Synthesis of Silver Nanoparticles by Using Quercus Robur Knopper Gall Extracts
Abstract
1. Introduction
2. Results and Discussion
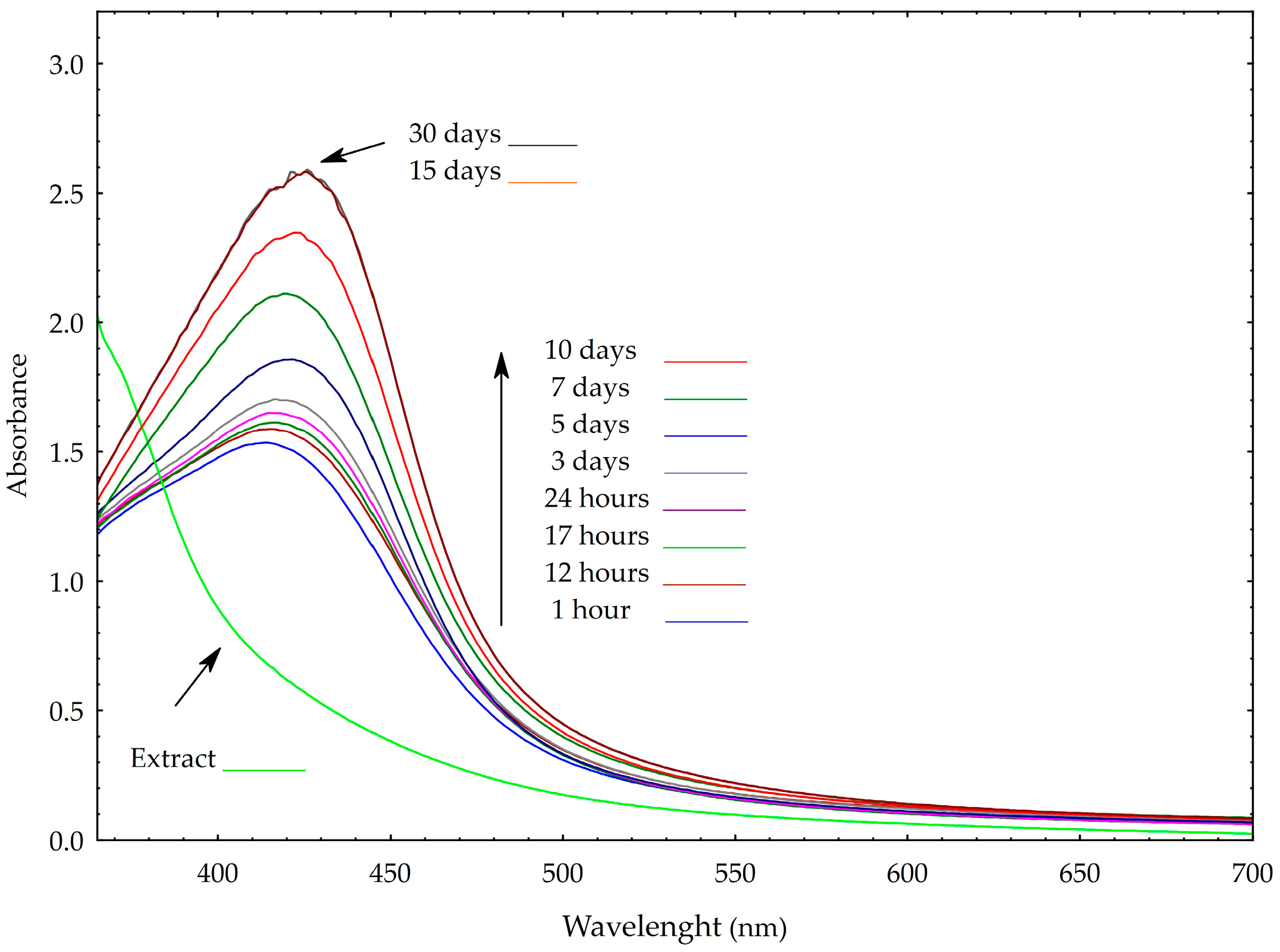
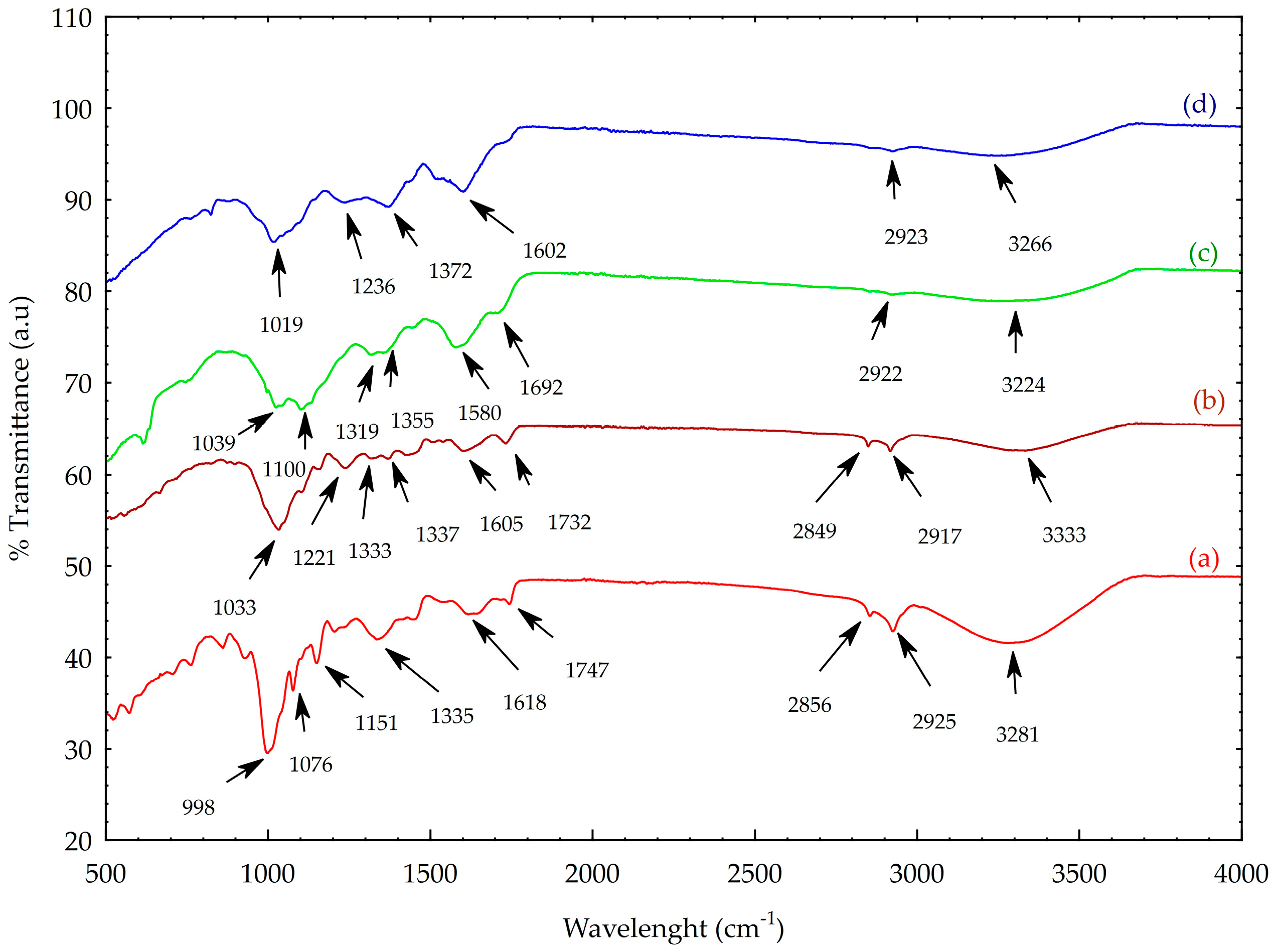
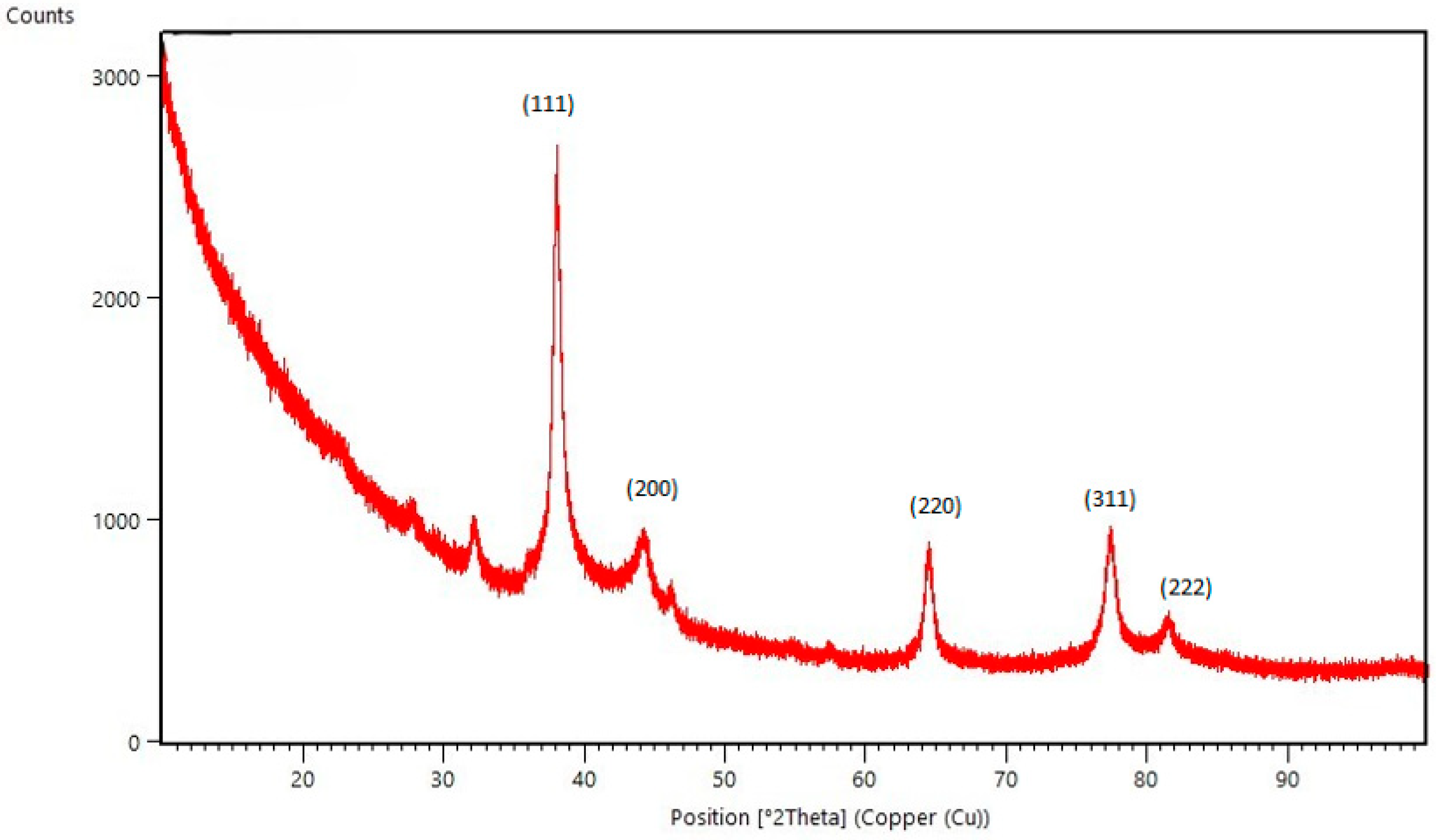
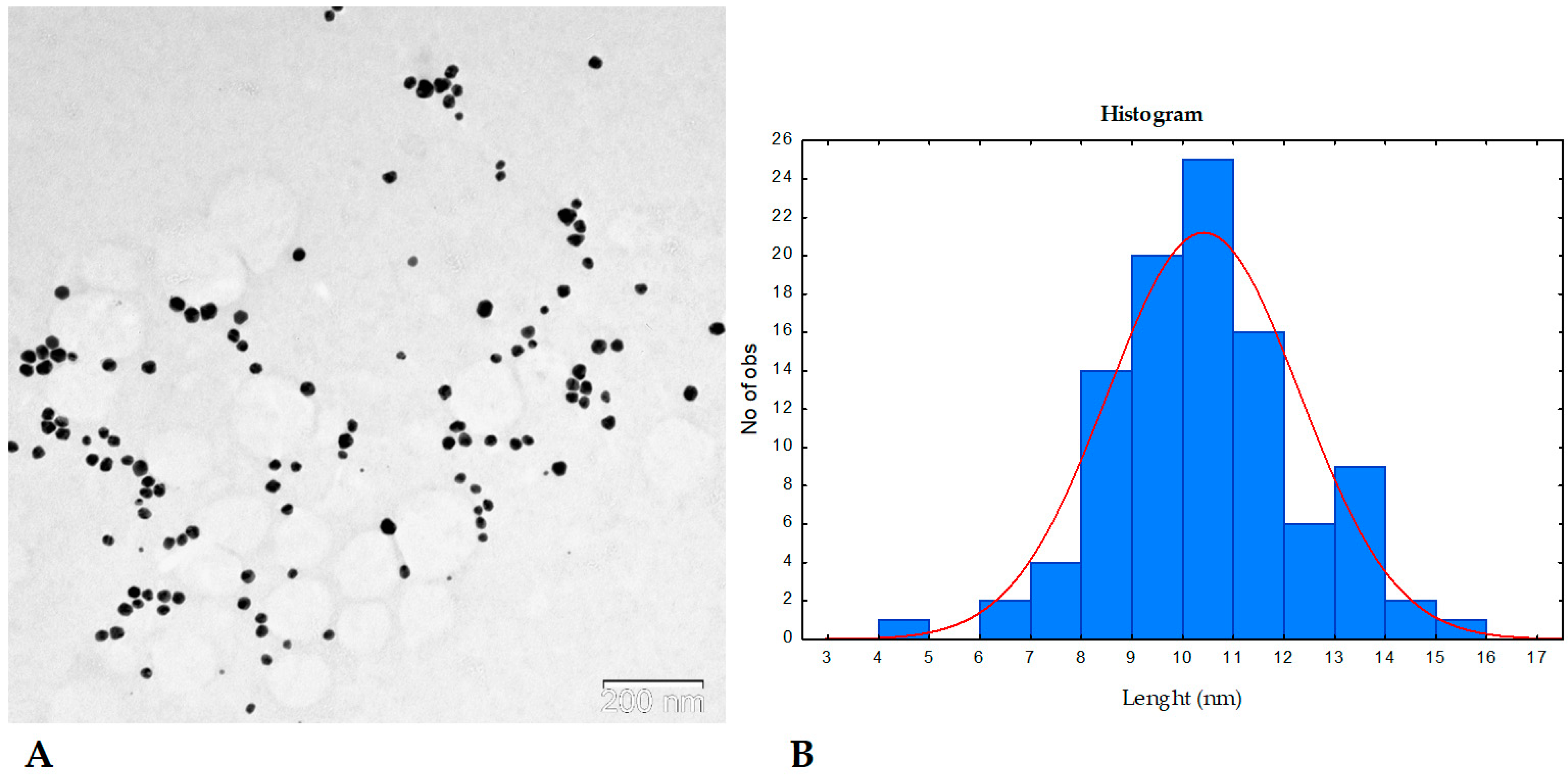
2.1. AgNP Characterization Results
2.2. Characterization of the Knopper Gall Extracts
2.3. Minimum Inhibitory Concentrations (MICs) of Synthesized AgNPs
2.4. Comparative Discussion and Future Perspectives
3. Materials and Methods
3.1. Material Collection
3.2. Extract Preparation
3.3. Analyses of Antioxidative Activity and Total Polyphenols in Knopper Gall Extracts
3.4. HPLC Characterization of the Extracts and Hydrolysable Tannin Content
3.5. AgNP Synthesis
3.6. AgNP Characterization Procedures
3.6.1. UV-Vis Spectroscopy
3.6.2. ATR-FTIR Spectroscopy
3.6.3. Powder X-Ray Diffraction (PXRD)
3.6.4. Transmission Electron Microscopy (TEM)
3.7. Determination of Minimum Inhibitory Concentrations (MICs) of Synthesized AgNPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, S.; Ahmad, M.; Swami, B.L.; Ikram, S. A Review on Plants Extract Mediated Synthesis of Silver Nanoparticles for Antimicrobial Applications: A Green Expertise. J. Adv. Res. 2016, 7, 17–28. [Google Scholar] [CrossRef]
- Sharma, V.K.; Yngard, R.A.; Lin, Y. Silver Nanoparticles: Green Synthesis and Their Antimicrobial Activities. Adv. Colloid Interface Sci. 2009, 145, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Iravani, S. Green Synthesis of Metal Nanoparticles Using Plants. Green Chem. 2011, 13, 2638–2650. [Google Scholar] [CrossRef]
- Mittal, A.K.; Chisti, Y.; Banerjee, U.C. Synthesis of Metallic Nanoparticles Using Plant Extracts. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar] [CrossRef]
- Ahmed, S.; Saifullah; Ahmad, M.; Swami, B.L.; Ikram, S. Green Synthesis of Silver Nanoparticles Using Azadirachta indica Aqueous Leaf Extract. J. Radiat. Res. Appl. Sci. 2016, 9, 1–7. [Google Scholar] [CrossRef]
- Thakkar, K.N.; Mhatre, S.S.; Parikh, R.Y. Biological Synthesis of Metallic Nanoparticles. Nanomed. Nanotechnol. Biol. Med. 2010, 6, 257–262. [Google Scholar] [CrossRef]
- Stone, G.N.; Schönrogge, K.; Atkinson, R.J.; Bellido, D.; Pujade-Villar, J. The Population Biology of Oak Gall Wasps (Hymenoptera: Cynipidae). Annu. Rev. Entomol. 2002, 47, 633–668. [Google Scholar] [CrossRef]
- Schonrogge, K.; Stone, G.N.; Crawley, M.J. Abundance Patterns and Species Richness of the Parasitoids and Inquilines of the Alien Gall-Former Andricus quercuscalicis (Hymenoptera: Cynipidae). Oikos 1996, 77, 507–518. [Google Scholar] [CrossRef]
