Copper-Mediated Homocoupling of N-propargylcytisine—Synthesis and Spectral Characterization of Novel Cytisine-Based Diyne Dimer
Abstract
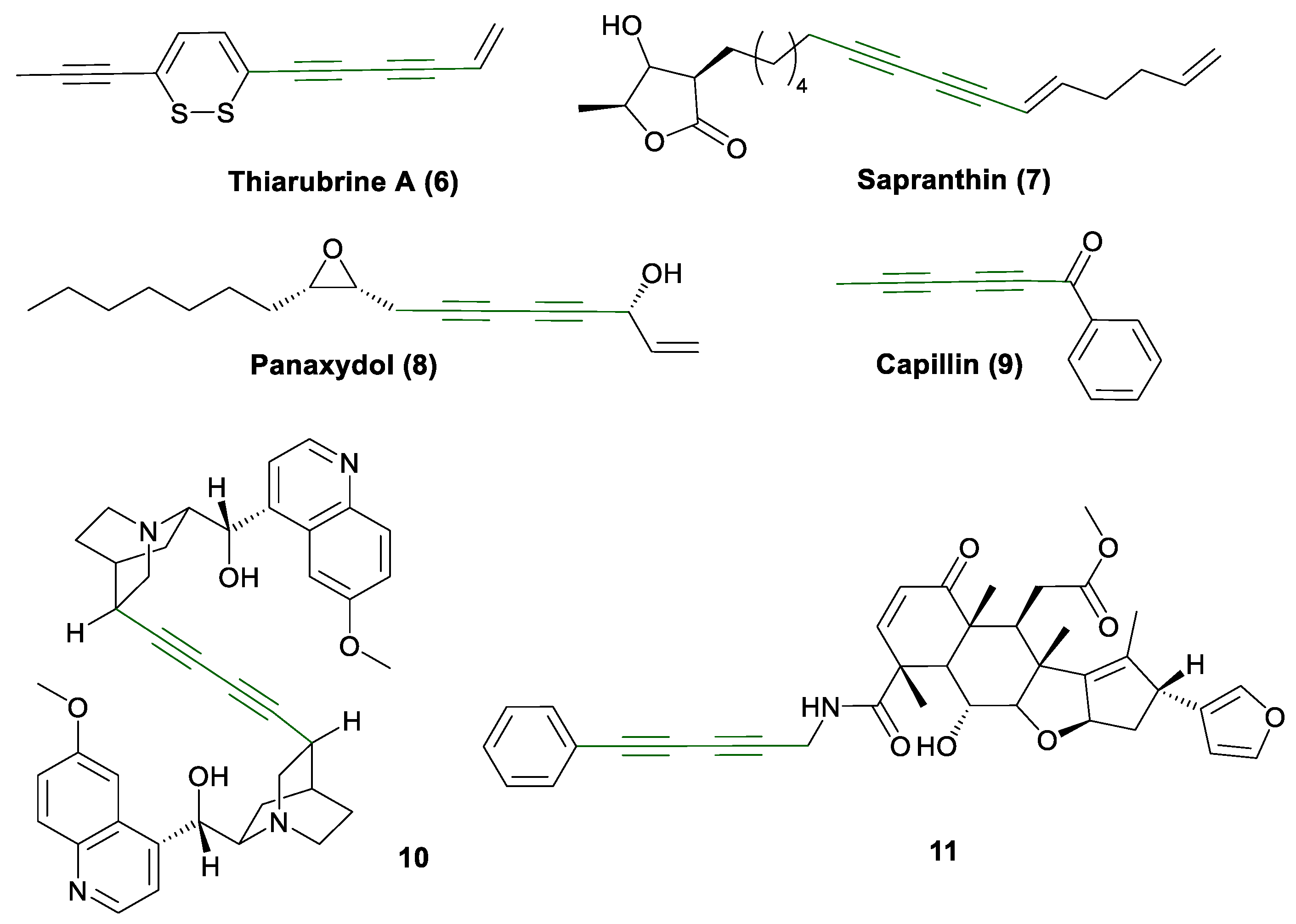
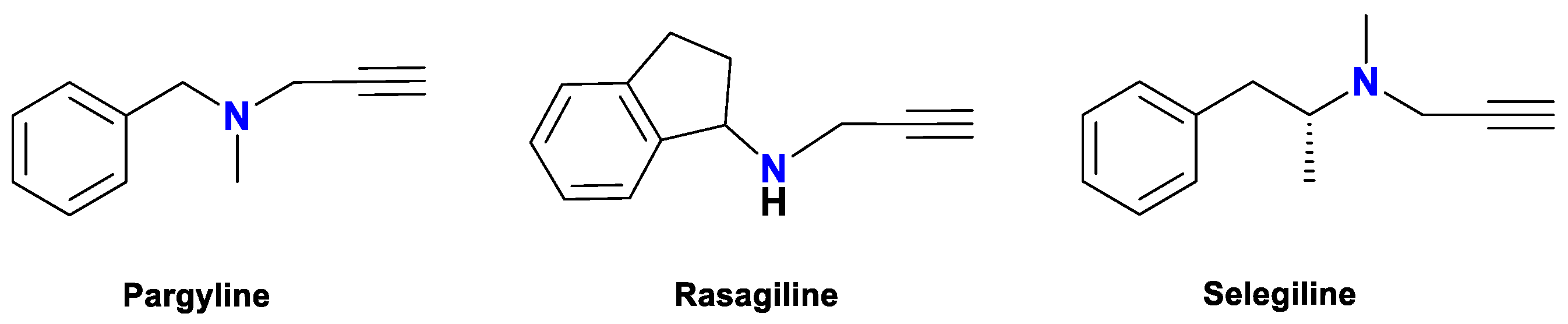
1. Introduction
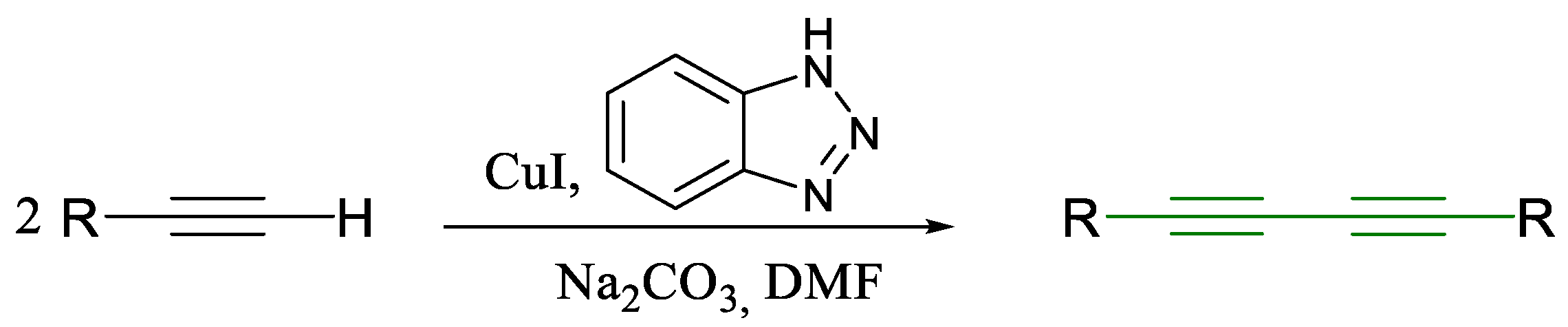
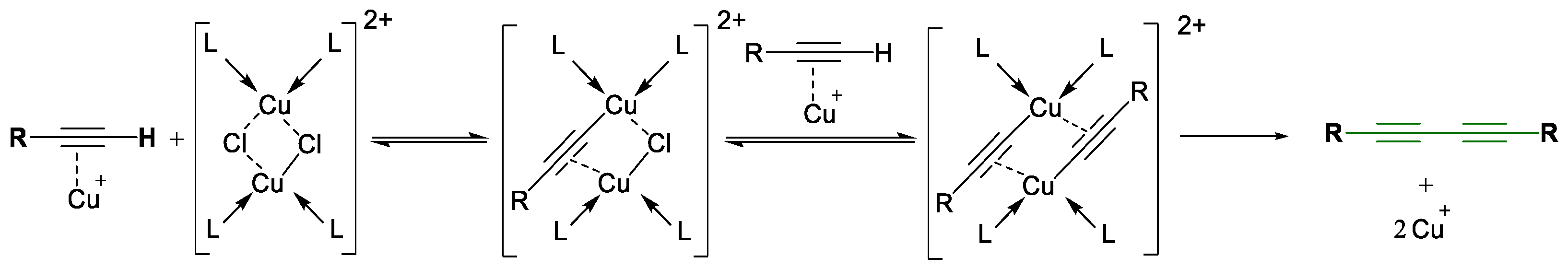
1.1. Glaser Coupling Reaction and Its Developments
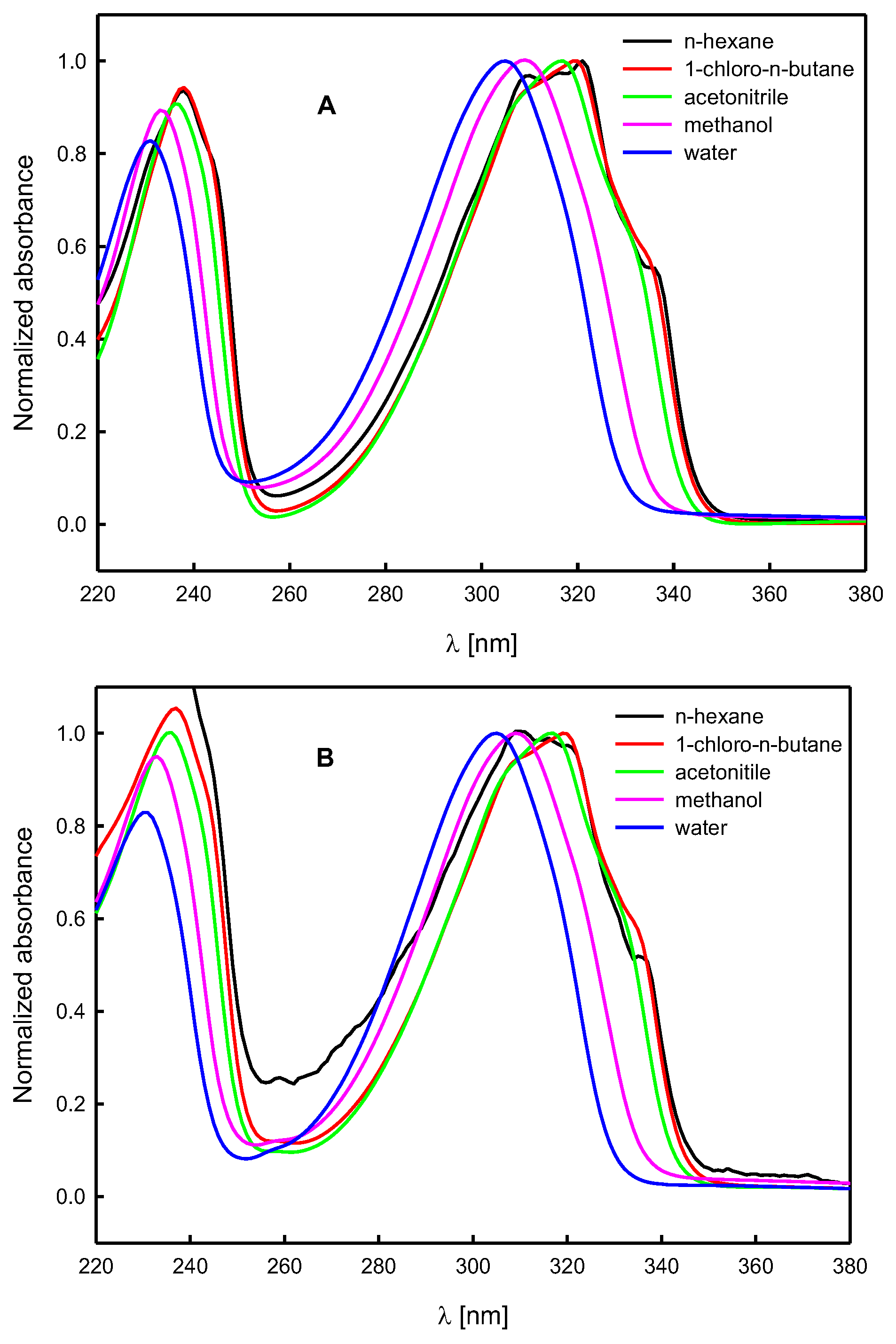
1.2. Solvatochromic Study
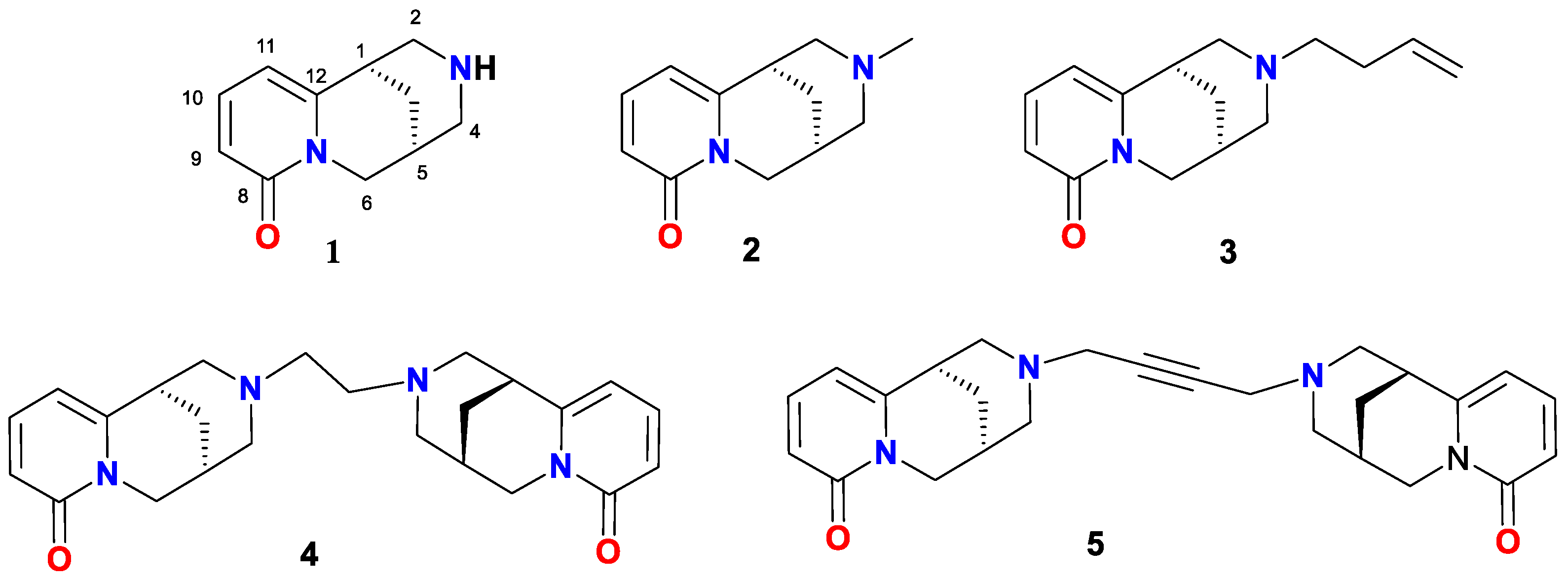
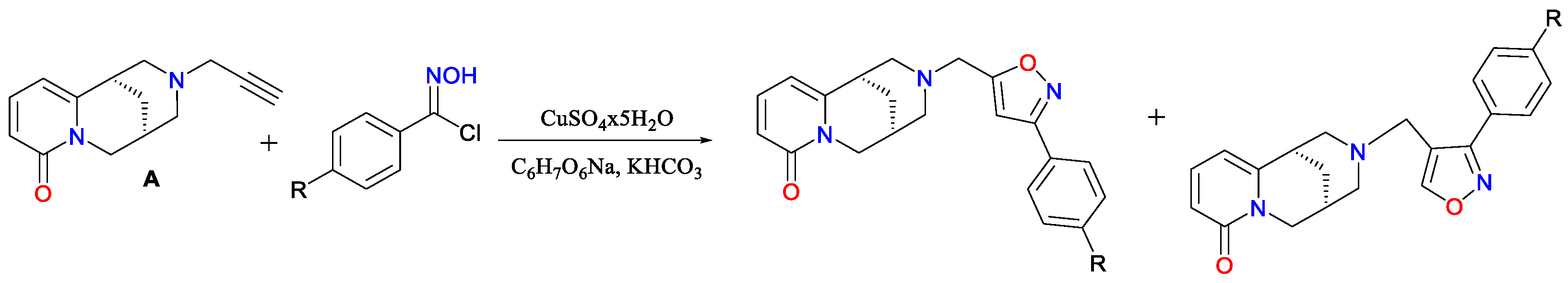
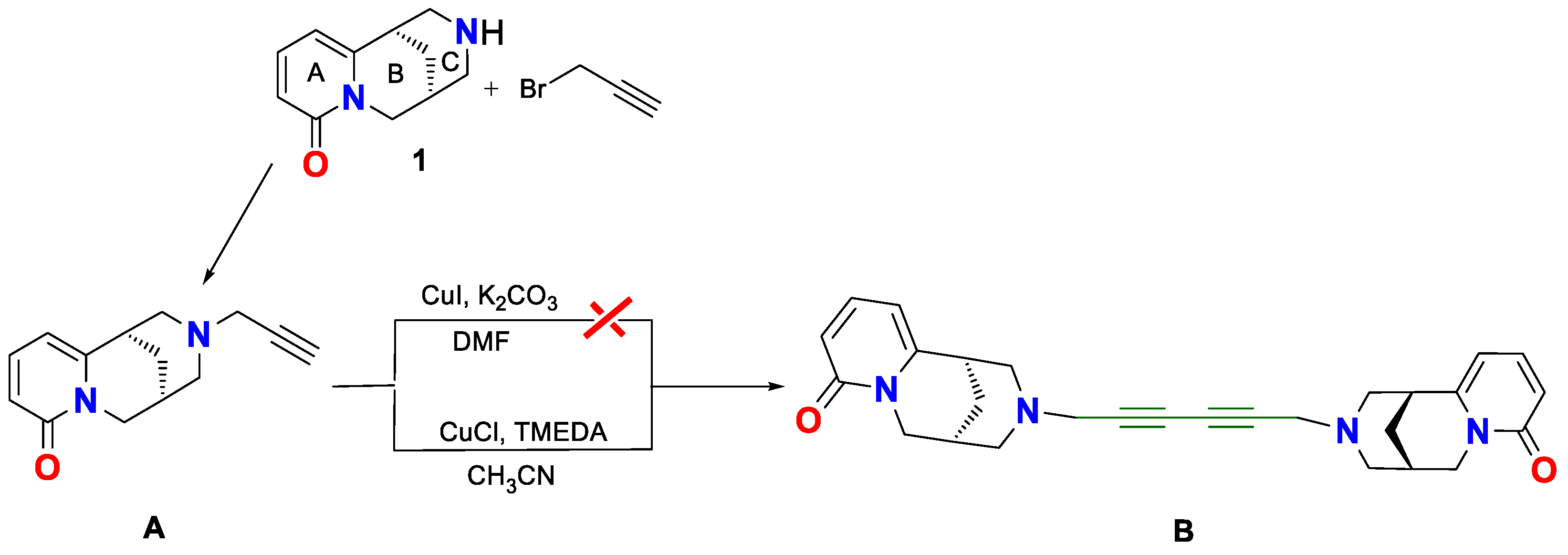
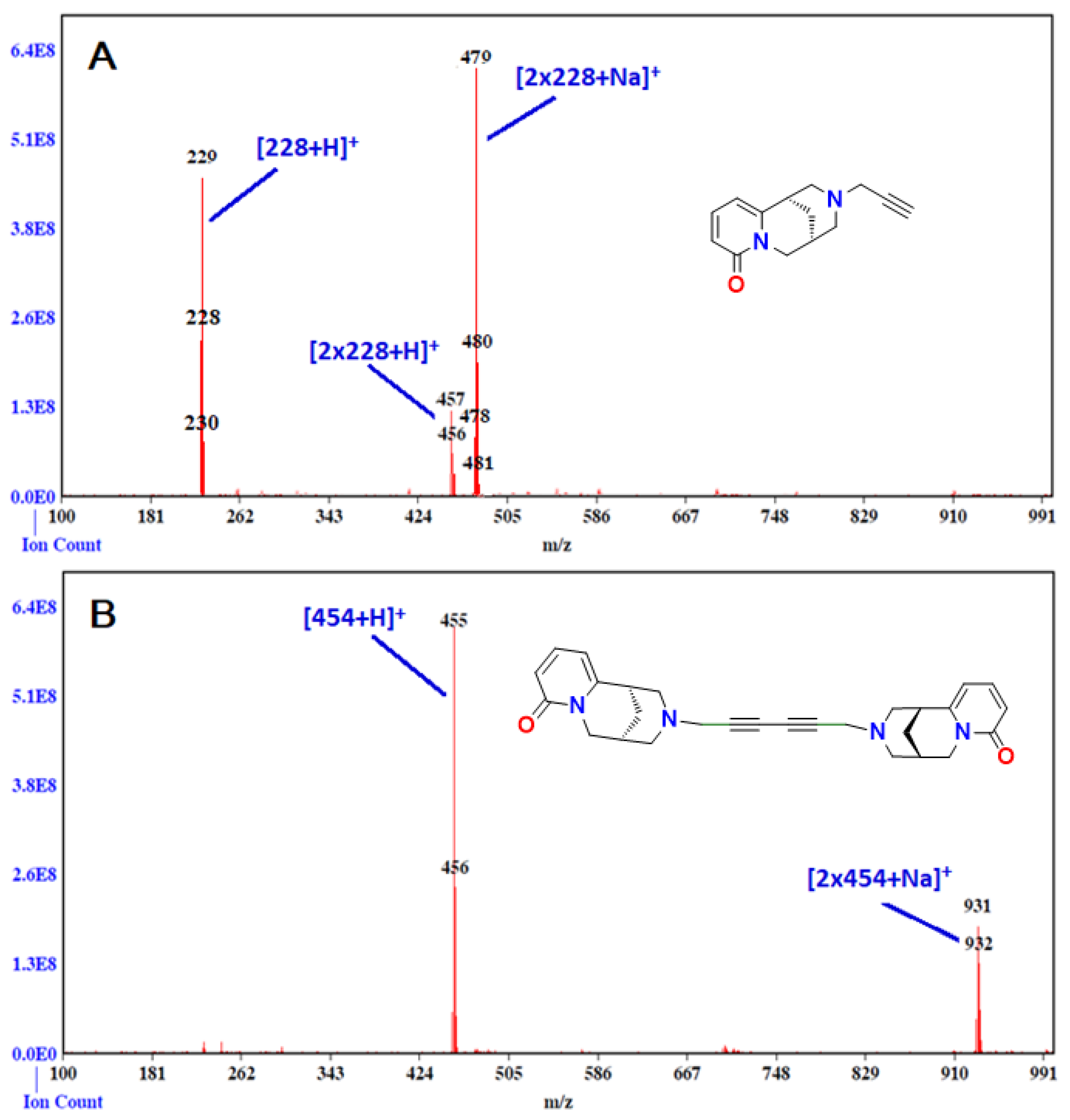
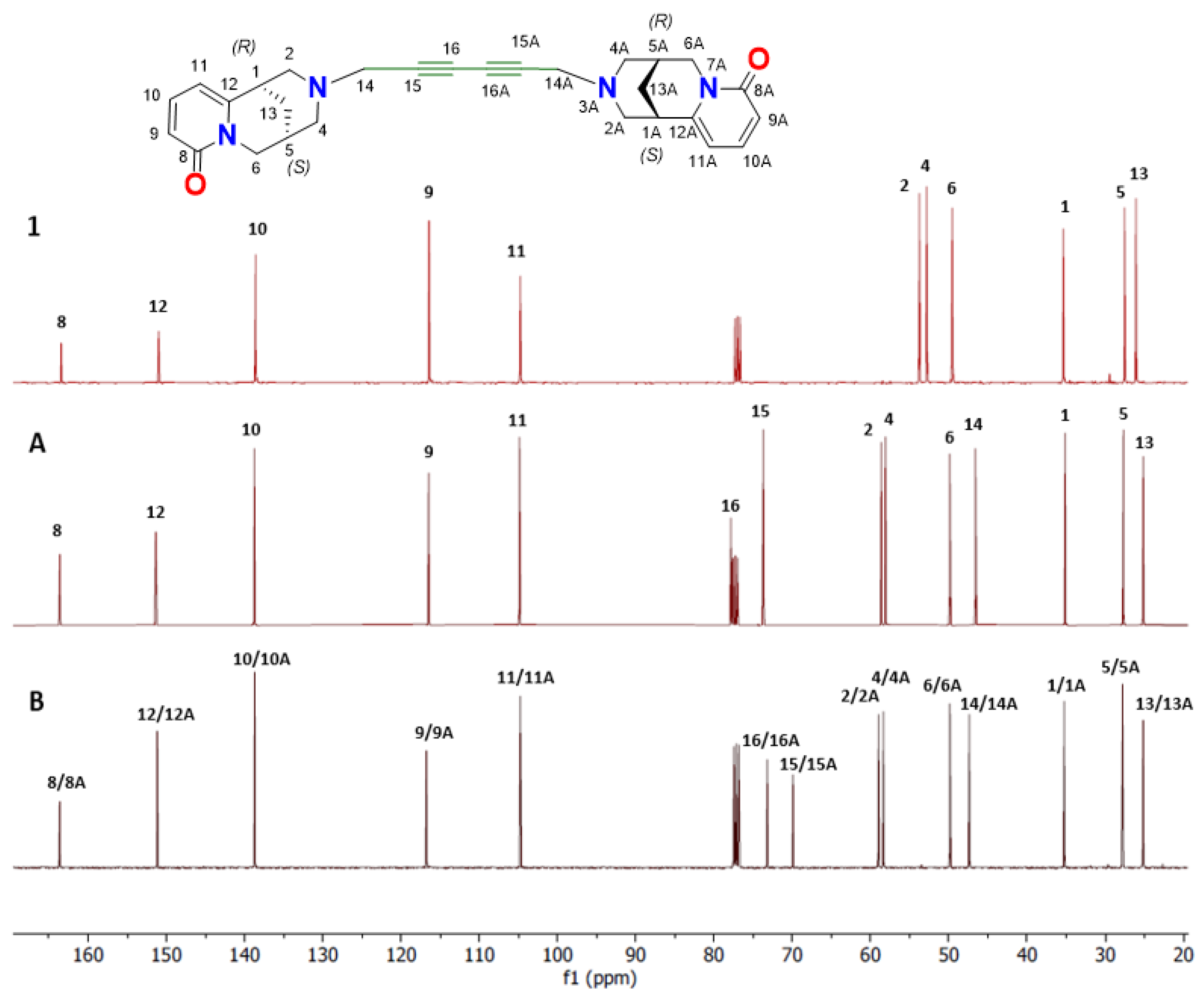
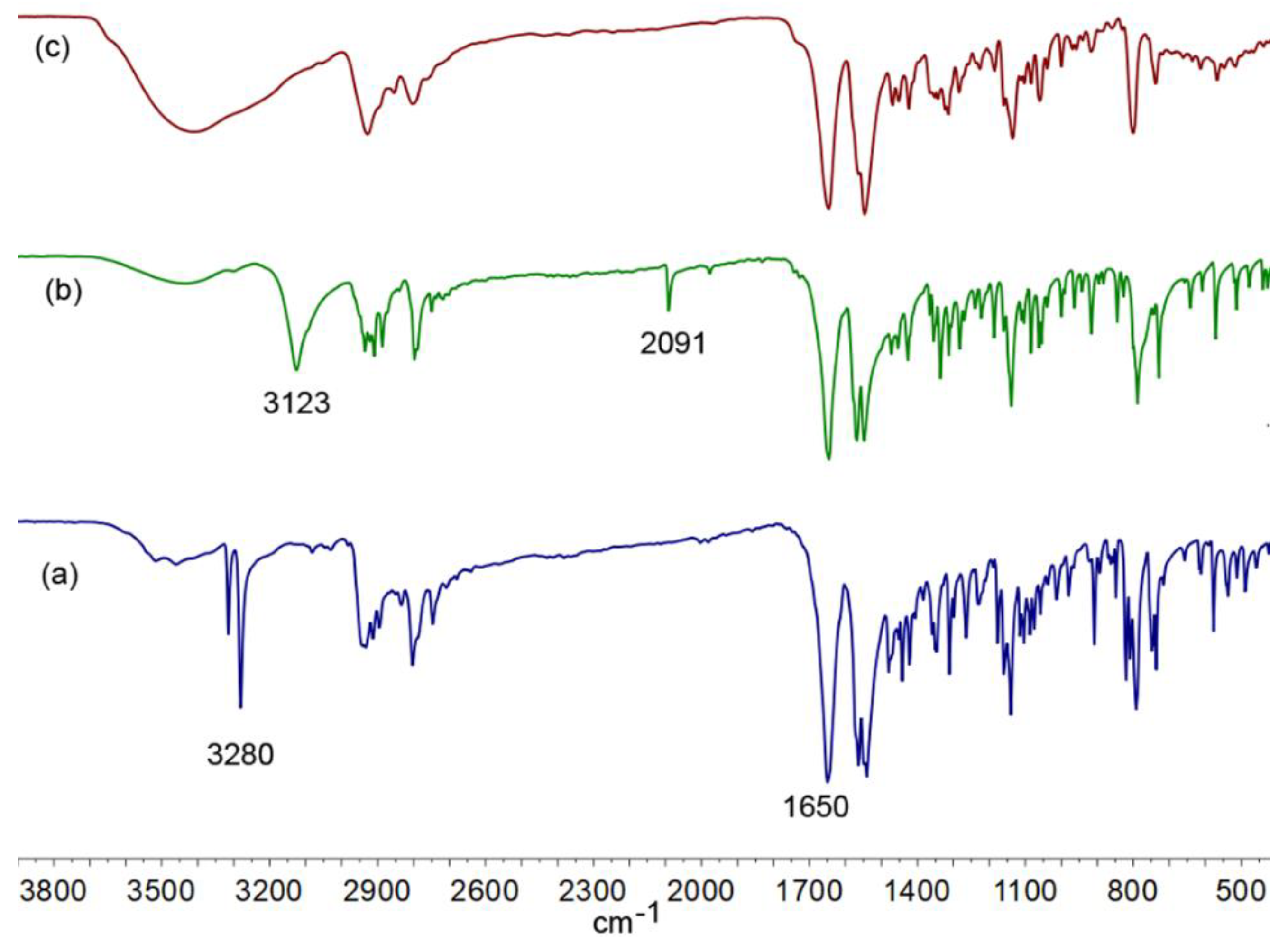
2. Results and Discussion
Spectroscopic Analysis
3. Materials and Methods
3.1. General
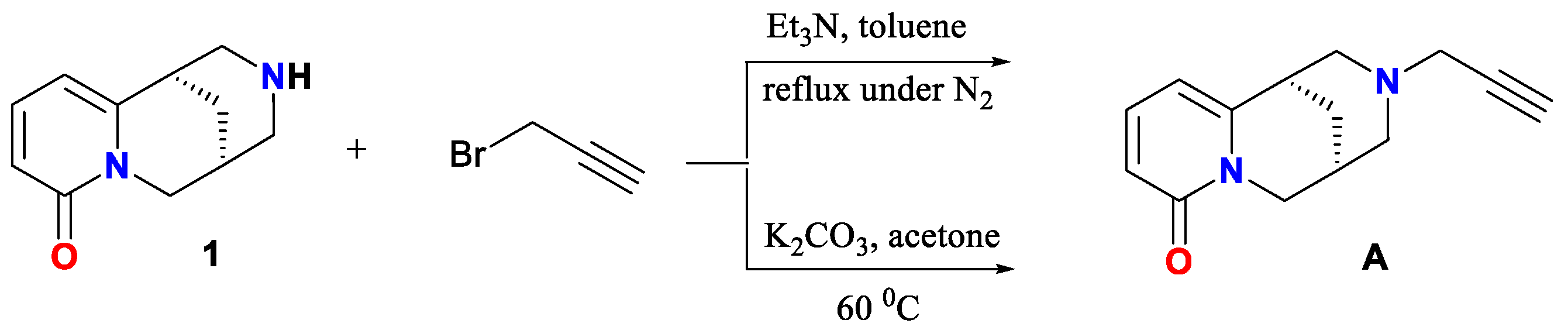
3.2. Synthesis of (−)-Cytisine Derivatives A and B
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rouden, J.; Lasne, M.-C.; Blanchet, J.; Baudoux, J. (−)-Cytisine and Derivatives: Synthesis, Reactivity, and Applications. Chem. Rev. 2014, 114, 712–778. [Google Scholar] [CrossRef] [PubMed]
- Bartusik, D.; Aebisher, D.; Tutka, P. A Review of the Organic Synthesis and Medicinal Applications of the Natural Product Cytisine. Mod. Org. Chem. Res. 2016, 1, 10–23. [Google Scholar] [CrossRef]
- Gotti, C.; Clementi, F. Cytisine and cytisine derivatives. More than smoking cessation aids. Pharmacol. Res. 2021, 170, 105700. [Google Scholar] [CrossRef] [PubMed]
- Gray, D.; Gallagher, T. A flexible strategy for the synthesis of tri- and tetracyclic lupin alkaloids: Synthesis of (+)-cytisine, (±)-anagyrine, and (±)-thermopsine. Angew. Chem. Int. Ed. 2006, 45, 2419–2423. [Google Scholar] [CrossRef]
- Ofori, S.; Lu, C.; Olasupo, O.O.; Dennis, B.B.; Fairbairn, N.; Devereaux, P.J.; Mbuagbaw, L. Cytisine for smoking cessation: A systematic review and meta-analysis. Drug Alcohol Depend. 2023. [Google Scholar] [CrossRef]
- Sala, M.; Braida, D.; Pucci, L.; Manfredi, I.; Marks, M.J.; Wageman, C.R.; Grady, S.R.; Loi, B.; Fucile, S.; Fasoli, F.; et al. CC4, a dimer of cytisine, is a selective partial agonist at α4β2/α6β2 nAChR with improved selectivity for tobacco smoking cessation. Br. J. Pharmacol. 2013, 168, 835–849. [Google Scholar] [CrossRef]
- Harpsøe, K.; Hald, H.; Timmermann, D.B.; Jensen, M.L.; Dyhring, T.; Nielsen, E.Ø.; Peters, D.; Balle, T.; Gajhede, M.; Kastrup, J.S.; et al. Molecular Determinants of Subtype-selective Efficacies of Cytisine and the Novel Compound NS3861 at Heteromeric Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2013, 288, 2559–2570. [Google Scholar] [CrossRef]
