Abstract
The review is focused on dictyopterenes A, B, C, and D found in marine algae, covering their (a) distribution; (b) methods of isolation and identification; (c) absolute configuration; and (d) biosynthesis considerations. Dictyopterenes A and B are usually present in high amounts in Dictyopteris spp. Dictyopterene A was found to be abundant in D. prolifera, D. undulata, D. latiscula, D. polypodioides, and D. membranacea. Dictyopterene B (hormosirene) was found as the major compound in D. plagiogramma, D. australis, Hormosira banksii, D. potatorum, D. willana, D. antarctica, Xiphophora chondrophylla, X. gladiata, Scytosiphon lomentaria, Colpomenia peregrina, and Haplospora globosa. Dictyopterene C (dictyotene) was a major compound in D. undulata, D. prolifera, D. membranacea, Gomphonema parvulum, Amphora veneta, Phaeodactylum tricornutum, and D. vermicularis. Dictyopterene D (ectocarpene) was present in Ectocarpus siliculosus, Analipus japonicus, D. prolifera, D. undulata, and Sargassum linifolium. The following enantiomers were found: (1S,2R)-dictyopterene A, (1R,2R)-dictyopterene B, (1S,2S)-dictyopterene B, (1S,2R)-dictyopterene B, (R)-dictyopterene C, and (S)-dictyopterene D. In marine algae, C11-hydrocarbons are derived from C20 polyunsaturated fatty acids by the oxidative cleavage via, e.g., 9-hydroperoxyicosa-(5Z,7E,11Z,14Z,17Z)-pentaenoic acid. An alternative biosynthetic pathway for dictyopterene A and B via the proposed intermediates (S)-dictyoprolenols was considered by oxidative cleavage of hydroperoxyicosatetraenoic acid.
1. Introduction
Marine algae volatile organic compounds (VOCs) are small, low-molecular compounds with low to moderate hydrophilicity and high vapour pressure. Among others, they have been reviewed regarding (a) their emission and roles in algae [1]; (b) the production and role of algal volatile halogenated compounds [2]; (c) the role of algae and cyanobacteria in the production and release of odorants [3]; and (d) the current understanding of algal VOC production and emission in the extreme environments [4].
The trend in marine fragrance (“sea-breeze” or “ocean-smell”) is relatively recent, and its chemistry has been mainly associated with four groups of natural organic compounds [5], including cyclic and alicyclic C11-hydrocarbons. According to their molecular structures, non-isoprenic C11-compounds can be classified into the following groups: acyclic olefins, cyclopentenes, dictyopterenes, related cyclopropanes, and cycloheptadienes. The C11-hydrocarbons and their derivatives have been isolated from different genera of brown algae (e.g., Desmarestia, Zonaria, Dictyota, Laminaria, Ectocarpus, or Fucus genera), and have also been detected in diatom cultures, blooms of freshwater microalgae, and higher plants [6]. However, they appear to be most abundant in brown algae of the genus Dictyopteris [7]. The genus Dictyopteris J.V. Lamouroux (from Greek Dictyon = network, and Pteris = fern) was first proposed by Lamouroux in 1809 [8] and belongs to the Dictyotales order [9] and is widely distributed in tropical, subtropical, and temperate regions. The brown algae of the genus Dictyopteris are odoriferous seaweeds [10] containing C11-hydrocarbons.
The chemical diversity, biological potential, and ecological roles of marine seaweeds of the Dictyopteris genus were reviewed [11,12,13,14]. Some species of this genus show a distinct phytochemistry, with specific secondary metabolites, including C11-hydrocarbons, sulphur compounds, quinone derivatives, terpenes, steroids, and halogenated compounds. This chemical diversity gives them interesting biological properties, including cytotoxic, antioxidant, anti-inflammatory, antimicrobial, and anti-herbivory activities [11]. In distinction from the previous reviews, the present review is, for the first time, focused entirely on dictyopterenes A, B, C, and D found in marine algae, covering their different aspects as follows: (a) distribution among marine algae; (b) methods of isolation and identification; (c) absolute configuration and the presence of enantiomers; and (d) biosynthesis considerations. The used database for search was Web of Science, and keywords were dictyopterene A, dictyopterene B, dictyopterene C, dictyopterene D, dictyotene, ectocarpene, and hormosirene.
2. Results
The chemical structures of selected dictyopterenes are presented in Figure 1 as follows: dictyopterene A (trans-1-(trans-hex-1′-enyl)-2-vinylcyclopropane), dictyopterene B (trans-l-(trans,cis-hexa-1′,3′-dienyl)-2-vinylcyclopropane), dictyopterene C (6-butylcyclohepta-1,4-diene), and dictyopterene D (6-(cis-but-l’-enyl)cyclohepta-1,4-diene). Although some papers reported dictyopterenes C’ or D’ (most probably referring to their isomers), in this review only the symbols A, B, C, and D are used. In the case when the compound’s absolute configuration and correct isomer were determined, that was highlighted.
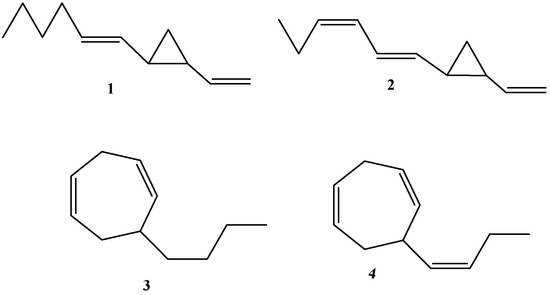
Figure 1.
The chemical structures of dictyopterene A (1), dictyopterene B (2), dictyopterene C (3), and dictyopterene D (4).
2.1. Distribution of Dictyopterenes A, B, C, and D in Marine Algae
Dictyopterenes A and B (also known as hormosirene) are usually present in high amounts in Dictyopteris spp. [15,16,17], while in the female gametes of the marine brown alga Analipus japonicus dictyopterene D (also known as ectocarpene) is the most abundant [18]. Dictyopterene C (also known as dictyotene) was found as the major compound in Dictyopteris undulata [19].
Detailed distribution of dictyopterenes A, B, C, and D in marine algae is presented in Table 1, according to the year of publication.

