Efficient Approaches to the Design of Six-Membered Polyazocyclic Compounds—Part 2: Nonaromatic Frameworks
Abstract
1. Introduction
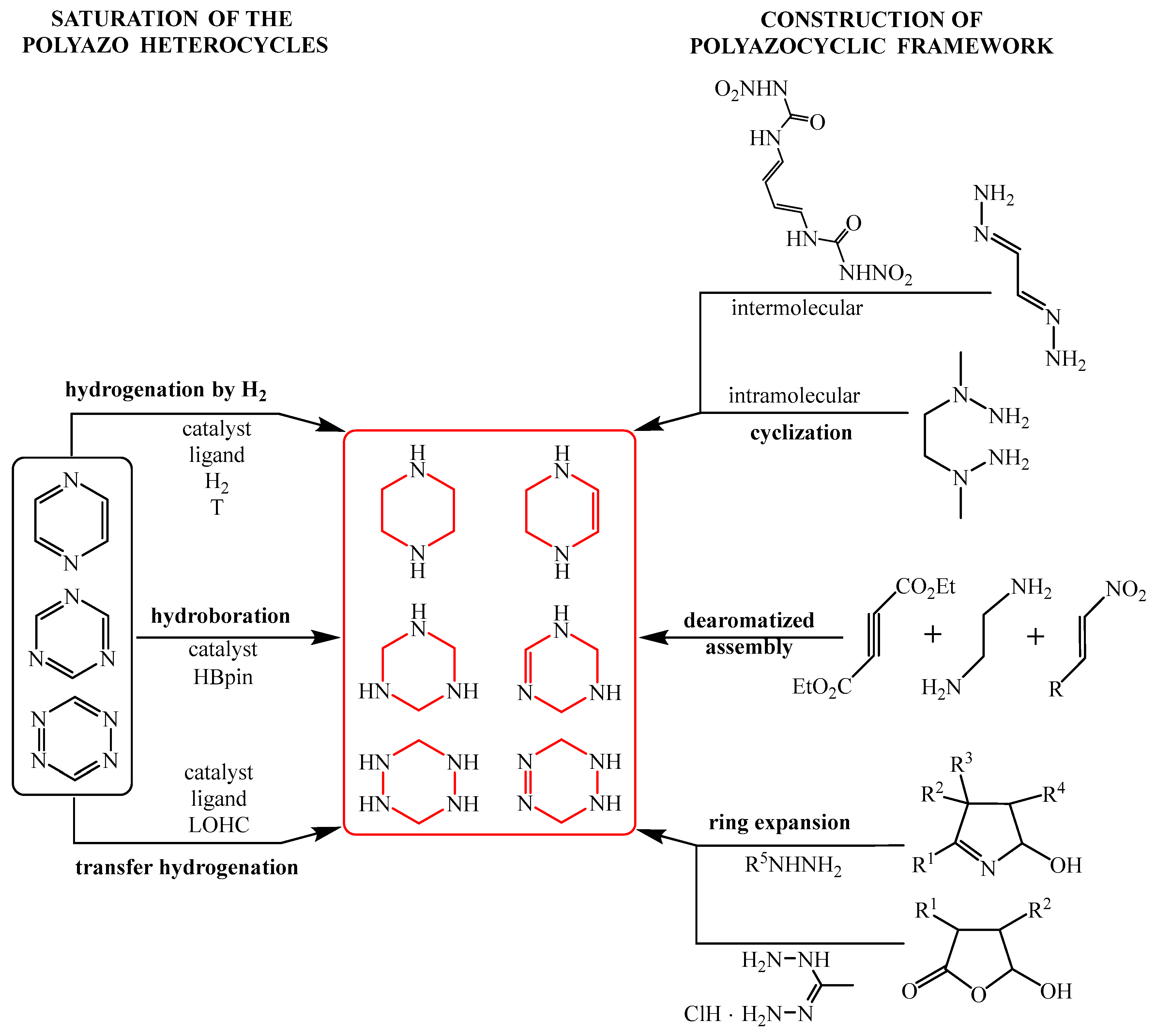
2. Saturation of the Six-Membered Polyazo Heterocycles
2.1. Methodology of Classical Hydrogenation
2.1.1. Pyridazine Moiety
2.1.2. Pyrimidine Moiety
2.1.3. Pyrazine Moiety
2.2. Other Methodology of the Saturation
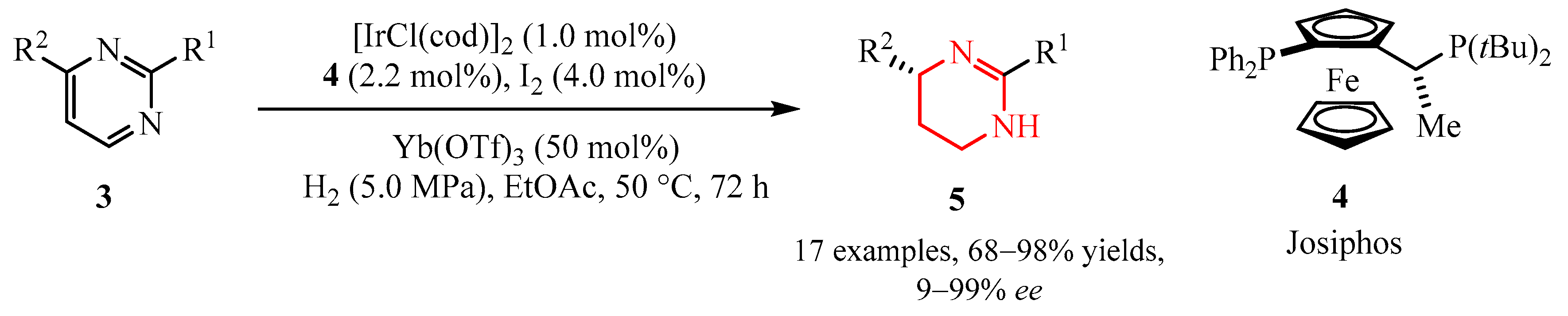
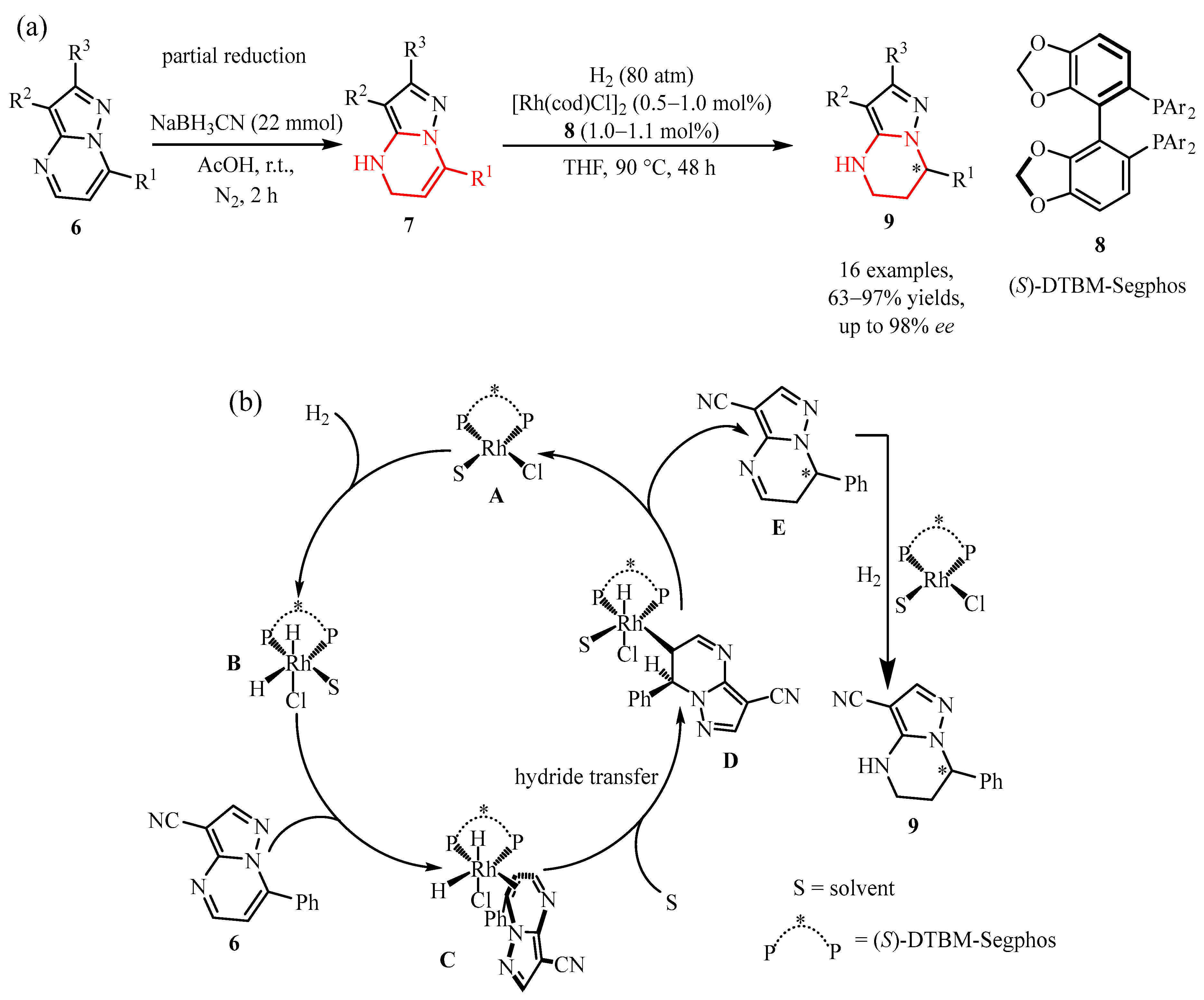
2.2.1. Pyrimidine Moiety
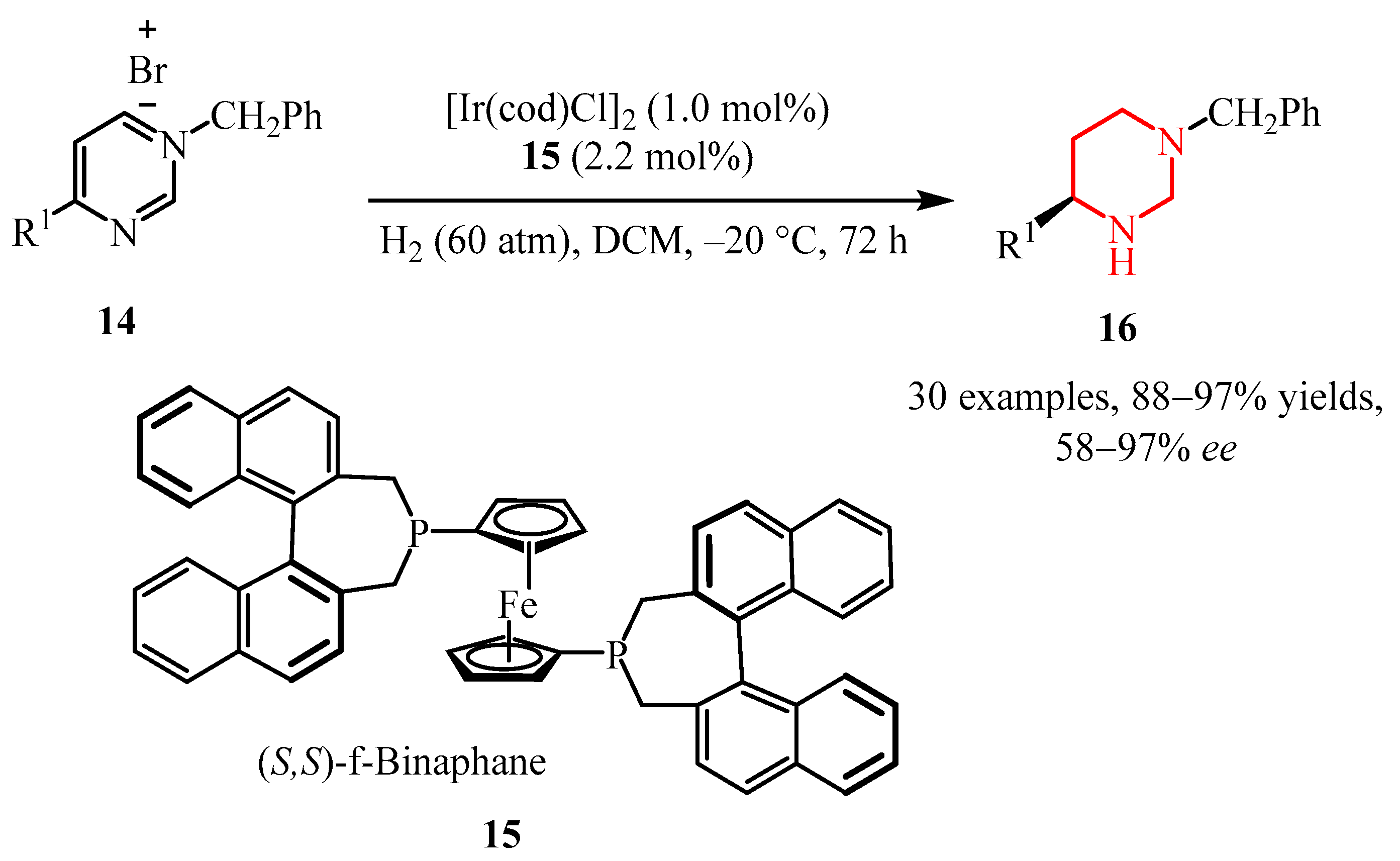
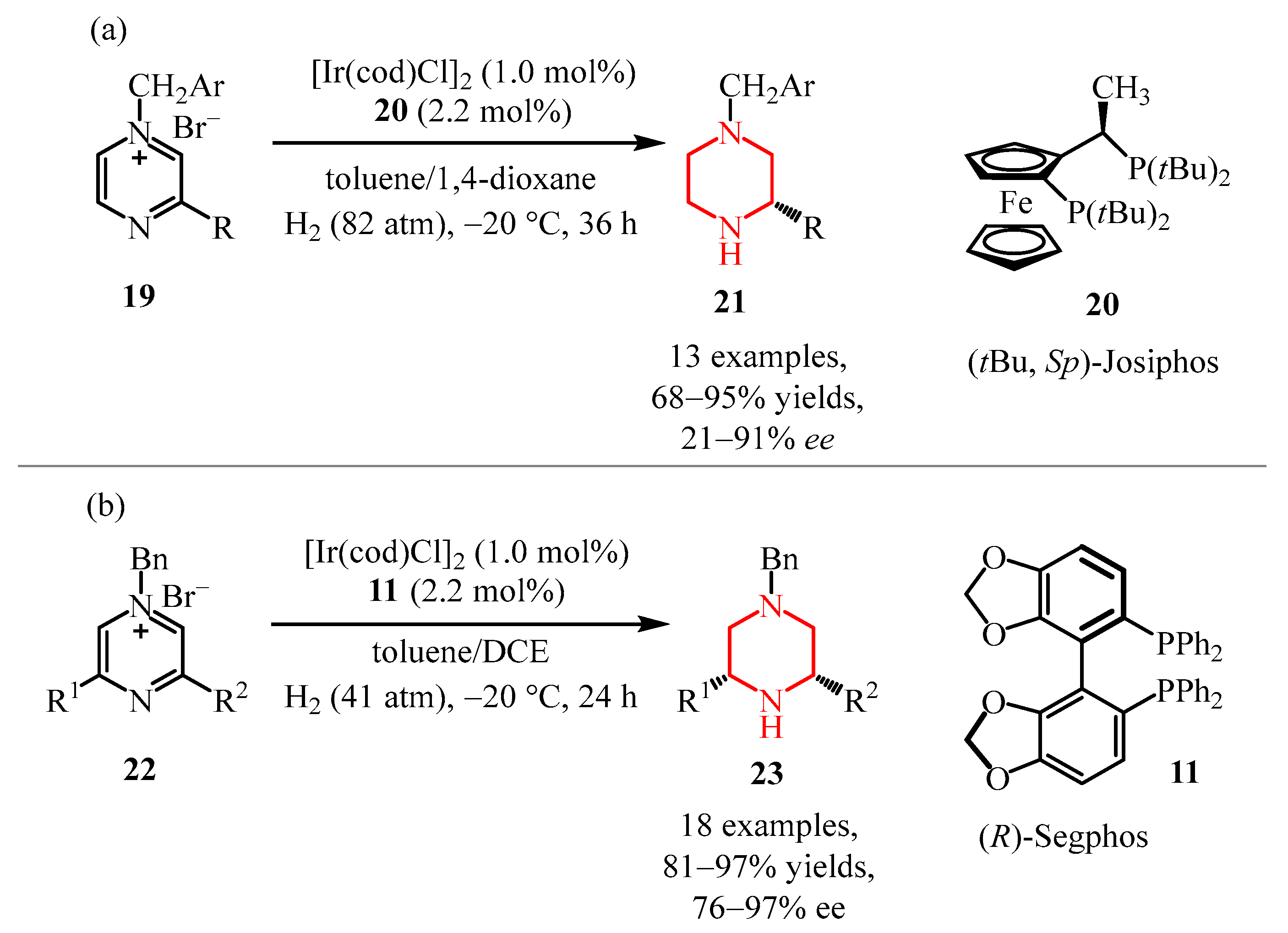
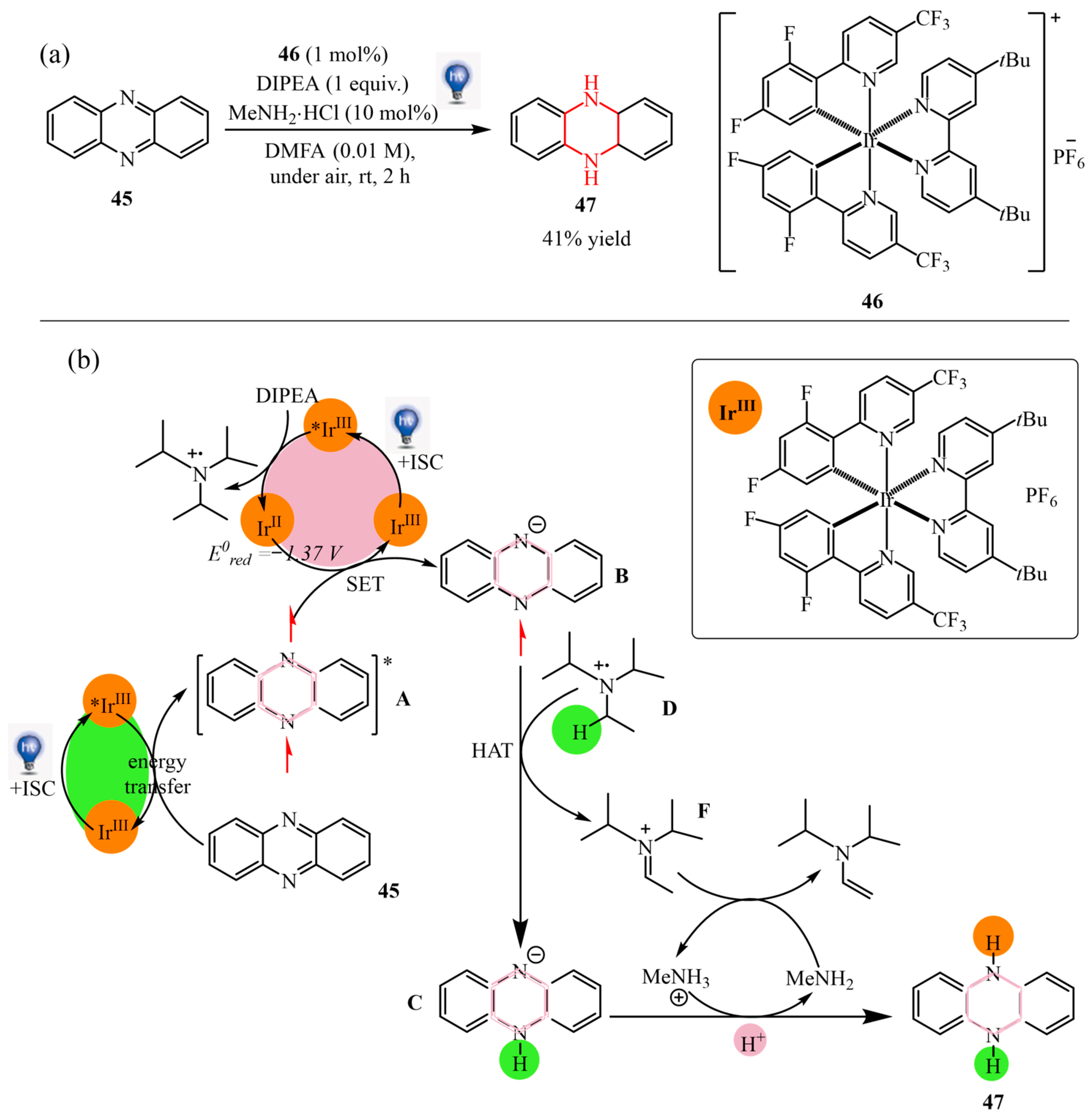
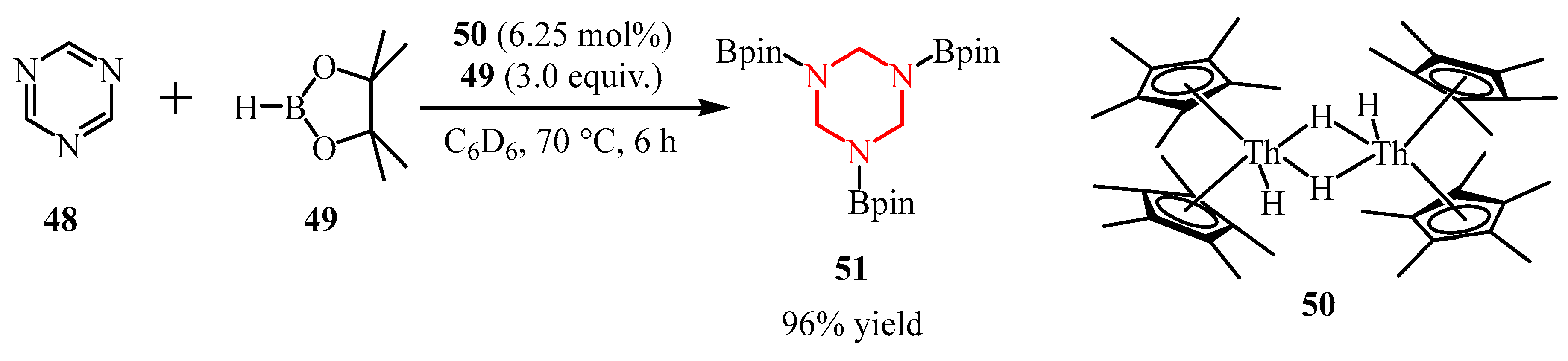
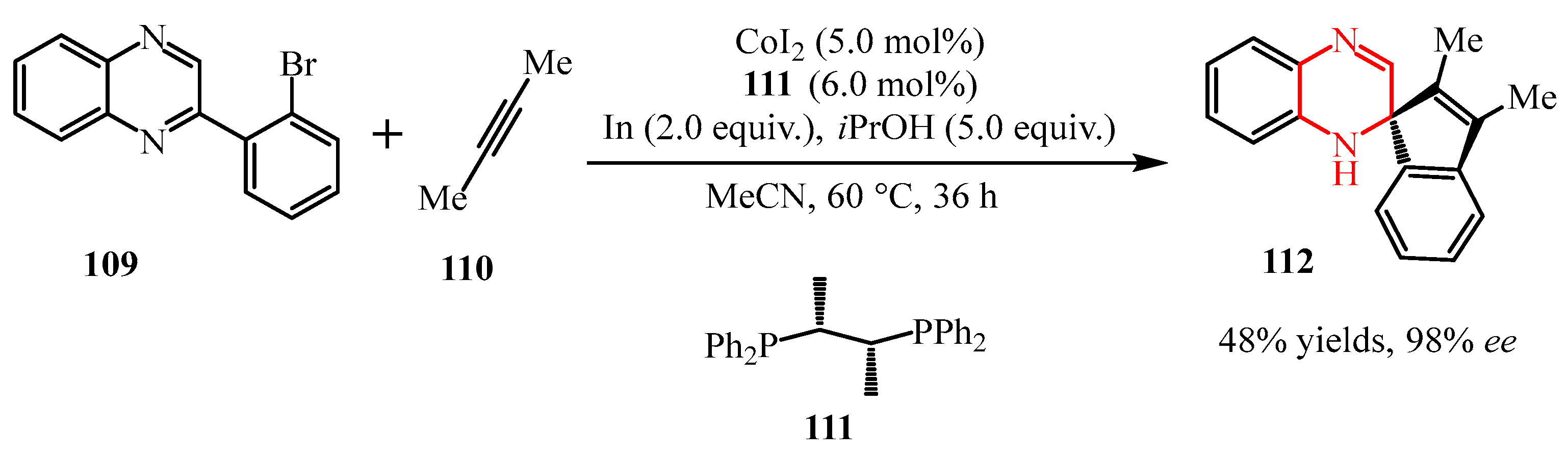
2.2.2. Pyrazine Moiety
2.2.3. Triazine Moiety
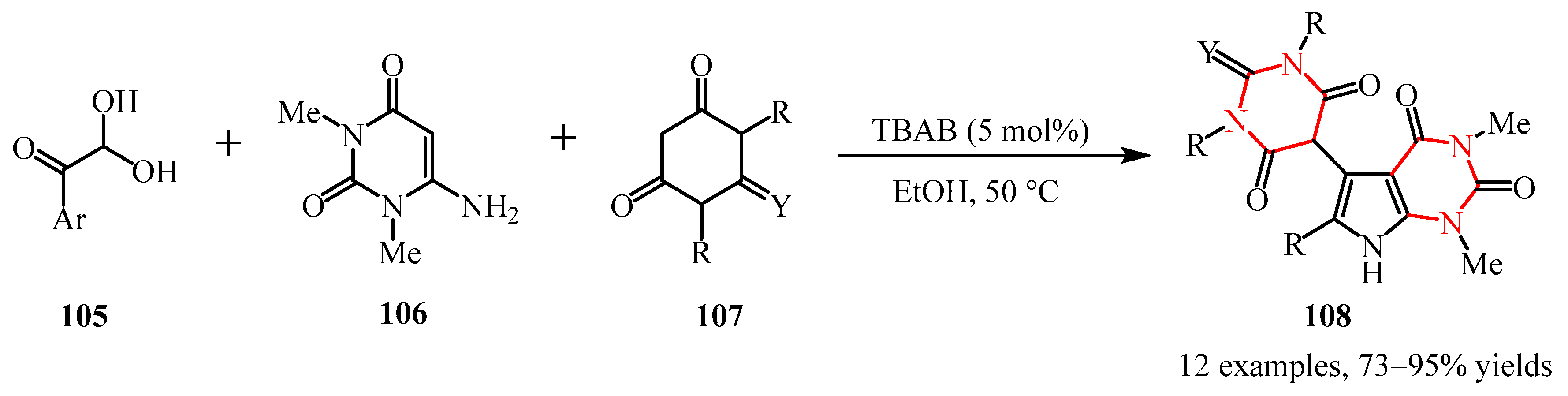
3. Construction of the Six-Membered Nonaromatic Polyazole Cores
3.1. Diazine Compounds
3.1.1. Pyridazine Moiety
3.1.2. Pyrimidine Moiety
3.1.3. Pyrazine Moiety
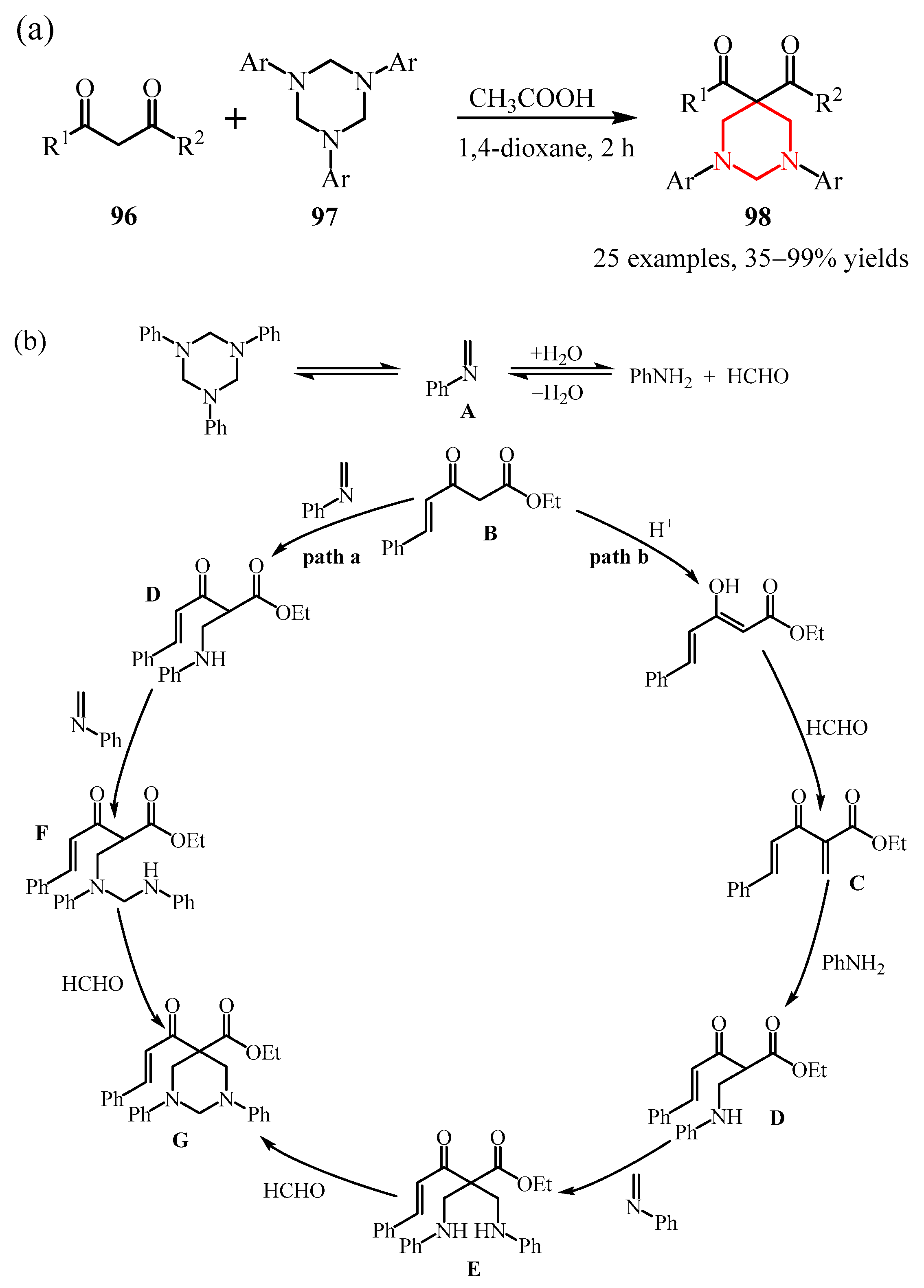
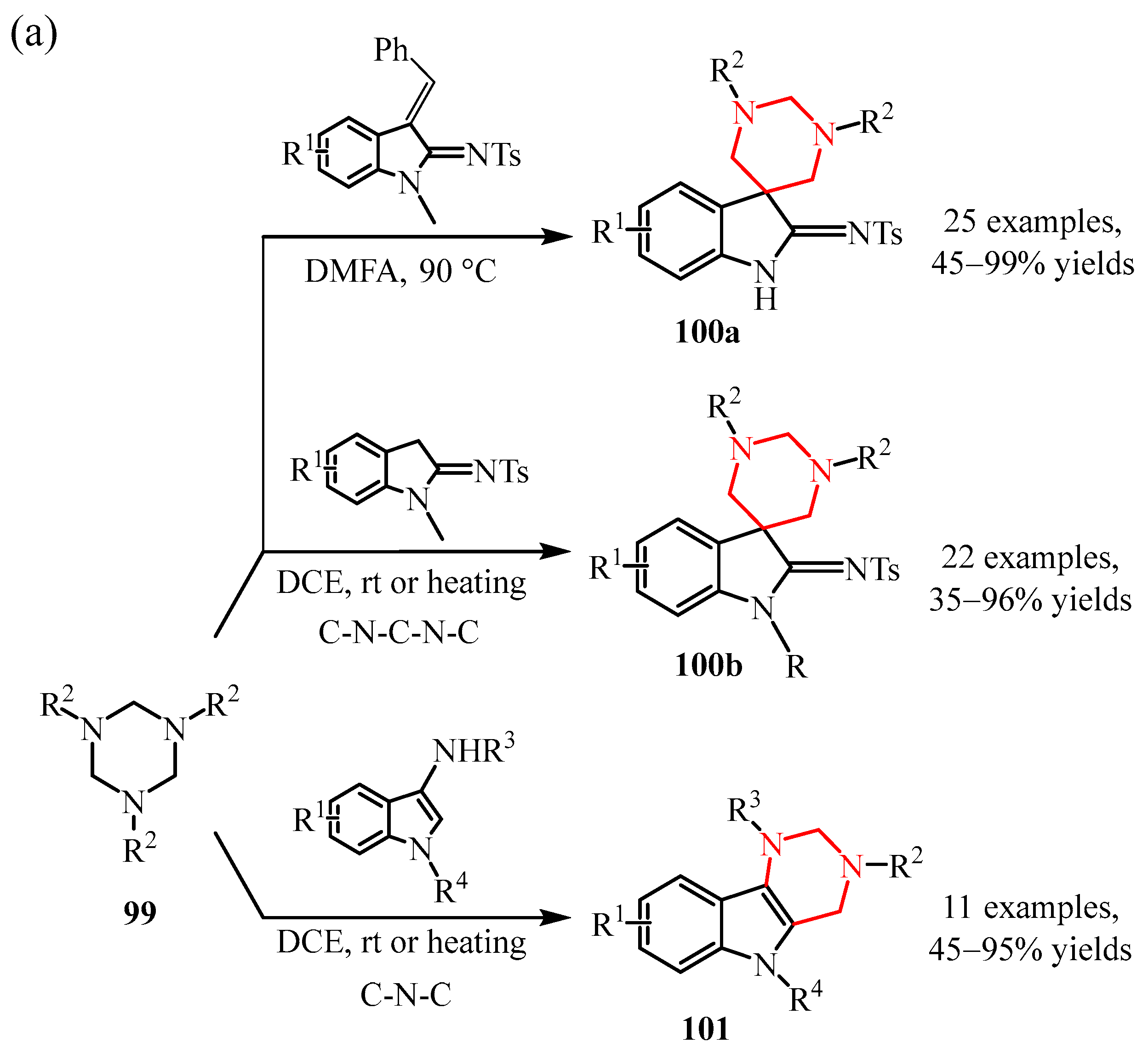
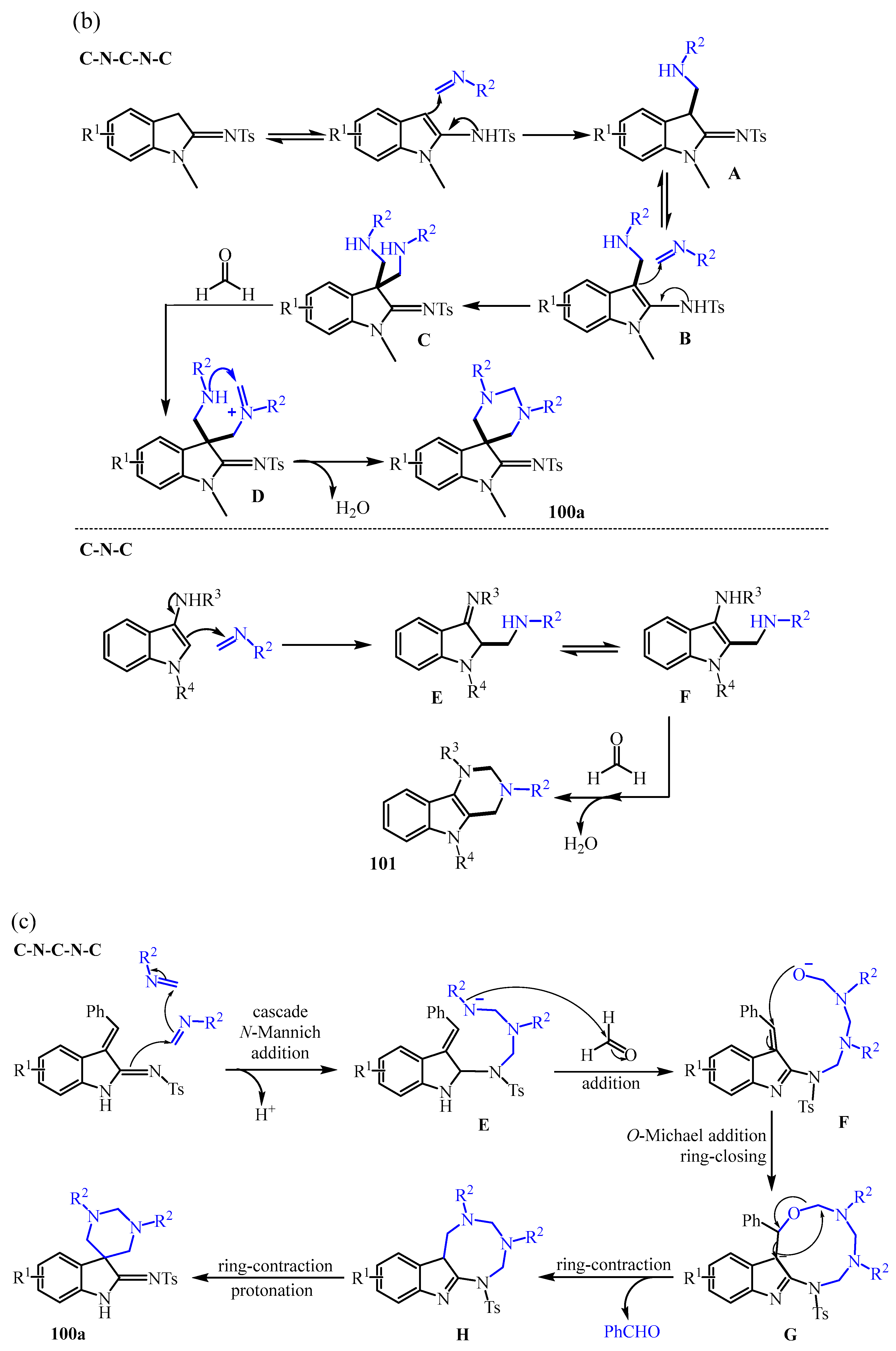
3.2. Triazine Compounds
3.2.1. 1,2,3-Triazines
3.2.2. 1,2,4-Triazines
3.2.3. 1,3,5-Triazines
3.3. Tetrazine Compounds
3.3.1. 1,2,3,4-Tetrazine
3.3.2. 1,2,3,5-Tetrazine
3.3.3. 1,2,4,5-Tetrazine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACN | Acetonitrile |
| AcOH | Acetic acid |
| anti-HIV-1 | Active against human immunodeficiency virus |
| CbzCl | Benzyl chloroformate |
| cod | 1,5-Cyclooctadiene |
| DCE | 1,2-Dichloroethane |
| DCM | Dichloromethane |
| DCC | 1,3-Dicyclohexylcarbodiimine |
| dF(CF3)ppy | 2-(2,4-difluorophenyl)-5-(trifluoromethyl)pyridine |
| DIPEA | N,N-Diisopropylethylamine |
| DMA | N,N-Dimethylaniline |
| DMFA | Dimethylformamide |
| dtbpy | 4,4′-Di-tert-butyl-2,2′-bipyridine) |
| ee | Enantiomeric excess |
| er | Enantiomeric ratio |
| EtOAc | Ethyl acetate |
| HAT | Hydrogen-atom transfer |
| HBpin | Pinacolborane |
| HRMS | High-resolution mass spectrometry |
| ISC | Intersystem crossing |
| LED | Light-emitting diode |
| LOHC | Liquid organic hydrogen carriers |
| MeOCOCl | Methyl chloroformate |
| MTBE | Methyl tert-butyl ether |
| MW | Microwave |
| NMP | N-Methyl-2-pyrrolidone |
| OTf | Triflate group |
| PMB | 4-Methoxybenzyl |
| Py | Pyridine |
| (R)-Segphos | (R)-(+)-5,5′-Bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole, [4(R)-(4,4′-Bi-1,3-benzodioxole)-5,5′-diyl]bis[diphenylphosphine] |
| (R,S)-PPF-P-t-Bu2 | (R)-1-[(SP)-2-(Diphenylphosphino)ferrocenyl]ethyldi-tert-butylphosphine |
| (R Sp)-Josiphos | {(R)-1-[(Sp)-2-(Dicyclohexylphosphino)ferrocenyl]ethyldi-tert-butylphosphine}[2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate |
| (S,S)-f-Binaphane | 1,1′-Bis{(S)-4,5-dihydro-3H-binaphtho [1,2-c:2′,1′-e]phosphino}-ferrocene |
| (S)-BINAP | (S)-(−)-2,2′-Bis(diphenylphosphino)-1,1′-binaphthalene |
| (S)-tol-BINAP | (S)-(−)-2,2′-p-Tolyl-phosphino)-1,1′-binaphthyl, (S)-Tol-BINAP |
| SET | Single-electron transfer |
| (S)-Segphos | (S)-(−)-5,5′-Bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole |
| (S)-Synphos | 6,6′-Bis(diphenylphosphino)-2,2′,3,3′-tetrahydro-5,5′-bibenzo[b][1,4]dioxine |
| (S)-DM-Segphos | (S)-(−)-5,5′-Bis(diphenylphosphino)-4,4′-bi-1,3-benzodioxole |
| (S)-DTBM-Segphos | (S)-(+)-5,5′-Bis[di(3,5-di-tert-butyl-4-methoxyphenyl)phosphino]-4,4′-bi-1,3-benzodioxole |
| (S)-Difluorphos | (2,2,2′,2′-Tetrafluoro-4,4′-bibenzo[d][1,3]dioxole-5,5′-diyl)bis(diphenylphosphine) |
| TBAB | Tetrabutylammonium bromide |
| temp | Temperature |
| TFA | Trifluoroacetic acid |
| Troc | 2,2,2-Trichloroethoxycarbonyl |
| TrocCl | 2,2,2-Trichloroethoxycarbonyl chloride |
| Xantphos | (9,9-Dimethyl-9H-xanthene-4,5-diyl)bis(diphenylphosphane) |
| US | Ultrasound |
| Δ | Boiling or reflux |
| * | Optically active fragment or center |
References
