2. Pauson–Khand Annulation: Forging the Core via Metal-Catalyzed Cycloaddition
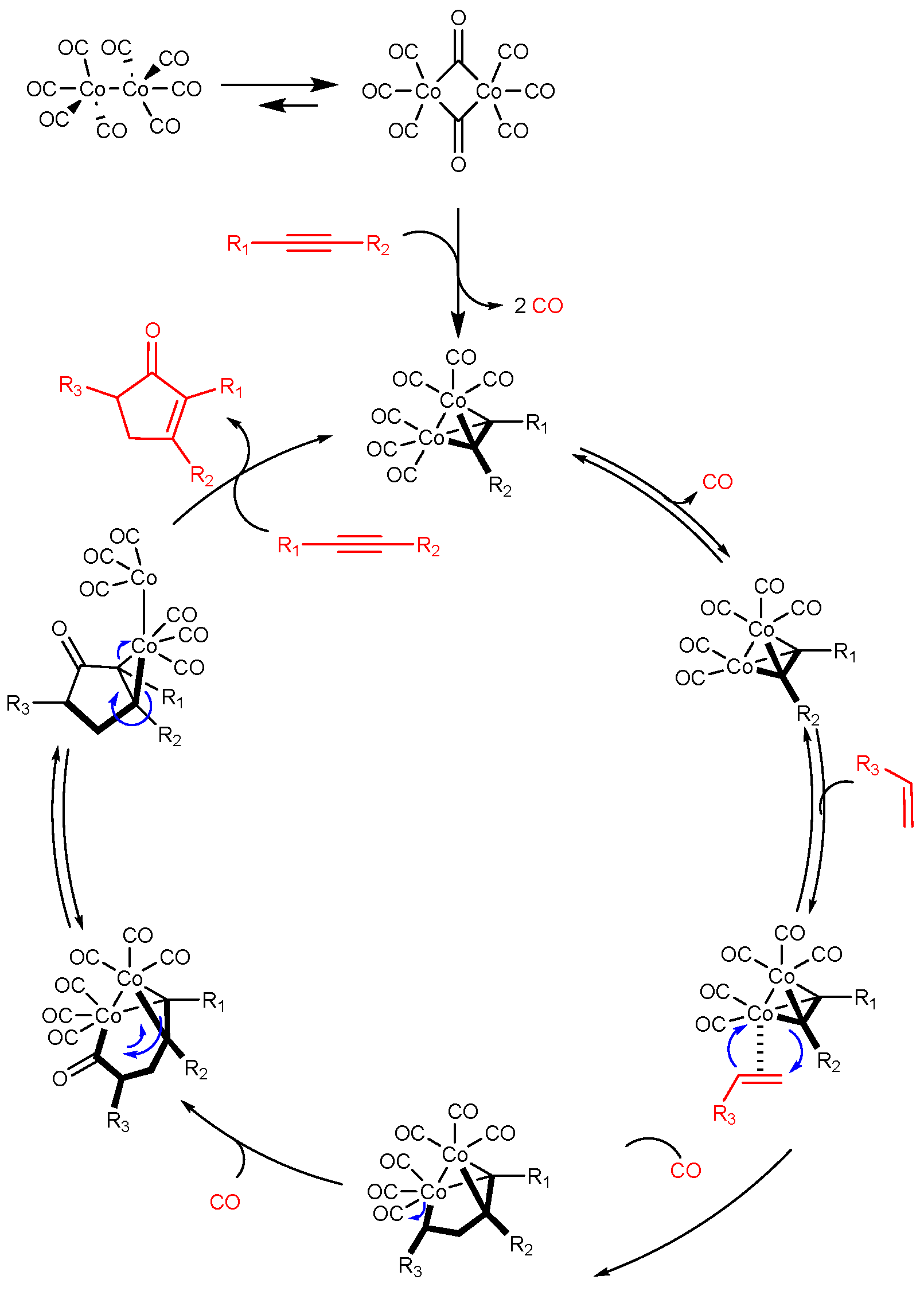
The Pauson–Khand reaction is a [2 + 2 + 1] cycloaddition of an alkyne, an alkene and carbon monoxide (
Figure 2). This reaction is highly valuable for constructing complex structures, particularly cyclopentenones, and allows for better control of stereochemistry owing to its intramolecular nature.
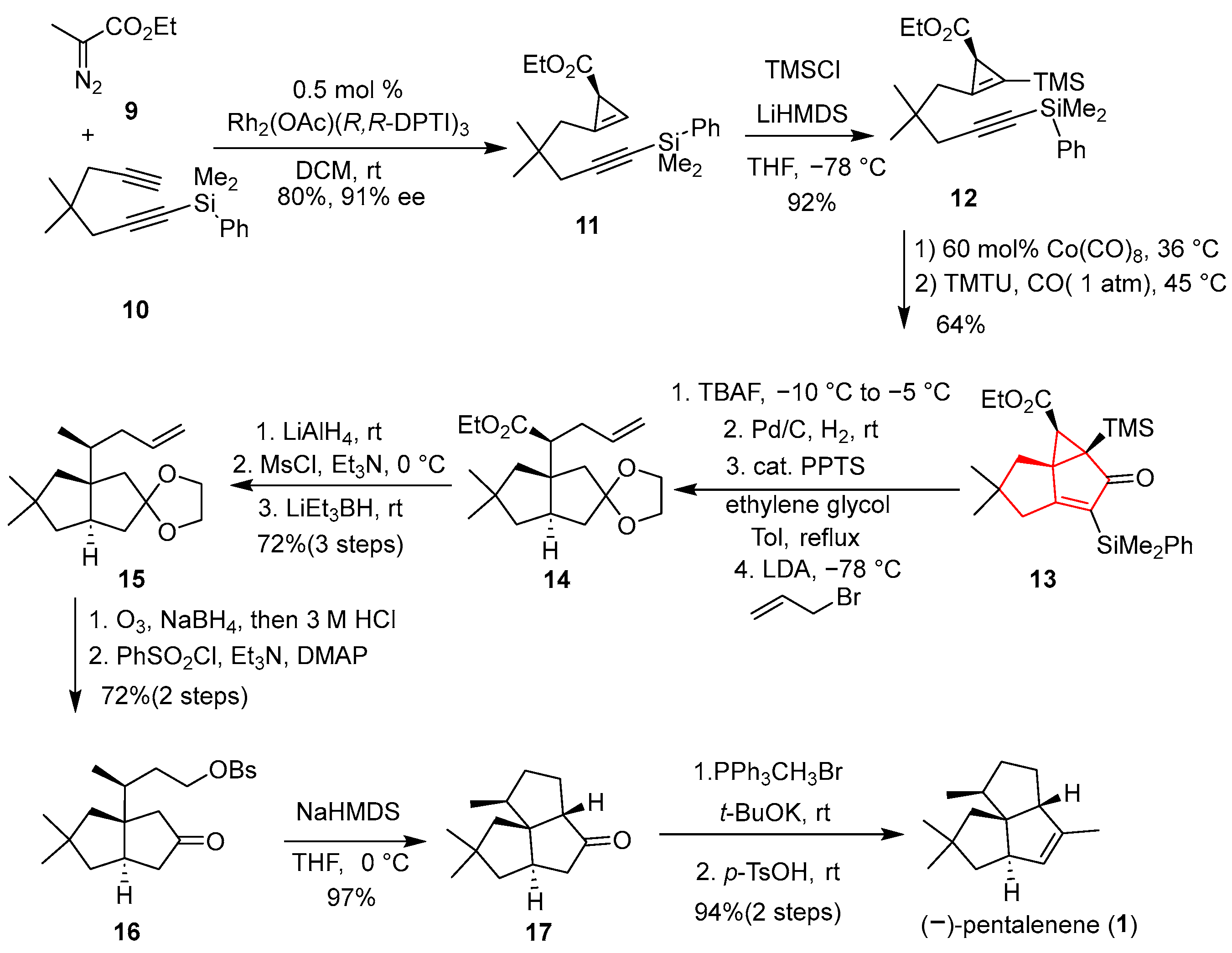
In 2007, Fox’s team reported the first enantioselective synthesis of (−)-pentalenene, employing a cascade intramolecular Pauson–Khand reaction catalyzed by noble metal rhodium as the key step to construct a [
5,
5,
3] tricyclic framework [
21]. As shown in
Scheme 1, ethyl diazoacetate (
9) underwent stereoselective cyclopropenylation with diyne compound
10 under the catalysis of divalent rhodium to deliver compound
11, with a yield of up to 80% and an enantioselectivity of 91% ee. Subsequently, the introduction of the TMS group facilitated the Pauson–Khand reaction, resulting in the formation of tricyclic compound
13. Following seven steps, including deprotection, cyclopropane opening, and introduction of side chains, provided compound
15, which was converted to ketone
16 via two-step conversion. Under the action of strong base NaHMDS, an intramolecular S
N2 reaction occurred with high yield, and the tricyclic compound
17 was constructed. Then asymmetric total synthesis of sesquiterpenes (−)-pentalenene (
1) was accomplished through a Wittig reaction and an isomerization reaction.
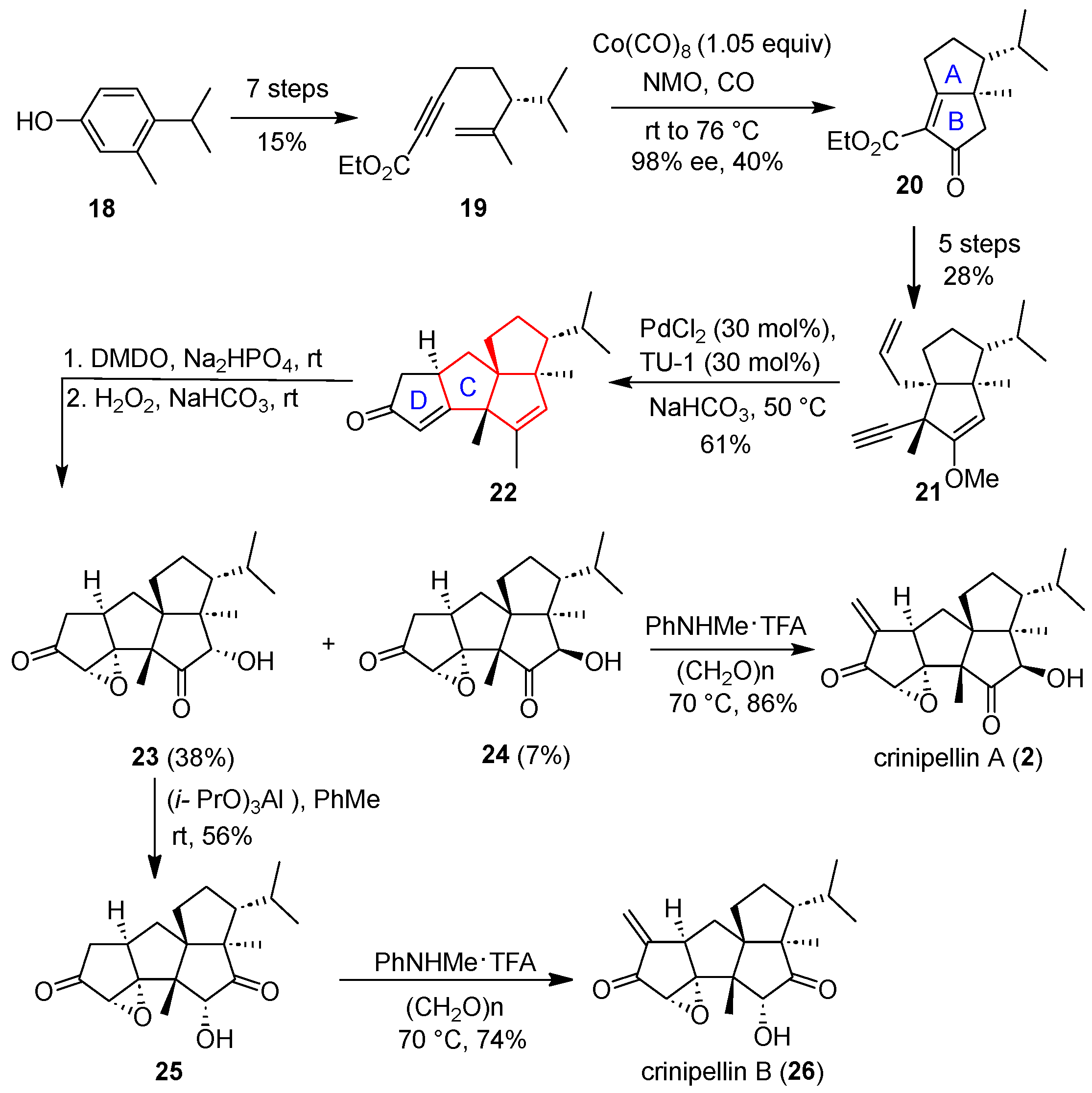
In 2018, Yang’s group reported a strategy for diastereoselective construction of the tetraquinane core of diterpene compounds crinipellin A and crinipellin B via two Pauson–Khand reactions promoted by octacarbonyl cobalt and thiourea/palladium chloride, respectively [
22]. As shown in
Scheme 2, converting commercially available phenol
18 into alkynyl ester
19 was successful through seven steps, with a 15% yield. Compound
19 underwent the first Pauson–Khand reaction under the action of Cobalt carbonyl, constructing the A and B rings of the diterpene and producing cyclopentenone
20. A series of functional group conversions of
12 were carried out to obtain alkynyl compound
21. Under the catalysis of thiourea/palladium chloride [
23], the second Pauson–Khand reaction occurred to provide tetracyclic compound
22. In this case, the C and D rings of the target natural product were constructed in one step. After subsequent adjustments of oxidation state and functional groups of
23 and
24, asymmetric total synthesis of crinipellin B (
26) and crinipellin A (
2), respectively, was achieved. Notably, this strategy will be helpful in the collective synthesis of crinipellin analogs, since it allows the protocol developed to be used to synthesize tetraquinane cores with different substituents through tactical changes in substituents and functionalities.
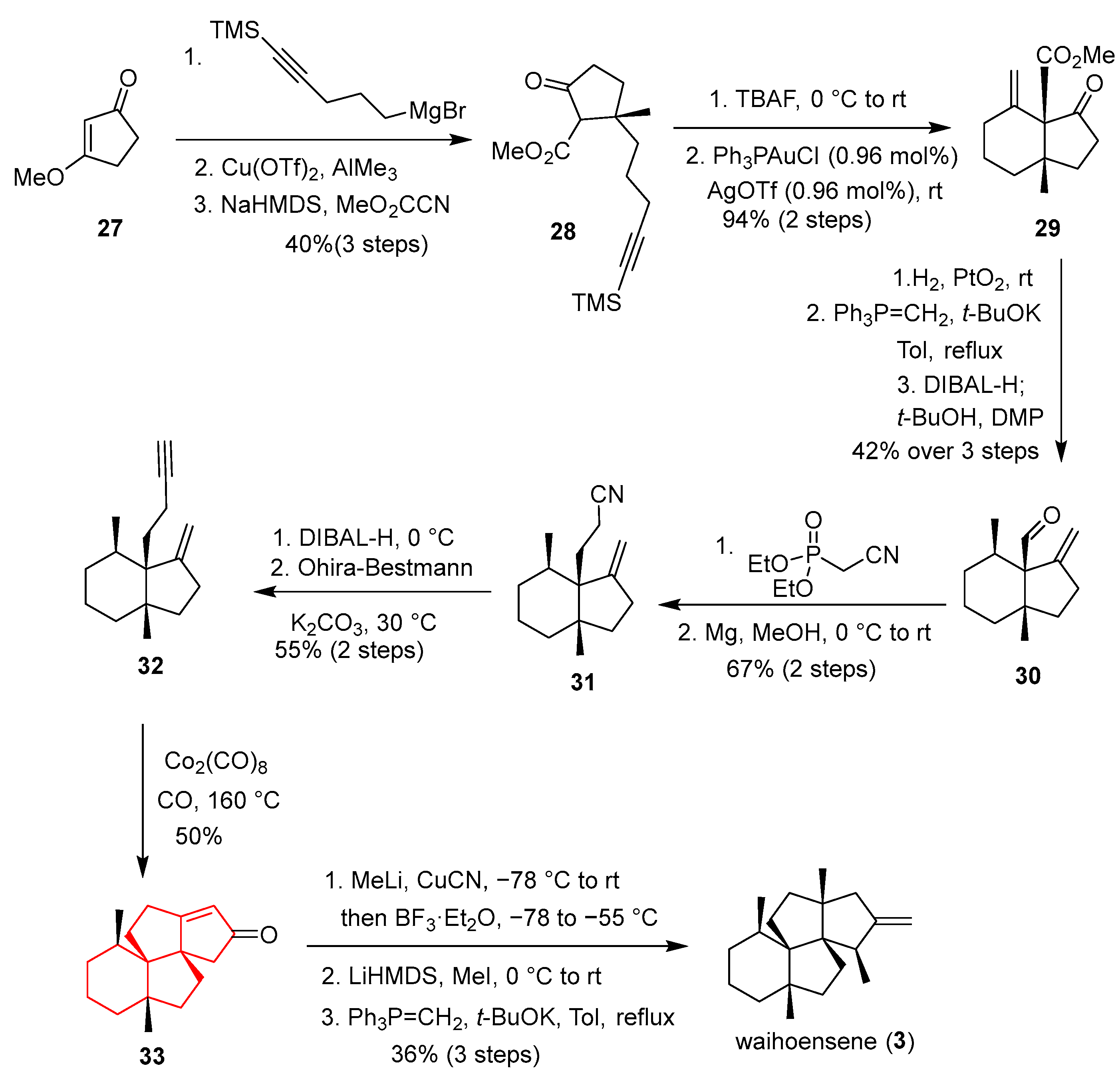
In 2020, Snyder’s group reported a highly concise, enantioselective total synthesis of the angular triquinane natural product (+)-waihoensene, a molecule featuring four contiguous all-carbon quaternary centers [
24] (
Scheme 3). Compound
27 was elaborated to ester
28, which served as the direct precursor for a pivotal gold-catalyzed Conia-ene reaction (Ph
3PAuCl, AgOTf), forging a six-membered ring and the second vicinal quaternary center to provide the bicyclic framework
29 in quantitative yield. The key transformation was a challenging intramolecular Pauson–Khand reaction of enyne
32 (Co
2(CO)
8, CO, mesitylene, 160 °C), which successfully constructed the third ring and the final quaternary carbon center of the angular triquinane core, delivering intermediate
33. The synthesis was concluded by installing the fourth quaternary center via a cuprate-mediated methylation and a final Wittig olefination to yield (+)-waihoensene (
3). This route is remarkable for its strategic use of minimal functional groups to orchestrate multiple critical C–C bond formations, its high convergency, and its innovative application of a Conia-ene reaction to assemble a vicinal quaternary center pair.
