Factors Affecting Distribution of Pharmaceutically Active Compounds in Bottom Sediments of Odra River Estuary (SW Baltic Sea)
Abstract
1. Introduction
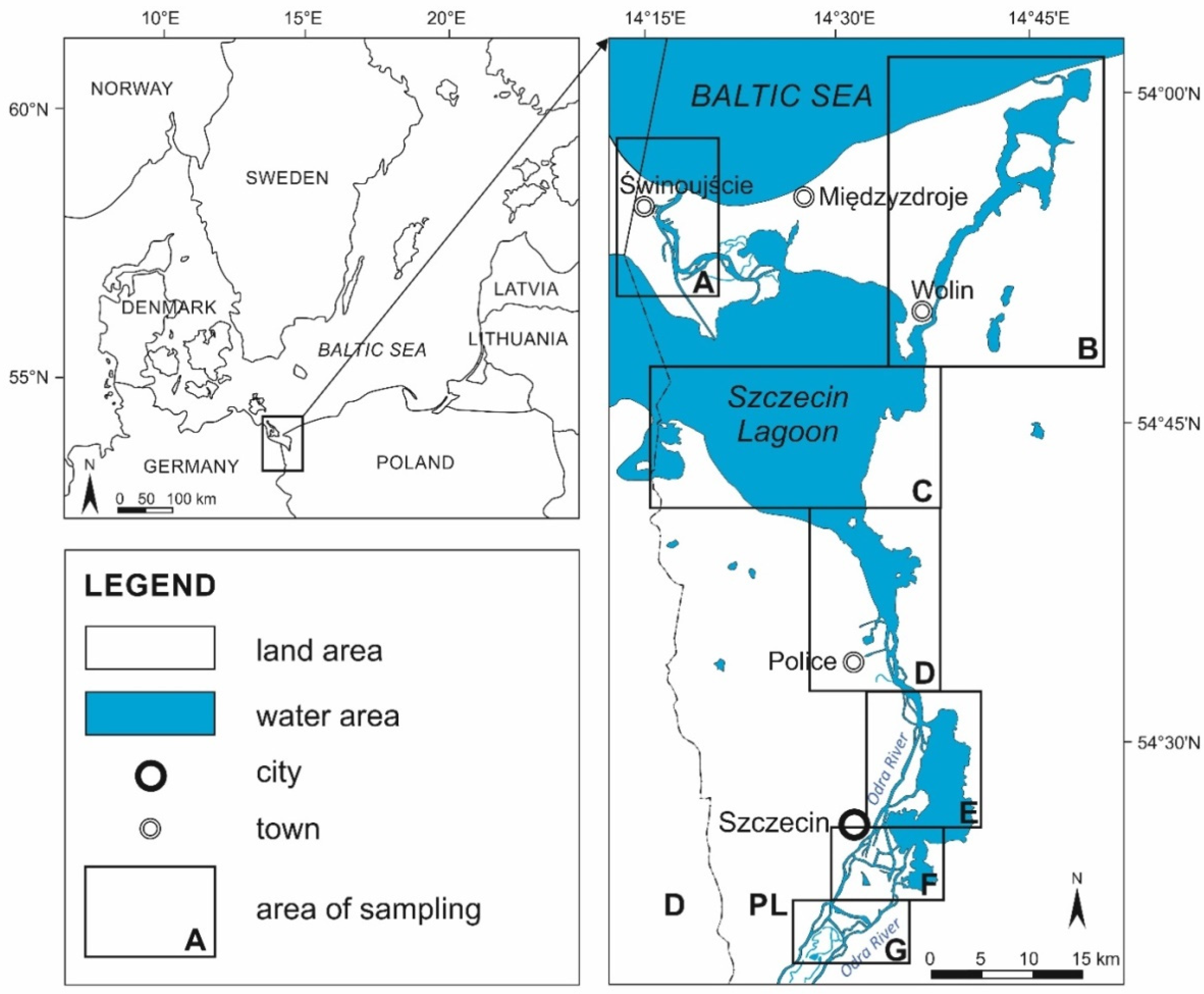
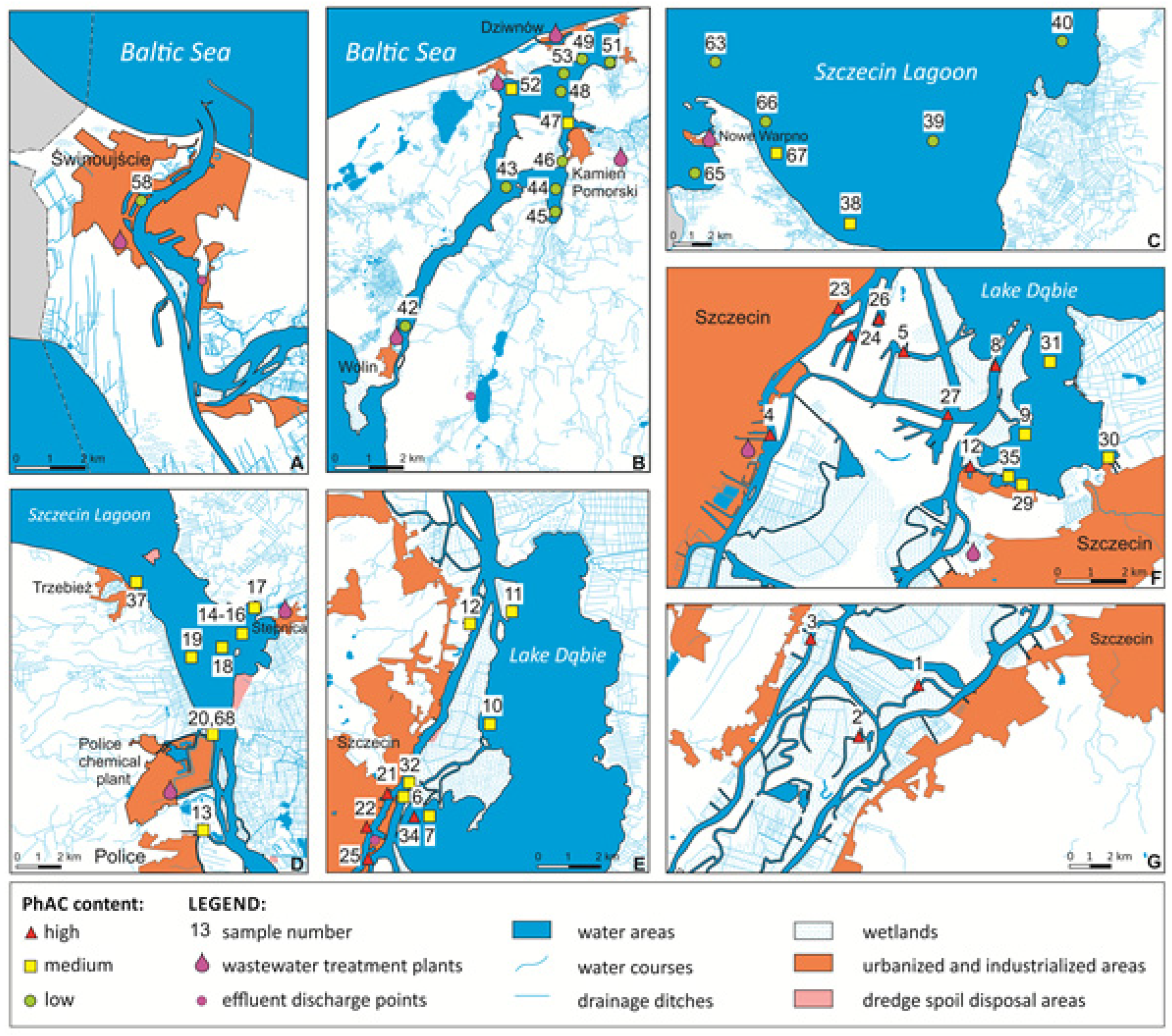
2. Study Area
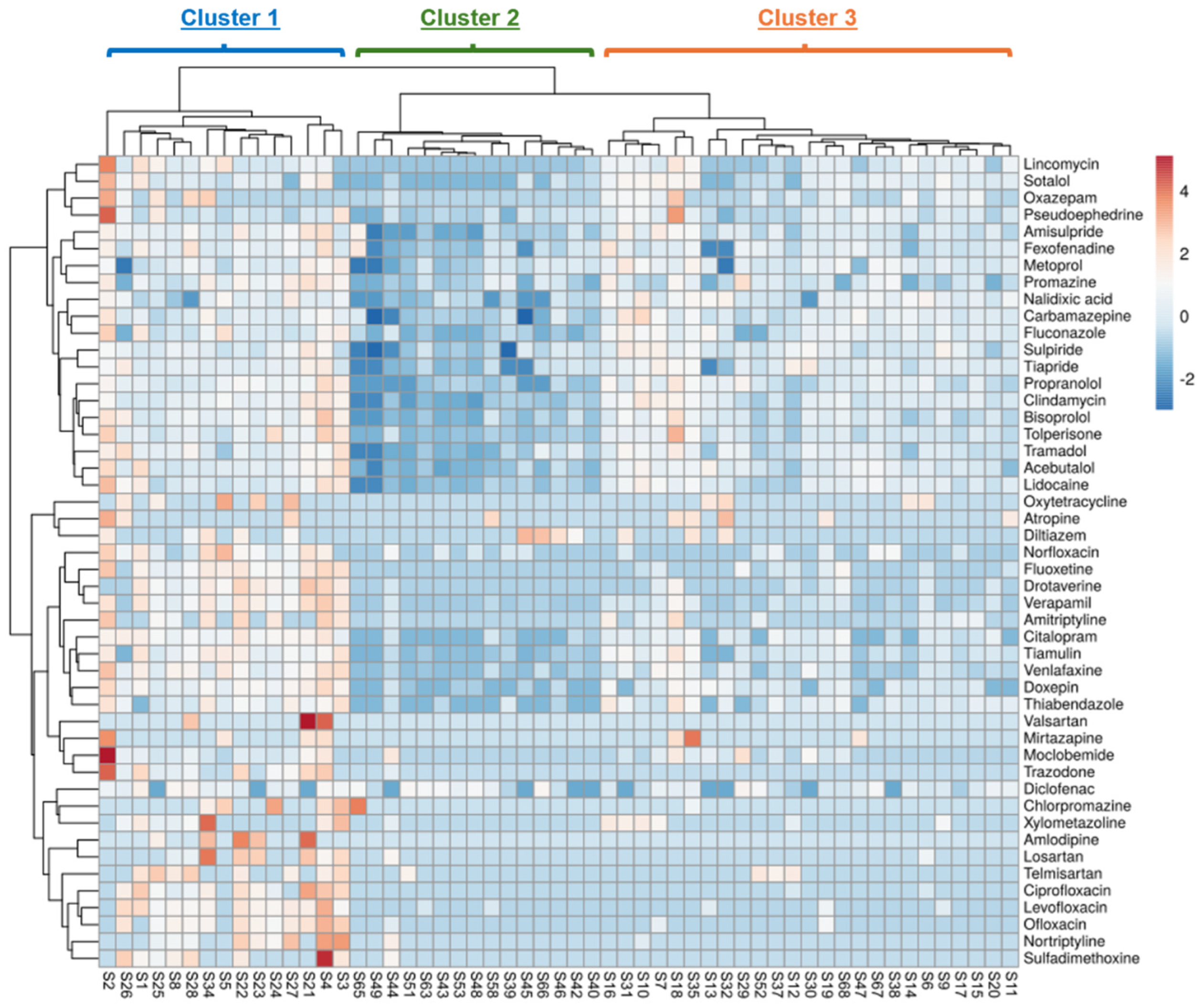
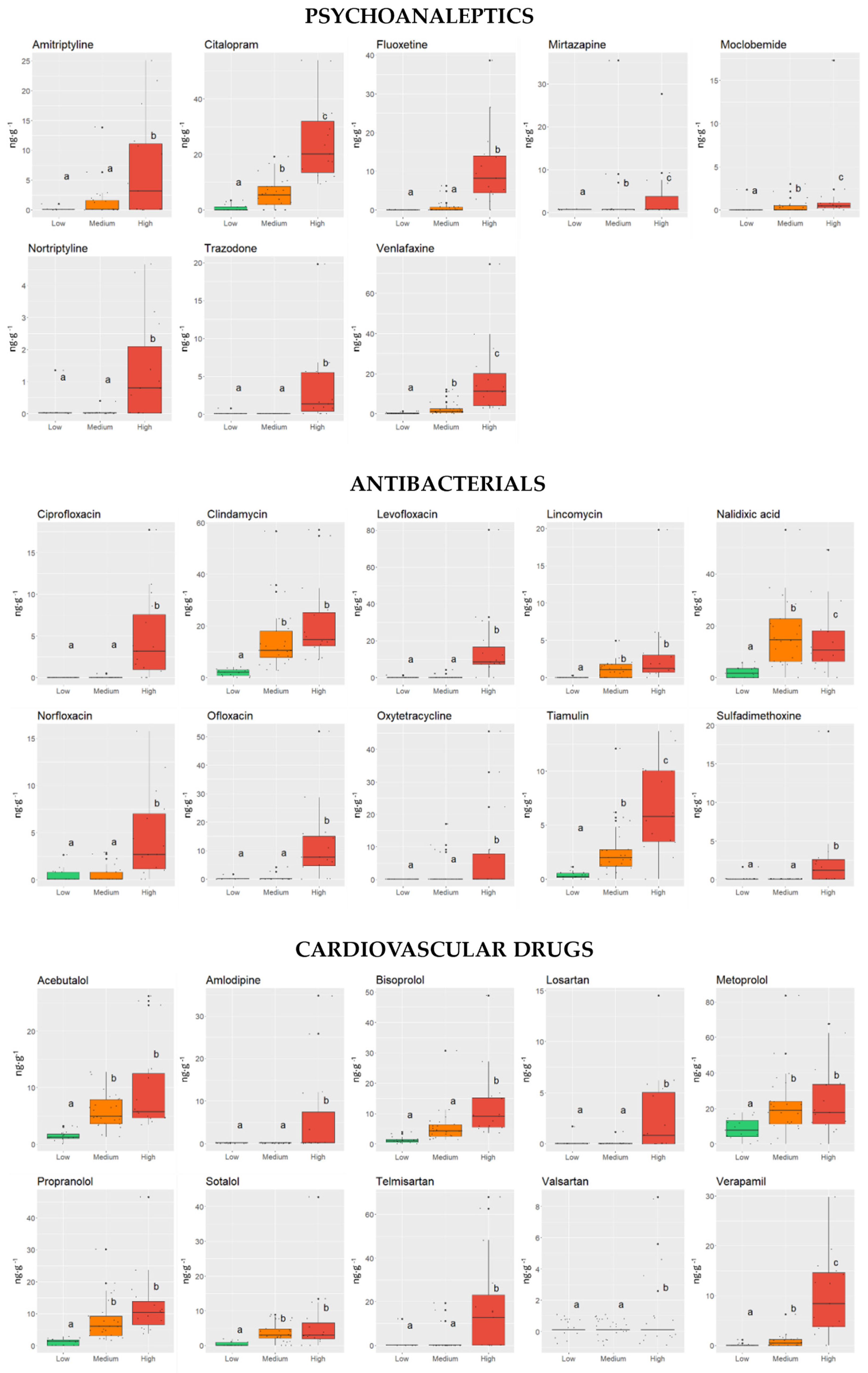
3. Results and Discussion
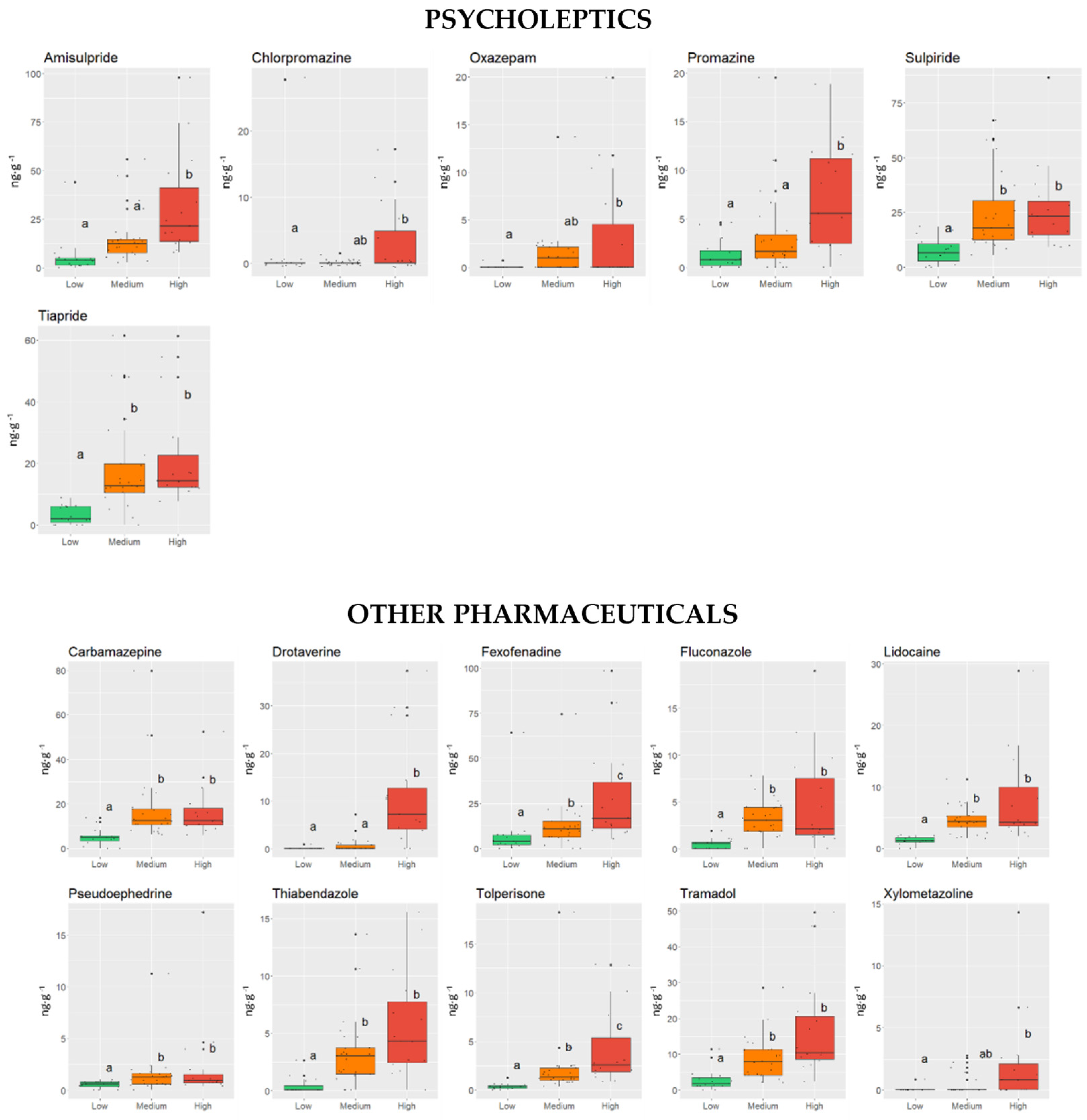
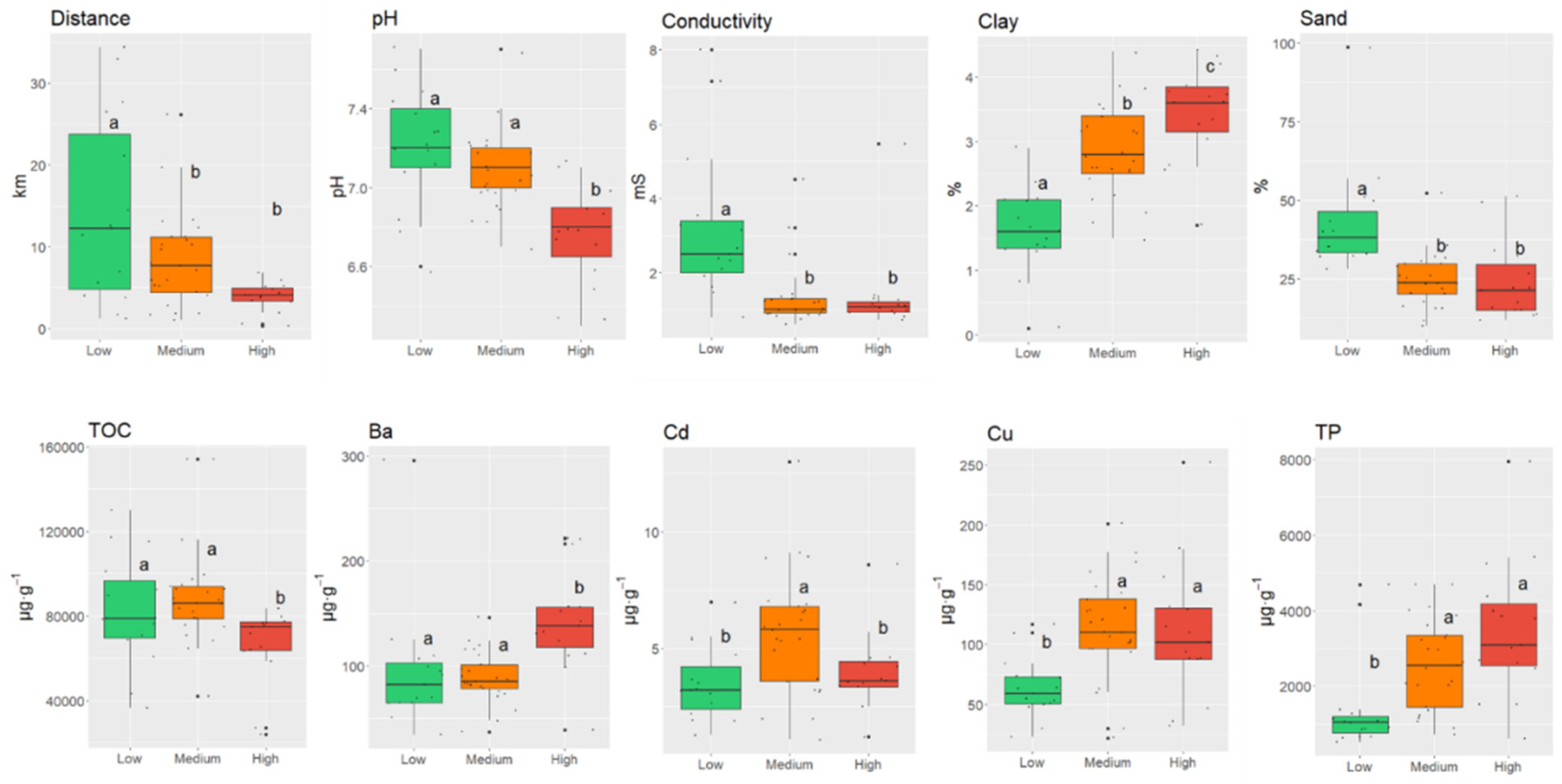
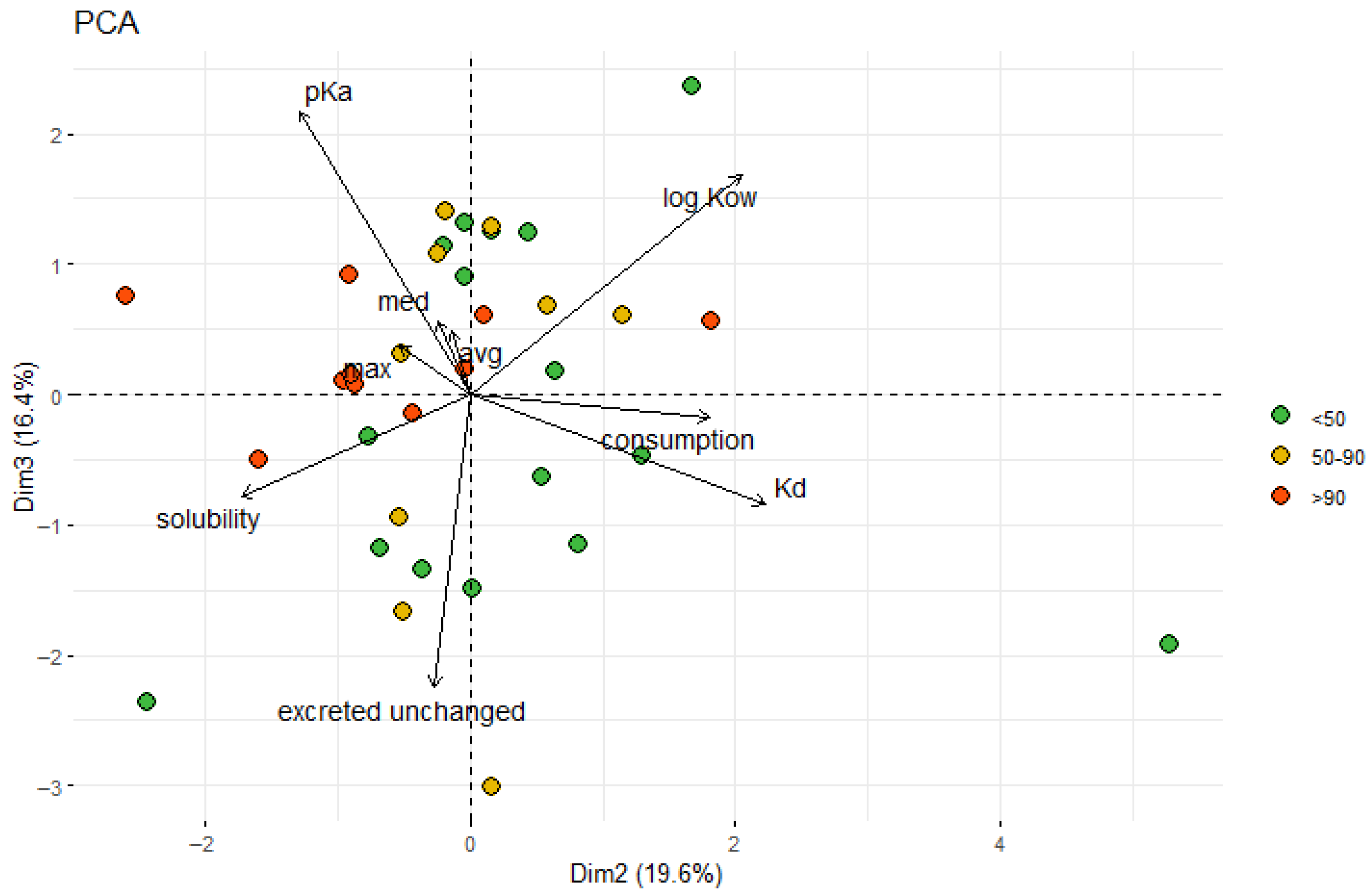
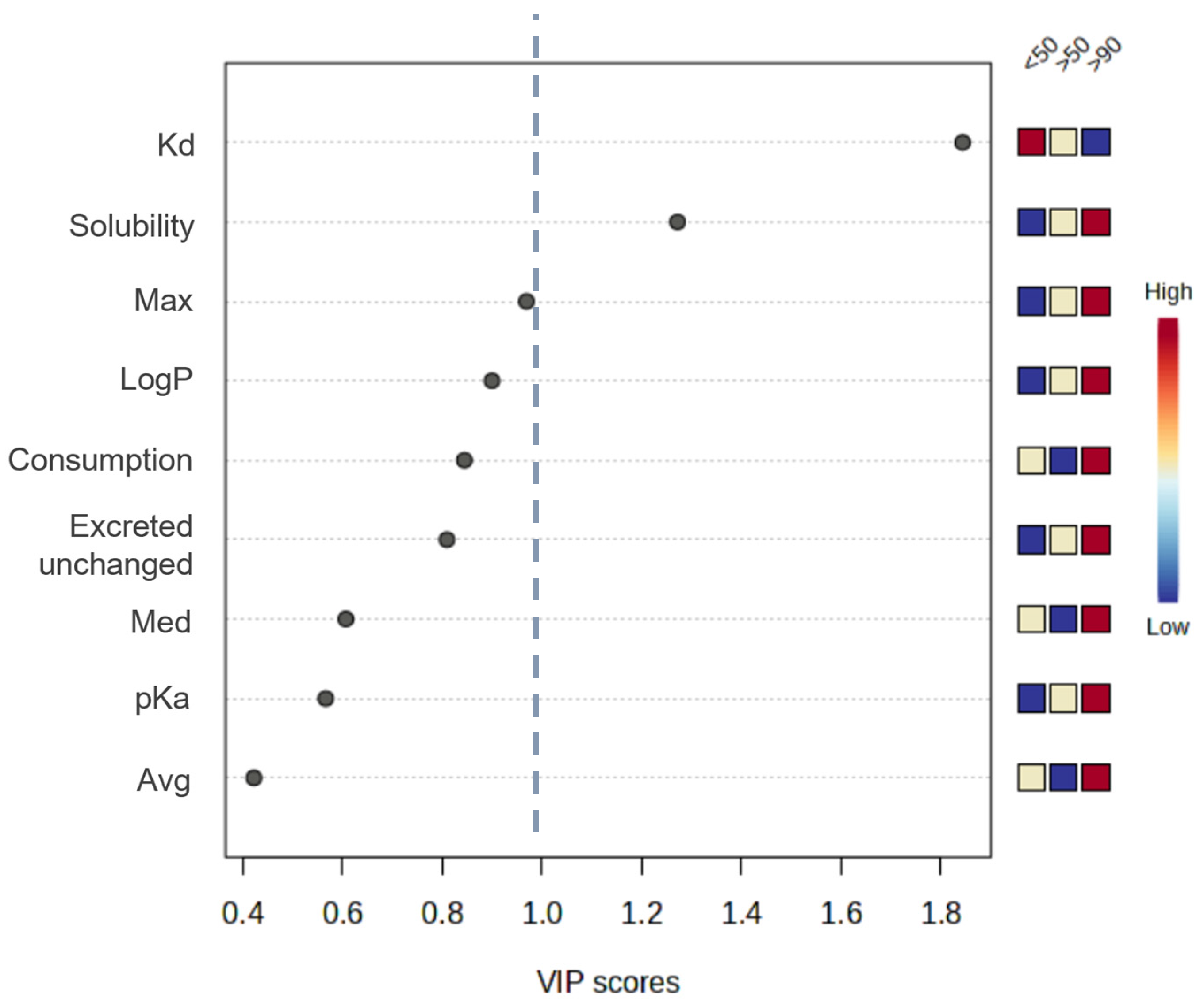
3.1. Influence of Physical and Chemical Characteristics of Sediments on the Occurrence of PhACs
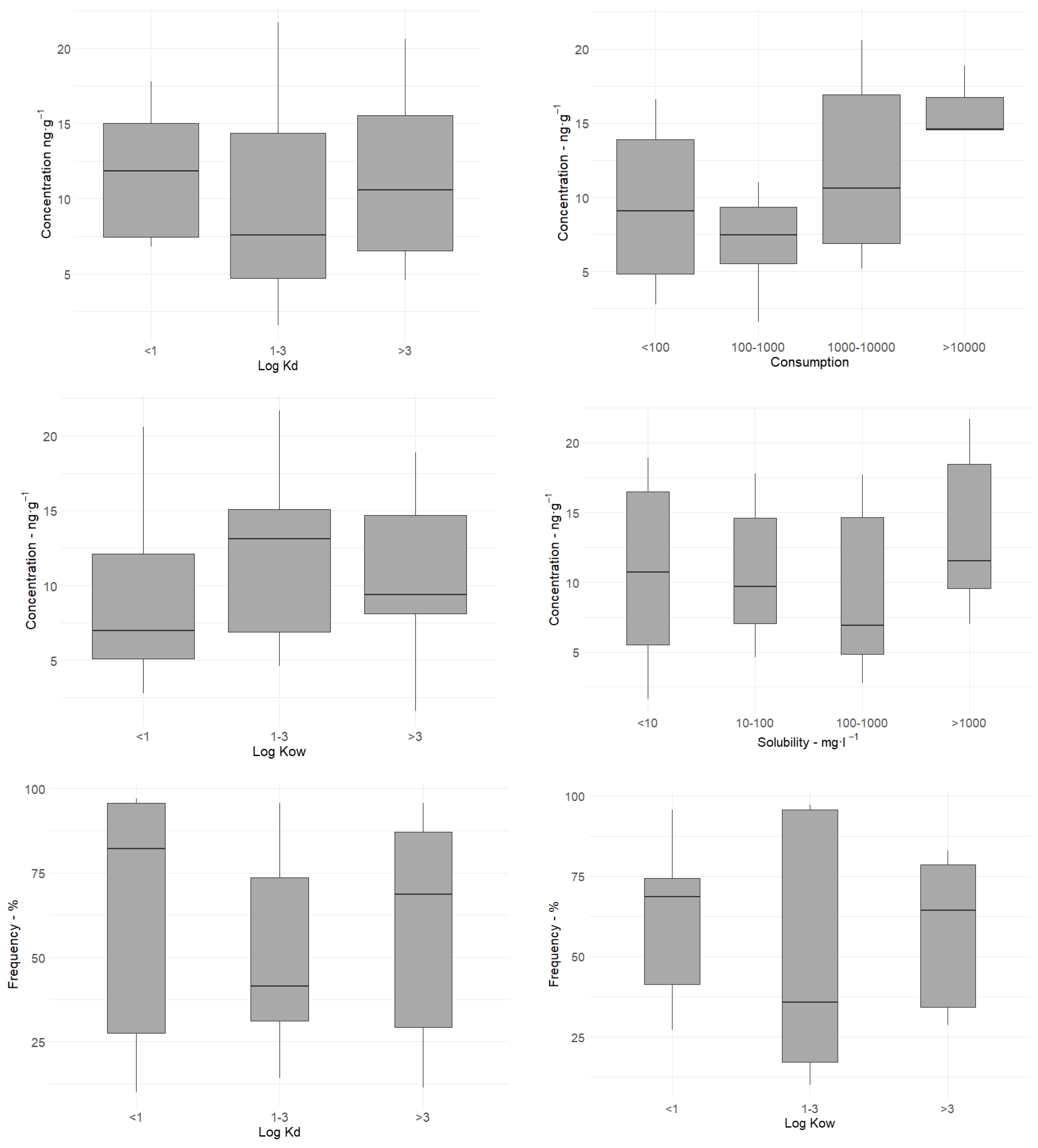
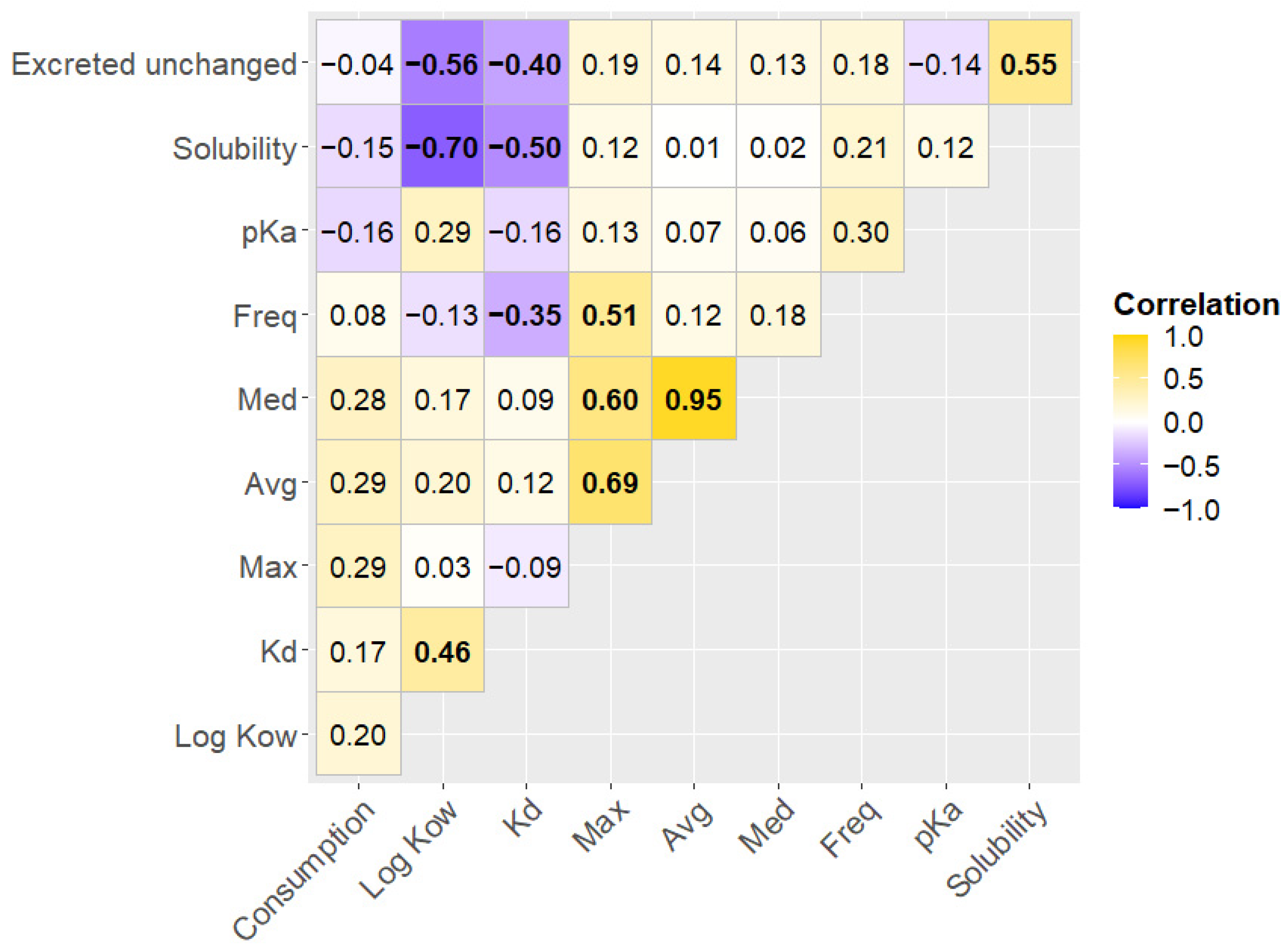
3.2. Influence of Physical and Chemical Parameters of PhACs on Their Occurrence in Sediments
4. Methodology
4.1. The Analysis of Clay Minerals in Sediments
4.2. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adeleye, A.S.; Xue, J.; Zhao, Y.; Taylor, A.A.; Zenobio, J.E.; Sun, Y.; Han, Z.; Salawu, O.A.; Zhu, Y. Abundance, fate, and effects of pharmaceuticals and personal care products in aquatic environments. J. Hazard. Mater. 2022, 424, 127284. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Kumar, R.; Kishor, K.; Mlsna, T.; Pittman, C.U., Jr.; Mohan, D. Pharmaceuticals of Emerging Concern in Aquatic Systems: Chemistry, Occurrence, Effects, and Removal Methods. Chem. Rev. 2019, 119, 3510–3673. [Google Scholar] [CrossRef] [PubMed]
- Mompelat, S.; Le Bot, B.; Thomas, O. Occurrence and fate of pharmaceutical products and by-products, from resource to drinking water. Environ. Int. 2009, 35, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Azuma, T.; Ishida, M.; Hisamatsu, K.; Yunoki, A.; Otomo, K.; Kunitou, M.; Shimizu, M.; Hosomaru, K.; Mikata, S.; Mino, Y. A method for evaluating the pharmaceutical deconjugation potential in river water environments. Chemosphere 2017, 180, 476–482. [Google Scholar] [CrossRef] [PubMed]
- Heberer, T. Occurrence, fate, and removal of pharmaceutical residues in the aquatic environment: A review of recent research data. Toxicol. Lett. 2002, 131, 5–17. [Google Scholar] [CrossRef]
- Heberer, T. Tracking persistent pharmaceutical residues from municipal sewage to drinking water. J. Hydrol. 2002, 266, 175–189. [Google Scholar] [CrossRef]
- Shaheen, J.F.; Sizirici, B.; Yildiz, I. Fate, transport, and risk assessment of widely prescribed pharmaceuticals in terrestrial and aquatic systems: A review. Emerg. Contam. 2022, 8, 216–228. [Google Scholar] [CrossRef]
- Gaw, S.; Thomas, K.V.; Hutchinson, T.H. Sources, impacts and trends of pharmaceuticals in the marine and coastal environment. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130572. [Google Scholar] [CrossRef]
