Dictyopterenes A, B, C, and D from Marine Algae
Abstract
1. Introduction
2. Results
2.1. Distribution of Dictyopterenes A, B, C, and D in Marine Algae
2.2. Abundance of Dictyopterene A
Enantiomeric Distribution of Dictyopterene A
2.3. Abundance of Dictyopterene B
Enantiomeric Distribution of Dictyopterene B
2.4. Abundance of Dictyopterene C
Enantiomeric Distribution of Dictyopterene C
2.5. Abundance of Dictyopterene D
Enantiomeric Distribution of Dictyopterene D
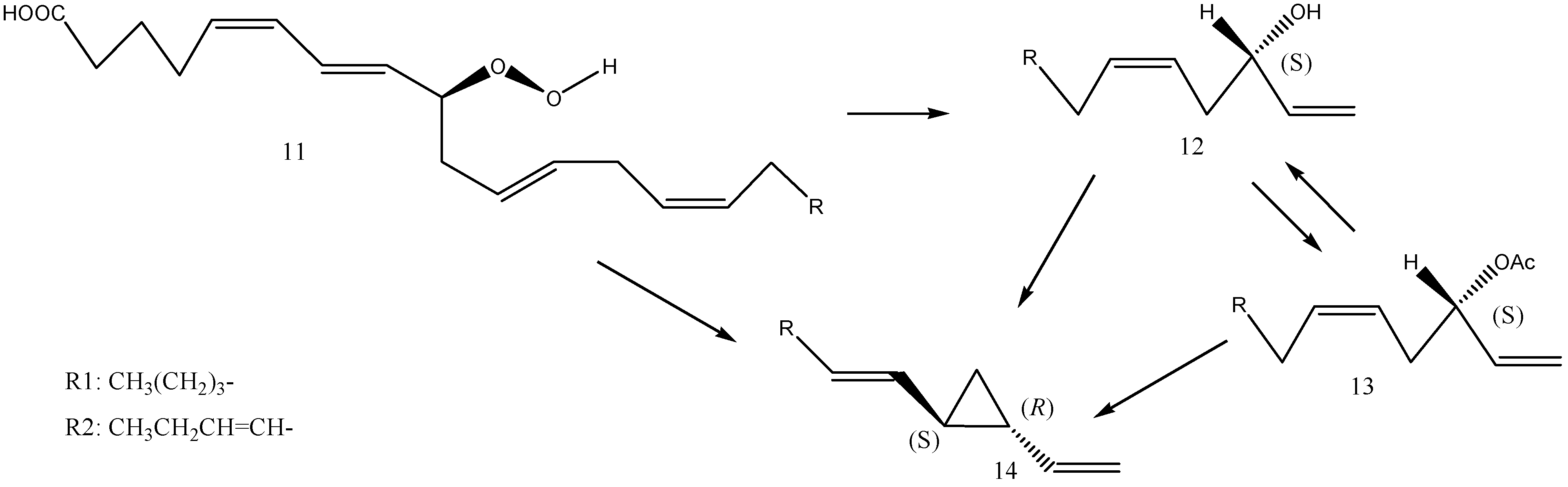
2.6. Biosynthesis of Dictyopterenes A, B, C, and D
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zuo, Z. Why algae release volatile organic compounds—The emission and roles. Front. Microbiol. 2019, 10, 491. [Google Scholar] [CrossRef]
- Paul, C.; Pohnert, G. Production and role of volatile halogenated compounds from marine algae. Nat. Prod. Rep. 2011, 28, 186–195. [Google Scholar] [CrossRef]
- Zuo, Z. Emission of cyanobacterial volatile organic compounds and their roles in blooms. Front. Microbiol. 2023, 14, 1097712. [Google Scholar] [CrossRef]
- Rinnan, R.; Steinke, M.; McGenity, T.; Loreto, F. Plant volatiles in extreme terrestrial and marine environments. Plant Cell Environ. 2014, 37, 1776–1789. [Google Scholar] [CrossRef]
- Oigman, S.S.; Fernandes, Y.F.M.; Teles, D.; Maia, L.F.; Epifanio, R.A.; Rezende, C.M. Brazilian gorgonians: A source of odoriferous compounds? Rev. Bras. Farmacogn. 2015, 25, 612–618. [Google Scholar] [CrossRef]
- Boland, W. The chemistry of gamete attraction: Chemical structures, biosynthesis, and (a)biotic degradation of algal pheromones. Proc. Natl. Acad. Sci. USA 1995, 92, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Moore, R.E. Volatile compounds from marine algae. Acc. Chem. Res. 1977, 10, 40–47. [Google Scholar] [CrossRef]
- Nizamuddin, M.; Saifullah, S.M. Studies on marine algae of Karachi: Dictyopteris Lamouroux. Bot. Mar. 1966, 10, 169–179. [Google Scholar] [CrossRef]
- Silberfeld, T.; Rousseau, F.; Reviers, B. An updated classification of brown algae (Ochrophyta, Phaeophyceae). Cryptogam. Algol. 2014, 35, 117–156. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr. Isolation and structure determination of dictyopterenes C’ and D’ from Dictyopteris. Stereospecificity in the cope rearrangement of dictyopterenes A and B. J. Am. Chem. Soc. 1971, 93, 3087–3088. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.; Saber, H.; Attia, E.Z.; Abdelmohsen, U.R. The natural products and pharmacological biodiversity of brown algae from the genus Dictyopteris. J. Mex. Chem. Soc. 2022, 66, 154–180. [Google Scholar] [CrossRef]
- Zatelli, G.A.; Philippus, A.C.; Falkenberg, M. An overview of odoriferous marine seaweeds of the Dictyopteris genus: Insights into their chemical diversity, biological potential and ecological roles. Rev. Bras. Farmacogn. 2018, 28, 243–260. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kodama, K.; Hatanaka, A.; Matsui, K. Volatile compounds from Japanese marine brown-algae. ACS Symp. Ser. Am. Chem. Soc. 1993, 525, 103–120. [Google Scholar] [CrossRef]
- Jaenicke, L.; Boland, W. Signal Substances and their reception in the sexual cycle of marine brown algae. Angew. Chem. 1982, 21, 643–653. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr.; Mistysyn, J. Odoriferous C11 hydrocarbons from Hawaiian Dictyopteris. J. Org. Chem. 1974, 39, 2201–2207. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Akakabe, Y.; Matsui, K.; Shimizu, H.; Kajiwara, T. Neodictyoprolenol and dictyoprolenol, the possible biosynthetic intermediates of dictyopterenes, in the Japanese brown algae Dictyopteris. Z. Naturforsch. C 2001, 56, 6–12. [Google Scholar] [CrossRef]
