Natural Biological Properties Inherited from Native Endemic Flora in Honeys from Lake Ranco Area of Southern Chile: A Botanical and Physicochemical Approach
Abstract
1. Introduction
2. Results
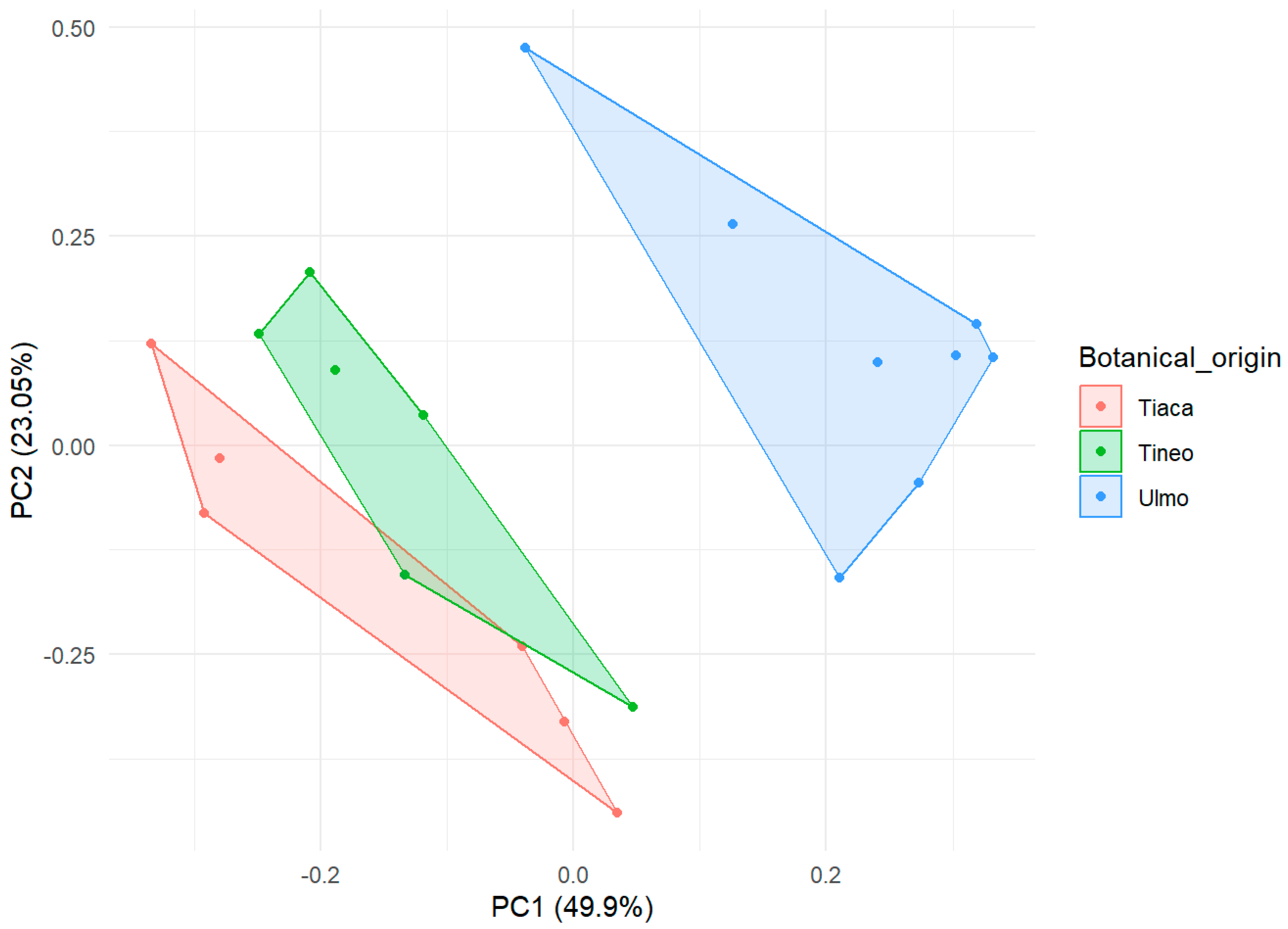
2.1. Botanical Origin of Honey Samples
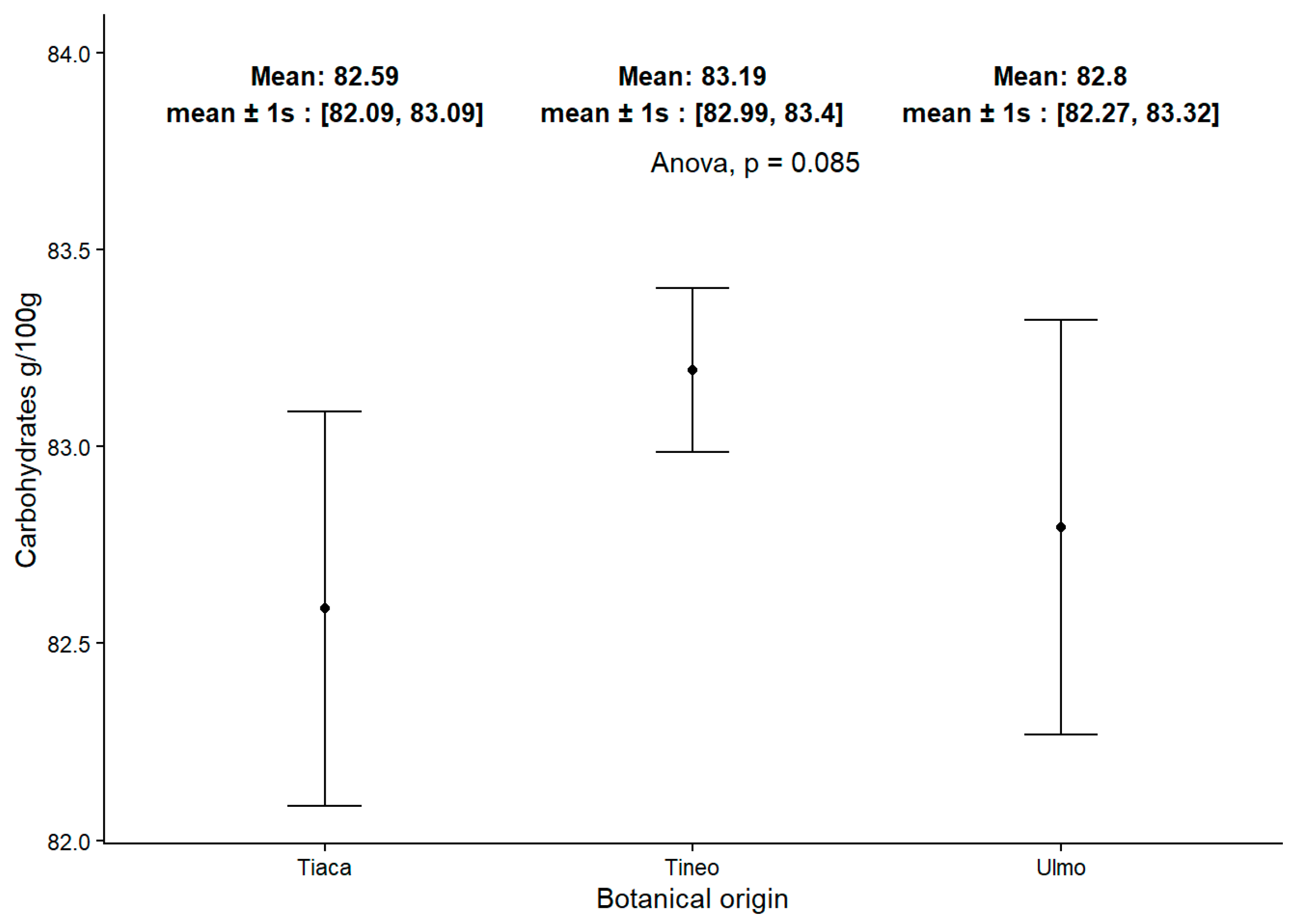
2.2. Carbohydrate Content in Honey Samples
2.3. Glucose and Fructose Content in Honey Samples
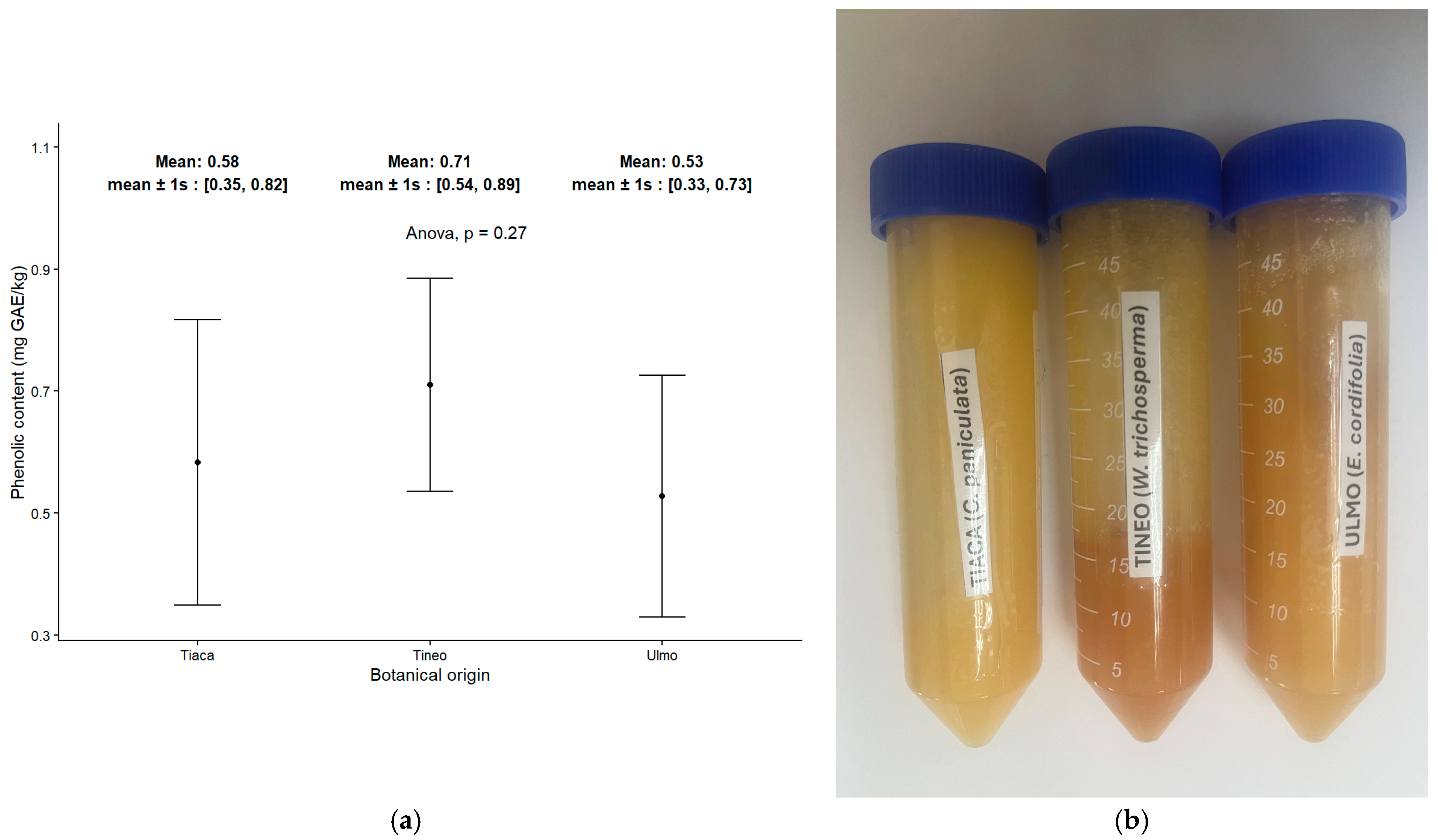
2.4. Content of Total Phenolic Compounds in Monofloral Honey Samples
2.5. Antiradical and Antioxidant Activities of Monofloral Honeys
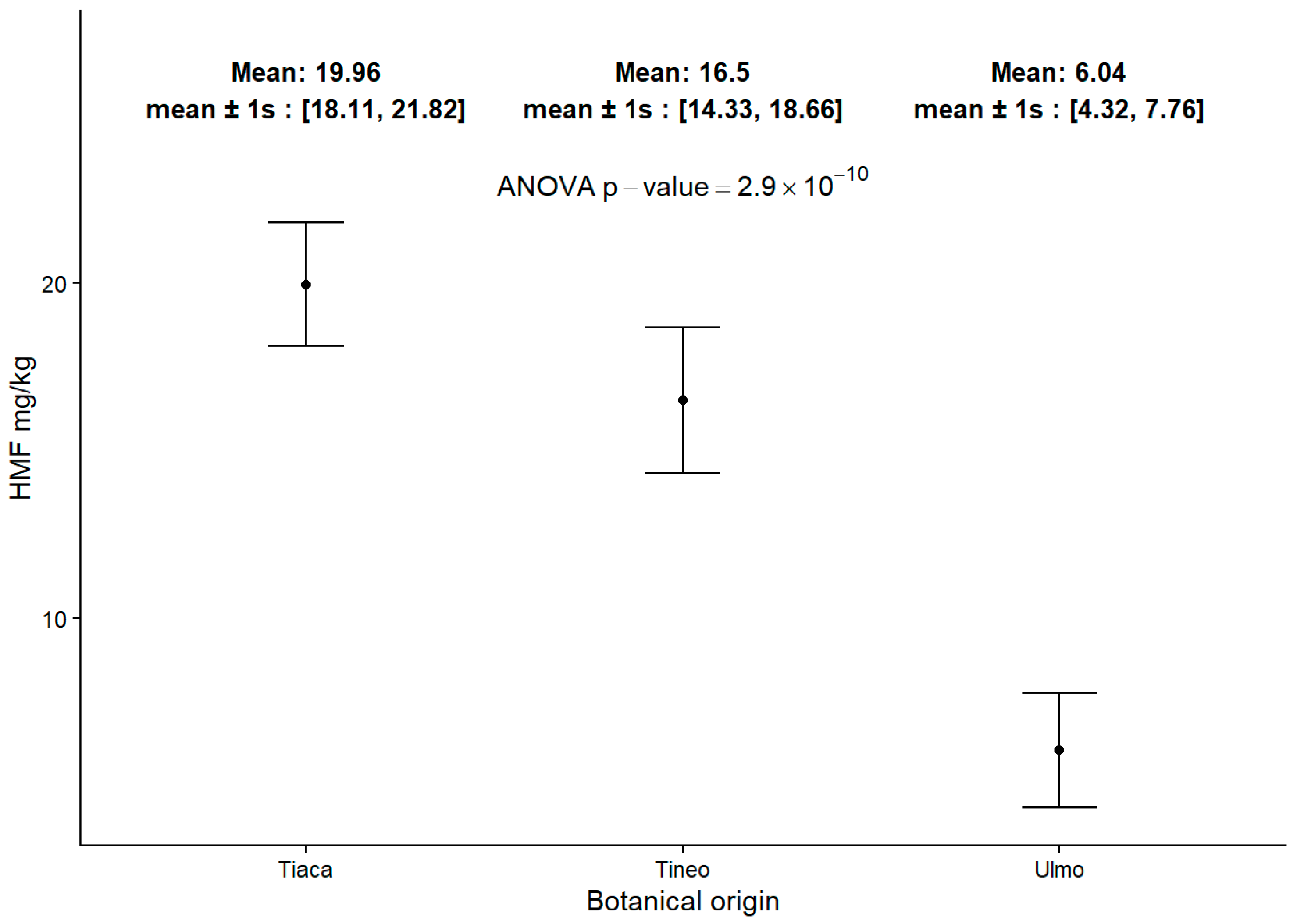
2.6. 5-Hydroxymethylfurfural (HMF) Content in Honey
3. Discussion
4. Materials and Methods
4.1. Honey Samples
4.2. Mellisopalynological Analysis
