Cucurbitane Glycosides from Siraitia Grosvenorii and Their Hepatoprotective Activities
Abstract
1. Introduction
2. Results and Discussion
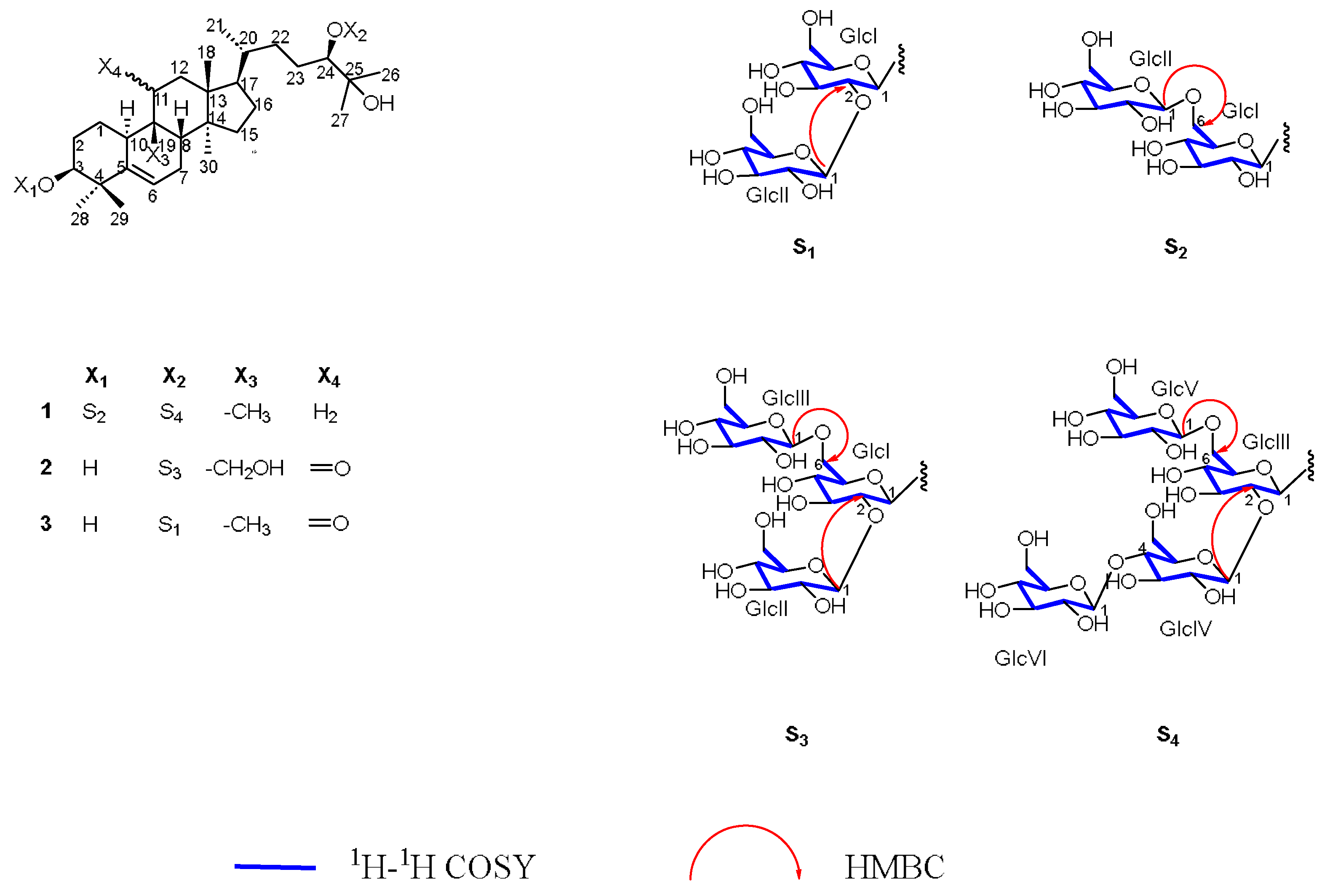
2.1. Detailed Information and Elucidation for New Compounds
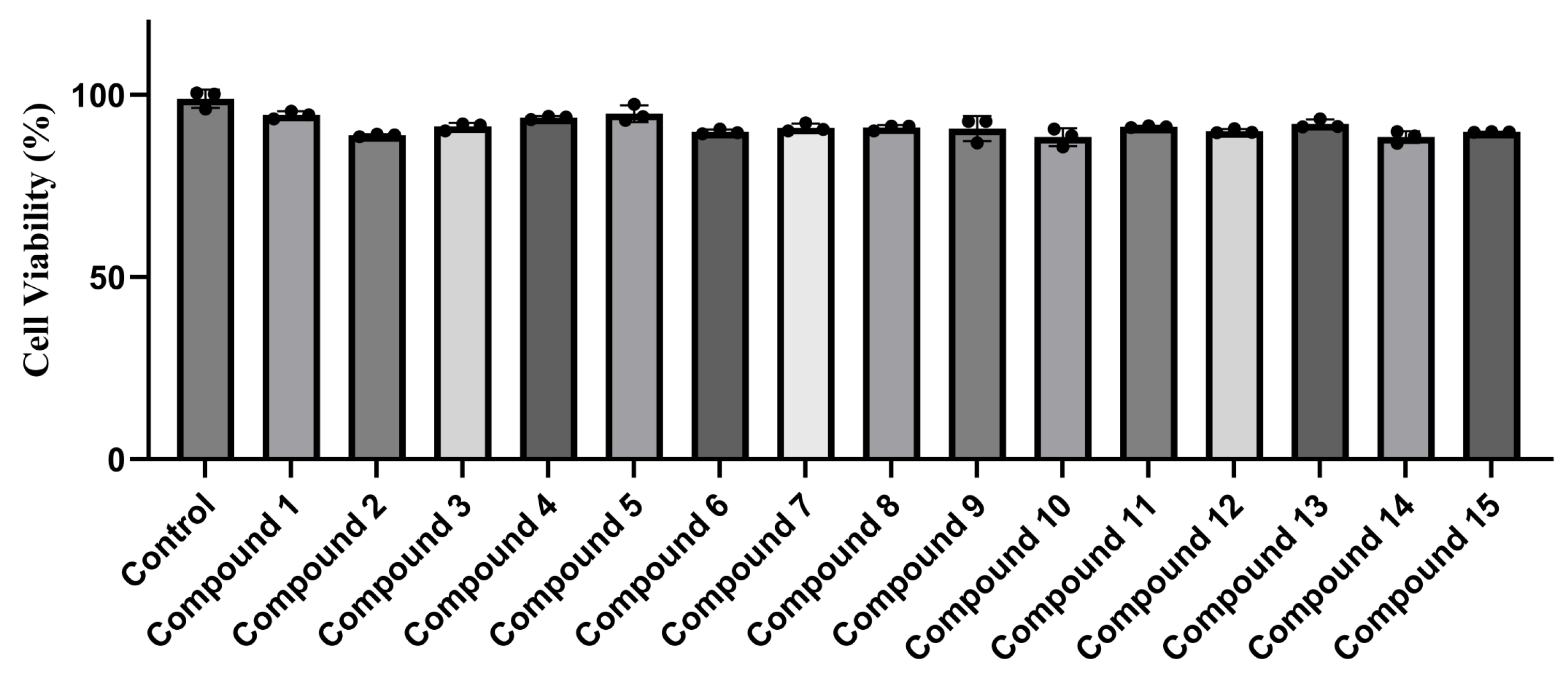
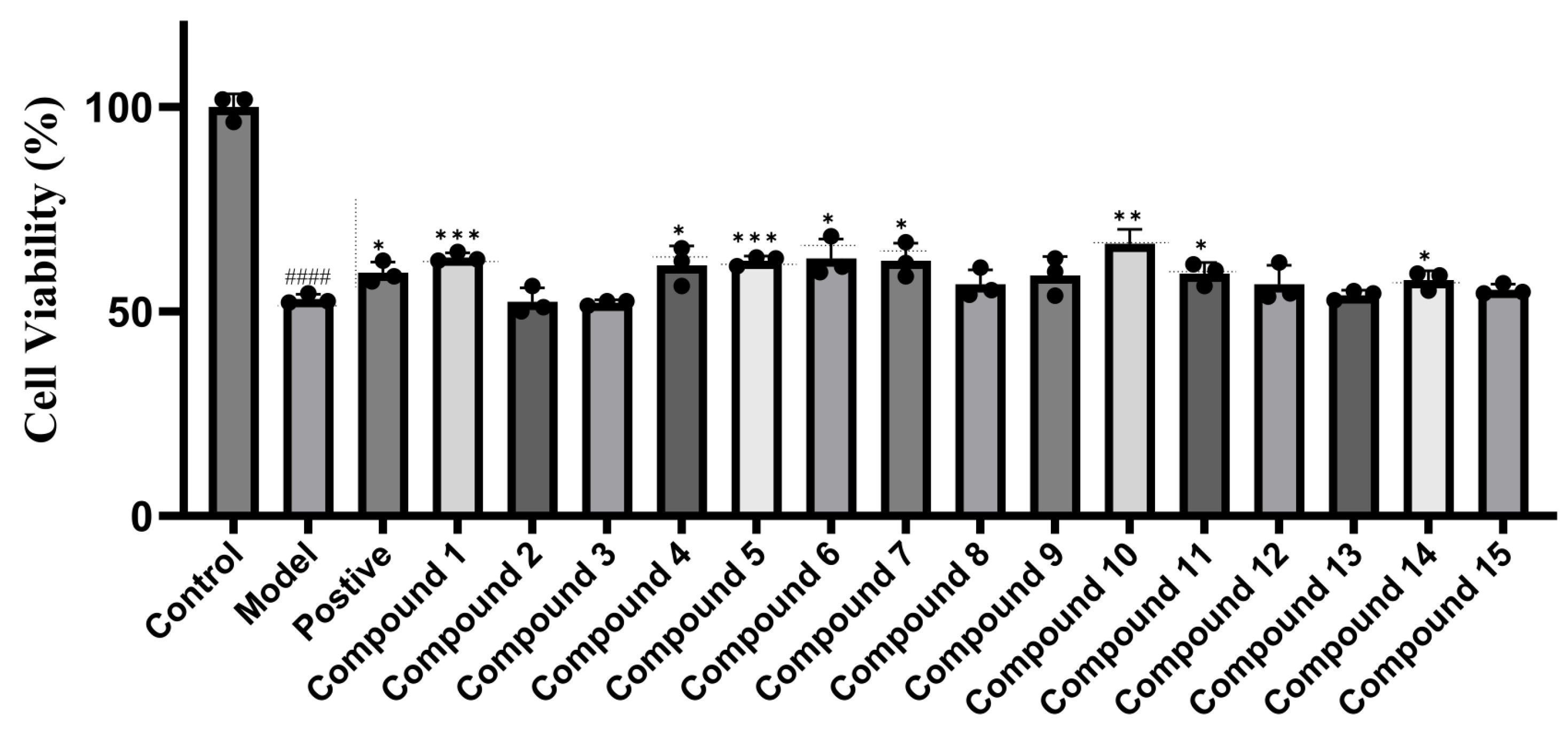
2.2. Cytotoxicity and Hepatoprotective Activity
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Isolation and Purification
3.3.1. Luohanguoside A (1)
3.3.2. Luohanguoside B (2)
3.3.3. Luohanguoside C (3)
3.4. Acid Hydrolysis
3.5. Cytotoxicity Assay
3.6. Hepatoprotective Activity Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 1H NMR | 1H Nuclear Magnetic Resonance |
| 13C NMR | 13C Nuclear Magnetic Resonance |
| 1H-1H COSY | 1H-1H Correlation Spectroscopy |
| HSQC | Heteronuclear Single Quantum Coherence |
| HMBC | Heteronuclear Multiple Bond Correlation |
| NOESY | Nuclear Overhauser Effect Spectroscopy |
| TOCSY | Total Correlation Spectroscopy |
| HRESIMS | High Resolution Electrospray Ionization Mass Spectrometry |
| UV | Ultraviolet |
| IR | Infrared |
| HPLC | High-Performance Liquid Chromatography |
| tR | retention time |
References
- Li, C.; Lin, L.-M.; Sui, F.; Wang, Z.-M.; Huo, H.-R.; Dai, L.; Jiang, T.-L. Chemistry and pharmacology of Siraitia grosvenorii: A review. Chin. J. Nat. Med. 2014, 12, 89–102. [Google Scholar] [CrossRef]
- Jin, J.-S.; Lee, J.-H. Phytochemical and pharmacological aspects of Siraitia grosvenorii, luo han kuo. Orient. Pharm. Exp. Med. 2012, 12, 233–239. [Google Scholar] [CrossRef]