- Rollema, H.; Shrikhande, A.; Ward, K.; Tingley III, F.; Coe, J.; O’Neill, B.; Tseng, E.; Wang, E.; Mather, R.; Hurst, R.; et al. Pre-clinical properties of the α4β2 nicotinic acetylcholine receptor partial agonists varenicline, cytisine and dianicline translate to clinical efficacy for nicotine dependence. Br. J. Pharmacol. 2010, 160, 334–345. [Google Scholar] [CrossRef]
- Tutka, P.; Zatoński, W. Cytisine for the treatment of nicotine addiction: From a molecule to therapeutic efficacy. Pharmacol. Rep. 2005, 58, 777–798. [Google Scholar]
- Pérez, E.G.; Méndez-Gálvez, C.; Cassels, B.K. Cytisine: A natural product lead for the development of drugs acting at nicotinic acetylcholine receptors. Nat. Prod. Rep. 2012, 29, 555–567. [Google Scholar] [CrossRef]
- Wang, X.; Yang, J.; Huang, P.; Wang, D.; Zhang, Z.; Zhou, Z.; Liang, L.; Yao, R.; Yang, L. Cytisine: State of the art in pharmacological activities and pharmacokinetics. Biomed. Pharmacother. 2024, 171, 116210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhang, T.; Liu, H.; Huang, Z.; Teng, J.; Deng, J.; Zhong, J.; Qian, X.; Sheng, X.; Ding, J.; et al. Cytisine Exerts an Anti-Epileptic Effect via α7nAChRs in a Rat Model of Temporal Lobe Epilepsy. Front. Pharmacol. 2021, 12, 706225. [Google Scholar] [CrossRef]
- Slater, Y.E.; Houlihan, L.M.; Maskell, P.D.; Exley, R.; Bermúdez, I.; Lukas, R.J.; Valdivia, A.C.; Cassels, B.K. Halogenated cytisine derivatives as agonists at human neuronal nicotinic acetylcholine receptor subtypes. Neuropharmacology 2003, 44, 503–515. [Google Scholar] [CrossRef]
- Zambrano, C.A.; Marks, M.J.; Cassels, B.K.; Maccioni, R.B. In vivo effects of 3-iodocytisine: Pharmacological and genetic analysis of hypothermia and evaluation of chronic treatment on nicotinic binding sites. Neuropharmacology 2009, 57, 332–342. [Google Scholar] [CrossRef]
- Abin-Carriquiry, J.A.; Zunini, M.P.; Cassels, B.K.; Wonnacott, S.; Dajas, F. In silico characterization of cytisinoids docked into an acetylcholine binding protein. Bioorg. Med. Chem. Lett. 2010, 20, 3683–3687. [Google Scholar] [CrossRef]
- Chellappan, S.K.; Xiao, Y.; Tueckmantel, W.; Kellar, K.J.; Kozikowski, A.P. Synthesis and pharmacological evaluation of novel 9- and 10-substituted cytisine derivatives. Nicotinic ligands of enhanced subtype selectivity. J. Med. Chem. 2006, 49, 2673–2676. [Google Scholar] [CrossRef]
- Houllier, N.; Gopisetti, J.; Lestage, P.; Lasne, M.C.; Rouden, J. Identification of 9-fluoro substituted (−)-cytisine derivatives as ligands with high affinity for nicotinic receptors. Bioorg. Med. Chem. Lett. 2010, 20, 6667–6670. [Google Scholar] [CrossRef]