4.3. Total Carbohydrate Content in Honey Samples
4.4. Glucose/Fructose Determination by LC-MS/MS
4.5. Honey Bioactive Compounds Extraction for Colorimetric Assays
4.6. Total Phenolic Compounds Content
4.7. DPPH Assay for Antiradical Activity of Honey Samples
4.8. FRAP Assay for Antioxidant Activity
4.9. Determination of 5-Hydroxymethylfurfural (HMF) in Honey
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Agriculture Organization of the United Nations—FAO; IZSLT; Apimondia; CAAS. Good Beekeeping Practices for Sustainable Apiculture. FAO Animal Production and Health Guidelines. 2021. Available online: https://openknowledge.fao.org/handle/20.500.14283/cb5353en (accessed on 31 July 2025).
- The European Parliament; The Council of The European Union. Directive (EU) 2024/1438 of the European Parliament and of the Council. Off. J. Eur. Union 2024. Available online: http://data.europa.eu/eli/dir/2024/1438/oj (accessed on 31 July 2025).
- Addorisio, R.; Spadoni, R.; Maesano, G. Adoption of Innovative Technologies for Sustainable Agriculture: A Scoping Review of the System Domain. Sustainability 2025, 17, 4224. [Google Scholar] [CrossRef]
- Almutlaq, A.M.; Al-Shayaa, M.S.; Dabiah, A.T.; Alfridi, J.S.; Alsanhani, A.N. Adoption of Sustainable Beekeeping Practices Among Rural Women in Hail Region, Kingdom of Saudi Arabia: Implications for Agricultural Extension. Sustainability 2025, 17, 4186. [Google Scholar] [CrossRef]
- Verbeke, W.; Diallo, M.A.; Van Dooremalen, C.; Schoonman, M.; Williams, J.H.; Van Espen, M.; D’Haese, M.; De Graaf, D.C. European beekeepers’ interest in digital monitoring technology adoption for improved beehive management. Comput. Electron. Agric. 2024, 227, 109556. [Google Scholar] [CrossRef]
- Vanbergen, A.J.; Insect Pollinators Initiative. Threats to an ecosystem service: Pressures on pollinators. Front. Ecol. Environ. 2013, 11, 251–259. [Google Scholar] [CrossRef]
- Van Espen, M.; Williams, J.H.; Alves, F.; Hung, Y.; De Graaf, D.C.; Verbeke, W. Beekeeping in Europe facing climate change: A mixed methods study on perceived impacts and the need to adapt according to stakeholders and beekeepers. Sci. Total Environ. 2023, 888, 164255. [Google Scholar] [CrossRef] [PubMed]
- European Food Safety Authority (EFSA). Revised guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA J. 2023, 21, e07989.
