The Synthesis of Novel Glucosylamide Organosilicon Quaternary Ammonium Salts and Long-Lasting Modification of Different Materials
Abstract
1. Introduction
2. Results and Discussion
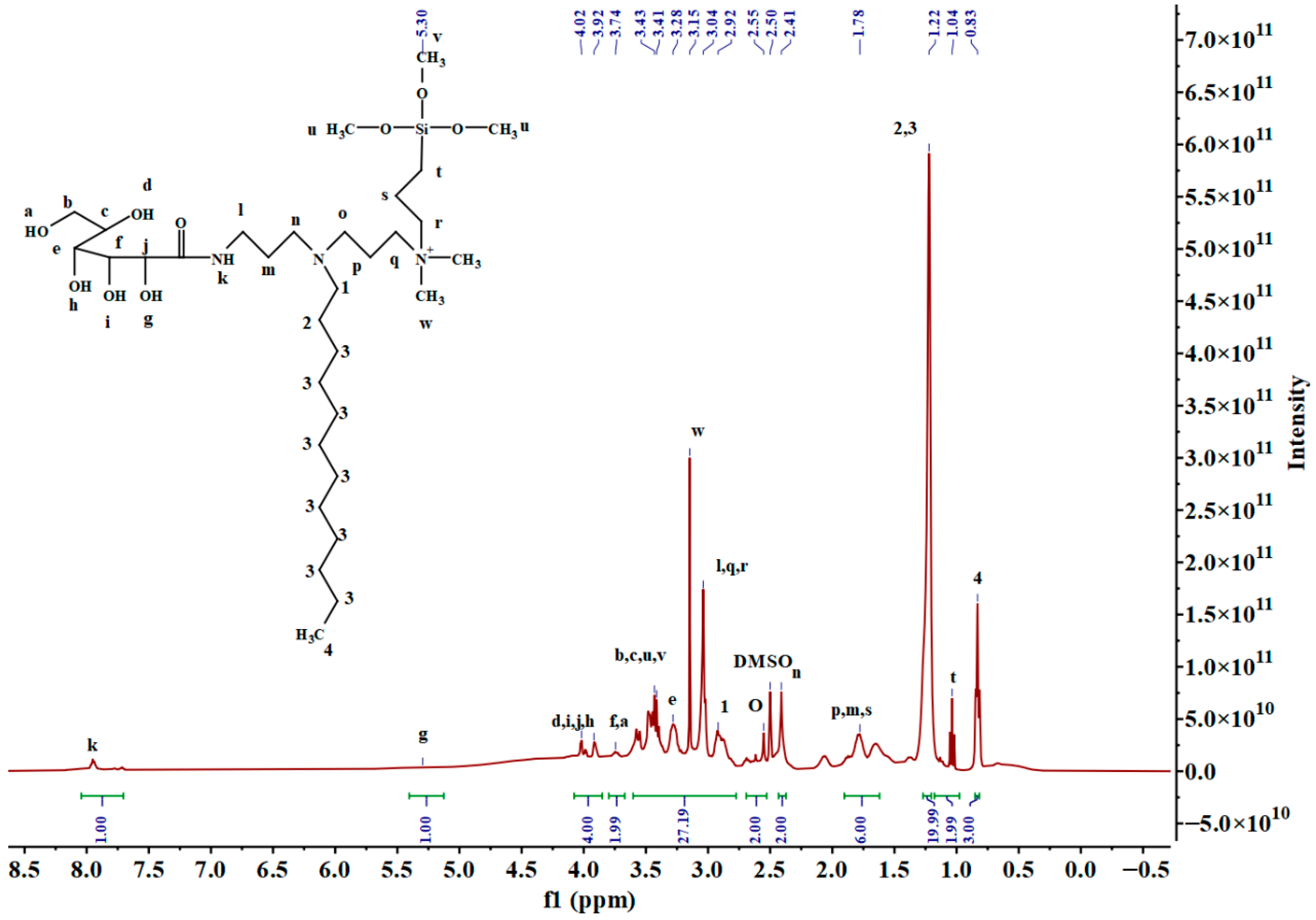
2.1. Structural Identification
2.2. Surface Activity
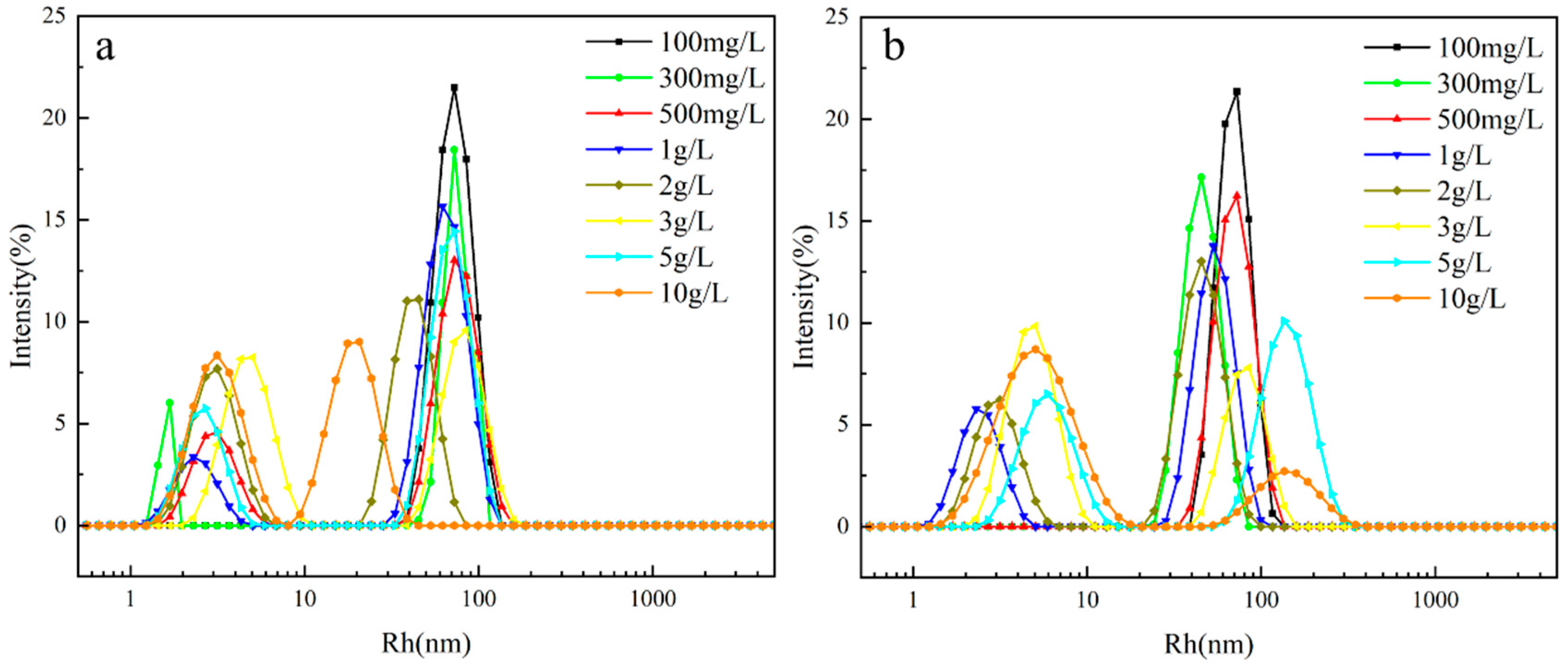
2.3. Aggregation Behavior in an Aqueous Solution
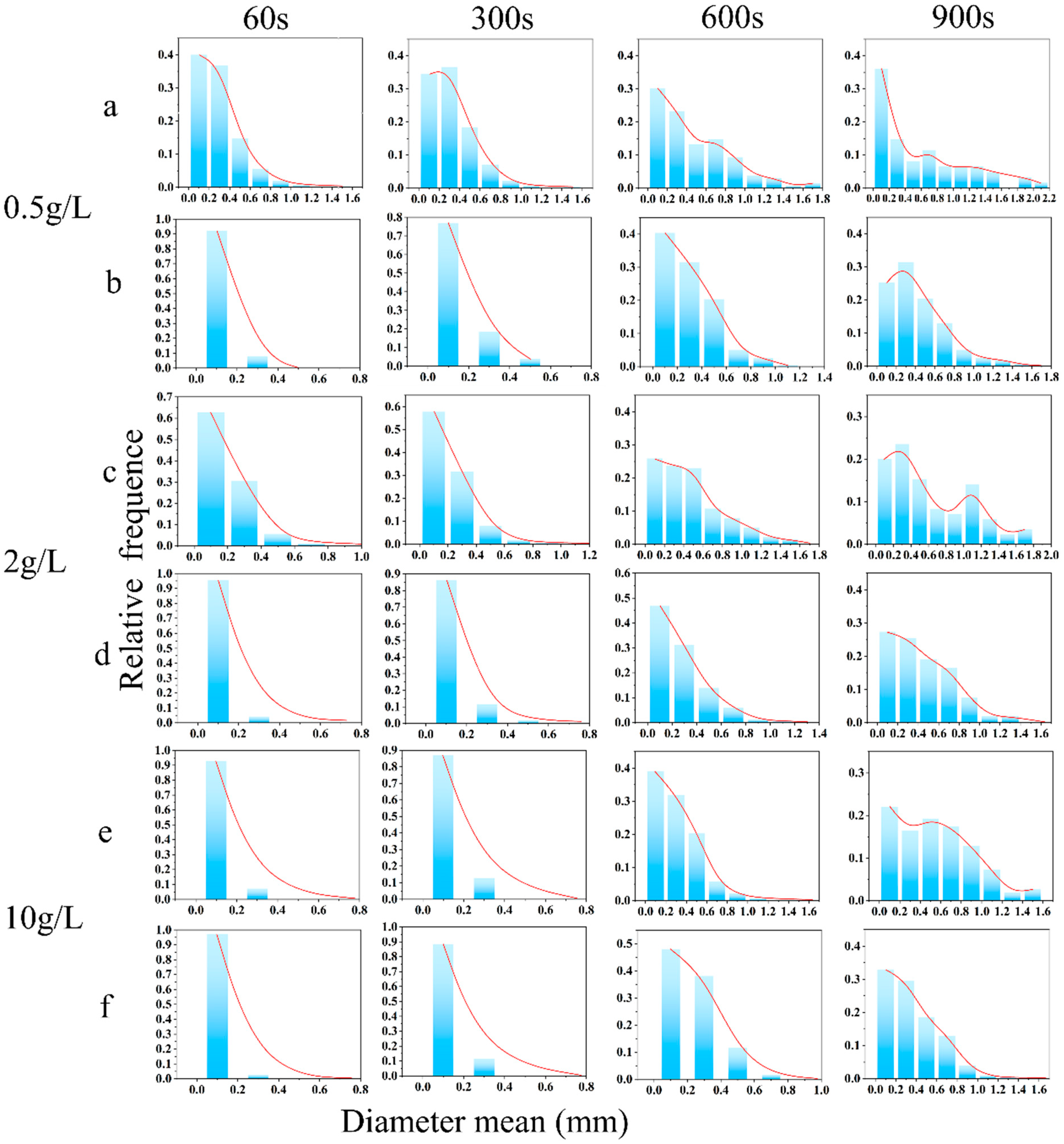
2.4. Foam Properties
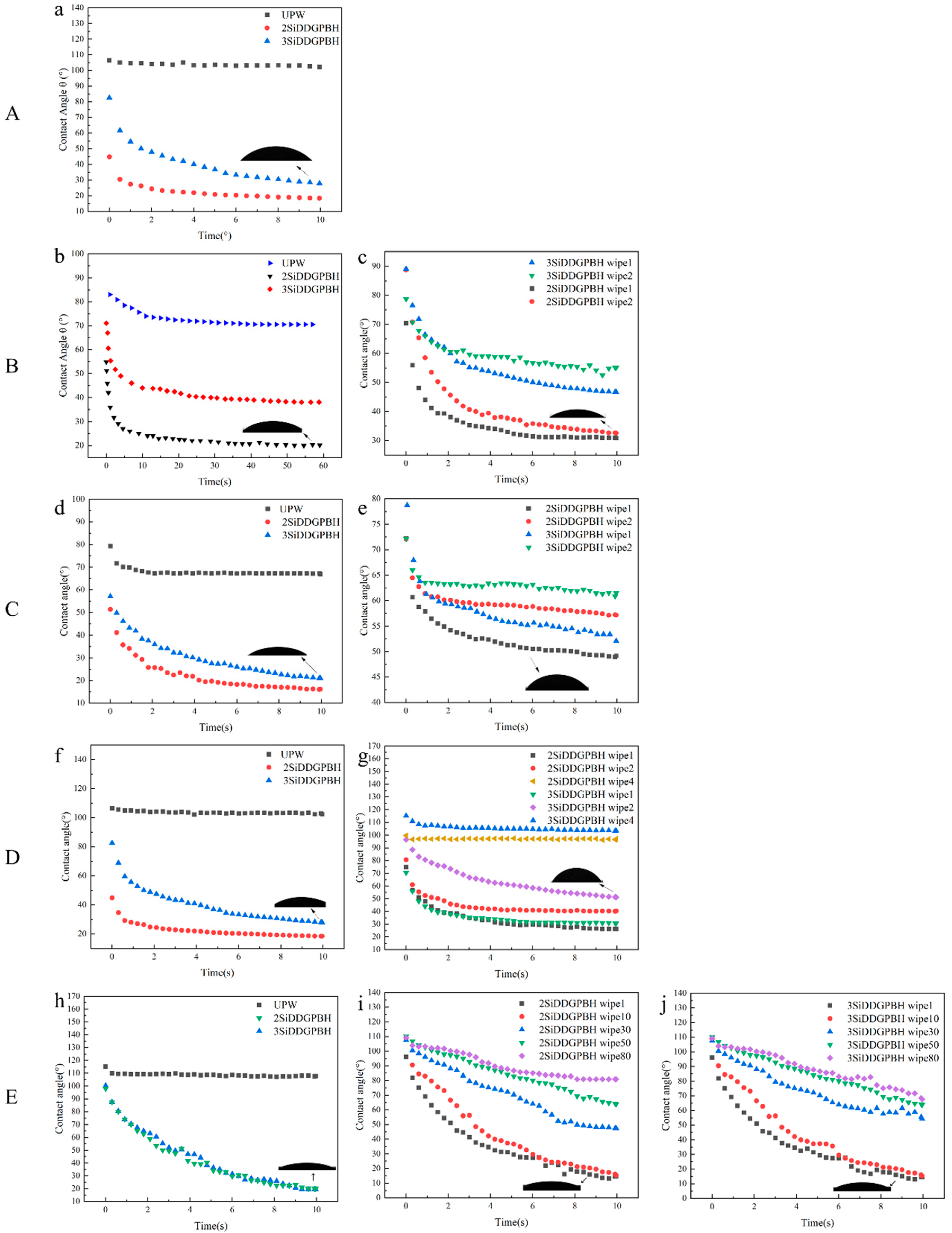
2.5. Spreading Performance
3. Materials and Methods
3.1. Materials and Apparatus
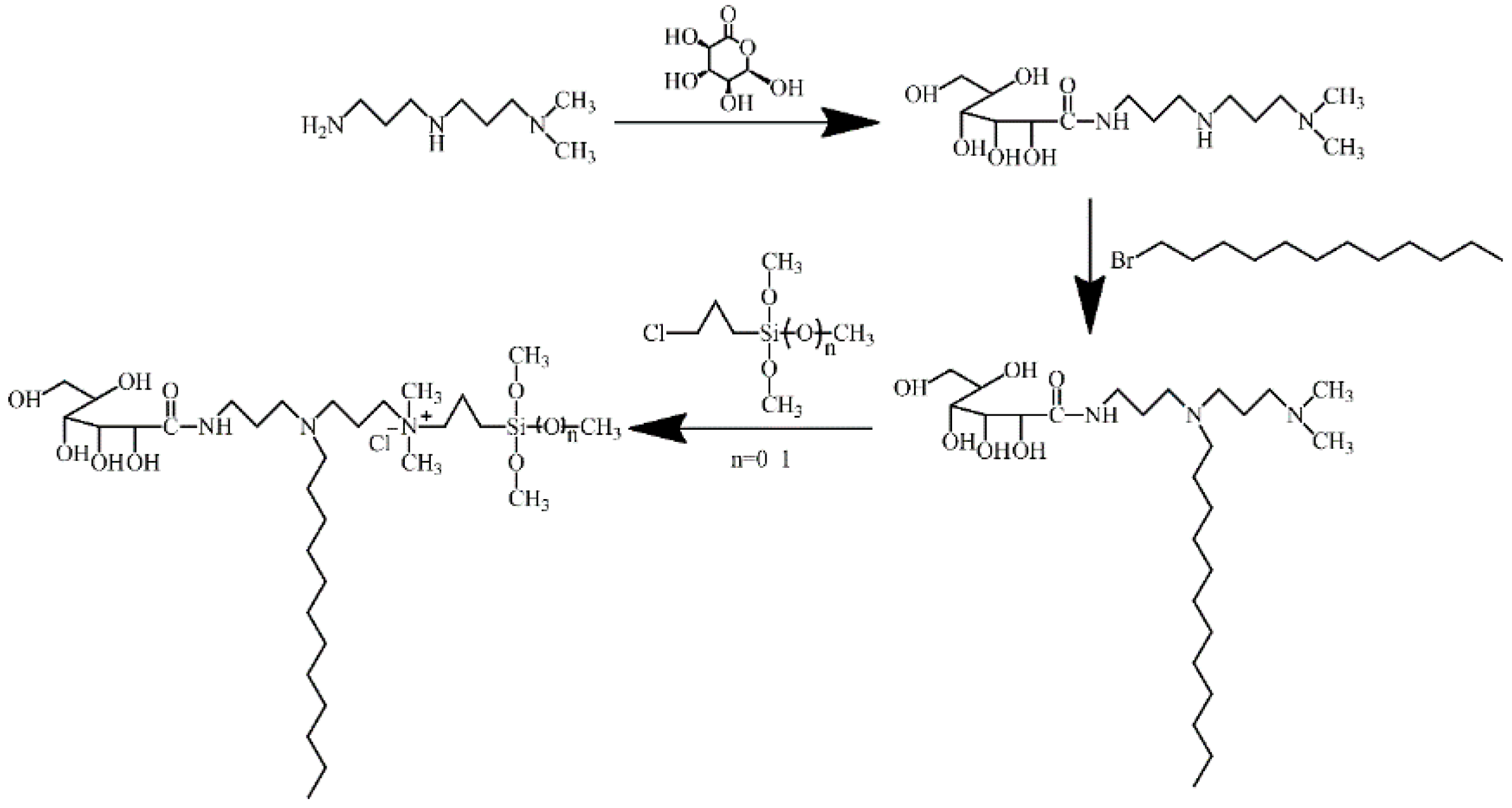
3.2. Synthesis Methods of 2/3SiDDGPBH
3.3. Characterization of 2/3SiDDGPBH
3.4. Surface Tension
3.5. Dynamic Surface Tension
3.6. Transmission Electron Microscopy
3.7. Dynamic Light Scattering
3.8. Foam Morphology Characterization
3.9. Contact Angle
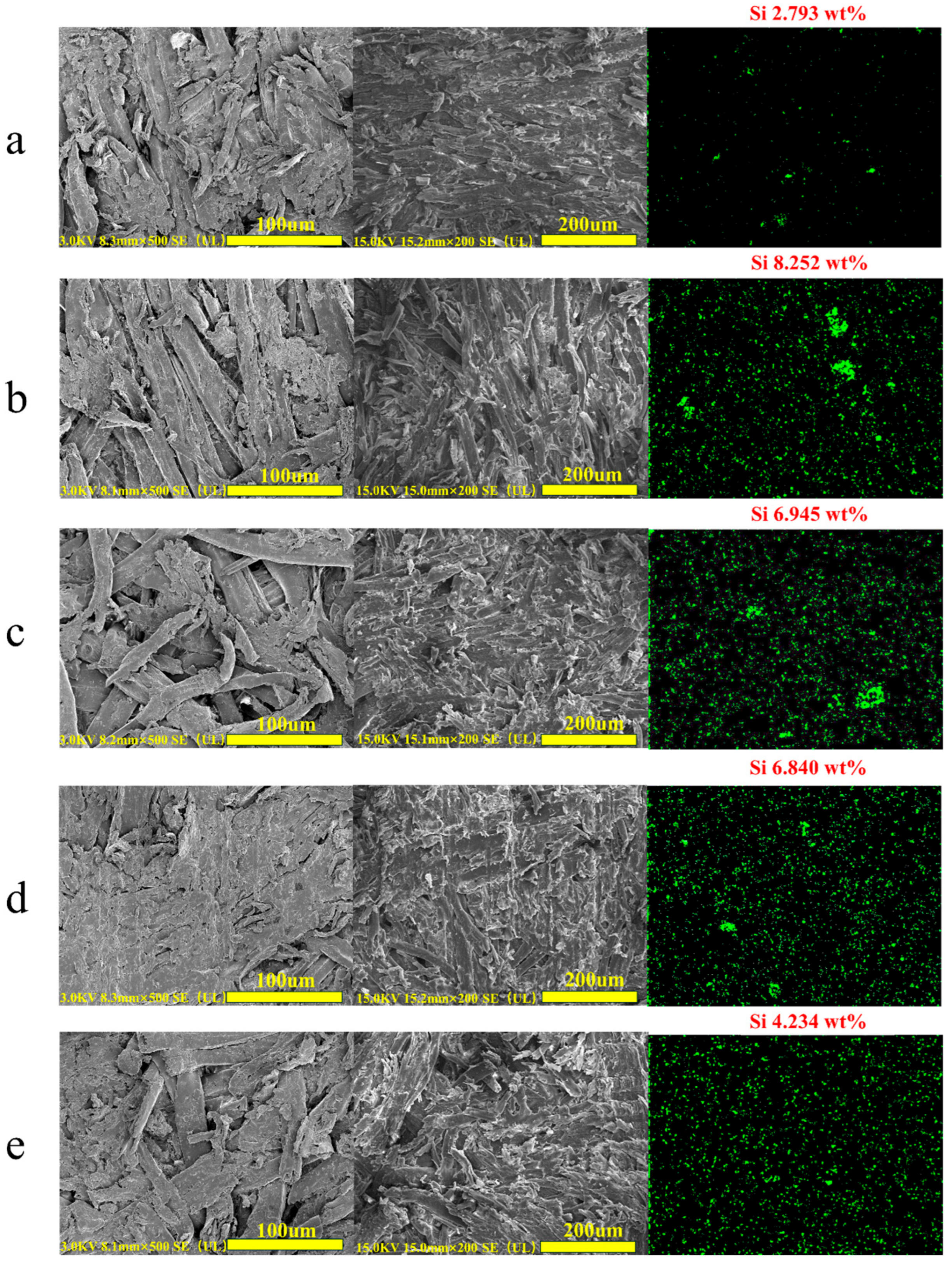
3.10. Scanning Electron Microscopy (SEM)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bao, Z.; Wu, Y.; Liu, R.; Zhang, S.; Chen, Y.; Wu, T.; Gao, Y.; Zhang, C.; Du, F. Molecular selection and environmental evaluation of eco-friendly surfactants to efficiently reduce pesticide pollution. J. Clean. Prod. 2023, 416, 137954. [Google Scholar] [CrossRef]
- Li, J.; Huang, X.; Zhao, Y. Research on the evolution of supervision strategy of renewable energy+ energy storage under China’s carbon peaking and carbon neutrality goal. Environ. Sci. Pollut. Res. 2023, 30, 69221–69240. [Google Scholar] [CrossRef]