- Harper, L.J.; Schönrogge, K.; Lim, K.Y.; Francis, P.; Lichtenstein, C.P. Cynipid Galls: Insect-Induced Modifications of Plant Development Create Novel Plant Organs. Plant Cell Environ. 2004, 27, 327–335. [Google Scholar] [CrossRef]
- Hough, J.S. Studies on the Common Spangle Gall of Oak. New Phytol. 1953, 52, 149–177. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of Silver Nanoparticles: Chemical, Physical and Biological Methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar]
- Singh, P.; Kim, Y.-J.; Zhang, D.; Yang, D.-C. Biological Synthesis of Nanoparticles from Plants and Microorganisms. Trends Biotechnol. 2016, 34, 588–599. [Google Scholar] [CrossRef] [PubMed]
- Banc, R.; Rusu, M.E.; Filip, L.; Popa, D.-S. Phytochemical Profiling and Biological Activities of Quercus Sp. Galls (Oak Galls): A Systematic Review of Studies Published in the Last 5 Years. Plants 2023, 12, 3873. [Google Scholar] [CrossRef]
- Khatamifar, M.; Fatemi, S.J.; Torkzadeh-Mahani, M.; Mohammadi, M.; Hassanshahian, M. Green and Eco-Friendly Synthesis of Silver Nanoparticles by Quercus infectoria Galls Extract: Thermal Behavior, Antibacterial, Antioxidant and Anticancer Properties. Part. Sci. Technol. 2022, 40, 281–289. [Google Scholar] [CrossRef]
- Falakdin, P.; Dastan, D.; Pourmoslemi, S. Combined Antimicrobial Activity of Extracts from Quercus infectoria Galls and Scrophularia striata Aerial Parts for an Anticariogenic Herbal Mouthwash. J. Pharmacopunct. 2023, 26, 44–52. [Google Scholar] [CrossRef]
- Kocadag Kocazorbaz, E.; Moulahoum, H.; Tut, E.; Sarac, A.; Tok, K.; Yalcin, H.T.; Zihnioglu, F. Kermes Oak (Quercus coccifera L.) Extract for a Biogenic and Eco-Benign Synthesis of Silver Nanoparticles with Efficient Biological Activities. Environ. Technol. Innov. 2021, 24, 102067. [Google Scholar] [CrossRef]
- Heydari, R.; Rashidipour, M. Green Synthesis of Silver Nanoparticles Using Extract of Oak Fruit Hull (Jaft): Synthesis and In Vitro Cytotoxic Effect on MCF-7 Cells. Int. J. Breast Cancer 2015, 2015, 846743. [Google Scholar] [CrossRef] [PubMed]
- Kaur, H.; Kaur, H.; Sharma, A. Study of SPR Peak Shifting of Silver Nanoparticles with Change in Surrounding Medium. Mater. Today Proc. 2021, 37, 3574–3576. [Google Scholar] [CrossRef]
- Chutrakulwong, F.; Thamaphat, K.; Intarasawang, M. Investigating UV-Irradiation Parameters in the Green Synthesis of Silver Nanoparticles from Water Hyacinth Leaf Extract: Optimization for Future Sensor Applications. Nanomaterials 2024, 14, 1018. [Google Scholar] [CrossRef]
- Car, J.; Krstulović, N. Fitting Procedure to Reconstruct the Size Distribution and the Concentration of Silver Colloidal Nanoparticles from UV-Vis Spectra. Nanomaterials 2022, 12, 3302. [Google Scholar] [CrossRef] [PubMed]