- Hattab, M.; Culioli, G.; Piovetti, L.; Chitour, S.E.; Valls, R. Comparison of various extraction methods for identification and determination of volatile metabolites from brown alga Dictyopteris membranacea. J. Chromatogr. A 2007, 1143, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Müller, D.G.; Kawai, H.; Stache, B.; Folster, E.; Boland, W. Sexual pheromones and gamete chemotaxis in Analipus japonicus (Phaeophyeae). Experientia 1990, 46, 534–536. [Google Scholar] [CrossRef]
- Kajiwara, T.; Akakabe, Y.; Matsui, K.; Kodama, K.; Koga, H.; Nagakura, T. (+)-(3S,4S)-3-butyl-4-vinylcyclopentene in brown algae of the genus Dictyopteris. Phytochemistry 1997, 45, 529–532. [Google Scholar] [CrossRef]
- Moore, R.E.; Pettus, J.A., Jr.; Doty, M.S. Dictyopterene A. An odoriferous constituent from algae of the genus Dictyopteris. Tetrahedron Lett. 1968, 9, 4787–4790. [Google Scholar] [CrossRef]
- Pettus, J.A., Jr.; Moore, R.E. Isolation and structure determination of an undeca-1,3,5,8-tetraene and dictyopterene B from algae of the genus Dictyopteris. J. Chem. Soc. D 1970, 17, 1093–1094. [Google Scholar] [CrossRef]
- Müller, D.G.; Jaenicke, L.; Donike, M.; Akintobi, T. Sex attractant in a brown alga: Chemical structure. Science 1971, 171, 815–817. [Google Scholar] [CrossRef]
- Yamada, K.; Tan, H.; Tatematsu, H. Isolation and structure of dictyoprolene, a possible precursor of various undecanes in brown algae from Dictyopteris prolifera. J. Chem. Soc. Chem. Commun. 1979, 13, 572–573. [Google Scholar] [CrossRef]
- Müller, D.; Gassmann, G.; Lüning, K. Isolation of a spermatozoid-releasing and -attracting substance from female gametophytes of Laminaria digitata. Nature 1979, 279, 430–431. [Google Scholar] [CrossRef]
- Kajiwara, T.; Kodama, K.; Hatanaka, A. Male-attracting substance in marine brown algae the genus Dictyopteris. Bull. Jpn. Soc. Sci. Fish. 1980, 46, 771–775. [Google Scholar] [CrossRef]
- Müller, D.G.; Gassmann, G. Sexual hormone specificity in Ectocarpus and Laminaria (Phaeophyceae). Naturwissenschaften 1980, 67, 462–463. [Google Scholar] [CrossRef]
- Müller, D.G.; Gassmann, G.; Boland, W.; Marner, F.; Jaenicke, L. Dictyota dichotoma (Phaeophyceae): Identification of the sperm attractant. Science 1981, 212, 1040–1041. [Google Scholar] [CrossRef]
- Müller, D.G.; Clayton, M.N.; Gassmann, G.; Boland, W.; Marner, F.-J.; Jaenicke, L. The sperm attractant of Hormosira banksii (Phaeophyceae, Fucales), a seaweed common to Australia and New Zealand. Experientia 1984, 40, 211–212. [Google Scholar] [CrossRef]
- Müller, D.G.; Clayton, M.N.; Gassmann, G.; Boland, W.; Marner, F.J.; Schotten, T.; Jaenicke, L. Cystophorene and hormosirene, sperm attractants in Australian brown algae. Naturwissenschaften 1985, 72, 97–99. [Google Scholar] [CrossRef]
- Kuhlenkamp, R.; Müller, D.G. Culture studies on the life history of Haplospora globosa and Tilopteris mertensii (Tilopteridales, Phaeophyceae). Br. Phycol. J. 1985, 20, 301–312. [Google Scholar] [CrossRef]