- Nicolotti, O.; Canu Boido, C.; Sparatore, F.; Carotti, A. Cytisine derivatives as high affinity nAChR ligands: Synthesis and comparative molecular field analysis. Farmaco 2002, 57, 469–478. [Google Scholar] [CrossRef]
- Ponzoni, L.; Braida, D.; Pucci, L.; Andrea, D.; Fasoli, F.; Manfredi, I.; Papke, R.L.; Stokes, C.; Cannazza, G.; Clementi, F.; et al. The cytisine derivatives, CC4 and CC26, reduce nicotine-induced conditioned place preference in zebrafish by acting on heteromeric neuronal nicotinic acetylcholine receptors. Psychopharmacology 2014, 231, 4681–4693. [Google Scholar] [CrossRef] [PubMed]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature 2016, 538, 411–428. [Google Scholar] [CrossRef] [PubMed]
- Blom, A.E.M.; Campello, H.R.; Lester, H.A.; Gallagher, T.; Dougherty, D.A. Probing Binding Interactions of Cytisine Derivatives to the α4β2 Nicotinic Acetylcholine Receptor. J. Am. Chem. Soc. 2019, 141, 15840–15849. [Google Scholar] [CrossRef]
- Etter, J.F.; Lukas, R.J.; Benowitz, N.L.; West, R.; Dresler, C.M. Cytisine for smoking cessation: A research agenda. Drug Alcohol Depend. 2008, 92, 3–8. [Google Scholar] [CrossRef]
- Sajja, R.K.; Rahman, S. Cytisine modulates chronic voluntary ethanol consumption and ethanol-induced striatal up-regulation of ΔFosB in mice. Alcohol 2013, 47, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Sotomayor-Zárate, R.; Gysling, K.; Busto, U.E.; Cassels, B.K.; Tampier, L.; Quintanilla, E. Varenicline and cytisine: Two nicotinic acetylcholine receptor ligands reduce ethanol intake in University of Chile bibulous rats. Psychopharmacology 2013, 227, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Jutkiewicz, E.M.; Rice, K.C.; Carroll, F.I.; Woods, J.H. Patterns of nicotinic receptor antagonism II: Cardiovascular effects in rats. Drug Alcohol Depend. 2013, 131, 284–297. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.; Steward, R.A.; Willis, A.G. Succinate Salt of Cytisine and Use Thereof. U.S. Patent 10,300,050, 2019. [Google Scholar]
- Przybył, A.K.; Janczak, J.; Huczyński, A. Molecular structure and spectroscopic studies of (−)-cytisine salt with (+)-tartaric acid. J. Mol. Struct. 2024, 1304, 137704. [Google Scholar] [CrossRef]
- Przybył, A.K.; Maj, E.; Wietrzyk, J.; Kubicki, M. Spectroscopic, structural and anticancer activity studies of (−)-cytisine halogenated N-benzyl derivatives. J. Mol. Struct. 2019, 1176, 871–880. [Google Scholar] [CrossRef]