- Alzoubi, F.Y.; Ahmad, A.A.; Aljarrah, I.A.; Migdadi, A.B.; Al-Bataineh, Q.M. Localize Surface Plasmon Resonance of Silver Nanoparticles Using Mie Theory. J. Mater. Sci. Mater. Electron. 2023, 34, 2128. [Google Scholar] [CrossRef]
- Abdel-Raouf, N.; Al-Enazi, N.M.; Ibraheem, I.B.M.; Alharbi, R.M.; Alkhulaifi, M.M. Biosynthesis of Silver Nanoparticles by Using of the Marine Brown Alga Padina pavonia and Their Characterization. Saudi J. Biol. Sci. 2019, 26, 1207–1215. [Google Scholar] [CrossRef] [PubMed]
- Barth, A. Infrared Spectroscopy of Proteins. Biochim. Biophys. Acta (BBA)—Bioenerg. 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Mirski, R.; Kawalerczyk, J.; Dziurka, D.; Siuda, J.; Wieruszewski, M. The Application of Oak Bark Powder as a Filler for Melamine-Urea-Formaldehyde Adhesive in Plywood Manufacturing. Forests 2020, 11, 1249. [Google Scholar] [CrossRef]
- Zarroug, Y.; Boulares, M.; Mejri, J.; Slimi, B.; Hamdaoui, G.; Djebi, S.; Saidi, F.; Nasri, H.; Sfayhi, D.T.; Kharrat, M. Extraction and Characterization of Tunisian Quercus ilex Starch and Its Effect on Fermented Dairy Product Quality. Int. J. Anal. Chem. 2020, 2020, 8868673. [Google Scholar] [CrossRef]
- Nikonenko, N.A.; Buslov, D.K.; Sushko, N.I.; Zhbankov, R.G. Investigation of Stretching Vibrations of Glycosidic Linkages in Disaccharides and Polysaccarides with Use of IR Spectra Deconvolution. Biopolymers 2000, 57, 257–262. [Google Scholar] [CrossRef]
- Pavia, D.L.; Lampman, G.M.; Kriz, G.S.; Vyvyan, J.R. Introduction to Spectroscopy, 5th ed.; Brooks/Cole Cengage Learning: Washington, DC, USA, 2015. [Google Scholar]
- Coman, N.-A.; Nicolae-Maranciuc, A.; Berța, L.; Nicolescu, A.; Babotă, M.; Man, A.; Chicea, D.; Farczadi, L.; Jakab-Farkas, L.; Silva, B.; et al. Green Synthesis of Metallic Nanoparticles from Quercus Bark Extracts: Characterization and Functional Properties. Antioxidants 2024, 13, 822. [Google Scholar] [CrossRef]
- Khalil, M.M.H.; Ismail, E.H.; El-Baghdady, K.Z.; Mohamed, D. Green Synthesis of Silver Nanoparticles Using Olive Leaf Extract and Its Antibacterial Activity. Arab. J. Chem. 2013, 7, 1131–1139. [Google Scholar] [CrossRef]
- Abdullah, A.R.; Hapidin, H.; Abdullah, H. Phytochemical Analysis of Quercus infectoria Galls Extracts Using FTIR, LC-MS and MS/MS Analysis. Res. J. Biotechnol. 2017, 12, 55–61. [Google Scholar]
- Prathna, T.C.; Chandrasekaran, N.; Raichur, A.M.; Mukherjee, A. Biomimetic Synthesis of Silver Nanoparticles by Citrus limon (Lemon) Aqueous Extract and Theoretical Prediction of Particle Size. Colloids Surf. B Biointerfaces 2011, 82, 152–159. [Google Scholar] [CrossRef]