- Yamada, K.; Tan, H.; Tatematsu, H.; Ojika, M. Dictyoprolene and neodictyoprolene, two new odoriferous compounds from the brown alga Dictyopteris prolifera: Structures and synthesis. Tetrahedron 1986, 42, 3775–3780. [Google Scholar] [CrossRef]
- Derenbach, J.B.; Pesando, D. Investigations into a small fraction of volatile hydrocarbons: III. Two diatom cultures produce ectocarpene, a pheromone of brown algae. Mar. Chem. 1986, 19, 337–341. [Google Scholar] [CrossRef]
- Boland, W.; Flegel, U.; Jordt, G.; Müller, D.G. Absolute configuration and enantiomer composition of hormosirene. Naturwissenschaften 1987, 74, 448–449. [Google Scholar] [CrossRef]
- Boland, W.; Müller, D.G. On the odor of the Mediterranean seaweed Dictyopteris membranacea; New C11 hydrocarbon from marine brown algae—III. Tetrahedron Lett. 1987, 28, 307–310. [Google Scholar] [CrossRef]
- Müller, D.G.; Schmidt, C.E. Qualitative and quantitative determination of pheromone secretion in female gametes of Ectocarpus siliculosus (Phaeophyceae). Biol. Chem. Hoppe-Seyler 1988, 369, 647–653. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Tanaka, Y.; Kawai, T.; Ishihara, M.; Tsuneya, T.; Fujimura, T. Volatile constituents from marine brown algae of Japanese Dictyopteris. Phytochemistry 1989, 28, 636–639. [Google Scholar] [CrossRef]
- Phillips, J.A.; Clayton, M.N.; Maier, I.; Boland, W.; Müller, D.G. Sexual reproduction in Dictyota diemensis (Dictyotales, Phaeophyta). Phycologia 1990, 29, 367–379. [Google Scholar] [CrossRef]
- Keitel, J.; Fischer-Lui, I.; Boland, W.; Müller, D.G. Novel C9 and C11 hydrocarbons from the brown alga Cutleria multifida; Sigmatropic and electrocyclic reactions in nature. Part VI. Helvetica 1990, 73, 2101–2112. [Google Scholar] [CrossRef]
- Kajiwara, T.; Hatanaka, A.; Kodama, K.; Ochi, S.; Fujimura, T. Dictyopterenes from three Japanese brown algae. Phytochemistry 1991, 30, 1805–1807. [Google Scholar] [CrossRef]
- Stratmann, K.; Boland, W.; Müller, D.G. Pheromones of marine brown-algae—A new branch of the eicosanoid metabolism. Angew. Chem. Int. Ed. Engl. 1992, 31, 1246–1248. [Google Scholar] [CrossRef]
- Maier, I.; Clayton, M.N. Quantitative evaluation of erotactin secretion in eggs of Hormosira banksii (Fucales, Phaeophyceae). Bot. Acta 1993, 106, 344–349. [Google Scholar] [CrossRef]
- Kodama, K.; Matsui, K.; Hatanaka, A.; Ishihara, M.; Kajiwara, T. A female gamete-characteristic (3Z,6Z,9Z)-dodecatrienoic acid from Analipus japonicus. Phytochemistry 1993, 33, 1039–1042. [Google Scholar] [CrossRef]
- Fujimura, T.; Kawai, T.; Kajiwara, T.; Ishida, Y. Volatile components in protoplasts isolated from the marine brown alga Dictyopteris prolifera (Dictyotales). Plant Tissue Cult. Lett. 1994, 11, 34–39. [Google Scholar] [CrossRef]
- Boland, W.; Pohnert, G.; Maier, I. Pericyclic reactions in nature: Spontaneous Cope rearrangement inactivates algae pheromones. Angew. Chem. Int. Ed. Engl. 1995, 34, 1602–1604. [Google Scholar] [CrossRef]
- Wendel, T.; Jüttner, F. Lipoxygenase-mediated formation of hydrocarbons and unsaturated aldehydes in freshwater diatoms. Phytochemistry 1996, 41, 1445–1449. [Google Scholar] [CrossRef]
- Hombeck, M.; Pohnert, G.; Boland, W. Biosynthesis of dictyopterene A: Stereoselectivity of a lipoxygenase/hydroperoxide lyase from Gomphonema parvulum (Bacillariophyceae). Chem. Commun. 1999, 3, 243–244. [Google Scholar] [CrossRef]