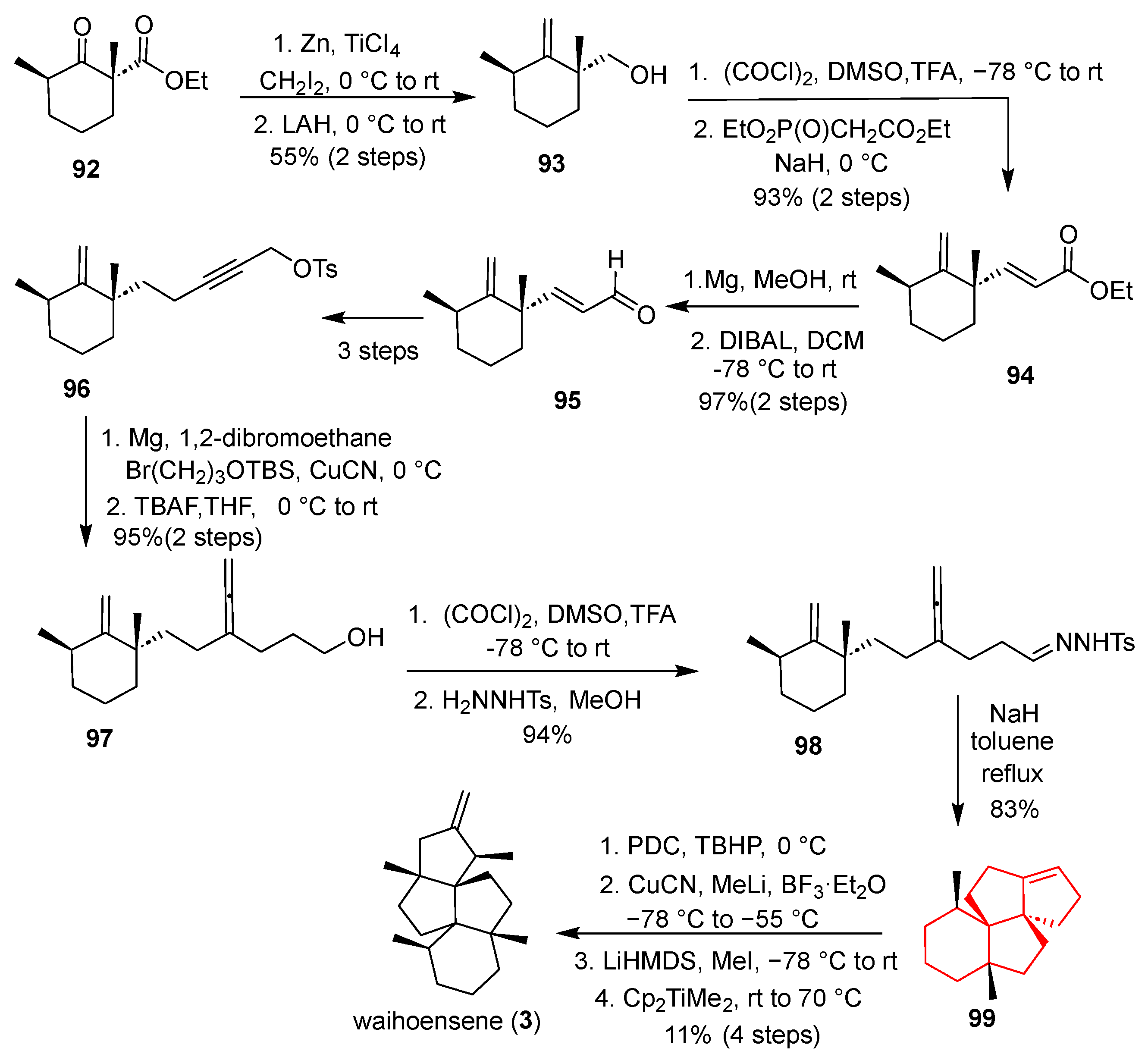
In 2020, Yang’s group accomplished an asymmetric total synthesis of the highly congested angular triquinane natural product (+)-waihoensene in 15 steps [
25] (
Scheme 4). The synthesis commenced with enone
34, which underwent a copper-catalyzed asymmetric conjugate addition and then a Sakurai allylation and ozonolysis to provide the key diyne precursor
35. The core transformation involved a diastereoselective Conia-ene type cyclization of
35 (
tBuOK, DMSO, rt) to construct the bicyclic framework
36, with a new quaternary center. A critical intramolecular Pauson–Khand reaction of enyne
36 (Co
2(CO)
8, N
2O, DCE, 80 °C) then forged the angular triquinane core, delivering tricycle
37 bearing three contiguous all-carbon quaternary centers. A nickel-catalyzed conjugate methylation installed the fourth quaternary center, producing diketone
38. After ketone protection, Wittig olefination occurred, followed by a highly diastereoselective Fe(acac)
3-catalyzed hydrogen atom transfer (HAT) reduction (PhSiH
3, EtOH, 60 °C) to saturate the double bond and set the desired stereochemistry, yielding
39. Final methylation (LiHMDS, MeI) and Wittig methylenation completed the synthesis of waihoensene (
3). This route is notable for its high step economy, exceptional diastereoselectivity in critical cyclizations, and the innovative use of a radical HAT process to solve a challenging stereochemical problem.
In 2021, the Gaich’s group reported the total synthesis of the same molecule waihoensene [
26] (
Scheme 5). They employed commercially available cyclohexenone derivative
40 as the starting material and utilized aldol addition and an elimination reaction to generate
41. Under the action of tributyltin hydride and AIBN, a radical cyclization reaction occurred to generate cyclohexanone
42. Then, through oxidation state adjustment and alkylation reaction, enyne compound
43 was obtained. Under the condition of octacarbonyl cobalt, enyne underwent the key Pauson–Khand reaction to generate
44. In this way, the four-ring skeleton was constructed, and the total synthesis of waihoensene was completed by following the reference process in the literature within three steps.
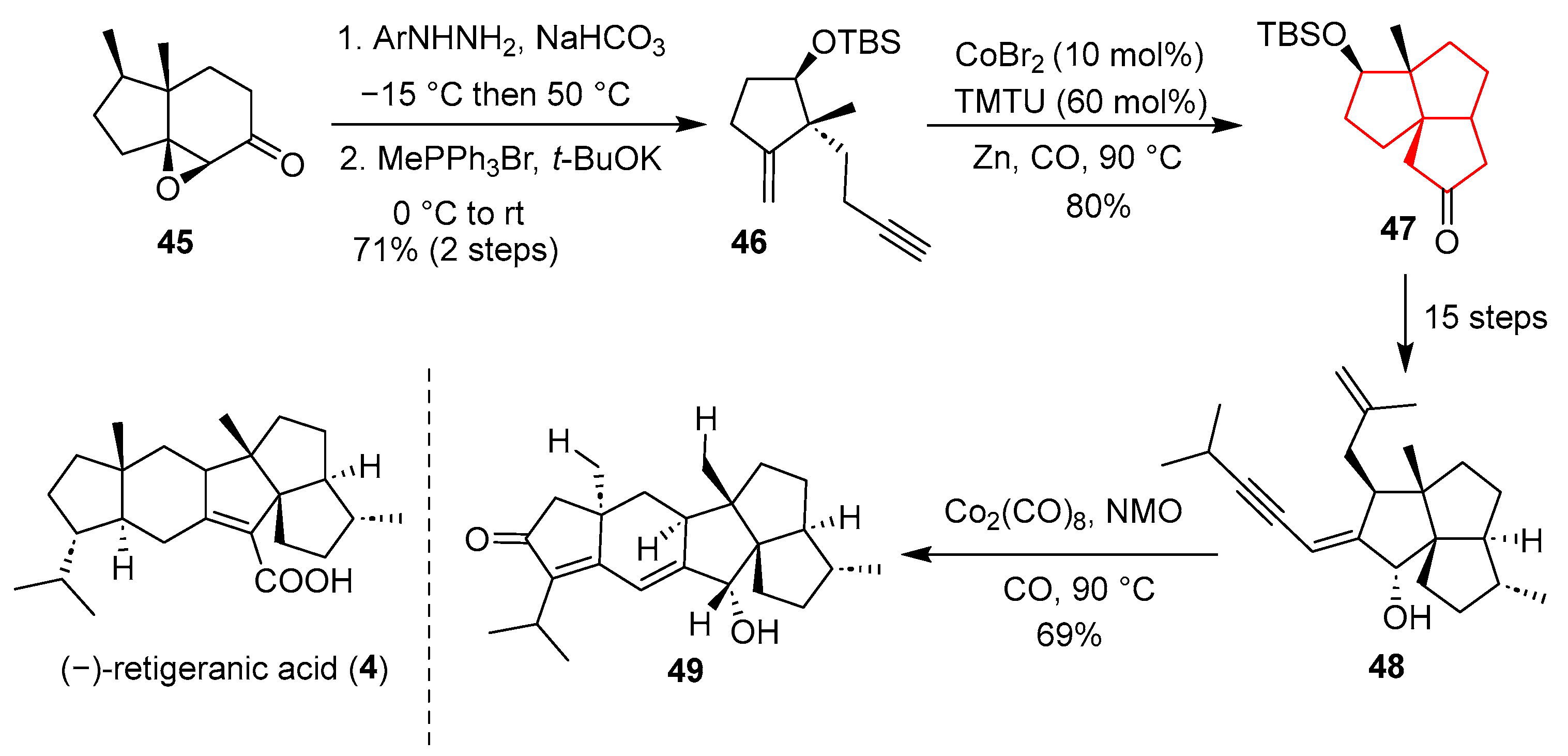
In 2021, Yang’s group utilized a strategy of two intramolecular Pauson–Khand reactions to synthesize the five-ring core skeleton of sesquiterpene (−)-retigeranic acid [
27] (
Scheme 6). Starting from the known compound
45, they synthesized the alkyne compound
46 through Eschenmoser Tanabe fragmentation and a Wittig reaction. The compound underwent the first intramolecular Pauson–Khand reaction under the conditions of cobalt bromide, TMTU, and zinc powder [
28] to construct the tricyclic structure
47. Then, the alkenone
47 underwent a long-step transformation to generate the cyclopentanone
48 with two side chains. Subsequently, the second Pauson–Khand reaction occurred between octacarbonyl cobalt and NMO, completing the construction of the core pentacyclic skeleton of (−)-retigeranic acid (
4).
The intramolecular Pauson–Khand reaction offers exceptional atom economy and convergence in constructing a cyclopentenone core ubiquitous in angular triquinanes. Its inherent cis-fused stereoselectivity reliably installs vicinal quaternary centers with predictable relative configuration. Recent advances in asymmetric catalysis further enhance enantiocontrol (>90% ee), positioning PKR as a stereodivergent platform for complex terpenoid synthesis. Substrate scope remains constrained to rigid alkyne–alkene tethers, often necessitating protective group manipulations. Toxic Co2(CO)8 reagents limit scalability, while competing side reactions cap yields at 40–60% for congested systems. Future catalysis designs must address chemo- and regioselectivity in polyunsaturated precursors.
3. Skeletal Reorganization Strategies: Structural Editing of Precursor Scaffolds
In 2008, Toste’s team presented an approach for constructing a tricyclic framework by intramolecular cycloisomerization of enynes containing an embedded cyclopropane unit catalyzed by Au (I) [
29]. As shown in
Scheme 7, Kulinkovich cyclopropanation of vinyl ester
50 was the first step in the process. Three-step functional group conversion of the resulting
51 produced vinyl compound
52. Gold(I) catalysis facilitated the cycloisomerization of enynes bearing a cyclopropane moiety, selectively generating ring systems with a cyclopropylmethyl cation. Intermediate
53 underwent a Wagner–Meerwein rearrangement, which triggered the diastereoselective formation of fused cyclobutanes to provide compound
54. Ring-expansion of the corresponding methyl ether
55 in the presence of catalytic PdCl
2(MeCN)
2 and DDQ delivered exo-methylene cyclopentanone
56. Finally, the total synthesis of ventricosene (
57) was achieved through functional group conversion. It is worth mentioning that the gold-catalyzed ring-expanding enyne cycloisomerizations enabled the efficient construction of architecturally complex polycyclic systems.
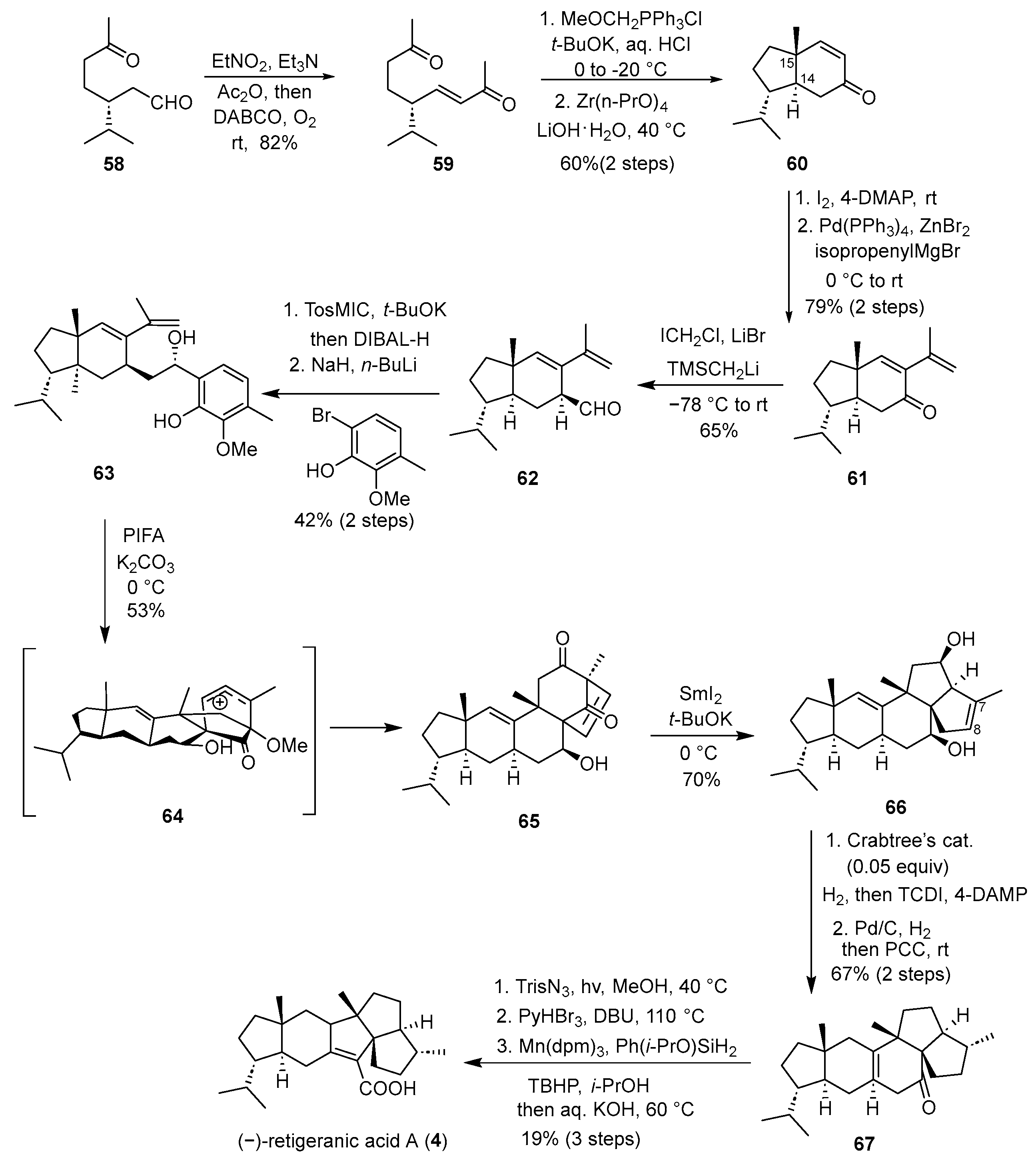
In 2023, Ding and co-workers achieved the first asymmetric total synthesis of (−)-retigeranic acid A. A pivotal strategy in their approach featured a reductive skeletal rearrangement cascade, enabling the precise construction of diverse angular triquinane frameworks [
30]. As shown in
Scheme 8, their approach commenced with a commercially available chiral aldehyde
58, which underwent a Henry condensation with ethyl nitroethane followed by a one-pot Nef reaction under DABCO/O
2 to produce enone
59 in 82% yield. Chemoselective Wittig olefination at the C15 ketone, followed by acid hydrolysis, provided a homologated keto-aldehyde. An intramolecular Michael/aldol cyclization mediated by Zr(n-PrO)
4 then established the trans-hydrindane framework, delivering
60 as the major diastereomer. Subsequent conversion to an α-iodoenone and Negishi coupling with isopropenylmagnesium bromide yielded keto-diene
61. A cascade epoxidation/Meinwald rearrangement initiated by ICH
2Cl and TMSCH
2Li·LiBr, followed by spontaneous epimerization, furnished aldehyde
62. Van Leusen homologation with TosMIC and
t-BuOK, DIBAL-H reduction, followed by 1,2-addition of an organolithium reagent, provided vinylphenol
63, which underwent an oxidative dearomatization-induced (ODI) [5 + 2] cycloaddition/pinacol rearrangement cascade to form pentacycle
65 as a single isomer. The core angular triquinane system was constructed via a reductive skeletal rearrangement cascade: treatment of
65 with SmI
2 in
t-BuOH promoted a Dowd–Beckwith rearrangement followed by an acyloin rearrangement, directly producing alcohol
66, with a 70% yield. Further elaboration included Crabtree’s catalyst-mediated stereoselective hydrogenation and Chugaev elimination, followed by hydrogenation and isomerization to install the double bond, and PCC oxidation to yield
67. A Wolff ring contraction via UV irradiation of the diazo precursor and a metal-hydride hydrogen atom transfer (MHAT) reduction using Ph(i-PrO)SiH
2, followed by saponification, delivered (–)-retigeranic acid A (
4). This synthesis features a concise and innovative reductive rearrangement to access the angular triquinane core, demonstrating broad applicability for constructing congested polyquinane systems.
