Synthesis and Characterization of Imidazolium-Based Ionenes
Abstract
1. Introduction
2. Results
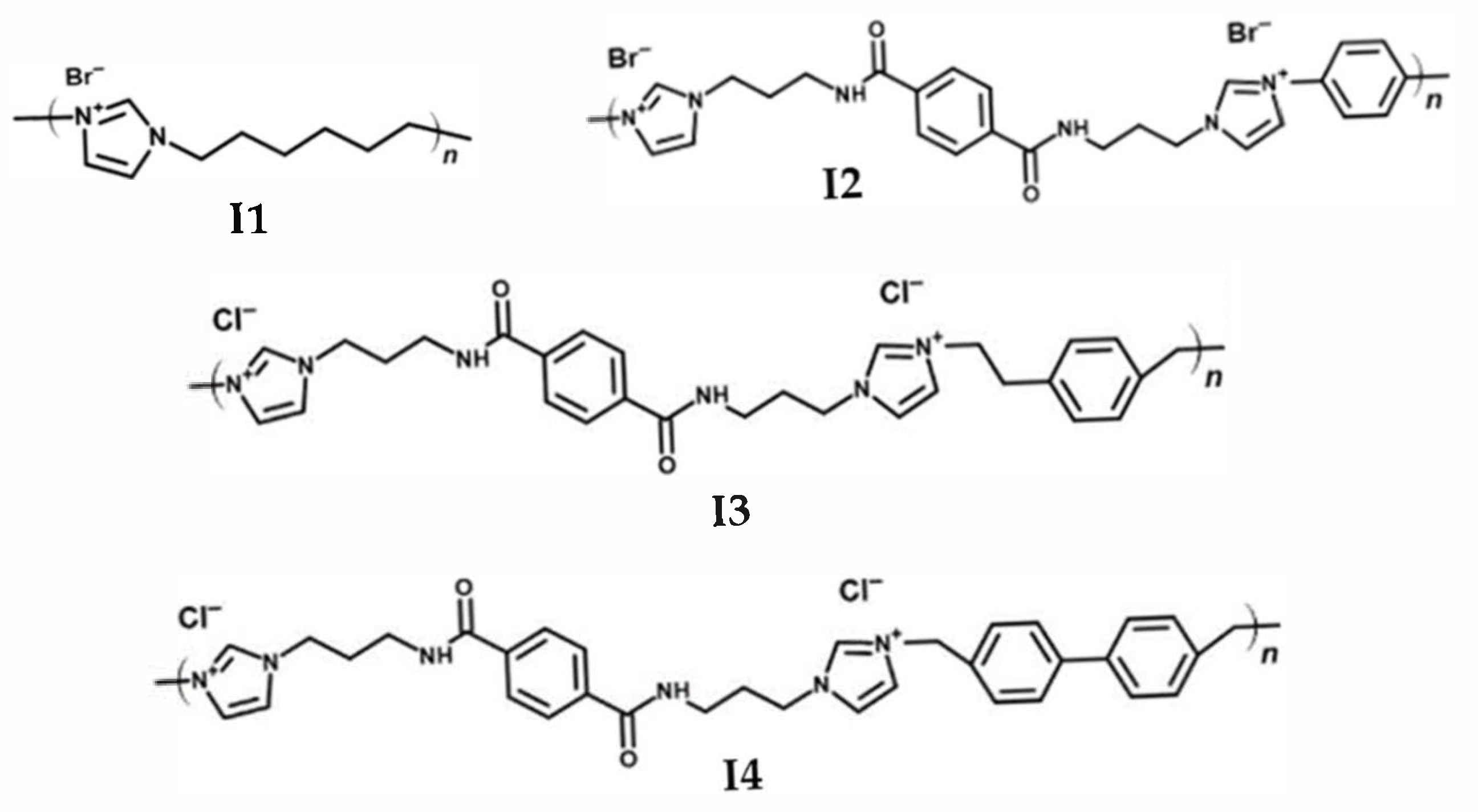
2.1. Synthesis of Ionenes
2.2. Thermal Properties of Ionenes
2.3. Possible Application as High-Pressure Hydrogen Gas Storage Liner
3. Materials and Methods
3.1. Materials and Chemicals
3.2. Synthesis of Monomers
3.2.1. Synthesis of 1,1′(1,6-Hexane)bisimidazole (M1)
3.2.2. Synthesis of 1,4-Benzenedicarboxamide N1,N4-Bis[3-(1H-imidazole-1-yl) Propyl] (M2)
3.3. Synthesis of Ionenes
3.3.1. Synthesis of Ionene 1 (I1)
3.3.2. Synthesis of Ionene 2 (I2)
3.3.3. Synthesis of Ionene 3 (I3)
3.3.4. Synthesis of Ionene 4 (I4)
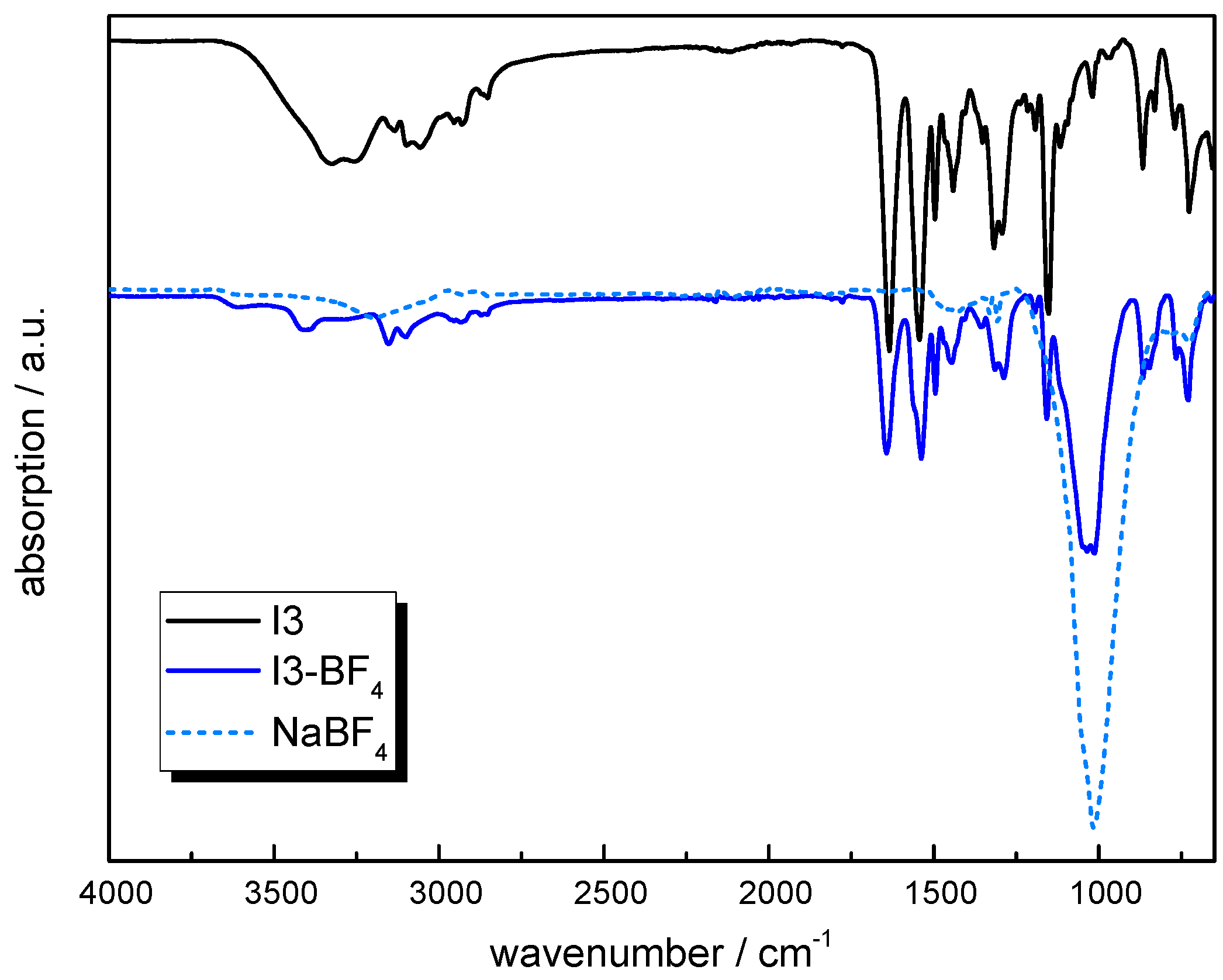
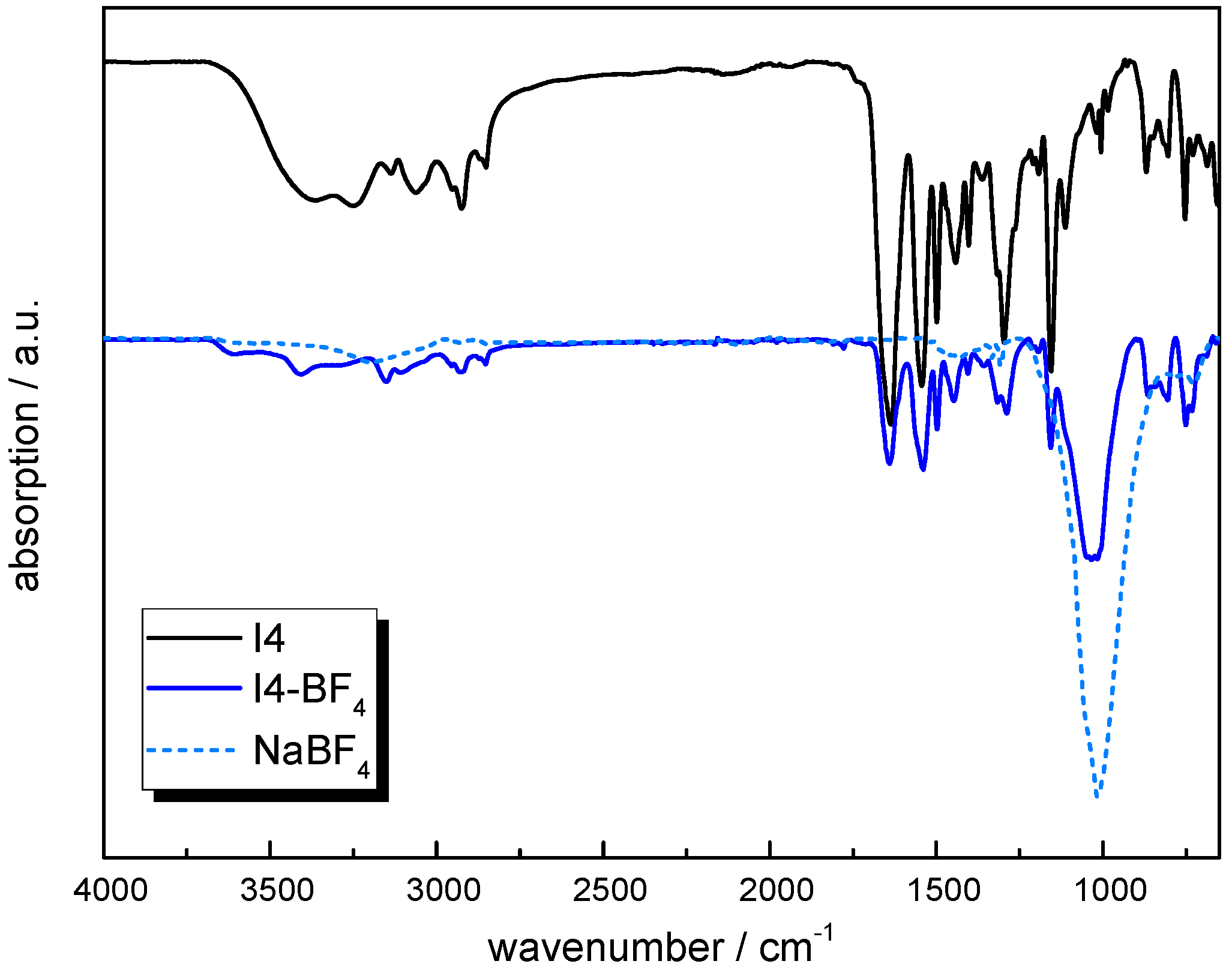
3.3.5. Anion Exchange
3.4. Characterization Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Yan, F.; Texter, J. Advanced applications of ionic liquids in polymer science. Prog. Polym. Sci. 2009, 34, 431–448. [Google Scholar] [CrossRef]
- Wanghofer, F.; Wolfberger, A.; Wolfahrt, M.; Schlögl, S. Cross-Linking and Evaluation of the Thermo-Mechanical Behavior of Epoxy Based Poly(ionic Liquid) Thermosets. Polymers 2021, 13, 3914. [Google Scholar] [CrossRef] [PubMed]
- Izgorodina, E.I.; Forsyth, M.; MacFarlane, D.R. Towards a Better Understanding of ‘Delocalized Charge’ in Ionic Liquid Anions. Aust. J. Chem. 2007, 60, 15. [Google Scholar] [CrossRef]
- Soares, B.G.; Livi, S.; Duchet-Rumeau, J.; Gerard, J.-F. Synthesis and Characterization of Epoxy/MCDEA Networks Modified with Imidazolium-Based Ionic Liquids. Macromol. Mater. Eng. 2011, 296, 826–834. [Google Scholar] [CrossRef]
- Zhao, Q.; Anderson, J.L. 2.11—Ionic Liquids. In Comprehensive Sampling and Sample Preparation: Analytical Techniques for Scientists; Pawliszyn, J., Ed.; Elsevier Academic Press: Amsterdam, The Netherlands, 2012; pp. 213–242. ISBN 978-0-12-381374-9. [Google Scholar]
- Walsh, D.A.; Goodwin, S. The Oxygen Reduction Reaction in Room-Temperature Ionic Liquids. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Wandelt, K., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK; Cambridge, MA, USA, 2018; pp. 898–907. ISBN 978-0-12-809894-3. [Google Scholar]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids—An Overview. Aust. J. Chem. 2004, 57, 113. [Google Scholar] [CrossRef]
- Greaves, T.L.; Drummond, C.J. Protic ionic liquids: Properties and applications. Chem. Rev. 2008, 108, 206–237. [Google Scholar] [CrossRef]
- Reisinger, D.; Kriehuber, M.U.; Bender, M.; Bautista-Anguís, D.; Rieger, B.; Schlögl, S. Thermally Latent Bases in Dynamic Covalent Polymer Networks and their Emerging Applications. Adv. Mater. 2023, 35, e2300830. [Google Scholar] [CrossRef]