- Caillol, S. Chemistry in 2025: Shaping the future of sustainable innovation and eco-friendly materials. Green Mater. 2025, 13, 1–2. [Google Scholar] [CrossRef]
- Morris, S.A.V.; Kasting, G.B.; Ananthapadmanabhan, K.P. Surfactant equilibria and its impact on penetration into stratum corneum. Curr. Opin. Colloid Interface Sci. 2022, 59, 101579. [Google Scholar] [CrossRef]
- Shi, X.; Zeng, M.; Xu, X.; Liu, Y.; Kou, J.; Bian, Q.; Song, H.; Zhang, J.; Wang, Q. Promotion of droplet deposition, diffusion-wetting and retention on hydrophobic surfaces by nonionic star-shaped oligomeric surfactants. J. Mol. Liq. 2023, 386, 122521. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Tu, Z.; Hua, W.; He, P.; Li, H.; Zhang, B.; Ren, T. Influence of the hydrophilic moiety of polymeric surfactant on their surface activity and physical stability of pesticide suspension concentrate. J. Mol. Liq. 2020, 317, 114136. [Google Scholar] [CrossRef]
- Li, Q.; Wei, B.; Ye, Q.; Xie, Q.; Wang, Y.; Wang, S.; Lu, J. Rapid design and optimization of complex surfactant microemulsions for high-efficiency oil extraction processes integrating experimental and modeling approaches. Sep. Purif. Technol. 2025, 354, 128930. [Google Scholar] [CrossRef]
- Zhu, B.Y.; Gu, T. Surfactant adsorption at solid-liquid interfaces. Adv. Colloid Interface Sci. 1991, 37, 1–32. [Google Scholar] [CrossRef]
- Ngo, I.; Srisuriyachai, F.; Sugai, Y.; Sasaki, K. Study of heterogeneous reservoir effects on surfactant flooding in consideration of surfactant adsorption reversibility. In SPWLA Formation Evaluation Symposium of Japan; SPWLA: Chiba, Japan, 2017; p. SPWLA-JFES-2017-A. [Google Scholar]