- Hattab, M.E.; Culioli, G.; Ortalo-Magné, A.; Piovetti, L.; Chitour, S.E. Isolation of the volatile compounds from the brown alga Dictyopteris membranacea by focused microwave-assisted hydrodistillation. J. Essent. Oil Res. 2002, 14, 422–424. [Google Scholar] [CrossRef]
- Awad, N.E.; Motawe, H.M.; Selim, M.A.; Matloub, A.A. Volatile constituents of the brown algae Padina pavonia (L.) Gaill. and Hydroclathrus clathratus (C. Agardh) Howe and their antimicrobial activity. Med. Aromat. Plant Sci. Biotechnol. 2009, 3, 12–15. [Google Scholar]
- Matloub, A.A.; Awad, N.E. Phycochemistry of some spp. and their cytotoxic and antimicrobial activities. Egypt. Pharm. J. 2012, 11, 99–108. [Google Scholar] [CrossRef]
- Jerković, I.; Marijanović, Z.; Roje, M.; Kuś, P.M.; Jokić, S.; Čož-Rakovac, R. Phytochemical study of the headspace volatile organic compounds of fresh algae and seagrass from the Adriatic Sea (single point collection). PLoS ONE 2018, 13, e0196462. [Google Scholar] [CrossRef]
- Jerković, I.; Kranjac, M.; Marijanović, Z.; Roje, M.; Jokić, S. Chemical diversity of headspace and volatile oil composition of two brown algae (Taonia atomaria and Padina pavonica) from the Adriatic Sea. Molecules 2019, 24, 495. [Google Scholar] [CrossRef]
- Riad, N.; Zahi, M.R.; Trovato, E.; Bouzidi, N.; Daghbouche, Y.; Utczás, M.; Mondello, L.; El Hattab, M. Chemical screening and antibacterial activity of essential oil and volatile fraction of Dictyopteris polypodioides. Microchem. J. 2020, 152, 104415. [Google Scholar] [CrossRef]
- Wang, P.; Chen, J.; Chen, L.; Shi, L.; Liu, H. Characteristic volatile composition of seven seaweeds from the Yellow sea of China. Mar. Drugs 2021, 19, 192. [Google Scholar] [CrossRef]
- Riad, N.; Bouzidi, N.; Zahi, M.R.; Touafek, O.; Daghbouche, Y.; Piovetti, L.; El Hattaba, M. Extraction of the volatile oils of Dictyopteris membranacea Batters 1902 by focused microwave-assisted hydrodistillation and supercritical carbon dioxide: Empirical kinetic modelling approach, apparent solubility and rate constants. Chem. Biochem. Eng. Q. 2021, 35, 319–331. [Google Scholar] [CrossRef]
- Cikoš, A.M.; Aladić, K.; Jokić, S.; Živković, D.; Jerković, I. Chemical profiles of less-volatile organic compounds from the Adriatic Sea macroalgae obtained by supercritical CO2 extraction. Croat. J. Food Sci. Technol. 2022, 14, 224–234. [Google Scholar] [CrossRef]
- Čagalj, M.; Radman, S.; Šimat, V.; Jerković, I. Detailed chemical prospecting of volatile organic compounds variations from Adriatic macroalga Halopteris scoparia. Molecules 2022, 27, 4997. [Google Scholar] [CrossRef]
- Radman, S.; Cikoš, A.M.; Babić, S.; Čižmek, L.; Čož-Rakovac, R.; Jokić, S.; Jerković, I. In vivo and in vitro antioxidant activity of less polar fractions of Dasycladus vermicularis (Scopoli) Krasser 1898 and the chemical composition of fractions and macroalga volatilome. Pharmaceuticals 2022, 15, 743. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal variability of volatilome from Dictyota dichotoma. Molecules 2022, 27, 3012. [Google Scholar] [CrossRef] [PubMed]
- Radman, S.; Čagalj, M.; Šimat, V.; Jerković, I. Seasonal monitoring of volatiles and antioxidant activity of brown alga Cladostephus spongiosus. Mar. Drugs 2023, 21, 415. [Google Scholar] [CrossRef]
- Cook, A.H.; Elvidge, J.A.; Bentley, R. Fertilization in the Fucaceae: Investigations on the nature of the chemotactic substance produced by eggs of Fucus serratus and F. vesiculosus. Proc. R. Soc. B 1951, 138, 97–114. [Google Scholar] [CrossRef]
