25, 3999. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, J.P.; Hill, K.P.; Jiang, Q.; Sebastian, A.; Archer, S.; Bidlack, J.M. Nitrocinnamoyl and chlorocinnamoyl derivatives of dihydrocodeinone: In vivo and in vitro characterization of mu-selective agonist and antagonist activity. J. Pharmacol. Exp. Ther. 1999, 289, 304–311. [Google Scholar] [CrossRef]
- Bidlack, J.M.; Cohen, D.J.; McLaughlin, J.P.; Lou, R.; Ye, Y.; Wentland, M.P. 8-Carboxamidocyclazocine: A long-acting, novel benzomorphan. J. Pharmacol. Exp. Ther. 2002, 302, 374–380. [Google Scholar] [CrossRef]
- Patkar, K.A.; Wu, J.; Ganno, M.L.; Singh, H.D.; Ross, N.C.; Rasakham, K.; Toll, L.; McLaughlin, J.P. Physical presence of nor-binaltorphimine in mouse brain over 21 days after a single administration corresponds to its long-lasting antagonistic effect on kappa-opioid receptors. J. Pharmacol. Exp. Ther. 2013, 346, 545–554. [Google Scholar] [CrossRef] [PubMed]
- Bardo, M.T.; Rowlett, J.K.; Harris, M.J. Conditioned place preference using opiate and stimulant drugs: A meta-analysis. Neurosci. Biobehav. Rev. 1995, 19, 39–51. [Google Scholar] [CrossRef]
- Toth, G.; Kramer, T.H.; Knapp, R.; Lui, G.; Davis, P.; Burks, T.F.; Yamamura, H.I.; Hruby, V.J. [D-Pen2,D-Pen5]enkephalin Analogues with Increased Affinity and Selectivity for d Opioid Receptors. J. Med. Chem. 1990, 33, 249–253. [Google Scholar] [CrossRef]
- Heyl, D.L.; Mosberg, H.I. Substitution on the Phe3 Aromatic Ring in Cyclic d Opioid Receptor-Selective Dermorphin/Deltorphin Tetrapeptide Analogues: Electronic and Lipophilic Requirements for Receptor Affinity. J. Med. Chem. 1992, 35, 1535–1541. [Google Scholar] [CrossRef]
- Salvadori, S.; Bianchi, C.; Lazarus, L.H.; Scaranari, V.; Attila, M.; Tomatis, R. Para-Substituted Phe3 Deltorphin Analogues: Enhanced Selectivity of Halogenated Derivatives for d Opioid Receptors. J. Med. Chem. 1992, 35, 4651–4657. [Google Scholar] [CrossRef]
- Heyl, D.L.; Dandabathula, M.; Kurtz, K.R.; Mousigian, C. Opioid Receptor Binding Requirements For the Delta-Selective Peptide Deltorphin I: Phe3 Replacement With Ring-Substituted and Heterocyclic Amino Acids. J. Med. Chem. 1995, 38, 1242–1246. [Google Scholar] [CrossRef]
- Piekielna, J.; Perlikowska, R.; do-Rego, J.C.; do-Rego, J.L.; Cerlesi, M.C.; Calo, G.; Kluczyk, A.; Lapinski, K.; Tomboly, C.; Janecka, A. Synthesis of mixed opioid affinity cyclic endomorphin-2 analogues with fluorinated phenylalanines. ACS Med. Chem. Lett. 2015, 6, 579–583. [Google Scholar] [CrossRef] [PubMed]
- Perlikowska, R.; Malfacini, D.; Cerlesi, M.C.; Calo, G.; Piekielna, J.; Floriot, L.; Henry, T.; do-Rego, J.C.; Tomboly, C.; Kluczyk, A.; et al. Pharmacological characterization of endomorphin-2-based cyclic pentapeptides with methylated phenylalanine residues. Peptides 2014, 55, 145–150. [Google Scholar] [CrossRef]
- Haaseth, R.C.; Zalewska, T.; Davis, P.; Yamamura, H.I.; Porreca, F.; Hruby, V.J. Para-Substituted Phenylalanine-4 Analogues of [L-Ala3]DPDPE: Highly Selective d Opioid Receptor Ligands. J. Pept. Res. 1997, 50, 171–177. [Google Scholar] [CrossRef]