- Huang, H.; Peng, Z.; Zhan, S.; Li, W.; Liu, D.; Huang, S.; Zhu, Y.; Wang, W. A comprehensive review of Siraitia grosvenorii (Swingle) C. Jeffrey: Chemical composition, pharmacology, toxicology, status of resources development, and applications. Front. Pharmacol. 2024, 15, 1388747. [Google Scholar] [CrossRef]
- Muñoz-Labrador, A.; Hernandez-Hernandez, O.; Moreno, F.J. A review of the state of sweeteners science: The natural versus artificial non-caloric sweeteners debate. Stevia rebaudiana and Siraitia grosvenorii into the spotlight. Crit. Rev. Biotechnol. 2023, 44, 1080–1102. [Google Scholar] [CrossRef] [PubMed]
- Di, R.; Huang, M.-T.; Ho, C.-T. Anti-inflammatory Activities of Mogrosides from Momordica grosvenori in Murine Macrophages and a Murine Ear Edema Model. J. Agric. Food Chem. 2011, 59, 7474–7481. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shi, M.; Sarengaowa; Xiao, Z.; Xiao, Y.; Feng, K. Recent Advances in the Distribution, Chemical Composition, Health Benefits. and Application of the Fruit of Siraitia grosvenorii. Foods 2024, 13, 2278. [Google Scholar] [CrossRef]
- Chen, N.; Cao, W.; Yuan, Y.; Wang, Y.; Zhang, X.; Chen, Y.; Yiasmin, M.N.; Tristanto, N.A.; Hua, X. Recent advancements in mogrosides: A review on biological activities, synthetic biology, and applications in the food industry. Food Chem. 2024, 449, 139277. [Google Scholar] [CrossRef]
- Liu, H.; Wang, C.; Qi, X.; Zou, J.; Sun, Z. Antiglycation and antioxidant activities of mogroside extract from Siraitia grosvenorii (Swingle) fruits. J. Food Sci. Technol. 2018, 55, 1880–1888. [Google Scholar] [CrossRef]
