Discovery of Primaquine–Indole Carboxamides with Cancer-Cell-Selective Antiproliferative Activity
Abstract
1. Introduction
2. Results and Discussion
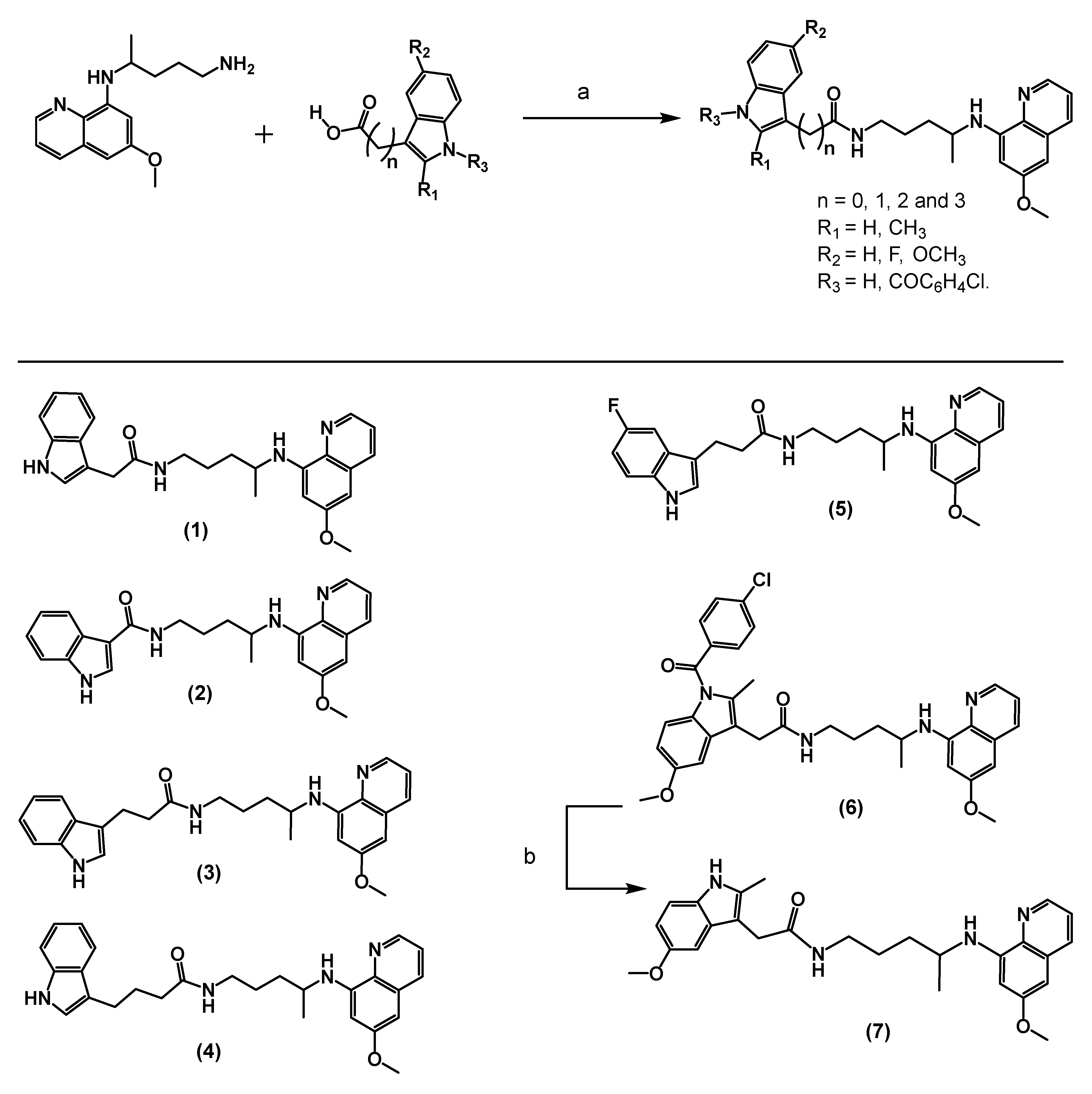
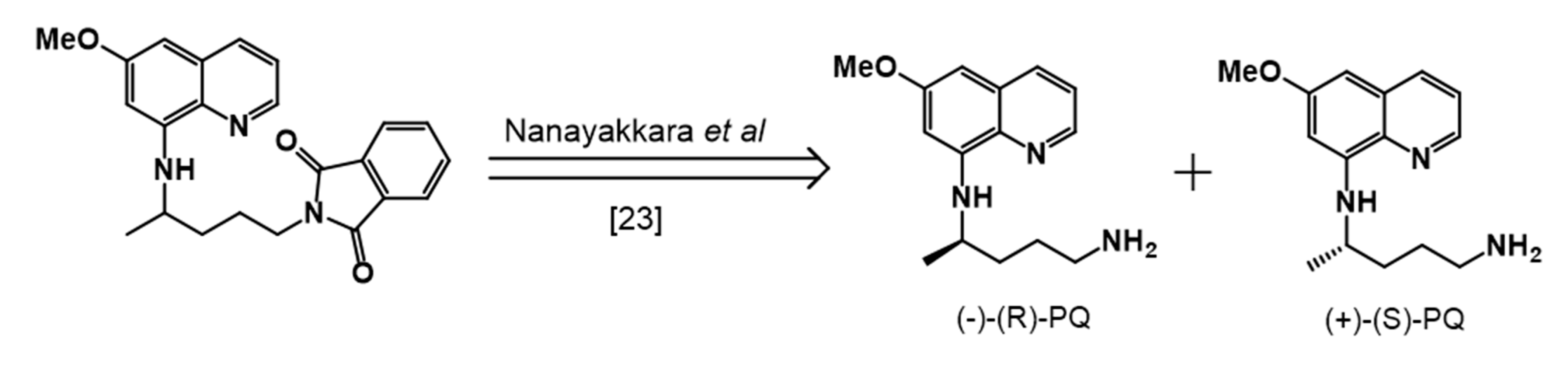
2.1. Synthesis
2.2. Antiproliferative Activity of Primaquine–Indole Carboxamide
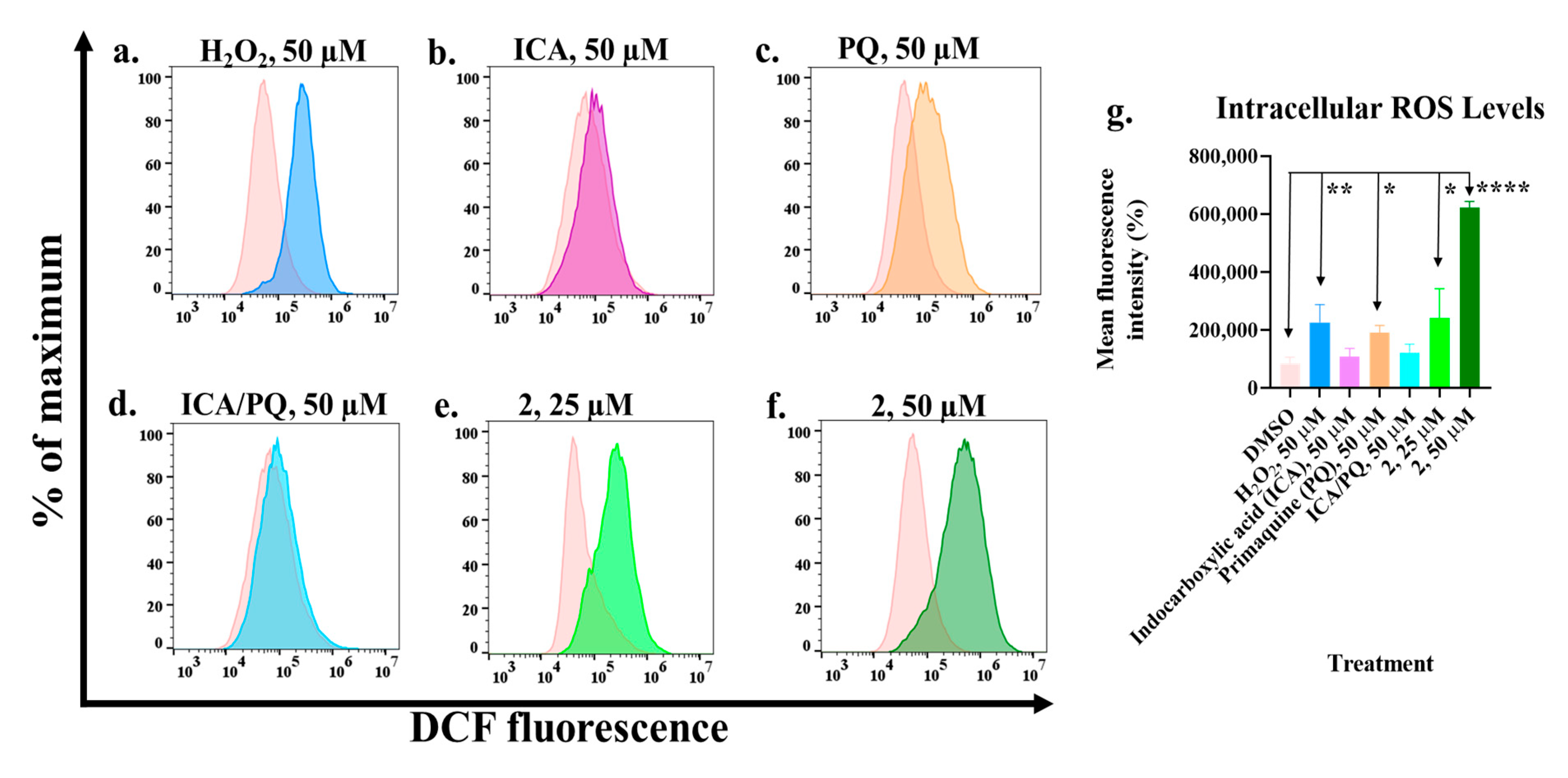
2.3. Primaquine–Indole Carboxamide Generates Reactive Oxygen Species
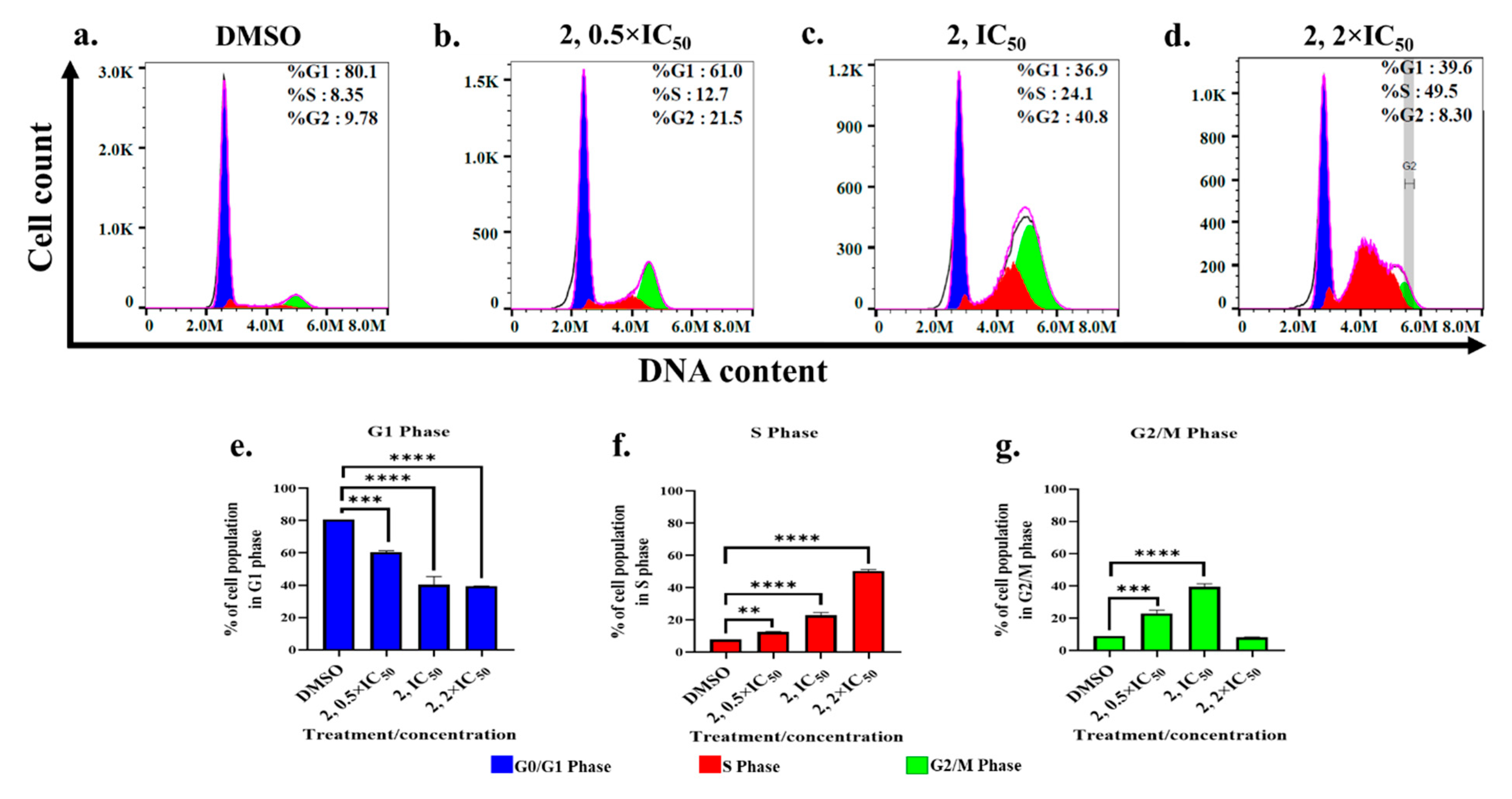
2.4. Cell Cycle Progression Is Modulated by Primaquine-Indole Carboxamide
3. Materials and Methods
3.1. Chemicals, Synthesis, and Charaterization
3.2. Molecular Docking Study
3.3. Cell Culture
3.4. MTS Cell Viability Assay
3.5. Quantification of Reactive Oxygen Species
3.6. Cell Cycle Analysis
3.7. AR-Binding Assay
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PQ | Primaquine |
| ICA | indole-3-carboxylic acid |
| IAA | indole-3-acetic acid |
| IPA | indole-3-propionic acid |
| IBA | indole-4-butyric acid |
| AR | androgen receptor |
| LBD | ligand binding domain |
| BF3 | binding function 3 |
References
- Sinha, A.K.; Laursen, M.F.; Brinck, J.E.; Rybtke, M.L.; Hjørne, A.P.; Procházková, N.; Pedersen, M.; Roager, H.M.; Licht, T.R. Dietary fibre directs microbial tryptophan metabolism via metabolic interactions in the gut microbiota. Nat. Microbiol. 2024, 9, 1964–1978. [Google Scholar] [CrossRef]
- Roager, H.M.; Licht, T.R. Microbial tryptophan catabolites in health and disease. Nat. Commun. 2018, 9, 3294. [Google Scholar] [CrossRef]
- Jia, D.; Kuang, Z.; Wang, L. The role of microbial indole metabolites in tumor. Gut Microbes 2024, 16, 2409209. [Google Scholar] [CrossRef] [PubMed]
