The Biologically Active Compounds in Fruits of Cultivated Varieties and Wild Species of Apples
Abstract
1. Introduction
2. Effects of Apple and Apple Extract Consumption on Human Diseases
3. Description of the Beneficial Properties of Biologically Active Compounds
3.1. Polyphenolic Compounds
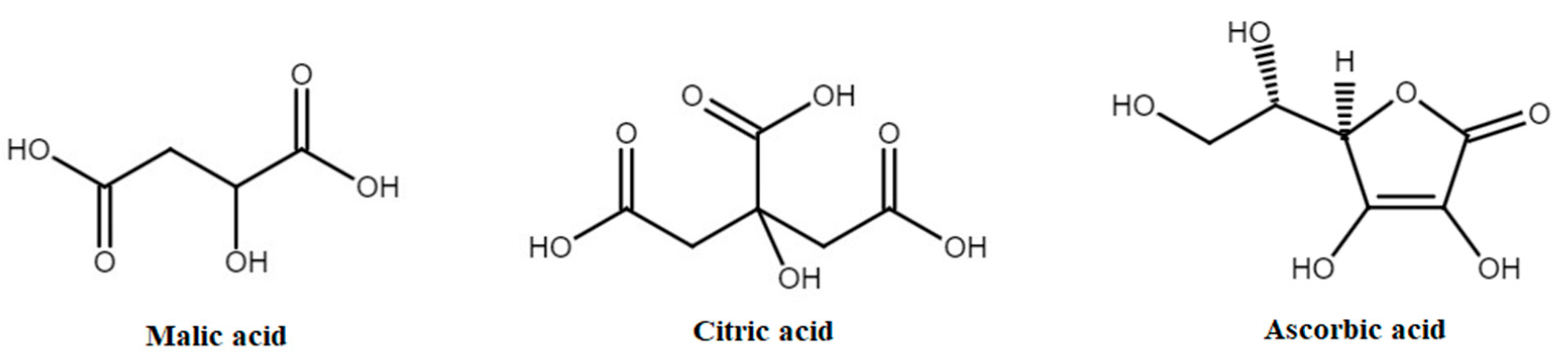
3.2. Organic Acids
3.3. Pigments
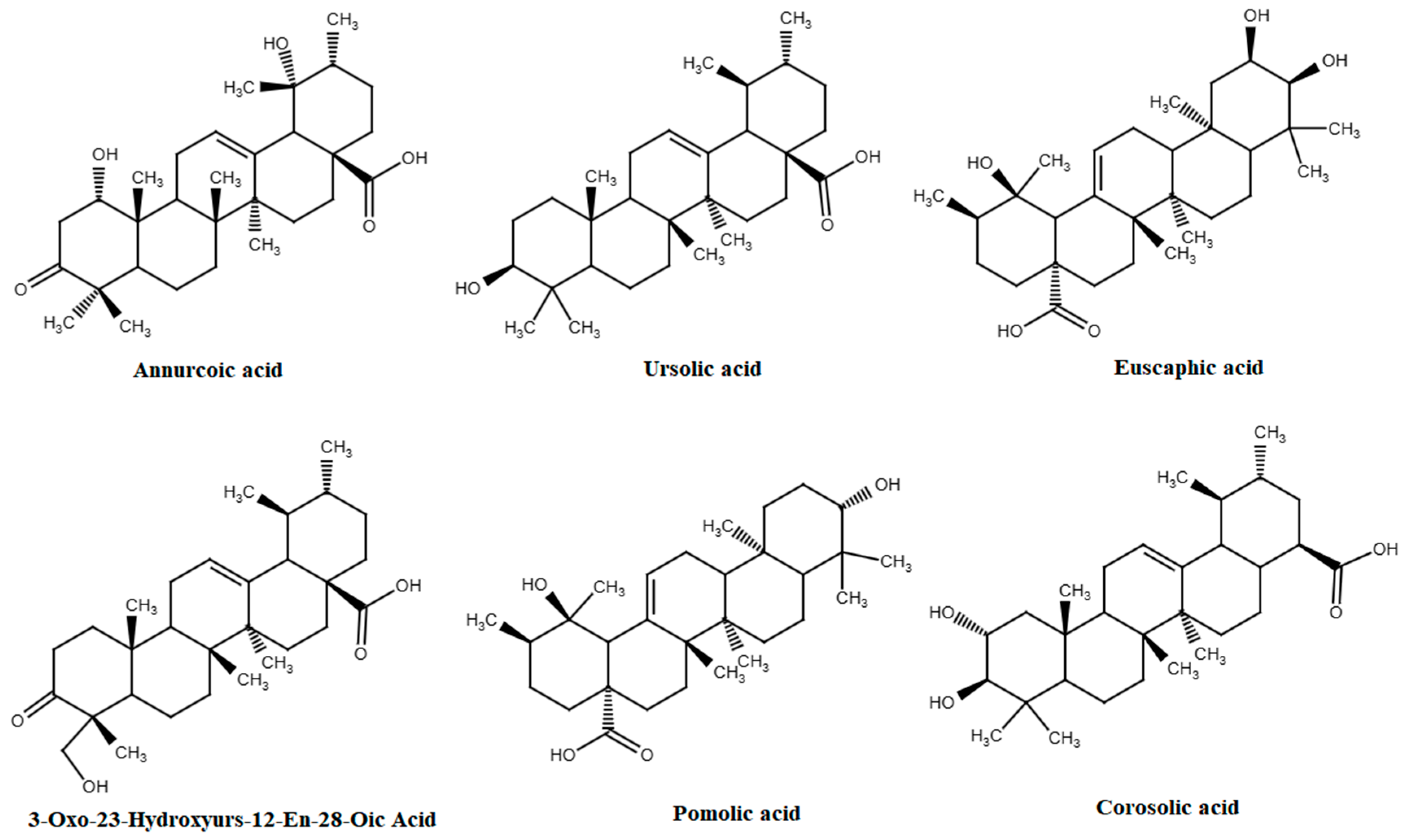
3.4. Triterpenoids
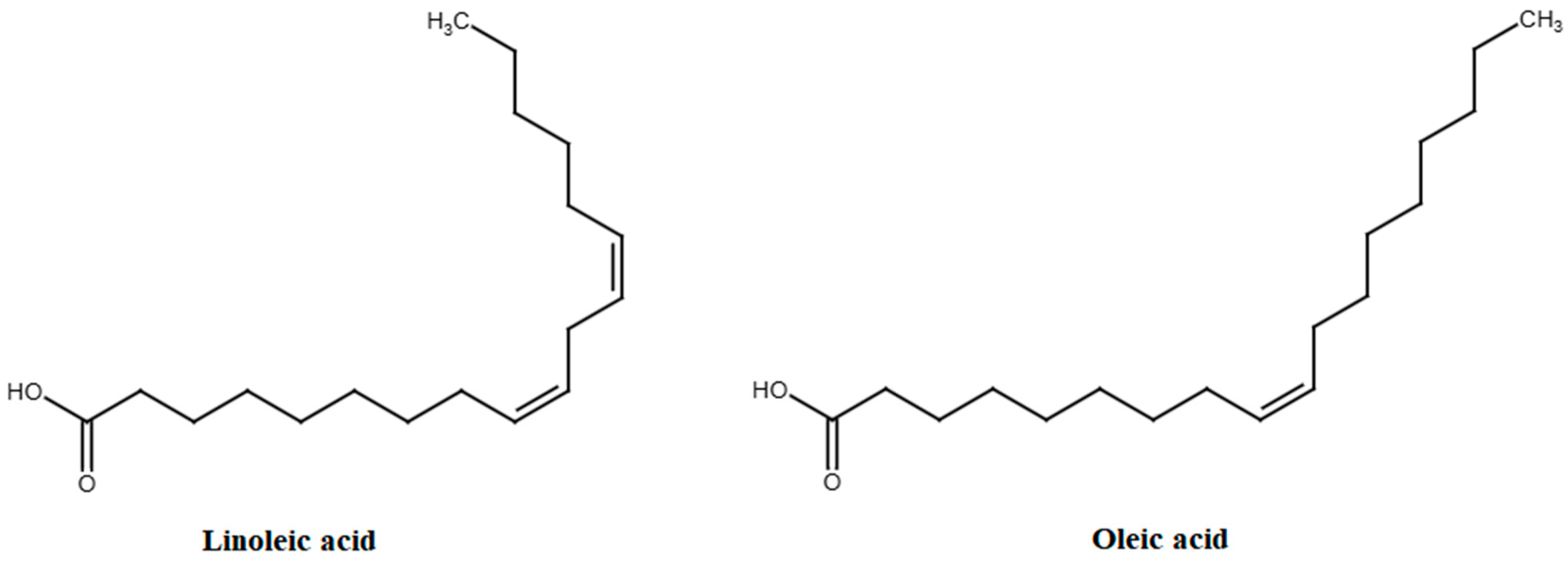
3.5. Fatty Acids
4. A Comparative Analysis of the Biochemical Composition of Cultivated and Wild Apple Species
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Yan, P.; Chen, W.; Wei, W.; Thomson, B.; Ruan, S.; Cao, Z.; Ou, C.; Geldsetzer, P.; Han, T.; et al. The Global Burden of Disease Attributable to Suboptimal Fruit and Vegetable Intake, 1990–2021: A Systematic Analysis of the Global Burden of Disease Study. BMC Med. 2025, 23, 456. [Google Scholar] [CrossRef]
- Carroll, B.T.; McNaughton, S.A.; Parker, K.E.; Marchese, L.E.; Livingstone, K.M. Identifying the Barriers and Facilitators to Fruit and Vegetable Consumption in Rural Australian Adults: A Mixed Methods Analysis. Nutr. J. 2024, 23, 69. [Google Scholar] [CrossRef]
- Scarano, A.; Chieppa, M.; Santino, A. Plant Polyphenols-Biofortified Foods as a Novel Tool for the Prevention of Human Gut Diseases. Antioxidants 2020, 9, 1225. [Google Scholar] [CrossRef] [PubMed]
- Linda Kantor, A.B. Apples and Oranges Are the Top U.S. Fruit Choices. United States Department of Agriculture, Economic Research Service (USDA ERS). Available online: https://www.ers.usda.gov/data-products/chart-gallery/chart-detail?chartId=58322&utm_source=chatgpt.com (accessed on 4 September 2025).
- Kalkisim, O.; Ozdes, D.; Okcu, Z.; Karabulut, B.; Senturk, H.B. Determination of Pomological and Morphological Characteristics and Chemical Compositions of Local Apple Varieties Grown in Gumushane, Turkey. Erwerbs-Obstbau 2016, 58, 41–48. [Google Scholar] [CrossRef]
- Aprea, E.; Charles, M.; Endrizzi, I.; Laura Corollaro, M.; Betta, E.; Biasioli, F.; Gasperi, F. Sweet Taste in Apple: The Role of Sorbitol, Individual Sugars, Organic Acids and Volatile Compounds. Sci. Rep. 2017, 7, 44950. [Google Scholar] [CrossRef] [PubMed]
- Mureşan, E.A.; Muste, S.; Mureşan, C.C.; Mudura, E.; Păucean, A.; Stan, L.; Vlaic, R.A.; Cerbu, C.G.; Mureşan, V. Assessment of Polyphenols, Chlorophylls and Carotenoids during Developmental Phases of Three Apple Varieties. Rom. Biotechnol. Lett. 2017, 22, 12546–12553. [Google Scholar]
- Butkeviciute, A.; Viskelis, J.; Viskelis, P.; Liaudanskas, M.; Janulis, V. Changes in the Biochemical Composition and Physicochemical Properties of Apples Stored in Controlled Atmosphere Conditions. Appl. Sci. 2021, 11, 6215. [Google Scholar] [CrossRef]
- Zhou, K.; Hu, L.; Liu, B.; Li, Y.; Gong, X.; Ma, F. Identification of Apple Fruits Rich in Health-Promoting Dihydrochalcones by Comparative Assessment of Cultivated and Wild Accessions. Sci. Hortic. 2018, 233, 38–46. [Google Scholar] [CrossRef]
- Spengler, R.N. Origins of the Apple: The Role of Megafaunal Mutualism in the Domestication of Malus and Rosaceous Trees. Front. Plant Sci. 2019, 10, 617. [Google Scholar] [CrossRef]
- Liao, L.; Zhang, W.; Zhang, B.; Fang, T.; Wang, X.-F.; Cai, Y.; Ogutu, C.; Gao, L.; Chen, G.; Nie, X.; et al. Unraveling a Genetic Roadmap for Improved Taste in the Domesticated Apple. Mol. Plant 2021, 14, 1454–1471. [Google Scholar] [CrossRef]
- Duan, N.; Bai, Y.; Sun, H.; Wang, N.; Ma, Y.; Li, M.; Wang, X.; Jiao, C.; Legall, N.; Mao, L.; et al. Genome Re-Sequencing Reveals the History of Apple and Supports a Two-Stage Model for Fruit Enlargement. Nat. Commun. 2017, 8, 249. [Google Scholar] [CrossRef]
- Beauchamp, G.K. Why Do We like Sweet Taste: A Bitter Tale? Physiol. Behav. 2016, 164, 432–437. [Google Scholar] [CrossRef]
- Ruiz-Cruz, S.; Chaparro-Hernández, S.; Ruiz, K.L.H.; Cira-Chávez, L.A.; Estrada-Alvarado, M.I.; Ortega, L.E.G.; Ornelas-Paz, J.D.J.; Mata, M.A.L. Food. In Flavonoids–From Biosynthesis to Human Health; Justino, G.C., Ed.; InTech: Toulon, France, 2017. [Google Scholar]
- Davies, T.; Watts, S.; McClure, K.; Migicovsky, Z.; Myles, S. Phenotypic Divergence between the Cultivated Apple (Malus domestica) and Its Primary Wild Progenitor (Malus sieversii). PLoS ONE 2022, 17, e0250751. [Google Scholar] [CrossRef]
- Ihwah, A.; Sucipto; Puspaningtyas, S.D. Quality Control Analysis of Apple Juice Drink Using the T2 Hotelling Control Diagram. In Proceedings of the International Conference on Biology and Applied Science (ICOBAS), Malang, Indonesia, 13–14 March 2019; p. 50002. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, M.; Zhang, X.; Yu, Q.; Zeng, W.; Yu, B.; Gan, J.; Zhang, S.; Jiang, X. Does an Apple a Day Keep Away Diseases? Evidence and Mechanism of Action. Food Sci. Nutr. 2023, 11, 4926–4947. [Google Scholar] [CrossRef] [PubMed]
- The Top 10 Causes of Death. World Health Organization. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death (accessed on 29 May 2025).