- Nikolaou, A.; Meric, S.; Fatta, D. Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem. 2007, 387, 1225–1234. [Google Scholar] [CrossRef]
- Li, W.C. Occurrence, sources, and fate of pharmaceuticals in aquatic environment and soil. Environ. Pollut. 2014, 187, 193–201. [Google Scholar] [CrossRef]
- Pulicharla, R.; Hegde, K.; Brar, S.K.; Surampalli, R.Y. Tetracyclines metal complexation: Significance and fate of mutual existence in the environment. Environ. Pollut. 2017, 221, 1–14. [Google Scholar] [CrossRef]
- Cuprys, A.; Pulicharla, R.; Brar, S.K.; Drogui, P.; Verma, M.; Surampalli, R.Y. Fluoroquinolones metal complexation and its environmental impacts. Coord. Chem. Rev. 2018, 376, 46–61. [Google Scholar] [CrossRef]
- Khurana, P.; Pulicharla, R.; Brar, S.K. Antibiotic-metal complexes in wastewaters: Fate and treatment trajectory. Environ. Int. 2021, 157, 106863. [Google Scholar] [CrossRef]
- Giebułtowicz, J.; Nałęcz-Jawecki, G. Occurrence of antidepressant residues in the sewage-impacted Vistula and Utrata rivers and in tap water in Warsaw (Poland). Ecotoxicol. Environ. Saf. 2014, 104, 103–109. [Google Scholar] [CrossRef]
- Stankiewicz, A.; Giebułtowicz, J.; Stankiewicz, U.; Wroczyński, P.; Nałęcz-Jawecki, G. Determination of selected cardiovascular active compounds in environmental aquatic samples–Methods and results, a review of global publications from the last 10 years. Chemosphere 2015, 138, 642–656. [Google Scholar] [CrossRef] [PubMed]
- Joseph, N.K.; Azimov, A.M.; Iztleuov, G.M.; Koshy, V.J.; Aravind, U.K.; Sataev, M.I.; Aravindakumar, C.T. Role of the aquatic environment in enhancing and inhibiting phototransformation of pharmaceutically active compounds. Emerg. Contam. 2024, 10, 100371. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nakamura, Y.; Moriguchi, S.; Honda, Y.; Tamura, I.; Hirata, Y.; Hayashi, A.; Sekizawa, J. Persistence and partitioning of eight selected pharmaceuticals in the aquatic environment: Laboratory photolysis, biodegradation, and sorption experiments. Water Res. 2009, 43, 351–362. [Google Scholar] [CrossRef]
- Bagnis, S.; Fitzsimons, M.F.; Snape, J.; Tappin, A.; Comber, S. Processes of distribution of pharmaceuticals in surface freshwaters: Implications for risk assessment. Environ. Chem. Lett. 2018, 16, 1193–1216. [Google Scholar] [CrossRef]
- Liang, X.; Chen, B.; Nie, X.; Shi, Z.; Huang, X.; Li, X. The distribution and partitioning of common antibiotics in water and sediment of the Pearl River Estuary, South China. Chemosphere 2013, 92, 1410–1416. [Google Scholar] [CrossRef]
- Halling-Sørensen, B.; Nielsen, S.N.; Lanzky, P.; Ingerslev, F.; Lützhøft, H.H.; Jørgensen, S. Occurrence, fate and effects of pharmaceutical substances in the environment—A review. Chemosphere 1998, 36, 357–393. [Google Scholar] [CrossRef]