- Shen, J.; Shen, D.; Tang, Q.; Li, Z.; Jin, X.; Li, C. Mogroside V exerts anti-inflammatory effects on fine particulate matter-induced inflammation in porcine alveolar macrophages. Toxicol. Vitr. 2022, 80, 105326. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, Z.; Ye, F.; Guo, T.; Luo, Y.; Zhou, W.; Qin, D.; Tang, Y.; Cao, F.; Luo, F.; et al. Mogroside V exerts anti-inflammatory effect via MAPK-NF-κB/AP-1 and AMPK-PI3K/Akt/mTOR pathways in ulcerative colitis. J. Funct. Foods 2021, 87, 104807. [Google Scholar] [CrossRef]
- Zhang, X.; Song, Y.; Ding, Y.; Wang, W.; Liao, L.; Zhong, J.; Sun, P.; Lei, F.; Zhang, Y.; Xie, W. Effects of Mogrosides on High-Fat-Diet-Induced Obesity and Nonalcoholic Fatty Liver Disease in Mice. Molecules 2018, 23, 1894. [Google Scholar] [CrossRef]
- Cao, F.; Zhang, Y.; Li, W.; Shimizu, K.; Xie, H.; Zhang, C. Mogroside IVE attenuates experimental liver fibrosis in mice and inhibits HSC activation through downregulating TLR4-mediated pathways. Int. Immunopharmacol. 2018, 55, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Peng, Y.; Zhao, L.; Zhou, G.; Li, X. Regulating the gut microbiota and SCFAs in the faeces of T2DM rats should be one of antidiabetic mechanisms of mogrosides in the fruits of Siraitia grosvenorii. J. Ethnopharmacol. 2021, 274, 114033. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.; Zhang, Q.; Zhang, S. Mogroside IIIE attenuates gestational diabetes mellitus through activating of AMPK signaling pathway in mice. J. Pharmacol. Sci. 2018, 138, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, G.; Peng, Y.; Wang, M.; Li, X. Anti-hyperglycemic and anti-hyperlipidemic effects of a special fraction of Luohanguo extract on obese T2DM rats. J. Ethnopharmacol. 2020, 247, 112273. [Google Scholar] [CrossRef]