- Mierczak, K.; Garus-Pakowska, A. An Overview of Apple Varieties and the Importance of Apple Consumption in the Prevention of Non-Communicable Diseases—A Narrative Review. Nutrients 2024, 16, 3307. [Google Scholar] [CrossRef] [PubMed]
- Vallée Marcotte, B.; Verheyde, M.; Pomerleau, S.; Doyen, A.; Couillard, C. Health Benefits of Apple Juice Consumption: A Review of Interventional Trials on Humans. Nutrients 2022, 14, 821. [Google Scholar] [CrossRef] [PubMed]
- Nezbedova, L.; McGhie, T.; Christensen, M.; Heyes, J.; Nasef, N.A.; Mehta, S. Onco-Preventive and Chemo-Protective Effects of Apple Bioactive Compounds. Nutrients 2021, 13, 4025. [Google Scholar] [CrossRef]
- Koutsos, A.; Lima, M.; Conterno, L.; Gasperotti, M.; Bianchi, M.; Fava, F.; Vrhovsek, U.; Lovegrove, J.; Tuohy, K. Effects of Commercial Apple Varieties on Human Gut Microbiota Composition and Metabolic Output Using an In Vitro Colonic Model. Nutrients 2017, 9, 533. [Google Scholar] [CrossRef]
- Shinohara, K.; Ohashi, Y.; Kawasumi, K.; Terada, A.; Fujisawa, T. Effect of Apple Intake on Fecal Microbiota and Metabolites in Humans. Anaerobe 2010, 16, 510–515. [Google Scholar] [CrossRef]
- Wassermann, B.; Müller, H.; Berg, G. An Apple a Day: Which Bacteria Do We Eat With Organic and Conventional Apples? Front. Microbiol. 2019, 10, 1629. [Google Scholar] [CrossRef]
- Yang, Y.; Zhou, X.; Chen, L.; Guo, Y. Effects of Young Apple Powder on Simulated Human Colonic Microbiota in Vitro. J. Food Sci. Technol. 2024, 42, 81–92. [Google Scholar] [CrossRef]
- Ward-Ritacco, C.L.; Wilson, A.R.; O’Connor, P.J. An Apple Extract Beverage Combined with Caffeine Can Improve Alertness, Mental Fatigue, and Information Processing Speed. J. Cogn. Enhanc. 2021, 5, 267–279. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.A.; Catalán, Ú.; Calderón-Pérez, L.; Companys, J.; Pla-Pagà, L.; Ludwig, I.A.; Romero, M.P.; Solà, R. The Effects and Associations of Whole-Apple Intake on Diverse Cardiovascular Risk Factors. A Narrative Review. Crit. Rev. Food Sci. Nutr. 2020, 60, 3862–3875. [Google Scholar] [CrossRef]
- Oyenihi, A.B.; Belay, Z.A.; Mditshwa, A.; Caleb, O.J. “An Apple a Day Keeps the Doctor Away”: The Potentials of Apple Bioactive Constituents for Chronic Disease Prevention. J. Food Sci. 2022, 87, 2291–2309. [Google Scholar] [CrossRef] [PubMed]
- Kołton, A.; Długosz-Grochowska, O.; Wojciechowska, R.; Czaja, M. Biosynthesis Regulation of Folates and Phenols in Plants. Sci. Hortic. 2022, 291, 110561. [Google Scholar] [CrossRef]
- He, W.; Laaksonen, O.; Tian, Y.; Heinonen, M.; Bitz, L.; Yang, B. Phenolic Compound Profiles in Finnish Apple (Malus × domestica Borkh.) Juices and Ciders Fermented with Saccharomyces cerevisiae and Schizosaccharomyces pombe Strains. Food Chem. 2022, 373, 131437. [Google Scholar] [CrossRef] [PubMed]
- Das, G.; Gonçalves, S.; Basilio Heredia, J.; Romano, A.; Jiménez-Ortega, L.A.; Gutiérrez-Grijalva, E.P.; Shin, H.S.; Patra, J.K. Cardiovascular Protective Effect of Cinnamon and Its Major Bioactive Constituents: An Update. J. Funct. Foods 2022, 97, 105045. [Google Scholar] [CrossRef]
- Qi, G.; Chen, J.; Shi, C.; Wang, Y.; Mi, S.; Shao, W.; Yu, X.; Ma, Y.; Ling, J.; Huang, J. Cinnamic Acid (CINN) Induces Apoptosis and Proliferation in Human Nasopharyngeal Carcinoma Cells. Cell. Physiol. Biochem. 2016, 40, 589–596. [Google Scholar] [CrossRef]
- Adisakwattana, S. Cinnamic Acid and Its Derivatives: Mechanisms for Prevention and Management of Diabetes and Its Complications. Nutrients 2017, 9, 163. [Google Scholar] [CrossRef]
- Nguyen, V.; Taine, E.G.; Meng, D.; Cui, T.; Tan, W. Chlorogenic Acid: A Systematic Review on the Biological Functions, Mechanistic Actions, and Therapeutic Potentials. Nutrients 2024, 16, 924. [Google Scholar] [CrossRef]
- Safaeian, L.; Asghari-Varzaneh, M.; Alavi, S.-S.; Halvaei-Varnousfaderani, M.; Laher, I. Cardiovascular Protective Effects of Cinnamic Acid as a Natural Phenolic Acid: A Review. Arch. Physiol. Biochem. 2025, 131, 52–62. [Google Scholar] [CrossRef]
- Jiang, Y.; Fang, Z.; Leonard, W.; Zhang, P. Phenolic Compounds in Lycium Berry: Composition, Health Benefits and Industrial Applications. J. Funct. Foods 2021, 77, 104340. [Google Scholar] [CrossRef]
- Adeosun, W.B.; More, G.K.; Steenkamp, P.; Prinsloo, G. Influence of Seasonal and Geographic Variation on the Anti-HSV-1 Properties and Chlorogenic Acids Content of Helichrysum aureonitens Sch. Bip. Front. Mol. Biosci. 2022, 9, 961859. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Liang, Q.; Xiong, X.; Wang, Y.; Zhang, Z.; Sun, M.; Lu, X.; Wu, D. Anti-Inflammatory Effects of P-Coumaric Acid, a Natural Compound of Oldenlandia diffusa, on Arthritis Model Rats. Evid.-Based Complement. Altern. Med. 2018, 2018, 5198594. [Google Scholar] [CrossRef]
- Mozaffari Godarzi, S.; Valizade Gorji, A.; Gholizadeh, B.; Mard, S.A.; Mansouri, E. Antioxidant Effect of P-Coumaric Acid on Interleukin 1-β and Tumor Necrosis Factor-α in Rats with Renal Ischemic Reperfusion. Nefrología 2020, 40, 311–319. [Google Scholar] [CrossRef]
- Pavlíková, N. Caffeic Acid and Diseases—Mechanisms of Action. Int. J. Mol. Sci. 2022, 24, 588. [Google Scholar] [CrossRef]
- Jafarinia, M.; Sadat Hosseini, M.; Kasiri, N.; Fazel, N.; Fathi, F.; Ganjalikhani Hakemi, M.; Eskandari, N. Quercetin with the Potential Effect on Allergic Diseases. Allergy Asthma Clin. Immunol. 2020, 16, 36. [Google Scholar] [CrossRef]
- Park, J.-E.; Han, J.-S. Improving the Effect of Ferulic Acid on Inflammation and Insulin Resistance by Regulating the JNK/ERK and NF-ΚB Pathways in TNF-α-Treated 3T3-L1 Adipocytes. Nutrients 2024, 16, 294. [Google Scholar] [CrossRef]
- Kaur, J.; Kaur, R. p-Coumaric Acid: A Naturally Occurring Chemical with Potential Therapeutic Applications. Curr. Org. Chem. 2022, 26, 1333–1349. [Google Scholar] [CrossRef]
- Zduńska, K.; Dana, A.; Kolodziejczak, A.; Rotsztejn, H. Antioxidant Properties of Ferulic Acid and Its Possible Application. Ski. Pharmacol. Physiol. 2018, 31, 332–336. [Google Scholar] [CrossRef]
- Naghavi, M.; Tamri, P.; Soleimani Asl, S. Investigation of Healing Effects of Cinnamic Acid in a Full-Thickness Wound Model in Rabbit. Jundishapur J. Nat. Pharm. Prod. 2021, 16, e97669. [Google Scholar] [CrossRef]
- Kageyama, T.; Seo, J.; Yan, L.; Fukuda, J. Cinnamic Acid Promotes Elongation of Hair Peg-like Sprouting in Hair Follicle Organoids via Oxytocin Receptor Activation. Sci. Rep. 2024, 14, 4709. [Google Scholar] [CrossRef] [PubMed]
- Yuan, X.; Wu, Y.; Liang, J.; Yuan, H.; Zhao, X.; Zhu, D.; Liu, H.; Lin, J.; Huang, S.; Lai, X.; et al. Phlorizin Treatment Attenuates Obesity and Related Disorders through Improving BAT Thermogenesis. J. Funct. Foods 2016, 27, 429–438. [Google Scholar] [CrossRef]
- Zawiła, T.; Swolana, D.; Rok, J.; Rzepka, Z.; Wojtyczka, R.D. Evaluation of the Antibacterial Activity of Cinnamic Acid and Its Derivatives: Synergistic Effects with Cloxacillin. Molecules 2025, 30, 660. [Google Scholar] [CrossRef]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health Effects of Phloretin: From Chemistry to Medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Chahardoli, A.; Jalilian, F.; Memariani, Z.; Farzaei, M.H.; Shokoohinia, Y. Analysis of Organic Acids. In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 767–823. [Google Scholar] [CrossRef]
- Weide, J.V.; Nocker, S.V.; Gottschalk, C. Meta-analysis of Apple (Malus × domestica Borkh.) Fruit and Juice Quality Traits for Potential Use in Hard Cider Production. Plants People Planet 2022, 4, 463–475. [Google Scholar] [CrossRef]
- De Carvalho, J.F.; Lerner, A. Malic Acid for the Treatment of Rheumatic Diseases. Mediterr. J. Rheumatol. 2023, 34, 592. [Google Scholar] [CrossRef]
- Tang, X.; Liu, J.; Dong, W.; Li, P.; Li, L.; Lin, C.; Zheng, Y.; Hou, J.; Li, D. The Cardioprotective Effects of Citric Acid and L-Malic Acid on Myocardial Ischemia/Reperfusion Injury. Evid.-Based Complement. Altern. Med. 2013, 2013, 820695. [Google Scholar] [CrossRef]
- Koriem, K.M.M.; Tharwat, H.A.K. Malic Acid Improves Behavioral, Biochemical, and Molecular Disturbances in the Hypothalamus of Stressed Rats. J. Integr. Neurosci. 2023, 22, 98. [Google Scholar] [CrossRef]
- Benali, T.; Bakrim, S.; Ghchime, R.; Benkhaira, N.; El Omari, N.; Balahbib, A.; Taha, D.; Zengin, G.; Hasan, M.M.; Bibi, S.; et al. Pharmacological Insights into the Multifaceted Biological Properties of Quinic Acid. Biotechnol. Genet. Eng. Rev. 2024, 40, 3408–3437. [Google Scholar] [CrossRef]
- Moser, M.; Chun, O. Vitamin C and Heart Health: A Review Based on Findings from Epidemiologic Studies. Int. J. Mol. Sci. 2016, 17, 1328. [Google Scholar] [CrossRef] [PubMed]
- Peerapen, P.; Thongboonkerd, V. Kidney Stone Prevention. Adv. Nutr. 2023, 14, 555–569. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Kaldate, R.; Bisht, A. Citric Acid, Antioxidant Effects in Health. In Antioxidants Effects in Health; Elsevier: Amsterdam, The Netherlands, 2022; pp. 309–322. [Google Scholar]
- Camarena, V.; Wang, G. The Epigenetic Role of Vitamin C in Health and Disease. Cell. Mol. Life Sci. 2016, 73, 1645–1658. [Google Scholar] [CrossRef] [PubMed]
- Turcu-Stiolica, A.; Ionele, C.M.; Ungureanu, B.S.; Subtirelu, M.-S. The Effects of Arginine-Based Supplements on Fatigue Levels Following COVID-19 Infection: A Prospective Study in Romania. Healthcare 2023, 11, 1477. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, Y.; Xia, J.; Liu, H.; Liu, J.P.; Li, D.; Wang, R.; Cao, H.; Sang, H. Topical, Light-Based, and Complementary Interventions for Acne: An Overview of Systematic Reviews. Cochrane Database Syst. Rev. 2021, 2021, CD014918. [Google Scholar] [CrossRef]
- Muhamed, S.A.; Moussa, E.M.; Aboasy, N.K.; Gaweesh, Y.Y. Effect of 1% Malic Acid Spray on Diabetes Mellitus-induced Xerostomia: A Randomized Clinical Trial. Oral Dis. 2024, 30, 631–638. [Google Scholar] [CrossRef]
- Coker, S.J.; Smith-Díaz, C.C.; Dyson, R.M.; Vissers, M.C.M.; Berry, M.J. The Epigenetic Role of Vitamin C in Neurodevelopment. Int. J. Mol. Sci. 2022, 23, 1208. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Shen, X.; Xu, Y.; Hu, X.; Shen, D.; Chen, C.; Sun, X. Diversity in Plastids Contributes to Variation in Fruit Color. Sci. Hortic. 2024, 337, 113471. [Google Scholar] [CrossRef]