- Tintelnot, J.; Xu, Y.; Lesker, T.R.; Schönlein, M.; Konczalla, L.; Giannou, A.D.; Pelczar, P.; Kylies, D.; Puelles, V.G.; Bielecka, A.A.; et al. Microbiota-derived 3-IAA influences chemotherapy efficacy in pancreatic cancer. Nature 2023, 615, 168–174. [Google Scholar] [CrossRef]
- Folkes, L.K.; Dennis, M.F.; Stratford, M.R.; Candeias, L.P.; Wardman, P. Peroxidase-catalyzed effects of indole-3-acetic acid and analogues on lipid membranes, DNA, and mammalian cells in vitro. Biochem. Pharmacol. 1999, 57, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Folkes, L.K.; Wardman, P. Oxidative activation of indole-3-acetic acids to cytotoxic species- a potential new role for plant auxins in cancer therapy. Biochem. Pharmacol. 2001, 61, 129–136. [Google Scholar] [CrossRef]
- Folkes, L.K.; Wardman, P. Enhancing the efficacy of photodynamic cancer therapy by radicals from plant auxin (indole-3-acetic acid). Cancer Res. 2003, 63, 776–779. [Google Scholar]
- Kim, S.Y.; Ryu, J.S.; Li, H.; Park, W.-J.; Yun, H.-Y.; Baek, K.J.; Kwon, N.S.; Sohn, U.D.; Kim, D.-S. UVB-activated indole-3-acetic acid induces apoptosis of PC-3 prostate cancer cells. Anticancer Res. 2010, 30, 4607–4612. [Google Scholar] [PubMed]
- Diengott, D.; Mirsky, I.A. Hypoglycemic action of indole-3-acetic acid by mouth in patients with diabetes mellitus. Proc. Soc. Exp. Biol. Med. 1956, 93, 109–110. [Google Scholar]
- Vasquez-Vivar, J.; Augusto, O. Hydroxylated metabolites of the antimalarial drug primaquine. Oxidation and redox cycling. J. Biol. Chem. 1992, 267, 6848–6854. [Google Scholar] [CrossRef]