- Takasaki, M.; Konoshima, T.; Murata, Y.; Sugiura, M.; Nishino, H.; Tokuda, H.; Matsumoto, K.; Kasai, R.; Yamasaki, K. Anticarcinogenic activity of natural sweeteners, cucurbitane glycosides, from Momordica grosvenori. Cancer Lett. 2003, 198, 37–42. [Google Scholar] [CrossRef]
- Luo, J.; Lu, D.; Zhang, R.; Long, B.; Chen, L.; Wang, W.; Tian, X. What exactly happens to rats that drink different types of sweetness water over a long time:A comparison with sucrose, artificial sweeteners and natural sweeteners. J. Funct. Foods 2025, 127, 106715. [Google Scholar] [CrossRef]
- Luo, Q.-J.; Zhou, W.-C.; Liu, X.-Y.; Li, Y.-J.; Xie, Q.-L.; Wang, B.; Liu, C.; Wang, W.-M.; Wang, W.; Zhou, X.-D. Chemical Constituents and α-Glucosidase Inhibitory, Antioxidant and Hepatoprotective Activities of Ampelopsis grossedentata. Molecules 2023, 28, 7956. [Google Scholar] [CrossRef]
- Jia, Y.Z.; Yang, Y.P.; Cheng, L.; Cao, S.W.; Xie, Q.L.; Wang, M.Y.; Li, B.; Jian, Y.Q.; Liu, B.; Peng, C.Y.; et al. Heilaohuguosus A-S from the fruits of Kadsura coccinea and their hepatoprotective activity. Phytochemistry 2021, 184, 112678. [Google Scholar] [CrossRef]
- Li, F.; Yang, F.; Liu, X.; Wang, L.; Chen, B.; Li, L.; Wang, M. Cucurbitane glycosides from the fruit of Siraitia grosvenori and their effects on glucose uptake in human HepG2 cells in vitro. Food Chem. 2017, 228, 567–573. [Google Scholar] [CrossRef]
- Chaturvedula, V.S.P.; Prakash, I. Cucurbitane Glycosides from Siraitia grosvenorii. J. Carbohydr. Chem. 2011, 30, 16–26. [Google Scholar] [CrossRef]
- Akihisa, T.; Hayakawa, Y.; Tokuda, H.; Banno, N.; Shimizu, N.; Suzuki, T.; Kimura, Y. Cucurbitane Glycosides from the Fruits of Siraitia grosvenorii and Their Inhibitory Effects on Epstein−Barr Virus Activation. J. Nat. Prod. 2007, 70, 783–788. [Google Scholar] [CrossRef] [PubMed]