- Akhter, M.S.; Rahman, M.A.; Ripon, R.K.; Mubarak, M.; Akter, M.; Mahbub, S.; Al Mamun, F.; Sikder, M.T. A Systematic Review on Green Synthesis of Silver Nanoparticles Using Plants Extract and Their Bio-Medical Applications. Heliyon 2024, 10, e29766. [Google Scholar] [CrossRef] [PubMed]
- Dhoondia, Z.H.; Chakraborty, H. Lactobacillus Mediated Synthesis of Silver Oxide Nanoparticles. Nanomater. Nanotechnol. 2012, 2, 15. [Google Scholar] [CrossRef]
- Huang, Z.; Thomson, P.; Di, S. Lattice Contractions of a Nanoparticle Due to the Surface Tension: A Model of Elasticity. J. Phys. Chem. Solids 2007, 68, 530–535. [Google Scholar] [CrossRef]
- Ali, M.H.; Azad, M.A.K.; Khan, K.A.; Rahman, M.O.; Chakma, U.; Kumer, A. Analysis of Crystallographic Structures and Properties of Silver Nanoparticles Synthesized Using PKL Extract and Nanoscale Characterization Techniques. ACS Omega 2023, 8, 28133–28142. [Google Scholar] [CrossRef]
- Khatoon, J.; Mehmood, A.; Khalid, A.U.R.; Khan, M.A.R.; Ahmad, K.S.; Amjad, M.S.; Bashir, U.; Raffi, M.; Proćków, J. Green-Fabricated Silver Nanoparticles from Quercus incana Leaf Extract to Control the Early Blight of Tomatoes Caused by Alternaria solani. BMC Plant Biol. 2024, 24, 302. [Google Scholar] [CrossRef]
- Khattak, A.; Ahmad, B.; Rauf, A.; Bawazeer, S.; Farooq, U.; Ali, J.; Patel, S.; Ramadan El-Sharkawy, E.; Ikram, R.; Linfang, H. Green Synthesis, Characterisation and Biological Evaluation of Plant-Based Silver Nanoparticles Using Quercus semecarpifolia Smith Aqueous Leaf Extract. IET Nanobiotechnol. 2019, 13, 36–41. [Google Scholar] [CrossRef]
- Kılınçarslan Aksoy, Ö.; Seçme, M.; Mammadov, R. Antioxidant, Cytotoxicity, Apoptotic Properties of Extracts of Andricus sternlichti Galls and Their Phenolic Characterisation by HPLC. Chem. Biodivers. 2023, 20, e202200742. [Google Scholar] [CrossRef]
- Kılınçarslan Aksoy, Ö.; Mammadov, R.; Seçme, M. Antioxidant Activity, Phytochemical Composition of Andricus tomentosus and Its Antiproliferative Effect on Mia-Paca2 Cell Line. Mol. Biol. Rep. 2020, 47, 7633–7641. [Google Scholar] [CrossRef]
- Cosme, F.; Aires, A.; Pinto, T.; Oliveira, I.; Vilela, A.; Gonçalves, B. A Comprehensive Review of Bioactive Tannins in Foods and Beverages: Functional Properties, Health Benefits, and Sensory Qualities. Molecules 2025, 30, 800. [Google Scholar] [CrossRef]
- Truong, V.-L.; Jeong, W.-S. Cellular Defensive Mechanisms of Tea Polyphenols: Structure-Activity Relationship. Int. J. Mol. Sci. 2021, 22, 9109. [Google Scholar] [CrossRef]
- Albahnasawi, A.; Alazaiza, M.Y.D.; Gürbulak, E.; Eyvaz, M. Plant-Based Nanomaterials for Contaminated Environs Nanoremediation. In Sustainable Nanoremediation; Apple Academic Press: Palm Bay, FL, USA, 2024. [Google Scholar]