Table 1.
Distribution and abundance (GC peak area percentages unless indicated otherwise) of dictyopterenes A, B, C, and D in marine algae.
2.2. Abundance of Dictyopterene A
Dictyopterene A was isolated by preparative gas chromatography (GC) and reported as a novel hydrocarbon. The compound’s structure was determined by NMR as trans-1-(trans-1-hexenyl)-2-vinylcyclopropane by Moore, Pettus, and Doty in 1968 [20] from the essential oil of algae from the genus Dictyopteris and later reported among the major constituents of their essential oil (25%) [10,15,21].
The occurrence of dictyopterene A (0.24–32.9%) was also confirmed in the volatiles from Dictyopteris prolifera extracted by the closed-loop stripping technique without heating, and it was higher in the VOCs from the algae along the Japan sea coasts, Susa and Yoshimo, than in those from Osaka and Hikoshima [36]. The volatile compounds released from protoplasts and intact plants of D. prolifera from Japan were extracted by closed-looping headspace (CLHS), and dictyopterene A was found [43] with high abundance (19.08%; 18.67%).
Dictyopterene A was present in the essential oils of D. prolifera (63.3%), Dictyopteris undulata (20.9%), and Dictyopteris spp. (10.9%) from Japan [36]. D. prolifera and D. undulata were extracted with pentane saturated with methanol [25], and the extracts were chromatographed on an alumina column and re-chromatographed on a silica gel column. The GC-MS analysis revealed dictyopterene A in the extracts of D. prolifera with 65% and of D. undulata with 69%. Dictyopterene A was found as the major compound after simultaneous distillation extraction (SDE) in D. prolifera (48.9%), D. undulata (21.1%), D. latiscula (40.1%), and Dictyopteris sp. (10.9%) from Japan [16]. The volatile fraction of Dictyopteris polypodioides was prepared from the crude extract by hydrodistillation, and dictyopterene A was found (14.1%; 9.3%, respectively) [52].
Focused microwave-assisted hydrodistillation (FMAH) and hydrodistillation (HD) were applied to Dictyopteris membranacea from the Atlantic coast of Brittany, and GC and GC-MS revealed dictyopterene A as the major compound (32.3%—FMAH; 7.6%—HD) [47]. Supercritical carbon dioxide extraction (SC-CO2) and FMAH were used comparatively to isolate the volatile oils of D. membranacea (collected from the Mediterranean coast of Algeria) from the crude ether extract, and the major compound determined by GC-MS was dictyopterene A [54].
A minor abundance of dictyopterene A is presented in Table 1.
Enantiomeric Distribution of Dictyopterene A
Dictyopterene A is 1-[(1E)-1-hexen-1-yl]-2-vinylcyclopropane, containing a vinyl and an E-hexenyl substituted ring with two chiral centres at positions 1 and 2 of the cyclopropane. (1S,2R)-Dictyopterene A (Figure 2) was found in the extracts from D. prolifera (48.9%; enantiomeric excess (e.e.) 97.7%), D. latiscula (40.1%; e.e. 95.1%), D. undulata (21.1%; e.e. 96.6%), and Dictyopteris spp. (10.9%, e.e. 84.2%) [16]. The extracts were obtained by digestion with pentane containing 30% methanol and fractionated on silica gel, then re-chromatographed on AgNO3-silica gel and subjected directly to a chiral GC analysis using the Lipodex column.
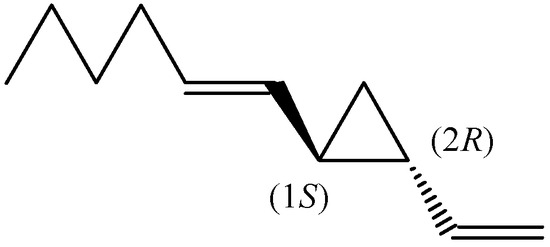
Figure 2.
The chemical structures of (1S,2R)-dictyopterene A.
2.3. Abundance of Dictyopterene B
Pettus and Moore in 1970 [21] determined the chemical structure of dictyopterene B (known also as hormosirene) by NMR (trans-1-(trans,cis-hexa-1′,3′-dienyl)-2-vinylcyclopropane) as the major compound (50%) of the essential oil from algae of the genus Dictyopteris [10,21]. Dictyopterene B was isolated for the first time as the major compound (50%) from the essential oil of Dictyopteris plagiogramma and Dictyopteris australis (Hawaiian Islands) after column silver nitrate-silica gel chromatography and characterized by NMR [15].
Hormosira banksii is a taxonomically isolated brown seaweed endemic to Australia and New Zealand. The sperm attractant of this species has been isolated [28] and identified as hormosirene. Hormosira is the first organism in which a cyclopropane derivative has been found to act as a hormone in sexual reproduction. The release of the spermatozoid attractant hormosirene by the eggs of Australian fucoid Hormosira banksii has been determined quantitatively by a closed-loop extraction technique [41], and the total amount of hormosirene produced per egg was 2.35 pmol (0.35 ng). The occurrence of hormosirene and dictyopterene A (ratio ca. 9:1) was reported [29] in female gametes of seven additional brown algae from different systematic groups: Durvillaea potatorum, Durvillaea willana, and Durvillaea antarctica (Durvillaeales); Xiphophora chondrophylla and Xiphophora gladiata (Fucales); Scytosiphon lomentaria and Colpomenia peregrina (Scytosiphonales).
Dictyopterene B was present in D. prolifera (10%) and D. undulata (9%) from Japan after the extraction with pentane saturated with methanol and chromatography on an alumina column and re-chromatography on a silica gel column [25]. It was found as the major constituent of the extract of Haplospora globosa [30]. The steam distillation was applied to D. prolifera, and dictyopterene B and C were not resolved well on the used DB-1 column; their sum percentage ranged from 12.3% to 59.9% [36]. CLHS isolated the volatiles from D. prolifera from Japan with dictyopterene B and dictyopterene C in the protoplast (15.82%) and intact plant (16.00%) [43]. SDE was performed on D. prolifera from Japan provided dictyopterene B (15.6%) in the isolate [16].
Table 1 contains data about dictyopterene B minor abundance.