- Asif, M. A Mini Review: Biological Significances of Nitrogen Hetero Atom Containing Heterocyclic Compounds. Int. J. Bioorganic Chem. 2017, 2, 146–152. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, Y.; Peng, Z.; Jiang, J.; Gao, Y.; Hao, X. Schoberine B, an Alkaloid with an Unprecedented Straight C5 Side Chain, and Myriberine B from Myrioneuron Faberi. RSC Adv. 2016, 6, 10180–10184. [Google Scholar] [CrossRef]
- Tageldin, G.N.; Ibrahim, T.M.; Fahmy, S.M.; Ashour, H.M.; Khalil, M.A.; Nassra, R.A.; Labouta, I.M. Synthesis, Modeling and Biological Evaluation of Some Pyrazolo [3, 4-d] Anti-Inflammatory Agents. Bioorg. Chem. 2019, 90, 102844. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, M.; Zhang, Y.; Li, X.; Gu, Y.; Li, X.N.; Di, Y.T.; Hao, X.J. Scalemic Myrionsumamide A, Tetracyclic Skeleton Alkaloids from Myrioneuron Effusum. RSC Adv. 2022, 12, 28147–28151. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Wandler, A.E.E.; Schepers, U.; Nieger, M.; Bräse, S. Synthesis of New Diketopiperazines, Thiolation to Thiodiketopiperazines, and Examination of Their ROS-Generating Properties. Eur. J. Org. Chem. 2015, 31, 6858–6871. [Google Scholar] [CrossRef]
- El-azab, A.S.; Abdel-aziz, A.A.; Bua, S.; Nocentini, A.; El-gendy, M.A. Bioorganic Chemistry Synthesis of Benzensulfonamides Linked to Quinazoline Scaffolds as Novel Carbonic Anhydrase Inhibitors. Bioorg. Chem. 2019, 87, 78–90. [Google Scholar] [CrossRef]
- Kirsanov, V.Y.; Rakhimova, E.B. Recent Advances in the Chemistry of Saturated Annulated Nitrogen-Containing Polycyclic Compounds. Int. J. Mol. Sci. 2022, 23, 15484. [Google Scholar] [CrossRef]
- Wang, R.; Yu, S.; Zhao, X.; Chen, Y.; Yang, B.; Wu, T.; Hao, C.; Zhao, D.; Cheng, M. Design, Synthesis, Biological Evaluation and Molecular Docking Study of Novel Thieno [3,2-d] Pyrimidine Derivatives as Potent FAK Inhibitors. Eur. J. Med. Chem. 2020, 188, 112024. [Google Scholar] [CrossRef]
- Zhang, Y.; Hou, Q.; Li, X.; Zhu, J.; Wang, W.; Li, B.; Zhao, L.; Xia, H. Enrichment of Novel Quinazoline Derivatives with High Antitumor Activity in Mitochondria Tracked by Its Self-Fluorescence. Eur. J. Med. Chem. 2019, 178, 417–432. [Google Scholar] [CrossRef]
- Sana, S.; Tokala, R.; Madanlal, D.; Nagesh, N.; Kumar, K. Bioorganic Chemistry Design and Synthesis of Substituted Dihydropyrimidinone Derivatives as Cytotoxic and Tubulin Polymerization Inhibitors. Bioorg. Chem. 2019, 93, 103317. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yu, L.; Diao, P.; Jian, X.; Zhou, M.; Jianga, C.S.; Youa, W.W.; Mab, W.F.; Zhao, P.L. Novel [1,2,4] Triazolo[1,5-a] Pyrimidine Derivatives as Potent Antitubulin Agents: Design, Multicomponent Synthesis and Antiproliferative Activities. Bioorg. Chem. 2019, 92, 103260. [Google Scholar] [CrossRef]
- Kerru, N.; Gummidi, L.; Maddila, S.; Gangu, K.K.; Jonnalagadda, S.B. A Review on Recent Advances in Nitrogen-Containing Molecules and Their Biological Applications. Molecules 2020, 25, 1909. [Google Scholar] [CrossRef]
- Sanu, M.C.; Joseph, J.; Chacko, D.; Vinod, B.; Daisy, P.A. A Review on Six Membered Nitrogen Containing Heterocyclic Compounds with Various Biological Activities. Int. J. Pharm. Sci. Rev. Res. 2021, 69, 64–68. [Google Scholar] [CrossRef]
- Phei, F.; Lim, L.; Dolzhenko, A. V 1,3,5-Triazine-Based Analogues of Purine: From Isosteres to Privileged Scaffolds in Medicinal Chemistry. Eur. J. Med. Chem. 2014, 85, 371–390. [Google Scholar] [CrossRef]
- Bai, L.; Wei, C.; Zhang, J.; Song, R. Design, Synthesis, and Anti-PVY Biological Activity of 1,3,5-Triazine Derivatives Containing Piperazine Structure. Int. J. Mol. Sci. 2023, 24, 8280. [Google Scholar] [CrossRef]
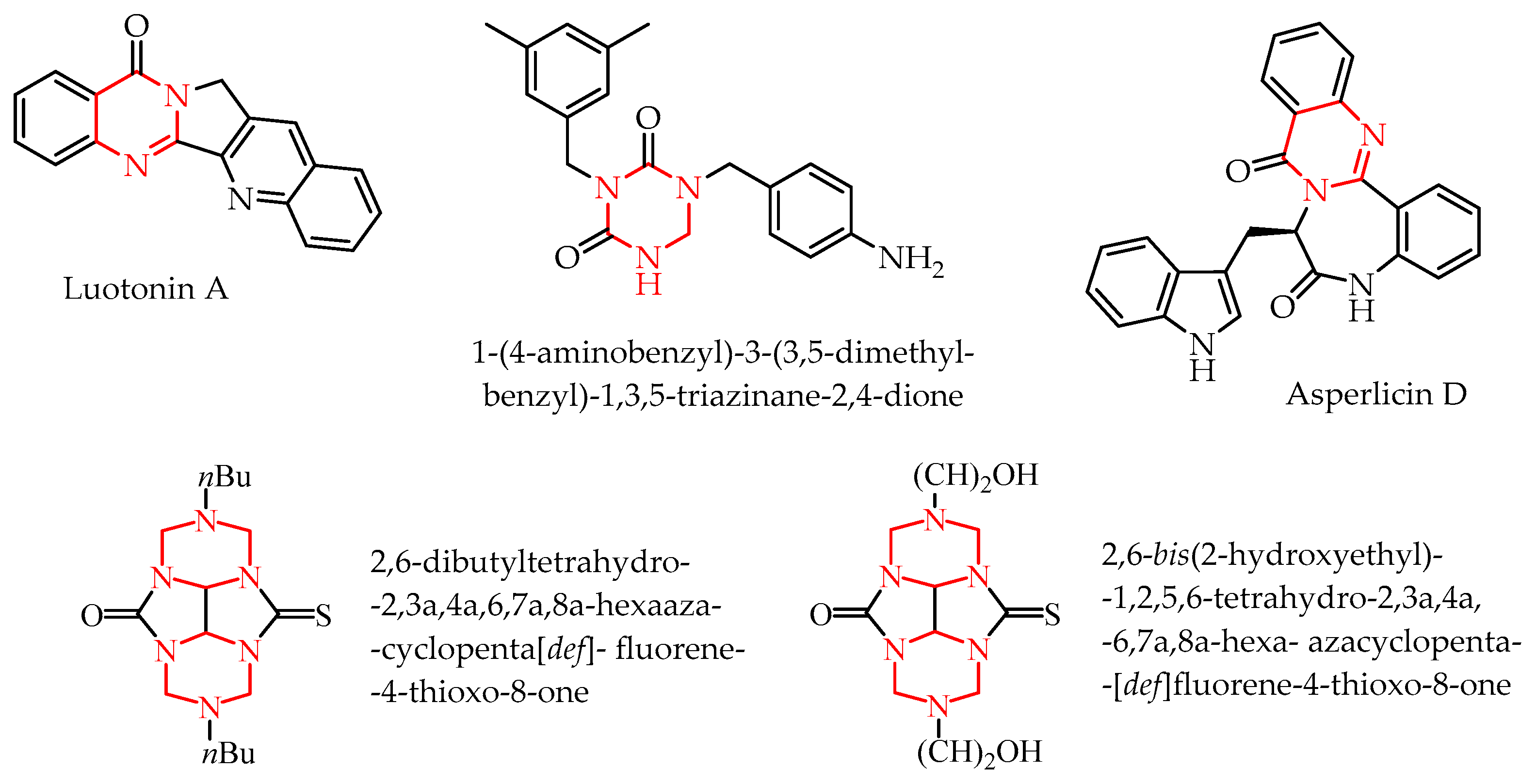
- Hao, Y.; Wang, K.; Wang, Z.; Liu, Y.; Ma, D.; Wang, Q. Luotonin A and Its Derivatives as Novel Antiviral and Antiphytopathogenic Fungus Agents. J. Agric. Food Chem. 2020, 68, 8764–8773. [Google Scholar] [CrossRef]