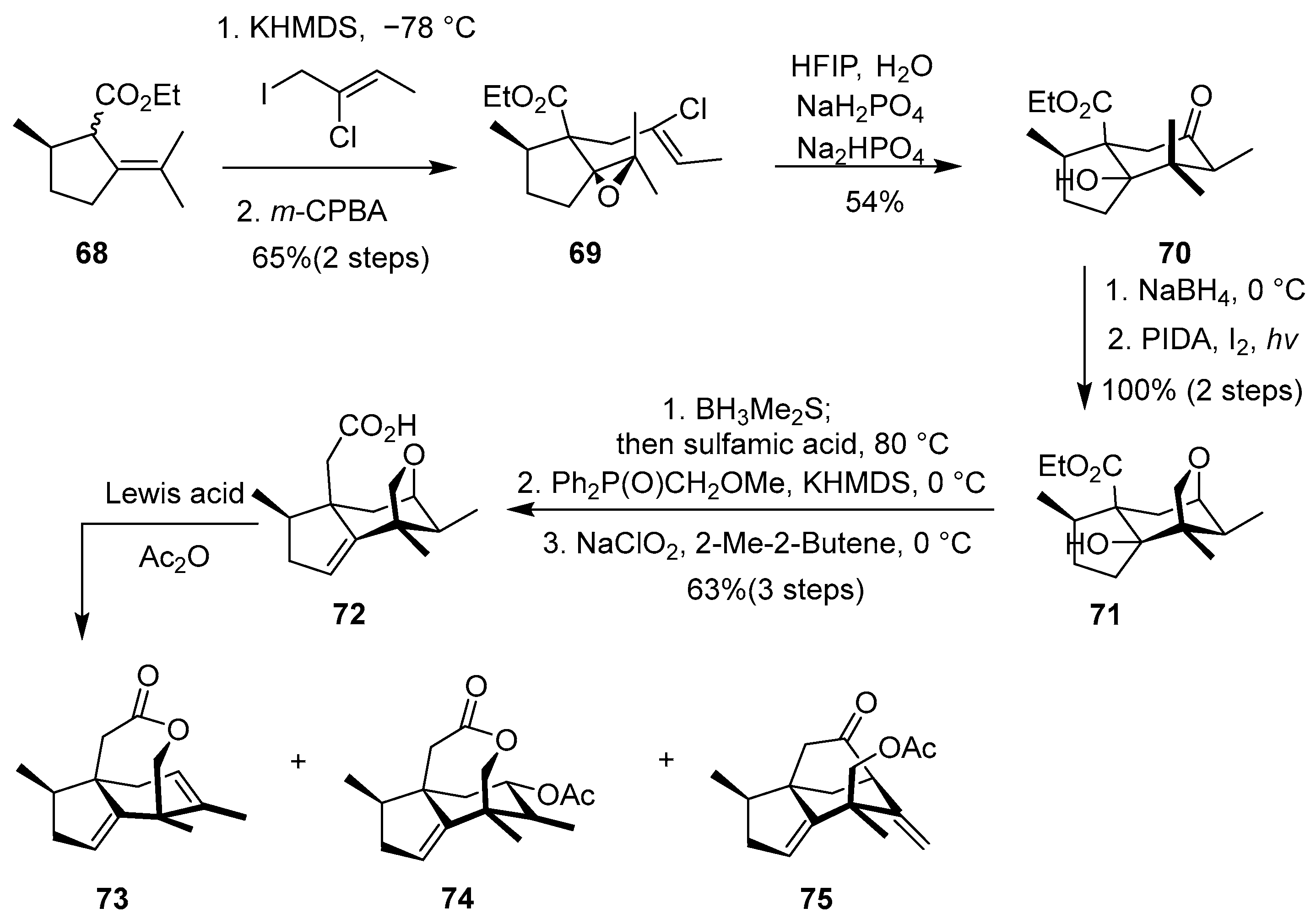
In 2023, Zhang and co-workers reported protecting-group-divergent syntheses of illicium sesquiterpenes [
31]. As shown in
Scheme 9, starting from chiral ester
68, alkylation with (
Z)-2-chloro-1-iodobut-2-ene and epoxidation formed epoxide
69. HFIP-promoted cationic epoxide-ene cyclization afforded bicyclic ketone
70. NaBH
4 reduction, Suárez reaction (HAT oxygenation), and homologation gave rapid access to the 15-carbon tricyclic carboxylic acid
72. Lewis acid (BF
3·OEt
2, Bi(OTf)
3, or TMSOTf)-mediated skeletal reorganization of
72 divergently generated three distinct tricyclic precursors (
73,
74,
75). Subsequent oxidations (e.g., Baeyer–Villiger, dihydroxylation, desaturation) and biomimetic rearrangements yielded eight allo-cedrane/seco-prezizaane sesquiterpenes (e.g., pseudoanisatin in 11 steps, 11-O-debenzoylashironin) and enabled the formal syntheses of five anislactones (e.g., merrilactone A).
In 2025, Wu’s group accomplished the first total synthesis of
abeo-cucurbitane triterpenoids cucurbalsaminone C via a bioinspired route featuring two key rearrangements [
32]. As shown in
Scheme 10, starting from lanosterol
76, the synthesis firstly installed the cucurbitane skeleton through a biomimetic tandem Wagner–Meerwein rearrangement of epoxide
77 to afford
78. After oxidation to enone
79, the pivotal photochemical oxa-di-π-methane (ODPM) rearrangement forged the 5/6/3-fused core
80 with complete diastereoselectivity, overcoming limitations observed in steroid substrates. Subsequent Eosin Y-photocatalyzed Barton–McCombie deoxygenation [(TMS)
3SiH, blue light] efficiently removed the sterically hindered C11-OH of
81 to give
82, suppressing competitive Chugaev elimination. Late-stage Burgess dehydration of allylic alcohols
82 furnished cucurbalsaminone C (
83) (71% over two steps). This synthesis demonstrated the power of photochemical strategies for constructing congested polycyclic scaffolds, though late-stage oxidations remain non-ideal for scalability.
Skeletal editing transcends conventional bond formation logic, enabling the topological metamorphosis of accessible precursors into strained frameworks. Hudlicky’s ring-expansion and Toste’s gold-catalyzed cycloisomerization exemplify how linear or monocyclic substrates undergo concerted reorganization to forge angular triquinanes with >50% yield. Such strategies often mimic biosynthetic pathways, offering step-count advantages. Predictive control over regioselectivity remains elusive due to competing rearrangement pathways. These strategies often require substrates with precise stereoelectronic alignment, and efficient transfer of chiral information remains a challenge. Scalability suffers from stoichiometric reagents (PbCO3, SmI2) and harsh conditions. Computational modeling of transition states could mitigate these issues.
4. Tandem and Cascade Cyclization: Convergent Assembly of the Tricyclic Framework
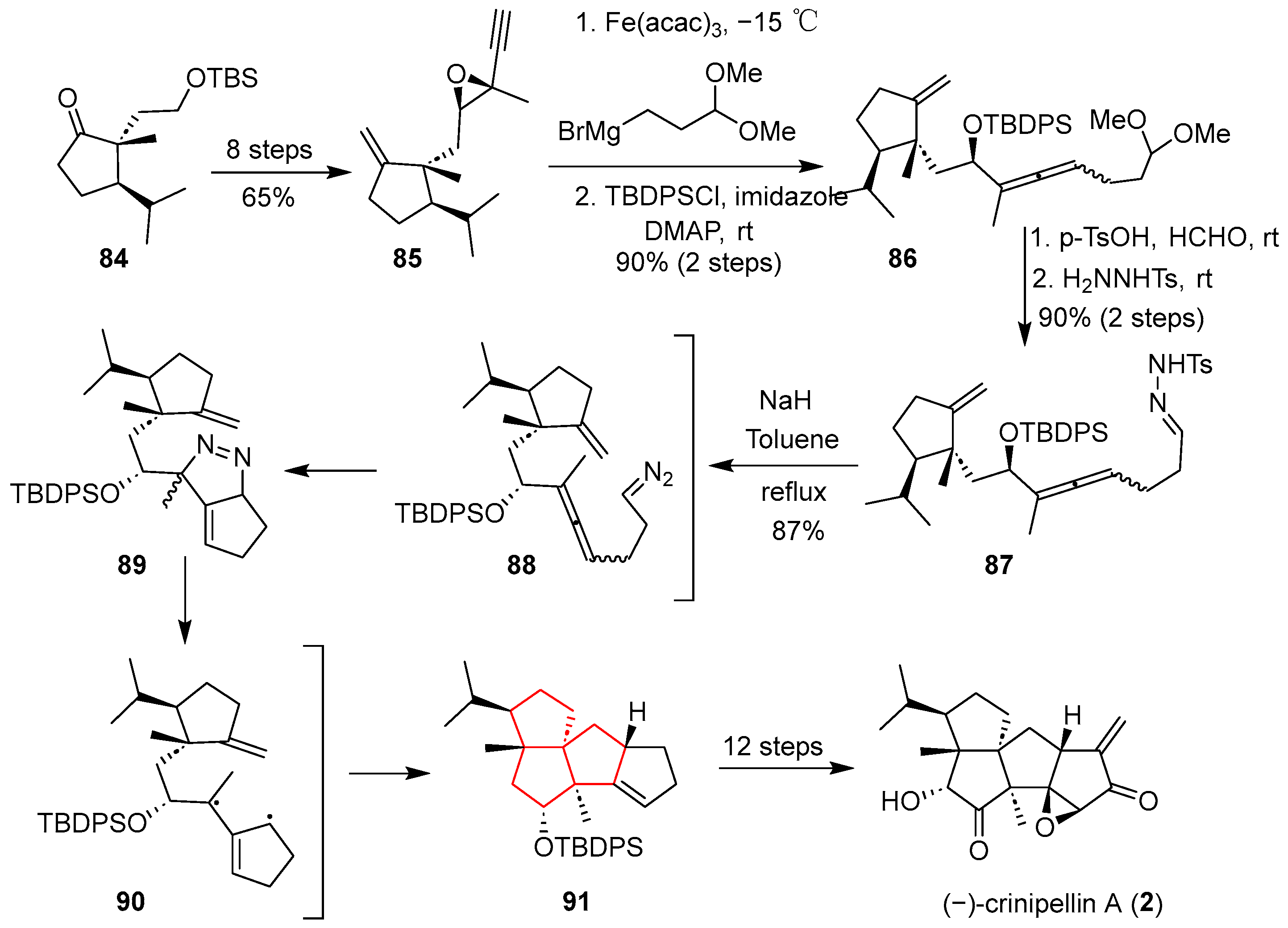
In 2014, the inaugural total synthesis of (−)-crinipellin A was achieved by Lee’s group. A pivotal step in this synthesis involved the construction of the diterpene’s tetracyclic core via an intramolecular radical tandem [2 + 3] cycloaddition of an allenyl diazo substrate [
33]. As delineated in
Scheme 11, the synthesis commenced with commercially available compound
84, which was converted to epoxyalkyne
85. Subjecting epoxyalkyne
85 to an iron catalyzed S
N2′ reaction with the requisite Grignard reagent, followed by silylation of the resultant secondary alcohol, yielded allene
86. Subsequent acidic hydrolysis cleaved the acetal moiety in
86, unmasking the aldehyde. Treatment of this aldehyde with
p-toluenesulfonylhydrazide prepared hydrazone
87, a perfect tandem cycloaddition precursor. Heating the sodium salt of the hydrazone triggered the conversion of
87 into diazo compound
88, thereby initiating the formation of the tetracyclic framework. An ensuing intramolecular [2 + 3] cycloaddition between the diazo functionality and the allene group furnished methylenepyrazole intermediate
89, which rapidly expelled nitrogen molecules to yield the transient TMM diyl species
90. Despite considerable steric encumbrance, this highly reactive diyl intermediate underwent a final [2 + 3] dipolar cycloaddition with the olefin, affording the tetracyclic product
91. Late-stage functional group modifications of compound
91 ultimately achieved the total synthesis of (−)-crinipellin A (
2).
In 2017, Lee’s group achieved the total synthesis of waihoensene by employing a [2 + 3] cycloaddition approach consistent with earlier methodologies shown in
Scheme 11. The synthesis of the cycloaddition precursor
98 was depicted in
Scheme 12 [
34]. Beginning with racemic ketoester
92, a Lombardo–Takai olefination was performed, and the resulting product was reduced to give alcohol
93. Oxidation under Swern conditions followed by a Horner–Wadsworth–Emmons olefination yielded α, β-unsaturated ester
94, which was then selectively reduced using magnesium in methanol to afford aldehyde
95. A three-step sequence comprising a Corey–Fuchs reaction, in situ hydroxymethylation, and tosylation delivered the functionalized propargylic alcohol
96. This intermediate was transformed into allenyl alcohol
97 via a copper(I)-catalyzed S
N2′ reaction and subsequent desilylation. Subjecting hydrazone
98 to a tandem [2 + 3] cycloaddition reaction constructed the core skeleton
99, which underwent late-stage functionalization in four additional steps to complete the synthesis of waihoensene.
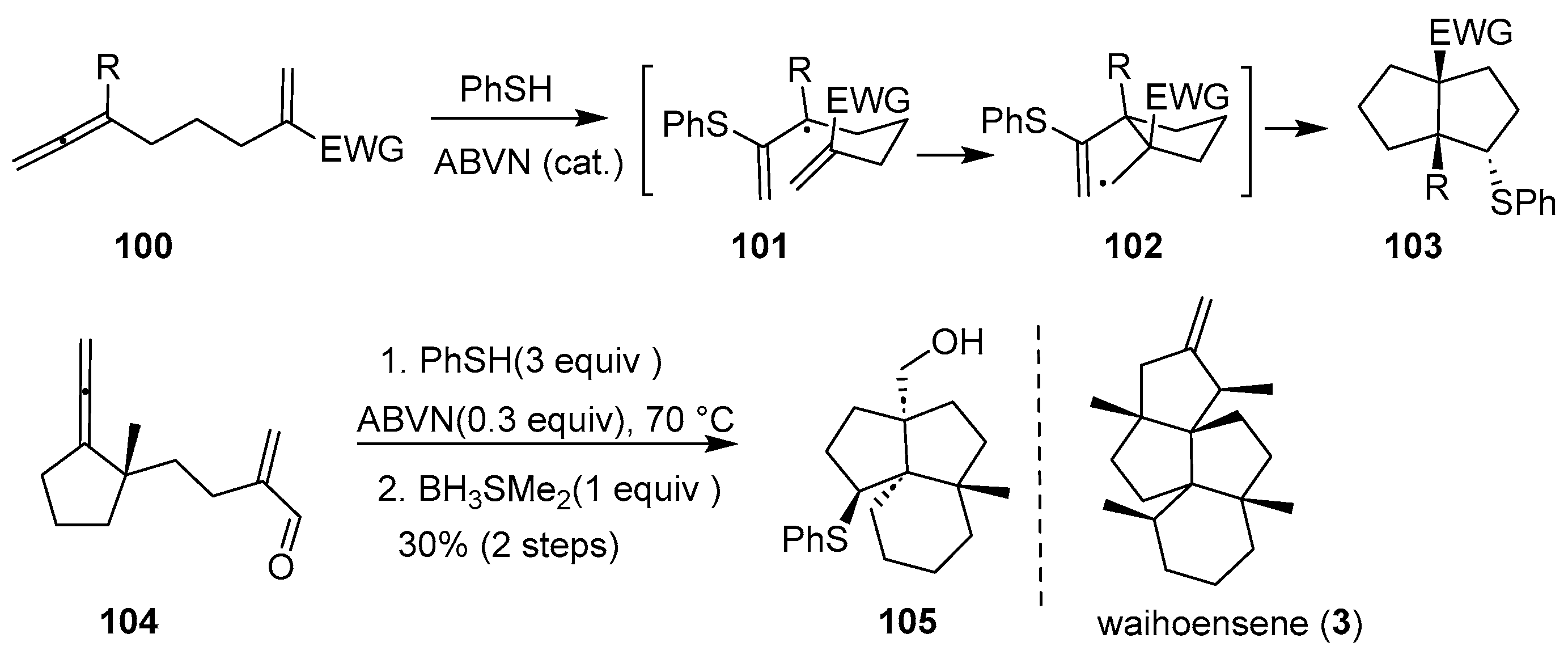
In the same year, Yang’s group developed a benzenethiol-mediated cascade reaction involving intramolecular [3 + 2] cycloaddition between an allene and an α,β-unsaturated carbonyl compound, leading to the diastereoselective construction of a [3.3.0] bicyclic system featuring two quaternary bridgehead carbon atoms (
Scheme 13) [
35]. Using substrate
100, the electrophilic benzenethiol radical was added regioselectively to the central sp-hybridized carbon of the allene, forming the stabilized tertiary radical
101. This intermediate underwent 5-exo-trig cyclization onto the α, β-unsaturated system to give adduct
102, which then participated in a 5-endo-trig cyclization, yielding a nucleophilic carbon-centered radical. Hydrogen abstraction from PhSH furnished product
103—bearing vicinal quaternary centers at the bridgeheads—and regenerated the benzenethiol radical. Inspired by this efficient strategy for assembling diquinanes with two adjacent bridgehead quaternary stereocenters, the group extended the methodology to the synthesis of angularly fused triquinanes containing similar structural motifs. Accordingly, substrate
104 was successfully transformed into the corresponding triquinane
105, with a 30% yield.