- Shahzadi, S.; Fatima, S.; ul Ain, Q.; Shafiq, Z.; Ashraf Janjua, M.R.S. A Review on Green Synthesis of Silver Nanoparticles (SNPs) Using Plant Extracts: A Multifaceted Approach in Photocatalysis, Environmental Remediation, and Biomedicine. RSC Adv. 2025, 15, 3858–3903. [Google Scholar] [CrossRef]
- Paaver, U.; Matto, V.; Raal, A. Total Tannin Content in Distinct Quercus robur L. Galls. JMPR 2010, 4, 702–705. [Google Scholar]
- Vattem, D.A.; Shetty, K. Biological Functionality of Ellagic Acid: A Review. J. Food Biochem. 2005, 29, 234–266. [Google Scholar] [CrossRef]
- Pascual-Alvarado, E.; Cuevas-Reyes, P.; Quesada, M.; Oyama, K. Interactions between Galling Insects and Leaf-Feeding Insects: The Role of Plant Phenolic Compounds and Their Possible Interference with Herbivores. J. Trop. Ecol. 2008, 24, 329–336. [Google Scholar] [CrossRef]
- Taper, M.L.; Case, T.J. Interactions between Oak Tannins and Parasite Community Structure: Unexpected Benefits of Tannins to Cynipid Gall-Wasps. Oecologia 1987, 71, 254–261. [Google Scholar] [CrossRef]
- Corciovă, A.; Fifere, A.; Moleavin, I.T.; Tuchiluș, C.; Mircea, C.; Macovei, I.; Burlec, A.F. In Vitro Evaluation of Antioxidant and Antibacterial Activities of Eco-Friendly Synthesized Silver Nanoparticles Using Quercus Robur Bark Extract. Curr. Pharm. Biotechnol. 2023, 24, 460–470. [Google Scholar] [CrossRef] [PubMed]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef]
- Yacamán, M.J.; Ascencio, J.A.; Liu, H.B.; Gardea-Torresdey, J. Structure Shape and Stability of Nanometric Sized Particles. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. Process. Meas. Phenom. 2001, 19, 1091–1103. [Google Scholar] [CrossRef]
- Morones, J.R.; Elechiguerra, J.L.; Camacho, A.; Holt, K.; Kouri, J.B.; Ramírez, J.T.; Yacaman, M.J. The Bactericidal Effect of Silver Nanoparticles. Nanotechnology 2005, 16, 2346. [Google Scholar] [CrossRef]
- Zheng, K.; Setyawati, M.I.; Leong, D.T.; Xie, J. Antimicrobial Silver Nanomaterials. Coord. Chem. Rev. 2018, 357, 1–17. [Google Scholar] [CrossRef]
- Hsueh, Y.-H.; Lin, K.-S.; Ke, W.-J.; Hsieh, C.-T.; Chiang, C.-L.; Tzou, D.-Y.; Liu, S.-T. The Antimicrobial Properties of Silver Nanoparticles in Bacillus Subtilis Are Mediated by Released Ag+ Ions. PLoS ONE 2015, 10, e0144306. [Google Scholar] [CrossRef]
- Durán, N.; Nakazato, G.; Seabra, A.B. Antimicrobial Activity of Biogenic Silver Nanoparticles, and Silver Chloride Nanoparticles: An Overview and Comments. Appl. Microbiol. Biotechnol. 2016, 100, 6555–6570. [Google Scholar] [CrossRef]
- Yang, W.; Shen, C.; Ji, Q.; An, H.; Wang, J.; Liu, Q.; Zhang, Z. Food Storage Material Silver Nanoparticles Interfere with DNA Replication Fidelity and Bind with DNA. Nanotechnology 2009, 20, 085102. [Google Scholar] [CrossRef]