- Pohnert, G.; Boland, W. Pericyclic reactions in nature: Synthesis and Cope rearrangement of thermolabile bis-alkenylcyclopropanes from female gametes of marine brown algae (Phaeophyceae). Tetrahedron 1997, 53, 13681–13694. [Google Scholar] [CrossRef]
- Rui, F.; Boland, W. Algal pheromone biosynthesis: Stereochemical analysis and mechanistic implications in gametes of Ectocarpus siliculosus. J. Org. Chem. 2010, 75, 3958–3964. [Google Scholar] [CrossRef] [PubMed]
- Pickenhagen, W.; Niif, F.; Ohloff, G.; Muller, P.; Perlberger, J.-C. Thermal and photochemical rearrangements of divinylcyclopropanes to cycloheptadienes.—A model for the biosynthesis of the cycloheptadiene derivatives found in a seaweed (Dictyopteris). Helv. Chim. Acta 1973, 56, 1868–1874. [Google Scholar] [CrossRef]
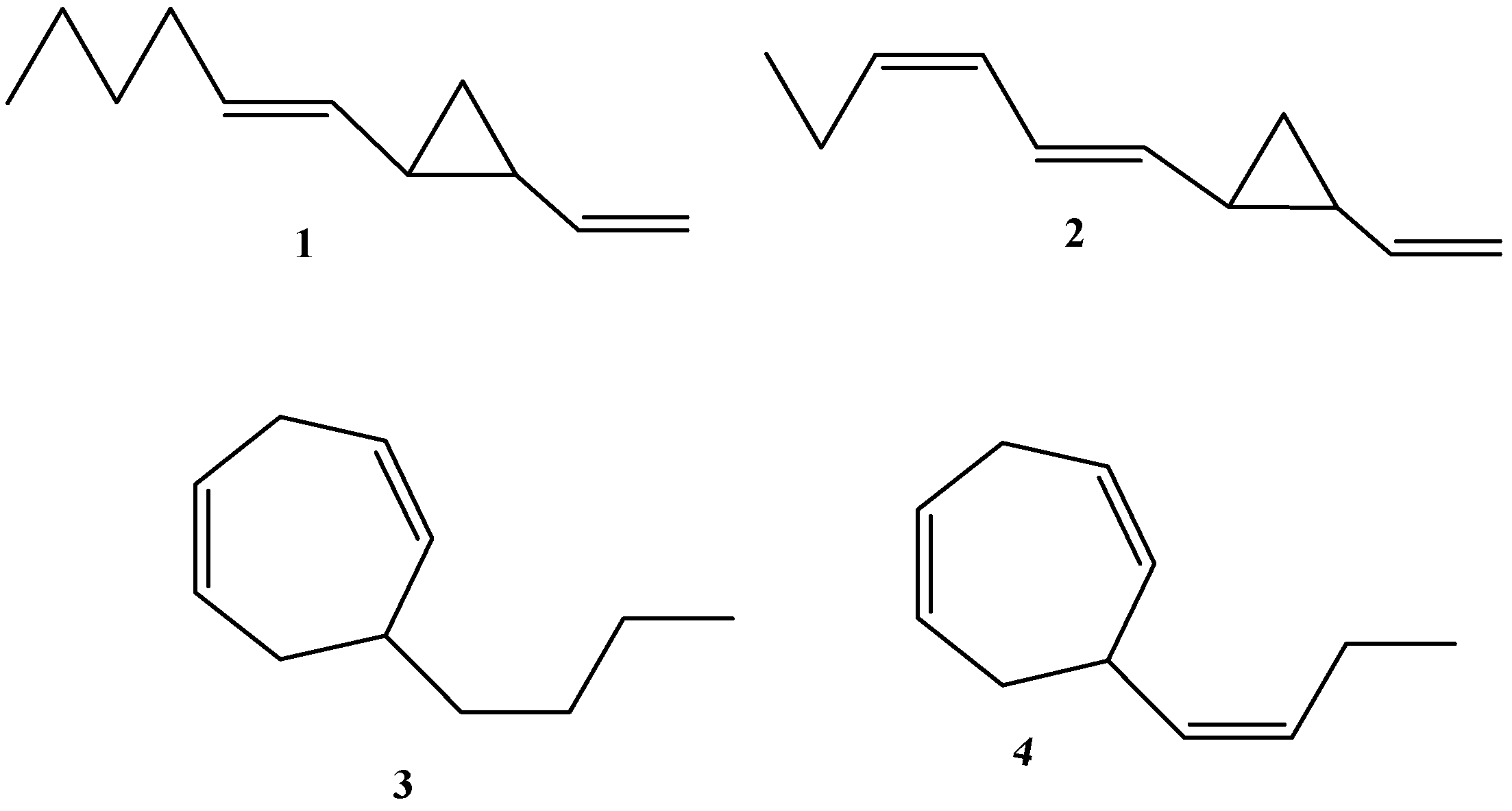
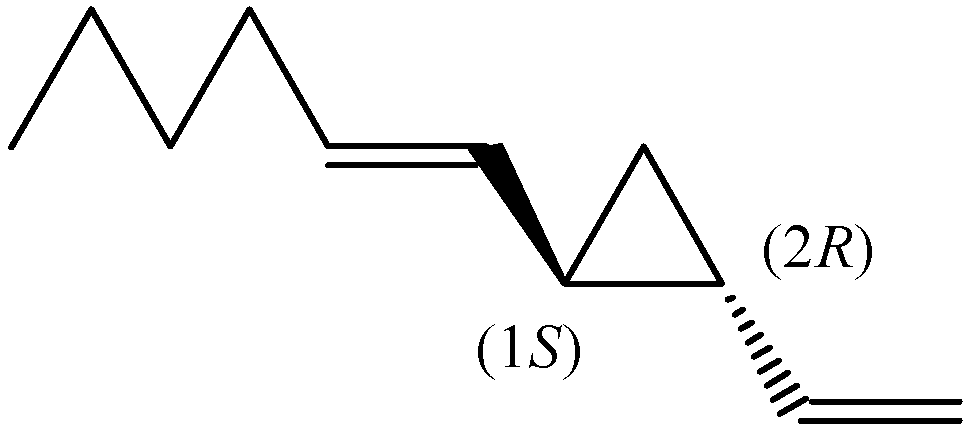
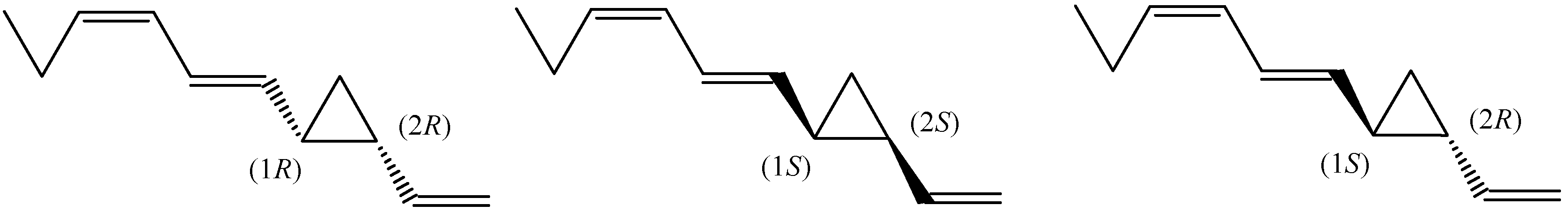
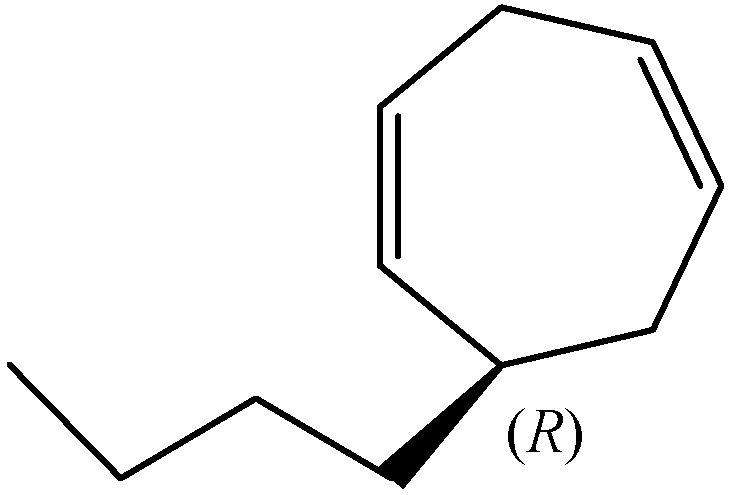
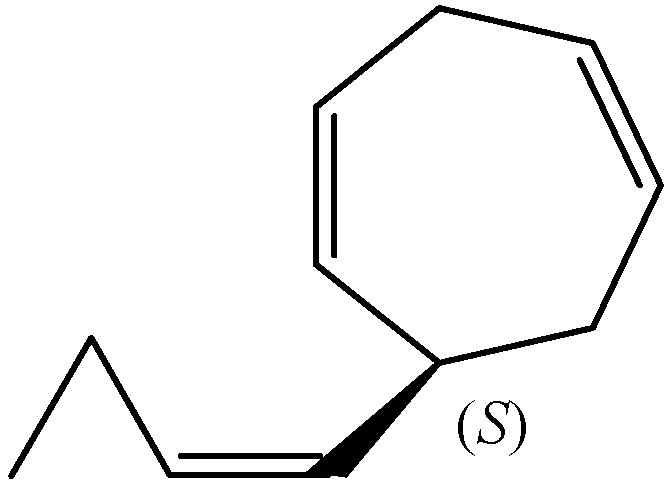

| Species | Method of Isolation and Identification | Dictyopterenes Abundance | Reference | |
|---|---|---|---|---|
| 1. | Dictyopteris plagiogramma (Montagne) Vickers | short-path distillation; preparative gas chromatography, nuclear magnetic resonance (NMR) | dictyopterene A | Moore et al., 1968 [20] |
| 2. | Dictyopteris australis (Sonder) Askenasy | |||
| 3. | Dictyopteris spp. | essential oil; gradient chromatography on 25% silver nitrate-silica gel followed by NMR | dictyopterene A (25%) dictyopterene B (50%) | Pettus & Moore, 1970 [21] |
| 4. | Dictyopteris spp. | essential oil; chromatography on 25% silver nitrate-silica gel followed by GC and NMR | dictyopterene A and dictyopterene B are the major constituents; dictyopterene C | Moore & Pettus, 1971 [10] |
| 5. | Ectocarpus siliculosus (Dillwyn) Lyngbye | the use of a stream of purified air and condensation; gas chromatography and mass spectrometry (GC-MS), NMR | dictyopterene D | Müller et al., 1971 [22] |
| 6. | Dictyopteris plagiogramma (Montagne) Vickers Dictyopteris australis Sonder | chromatography of the essential oil on silica impregnated with silver nitrate; GC | dictyopterene A (around 25%) dictyopterene B (around 50%) dictyopterene C dictyopterene D | Moore et al., 1974 [15] |
| 7. | Dictyopteris prolifera (Okamura) Okamura | hexane-soluble fraction of the acetone extract was chromatographed on silica gel and preparative thin layer chromatography (TLC) on silica gel; NMR | dictyopterene A (yield 0.003%) dictyopterene B (yield 0.001%) | Yamada et al., 1979 [23] |
| 8. | Laminaria digitata (Hudson) J.V.Lamouroux | closed-loop stripping technique; GC-MS | dictyopterene D | Müller et al., 1979 [24] |
| 9. | Dictyopteris prolifera (Okamura) Okamura | extraction with pentane saturated with methanol; GC-MS | dictyopterene A (65%) dictyopterene C (3%) dictyopterene B (10%) | Kajiwara et al., 1980 [25] |
| 10. | Dictyopteris undulata Holmes | dictyopterene A (69%) dictyopterene C (5%) dictyopterene B (9%) | ||
| 11. | Ectocarpus siliculosus | closed-loop stripping technique; GC-MS | dictyopterene D | Müller et al., 1980 [26] |
| 12. | Ectocarpus siliculosus (Dillwyn) Lyngbye | closed-loop stripping technique; GC-MS | dictyopterene D (major compound) | Müller & Gassmann, 1980 [26] |