- European Food Safety Authority (EFSA). Supplementary information to the revised guidance on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Support. Publ. 2023, 20, EN-7982. [Google Scholar] [CrossRef]
- Ulgezen, Z.N.; Van Dooremalen, C.; Van Langevelde, F. Understanding social resilience in honeybee colonies. Curr. Res. Insect Sci. 2021, 1, 100021. [Google Scholar] [CrossRef]
- Zioga, E.; White, B.; Stout, J.C. Honey bees and bumble bees may be exposed to pesticides differently when foraging on agricultural areas. Sci. Total Environ. 2023, 896, 166214. [Google Scholar] [CrossRef]
- Tosi, S.; Sfeir, C.; Carnesecchi, E.; van Engelsdorp, D.; Chauzat, M.P. Lethal, sublethal, and combined effects of pesticides on bees: A meta-analysis and new risk assessment tools. Sci. Total Environ. 2023, 844, 156857. [Google Scholar] [CrossRef] [PubMed]
- Stevanović, N.; Idbeaa, W.; Bošković, J.; Prodanović, R.; Vapa, I.; Bursić, V.; Puvača, N.; Vještica, S. Pesticide residues in different honey types and public health risk assessment. Acta Vet. Brno 2024, 93, 105–114. [Google Scholar] [CrossRef]
- Eissa, F.; Taha, E.K.A. Contaminants in honey: An analysis of EU RASFF notifications from 2002 to 2022. J. Consum. Prot. Food Saf. 2023, 18, 393–402. [Google Scholar] [CrossRef]
- Inaudi, P.; Garzino, M.; Abollino, O.; Malandrino, M.; Giacomino, A. Honey: Inorganic composition as possible marker for botanical and geological assignment. Molecules 2025, 30, 1466. [Google Scholar] [CrossRef] [PubMed]
- Zawawi, N.; Chong, P.J.; Mohd Tom, N.N.; Saiful Anuar, N.S.; Mohammad, S.M.; Ismail, N.; Jusoh, A.Z. Establishing relationship between vitamins, total phenolic and total flavonoid content and antioxidant activities in various honey types. Molecules 2021, 26, 4399. [Google Scholar] [CrossRef]
- Tlak Gajger, I.; Dar, S.A.; Ahmed, M.M.M.; Aly, M.M.; Vlainic, J. Antioxidant Capacity and Therapeutic Applications of Honey: Health Benefits, Antimicrobial Activity and Food Processing Roles. Antioxidants 2025, 14, 959. [Google Scholar] [CrossRef]
- Shakoori, Z.; Salaseh, E.; Mehrabian, A.R.; Tehrani, D.M.; Dardashti, N.F.; Salmanpour, F. The amount of antioxidants in honey has a strong relationship with the plants selected by honeybees. Sci. Rep. 2024, 14, 351. [Google Scholar] [CrossRef]
- Jaskiewicz, K.; Szczesna, T.; Jachuła, J. How phenolic compounds profile and antioxidant activity depend on botanical origin of honey—A case of polish varietal honeys. Molecules 2025, 30, 360. [Google Scholar] [CrossRef]
- Hernanz, D.; Jara-Palacios, M.J.; Santos, J.L.; Gómez Pajuelo, A.; Heredia, F.J.; Terrab, A. The profile of phenolic compounds by HPLC-MS in Spanish oak (Quercus) honeydew honey and their relationships with color and antioxidant activity. LWT 2023, 180, 114724. [Google Scholar] [CrossRef]
- Nyarko, K.; Boozer, K.; Greenlief, C.M. Profiling of the Polyphenol Content of Honey from Different Geographical Origins in the United States. Molecules 2023, 28, 5011. [Google Scholar] [CrossRef]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef]
- Peng Kek, S.; Ling Chin, N.; Yusof, Y.A.; Tan, S.W.; Chua, L.S. Total Phenolic Contents and Colour Intensity of Malaysian Honeys from the Apis spp. and Trigona spp. Bees. Agric. Agric. Sci. Procedia 2014, 2, 150–155. [Google Scholar] [CrossRef]
- Nesovic, M.; Gasic, U.; Tosti, T.; Trifkovic, J.; Baosic, R.; Blagojevic, S.; Ignjatovic, L.; Tesic, Z. Physicochemical analysis and phenolic profile of polyfloral and honeydew honey from Montenegro. RSC Adv. 2020, 10, 2462–2471. [Google Scholar] [CrossRef] [PubMed]
- Perna, A.; Intaglietta, I.; Simonetti, A.; Gambacorta, E. A comparative study on phenolic profile, vitamin C content and antioxidant activity of Italian honeys of different botanical origin. Int. J. Food Sci. Technol. 2013, 48, 1899–1908. [Google Scholar] [CrossRef]
- Stefanis, C.; Stavropoulou, E.; Giorgi, E.; Voidarou, C.; Constantinidis, T.C.; Vrioni, G.; Tsakris, A. Honey’s Antioxidant and Antimicrobial Properties: A Bibliometric Study. Antioxidants 2023, 12, 414. [Google Scholar] [CrossRef] [PubMed]