- Kishimoto, S.; Tamura, T.; Okamoto, T.; Watanabe, K. Enantioselective Biosynthesis of (+)- and (−)-Auranthines. J. Am. Chem. Soc. 2025, 147, 10612–10617. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Ghosh, S.K.; Mandal, M.K.; Masih, A.; Bhat, H.R.; Singh, U.P. 1,3,5-Triazine: A Versatile Pharmacophore with Diverse Biological Activities. Arch Pharm. 2021, 354, e2000363. [Google Scholar] [CrossRef] [PubMed]
- Barsegyan, Y.A.; Baranov, V.V.; Strelenko, Y.A.; Anikina, L.V.; Kravchenko, A.N.; Karnoukhova, V.A.; Kolotyrkina, N.G. Synthesis, Structure, and Biological Activity of 2,6-Disubstituted 2,3a,4a,6,7a,8a-Hexaazaperhydrocyclopenta [Def] Fluorene-4- Thioxo-8-Ones. Synthesis 2018, 50, 2099–2105. [Google Scholar] [CrossRef]
- Wang, D.; Chen, Q.A.; Lu, S.; Zhou, Y.G. Asymmetric Hydrogenation of Heteroarenes and Arenes. Chem. Rev. 2012, 112, 2557–2590. [Google Scholar] [CrossRef]
- Balakrishna, B.; Núñez-rico, J.L.; Vidal-ferran, A. Substrate Activation in the Catalytic Asymmetric Hydrogenation of N-Heteroarenes. Eur. J. Org. Chem. 2015, 2015, 5293–5303. [Google Scholar] [CrossRef]
- Titova, Y.Y.; Ivanov, A.V. Non-Classical Effective Methods for Reduction of Heteroaromatic Compounds. Russ. Chem. Rev. 2024, 93, RCR5149. [Google Scholar] [CrossRef]
- Kim, A.N.; Stoltz, B.M. Recent Advances in Homogeneous Catalysts for the Asymmetric Hydrogenation of Heteroarenes. ACS Catal. 2020, 10, 13834–13851. [Google Scholar] [CrossRef]
- Mamedov, V.A.; Zhukova, N.A. Recent Advances in the Synthesis of Indoles with Partially Hydro- Genated Benzene Ring (Tetrahydroindoles). Synthesis 2024, 56, 1207–1243. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, Y.G. Catalytic, Enantioselective Hydrogenation of Heteroaromatic Compounds. In Organic Reactions; Denmark, S.E., Ed.; WILEY: Oxford, UK, 2018; Volume 96, pp. 1–226. [Google Scholar]
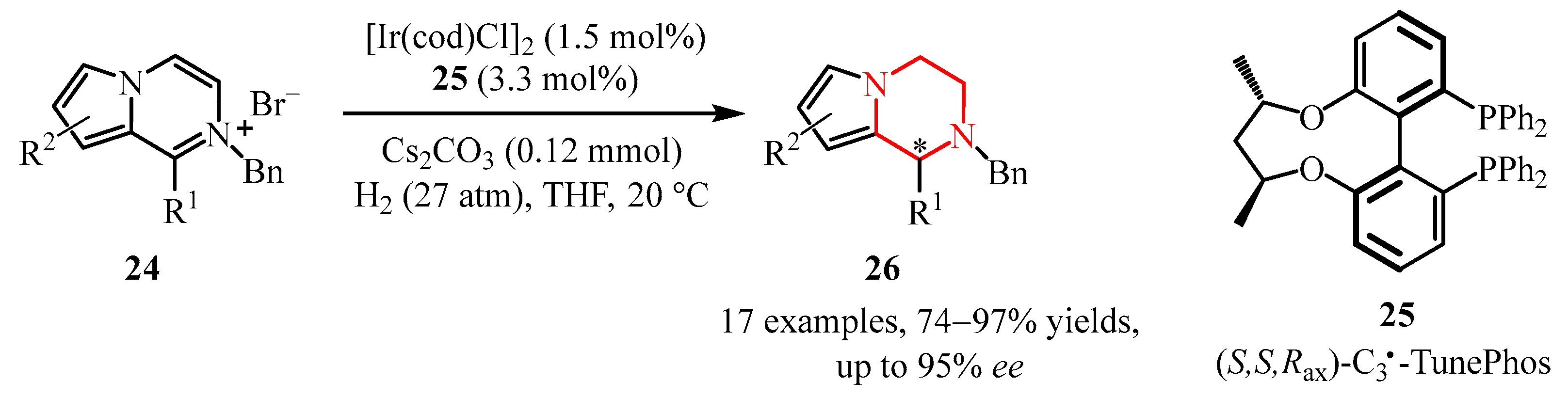
- Huang, W.; Yu, C.; Shi, L.; Zhou, Y. Iridium-Catalyzed Asymmetric Hydrogenation of Pyrrolo [1,2-a] Pyrazinium Salts. Org. Lett. 2014, 16, 3324–3327. [Google Scholar] [CrossRef]
- Zhang, Z.; Du, H. A Highly Cis-Selective and Enantioselective Metal-Free Hydrogenation of 2,3-Disubstituted Quinoxalines. Angew. Chem. Int. Ed. 2014, 54, 623–626. [Google Scholar] [CrossRef]
- Zhao, D.; Candish, L.; Paul, D.; Glorius, F. N-Heterocyclic Carbenes in Asymmetric Hydrogenation. ACS Catal. 2016, 6, 5978–5988. [Google Scholar] [CrossRef]
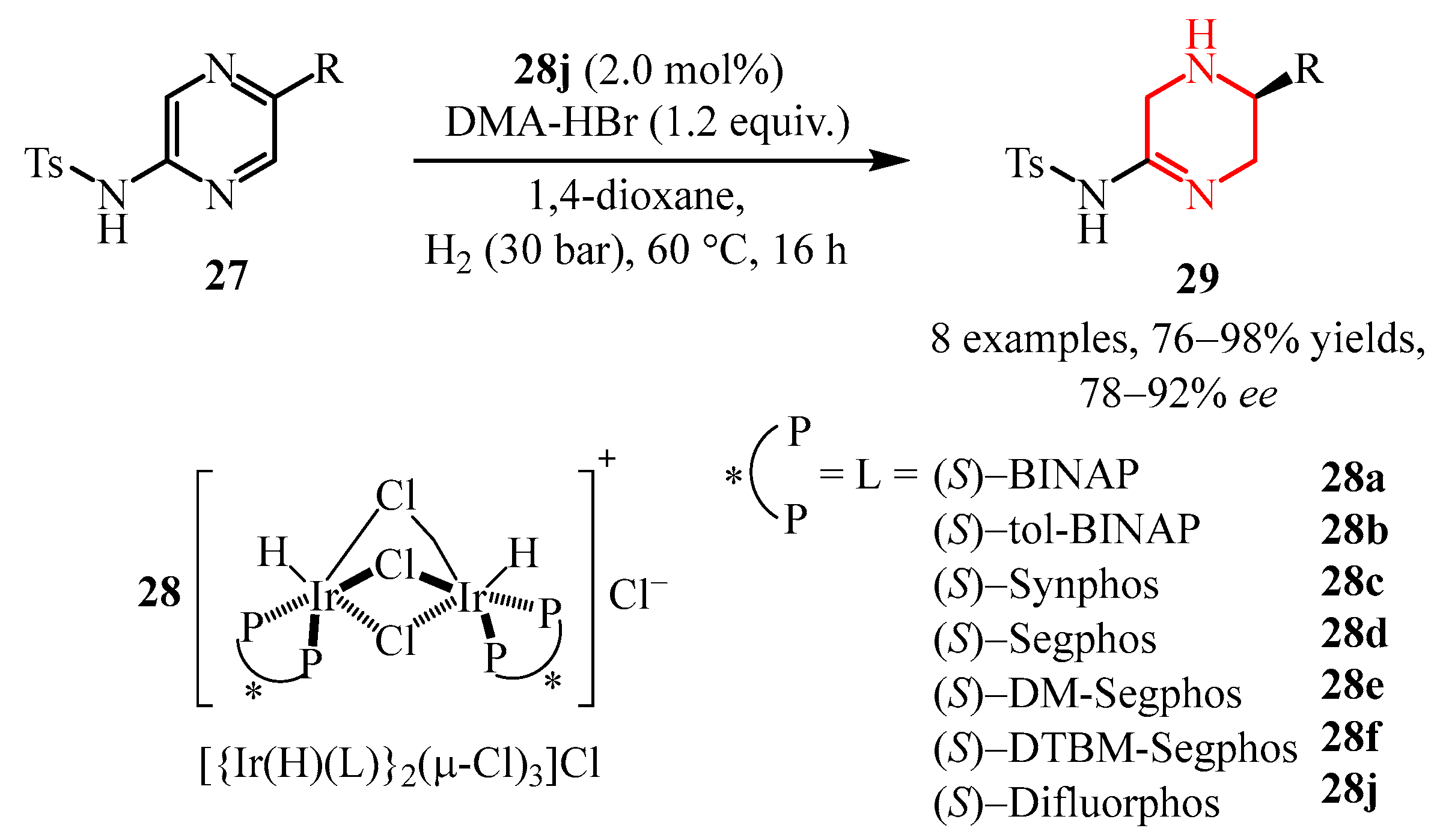
- Higashida, K.; Nagae, H.; Mashima, K. Iridium-Catalyzed Asymmetric Hydrogenation of Tosylamido- Substituted Pyrazines for Constructing Chiral Tetrahydropyra- Zines with an Amidine Skelton. Adv. Synth. Catal. 2016, 358, 3949–3954. [Google Scholar] [CrossRef]
- Wu, X.; Li, X.; Zanotti-Gerosa, A.; Pettman, A.; Liu, J.; Mills, A.J.; Xiao, J. RhIII- and IrIII-Catalyzed Asymmetric Transfer Hydrogenation of Ketones in Water. Chem. A Eur. J. 2008, 14, 2209–2222. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.; Bamberger, J.; Mancheño, O.G. Asymmetric Nucleophilic Dearomatization of Diazarenes by Anion-Binding Catalysis. Org. Biomol. Chem. 2016, 14, 5794–5802. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chang, S. Catalytic Dearomatization of N-Heteroarenes with Silicon and Boron Compounds. Angew. Chem. Int. Ed. 2017, 56, 2–21. [Google Scholar] [CrossRef]
- Liu, H.; Khononov, M.; Eisen, M.S. Catalytic 1,2-Regioselective Dearomatization of N-Heteroaromatics via a Hydroboration. ACS Catal. 2018, 8, 3673–3677. [Google Scholar] [CrossRef]
- Ni, Q.; Chen, Y.; Ma, Y. Synthesis Recent Progress in Base-Metal-Catalyzed Dearomative Reaction of N -Heteroarenes. Synthesis 2025, 57, 2289–2306. [Google Scholar] [CrossRef]
- Ketelboeter, D.R.; Pappoppula, M.; Aponick, A. Chemoselective Diazine Dearomatizationn: The Catalytic Enantioselective Dearomatization of Pyrazine. J. Am. Chem. Soc. 2024, 146, 11610–11615. [Google Scholar] [CrossRef]
- Resch, V.; Seidler, C.; Chen, B.; Degeling, I.; Hanefeld, U. On the Michael Addition of Water to α,β-Unsaturated Ketones Using Amino Acids. Eur. J. Org. Chem. 2013, 2013, 7697–7704. [Google Scholar] [CrossRef]