- Vashchuk, A.; Fainleib, A.M.; Starostenko, O.; Grande, D. Application of ionic liquids in thermosetting polymers: Epoxy and cyanate ester resins. Express Polym. Lett. 2018, 12, 898–917. [Google Scholar] [CrossRef]
- Cao, Y.; Mu, T. Comprehensive Investigation on the Thermal Stability of 66 Ionic Liquids by Thermogravimetric Analysis. Ind. Eng. Chem. Res. 2014, 53, 8651–8664. [Google Scholar] [CrossRef]
- Maton, C.; Vos, N.D.; Stevens, C.V. Ionic liquid thermal stabilities: Decomposition mechanisms and analysis tools. Chem. Soc. Rev. 2013, 42, 5963–5977. [Google Scholar] [CrossRef]
- Hendy, B.N. Ionic Polymers. In Specialty Polymers; Dyson, R.W., Ed.; Springer: Boston, MA, USA, 1987; pp. 110–149. ISBN 978-0-216-92248-8. [Google Scholar]
- Matsumoto, K.; Endo, T. Design and synthesis of ionic-conductive epoxy-based networked polymers. React. Funct. Polym. 2013, 73, 278–282. [Google Scholar] [CrossRef]
- Hess, M.; Jones, R.G.; Kahovec, J.; Kitayama, T.; Kratochvíl, P.; Kubisa, P.; Mormann, W.; Stepto, R.F.T.; Tabak, D.; Vohlídal, J.; et al. Terminology of polymers containing ionizable or ionic groups and of polymers containing ions (IUPAC Recommendations 2006). Pure Appl. Chem. 2006, 78, 2067–2074. [Google Scholar] [CrossRef]
- Ribot, J.C.; Guerrero-Sanchez, C.; Greaves, T.L.; Kennedy, D.F.; Hoogenboom, R.; Schubert, U.S. Amphiphilic oligoether-based ionic liquids as functional materials for thermoresponsive ion gels with tunable properties via aqueous gelation. Soft Matter 2012, 8, 1025–1032. [Google Scholar] [CrossRef]
- Ribot, J.C.; Guerrero-Sanchez, C.; Hoogenboom, R.; Schubert, U.S. Thermoreversible ionogels with tunable properties via aqueous gelation of an amphiphilic quaternary ammonium oligoether-based ionic liquid. J. Mater. Chem. 2010, 20, 8279. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa, M.; Ogihara, W. Development of new class of ion conductive polymers based on ionic liquids. Electrochim. Acta 2004, 50, 255–261. [Google Scholar] [CrossRef]
- Green, M.D.; La Salas-de Cruz, D.; Ye, Y.; Layman, J.M.; Elabd, Y.A.; Winey, K.I.; Long, T.E. Alkyl-Substituted N-Vinylimidazolium Polymerized Ionic Liquids: Thermal Properties and Ionic Conductivities. Macromol. Chem. Phys. 2011, 212, 2522–2528. [Google Scholar] [CrossRef]
- Gu, Y.; Lodge, T.P. Synthesis and Gas Separation Performance of Triblock Copolymer Ion Gels with a Polymerized Ionic Liquid Mid-Block. Macromolecules 2011, 44, 1732–1736. [Google Scholar] [CrossRef]
- Williams, S.R.; La Salas-de Cruz, D.; Winey, K.I.; Long, T.E. Ionene segmented block copolymers containing imidazolium cations: Structure–property relationships as a function of hard segment content. Polymer 2010, 51, 1252–1257. [Google Scholar] [CrossRef]