- Wang, S.H.; Tang, T.W.H.; Wu, E.; Wang, D.W.; Wang, C.F.; Liao, Y.D. Inhibition of bacterial adherence to biomaterials by coating antimicrobial peptides with anionic surfactant. Colloid Surf. B 2020, 196, 111364. [Google Scholar] [CrossRef]
- Zhang, R.G.; Feng, R.; Wang, F.; Li, H.L.; Sun, R.Y.; Gao, H.H.; Li, C.B.; Wang, Y.Z.; Song, F. A surfactant-mediated dynamic protection strategy for 100% waterborne fabrication of durable superhydrophobic coatings. Chem. Eng. J. 2024, 499, 156683. [Google Scholar] [CrossRef]
- Gao, W.; Dang, Z.; Liu, F.; Wang, S.; Zhang, D.; Yan, M. Preparation of antistatic epoxy resin coatings based on double comb-like quaternary ammonium salt polymers. RSC Adv. 2020, 10, 43523–43532. [Google Scholar] [CrossRef] [PubMed]
- Gizer, S.G.; Bhethanabotla, V.R.; Ayyala, R.S.; Sahiner, N. Low-pressure plasma treated polycarbonate and polymethyl methacrylate (PMMA) sheets with different surface patterns to change their surface properties. Surf. Interfaces 2023, 37, 102646. [Google Scholar] [CrossRef]
- Zafar, M.S. Prosthodontic Applications of Polymethyl Methacrylate (PMMA): An Update. Polymers 2020, 12, 2299. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Ndudi, W.; Ali, A.B.M.; Yousif, E.; Zainulabdeen, K.; Onyibe, P.N.; Akpoghelie, P.O.; Ekokotu, H.A.; Isoje, E.F.; Igbuku, U.A.; et al. An updated review on the modifications, recycling, polymerization, and applications of polymethyl methacrylate (PMMA). J. Mater. Sci. 2024, 59, 20496–20539. [Google Scholar] [CrossRef]