- Ghosh, S.; Sarkar, T.; Das, A.; Chakraborty, R. Natural Colorants from Plant Pigments and Their Encapsulation: An Emerging Window for the Food Industry. LWT 2022, 153, 112527. [Google Scholar] [CrossRef]
- Carvalho, A.M.S.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antoniolli, A.R.; Quintans, J.S.S.; Quintans-Júnior, L.J. Phytol, a Chlorophyll Component, Produces Antihyperalgesic, Anti-Inflammatory, and Antiarthritic Effects: Possible NFκB Pathway Involvement and Reduced Levels of the Proinflammatory Cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef]
- Martins, T.; Barros, A.N.; Rosa, E.; Antunes, L. Enhancing Health Benefits through Chlorophylls and Chlorophyll-Rich Agro-Food: A Comprehensive Review. Molecules 2023, 28, 5344. [Google Scholar] [CrossRef]
- Nishino, A.; Maoka, T.; Yasui, H. Preventive Effects of β-Cryptoxanthin, a Potent Antioxidant and Provitamin A Carotenoid, on Lifestyle-Related Diseases—A Central Focus on Its Effects on Non-Alcoholic Fatty Liver Disease (NAFLD). Antioxidants 2021, 11, 43. [Google Scholar] [CrossRef]
- Lopresti, A.L.; Smith, S.J.; Drummond, P.D. The Effects of Lutein and Zeaxanthin Supplementation on Cognitive Function in Adults With Self-Reported Mild Cognitive Complaints: A Randomized, Double-Blind, Placebo-Controlled Study. Front. Nutr. 2022, 9, 843512. [Google Scholar] [CrossRef] [PubMed]
- Bakac, E.R.; Percin, E.; Gunes-Bayir, A.; Dadak, A. A Narrative Review: The Effect and Importance of Carotenoids on Aging and Aging-Related Diseases. Int. J. Mol. Sci. 2023, 24, 15199. [Google Scholar] [CrossRef] [PubMed]
- Buscemi, S.; Corleo, D.; Di Pace, F.; Petroni, M.; Satriano, A.; Marchesini, G. The Effect of Lutein on Eye and Extra-Eye Health. Nutrients 2018, 10, 1321. [Google Scholar] [CrossRef]
- Woźniak, Ł.; Szakiel, A.; Głowacka, A.; Rozpara, E.; Marszałek, K.; Skąpska, S. Triterpenoids of Three Apple Cultivars—Biosynthesis, Antioxidative and Anti-Inflammatory Properties, and Fate during Processing. Molecules 2023, 28, 2584. [Google Scholar] [CrossRef]
- Zhao, J.; Zhou, H.; An, Y.; Shen, K.; Yu, L. Biological Effects of Corosolic Acid as an Anti-inflammatory, Anti-metabolic Syndrome and Anti-neoplasic Natural Compound (Review). Oncol. Lett. 2020, 21, 84. [Google Scholar] [CrossRef]
- Seo, D.Y.; Lee, S.R.; Heo, J.-W.; No, M.-H.; Rhee, B.D.; Ko, K.S.; Kwak, H.-B.; Han, J. Ursolic Acid in Health and Disease. Korean J. Physiol. Pharmacol. 2018, 22, 235. [Google Scholar] [CrossRef]
- Chan, E.W.C.; Ng, Y.K.; Lim, C.S.S.; Anggraeni, V.S.; Siew, Z.Z.; Wong, C.W.; Wong, S.K. Pomolic Acid: A Short Review on Its Chemistry, Plant Sources, Pharmacological Properties, and Patents. J. Appl. Pharm. Sci. 2023, 13, 58–65. [Google Scholar] [CrossRef]
- Erdmann, J.; Kujaciński, M.; Wiciński, M. Beneficial Effects of Ursolic Acid and Its Derivatives—Focus on Potential Biochemical Mechanisms in Cardiovascular Conditions. Nutrients 2021, 13, 3900. [Google Scholar] [CrossRef]
- Lozano-Mena, G.; Sánchez-González, M.; Juan, M.; Planas, J. Maslinic Acid, a Natural Phytoalexin-Type Triterpene from Olives —A Promising Nutraceutical? Molecules 2014, 19, 11538–11559. [Google Scholar] [CrossRef]
- Jeong, N.-H.; Lee, S.; Choi, Y.-A.; Song, K.-S.; Kim, S.-H. Inhibitory Effects of Euscaphic Acid in the Atopic Dermatitis Model by Reducing Skin Inflammation and Intense Pruritus. Inflammation 2022, 45, 1680–1691. [Google Scholar] [CrossRef] [PubMed]
- Xia, D.; Qian, Q.; Wang, S.; Dong, X.; Liu, Y. Alendronate Functionalized Bone-Targeting Pomolic Acid Liposomes Restore Bone Homeostasis for Osteoporosis Treatment. Int. J. Nanomed. 2024, 19, 7983–7996. [Google Scholar] [CrossRef] [PubMed]
- Ghaffari Moghaddam, M.; Bin, H.; Ahmad, F.; Samzadeh-Kermani, A. Biological Activity of Betulinic Acid: A Review. Pharmacol. Pharm. 2012, 03, 119–123. [Google Scholar] [CrossRef]
- Liou, C.; Dai, Y.; Wang, C.; Fang, L.; Huang, W. Maslinic Acid Protects against Obesity-induced Nonalcoholic Fatty Liver Disease in Mice through Regulation of the Sirt1/AMPK Signaling Pathway. FASEB J. 2019, 33, 11791–11803. [Google Scholar] [CrossRef]
- Kunkel, S.D.; Suneja, M.; Ebert, S.M.; Bongers, K.S.; Fox, D.K.; Malmberg, S.E.; Alipour, F.; Shields, R.K.; Adams, C.M. mRNA Expression Signatures of Human Skeletal Muscle Atrophy Identify a Natural Compound That Increases Muscle Mass. Cell Metab. 2011, 13, 627–638. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Jung, H.; Kweon, M.; Kim, J.; Choi, S.-Y.; Ahn, H.-J.; Park, C.-S.; Kim, H.-M.; Jeong, H.-J. Euscaphic Acid Relieves Fatigue by Enhancing Anti-Oxidative and Anti-Inflammatory Effects. Immunopharmacol. Immunotoxicol. 2023, 45, 114–121. [Google Scholar] [CrossRef]
- Ohata, Y.; Tetsumoto, Y.; Morita, S.; Mori, N.; Ishiguri, Y.; Yoshinaga, N. Triterpenes Induced by Young Apple Fruits in Response to Herbivore Attack. Biosci. Biotechnol. Biochem. 2021, 85, 1594–1601. [Google Scholar] [CrossRef]
- Akšić, M.F.; Lazarević, K.; Šegan, S.; Natić, M.; Tosti, T.; Ćirić, I.; Meland, M. Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Seeds from Apple Cultivars (Malus domestica Borkh.) Grown in Norway. Foods 2021, 10, 1956. [Google Scholar] [CrossRef]
- Taha, A.Y. Linoleic Acid–Good or Bad for the Brain? NPJ Sci. Food 2020, 4, 1. [Google Scholar] [CrossRef]
- Piccinin, E.; Cariello, M.; De Santis, S.; Ducheix, S.; Sabbà, C.; Ntambi, J.M.; Moschetta, A. Role of Oleic Acid in the Gut-Liver Axis: From Diet to the Regulation of Its Synthesis via Stearoyl-CoA Desaturase 1 (SCD1). Nutrients 2019, 11, 2283. [Google Scholar] [CrossRef] [PubMed]
- Farvid, M.S.; Ding, M.; Pan, A.; Sun, Q.; Chiuve, S.E.; Steffen, L.M.; Willett, W.C.; Hu, F.B. Dietary Linoleic Acid and Risk of Coronary Heart Disease: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. Circulation 2014, 130, 1568–1578. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jia, Y.; He, H. The Role of Linoleic Acid in Skin and Hair Health: A Review. Int. J. Mol. Sci. 2024, 26, 246. [Google Scholar] [CrossRef] [PubMed]
- Jalali, M.; Abedi, M.; Tabarsa, M.; Moreno, D.A. Morphological and Biochemical Characteristics of Wild Red-Fleshed Apples (Malus sieversii f. niedzwetzkyana) in the North and Northeast of Iran. BMC Plant Biol. 2024, 24, 899. [Google Scholar] [CrossRef]
- Angeli, L.; Populin, F.; Morozova, K.; Ding, Y.; Asma, U.; Bolchini, S.; Cebulj, A.; Busatto, N.; Costa, F.; Ferrentino, G.; et al. Comparative Analysis of Antioxidant Activity and Capacity in Apple Varieties: Insights from Stopped Flow DPPH• Kinetics, Mass Spectrometry and Electrochemistry. Food Biosci. 2024, 58, 103729. [Google Scholar] [CrossRef]
- Mitić, S.S.; Stojanović, B.T.; Stojković, M.B.; Mitić, M.N.; Pavlović, J.L. Total Phenolics, Flavonoids and Antioxidant Activity of Different Apple Cultivars. Bulg. Chem. Commun. 2013, 45, 326–331. [Google Scholar]
- Liu, F.; Wang, M.; Wang, M. Phenolic Compounds and Antioxidant Activities of Flowers, Leaves and Fruits of Five Crabapple Cultivars (Malus Mill. species). Sci. Hortic. 2018, 235, 460–467. [Google Scholar] [CrossRef]
- Zhang, Y.; Balasooriya, H.; Sirisena, S.; Ng, K. The Effectiveness of Dietary Polyphenols in Obesity Management: A Systematic Review and Meta-Analysis of Human Clinical Trials. Food Chem. 2023, 404, 134668. [Google Scholar] [CrossRef]
- Yingxiang, Y.; Zhida, Z.; Cuiqing, C. Chlorogenic Acid Intake Guidance: Sources, Health Benefits, and Safety. Asia Pac. J. Clin. Nutr. 2022, 31, 602–610. [Google Scholar] [CrossRef]
- Li, Y.; Sun, H.; Li, J.; Qin, S.; Niu, Z.; Qiao, X.; Yang, B. Influence of Genetic Background, Growth Latitude and Bagging Treatment on Phenolic Compounds in Fruits of Commercial Cultivars and Wild Types of Apples (Malus sp.). Eur. Food Res. Technol. 2021, 247, 1149–1165. [Google Scholar] [CrossRef]
- Średnicka-Tober, D.; Barański, M.; Kazimierczak, R.; Ponder, A.; Kopczyńska, K.; Hallmann, E. Selected Antioxidants in Organic vs. Conventionally Grown Apple Fruits. Appl. Sci. 2020, 10, 2997. [Google Scholar] [CrossRef]
- Kumar, S.; Molloy, C.; Hunt, M.; Deng, C.H.; Wiedow, C.; Andre, C.; Dare, A.; McGhie, T. GWAS Provides New Insights into the Genetic Mechanisms of Phytochemicals Production and Red Skin Colour in Apple. Hortic. Res. 2022, 9, uhac218. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Shi, J.; Wang, K. Profile and Antioxidant Activity of Phenolic Extracts from 10 Crabapples (Malus wild species). J. Agric. Food Chem. 2014, 62, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Karaman, Ş.; Tütem, E.; Başkan, K.S.; Apak, R. Comparison of Antioxidant Capacity and Phenolic Composition of Peel and Flesh of Some Apple Varieties. J. Sci. Food Agric. 2013, 93, 867–875. [Google Scholar] [CrossRef]
- Akagić, A.; Murtić, S.; Musić, O.; Kurtović, M.; Oručević Žuljević, S.; Spaho, N.; Drkenda, P.; Gaši, F.; Vranac, A.; Hudina, M. Sugars, Acids and Polyphenols Profile of Commercial and Traditional Apple Cultivars for Processing. Acta Agric. Slov. 2019, 113, 239–250. [Google Scholar] [CrossRef]
- Stojiljković, D.; Nešić, I.; Tadić, V.; Najman, S.; Stojanović, S. Standardized Wild Apple Fruit Extract as a Bioactive Agent in Dermocosmetic Products for Efficacy Skin Hydration—In Vitro and in Vivo Evaluation. J. Cosmet. Dermatol. 2022, 21, 4788–4795. [Google Scholar] [CrossRef]
- Yanar, M.; KAÇAR, Y.A.; UĞUR, Y. Chemical Properties of Some Wild Fruit Species in Turkey. In Proceedings of the III. International Agricultural, Biological & Life Science Conference, Edirne, Turkey, 1–3 September 2021. [Google Scholar]
- Grosso, G.; Stepaniak, U.; Micek, A.; Kozela, M.; Stefler, D.; Bobak, M.; Pajak, A. Dietary Polyphenol Intake and Risk of Hypertension in the Polish Arm of the HAPIEE Study. Eur. J. Nutr. 2018, 57, 1535–1544. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of Polyphenols in Different Apple Varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Arts, I.C.W.; Van De Putte, B.; Hollman, P.C.H. Catechin Contents of Foods Commonly Consumed in The Netherlands. 1. Fruits, Vegetables, Staple Foods, and Processed Foods. J. Agric. Food Chem. 2000, 48, 1746–1751. [Google Scholar] [CrossRef]