- Neo, R.; Gaonkar, P.; Huber, L.; Hlusko, K.C. Medical-grade honey has superior antibacterial properties against common bacterial isolates in wound cultures of dogs and cats in comparison to non-medical-grade honey types. Am. J. Vet. Res. 2024, 85, ajvr.24.07.0188. [Google Scholar] [CrossRef]
- Barreiros, J.; Cepeda, A.; Franco, C.; Nebot, C.; Vázquez, B. Analysis of minerals in honey and their nutritional implications. J. Food Compos. Anal. 2024, 136, 106733. [Google Scholar] [CrossRef]
- Khan, S.U.; Anjum, S.I.; Rahman, K.; Ansari, M.J.; Khan, W.U.; Kamal, S.; Khattak, B.; Muhammad, A.; Khan, H.U. Honey: Single food stuff comprises many drugs. Saudi J. Biol. Sci. 2018, 25, 320–325. [Google Scholar] [CrossRef]
- Palma-Morales, M.; Huertas, J.R.; Rodríguez-Pérez, C. A Comprehensive Review of the Effect of Honey on Human Health. Nutrients 2023, 15, 3056. [Google Scholar] [CrossRef]
- Tashkandi, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Ranneh, Y.; Akim, A.M.; Hamid, H.A.; Hamid, H.A.; Khazaai, H.; Fadel, A.; Zakaria, Z.A.; Albujja, M.; Abu Bakar, M.F. Honey and its nutritional and anti-inflammatory value. BMC Complement. Med. Ther. 2021, 21, 30. [Google Scholar] [CrossRef]
- Albaridi, N.A. Antibacterial Potency of Honey. Int. J. Microbiol. 2019, 2019, 2464507. [Google Scholar] [CrossRef]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a Potential Natural Antioxidant Medicine: An Insight into Its Molecular Mechanisms of Action. Oxidative Med. Cell. Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed]
- Eteraf-Oskouei, T.; Najafi, M. Uses of Natural Honey in Cancer: An Updated Review. Adv. Pharm. Bull. 2022, 12, 248–261. [Google Scholar] [CrossRef]
- Montenegro Rizzardini, G. (Ed.) Chile, Nuestra Flora Útil: Guía de Plantas de Uso Apícola Alimentario, Medicinal Folclórico, Artesanal y Ornamental; Universidad Católica de Chile: Santiago, Chile, 2000; p. 267. [Google Scholar]
- García, S.; Troncoso, J.M.; Rondanelli-Reyes, M. Study of honey according to botanical origin and physicochemical parameters in the Biobío Region, Chile. Chil. J. Agric. Res. 2020, 80, 675–685. [Google Scholar] [CrossRef]
- Montenegro, G.; Mejías, E. Biological applications of honeys produced by Apis mellifera. Biol. Res. 2013, 46, 341–345. [Google Scholar] [CrossRef]
- Biblioteca del Congreso Nacional de Chile BCN. Ley 21489: Ley de Promoción, Protección y Fomento de la Actividad Apícola; Ministerio de Agricultura, Biblioteca del Congreso Nacional de Chile: Valparaíso, Chile, 2022; Available online: https://bcn.cl/37xy9 (accessed on 31 July 2025).
- Oficina de Estudios y Políticas Agrarias ODEPA; Ministerio de Agricultura de Chile. Boletín Interactivo Apícola. 2025. Available online: https://apps.odepa.gob.cl/powerBI/boletin_apicola.html (accessed on 31 July 2025).
- Montenegro, G.; Gomez, M.; Diaz-Forestier, J.; Pizarro, R. Application of the Chilean Official Standard to designate the botanical origins of honey for the characterization of the apicultural production. Cienc. Investig. Agrar. 2008, 35, 181–190. [Google Scholar]
- Mejías, E.; Gómez, C.; Garrido, T. Suitable Areas for Apiculture Expansion Determined by Antioxidant Power, Chemical Profiles, and Pesticide Residues in Caldcluvia paniculata Honey and Beeswax Samples. Insects 2022, 13, 31. [Google Scholar] [CrossRef]
- Velásquez, P.; Montenegro, G.; Leyton, F.; Ascar, L.; Ramirez, O.; Giordano, A. Bioactive compounds and antibacterial properties of monofloral Ulmo honey. CyTA—J. Food 2019, 18, 11–19. [Google Scholar] [CrossRef]
- Muñoz, M.; Del Sol, M.; Vásquez, B. Antibacterial and wound-healing action of Ulmo honey (Eucryphia cordifolia) of differing degrees of purity. Front. Vet. Sci. 2023, 10, 1172025. [Google Scholar] [CrossRef]
- CXS 12-19811; Codex Alimentarius, International Foods Standards. Adopted in 1981. Amended in 2019. Food and Agriculture Organization of the United Nations: Rome, Italy, 2022.
- United Nations Development Programme—PNUD. Manejo Sustentable Del Bosque Nativo. 2016. Available online: https://www.estudiospnud.cl/publicaciones/manejo-sustentable-del-bosque-nativo/ (accessed on 31 July 2025).