- Lauder, K.; Toscani, A.; Scalacci, N.; Castagnolo, D. Synthesis and Reactivity of Propargylamines in Organic Chemistry. Chemical Reviews 2017, 117, 14091–14200. [Google Scholar] [CrossRef]
- Glaser, C. Beiträge zur Kenntniss des Acetenylbenzols. Eur. J. Inorg. Chem. 1869, 2, 422–424. [Google Scholar] [CrossRef]
- Eglinton, G.; Galbraith, A.R. 182. Macrocyclic Acetglenic Compounds. Part, I. cycloTetradecu-1: 3-diyne and Belated Compounds. J. Chem. Soc. (Resumed) 1959, 889–896. [Google Scholar] [CrossRef]
- Hay, A.S. Oxidative Coupling of Acetylenes. II1. J. Org. Chem. 1962, 27, 3320–3321. [Google Scholar] [CrossRef]
- Li, L.; Wang, J.; Zhang, G.; Liu, Q. A mild copper-mediated Glaser-type coupling reaction under the novel CuI/NBS/DIPEA promoting system. Tetrahedron Lett. 2009, 50, 4033–4036. [Google Scholar] [CrossRef]
- Singh, M.; Singh, A.S.; Mishra, N.; Agrahari, A.K.; Tiwari, V.K. Benzotriazole as an Efficient Ligand in Cu-Catalyzed Glaser Reaction. ACS Omega 2019, 4, 2418–2424. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.; Singh, S.K.; Singh, A.S.; Agrahari, A.K.; Tiwari, V.K. Glycosyl Triazole Ligand for Temperature-Dependent Competitive Reactions of Cu-Catalyzed Sonogashira Coupling and Glaser Coupling. J. Org. Chem. 2021, 86, 17884–17895. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhu, Y.; Luo, J.; Zhu, Z.; Zhao, L.; Zeng, X.; Li, D.; Chen, J.; Lan, X. A Simple and Practical Bis-N-Heterocyclic Carbene as an Efficient Ligand in Cu-Catalyzed Glaser Reaction. Molecules 2023, 28, 5083. [Google Scholar] [CrossRef]
- Tripp, V.T.; Lampkowski, J.S.; Tyler, R.; Young, D.D. Development of solid-supported Glaser-Hay couplings. ACS Comb. Sci. 2014, 16, 164–167. [Google Scholar] [CrossRef]
- Bohlmann, F.; Schönowsky, H.; Inhoffen, E.; Grau, G. Polyacetylenverbindungen, LII. Über den Mechanismus der oxydativen Dimerisierung von Acetylenverbindungen. Chem. Ber. 1964, 97, 794–800. [Google Scholar] [CrossRef]
- Kurpanik, A.; Matussek, M.; Szafraniec-Gorol, G.; Filapek, M.; Lodowski, P.; Marcol-Szumilas, B.; Ignasiak, W.; Małecki, J.G.; Machura, B.; Małecka, M.; et al. APEX Strategy Represented by Diels–Alder Cycloadditions—New Opportunities for the Syntheses of Functionalised PAHs. Chem. Eur. J. 2020, 26, 12150. [Google Scholar] [CrossRef]
- Filatova, E.A.; Tsybulin, S.V.; Rybin, D.A.; Ozeryanskii, V.A.; Gulevskaya, A.V.; Pozharskii, A.F.; Borodkin, G.S. A new family of 1,4-diaryl-1,3-butadiynes based on the “proton sponge”: Synthesis, electronic and chemical properties. New J. Chem. 2022, 46, 1829–1838. [Google Scholar] [CrossRef]