- Podsędek, A.; Wilska-Jeszka, J.; Anders, B.; Markowski, J. Compositional Characterisation of Some Apple Varieties. Eur. Food Res. Technol. 2000, 210, 268–272. [Google Scholar] [CrossRef]
- Harada, U.; Chikama, A.; Saito, S.; Takase, H.; Nagao, T.; Hase, T.; Tokimitsu, I. Effects of the Long-Term Ingestion of Tea Catechins on Energy Expenditure and Dietary Fat Oxidation in Healthy Subjects. J. Health Sci. 2005, 51, 248–252. [Google Scholar] [CrossRef][Green Version]
- Boyer, J.; Liu, R.H. Apple Phytochemicals and Their Health Benefits. Nutr. J. 2004, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Alañón, M.E.; Castle, S.M.; Serra, G.; Lévèques, A.; Poquet, L.; Actis-Goretta, L.; Spencer, J.P.E. Acute Study of Dose-Dependent Effects of (−)-Epicatechin on Vascular Function in Healthy Male Volunteers: A Randomized Controlled Trial. Clin. Nutr. 2020, 39, 746–754. [Google Scholar] [CrossRef] [PubMed]
- Rudikovskaya, E.G.; Dudareva, L.V.; Shishparenok, A.A.; Rudikovskii, A.V. Peculiarities of Polyphenolic Profile of Fruits of Siberian Crab Apple and Its Hybrids with Malus × domestica Borkh. Acta Physiol. Plant 2015, 37, 238. [Google Scholar] [CrossRef]
- Duda-Chodak, A.; Tarko, T.; Tuszyński, T. Antioxidant Activity of Apples—An Impact of Maturity Stage and Fruit Part. Acta Sci. Pol. Technol. Aliment. 2011, 10, 443–454. [Google Scholar]
- Jakobek, L.; Barron, A.R. Ancient Apple Varieties from Croatia as a Source of Bioactive Polyphenolic Compounds. J. Food Compos. Anal. 2016, 45, 9–15. [Google Scholar] [CrossRef]
- Sun, Y.; Zimmermann, D.; De Castro, C.A.; Actis-Goretta, L. Dose–Response Relationship between Cocoa Flavanols and Human Endothelial Function: A Systematic Review and Meta-Analysis of Randomized Trials. Food Funct. 2019, 10, 6322–6330. [Google Scholar] [CrossRef]
- Lin, L.; Peng, A.; Yang, K.; Zou, Y. Monomeric Phenolics in Different Parts of High-acid Apple (Malus sieversii f. niedzwetzkyana (Dieck) Langenf): A Promising Source of Antioxidants for Application in Nutraceuticals. Int. J. Food Sci. Technol. 2018, 53, 1503–1509. [Google Scholar] [CrossRef]
- Niederberger, K.E.; Tennant, D.R.; Bellion, P. Dietary Intake of Phloridzin from Natural Occurrence in Foods. Br. J. Nutr. 2020, 123, 942–950. [Google Scholar] [CrossRef]
- Serban, M.; Sahebkar, A.; Zanchetti, A.; Mikhailidis, D.P.; Howard, G.; Antal, D.; Andrica, F.; Ahmed, A.; Aronow, W.S.; Muntner, P.; et al. Effects of Quercetin on Blood Pressure: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Am. Heart Assoc. 2016, 5, e002713. [Google Scholar] [CrossRef]
- Tian, L.; Cao, J.; Zhao, T.; Liu, Y.; Khan, A.; Cheng, G. The Bioavailability, Extraction, Biosynthesis and Distribution of Natural Dihydrochalcone: Phloridzin. Int. J. Mol. Sci. 2021, 22, 962. [Google Scholar] [CrossRef]
- Wu, X.; Beecher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Concentrations of Anthocyanins in Common Foods in the United States and Estimation of Normal Consumption. J. Agric. Food Chem. 2006, 54, 4069–4075. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Thilakarathna, S.; Nair, S. Polyphenols of Apples and Their Potentioal Health Benefits. In Polyphenols: Chemistry, Dietary Sources and Health Benefits; Sun, J., Ed.; Nutrition and diet research progress; Nova Science Publishers, Inc.: New York, NY, USA, 2013; pp. 333–367. [Google Scholar]
- Celik, F.; Gundogdu, M.; Ercisli, S.; Kaki, B.; Berk, S.; Ilhan, G.; Sagbas, H.I. Variation in Organic Acid, Sugar and Phenolic Compounds in Fruits of Historical Apple Cultivars. Not. Bot. Horti Agrobot. Cluj. Napoca 2018, 46, 622–629. [Google Scholar] [CrossRef]
- Maurya, H.; Mangal, V.; Gandhi, S.; Prabhu, K.; Ponnudurai, K. Prophylactic Antioxidant Potential of Gallic Acid in Murine Model of Sepsis. Int. J. Inflam. 2014, 2014, 580320. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Prahalathan, P.; Raja, B. Antihypertensive and Antioxidant Potential of Vanillic Acid, a Phenolic Compound in l-NAME-Induced Hypertensive Rats: A Dose-Dependence Study. Redox Rep. 2011, 16, 208–215. [Google Scholar] [CrossRef] [PubMed]
- DiNicolantonio, J.J.; McCarty, M.F.; Assanga, S.I.; Lujan, L.L.; O’Keefe, J.H. Ferulic Acid and Berberine, via Sirt1 and AMPK, May Act as Cell Cleansing Promoters of Healthy Longevity. Open Heart 2022, 9, e001801. [Google Scholar] [CrossRef] [PubMed]
- Cvetković, B.; Bajić, A.; Belović, M.; Pezo, L.; Dragojlović, D.; Šimurina, O.; Djordjević, M.; Korntheuer, K.; Philipp, C.; Eder, R. Assessing Antioxidant Properties, Phenolic Compound Profiles, Organic Acids, and Sugars in Conventional Apple Cultivars (Malus domestica): A Chemometric Approach. Foods 2024, 13, 2291. [Google Scholar] [CrossRef]
- Luceri, C.; Giannini, L.; Lodovici, M.; Antonucci, E.; Abbate, R.; Masini, E.; Dolara, P. p-Coumaric Acid, a Common Dietary Phenol, Inhibits Platelet Activity in Vitro and in Vivo. Br. J. Nutr. 2007, 97, 458–463. [Google Scholar] [CrossRef]
- Shafi, W.; Mansoor, S.; Jan, S.; Singh, D.; Kazi, M.; Raish, M.; Alwadei, M.; Mir, J.; Ahmad, P. Variability in Catechin and Rutin Contents and Their Antioxidant Potential in Diverse Apple Genotypes. Molecules 2019, 24, 943. [Google Scholar] [CrossRef]
- Bazyar, H.; Zare Javid, A.; Ahangarpour, A.; Zaman, F.; Hosseini, S.A.; Zohoori, V.; Aghamohammadi, V.; Yazdanfar, S.; Ghasemi Deh Cheshmeh, M. The Effects of Rutin Supplement on Blood Pressure Markers, Some Serum Antioxidant Enzymes, and Quality of Life in Patients with Type 2 Diabetes Mellitus Compared with Placebo. Front. Nutr. 2023, 10, 1214420. [Google Scholar] [CrossRef]
- Liang, T.; Liu, E.; Zhao, C.; Ban, S.; Li, Q. Simultaneous Determination of Four Flavonoids in Malus prunifolia from Shanxi Province by RP-HPLC. Zhongguo Zhong Yao Za Zhi 2009, 34, 2217–2219. [Google Scholar]
- Hoffmann-Ribani, R.; Huber, L.S.; Rodriguez-Amaya, D.B. Flavonols in Fresh and Processed Brazilian Fruits. J. Food Compos. Anal. 2009, 22, 263–268. [Google Scholar] [CrossRef]
- Zielinska, D.; Laparra-Llopis, J.M.; Zielinski, H.; Szawara-Nowak, D.; Giménez-Bastida, J.A. Role of Apple Phytochemicals, Phloretin and Phloridzin, in Modulating Processes Related to Intestinal Inflammation. Nutrients 2019, 11, 1173. [Google Scholar] [CrossRef]
- Birru, R.L.; Bein, K.; Wells, H.; Bondarchuk, N.; Barchowsky, A.; Di, Y.P.; Leikauf, G.D. Phloretin, an Apple Polyphenol, Inhibits Pathogen-Induced Mucin Overproduction. Mol. Nutr. Food Res. 2021, 65, 2000658. [Google Scholar] [CrossRef]
- Dadwal, V.; Agrawal, H.; Sonkhla, K.; Joshi, R.; Gupta, M. Characterization of Phenolics, Amino Acids, Fatty Acids and Antioxidant Activity in Pulp and Seeds of High Altitude Himalayan Crab Apple Fruits (Malus baccata). J. Food Sci. Technol. 2018, 55, 2160–2169. [Google Scholar] [CrossRef]
- Habtemariam, S. The Molecular Pharmacology of Phloretin: Anti-Inflammatory Mechanisms of Action. Biomedicines 2023, 11, 143. [Google Scholar] [CrossRef] [PubMed]
- Jakobek, L.; Ištuk, J.; Buljeta, I.; Voća, S.; Žlabur, J.Š.; Babojelić, M.S. Traditional, Indigenous Apple Varieties, a Fruit with Potential for Beneficial Effects: Their Quality Traits and Bioactive Polyphenol Contents. Foods 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Mattila, P.; Hellström, J.; Törrönen, R. Phenolic Acids in Berries, Fruits, and Beverages. J. Agric. Food Chem. 2006, 54, 7193–7199. [Google Scholar] [CrossRef] [PubMed]
- Veberic, R.; Trobec, M.; Herbinger, K.; Hofer, M.; Grill, D.; Stampar, F. Phenolic Compounds in Some Apple (Malus domestica Borkh) Cultivars of Organic and Integrated Production. J. Sci. Food Agric. 2005, 85, 1687–1694. [Google Scholar] [CrossRef]
- Punvittayagul, C.; Luangsuphabool, T.; Wongpoomchai, R. Protocatechuic Acid as a Potent Anticarcinogenic Compound in Purple Rice Bran against Diethylnitrosamine-Initiated Rat Hepatocarcinogenesis. Sci. Rep. 2022, 12, 10548. [Google Scholar] [CrossRef]
- Oszmiański, J.; Lachowicz, S.; Gławdel, E.; Cebulak, T.; Ochmian, I. Determination of Phytochemical Composition and Antioxidant Capacity of 22 Old Apple Cultivars Grown in Poland. Eur. Food Res. Technol. 2018, 244, 647–662. [Google Scholar] [CrossRef]
- Li, Q.; Song, F.; Zhu, M.; Wang, Q.; Han, Y.; Ling, Y.; Qiao, L.; Zhong, N.; Zhang, L. Hyperoside: A Review of Pharmacological Effects. F1000Res 2022, 11, 635. [Google Scholar] [CrossRef]
- Chinnici, F.; Gaiani, A.; Natali, N.; Riponi, C.; Galassi, S. Improved HPLC Determination of Phenolic Compounds in Cv. Golden Delicious Apples Using a Monolithic Column. J. Agric. Food Chem. 2004, 52, 3–7. [Google Scholar] [CrossRef]
- Babotă, M.; Frumuzachi, O.; Tanase, C.; Mocan, A. Efficacy of Myricetin Supplementation on Glucose and Lipid Metabolism: A Systematic Review and Meta-Analysis of In Vivo Mice Studies. Nutrients 2024, 16, 3730. [Google Scholar] [CrossRef]
- Eichenmüller, M.; Hemmerlein, B.; Von Schweinitz, D.; Kappler, R. Betulinic Acid Induces Apoptosis and Inhibits Hedgehog Signalling in Rhabdomyosarcoma. Br. J. Cancer 2010, 103, 43–51. [Google Scholar] [CrossRef]
- Miura, T.; Itoh, Y.; Kaneko, T.; Ueda, N.; Ishida, T.; Fukushima, M.; Matsuyama, F.; Seino, Y. Corosolic Acid Induces GLUT4 Translocation in Genetically Type 2 Diabetic Mice. Biol. Pharm. Bull. 2004, 27, 1103–1105. [Google Scholar] [CrossRef]
- Chen, J.; Han-Qing, Z.; Wei-Lin, L. Euscaphic Acid, a New Hypoglycemic Natural Product from Folium Eriobotryae. Pharmazie 2008, 64, 765–767. [Google Scholar] [CrossRef]
- Qian, Z.; Wang, X.; Song, Z.; Zhang, H.; Zhou, S.; Zhao, J.; Wang, H. A Phase I Trial to Evaluate the Multiple-Dose Safety and Antitumor Activity of Ursolic Acid Liposomes in Subjects with Advanced Solid Tumors. Biomed. Res. Int. 2015, 2015, 809714. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, Y.; Kinoshita, T.; Fukumitsu, S.; Aida, K.; Maruyama, K.; Saito, I.; Yamamoto, N. Pharmacokinetics and Effect of Maslinic Acid with Physical Exercise on Grip Strength and Trunk Muscle Mass in Healthy Japanese Individuals. J. Clin. Biochem. Nutr. 2023, 72, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Estrada, O.; González-Guzmán, J.M.; Salazar-Bookaman, M.; Fernández, A.Z.; Cardozo, A.; Alvarado-Castillo, C. Pomolic Acid of Licania pittieri Elicits Endothelium-Dependent Relaxation in Rat Aortic Rings. Phytomedicine 2011, 18, 464–469. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, H.; Jin, Q.; Wang, X. Effects of Dietary Linoleic Acid on Blood Lipid Profiles: A Systematic Review and Meta-Analysis of 40 Randomized Controlled Trials. Foods 2023, 12, 2129. [Google Scholar] [CrossRef]