- Pybus, B.S.; Marcsisin, S.R.; Jin, X.; Deye, G.; Sousa, J.C.; Li, Q.; Caridha, D.; Zeng, Q.; Reichard, G.A.; Ockenhouse, C.; et al. The metabolism of primaquine to its active metabolite is dependent on CYP 2D6. Malar. J. 2013, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Camarda, G.; Jirawatcharadech, P.; Priestley, R.S.; Saif, A.; March, S.; Wong, M.H.L.; Leung, S.; Miller, A.B.; Baker, D.A.; Alano, P.; et al. Antimalarial activity of primaquine operates via a two-step biochemical relay. Nat. Commun. 2019, 10, 3226. [Google Scholar] [CrossRef]
- Marcsisin, S.R.; Reichard, G.; Pybus, B.S. Primaquine pharmacology in the context of CYP 2D6 pharmacogenomics: Current state of the art. Pharmacol. Ther. 2016, 161, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Choi, H.S.; Lee, D.S. Primaquine Inhibits the Endosomal Trafficking and Nuclear Localization of EGFR and Induces the Apoptosis of Breast Cancer Cells by Nuclear EGFR/Stat3-Mediated c-Myc Downregulation. Int. J. Mol. Sci. 2021, 22, 12961. [Google Scholar] [CrossRef]
- Pavic, K.; Rubinic, B.; Rajic, Z.; Fontinha, D.; Prudêncio, M.; Uzelac, L.; Kralj, M.; Held, J.; Zorc, B. Primaquine homodimers as potential antiplasmodial and anticancer agents. Bioorg. Med. Chem. Lett. 2019, 29, 126614. [Google Scholar] [CrossRef]
- Duarte, D.; Vale, N. New Trends for Antimalarial Drugs: Synergism between Antineoplastics and Antimalarials on Breast Cancer Cells. Biomolecules 2020, 10, 1623. [Google Scholar] [CrossRef]
- Nordor, A.V.; Bellet, D.; Siwo, G.H. Cancer-malaria: Hidden connections. Open Biol. 2018, 8, 180127. [Google Scholar] [CrossRef]
- Qin, L.; Chen, C.; Chen, L.; Xue, R.; Ou-Yang, M.; Zhou, C.; Zhao, S.; He, Z.; Xia, Y.; He, J.; et al. Worldwide malaria incidence and cancer mortality are inversely associated. Infect. Agents Cancer 2017, 12, 14. [Google Scholar] [CrossRef]