- Marshall, L.; Ascher, J.S.; Villagra, C.; Beaugendre, A.; Herrera, V.; Henríquez-Piskulich, P.; Vera, A.; Vereecken, N.J. Chilean bee diversity: Contrasting patterns of species and phylogenetic turnover along a large-scale ecological gradient. Ecosphere 2023, 14, e4535. [Google Scholar] [CrossRef]
- Lobos, I.; Silva, M.; Ulloa, P.; Pavez, P. Mineral and Botanical Composition of Honey Produced in Chile’s Central-Southern Region. Foods 2022, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Parada, T.; Jara, C.; Lusk, C. Distribution of maximum heights of tree species in an old-growth Chilean rain forest. BOSQUE 2003, 24, 63–67. [Google Scholar]
- Servicio Agrícola y Ganadero–División de Protección Pecuaria. Boletin Apícola N 9. 2024. Available online: https://www.sag.gob.cl/sites/default/files/BOLET%C3%8DN%20AP%C3%8DCOLA%20N%C2%B09.pdf (accessed on 31 July 2025).
- Acevedo, F.; Torres, P.; Oomah, B.D.; De Alencar, S.M.; Prado-Massarioli, A.; Venegas, R.M.; Albarral-Ávila, V.; Burgos-Díaz, C.; Ferrer, R.; Rubilar, M. Volatile and non-volatile/semi-volatile compounds and in vitro bioactive properties of Chilean Ulmo (Eucryphia cordifolia Cav.) honey. Food Res. Int. 2017, 94, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Llorente, E.J.; Alvarado-Álvarez, H.J.; Castro-Cano, J.M.; Sosa-Arias, B.M.; Puga-Lascano, S.A. Evaluación del contenido de hidroximetilfurfural en miel comercial y artesanal de Los Ríos-Babahoyo. J. Sci. Res. 2023, 8, 57–79. [Google Scholar]
- Shapla, U.M.; Solayman, M.; Alam, N.; Khalil, M.I.; Gan, H.S. 5-Hydroxymethylfurfural (HMF) levels in honey and other food products: Effects on bees and human health. Chem. Cent. J. 2018, 12, 35. [Google Scholar] [CrossRef] [PubMed]
- Abraham, K.; Gürtler, R.; Berg, K.; Heinemeyer, G.; Lampen, A.; Appel, K.E. Toxicology and risk assessment of 5-Hydroxymethylfurfural in food. Mol. Nutr. Food Res. 2011, 55, 667–678. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef]
- Poulsen-Silva, E.; Gordillo-Fuenzalida, F.; Velásquez, P.; Llancalahuen, F.M.; Carvajal, R.; Cabaña-Brunod, M.; Otero, M.C. Antimicrobial, Antioxidant, and Anti-Inflammatory Properties of Monofloral Honeys from Chile. Antioxidants 2023, 12, 1785. [Google Scholar] [CrossRef]
- Sharaf El-Din, M.G.; Farrag, A.F.S.; Wu, L.; Huang, Y.; Wang, K. Health benefits of honey: A critical review on the homology of medicine and food in traditional and modern contexts. J. Tradit. Chin. Med. Sci. 2025, 12, 147–164. [Google Scholar] [CrossRef]
- Wilczynska, A.; Zak, N. Polyphenols as the Main Compounds Influencing the Antioxidant Effect of Honey—A Review. Int. J. Mol. Sci. 2024, 25, 10606. [Google Scholar] [CrossRef]
- Mandal, M.D.; Mandal, S. Honey: Its medicinal property and antibacterial activity. Asian Pac. J. Trop. Biomed. 2011, 2, 154–160. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978, 59, 139–157. [Google Scholar] [CrossRef]
- Abashidze, N.; Djafaridze, I.; Vanidze, M.; Khakhutaishvili, M.; Kharadze, M.; Kartsivadze, I.; Davitadze, R.; Kalandia, A. Physicochemical Characterization and Antioxidant Activity of Jara Honey Produced in Western Georgia. Appl. Sci. 2024, 14, 6874. [Google Scholar] [CrossRef]
- Lin, H.; Chang, Y.A. Carbohydrates Are Associated with the Flowering Ability of Oncidesa Gower Ramsey ‘Honey Angel’. HortScience 2024, 59, 348–354. [Google Scholar] [CrossRef]
- Lupu, D.; Sammani, M.S.; Palacio, E.; Hancu, G.; Ferrer, L. Comprehensive honey analysis: A novel HPLC-DAD method for hydroxymethylfurfural quantification and quality evaluation via diastase activity and sugar profile determination. J. Food Compos. Anal. 2025, 137, 106925. [Google Scholar] [CrossRef]
- Kamal, M.A.; Klein, P. Determination of sugars in honey by liquid chromatography. Saudi J. Biol. Sci. 2011, 18, 17–21. [Google Scholar] [CrossRef]
