Abstract
Photocatalytic conversion of CO2 to hydrocarbon fuels offers a promising pathway for sustainable renewable energy production. In this study, a ZnO/ZnFe2O4 composite featuring a Type-II heterojunction was synthesized through a facile one-step hydrothermal approach, significantly enhancing visible-light-driven CO2 reduction activity. The optimized catalyst exhibits CH4 and CO production rates that are 3.3 and 4.9 times higher, respectively, than those of pristine ZnFe2O4 over 6 h. This significant enhancement in photocatalytic performance is attributed to the Type-II band alignment, which not only broadens light absorption but also greatly promotes efficient charge separation. It is corroborated by a series of experimental evidence: a two-fold enhancement in photocurrent response, a 15.1% reduction in PL intensity, decreased electrochemical impedance, and an extended charge carrier lifetime. Furthermore, in situ FTIR spectroscopy confirms that the heterojunction facilitates the formation of key intermediates (specifically *COOH and HCOO−). This study highlights the importance of precise interface design based on a Type-II heterojunction in heterostructured composite catalysts and provides mechanistic insights for developing highly efficient CO2 photoreduction systems.
1. Introduction
The rapid development of global industrialization and urbanization has led to a severe environmental crisis caused by CO2, posing significant environmental challenges for researchers. Currently, mitigating greenhouse gases primarily through photocatalysis, electrocatalysis, and carbon capture is anticipated to be a key strategy for addressing this crisis [1,2,3,4]. Numerous semiconductor and photocatalytic materials, such as TiO2, ZnO, ZnIn2S4, CeO2, and ZnFe2O4, have been developed [5,6,7,8,9,10]. Among these, photocatalysis represents a widely used green synthesis method in environmental science. It offers advantages including environmental friendliness, lower production costs, and safety. The synthesized nanoparticles exhibit enhanced biocompatibility and good performance, making them suitable for various applications like wastewater treatment, environmental protection, and other bio-related fields [11,12].
However, utilizing light energy to drive the conversion of CO2 into carbon-based fuels is challenging due to the high stability and low reactivity of the CO2 molecule, attributed to the high dissociation energy of its C=O bond. The conversion of solar energy through mild light-driven chemical reactions is of profound importance for developing green and sustainable energy [13]. Related studies have demonstrated that ZnFe2O4 nanoparticles possess strong antioxidant activity and high safety. As a novel narrow-bandgap semiconductor material, nano-sized zinc ferrite has been extensively researched and applied in areas such as magnetism, photocatalysis, and energy storage [14,15,16]. Deng et al. [17] prepared monodisperse ferrite microspheres using a simple one-step solvothermal method. Modulating the hydrophilicity and biocompatibility of these micro/nanomaterials is expected to broaden their applications in advanced magnetic materials, ferromagnetic fluid technology, and biomedicine. Zinc ferrite nanomaterials are synthesized via a variety of methods, including mechanical ball milling, sol-gel, solvothermal synthesis, chemical vapor deposition, and microwave-assisted combustion. The advantages of each method are accompanied by certain trade-offs, such as encompassing elevated impurity levels in the final product, procedures that are both cumbersome and time-consuming, or a dependence on stringent conditions like high temperature and pressure [18]. Therefore, more effective synthesis methods are needed to address these material shortcomings.
Meanwhile, ZnO has a narrow light absorption range, primarily limited to ultraviolet (UV) light, which constitutes only about 4% of solar radiation, thus restricting the full utilization of sunlight. Furthermore, the rapid recombination of photogenerated carriers prevents effective separation of electron–hole pairs and their transfer to the catalyst surface for participation in photocatalytic reactions, leading to reduced photocatalytic activity. Strategies reported to enhance the catalytic activity of ZnO include morphology control, ion doping, semiconductor coupling, and surface noble metal deposition. However, these approaches often suffer from drawbacks such as complex preparation processes and the requirement for specialized equipment like xenon lamps or microwaves during catalysis [19,20,21,22].
ZnO is an excellent photocatalyst due to its high electron mobility and fast photogenerated electron transfer rate. However, its wide bandgap restricts light absorption to the UV region. Conversely, ZnFe2O4 has a narrower bandgap and strong visible light absorption capability, but suffers from rapid electron–hole recombination and low photocatalytic activity. In this study, a ZnO/ZnFe2O4 composite was synthesized via a simple and feasible hydrothermal method. Interface engineering was employed to enhance the visible-light photocatalytic activity, and the corresponding CO2 reduction performance was systematically evaluated [23,24,25,26].
2. Results and Discussion
2.1. Interface Structure Construction and Physicochemical Properties
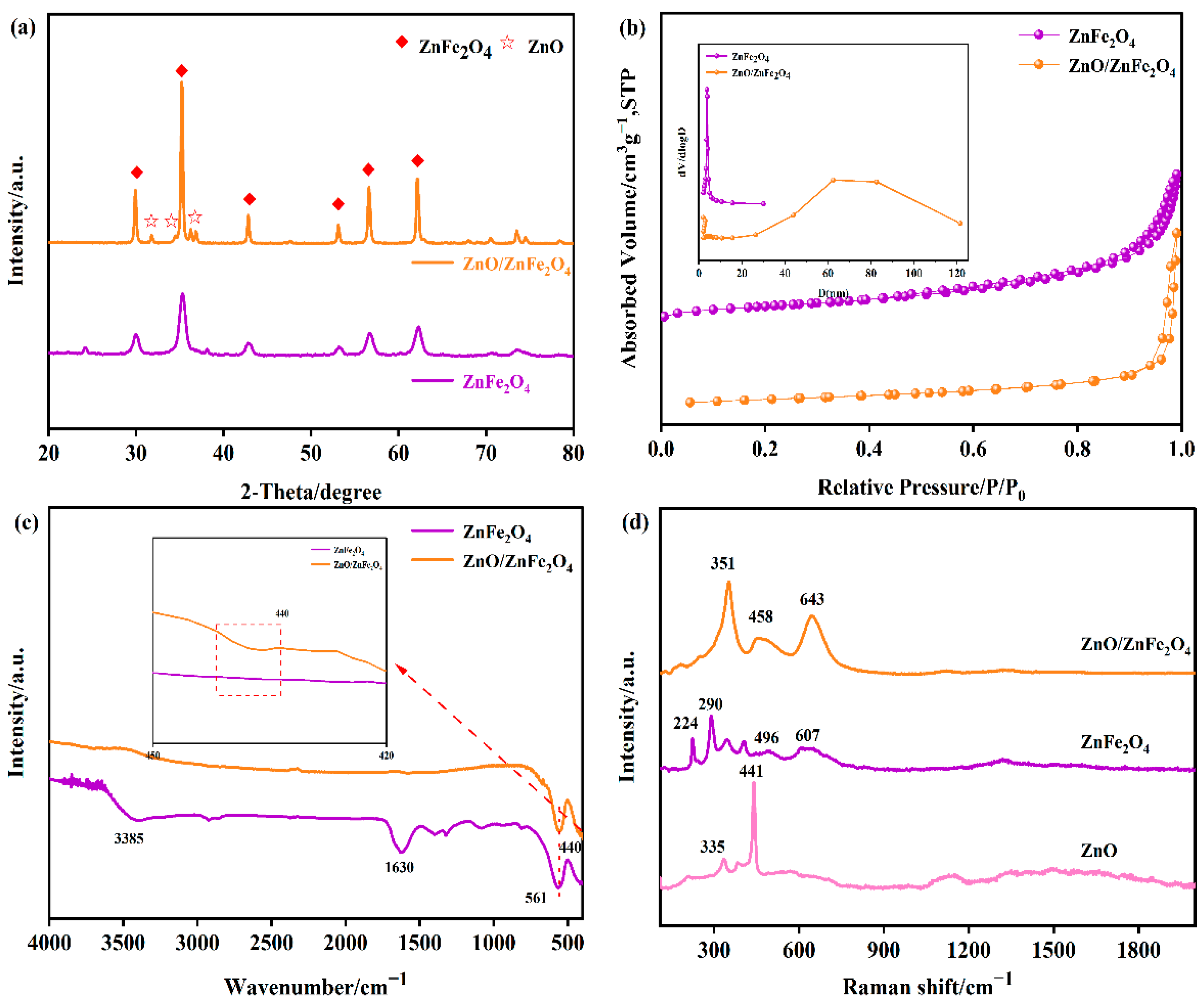
Figure 1a presents the XRD patterns of the synthesized materials. For pure ZnFe2O4, the characteristic diffraction peaks observed at 2θ = 29.9°, 35.3°, 42.8°, 53.1°, 56.6°, and 62.2° can be assigned to the (220), (311), (400), (422), (511), and (440) crystal planes, respectively. These peaks match well with the standard pattern for cubic spinel ZnFe2O4 (JCPDS No. 79-1105), confirming its phase purity and structure. In the XRD patterns of the ZnO/ZnFe2O4 composites, additional distinct diffraction peaks appear at 2θ = 31.8°, 34.4°, 36.2°, and 67.9°. These peaks are indexed to the (100), (002), (101), and (112) planes of hexagonal wurtzite ZnO (JCPDS No. 36-1451), confirming the successful formation of the composite [14,15]. Alongside these ZnO peaks, the characteristic peaks of the cubic spinel ZnFe2O4 phase (e.g., (311), (400), (422), (511), (440)) remain present. More importantly, the composite exhibits notable structural improvements in the ZnFe2O4 phase compared to pure ZnFe2O4. Among these, the crystallinity of ZnO/ZnFe2O4 is significantly enhanced, as evidenced by the markedly reduced full width at half maximum (FWHM) of the (311) peak from 0.84 to 0.27, accompanied by a substantial increase in peak intensity. These changes suggest improved crystallite growth and structural ordering within the composite, as summarized in Table S1. Applying Scherrer’s formula to this peak reveals a substantial increase in the average crystallite size of ZnFe2O4, from approximately 10.7 nm in the pure phase to 32.6 nm in the composite (Table 1). The diffraction peaks associated with ZnFe2O4 in the composite become noticeably sharper. These observed changes-reduced FWHM (increased crystallite size), lattice contraction, and peak sharpening-collectively indicate enhanced crystallinity and reduced lattice strain within the ZnFe2O4 phase in the composite. These results suggest that the presence of ZnO induces interfacial tensile strain and simultaneously promotes the growth of ZnFe2O4 crystallites [10,17,19]. The presence of ZnO promotes stress release at the interface, facilitates the formation of a well-defined heterojunction, lowers the surface energy, and drives the orderly growth of ZnFe2O4 crystallites. The successful integration of ZnO with ZnFe2O4, leading to these distinct structural modifications, is thus clearly evidenced by the XRD analysis.