- Kim, J.H.; Choi, A.R.; Kim, Y.K.; Yoon, S. Co-treatment with the anti-malarial drugs mefloquine and primaquine highly sensitizes drug-resistant cancer cells by increasing P-gp inhibition. Biochem. Biophys. Res. Commun. 2013, 441, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.R.; Kim, J.H.; Woo, Y.H.; Kim, H.S.; Yoon, S. Anti-malarial Drugs Primaquine and Chloroquine Have Different Sensitization Effects with Anti-mitotic Drugs in Resistant Cancer Cells. Anticancer Res. 2016, 36, 1641–1648. [Google Scholar] [CrossRef]
- Gryder, B.E.; Akbashev, M.J.; Rood, M.K.; Raftery, E.D.; Meyers, W.M.; Dillard, P.; Khan, S.; Oyelere, A.K. Selectively targeting prostate cancer with antiandrogen equipped histone deacetylase inhibitors. ACS Chem. Biol. 2013, 8, 2550–2560. [Google Scholar] [CrossRef][Green Version]
- Leblanc, E.; Ban, F.; Cavga, A.D.; Lawn, S.; Huang, C.-C.F.; Mohan, S.; Chang, M.E.K.; Flory, M.R.; Ghaidi, F.; Lingadahalli, S.; et al. Development of 2-(5,6,7-Trifluoro-1H-Indol-3-yl)-quinoline-5-carboxamide as a Potent, Selective, and Orally Available Inhibitor of Human Androgen Receptor Targeting Its Binding Function-3 for the Treatment of Castration-Resistant Prostate Cancer. J. Med. Chem. 2021, 64, 14968–14982. [Google Scholar] [CrossRef]
- Nanayakkara, N.P.; Tekwani, B.L.; Herath, H.M.; Sahu, R.; Gettayacamin, M.; Tungtaeng, A.; van Gessel, Y.; Baresel, P.; Wickham, K.S.; Bartlett, M.S.; et al. Scalable preparation and differential pharmacologic and toxicologic profiles of primaquine enantiomers. Antimicrob. Agents Chemother. 2014, 58, 4737–4744. [Google Scholar] [CrossRef] [PubMed]