- Cicero, A.F.G.; Nascetti, S.; López-Sabater, M.C.; Elosua, R.; Salonen, J.T.; Nyyssönen, K.; Poulsen, H.E.; Zunft, H.-J.F.; Kiesewetter, H.; de la Torre, K.; et al. Changes in LDL Fatty Acid Composition as a Response to Olive Oil Treatment Are Inversely Related to Lipid Oxidative Damage: The EUROLIVE Study. J. Am. Coll. Nutr. 2008, 27, 314–320. [Google Scholar] [CrossRef]
- Kim, I.; Ku, K.; Jeong, M.; Kim, S.S.; Mitchell, A.E.; Lee, J. A Comparison of the Chemical Composition and Antioxidant Activity of Several New Early- to Mid-season Apple Cultivars for a Warmer Climate with Traditional Cultivars. J. Sci. Food Agric. 2019, 99, 4712–4724. [Google Scholar] [CrossRef]
- Ma, B.; Yuan, Y.; Gao, M.; Li, C.; Ogutu, C.; Li, M.; Ma, F. Determination of Predominant Organic Acid Components in Malus species: Correlation with Apple Domestication. Metabolites 2018, 8, 74. [Google Scholar] [CrossRef]
- Rodgers, A.L.; Webber, D.; De Charmoy, R.; Jackson, G.E.; Ravenscroft, N. Malic Acid Supplementation Increases Urinary Citrate Excretion and Urinary PH: Implications for the Potential Treatment of Calcium Oxalate Stone Disease. J. Endourol. 2014, 28, 229–236. [Google Scholar] [CrossRef]
- Fang, T.; Zhen, Q.; Liao, L.; Owiti, A.; Zhao, L.; Korban, S.S.; Han, Y. Variation of Ascorbic Acid Concentration in Fruits of Cultivated and Wild Apples. Food Chem. 2017, 225, 132–137. [Google Scholar] [CrossRef]
- Doseděl, M.; Jirkovský, E.; Macáková, K.; Krčmová, L.; Javorská, L.; Pourová, J.; Mercolini, L.; Remião, F.; Nováková, L.; Mladěnka, P.; et al. Vitamin C—Sources, Physiological Role, Kinetics, Deficiency, Use, Toxicity, and Determination. Nutrients 2021, 13, 615. [Google Scholar] [CrossRef] [PubMed]
- Demircan, H.; Sarioğlu, K.; Sağdiç, O.; Özkan, K.; Kayacan, S.; Us, A.A.; Oral, R.A. Deer Apple (Malus trilobata) Fruit Grown in the Mediterranean Region: Identification of Some Components and Pomological Features. Food Sci. Technol. 2022, 42, e116421. [Google Scholar] [CrossRef]
- Lee, J.; Jeong, M.-C.; Ku, K.-H. Chemical, Physical, and Sensory Properties of 1-MCP-Treated Fuji Apple (Malus domestica Borkh.) Fruits after Long-Term Cold Storage. Appl. Biol. Chem. 2017, 60, 363–374. [Google Scholar] [CrossRef]
- Sugino, T.; Aoyagi, S.; Shirai, T.; Kajimoto, Y.; Kajimoto, O. Effects of Citric Acid and L-Carnitine on Physical Fatigue. J. Clin. Biochem. Nutr. 2007, 41, 224–230. [Google Scholar] [CrossRef]
- Dong, J.; Zheng, H.; Zeng, Q.; Zhang, X.; Du, L.; Bais, S. Protective Effect of D-(−)-Quinic Acid as Food Supplement in Modulating AMP-Activated Protein Kinase Signalling Pathway Activation in HFD Induced Obesity. Hum. Exp. Toxicol. 2022, 41, 9603271221119804. [Google Scholar] [CrossRef]
- Delgado-Pelayo, R.; Gallardo-Guerrero, L.; Hornero-Méndez, D. Chlorophyll and Carotenoid Pigments in the Peel and Flesh of Commercial Apple Fruit Varieties. Food Res. Int. 2014, 65, 272–281. [Google Scholar] [CrossRef]
- Ponder, A.; Jariené, E.; Hallmann, E. The Effect of Storage Conditions on the Content of Molecules in Malus domestica ‘Chopin’ Cv. and Their In Vitro Antioxidant Activity. Molecules 2022, 27, 6979. [Google Scholar] [CrossRef]
- Petkova, N.; Ognyanov, M.; Inyutin, B.; Zhelev, P.; Denev, P. Phytochemical Composition and Antioxidant Activity of Malus baccata (L.) Borkh. Fruits. Food Sci. Appl. Biotechnol. 2020, 3, 47. [Google Scholar] [CrossRef]
- Jubert, C.; Mata, J.; Bench, G.; Dashwood, R.; Pereira, C.; Tracewell, W.; Turteltaub, K.; Williams, D.; Bailey, G. Effects of Chlorophyll and Chlorophyllin on Low-Dose Aflatoxin B1 Pharmacokinetics in Human Volunteers. Cancer Prev. Res. 2009, 2, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Kishor, A.; Attri, B.L.; Brijwal, M.; Kumar, A.; Narayan, R.; Singh, D.B.; Debnath, S.; Mer, M.S.; Tiwari, V.K. Physico-Chemical Characterization of Wild Apple (Malus baccata) in Kumaon Hills of Uttarakhand. Ecol. Environ. Conserv. 2016, 22, S287–S291. [Google Scholar]
- Hallmann, E.; Orpel, E.; Rembiałkowska, E. The Content of Biologically Active Compounds in Some Fruits from Natural State. J. Fruit Ornam. Plant Res. 2011, 75, 81–90. [Google Scholar] [CrossRef]
- Darvin, M.E.; Lademann, J.; Von Hagen, J.; Lohan, S.B.; Kolmar, H.; Meinke, M.C.; Jung, S. Carotenoids in Human Skin In Vivo: Antioxidant and Photo-Protectant Role against External and Internal Stressors. Antioxidants 2022, 11, 1451. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; He, J.; Li, X.; Hou, N.; Guo, J.; Niu, C.; Li, C.; Liu, S.; Xu, J.; et al. Chromosome-scale Reference Genome Provides Insights into the Genetic Origin and Grafting-mediated Stress Tolerance of Malus prunifolia. Plant Biotechnol. J. 2022, 20, 1015–1017. [Google Scholar] [CrossRef]
- McClure, K.A.; Gong, Y.; Song, J.; Vinqvist-Tymchuk, M.; Campbell Palmer, L.; Fan, L.; Burgher-MacLellan, K.; Zhang, Z.; Celton, J.-M.; Forney, C.F.; et al. Genome-Wide Association Studies in Apple Reveal Loci of Large Effect Controlling Apple Polyphenols. Hortic. Res. 2019, 6, 107. [Google Scholar] [CrossRef]
- Rudikovskaya, E.G.; Dudareva, L.V.; Shishparenok, A.A.; Mitanova, N.B.; Petrova, I.G.; Rudikovskii, A.V. Phenolic Composition of Malus baccata Fruit. Chem. Nat. Compd. 2014, 50, 739–740. [Google Scholar] [CrossRef]
- Sharma, R.; Nath, A.K. Antioxidant Levels and Activities of Reactive Oxygen-Scavenging Enzymes in Crab Apple Fruits (Malus baccata). Proc. Natl. Acad. Sci. India Sect. B: Biol. Sci. 2016, 86, 877–885. [Google Scholar] [CrossRef]
- Ji, X.; Hou, C.; Guo, X. The Fruit Malus prunifolia (Malus micromalus Mak.): A Minireview of Current Knowledge of Fruit Composition and Health Benefits. J. Chem. 2020, 2020, 1–8. [Google Scholar] [CrossRef]
- Mustafa, B.; Nebija, D.; Hajdari, A. Evaluation of Essential Oil Composition, Total Phenolics, Total Flavonoids and Antioxidant Activity of Malus sylvestris (L.) Mill. fruits. Fruits. Res. 2018, 23, 71–85. [Google Scholar]
- Assaleh, M.H.; Bjelogrlic, S.K.; Prlainovic, N.; Cvijetic, I.; Bozic, A.; Arandjelovic, I.; Vukovic, D.; Marinkovic, A. Antimycobacterial and Anticancer Activity of Newly Designed Cinnamic Acid Hydrazides with Favorable Toxicity Profile. Arab. J. Chem. 2022, 15, 103532. [Google Scholar] [CrossRef]
- Purushothaman, A.; Babu, S.S.; Naroth, S.; Janardanan, D. Antioxidant Activity of Caffeic Acid: Thermodynamic and Kinetic Aspects on the Oxidative Degradation Pathway. Free Radic. Res. 2022, 56, 617–630. [Google Scholar] [CrossRef]
- Maity, S.; Kinra, M.; Nampoothiri, M.; Arora, D.; Pai, K.S.R.; Mudgal, J. Caffeic Acid, a Dietary Polyphenol, as a Promising Candidate for Combination Therapy. Chem. Pap. 2022, 76, 1271–1283. [Google Scholar] [CrossRef]
- Panzella, L. Polyphenols and Their Impact on Human Health. Int. J. Mol. Sci. 2023, 24, 16683. [Google Scholar] [CrossRef]
- Wang, L.; Pan, X.; Jiang, L.; Chu, Y.; Gao, S.; Jiang, X.; Zhang, Y.; Chen, Y.; Luo, S.; Peng, C. The Biological Activity Mechanism of Chlorogenic Acid and Its Applications in Food Industry: A Review. Front. Nutr. 2022, 9, 943911. [Google Scholar] [CrossRef]
- Che, J.; Zhao, T.; Liu, W.; Chen, S.; Yang, G.; Li, X.; Liu, D. Neochlorogenic Acid Enhances the Antitumor Effects of Pingyangmycin via Regulating TOP2A. Mol. Med. Rep. 2020, 23, 158. [Google Scholar] [CrossRef]
- Shen, Y.; Song, X.; Li, L.; Sun, J.; Jaiswal, Y.; Huang, J.; Liu, C.; Yang, W.; Williams, L.; Zhang, H.; et al. Protective Effects of P-Coumaric Acid against Oxidant and Hyperlipidemia-an in Vitro and in Vivo Evaluation. Biomed. Pharmacother. 2019, 111, 579–587. [Google Scholar] [CrossRef]
- Zaman, A.; Hasnat, H.; Noman, Z.A.; Islam, M.M.; Nakib, A.A.; Mukherjee, S.; Saha, K.; Ahmed, N.U.; Ashrafi, S.; Saha, T.; et al. Exploring Pharmacological Potentials of P-Coumaric Acid: A Prospective Phytochemical for Drug Discovery. Bangladesh Pharm. J. 2023, 26, 185–194. [Google Scholar] [CrossRef]
- Yu, X.-D.; Zhang, D.; Xiao, C.-L.; Zhou, Y.; Li, X.; Wang, L.; He, Z.; Reilly, J.; Xiao, Z.-Y.; Shu, X. P-Coumaric Acid Reverses Depression-Like Behavior and Memory Deficit Via Inhibiting AGE-RAGE-Mediated Neuroinflammation. Cells 2025, 11, 1594. [Google Scholar] [CrossRef] [PubMed]
- Sova, M.; Saso, L. Natural Sources, Pharmacokinetics, Biological Activities and Health Benefits of Hydroxycinnamic Acids and Their Metabolites. Nutrients 2020, 12, 2190. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, T.A.; Hassen, A.; Apostolides, Z. The Antimethanogenic Potentials of Plant Extracts: Their Yields and Phytochemical Compositions as Affected by Extractive Solvents. Plants 2022, 11, 3296. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, M.; Sudheer, A.R.; Menon, V.P. Ferulic Acid: Therapeutic Potential Through Its Antioxidant Property. J. Clin. Biochem. Nutr. 2007, 40, 92–100. [Google Scholar] [CrossRef]
- Purushothaman, J.R.; Rizwanullah, M. Ferulic Acid: A Comprehensive Review. Cureus 2024, 16, e68063. [Google Scholar] [CrossRef]
- Mu, M.; Zuo, S.; Wu, R.-M.; Deng, K.-S.; Lu, S.; Zhu, J.-J.; Zou, G.-L.; Yang, J.; Cheng, M.-L.; Zhao, X.-K. Ferulic Acid Attenuates Liver Fibrosis and Hepatic Stellate Cell Activation via Inhibition of TGF-β/Smad Signaling Pathway. Drug Des. Dev. Ther. 2018, 12, 4107–4115. [Google Scholar] [CrossRef]
- Luo, Z.; Li, M.; Yang, Q.; Zhang, Y.; Liu, F.; Gong, L.; Han, L.; Wang, M. Ferulic Acid Prevents Nonalcoholic Fatty Liver Disease by Promoting Fatty Acid Oxidation and Energy Expenditure in C57BL/6 Mice Fed a High-Fat Diet. Nutrients 2022, 14, 2530. [Google Scholar] [CrossRef]
- Pandi, A.; Raghu, M.H.; Chandrashekar, N.; Kalappan, V.M. Cardioprotective Effects of Ferulic Acid against Various Drugs and Toxic Agents. Beni Suef Univ. J. Basic. Appl. Sci. 2022, 11, 92. [Google Scholar] [CrossRef]
- Zhang, S.; Gai, Z.; Gui, T.; Chen, J.; Chen, Q.; Li, Y. Antioxidant Effects of Protocatechuic Acid and Protocatechuic Aldehyde: Old Wine in a New Bottle. Evid.-Based Complement. Altern. Med. 2021, 2021, 6139308. [Google Scholar] [CrossRef]