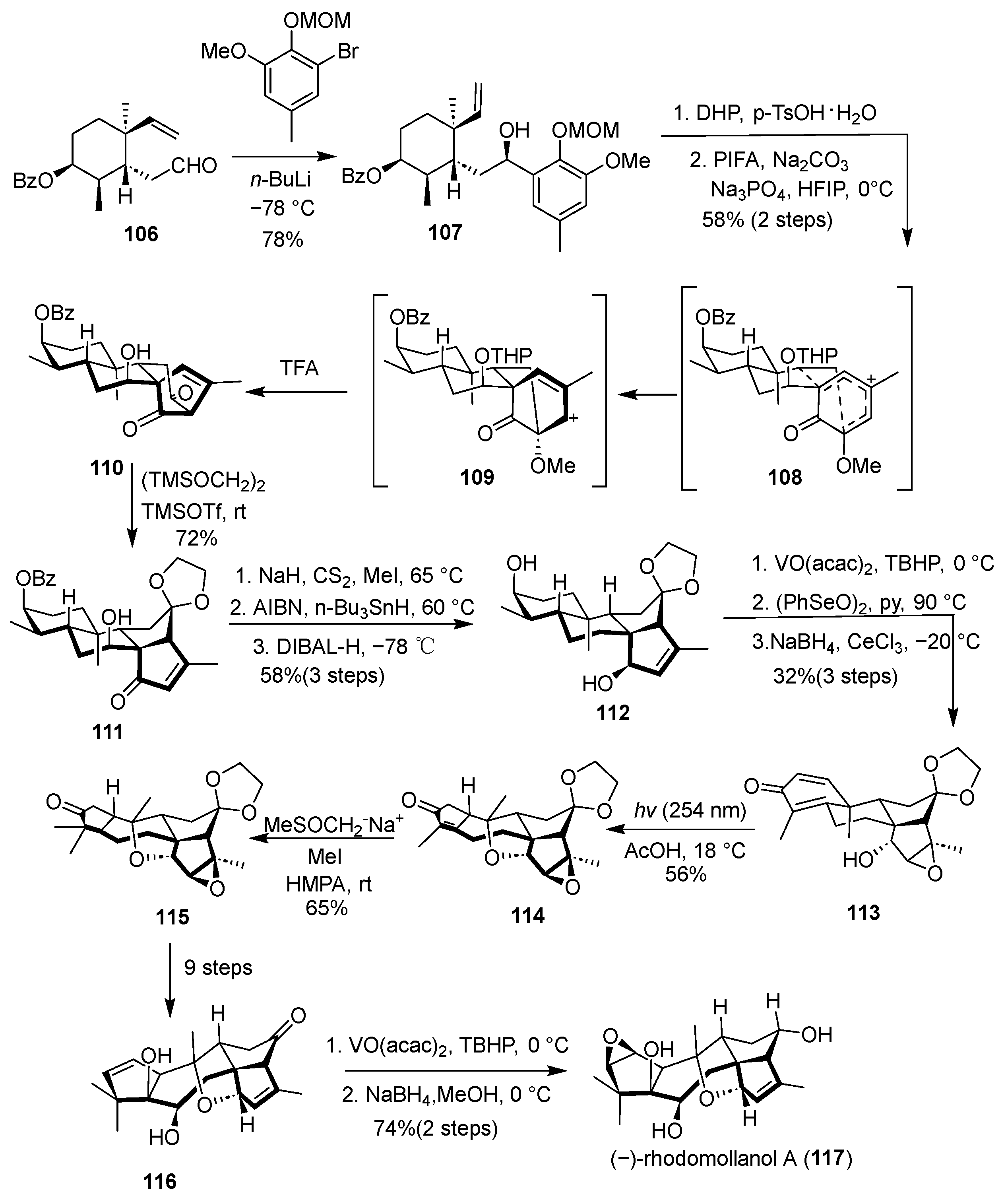
(–)-Rhodomollanol A, a natural product featuring a highly complex [3.5.7.5.5] hexacyclic framework with a distinctive 7-oxabicyclo [4.2.1] nonane core and eleven contiguous stereocenters, was first isolated from the Rhododendron species by Yao’s group in 2017 [
36]. In 2020, Ding et al. accomplished the first asymmetric total synthesis of this structurally challenging molecule, as outlined in
Scheme 14 [
37]. Their synthesis commenced with compound
107, which, upon oxidation with PIFA, underwent a one-step ODI-[5 + 2] cyclization tandem rearrangement to directly assemble the tetracyclic dicarbonyl compound
108. This intermediate was then subjected to a tandem reverse Dieckmann cleavage/insertion–Dieckmann condensation sequence, effectively reorganizing the molecular skeleton to afford tricyclic ketone
110. Subsequent elaboration of
110 over seven steps yielded cyclohexanedione
113, which was further transformed via a light-promoted tandem Yamaguchi rearrangement/intramolecular etherification reaction to give ketone
114, thereby establishing the core structure of the natural product. Finally, ketone
115 was advanced through an additional eleven steps to complete the inaugural asymmetric total synthesis of (–)-rhodomollanol A (
117) from resveratrol-derived diterpenoid precursors.
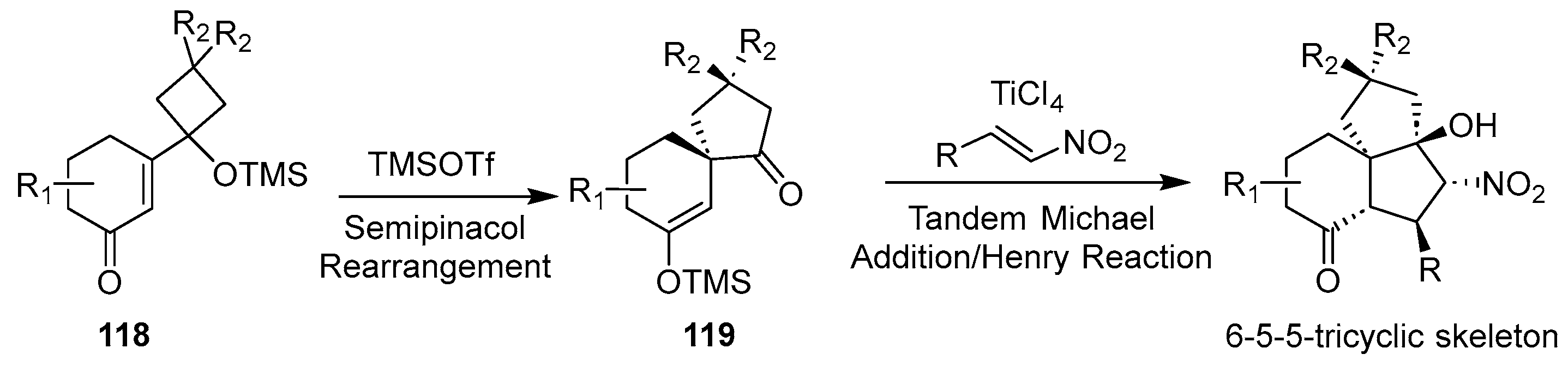
In 2020, Tu and co-workers disclosed a novel one-pot cascade reaction sequence involving semi-pinacol rearrangement, Michael addition, and Henry reaction [
38]. This transformation, catalyzed cooperatively by the dual Lewis acid system TMSOTf/TiCl
4, utilized vinylogous α-ketols and nitroolefins as substrates to efficiently construct synthetically challenging 6-5-5 and 7-5-5 fused tricyclic carbocyclic frameworks. The methodology confers high structural complexity, generating products featuring up to five contiguous stereogenic centers, including a quaternary carbon stereocenter, within a convergent sequence. As shown in
Scheme 15, the ketene compound
118 underwent a semi-pinacol rearrangement reaction under the action of Lewis acid TMSOTf to generate intermediate
119. A Michael addition of compound
119 with the nitropropene compound under titanium tetrachloride conditions, and a subsequent Henry reaction, closed the last ring. This process featured a broad scope of substrates, providing a novel and efficient method for the synthesis of natural products containing this skeleton.
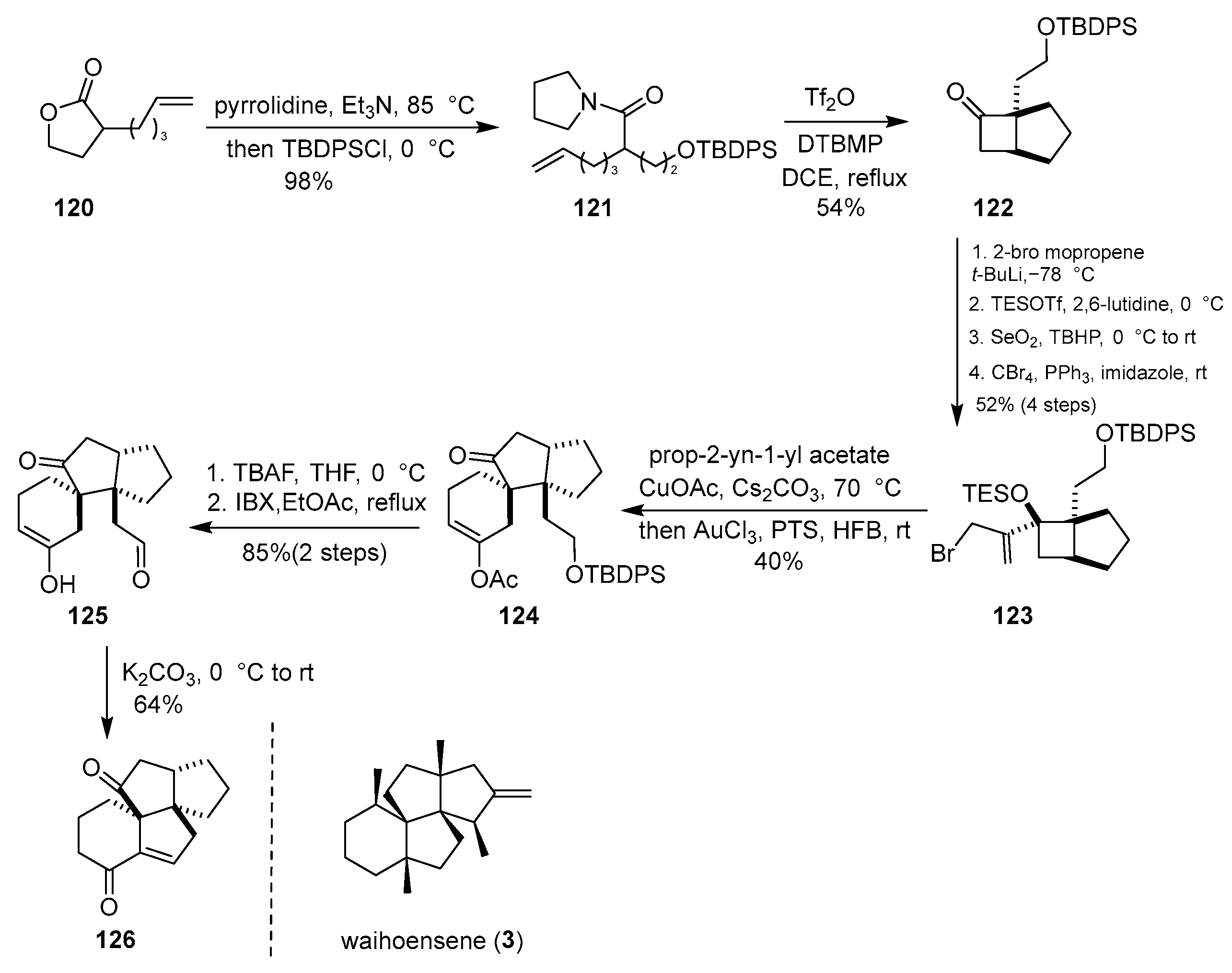
In the same year, Tu and colleagues reported a continuous cascade process comprising Castro–Stephens coupling, 1,3-acetate migration, cyclization, and hemipinacol rearrangement for the construction of functionally rich spirocyclic [4.5] decanes from readily accessible starting materials [
39]. This strategy was applied to the skeletal synthesis of the diterpenoid waihoensene. As illustrated in
Scheme 16, commencing from compound
120, allylic bromide
123 was synthesized over six steps, including a [2 + 2] cycloaddition and an aldol addition. Treatment of
123 with cuprous acetate and cesium carbonate afforded the tricyclic intermediate
124, with a 40% yield, efficiently assembling the core framework. Late-stage manipulation, including an intramolecular aldol condensation, then delivered the tetracyclic core
126 of waihoensene (
3).
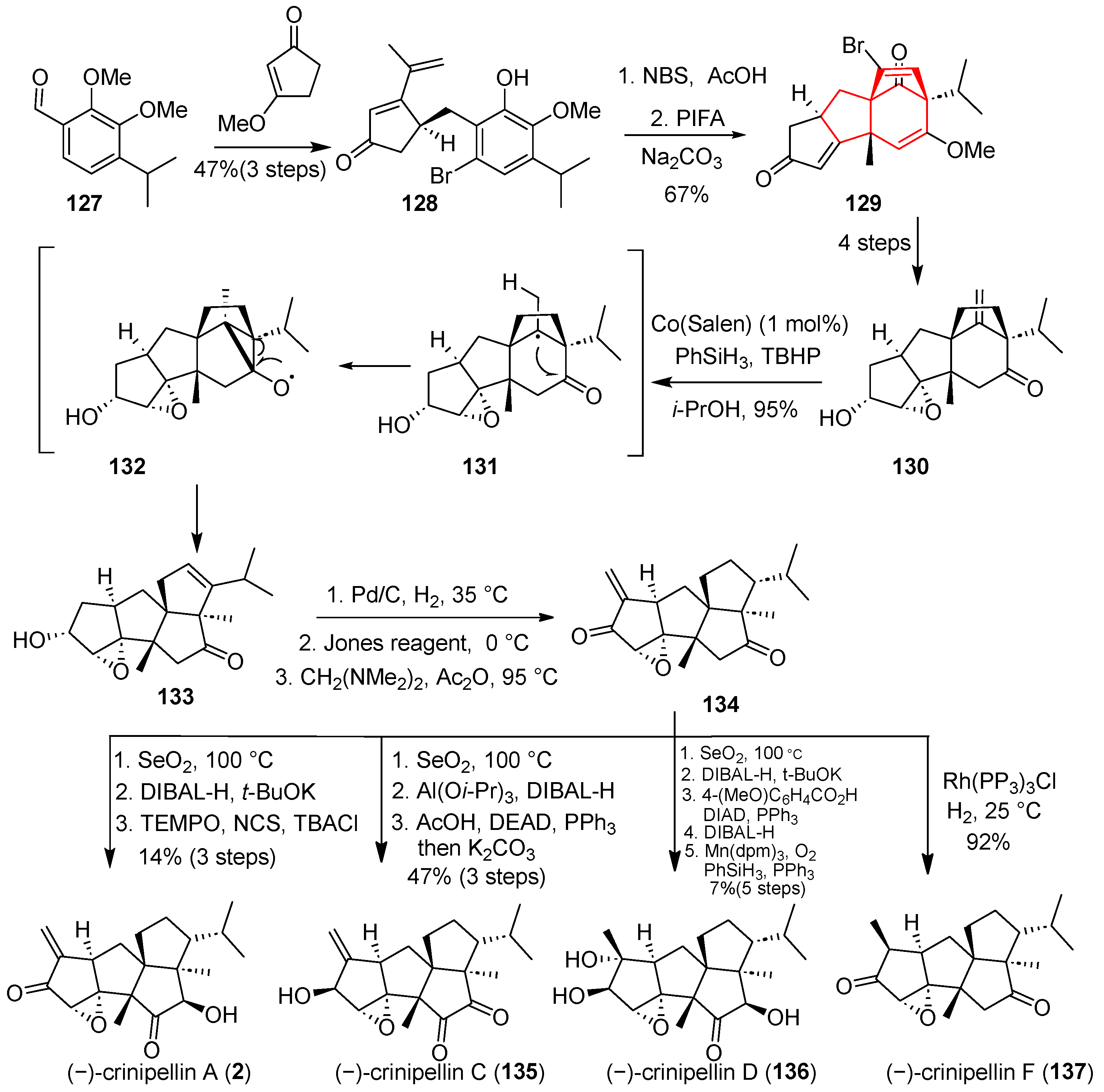
In 2022, Ding and co-workers disclosed a novel HAT-initiated Dowd–Beckwith rearrangement, enabling efficient access to diversely functionalized polyquinane scaffolds [
40] (
Scheme 17). Their synthetic approach featured an iridium-catalyzed regio- and enantioselective hydrogenation, followed by a diastereoselective ODI-[5 + 2] cycloaddition/pinacol rearrangement cascade. Starting from multisubstituted benzaldehyde
127, condensation, asymmetric hydrogenation, and bromination afforded the key brominated intermediate
128. Subsequent oxidation with hypervalent iodine reagent (PIFA) triggered an ODI-[5 + 2] cyclization and rearrangement sequence, smoothly delivering the tetracyclic framework
129. Four additional steps converted
129 into olefin
130, which then underwent an intramolecular Dowd–Beckwith rearrangement mediated by Co(Salen) and PhSiH
3 to yield
133. This critical rearrangement transformed the original [3.2.1] bridged system into a 5-5 fused ring skeleton. Notably, leveraging intermediate
134, the team accomplished concise and collective asymmetric total syntheses of eight members of the Crinipellin family, highlighting the efficiency and versatility of this strategy.