- Pal, A.; Gin, K.Y.-H.; Lin, A.y.-C.; Reinhard, M. Impacts of emerging organic contaminants on freshwater resources: Review of recent occurrences, sources, fate and effects. Sci. Total Environ. 2010, 408, 6062–6069. [Google Scholar] [CrossRef]
- Dawood, A.; Drage, D.S.; Harrad, S.; Abdallah, M.A.-E. Concentrations, partitioning and ecological risk of pharmaceuticals and personal care products in UK freshwater sediment. Environ. Pollut. Manag. 2024, 1, 87–98. [Google Scholar] [CrossRef]
- Martínez-Hernández, V.; Meffe, R.; Herrera, S.; Arranz, E.; de Bustamante, I. Sorption/desorption of non-hydrophobic and ionisable pharmaceutical and personal care products from reclaimed water onto/from a natural sediment. Sci. Total. Environ. 2014, 472, 273–281. [Google Scholar] [CrossRef] [PubMed]
- Al-Khazrajy, O.S.; Boxall, A.B. Impacts of compound properties and sediment characteristics on the sorption behaviour of pharmaceuticals in aquatic systems. J. Hazard. Mater. 2016, 317, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Le Gaudu, M.; Thiebault, T.; Quénéa, K.; Alliot, F.; Guigon, E.; Le Callonnec, L. Trace organic contaminants within solid matrices along an anthropized watercourse: Organo-mineral controls on their spatial distribution. Sci. Total. Environ. 2022, 822, 153601. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Santos, L. Antibiotics in the aquatic environments: A review of the European scenario. Environ. Int. 2016, 94, 736–757. [Google Scholar] [CrossRef]
- Wilkinson, J.L.; Boxall, A.B.A.; Kolpin, D.W.; Leung, K.M.Y.; Lai, R.W.S.; Galbán-Malagón, C.; Adell, A.D.; Mondon, J.; Metian, M.; Marchant, R.A.; et al. Pharmaceutical pollution of the world’s rivers. Proc. Natl. Acad. Sci. USA 2022, 119, e2113947119. [Google Scholar] [CrossRef]
- Vaudreuil, M.-A.; Munoz, G.; Duy, S.V.; Sauvé, S. Tracking down pharmaceutical pollution in surface waters of the St. Lawrence River and its major tributaries. Sci. Total. Environ. 2023, 912, 168680. [Google Scholar] [CrossRef]
- Singh, P.K.; Ranjan, N. Ecological impact of pharmaceutical pollutants and options of river health improvements–A risk analysis-based approach. Sci. Total. Environ. 2024, 928, 172358. [Google Scholar] [CrossRef]
- EC. Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for community action in the field of water policy. Off. J. Eur. Union 2000, L327, 1–73. [Google Scholar]
- EC. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 Amending Directives 2000/60/EC and 2008/105/EC as Regards Priority Substances in the Field of Water Policy. Off. J. Eur. Union 2013, L226, 1–17. [Google Scholar]
