Cerium Phosphate Nanoparticles: Synthesis, Characterization, Biocompatibility, Regenerative Potential, and Antioxidant Activity
Abstract
1. Introduction
2. Results
2.1. Results of Physicochemical Examinations
2.1.1. Results of Transmission Electron Microscopy
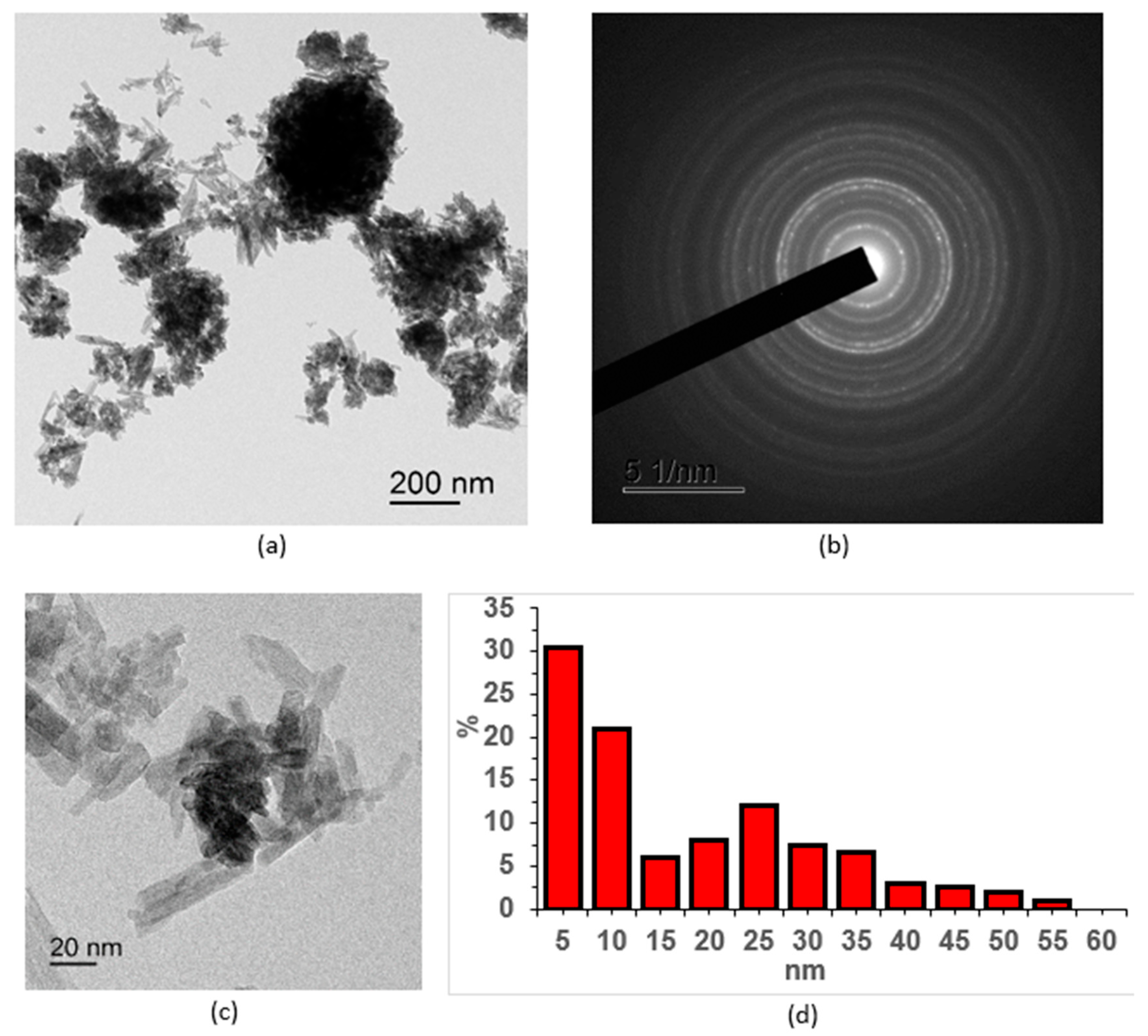
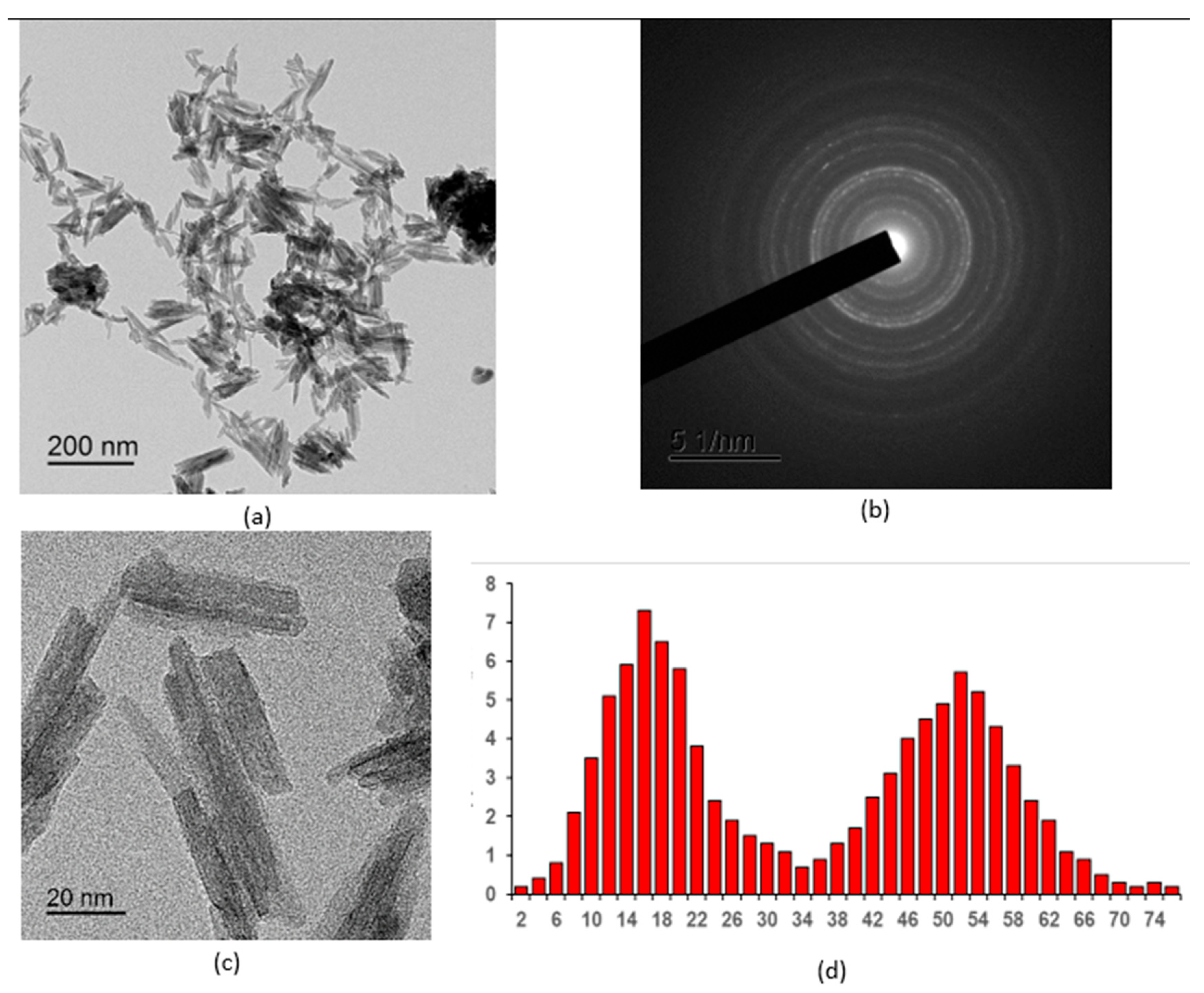
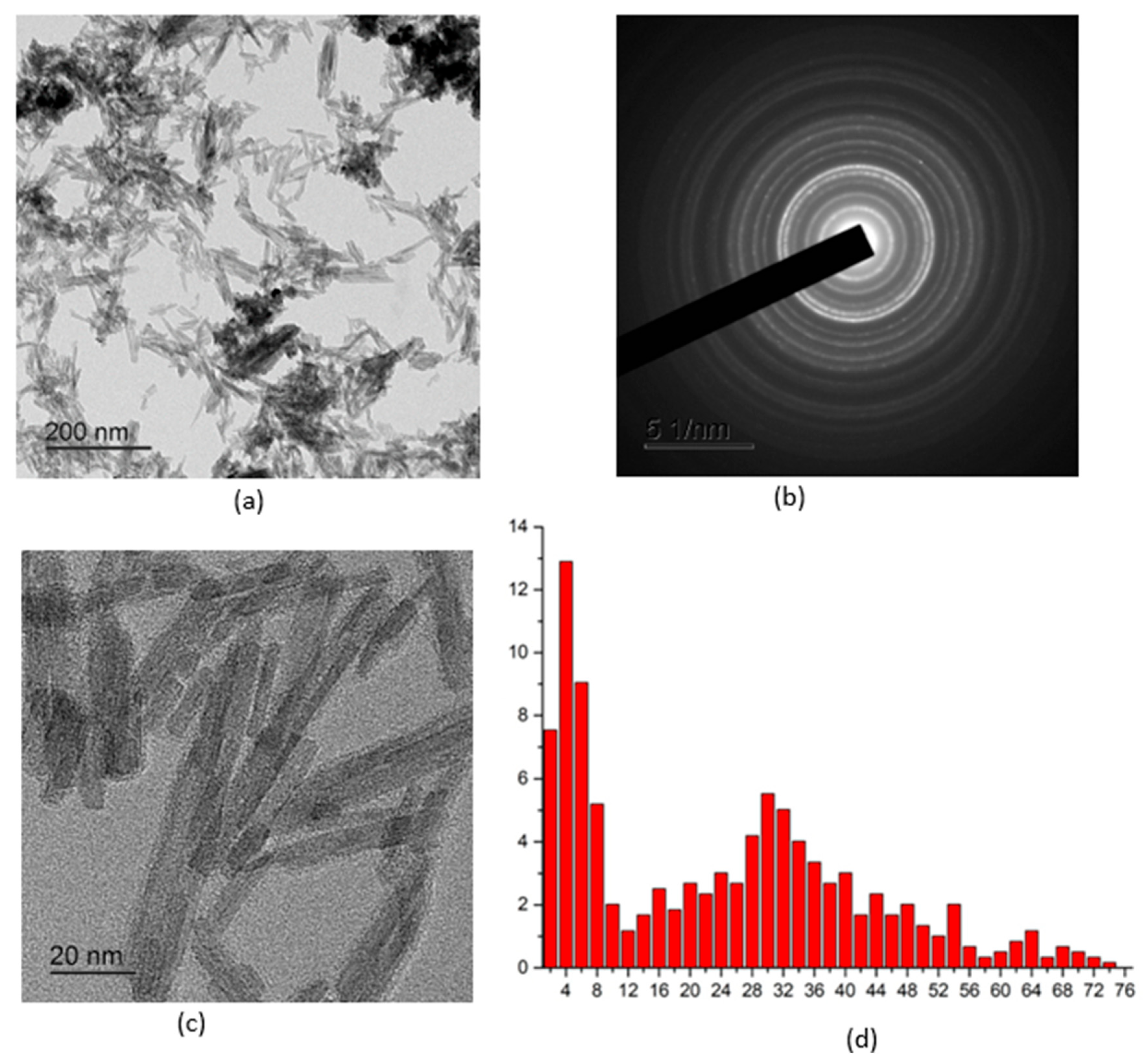
2.1.2. Results of X-Ray Diffraction Analysis

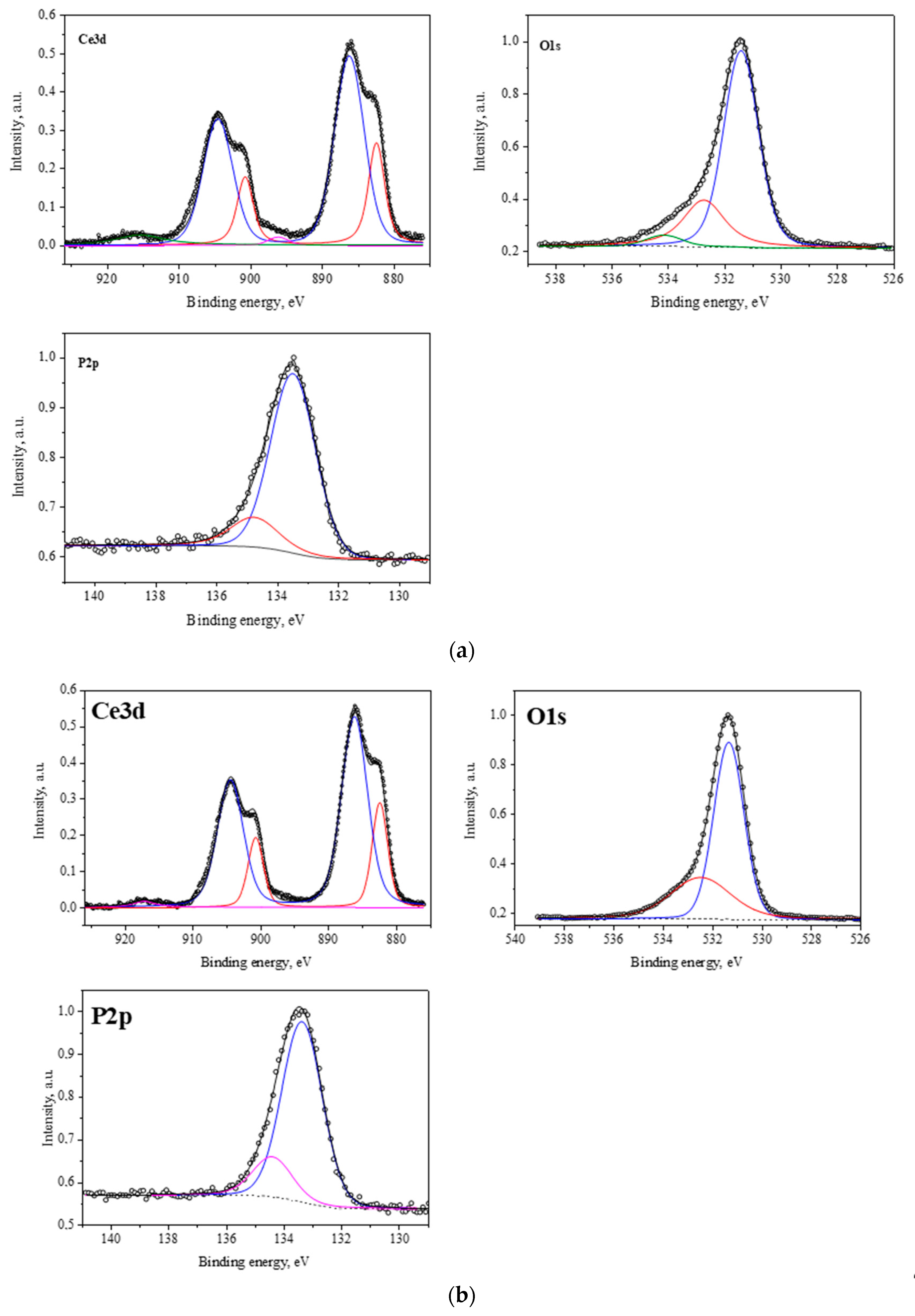
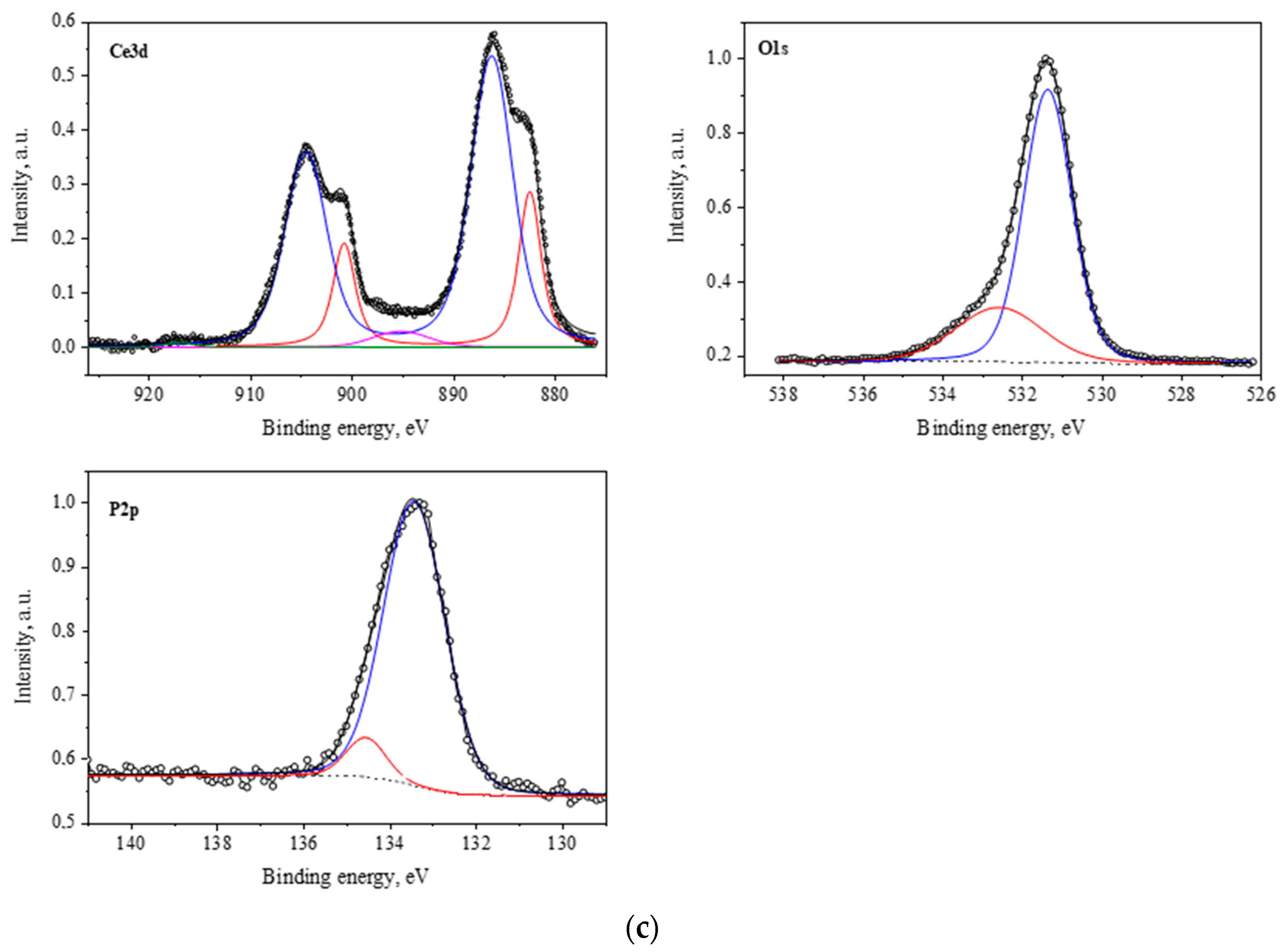
2.1.3. Results of X-Ray Photoelectron Spectroscopy