- Mieszczanin, A.; Filipek, P.; Chrobok, A.; Brzęczek-Szafran, A.; Filapek, M.; Matussek, M.; Kula, S.; Szłapa-Kula, A.; Pietraszuk, C.; Rogalski, S.; et al. FromKetoesters, Diynes, and Arynes to Functionalized Pyranones, Naphthalenes, and Anthracenes. Chem. Eur. J. 2025, 31, e01917. [Google Scholar] [CrossRef]
- Shi, W.; Lei, A. 1,3-Diyne chemistry: Synthesis and derivations. Tetrahedron Lett. 2014, 55, 2763–2772. [Google Scholar] [CrossRef]
- Goswami, L.; Gupta, L.; Paul, S.; Vijayaraghavan, P.; Bhattacharya, A.K. Design and Synthesis of 1,3-Diynes as Potent Antifungal Agents against Aspergillus fumigatus. ChemMedChem 2023, 18, e202300013. [Google Scholar] [CrossRef]
- Shi Shun, A.L.K.; Tykwinski, R.R. Synthesis of Naturally Occurring Polyynes. Angew. Chem. Int. Ed. 2006, 45, 1034–1057. [Google Scholar] [CrossRef]
- Frackenpohl, J.; Braje, W.M.; Hoffmann, H.M.R. Cross-coupling reactions in Cinchona alkaloid chemistry: Aryl-substituted and dimeric quinine, quinidine, as well as quincorine and quincoridine derivatives. J. Chem. Soc. Perkin 1 2001, 47–65. [Google Scholar] [CrossRef]
- Manga, B.; Venkateswara Rao, B.; Sudeshna, K.; Andugulapati, S.B.; Jadav, S.S.; Ramalingam, V.; Suresh Babu, K. Design, synthesis and cytotoxic activity studies of alkyne linked analogues of Nimbolide. Fitoterapia 2022, 161, 105246. [Google Scholar] [CrossRef]
- Nurkenov, O.A.; Baikenova, G.G.; Turdybekov, D.M.; Ibraev, M.K.; Gazaliev, A.M.; Turdybekov, K.M. Synthesis, structure, and transformations of 3-(N-Cytisinyl)propyne. Russ. J. Gen. Chem. 2006, 76, 129–132. [Google Scholar] [CrossRef]
- Brel, V.K. Click chemistry methodology in the synthesis of anabasine and cytisine conjugates with isoxazole derivatives. Russ. J. Org. Chem. 2016, 52, 54–60. [Google Scholar] [CrossRef]
- Sánchez-Velasco, O.A.; Saavedra-Olavarría, J.; Araya-Santelices, D.A.A.; Hermosilla-Ibáñez, P.; Cassels, B.K.; Pérez, E.G. Synthesis of N-Arylcytisine Derivatives Using the Copper-Catalyzed Chan-Lam Coupling. J. Nat. Prod. 2021, 84, 1985–1992. [Google Scholar] [CrossRef] [PubMed]
- Przybył, A.K.; Kubicki, M.; Jatrząb, R. Complexing ability (−)-cytisine–Synthesis, spectroscopy and crystal structures of the new copper and zinc compexes. J. Inorg. Biochem. 2014, 138, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Miotke, M.M.; Józefowicz, M. Solvatochromism of antiinflammatory drug—Naproxen sodium. J. Mol. Liq. 2017, 230, 129–136. [Google Scholar] [CrossRef]
- Katritzky, A.R.; Fara, D.C.; Yang, H.; Tämm, K.; Tamm, T.; Karelson, M. Quantitative Measures of Solvent Polarity. Chem. Rev. 2004, 104, 175–198. [Google Scholar] [CrossRef]