- Pavic, K.; Poje, G.; Pessanha de Carvalho, L.; Tandarić, T.; Marinović, M.; Fontinha, D.; Held, J.; Prudêncio, M.; Piantanida, I.; Vianello, R.; et al. Discovery of harmiprims, harmine-primaquine hybrids, as potent and selective anticancer and antimalarial compounds. Bioorg. Med. Chem. 2024, 105, 117734. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef]
- Schiewer, M.J.; Augello, M.A.; Knudsen, K.E. The AR dependent cell cycle: Mechanisms and cancer relevance. Mol. Cell. Endocrinol. 2012, 352, 34–45. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, S.; Chaurasiya, N.D.; Sahu, R.; Walker, L.A.; Tekwani, B.L. Understanding the mechanisms for metabolism-linked hemolytic toxicity of primaquine against glucose 6-phosphate dehydrogenase deficient human erythrocytes: Evaluation of eryptotic pathway. Toxicology 2012, 294, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Gong, T.; Hong, Y. Hydrogen peroxide initiates oxidative stress and proteomic alterations in meningothelial cells. Sci. Rep. 2022, 12, 14519. [Google Scholar] [CrossRef]
- Manchado, E.; Guillamot, M.; Malumbres, M. Killing cells by targeting mitosis. Cell Death Differ. 2012, 19, 369–377. [Google Scholar] [CrossRef]
- Curtin, N.J. DNA repair dysregulation from cancer driver to therapeutic target. Nat. Rev. Cancer 2012, 12, 801–817. [Google Scholar] [CrossRef] [PubMed]


| Structure | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| DU-145 | LNCaP | Hep-G2 | MCF-7 | MDA-MB-231 | MDA-MB-453 | Vero | |
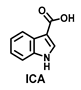 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
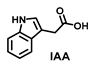 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