- Paula, V.B.; Sousa-Dias, M.L.; Seixas, N.L.; Combarros-Fuertes, P.; Estevinho, L.M.; Dias, L.G. Phenolic Class Analysis Honey: Comparison of Classical and Single UV Spectrum Methodologies. Processes 2024, 12, 2297. [Google Scholar] [CrossRef]
- Deflaoui, L.; Setyaningsih, W.; Palma, M.; Mekhoukhe, A.; Tamendjari, A. Phenolic compounds in olive oil by solid phase extraction—Ultra performance liquid chromatography—Photodiode array detection for varietal characterization. Arab. J. Chem. 2021, 14, 103102. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar] [CrossRef]
- Mejías, E.; Montenegro, G. The Antioxidant Activity of Chilean Honey and Bee Pollen Produced in the Llaima Volcano’s Zones. J. Food Qual. 2012, 35, 315–322. [Google Scholar] [CrossRef]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Dimitriu, L.; Constantinescu-Aruxandei, D.; Preda, D.; Nichițean, A.-L.; Nicolae, C.-A.; Faraon, V.A.; Ghiurea, M.; Ganciarov, M.; Băbeanu, N.E.; Oancea, F. Honey and its biomimetic deep eutectic solvent modulate the antioxidant activity of polyphenols. Antioxidants 2022, 11, 2194. [Google Scholar] [CrossRef] [PubMed]
- Bertoncelj, J.; Doberšek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Nobari Moghaddam, H.; Alaeepajouh, S.; Behzad, M.; Hajimahmoodi, M.; Sadeghi, N. Evaluating the antioxidant activity and the level of 5-hydroxymethylfurfural in honey. J. Food Saf. Hyg. 2024, 9, 241–251. [Google Scholar] [CrossRef]


| Predominant Botanical Species | ||||
|---|---|---|---|---|
| Sample | Geographical Origin | Scientific Name | Common Name | Predominance (%) |
| 1 | Futrono Futrono | Caldcluvia paniculata | Tiaca | 63 |
| 2 | Futrono Futrono | Caldcluvia paniculata | Tiaca | 71 |
| 3 | Cerrillos Futrono | Eucryphia cordifolia | Ulmo | 88 |
| 4 | Llifén Futrono | Weinmannia trichosperma | Tineo | 58 |
| 5 | Llifén Futrono | Weinmannia trichosperma | Tineo | 61 |
| 6 | Cerrillos Futrono | Eucryphia cordifolia | Ulmo | 93 |
| 7 | Santa Juana Futrono | Caldcluvia paniculata | Tiaca | 78 |
| 8 | Futrono Futrono | Eucryphia cordifolia | Ulmo | 86 |
| 9 | Cerrillos Futrono | Eucryphia cordifolia | Ulmo | 79 |
| 10 | Llifén Futrono | Weinmannia trichosperma | Tineo | 55 |
| 11 | Llifén Futrono | Caldcluvia paniculata | Tiaca | 68 |
| 12 | Futrono Futrono | Weinmannia trichosperma | Tineo | 54 |
| 13 | Santa Juana Futrono | Eucryphia cordifolia | Ulmo | 95 |
| 14 | Llifén Futrono | Eucryphia cordifolia | Ulmo | 84 |
| 15 | Futrono Futrono | Eucryphia cordifolia | Ulmo | 89 |
| 16 | Futrono Futrono | Weinmannia trichosperma | Tineo | 52 |
| 17 | Santa Juana Futrono | Caldcluvia paniculata | Tiaca | 77 |
| 18 | Cerrillos Futrono | Caldcluvia paniculata | Tiaca | 71 |
| 19 | Llifén Futrono | Weinmannia trichosperma | Tineo | 60 |
| 20 | Cerrillos Futrono | Eucryphia cordifolia | Ulmo | 91 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mejías, E.; Gómez, C.; Díaz, P.; Garrido, T. Natural Biological Properties Inherited from Native Endemic Flora in Honeys from Lake Ranco Area of Southern Chile: A Botanical and Physicochemical Approach. Molecules 2025, 30, 3984. https://doi.org/10.3390/molecules30193984
Mejías E, Gómez C, Díaz P, Garrido T. Natural Biological Properties Inherited from Native Endemic Flora in Honeys from Lake Ranco Area of Southern Chile: A Botanical and Physicochemical Approach. Molecules. 2025; 30(19):3984. https://doi.org/10.3390/molecules30193984
Chicago/Turabian StyleMejías, Enrique, Carlos Gómez, Pablo Díaz, and Tatiana Garrido. 2025. "Natural Biological Properties Inherited from Native Endemic Flora in Honeys from Lake Ranco Area of Southern Chile: A Botanical and Physicochemical Approach" Molecules 30, no. 19: 3984. https://doi.org/10.3390/molecules30193984
APA StyleMejías, E., Gómez, C., Díaz, P., & Garrido, T. (2025). Natural Biological Properties Inherited from Native Endemic Flora in Honeys from Lake Ranco Area of Southern Chile: A Botanical and Physicochemical Approach. Molecules, 30(19), 3984. https://doi.org/10.3390/molecules30193984