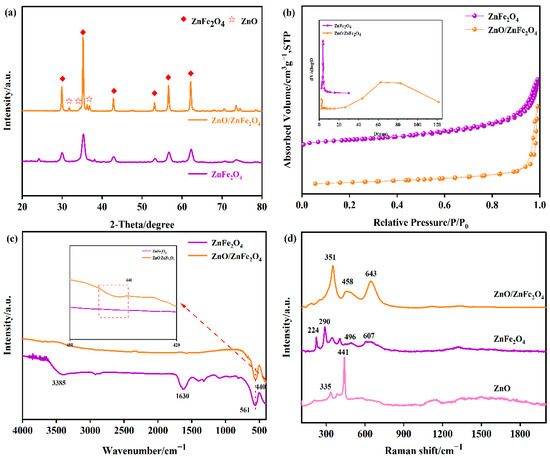
Figure 1.
(a) XRD patterns of the synthesized ZnFe2O4, ZnO/ZnFe2O4, (b) N2 adsorption-desorption isotherms and PSD curves (inset), (c) FT-IR spectra of samples ZnFe2O4 and ZnO/ZnFe2O4 catalysts, (d) Raman spectra of samples ZnFe2O4 and ZnO/ZnFe2O4 catalysts with different nanomorphologies.

Table 1.
XRD structural parameter of samples ZnO and ZnFe2O4.
Figure 1b displays the N2 adsorption-desorption isotherms along with the corresponding pore size distribution curves for both ZnFe2O4 and ZnO/ZnFe2O4 samples. The isotherm for pure ZnFe2O4 is classified as type IV, showing an H1-type hysteresis loop occurring at relative pressures (P/Po) between 0.9 and 1.0. This characteristic loop signifies the occurrence of capillary condensation, and its steep profile suggests the presence of relatively uniform cylindrical mesopores. The specific surface area (SSA) and total pore volume of ZnFe2O4 were determined to be 115.97 m2/g and 0.137 cm3/g, respectively. The pore size distribution (PSD) analysis (Table S2) indicates that the pores are predominantly concentrated within the 1–10 nm range, with an average pore size of 4.73 nm. The ZnO/ZnFe2O4 composite also displays a type IV isotherm with an H1-type hysteresis loop (P/Po = 0.9–1.0). Notably, the hysteresis loop appears steeper and narrower compared to that of pure ZnFe2O4, suggesting improved pore uniformity due to the homogeneous distribution of ZnFe2O4 nanoparticles on ZnO. However, a significant alteration is evident in the PSD curve of ZnO/ZnFe2O4. While mesopores (1–10 nm) remain present, a distinct new peak emerges in the 50–80 nm range, indicative of macropore formation. This shift is presumably attributable to the formation of interstitial voids resulting from the stacking of ZnFe2O4 nanoparticles on the lamellar ZnO structures with varied thicknesses during the multi-phase modification process. Consequently, the composite exhibits a significantly reduced SSA and an increased average pore size of 33.44 nm. The coexistence of mesopores and macropores, along with the larger average pore size in ZnO/ZnFe2O4, may facilitate the diffusion of reactant molecules within the catalyst structure [22].
As evidenced by the FT-IR spectrum (Figure 1c), the broad absorption band centered at 3385 cm−1 in ZnFe2O4 is associated with the O-H stretching vibration of adsorbed surface water. The distinct medium-intensity peak at 561 cm−1 is assigned to the Fe-O stretching vibration, confirming the successful synthesis of ZnFe2O4. In the spectra of the ZnO/ZnFe2O4 composites, the Fe-O stretching vibration remains clearly visible at 561 cm−1. Additionally, a distinct peak appears at 440 cm−1, assigned to the Zn-O stretching vibration within the tetrahedral sites of ZnO. The presence of both characteristic Zn-O and Fe-O vibration bands, coupled with the absence of other significant impurity peaks, confirms the successful synthesis of high-purity ZnO/ZnFe2O4 composites.
Raman spectra (Figure 1d) further reveal the coexistence of spinel-type ZnFe2O4 (A1g mode at 607 cm−1) and wurtzite ZnO (E2(high) mode at 441 cm−1) in the composite. Notably, both peaks exhibit significant sharpening and blue shifts (Δν = +36 cm−1 for A1g, Δν = +17 cm−1 for E2(high)), primarily resulting from interfacial lattice strain and strengthened Zn-O-Fe bonding at the heterojunction interface.
These observations are strongly supported by XRD analysis (Figure 1a), which shows lattice contraction, peak sharpening, and enhanced intensity, collectively indicating that interfacial engineering optimizes crystallinity while introducing controlled microstrain [23].
