Traditional Applications, Phytochemical Constituents, and Pharmacological Properties of Lavandula multifida L.: A Review
Abstract
1. Introduction
2. Methodology
3. Botanical Characterization and Geographical Distribution
3.1. Taxonomy
3.2. Taxonomic Classification
| Kingdom | Plantae |
| Division | Streptophyta |
| Class | Equisetopsida |
| Subclass | Magnoliidae |
| Order | Lamiales |
| Family | Lamiaceae |
| Genus | Lavandula |
| Species | Lavandula multifida L. |
3.3. Botanical Description
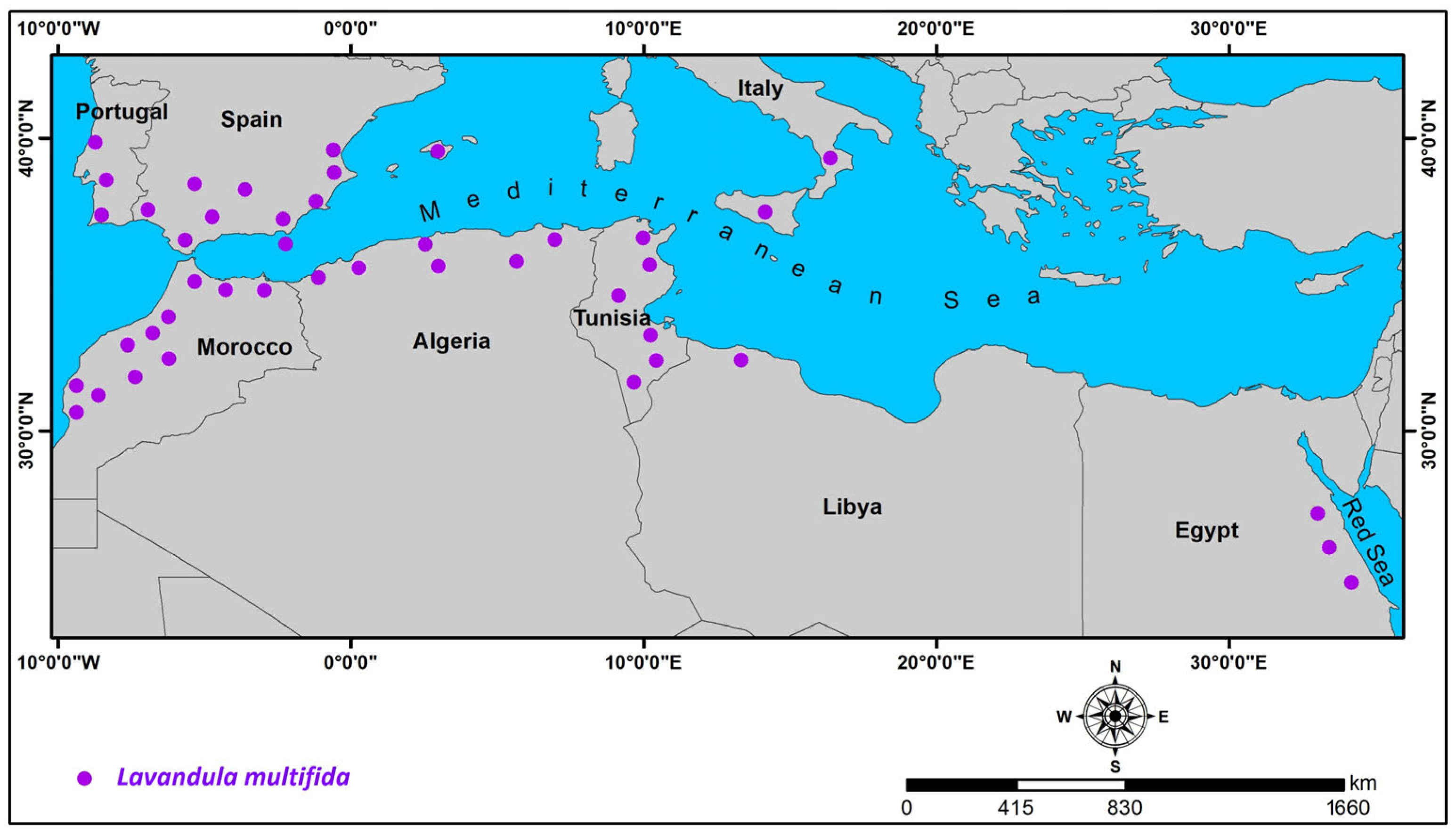
3.4. Geographical Distribution
4. Traditional Uses
5. Phytochemicals
Chemical Constituents of L. multifida Essential Oils
| Country of Origin | Plant Part | Extraction Method | Analytical Method | Oil (%,v/w) | Nb. of Compounds/Oil% | Major Compounds | Compound Category | Ref. |
|---|---|---|---|---|---|---|---|---|
| Algeria (Chlef—northern Algeria) | Inflorescences | SD | GC-FID; GC-MS | 1% | 29/(92%) | carvacrol (61.73%) linalool (5.69%) 1-octen-3-ol (3%) | OM (74.68%) MH (1.84%) OS (0.64%) SH (2.82%) | [42] |
| Leaves | SD | GC-FID; GC-MS | 0.8% | 43/(96.25%) | carvacrol (50.92%) anethole (17.37%) β-bisabolene (5.81%) | OM (58.25%) MH (1.47%) OS (0.59%) SH (8.02%) | ||
| Algeria (Tlemcen—North-West of Algeria) | Aerial parts | HD | GC-FID; GC-MS | 0.2% | 23/(98.4%) | carvacrol (57.1%) β-bisabolene (25.2%) caryophyllene oxide (3.7%) spathulenol (3.4%) | OM (60.1%) MH (3.8%) OS (7.1%) SH (27.4%) | [78] |
| Egypt | Leaves | HD | GC-MS | NI | 23/(99.72%) | 1,8-cineole (39.84%) camphor (18.86%) α-pinene (8.86%) | OM (65.52%) MH (23.18%) OS (3.40%) SH (6.10%) | [86] |
| Morocco (Errachidia- South-Eastern Morocco) | Aerial parts | HD | GC-FID; GC-MS | 0.7% | 34/(96.5%) | carvacrol (66.2%) spathulenol (4.9%) p-cymene-8-ol (4.2%) caryophyllene oxide (2.7%) terpinolene (2.6%) | OM (73.8%) MH (8.2%) OS (7.7%) SH (1.1%) | [45] |
| Morocco (Tetuan-North-western Morocco) | Aerial parts | HD | GC-FID; GC-MS | 0.097% | 34/(95.25%) | carvacrol (47.62%) β-bisabolene (9.01%) dodecyl acrylate (8.37%) linalol (7.42%) | OM (62.51%) MH (11.09%) OS (9.27%) SH (12.38%) | [81] |
| Morocco (Errachidia- South-Eastern Morocco) | Leaves and flowers | HD | GC-FID; GC-MS | 2.4% | 28/(86.2 ± 86.8%) | carvacrol (57.9 ± 59.0%) carvacrol methyl ether (7.0 ± 7.6%) p-cymen-8-ol (3.9 ± 4.7%) | OM (72.9 ± 81.1%) MH (1.8 ± 8.2%) OS (2.4 ± 3.0%) SH (0.2 ± 1.2%) | [82] |
| Morocco Rabat- North- western Morocco | Leaves and stems | HD | GC-FID; GC-MS | 0.46% | 20/(97.8%) | carvacrol (44.3%) β-bisabolene (31.9%) careophylene oxide (5.8%) fenchol (3.2%) | OM (48.7%) MH (6.8%) OS (6.2%) SH (35.6%) | [44] |
| Morocco (Anti Atlas region) | Aerial parts | SD | GC; GC-MS | 2.01% | 22/(99.59%) | durenol (89.97%) caryophyllene oxide (2.43%) sphatulenol (1.83%) aromadendrene (1.68%) carvacrol methyl ether (1%) | OM (92.58%) MH (0.25%) OS (0.6%) SH (6.48%) | [72] |
| Morocco (Errachidia- South-Eastern Morocco) | Leaves and flowers | SD | GC-FID; GC-MS | 1.2% | 39/(99.76%) | thymol (32.00%) carvacrol (27.77%) p-cymene (15.72%) γ-terpinene (9.54%) | OM (81.49%) MH (2.75%) OS (11.81%) SH (0.23%) | [64] |