- Jin, X.C.; Guo, L.Y.; Deng, L.L.; Wu, H. Study on epoxy resin modified by polyether ionic liquid. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 12037. [Google Scholar] [CrossRef]
- Mecerreyes, D. Applications of Ionic Liquids in Polymer Science and Technology; Springer: Berlin/Heidelberg, Germany, 2015; ISBN 978-3-662-44902-8. [Google Scholar]
- Bernard, F.L.; Polesso, B.B.; Cobalchini, F.W.; Donato, A.J.; Seferin, M.; Ligabue, R.; Chaban, V.V.; do Nascimento, J.F.; Dalla Vecchia, F.; Einloft, S. CO2 capture: Tuning cation-anion interaction in urethane based poly(ionic liquids). Polymer 2016, 102, 199–208. [Google Scholar] [CrossRef]
- Vlassi, E.; Pispas, S. Imidazolium Quaternized Polymers Based On Poly(Chloromethyl Styrene) and their Complexes with FBS Proteins and DNA. Macromol. Chem. Phys. 2015, 216, 1718–1728. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Gin, D.L.; Noble, R.D.; Deng, L. Facile fabrication of CO2 separation membranes by cross-linking of poly(ethylene glycol) diglycidyl ether with a diamine and a polyamine-based ionic liquid. J. Membr. Sci. 2017, 523, 551–560. [Google Scholar] [CrossRef]
- McDanel, W.M.; Cowan, M.G.; Carlisle, T.K.; Swanson, A.K.; Noble, R.D.; Gin, D.L. Cross-linked ionic resins and gels from epoxide-functionalized imidazolium ionic liquid monomers. Polymer 2014, 55, 3305–3313. [Google Scholar] [CrossRef]
- Wanghofer, F.; Trausner, C.; Reisinger, D.; Wolfahrt, M.; Schlögl, S. Influence of Anion and Cation Structures on Ionic Liquid Epoxy-Based Vitrimers. ACS Appl. Polym. Mater. 2024, 6, 649–657. [Google Scholar] [CrossRef]
- Wanghofer, F.; Kriehuber, M.; Reisinger, D.; Floh, F.; Wolfahrt, M.; Schlögl, S. Design of Reversible Adhesives by Using a Triple Function of Ionic Liquids. Macro Mater. Eng. 2024, 309, 2400011. [Google Scholar] [CrossRef]
- Radchenko, A.V.; Chabane, H.; Demir, B.; Searles, D.J.; Duchet-Rumeau, J.; Gérard, J.-F.; Baudoux, J.; Livi, S. New Epoxy Thermosets Derived from a Bisimidazolium Ionic Liquid Monomer: An Experimental and Modeling Investigation. ACS Sustain. Chem. Eng. 2020, 8, 12208–12221. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Rewar, A.S.; Kharul, U.K. Polybenzimidazole based film forming polymeric ionic liquids: Synthesis and effects of cation–anion variation on their physical properties. Polym. Chem. 2014, 5, 4083. [Google Scholar] [CrossRef]
- Livi, S.; Lins, L.C.; Capeletti, L.B.; Chardin, C.; Halawani, N.; Baudoux, J.; Cardoso, M.B. Antibacterial surface based on new epoxy-amine networks from ionic liquid monomers. Eur. Polym. J. 2019, 116, 56–64. [Google Scholar] [CrossRef]