- da Silva, B.F.; Jelic, A.; López-Serna, R.; Mozeto, A.A.; Petrovic, M.; Barceló, D. Occurrence and distribution of pharmaceuticals in surface water, suspended solids and sediments of the Ebro river basin, Spain. Chemosphere 2011, 85, 1331–1339. [Google Scholar] [CrossRef]
- Zhou, J.; Broodbank, N. Sediment-water interactions of pharmaceutical residues in the river environment. Water Res. 2014, 48, 61–70. [Google Scholar] [CrossRef]
- Wang, X.-H.; Lin, A.Y.-C. Is the phototransformation of pharmaceuticals a natural purification process that decreases ecological and human health risks? Environ. Pollut. 2014, 186, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Grabicova, K.; Grabic, R.; Blaha, M.; Kumar, V.; Cerveny, D.; Fedorova, G.; Randak, T. Presence of pharmaceuticals in benthic fauna living in a small stream affected by effluent from a municipal sewage treatment plant. Water Res. 2015, 72, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Puckowski, A.; Mioduszewska, K.; Łukaszewicz, P.; Borecka, M.; Caban, M.; Maszkowska, J.; Stepnowski, P. Bioaccumulation and analytics of pharmaceutical residues in the environment: A review. J. Pharm. Biomed. Anal. 2016, 127, 232–255. [Google Scholar] [CrossRef]
- Mankiewicz-Boczek, J.; Nałęcz-Jawecki, G.; Drobniewska, A.; Kaza, M.; Sumorok, B.; Izydorczyk, K.; Zalewski, M.; Sawicki, J. Application of a microbiotests battery for complete toxicity assessment of rivers. Ecotoxicol. Environ. Saf. 2008, 71, 830–836. [Google Scholar] [CrossRef] [PubMed]
- Niemirycz, E.; Nichthauser, J.; Staniszewska, M.; Nałęcz-Jawecki, G.; Bolałek, J. The Microtox® biological test: Application in toxicity evaluation of surface waters and sediments in Poland. Oceanol. Hydrobiol. Stud. 2007, 36, 151–163. [Google Scholar] [CrossRef]
- Drobniewska, A.; Sumorok, B.; Nałęcz-Jawecki, G.; Sawicki, J. Toxicity assessment of sediments and soil from rivers and floodplains in central Poland using a battery of microbiotests—A case study. Fresenius Environ. Bull. 2007, 16, 109–117. [Google Scholar]
- Wagil, M.; Maszkowska, J.; Białk-Bielińska, A.; Caban, M.; Stepnowski, P.; Kumirska, J. Determination of metronidazole residues in water, sediment and fish tissue samples. Chemosphere 2015, 119, S28–S34. [Google Scholar] [CrossRef]
- Silva, L.J.; Pereira, A.M.; Meisel, L.M.; Lino, C.M.; Pena, A. Reviewing the serotonin reuptake inhibitors (SSRIs) footprint in the aquatic biota: Uptake, bioaccumulation and ecotoxicology. Environ. Pollut. 2015, 197, 127–143. [Google Scholar] [CrossRef]
- Michorowska, S.; Kucharski, D.; Chojnacka, J.; Nałęcz-Jawecki, G.; Marek, D.; Giebułtowicz, J. Metabolomic study on ostracods exposed to environmentally relevant concentrations of five pharmaceuticals selected via a novel approach. Sci. Total. Environ. 2024, 946, 174036. [Google Scholar] [CrossRef]