- Choi, H.; Murray, T.F.; Aldrich, J.V. Synthesis and evaluation of potential affinity labels derived from endomorphin-2. J. Pept. Res. 2003, 61, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, J.V.; Senadheera, S.N.; Ross, N.C.; Ganno, M.L.; Eans, S.O.; McLaughlin, J.P. The macrocyclic peptide natural product CJ-15,208 is orally active and prevents reinstatement of extinguished cocaine-seeking behavior. J. Nat. Prod. 2013, 76, 433–438. [Google Scholar] [CrossRef] [PubMed]
- Brice-Tutt, A.C.; Eans, S.O.; Yakovlev, D.; Aldrich, J.V.; McLaughlin, J.P. An analog of [D-Trp]CJ-15,208 exhibits kappa opioid receptor antagonism following oral administration and prevents stress-induced reinstatement of extinguished morphine conditioned place preference. Pharmacol. Biochem. Behav. 2022, 217, 173405. [Google Scholar] [CrossRef]
- Clarke, T.K.; Ambrose-Lanci, L.; Ferraro, T.N.; Berrettini, W.H.; Kampman, K.M.; Dackis, C.A.; Pettinati, H.M.; O’Brien, C.P.; Oslin, D.W.; Lohoff, F.W. Genetic association analyses of PDYN polymorphisms with heroin and cocaine addiction. Genes. Brain Behav. 2012, 11, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Rasakham, K.; Liu-Chen, L.Y. Sex differences in kappa opioid pharmacology. Life Sci. 2011, 88, 2–16. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.B.; Chartoff, E. Sex differences in neural mechanisms mediating reward and addiction. Neuropsychopharmacology 2019, 44, 166–183. [Google Scholar] [CrossRef]
- Liu, S.S.; Pickens, S.; Burma, N.E.; Ibarra-Lecue, I.; Yang, H.; Xue, L.; Cook, C.; Hakimian, J.K.; Severino, A.L.; Lueptow, L.; et al. Kappa Opioid Receptors Drive a Tonic Aversive Component of Chronic Pain. J. Neurosci. 2019, 39, 4162–4178. [Google Scholar] [CrossRef]
- Pina, M.M.; Pati, D.; Neira, S.; Taxier, L.R.; Stanhope, C.M.; Mahoney, A.A.; D’Ambrosio, S.; Kash, T.L.; Navarro, M. Insula Dynorphin and Kappa Opioid Receptor Systems Regulate Alcohol Drinking in a Sex-Specific Manner in Mice. J. Neurosci. 2023, 43, 5158–5171. [Google Scholar] [CrossRef]
- Mantsch, J.R.; Baker, D.A.; Funk, D.; Le, A.D.; Shaham, Y. Stress-Induced Reinstatement of Drug Seeking: 20 Years of Progress. Neuropsychopharmacology 2016, 41, 335–356. [Google Scholar] [CrossRef]
- Nygard, S.K.; Hourguettes, N.J.; Sobczak, G.G.; Carlezon, W.A.; Bruchas, M.R. Stress-Induced Reinstatement of Nicotine Preference Requires Dynorphin/Kappa Opioid Activity in the Basolateral Amygdala. J. Neurosci. 2016, 36, 9937–9948. [Google Scholar] [CrossRef]
- Gillett, K.; Harshberger, E.; Valdez, G.R. Protracted withdrawal from ethanol and enhanced responsiveness stress: Regulation via the dynorphin/kappa opioid receptor system. Alcohol 2013, 47, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Haun, H.L.; Lebonville, C.L.; Solomon, M.G.; Griffin, W.C.; Lopez, M.F.; Becker, H.C. Dynorphin/Kappa Opioid Receptor Activity Within the Extended Amygdala Contributes to Stress-Enhanced Alcohol Drinking in Mice. Biol. Psychiatry 2022, 91, 1019–1028. [Google Scholar] [CrossRef]
- Dixon, H.B.F. Nomenclature and symbolism for amino acids and peptides. Eur. J. Biochem. 1984, 56, 595–624. Available online: https://febs.onlinelibrary.wiley.com/doi/10.1111/j.1432-1033.1984.tb07877.x (accessed on 28 July 2025).