Enantiomeric Distribution of Dictyopterene B
Dictyopterene B contains two chiral centres at C1 and C2 of the cyclopropane ring. The inclusion chromatography on cellulose tribenzoate coated on silica resulted in the separation of (±)-hormosirene [33]. With this method, the enantiomeric excess and the absolute configuration of Hormosira banksii, Durvillaea potatorum, Xiphophora chondrophylla, Dictyopteris membranacea, Haplospora globosa, and Xiphophora gladiata were determined, and none of these species produce enantiomeric pure hormosirene [33]. The highest e.e. of (1R,2R)-dictyopterene B (Figure 3) was found in H. banksii (83% e.e.), followed by D. potatorum (52% e.e.). This isomer was present in D. membranacea (71% e.e.), while H. globosa produced opposite enantiomer (1S,2S)-dictyopterene B (83% e.e.) [33].
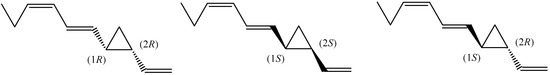
Figure 3.
The chemical structures of (1R,2R)-, (1S,2S)-, and (1S,2R)-dictyopterene B.
(1S,2S)-dictyopterene B enantiomer was discovered by the chiral HPLC analysis as a minor component in the female gametes and essential oils of Scytosiphonaceae (S. lomentaria, C. bullosa), Chordariaceae (A. japonicus), and Dictyotaceae (D. prolifera and D. undulata) families from Japan [39], with the highest enantiomeric composition (17.0%) in A. japonicus. The enantiomeric composition of (1R,2R)-dictyopterene B in female gametes and in essential oil was the following: S. lomentaria (97.0%), C. bullosa (96.5%), A. japonicus (83.0%), D. prolifera (95.0%), and D. undulata (96.5%). The optical purities of dictyopterene B in S. lomentaria, C. bullosa, D. prolifera, and D. undulata were much higher than that in A. japonicus. The amount of (1S,2S)-hormosirene (66% e.e.) was determined by a HPLC (Zorbax ODS) after the extraction (CH2Cl2-MeOH) from female gamete suspensions of A. japonicus [42].
(1S,2R)-dictyopterene B was found among volatile components in the essential oils from D. prolifera (15.6%, e.e. 98.9%), Dictyopteris latiscula (3.0%, e.e. 98.7%), D. undulata (2.3%, e.e. 90.1%), and Dictyopteris spp. (0.6%, e.e. 78.8%) [16].
2.4. Abundance of Dictyopterene C
Dictyopterene C is also known as dictyotene. Dictyopteris plagiogramma and Dictyopteris australis from the Hawaiian Islands contained dictyopterene C (10%) [15].
The steam distillation of D. prolifera provided dictyopterene B and C (their sum percentages were 12.3% to 59.9%) in the volatile fraction, but they were not resolved well on the DB-1 column [36]. The volatiles from D. prolifera from Japan were isolated by CLHS with a high abundance of dictyopterene B, with dictyopterene C in the protoplast (15.82%) and intact plant (16.00%) [43]. Dictyopterene C was found in the essential oils of D. prolifera (18.2%), Dictyopteris undulata (38.5%), and Dictyopteris sp. (10.5%) from Japan [19]. VOCs secreted by eggs from Dictyota diemensis were collected using the Grob–Hersch closed-loop extraction method, and dictyotene was found (31%) [37]. In Dictyopteris membranacea, dictyopterene C (74.1%) was identified by the closed-loop stripping technique [34].
The freshwater diatoms were cultivated in batch cultures [45]. The VOCs liberated by diatoms after initiation of the lipoxygenase reactions by NaCl treatment included unsaturated acyclic and alicyclic hydrocarbons. The VOCs stripped in a closed-loop stripping device were adsorbed on Tenax TA and analyzed using GC-MS by thermodesorption. Dictyopterene C was found in Gomphonema parvulum, Amphora veneta, and Phaeodactylum tricornutum [45].
Dasycladus vermicularis collected in Croatia in the headspace (determined by HS-SPME/GC-MS) contained dictyopterene C (8.65%; 9.34%) [57].
Dictyopterene C, as a minor constituent of marine algae, is presented in Table 1.
Enantiomeric Distribution of Dictyopterene C
Dictyopterene C (6-butylcyclohepta-1,4-diene) contains one chiral centre at C6. The fraction 2 from the chromatography of the essential oil of Dictyopteris spp. on silver nitrate-silica gel revealed dictyopterene C as (R)-butylcyclohepta-1,4-diene. The ozonolysis to (R)-butylsuccinic acid established the configuration at C-6 as R [10].
(R)-Dictyopterene C (Figure 4) was found in the essential oil of D. prolifera (5.8%; e.e. 97.1%), in the oil of D. latiscula (2.2%; e.e. 92.3%), the oil of D. undulata (2.0%; e.e. 94.2%), and the oil of Dictyopteris spp. (0.9%; e.e. 82.7%) [16].
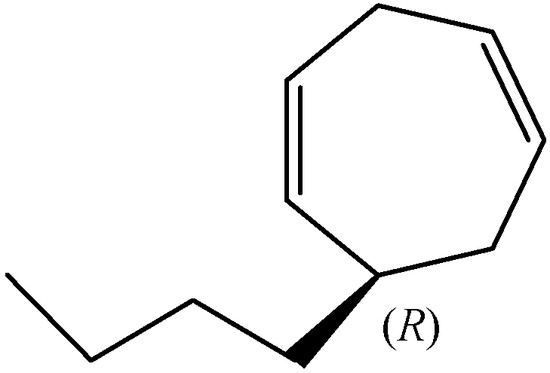
Figure 4.
The chemical structure of (R)-dictyopterene C.
(+)- and (−)-Dictyotene were equally effective in stimulating sperm attraction of D. diemensis, and the comparison of the chemotactic response to (+)- and (−)-dictyotene revealed an apparently greater effect for the (+) enantiomer in seawater [37].