- Niu, B.; Ke, C.-Q.; Li, B.-H.; Li, Y.; Yi, Y.; Luo, Y.; Shuai, L.; Yao, S.; Lin, L.-G.; Li, J.; et al. Cucurbitane Glucosides from the Crude Extract of Siraitia grosvenorii with Moderate Effects on PGC-1α Promoter Activity. J. Nat. Prod. 2017, 80, 1428–1435. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.; Yaseen, A.; Wang, L.; Chen, B.; Wang, M.; Hu, W.; Li, F. Two New Cucurbitane Glycosides from the Fruits of Siraitia grosvenori. Chem. Pharm. Bull. 2019, 67, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.A.; Murata, Y.; Inui, H.; Sugiura, M.; Nakano, Y. Triterpene Glycosides of Siraitia grosvenori Inhibit Rat Intestinal Maltase and Suppress the Rise in Blood Glucose Level after a Single Oral Administration of Maltose in Rats. J. Agric. Food Chem. 2005, 53, 2941–2946. [Google Scholar] [CrossRef]
- Matsumoto, K.; Kasai, R.; Ohtani, K.; Tanaka, O. Minor cucurbitane-glycosides from fruits of Siraitia grosvenori (Cucurbitaceae). Chem. Pharm. Bull. 1990, 38, 2030–2032. [Google Scholar] [CrossRef]
- Prakash, I.; Chaturvedula, V. Additional New Minor Cucurbitane Glycosides from Siraitia grosvenorii. Molecules 2014, 19, 3669–3680. [Google Scholar] [CrossRef]
- Oobayashi, K.; Yoshikawa, K.; Arihara, S. Structural revision of bryonoside and structure elucidation of minor saponins from Bryonia dioica. Phytochemistry 1992, 31, 943–946. [Google Scholar] [CrossRef]

| Position | 1 | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |||
| 1 | 2.02, m 3.00, m | 26.9 | 1.83, m 2.02, m | 20.5 | 1.66, m 2.11, m | 21.6 | ||
| 2 | 2.23, m 2.52, m | 29.5 | 1.27, m 1.84, m | 30.2 | 1.85, m 1.93, m | 30.2 | ||