- Williams, M.; Ong, P.L.; Williams, D.B.; Kookana, R.S. Estimating the sorption of pharmaceuticals based on their phar-macological distribution. Environ. Toxicol. Chem. 2009, 28, 2572–2579. [Google Scholar] [CrossRef]
- Harrower, J.; McNaughtan, M.; Hunter, C.; Hough, R.; Zhang, Z.; Helwig, K. Chemical Fate and Partitioning Behavior of Antibiotics in the Aquatic Environment—A Review. Environ. Toxicol. Chem. 2021, 40, 3275–3298. [Google Scholar] [CrossRef] [PubMed]
- Drobniewska, A.; Giebułtowicz, J.; Wawryniuk, M.; Kierczak, P.; Nałęcz-Jawecki, G. Toxicity and bioaccumulation of selected antidepressants in Lemna minor (L.). Ecohydrol. Hydrobiol. 2024, 24, 262–270. [Google Scholar] [CrossRef]
- Nowakowska, K.; Giebułtowicz, J.; Kamaszewski, M.; Adamski, A.; Szudrowicz, H.; Ostaszewska, T.; Solarska-Dzięciołowska, U.; Nałęcz-Jawecki, G.; Wroczyński, P.; Drobniewska, A. Acute exposure of zebrafish (Danio rerio) larvae to environmental concentrations of selected antidepressants: Bioaccumulation, physiological and histological changes. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 229, 108670. [Google Scholar] [CrossRef] [PubMed]
- Kaza, M.; Nałęcz-Jawecki, G.; Sawicki, J. The toxicity of selected pharmaceuticals to the aquatic plant Lemna minor. Fresenius Environ Bull. 2007, 16, 524–531. [Google Scholar]
- Topal, M.; Öbek, E.; Şenel, G.U.; Topal, E.I.A. Removal of tetracycline antibiotic by Lemna gibba L. from aqueous solutions. Water Environ. J. 2018, 34, 37–44. [Google Scholar] [CrossRef]
- Nałęcz-Jawecki, G.; Wawryniuk, M.; Giebułtowicz, J.; Olkowski, A.; Drobniewska, A. Influence of Selected Antidepressants on the Ciliated Protozoan Spirostomum ambiguum: Toxicity, Bioaccumulation, and Biotransformation Products. Molecules 2020, 25, 1476. [Google Scholar] [CrossRef]
- Hubeny, J.; Harnisz, M.; Korzeniewska, E.; Buta, M.; Zieliński, W.; Rolbiecki, D.; Giebułtowicz, J.; Nałęcz-Jawecki, G.; Płaza, G.; Zhou, Z. Industrialization as a source of heavy metals and antibiotics which can enhance the antibiotic resistance in wastewater, sewage sludge and river water. PLoS ONE 2021, 16, e0252691. [Google Scholar] [CrossRef]
- Archer, E.; Petrie, B.; Kasprzyk-Hordern, B.; Wolfaardt, G.M. The fate of pharmaceuticals and personal care products (PPCPs), endocrine disrupting contaminants (EDCs), metabolites and illicit drugs in a WWTW and environmental waters. Chemosphere 2017, 174, 437–446. [Google Scholar] [CrossRef]
- Bączkowska, E.; Jankowska, K.; Artichowicz, W.; Fudala-Ksiazek, S.; Szopińska, M. Emerging Contaminants in Coastal Landscape Park, South Baltic Sea Region: Year-Round Monitoring of Treated Wastewater Discharge into Czarna Wda River. Resources 2025, 14, 123. [Google Scholar] [CrossRef]