2.5. Abundance of Dictyopterene D
It took ca. 100 years since the initial observation to prove the existence of a female-released attractant for male gametes [60]. Another 20 years were required to determine the chemical structure of the first brown algal pheromone [22] when gynogametes (female eggs) of the brown alga Ectocarpus siliculosus from more than 1000 culture dishes were extracted by a stream of air passing a cold trap for condensation. After bioassay-guided chromatographic fractionation, the infrared spectrum indicated the presence of cis-configuration of the unsaturated side chain, and the collected compound was identified by NMR and mass spectroscopy as 6-(1(Z)-butenyl)-cyclohepta-1,4-diene and was named Ectocarpus sirenine [22], but later was revised to ectocarpene (dictyopterene D). Later, it was found by the closed-loop stripping technique and GC-MS from E. siliculosus [26].
The most prominent fraction of low-molecular volatile secretion products of E. siliculosus gametes collected on an adsorbent bed of activated carbon in a Grob–Hersch closed-loop system was identified as ectocarpene as the main compound, the pheromone bouquet of E. siliculosus [35]. In Analipus japonicus dictyopterene D was found as the major constituent by the closed-loop stripping system [42].
Furthermore, it was determined that ectocarpene was a moderately active pheromone in comparison to pre-ectocarpene (cis-disubstituted bis-alkenylcyclopropane: cis-1-alyl-2-hex-1,3-dienylcyclopropane) with ca. 10,000-fold higher activity [44]. Pre-ectocarpene was extracted at 0 °C from a dense suspension of freshly released female gametes of E. siliculosus, as analyzed by HPLC [44]. However, the half-life for pre-ectorapene rearrangement is significantly longer than the time required for sexual encounter in algae [44], but rapid, temperature-dependent degradation (Cope rearrangement) of the cyclopropyl compound to ectocarpene was noticed. These results suggest that systems in which cycloheptadienes were identified as pheromones or release factors should be re-examined. In addition, when identification of the volatile organic compounds relies entirely on GC methods, the rearranged products (artefacts) of the genuine cyclopropyl precursors could be detected.
Later, different studies reported the occurrence of dictyopterene D in marine algae among the major constituents of the volatile fraction. Dictyopterene D was found in the essential oil of D. prolifera (10.4–30.4%) from Japan [36]. CLHS from D. prolifera from Japan determined dictyopterene D (4.62%; 8.91%) [43]. Dictyopterene D was found in the essential oils of D. prolifera (15.9%), D. undulata (40.7%), and Dictyopteris sp. (20.5%) from Japan [19]. Dictyopterene D was a major constituent (20.26%) in the essential oil of Sargassum linifolium [49]. Dictyota dichotoma contained dictyopterene D (0.07–8.22%) in the headspace (HS-SPME/GC-MS) [58].
Table 1 presents the species with minor dictyopterene D abundance.
Enantiomeric Distribution of Dictyopterene D
Dictyopterene D (6-(1(Z)-butenyl)-cyclohepta-1,4-diene) contains one chiral centre at C6. The absolute configuration of dictyopterene D was determined as (+)-(S)-6-(1(Z)-butenyl)-cyclohepta-1,4-diene (Figure 5) after silver nitrate-silica gel column chromatographic separation and NMR analysis of the essential oils from Dictyopteris plagiogramma and D. australis collected at the Hawaiian Islands [15]. Dictyopterene D was identical in all respects, including optical properties, with the male-attracting substance, ectocarpene, excreted by the female gametes of E. siliculosus [15].
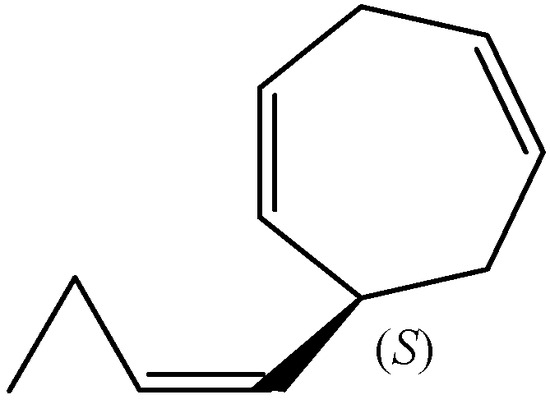
Figure 5.
The chemical structure of (S)-dictyopterene D.
Enantiomeric compositions of dictyopterene D, which cannot be separated by commercially available chiral GC or LC columns, was successfully determined by chiral GC analysis using Lipodex after side-chain reduction with hydrazine as a form of hydrogenated dictyopterene D as follows: (S)-dictyopterene D present with 2.2% (e.e. 99.9%) in the essential oil of D. prolifera, 0.1% (e.e. 99.1%) in the oil of D. latiscula, and 0.2% (e.e. 99.1%) in the oil of D. undulata [16].
2.6. Biosynthesis of Dictyopterenes A, B, C, and D
The structural similarities among C11-hydrocarbons suggest a common biosynthetic origin. In terrestrial plants, these compounds are generated from unsaturated C12 precursors [40] by three consecutive β-oxidations and a final oxidative decarboxylation of the resulting dodeca-3,6,9-trienoic acid [6].
In marine algae, C11-hydrocarbons are derived from the aliphatic terminus of C20 polyunsaturated fatty acids by the oxidative cleavage [40,61,62]. Female gametes of Ectocarpus silliculosus and Sphacelaria rigidula, as well as thalli of Giffordia mitchelliae, metabolized externally added [2Hn]icosanoic acids into the hydrocarbon pheromones ectocarpene, dictyotene, and finavarrene [40]. The series of C11H16 hydrocarbons originated from all-cis-5,8,11,14,17-eicosapentaenoic acid, and the C11H18 compound dictyotene was produced from all-cis-5,8,11,14-eicosatetraenoic acid (arachidonic acid). Externally supplied [2H8]arachidonic acid can be converted into labelled 6-butylcyclohepta-1,4-diene (dictyotene) in high yield by female gametes of E. siliculosus [40]. Ectocarpene should arise from the aliphatic terminus of the more unsaturated eicosapentaenoic acid that was established through the deuteration pattern with the [2H8]-C19 carboxylic acid, which was designed as a short-chain analogue of natural eicosapentaenoic acid [40].