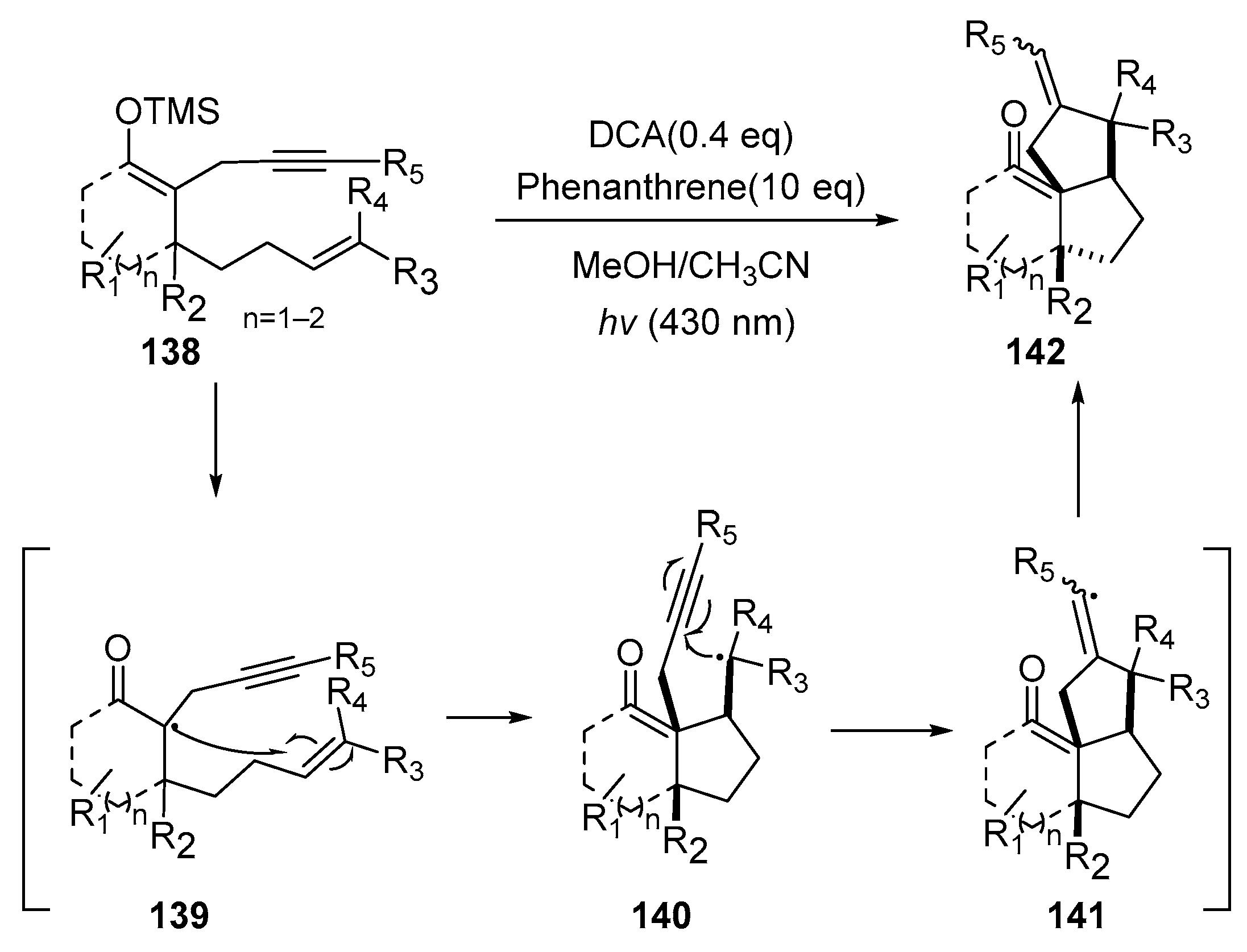
In 2023, Liu’s research group reported a photo-promoted enol silyl ether radical tandem cyclization reaction, which efficiently constructed a tricyclic framework compound [
41]. As shown in
Scheme 18, they used enol silyl ether
138 as the substrate and, under light conditions, first generated radical species
139 at the carbonyl ortho position. This radical underwent a 5-exo-trig cyclization, generating tertiary carbon radical
140, which cyclized alkynes in the 5-exo-dig form, thus constructing skeleton compound
141 containing tricyclic rings. This method exhibited significant synthetic versatility, delivering products with exceptional stereoselectivity across diverse substrates.
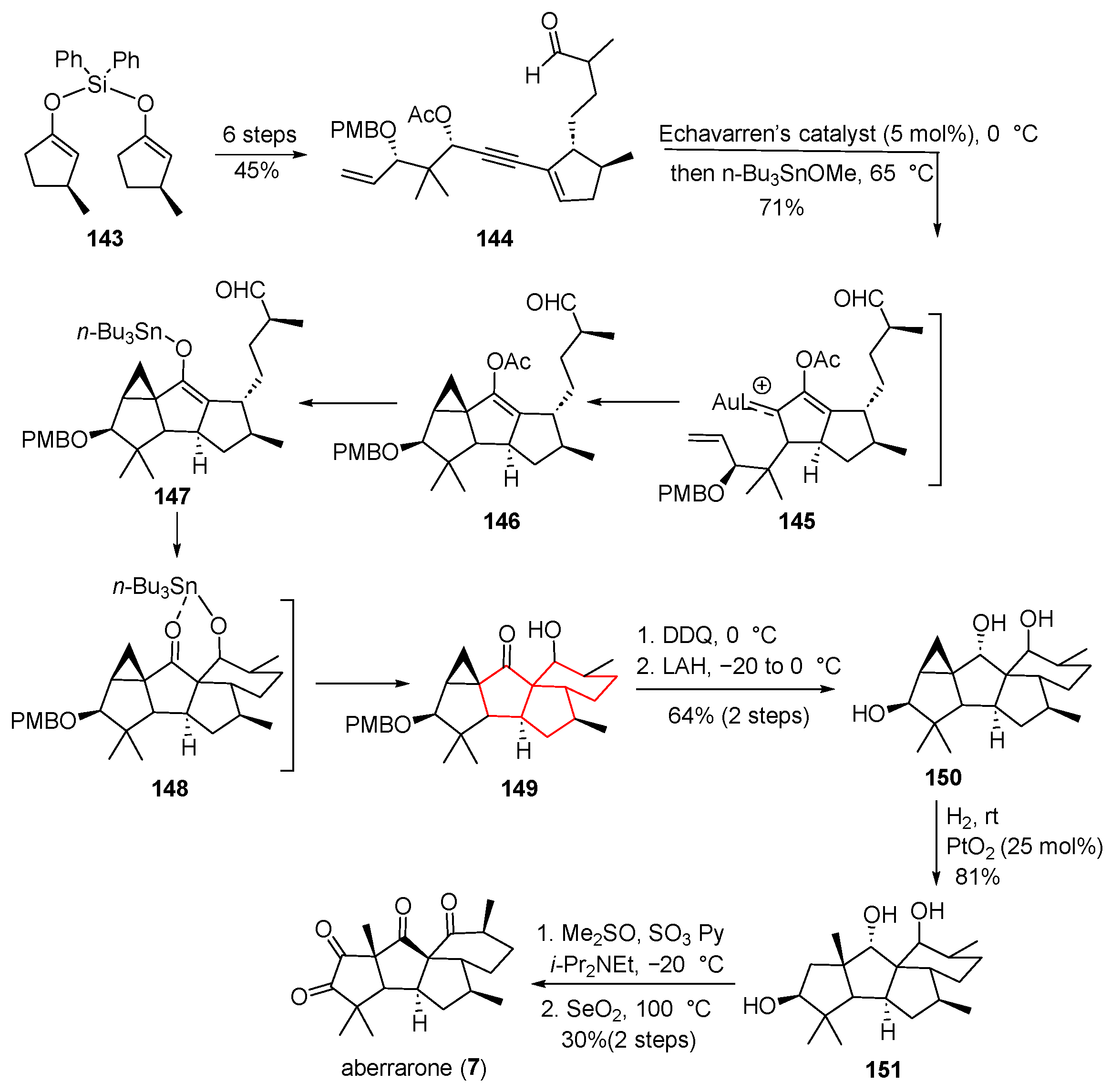
In 2009, Rodríguez and colleagues isolated (+)-aberrarone from the Caribbean willow coral Pseudopterogorgia elisabethae; biological evaluation revealed potent in vitro anti-malarial activity [
12]. Structurally, (+)-aberrarone possesses a distinctive 6-5-5-5 fused ring system incorporating four carbonyl groups and seven stereocenters, three of which are all-carbon quaternary centers. The first asymmetric total synthesis of this complex natural product was achieved by Carreira’s group in 2022 [
42], pivoting on a Au/Sn-catalyzed Meyer–Schuster–Nazarov/cyclopropanation/aldol addition tandem sequence. As delineated in
Scheme 19, the synthesis began with chiral enol silyl ether
143, which was elaborated over six steps—including a MeLi-promoted aldol addition and Barton–McCombie deoxygenation—to furnish alkyne
144. Exposure of
144 to Echavarren’s catalyst induced Meyer–Schuster rearrangement, generating allenol
146. Subsequent treatment with
n-Bu
3SnOMe at 65 °C promoted cyclopropanation and aldol addition, delivering the pivotal pentacyclic intermediate
149 as a single diastereomer in 71% yield. Oxidative removal of the PMB group in
149, followed by reduction with LAH, afforded triol
150. Hydrogenation over PtO
2 yielded
151, which was then oxidized under Parikh–Doering conditions to the corresponding triketone. Finally, Riley oxidation using SeO
2 completed the asymmetric total synthesis of (+)-aberrarone (
7).
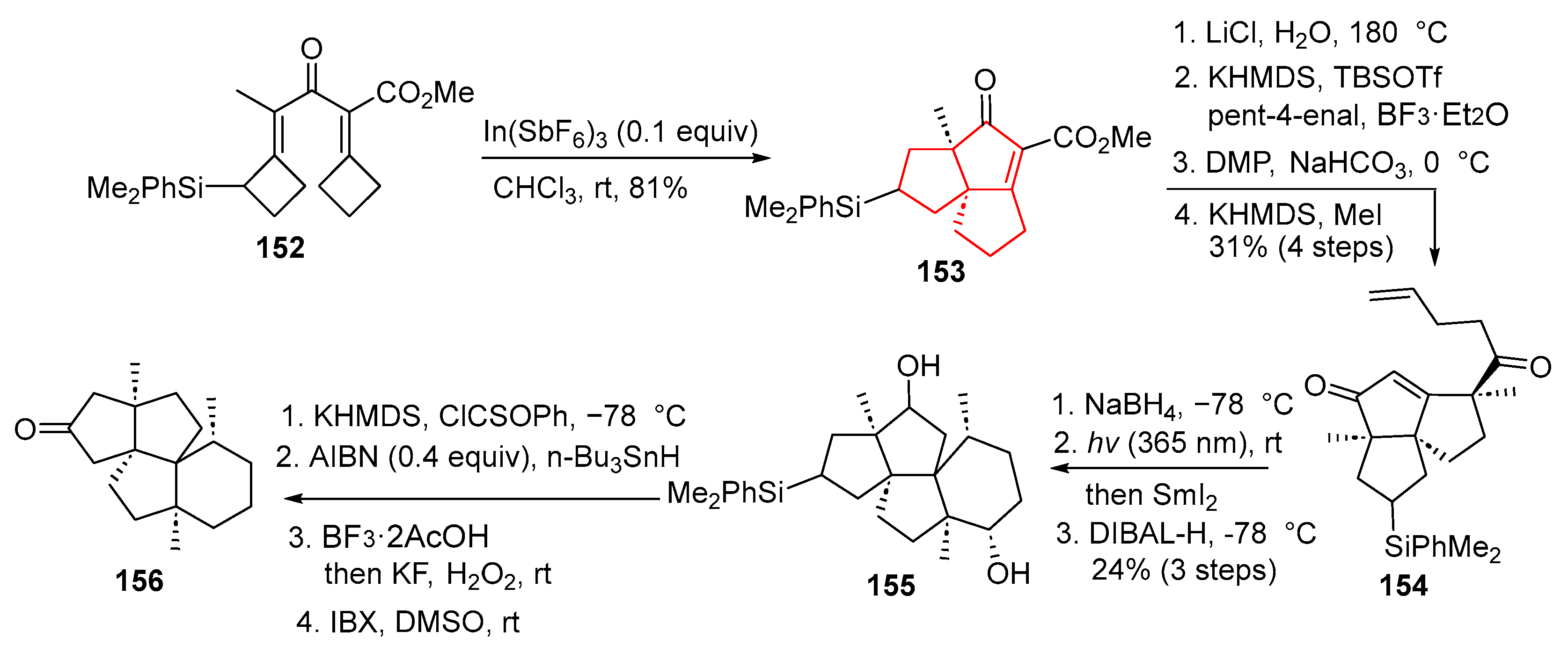
In 2022, Tu and colleagues established a modular and efficient route to angular triquinane-type frameworks containing quaternary carbon centers [
43]. Their approach employed 1,3-dicyclobutylidene ketone (
152) as a precursor, engaging it in an innovative cascade process consisting of a Nazarov cyclization and two successive ring expansions. As illustrated in
Scheme 20, under In(SbF
6)
3 catalysis, compound
152 underwent cyclization to deliver the key tricyclic intermediate
153. Subsequent decarboxylation, followed by a critical vinylogous aldol addition, Dess–Martin oxidation, and regioselective methylation, afforded enone
154. This intermediate was then selectively reduced to generate a substrate suitable for a photoinduced intramolecular [2 + 2] cycloaddition. The resulting cycloadduct was subjected to SmI
2-mediated ring-opening, yielding diol
155. Finally, Barton–McCombie deoxygenation, Tamao–Fleming oxidation, and IBX oxidation completed compound
156, which was finally transformed into (±)-waihoensene (
3) following known procedures [
25]. This synthesis highlights the power of a tandem cyclization–expansion cascade to rapidly build complex angular triquinane systems under mild conditions.
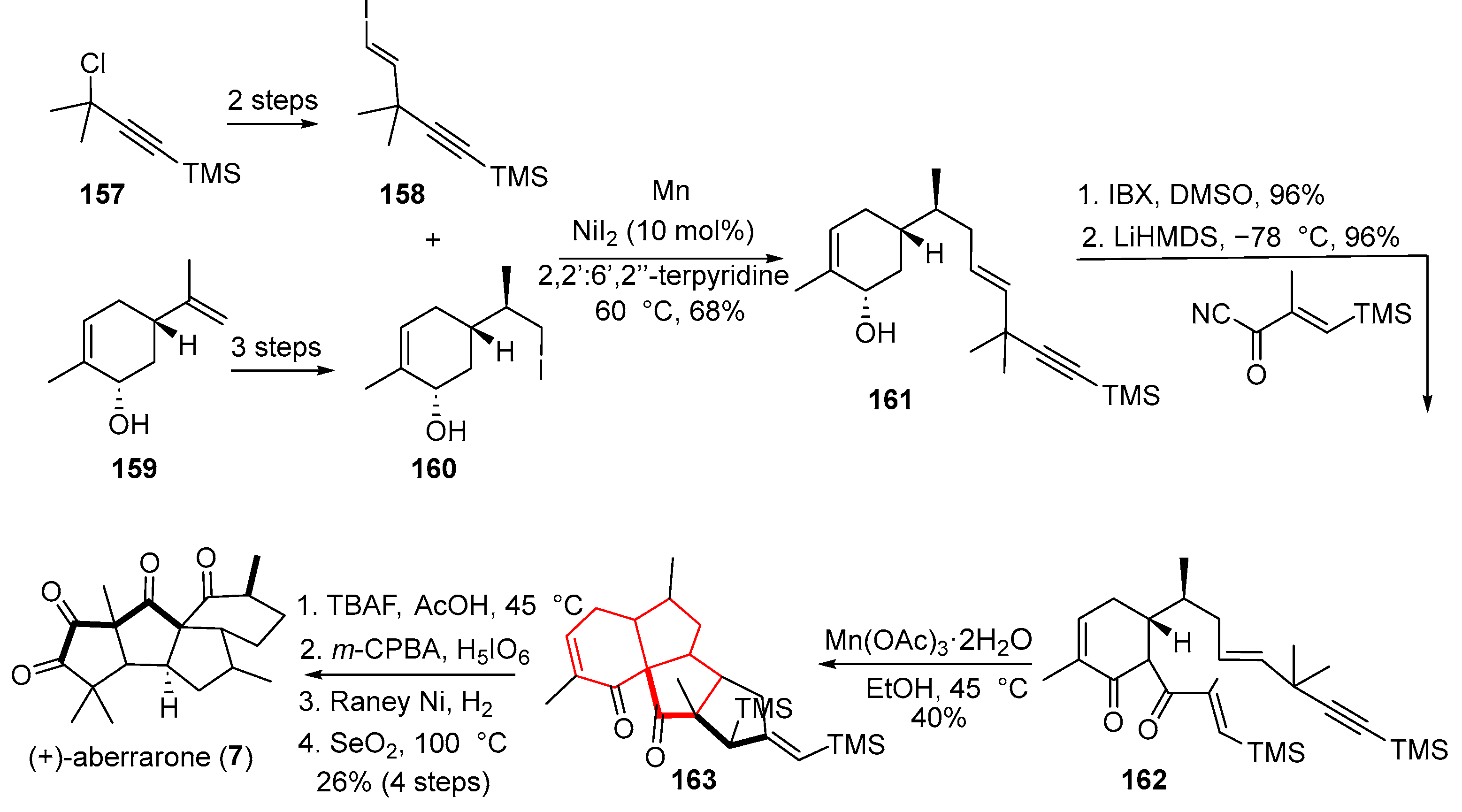
In 2023, Jia and colleagues accomplished a protecting-group-free total synthesis of the natural product (+)-aberrarone [
44] (
Scheme 21). A pivotal radical cascade cyclization enabled the simultaneous formation of three carbon–carbon bonds in a single operation, constructing three rings while precisely installing four new stereocenters. The synthesis commenced from commercially available (S,S)-carvacrol (
159), which was converted to alkyl iodide
160 via a three-step sequence. A nickel iodide-catalyzed coupling between
160 and
158 then afforded compound
161. Subsequent oxidation and acylation yielded radical precursor
162. Treatment of this 1,3-dicarbonyl compound with manganese (III) acetate triggered an intramolecular 5-exo/5-exo/5-exo radical cascade, delivering the polycyclic product
163 in 40% yield. Further functionalization of
163 completed the total synthesis of (+)-aberrarone (
7).