- Li, K.; Meng, F.; Jiang, W.; Shi, L. ZrCl4-Catalyzed Nucleophilic Dearomatization of 2-Hydroxy-Pyrimidines: A Concise Synthesis of Novel 3,4-Dihydropyrimidin-2 (1H)-Ones Containing a Phosphonic Ester Group. Tetrahedron Lett. 2021, 73, 153149. [Google Scholar] [CrossRef]
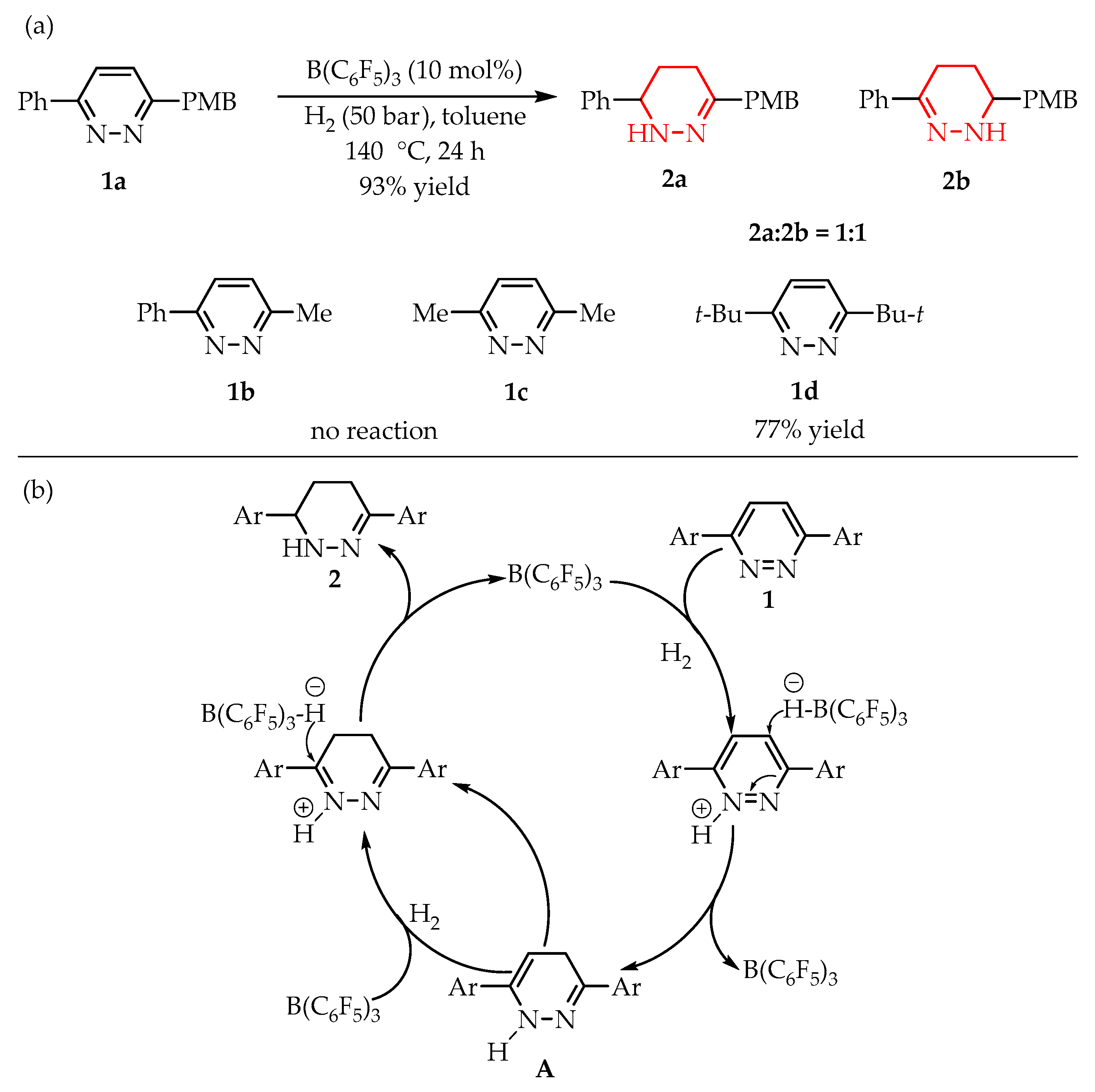
- Wanga, W.; Menga, W.; Du, H. B(C6F5)3-Catalyzed Metal-Free Hydrogenations of 3,6- Diarylpyridazines. Dalt. Trans. 2016, 45, 5945–5948. [Google Scholar] [CrossRef] [PubMed]
- Kuwano, R.; Hashiguchi, Y.; Ikeda, R.; Ishizuka, K. Catalytic Asymmetric Hydrogenation of Pyrimidines. Angew. Chem. Int. Ed. 2015, 54, 2393–2396. [Google Scholar] [CrossRef]
- Xie, C.; Xiao, G.; Guo, Q.; Wu, X.; Zi, G.; Ding, W.; Hou, G. Highly Enantioselective Rh-Catalyzed Asymmetric Reductive Dearomatization of Multi-Nitrogen. Chem. Sci. 2023, 14, 9048–9054. [Google Scholar] [CrossRef]
- Chen, M.; Li, H.; Wang, Y.; Wu, B.; Liu, Z.; Lai, X.; Deerberg, J.; Zhou, Y. Iridium-Catalyzed Asymmetric Hydrogenation of Heteroaromatics with Multiple N Atoms via Substrate Activation: An Entry to 4,5,6,7- Tetrahydropyrazolo [1,5-a] Pyrimidine-3-Carbonitrile Core of a Potent BTK Inhibitor. J. Org. Chem. 2024, 89, 4336–4348. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Y.; Wan, L.; Li, Y.; Chen, D.; Tao, J.; Tang, P.; Chen, F. Iridium-Catalyzed Asymmetric, Complete Hydrogenation of Pyrimidinium Salts under Batch and Flow. Green Chem. 2024, 26, 317–322. [Google Scholar] [CrossRef]
- Tautomers, P.-H.; Feng, G.; Chen, M.; Shi, L.; Zhou, Y. Asymmetric Hydrogenation Facile Synthesis of Chiral Cyclic Ureas through Hydrogenation of 2-Hydroxypyrimidine/Pyrimidin-2(1H)-One Tautomers. Angew. Chem. Int. Ed. 2018, 57, 5853–5857. [Google Scholar] [CrossRef]
- Huang, W.; Liu, L.; Wu, B.; Feng, G.; Wang, B.; Zhou, Y. Synthesis of Chiral Piperazines via Hydrogenation of Pyrazines Activated by Alkyl Halides. Org. Lett. 2016, 18, 3082–3085. [Google Scholar] [CrossRef]
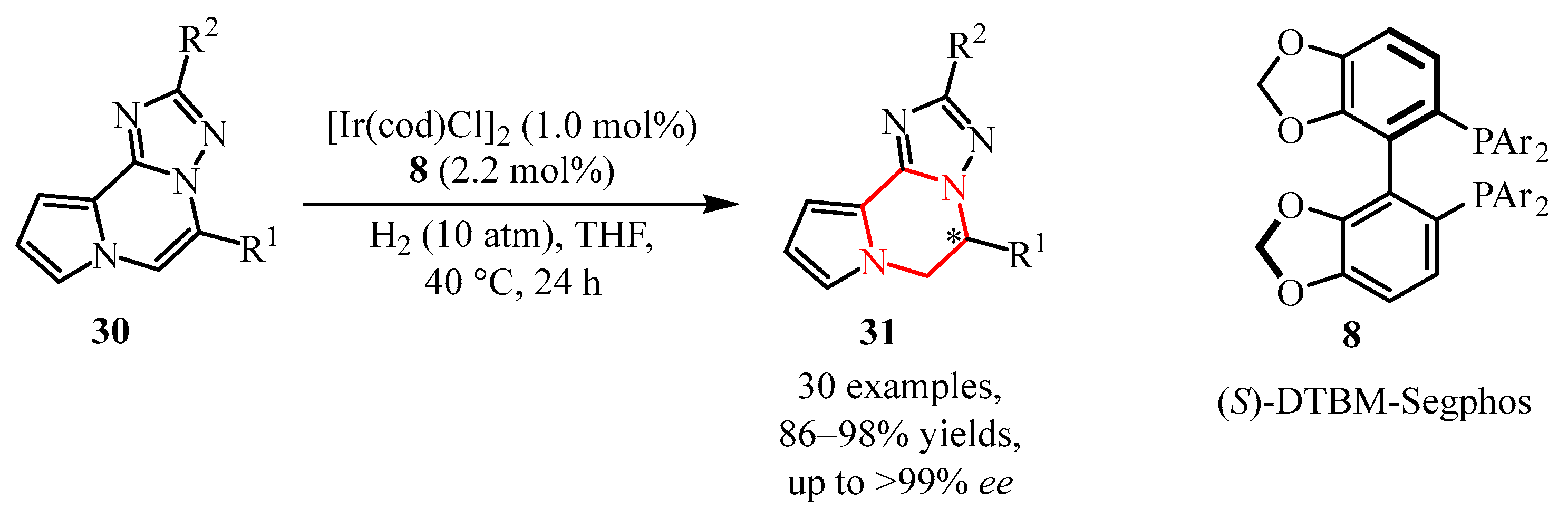
- Guo, Q.; Xie, C.; Zi, G.; Lai, X.; Deerberg, J.; Hou, G. Ir-Catalyzed Asymmetric Hydrogenation of N-Fused Heteroarenes with High Nitrogen Density: An Access to Chiral 2,5-Disubstituted 5,6-Dihydropyrrolo [1,2-a] [1,2,4] Triazolo [5,1-c] Pyrazines. Org. Lett. 2024, 26, 7363–7369. [Google Scholar] [CrossRef]
- Chen, A.Y.; He, Y.; Zhang, S.; Miao, T.; Fan, Q. Rapid Construction of Structurally Diverse Quinolizidines, Indolizidines and Their Analogues via Ruthenium-Catalyzed Asymmetric Cascade Hydrogenation/Reductive Amination. Angew. Chemie Int. Ed. 2019, 58, 3809–3813. [Google Scholar] [CrossRef]
- Feng, G.; Zhao, Z.; Shi, L.; Zhou, Y. Synthesis of Chiral Piperazin-2-Ones through Palladium-Catalyzed Asymmetric Hydrogenation of Pyrazin-2-Ols. Org. Chem. Front. 2021, 8, 6273–6278. [Google Scholar] [CrossRef]
- Voutchkova, A.M.; Gnanamgari, D.; Jakobsche, C.E.; Butler, C.; Miller, S.J.; Parr, J.; Crabtree, R.H. Selective Partial Reduction of Quinolines: Hydrosilylation vs. Transfer Hydrogenation. J. Organomet. Chem. 2008, 693, 1815–1821. [Google Scholar] [CrossRef]
- Manas, M.G.; Sharninghausen, L.S.; Crabtree, R.H. Experimental and Computational Studies of Borohydride Catalyzed Hydrosilylation of a Variety of C=O and C=N Functionalities Including Esters, Amides and Heteroarenes. New J. Chem. 2014, 38, 1694–1700. [Google Scholar] [CrossRef]
- Liu, T.; He, J.; Zhang, Y. Regioselective 1,2-Hydroboration of N-Heteroarenes Using a Potassium-Based Catalyst. Org. Chem. Front. 2019, 6, 2749–2755. [Google Scholar] [CrossRef]
- Yang, C.H.; Chen, X.; Li, H.; Wei, W.; Yang, Z.; Chang, J. Iodine Catalyzed Reduction of Quinolines under Mild Reaction Conditions. Chem. Commun. 2018, 54, 8622–8625. [Google Scholar] [CrossRef]
- Shakhman, D.; Dmitrienko, A.; Pilkington, M.; Nikonov, G.I. Selective Zinc-Catalyzed 1,2-Hydroboration of N-Heteroaromatics via a Non-Hydride Mechanism. Eur. J. Inorg. Chem. 2023, 26, e202300293. [Google Scholar] [CrossRef]