| 3 | 3.69, br s | 87.6 | 3.70, br s | 75.6 | 3.73, br s | 75.9 | ||
| 4 | - | 42.4 | - | 42.0 | - | 42.2 | ||
| 5 | - | 144.4 | - | 141.3 | - | 141.7 | ||
| 6 | 5.49, m | 118.3 | 5.77, m | 120.1 | 5.68, m | 119.3 | ||
| 7 | 1.69, m 2.30, m | 24.6 | 1.93, m 2.03, m | 24.1 | 1.82, m 2.31, m | 24.5 | ||
| 8 | 1.66, m | 43.6 | 3.21, m | 34.4 | 1.84, m | 44.4 | ||
| 9 | - | 40.2 | - | 54.2 | - | 49.4 | ||
| 10 | 2.85, d (12.2) | 36.7 | 2.67, d (14.7) | 35.9 | 2.55, m | 36.3 | ||
| 11 | 1.46, m 2.12, m | 28.6 | - | 213.0 | - | 214.4 | ||
| 12 | 1.08, m 1.15, m | 34.6 | 2.68, m 3.08, m | 49.2 | 2.55, m 3.01, m | 49.1 | ||
| 13 | - | 49.7 | - | 49.6 | - | 49.4 | ||
| 14 | - | 47.5 | - | 48.9 | - | 50.0 | ||
| 15 | 2.16, m 2.21, m | 41.1 | 1.36, m 1.36, m | 35.1 | 1.17, m 1.31, m | 34.9 | ||
| 16 | 1.46, m 2.12, m | 28.4 | 1.57, m 2.24, m | 29.3 | 1.84, m 2.05, m | 28.6 | ||
| 17 | 1.79, m | 51.2 | 1.92, m | 50.4 | 1.83, m | 50.1 | ||
| 18 | 0.95, s | 17.1 | 1.12, s | 16.2 | 0.74, s | 17.3 | ||
| 19 | 1.52, s | 26.3 | 3.17, m 4.96, m | 60.4 | 1.27, s | 20.5 | ||
| 20 | 1.53, m | 36.6 | 1.46, m | 36.3 | 1.44, m | 36.9 | ||
| 21 | 1.12, d (6.4) | 19.1 | 1.03, d (6.4) | 18.8 | 1.01, d (6.5) | 18.7 | ||
| 22 | 1.79, m 1.87, m | 33.3 | 1.96, m 1.77, m | 33.2 | 1.83, m 1.83, m | 33.9 | ||
| 23 | 1.58, m 1.89, m | 29.8 | 1.50, m 1.90, m | 28.5 | 2.05, m 2.22, m | 28.7 | ||
| 24 | 3.77, m | 92.3 | 3.77, d (9.5) | 92.2 | 3.93, d (8.3) | 88.4 | ||
| 25 | - | 72.8 | - | 72.8 | - | 72.7 | ||
| 26 | 1.43, s | 24.6 | 1.47, s | 24.6 | 1.48, s | 26.2 | ||
| 27 | 1.34, s | 27.0 | 1.35, s | 27.1 | 1.51, s | 26.6 | ||
| 28 | 1.17, s | 27.9 | 1.14, s | 27.8 | 1.15, s | 28.3 | ||