2.2. Results of Culture Studies on Human Mesenchymal Stem Cells
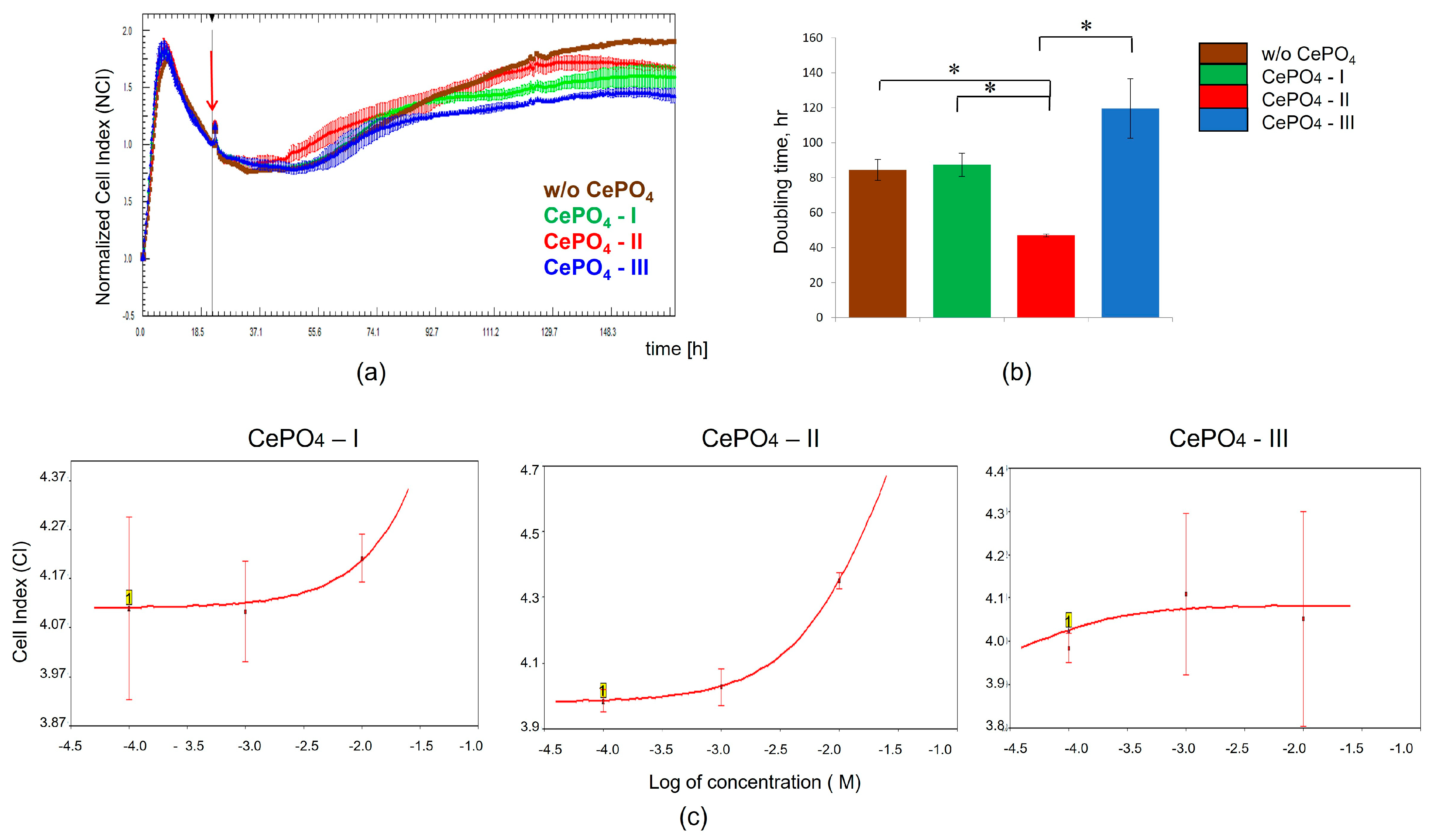
2.2.1. Monitoring MSC Growth Curves Using Real-Time Cell Analysis (RTCA)
2.2.2. Monitoring of CePO4-Induced Responses in MSCs Using RTCA
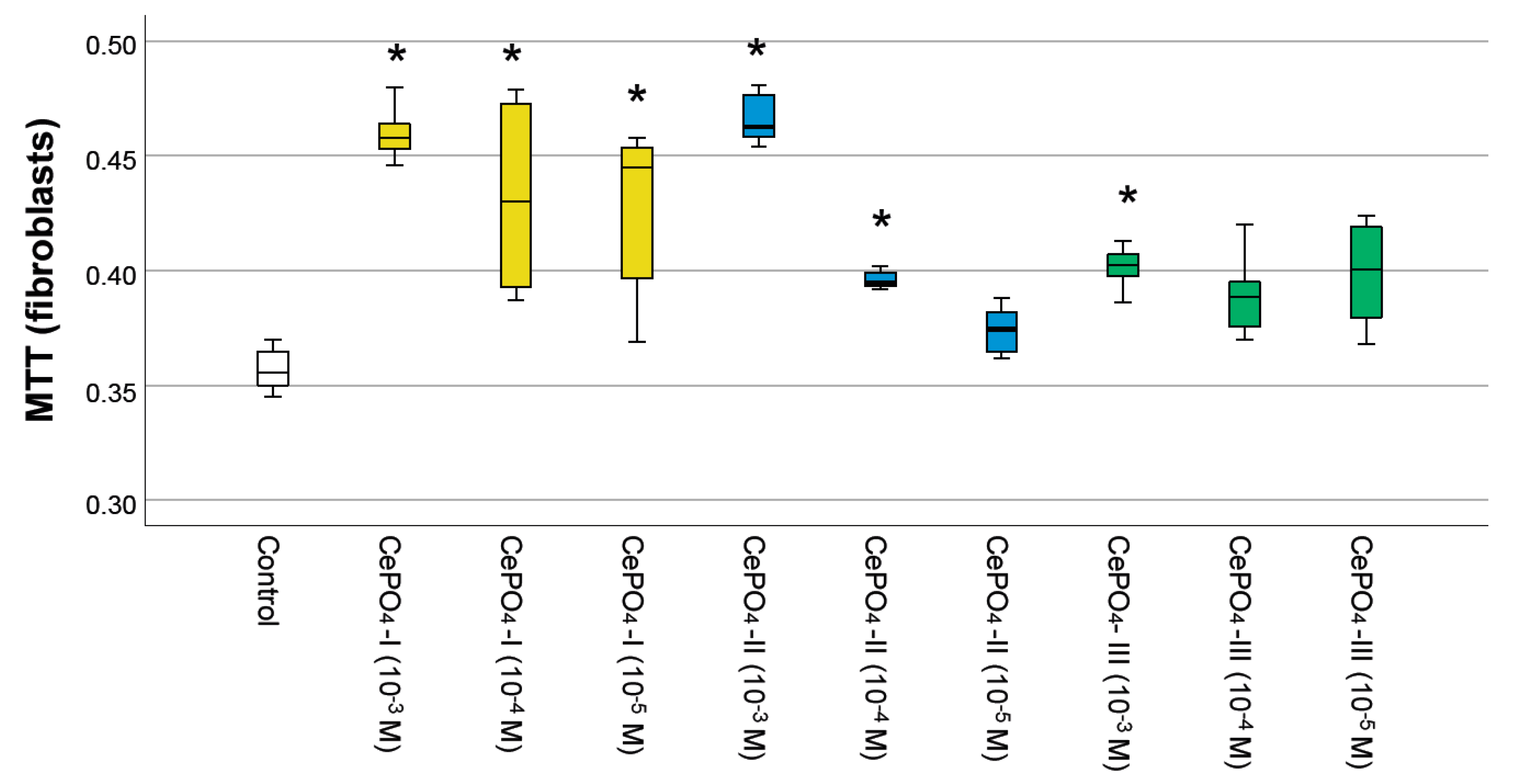
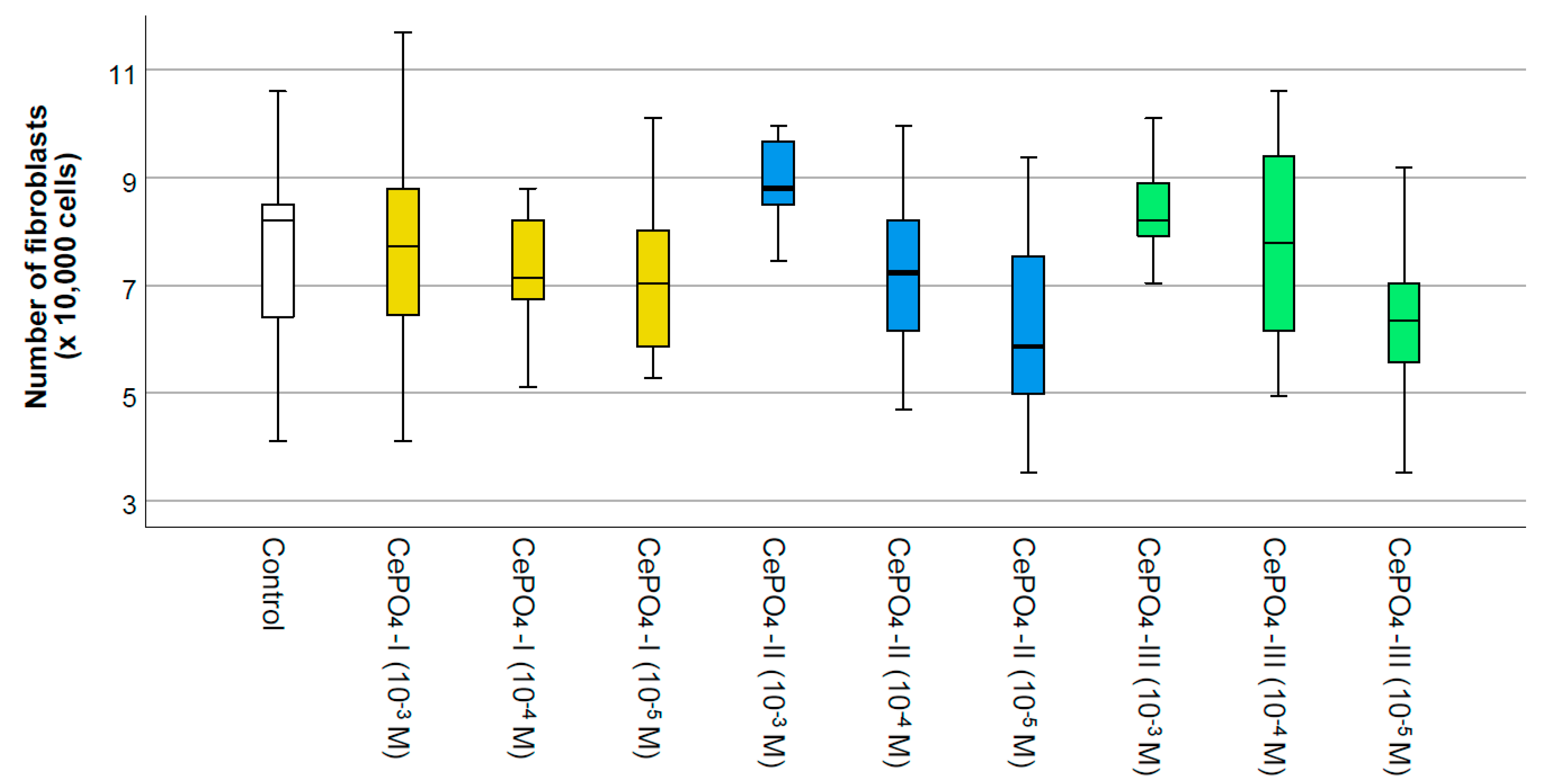
2.3. Results of Culture Studies on Human Fibroblasts
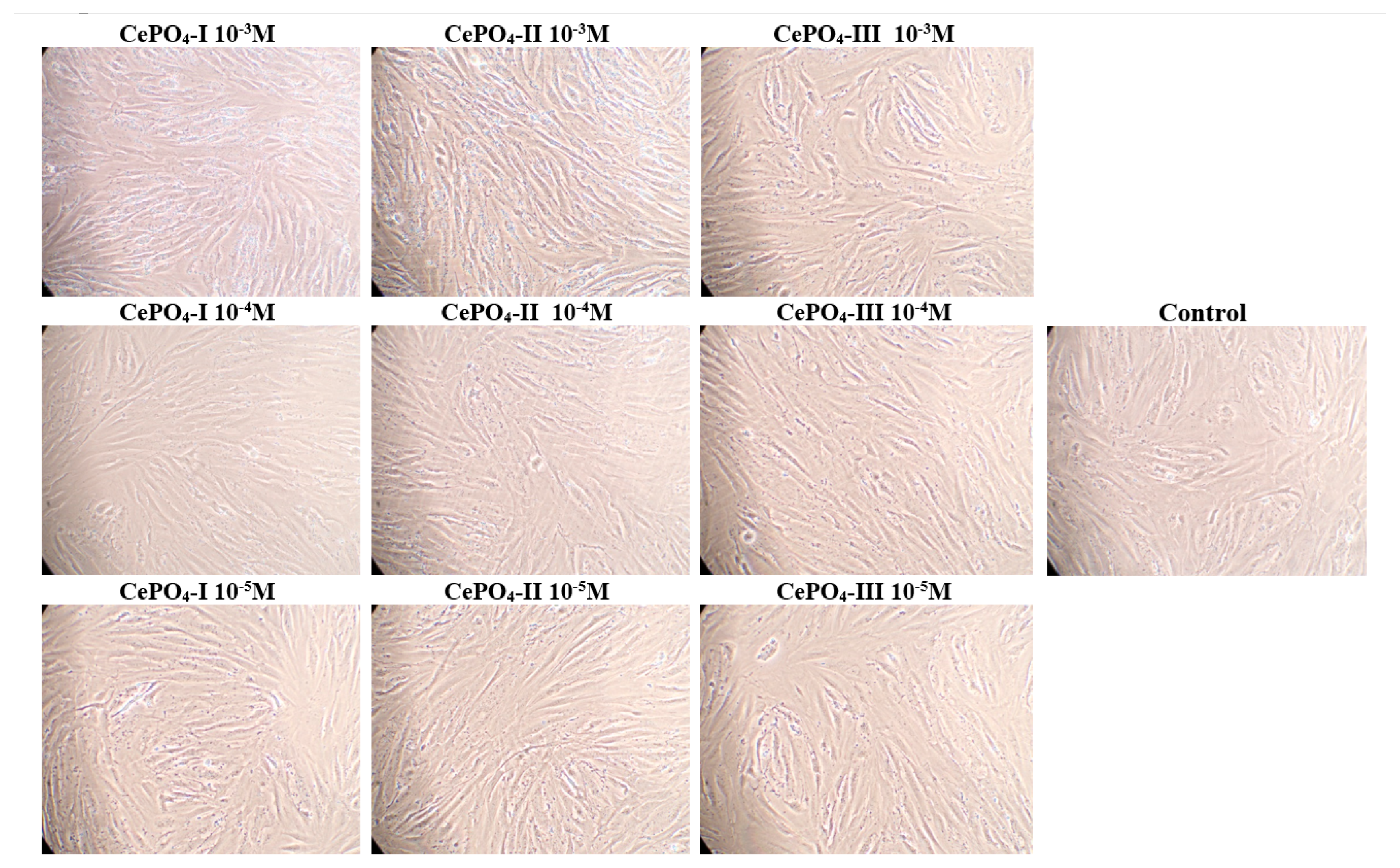
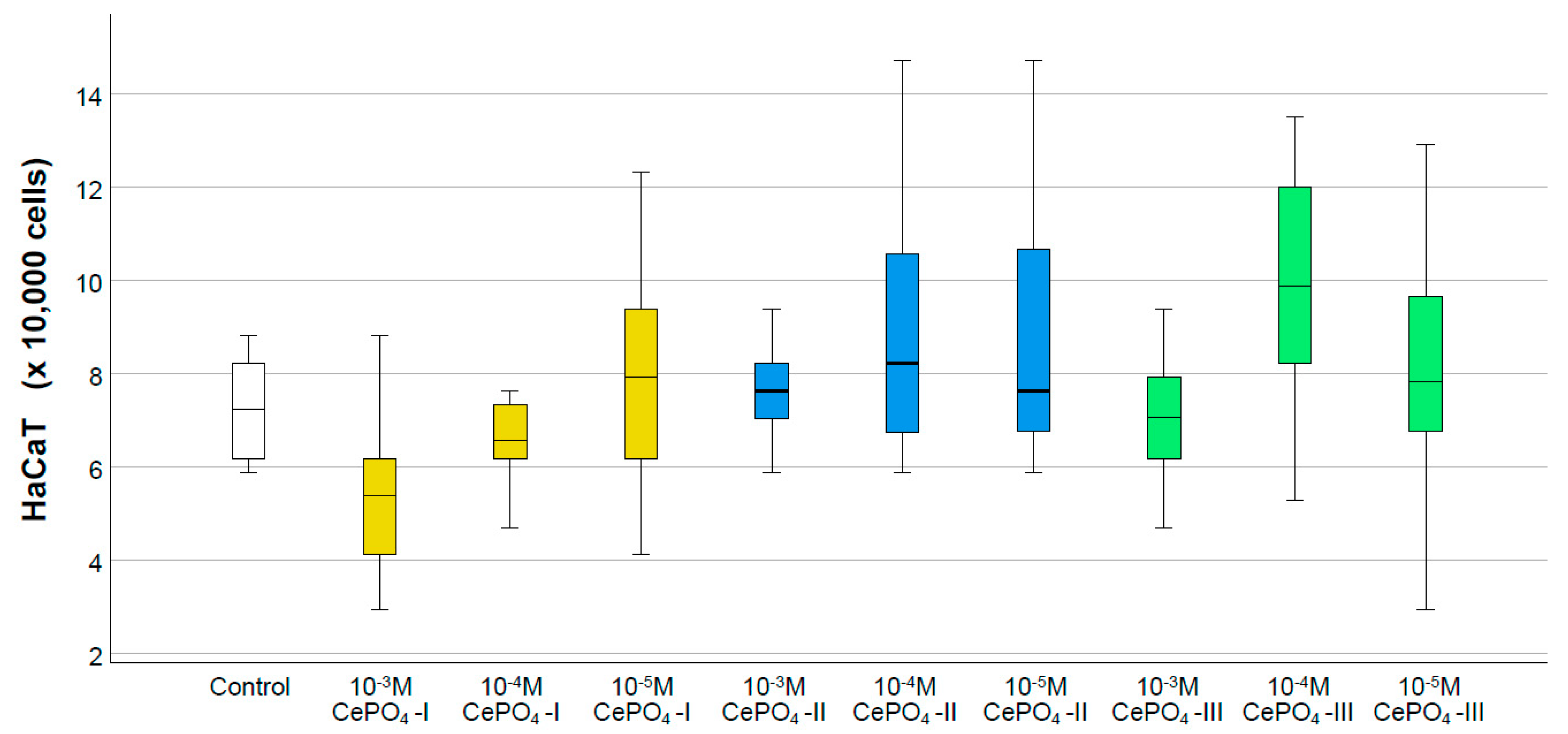
2.4. Results of Culture Studies on Human Keratinocytes
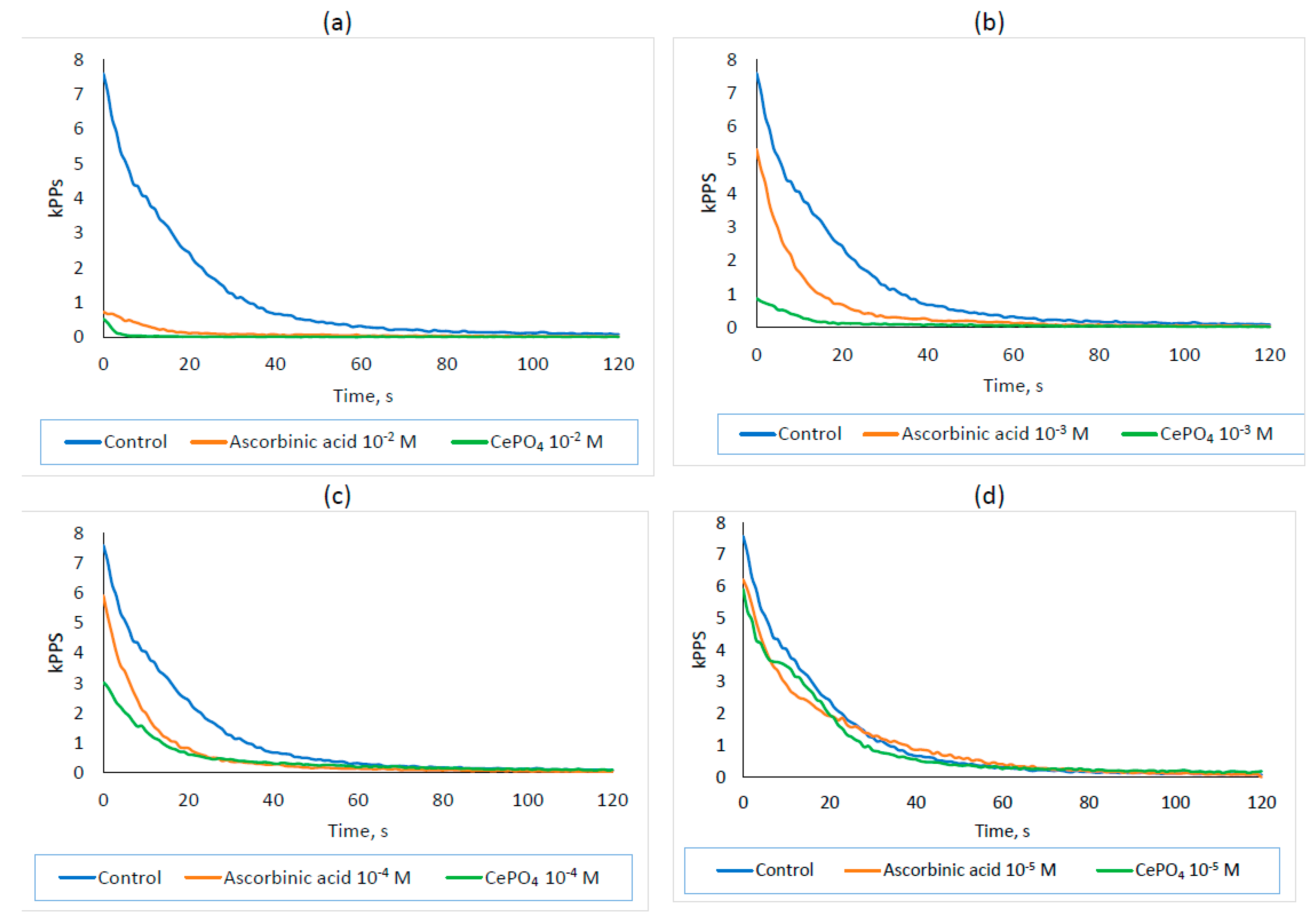