| Morocco (Errachidia- South-Eastern Morocco) | Aerial parts | HS-SPME HD | GC-FID; GC-MS GC-FID; GC-MS | NI* 2.4% | 21/(90.2%) 29/(90.6%) | carvacrol (65.6%) spathulenol (8.6%) p-cymene-8-ol (4.8%) carvacrol methyl ether (4.6%) carvacrol (57.9%) carvacrol methyl ether (7.6%) p-cymene-8-ol (3.9%) spathulenol (3.8%) | OM (75.8%) MH (3.1%) OS (8.7%) SH (1.7%) OM (72.9%) MH (8.2%) OS (6.6%) SH (1%) | [53,83] |
| Portugal (Sesimbra/Arrábida and Mértola—south of Portugal) | Aerial parts | HD | GC; GC-MS | NI | 33/(97.9%) | carvacrol (42.8% and 41.5%) cis-β-ocimene (27.4% and 27.0%) myrcene (5.7% and 5.5%) β-bisabolene (5.6% and 5.0%) | OM (43.2% and 41.8%) MH (41.4% and 38.5%) OS (1.5% and1.8%) SH (10.8%and 10.5%) | [34] |
| Portugal Sesimbra—south of Portugal) | Aerial parts | HD | GC; GC–MS | NI | 31/(95.2%) | carvacrol (46.4%) cis-β-ocimene (12.7%) β-bisabolene (10.1%) myrcene (5.9%) | OM (48.8%) MH (25.1%) OS (3.7%) SH (14.9%) | [79] |
| Tunisia | Leaves | HD | GC-FID; GC-MS | NI | 36/(83.48%) | carvacrol (31.81%) β -bisabolene (14.89%) acrylic acid dodecanyl ester (11.43%) | OM (33.70%) MH (15.76%) OS (6.38%) SH (14.89%) | [62] |
| Tunisia (Sidi Bouzid- Central West of Tunisia) | Stems leaves | HD | GC-FID; GC-MS | 0.26% | 29/(98.3%) | carvacrol (65.1%) β-bisabolene (24.7%) β-caryophyllene (2.4%) myrcene (5.7% and 5.5%) | OM (65.7%) MH (1.5%) OS (1.0%) SH (30.1%) | [80] |
| Tunisia (Grombalia—North-Eastern Tunisia) | Aerial parts | HD | GC-FID; GC-MS | 1.62% | 52/(98.21%) | linalool (50.05 ± 6.52%) camphene (10.06 ± 1.21%) linalyl acetate (7.30 ± 0.65%) α-thujene (3.83 ± 0.41%) | OM (71.40%) MH (20.70%) OS (0.10%) SH (5.27%) | [55] |
| Tunisia (Agareb—North-Eastern Tunisia) | Aerial parts | HD | GC-MS | NI | 58/(98.98) | camphor (15.68%) 1,8-cineole (14.15%) α-pinene (13.82%) linalool (9%) | OM (45.98%) MH (11.79%) OS (7.37%) SH (0.14%) | [43] |
6. Pharmacological Activities
6.1. Antimicrobial Activity
| Activity | Test | Extracts/Compounds | Targets; Model | Effects; Key Findings | Ref. |
|---|---|---|---|---|---|
| Antibacterial | In vitro | EO; isolated camphor | E. coli, S. aureus | - EO: Strong antibacterial activity; 22.6 mm (E. coli), 17.8 mm (S. aureus); effect due to synergy of multiple components. - Camphor: Weaker (13.2 mm E. coli, 14.1 mm S. aureus at 2.5 µg/mL). | [43] |
| EO | S. aureus, S. epidermidis, E. coli, K. pneumoniae, A. baumannii | - Strong antibacterial activity (IZ 9–20 mm; MIC 0.5–4 µg/mL); S. aureus most sensitive. - Effect linked to carvacrol and β-bisabolene, with possible synergistic action. | [44] | ||
| EO | S. aureus, B. subtilis, L. innocua, L. monocytogenes, E. coli, P. vulgaris, P. mirabilis, P. aeruginosa | - Moderate antibacterial activity (IZ 8.5–16 mm; MIC 1–>4% v/v); S. aureus most sensitive. - Effect linked to carvacrol and β-bisabolene, with possible synergistic action. | [82] | ||
| EO | E. coli, K. pneumoniae, P. aeruginosa | - Strong antibacterial activity (IZ 10.6–37.3 mm; MIC 0.6–4.8 µL/mL). - Most sensitive: E. coli, K. pneumoniae; P. aeruginosa was less sensitive. - Effect linked to durenol with possible synergy from other compounds. | [72] | ||
| EO; ethanol extract | Methicillin-resistant S. aureus strains (MRSA), Methicillin-sensitive S. aureus strains (MSSA) | - EO: Strong anti-MRSA activity (inhibition zones 14–27 mm; MIC 0.6–5 µL/mL), with MSSA and some MRSA strains being most sensitive. - The ethanol extract was less active. - Effect linked to carvacrol, β-bisabolene and caryophyllene oxide, with possible synergistic action. | [79] | ||