- Eggleton, J.; Thomas, K.V. A review of factors affecting the release and bioavailability of contaminants during sediment disturbance events. Environ. Int. 2004, 30, 973–980. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture 2022, 12, 321. [Google Scholar] [CrossRef]
- Kucharski, D.; Nałęcz-Jawecki, G.; Drzewicz, P.; Skowronek, A.; Mianowicz, K.; Strzelecka, A.; Giebułtowicz, J. The assessment of environmental risk related to the occurrence of pharmaceuticals in bottom sediments of the Odra River estuary (SW Baltic Sea). Sci. Total. Environ. 2022, 828, 154446. [Google Scholar] [CrossRef]
- Kucharski, D.; Giebułtowicz, J.; Drobniewska, A.; Nałęcz-Jawecki, G.; Skowronek, A.; Strzelecka, A.; Mianowicz, K.; Drzewicz, P. The study on contamination of bottom sediments from the Odra River estuary (SW Baltic Sea) by tributyltin using environmetric methods. Chemosphere 2022, 308, 136133. [Google Scholar] [CrossRef]
- Majewski, A. The Szczecin Lagoon; Wydawnictwa Komunikacji i Łączności: Warszawa, Poland, 1980. [Google Scholar]
- Jasińska, E.; Massel, S. Water dynamics in estuaries along the Polish Baltic coast. Oceanol. Hydrobiol. Stud. 2007, 36, 101–133. [Google Scholar] [CrossRef]
- Kowalewska-Kalkowska, H. Storm-Surge Induced Water Level Changes in the Odra River Mouth Area (Southern Baltic Coast). Atmosphere 2021, 12, 1559. [Google Scholar] [CrossRef]
- Weyrauch, P.; Matzinger, A.; Pawlowsky-Reusing, E.; Plume, S.; von Seggern, D.; Heinzmann, B.; Schroeder, K.; Rouault, P. Contribution of combined sewer overflows to trace contaminant loads in urban streams. Water Res. 2010, 44, 4451–4462. [Google Scholar] [CrossRef] [PubMed]
- Perry, W.B.; Ahmadian, R.; Munday, M.; Jones, O.; Ormerod, S.J.; Durance, I. Addressing the challenges of combined sewer overflows. Environ. Pollut. 2023, 343, 123225. [Google Scholar] [CrossRef]
- Bojakowska, I.; Sokołowska, G.; Konieczyńska, M. Impact of mine water disposal on concentration of barium and strontium in waters and sediments of the Odra River. Geol. Q. 1998, 42, 113–120. [Google Scholar]
- Atun, G.; Bascetin, E. Adsorption of barium on kaolinite, illite and montmorillonite at various ionic strengths. Radiochim. Acta 2003, 91, 223–228. [Google Scholar] [CrossRef]
- Bharate, S.S. Modulation of biopharmaceutical properties of acidic drugs using cationic counterions: A critical analysis of FDA-approved pharmaceutical salts. Int. J. Pharm. 2021, 607, 120993. [Google Scholar] [CrossRef]
- Radziejewska, T.; Schernewski, G. The Szczecin (Oder-) Lagoon. In Ecological Studies; Schiewer, U., Ed.; Ecology of Baltic Coastal Waters; Springer: Berlin/Heidelberg, Germany, 2008; Volume 197. [Google Scholar] [CrossRef]