- Velmurugan, P.; Eisen, M.S.; Ghatak, T. Recent Strategies and Developments in the Hydroboration of N-Heteroarenes Mediated by Transition and Rare-Earth Metal Complexes. Inorganica Chim. Acta 2025, 580, 122594. [Google Scholar] [CrossRef]
- Shida, N.; Shimizu, Y.; Yonezawa, A.; Harada, J.; Furutani, Y.; Muto, Y.; Kurihara, R.; Kondo, J.N.; Sato, E.; Mitsudo, K.; et al. Electrocatalytic Hydrogenation of Pyridines and Other Nitrogen- Containing Aromatic Compounds. J. Am. Chem. Soc. 2024, 146, 30212–30221. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Konig, B. Birch-Type Photoreduction of Arenes and Heteroarenes by Sensitized Electron Transfer. Angew. Chem. Int. Ed. 2019, 58, 14289–14294. [Google Scholar] [CrossRef] [PubMed]
- Moore, J.; Volker, E.J.; Kopay, C. Heterocyclic Studies. 34. Toluenesulfonyl Derivatives of 2,3-Dihydro-5-Methyl-(i-Phenyl-1,2-Diazepin-4-One, Rearrangement to a 1,4-Dihydropyridazine. J. Org. Chem. 1971, 36, 2676–2680. [Google Scholar] [CrossRef]
- Shabalin, D.A.; Dvorko, Y.; Zolotareva, E.E.; Ushakov, I.A.; Vashchenko, A.V.; Schmidt, E.Y.; Trofimov, B.A. Metal-Free Selective Synthesis of 1,4-Dihydropyridazines from Hydroxypyrrolines and Hydrazines. Eur. J. Org. Chem. 2017, 2017, 4004–4010. [Google Scholar] [CrossRef]
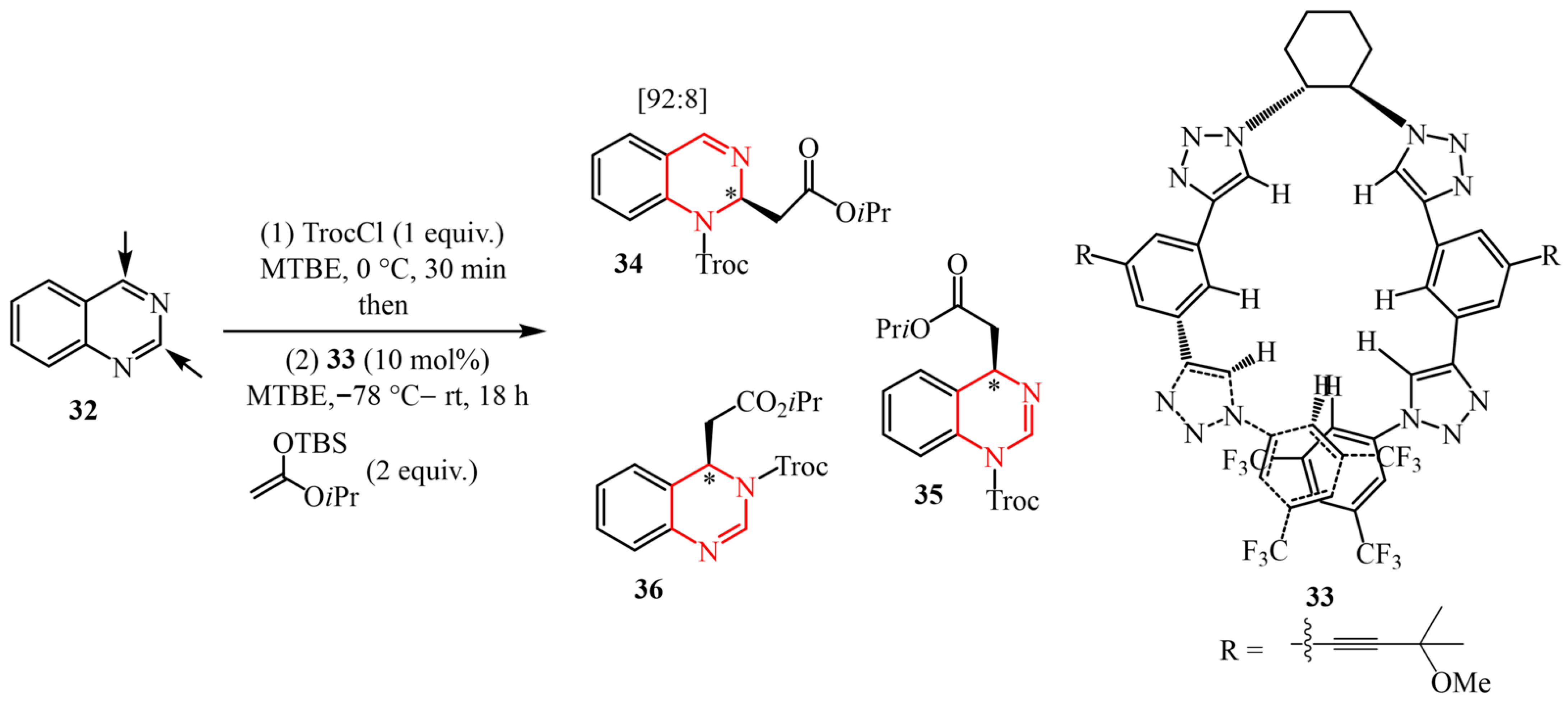
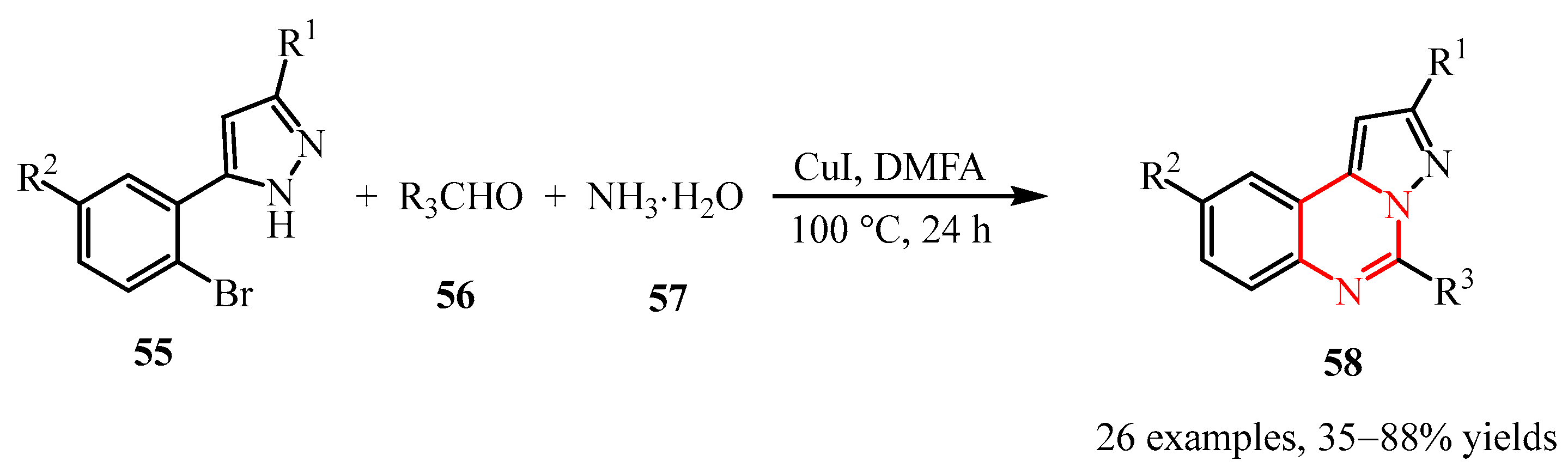
- Guo, S.; Wang, J.; Fan, X.; Zhang, X.; Guo, D. Synthesis of Pyrazolo [1,5-c] Quinazoline Derivatives through Copper-Catalyzed Tandem Reaction of 5-(2-Bromoaryl)-1H-pyrazoles with Carbonyl Compounds and Aqueous Ammonia. J. Org. Chem. 2013, 78, 3262–3270. [Google Scholar] [CrossRef]
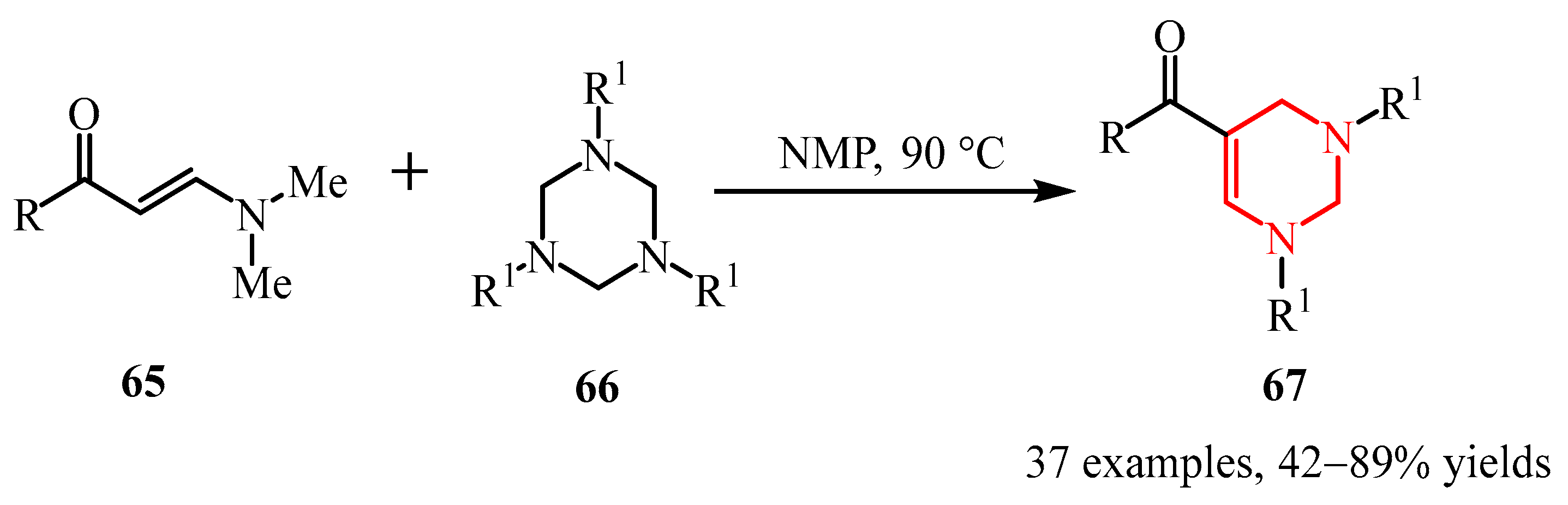
- Shi, R.; Gao, L.; Chen, W.; Shi, Y.; Cao, Z.; Zheng, Y.; Liu, J. Formal [2 + 2 + 2] Cycloaddition Reaction of 1,3,5-Triazinanes with Diethyl Acetylene Dicarboxylate: Approach to Tetrahydropyrimidines. Eur. J. Org. Chem. 2021, 2021, 5941–5945. [Google Scholar] [CrossRef]
- Chen, L.; Liu, K.; Sun, J. Catalyst-Free Synthesis of Tetrahydropyrimidines via Formal [3+3]-Cycloaddition of Imines with 1,3,5- Hexahydro-1,3,5-Triazines. RSC Adv. 2018, 8, 5532–5535. [Google Scholar] [CrossRef]
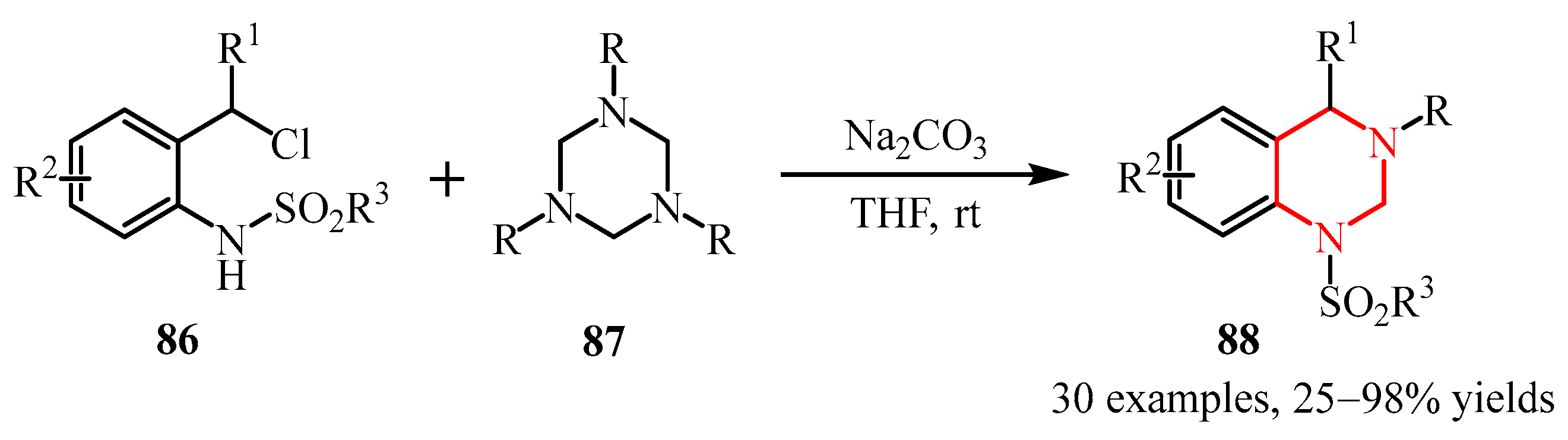
- Rong, L.; Lai, C.; Han, S.; Liao, J.; Liu, C.; Li, X.; Huang, J. Additive-Free Construction of Tetrahydropyrimidine Skeleton by Using 1,3,5-Triazinane as Four-Atom Synthon. J. Org. Chem. 2024, 89, 9496–9501. [Google Scholar] [CrossRef]
- Han, S.; Fang, C.; Li, S.; Mo, X.; Xu, Y.; An, B.; Cheng, B. Divergent Sc (OTf)3 -Catalyzed Tandem Cyclization of o-Hydroxyphenyl Enaminones with 1,3,5-Triazinanes: Access to C3-Aminomethyl Chromones and Tetrahydropyrimidines. Asian J. Org. Chem. 2024, 13, e202400391. [Google Scholar] [CrossRef]
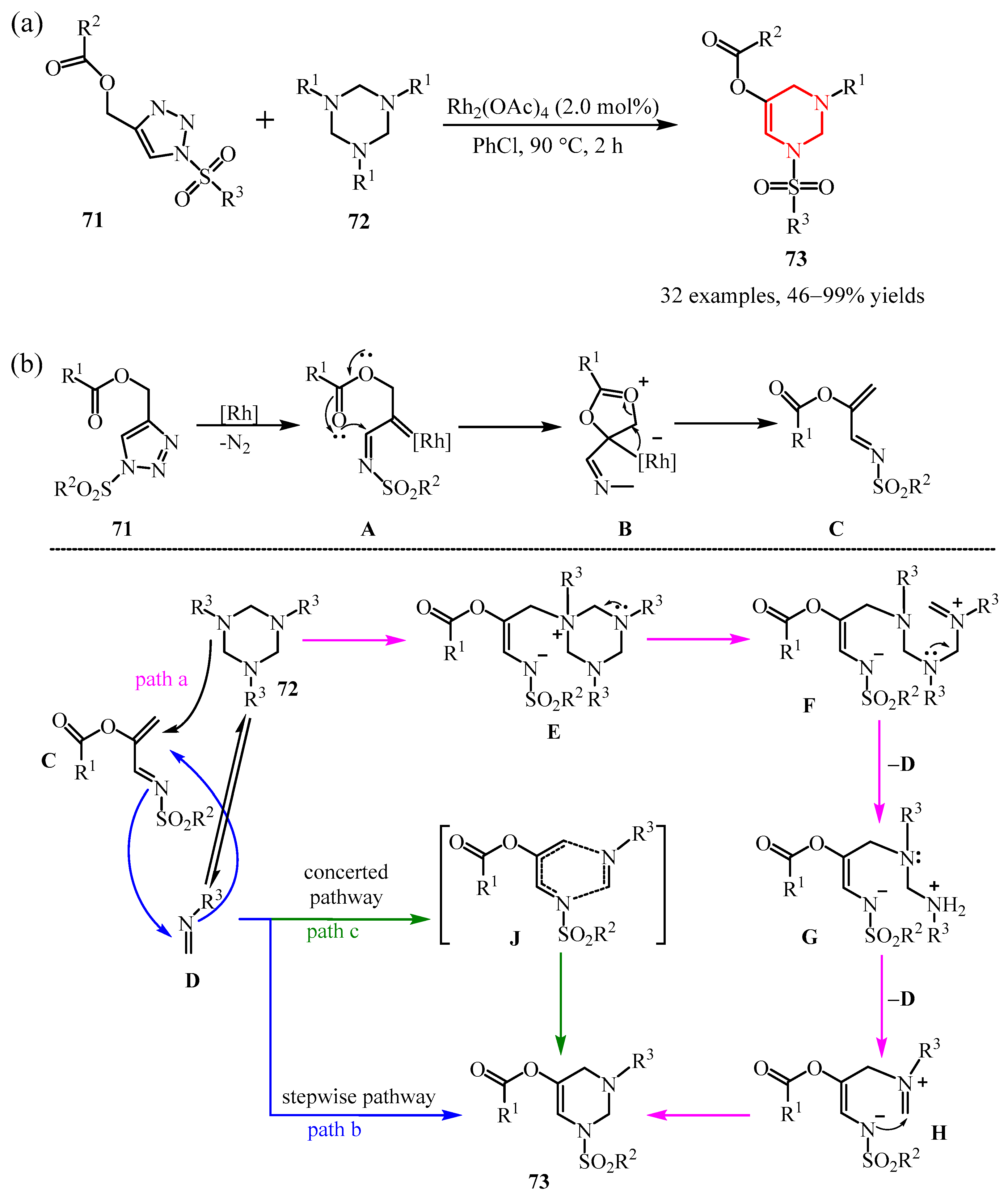
- Duan, S.; Meng, H.; Jablasone, S.T.; Luo, H.; Xu, Z.; Li, C. Rhodium (II)-Catalyzed [4+2] Annulation of Ester-Tethered 1-Sulfonyl-1,2,3-Triazoles and Hexahydro-1,3,5-Triazines. Asian J. Org. Chem. 2021, 10, 1076–1080. [Google Scholar] [CrossRef]
- Xie, W.; Wang, C.; Xu, J. Reaction of 1,3,5-Triazinanes with Phosphoryl Diazomethanes: Access to 5-Phosphoryl-1,2,3,4-Tetrahydropyrimidines. Org. Lett. 2024, 26, 3391–3396. [Google Scholar] [CrossRef]
- Tabor, W.; Katsogiannou, A.; Karta, D.; Andrianopoulou, E.; Berlicki, Ł.; Vassiliou, S.; Grabowiecka, A. Exploration of Thiourea-Based Scaffolds for the Construction of Bacterial Ureases Inhibitors. ACS Omega 2023, 8, 28783–28796. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zheng, J.; Zhao, S. Chemoselective Synthesis of Tetrahydropyrimidines and Dihydropyrrolidones through Three-Component Reaction of Amines, 1,3, 5-Triazinanes, and Alkyne Esters. Eur. J. Org. Chem. 2025, 28, e202401400. [Google Scholar] [CrossRef]
- Wang, T.; Zhang, B.; Hu, L.; Sun, H.; Wang, Y.; Zhai, H.; Cheng, B. Synthesis of Chromeno [2,3-d] Pyrimidin-5-One Derivatives from 1,3,5-Triazinanes via Two Different Reaction Pathways. J. Org. Chem. 2022, 87, 1348–1356. [Google Scholar] [CrossRef]