| EO; isolated linalool | S. epidermidis (biofilm-forming strain) | - EO: Strong anti-biofilm activity (1.3–1.6 log at 10–30%) and synergized with CHG (~3.3–3.7 log) and CHG+IPA (up to 5.5 log). - Linalool: No significant difference in anti-biofilm effect or in synergy with disinfectants. - Effect linked to linalool. | [110] | ||
| Antifungal | In vitro | EO; isolated Carvacrol, cis-β-Ocimene | Candida spp., Cryptococcus neoformans, dermatophytes (Trichophyton spp., Microsporum spp., Epidermophyton floccosum), Aspergillus spp. | - EO: Broad antifungal activity; most active against dermatophytes and C. neoformans (MIC 0.16 µL/mL). - Carvacrol more active (MIC 0.04–0.16 µL/mL); cis-β-ocimene less active but contributed to filamentation inhibition. | [34] |
| EO | Alternaria sp., P. expansum, R. stolonifer, B. cinerea | - Strong antifungal activity; complete inhibition of R. stolonifer and Alternaria sp. (100 µg/mL). - Effect linked to carvacrol with possible synergy. | [45] | ||
| EO | Alternaria sp., P. expansum, R. stolonifer | - Strong antifungal; MIC 0.25–0.5 µL/mL; fungicidal at 0.5–1 µL/mL. - Effect mainly linked to carvacrol with synergy from other constituents. | [84] | ||
| EO | C. albicans, dermatophytes (E. floccosum, M. gypseum, M. canis, T. mentagrophytes, T. interdigitale, T. rubrum) | - Strong antibiofilm activity; inhibited biofilm formation (from 0.32 µL/mL) and disrupted mature biofilms (46% biomass, 49% matrix, 30% viability); most active against E. floccosum. - Effect mainly linked to carvacrol with contribution from cis-β-ocimene and β-bisabolene. | [80] | ||
| Antioxidant | in vitro; in vivo | Hydro-methanolic extract | Radical scavenging (DPPH, TEAC, ORAC), reducing power (FRAP); oxidative stress model (mice fed a high-fat diet) | - In vitro Strong antioxidant (DPPH IC50 = 8.06 µg/mL; FRAP = 2.58 mmol Fe2+/g; TEAC = 1.30 mmol Trolox/g; ORAC = 2.08 mmol Trolox/g). - In vivo, reduction in oxidative stress and liver lipid peroxidation (decreased TBARS). - Effect linked to polyphenols, triterpenes, and flavonoids. | [73] |
| In vitro | Crude extracts; fractions (dichloromethane, ethyl acetate, butanol) | Radical scavenging (DPPH, Galvinoxyl, ABTS), reducing power (CUPRAC, FRAP, phenanthroline), metal chelation assays. | - Ethyl acetate fraction: Strong antioxidant activity; DPPH (EC50 = 12.32 µg/mL, < BHA 5.73); superior in ABTS (4.89 µg/mL), Galvinoxyl (9.60 µg/mL), and reducing power (FRAP, CUPRAC, phenanthroline) - All extracts and fractions weak in metal chelation (>800 µg/mL). - Effect linked to polyphenols and flavonoids. | [75] | |
| EO | Radical scavenging (DPPH), lipid peroxidation inhibition (β-carotene bleaching) | - Moderate DPPH scavenging activity (IC50 = 16.83 µg/mL; BHT = 7.73 µg/mL). - Strong β-carotene inhibition (78.4%; BHT = 86.2%). - Effect linked to carvacrol and phenolics. | [45] | ||
| Aqueous extract | Radical scavenging (DPPH), reducing power (FRAP) | - Strong DPPH scavenging (IC50 = 2.6 mg/mL); FRAP = 12.76 mmol Trolox/g. - Effect linked to polyphenols and flavonoids. | [74] | ||