- Reichardt, C.; Welton, T. Solvents and Solvent Effects in Organic Chemistry, 4th ed.; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Kamlet, M.J.; Abboud, J.L.M.; Abraham, M.H.; Taft, R.W. Linear solvation energy relationships. 23. A comprehensive collection of the solvatochromic parameters, π*, α, and β, and some methods for simplifying the generalized solvatochromic equation. J. Org. Chem. 1983, 48, 2877–2887. [Google Scholar] [CrossRef]
- Catalan, J. Toward a generalized treatment of the solvent effect based on four empirical scales: Dipolarity (SdP, a new scale), polarizability (SP), acidity (SA), and basicity (SB) of the medium. J. Phys. Chem. B 2009, 113, 5951–5960. [Google Scholar] [CrossRef] [PubMed]
- Marcus, Y. The Properties of Solvents; Wiley: Chichester, UK, 1998. [Google Scholar]
- Krystkowiak, E. Effect of Solute–Solvent Hydrogen-Bonding on Spectral and Photophysical Properties of Aromatic Probes. In Hydrogen-Bonding Research in Photochemistry, Photobiology, and Optoelectronic Materials; Han, K.-L., Zhao, G.-J., Eds.; World Scientific: Hackensack, NJ, USA, 2019. [Google Scholar]
- Krystkowiak, E.; Przybył, A.K.; Bayda, M.; Józkowiak, J.; Maciejewski, A. Spectral and Photophysical Behaviour of Cytisine in n-Hexane. Experimental Evidence for the S1(n,π*) → S0 Fluorescence. J. Phys. Chem. A 2017, 121, 5597–5604. [Google Scholar] [CrossRef] [PubMed]
- Krystkowiak, E.; Przybył, A.K.; Bayda-Smykaj, M.; Koput, J.; Maciejewski, A. Spectral and photophysical properties of cytisine in acetonitrile–theory and experiment. Spectrochim. Acta A 2018, 203, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Alvarez Escalada, F.C.; Romano, E.; Brandán, S.A.; Ledesma, A.E. Experimental and computational analysis of N-methylcytisine alkaloid in solution and prediction of biological activity by docking calculations. Mol. Phys. 2022, 120, e1987544. [Google Scholar] [CrossRef]
- Petruczynik, A.; Wróblewski, K.; Misiurek, J.; Plech, T.; Szalast, K.; Wojtanowski, K.; Mroczek, T.; Szymczak, G.; Waksmundzka-Hajnos, M.; Tutka, P. Determination of Cytisine and N-Methylcytisine from Selected Plant Extracts by High-Performance Liquid Chromatography and Comparison of Their Cytotoxic Activity. Toxins 2020, 12, 557. [Google Scholar] [CrossRef]
- Wróblewski, K.; Petruczynik, A.; Tuzimski, T.; Przygodzka, D.; Buszewicz, G.; Kołodziejczyk, P.; Tutka, P. Comparison of Various Chromatographic Systems for Analysis of Cytisine in Human Serum, Saliva and Pharmaceutical Formulation by HPLC with Diode Array, Fluorescence or Mass Spectrometry Detection. Molecules 2019, 24, 2580. [Google Scholar] [CrossRef]