- Appah, S.; Jia, W.; Ou, M.; Wang, P.; Asante, E.A. Analysis of potential impaction and phytotoxicity of surfactant-plant surface interaction in pesticide application. Crop Prot. 2020, 127, 104961. [Google Scholar] [CrossRef]
- Sar, P.; Ghosh, A.; Scarso, A.; Saha, B. Surfactant for better tomorrow: Applied aspect of surfactant aggregates from laboratory to industry. Res. Chem. Intermed. 2019, 45, 6021–6041. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Yuan, C.; Cao, P.; Bai, X. Antifouling applications and fabrications of biomimetic micro-structured surfaces: A review. J. Adv. Res. 2024, 59, 201–221. [Google Scholar] [CrossRef]
- Jin, L.; Xu, W.; Wen, H.; Wang, Y.; Zhang, F. Imparting waterproofing properties to leather by polymer nanoemulsion based on long-chain alkyl acrylate. Materials 2023, 16, 1464. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Jin, Y.; Shen, Y.; Zhao, P.; Zhou, Y. Synthesis and application of non-bioaccumulable fluorinated surfactants: A review. Leather Sci. Eng. 2021, 3, 6. [Google Scholar] [CrossRef]
- Merline, D.J.; Vukusic, S.; Abdala, A.A. Melamine formaldehyde: Curing studies and reaction mechanism. Polym. J. 2013, 45, 413–419. [Google Scholar] [CrossRef]
- Dorieh, A.; Pour, M.F.; Movahed, S.G.; Pizzi, A.; Selakjani, P.P.; Kiamahalleh, M.V.; Hatefnia, H.; Shahavi, M.H.; Aghaei, R. A review of recent progress in melamine-formaldehyde resin based nanocomposites as coating materials. Prog. Org. Coat. 2022, 165, 106768. [Google Scholar] [CrossRef]