| EO; methanol extract | Radical scavenging (DPPH), reducing power (FRAP), metal chelation | - Methanol extract: Strong antioxidant; DPPH IC50 = 19.3 µg/mL (BHT 26.5; Trolox 12.8); FRAP = 377.8 mmol/g; chelation IC50 = 0.8 µg/mL. - Essential oil: Weaker antioxidant; DPPH IC50 = 201.6 µg/mL; FRAP = 39.1 mmol/g; chelation IC50 = 7.9 µg/mL. - Effect linked to polyphenols and carvacrol. | [81] | ||
| Methanolic extract | Radical scavenging (DPPH assay) | - Moderate DPPH scavenging (IC50 = 17.36 mg/mL) - Stronger than L. stoechas, weaker than L. dentate. - Effect linked to polyphenols. | [46] | ||
| Anti-inflammatory | Aqueous and ethanol extracts; isolated compounds from ethanol extract | Croton oil-induced ear edema in mice | - Ethanol extract: strong topical anti-inflammatory (17–62% edema reduction, ID50 = 510 µg/cm2; ~5× weaker than indomethacin); - Aqueous extract: Weaker, active only at high doses (24–33% reduction). - Isolated compounds (ursolic acid, oleanolic acid, maslinic acid) highly active (41–74% reduction) - Effect linked to ethanol-soluble triterpenoids, ursolic acid, oleanolic acid, and maslinic acid. | [77] | |
| in vivo | Aqueous extract | formaldehyde-induced paw edema in Rats | - Moderate anti-inflammatory (10–44% inhibition of paw edema, 200 mg/kg); weaker than diclofenac. - Effect linked to flavonoid and phenolic constituents. | [75] | |
| Insecticidal | in vitro | EO | Spodoptera littoralis, Agrotis ipsilon | - Strong toxicity (LC50 = 2.35 mg/mL for S. littoralis; 2.99 mg/mL for A. ipsilon); Shortening of larval and pupal stages and alteration of sex ratio in S. littoralis. - Effect linked mainly to eucalyptol and camphor, with contributions from α- and β-pinene. | [85] |
| in vitro; in silico | EO | Spodoptera frugiperda | - Weak toxicity (LC50 = 2701.5 mg/L); synergy with cyantraniliprole (53.3%), antagonism with emamectin (36.7%). - Effect linked mainly to eucalyptol, camphor, and α- and β-pinene. - Docking confirmed binding of EO constituents, strongest for eucalyptol. | [111] | |
| in vitro | Acetone, hexane extracts | Culex pipiens | - Hexane extract: Strong larvicidal activity (LC50 = 0.08–0.15 ppm); acetone extract weaker (54–79 ppm). - Effect linked to: Non-polar compounds in the hexane extract | [112] | |
| anti-hemolytic | in vitro | Aqueous extract | AAPH-induced hemolysis of rabbit erythrocytes | - Strong antihemolytic activity; increased half-time hemolysis by 479%. - Effect linked to polyphenols and flavonoids. | [74] |
| Cytotoxic | in vitro | Hexane, dichloromethane, methanol extracts | RD (embryonal rhabdomyosarcoma), BSR (hamster kidney adenocarcinoma), Vero (monkey kidney) cell lines | - Hexane and dichloromethane: Moderate cytotoxicity (IC50 = 115–300 µg/mL); methanol extract being inactive (>300 µg/mL). - Effect linked to Bioactive fatty acids (oleic acid, methyl linolenate) with possible synergistic effects. | [41] |
| Enzymes inhibition | in vitro | Crude extracts and fractions (dichloromethane, ethyl acetate, n-butanol) | α-amylase (anti-diabetic target), Butyrylcholinesterase (BuChE) (Alzheimer’s-related enzyme) | - α-amylase: Strong inhibition (antidiabetic potential) by crude extract (IC50 = 64.17 µg/mL), much stronger than acarbose (IC50 = 3650.93 µg/mL). - BuChE: Moderate inhibition (Alzheimer’s-related) by crude extract (IC50 = 83.55 µg/mL) and dichloromethane fraction (IC50 = 152.44 µg/mL). - Effect linked to polyphenols and tannins (α-amylase), alkaloids/terpenes (BuChE). | [75] |
6.2. Antioxidant Activity