- Zheng, Y.; Tu, L.; Li, N.; Huang, R.; Feng, T.; Sun, H.; Li, Z.; Liu, J. Inverse-Electron-Demand [4+2]-Cycloaddition of 1,3,5- Triazinanes: Facile Approaches to Tetrahydroquinazolines Yongsheng. Adv. Synth. Catal. 2019, 361, 44–48. [Google Scholar] [CrossRef]
- Ji, D.; Wang, C.; Sun, J. Asymmetric [4+2]-Cycloaddition of Copper−Allenylidenes with Hexahydro-1,3,5-Triazines: Access to Chiral Tetrahydroquinazolines. Org. Lett. 2018, 20, 3710–3713. [Google Scholar] [CrossRef]
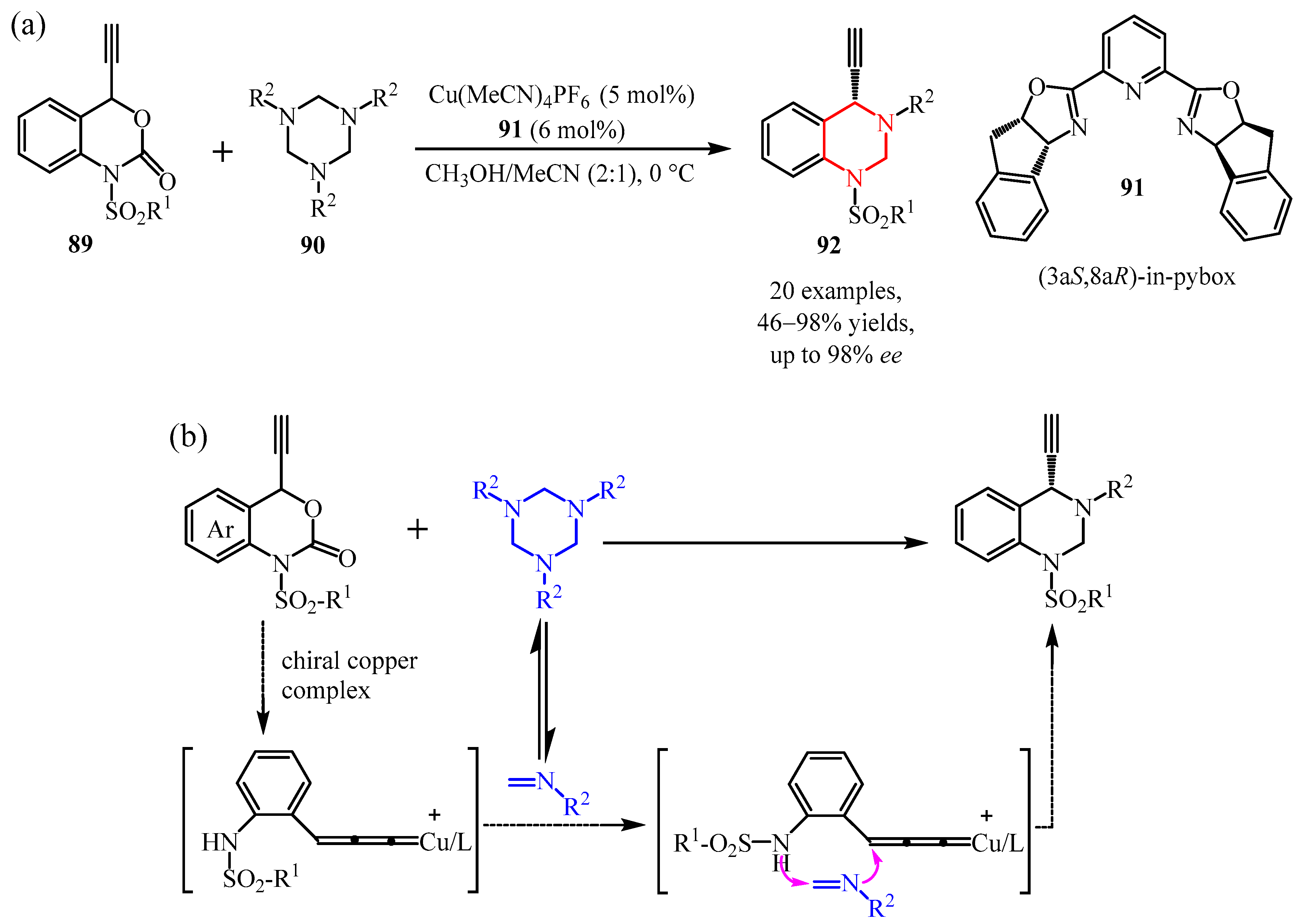
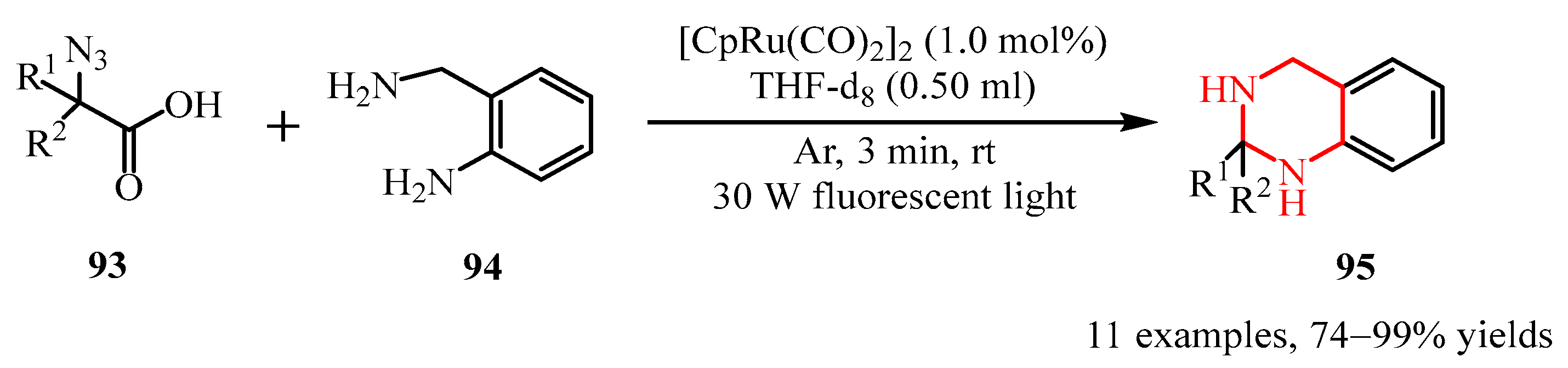
- Jo, H.Y.; Lee, J.M.; Pietrasiak, E.; Lee, E.; Rhee, Y.H.; Park, J. Generation of N−H Imines from A-Azidocarboxylic Acids through Ru-Catalyzed Decarboxylation. J. Org. Chem. 2021, 86, 17409–17417. [Google Scholar] [CrossRef]
- Tian, Y.; Chen, Z.; Su, K.; Zheng, Y.; Liu, J. [2 + 2 + 1 + 1] Cycloaddition Reaction of 1,3,5-Triazinanes with Methylene Compounds: Approach to Hexahydropyrimidines. Adv. Synth. Catal. 2023, 365, 3909–3914. [Google Scholar] [CrossRef]
- Yang, D.; Wang, Y.; Zhang, C.; Luo, Y.; Zhu, X.; Zhao, S.; Fu, W.; Cheng, B.; Zhai, H.; Wang, T. Shifting Access from Pyrimidine-Spirofused to Fused Benzoheterocycles by Modifying the Activated Group Position. Adv. Synth. Catal. 2024, 366, 1096–1100. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Q.; Ren, M.; Xue, Y.; Wang, Z.; Hou, X.H.; Ma, Z.W.; Xie, Y.X.; Chen, Y.J. [1 + 5] Cyclization of Indoline-Derived Azadienes with 1,3,5- Triazinanes: An Efficient Protocol for the Synthesis of Indoline-Spiro-Hexahydropyrimidines. Chem. Eur. J. 2025, 31, e202404277. [Google Scholar] [CrossRef]
- Shipilovskikh, S.A.; Rubtsov, A.E. One-Pot Synthesis of Thieno [3,2-e] Pyrrolo [1,2-a] Pyrimidine Derivative Scaffold: A Valuable Source of PARP-1 Inhibitors. J. Org. Chem. 2019, 84, 15788–15796. [Google Scholar] [CrossRef] [PubMed]
- Javahershenas, R.; Khalafy, J. One-Pot, Three-Component Synthesis of Pyrrolo [2,3-d] Pyrimidine Derivatives. J. Mex. Chem. Soc. 2018, 62, 1–9. [Google Scholar] [CrossRef]
- Yang, P.; Wang, Q.; Cui, B.H.; Zhang, X.D.; Liu, H.; Zhang, Y.Y.; Liu, J.L.; Huang, W.Y.; Liang, R.X.; Jia, Y.X. Enantioselective Dearomative [3 + 2] Umpolung Annulation of N-Heteroarenes with Alkynes. J. Am. Chem. Soc. 2022, 144, 1087–1093. [Google Scholar] [CrossRef] [PubMed]
- Xiong, B.; Zhang, S.; Chen, L.; Li, B.; Jiang, H.; Zhang, M. An Annulative Transfer Hydrogenation Strategy Enables Straightforward Access to Tetrahydro Fused-Pyrazine Derivatives. Chem. Commun. 2016, 52, 10636–10639. [Google Scholar] [CrossRef]
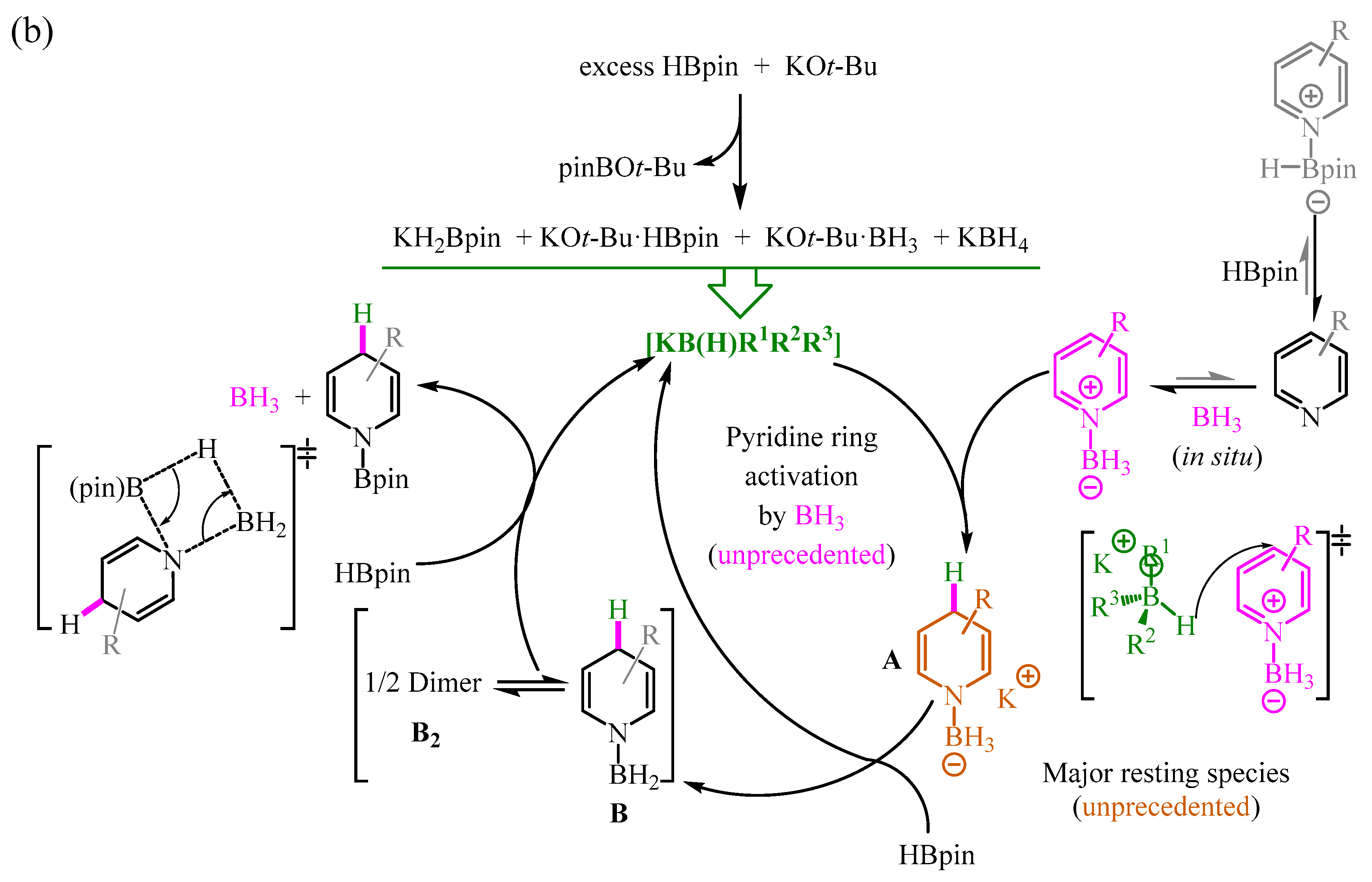
- Jeong, E.; Heo, J.; Park, S.; Chang, S. Alkoxide-Promoted Selective Hydroboration of N-Heteroarenes: Pivotal Roles of in Situ Generated BH3 in the Dearomatization Process. Chem. A Eur. J. 2019, 25, 6320–6325. [Google Scholar] [CrossRef]
- Moradi, L.; Piltan, M.; Rostami, H.; Abasi, G. One-Pot Synthesis of Pyrrolo [1,2-a] Pyrazines via Three Component Reaction of Ethylenediamine, Acetylenic Esters and Nitrostyrene Derivatives. Chinese Chem. Lett. 2013, 24, 740–742. [Google Scholar] [CrossRef]
- Wippert, N.; Nieger, M.; Herlan, C.; Jung, N.; Bräse, S. Synthesis of New Pyrazolo [1,2,3] Triazines by Cyclative Cleavage of Pyrazolyltriazenes. Beilstein J. Org. Chem. 2021, 17, 2773–2780. [Google Scholar] [CrossRef]
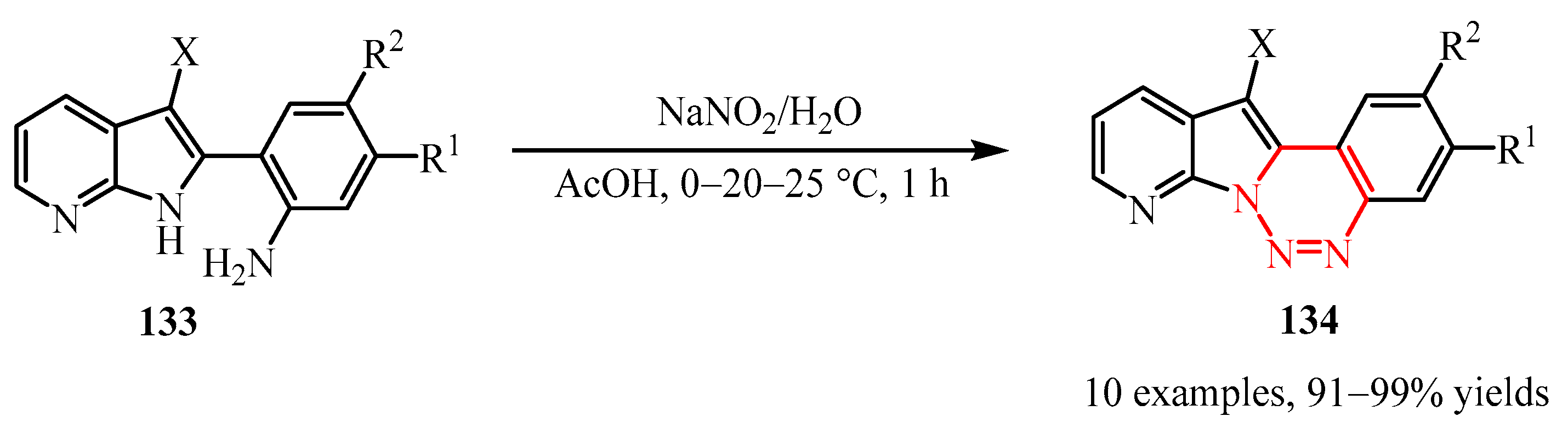
- Parrino, B.; Carbone, A.; Muscarella, M.; Spano, V.; Montalbano, A.; Barraja, P.; Salvador, A.; Vedaldi, D.; Cirrincione, G.; Diana, P. 11H-Pyrido [3’,2’:4,5] Pyrrolo [3,2-c] Cinnoline and Pyrido [3’,2’:4,5] Pyrrolo [1,2- c] [1,2,3] Benzotriazine: Two New Ring Systems with Antitumor Activity. J. Med. Chem. 2014, 57, 9495–9511. [Google Scholar] [CrossRef]