According to the current biogenetic concept (Figure 6), 9-lipoxygenase is believed to activate, for example, the eicosanoid (e.g., eicosapentaenoic acid, 5) as 9-hydroperoxyicosa-(5Z,7E,11Z,14Z,17Z)-pentaenoic acid (9-HPEPE, 6). Subsequent cleavage of 9-HPEPE by a hydroperoxide lyase may generate C11 hydrocarbons from the aliphatic segment and 9-oxo-nonadienoic acid (9) from the fatty acid part. The same mechanistic implications, in conjunction with a different conformation of 9-HPEP at the active centre of the hydroperoxide lyase, could result in cis-disubstituted cyclopropane (7), identified as pre-ectocarpene in the E. siliculosus pheromone bouquet [44], with some amount of hormosirene (8). Furthermore, cis-cyclopropane (7) is thermolabile, and thus a subsequent spontaneous [3.3]-sigmatropic rearrangement (Cope rearrangement) is assumed to proceed [38,60] at room temperature via a cis–endo transition state to give (S)-ectocarpene (10). This hypothesis was verified by the synthesis and rearrangement reactions of thermally labile cis-divinylcyclopropane (7) and its analogues [38]. For instance, the Cope rearrangement of 7 occurred spontaneously at ambient temperatures to afford 10. Surprisingly, comparative biological assays of E. siliculosus revealed that the unstable cis-cyclopropane 7 was much more active than the stable cycloheptadiene (10). The above enzymes can principally act on all types of naturally occurring eicosanoids and, thus, produce the whole variety of cyclohepta-1,4-dienes and cyclopropanes which are currently known as gamete-releasing and/or gamete-attracting pheromones of brown algae.
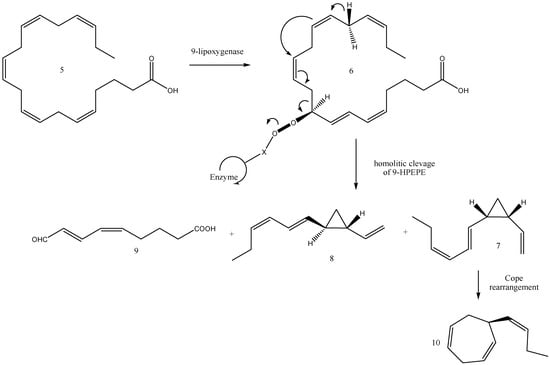
Figure 6.
Biosynthetic concept starting from eicosapentaenoic acid (5): 9-hydroperoxyicosa-(5Z,7E,11Z,14Z,17Z)-pentaenoic acid (9-HPEPE, 6); pre-ectocarpene (7), hormosirene (8), oxo-nonadienoic acid (9), (S)-ectocarpene (10).
An alternative biosynthetic pathway for (1S,2R)-dictyopterene A and (1S,2R)-dictyopterene B via the proposed intermediates (S)-dictyoprolenols (dictyoprolenol and neodictyoprolenol) was also investigated [16]. D. prolifera was incubated with synthetic (±)-dictyoprolenol and (±)-neodictyoprolenol as substrates, and the product content and enantiomeric composition were analyzed by GC. Added (S)-enantiomers in racemic substrates were selectively consumed, and a significant increase in dictyopterenes (1S,2R)-dictyopterene A and (S)-dictyopterene D was observed. Based on the obtained result, (S)-dictyoprolenol and (S)-neodictyoprolenol are assumed to be the possible biosynthetic intermediates for dictyopterenes. Hombeck et al. [46] reported a possible biosynthetic pathway of dictyopterene A in Gomphonema parvulum by oxidative cleavage of (9S)-hydroperoxyicosatetraenoic acid [(9S)-HPITE]. From (9S)-hydroperoxides such as (9S)-HPITE and (9S)-hydroperoxyicosapentaenoic acid [(9S)-HPIPE], dictyopterenes via dictyoprolenols and dictyoprolenes might be formed by stereo-specific shifting of the hydroxy group at C-9 to C-12 via a six-membered ring, as shown in Figure 7.
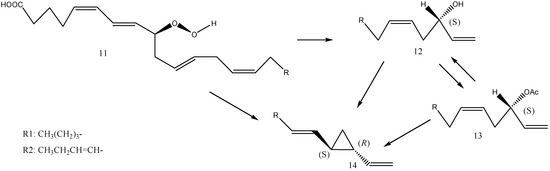
Figure 7.
Part of the hypothetical biosynthetic pathway of dictyopterene A (14, R = R1), dictyopterene B (14, R = R2): 11-(9S)-hydroperoxyicosatetraenoic acid (HPITE, R = R1), 11-(9S)-hydroperoxyicosapentaenoic acid (HPIPE, R = R2), 12-dictyoprenol (R = R1), 12-neodictyoprolenol (R = R2), 13-dictyoprolene A (R = R1), 13-dictyoprolene B (R = R2).
In addition, photochemical divinylcyclopropane trans-cis-isomerization provided strong evidence for possible in vivo photochemical steps of cis-trans-isomerization of diphenylcyclopropane during the trans-divinylcyclopropane-cycloheptadiene rearrangement [63].
3. Conclusions
The present review summarizes dictyopterenes A, B, C, and D found in marine algae for the first time, covering different aspects: (a) distribution among marine algae; (b) methods of isolation and identification; (c) absolute configuration and the presence of enantiomers; and (d) biosynthesis considerations. These dictyopterenes are important molecules as signalling substances (e.g., attractants, pheromones) and contribute to the ocean and beach smell.
Dictyopterenes A and B (hormosirene) are usually present in high amounts in Dictyopteris spp., but not exclusively. Dictyopterene A was found to be abundant in D. prolifera, D. undulata, D. latiscula, D. polypodioides, and D. membranacea. Dictyopterene B (hormosirene) was found as the major compound in D. plagiogramma, D. australis, H. banksii, D. potatorum, D. willana, D. antarctica, X. chondrophylla, X. gladiata, S. lomentaria, C. peregrina, and H. globosa. Dictyopterene C (dictyotene) was a major compound in D. undulata, D. prolifera, D. membranacea, G. parvulum, A. veneta, P. tricornutum, and D. vermicularis. Dictyopterene D was abundantly present in E. siliculosus, A. japonicus, D. prolifera, D. undulata, and S. linifolium. Since dictyopterenes A, B, C, and D were not found only in Dictyopteris spp., they cannot be considered specific chemical biomarkers of this genus.