- Blanchet, P.; Pepin, S. Trends in chemical wood surface improvements and modifications: A review of the last five years. Coatings 2021, 11, 1514. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Sharma, R.; Mahajan, S.; Mahajan, R.K. Surface adsorption and mixed micelle formation of surface active ionic liquid in cationic surfactants: Conductivity, surface tension, fluorescence and NMR studies. Colloid Surf. A 2013, 427, 62–75. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, Z.; Zhang, E.; Zhi, L.; Di Serio, M.; Wang, G.; Wang, Y.; Li, X.; Liu, X.; Huang, Y. Aggregation Behavior and Application Properties of Novel Glycosylamide Quaternary Ammonium Salts in Aqueous Solution. Molecules 2024, 29, 2749. [Google Scholar] [CrossRef]
- Wang, Y.; Dou, J.; Bian, Q.; Zhi, L.; Han, Z.; Wang, G. Physicochemical Properties of Polyether-Modified Fluorosilicone Surfactants in Ethanol/Water Mixed Solutions. Langmuir 2025, 41, 11173–11182. [Google Scholar] [CrossRef]
- Shaban, S.M.; Fouda, A.S.; Elmorsi, M.A.; Fayed, T.; Azazy, O. Adsorption and micellization behavior of synthesized amidoamine cationic surfactants and their biological activity. J. Mol. Liq. 2016, 216, 284–292. [Google Scholar] [CrossRef]
- Dou, J.; Liu, J.; Wang, Y.; Zhi, L.; Shen, J.; Wang, G. Surface activity, wetting, and aggregation of a perfluoropolyether quaternary ammonium salt surfactant with a hydroxyethyl group. Molecules 2023, 28, 7151. [Google Scholar] [CrossRef]
- Peng, M.; Duignan, T.T.; Nguyen, C.V.; Nguyen, A.V. From surface tension to molecular distribution: Modeling surfactant adsorption at the air–water interface. Langmuir 2021, 37, 2237–2255. [Google Scholar] [CrossRef]