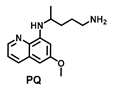 | 97.20 ± 5.6 | 29.50 ± 6.5 | 71.40 ± 12.4 | 90.92 ± 8.9 | 65.42 ± 2.3 | 13.72 ± 3.2 | 87. 55 ± 21.6 |
| ICA + PQ | 35.97 | 40.73 ± 1.1 | 82.57 | NT | NT | NT | NT |
| IAA + PQ | >100 | >100 | 82.65 ± 17.0 | 40.40 ± 8.5 | 44.94 ± 2.2 | 26.78 ± 5.7 | >100 |
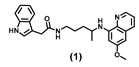 | 46.43 ± 9.3 | 48.67 ± 10.1 | 48.77 ± 1.3 | 48.27 ± 4.0 | 57.89 | 42.53 ± 2.5 | 91.50 ± 12.0 |
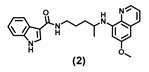 | 14.75 ± 3.8 | 11.90 ± 4.7 | 34.41 ± 4.3 | 43.68 ± 7.6 | 38.74 ± 3.4 | 23.83 ± 2.6 | 82.55 ± 15.7 |
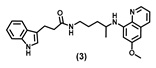 | 56.26 ± 11.7 | 33.47 ± 5.3 | 32.22 ± 1.6 | 67.10 ± 6.6 | 99.52 ± 1.6 | 43.54 ± 5.2 | >100 |
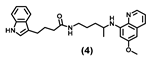 | 67.69 ± 11.0 | 25.93 ± 4.7 | 82.56 ± 13.1 | 90.55 ± 16.4 | >100 | 30.41 ± 8.2 | >100 |
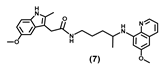 | 37.71 ± 4.0 | 32.90 ± 9.58 | 29.40 ± 10.0 | 42.51 ± 4.4 | >100.00 | 33.57 ± 0.09 | >100 |
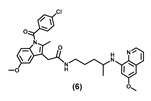 | >100 | >100 | >100 | >100 | >100 | >100 | >100 |
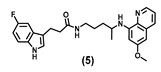 | 40.17 ± 4.0 | 24.28 ± 4.7 | 43.87 ± 3.8 | 48.77 ± 10.9 | 45.83 ± 5.1 | 36.20 ± 1.0 | 83.42 ± 2.3 |
| Enzalutamide | 21.26 ± 1.5 | 55.55 ± 7.9 | >100 | >100 | >100 | 74.33 ± 22.7 | >100 |
| Structure | IC50 (µM) | ||||||
|---|---|---|---|---|---|---|---|
| DU-145 | LNCaP | Hep-G2 | MCF-7 | MDA-MB-231 | MDA-MB-453 | Vero | |
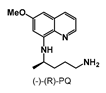 | >100 | >100 | >100 | >100 | 56.22 ± 7.1 | 23.45 ± 1.5 | >100 |
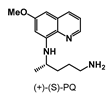 | >100 | >100 | >100 | >100 | 87.50 ± 12.5 | 28.19 ± 1.3 | >100 |
| (+)-(S)-PQ + (-)-(R)-PQ | 41.37 | 52.39 | 57.95 ± 0.1 | 45.31 ± 5.5 | 38.46 ± 9.9 | 21.45 ± 2.6 | >100 |