- Zhang, S.-Y.; Zhuang, Q.; Zhang, M.; Wang, H.; Gao, Z.; Sun, J.-K.; Yuan, J. Poly(ionic liquid) composites. Chem. Soc. Rev. 2020, 49, 1726–1755. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vlasov, P.S.; Lozinskaya, E.I.; Malyshkina, I.A.; Vidal, F.; Aubert, P.-H.; Armand, M.; Vygodskii, Y.S. Solid-state electrolytes based on ionic network polymers. Polym. Sci. Ser. B 2014, 56, 164–177. [Google Scholar] [CrossRef]
- Perli, G.; Wylie, L.; Demir, B.; Gerard, J.-F.; Pádua, A.A.H.; Gomes, M.C.; Duchet-Rumeau, J.; Baudoux, J.; Livi, S. From the Design of Novel Tri- and Tetra-Epoxidized Ionic Liquid Monomers to the End-of-Life of Multifunctional Degradable Epoxy Thermosets. ACS Sustain. Chem. Eng. 2022, 10, 15450–15466. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Z.; Li, S.; Yang, L.; Hirano, S. Polymeric ionic liquid-plastic crystal composite electrolytes for lithium ion batteries. J. Power Sources 2016, 307, 678–683. [Google Scholar] [CrossRef]
- Pont, A.-L.; Marcilla, R.; de Meatza, I.; Grande, H.; Mecerreyes, D. Pyrrolidinium-based polymeric ionic liquids as mechanically and electrochemically stable polymer electrolytes. J. Power Sources 2009, 188, 558–563. [Google Scholar] [CrossRef]
- Tomé, L.C.; Isik, M.; Freire, C.S.; Mecerreyes, D.; Marrucho, I.M. Novel pyrrolidinium-based polymeric ionic liquids with cyano counter-anions: High performance membrane materials for post-combustion CO2 separation. J. Membr. Sci. 2015, 483, 155–165. [Google Scholar] [CrossRef]
- Hu, X.; Tang, J.; Blasig, A.; Shen, Y.; Radosz, M. CO2 permeability, diffusivity and solubility in polyethylene glycol-grafted polyionic membranes and their CO2 selectivity relative to methane and nitrogen. J. Membr. Sci. 2006, 281, 130–138. [Google Scholar] [CrossRef]
- Bhavsar, R.S.; Kumbharkar, S.C.; Kharul, U.K. Polymeric ionic liquids (PILs): Effect of anion variation on their CO2 sorption. J. Membr. Sci. 2012, 389, 305–315. [Google Scholar] [CrossRef]
- Shaplov, A.S.; Ponkratov, D.O.; Vlasov, P.S.; Lozinskaya, E.I.; Komarova, L.I.; Malyshkina, I.A.; Vidal, F.; Nguyen, G.T.M.; Armand, M.; Wandrey, C.; et al. Synthesis and properties of polymeric analogs of ionic liquids. Polym. Sci. Ser. B 2013, 55, 122–138. [Google Scholar] [CrossRef]
- Kammakakam, I.; O’Harra, K.E.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Self-healing imidazolium-based ionene-polyamide membranes: An experimental study on physical and gas transport properties. Polym. Int. 2019, 68, 1123–1129. [Google Scholar] [CrossRef]