- Kakkar, S.; Bais, S. A Review on Protocatechuic Acid and Its Pharmacological Potential. ISRN Pharmacol. 2014, 2014, 952943. [Google Scholar] [CrossRef] [PubMed]
- Aissani, N.; Albouchi, F.; Sebai, H. Anticancer Effect in Human Glioblastoma and Antioxidant Activity of Petroselinum crispum L. Methanol Extract. Nutr. Cancer 2021, 73, 2605–2613. [Google Scholar] [CrossRef] [PubMed]
- Semaming, Y.; Pannengpetch, P.; Chattipakorn, S.C.; Chattipakorn, N. Pharmacological Properties of Protocatechuic Acid and Its Potential Roles as Complementary Medicine. Evid.-Based Complement. Altern. Med. 2015, 2015, 593902. [Google Scholar] [CrossRef]
- Ud Din, S.R.; Saeed, S.; Khan, S.U.; Kiani, F.A.; Alsuhaibani, A.M.; Zhong, M. Bioactive Compounds (BACs): A Novel Approach to Treat and Prevent Cardiovascular Diseases. Curr. Probl. Cardiol. 2023, 48, 101664. [Google Scholar] [CrossRef]
- Magiera, A.; Kołodziejczyk-Czepas, J.; Olszewska, M.A. Antioxidant and Anti-Inflammatory Effects of Vanillic Acid in Human Plasma, Human Neutrophils, and Non-Cellular Models In Vitro. Molecules 2025, 30, 467. [Google Scholar] [CrossRef]
- Ullah, R.; Ikram, M.; Park, T.J.; Ahmad, R.; Saeed, K.; Alam, S.I.; Rehman, I.U.; Khan, A.; Khan, I.; Jo, M.G.; et al. Vanillic Acid, a Bioactive Phenolic Compound, Counteracts LPS-Induced Neurotoxicity by Regulating c-Jun N-Terminal Kinase in Mouse Brain. Int. J. Mol. Sci. 2020, 22, 361. [Google Scholar] [CrossRef]
- Hadidi, M.; Liñán-Atero, R.; Tarahi, M.; Christodoulou, M.C.; Aghababaei, F. The Potential Health Benefits of Gallic Acid: Therapeutic and Food Applications. Antioxidants 2024, 13, 1001. [Google Scholar] [CrossRef]
- Fitzpatrick, L.R.; Woldemariam, T. Small-Molecule Drugs for the Treatment of Inflammatory Bowel Disease. In Comprehensive Medicinal Chemistry III; Elsevier: Amsterdam, The Netherlands, 2017; pp. 495–510. [Google Scholar]
- Zbikowska, H.M.; Antosik, A.; Szejk, M.; Bijak, M.; Olejnik, A.K.; Saluk, J.; Nowak, P. Does Quercetin Protect Human Red Blood Cell Membranes against γ-Irradiation? Redox Rep. 2014, 19, 65–71. [Google Scholar] [CrossRef]
- Yu, K.-H.; Jheng, C.-P.; Lee, C.-I. Quercetin Binding Accelerates Prion Fibrillation into Proteinase Sensitive and Loosely Structured Amyloids. Biomed. Pharmacother. 2022, 151, 113177. [Google Scholar] [CrossRef]
- Aghababaei, F.; Hadidi, M. Recent Advances in Potential Health Benefits of Quercetin. Pharmaceuticals 2023, 16, 1020. [Google Scholar] [CrossRef]
- Jang, E. Hyperoside as a Potential Natural Product Targeting Oxidative Stress in Liver Diseases. Antioxidants 2022, 11, 1437. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, H.; Yuan, L.; Li, X.; Cai, Y. Potential Implications of Hyperoside on Oxidative Stress-Induced Human Diseases: A Comprehensive Review. J. Inflamm. Res. 2023, 16, 4503–4526. [Google Scholar] [CrossRef]
- Xu, S.; Chen, S.; Xia, W.; Sui, H.; Fu, X. Hyperoside: A Review of Its Structure, Synthesis, Pharmacology, Pharmacokinetics and Toxicity. Molecules 2022, 27, 3009. [Google Scholar] [CrossRef]
- Yuan, J.; Dong, X.; Zhou, S.; Nao, J. Pharmacological Activities and Therapeutic Potential of Hyperoside in the Treatment of Alzheimer’s and Parkinson’s Diseases: A Systemic Review. Neuroscience 2024, 563, 136–147. [Google Scholar] [CrossRef]
- Lan, Z.; Wang, H.; Wang, S.; Zhu, T.; Ma, S.; Song, Y.; Cui, C.; Liu, M.; Tian, C. Rutin Protects against Cyclophosphamide Induced Immunological Stress by Inhibiting TLR4-NF-ΚB-Mediated Inflammation and Activating the Nrf2- Mediated Antioxidant Responses. Pharmacol. Res.-Mod. Chin. Med. 2022, 4, 100135. [Google Scholar] [CrossRef]
- Baliga, M.S.; Saxena, A.; Kaur, K.; Kalekhan, F.; Chacko, A.; Venkatesh, P.; Fayad, R. Polyphenols in the Prevention of Ulcerative Colitis. In Polyphenols in Human Health and Disease; Elsevier: Amsterdam, The Netherlands, 2014; pp. 655–663. [Google Scholar]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Chen, Y.C. A Review of the Dietary Flavonoid, Kaempferol on Human Health and Cancer Chemoprevention. Food Chem. 2013, 138, 2099–2107. [Google Scholar] [CrossRef]
- Ren, J.; Lu, Y.; Qian, Y.; Chen, B.; Wu, T.; Ji, G. Recent Progress Regarding Kaempferol for the Treatment of Various Diseases (Review). Exp. Ther. Med. 2019, 18, 2759–2776. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, M.; Imran, M.; Alsagaby, S.A.; Naeem, H.; Al Abdulmonem, W.; Hussain, M.; Abdelgawad, M.A.; El-Ghorab, A.H.; Ghoneim, M.M.; El-Sherbiny, M.; et al. Anticancer, Antioxidant, Ameliorative and Therapeutic Properties of Kaempferol. Int. J. Food Prop. 2023, 26, 1140–1166. [Google Scholar] [CrossRef]
- Kamisah, Y.; Jalil, J.; Yunos, N.M.; Zainalabidin, S. Cardioprotective Properties of Kaempferol: A Review. Plants 2023, 12, 2096. [Google Scholar] [CrossRef]
- Wielgus, M.; Zaniewicz, N. Selected Biological Properties of Quercetin, Curcumin, and Kaempferol. Acta Univ. Lodziensis. Folia Biol. Oecologica 2024, 18, 48–65. [Google Scholar] [CrossRef]
- Periferakis, A.; Periferakis, K.; Badarau, I.A.; Petran, E.M.; Popa, D.C.; Caruntu, A.; Costache, R.S.; Scheau, C.; Caruntu, C.; Costache, D.O. Kaempferol: Antimicrobial Properties, Sources, Clinical, and Traditional Applications. Int. J. Mol. Sci. 2022, 23, 15054. [Google Scholar] [CrossRef] [PubMed]
- Mostafa-Hedeab, G.; Ewaiss Hassan, M.; Halawa, T.F.; Wani, F.A. Epigallocatechin Gallate Ameliorates Tetrahydrochloride-Induced Liver Toxicity in Rats via Inhibition of TGFβ/p-ERK/p-Smad1/2 Signaling, Antioxidant, Anti-Inflammatory Activity. Saudi Pharm. J. 2022, 30, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Zheng, Y. Myricetin Protects Keratinocyte Damage Induced by UV through IκB/NFκb Signaling Pathway. J. Cosmet. Dermatol. 2017, 16, 444–449. [Google Scholar] [CrossRef]
- Cho, B.O.; Yin, H.H.; Park, S.H.; Byun, E.B.; Ha, H.Y.; Jang, S.I. Anti-Inflammatory Activity of Myricetin from Diospyros lotus through Suppression of NF-ΚB and STAT1 Activation and Nrf2-Mediated HO-1 Induction in Lipopolysaccharide-Stimulated RAW264.7 Macrophages. Biosci. Biotechnol. Biochem. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Latief, N.; Anand, S.; Lingaraju, M.C.; Balaganur, V.; Pathak, N.N.; Kalra, J.; Kumar, D.; Bhadoria, B.K.; Tandan, S.K. Effect of Trimeric Myricetin Rhamnoside (TMR) in Carrageenan-induced Inflammation and Caecal Ligation and Puncture-induced Lung Oxidative Stress in Mice. Phytother. Res. 2015, 29, 1798–1805. [Google Scholar] [CrossRef]
- Lee, D.H.; Lee, C.S. Flavonoid Myricetin Inhibits TNF-α-Stimulated Production of Inflammatory Mediators by Suppressing the Akt, MTOR and NF-ΚB Pathways in Human Keratinocytes. Eur. J. Pharmacol. 2016, 784, 164–172. [Google Scholar] [CrossRef]
- Imran, M.; Saeed, F.; Hussain, G.; Imran, A.; Mehmood, Z.; Gondal, T.A.; El-Ghorab, A.; Ahmad, I.; Pezzani, R.; Arshad, M.U.; et al. Myricetin: A Comprehensive Review on Its Biological Potentials. Food Sci. Nutr. 2021, 9, 5854–5868. [Google Scholar] [CrossRef]
- Niisato, N.; Marunaka, Y. Therapeutic Potential of Multifunctional Myricetin for Treatment of Type 2 Diabetes Mellitus. Front. Nutr. 2023, 10, 1175660. [Google Scholar] [CrossRef]
- Hu, T.; Yuan, X.; Wei, G.; Luo, H.; Lee, H.J.; Jin, W. Myricetin-Induced Brown Adipose Tissue Activation Prevents Obesity and Insulin Resistance in Db/Db Mice. Eur. J. Nutr. 2018, 57, 391–403. [Google Scholar] [CrossRef] [PubMed]
- Chao, H.; Tsai, P.; Lee, S.; Lin, Y.; Wu, M. Effects of Myricetin-Containing Ethanol Solution on High-Fat Diet Induced Obese Rats. J. Food Sci. 2017, 82, 1947–1952. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; An, B.; Yu, Q.; Cao, Y.; Liu, Y.; Li, S. The Hepatoprotective Effect of Myricetin against Lipopolysaccharide and D-Galactosamine-Induced Fulminant Hepatitis. Int. J. Biol. Macromol. 2020, 155, 1092–1104. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, P.K.; Agrawal, C.; Blunden, G. Antiviral and Possible Prophylactic Significance of Myricetin for COVID-19. Nat. Prod. Commun. 2023, 18, 1934578X231166283. [Google Scholar] [CrossRef]
- Ruan, W.; Shen, S.; Xu, Y.; Ran, N.; Zhang, H. Mechanistic Insights into Procyanidins as Therapies for Alzheimer’s Disease: A Review. J. Funct. Foods 2021, 86, 104683. [Google Scholar] [CrossRef]
- Orisakeye, O.T.; Olugbade, T.A. Epicatechin and Procyanidin B2 in the Stem and Root Bark of Sterculia tragacantha Lindl (Sterculiaceae). Med. Chem. 2014, 4, 334–337. [Google Scholar] [CrossRef]
- Liang, Z.; Liang, H.; Guo, Y.; Yang, D. Cyanidin 3-O-Galactoside: A Natural Compound with Multiple Health Benefits. Int. J. Mol. Sci. 2021, 22, 2261. [Google Scholar] [CrossRef]
- Cerletti, C.; De Curtis, A.; Bracone, F.; Digesù, C.; Morganti, A.G.; Iacoviello, L.; de Gaetano, G.; Donati, M.B. Dietary Anthocyanins and Health: Data from FLORA and ATHENA EU Projects: Dietary Anthocyanins and Health. Br. J. Clin. Pharmacol. 2017, 83, 103–106. [Google Scholar] [CrossRef]
- Cremonini, E.; Daveri, E.; Iglesias, D.E.; Kang, J.; Wang, Z.; Gray, R.; Mastaloudis, A.; Kay, C.D.; Hester, S.N.; Wood, S.M.; et al. A Randomized Placebo-Controlled Cross-over Study on the Effects of Anthocyanins on Inflammatory and Metabolic Responses to a High-Fat Meal in Healthy Subjects. Redox Biol. 2022, 51, 102273. [Google Scholar] [CrossRef]
- Li, D.; Wang, P.; Luo, Y.; Zhao, M.; Chen, F. Health Benefits of Anthocyanins and Molecular Mechanisms: Update from Recent Decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Li, S.; Wang, T.; Wu, B.; Fu, W.; Xu, B.; Pamuru, R.R.; Kennett, M.; Vanamala, J.K.P.; Reddivari, L. Anthocyanin-Containing Purple Potatoes Ameliorate DSS-Induced Colitis in Mice. J. Nutr. Biochem. 2021, 93, 108616. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and Hypolipidemic Effects of Blueberry Anthocyanins by AMPK Activation: In Vitro and in Vivo Studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, J.; Zhuang, Y.; Fei, P. Acylating Blueberry Anthocyanins with Fatty Acids: Improvement of Their Lipid Solubility and Antioxidant Activities. Food Chem. X 2022, 15, 100420. [Google Scholar] [CrossRef] [PubMed]
- Yousuf, B.; Gul, K.; Wani, A.A.; Singh, P. Health Benefits of Anthocyanins and Their Encapsulation for Potential Use in Food Systems: A Review. Crit. Rev. Food Sci. Nutr. 2016, 56, 2223–2230. [Google Scholar] [CrossRef]