| 13. | Dictyota dichotoma (Hudson) Lamour | extraction procedure by closed-loop technique; GC-MS | dictyopterene C | Müller et al., 1981 [27] |
| 14. | Hormosira banksii (Turner) Decaisne | closed-loop stripping technique; GC-MS | dictyopterene B | Müller et al., 1984 [28] |
| 15. | Durvillaea potatorum (Labillard) Aresch. Durvillaea willana Lindauer Durvillaea antarctica (Chamisso) Hariot Xiphophora chondrophylla (R. Brown ex Turn.) Mont. ex Harv. Xiphophora gladiata (Labillard) Mont. ex Kjellm. Scytosiphon lomentaria (Lyngb.) C. Ag. Colpomenia peregrina (Sauv.) Hareel | closed-loop stripping technique; GC-MS | dictyopterene B and dictyopterene A (ratio ca. 9:1) | Müller et al., 1985 [29] |
| 16. | Haplospora globosa Kjellman | extraction procedures for low-molecular hydrophobic substances; GC | dictyopterene B (major compound) dictyopterene D | Kuhlenkamp & Müller, 1985 [30] |
| 17. | Dictyopteris prolifera (Okamura) Okamura | the hexane-soluble fraction of the acetone extract was chromatographed on silica gel; NMR | dictyopterene A (yield 0.0021%) dictyopterene B (yield 0.0019%) | Yamada et al., 1986 [31] |
| 18. | Skeletonema costatum (Greville) Cleve | purging with ultrapure air in a cold trap; GC | dictyopterene D (mainly in S. costatum) | Derenbach & Pesando (1986) [32] |
| 19. | Lithodesmium undulatum Ehrenberg | |||
| 20. | Hormosira banksii (Turner) Decaisne Durvillaea potatorum (Chamisso) Hariot Xiphophora chondrophylla (R. Brown ex Turn.) Mont. ex Harv. Xiphophora gladiata (Labillard) Mont. ex Kjellm. Dictyopteris membranacea (Stackh.) Batt. Haplospora globosa Kjellman | inclusion chromatography | dictyopterene B | Boland et al., 1987 [33] |
| 21. | Dictyopteris membranacea (Stackh.) Batt. | closed-loop stripping technique; GC-MS | dictyopterene A (2.2%) dictyopterene B (2.3%) dictyopterene C (74.1%) dictyopterene D (13.4%) | Boland et al., 1987 [34] |
| 22. | Ectocarpus siliculosus (Dillwyn) Lyngbye | Grob–Hersch closed-loop extraction; GC | dictyopterene D (17.4%) | Muller and Schmidt, 1988 [35] |
| 23. | Dictyopteris prolifera (Okamura) Okamura | close-looping headspace (CLHS) procedure on charcoal; GC-MS | dictyopterene A (0.24–32.9%) dictyopterene D (10.4–30.4%) dictyopterene B + C (3.14–59.9%) | Kajiwara et al., 1989 [36] |
| 24. | Dictyota diemensis Kützing | Grob–Hersch closed-loop extraction method; GC-MS | dictyopterene C (31%) dictyopterene D (0.5%) dictyopterene A (0.5%) | Philips et al., 1990 [37] |
| 25. | Cutleria multifida (Smith) Greville | VOCs were entrapped on charcoal filters using air circulation in a closed system; GC-MS | dictyopterene C dictyopterene D | Keitel et al., 1990 [38] |
| 26. | Scytosiphon lomentaria (Lyngbye) Link | steam distillate; GC, GC-MS | dictyopterene A (0.26%) dictyoptercne D (0.13%) dictyopterene C (0.32%) | Kajiwara et al., 1991 [39] |
| 27. | Scytosiphon lomentaria (Lyngbye) Link | activated carbon fibre in a closed-loop stripping system for VOCs; female gametes suspension extracted with CH2Cl2; HPLC on chiral column | dictyopterene B | |
| 28. | Colpomenia bullosa (D.A.Saunders) Yamada | |||
| 29. | Analipus japonicus (Harvey) M.J.Wynne | |||
| 30. | Dictyopteris prolifera (Okamura) Okamura | |||
| 31. | Dictyopteris undulata Holmes | |||
| 32. | Ectocarpus siliculosus (Dillwyn) Lyngbye | closed-loop extraction; GC-MS | dictyopterene C Dictyopterene D | Stratmann et al., 1992 [40] |