In 2024, Tu’s group developed a cascade Nazarov cyclization/dicycloexpansion reaction for the precise synthesis of the angularly fused tricyclic skeletons and executed a succinct total synthesis of madreporanone for the first time [
45]. As shown in
Scheme 22, the synthesis featured a pivotal, optimized cascade Nazarov cyclization/dicycloexpansion reaction of dienone
165 (prepared via Horner–Wadsworth–Emmons reactions from
164) to construct the angular 5/5/7 tricyclic core (
166) bearing vicinal quaternary stereocenters using B(C
6F
5)
3 catalysis. Ozonolysis of compound
166, followed by protection, led to acetal
167. Selective reduction with DIBAL-H and epoxidation with m-CPBA afforded epoxide
168 diastereoselectively. Ley–Griffith oxidation and deprotection yielded a diketone, which underwent hydrogenation to remove an isopropyl group. A second Ley–Griffith oxidation and Saegusa–Ito oxidation then provided enone
169. Bromination and Stille coupling installed a methyl group, yielding
170. Subsequent 1,4-boronation/oxidation gave β-hydroxy ketone, which was converted to alcohol
171 via CeCl
3-mediated MeMgBr addition. Wharton transposition and hydrogenation afforded triol, and final oxidation yielded madreporanone (
6).
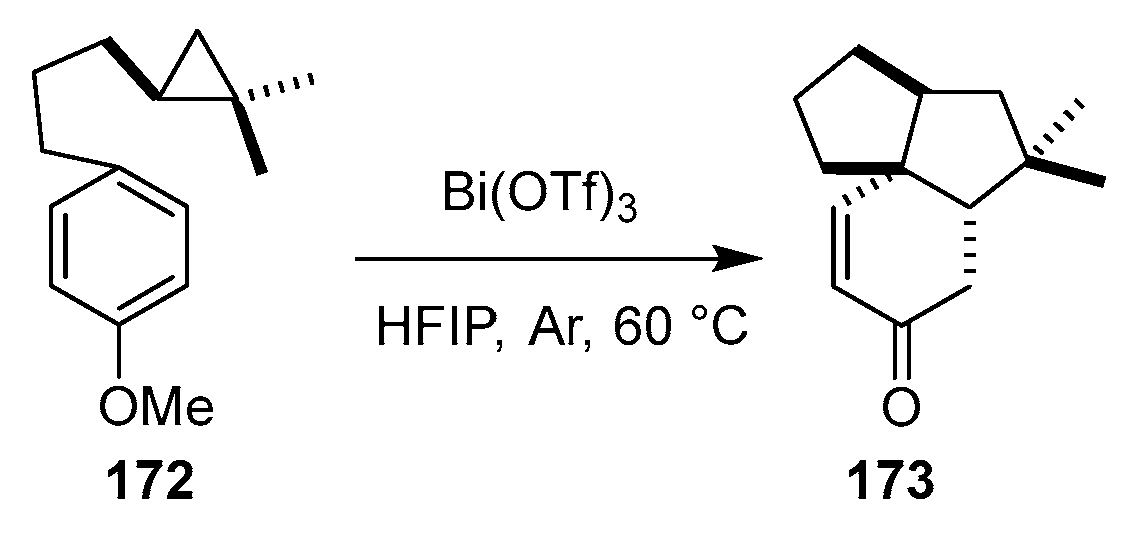
In 2024, Wang’s group reported a Lewis acid-catalyzed dearomative (3 + 2) intramolecular parallel cycloaddition (IMPC) strategy to construct angular 5-5-6 tricyclic cores relevant to natural products like norascyronone A, dankasterone A, and emervaridone A [
46]. As shown in
Scheme 23, the pivotal step employed donor–acceptor cyclopropanes (DACPs) tethered to benzene rings (e.g., 172). Under optimized conditions [Bi(OTf)
3 (20 mol%), HFIP, 60 °C, 2–12 h], compound
172 underwent ipso-Friedel–Crafts-initiated ring-opening followed by Michael addition, efficiently forging the angular tricyclic skeleton (e.g., 173) with an embedded cyclohexenone.
Radical- or cation-initiated cascades achieve unparalleled step economy by assembling multiple rings/quaternary centers in a single operation. The “zipper effect” minimizes intermediate isolation, thereby improving overall yields. Photoredox variants enable chemoselective C–C bond formation under mild conditions. Cascade efficiency hinges on precise radical/ion stability gradients, often requiring tailored substrates. Diastereocontrol is compromised when multiple stereocenters form concurrently. Strict exclusion of protic impurities and stoichiometric radical mediators (Bu3SnH) impede industrial translation.
5. Emerging Frontiers: Photochemical and Ring-Expansion Tactics
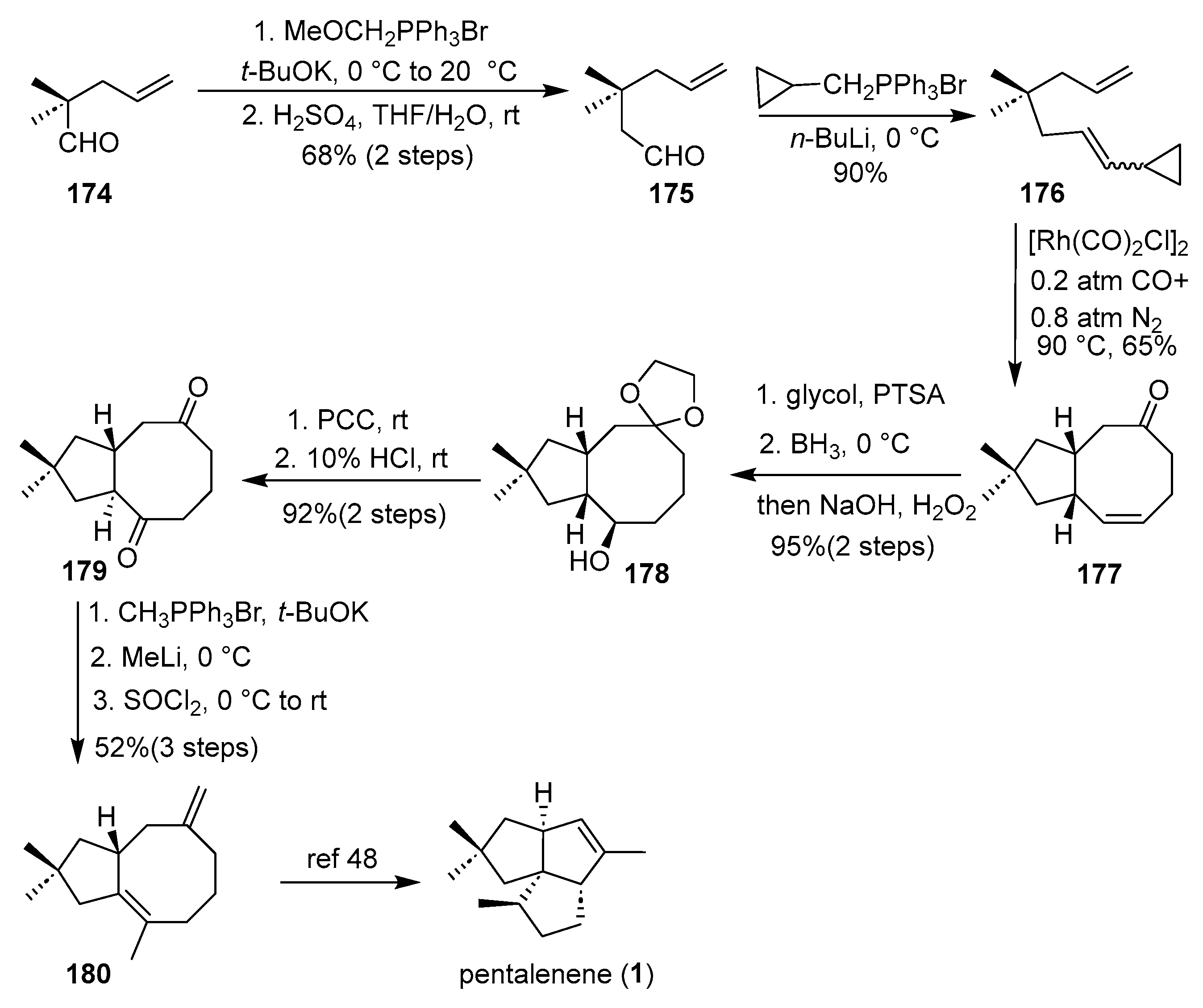
In 2009, Yu’s group achieved a formal synthesis of the angular triquinane natural product (±)-pentalenene using a Rh(I)-catalyzed [(5 + 2) + 1] cycloaddition as the pivotal step [
47]. As shown in
Scheme 24, the synthesis commenced with ene-vinylcyclopropane substrate
176, which underwent cyclization under [Rh(CO)
2Cl]
2 catalysis in dioxane under a mixed CO/N
2 atmosphere to furnish the bicyclic cyclooctenone
177. This key intermediate was subsequently transformed through a sequence involving ketalization, hydroboration–oxidation (BH
3·THF, then H
2O
2/NaOH), oxidation with PCC, and acid-mediated isomerization (10% HCl) to yield the advanced trans-diketone
179. Further elaboration was carried out via Wittig olefination, methylation with MeLi, and dehydration using SOCl
2/pyridine afforded diene
180—a known precursor to pentalenene (
1) [
48]. This strategy highlighted the power of transition metal-catalyzed higher-order cycloadditions to rapidly build complex polycyclic cores, though the route was somewhat hampered by moderate regioselectivity in hydroboration and a modest overall yield.
In 2011, Srikrishna and colleagues developed an enantiosecific route to angular triquinane natural products starting from the allylic alcohol
157, readily available from (R)-limonene [
49] (
Scheme 25). The synthesis proceeded via Johnson orthoester Claisen rearrangement of
181 with triethyl orthoacetate to install the first quaternary center, yielding diazoketone
182. Subsequent copper-catalyzed intramolecular cyclopropanation furnished the tricyclic ketone
183, which underwent regioselective cyclopropane cleavage, ketalization, and ozonolysis, leading to the α-diazo-β-keto ester
184. Treatment with Rh
2(OAc)
4 in refluxing CH
2Cl
2 effected highly regioselective intramolecular insertion into the tertiary methyl group, directly constructing the angular triquinane framework in
185. Further steps including Krapcho decarbalkoxylation and double Wittig olefination yielded the advanced diene
186, common to norsilphiperfolane and norcameroonane. This strategy showcased a powerful, stereocontrolled assembly of congested triquinanes via transition metal-catalyzed C–H functionalization, though the multi-step sequence and need for stereospecific starting materials may limit broad applicability.
In 2011, Tu’s group accomplished the first total synthesis of the complex alkaloid (±)-sieboldine A, featuring an angular triquinane-type core, via a biomimetic route from alopecuridine [
50]. As shown in
Scheme 26, the synthesis commenced with the coupling of iodoalkene
187 and carbamate
188 to form advanced intermediate. This was followed by stereoselective epoxidation with m-CPBA to furnish epoxy alcohol
189. The pivotal semipinacol rearrangement of
189, mediated by BF
3·Et
2O in diethyl ether at −30 to −15 °C, constructed the aza-cyclononane ring and established the all-carbon quaternary center, yielding key intermediates
190. Subsequent MOM protection, ozonolysis, and a SmI
2-promoted pinacol coupling forged the oxa-quaternary center and closed the B ring, delivering the tricyclic core
193. Further functional group manipulations, including deprotection, oxidation (Ley oxidation), and Boc cleavage, afforded alopecuridine·TFA (
194), which underwent biomimetic oxidation with m-CPBA and HgO to introduce the N-hydroxy group and form the tetrahydrofuran ring, completing the synthesis of sieboldine A (
195). This route demonstrated the innovative use of semipinacol and pinacol couplings to efficiently assemble highly congested polycyclic architectures with contiguous quaternary centers.
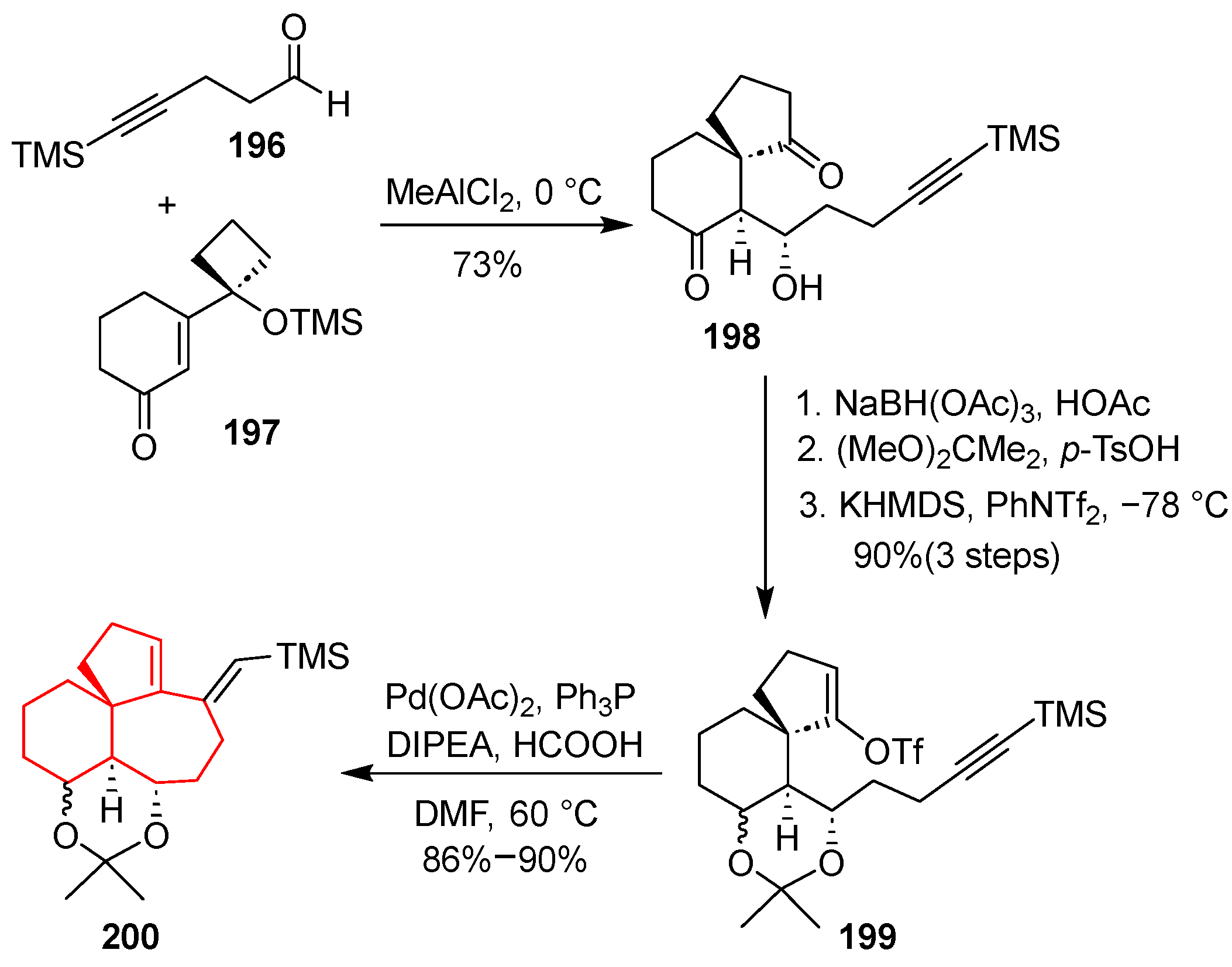
In 2012, Tu’s group developed a concise synthetic route to the [
5,
6,
7] all-carbon tricyclic core characteristic of Calyciphylline A-type alkaloids, utilizing a tandem semipinacol-type 1,2-carbon migration/aldol reaction as the key transformation [
51]. The synthesis commenced with the Lewis acid-mediated coupling of aldehyde
196 and trimethylsilane-protected vinylogous α-ketol
197 using MeAlCl
2 in CH
2Cl
2 at 0 °C, which afforded 6-substituted spiro [4.5]decane-1,7-dione
198. This one-pot tandem process efficiently established the spirocyclic quaternary center. The resulting diketone
198 was then subjected to selective reduction with NaBH(OAc)
3 in acetic acid and protection with 2,2-dimethoxypropane, followed by enol triflation with PhNTf
2, resulting in enol triflate
199. Palladium-catalyzed reductive cyclization using Pd(OAc)
2 successfully constructed the angular [
5,
6,
7] tricyclic framework in
200 with high efficiency. This strategy demonstrated a powerful and rapid approach to access highly congested polycyclic architectures via a novel tandem rearrangement–aldol sequence, enabling efficient installation of the spiro center and subsequent ring fusion (
Scheme 27).