- Bidlack, J.M.; Knapp, B.I.; Deaver, D.R.; Plotnikava, M.; Arnelle, D.; Wonsey, A.M.; Fern Toh, M.; Pin, S.S.; Namchuk, M.N. In Vitro Pharmacological Characterization of Buprenorphine, Samidorphan, and Combinations Being Developed as an Adjunctive Treatment of Major Depressive Disorder. J. Pharmacol. Exp. Ther. 2018, 367, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Prusoff, W.H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 1973, 22, 3099–3108. [Google Scholar]
- Ko, W.; Porter, J.J.; Sipple, M.T.; Edwards, K.M.; Lueck, J.D. Efficient suppression of endogenous CFTR nonsense mutations using anticodon-engineered transfer RNAs. Mol. Ther. Nucleic Acids 2022, 28, 685–701. [Google Scholar] [CrossRef]
- Mogil, J.S.; Kest, B.; Sadowski, B.; Belknap, J.K. Differential genetic mediation of sensitivity to morphine in genetic models of opiate antinociception: Influence of nociceptive assay. J. Pharmacol. Exp. Ther. 1996, 276, 532–544. [Google Scholar] [CrossRef]
- Wilson, S.G.; Smith, S.B.; Chesler, E.J.; Melton, K.A.; Haas, J.J.; Mitton, B.; Strasburg, K.; Hubert, L.; Rodriguez-Zas, S.L.; Mogil, J.S. The heritability of antinociception: Common pharmacogenetic mediation of five neurochemically distinct analgesics. J. Pharmacol. Exp. Ther. 2003, 304, 547–559. [Google Scholar] [CrossRef]
- Orsini, C.; Bonito-Oliva, A.; Conversi, D.; Cabib, S. Susceptibility to conditioned place preference induced by addictive drugs in mice of the C57BL/6 and DBA/2 inbred strains. Psychopharmacology 2005, 181, 327–336. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef] [PubMed]



| KOR Ki | MOR Ki | KOR | |||
|---|---|---|---|---|---|
| Analog 1 | (nM ± S.E.M) 2 | (nM ± S.E.M) 2 | Selectivity 3 | Efficacy (%) 4 | Antag. IC50 5 |
| [D-Trp]CJ-15,208 and its analogs: | |||||
| 1 | 6.9 ± 1.1 | 58 ± 7.5 | 8 | 16 ± 2.4 | 66 ± 11 |
| 2, D-Trp(Me) | 16 ± 0.8 | 36 ± 2.6 | 2 | 14 ± 7.1 | 220 ± 32 |
| 3, D-Trp(form-amide) | 2.2 ± 0.15 | 90 ± 14 | 41 | 9.2 ± 4.9 | 49 ± 5.4 |
| [D-Phe(X)4]CJ-15,208: | |||||
| 4, X = H | 24 ± 0.97 | 270 ± 23 | 11 | 19 ± 8.1 | 460 ± 100 |
| 5, X = o-F | 39 ± 0.57 | 350 ± 22 | 9 | ND 6 | ND |
| 6, X = m-F | 32 ± 5.8 | 160 ± 15 | 5 | 11 ± 5.6 | 340 ± 58 |
| 7, X = p-F | 15 ± 1.8 | 45 ± 1.7 | 3 | 5.5 ± 7.9 | 170 ± 13 |