| 33. | Hormosira banksii (Turner) Decaisne | closed-loop extraction technique with activated carbon filter and eluted with dichloromethane; GC | dictyopterene B | Maier & Clayton, 1993 [41] |
| 34. | Analipus japonicus (Harvey) M.J.Wynne | closed-loop stripping system; GC, GC-MS, chiral HPLC | dictyopterene B: dictyopterene D: dictyopterene C (ratio 11:87:2) | Kodama et al., 1993 [42] |
| 35. | Dictyopteris prolifera (Okamura) Okamura | closed-looping headspace (CLHS) procedure for isolation from protoplasts and intact plant; GC, GC-MS | dictyopterene A (19.08%; 18.67%) dictyopterene B + C (15.82%; 16.00%) dictyopterene D (4.62%; 8.91%) | Fujimura et al., 1994 [43] |
| 36. | Ectocarpus siliculosus (Dillwyn) Lyngbye | extraction with MeOH and CH2Cl2; HPLC | dictyopterene D | Boland et al., 1995 [44] |
| 37. | Gomphonema parvulum (Kützing) Kützing | VOC stripped in a closed-loop stripping device and adsorbed on Tenax TA and subsequently transferred to GC-MS by thermodesorption; GC-Fourier transform infrared spectroscopy (FTIR) | dictyopterene A dictyopterene C | Wendel & Jüttner, 1996 [45] |
| 38. | Amphora veneta Kützing | dictyopterene C | ||
| 39. | Phaeodactylum tricornutum Bohlin | dictyopterene C | ||
| 40. | Dictyopteris prolifera (Okamura) Okamura | essential oil; GC-MS; chiral GC | dictyopterene A (63.3%), C (18.2%), D (15.9%) | Kajiwara, 1997 [19] |
| 41. | Dictyopteris undulata Holmes | dictyopterene A (20.9%), C (38.5%), D (40.7%) | ||
| 42. | Dictyopteris divaricata (Okamura) Okamura | dictyopterene C (trace) dictyopterene D (trace) | ||
| 43. | Dictyopteris spp. | dictyopterene A (10.9%), C (10.5%), D (20.5%) | ||
| 44. | Gomphonema parvulum (Kützing) Kützing | solid phase microextraction (SPME); chiral GC-MS | dictyopterene A | Hombeck et al., 1999 [46] |
| 45. | Dictyopteris prolifera (Okamura) Okamura | SDE (simultaneous distillation extraction), solvent extraction with pentane solution with 30% of ethanol; chromatography on silica gel and then on AgNO3-silica gel; GC, GC-MS, chiral GC | dictyopterene A (48.9%), B (15.6%), C (5.8%), D (2.2%) | Yamamoto et al., 2001 [16] |
| 46. | Dictyopteris undulata Holmes | dictyopterene A (21.1%), B (2.3%), C (2.0%), D (0.2%) | ||
| 47. | Dictyopteris latiscula (Okamura) Okamura | Dictyopterene A (40.1%), B (3.0%), C (2.2%), D (0.1%) | ||
| 48. | Dictyopteris sp. | Dictyopterene A (10.9%), B (0.6%), C (0.9%), D (trace) | ||
| 49. | Dictyopteris membranacea (Stackhouse) Batters | focused microwave-assisted hydrodistillation (FMAH) and hydrodistillation (HD); GC and GC-MS | dictyopterene A (32.3%—FMAH; 7.6%—HD) dictyopterene C (3.3%—FMAH; 0.8%—HD) | El Hattab et al., 2002 [47] |
| 50. | Padina pavonia (L.) Gaill Hydroclathrus clathratus (C. Agardh) | SDE; GC-MS | dictyopterene D (3.22%) dictyopterene A (0.60%) | Awad et al., 2009 [48] |
| 51. | Sargassum asperifolium Hering & G. Martens ex J. Agardh | SDE; GC-MS | dictyopterene D (2.02%), dictyopterene C (0.32%) | Matloub et al., 2012 [49] |
| 52. | Sargassum dentifolium (Turner) C. Agardh | dictyopterene D (4.63%), dictyopterene A (1.02%) | ||
| 53. | Sargassum linifolium C. Agardh | dictyopterene D (20.26%) dictyopterene A (3.18%) | ||
| 54. | Halopteris filicina (Grateloup) Kutzing | headspace solid-phase microextraction (HS-SPME), GC, GC-MS | dictyopterene D (1.9%) and C (0.7%) | Jerković et al., 2018 [50] |