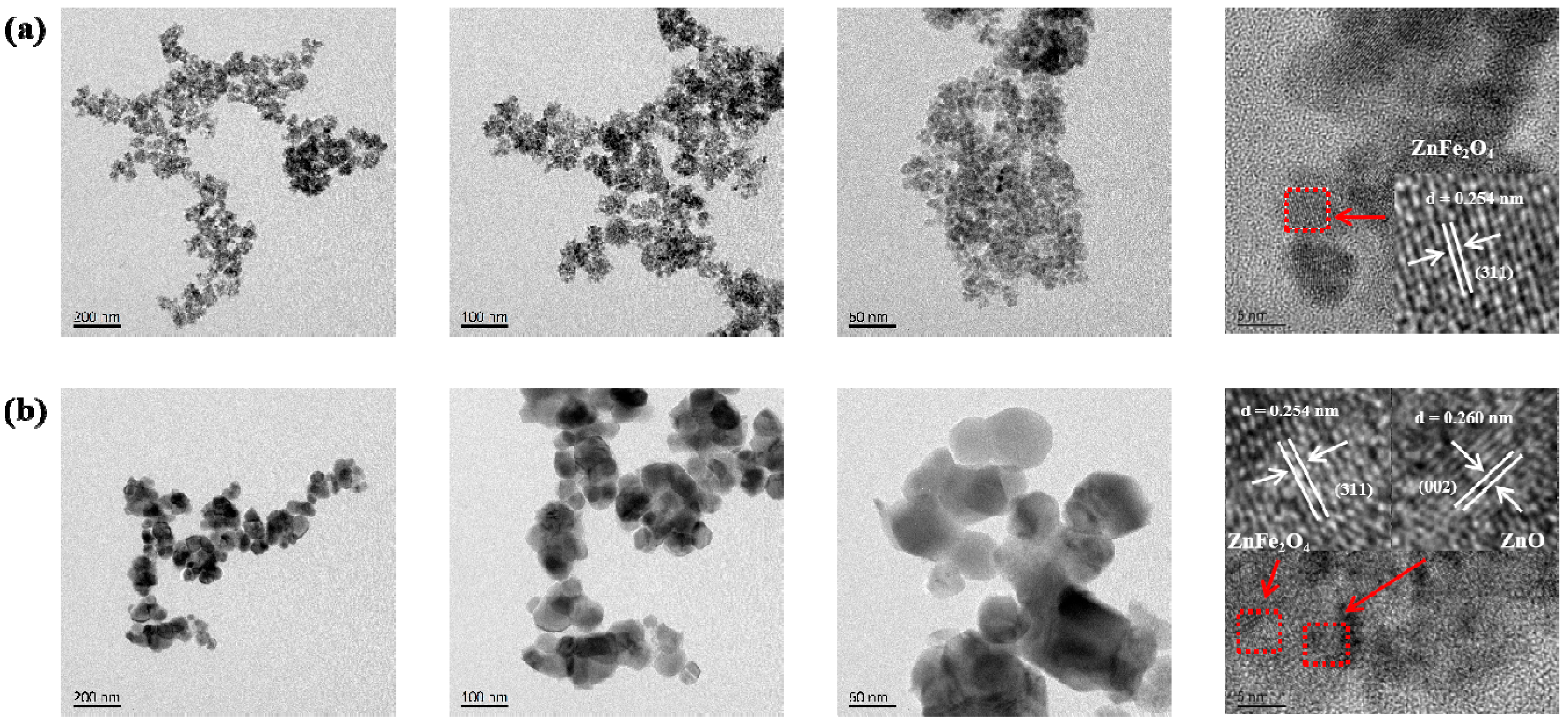
As shown in the TEM image of the pristine ZnFe2O4 (Figure 2a), the material consists of an aggregation of quasi-spherical particles. These particles possess a small grain size, predominantly within the 6–12 nm range. High-resolution imaging identifies distinct lattice fringes with a d-spacing of 0.254 nm, corresponding to the (311) plane of ZnFe2O4. Figure 2b displays the TEM image of the ZnO/ZnFe2O4 composite. In contrast to the pure phase, the composite exhibits a ZnO substrate decorated with well-dispersed ZnFe2O4 nanoparticles, with particle sizes ranging from approximately 30 to 150 nm. Well-defined lattice fringes are observed, with spacings of 0.260 nm and 0.254 nm, assignable to the (002) plane of ZnO and the (311) plane of ZnFe2O4, respectively. These clear lattice resolutions indicate high crystallinity within the composite, consistent with the sharpening of ZnFe2O4 diffraction peaks observed by XRD. The TEM results confirm the successful construction of a ZnO/ZnFe2O4 composite with intimate interfacial contact. The distinct lattice mismatch (d = 0.260 nm vs. d = 0.254 nm) and the observed interfacial coupling provide clear evidence for the formation of a well-coupled interface between the ZnO and ZnFe2O4 phases [27].
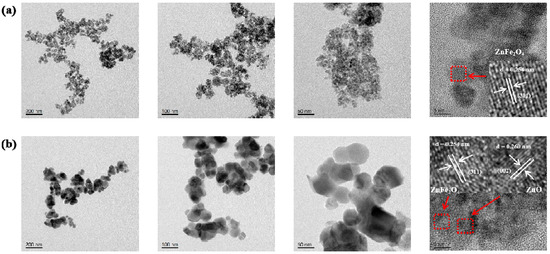
Figure 2.
TEM and HRTEM images of (a) ZnFe2O4, (b) ZnO/ZnFe2O4.
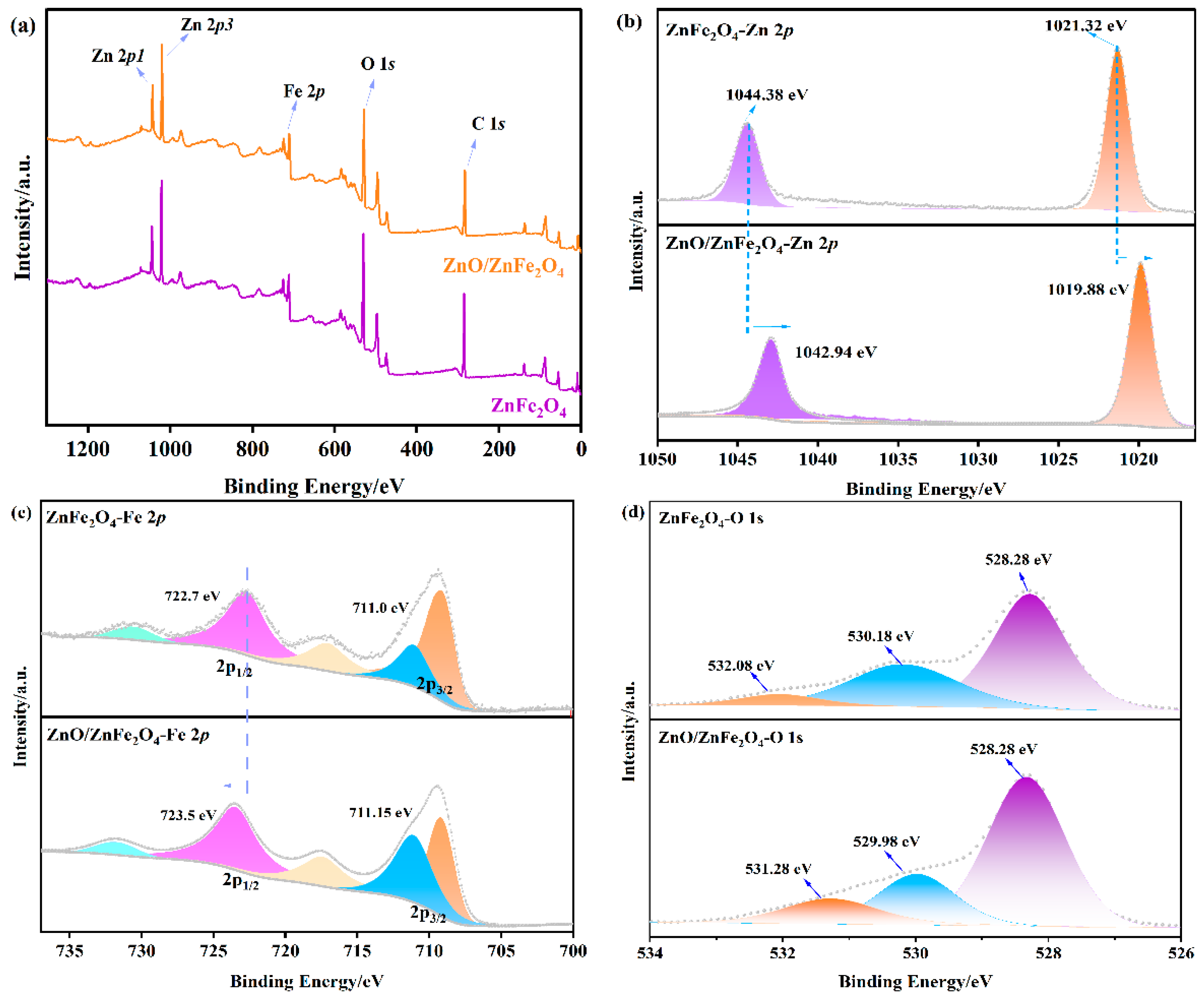
XPS measurements were conducted to examine the chemical states and interfacial characteristics of the samples. The survey scan (Figure 3a) verifies the existence of Zn, Fe, O, and C. In the Fe 2p region (Figure 3c), the peaks observed at 722.7 eV (Fe 2p1/2) and 711.0 eV (Fe 2p3/2) are indicative of Fe2+ and Fe3+ species, respectively, consistent with the Fe-O and Fe-O-Zn bonding environments in ZnFe2O4. Notably, in the ZnO/ZnFe2O4 composite, the Fe 2p peaks exhibit a positive shift of approximately 0.8 eV, while the Zn 2p peaks show a negative shift of about 1.4 eV. The Zn 2p spectrum of pristine ZnFe2O4 (Figure 3b) shows binding energies of 1021.32 eV (Zn 2p3/2) and 1044.38 eV (Zn 2p1/2), characteristic of Zn2+. In the ZnO/ZnFe2O4 composite, these peaks shift negatively to 1019.88 eV and 1042.94 eV, respectively. The large negative shift in Zn 2p (1.4 eV) exceeds typical heterojunction values (0.1−0.5 eV), suggesting contributions from interfacial dipole formation and local coordination environment changes in addition to charge transfer. These opposing shifts suggest a redistribution of electron density at the interface, consistent with electron transfer from ZnFe2O4 to ZnO. Quantitative analysis of XPS-derived elemental molar ratios reveals Zn and Fe ratios of ≈0.7:1 and 3.0:1, respectively (Table S3). The Fe ratio (3.0:1) closely corresponds to the CO/CH4 production rate ratio, providing a quantitative correlation between electronic structure modification and catalytic performance [19,22]. The observed increase in electron density around Zn atoms further supports this charge transfer mechanism. The O 1s spectrum (Figure 3d) exhibits three contributions: lattice oxygen in Zn-O (528.28 eV), Fe-O (530.18 eV), and surface-adsorbed water (532.08 eV). After the formation of the composite, the relative concentration of oxygen vacancies (associated with the 530.18 eV peak) increases from 21.3% to 25.3% (Table 2), providing more active sites for CO2 reduction. These results collectively demonstrate strong interfacial electronic interaction between ZnO and ZnFe2O4, which enhances the material’s catalytic properties [28].