6.3. Anti-Inflammatory Activity
6.4. Insecticidal Activity
6.5. Other Pharmacological Activities
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jindal, A.; Seth, C.S. Medicinal plants: The rising strategy for synthesis of modern medicine. Int. J. Plant Environ. 2022, 8, 76–80. [Google Scholar] [CrossRef]
- Dhayalan, M.; Anitha Jegadeeshwari, L.; Nagendra Gandhi, N. Biological activity sources from traditionally used tribe and herbal plants material. Asian J. Pharm. Clin. Res. 2015, 8, 11–23. [Google Scholar]
- Ravichandran, S.; Bhargavi, K.M.; Rai, A.; Pandey, T.; Rajput, J.; Sri, R.M.M. Medicinal plants for curing human diseases. Insight-Chin. Med. 2023, 6, 570. [Google Scholar] [CrossRef]
- Sadiq, I.Z.; Abubakar, F.S.; Ibrahim, B.; Usman, M.A.; Kudan, Z.B. Medicinal plants for management and alternative therapy of common ailments in Dutsin-Ma (Katsina State) in Nigeria. Herba Pol. 2019, 65, 45–55. [Google Scholar] [CrossRef]
- Jamshidi-Kia, F.; Lorigooini, Z.; Amini-Khoei, H. Medicinal plants: Past history and future perspective. J. Herbmed Pharmacol. 2018, 7, 1–7. [Google Scholar] [CrossRef]
- Ogunrinola, O.O.; Kanmodi, R.I.; Ogunrinola, O.A. Medicinal plants as immune booster in the palliative management of viral diseases: A perspective on coronavirus. Food Front. 2022, 3, 83–95. [Google Scholar] [CrossRef]
- González-Tejero, M.R.; Casares-Porcel, M.; Sánchez-Rojas, C.P.; Ramiro-Gutiérrez, J.M.; Molero-Mesa, J.; Pieroni, A.; Giusti, M.E.; Censorii, E.; de Pasquale, C.; Della, A.; et al. Medicinal plants in the Mediterranean area: Synthesis of the results of the project Rubia. J. Ethnopharmacol. 2008, 116, 341–357. [Google Scholar] [CrossRef]
- Miara, M.D.; Bendif, H.; Ait Hammou, M.; Teixidor-Toneu, I. Ethnobotanical survey of medicinal plants used by nomadic peoples in the Algerian steppe. J. Ethnopharmacol. 2018, 219, 248–256. [Google Scholar] [CrossRef]
- Aboukhalaf, A.; Tbatou, M.; Kalili, A.; Naciri, K.; Moujabbir, S.; Sahel, K.; Rocha, J.M.; Belahsen, R. Traditional knowledge and use of wild edible plants in Sidi Bennour region (Central Morocco). Ethnobot. Res. Appl. 2022, 23, 265–273. [Google Scholar] [CrossRef]
- Savvides, A.M.; Stavridou, C.; Ioannidou, S.; Zoumides, C.; Stylianou, A. An ethnobotanical investigation into the traditional uses of mediterranean medicinal and aromatic plants: The case of troodos mountains in Cyprus. Plants 2023, 12, 1119. [Google Scholar] [CrossRef]
- Marín, J.; Garnatje, T.; Vallès, J. Traditional knowledge 10 min far from Barcelona: Ethnobotanical study in the Llobregat river delta (Catalonia, NE Iberian Peninsula), a heavily anthropized agricultural area. J. Ethnobiol. Ethnomed. 2023, 19, 41. [Google Scholar] [CrossRef]
- Patti, M.; Musarella, C.M.; Spampinato, G. Ethnobotanical knowledge in Calabria (southern Italy): A summary review. Heliyon 2025, 11, e42050. [Google Scholar] [CrossRef] [PubMed]
- Fakir, H.; Korkmaz, M.; Güller, B. Medicinal plant diversity of western Mediterranean region in Turkey. J. Appl. Biol. Sci. 2009, 3, 30–40. [Google Scholar]
- Nawash, O.S.; Al-Assaf, A.; El-Oqlah, A.; Omari, M. Floristic features, distribution, and ethnobotany of plants gathered and used by local people from the Mediterranean forest in northern Jordan. Ethnobot. Res. Appl. 2014, 12, 385–396. [Google Scholar] [CrossRef]