- Garcia, C.; Blesso, C.N. Antioxidant Properties of Anthocyanins and Their Mechanism of Action in Atherosclerosis. Free Radic. Biol. Med. 2021, 172, 152–166. [Google Scholar] [CrossRef]
- Kent, K.; Yousefi, M.; Do Rosario, V.A.; Fitzgerald, Z.; Broyd, S.; Visentin, D.; Roodenrys, S.; Walton, K.; Charlton, K.E. Anthocyanin Intake Is Associated with Improved Memory in Older Adults with Mild Cognitive Impairment. Nutr. Res. 2022, 104, 36–43. [Google Scholar] [CrossRef]
- Nguyen, N.A.; Cao, N.T.; Nguyen, T.H.H.; Ji, J.-H.; Cha, G.S.; Kang, H.-S.; Yun, C.-H. Enzymatic Production of 3-OH Phlorizin, a Possible Bioactive Polyphenol from Apples, by Bacillus megaterium CYP102A1 via Regioselective Hydroxylation. Antioxidants 2021, 10, 1327. [Google Scholar] [CrossRef]
- Li, P.; Tan, J.; Xiao, M.; Cai, X.; Xue, H.; Yu, H. Bioactive Substances and Biological Functions in Malus hupehensis: A Review. Molecules 2023, 28, 658. [Google Scholar] [CrossRef]
- Vandorou, M.; Plakidis, C.; Tsompanidou, I.M.; Adamantidi, T.; Panagopoulou, E.A.; Tsoupras, A. A Review on Apple Pomace Bioactives for Natural Functional Food and Cosmetic Products with Therapeutic Health-Promoting Properties. Int. J. Mol. Sci. 2024, 25, 10856. [Google Scholar] [CrossRef]
- Hutabarat, O.S.; Flachowsky, H.; Regos, I.; Miosic, S.; Kaufmann, C.; Faramarzi, S.; Alam, M.Z.; Gosch, C.; Peil, A.; Richter, K.; et al. Transgenic Apple Plants Overexpressing the Chalcone 3-Hydroxylase Gene of Cosmos Sulphureus Show Increased Levels of 3-Hydroxyphloridzin and Reduced Susceptibility to Apple Scab and Fire Blight. Planta 2016, 243, 1213–1224. [Google Scholar] [CrossRef]
- Shelke, V.; Kale, A.; Kulkarni, Y.A.; Gaikwad, A.B. Phloretin: A Comprehensive Review of Its Potential against Diabetes and Associated Complications. J. Pharm. Pharmacol. 2024, 76, 201–212. [Google Scholar] [CrossRef]
- Wu, K.-H.; Ho, C.-T.; Chen, Z.-F.; Chen, L.-C.; Whang-Peng, J.; Lin, T.-N.; Ho, Y.-S. The Apple Polyphenol Phloretin Inhibits Breast Cancer Cell Migration and Proliferation via Inhibition of Signals by Type 2 Glucose Transporter. J. Food Drug Anal. 2018, 26, 221–231. [Google Scholar] [CrossRef] [PubMed]
- Ni, T.; Zhang, S.; Rao, J.; Zhao, J.; Huang, H.; Liu, Y.; Ding, Y.; Liu, Y.; Ma, Y.; Zhang, S.; et al. Phlorizin, an Important Glucoside: Research Progress on Its Biological Activity and Mechanism. Molecules 2024, 29, 741. [Google Scholar] [CrossRef] [PubMed]
- Abraham, G.E.; Flechas, J.D. Management of Fibromyalgia: Rationale for the Use of Magnesium and Malic Acid. J. Nutr. Med. 1992, 3, 49–59. [Google Scholar] [CrossRef]
- Tang, S.-C.; Yang, J.-H. Dual Effects of Alpha-Hydroxy Acids on the Skin. Molecules 2018, 23, 863. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Moreno, G.; Guardia, J.; Aguilar-Salvatierra, A.; Cabrera-Ayala, M.; Mate-Sanchez de-Val, J.E.; Calvo-Guirado, J.L. Effectiveness of Malic Acid 1% in Patients with Xerostomia Induced by Antihypertensive Drugs. Med. Oral 2013, 18, e49–e55. [Google Scholar] [CrossRef]
- Wu, X.; Dai, H.; Xu, C.; Liu, L.; Li, S. Citric Acid Modification of a Polymer Exhibits Antioxidant and Anti-inflammatory Properties in Stem Cells and Tissues. J. Biomed. Mater. Res. A 2019, 107, 2414–2424. [Google Scholar] [CrossRef]
- Ceresnakova, M.; Murray, D.; Soulimane, T.; Hudson, S.P. Candidates for Smart Cardiovascular Medical Device Coatings: A Comparative Study with Endothelial and Smooth Muscle Cells. Eur. J. Pharmacol. 2021, 910, 174490. [Google Scholar] [CrossRef]
- Tsiaoussis, G.I.; Christaki, E.; Apidianakis, Y. I Can C Clearly Now: How EPEC Inhibits Gut Vitamin C Transport by Dysregulating SVCT. Dig. Dis. Sci. 2021, 66, 2140–2142. [Google Scholar] [CrossRef]
- Larsson, S.C.; Mason, A.M.; Vithayathil, M.; Carter, P.; Kar, S.; Zheng, J.-S.; Burgess, S. Circulating Vitamin C and Digestive System Cancers: Mendelian Randomization Study. Clin. Nutr. 2022, 41, 2031–2035. [Google Scholar] [CrossRef]
- Ashor, A.W.; Shannon, O.M.; Werner, A.-D.; Scialo, F.; Gilliard, C.N.; Cassel, K.S.; Seal, C.J.; Zheng, D.; Mathers, J.C.; Siervo, M. Effects of Inorganic Nitrate and Vitamin C Co-Supplementation on Blood Pressure and Vascular Function in Younger and Older Healthy Adults: A Randomised Double-Blind Crossover Trial. Clin. Nutr. 2020, 39, 708–717. [Google Scholar] [CrossRef]
- Alnaimat, S.; Oseni, A.; Yang, Y.; Melvani, V.; Aronson, A.; Harris, K.; Panaich, S. Missing Vitamin C. JACC Case Rep. 2019, 1, 192–196. [Google Scholar] [CrossRef] [PubMed]
- Turck, D.; Siani, A. Vitamin C and Protection of DNA, Proteins and Lipids from Oxidative Damage: Evaluation of a Health Claim Pursuant to Article 14 of Regulation (EC) No 1924/2006. EFSA J. 2017, 15, 4685. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, S.; Ali, S.; Tahir, H.M.; Kazmi, S.A.R.; Shakir, H.A.; Mughal, T.A.; Mumtaz, S.; Summer, M.; Farooq, M.A. Aging and Its Treatment with Vitamin C: A Comprehensive Mechanistic Review. Mol. Biol. Rep. 2021, 48, 8141–8153. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, Y.-H. Physical Activity, Dietary Vitamin C, and Metabolic Syndrome in the Korean Adults: The Korea National Health and Nutrition Examination Survey 2008 to 2012. Public Health 2016, 135, 30–37. [Google Scholar] [CrossRef]
- Liu, M.; Park, S. A Causal Relationship between Vitamin C Intake with Hyperglycemia and Metabolic Syndrome Risk: A Two-Sample Mendelian Randomization Study. Antioxidants 2022, 11, 857. [Google Scholar] [CrossRef]
- Li, S.; Cai, Y.; Guan, T.; Zhang, Y.; Huang, K.; Zhang, Z.; Cao, W.; Guan, X. Quinic Acid Alleviates High-Fat Diet-Induced Neuroinflammation by Inhibiting DR3/IKK/NF-ΚB Signaling via Gut Microbial Tryptophan Metabolites. Gut Microbes 2024, 16, 2374608. [Google Scholar] [CrossRef]
- Lin, K.-H.; Hsu, C.-Y.; Huang, Y.-P.; Lai, J.-Y.; Hsieh, W.-B.; Huang, M.-Y.; Yang, C.-M.; Chao, P.-Y. Chlorophyll-Related Compounds Inhibit Cell Adhesion and Inflammation in Human Aortic Cells. J. Med. Food 2013, 16, 886–898. [Google Scholar] [CrossRef]
- Sahin, N.; Akdemir, F.; Orhan, C.; Tuzcu, M.; Yilmaz, I.; Juturu, V. 3R, 3′R Zeaxanthin Protects Retina from Photo-Oxidative Damage: In Vivo Model. FASEB J. 2017, 31, 646–648. [Google Scholar] [CrossRef]
- Li, X.; Holt, R.R.; Keen, C.L.; Morse, L.S.; Zivkovic, A.M.; Yiu, G.; Hackman, R.M. Potential Roles of Dietary Zeaxanthin and Lutein in Macular Health and Function. Nutr. Rev. 2022, 81, 670–683. [Google Scholar] [CrossRef]
- Kim, J.; Lee, J.; Oh, J.H.; Chang, H.J.; Sohn, D.K.; Kwon, O.; Shin, A.; Kim, J. Dietary Lutein Plus Zeaxanthin Intake and DICER1 Rs3742330 A > G Polymorphism Relative to Colorectal Cancer Risk. Sci. Rep. 2024, 9, 3406. [Google Scholar] [CrossRef]
- Varghese, R.; Efferth, T.; Ramamoorthy, S. Carotenoids for Lung Cancer Chemoprevention and Chemotherapy: Promises and Controversies. Phytomedicine 2023, 116, 154850. [Google Scholar] [CrossRef] [PubMed]
- Sengngam, K.; Hoc, T.H.; Hang, D.V.; Tran Ngoan, L. Trans-Lycopene and β-Cryptoxanthin Intake and Stomach Cancer in Vietnamese Men: A Pilot Case-Control Study. Asian Pac. J. Cancer Prev. 2022, 23, 861–865. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Tang, R.; Zhou, R.; Qian, Y.; Di, D. The Protective Effect of Serum Carotenoids on Cardiovascular Disease: A Cross-Sectional Study from the General US Adult Population. Front. Nutr. 2024, 10, 1154239. [Google Scholar] [CrossRef]
- Mrowicka, M.; Mrowicki, J.; Kucharska, E.; Majsterek, I. Lutein and Zeaxanthin and Their Roles in Age-Related Macular Degeneration—Neurodegenerative Disease. Nutrients 2022, 14, 827. [Google Scholar] [CrossRef]
- Zhao, M.; Wu, F.; Tang, Z.; Yang, X.; Liu, Y.; Wang, F.; Chen, B. Anti-Inflammatory and Antioxidant Activity of Ursolic Acid: A Systematic Review and Meta-Analysis. Front. Pharmacol. 2023, 14, 1256946. [Google Scholar] [CrossRef]
- Li, L.; Zhang, X.; Cui, L.; Wang, L.; Liu, H.; Ji, H.; Du, Y. Ursolic Acid Promotes the Neuroprotection by Activating Nrf2 Pathway after Cerebral Ischemia in Mice. Brain Res. 2013, 1497, 32–39. [Google Scholar] [CrossRef]
- D’Abrosca, B.; Fiorentino, A.; Monaco, P.; Oriano, P.; Pacifico, S. Annurcoic Acid: A New Antioxidant Ursane Triterpene from Fruits of Cv. Annurca apple. Food Chem. 2006, 98, 285–290. [Google Scholar] [CrossRef]
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Kinghorn, A.D.; Brown, D.M.; et al. Discovery of Betulinic Acid as a Selective Inhibitor of Human Melanoma That Functions by Induction of Apoptosis. Nat. Med. 1995, 1, 1046–1051. [Google Scholar] [CrossRef]
- Fulda, S.; Debatin, K.-M. Betulinic Acid Induces Apoptosis through a Direct Effect on Mitochondria in Neuroectodermal Tumors. Med. Pediatr. Oncol. 2000, 35, 616–618. [Google Scholar] [CrossRef]
- Cai, Y.; Zheng, Y.; Gu, J.; Wang, S.; Wang, N.; Yang, B.; Zhang, F.; Wang, D.; Fu, W.; Wang, Z. Betulinic Acid Chemosensitizes Breast Cancer by Triggering ER Stress-Mediated Apoptosis by Directly Targeting GRP78. Cell Death Dis. 2018, 9, 636. [Google Scholar] [CrossRef]
- Lu, J.; Wang, C. Medicinal Components and Pharmacological Effects of Rosa rugosa. Rec. Nat. Prod. 2018, 12, 535–543. [Google Scholar] [CrossRef]
- Tan, H.; Sonam, T.; Shimizu, K. The Potential of Triterpenoids from Loquat Leaves (Eriobotrya japonica) for Prevention and Treatment of Skin Disorder. Int. J. Mol. Sci. 2017, 18, 1030. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, K.; Rufino-Palomares, E.E.; Pérez-Jiménez, A.; Reyes-Zurita, F.J.; Figuera, C.; García-Salguero, L.; Medina, P.P.; Peragón, J.; Lupiáñez, J.A. Maslinic Acid, a Triterpene from Olive, Affects the Antioxidant and Mitochondrial Status of B16F10 Melanoma Cells Grown under Stressful Conditions. Evid.-Based Complement. Altern. Med. 2015, 2015, 272457. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Guo, Y.; Hao, Q.; Li, D.; Qiao, Z.; Cui, Y.; Li, J. Pomolic Acid Inhibits Proliferation of Human Lung Carcinoma Cells via Induction of Apoptosis and Suppression of Cell Migration and Invasion. Trop. J. Pharm. Res. 2022, 21, 1201–1207. [Google Scholar] [CrossRef]