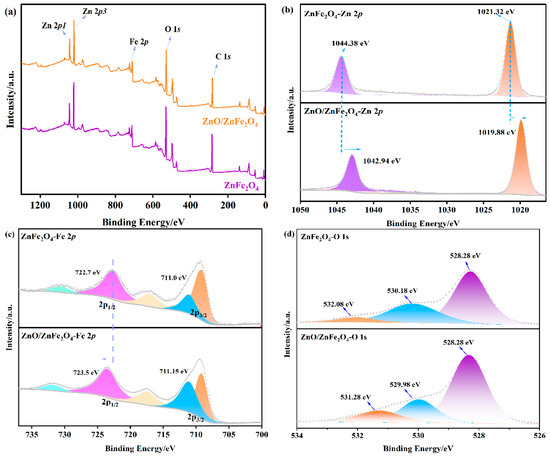
Figure 3.
XPS of (a) survey spectra, (b) Fe 2p, (c) Zn 2p, and (d) O 1s of ZnFe2O4 and ZnO/ZnFe2O4.

Table 2.
The chemical state of Zn, Fe and O in the catalysts.
2.2. Photoelectric Characteristics and Charge Separation Effect
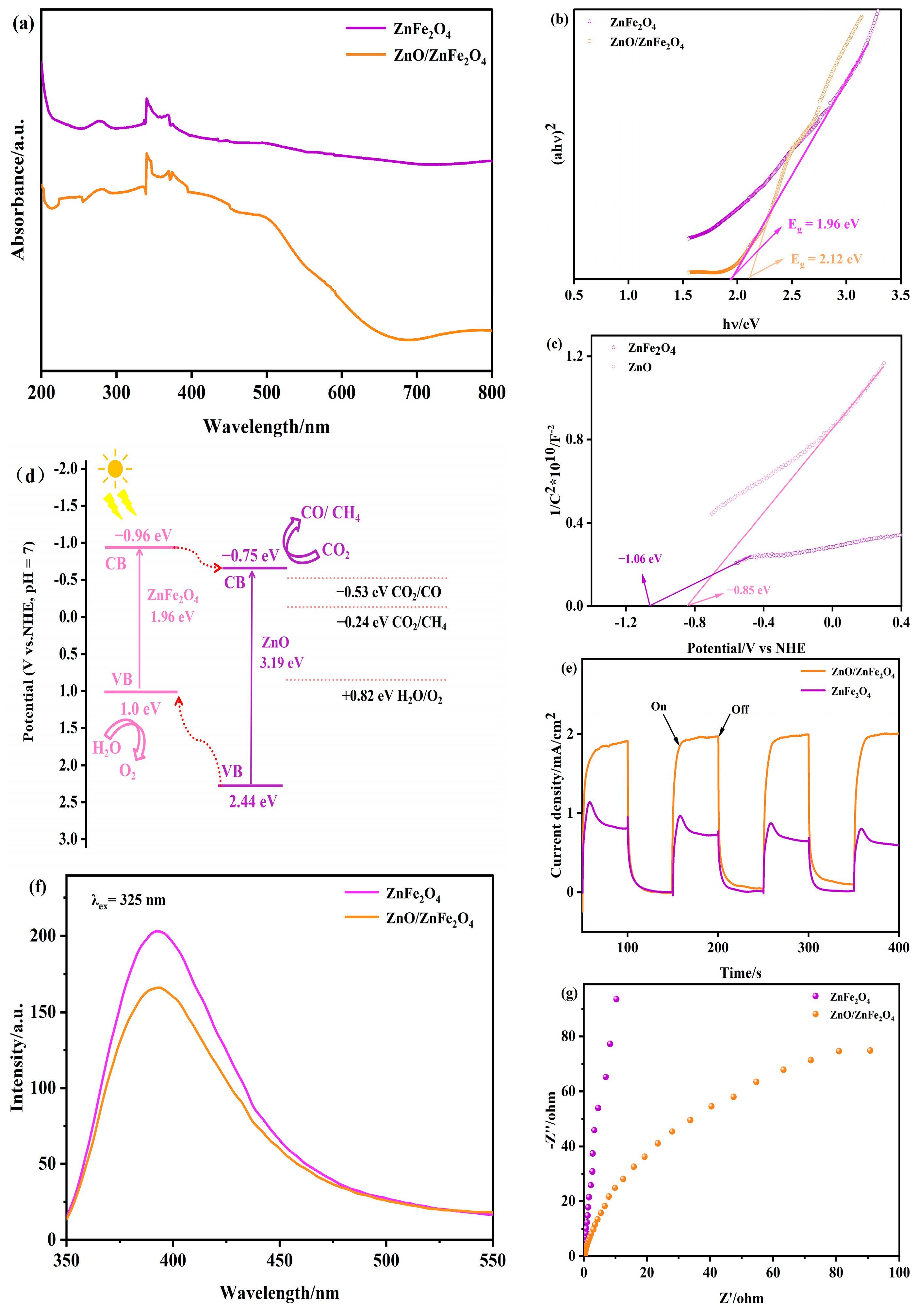
Figure 4a–c presents the UV-visible diffuse reflectance spectra (DRS) of ZnFe2O4 and ZnO/ZnFe2O4 composites. The ZnO/ZnFe2O4 sample exhibits significantly enhanced light absorption across both UV and visible regions (500−700 nm) compared to pristine ZnFe2O4, along with a broader visible-light response range. This pronounced absorption enhancement indicates improved photocatalytic potential. The optical bandgaps (Eg) determined from the absorption onset (λg) via the tangent method (Figure 4b) are 1.96 eV for ZnFe2O4 and 2.12 eV for ZnO/ZnFe2O4, calculated using Eg = 1240/λg (eV). The observed Eg widening in the composite is attributed to interfacial lattice strain (consistent with the Raman blue-shift). While bandgap widening typically reduces photoabsorption, the introduction of ZnO (Eg ≈ 3.2 eV) extends the UV response. Importantly, the net enhancement in visible-light absorption originates from two synergistic effects: an increase in oxygen vacancies introducing defect states within the bandgap, as corroborated by XPS O 1s analysis, and interfacial engineering-induced band tailing that broadens the optical response range [25,27,28].
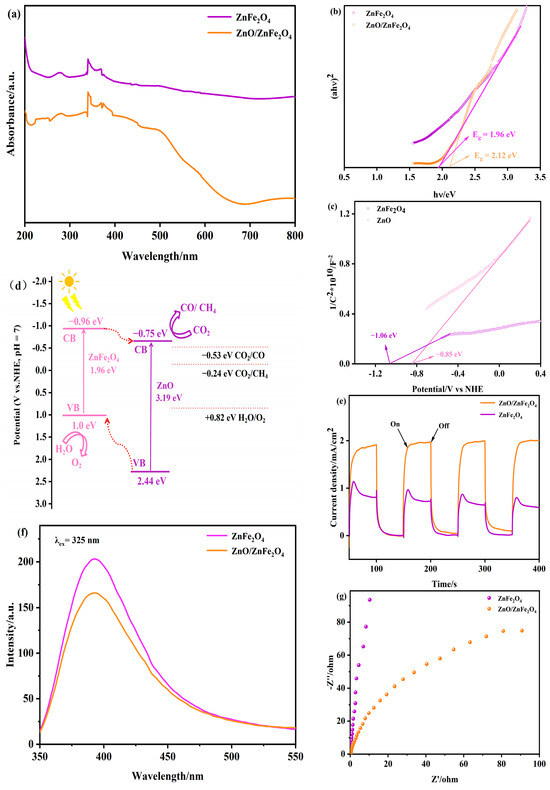
Figure 4.