- de Cortes Sánchez-Mata, M.; Tardío, J. Mediterranean Wild Edible Plants: Ethnobotany and Food Composition Tables; Springer: New York, NY, USA, 2016. [Google Scholar]
- Batiha, G.E.-S.; Teibo, J.O.; Wasef, L.; Shaheen, H.M.; Akomolafe, A.P.; Teibo, T.K.A.; Al-Kuraishy, H.M.; Al-Garbeeb, A.I.; Alexiou, A.; Papadakis, M. A Review of the bioactive components and pharmacological properties of Lavandula species. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2023, 396, 877–900. [Google Scholar] [CrossRef]
- Bayındır, D.; Uysal, G.; Erbaş, S.; Devran, Z. The response of lavender and lavandin cultivars to Meloidogyne incognita and Meloidogyne arenaria. J. Plant Dis. Prot. 2023, 130, 1049–1055. [Google Scholar] [CrossRef]
- Sun, M.; Zhu, L.; Zhang, Y.; Liu, N.; Zhang, J.; Li, H.; Bai, H.; Shi, L. Creation of new germplasm resources, development of SSR markers, and screening of monoterpene synthases in thyme. BMC Plant Biol. 2023, 23, 13. [Google Scholar] [CrossRef]
- Ricardo-Rodrigues, S.; Rouxinol, M.I.; Agulheiro-Santos, A.C.; Potes, M.E.; Laranjo, M.; Elias, M. The antioxidant and antibacterial potential of thyme and clove essential oils for meat preservation—An overview. Appl. Biosci. 2024, 3, 87–101. [Google Scholar] [CrossRef]
- Boulares, M.; Bezzezi, A.; Arfaoui, M.; Boulares, A.; Ghrab, M.; Ben Moussa, O.; Hassouna, M.; Boudiche, S. Improvement of Tunisian ‘Chemlali’ extra virgin olive oil stability with rosemary and laurel herbs and essential oils. Riv. Ital. Sostanze Grasse 2022, 99, 131–140. [Google Scholar]
- Barak, T.H.; Bölükbaş, E.; Bardakci, H. Evaluation of marketed rosemary essential oils (Rosmarinus officinalis L.) in terms of European Pharmacopoeia 10.0 criteria. Turk. J. Pharm. Sci. 2023, 20, 253–260. [Google Scholar] [CrossRef]
- Silva, B.N.; Cadavez, V.; Caleja, C.; Pereira, E.; Calhelha, R.C.; Añibarro-Ortega, M.; Finimundy, T. Phytochemical composition and bioactive potential of Melissa officinalis L., Salvia officinalis L., and Mentha spicata L. extracts. Foods 2023, 12, 947. [Google Scholar] [CrossRef]
- Napoli, E.; Kim, M.; Sowndhararajan, K.; Ruberto, G.; Kim, S. The effect of exposure to Mentha suaveolens Ehrh. essential oil on the electroencephalographic activity according to gender difference. J. Essent. Oil Res. 2023, 35, 486–499. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.S.; Aldarhami, A.; Alharbi, B.; Almashjary, M.N.; Hazzazi, M.S.; Felemban, H.R.; Abdelghany, T.M. Phytochemical characterization and efficacy of Artemisia judaica extract loaded chitosan nanoparticles as inhibitors of cancer proliferation and microbial growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Lantzouraki, D.Z.; Amerikanou, C.; Karavoltsos, S.; Kafourou, V.; Sakellari, A.; Tagkouli, D.; Zoumpoulakis, P.; Makris, D.P.; Kalogeropoulos, N.; Kaliora, A.C. Artemisia arborescens and Artemisia inculta from Crete; secondary metabolites, trace metals and in vitro antioxidant activities. Life 2023, 13, 1416. [Google Scholar] [CrossRef] [PubMed]
- Barut, M.; Tansi, L.S. Elucidating the flower and seed yield and phytochemical variability of marigold (Calendula officinalis L.) in response to winter sowing at different harvest intervals and dates. S. Afr. J. Bot. 2024, 166, 191–207. [Google Scholar]