| 8, X = 3,4-F2 | 6.6 ± 0.73 | 57 ± 5.6 | 9 | 13 ± 2.7 | 170 ± 28 |
| 9, X = 3,5-F2 | 25 ± 4.3 | 290 ± 27 | 12 | 14 ± 2.4 | 320 ± 49 |
| 10, X = 2,4,5-F3 | 27 ± 3.3 | 270 ± 30 | 10 | 19 ± 6.8 | 290 ± 40 |
| 11, X = 3,4,5-F3 | 12 ± 0.44 | 46 ± 5.4 | 4 | 2.2 ± 6.2 | 160 ± 19 |
| 12, X = F5 | 310 ± 9.9 | ND | ND | ND | ND |
| 13, X = o-Me | 110 ± 13 | 280 ± 8 | 2.5 | ND | ND |
| 14, X = m-Me | 26 ± 3.0 | 130 ± 11 | 5 | 0.74 ± 5.7 | 320 ± 67 |
| 15, X = p-Me | 11 ± 0.67 | 110 ± 15 | 10 | 18 ± 1.4 | 190 ± 17 |
| 16, X = p-NO2 | 7.0 ± 0.99 | 61 ± 2.7 | 9 | 7.3 ± 7.3 | 210 ± 13 |
| 17, X = p-NH2 | 7 | ND | ND | ND | ND |
| 18, X = p-OMe | 150 ± 16 | ND | ND | ND | ND |
| Aticaprant | 0.77 ± 0.10 | 15 ± 2.0 | 19 | −48 ± 3.0 8 | 8.0 ± 1.7 |
| Navacaprant | 21 ± 3.1 | 850 ± 73 | 40 | 7.4 ± 7.5 | 69 ± 17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldrich, J.V.; Yakovlev, D.Y.; Coleman, J.S.; Senadheera, S.N.; Stacy, H.M.; Eans, S.O.; Knapp, B.I.; Bidlack, J.M.; McLaughlin, J.P. Optimization of the Macrocyclic Tetrapeptide [D-Trp]CJ-15,208 to Prevent Stress-Induced Relapse of Cocaine-Seeking Behavior. Molecules 2025, 30, 3993. https://doi.org/10.3390/molecules30193993
Aldrich JV, Yakovlev DY, Coleman JS, Senadheera SN, Stacy HM, Eans SO, Knapp BI, Bidlack JM, McLaughlin JP. Optimization of the Macrocyclic Tetrapeptide [D-Trp]CJ-15,208 to Prevent Stress-Induced Relapse of Cocaine-Seeking Behavior. Molecules. 2025; 30(19):3993. https://doi.org/10.3390/molecules30193993
Chicago/Turabian StyleAldrich, Jane V., Dmitry Y. Yakovlev, Jeremy S. Coleman, Sanjeewa N. Senadheera, Heather M. Stacy, Shainnel O. Eans, Brian I. Knapp, Jean M. Bidlack, and Jay P. McLaughlin. 2025. "Optimization of the Macrocyclic Tetrapeptide [D-Trp]CJ-15,208 to Prevent Stress-Induced Relapse of Cocaine-Seeking Behavior" Molecules 30, no. 19: 3993. https://doi.org/10.3390/molecules30193993
APA StyleAldrich, J. V., Yakovlev, D. Y., Coleman, J. S., Senadheera, S. N., Stacy, H. M., Eans, S. O., Knapp, B. I., Bidlack, J. M., & McLaughlin, J. P. (2025). Optimization of the Macrocyclic Tetrapeptide [D-Trp]CJ-15,208 to Prevent Stress-Induced Relapse of Cocaine-Seeking Behavior. Molecules, 30(19), 3993. https://doi.org/10.3390/molecules30193993