- Eastoe, J.; Dalton, J.S. Dynamic surface tension and adsorption mechanisms of surfactants at the air-water interface. Adv. Colloid Interface Sci. 2000, 85, 103–144. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.H.; Franses, E.I. Adsorption dynamics of surfactants at the air/water interface: A critical review of mathematical models, data, and mechanisms. Colloid Surf. A 1995, 100, 1–45. [Google Scholar] [CrossRef]
- Gao, T.; Rosen, M.J. Dynamic surface tension of aqueous surfactant solutions: 7. Physical significance of dynamic parameters and the induction period. J. Colloid Interface Sci. 1995, 172, 242–248. [Google Scholar] [CrossRef]
- Vinogradov, A.V.; Kuprin, D.S.; Abduragimov, I.M.; Kuprin, G.N.; Serebriyakov, E.; Vinogradov, V.V. Silica Foams for Fire Prevention and Firefighting. ACS Appl. Mater. Interfaces 2016, 8, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Bois, R.; Pezron, I.; Rotureau, P.; Van Hecke, E.; Fayet, G.; Nesterenko, A. Foaming behavior of sugar-based surfactants: Influence of molecular structure and anticipation from surface properties. J. Dispers. Sci. Technol. 2023, 44, 840–851. [Google Scholar] [CrossRef]
- Kovalchuk, N.M.; Trybala, A.; Starov, V.; Ivanova, N. Fluoro-vs hydrocarbon surfactants: Why do they differ in wetting performance. Adv. Colloid Interface Sci. 2014, 210, 65–71. [Google Scholar] [CrossRef]