The presence of exact enantiomers of dictyopterenes A, B, C, and D has been investigated rarely, and this gap can be researched further, particularly for dictyopterenes A and B that contain 2 chiral centres. (1S,2R)-Dictyopterene A was found in D. prolifera, D. latiscula, D. undulata, and Dictyopteris spp. The highest e.e. of (1R,2R)-dictyopterene B was found in H. banksii, followed by D. potatorum. This isomer was present in D. membranacea, while H. globosa produced opposite enantiomer (1S,2S)-dictyopterene B. (R)-dictyopterene C with high e.e. was identified in D. prolifera, D. latiscula, and D. undulata. (S)-dictyopterene D was present with e.e. 99.9% in D. prolifera, D. latiscula, and D. undulata.
The methods used for the isolation of dictyopterenes A, B, C, and D include distillation (e.g., hydrodistillation (HD), simultaneous distillation extraction (SDE), focused microwave-assisted hydrodistillation (FMAH)), extraction (e.g., solvent extraction, supercritical CO2 (SC-CO2) extraction), and headspace methods (e.g., closed-loop stripping technique, headspace solid phase microextraction), while their identification is made in the beginning by NMR and later by GC-MS or GC. For the determination of enantiomers, the chiral columns were used for GC or HPLC. There is potential to use the chiral analysis more abundantly for the determination of the presence of exact enantiomers in different marine algae.
In marine algae, C11-hydrocarbons are derived from the aliphatic terminus of C20 polyunsaturated fatty acids by oxidative cleavage, but the biosynthesis studies of dictyopterenes A, B, C, and D by labelled fatty acids are not so abundant. In contrast, C11 hydrocarbons from terrestrial plants are generated from unsaturated C12 precursors. According to the current biogenetic concept, 9-lipoxygenase is believed to activate, for example, the eicosanoid (9-HPEPE). Subsequent cleavage of 9-HPEPE by a hydroperoxide lyase may generate C11 hydrocarbons. Formed cis-cyclopropane is thermolabile, and thus a subsequent spontaneous Cope rearrangement is assumed to proceed at room temperature to produce (S)-ectocarpene. The above enzymes can principally act on all types of naturally occurring eicosanoids and, thus, produce the whole variety of cyclohepta-1,4-dienes and cyclopropanes. An alternative biosynthetic pathway for dictyopterene A and B via the proposed intermediates (S)-dictyoprolenols was considered by oxidative cleavage of hydroperoxyicosatetraenoic acid.
Author Contributions
Conceptualization, I.J.; methodology, I.J.; validation, I.J. and S.J.; formal analysis, D.B., A.G.L., I.J. and S.J.; investigation, D.B., A.G.L., I.J. and S.J.; data curation, D.B., A.G.L. and I.J.; writing—original draft preparation, I.J.; writing—review and editing, I.J., D.B., A.G.L. and S.J.; supervision, I.J.; project administration, I.J. All authors have read and agreed to the published version of the manuscript.
Funding
The Croatian Government and the European Union through the European Regional Development Fund—the Competitiveness and Cohesion Operational Programme (KK.01.1.1.01) funded The Scientific Centre of Excellence for Marine Bioprospecting-BioProCro and also the project Sustainable Bioprospecting of Organisms from the Adriatic Sea for Innovative Natural Products-BioProCro (PK.1.1.10.0001). The APC was funded by I.J.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
We would like to thank the Croatian Government and the European Union through the European Regional Development Fund—the Competitiveness and Cohesion Operational Programme (KK.01.1.1.01) for funding The Scientific Centre of Excellence for Marine Bioprospecting-BioProCro and also to the project Sustainable Bioprospecting of Organisms from the Adriatic Sea for Innovative Natural Products-BioProCro (PK.1.1.10.0001).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zuo, Z. Why algae release volatile organic compounds—The emission and roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef]
- Zuo, Z. Emission of cyanobacterial volatile organic compounds and their roles in blooms. Front. Microbiol. 2023, 14, 1097712. [Google Scholar] [CrossRef]
- Rinnan, R.; Steinke, M.; McGenity, T.; Loreto, F. Plant volatiles in extreme terrestrial and marine environments. Plant Cell Environ. 2014, 37, 1776–1789. [Google Scholar] [CrossRef]
- Oigman, S.S.; Fernandes, Y.F.M.; Teles, D.; Maia, L.F.; Epifanio, R.A.; Rezende, C.M. Brazilian gorgonians: A source of odoriferous compounds? Rev. Bras. Farmacogn. 2015, 25, 612–618. [Google Scholar] [CrossRef][Green Version]
- Boland, W. The chemistry of gamete attraction: Chemical structures, biosynthesis, and (a)biotic degradation of algal pheromones. Proc. Natl. Acad. Sci. USA 1995, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E. Volatile compounds from marine algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Nizamuddin, M.; Saifullah, S.M. Studies on marine algae of Karachi: Dictyopteris Lamouroux. Bot. Mar. 1966, 10, 169–179. [Google Scholar] [CrossRef]
- Silberfeld, T.; Rousseau, F.; Reviers, B. An updated classification of brown algae (Ochrophyta, Phaeophyceae). Cryptogam. Algol. 2014, 35, 117–156. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr. Isolation and structure determination of dictyopterenes C’ and D’ from Dictyopteris. Stereospecificity in the cope rearrangement of dictyopterenes A and B. J. Am. Chem. Soc. 1971, 93, 3087–3088. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Saber, H.; Attia, E.Z.; Abdelmohsen, U.R. The natural products and pharmacological biodiversity of brown algae from the genus Dictyopteris. J. Mex. Chem. Soc. 2022, 66, 154–180. [Google Scholar] [CrossRef]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An overview of odoriferous marine seaweeds of the Dictyopteris genus: Insights into their chemical diversity, biological potential and ecological roles. Rev. Bras. Farmacogn. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kodama, K.; Hatanaka, A.; Matsui, K. Volatile compounds from Japanese marine brown-algae. ACS Symp. Ser. Am. Chem. Soc. 1993, 525, 103–120. [Google Scholar] [CrossRef]