2.5. Antioxidant Activity Results
3. Discussion
Limitations and Prospects
4. Materials and Methods
4.1. Synthesis of Cerium Orthophosphate Nanoparticles
The Studied Samples
4.2. Physicochemical Characterization of Nanoparticles
4.3. Biomedical Research on Cell Lines
4.3.1. Study of the Toxicity/Biocompatibility and Effect of CePO4 Nanoparticles on the Activity of Human Mesenchymal Stem Cells
Culture of Human Adipose-Derived Mesenchymal Stromal Cells
Monitoring MSC Growth Using the xCELLigence DP System
4.3.2. Study of the Toxicity/Biocompatibility and Effect of CePO4 Nanoparticles on the Activity of Human Keratinocytes and Fibroblasts
4.4. Study of Antioxidant Properties of CePO4 Using Chemiluminescence Method
4.5. Statistical Analysis
5. Conclusions
- Cerium(III) orthophosphate with a rhabdophane structure was obtained by precipitating ammonium dihydrogen phosphate from a cerium nitrate solution. Optimal conditions for obtaining the CePO4 nanopowders were established by varying the initial solution concentrations and drying and annealing temperatures. Their particle size ranges from 2 to 10 nm in the transverse direction and 20 to 50 nm in the longitudinal direction.
- Cell line studies demonstrated a high level of safety and biocompatibility across a wide concentration range (10−2 or 10−3 to 10−5 M).
- The regenerative potential of CePO4 nanoparticles on different cells was proven: significant enhancement of MSC proliferation at concentrations of 10−2–10−3 M within 48 h post-application and stimulation of human keratinocyte and fibroblast metabolism at concentrations of 10−3–10−5 M within 72 h post-application.