- Le Ouay, B.; Stellacci, F. Antibacterial Activity of Silver Nanoparticles: A Surface Science Insight. Nano Today 2015, 10, 339–354. [Google Scholar] [CrossRef]
- Mateș, L.; Rusu, M.E.; Popa, D.-S. Phytochemicals and Biological Activities of Walnut Septum: A Systematic Review. Antioxidants 2023, 12, 604. [Google Scholar] [CrossRef]
- Piao, M.J.; Kang, K.A.; Lee, I.K.; Kim, H.S.; Kim, S.; Choi, J.Y.; Choi, J.; Hyun, J.W. Silver Nanoparticles Induce Oxidative Cell Damage in Human Liver Cells through Inhibition of Reduced Glutathione and Induction of Mitochondria-Involved Apoptosis. Toxicol. Lett. 2011, 201, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Singh, P.; Pandit, S.; Jers, C.; Joshi, A.S.; Garnæs, J.; Mijakovic, I. Silver Nanoparticles Produced from Cedecea Sp. Exhibit Antibiofilm Activity and Remarkable Stability. Sci. Rep. 2021, 11, 12619. [Google Scholar] [CrossRef] [PubMed]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver Nanoparticles as Potential Antibacterial Agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Khan, T.; Adil, M.; Khan, A. Mechanistic Aspects of Plant-Based Silver Nanoparticles against Multi-Drug Resistant Bacteria. Heliyon 2021, 7, e07448. [Google Scholar] [CrossRef]
- Vasiliev, G.; Kubo, A.-L.; Vija, H.; Kahru, A.; Bondar, D.; Karpichev, Y.; Bondarenko, O. Synergistic Antibacterial Effect of Copper and Silver Nanoparticles and Their Mechanism of Action. Sci. Rep. 2023, 13, 9202. [Google Scholar] [CrossRef]
- Haji, S.H.; Ali, F.A.; Aka, S.T.H. Synergistic Antibacterial Activity of Silver Nanoparticles Biosynthesized by Carbapenem-Resistant Gram-Negative Bacilli. Sci. Rep. 2022, 12, 15254. [Google Scholar] [CrossRef] [PubMed]
- Aisida, S.O.; Ugwu, K.; Akpa, P.A.; Nwanya, A.C.; Nwankwo, U.; Botha, S.S.; Ejikeme, P.M.; Ahmad, I.; Maaza, M.; Ezema, F.I. Biosynthesis of Silver Nanoparticles Using Bitter Leave (Veronica amygdalina) for Antibacterial Activities. Surf. Interfaces 2019, 17, 100359. [Google Scholar] [CrossRef]
- Ahmed, B.; Hashmi, A.; Khan, M.S.; Musarrat, J. ROS Mediated Destruction of Cell Membrane, Growth and Biofilms of Human Bacterial Pathogens by Stable Metallic AgNPs Functionalized from Bell Pepper Extract and Quercetin. Adv. Powder Technol. 2018, 29, 1601–1616. [Google Scholar] [CrossRef]
- Alsamhary, K.I. Eco-Friendly Synthesis of Silver Nanoparticles by Bacillus Subtilis and Their Antibacterial Activity. Saudi J. Biol. Sci. 2020, 27, 2185–2191. [Google Scholar] [CrossRef]
- Yu, J.; Nan, J.; Zeng, H. Size Control of Nanoparticles by Multiple-Pulse Laser Ablation. Appl. Surf. Sci. 2017, 402, 330–335. [Google Scholar] [CrossRef]