- Pang, X.; Zhao, L.; Zhou, D.; He, P.Y.; An, Z.; Ni, J.X.; Yan, R. Tert-Butyl Nitrite (TBN) as the N Atom Source for the Synthesis of Substituted Cinnolines with 2-Vinylanilines and a Relevant Mechanism Was Studied. Org. Biomol. Chem. 2017, 15, 6318–6322. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Li, B.; Yu, F.; Zhang, X.; Fan, X. Synthesis of Pyrazolidinone-Fused Benzotriazines through C−H/ N−H Bond Functionalization of 1-Phenylpyrazolidinones with Oxadiazolones. J. Org. Chem. 2023, 88, 8179–8191. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, J.; Guan, Y.; Guo, H. Dearomative [4+2] Annulation of Electron-Poor N-Heteroarenes with Azoalkenes for Access to Polycyclic Tetrahydro-1,2,4-Triazines. Org. Lett. 2023, 25, 3543–3547. [Google Scholar] [CrossRef]
- Li, X.; Romero, M.D.; Tcaturian, S.; Kurpiewska, K.; Dömling, A. N-Edited Guanine Isosteres. J. Org. Chem. 2023, 88, 9823–9834. [Google Scholar] [CrossRef]
- Fang, Z.; Jin, Q.; Wang, X.; Ning, Y. Metal-Free [2+1+3] Cycloaddition of Trifluoroacetaldehyde N-Sulfonylhydrazones with Hexahydro-1,3,5-Triazines Leading to Trifluoromethylated 2,3,4,5-Tetrahydro-1,2,4-Triazines. J. Org. Chem. 2022, 87, 2966–2974. [Google Scholar] [CrossRef]
- Tahmasby, M.; Darehkordi, A.; Mohammadi, M.; Nejadkhorasani, F. Pyrido Triazin-Nucleus Synthesis and Theoretical Studies: 2,3,6-Trioxo-8-Aryl-1,3,4,6-Tetrahydro-] 2H [Pyrido] 1,2-b][1,2,4 [Triazin-7,9-Dicarbonitryl Derivatives. J. Mol. Struct. 2020, 1224, 129032. [Google Scholar] [CrossRef]
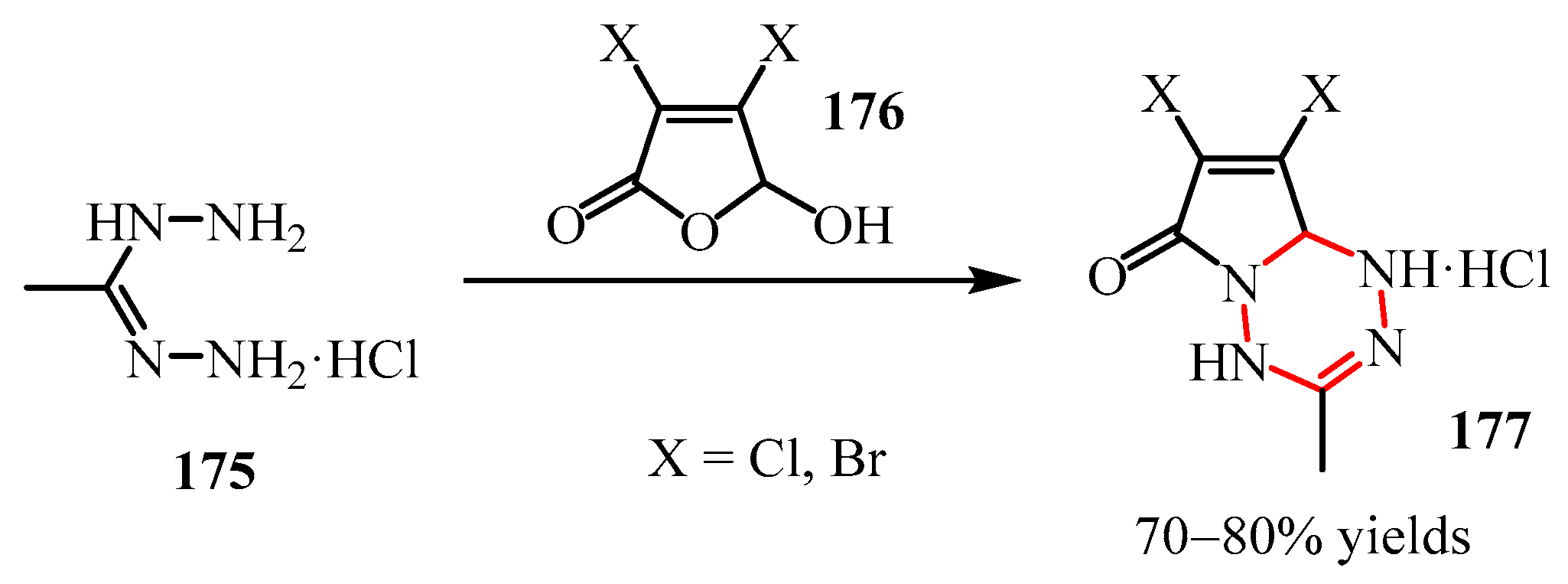
- Traynor, J.R.; Wibberley, D.G. Pyrrolo [1,2-a] [1,3,5] Triazine-2,4 (1H,3H)-Diones. Part II. Synthesis from 3,4-Disubstituted 2-Aminopyrroles. J. Chem. Soc. Perkin Trans. 1 1974, 1974, 1786–1788. [Google Scholar] [CrossRef]
- Verhoeven, J.; Reddy, B.N.; Meerpoel, L.; Willem, J.; Verniest, G. Synthesis and Transformations of Pyrrolo [1,2-a] [1,3,5]-Triazines. Tetrahedron Lett. 2018, 59, 4537–4539. [Google Scholar] [CrossRef]
- Wingard, L.A.; Johnson, E.C.; Sabatini, J.J. Efficient Method for the Cycloaminomethylation of Glycoluril. Tetrahedron Lett. 2016, 57, 1681–1682. [Google Scholar] [CrossRef]
- Salkeeva, L.K.; Shibaeva, A.K.; Bakibaev, A.A.; Taishibekova, E.K.; Minaevaa, E.V.; Zhortarovaa, A.A.; Sal’keeva, A.K. New Heterocycles Based on Tetramethylol Glycoluril. Russ. J. Gen. Chem. 2014, 84, 344–345. [Google Scholar] [CrossRef]
- Khairullina, R.R.; Geniyatova, A.R.; Meshcheryakova, E.S.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemilev, U.M. Catalytic Cycloaminomethylation of Ureas and Thioureas with N,N-Bis (Methoxymethyl) Alkanamines. Russ. J. Org. Chem. 2015, 51, 118–122. [Google Scholar] [CrossRef]
- Kravchenko, A.N.; Chikunov, I.E.; Lyssenko, K.A.; Baranov, V. V Glycolurils in α-Ureido- and α-Aminoalkylation Reactions. 3**. N-(Hydroxymethyl) Glycolurils in Reactions with Aliphatic Amines and Amino Acids. Chem. Heterocycl. Compd. 2014, 50, 1322–1331. [Google Scholar] [CrossRef]
- Rakhimova, E.B.; Ismagilov, R.A.; Meshcheryakova, E.S.; Khalilov, L.M.; Ibragimov, A.G.; Dzhemilev, U.M. An Efficient Catalytic Method for the Synthesis of 2, 7-Dialkyl-2,3a,5a,7,8a,10a-Hexaazaperhydropyrenes. Tetrahedron Lett. 2014, 55, 6367–6369. [Google Scholar] [CrossRef]
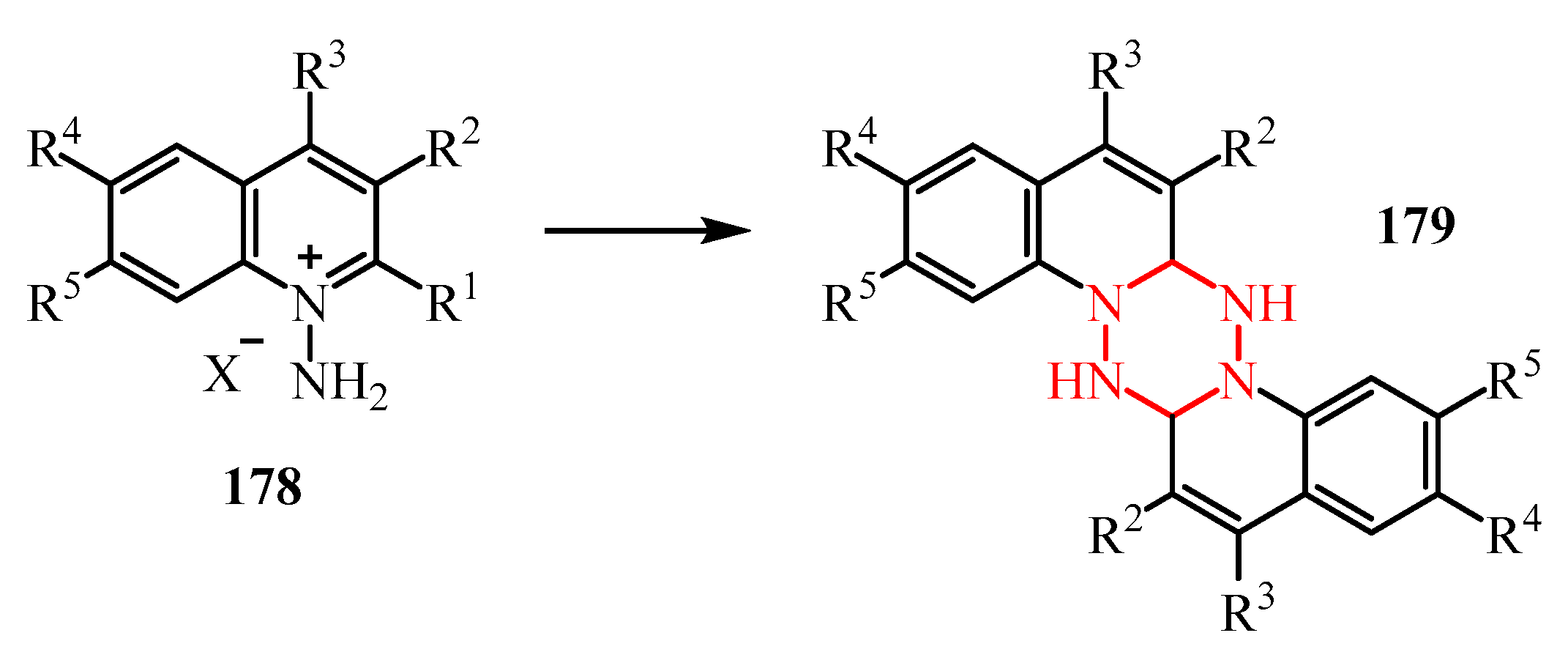
- Rakhimova, E.B.; Kirsanov, V.Y.; Meshcheryakova, E.S.; Khalilov, L.M.; Kutepov, B.I.; Ibragimov, A.G.; Dzhemilev, U.M. One-Pot Catalytic Synthesis of 2,7-Bis-Substituted 4,9(10)-Dimethyl-2,3a,5a,7,8a,10a-Hexaazaperhydropyrenes. Tetrahedron 2017, 73, 6880–6886. [Google Scholar] [CrossRef]
- Rakhimova, E.B.; Ibragimov, A.G.; Dzhemilev, U.M. Efficient Catalytic Synthesis of 2,7-Diaryl (Hetaryl)-4,9-Dimethyl-Perhydro-2,3a,5a,7,8a,10a-Hexaazapyrenes. Russ. J. Org. Chem. 2018, 54, 1085–1089. [Google Scholar] [CrossRef]
- Klenov, M.S.; Voronin, A.A.; Churakov, A.M.; Tartakovsky, V.A. Advances in the Chemistry of 1,2,3,4-Tetrazines. Russ. Chem. Rev. 2023, 92, RCR5089. [Google Scholar] [CrossRef]
- Renault, A.; Joucla, L.; Lacôte, E. Catalytic Aerobic Oxidation of Hydrazines into 2-Tetrazenes. Eur. J. Org. Chem. 2022, 2022, e202200265. [Google Scholar] [CrossRef]