| 55. | Flabellia petiolata (Turra) Nizamuddin | dictyopterene D (7.4%) and C (0.7%) | ||
| 56. | Padina pavonica (Linnaeus) Thivy | HS-SPME, HD; GC, GC-MS | dictyopterene A (0.87–1.27%) dictyopterene D (1.15%) | Jerković et al., 2019 [51] |
| 57. | Dictyopteris polypodioides (A.P. De Candolle) J.V. Lamouroux | essential oil (EO), steam distillation of diethyl ether extract (volatile fraction, VF), HD; GC-FID, GC-MS | dictyopterene A (14.1% in VF, 9.3% in EO) dictyopterene B’ (1.1% in VF, 0.4% in EO) dictyopterene C (trace in VF, 1.6% in EO) dictyopterene D (1.8% in VF, 0.2% in EO) | Riad, 2020 [52] |
| 58. | Polysiphonia senticulosa Harvey | HS-SPME; GC–MS | dictyopterene D (0.95–1.74%) | Wang et al., 2021 [53] |
| 59. | Dictyopteris membranacea (Stackhouse) Batters | FMAHD, supercritical CO2 (SC-CO2) extraction of crude diethyl ether extract; GC-MS | dictyopterene A (major compound) | Riad et al., 2021 [54] |
| 60. | Ericaria amentacea (C. Agardh) Molinari & Guiry | SC-CO2 extraction; GC-MS | C11 unsaturated hydrocarbons were found in traces | Cikoš et al., 2022 [55] |
| 61. | Dictyopteris polypodioides (A.P.De Candolle) J.V.Lamouroux | dictyopterene A (8.00%) dictyopterene C (0.87%) dictyopterene D (0.08%) | ||
| 62. | Halopteris scoparia (Linnaeus) Sauvageau | HS-SPME (divinylbenzene (DVB)/carboxene (CAR)/polydimethylsiloxane (PDMS) fibre); GC–MS | dictyopterene D (0.27–2.52%) dictyopterene C (0.92%) | Čagalj et al., 2022 [56] |
| HS-SPME (PDMS/DVB fibre); GC–MS | dictyopterene D (0.35–1.23%) dictyopterene C (0.31%) | |||
| HD; GC–MS | dictyopterene D (0.06%) dictyopterene C (0.10–0.14%) | |||
| 63. | Dasycladus vermicularis (Scopoli) Krasser | HS-SPME; GC–MS | dictyopterene D (1.41–7.78%) dictyopterene C (8.65–9.34%) | Radman et al., 2022 [57] |
| HD; GC–MS | dictyopterene D (0.17%) dictyopterene C (0.13%) | |||
| 64. | Dictyota dichotoma (Hudson) J.V. Lamouroux | HS-SPME (DVB/CAR)/PDMS) fibre); GC-MS | dictyopterene D (0.46–4.42%) dictyopterene C (0.46–1.03%) | Radman et al., 2022 [58] |
| HS-SPME (PDMS/DVB fibre); GC-MS | dictyopterene D (0.07–8.22%) dictyopterene C (0.76–1.69%) | |||
| HD; GC-MS | dictyopterene C (0.04–0.13%) | |||
| 65. | Cladostephus spongiosus (Hudson) C. Agardh | HS-SPME with DVB/CAR/PDMS fibre; GC-MS | dictyopterene D (0.81–2.94%) dictyopterene C (0.49–1.3%) | Radman et al., 2023 [59] |
| HS-SPME with PDMS/DVB fibre; GC-MS | dictyopterene D (0.44–1.34%) dictyopterene C (0.2%) | |||
| HD; GC-MS | dictyopterene A (0.07–0.08%) dictyopterene D (0.06–0.11%) dictyopterene (0.05%) | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jerković, I.; Golemac Lipovac, A.; Balaić, D.; Jokić, S. Dictyopterenes A, B, C, and D from Marine Algae. Molecules 2025, 30, 3987. https://doi.org/10.3390/molecules30193987
Jerković I, Golemac Lipovac A, Balaić D, Jokić S. Dictyopterenes A, B, C, and D from Marine Algae. Molecules. 2025; 30(19):3987. https://doi.org/10.3390/molecules30193987
Chicago/Turabian StyleJerković, Igor, Anja Golemac Lipovac, Dina Balaić, and Stela Jokić. 2025. "Dictyopterenes A, B, C, and D from Marine Algae" Molecules 30, no. 19: 3987. https://doi.org/10.3390/molecules30193987
APA StyleJerković, I., Golemac Lipovac, A., Balaić, D., & Jokić, S. (2025). Dictyopterenes A, B, C, and D from Marine Algae. Molecules, 30(19), 3987. https://doi.org/10.3390/molecules30193987