- Arjmandi-Tash, O.; Kovalchuk, N.M.; Trybala, A.; Kuchin, I.V.; Starov, V. Kinetics of wetting and spreading of droplets over various substrates. Langmuir 2017, 33, 4367–4385. [Google Scholar] [CrossRef]
- Shen, J.; Bai, Y.; Tai, X.; Wang, G. Surface activity, spreading, and aggregation behavior of ecofriendly perfluoropolyether amide propyl betaine in aqueous solution. ACS Sustain. Chem. Eng. 2018, 6, 6183–6191. [Google Scholar] [CrossRef]
- Li, J.; Bai, Y.; Wang, W.; Tai, X.; Wang, G. Green glucamine-based trisiloxane surfactant: Surface activity, aggregate behavior, and superspreading on hydrophobic surfaces. ACS Sustain. Chem. Eng. 2019, 7, 4390–4398. [Google Scholar] [CrossRef]
- Van Ooij, W.J.; Zhu, D.; Stacy, M.; Mugada, T.; Gandhi, J.; Puomi, P. Corrosion protection properties of organofunctional silanes—An overview. Tsinghua Sci. Technol. 2005, 10, 639–664. [Google Scholar] [CrossRef]
- Mader, A.; Schirò, A.; Brischetto, M.; Pizzo, B. Interactions and penetration of polymers and nanolatexes into wood: An overview. Prog. Org. Coat. 2011, 71, 123–135. [Google Scholar] [CrossRef]
- Lee, K.S.; Ivanova, N.; Starov, V.M.; Dutschk, V. Kinetics of wetting and spreading by aqueous surfactant solutions. Adv. Colloid Interface Sci. 2008, 144, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Weiss, S.; Seidl, R.; Kessler, W.; Kessler, R.W.; Zikulnig-Rusch, E.M.; Kandelbauer, A. Unravelling the phases of melamine formaldehyde resin cure by infrared spectroscopy (FTIR) and multivariate curve resolution (MCR). Polymers 2020, 12, 2569. [Google Scholar] [CrossRef] [PubMed]


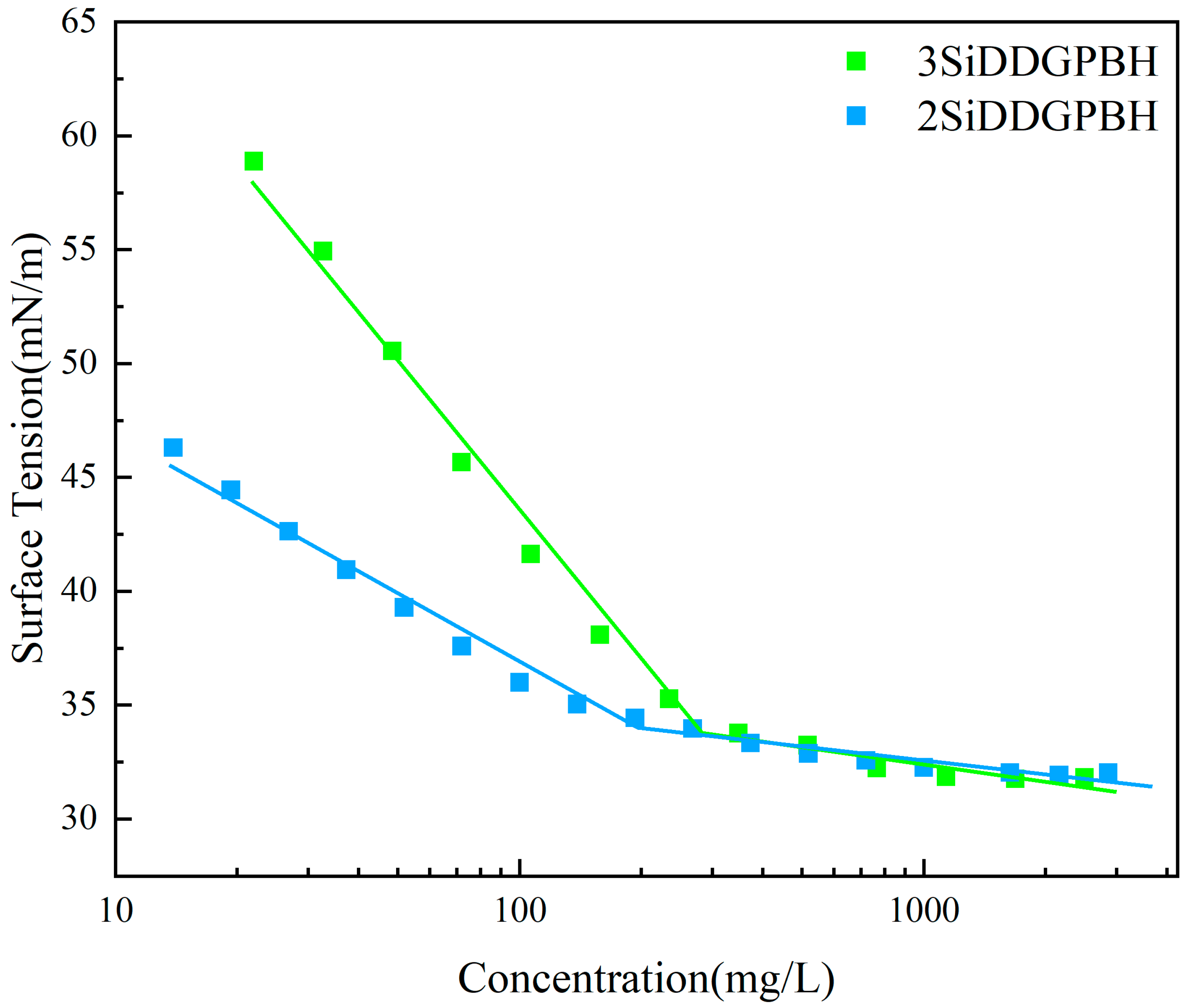