- Jaenicke, L.; Boland, W. Signal Substances and their reception in the sexual cycle of marine brown algae. Angew. Chem. 1982, 21, 643–653. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr.; Mistysyn, J. Odoriferous C11 hydrocarbons from Hawaiian Dictyopteris. J. Org. Chem. 1974, 39, 2201–2207. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Akakabe, Y.; Matsui, K.; Shimizu, H.; Kajiwara, T. Neodictyoprolenol and dictyoprolenol, the possible biosynthetic intermediates of dictyopterenes, in the Japanese brown algae Dictyopteris. Z. Naturforsch. C 2001, 56, 6–12. [Google Scholar] [CrossRef][Green Version]
- Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of various extraction methods for identification and determination of volatile metabolites from brown alga Dictyopteris membranacea. J. Chromatogr. A 2007, 1143, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.G.; Kawai, H.; Stache, B.; Folster, E.; Boland, W. Sexual pheromones and gamete chemotaxis in Analipus japonicus (Phaeophyeae). Experientia 1990, 46, 534–536. [Google Scholar] [CrossRef]
- Kajiwara, T.; Akakabe, Y.; Matsui, K.; Kodama, K.; Koga, H.; Nagakura, T. (+)-(3S,4S)-3-butyl-4-vinylcyclopentene in brown algae of the genus Dictyopteris. Phytochemistry 1997, 45, 529–532. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr.; Doty, M.S. Dictyopterene A. An odoriferous constituent from algae of the genus Dictyopteris. Tetrahedron Lett. 1968, 9, 4787–4790. [Google Scholar] [CrossRef]
- Pettus, J.A., Jr.; Moore, R.E. Isolation and structure determination of an undeca-1,3,5,8-tetraene and dictyopterene B from algae of the genus Dictyopteris. J. Chem. Soc. D 1970, 17, 1093–1094. [Google Scholar] [CrossRef]
- Müller, D.G.; Jaenicke, L.; Donike, M.; Akintobi, T. Sex attractant in a brown alga: Chemical structure. Science 1971, 171, 815–817. [Google Scholar] [CrossRef]
- Yamada, K.; Tan, H.; Tatematsu, H. Isolation and structure of dictyoprolene, a possible precursor of various undecanes in brown algae from Dictyopteris prolifera. J. Chem. Soc. Chem. Commun. 1979, 13, 572–573. [Google Scholar] [CrossRef]
- Müller, D.; Gassmann, G.; Lüning, K. Isolation of a spermatozoid-releasing and -attracting substance from female gametophytes of Laminaria digitata. Nature 1979, 279, 430–431. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kodama, K.; Hatanaka, A. Male-attracting substance in marine brown algae the genus Dictyopteris. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 771–775. [Google Scholar] [CrossRef][Green Version]
- Müller, D.G.; Gassmann, G. Sexual hormone specificity in Ectocarpus and Laminaria (Phaeophyceae). Naturwissenschaften 1980, 67, 462–463. [Google Scholar] [CrossRef]
- Müller, D.G.; Gassmann, G.; Boland, W.; Marner, F.; Jaenicke, L. Dictyota dichotoma (Phaeophyceae): Identification of the sperm attractant. Science 1981, 212, 1040–1041. [Google Scholar] [CrossRef]
- Müller, D.G.; Clayton, M.N.; Gassmann, G.; Boland, W.; Marner, F.-J.; Jaenicke, L. The sperm attractant of Hormosira banksii (Phaeophyceae, Fucales), a seaweed common to Australia and New Zealand. Experientia 1984, 40, 211–212. [Google Scholar] [CrossRef]
- Müller, D.G.; Clayton, M.N.; Gassmann, G.; Boland, W.; Marner, F.J.; Schotten, T.; Jaenicke, L. Cystophorene and hormosirene, sperm attractants in Australian brown algae. Naturwissenschaften 1985, 72, 97–99. [Google Scholar] [CrossRef]
- Kuhlenkamp, R.; Müller, D.G. Culture studies on the life history of Haplospora globosa and Tilopteris mertensii (Tilopteridales, Phaeophyceae). Br. Phycol. J. 1985, 20, 301–312. [Google Scholar] [CrossRef]
- Yamada, K.; Tan, H.; Tatematsu, H.; Ojika, M. Dictyoprolene and neodictyoprolene, two new odoriferous compounds from the brown alga Dictyopteris prolifera: Structures and synthesis. Tetrahedron 1986, 42, 3775–3780. [Google Scholar] [CrossRef]
- Derenbach, J.B.; Pesando, D. Investigations into a small fraction of volatile hydrocarbons: III. Two diatom cultures produce ectocarpene, a pheromone of brown algae. Mar. Chem. 1986, 19, 337–341. [Google Scholar] [CrossRef]
- Boland, W.; Flegel, U.; Jordt, G.; Müller, D.G. Absolute configuration and enantiomer composition of hormosirene. Naturwissenschaften 1987, 74, 448–449. [Google Scholar] [CrossRef]
- Boland, W.; Müller, D.G. On the odor of the Mediterranean seaweed Dictyopteris membranacea; New C11 hydrocarbon from marine brown algae—III. Tetrahedron Lett. 1987, 28, 307–310. [Google Scholar] [CrossRef]
- Müller, D.G.; Schmidt, C.E. Qualitative and quantitative determination of pheromone secretion in female gametes of Ectocarpus siliculosus (Phaeophyceae). Biol. Chem. Hoppe-Seyler 1988, 369, 647–653. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Tanaka, Y.; Kawai, T.; Ishihara, M.; Tsuneya, T.; Fujimura, T. Volatile constituents from marine brown algae of Japanese Dictyopteris. Phytochemistry 1989, 28, 636–639. [Google Scholar] [CrossRef]
- Phillips, J.A.; Clayton, M.N.; Maier, I.; Boland, W.; Müller, D.G. Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta). Phycologia 1990, 29, 367–379. [Google Scholar] [CrossRef]