- Borisevich, S.S.; Kayumova, R.R.; Tsypysheva, I.P.; Ostakhov, S.S.; Khursan, S.L. Luminescent characterization of interaction efficiency between (−)-cytisine and amino acids an indicator of anti-inflammatory of some 12-N-substituted (−)-cytisine derivatives. J. Photochem. Photobiol. A Chem. 2017, 344, 192–198. [Google Scholar] [CrossRef]
- Wang, L.; Yan, J.; Li, P.; Wang, M.; Su, C. The effects of amines on oxidative homo-coupling of terminal alkynes promoted by copper salts. J. Chem. Res. 2005, 2005, 112–115. [Google Scholar] [CrossRef]
- Akhtar, R.; Zahoor, A.F. Transition metal catalyzed Glaser and Glaser-Hay coupling reactions: Scope, classical/green methodologies and synthetic applications. Synth. Commun. 2020, 50, 3337–3368. [Google Scholar] [CrossRef]
- Malafaia, D.; Sousa, J.L.C.; Silva, A.M.S.; Albuquerque, H.M.T. 2,2′-[(1E,1′E)-{[Hexa-2,4-diyne-1,6-diylbis(oxy)]bis(2,1-phenylene)}bis(ethene-2,1-diyl)]bis(4H-chromen-4-one). Molbank 2023, 2023, M1621. [Google Scholar] [CrossRef]
- Abdusalamov, B.A.; Khoroshkova, O.A.; Aslanov, K.A. The structure of sophorbenzamine. Chem. Nat. Compd. 1976, 12, 60–62. [Google Scholar] [CrossRef]
- Atta-ur-Rahman; Pervin, A.; Choudhary, M.I. Sophazrine — A Novel Quinolizidine Alkaloid from Sophoragriffithii. J. Nat. Prod. 1991, 54, 929–935. [Google Scholar] [CrossRef]
- Al-Azizi, M.M.; Al-Said, M.S.; El-Olemy, M.M.; Abdel Saltar, E.; Khalifa, A.S. Rhombifoline and 5,6-Dehydrolupanine from Anagyrus foetida L. Arch. Pharm. Res. 1994, 17, 393–397. [Google Scholar]
- Sagen, A.-L.; Gertsch, J.; Becker, R.; Heilmann, J.; Sticher, O. Quinolizidine alkaloids from the curare adjuvant Clathrotropis glaucophylla. Phytochemistry 2002, 61, 975–978. [Google Scholar] [CrossRef]
- Aslanov, K.A.; Kushmuradov, Y.K.; Sadykov, A.S. Lupine Alkaloids. In The Alkaloids: Chemistry and Pharmacology; Brossi, A., Ed.; Academic Press Inc.: London, UK, 1987; Volume 31, pp. 117–192. [Google Scholar]
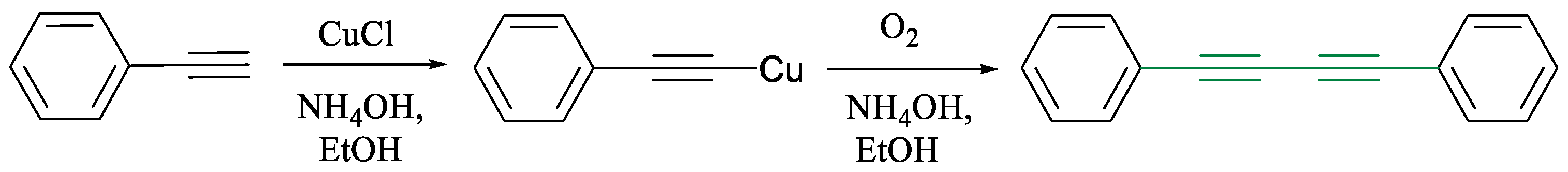
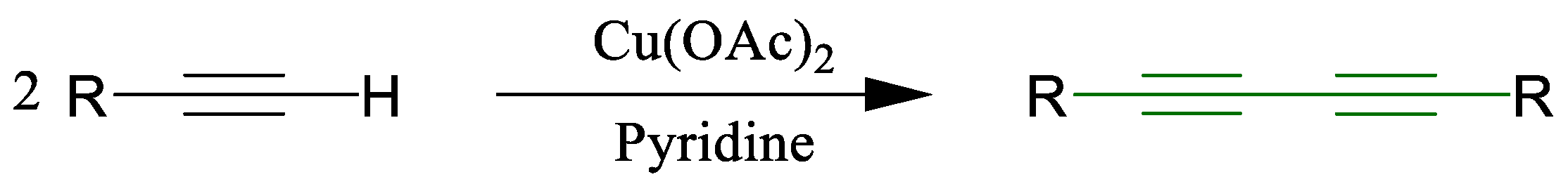
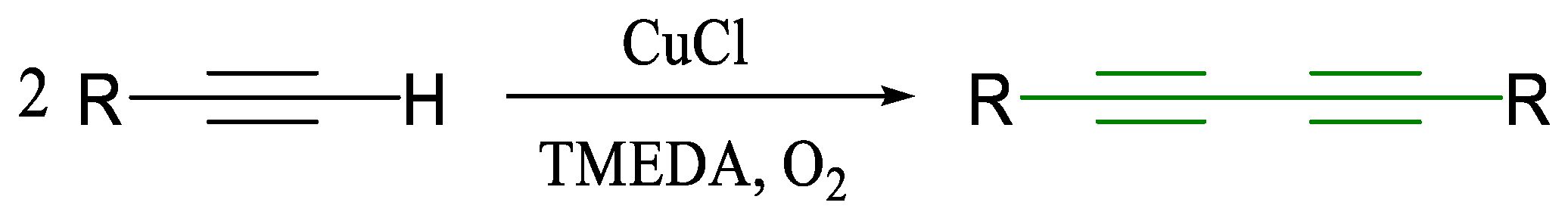
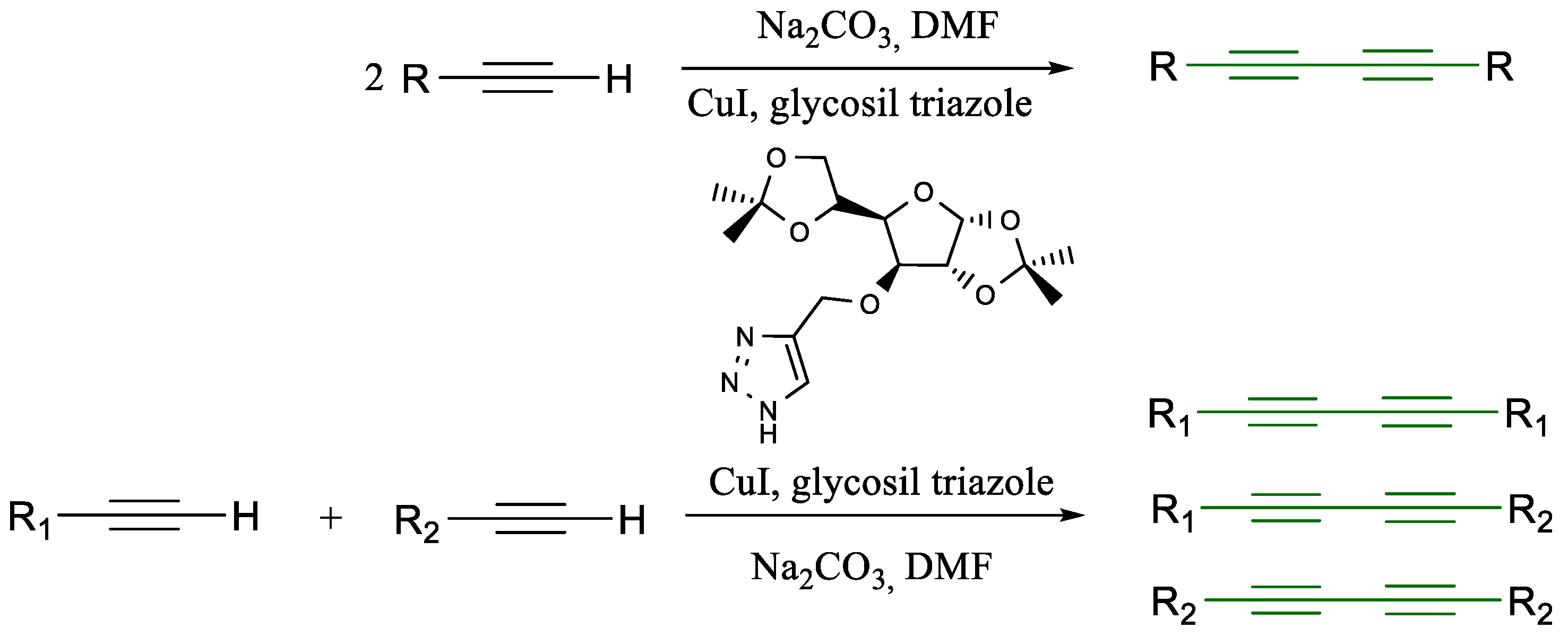
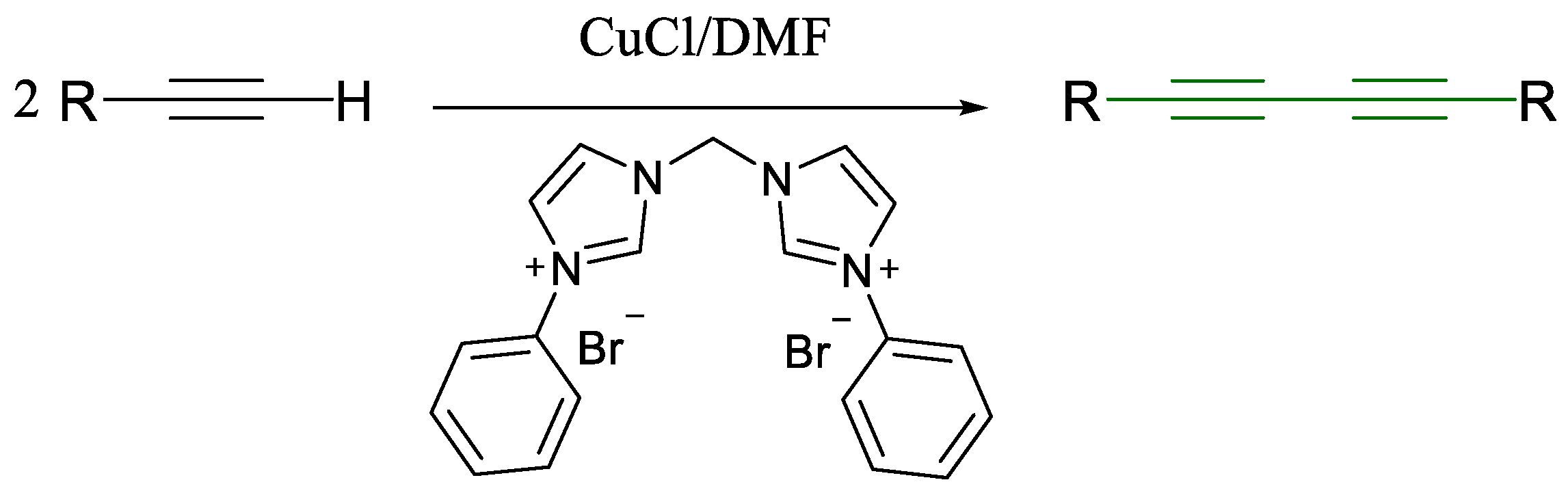
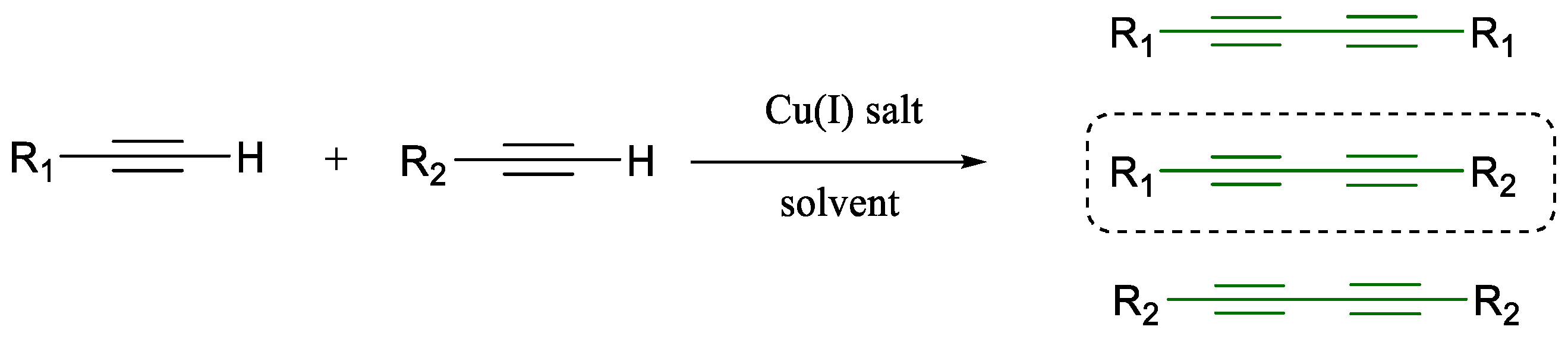
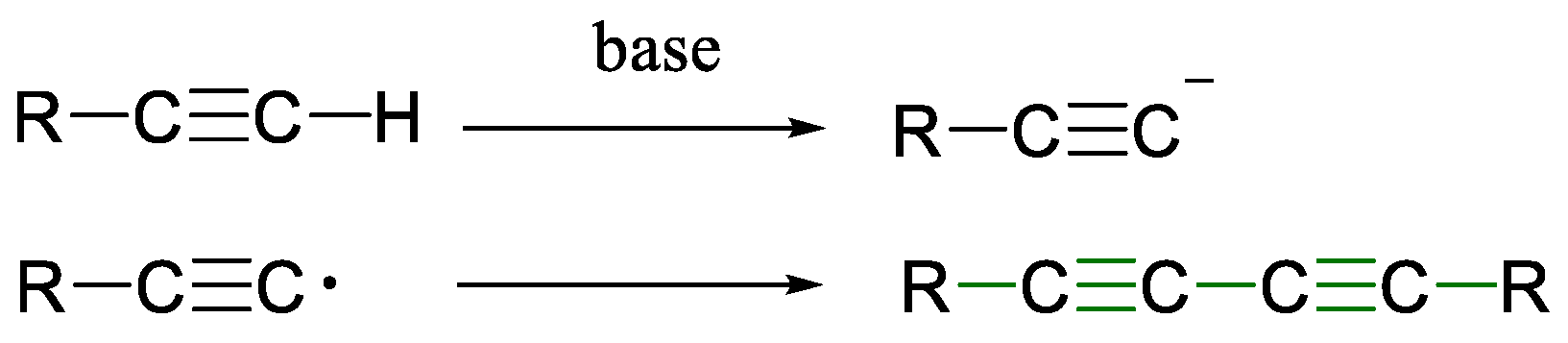
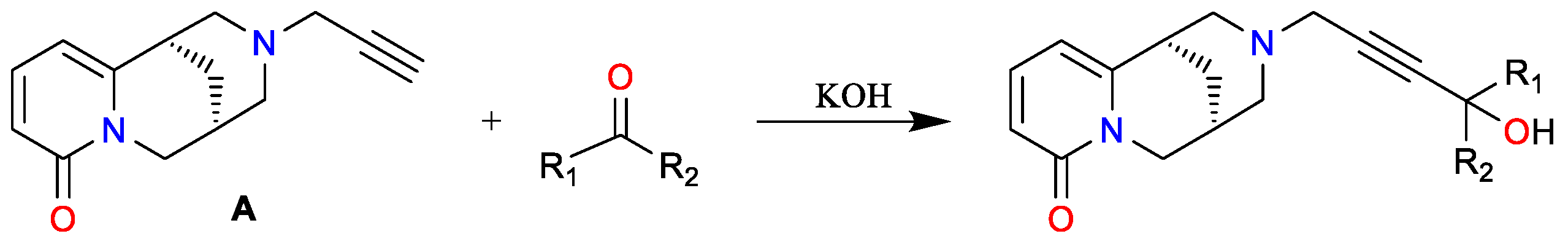
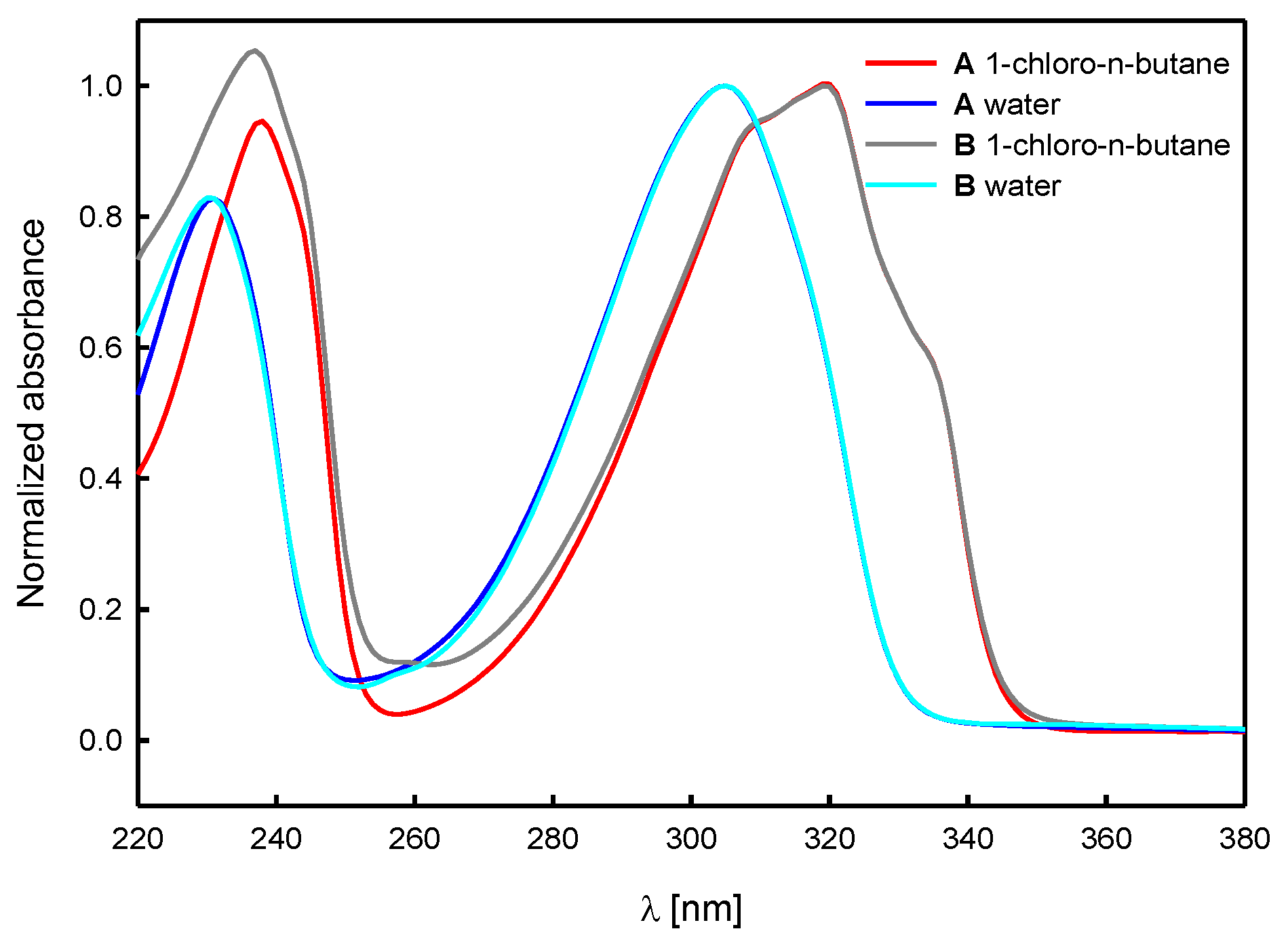
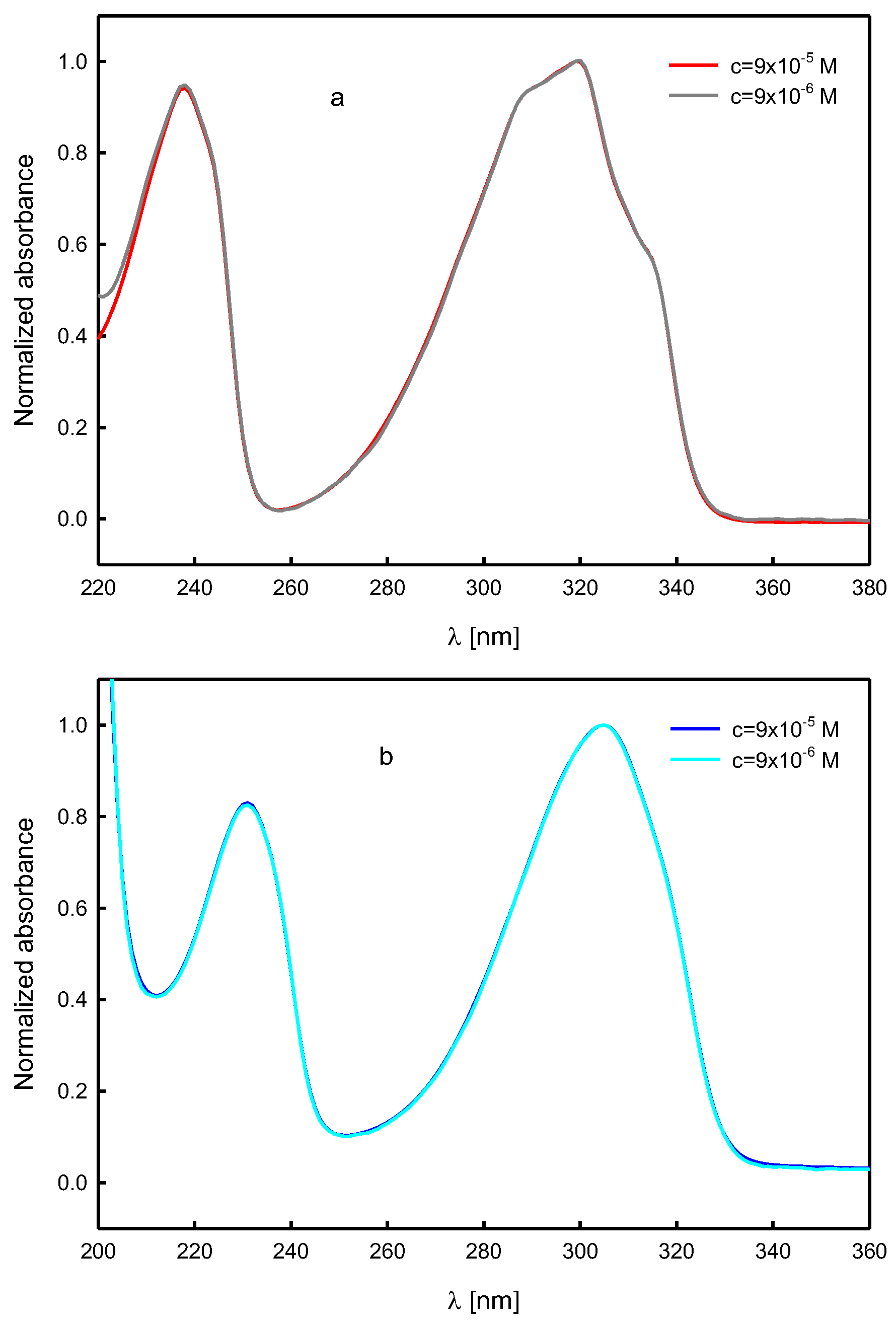
| Solvent | f(ε,n2) | ε a | n a | α a | β a |
|---|---|---|---|---|---|