 | 35.56 ± 1.8 | 40.38 ± 7.0 | 52.07 | 94.21 ± 5.0 | 53.09 ± 1.8 | 38.47 ± 4.6 | >100 |
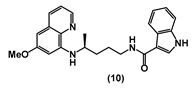 | 37.89 ± 2.5 | 32.09 ± 3.8 | 47.99 ± 2.7 | 78.51 ± 6.1 | 51.06 ± 4.7 | 16.06 ± 3.0 | >100 |
| 8 + 10 | 25.15 ± 8.8 | 18.83 ± 1.7 | 29.96 ± 5.9 | 37.45 ± 8.8 | 26.31 ± 1.0 | 17.79 ± 0.9 | >100 |
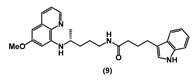 | 91.75 ± 11.7 | 24.75 ± 0.3 | 64.82 ± 5.6 | 99.72 ± 0.4 | >100 | 31.82 ± 2.9 | >100 |
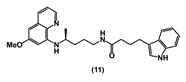 | 65.81 ± 5.4 | 21.29 ± 5.5 | 43.22 ± 2.6 | 98.50 ± 2.1 | >100 | 19.30 ± 0.9 | >100 |
| 9 + 11 | 49.80 ± 8.5 | 8.83 ± 2.0 | 26.90 ± 1.5 | >50 | >50 | 12.14 ± 0.8 | >100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peer, B.H.; Olugbami, J.O.; Walunj, D.T.; Oyelere, A.K. Discovery of Primaquine–Indole Carboxamides with Cancer-Cell-Selective Antiproliferative Activity. Molecules 2025, 30, 3988. https://doi.org/10.3390/molecules30193988
Peer BH, Olugbami JO, Walunj DT, Oyelere AK. Discovery of Primaquine–Indole Carboxamides with Cancer-Cell-Selective Antiproliferative Activity. Molecules. 2025; 30(19):3988. https://doi.org/10.3390/molecules30193988
Chicago/Turabian StylePeer, Benjamin H., Jeremiah O. Olugbami, Dipak T. Walunj, and Adegboyega K. Oyelere. 2025. "Discovery of Primaquine–Indole Carboxamides with Cancer-Cell-Selective Antiproliferative Activity" Molecules 30, no. 19: 3988. https://doi.org/10.3390/molecules30193988
APA StylePeer, B. H., Olugbami, J. O., Walunj, D. T., & Oyelere, A. K. (2025). Discovery of Primaquine–Indole Carboxamides with Cancer-Cell-Selective Antiproliferative Activity. Molecules, 30(19), 3988. https://doi.org/10.3390/molecules30193988