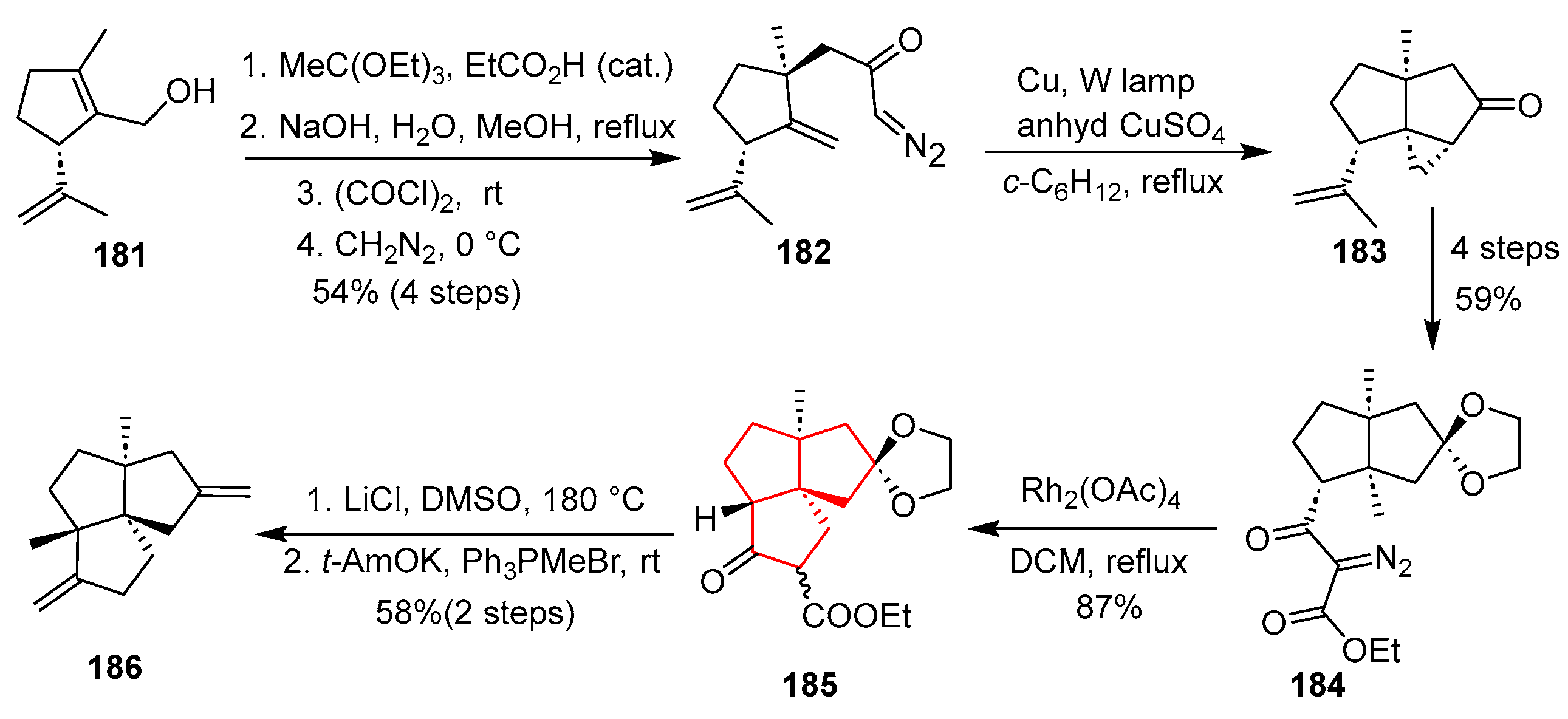
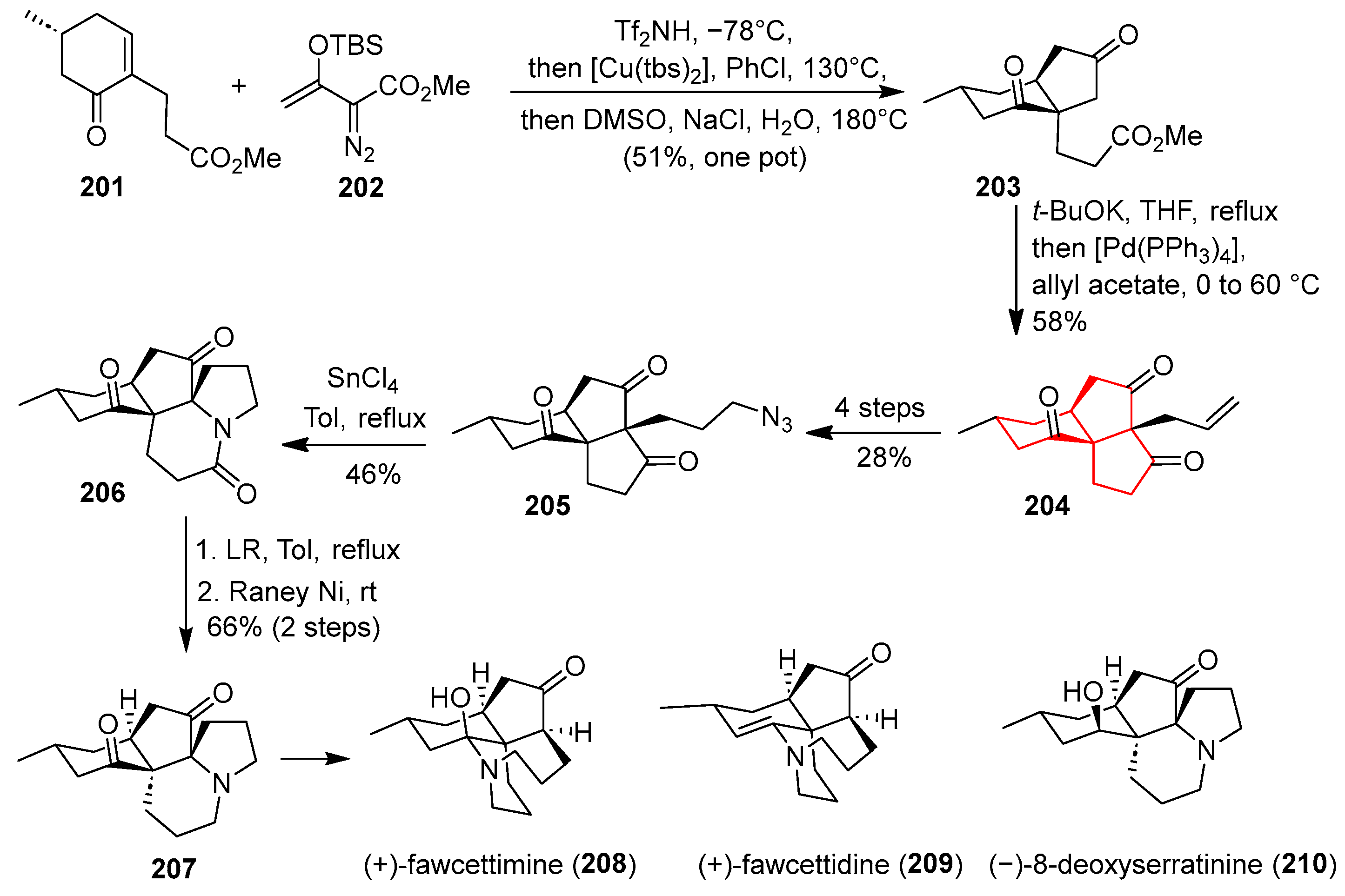
In 2013, Tu’s group accomplished an efficient and divergent synthesis of the fawcettimine-class alkaloids, including the angular triquinane-containing (+)-fawcettimine, starting from chiral enone
201 and vinyl diazoacetate
202 [
52]. As shown in
Scheme 28, the synthesis commenced with a Mukaiyama–Michael addition and an intramolecular carbene addition/cyclization mediated by [Cu(tbs)
2] in chlorobenzene at 130 °C, followed by decarboxylation, which furnished the cis-bicyclic dione
203 in a one-pot process. Subsequent Dieckmann condensation with
t-BuOK and Tsuji–Trost allylation with allyl acetate constructed the 6/5/5 tricyclic system in
204. After protection of the ketone, hydroboration–oxidation and Mitsunobu azidation provided azidotrione
205. The regioselective intramolecular Schmidt reaction of
205 using SnCl
4 in refluxing toluene effected N-insertion to form lactam
206, which was converted via thionation and reduction into the common intermediate
207. Finally, three members of fawcettimine (
208,
209,
210) were synthesized. This route exemplifies a highly convergent and modular strategy for constructing congested angular triquinane frameworks, leveraging powerful metal-catalyzed cyclizations and rearrangement reactions to achieve brevity (ten steps total) and functional group compatibility.
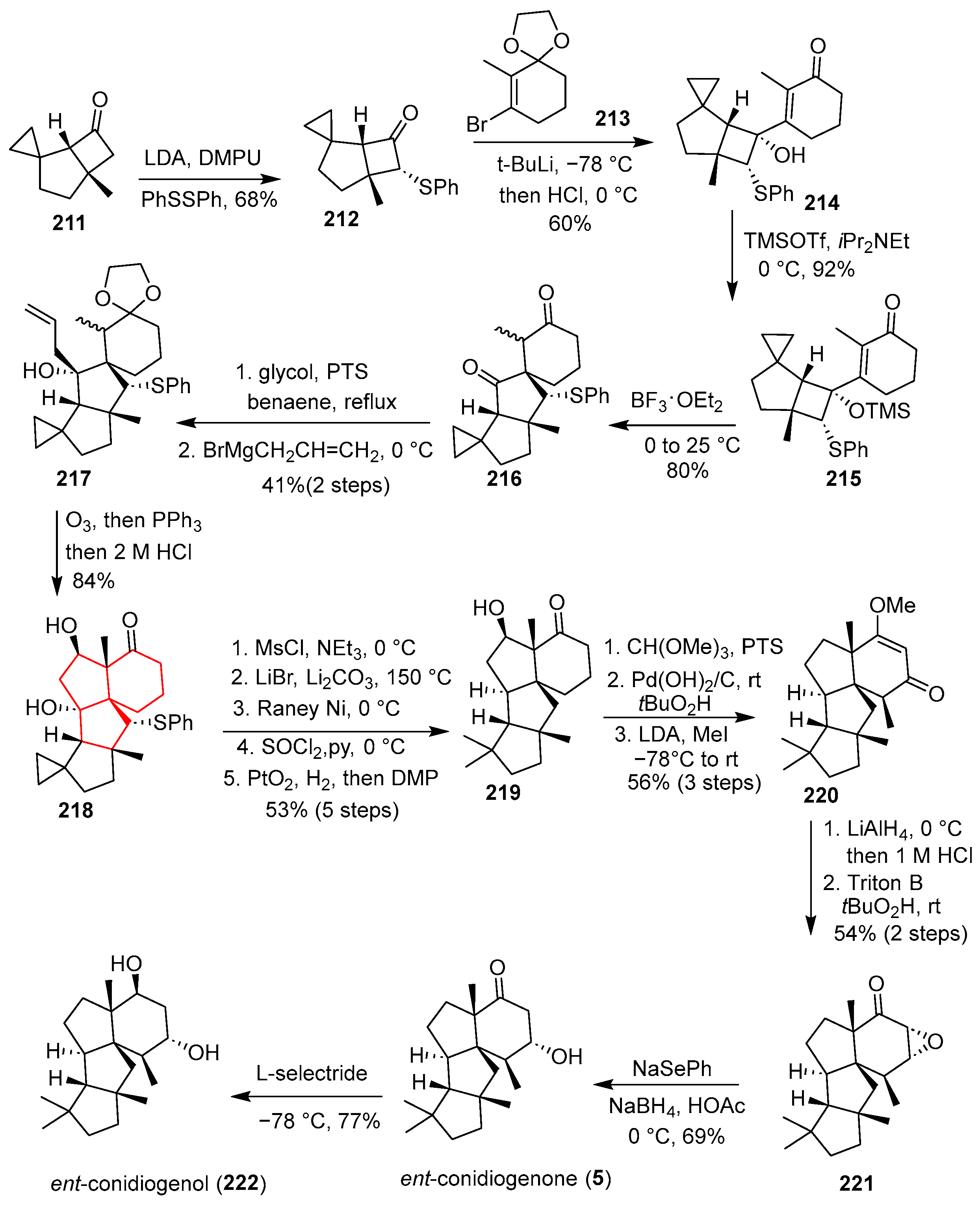
In 2016, Tu’s group reported the first total synthesis of the cyclopiane-type tetracyclic diterpenes conidiogenone and conidiogenol [
53] (
Scheme 29). Their synthetic approach commenced with the coupling of vinyl bromide
213 and the phenylthio-substituted cyclobutanone
212 via lithium–halogen exchange followed by nucleophilic addition, yielding the key tertiary alcohol intermediate
214. This was subsequently protected to form the rearrangement precursor
215. The cornerstone of their strategy was a Lewis acid-mediated semipinacol-type cycloenlargement, specifically using BF
3·OEt
2 in CH
2Cl
2, which effectively constructed the spirocyclic A/C ring system with the desired regioselectivity and diastereoselectivity, affording compound
216. This critical step established the angular triquinane core bearing the requisite quaternary centers. Compound
216 was subjected to a ketal protection and chiral resolution sequence, followed by a diastereoselective allylation with allylmagnesium bromide to afford alcohol
217 as a single diastereomer. Ozonolysis of the alkene in
217 generated an aldehyde, which underwent spontaneous intramolecular aldol cyclization under acidic conditions to construct the pentacyclic framework in
218 with high stereocontrol. Further functional group manipulations, including elimination, hydrogenation, and oxidation, provided ketone
219. Compound
220 was then transformed into epoxide
221, and subsequent reductive ring-opening with NaSePh afforded conidiogenone (
5). Finally, reduction of
5 with L-selectride produced conidiogenol (
222). The synthesis featured a highly efficient and stereocontrolled assembly of the congested tetracyclic framework, though the necessity for chiral resolution and multiple steps to introduce and remove the phenylthio auxiliary slightly diminished the overall atom economy.
In 2017, Yang’s group developed a formal total synthesis of (±)-lycojaponicumin C, constructing its angular triquinane core through a highly strategic sequence [
54] (
Scheme 30). Starting from commercially available ethyl acetoacetate
223, the key enyne precursor
192 was prepared via Waser alkynylation and Luche reduction on a decagram scale. The pivotal step involved a Rh(I)-catalyzed formal [3 + 2] cycloaddition of
224 under a CO atmosphere, efficiently forging the bicyclic [3.3.0] scaffold
225 with two adjacent all-carbon quaternary centers in excellent diastereoselectivity. Aldehyde
225 was then elaborated through a double-Wittig reaction, reduction, and mesylation-azidation to give keto-azide
226. A Staudinger/reductive amination cascade on
226 constructed the piperidine ring, yielding intermediate
227. Subsequent functional group manipulations, including tosylation, an ingenious γ-OH-directed vinyl Michael addition, and Ley oxidation, installed the requisite stereochemistry and functionality to deliver aldehyde
229. Grignard addition with 2-methylallyl magnesium chloride then provided diene, which underwent ring-closing metathesis (RCM) and double bond migration to form the cyclohexanone ring, affording advanced intermediate
230 and completing the tetracyclic skeleton of the natural product. This synthesis is notable for its innovative use of transition metal catalysis to build sterically congested systems and directed reactions to control stereochemistry in highly hindered environments. However, the route involved multiple functional group interconversions and a late-stage RCM, which somewhat diminished its overall atom economy and step efficiency.
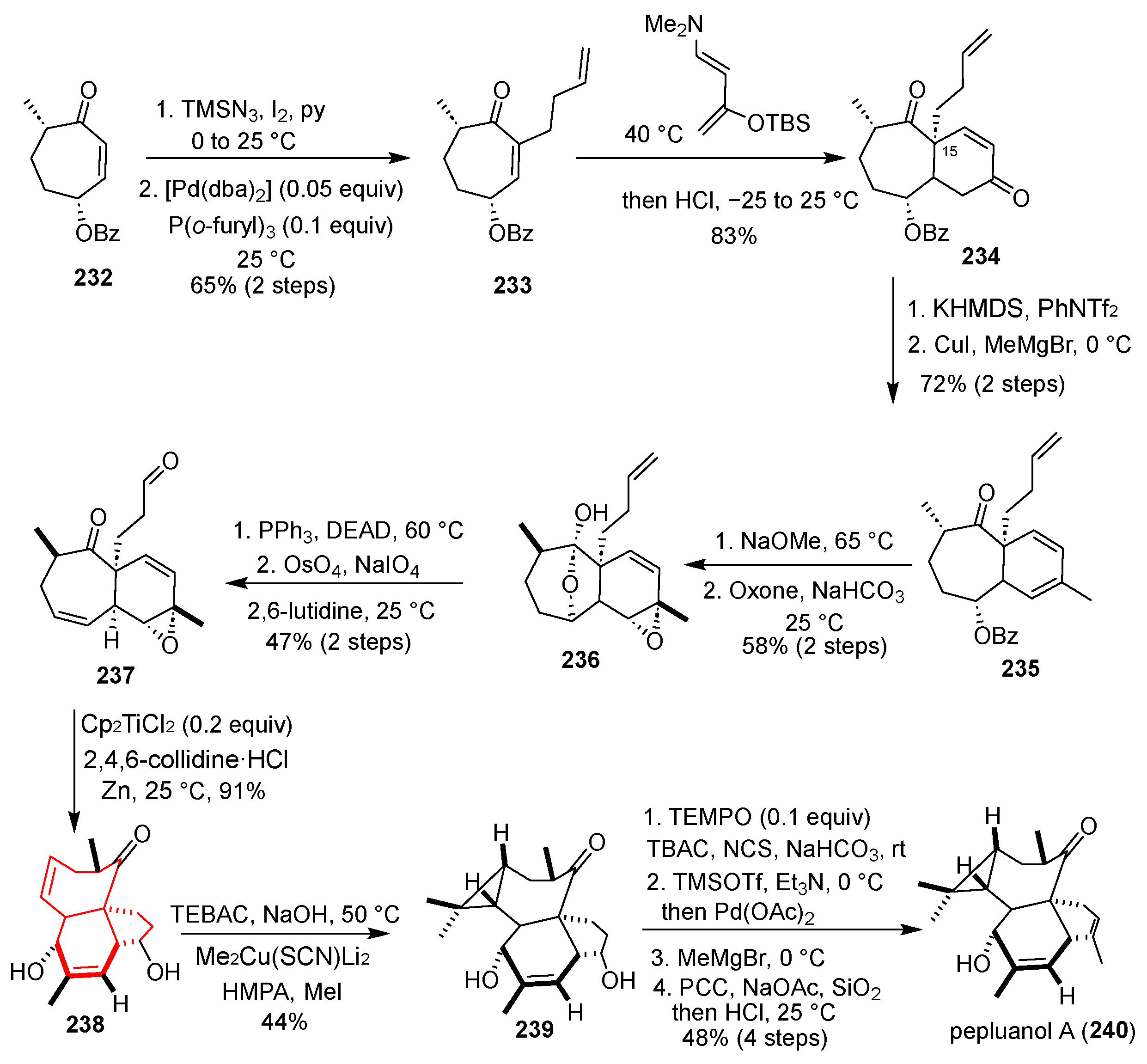
Pepluanol A (
240) was isolated from Euphorbia peplus, a plant in the
Euphorbia genus, in 2016 with a [
5,
6,
7,
3] four-ring skeleton with seven chiral centers, including six continuously distributed centers and one quaternary carbon center. The synthesis of this molecule presents significant challenges. In 2017, Ding’s group achieved the first total synthesis of the complex Euphorbia diterpenoid (±)-pepluanol A, utilizing an innovative titanium(III)-catalyzed reductive annulation as a pivotal strategy [
55] (
Scheme 31). Their synthesis commenced with readily available cycloheptenone
232, which was converted to enone
233 in two steps. A high-pressure Diels–Alder cycloaddition between
233 and the Rawal diene constructed the bicyclic framework
234, introducing the crucial C15 quaternary carbon center as a single diastereomer. Subsequent functionalization, including regioselective enol triflation, methyl cuprate addition, and base-induced epimerization, yielded vinyl epoxide
236. Dehydration and Lemieux–Johnson oxidation then afforded the key vinyl epoxide–aldehyde coupling partner
237. The core transformation was a titanium(III)-catalyzed reductive annulation of
237 (Cp
2TiCl
2/Zn, collidine·HCl, THF), which efficiently forged the [5,6,7]tricyclic 1,5-diol
238—the angular triquinane core precursor—in excellent yield, albeit with moderate diastereoselectivity. Late-stage synthesis involved a diastereoselective cyclopropanation via dibromocarbene addition to
238 and reductive bismethylation to assemble the tetracyclic skeleton
239. Finally, a TEMPO/NCS oxidation, Saegusa–Ito dehydrogenation, regioselective Grignard addition, and a Dauben–Michno oxidation completed the synthesis of pepluanol A (
240). This route is notable for its convergent design and the innovative, atom-economical reductive annulation to construct the challenging 1,5-diol moiety. However, the moderate diastereoselectivity in the key cyclization and the requirement for extensive functional group manipulations in the late stage present opportunities for further refinement.