- Walaszek, D.; Senn, M.; Wichser, A.; Faller, M.; Wagner, B.; Bulska, E.; Ulrich, A. Minimally-Invasive Laser Ablation Inductively Coupled Plasma Mass Spectrometry Analysis of Model Ancient Copper Alloys. Spectrochim. Acta Part B At. Spectrosc. 2014, 99, 115–120. [Google Scholar] [CrossRef]
- Sportelli, M.C.; Izzi, M.; Volpe, A.; Clemente, M.; Picca, R.A.; Ancona, A.; Lugarà, P.M.; Palazzo, G.; Cioffi, N. The Pros and Cons of the Use of Laser Ablation Synthesis for the Production of Silver Nano-Antimicrobials. Antibiotics 2018, 7, 67. [Google Scholar] [CrossRef]
- Naaz, R.; Siddiqui, V.U.; Qadir, S.U.; Siddiqi, W.A. Green Synthesis of Silver Nanoparticles Using Syngonium podophyllum Leaf Extract and Its Antibacterial Activity. Mater. Today Proc. 2021, 46, 2352–2358. [Google Scholar] [CrossRef]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, 1701503. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A.; Hussein, M.H.; Shaaban-Dessuuki, S.A.; Dalal, S.R. Production, Extraction and Characterization of Chlorella Vulgaris Soluble Polysaccharides and Their Applications in AgNPs Biosynthesis and Biostimulation of Plant Growth. Sci. Rep. 2020, 10, 3011. [Google Scholar] [CrossRef]
- Kim, D.-Y.; Saratale, R.G.; Shinde, S.; Syed, A.; Ameen, F.; Ghodake, G. Green Synthesis of Silver Nanoparticles Using Laminaria japonica Extract: Characterization and Seedling Growth Assessment. J. Clean. Prod. 2018, 172, 2910–2918. [Google Scholar] [CrossRef]
- Kumar, L.; Mohan, L.; Anand, R.; Bharadvaja, N. Chlorella Minutissima-Assisted Silver Nanoparticles Synthesis and Evaluation of Its Antibacterial Activity. Syst. Microbiol. Biomanufacturing 2024, 4, 230–239. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Turovsky, E.A.; Blinova, E.V. Therapeutic Potential and Main Methods of Obtaining Selenium Nanoparticles. Int. J. Mol. Sci. 2021, 22, 10808. [Google Scholar] [CrossRef]
- Varlamova, E.G.; Gudkov, S.V.; Turovsky, E.A. Differential Effect of Cerium Nanoparticles on the Viability, Redox-Status and Ca2+-Signaling System of Cancer Cells of Various Origins. Arch. Biochem. Biophys. 2025, 764, 110261. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Varlamova, E.G. Mechanism of Ca2+-Dependent Pro-Apoptotic Action of Selenium Nanoparticles, Mediated by Activation of Cx43 Hemichannels. Biology 2021, 10, 743. [Google Scholar] [CrossRef] [PubMed]
- Nemes, A.; Szőllősi, E.; Stündl, L.; Biró, A.; Homoki, J.R.; Szarvas, M.M.; Balogh, P.; Cziáky, Z.; Remenyik, J. Determination of Flavonoid and Proanthocyanidin Profile of Hungarian Sour Cherry. Molecules 2018, 23, 3278. [Google Scholar] [CrossRef] [PubMed]
- Kovač, M.J.; Jokić, S.; Jerković, I.; Molnar, M. Optimization of Deep Eutectic Solvent Extraction of Phenolic Acids and Tannins from Alchemilla vulgaris L. Plants 2022, 11, 474. [Google Scholar] [CrossRef]