- Diana, P.; Barraja, P.; Lauria, A.; Montalbano, A.; Almerico, A.M.; Dattolo, G.; Cirrincione, G. Pyrrolo [2,1-d] [1,2,3,5] Tetrazine-4(3H)-Ones, a New Class of Azolotetrazines with Potent Antitumor Activity. Bioorg. Med. Chem. 2003, 11, 2371–2380. [Google Scholar] [CrossRef] [PubMed]
- Degen, H.-J.; Hailer, S.; Heeg, K.; Neunhoeffer, H. Hydrazidine, III. Synthese von 1,2,4,5-Tetrazino [3,2-a] Isoindolen. Chem. Ber. 1979, 112, 1981–1990. [Google Scholar] [CrossRef]
- Neunhoeffer, H.; Karafiat, U.; Kohler, G.; Sowa, B.; Hydrazidine, V. Synthese Und Reaktionen Aromatischer Und Aliphatischer Hydrazidine Und Deren N1-Substituierter Derivate. Liebigs Ann. Chemie 1992, 1992, 115–126. [Google Scholar] [CrossRef]
- Powell, B.F.; Overberger, C.G.; Anselme, J.-P. Hydrosulfite Reduction of N-Nitroso-1,2,3,4-Etrahydroisoquinolines and Oxidation of N-Amino-l,2,3,4 Tetrahydroisoquinolines. J. Heterocycl. Chem. 1983, 20, 121–128. [Google Scholar] [CrossRef]
- Lempert, K.; Fetter, J.; Nyitrai, J.; Bertha, F.; Møller, J. Electron-Deficient Heteroaromatic Ammonioamidates. Part 27. Quinazolinioamidates. Part 14. N-Amination of Some Quinazoline Derivatives and Some Reactions of the Resulting Quinazolinioamides. J. Chem. Soc. Perkin Trans. 1 1986, 1986, 269–275. [Google Scholar] [CrossRef]
- Glukhacheva, V.S.; Il’yasov, S.G.; Obraztsov, A.A.; Gatilov, Y.V.; Eltsov, I.V. A New Synthetic Route to Heteroanthracenes. Eur. J. Org. Chem. 2018, 2018, 1265–1273. [Google Scholar] [CrossRef]
- Daher, A.; Bousfiha, A.; Tolbatov, I.; Mboyi, C.D.; Cattey, H.; Roisnel, T.; Fleurat-lessard, P.; Hissler, M.; Hierso, J.; Bouit, P.-A.; et al. Tetrazo [1,2-b] Indazoles: Straightforward Access to Nitrogen-Rich Polyaromatics from s-Tetrazines Angewandte. Angew. Chem. Int. Ed. 2023, 62, e202300571. [Google Scholar] [CrossRef] [PubMed]
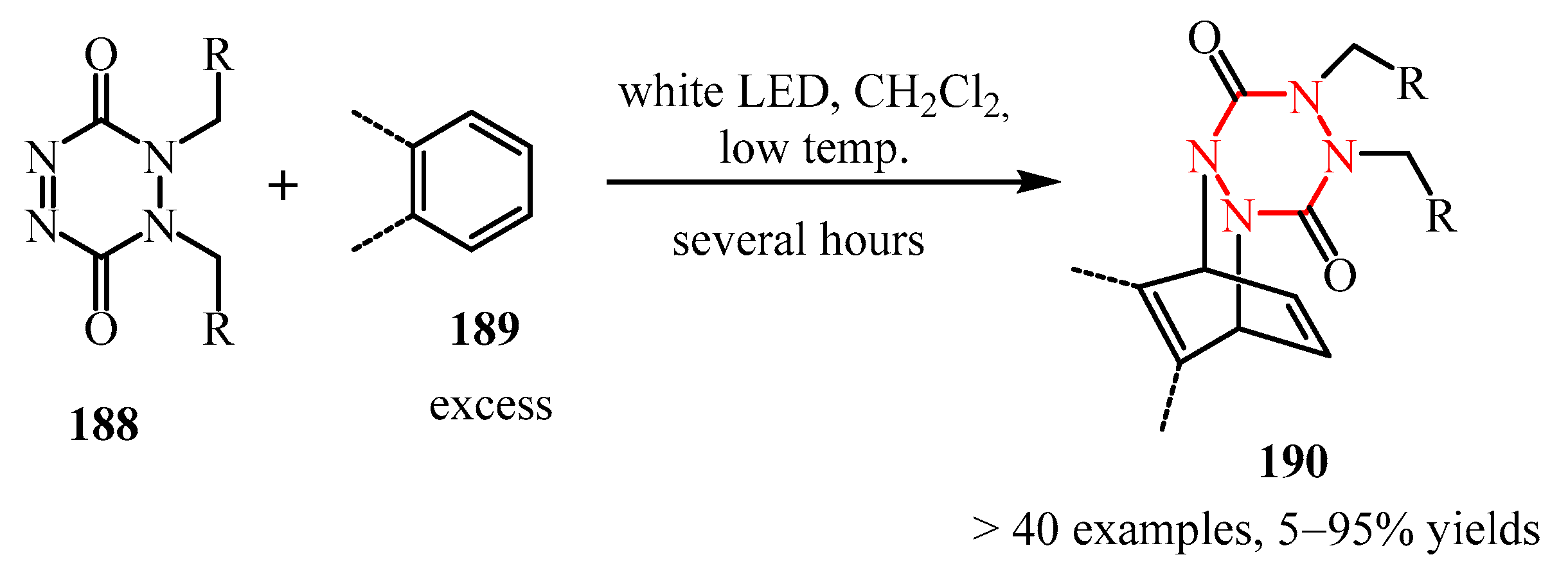
- Ikeda, K.; Kojima, R.; Kawai, K.; Murakami, T.; Kikuchi, T.; Kojima, M.; Yoshino, T.; Matsunaga, S. Formation of Isolable Dearomatized [4+2] Cycloadducts from Benzenes, Naphthalenes, and N-Heterocycles Using 1,2-Dihydro-1,2,4,5-Tetrazine-3,6-Diones as Arenophiles under Visible Light Irradiation. J. Am. Chem. Soc. 2023, 145, 9326–9333. [Google Scholar] [CrossRef]



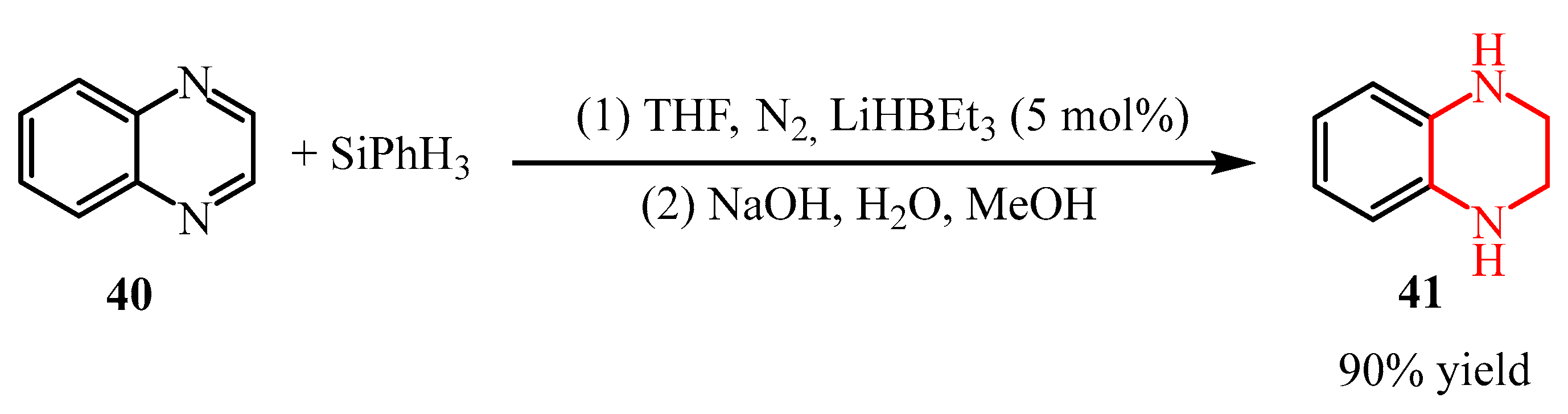
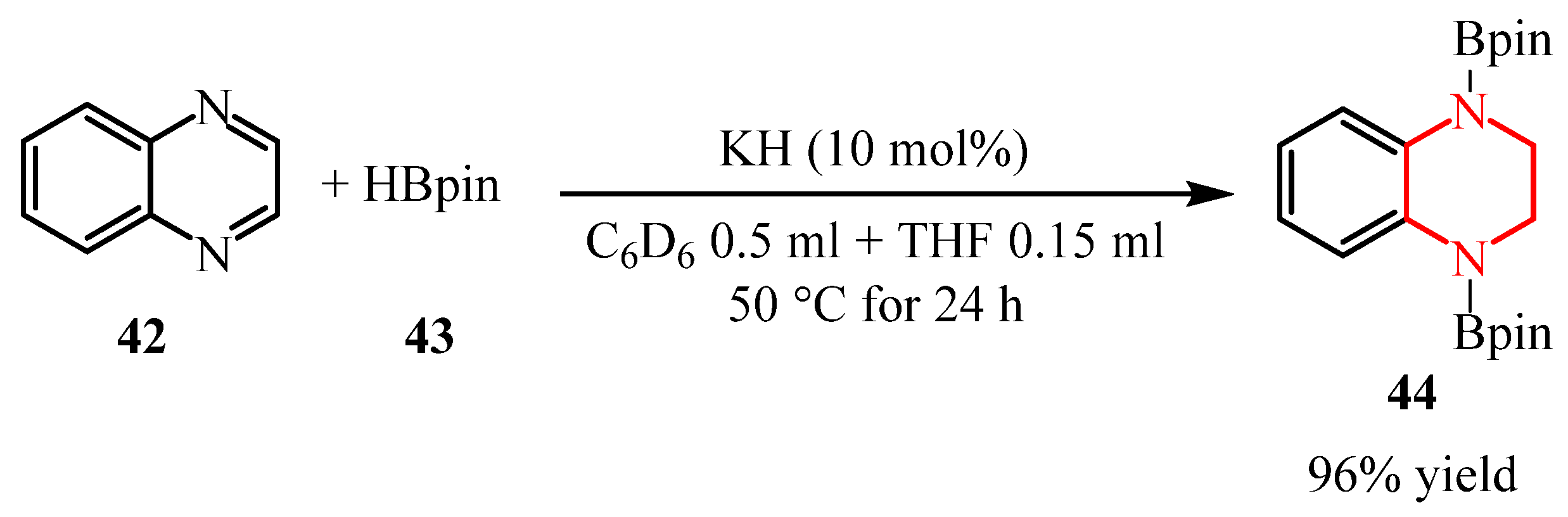




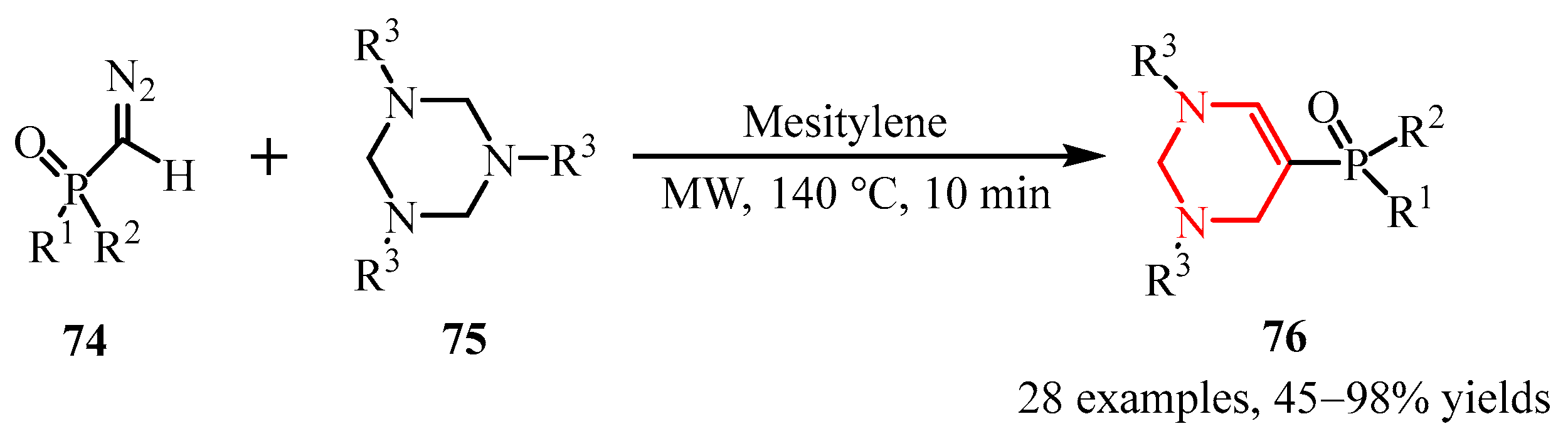

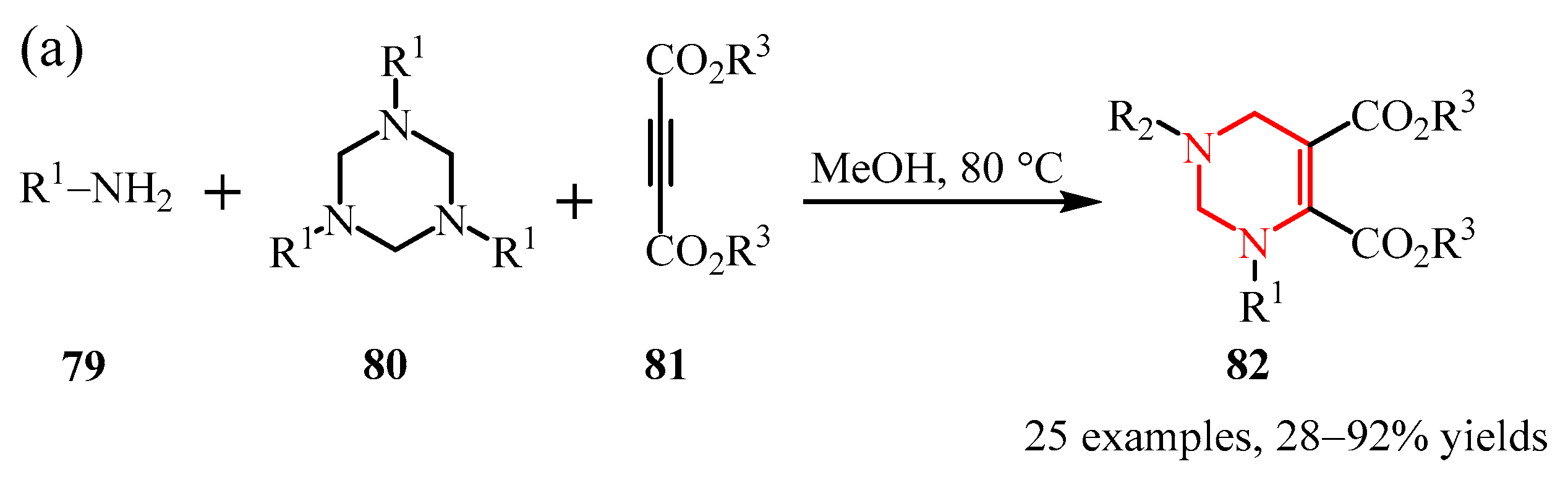


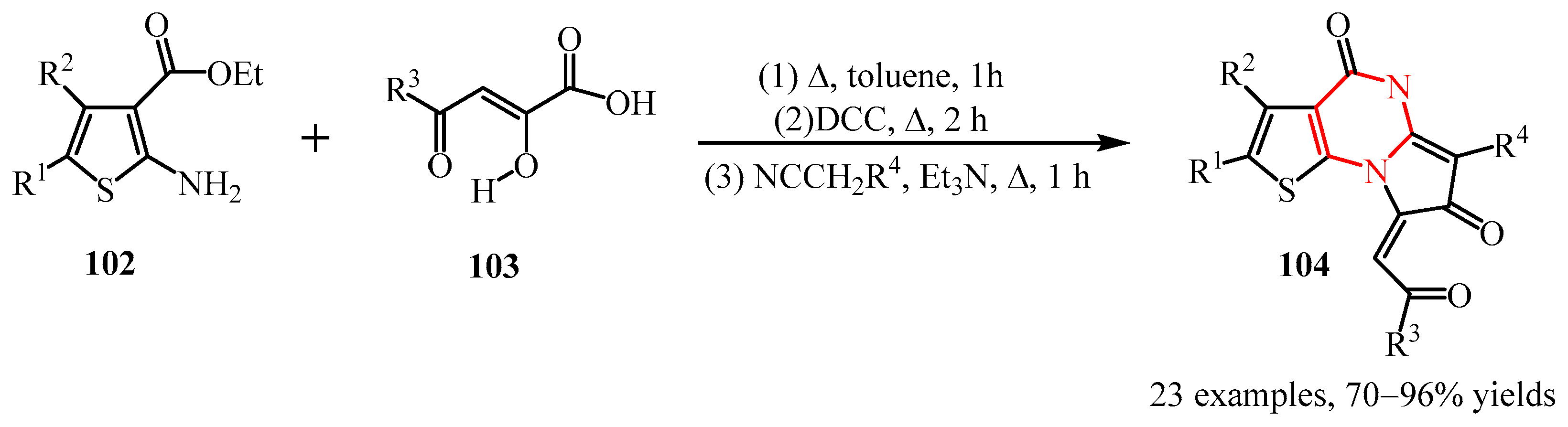
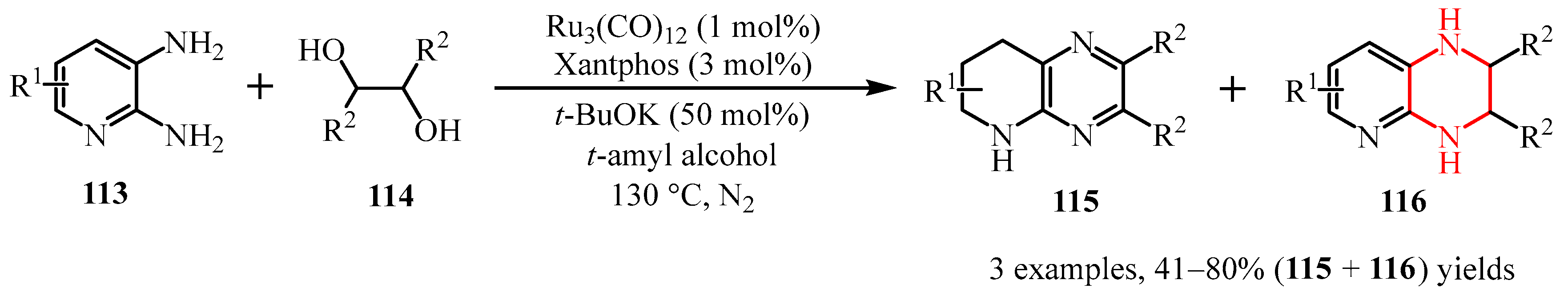
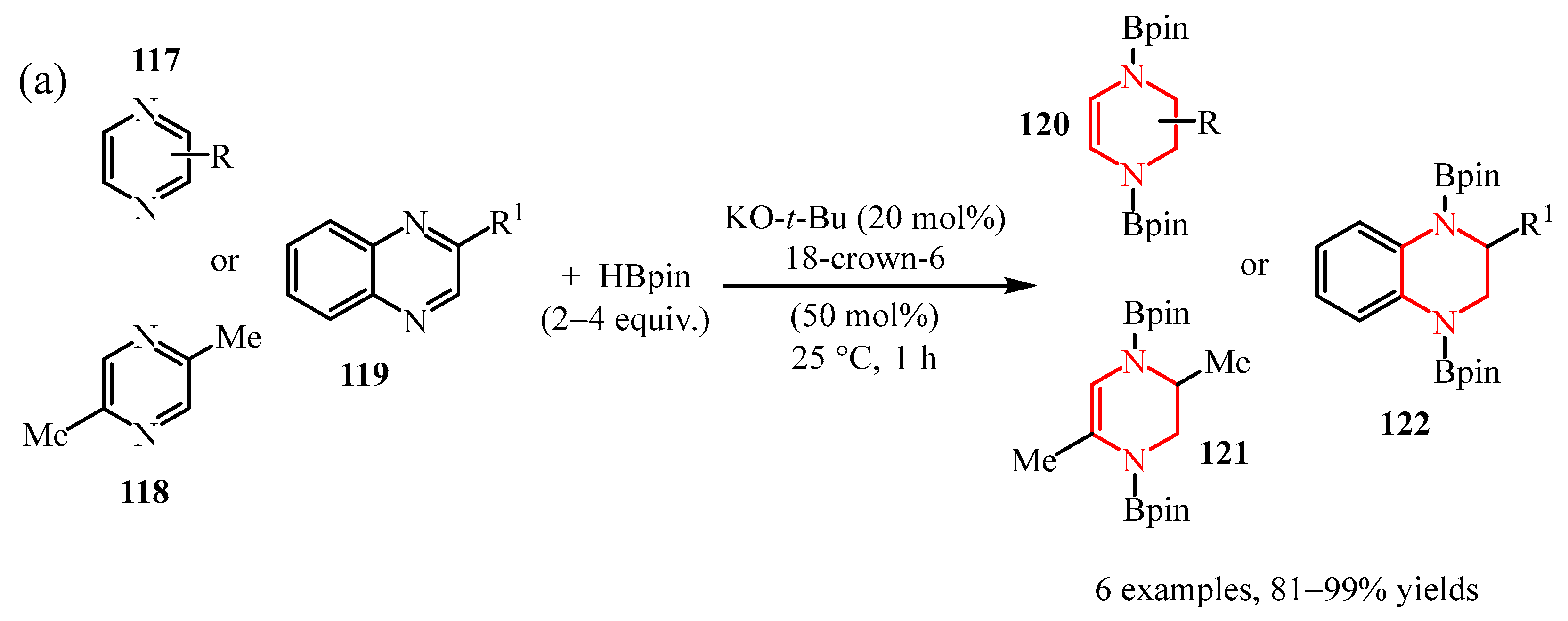

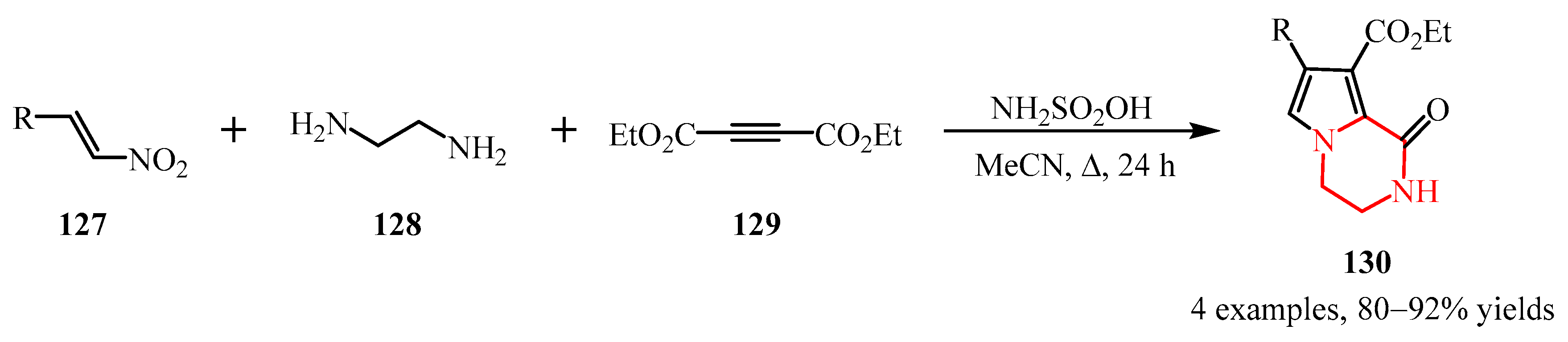
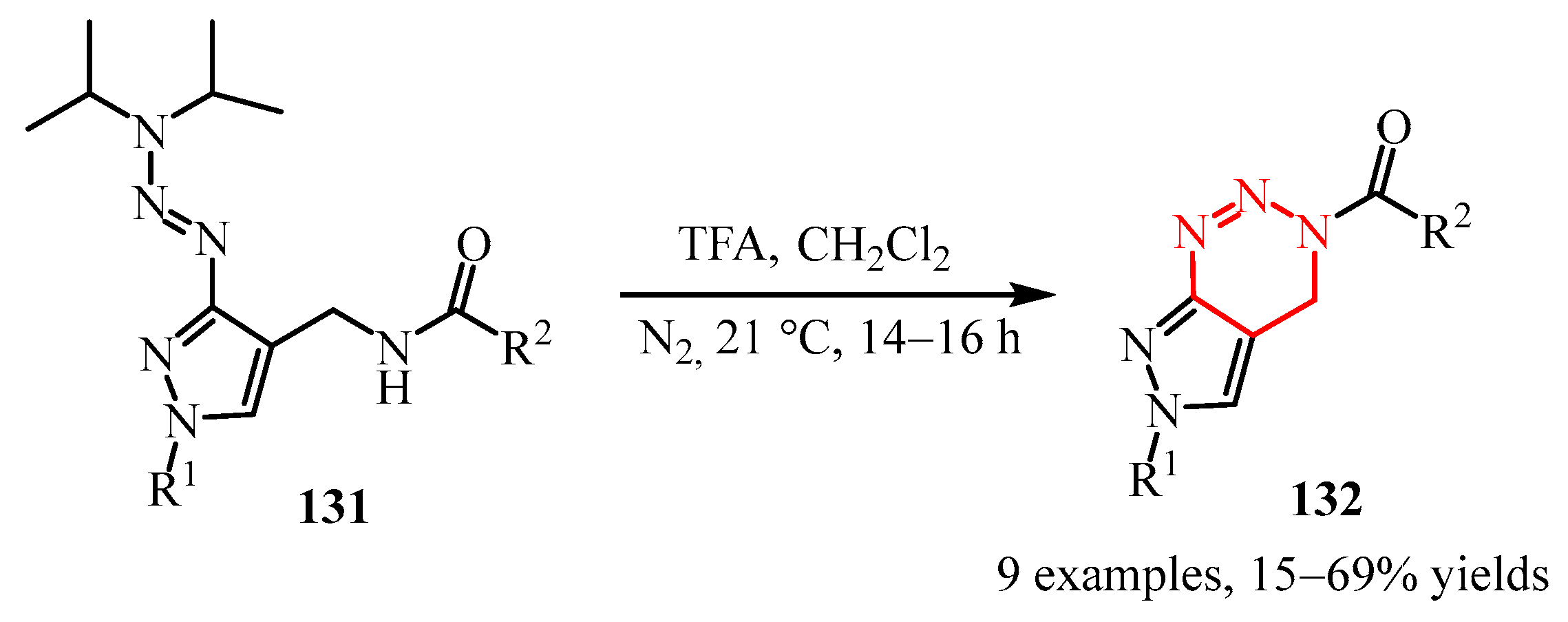
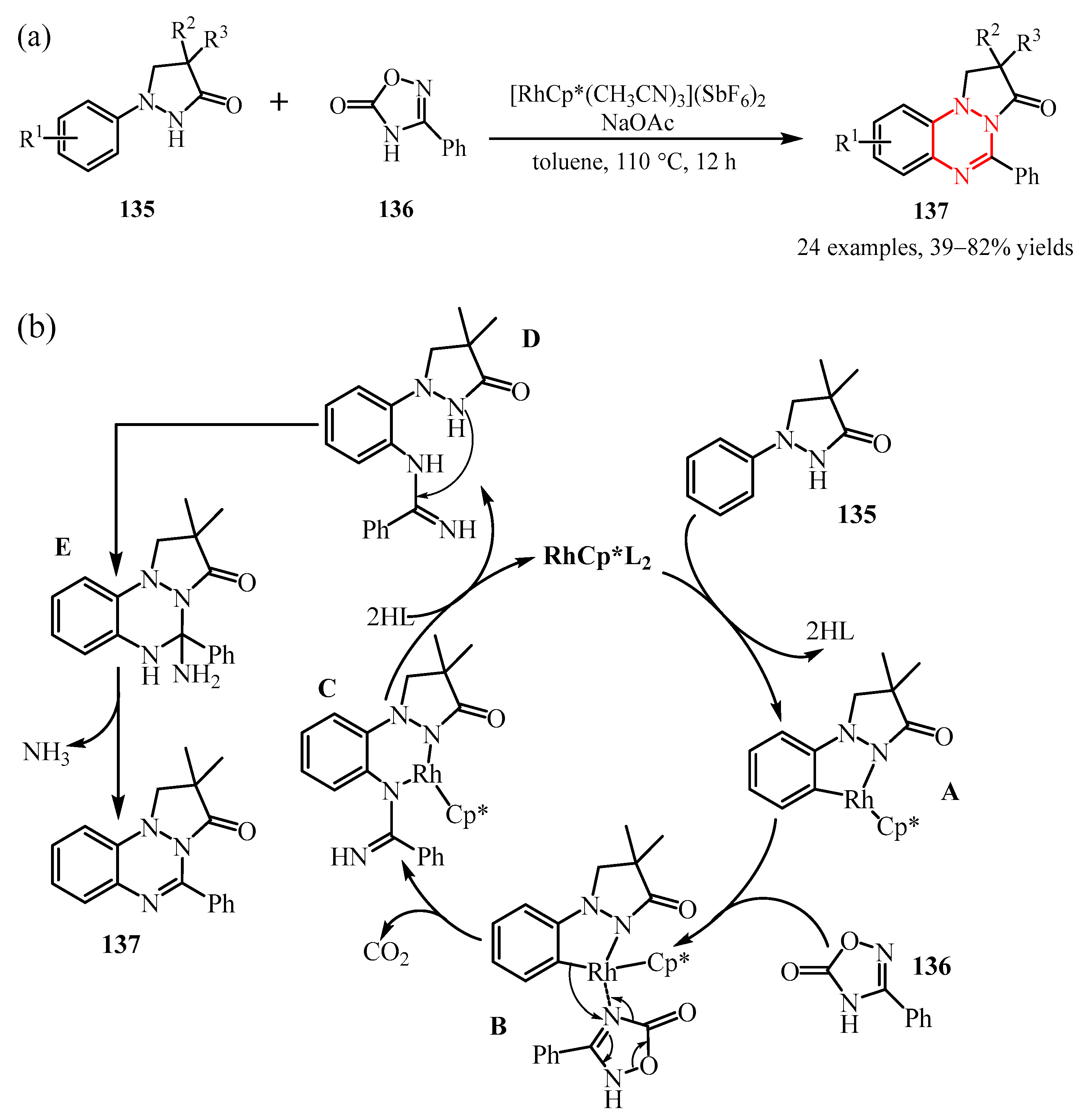
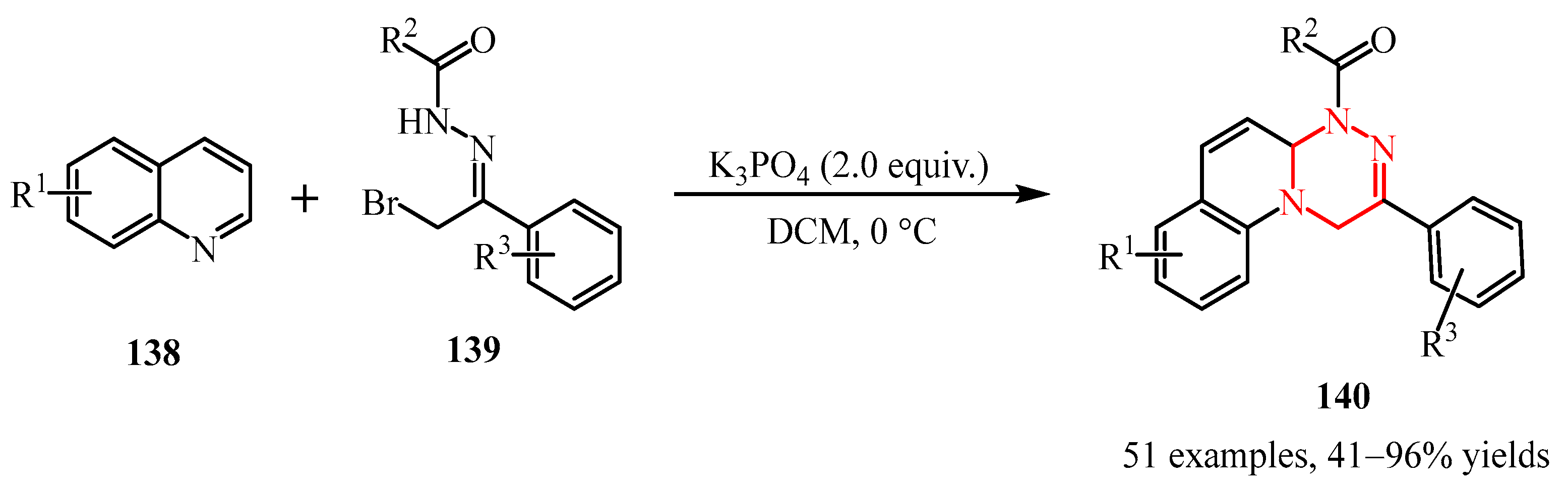
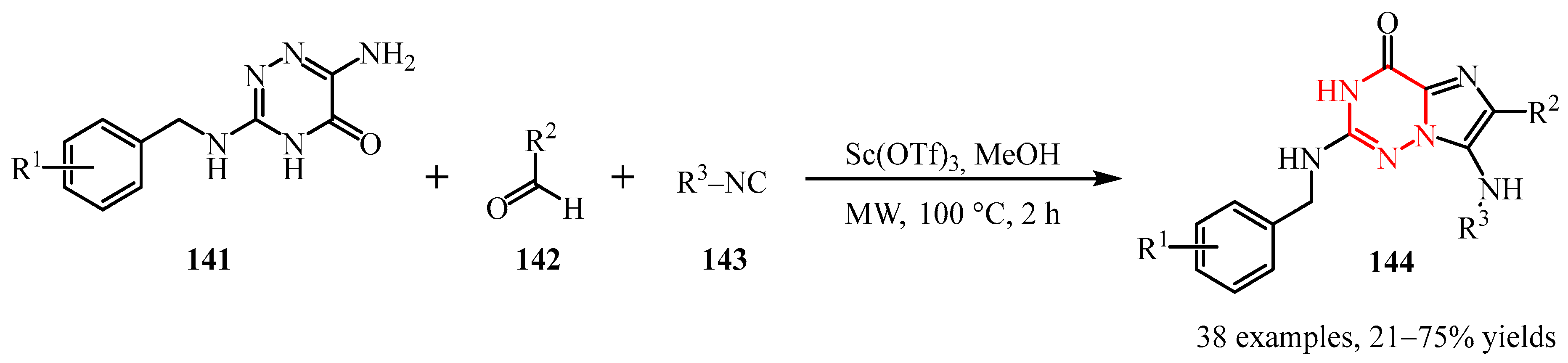
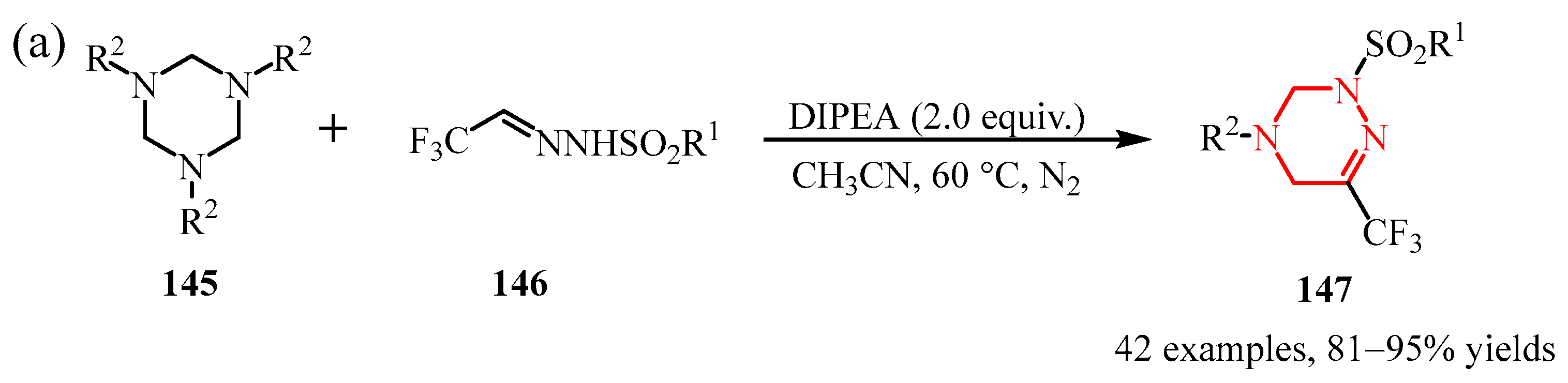
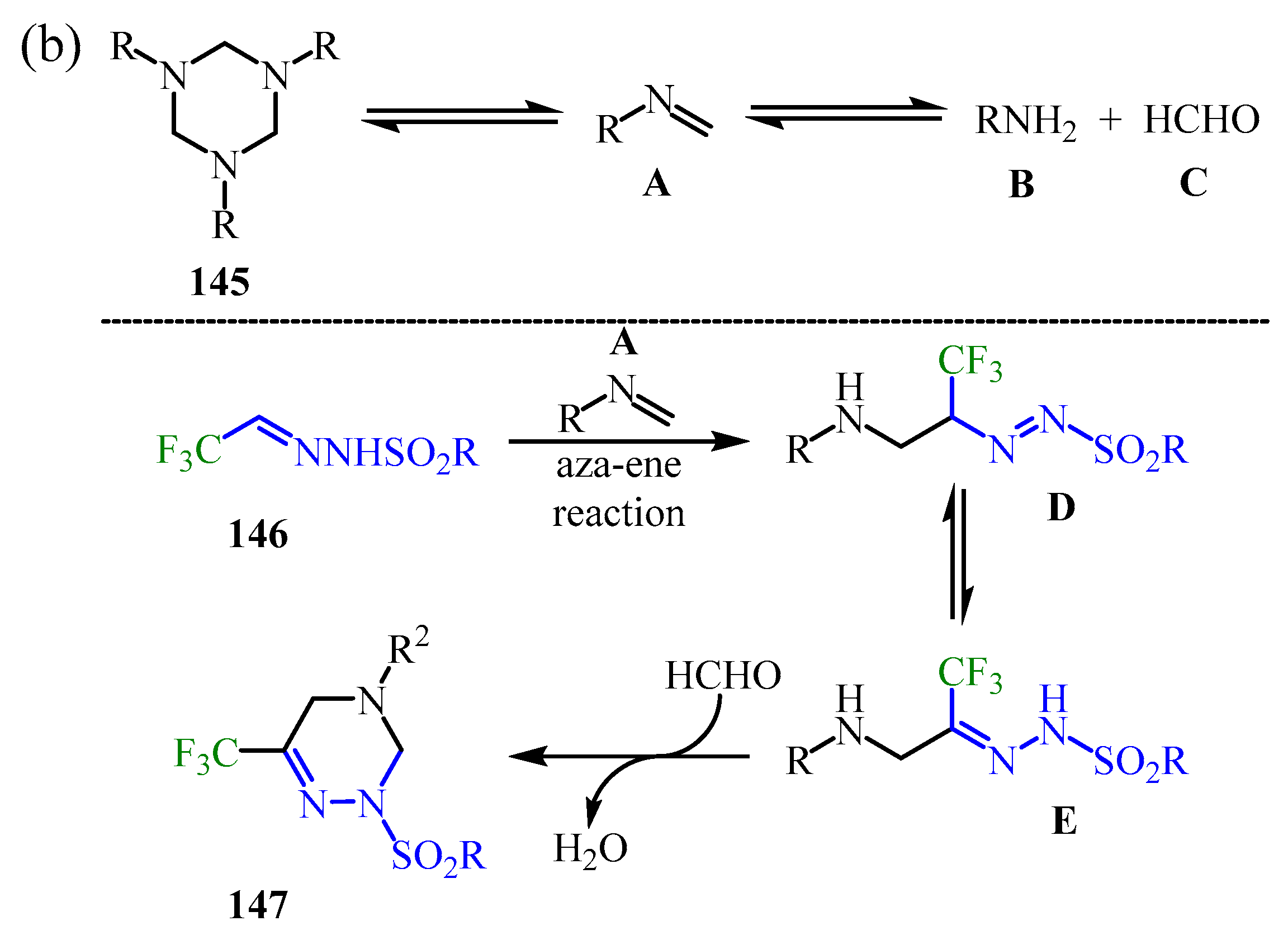



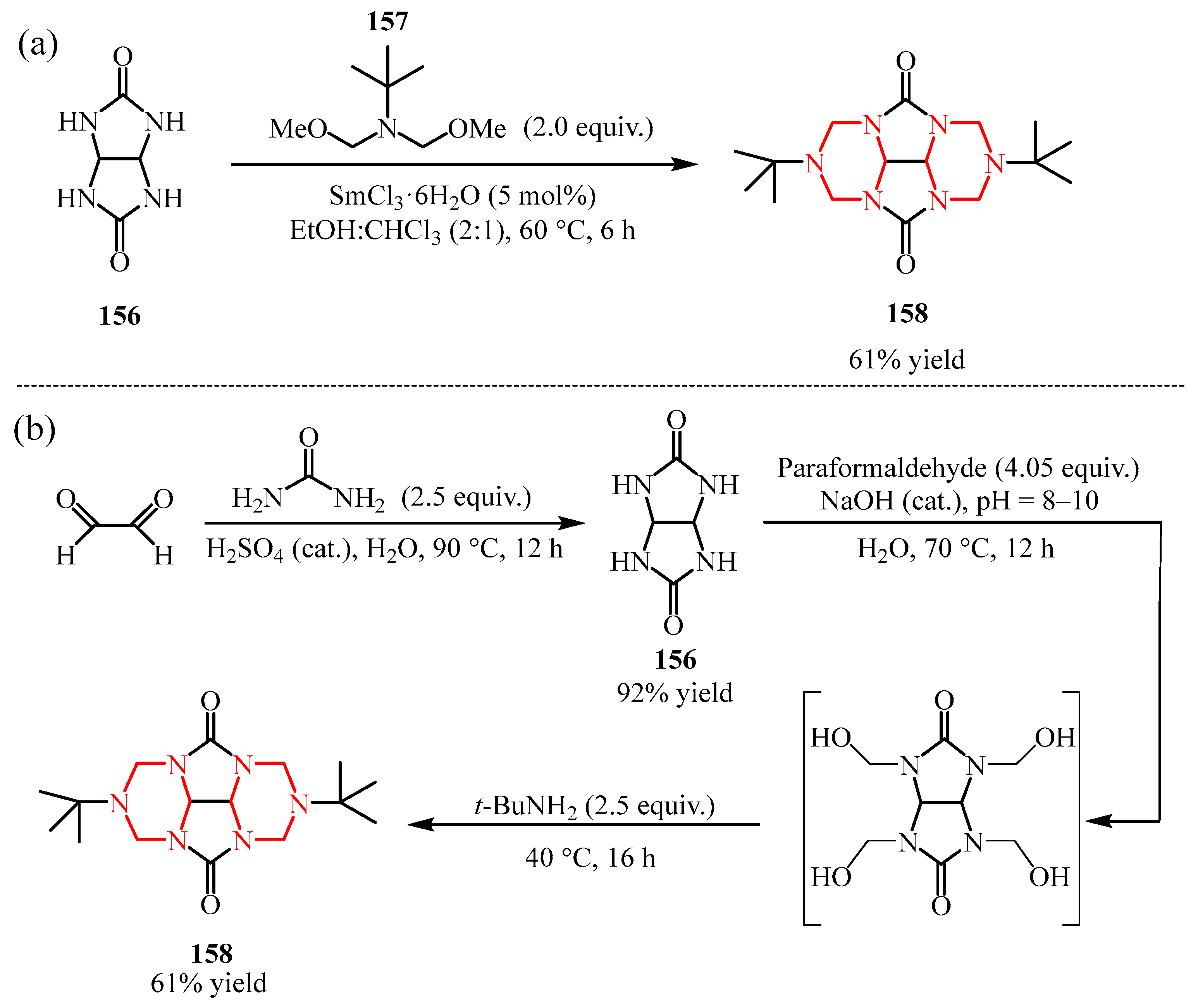

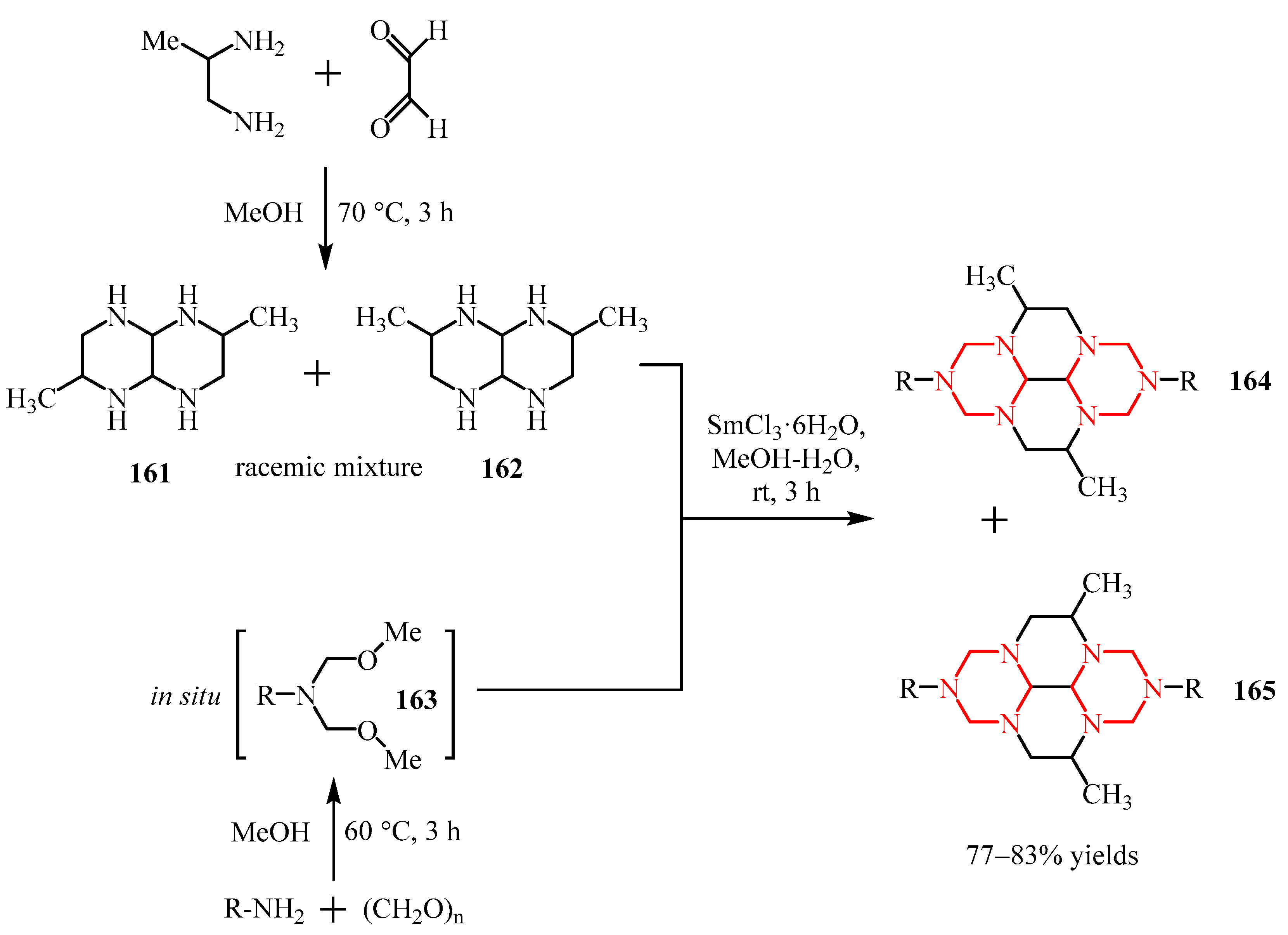


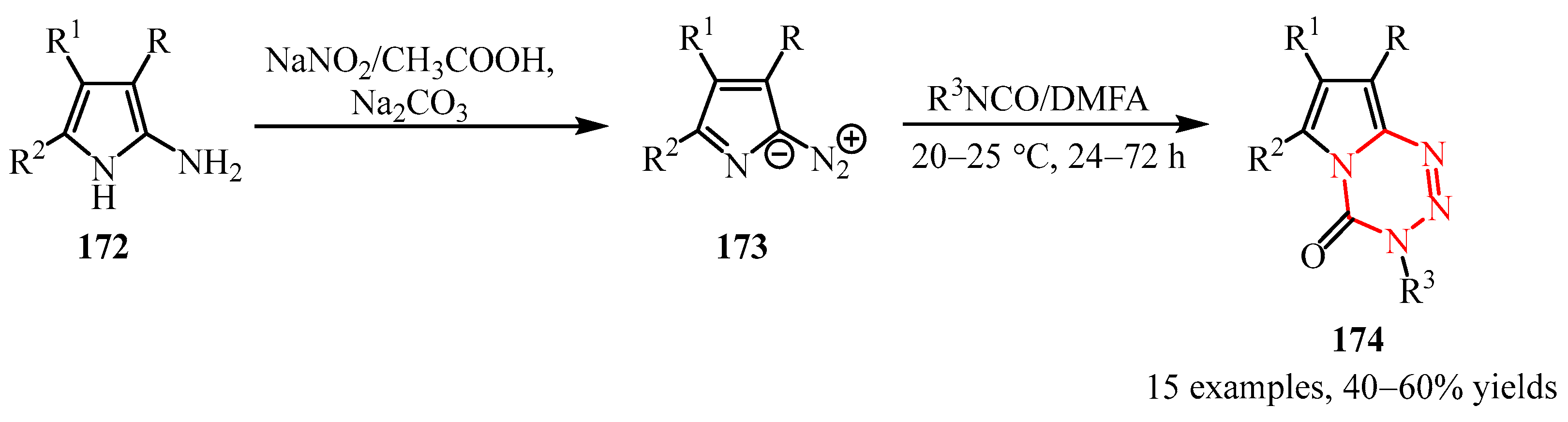


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Titova, Y.Y.; Gyrgenova, E.A.; Ivanov, A.V. Efficient Approaches to the Design of Six-Membered Polyazocyclic Compounds—Part 2: Nonaromatic Frameworks. Molecules 2025, 30, 3911. https://doi.org/10.3390/molecules30193911
Titova YY, Gyrgenova EA, Ivanov AV. Efficient Approaches to the Design of Six-Membered Polyazocyclic Compounds—Part 2: Nonaromatic Frameworks. Molecules. 2025; 30(19):3911. https://doi.org/10.3390/molecules30193911
Chicago/Turabian StyleTitova, Yuliya Yu., Elena A. Gyrgenova, and Andrey V. Ivanov. 2025. "Efficient Approaches to the Design of Six-Membered Polyazocyclic Compounds—Part 2: Nonaromatic Frameworks" Molecules 30, no. 19: 3911. https://doi.org/10.3390/molecules30193911
APA StyleTitova, Y. Y., Gyrgenova, E. A., & Ivanov, A. V. (2025). Efficient Approaches to the Design of Six-Membered Polyazocyclic Compounds—Part 2: Nonaromatic Frameworks. Molecules, 30(19), 3911. https://doi.org/10.3390/molecules30193911