| n-hexane | −0.0003 | 1.88 | 1.372 | 0.00 | 0.00 |
| 1-chloro-n-butane | 0.209 | 7.39 | 1.400 | 0.00 | 0.00 |
| HMPA | 0.261 | 29.30 | 1.457 | 0.00 | 1.00 |
| acetonitrile | 0.306 | 35.94 | 1.341 | 0.19 | 0.40 |
| methanol | 0.309 | 32.66 | 1.328 | 0.98 | 0.66 |
| water | 0.320 | 78.00 | 1.333 | 1.17 | 0.47 |
| HFIP | 0.308 | 16.62 | 1.277 | 1.96 | 0.00 |
| C Atoms | 1 | Δδ (1-A) | A | B | Δδ (A-B) |
|---|---|---|---|---|---|
| 1 | 35.6 | 0.4 | 35.2 | 35.2 (1/1A) | 0 |
| 2 | 54.0 | −4.6 | 58.6 a | 58.9 (2/2A) a | −0.3 |
| 4 | 53.0 | −5.1 | 58.1 a | 58.3 (4/4A) a | −0.2 |
| 5 | 27.7 | 0 | 27.7 | 27.7 (5/5A) | 0.1 |
| 6 | 49.7 | 0.1 | 49.8 | 49.8 (6/6A) | 0 |
| 8 | 163.6 | 0.1 | 163.5 | 163.5 (8/8A) | 0 |
| 9 | 116.6 | 0.2 | 116.7 | 116.7 (9/9A) | 0 |
| 10 | 138.8 | 0.3 | 138.5 | 138.6 (10/10A) | 0.1 |
| 11 | 104.9 | 0.4 | 104.5 | 104.6 (11/11A) | −0.1 |
| 12 | 151.1 | 0.1 | 151.2 | 151.0 (12/12A) | 0.2 |
| 13 | 26.3 | 1.1 | 25.2 | 25.1 (13/13A) | −0.1 |
| 14 | 46.6 | 47.3 (14/14A) | −0.7 | ||
| 15 | 77.8 | 73.1 (15/15A) | 4.5 | ||
| 16 | 73.5 | 69.8 (16/16A) | 3.7 |
| Solvent | (1) | A | B | |||
|---|---|---|---|---|---|---|
| λmax [nm] | εmax [M−1cm−1] | λmax [nm] | εmax [M−1cm−1] | λmax [nm] | εmax [M−1cm−1] | |
| n-hexane | 322 a | 6070 a | 321 | 7100 | 321 | - |
| 1-chloro- n-butane | 320 | 6240 | 319 | 6950 | 319 | 12,100 |
| acetonitrile | 318 b | 7240 b | 317 | 7250 | 317 | 13,300 |
| methanol | 310 | 7760 | 309 | 8300 | 309 | 15,800 |
| water | 307 | 8090 | 305 | 8200 | 305 | 13,800 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przybył, A.K.; Huczyński, A.; Krystkowiak, E. Copper-Mediated Homocoupling of N-propargylcytisine—Synthesis and Spectral Characterization of Novel Cytisine-Based Diyne Dimer. Molecules 2025, 30, 3955. https://doi.org/10.3390/molecules30193955
Przybył AK, Huczyński A, Krystkowiak E. Copper-Mediated Homocoupling of N-propargylcytisine—Synthesis and Spectral Characterization of Novel Cytisine-Based Diyne Dimer. Molecules. 2025; 30(19):3955. https://doi.org/10.3390/molecules30193955
Chicago/Turabian StylePrzybył, Anna K., Adam Huczyński, and Ewa Krystkowiak. 2025. "Copper-Mediated Homocoupling of N-propargylcytisine—Synthesis and Spectral Characterization of Novel Cytisine-Based Diyne Dimer" Molecules 30, no. 19: 3955. https://doi.org/10.3390/molecules30193955
APA StylePrzybył, A. K., Huczyński, A., & Krystkowiak, E. (2025). Copper-Mediated Homocoupling of N-propargylcytisine—Synthesis and Spectral Characterization of Novel Cytisine-Based Diyne Dimer. Molecules, 30(19), 3955. https://doi.org/10.3390/molecules30193955