- Shaligram, S.V.; Wadgaonkar, P.P.; Kharul, U.K. Polybenzimidazole-based polymeric ionic liquids (PILs): Effects of ‘substitution asymmetry’ on CO2 permeation properties. J. Membr. Sci. 2015, 493, 403–413. [Google Scholar] [CrossRef]
- Hamori, H.; Kumazawa, H.; Higuchi, R.; Yokozeki, T. Numerical and experimental evaluation of the formation of leakage paths through CFRP cross-ply laminates with leak barrier layers. Compos. Struct. 2019, 230, 111530. [Google Scholar] [CrossRef]
- Kumazawa, H.; Susuki, I.; Aoki, T. Gas Leakage Evaluation of CFRP Cross-ply Laminates under Biaxial Loadings. J. Compos. Mater. 2006, 40, 853–871. [Google Scholar] [CrossRef]
- Condé-Wolter, J.; Ruf, M.G.; Liebsch, A.; Lebelt, T.; Koch, I.; Drechsler, K.; Gude, M. Hydrogen permeability of thermoplastic composites and liner systems for future mobility applications. Compos. Part. A Appl. Sci. Manuf. 2023, 167, 107446. [Google Scholar] [CrossRef]
- Ruf, M.; Stahl, H.-U.; Kunze, K.; Zaremba, S.; Horoschenkoff, A.; von Unwerth, T.; Drechsler, K. Neue Bauweisen Von Wasserstoffdruckbehältern Für Die Integration in Zukünftige Fahrzeugarchitekturen. In Proceedings of the Munich Symposium on Lightweight Design 2020; Pfingstl, S., Horoschenkoff, A., Höfer, P., Zimmermann, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2021; pp. 74–85. ISBN 978-3-662-63142-3. [Google Scholar]
- Amovilli, C. VB analysis of wavefunctions calculated for chemical reactions in solution. In Valence Bond Theory; Elsevier: Amsterdam, The Netherlands, 2002; pp. 415–445. ISBN 9780444508898. [Google Scholar]
- O’Harra, K.E.; Kammakakam, I.; Noll, D.M.; Turflinger, E.M.; Dennis, G.P.; Jackson, E.M.; Bara, J.E. Synthesis and Performance of Aromatic Polyamide Ionenes as Gas Separation Membranes. Membranes 2020, 10, 51. [Google Scholar] [CrossRef] [PubMed]
- O’Harra, K.E.; Bara, J.E. Toward controlled functional sequencing and hierarchical structuring in imidazolium ionenes. Polym. Int. 2021, 70, 944–950. [Google Scholar] [CrossRef]
- Anderson, E.B.; Long, T.E. Imidazole- and imidazolium-containing polymers for biology and material science applications. Polymer 2010, 51, 2447–2454. [Google Scholar] [CrossRef]
- Ziyada, A.K.; Bustam, M.A.; Murugesan, T.; Wilfred, C.D. Effect of sulfonate-based anions on the physicochemical properties of 1-alkyl-3-propanenitrile imidazolium ionic liquids. New J. Chem. 2011, 35, 1111. [Google Scholar] [CrossRef]
- Carlisle, T.K.; Bara, J.E.; Lafrate, A.L.; Gin, D.L.; Noble, R.D. Main-chain imidazolium polymer membranes for CO2 separations: An initial study of a new ionic liquid-inspired platform. J. Membr. Sci. 2010, 359, 37–43. [Google Scholar] [CrossRef]