- Keitel, J.; Fischer-Lui, I.; Boland, W.; Müller, D.G. Novel C9 and C11 hydrocarbons from the brown alga Cutleria multifida; Sigmatropic and electrocyclic reactions in nature. Part VI. Helvetica 1990, 73, 2101–2112. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Kodama, K.; Ochi, S.; Fujimura, T. Dictyopterenes from three Japanese brown algae. Phytochemistry 1991, 30, 1805–1807. [Google Scholar] [CrossRef]
- Stratmann, K.; Boland, W.; Müller, D.G. Pheromones of marine brown-algae—A new branch of the eicosanoid metabolism. Angew. Chem. Int. Ed. Engl. 1992, 31, 1246–1248. [Google Scholar] [CrossRef]
- Maier, I.; Clayton, M.N. Quantitative evaluation of erotactin secretion in eggs of Hormosira banksii (Fucales, Phaeophyceae). Bot. Acta 1993, 106, 344–349. [Google Scholar] [CrossRef]
- Kodama, K.; Matsui, K.; Hatanaka, A.; Ishihara, M.; Kajiwara, T. A female gamete-characteristic (3Z,6Z,9Z)-dodecatrienoic acid from Analipus japonicus. Phytochemistry 1993, 33, 1039–1042. [Google Scholar] [CrossRef]
- Fujimura, T.; Kawai, T.; Kajiwara, T.; Ishida, Y. Volatile components in protoplasts isolated from the marine brown alga Dictyopteris prolifera (Dictyotales). Plant Tissue Cult. Lett. 1994, 11, 34–39. [Google Scholar] [CrossRef]
- Boland, W.; Pohnert, G.; Maier, I. Pericyclic reactions in nature: Spontaneous Cope rearrangement inactivates algae pheromones. Angew. Chem. Int. Ed. Engl. 1995, 34, 1602–1604. [Google Scholar] [CrossRef]
- Wendel, T.; Jüttner, F. Lipoxygenase-mediated formation of hydrocarbons and unsaturated aldehydes in freshwater diatoms. Phytochemistry 1996, 41, 1445–1449. [Google Scholar] [CrossRef]
- Hombeck, M.; Pohnert, G.; Boland, W. Biosynthesis of dictyopterene A: Stereoselectivity of a lipoxygenase/hydroperoxide lyase from Gomphonema parvulum (Bacillariophyceae). Chem. Commun. 1999, 3, 243–244. [Google Scholar] [CrossRef]
- Hattab, M.E.; Culioli, G.; Ortalo-Magné, A.; Piovetti, L.; Chitour, S.E. Isolation of the volatile compounds from the brown alga Dictyopteris membranacea by focused microwave-assisted hydrodistillation. J. Essent. Oil Res. 2002, 14, 422–424. [Google Scholar] [CrossRef]
- Awad, N.E.; Motawe, H.M.; Selim, M.A.; Matloub, A.A. Volatile constituents of the brown algae Padina pavonia (L.) Gaill. and Hydroclathrus clathratus (C. Agardh) Howe and their antimicrobial activity. Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 12–15. [Google Scholar]
- Matloub, A.A.; Awad, N.E. Phycochemistry of some spp. and their cytotoxic and antimicrobial activities. Egypt. Pharm. J. 2012, 11, 99–108. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- Riad, N.; Zahi, M.R.; Trovato, E.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Chemical screening and antibacterial activity of essential oil and volatile fraction of Dictyopteris polypodioides. Microchem. J. 2020, 152, 104415. [Google Scholar] [CrossRef]
- Wang, P.; Chen, J.; Chen, L.; Shi, L.; Liu, H. Characteristic volatile composition of seven seaweeds from the Yellow sea of China. Mar. Drugs 2021, 19, 192. [Google Scholar] [CrossRef]
- Riad, N.; Bouzidi, N.; Zahi, M.R.; Touafek, O.; Daghbouche, Y.; Piovetti, L.; El Hattaba, M. Extraction of the volatile oils of Dictyopteris membranacea Batters 1902 by focused microwave-assisted hydrodistillation and supercritical carbon dioxide: Empirical kinetic modelling approach, apparent solubility and rate constants. Chem. Biochem. Eng. Q. 2021, 35, 319–331. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Aladić, K.; Jokić, S.; Živković, D.; Jerković, I. Chemical profiles of less-volatile organic compounds from the Adriatic Sea macroalgae obtained by supercritical CO2 extraction. Croat. J. Food Sci. Technol. 2022, 14, 224–234. [Google Scholar] [CrossRef]
- Čagalj, M.; Radman, S.; Šimat, V.; Jerković, I. Detailed chemical prospecting of volatile organic compounds variations from Adriatic macroalga Halopteris scoparia. Molecules 2022, 27, 4997. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. In vivo and in vitro antioxidant activity of less polar fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the chemical composition of fractions and macroalga volatilome. Pharmaceuticals 2022, 15, 743. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal variability of volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal monitoring of volatiles and antioxidant activity of brown alga Cladostephus spongiosus. Mar. Drugs 2023, 21, 415. [Google Scholar] [CrossRef]
- Cook, A.H.; Elvidge, J.A.; Bentley, R. Fertilization in the Fucaceae: Investigations on the nature of the chemotactic substance produced by eggs of Fucus serratus and F. vesiculosus. Proc. R. Soc. B 1951, 138, 97–114. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. Pericyclic reactions in nature: Synthesis and Cope rearrangement of thermolabile bis-alkenylcyclopropanes from female gametes of marine brown algae (Phaeophyceae). Tetrahedron 1997, 53, 13681–13694. [Google Scholar] [CrossRef]
- Rui, F.; Boland, W. Algal pheromone biosynthesis: Stereochemical analysis and mechanistic implications in gametes of Ectocarpus siliculosus. J. Org. Chem. 2010, 75, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Pickenhagen, W.; Niif, F.; Ohloff, G.; Muller, P.; Perlberger, J.-C. Thermal and photochemical rearrangements of divinylcyclopropanes to cycloheptadienes.—A model for the biosynthesis of the cycloheptadiene derivatives found in a seaweed (Dictyopteris). Helv. Chim. Acta 1973, 56, 1868–1874. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).