- Barut, M.; Tansi, L.S.; Karaman, Ş. Unveiling the phytochemical variability of fatty acids in world marigold (Calendula officinalis L.) germplasm affected by genotype. Int. J. Agric. Environ. Food Sci. 2023, 7, 639–649. [Google Scholar] [CrossRef]
- Guitton, Y.; Nicolè, F.; Jullien, F.; Caissard, J.-C.; Saint-Marcoux, D.; Legendre, L.; Pasquier, B.; Moja, S. A comparative study of terpene composition in different clades of the genus Lavandula. Bot. Lett. 2018, 165, 494–505. [Google Scholar] [CrossRef]
- Salehi, B.; Mnayer, D.; Özçelik, B.; Altin, G.; Kasapoğlu, K.N.; Daskaya-Dikmen, C.; Sharifi-Rad, M. Plants of the genus Lavandula: From farm to pharmacy. Nat. Prod. Commun. 2018, 13, 1385–1402. [Google Scholar] [CrossRef]
- Aprotosoaie, A.C.; Gille, E.; Trifan, A.; Luca, V.S.; Miron, A. Essential oils of Lavandula genus: A systematic review of their chemistry. Phytochem. Rev. 2017, 16, 761–799. [Google Scholar] [CrossRef]
- Ez zoubi, Y.; Bousta, D.; Farah, A. A phytopharmacological review of a Mediterranean plant: Lavandula stoechas L. Clin. Phytosci. 2020, 6, 9. [Google Scholar] [CrossRef]
- Héral, B.; Stierlin, É.; Fernandez, X.; Michel, T. Phytochemicals from the genus Lavandula: A review. Phytochem. Rev. 2021, 20, 751–771. [Google Scholar] [CrossRef]
- Vairinhos, J.; Miguel, M.G. Essential oils of spontaneous species of the genus Lavandula from Portugal: A brief review. Z. Naturforsch. C 2020, 75, 233–245. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Vale-Silva, L.; Gonçalves, M.J.; Cavaleiro, C.; Vaz, S.; Canhoto, J.; Pinto, E.; Salgueiro, L. Antifungal activity of phenolic-rich Lavandula multifida L. essential oil. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1359–1366. [Google Scholar] [CrossRef] [PubMed]
- Benabdelkader, T.; Zitouni, A.; Guitton, Y.; Jullien, F.; Maitre, D.; Casabianca, H.; Legendre, L.; Kameli, A. Essential oils from wild populations of Algerian Lavandula stoechas L.: Composition, chemical variability, and in vitro biological properties. Chem. Biodivers. 2011, 8, 937–953. [Google Scholar] [CrossRef] [PubMed]
- Benbelaid, D.E.A.F.; Bendahou, M.; Khadir, A.; Abdoune, M.A.; Bellahsene, C.; Zenati, F.; Bouali, W. Antimicrobial activity of essential oil of Lavandula multifida L. J. Microbiol. Biotechnol. Res. 2012, 2, 244–247. [Google Scholar]
- Mechaala, S.; Bouatrous, Y.; Adouane, S. Traditional knowledge and diversity of wild medicinal plants in El Kantara’s area (Algerian Sahara gate): An ethnobotany survey. Acta Ecol. Sin. 2022, 42, 33–45. [Google Scholar] [CrossRef]
- Hendel, N.; Larous, L.; Sari, M.; Boudjelal, A.; Sarri, D. Place of labiates in folk medicine of the area of M’sila (Algeria). Glob. J. Res. Med. Plants Indig. Med. 2012, 1, 315–322. [Google Scholar]
- Neves, J.M.; Matos, C.; Moutinho, C.; Queiroz, G.; Gomes, L.R. Ethnopharmacological notes about ancient uses of medicinal plants in Trás-os-Montes (northern of Portugal). J. Ethnopharmacol. 2009, 124, 270–283. [Google Scholar] [CrossRef]
- Bachiri, L.; Labazi, N.; Daoudi, A.; Ibijbijien, J.; Nassiri, L.; Echchegadda, G.; Mokhtari, F. Ethnobotanical study of some spontaneous moroccan lavenders. Int. J. Biol. Chem. Sci. 2015, 9, 1308–1318. [Google Scholar] [CrossRef]