- Bara, J.E.; O’Harra, K.E.; Durbin, M.M.; Dennis, G.P.; Jackson, E.M.; Thomas, B.; Odutola, J.A. Synthesis and Characterization of Ionene-Polyamide Materials as Candidates for New Gas Separation Membranes. MRS Adv. 2018, 3, 3091–3102. [Google Scholar] [CrossRef]
- Butler, R.N. Tetrazoles. In Comprehensive Heterocyclic Chemistry II; Katrisky, A.R., Scriven, E.F.V., Eds.; Elsevier: Amsterdam, The Netherlands, 1996; Volume 4, pp. 621–678. ISBN 9780080965185. [Google Scholar]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts, 3rd ed.; Wiley: Chichester, UK, 2001; ISBN 978-0-470-09307-8. [Google Scholar]
- Hesse, M.; Meier, H.; Zeeh, B.; Bienz, S.; Bigler, L.; Fox, T. Spektroskopische Methoden in der Organischen Chemie, 8th ed.; Überarbeitete und Erweiterte Auflage; Georg Thieme Verlag: Stuttgart, Germany; New York, NY, USA, 2012; ISBN 978-3-13-576108-4. [Google Scholar]
- Larkin, P.J. Infrared and Raman Spectroscopy: Principles and Spectral Interpretation; Elsevier: Amsterdam, The Netherlands, 2011; ISBN 978-0-12-386984-5. [Google Scholar]
- Heimer, N.E.; del Sesto, R.E.; Meng, Z.; Wilkes, J.S.; Carper, W.R. Vibrational spectra of imidazolium tetrafluoroborate ionic liquids. J. Mol. Liq. 2006, 124, 84–95. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Rees, C.W.; Scriven, E.F.V. Comprehensive Heterocyclic Chemistry II; Elsevier: Amsterdam, The Netherlands, 1996; ISBN 9780080965185. [Google Scholar]
- Bara, J.E. Versatile and Scalable Method for Producing N-Functionalized Imidazoles. Ind. Eng. Chem. Res. 2011, 50, 13614–13619. [Google Scholar] [CrossRef]
- Hesse-Ertelt, S.; Heinze, T.; Kosan, B.; Schwikal, K.; Meister, F. Solvent Effects on the NMR Chemical Shifts of Imidazolium-Based Ionic Liquids and Cellulose Therein. Macromol. Symp. 2010, 294, 75–89. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, Y.; Shi, Y.; Xue, Z.; Mu, T. Quantitative Research on the Vaporization and Decomposition of [EMIM][Tf2N] by Thermogravimetric Analysis–Mass Spectrometry. Ind. Eng. Chem. Res. 2012, 51, 7418–7427. [Google Scholar] [CrossRef]
- Ehrenstein, G.W. Thermische Analyse: Brandprüfung, Wärme- und Temperaturleitfähigkeit, DSC, DMA, TMA; Hanser: München, Germany, 2020; ISBN 3446462589. [Google Scholar]
- Jia, N.; Fraenkel, H.A.; Kagan, V.A. Effects of Moisture Conditioning Methods on Mechanical Properties of Injection Molded Nylon 6. J. Reinf. Plast. Compos. 2004, 23, 729–737. [Google Scholar] [CrossRef]
- Launay, A.; Marco, Y.; Maitournam, M.H.; Raoult, I. Modelling the influence of temperature and relative humidity on the time-dependent mechanical behaviour of a short glass fibre reinforced polyamide. Mech. Mater. 2013, 56, 1–10. [Google Scholar] [CrossRef]