| 29 | 1.52, s | 26.3 | 1.43, s | 26.6 | 1.44, s | 27.5 | ||
| 30 | 0.94, s | 19.4 | 1.16, s | 18.9 | 1.03, s | 19.1 | ||
| Position | 1 | 2 | 3 | |||||
|---|---|---|---|---|---|---|---|---|
| δH | δC | δH | δC | δH | δC | |||
| GlcI | ||||||||
| GI-1 | 4.80, d (7.5) | 107.0 | 4.93, d (7.5) | 103.7 | 5.08, overlapped | 102.2 | ||
| GI-2 | 3.92, m | 75.6 | 4.23, m | 82.2 | 4.17, m | 84.1 | ||
| GI-3 | 4.15, m | 78.6 | 4.24, m | 78.6 | 4.34, m | 78.6 | ||
| GI-4 | 4.04, m | 71.7 | 3.95, m | 71.6 | 4.20, m | 71.7 | ||
| GI-5 | 4.07, m | 77.4 | 4.12, m | 76.4 | 3.98, m | 78.9 | ||
| GI-6 | 4.34, m 4.80, m | 70.3 | 3.98, m 4.93, m | 70.2 | 4.36, m 4.57, m | 62.8 | ||
| GlcII | ||||||||
| GII-1 | 5.19, d (7.8) | 105.4 | 5.54, d (7.8) | 105.5 | 5.40, d (7.7) | 106.6 | ||
| GII-2 | 4.05, m | 75.3 | 4.13, m | 75.6 | 4.15, m | 76.6 | ||
| GII-3 | 4.27, m | 78.2 | 4.25, m | 78.4 | 4.25, m | 78.7 | ||
| GII-4 | 4.26, m | 71.5 | 4.15, m | 72.5 | 4.19, m | 72.5 | ||
| GII-5 | 3.97, m | 78.5 | 3.97, m | 78.2 | 3.98, m | 78.8 | ||
| GII-6 | 4.40, m 4.53, m | 62.6 | 4.35, m 4.54, m | 63.5 | 4.40, m 4.57, m | 63.6 | ||
| GlcIII | ||||||||
| GIII-1 | 4.93, d (7.6) | 103.7 | 4.88, d (7.7) | 104.9 | ||||
| GIII-2 | 4.15, m | 82.6 | 4.08, m | 75.5 | ||||
| GIII-3 | 4.23, m | 76.3 | 4.27, m | 78.1 | ||||
| GIII-4 | 3.94, m | 71.5 | 4.27, m | 71.5 | ||||
| GIII-5 | 4.08, m | 76.4 | 3.93, m | 78.8 | ||||
| GIII-6 | 3.98, m 4.92, m | 70.2 | 4.37, m 4.52, m | 62.6 | ||||
| GlcIV | ||||||||
| GIV-1 | 5.44, d (7.9) | 105.4 | ||||||
| GIV-2 | 4.11, m | 75.4 | ||||||
| GIV-3 | 4.24, m | 76.7 | ||||||
| GIV-4 | 4.23, m | 82.4 | ||||||
| GIV-5 | 3.94, m | 78.6 | ||||||