| Surfactant | CMC (g/L) | γcmc (mN/m) | Γmax (mol·cm−2) | Amin (Å2) | (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|---|---|
| 2SiDDGPBH | 0.203 | 33.4 | 8.27 × 10−11 | 200.91 | −81.09 | −25.89 |
| 3SiDDGPBH | 0.285 | 33.64 | 7.95 × 10−11 | 208.87 | −80.99 | −25.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Wang, Y.; Wang, J.; Zhi, L.; Li, L.; Li, X.; Wu, C.; Jin, R.; Ma, Z.; Han, Z.; et al. The Synthesis of Novel Glucosylamide Organosilicon Quaternary Ammonium Salts and Long-Lasting Modification of Different Materials. Molecules 2025, 30, 3934. https://doi.org/10.3390/molecules30193934
Meng X, Wang Y, Wang J, Zhi L, Li L, Li X, Wu C, Jin R, Ma Z, Han Z, et al. The Synthesis of Novel Glucosylamide Organosilicon Quaternary Ammonium Salts and Long-Lasting Modification of Different Materials. Molecules. 2025; 30(19):3934. https://doi.org/10.3390/molecules30193934
Chicago/Turabian StyleMeng, Xiangji, Yunkai Wang, Jingru Wang, Lifei Zhi, Linfei Li, Xiaoming Li, Chan Wu, Rui Jin, Ziyong Ma, Zhiwang Han, and et al. 2025. "The Synthesis of Novel Glucosylamide Organosilicon Quaternary Ammonium Salts and Long-Lasting Modification of Different Materials" Molecules 30, no. 19: 3934. https://doi.org/10.3390/molecules30193934
APA StyleMeng, X., Wang, Y., Wang, J., Zhi, L., Li, L., Li, X., Wu, C., Jin, R., Ma, Z., Han, Z., & Liu, X. (2025). The Synthesis of Novel Glucosylamide Organosilicon Quaternary Ammonium Salts and Long-Lasting Modification of Different Materials. Molecules, 30(19), 3934. https://doi.org/10.3390/molecules30193934