- Sambale, A.; Kurkowski, M.; Stommel, M. Determination of moisture gradients in polyamide 6 using StepScan DSC. Thermochim. Acta 2019, 672, 150–156. [Google Scholar] [CrossRef]
- Gilbert, M. Brydson’s Plastics Materials, 8th ed.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780323370226. [Google Scholar]
- Rondinella, A.; Capurso, G.; Zanocco, M.; Basso, F.; Calligaro, C.; Menotti, D.; Agnoletti, A.; Fedrizzi, L. Study of the Failure Mechanism of a High-Density Polyethylene Liner in a Type IV High-Pressure Storage Tank. Polymers 2024, 16, 779. [Google Scholar] [CrossRef]
- Sun, Y.; Lv, H.; Zhou, W.; Zhang, C. Research on hydrogen permeability of polyamide 6 as the liner material for type IV hydrogen storage tank. Int. J. Hydrog. Energy 2020, 45, 24980–24990. [Google Scholar] [CrossRef]
- Hocker, S.J.; Kim, W.T.; Schniepp, H.C.; Kranbuehl, D.E. Polymer crystallinity and the ductile to brittle transition. Polymer 2018, 158, 72–76. [Google Scholar] [CrossRef]
- Bersted, B.H.; Anderson, T.G. Influence of molecular weight and molecular weight distribution on the tensile properties of amorphous polymers. J. Appl. Polym. Sci. 1990, 39, 499–514. [Google Scholar] [CrossRef]
- Boumediene, M.; Haddad, B.; Paolone, A.; Drai, M.; Villemin, D.; Rahmouni, M.; Bresson, S.; Abbas, O. Synthesis, thermal stability, vibrational spectra and conformational studies of novel dicationic meta-xylyl linked bis-1-methylimidazolium ionic liquids. J. Mol. Struct. 2019, 1186, 68–79. [Google Scholar] [CrossRef]
- Bevington, J.C.; Huckerby, T.N. Studies of end-groups in polystyrene using 1H NMR. Eur. Polym. J. 2006, 42, 1433–1436. [Google Scholar] [CrossRef]
- Donovan, A.R.; Moad, G. A novel method for determination of polyester end-groups by NMR spectroscopy. Polymer 2005, 46, 5005–5011. [Google Scholar] [CrossRef]
- Lappan, U.; Fuchs, B.; Geißler, U.; Scheler, U.; Lunkwitz, K. Number-average molecular weight of radiation-degraded poly(tetrafluoroethylene). An end group analysis based on solid-state NMR and IR spectroscopy. Polymer 2002, 43, 4325–4330. [Google Scholar] [CrossRef]
- EN ISO 527-1; Plastics—Determination of Tensile Properties—Part 1: General Principles. International Organization for Standardization: Vernier, Switzerland, 2019.
- ISO 527-2:2025; Plastics—Determination of Tensile Properties—Part 2: Test Conditions for Moulding and Extrusion Plastics. International Organization for Standardization: Vernier, Switzerland, 2025.
- ISO 306:2022; Plastics—Thermoplastic Materials—Determination of Vicat Softening Temperature (VST). International Organization for Standardization: Vernier, Switzerland, 2022.