- Under the conditions of the chemical experiment, a dose-dependent antioxidant effect of CePO4 nanoparticles at concentrations of 10−2–10−5 M was established, which suggests that this property will be preserved when they come into contact with living cellular objects and multicellular organisms.
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, R.G.; Stotts, C.; Gupta, A.; Prosperi-Porta, G.; Dhaliwal, S.; Motazedian, P.; Abdel-Razek, O.; Di Santo, P.; Parlow, S.; Belley-Cote, E.; et al. Prognostic Factors Associated with Mortality in Cardiogenic Shock—A Systematic Review and Meta-Analysis. NEJM Evid. 2024, 3, EVIDoa2300323. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.D.; Bohula, E.A.; Morrow, D.A. Epidemiology and causes of cardiogenic shock. Curr. Opin. Crit. Care 2021, 27, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Welt, F.G.P.; Batchelor, W.; Spears, J.R.; Penna, C.; Pagliaro, P.; Ibanez, B.; Drakos, S.G.; Dangas, G.; Kapur, N.K. Reperfusion Injury in Patients with Acute Myocardial Infarction: JACC Scientific Statement. J. Am. Coll. Cardiol. 2024, 83, 2196–2213. [Google Scholar] [CrossRef]
- Thiele, H.; de Waha-Thiele, S.; Freund, A.; Zeymer, U.; Desch, S.; Fitzgerald, S. Management of cardiogenic shock. EuroIntervention 2021, 17, 451–465. [Google Scholar] [CrossRef]
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; FGrupper, M.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Saini, V.; Guada, L.; Yavagal, D.R. Global Epidemiology of Stroke and Access to Acute Ischemic Stroke Interventions. Neurology 2021, 97 (Suppl. 2), S6–S16. [Google Scholar] [CrossRef]
- Tu, W.J.; Wang, L.D.; Special Writing Group of China Stroke Surveillance Report. China stroke surveillance report 2021. Mil. Med. Res. 2023, 10, 33. [Google Scholar] [CrossRef]
- Goldberg-Lamensdorf, S.; Amir, H. The importance and uniqueness of the oncological rehabilitation process. Harefuah 2024, 163, 631–635. [Google Scholar] [PubMed]
- Oral GBD 2019 Lip; da Cunha, A.R.; Compton, K.; Xu, R.; Mishra, R.; Drangsholt, M.T.; Antunes, J.L.F.; Kerr, A.R.; Acheson, A.R.; Lu, D.; et al. The Global, Regional, and National Burden of Adult Lip, Oral, and Pharyngeal Cancer in 204 Countries and Territories: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2023, 9, 1401–1416. [Google Scholar] [CrossRef]
- Global Burden of Disease 2019 Cancer Collaboration. Cancer Incidence, Mortality, Years of Life Lost, Years Lived with Disability, and Disability-Adjusted Life Years for 29 Cancer Groups from 2010 to 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [CrossRef] [PubMed]
- Speight, J.; Holmes-Truscott, E.; Garza, M.; Scibilia, R.; Wagner, S.; Kato, A.; Pedrero, V.; Deschênes, S.; Guzman, S.J.; Joiner, K.L.; et al. Bringing an end to diabetes stigma and discrimination: An international consensus statement on evidence and recommendations. Lancet Diabetes Endocrinol. 2024, 12, 61–82. [Google Scholar] [CrossRef]
- Li, C.; Bishop, T.R.P.; Imamura, F.; Sharp, S.J.; Pearce, M.; Brage, S.; Ong, K.K.; Ahsan, H.; Bes-Rastrollo, M.; Beulens, J.W.J.; et al. Meat consumption and incident type 2 diabetes: An individual-participant federated meta-analysis of 1·97 million adults with 100,000 incident cases from 31 cohorts in 20 countries. Lancet Diabetes Endocrinol. 2024, 12, 619–630. [Google Scholar] [CrossRef] [PubMed]
- Zöller, B.; Connors, J.M. Multimorbidity, comorbidity, frailty, and venous thromboembolism. Haematologica 2024, 109, 3852–3859. [Google Scholar] [CrossRef] [PubMed]
- Pepper, A.; Dening, K.H. Dementia, comorbidity and multimorbidity. Br. J. Community Nurs. 2024, 29, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Walther, R.; Huynh, T.H.; Monge, P.; Fruergaard, A.S.; Mamakhel, A.; Zelikin, A.N. Ceria Nanozyme and Phosphate Prodrugs: Drug Synthesis through Enzyme Mimicry. ACS Appl. Mater. Interfaces 2021, 13, 25685–25693. [Google Scholar] [CrossRef]
- Titova, S.A.; Kruglova, M.P.; Stupin, V.A.; Manturova, N.E.; Silina, E.V. Potential Applications of Rare Earth Metal Nanoparticles in Biomedicine. Pharmaceuticals 2025, 18, 154. [Google Scholar] [CrossRef]
- Pavelić, K.; Kraljević Pavelić, S.; Bulog, A.; Agaj, A.; Rojnić, B.; Čolić, M.; Trivanović, D. Nanoparticles in Medicine: Current Status in Cancer Treatment. Int. J. Mol. Sci. 2023, 24, 12827. [Google Scholar] [CrossRef] [PubMed]
- de la Rosa, S.Y.G.; Diaz, R.M.; Gutiérrez, P.T.V.; Patakfalvi, R.; Coronado, Ó.G. Functionalized Platinum Nanoparticles with Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 9404. [Google Scholar] [CrossRef]
- Neamati, F.; Kodori, M.; Feizabadi, M.M.; Abavisani, M.; Barani, M.; Khaledi, M.; Moghadaszadeh, M.; Azadbakht, M.K.; Zeinali, M.; Fathizadeh, H. Bismuth nanoparticles against microbial infections. Nanomedicine 2022, 17, 2109–2122. [Google Scholar] [CrossRef]
- Devi, R.S.; Girigoswami, A.; Siddharth, M.; Girigoswami, K. Applications of Gold and Silver Nanoparticles in Theranostics. Appl. Biochem. Biotechnol. 2022, 194, 4187–4219. [Google Scholar] [CrossRef]
- Lachowicz, J.I.; Lecca, L.I.; Meloni, F.; Campagna, M. Metals and Metal-Nanoparticles in Human Pathologies: From Exposure to Therapy. Molecules 2021, 26, 6639. [Google Scholar] [CrossRef]
- Lv, C.; Di, W.; Liu, Z.; Zheng, K.; Qin, W. Luminescent CePO4:Tb colloids for H2O2 and glucose sensing. Analyst 2014, 139, 4547–4555. [Google Scholar] [CrossRef]
- Vinothkumar, G.; Lalitha, A.I.; Suresh Babu, K. Cerium Phosphate-Cerium Oxide Heterogeneous Composite Nanozymes with Enhanced Peroxidase-Like Biomimetic Activity for Glucose and Hydrogen Peroxide Sensing. Inorg. Chem. 2019, 58, 349–358. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.W.; Liu, X.L.; Yu, D.G.; Zhu, Z.A.; Ke, Q.F.; Mao, Y.Q.; Guo, Y.P.; Zhang, J.W. Graphene-modified CePO4 nanorods effectively treat breast cancer-induced bone metastases and regulate macrophage polarization to improve osteo-inductive ability. J. Nanobiotechnology 2021, 19, 11. [Google Scholar] [CrossRef]
- Seixas, V.C.; Serra, O.A. Stability of sunscreens containing CePO4: Proposal for a new inorganic UV filter. Molecules 2014, 19, 9907–9925. [Google Scholar] [CrossRef]
- Kurajica, S.; Brleković, F.; Keser, S.; Dražić, G.; Mužina, K.; Mihajlović, V. Evaluation of Mechanochemically Prepared CePO4∙H2O Nanoparticles as UV Filter for Photoprotective Formulations. Molecules 2025, 30, 405. [Google Scholar] [CrossRef] [PubMed]
- Lima, J.F.; Filho, P.C.d.S.; Serra, O.A. Single crystalline rhabdophane-type CePO4 nanoparticles as efficient UV filters. Ceram. Int. 2016, 42, 7422–7431. [Google Scholar] [CrossRef]
- Molinari, M.; Symington, A.R.; Sayle, D.C.; Sakthivel, T.S.; Seal, S.; Parker, S.C. Computer-Aided Design of Nanoceria Structures as Enzyme Mimetic Agents: The Role of Bodily Electrolytes on Maximizing Their Activity. ACS. Appl. Bio Mater. 2019, 2, 1098–1106. [Google Scholar] [CrossRef]