(a–c) UV-vis DRS, (d) proposed photocatalytic mechanism, (e) transient photocurrent response plots, (f) PL, and (g) EIS of ZnO and ZnO/ZnFe2O4.
The Mott-Schottky curves (Figure 4c) exhibit positive slopes for both materials, confirming n-type semiconductor behavior [29]. The flat-band potential (EFB) approximates the conduction band potential (ECB) within ±0.1 eV for n-type semiconductors. The tangent extrapolation method yields ECB values of −0.96 V (vs. NHE) for ZnFe2O4 and −0.75 V (vs. NHE) for ZnO (Figure S1). Using the relation ECB = EVB − Eg [30], the valence band potentials (EVB) are calculated as +1.0 V and +2.44 V (vs. NHE) for ZnFe2O4 and ZnO, respectively.
Based on the band alignments, a type-II heterojunction is formed between ZnO and ZnFe2O4. This configuration promotes the transfer of photogenerated electrons from the conduction band of ZnFe2O4 (−0.96 eV) to that of ZnO (−0.75 eV), thereby facilitating CO2 reduction on the ZnO surface. Simultaneously, photogenerated holes migrate from the valence band of ZnO (+2.44 eV) to that of ZnFe2O4 (+1.0 eV), where oxidation reactions occur (Figure 4d). This charge separation mechanism is further supported by the enhanced photocurrent response (Figure 4e–g) and reduced charge transfer resistance observed in EIS measurements [31].
The efficient spatial separation of electrons and holes suppresses recombination, as confirmed by the 15.1% decrease in PL intensity (Figure 4f, Table S4). The synergistic effect of improved charge separation and widened visible-light absorption collectively enhances the photocatalytic CO2 reduction performance of the ZnO/ZnFe2O4 composite.
2.3. Photocatalytic Performance
The photocatalytic CO2 reduction performance was evaluated in a solid-gas phase reaction system containing CO2 and water vapor under irradiation from a 300 W xenon lamp (λ ≥ 420 nm). For each test, 20 mg of catalyst was evenly spread on a sample holder. The reaction atmosphere, consisting of high-purity CO2 and water vapor, was introduced by injecting 0.3 mL of deionized water into the reactor as the proton source. The products were analyzed using an online gas chromatograph (GC9790II) equipped with a flame ionization detector (FID) and a thermal conductivity detector (TCD), using high-purity argon as the carrier gas.
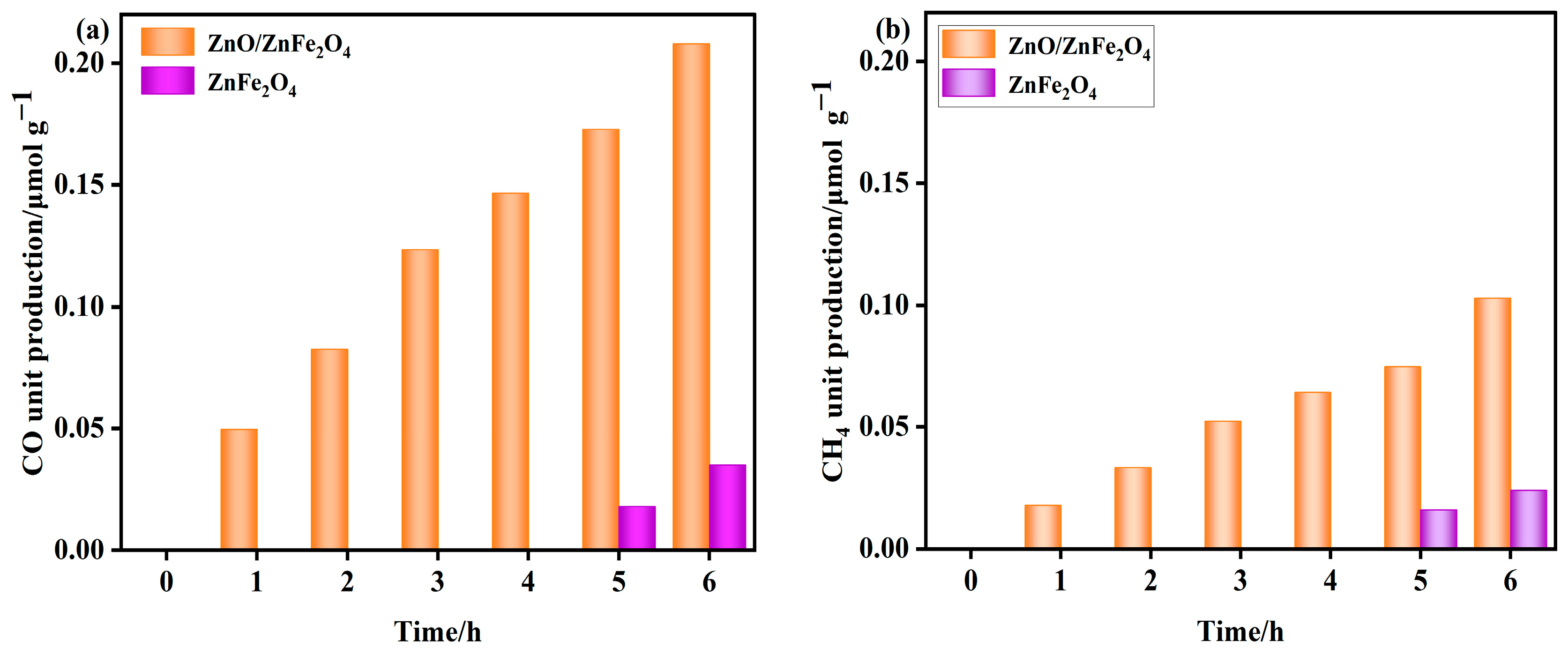
As shown in Figure 5, the ZnFe2O4 sample produced no detectable CH4 or CO during the initial 4 h. Trace amounts of CH4 (0.016−0.024 μmol·g−1) and CO (0.018−0.035 μmol·g−1) were observed between 5 and 6 h.
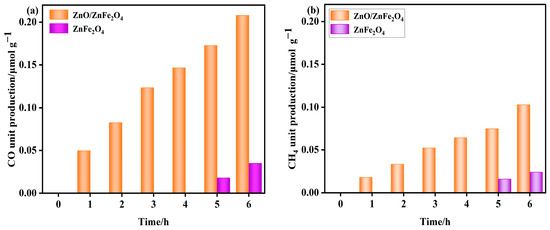
Figure 5.
(a,b) Product unit yields of ZnO and ZnO/ZnFe2O4 catalysts (unit yield of CO and CH4).
In contrast, the ZnO-decorated ZnFe2O4 composite exhibited significantly enhanced photocatalytic activity. CH4 and CO were detected from the first hour onward, with cumulative yields reaching 0.103 μmol·g−1 and 0.208 μmol·g−1 after 6 h, respectively. These values correspond to 3.3-fold and 4.9-fold enhancements in CH4 and CO production compared to pure ZnFe2O4. Moreover, the composite demonstrated excellent stability over the reaction period.
The markedly improved activity and earlier onset of CO2 reduction indicate that interface engineering through ZnO decoration plays a critical role in facilitating catalytic performance. The modified interface provides abundant active sites, enhances CO2 adsorption and activation, and promotes efficient charge separation by suppressing the recombination of photoinduced charge carriers throughout the ZnFe2O4 matrix. These synergistic effects collectively contribute to the superior CO2 reduction performance of the ZnO/ZnFe2O4 composite [32].