- Olsen, C.; Cutshall, N.; Larsen, I. Pollutant—Particle associations and dynamics in coastal marine environments: A review. Mar. Chem. 1982, 11, 501–533. [Google Scholar] [CrossRef]
- Owens, P.N.; Batalla, R.J.; Collins, A.J.; Gomez, B.; Hicks, D.M.; Horowitz, A.J.; Kondolf, G.M.; Marden, M.; Page, M.J.; Peacock, D.H.; et al. Fine-grained sediment in river systems: Environmental significance and management issues. River Res. Appl. 2005, 21, 693–717. [Google Scholar] [CrossRef]
- Droppo, I.G. Rethinking what constitutes suspended sediment. Hydrol. Process. 2001, 15, 1551–1564. [Google Scholar] [CrossRef]
- Liu, Y.; Alessi, D.S.; Flynn, S.L.; Alam, S.; Hao, W.; Gingras, M.; Zhao, H.; Konhauser, K.O. Acid-base properties of kaolinite, montmorillonite and illite at marine ionic strength. Chem. Geol. 2018, 483, 191–200. [Google Scholar] [CrossRef]
- Bavumiragira, J.P.; Ge, J.; Yin, H. Fate and transport of pharmaceuticals in water systems: A processes review. Sci. Total. Environ. 2022, 823, 153635. [Google Scholar] [CrossRef] [PubMed]
- Środoń, J.; Drits, V.A.; McCarty, D.K.; Hsieh, J.C.C.; Eberl, D.D. Quantitative X-Ray Diffraction Analysis of Clay-Bearing Rocks From Random Preparations. Clays Clay Miner. 2001, 49, 514–528. [Google Scholar] [CrossRef]
- Eberl, D.D. User’s Guide to Rockjock—A Program for Determining Quantitative Mineralogy from Powder X-Ray Diffraction Data; U.S. Geological Survey, Open-File Report 03-78; USGS: Boulder, CO, USA, 2003; pp. 1–48. [Google Scholar] [CrossRef]
- Sousa, J.C.; Ribeiro, A.R.; Barbosa, M.O.; Pereira, M.F.R.; Silva, A.M. A review on environmental monitoring of water organic pollutants identified by EU guidelines. J. Hazard. Mater. 2018, 344, 146–162. [Google Scholar] [CrossRef]


| PhACs | Sale *, kg | log Kow (calc.) | Kd, L/kg (calc.) | Freq., % | pKa | Solubility, mg/L | Excreted Unchanged, % |
|---|---|---|---|---|---|---|---|
| Acebutolol | 1001.3 | 1.70 | 1.8 | 94.3 | 13.9 | 259 | 40% |
| Amisulpride | 2472.2 | 1.10 | 46.8 | 97.1 | 9.4 | 293 | 60% |
| Amitriptyline | 355.3 | 4.92 | 37,045.0 | 28.6 | 9.4 | 10.8 | 5% |
| Amlodipine | 2508.9 | 3.00 | 220.2 | 10 | 9.4 | 75.3 | 5% |
| Atropine | 1.1 | 1.80 | 50.6 | 17.1 | 9.4 | 2200 | 50% |
| Bisoprolol | 621.0 | 1.87 | 2.4 | 95.7 | 9.5 | 71 | 50% |
| Carbamazepine | 25,964.6 | 2.45 | 284.1 | 97.1 | 7.0 | 18 | 3% |
| Ciprofloxacin | 6873.9 | 0.28 | 2.6 | 27.1 | 6.1 | 1350 | 50% |
| Citalopram | 3.3 | 3.74 | 1862.2 | 70 | 9.9 | 31 | 30% |
| Clindamycin | 6703.74 | 2.16 | 4.3 | 95.7 | 7.6 | 30.6 | 15% |
| Diclofenac | 6439.0 | 4.51 | 61.2 | 80 | 4.2 | 2.4 | 1% |
| Diltiazem | 1499.7 | 2.80 | 697.4 | 25.7 | 8.9 | 465 | 4% |
| Doxepin | 2.3 | 0.67 | 8426.3 | 71.4 | 9.0 | 31.6 | 3% |