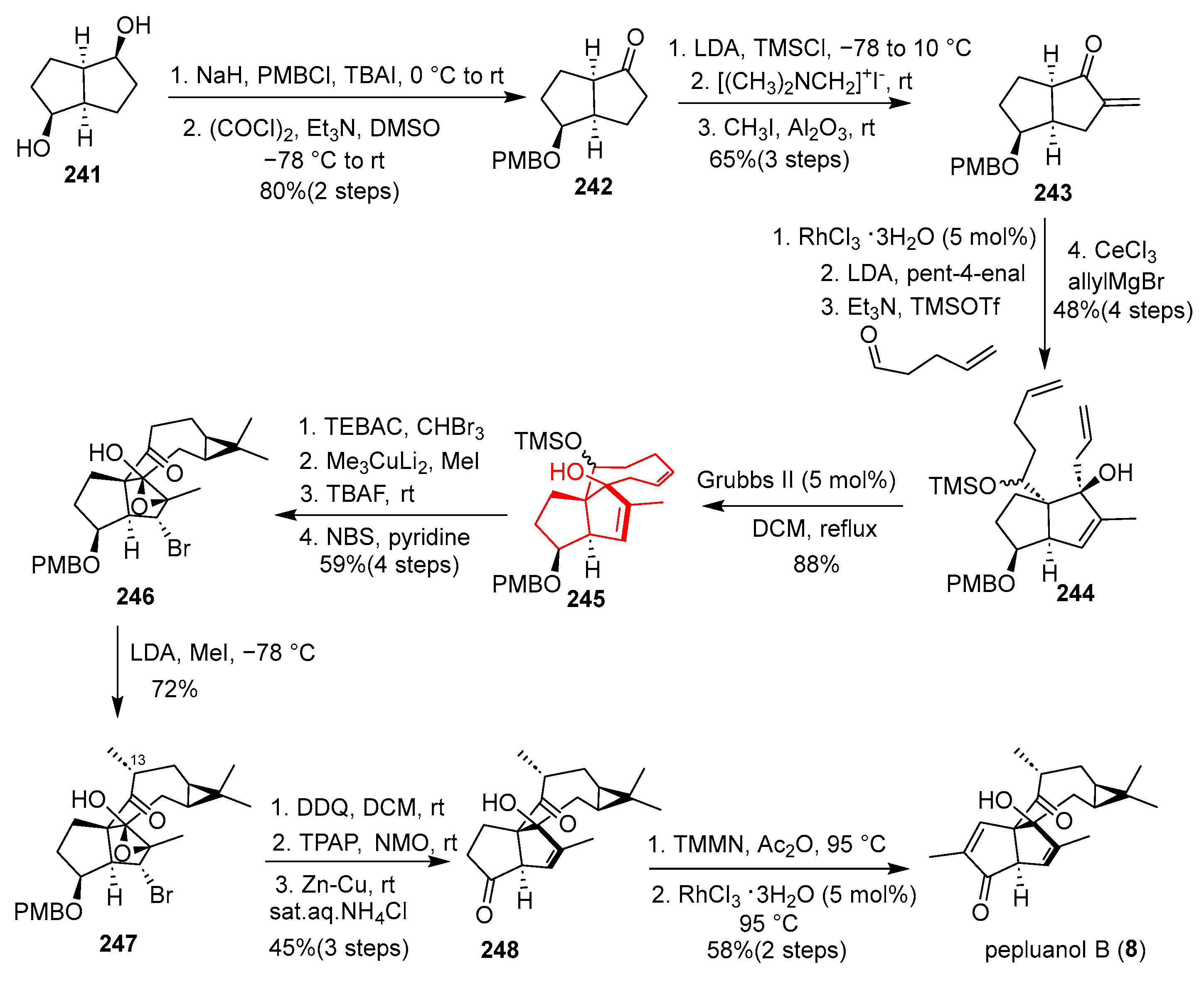
Pepluanol B (
8) is a structurally novel diterpene molecule isolated by Qiu’s group from Euphorbia Euphorbia in Southwest China [
14,
15]. Structurally, pepluanol B has a unique [
5,
5,
8,
3] four ring skeleton structure with six chiral centers (including a bridgehead all carbon quaternary carbon chiral center). In 2020, She’s group reported the first total synthesis of (–)-pepluanol B, which features a challenging [5-5-8-3] tetracyclic framework [
56] (
Scheme 32). The synthesis commenced with known bicyclic diol
241, which was elaborated through PMB protection and oxidation to afford ketone
242. Subsequent Eschenmoser methylenation provided exo-methylene intermediate
243. Rh-catalyzed isomerization, aldol reaction, TMS protection, and CeCl
3-mediated allylation obtained diene
244. The pivotal ring-closing metathesis (RCM) of
244 using Grubbs II catalyst forged the eight-membered ring, delivering cyclic Z-alkene
245 as a key intermediate. Dibromocyclopropanation of
245 and reductive bismethylation then constructed the gem-dimethyl cyclopropane unit, yielding advanced intermediate
246. Methylation with LDA proceeded smoothly, affording compound
247 with the established R configuration at the C13 stereocenter. Final Zn–Cu–mediated deprotection yielded allylic alcohol
248, and α-methylenation followed by Rh-catalyzed isomerization completed the synthesis of pepluanol B (
8). This route is notable for employing strategic C–C bond formations like aldol and RCM. However, the synthesis involves multiple functional group manipulations and sensitive intermediates, resulting in moderate overall yield, yet it provides efficient access to the complex angular triquinane framework and enables further biological studies.
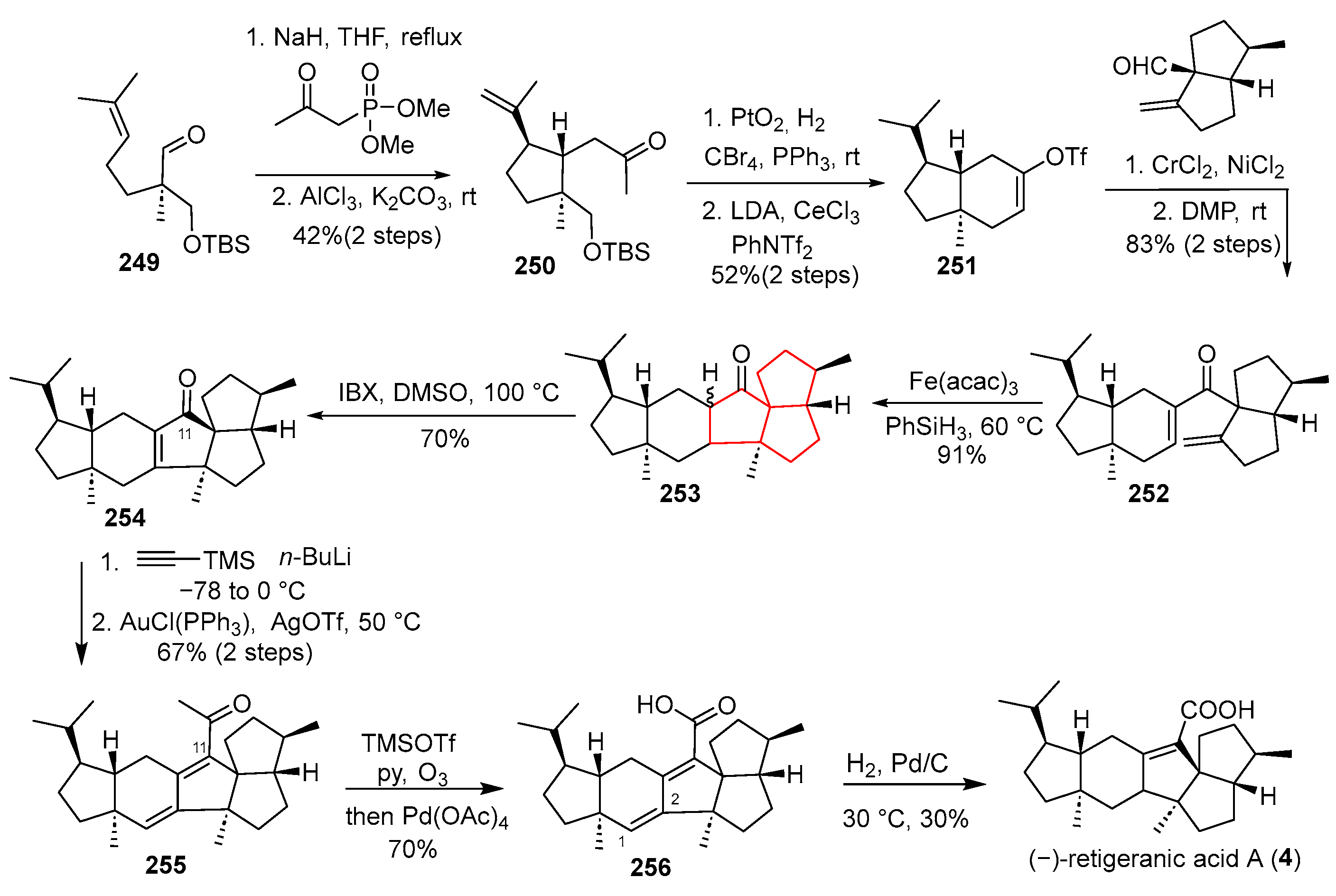
In 2023, Chen’s group reported an enantiocontrolled total synthesis of (–)-retigeranic acid A, which features a strategic construction of the angular triquinane core [
57] (
Scheme 33). Beginning from aldehyde
249, a Horner–Wadsworth–Emmons homologation and a diastereoselective Prins cyclization under AlCl
3 catalysis furnished the trans-hydrindane framework
250. Subsequent functional group manipulations, including hydrogenation and α-alkylation, converted
215 into vinyl triflate
251. A Nozaki–Hiyama–Kishi coupling between
251 and aldehyde yielded ketone
252. The pivotal angular triquinane formation was then attempted via an Fe-mediated hydrogen atom transfer (HAT)-initiated 5-endo-trig radical cyclization. The reaction yielded Paquette-type intermediate
253. Oxidative dehydrogenation of
253 with IBX provided enone
254. Introduction of the C11 carbon was achieved through addition of lithium (trimethylsilyl)acetylide, followed by hydrolysis to give methyl ketone
255. A multi-step sequence involving enolization, ozonolysis, and Pb(OAc)
4 oxidation then installed the C11 carboxylic acid, yielding diene acid
256. The synthesis culminated in a high-pressure hydrogenation over Pd/C that reduced the C1–C2 double bond and epimerized several centers, delivering a mixture from which (–)-retigeranic acid A (
4) was isolated. This route stands out for its innovative use of a Prins cyclization to assemble the trans-hydrindane system and a late-stage HAT-based radical cyclization to form the congested triquinane core.
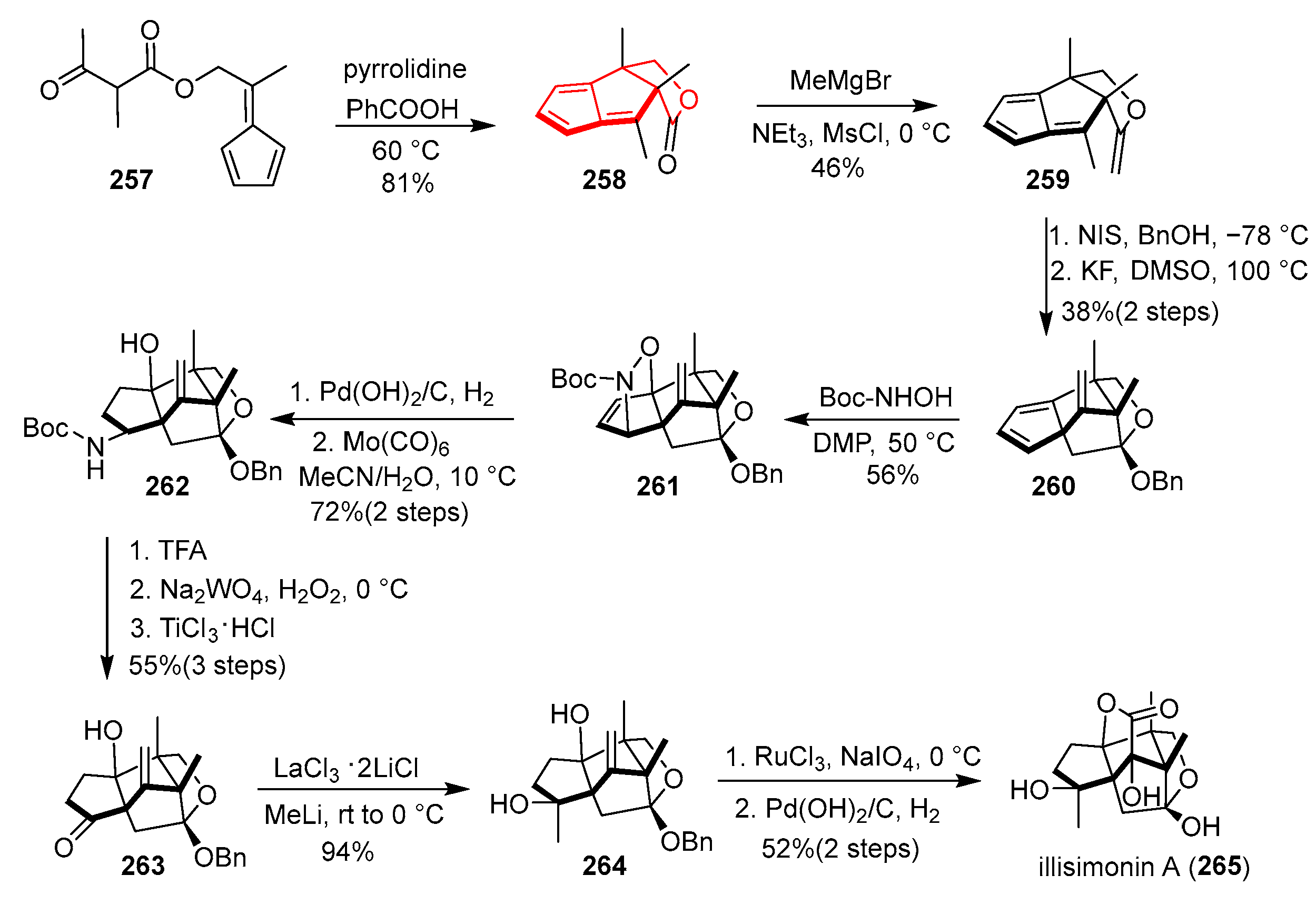
In 2025, Lu’s group reported a gram-scale total synthesis of the complex sesquiterpenoid (±)-illisimonin A, featuring a novel strategy centered on higher-order cycloadditions for constructing its challenging caged pentacyclic framework [
58]. As shown in
Scheme 34, beginning with fulvene ester
257, the key step involved an intramolecular [6 + 2] cycloaddition under pyrrolidine and benzoic acid catalysis at 60 °C, efficiently furnishing the stereochemically dense cis-fused 5/5/5 tricyclic lactone
258 with two adjacent quaternary centers in 81% yield. Subsequent conversion of
258 to enol ether
259 was achieved via methylation with MeMgBr followed by dehydration with MsCl/Et
3N. The pivotal angular triquinane precursor was then assembled through an iodoetherification of
259 followed by an intramolecular alkylation to deliver cyclopentadiene
260. A critical nitroso-Diels–Alder reaction with an in situ-generated acyl nitroso species afforded the stable nitroxide adduct
261 as the major thermodynamic product. Hydrogenation of
261 and Mo(CO)
6-mediated N–O bond cleavage provided carbamate
262. Deprotection of
262 with TFA, followed by sequential oxidation and TiCl
3·HCl reduction, yielded ketone
263. Diastereoselective methyl addition to
263 with MeLi/LaCl
3·2LiCl delivered diol
264 with excellent selectivity. Finally, lactonization via RuCl
3/NaIO
4-mediated oxidative cyclization constructed the α-hydroxy lactone motif, and global deprotection completed the synthesis of (±)-illisimonin A (
265). This route is notable for its innovative use of a pentafulvene-based [6 + 2] cycloaddition to rapidly build the polycyclic core and a nitroso-Diels–Alder reaction to overcome earlier peroxide instability issues, enabling a scalable and convergent synthesis in 14 steps.
Table 1 reveals nuanced trade-offs in waihoensene synthesis. Pauson–Khand approaches (Snyder, Yang, Gaich) excel in step efficiency (LLS 14–17) and yield (1.1–4.0%), with Gaich’s 14-step route leading at 4.0%. Their intramolecular Pauson–Khand reaction enables stereoselective tricyclic core construction (e.g., Snyder’s 50% yield) with inherent cis-fused control, leveraging atom economy. However, reliance on toxic Co
2(CO)
8 limits scalability, and substrate rigidity demands protective group manipulations. Tandem cyclization methods (Lee [
34], Tu [
43]) offer step economy—Lee’s one-pot tetracyclic assembly via [3 + 2] cycloaddition; Tu’s Nazarov/ring-expansion cascade—but suffer longer LLS (18) and lower yields (0.99–2.8%). Diastereocontrol challenges in concurrent stereocenter formation and regioselectivity issues in cascades hinder their performance, making Pauson–Khand strategies more robust for this target.