2.4. Mechanism of Photocatalytic CO2 Reduction
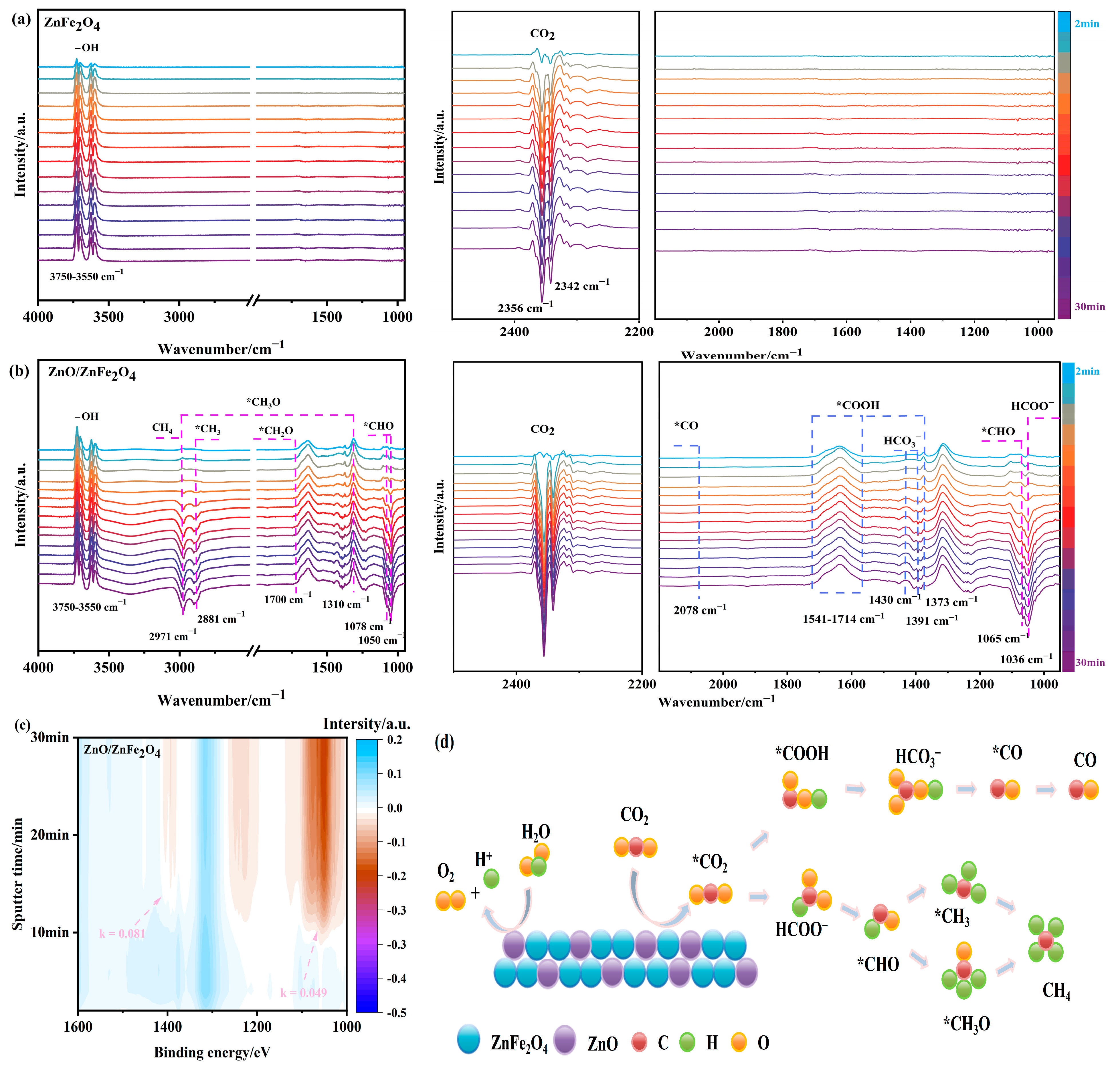
As shown in Figure 6a, ZnFe2O4 exhibits characteristic -OH stretching vibrations in the range of 3750–3550 cm−1, along with signals at 2356 cm−1 and 2342 cm−1 corresponding to physically adsorbed CO2. The intensity of these adsorption peaks increases over time; however, no clear evidence of CO2 reduction intermediates or products is observed within 30 min of light irradiation, indicating limited photocatalytic activity. This result is consistent with the delayed production of CO and CH4 until the 5th hour during activity tests.
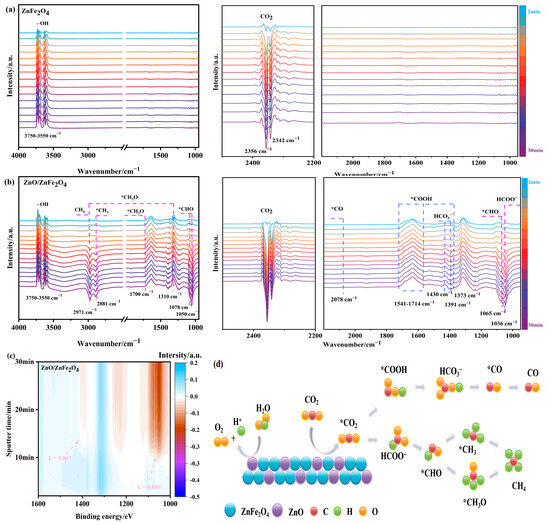
Figure 6.
(a–c) In situ FTIR and (d) the possible processes for CO2 reduction of ZnO/ZnFe2O4.
In contrast, the ZnO/ZnFe2O4 catalyst shows enhanced signals associated with -OH (3750–3550 cm−1) and CO2 (2356 cm−1 and 2342 cm−1) along with the rapid appearance of reduction intermediates under illumination, thereby facilitating the subsequent photocatalytic reduction process. As illustrated in Figure 6b, the appearance of a formate (HCOO−) peak at 1036 cm−1, as well as signals corresponding to key C1 pathway intermediates—such as *CHO at 1050, 1065, and 1078 cm−1, *CH3O at 1310 and 2971 cm−1, and formyl (*CHO) at 1700 cm−1—confirms active carbon hydrogenation. The emergence of a *CH3 peak at 2881 cm−1 and the gradual intensification of the 2971 cm−1 peak after 12 min indicate the formation of C-H-bonded species, suggesting CH4 generation (the adsorbed methyl vibration peak of CH4) [33]. Additionally, intermediates critical for CO formation are identified: *COOH at 1373 cm−1 and 1541–1714 cm−1, HCO3− at 1391 and 1430 cm−1 (protonated precursor), and weakly adsorbed *CO at 2078 cm−1 (Figure S2), which readily desorbs to facilitate CO release [34].
Time-dependent analysis of these characteristic peaks revealed formation rate constants of 0.081 mV·s/min for the CO pathway and 0.049 mV·s/min for the CH4 pathway (Figure 6c and Figure S3), indicating that the CO formation rate is 1.65 times faster than that of CH4. This kinetic result aligns well with the product distribution observed in photocatalytic tests (Section 2.3).
These results demonstrate that the ZnO/ZnFe2O4 interface promotes the formation and stabilization of reduction intermediates, substantially enhancing the CO2 photocatalytic reduction activity compared to ZnFe2O4 alone.
Based on the in situ FTIR evidence, two primary reaction pathways are proposed for product formation (Figure 6d): the CO pathway via *COOH intermediate and the CH4 pathway through progressive hydrogenation of *CH3O species.
CH4 formation may proceed via:
CO2 → HCOO− → *CHO → *CH3O → CH4
CO2 → HCOO− → *CHO → *CH3 → CH4
For CO production, the routes may proceed via:
CO2 → *CO2 → *CO → CO
CO2 → *CO2 → *COOH → HCO3− → *CO → CO
In conclusion, the introduction of ZnO facilitates interfacial engineering that enhances CO2 adsorption, promotes key intermediate formation, and accelerates reaction kinetics, providing valuable insights for the rational design of efficient CO2 reduction photocatalysts [35].
3. Experimental Section
3.1. Materials
Detailed materials are provided in S3.1 (Supplementary Materials).
3.2. Preparation of ZnFe2O4 and ZnO Microspheres via Solvothermal Method
ZnFe2O4 was synthesized via a solvothermal method. Briefly, Zn(NO3)2·6H2O and Fe(NO3)3·9H2O were dissolved in ethylene glycol with a molar ratio of Zn:Fe = 1:2. ZnO was prepared using a procedure similar to that of ZnFe2O4, but without the addition of Fe(NO3)3·9H2O. To adjust the alkalinity of the solution, 20 mL of NaOH aqueous solution (40 g/L) was added under continuous stirring. Detailed materials are provided in S3.2 (Supplementary Materials).
3.3. Preparation of ZnO/ZnFe2O4 Composite via Sodium Hydroxide-Assisted Method
The ZnO/ZnFe2O4 composite was synthesized with a nominal Zn:Fe = 1.5:2. Similarly, 20 mL of NaOH solution (40 g/L) was introduced to maintain alkaline conditions during the synthesis. The resulting product was collected, washed, and dried for further characterization. Detailed materials are provided in S3.3 (Supplementary Materials).