- Liu, L.-Y.; Yu, T.-H.; Liao, T.-S.; Xu, P.; Wang, Y.; Shi, M.; Li, B. Pomolic Acid and Its Glucopyranose Ester Promote Apoptosis through Autophagy in HT-29 Colon Cancer Cells. World J. Gastrointest. Oncol. 2025, 15, 1756–1770. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Wang, H.-K.; Nagao, T.; Kitanaka, S.; Yasuda, I.; Fujioka, T.; Yamagishi, T.; Cosentino, L.M.; Kozuka, M.; Okabe, H.; et al. Anti-AIDS Agents. 30. Anti-HIV Activity of Oleanolic Acid, Pomolic Acid, and Structurally Related Triterpenoids. J. Nat. Prod. 1998, 61, 1090–1095. [Google Scholar] [CrossRef]
- Parhizkar, S.; Latiff, L.A. Supplementary Health Benefits of Linoleic Acid by Improvement of Vaginal Cornification of Ovariectomized Rats. Adv. Pharm. Bull. 2013, 3, 31–36. [Google Scholar] [CrossRef]
- Sakurai, K.; Shen, C.; Shiraishi, I.; Inamura, N.; Hisatsune, T. Consumption of Oleic Acid on the Preservation of Cognitive Functions in Japanese Elderly Individuals. Nutrients 2021, 13, 284. [Google Scholar] [CrossRef]
- Massaro, M.; Scoditti, E.; Carluccio, M.A.; Calabriso, N.; Santarpino, G.; Verri, T.; De Caterina, R. Effects of Olive Oil on Blood Pressure: Epidemiological, Clinical, and Mechanistic Evidence. Nutrients 2020, 12, 1548. [Google Scholar] [CrossRef]
- Pant, A. Effective Dose (ED). In Dictionary of Toxicology; Springer Nature Singapore: Singapore, 2024; p. 325. [Google Scholar]
- Jacob, S.; Nair, A.B.; Morsy, M.A. Dose Conversion Between Animals and Humans: A Practical Solution. Indian J. Pharm. Educ. Res. 2022, 56, 600–607. [Google Scholar] [CrossRef]


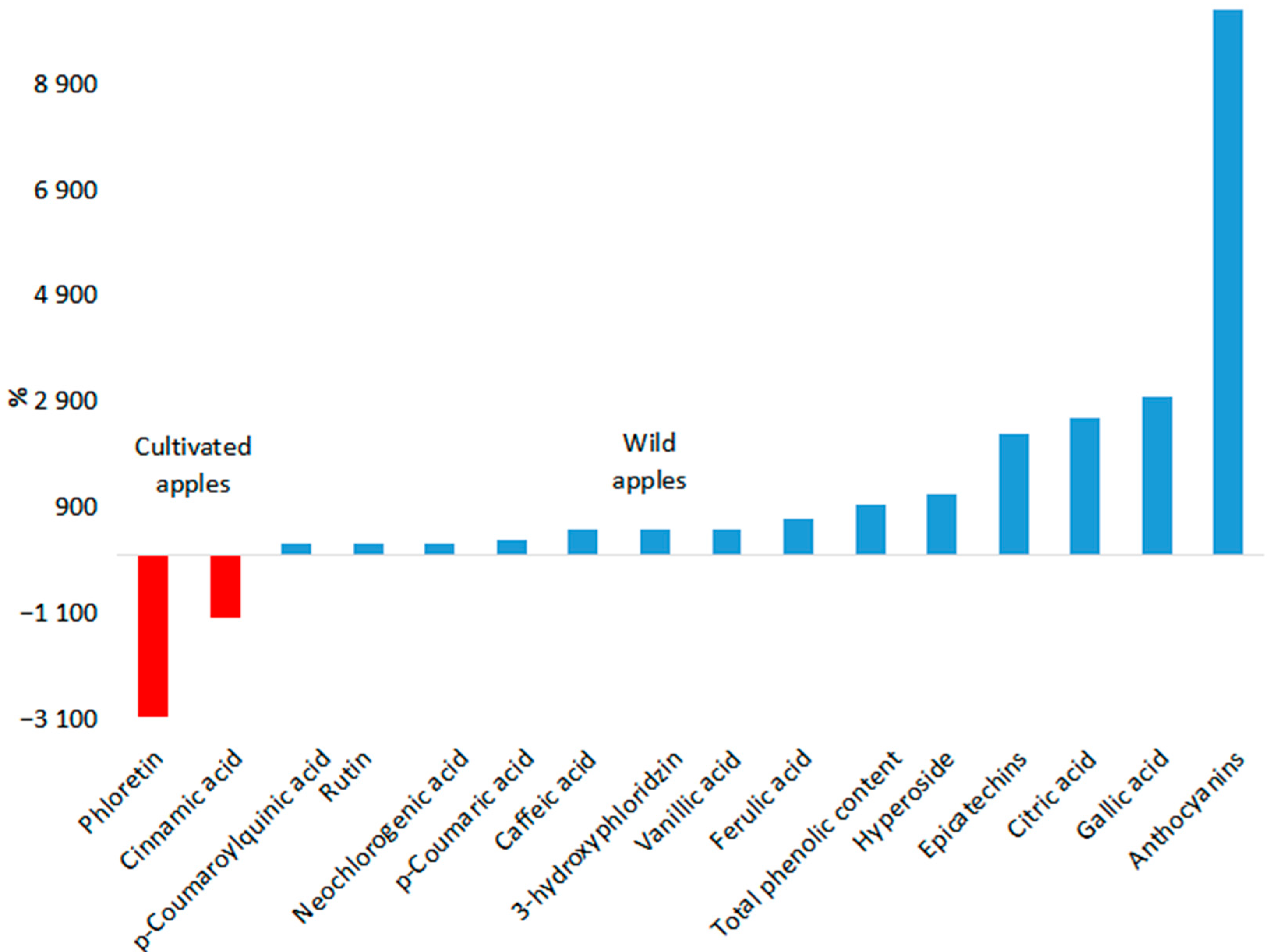
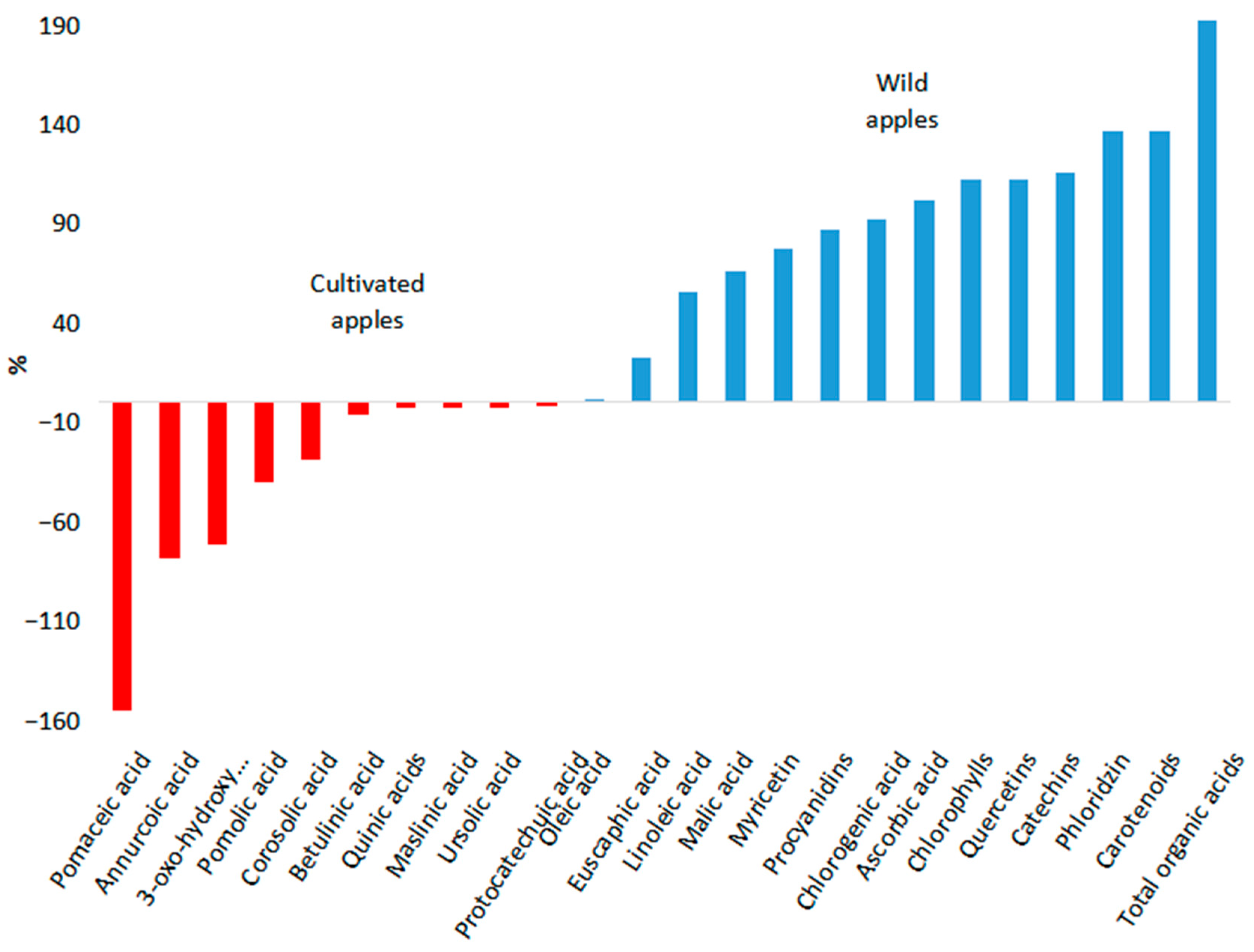
| Chemical Compounds | Type of Apple | Chemical Compound Content, Range (Mean Value) μg/g FW | Effective Dose for Humans | Study |
|---|---|---|---|---|
| Phenolic Compounds | ||||
| Total phenolic content | Cultivated apples | 404–2170 (1016.8) † | 220 mg/day | [90,91,92,93,94] |
| Wild apple species | 1460.98–2.28 × 104 (1.05 × 104) † | |||
| Chlorogenic acid | Cultivated apples | 28.25–1104 (141.06) † | 13.5 mg/day | [95,96,97,98,99] |
| Wild apple species | 25.02–2.75 × 104 (270.93) † | |||
| Caffeic acid | Cultivated apples | 1.1–18.88 (7.47) †‡ | 159.4 mg/day | [97,99,100,101,102,103,104] |
| Wild apple species | 0.14–138.1 (43.22) † | |||
| Catechins | Cultivated apples | 0.45–210.9 (36.06) † | 592.9 mg/day | [98,102,105,106,107,108] |
| Wild apple species | 20.9–134.9 (77.9) † | |||
| Epicatechins | Cultivated apples | 13.13–960.4 (88.87) † | 0.5 mg/kg | [96,98,102,109,110] |
| Wild apple species | 62.18–5816.3 (2108.98) † | |||
| Procyanidins | Cultivated apples | 57.8–895.3 (185.1) † | 704 mg/day | [98,111,112,113,114] |
| Wild apple species | 5.9–2284.4 (345.58) †‡ | |||
| Phlorizin | Cultivated apples | 5.61–115.5 (25.95) † | 60 mg/day | [96,98,102,115,116] |
| Wild apple species | 29.48–430.4 (61.46) † | |||
| Quercetins | Cultivated apples | 23.73–408.4 (40.73) † | >500 mg/day | [96,98,117] |
| Wild apple species | 69.65–290.1 (86.39) † | |||
| Anthocyanins | Cultivated apples | 4–123 (35.4) † | 80 mg/day | [90,111,118,119,120] |
| Wild apple species | 34.74–18930 (3677.37) †‡ | |||
| Gallic acid | Cultivated apples | 6.3–11.4 (8.93) † | 1.42 mg/kg ⱷ | [97,102,121,122] |
| Wild apple species | 0.91–547.2 (275.2) † | |||
| Vanillic acid | Cultivated apples | 15.03–15.55 (15.29) | 6.92 mg/kg ⱷ | [115,121,123] |
| Wild apple species | 11.1–314.26 (88,97) † | |||
| Ferulic acid | Cultivated apples | 1.05–1.37 (1.21) | 500–1000 mg/day | [115,121,124] |
| Wild apple species | 2.43–14.94 (9.46) † | |||
| p-Coumaric acid | Cultivated apples | 1.33–14.6 (6.41) † | 1.57 mg/kg ⱷ | [97,115,121,125,126] |
| Wild apple species | 9.52–37.18 (23.76) † | |||
| Rutin | Cultivated apples | 38.7–44.4 (41.89) † | 1000 mg/day | [97,115,127,128] |
| Wild apple species | 56.51–212.09 (134.3) † | |||
| Kaempferol | Cultivated apples | 1.6–86 (28.89) † | 0.36 mg/kg ⱷ | [97,118,129,130] |
| Wild apple species | 0.8–20 (n.d.) † | |||
| Cinnamic acid | Cultivated apples | 0.02–1.25 (0.51) ‡ | 159.4 mg/day | [104,111] |
| Wild apple species | n.d. (0.04) ‡ | |||
| Phloretin | Cultivated apples | 0.21–7.52 (19.97) †‡ | 0.71 mg/kg ⱷ | [111,131,132,133,134] |
| Wild apple species | n.d. (0.63) †‡ | |||
| 3-hydroxyphloridzin | Cultivated apples | n.d. (1.1) | n.d. | [98] |
| Wild apple species | n.d. (6.4) | |||
| p-Coumaroylquinic acid | Cultivated apples | 0.56–29 (9.28) † | n.d. | [105,112,135] |
| Wild apple species | n.d. (29) ‡ | |||
| Protocatechuic acid | Cultivated apples | 0.6–7.3 (3.43) †‡ | 0.55 mg/kg ⱷ | [99,121,136,137,138] |
| Wild apple species | 1.41–13.36 (3.37) † | |||
| Neochlorogenic acid | Cultivated apples | 0.45–10.37 (1.67) † | 200 mg/day | [95,96,125,139] |
| Wild apple species | n.d. (5.47) † | |||
| Hyperoside | Cultivated apples | 0.6–1.6 (0.95) ‡ | 3.46 mg/kg ⱷ | [99,140,141] |
| Wild apple species | 2.77–23.1 (11.99) | |||
| Myricetin | Cultivated apples | 5.81–12.1 (8.96) | 3.56 mg/kg/day ⱷ | [115,142] |
| Wild apple species | n.d. (15.93) | |||
| Triterpenoids | ||||
| 3-Oxo-23-hydroxyurs-12-en-28-oic acid | Cultivated apples | n.d. (21.9) | n.d. | [98] |
| Wild apple species | n.d. (12.8) | |||
| Annurcoic acid | Cultivated apples | n.d. (75.9) | n.d. | [98] |
| Wild apple species | n.d. (42.6) | |||
| Betulinic acid | Cultivated apples | n.d. (8.1) | 1.42 mg/kg/day ⱷ | [98,143] |
| Wild apple species | n.d. (7.6) | |||
| Corosolic acid | Cultivated apples | n.d. (21.2) | 10 mg/kg | [98,144] |
| Wild apple species | n.d. (16.5) | |||
| Euscaphic acid | Cultivated apples | n.d. (25.7) | 3.56 mg/kg ⱷ | [98,145] |
| Wild apple species | n.d. (31.6) | |||
| Ursolic acid | Cultivated apples | n.d. (59.6) | 2.65 mg/kg | [98,146] |
| Wild apple species | n.d. (58.1) | |||
| Maslinic acid | Cultivated apples | n.d. (11.9) | 30 mg/day | [98,147] |
| Wild apple species | n.d. (11.6) | |||
| Pomolic acid | Cultivated apples | n.d. (21.3) | 0.06 mg/kg ⱷ | [98,148] |
| Wild apple species | n.d. (15.2) | |||
| Pomaceic acid | Cultivated apples | n.d. (5.1) | n.d. | [98] |
| Wild apple species | n.d. (2) | |||
| Fatty acids | ||||
| Linoleic acid | Cultivated apples | n.d. (11.3) | 20,000 mg/day | [98,149] |
| Wild apple species | n.d. (17.6) | |||
| Oleic acid | Cultivated apples | n.d. (12.4) | 13,750–20,750 mg/day | [98,150] |
| Wild apple species | n.d. (12.5) | |||
| Organic acids | ||||
| Total organic acids | Cultivated apples | 1629–1.01 × 104 (5253) † | n.d. | [151,152] |
| Wild apple species | 2580–4.46 × 104 (1.54 × 104) | |||
| Malic acid | Cultivated apples | 1542–1.77 × 104 (6966) † | 1200 mg/day | [98,151,152,153] |
| Wild apple species | 2580–2.93 × 104 (1.16 × 104) † | |||
| Ascorbic acid | Cultivated apples | 10.48–220.5 (38.39) † | 40 mg/day | [98,154,155] |
| Wild apple species | 22.07–325 (77.41) † | |||
| Citric acid | Cultivated apples | 32.9–551.2 (84.22) † | 2700 mg/day | [151,152,156,157,158] |
| Wild apple species | 430–24210 (2254.5) † | |||
| Quinic acids | Cultivated apples | 1.5–571.9 (19.17) | 5.34 mg/kg | [98,159] |
| Wild apple species | 1.3–287.6 (18.6) | |||
| Pigments | ||||
| Chlorophylls | Cultivated apples | 0.2–8.08 (3.07) †‡ | 150 mg | [160,161,162,163] |
| Wild apple species | n.d. (6.51) | |||
| Carotenoids | Cultivated apples | 1.328–4.95 (15.38) †‡ | 6.45 mg/day | [160,161,162,163,164,165,166] |
| Wild apple species | 0.84–99 (36.38) † | |||
| Chemical Compounds | Cultivated Apple Varieties | Wild Apple Species |
|---|---|---|
| Epicatechins | 337.6 | 14.2 |
| Anthocyanins | 2259.89 | 21.75 |
| Chlorogenic acid | 95.7 | 49.8 |
| Malic acid | 172.27 | 103.89 |
| Carotenoids | 419.38 | 177.30 |
| Pomolic acid | 169.01 | 236.84 |
| Gallic acid | 9540.87 | 309.59 |
| Ascorbic acid | 1041.94 | 516.73 |
| Phloridzin | 2312.1 | 976.2 |
| Citric acid | 3.21 × 104 | 1197.60 |
| Procyanidins | 3803.3 | 2037.2 |
| Maslinic acid | 2521.01 | 2586.21 |
| Ursolic acid | 2667.79 | 2736.66 |
| Caffeic acid | 2.13 × 104 | 3688.1 |
| p-Coumaric acid | 1.47 × 104 | 3964.65 |
| Vanillic acid | 2.72 × 104 | 4666.74 |
| Quercetins | 1.23 × 104 | 5787.7 |
| Euscaphic acid | 8311.28 | 6759.49 |
| Rutin | 2.39 × 104 | 7446.02 |
| Catechins | 2.94 × 104 | 7611 |
| Protocatechuic acid | 9620.99 | 9792.28 |
| Betulinic acid | 1.05 × 104 | 1.12 × 104 |
| Myricetin | 2.38 × 104 | 1.34 × 104 |
| Quinic acids | 1.67 × 104 | 1.72 × 104 |
| Hyperoside | 2.19 × 105 | 1.73 × 104 |
| Chlorophylls | 4.89 × 104 | 2.30 × 104 |
| Corosolic acid | 2.83 × 104 | 3.64 × 104 |
| Neochlorogenic acid | 1.20 × 105 | 3.66 × 104 |
| Ferulic acid | 4.13 × 105 | 5.29 × 104 |
| Phloretin | 2133.20 | 6.76 × 104 |
| Oleic acid | 1.11 × 106 | 1.10 × 106 |
| Linoleic acid | 1.77 × 106 | 1.14 × 106 |
| Cinnamic acid | 3.13 × 105 | 3.99 × 106 |
| Wild Apple Species | Total Phenolic Content, µg GAE/g FW | Study |
|---|---|---|
| Malus baccata | 2033.33 † | [162,169,170] |
| M. sieversii f. niedzwetzkyana | 2975.7 † | [90,115] |
| Malus prunifolia | 8074–2.28 × 104 | [171] |
| Malus sylvestris | ≈1600 | [172] |
| Malus sieversii | 477.84 | [9] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shishparenok, A.A.; Shishparenok, A.N.; Harr, H.A.; Gulidova, V.A.; Rogozhin, E.A.; Markin, A.M. The Biologically Active Compounds in Fruits of Cultivated Varieties and Wild Species of Apples. Molecules 2025, 30, 3978. https://doi.org/10.3390/molecules30193978
Shishparenok AA, Shishparenok AN, Harr HA, Gulidova VA, Rogozhin EA, Markin AM. The Biologically Active Compounds in Fruits of Cultivated Varieties and Wild Species of Apples. Molecules. 2025; 30(19):3978. https://doi.org/10.3390/molecules30193978
Chicago/Turabian StyleShishparenok, Alexander A., Anastasiya N. Shishparenok, Heather A. Harr, Valentina A. Gulidova, Eugene A. Rogozhin, and Alexander M. Markin. 2025. "The Biologically Active Compounds in Fruits of Cultivated Varieties and Wild Species of Apples" Molecules 30, no. 19: 3978. https://doi.org/10.3390/molecules30193978
APA StyleShishparenok, A. A., Shishparenok, A. N., Harr, H. A., Gulidova, V. A., Rogozhin, E. A., & Markin, A. M. (2025). The Biologically Active Compounds in Fruits of Cultivated Varieties and Wild Species of Apples. Molecules, 30(19), 3978. https://doi.org/10.3390/molecules30193978