| Fluconazole | 691.7 | 0.50 | 3873.3 | 74.3 | 1.8 | 1000 | 80% |
| Fluoxetine | 574.4 | 4.05 | 15,223.2 | 34.3 | 9.8 | 14 | 1% |
| Levofloxacin | 6.6 | 2.10 | 3.3 | 35.7 | 6.2 | 54 | 100% |
| Lidocaine | 52.4 | 2.44 | 66.7 | 95.7 | 8.0 | 410 | 10% |
| Lincomycin | 0.6 | 0.20 | 4.3 | 57.1 | 7.6 | 927 | 33% |
| Losartan | 3788.2 | 1.19 | 66,794.0 | 15.7 | 5.0 | 8.2 | 4% |
| Metoprolol | 409.0 | 1.88 | 4.6 | 92.9 | 9.7 | 10,000 | 5% |
| Mirtazapine | 1.9 | 2.90 | 2070.6 | 14.3 | 7.9 | 0 | 4% |
| Moclobemide | 483.6 | 10.6 | 15.2 | 41.4 | 10.6 | 4 | 1% |
| Norfloxacin | 0.02 | −1.03 | 6.8 | 41.4 | 6.3 | 280 | 30% |
| Ofloxacin | 46.1 | −0.39 | 3.3 | 30 | 6.0 | 10,000 | 70% |
| Oxazepam | 0.4 | 2.24 | 88.6 | 27.1 | 1.7 | 20 | 2% |
| Propranolol | 1375.1 | 3.48 | 89.4 | 82.9 | 9.4 | 61.7 | 1% |
| Sotalol | 4155.3 | 0.24 | 2.8 | 68.6 | 8.2 | 782 | 90% |
| Sulpiride | 1829.9 | 0.57 | 105.9 | 95.7 | 9.1 | 2280 | 90% |
| Telmisartan | 12,696.3 | 7.70 | 3,319,882.0 | 31.4 | 3.6 | 0 | 97% |
| Tiapride | 6.9 | 0.90 | 44.3 | 91.4 | 8.8 | 252 | 50% |
| Tramadol | 141.9 | 1.34 | 59.0 | 95.7 | 9.4 | 1151 | 40% |
| Trazodone | 6180.1 | 2.68 | 3556.2 | 22.9 | 6.7 | 27.6 | 1% |
| Valsartan | 16,229.1 | 1.50 | 75,161.6 | 11.4 | 4.7 | 180 | 13% |
| Venlafaxine | 5577.2 | 3.80 | 107.5 | 78.6 | 10.1 | 267 | 5% |
| Verapamil | 462.9 | 3.79 | 340,135.6 | 64.3 | 8.9 | 4.5 | 4% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giebułtowicz, J.; Kucharski, D.; Nałęcz-Jawecki, G.; Skowronek, A.; Strzelecka, A.; Maciąg, Ł.; Drzewicz, P. Factors Affecting Distribution of Pharmaceutically Active Compounds in Bottom Sediments of Odra River Estuary (SW Baltic Sea). Molecules 2025, 30, 3935. https://doi.org/10.3390/molecules30193935
Giebułtowicz J, Kucharski D, Nałęcz-Jawecki G, Skowronek A, Strzelecka A, Maciąg Ł, Drzewicz P. Factors Affecting Distribution of Pharmaceutically Active Compounds in Bottom Sediments of Odra River Estuary (SW Baltic Sea). Molecules. 2025; 30(19):3935. https://doi.org/10.3390/molecules30193935
Chicago/Turabian StyleGiebułtowicz, Joanna, Dawid Kucharski, Grzegorz Nałęcz-Jawecki, Artur Skowronek, Agnieszka Strzelecka, Łukasz Maciąg, and Przemysław Drzewicz. 2025. "Factors Affecting Distribution of Pharmaceutically Active Compounds in Bottom Sediments of Odra River Estuary (SW Baltic Sea)" Molecules 30, no. 19: 3935. https://doi.org/10.3390/molecules30193935
APA StyleGiebułtowicz, J., Kucharski, D., Nałęcz-Jawecki, G., Skowronek, A., Strzelecka, A., Maciąg, Ł., & Drzewicz, P. (2025). Factors Affecting Distribution of Pharmaceutically Active Compounds in Bottom Sediments of Odra River Estuary (SW Baltic Sea). Molecules, 30(19), 3935. https://doi.org/10.3390/molecules30193935