3.4. Catalysts Characterization
Detailed materials are provided in S3.4 (Supplementary Materials).
3.5. Photocatalytic CO2 Reduction Reaction
Detailed materials of the photocatalytic CO2 reduction reaction were provided in S3.5 (Supplementary Materials).
4. Conclusions
In this study, a one-step modification strategy was developed to decorate ZnFe2O4 with ZnO nanoparticles, forming a unique interfacial architecture that significantly enhances visible-light photocatalytic CO2 reduction. Comprehensive multiscale characterization reveals the following key insights:
- (1)
- Interfacial Optimization of Crystalline Structure: XRD and TEM analyses indicate that the presence of ZnO promotes the growth of ZnFe2O4 crystallites, increasing the average grain size from 10.7 nm to 32.6 nm. This is accompanied by a sharpening of the (311) diffraction peak, suggesting improved crystallinity [36]. Lattice parameter analysis reveals interfacial tensile strain (a reduction in the lattice constant *a* from 8.452 Å in pure ZnFe2O4), further supported by a Raman blue shift in the A1g mode (+36 cm−1).
- (2)
- The opposite XPS binding energy shifts of Zn 2p (−1.4 eV) and Fe 2p (+0.8 eV) suggest an interfacial electron transfer from ZnFe2O4 to ZnO, leading to an increased electron density around Zn and a decreased density around Fe. Mott–Schottky analysis confirms that the conduction band potential of ZnO is −0.75 eV while that of ZnFe2O4 is −0.96 eV, which is consistent with the formation of a Type-II heterojunction. Additionally, the oxygen vacancy concentration increases to 25.3% (from O 1s spectra), which facilitates extended visible-light absorption.
- (3)
- Enhanced Charge Separation and Transport: The built-in electric field formed at the Type-II heterojunction interface promotes directional migration of photogenerated electrons, resulting in a two-fold increase in photocurrent intensity (Figure 4e) and a 15.1% reduction in PL intensity (Figure 4f). Electrochemical impedance spectroscopy (EIS) shows reduced charge transfer resistance, collectively contributing to suppressed electron–hole recombination and prolonged carrier lifetime.
- (4)
- Synergistic Effects of Interface Engineering: The ZnO/ZnFe2O4 system overcomes the inherent limitations of the individual components (e.g., rapid charge recombination in ZnFe2O4 and narrow light response of ZnO). Through the Type-II band alignment, the composite achieves synergistic broad visible-light absorption (Eg = 2.12 eV) and strong reduction capacity (ECB = −0.96 eV), leading to a dramatic improvement in CO2 photoreduction performance. After 6 h of visible-light irradiation, the production rates of CH4 and CO increased by 3.3 and 4.9 times, respectively, compared to pure ZnFe2O4.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30193980/s1.
Author Contributions
Conceptualization, Writing—original draft, Software, C.C.; Writing—review and editing, C.C., Y.S., M.P. and W.W.; Writing—review, W.W.; performed experiments, Y.X. and W.Z. All authors have read and agreed to the published version of the manuscript.
Funding
Provincial Young and Middle-aged Teachers Education and Research Project of Fujian (Science and Technology) (JAT241231); Provincial First-class Undergraduate Major Construction Project for “Environmental Science” in 2021 (SJZY-2022-02); Provincial First-class Undergraduate Program of Virtual Simulation Experimental Teaching in 2020 (SJKC-2020-04); Provincial Education and Teaching Research of Fujian in 2024 (FBJY20240020).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Zhang, Y.N.; Xu, M.Y.; Zhou, W.Q.; Song, X.H.; Liu, X.; Zhang, J.S.; Chen, S.T.; Huo, P.W. Fabricated ZnO@ZnIn2S4 S-scheme heterojunction photocatalyst for enhanced electron-transfer and CO2 reduction. J. Colloid Interface Sci. 2023, 650, 1762–1772. [Google Scholar] [CrossRef]
- Saira; Janna, F.; Le-Hussain, F. Effectiveness of modified CO2 injection at improving oil recovery and CO2 storage—Review and simulations. Energy Rep. 2020, 6, 1922–1941. [Google Scholar] [CrossRef]
- Li, R.F.; Dai, Z.H.; Huang, T.Y.; Zhang, Q.Q.; Zhou, X.F.; Peng, Z.L.; Liu, Z.T.; Fang, Y.P. In site constructed B-CdS/Cd Schottky junctions for efficiet photocatalytic CO2 reduction under visible light. Surf. Interfaces 2024, 45, 103926. [Google Scholar] [CrossRef]
- Liu, X.J.; Fan, X.F.; Wu, J.; Zhuge, Z.H.; Li, L.; Fan, J.C.; Shen, S.L.; Tang, Z.H.; Gong, Y.Y.; Xue, Y.H.; et al. CdS-based Schottky junctions for efficient visible light photocatalytichydrogen evolution. J. Colloid Interface Sci. 2024, 673, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Cheng, B.; Fan, J.J.; Yu, J.G.; Liu, G. Near-infrared absorbing 2D/3D ZnIn2S4/N-doped graphene photocatalyst for highly efficient CO2 capture and photocatalytic reduction. Sci. China Mater. 2020, 63, 552–565. [Google Scholar] [CrossRef]
- Jiang, J.W.; Zou, X.X.; Mei, Z.Y.; Cai, S.; An, Q.; Fu, Y.; Wang, H.; Liu, T.T.; Guo, H. Understanding rich oxygen vacant hollow CeO2@MoSe2 heterojunction for accelerating photocatalytic CO2 reduction. J. Colloid Interface Sci. 2022, 611, 644–653. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Q.; Labidi, A.; Luo, T.; Gao, T.; Nuraje, N.; Zvereva, I.; Wang, C.Y. In situ fabrication of TiO2 nanoparticles/2D porphyrin metal-organic frameworks for enhancing the photoreduction of CO2 to CO. J. Mater. Chem. A 2025, 13, 11389–11395. [Google Scholar] [CrossRef]
- Deng, H.Z.; Xu, F.Y.; Cheng, B.; Yu, J.G.; Ho, W.K. Photocatalytic CO2 reduction of C/ZnO nanofibers enhanced by an Ni-NiS cocatalyst. Nanoscale 2020, 12, 7206–7213. [Google Scholar] [CrossRef]
- Tang, Z.L.; He, W.J.; Wang, Y.L.; Wei, Y.C.; Yu, X.L.; Xiong, J.; Wang, X.; Zhang, X.; Zhao, Z.; Liu, J. Ternary heterojunction in rGO-coated Ag/Cu2O catalysts for boosting selective photocatalytic CO2 reduction into CH4. Appl. Catal. B Environ. Energy 2022, 311, 121371. [Google Scholar] [CrossRef]
- Yan, Y.L.; Fang, Q.J.; Pan, J.K.; Yang, J.; Zhang, L.L.; Zhang, W.; Zhuang, G.L.; Zhong, X.; Deng, S.W.; Wang, J.G. Efficient photocatalytic reduction of CO2 using Fe-based covalent triazine frameworks decorated with in situ grown ZnFe2O4 nanoparticles. Chem. Eng. J. 2020, 408, 127358. [Google Scholar] [CrossRef]
- Yang, T.; Yu, Q.M.; Wang, H.M. Photocatalytic Reduction of CO2 to CH3OH Coupling with the Oxidation of Amine to Imine. Catal. Lett. 2018, 148, 2382–2390. [Google Scholar] [CrossRef]