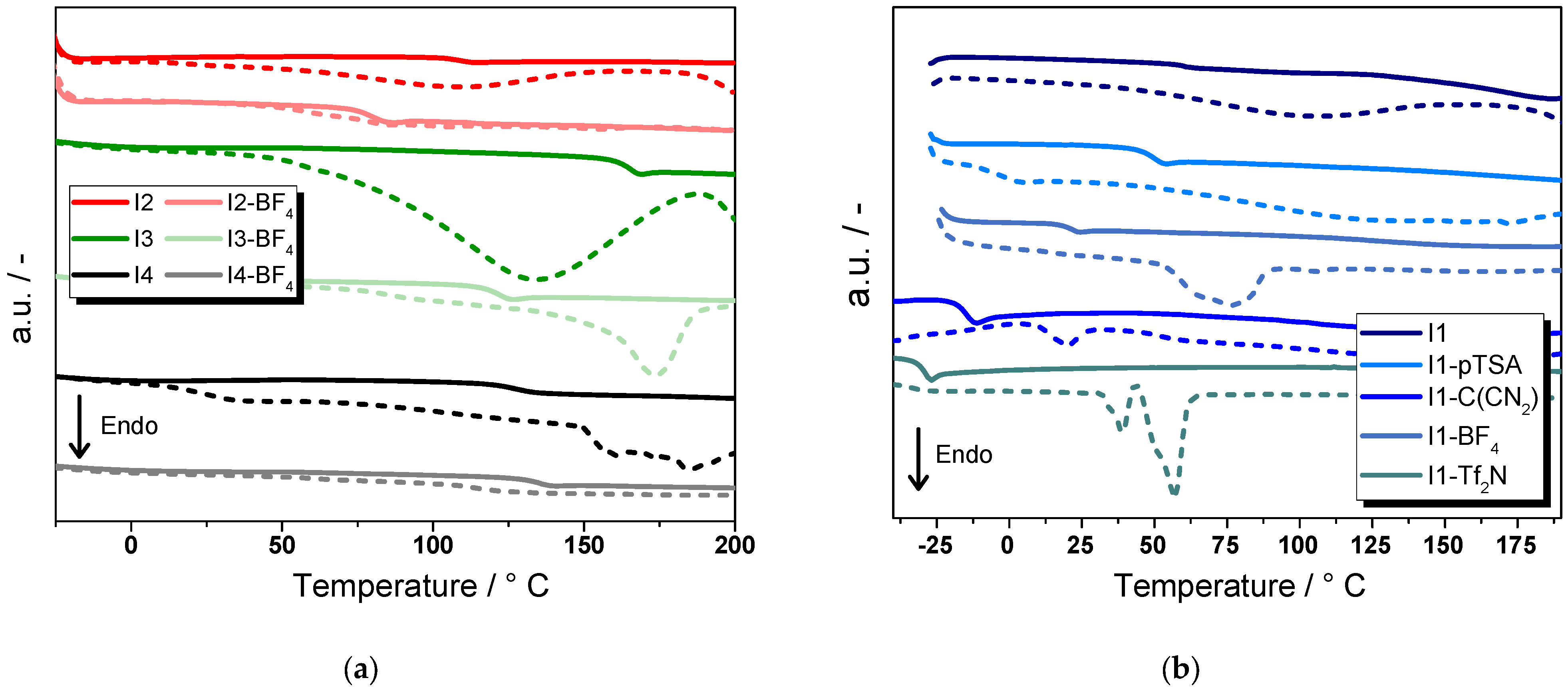
| Ionene | T5% (°C) | Tonset (°C) | Tgonset (°C) |
|---|---|---|---|
| I1 | 283 | 270 | 55 |
| I1-pTSA | 163 | 323 | 45 |
| I1-C(NCN)2 | 351 | 327 | −18 |
| I1-BF4− | 330 | 330 | 16 |
| I1-TF2N | 414 | 398 | −31 |
| I2 | 318 | 312 | 105 |
| I2-BF4− | 342 | 332 | 75 |
| I3 | 110 | 320 | 158 |
| I3-BF4− | 356 | 345 | 116 |
| I4 | 183 | 185 | 123 |
| I4-BF4− | 269 | 342 | 131 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kanatschnig, E.E.; Wanghofer, F.; Wolfahrt, M.; Schlögl, S. Synthesis and Characterization of Imidazolium-Based Ionenes. Molecules 2025, 30, 3961. https://doi.org/10.3390/molecules30193961
Kanatschnig EE, Wanghofer F, Wolfahrt M, Schlögl S. Synthesis and Characterization of Imidazolium-Based Ionenes. Molecules. 2025; 30(19):3961. https://doi.org/10.3390/molecules30193961
Chicago/Turabian StyleKanatschnig, Eveline Elisabeth, Florian Wanghofer, Markus Wolfahrt, and Sandra Schlögl. 2025. "Synthesis and Characterization of Imidazolium-Based Ionenes" Molecules 30, no. 19: 3961. https://doi.org/10.3390/molecules30193961
APA StyleKanatschnig, E. E., Wanghofer, F., Wolfahrt, M., & Schlögl, S. (2025). Synthesis and Characterization of Imidazolium-Based Ionenes. Molecules, 30(19), 3961. https://doi.org/10.3390/molecules30193961