- Meng, L.; Yang, L.; Zhou, B.; Cai, C. Cerium phosphate nanotubes: Synthesis, characterization and biosensing. Nanotechnology 2009, 20, 035502. [Google Scholar] [CrossRef]
- Li, M.; Fang, J.; Wang, C.; Zhang, J.; Liu, L.; Li, Y.; Cao, W.; Wei, Q. CePO4/CeO2 heterostructure and enzymatic action of D-Fe2O3 co-amplify luminol-based electrochemiluminescence immunosensor for NSE detection. Biosens. Bioelectron. 2022, 214, 114516. [Google Scholar] [CrossRef]
- Titova, S.A.; Kruglova, M.P.; Stupin, V.A.; Manturova, N.E.; Achar, R.R.; Deshpande, G.; Parfenov, V.A.; Silina, E.V. Excipients for Cerium Dioxide Nanoparticle Stabilization in the Perspective of Biomedical Applications. Molecules 2025, 30, 1210. [Google Scholar] [CrossRef]
- Tang, C.; Bando, Y.; Golberg, D.; Ma, R. Cerium phosphate nanotubes: Synthesis, valence state, and optical properties. Angew. Chem. Int. Ed. Engl. 2005, 44, 576–579. [Google Scholar] [CrossRef]
- Yokel, R.A.; Wohlleben, W.; Keller, J.G.; Hancock, M.L.; Unrine, J.M.; Butterfield, D.A.; Grulke, E.A. The preparation temperature influences the physicochemical nature and activity of nanoceria. Beilstein J. Nanotechnol. 2021, 12, 525–540. [Google Scholar] [CrossRef]
- Nilchi, A.; Khanchi, A.; Ghanadi Maragheh, M. The importance of cerium substituted phosphates as cation exchanger-some unique properties and related application potentials. Talanta 2002, 56, 383–393. [Google Scholar] [CrossRef]
- Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Chuvilina, E.L.; Gasanov, A.A.; Ostrovskaya, A.A.; Andreeva, O.I.; Tabachkova, N.Y.; Abakumov, M.A.; Nikitin, A.A.; et al. Development of Technology for the Synthesis of Nanocrystalline Cerium Oxide Under Production Conditions with the Best Regenerative Activity and Biocompatibility for Further Creation of Wound-Healing Agents. Pharmaceutics 2024, 16, 1365. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, Y.; Jiao, C.; Liu, M.; Luo, W.; Dong, C.; Fan, S.; He, X.; Yang, F.; Zhang, Z. Comparative toxicity of rod-shaped nano-CeO2 and nano-CePO4 to lettuce. Metallomics 2021, 13, mfab033. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.R.; Zhu, X.W.; Wei, L.S.; Peng, J.W.; Li, W.X. Controlled syntheses and photoluminescence characterization of monoclinic cerium orthophosphate with nanostructure. Spectrosc. Spectr. Anal. 2011, 31, 1463–1466. (In Chinese) [Google Scholar] [PubMed]
- Ta, K.M.; Cooke, D.J.; Gillie, L.J.; Parker, S.C.; Seal, S.; Wilson, P.B.; Phillips, R.M.; Skelton, J.M.; Molinari, M. Infrared and Raman Diagnostic Modeling of Phosphate Adsorption on Ceria Nanoparticles. J. Phys. Chem. C Nanomater. Interfaces 2023, 127, 20183–20193. [Google Scholar] [CrossRef]
- Römer, I.; Briffa, S.M.; Arroyo Rojas Dasilva, Y.; Hapiuk, D.; Trouillet, V.; Palmer, R.E.; Valsami-Jones, E. Impact of particle size, oxidation state and capping agent of different cerium dioxide nanoparticles on the phosphate-induced transformations at different pH and concentration. PLoS ONE 2019, 14, e0217483. [Google Scholar] [CrossRef]
- Silina, E.V.; Manturova, N.E.; Ivanova, O.S.; Baranchikov, A.E.; Artyushkova, E.B.; Medvedeva, O.A.; Kryukov, A.A.; Dodonova, S.A.; Gladchenko, M.P.; Vorsina, E.S.; et al. Cerium Dioxide–Dextran Nanocomposites in the Development of a Medical Product for Wound Healing: Physical, Chemical and Biomedical Characteristics. Molecules 2024, 29, 2853. [Google Scholar] [CrossRef]
- Gilboa, S.; Panz, L.; Arbell, N.; Paz, Y. Light-Assisted Formation of Nucleosides and Nucleotides from Formamide in the Presence of Cerium Phosphate. Life 2024, 14, 846. [Google Scholar] [CrossRef]
- Steblevskaya, N.I.; Belobeletskaya MVMedkov, M.A. Synthesis and Study of Double NaYP2O7 Phosphates Doped with Rare-Earth Elements. Theor. Found. Chem. Eng. 2025, 59, 162–167. [Google Scholar] [CrossRef]
- Kazeem, J.; Huang, L.; Majee, B.P.; Bryce, K.; Lian, J. Thermomechanical properties of rare earth phosphates as environmental barrier coatings. J. Am. Ceram. Soc. 2025, 108, e70017. [Google Scholar] [CrossRef]
- Majee, B.P.; Bryce, K.; Huang, L.; Lian, J. A high-entropy rare-earth phosphate and its principle single component REPO4 for environmental barrier coatings. J. Adv. Ceram. 2025, 14, 9221041. [Google Scholar] [CrossRef]
- Wang, E.X.; Ushakov, S.V.; Wang, L.; Matteucci, J.; Xu, H.; Opila, E.J.; Hong, Q.-J.; Navrotsky, A. Ab initio stability predictions for rare earth oxyphosphates and experimental confirmation of cerium (III) phases. Proc. Natl. Acad. Sci. USA 2025, 122, e2426921122. [Google Scholar] [CrossRef] [PubMed]
- Onoda, H.; Yamaoka, K.; Charoonsuk, T.; Pulphol, P.; Vittayakorn, N. Synthesis of cerium dioxide-based pigments with co-precipitated phosphate: Tuning oxidation catalytic activity for cosmetic and paint applications. J. Aust. Ceram. Soc. 2025, 61, 1537–1544. [Google Scholar] [CrossRef]
- Yang, C.; Ying, T.; Huang, A.; Huang, J.; Chen, P.; Chu, P.K.; Zeng, X. Enhancing corrosion resistance of MAO coatings on Al alloy LY12 through in situ co-doping with zinc phosphate and cerium phosphate. Corros. Commun. 2025, 17, 35–43. [Google Scholar] [CrossRef]
- Kastenhofer, J.; Spadiut, O.; Papangelakis, V.G.; Allen, D.G. Roles of pH and phosphate in rare earth element biosorption with living acidophilic microalgae. Appl. Microbiol. Biotechnol. 2024, 108, 262. [Google Scholar] [CrossRef]
- Yang, W.; Wu, K.; Chen, H.; Huang, J.; Yu, Z. Emerging role of rare earth elements in biomolecular functions. ISME J. 2025, 19, wrae241. [Google Scholar] [CrossRef]
- Peter, A.M.; Ramya, M.; Kailasnath, M. Investigation of Intensity Dependent Nonlinear Absorption in Cerium Phosphate Nanorods. J. Phys. Conf. Ser. 2022, 2357, 012013. [Google Scholar] [CrossRef]
- Besprozvannykh, V.K.; Nifant’eV, I.E.; Tavtorkin, A.N.; Ivchenko, P.V. Cerium-doped calcium phosphate materials for bone tissue engineering. Russ. Chem. Bull. 2024, 74, 1505–1540. [Google Scholar] [CrossRef]
- Ekram, B.; Mousa, S.M.; El-Bassyouni, G.T.; Abdel-Hady, B.M.; Moaness, M. Novel highly proliferative electrospun cerium-doped hydroxyapatite/polyamide/gelatin nanofibers for guided bone regeneration application. Mater. Chem. Phys. 2025, 342, 130975. [Google Scholar] [CrossRef]
- Trinh, C.D.; Le, D.N.; Truong, A.T.T.; Nguyen, N.P.; Mai, K.V.; Dang, C.M. Morphology and optical properties of wet chemistry synthesized submicron CePO4:Tb3+ hollow spheres. Chem. Pap. 2024, 78, 3907–3918. [Google Scholar] [CrossRef]
- Salman, M.; Mahmood, Z.; Aurangzaib; Akhtar, K.; Muhammad, A.D.; Tahir, W.S.; Iqbal, M.; Javeed, Y. Investigating the effects of cerium doping on the chemical and optical properties of lanthanum phosphate nanoparticles. Kashf J. Multidiscip. Res. 2025, 2, 31–39. [Google Scholar] [CrossRef]
- Hurtig, N.C.; Gysi, A.P.; Smith-Schmitz, S.E.; Harlov, D. Raman spectroscopic study of anhydrous and hydrous REE phosphates, oxides, and hydroxides. Dalton Trans. 2024, 53, 9964–9978. [Google Scholar] [CrossRef]