- Long, R.; Li, Y.; Liu, Y.; Chen, S.G.; Zheng, X.S.; Gao, C.; He, C.H.; Chen, N.S.; Qi, Z.M.; Song, L.; et al. Isolation of Cu Atoms in Pd Lattice: Forming Highly Selective Sites for Photocatalytic Conversion of CO2 to CH4. J. Am. Chem. Soc. 2017, 139, 4486–4492. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.G.; Huang, Z.X.; Gao, X.P.; Hong, X.; Zhu, J.F.; Wang, G.M.; Wu, Y.E.; Zeng, J.; Zheng, X.S. Synergy between Palladium Single Atoms and Nanoparticles via Hydrogen Spillover for Enhancing CO2 Photoreduction to CH4. Adv. Mater. 2022, 34, 2200057. [Google Scholar] [CrossRef] [PubMed]
- Rameshbabu, R.; Kumar, N.; Karthigeyan, A.; Neppolian, B. Visible light photocatalytic activities of ZnFe2O4/ZnO nanoparticles for the degradation of organic pollutants. Mater. Chem. Phys. 2016, 181, 106–115. [Google Scholar] [CrossRef]
- Tong, G.X.; Du, F.F.; Wu, W.H.; Wu, R.N.; Liu, F.T.; Liang, Y. Enhanced reactive oxygen species (ROS) yields and antibacterial activity of spongy ZnO/ZnFe2O4 hybrid micro-hexahedra selectively synthesized through a versatile glucose-engineered co-precipitation/annealing process. J. Mater. Chem. B 2013, 1, 2647–2657. [Google Scholar] [CrossRef]
- Renukadevi, S.; Jeyakumari, A.P. Rational design of ZnFe2O4/g-C3N4 heterostructures composites for high efficient visible-light photocatalysis for degradation of aqueous organic pollutants. Inorg. Chem. Commun. 2020, 118, 108047. [Google Scholar] [CrossRef]
- Deng, H.Z.; Fei, X.G.; Yang, Y.; Fan, J.J.; Yu, J.G.; Cheng, B.; Zhang, L.Y. S-scheme heterojunction based on p-type ZnMn2O4 and n-type ZnO with improved photocatalytic CO2 reduction activity. Chem. Eng. J. 2021, 409, 127377. [Google Scholar] [CrossRef]
- Harini, G.; Syed, A.; Rahiman, M.K.; Bahkali, A.H.; Elgorban, A.M.; Varma, R.S.; Khan, S.S. Enhanced photodegradation of rifampicin and co-trimoxazole by ZnO/ZnMn2O4/ZnS-PVA and its genotoxicity studies on Allium cepa. Chemosphere 2022, 308, 136238. [Google Scholar] [CrossRef]
- Yasmeen, H.; Zada, A.; Ali, S.; Khan, I.; Ali, W.; Khan, W.; Khan, M.; Anwar, N.; Ali, A.; Huerta-Flores, A.M.; et al. Visible light-excited surface plasmon resonance charge transfer significantly improves the photocatalytic activities of ZnO semiconductor for pollutants degradation. J. Chin. Chem. Soc. 2020, 67, 1611–1617. [Google Scholar] [CrossRef]
- Sun, H.; Lee, S.Y.; Park, S.J. Bimetallic CuPd alloy nanoparticles decorated ZnO nanosheets with enhanced photocatalytic degradation of methyl orange dye. J. Colloid Interface Sci. 2022, 629, 87–96. [Google Scholar] [CrossRef]
- Elamin, N.; Modwi, A.; Aissa, M.A.B.; Taha, K.K.; Al-Duaij, O.K.; Yousef, T.A. Fabrication of Cr–ZnO photocatalyst by starch-assisted sol–gel method for photodegradation of congo red under visible light. J. Mater. Sci. Mater. Electron. 2021, 32, 2234–2248. [Google Scholar] [CrossRef]
- Yilmaz, G.; Dindar, B. Non-metal doped ZnO photocatalyst prepared by sonication-assisted Sol-gel method and use for dye degradation. Inorg. Chem. Commun. 2023, 157, 111320. [Google Scholar] [CrossRef]
- Jiang, H.Y.; Chen, Y.J.; Li, L.; Liu, H.; Ren, C.; Liu, X.; Tian, G.H. Hierarchical ZnO nanorod/ZnFe2O4 nanosheet core/shell nanoarray decorated with PbS quantum dots for efficient photoelectrochemical water splitting. J. Alloys Compd. 2020, 828, 154449. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.; Li, X.L.; Huang, Y.H.; Wang, Z.P.; Han, Y.D.; Wu, J.B.; Meng, H.; Zhang, X. Synthesis, characterization, and photocatalytic degradation properties of ZnO/ZnFe2O4 magnetic heterostructures. New J. Chem. 2017, 41, 15433–15438. [Google Scholar] [CrossRef]
- Chakraborty, M.; Roy, D.; Sharma, A.; Thangavel, R. Post-treatment with ZnFe2O4 nanoparticles to improve photoelectrochemical performance of ZnO nanorods based photoelectrodes. Sol. Energy Mater. Sol. Cells 2019, 200, 109975. [Google Scholar] [CrossRef]
- Xiao, J.; Yang, W.Y.; Gao, S.; Sun, C.X.; Li, Q. Fabrication of ultrafine ZnFe2O4 nanoparticles for efficient photocatalytic reduction CO2 under visible light illumination. J. Mater. Sci. Technol. 2018, 34, 2331–2336. [Google Scholar] [CrossRef]
- Liu, X.; Ge, J.; Li, S.; Yang, H.; Tian, H.; Liu, H.; Li, Y.; Zheng, X.; Tian, Y.; Cui, X.; et al. Activating dynamic Zn-ZnO interface with controllable oxygen vacancy in CO2 electroreduction for boosting CO production. Green Chem. 2025, 27, 6133–6144. [Google Scholar] [CrossRef]
- Xiao, Z.; Wan, Z.Y.; Zhang, J.J.; Jiang, J.N.; Li, D.M.; Shen, J.N.; Dai, W.X.; Li, Y.; Wang, X.X.; Zhang, Z.Z. Interstitial Zinc Defects Enriched ZnO Tuning O2 Adsorption and Conversion Pathway for Superior Photocatalytic CH4 Oxygenation. ACS Catal. 2024, 14, 9104–9114. [Google Scholar] [CrossRef]
- Madhusudan, P.; Wang, Y.; Chandrashekar, B.N.; Wang, W.J.; Wang, J.W.; Miao, J.; Shi, R.; Liang, Y.X.; Mi, G.J.; Cheng, C. Nature inspired ZnO/ZnS nanobranch-like composites, decorated with Cu(OH)2 clusters for enhanced visible-light photocatalytic hydrogen evolution. Appl. Catal. B Environ. Energy 2019, 253, 379–390. [Google Scholar] [CrossRef]
- Wageh, S.; Al-Ghamdi, A.A.; Jafer, R.; Li, X.; Zhang, P. A new heterojunction in photocatalysis: S-scheme heterojunction. Chin. J. Catal. 2021, 42, 667–669. [Google Scholar] [CrossRef]
- Zheng, J.H.; Lei, Z. Incorporation of CoO nanoparticles in 3D marigold flower-like hierarchical architecture MnCo2O4 for highly boosting solar light photo-oxidation and reduction ability. Appl. Catal. B Environ. Energy 2018, 237, 1–8. [Google Scholar] [CrossRef]
- Pan, M.H.; Zheng, L.L.; Cai, C.Y.; Wang, W.W. Z-Scheme ZnO/ZnAl2O4 Heterojunction with Synergistic Effects for Enhanced Photocatalytic CO2 Reduction. Molecules 2025, 30, 2626. [Google Scholar] [CrossRef]
- Lan, R.M.; Hu, Z.F.; Liu, H.R.; Shen, K.; Wang, H.; Hou, T.T.; Li, Y.W. Passivating Lattice Oxygen in ZnO Nanocrystals to Reduce its Interactions with the Key Intermediates for a Selective Photocatalytic Methane Oxidation to Methanol. Angew. Chem. Int. Ed. 2025, 64, e202425186. [Google Scholar] [CrossRef]
- Lai, K.Z.; Sun, Y.X.; Li, N.; Gao, Y.Q.; Li, H.; Ge, L.; Ma, T.Y. Photocatalytic CO2-to-CH4 Conversion with Ultrahigh Selectivity of 95.93% on S-Vacancy Modulated Spatial In2S3/In2O3 Heterojunction. Adv. Funct. Mater. 2024, 34, 2409031. [Google Scholar] [CrossRef]
- Cui, Y.F.; Zhou, W.H.; Yang, H.; Ma, Y.Q.; Zheng, G.H.; Zhu, C.H.; Wang, M.L.; Chen, B. Largely Promoted C–H Activation in Methane with O2 via d-Orbital Hybridization Induced by CuOx Supported on ZnO. ACS Catal. 2025, 15, 1607–1615. [Google Scholar] [CrossRef]
- Zheng, X.H.; Li, B.; Huang, R.; Jiang, W.P.; Shen, L.J.; Lei, G.C.; Wang, S.P.; Zhan, Y.Y.; Jiang, L.L. Asymmetric Oxygen Vacancy-Promoted Synthesis of Aminoarenes from Nitroarenes Using Waste H2S as a “Hydrogen Donor”. ACS Catal. 2024, 14, 10245–10259. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).