- Rhazi, N.; Hannache, H.; Oumam, M.; Sesbou, A.; Charrier, B.; Pizzi, A.; Bouhtoury, F.C.-E. Green Extraction Process of Tannins Obtained from Moroccan Acacia Mollissima Barks by Microwave: Modeling and Optimization of the Process Using the Response Surface Methodology RSM. Arab. J. Chem. 2015, 12, 2668–2684. [Google Scholar] [CrossRef]
- Weinstein, M.P.; Patel, J.B. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically: M07-A11, 11th ed.; Documents/Clinical and Laboratory Standards Institute; Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2018; ISBN 978-1-56238-836-2. [Google Scholar]


| Parameter | Value |
|---|---|
| TPC mg GAE g−1 DW | 72.0 ± 1.9 |
| FRAP mg Trolox g−1 DW | 48.9 ± 5.2 |
| DPPH mg Trolox g−1 DW | 88.5 ± 1.3 |
| ABTS mg Trolox g−1 DW | 9.4 ± 0.1 |
| Phenolic Component | Concentration (µg g−1 DW) |
|---|---|
| Protochatechuic acid | 0.03 ± 0.001 |
| Ellagic acid | 13.15 ± 0.002 |
| Epigallocatechin | 2.41 ± 0.004 |
| Epigallocatechin gallate | 1.64 ± 0.002 |
| Herniarin | 0.01 ± 0.000 |
| Chrysin | 0.01 ± 0.000 |
| Rosmarinic acid | 0.01 ± 0.000 |
| Hydrolysable tannins | 11.66 ± 0.030 |
| MIC (mg mL−1) | ||||
|---|---|---|---|---|
| G+ | G− | |||
| B. subtilis | S. aureus | E. coli | P. aeruginosa | |
| AgNPs | 0.03125 | 0.0625 | 0.250 | 0.250 |
| C * | 0.244 | 1.953 | 1.953 | 0.488 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gvozdić, V.; Užarević, Z.; Andrić, E.K.; Galić, V.; Kalinić, L.; Kovač, M.J.; Ćorić, I.; Kirchbauer, K.; Vidosavljević, D.; Pavić, V. Synthesis of Silver Nanoparticles by Using Quercus Robur Knopper Gall Extracts. Molecules 2025, 30, 3979. https://doi.org/10.3390/molecules30193979
Gvozdić V, Užarević Z, Andrić EK, Galić V, Kalinić L, Kovač MJ, Ćorić I, Kirchbauer K, Vidosavljević D, Pavić V. Synthesis of Silver Nanoparticles by Using Quercus Robur Knopper Gall Extracts. Molecules. 2025; 30(19):3979. https://doi.org/10.3390/molecules30193979
Chicago/Turabian StyleGvozdić, Vlatka, Zvonimir Užarević, Elvira Kovač Andrić, Vlatko Galić, Lidija Kalinić, Martina Jakovljević Kovač, Ivan Ćorić, Klara Kirchbauer, Domagoj Vidosavljević, and Valentina Pavić. 2025. "Synthesis of Silver Nanoparticles by Using Quercus Robur Knopper Gall Extracts" Molecules 30, no. 19: 3979. https://doi.org/10.3390/molecules30193979
APA StyleGvozdić, V., Užarević, Z., Andrić, E. K., Galić, V., Kalinić, L., Kovač, M. J., Ćorić, I., Kirchbauer, K., Vidosavljević, D., & Pavić, V. (2025). Synthesis of Silver Nanoparticles by Using Quercus Robur Knopper Gall Extracts. Molecules, 30(19), 3979. https://doi.org/10.3390/molecules30193979