- Di, W.; Wang, X.; Zhao, H. Synthesis and characterization of LnPO4 x nH2O (Ln = La, Ce, Gd, Tb, Dy) nanorods and nanowires. J. Nanosci. Nanotechnol. 2007, 7, 3624–3628. [Google Scholar] [CrossRef]
- Zhoujin, Y.; Tisdale, H.B.; Keerthisinghe, N.; Morrison, G.; Smith, M.D.; Hadermann, J.; Besmann, T.M.; Amoroso, J.; Loye, H.Z. A low-temperature, one-step synthesis for monazite can transform monazite into a readily usable advanced nuclear waste form. Sci. Adv. 2025, 11, eadt3518. [Google Scholar] [CrossRef]
- Mesbah, A.; Clavier, N.; Elkaïm, E.; Szenknect, S.; Dacheux, N. In pursuit of the rhabdophane crystal structure: From the hydrated monoclinic LnPO4.0.667H2O to the hexagonal LnPO4 (Ln = Nd, Sm, Gd, Eu and Dy). J. Solid State Chem. 2017, 249, 221–227. [Google Scholar] [CrossRef]
- Wang, X.; Xie, H.; Wang, P.; Yin, H. Nanoparticles in Plants: Uptake, Transport and Physiological Activity in Leaf and Root. Materials 2023, 16, 3097. [Google Scholar] [CrossRef]
- Chen, Y.; Feng, X. Gold nanoparticles for skin drug delivery. Int. J. Pharm. 2022, 625, 122122. [Google Scholar] [CrossRef]
- Menichetti, A.; Mavridi-Printezi, A.; Mordini, D.; Montalti, M. Effect of Size, Shape and Surface Functionalization on the Antibacterial Activity of Silver Nanoparticles. J. Funct. Biomater. 2023, 14, 244. [Google Scholar] [CrossRef] [PubMed]
- Yan, G.; Du, Q.; Wei, X.; Miozzi, J.; Kang, C.; Wang, J.; Han, X.; Pan, J.; Xie, H.; Chen, J.; et al. Application of Real-Time Cell Electronic Analysis System in Modern Pharmaceutical Evaluation and Analysis. Molecules 2018, 23, 3280. [Google Scholar] [CrossRef]
- Kho, D.; MacDonald, C.; Johnson, R.; Unsworth, C.P.; O’Carroll, S.J.; du Mez, E.; Angel, C.E.; Graham, E.S. Application of xCELLigence RTCA Biosensor Technology for Revealing the Profile and Window of Drug Responsiveness in Real Time. Biosensors 2015, 5, 199–222. [Google Scholar] [CrossRef]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar] [CrossRef]
- Zhou, Y.; Yamamoto, Y.; Xiao, Z.; Ochiya, T. The Immunomodulatory Functions of Mesenchymal Stromal/Stem Cells Mediated via Paracrine Activity. J. Clin. Med. 2019, 8, 1025. [Google Scholar] [CrossRef]
- Suzdaltseva, Y.; Zhidkih, S.; Kiselev, S.L.; Stupin, V. Locally Delivered Umbilical Cord Mesenchymal Stromal Cells Reduce Chronic Inflammation in Long-Term Nonhealing Wounds: A Randomized Study. Stem Cells Int. 2020, 2020, 5308609. [Google Scholar] [CrossRef]
- Suzdaltseva, Y.; Kiselev, S.L. Mesodermal Derivatives of Pluripotent Stem Cells Route to Scarless Healing. Int. J. Mol. Sci. 2023, 24, 11945. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Discher, D.E.; Péault, B.M.; Phinney, D.G.; Hare, J.M.; Caplan, A.I. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen. Med. 2019, 4, 22. [Google Scholar] [CrossRef]
- Lebourgeois, S.; Fraisse, A.; Hennechart-Collette, C.; Guillier, L.; Perelle, S.; Martin-Latil, S. Development of a Real-Time Cell Analysis (RTCA) Method as a Fast and Accurate Method for Detecting Infectious Particles of the Adapted Strain of Hepatitis A Virus. Front. Cell. Infect. Microbiol. 2018, 8, 335. [Google Scholar] [CrossRef]
- Di, W.; Wang, X.; Ren, X. Nanocrystalline CePO4:Tb as a novel oxygen sensing material on the basis of its redox responsive reversible luminescence. Nanotechnology 2010, 21, 75709. [Google Scholar] [CrossRef] [PubMed]
- Ta, K.M.; Neal, C.J.; Coathup, M.J.; Seal, S.; Phillips, R.M.; Molinari, M. The interaction of phosphate species with cerium oxide: The known, the ambiguous and the unexplained. Biomater. Adv. 2025, 166, 214063. [Google Scholar] [CrossRef] [PubMed]
- Yokel, R.A.; Tseng, M.T.; Butterfield, D.A.; Hancock, M.L.; Grulke, E.A.; Unrine, J.M.; Stromberg, A.J.; Dozier, A.K.; Graham, U.M. Nanoceria distribution and effects are mouse-strain dependent. Nanotoxicology 2020, 14, 827–846. [Google Scholar] [CrossRef] [PubMed]
- Suzdaltseva, Y.; Goryunov, K.; Silina, E.; Manturova, N.; Stupin, V.; Kiselev, S.L. Equilibrium among Inflammatory Factors Determines Human MSC-Mediated Immunosuppressive Effect. Cells 2022, 11, 1210. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop Dj Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef] [PubMed]



| CePO4-I | CePO4-II | CePO4-III | |
|---|---|---|---|
| Group | 180:P6222 | 152:P3121 | 180:P6222 |
| Coherent scattering region, nm | 6.7 ± 0.6 | 12.6 ± 1.2 | 3.51 ± 0.2 |
| COD | 9,010,969 | 9,015,938 | 9,010,969 |
| Binding Energy, eV | CePO4-I | CePO4-II | CePO4-III | CePO4 [Database Reference Number] |
|---|---|---|---|---|
| Ce(3d3/2) | 904.47 | 904.86 | 904.01 | 904.00 |
| Ce(3d5/2) | 885.87 | 885.98 | 885.81 | 885.40 |
| P(2p3/2) | 133.47 | 133.67 | 133.41 | 133.30 |
| O(1s) | 531.37 | 531.37 | 531.41 | 531.00 |
| Control | Ascorbic Acid | CePO4 | |
|---|---|---|---|
| 133.82 ± 6.05 | Drug, 10−2 M | 12.18 ± 0.61 | 2.99 ± 0.13 |
| Drug, 10−3 M | 58.48 ± 2.54 | 13.11 ± 0.45 | |
| Drug, 10−4 M | 67.14 ± 3.27 | 54.06 ± 2.28 | |
| Drug, 10−5 M | 120.45 ± 4.95 | 113.70 ± 4.84 |
| Conditions | CePO4-I | CePO4-II | CePO4-III |
|---|---|---|---|
| CCe(NO3)3, g/L (by CeO2 content) | 25 | 10 | 25 |
| C(NH4H2PO4), g/L | 35 | 15 | 37.5 |
| C(NH4NO3), g/L | – | 75 | – |
| pH | 3–4 | 2–3 | 2 |
| TH2O, °C | 40–50 | 30–20 | 15–20 |
| Exposure time; h | 3 | 3 | 2 |
| Yield of finished product, % of theoretical | 97.5 | 95 | 98.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silina, E.V.; Stupin, V.A.; Manturova, N.E.; Chuvilina, E.L.; Gasanov, A.A.; Andreeva, O.I.; Korobko, E.V.; Andreeva, N.V.; Dodonova, S.A.; Tkachenko, D.D.; et al. Cerium Phosphate Nanoparticles: Synthesis, Characterization, Biocompatibility, Regenerative Potential, and Antioxidant Activity. Molecules 2025, 30, 3916. https://doi.org/10.3390/molecules30193916
Silina EV, Stupin VA, Manturova NE, Chuvilina EL, Gasanov AA, Andreeva OI, Korobko EV, Andreeva NV, Dodonova SA, Tkachenko DD, et al. Cerium Phosphate Nanoparticles: Synthesis, Characterization, Biocompatibility, Regenerative Potential, and Antioxidant Activity. Molecules. 2025; 30(19):3916. https://doi.org/10.3390/molecules30193916
Chicago/Turabian StyleSilina, Ekaterina V., Victor A. Stupin, Natalia E. Manturova, Elena L. Chuvilina, Akhmedali A. Gasanov, Olga I. Andreeva, Elena V. Korobko, Natalia V. Andreeva, Svetlana A. Dodonova, Daria D. Tkachenko, and et al. 2025. "Cerium Phosphate Nanoparticles: Synthesis, Characterization, Biocompatibility, Regenerative Potential, and Antioxidant Activity" Molecules 30, no. 19: 3916. https://doi.org/10.3390/molecules30193916
APA StyleSilina, E. V., Stupin, V. A., Manturova, N. E., Chuvilina, E. L., Gasanov, A. A., Andreeva, O. I., Korobko, E. V., Andreeva, N. V., Dodonova, S. A., Tkachenko, D. D., Izmailov, D. Y., Tabachkova, N. Y., & Suzdaltseva, Y. G. (2025). Cerium Phosphate Nanoparticles: Synthesis, Characterization, Biocompatibility, Regenerative Potential, and Antioxidant Activity. Molecules, 30(19), 3916. https://doi.org/10.3390/molecules30193916