| GIV-6 | 4.48, m 4.50, m | 63.2 | ||||||
| GlcV | ||||||||
| GV-1 | 4.88, d (7.6) | 104.9 | ||||||
| GV-2 | 4.07, m | 75.6 | ||||||
| GV-3 | 4.27, m | 78.3 | ||||||
| GV-4 | 4.26, m | 71.7 | ||||||
| GV-5 | 3.93, m | 78.5 | ||||||
| GV-6 | 4.31, m 4.55, m | 62.5 | ||||||
| GlcVI | ||||||||
| GVI-1 | 5.13, d (7.9) | 105.0 | ||||||
| GVI-2 | 4.09, m | 75.4 | ||||||
| GVI-3 | 4.20, m | 78.1 | ||||||
| GVI-4 | 4.23, m | 71.5 | ||||||
| GVI-5 | 4.01, m | 78.1 | ||||||
| GVI-6 | 4.31, m 4.55, m | 62.5 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mao, J.-N.; Huang, H.-X.; Xie, Q.-L.; Chen, G.-Y.; Wu, J.-J.; Deng, Y.; Zhan, S.; Peng, Z.; Zhou, X.-D.; Wang, W. Cucurbitane Glycosides from Siraitia Grosvenorii and Their Hepatoprotective Activities. Molecules 2025, 30, 3983. https://doi.org/10.3390/molecules30193983
Mao J-N, Huang H-X, Xie Q-L, Chen G-Y, Wu J-J, Deng Y, Zhan S, Peng Z, Zhou X-D, Wang W. Cucurbitane Glycosides from Siraitia Grosvenorii and Their Hepatoprotective Activities. Molecules. 2025; 30(19):3983. https://doi.org/10.3390/molecules30193983
Chicago/Turabian StyleMao, Jia-Nan, Hua-Xue Huang, Qing-Ling Xie, Guang-Yu Chen, Juan-Jiang Wu, Ying Deng, Shuang Zhan, Zhi Peng, Xu-Dong Zhou, and Wei Wang. 2025. "Cucurbitane Glycosides from Siraitia Grosvenorii and Their Hepatoprotective Activities" Molecules 30, no. 19: 3983. https://doi.org/10.3390/molecules30193983
APA StyleMao, J.-N., Huang, H.-X., Xie, Q.-L., Chen, G.-Y., Wu, J.-J., Deng, Y., Zhan, S., Peng, Z., Zhou, X.-D., & Wang, W. (2025). Cucurbitane Glycosides from Siraitia Grosvenorii and Their Hepatoprotective Activities. Molecules, 30(19), 3983. https://doi.org/10.3390/molecules30193983