- Aneb, M.; Talbaoui, A.; Bouyahya, A.; El Boury, H.; Amzazi, S.; Benjouad, A.; Dakka, N.; Bakri, Y. In vitro cytotoxic effects and antibacterial activity of moroccan medicinal plants Aristolochia longa and Lavandula multifida. Eur. J. Med. Plants 2016, 16, 1–13. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Fazio, A.; Papalia, T.; Barreca, D. Antioxidant properties and flavonoid profile in leaves of calabrian Lavandula multifida L., an autochthon plant of mediterranean southern regions. Chem. Biodivers. 2016, 13, 416–421. [Google Scholar] [CrossRef] [PubMed]
- Tofah, M.L.; Mseddi, K.; Al-Abbasi, O.K.; Ben Yazid, A.; Khechine, A.; Gdoura, R.; Khannous, L. A new lavender (Lavandula multifida L.) ecotype from arid Tunisia, with differential essential oil composition and higher antimicrobial potential. Life 2023, 13, 103. [Google Scholar]
- Elmakaoui, A.; Bourais, I.; Oubihi, A.; Nassif, A.; Bezhinar, T.; Shariati, M.A.; Blinov, A.V.; Hleba, L.; El Hajjaji, S. Chemical composition and antibacterial activity of essential oil of Lavandula multifida. J. Microbiol. Biotechnol. Food Sci. 2022, 11, 7–11. [Google Scholar] [CrossRef]
- Sellam, K.; Ramchoun, M.; Alem, C.; ElRhaffari, L. Biological investigations of antioxidant-antimicrobial properties and chemical composition of essential oil from Lavandula multifida. Oxid. Antioxid. Med. Sci. 2013, 2, 211. [Google Scholar]
- Dif, M.M.; Benyahia, M.; Toumi Benali, F.; Rahmani, M.; Bouazza, S. Teneur en composés phénoliques et activité antioxydantes de trois espèces algérienne de Lavandula. Phytotherapie 2017, 15, 367–372. [Google Scholar] [CrossRef]
- Gross, N.; Börger, L.; Soriano-Morales, S.I.; Le Bagousse-Pinguet, Y.; Quero, J.L.; García-Gómez, M.; Valencia-Gomez, E.; Maestre, F.T. Uncovering Multiscale Effects of Aridity and Biotic Interactions on the Functional Structure of Mediterranean Shrublands. J. Ecol. 2013, 101, 637–649. [Google Scholar] [CrossRef]
- García-Caparrós, P.; Llanderal, A.; Pestana, M.; Correia, P.J.; Lao, M.T. Lavandula multifida response to salinity: Growth, nutrient uptake, and physiological changes. J. Plant Nutr. Soil Sci. 2017, 180, 96–104. [Google Scholar] [CrossRef]
- Nuutinen, T. Medicinal Properties of Terpenes Found in Cannabis sativa and Humulus lupulus. Eur. J. Med. Chem. 2018, 157, 198–228. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A Natural Phenolic Compound with Antimicrobial Properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef] [PubMed]
- Hoch, C.C.; Petry, J.; Griesbaum, L.; Weiser, T.; Werner, K.; Ploch, M.; Verschoor, A.; Multhoff, G.; Dezfouli, A.B.; Wollenberg, B.; et al. 1,8-Cineole (Eucalyptol): A Versatile Phytochemical with Therapeutic Applications across Multiple Diseases. Biomed. Pharmacother. 2023, 167, 115467. [Google Scholar] [CrossRef]
- Zuzarte, M.; Dinis, A.M.; Cavaleiro, C.; Canhoto, J.; Salgueiro, L. Trichomes morphology and essential oils characterization of field-growing and in vitro propagated plants of Lavandula pedunculata. Microsc. Microanal. 2008, 14, 148–149. [Google Scholar] [CrossRef]
- Znini, M.; Laghchimi, A.; Paolini, J.; Costa, J.; Majidi, L. Characterization of Lavandula multifida volatile composition from Morocco by headspace solid-phase microextraction (HS-SPME) and hydrodistillation coupled to GC–MS. Arab. J. Med. Aromat. Plants 2019, 5, 18–31. [Google Scholar]
- Lacaze, R. La culture de la lavande dans le Quercy. Rev. Géogr. Pyrénées Sud-Ouest 1965, 36, 39–52. [Google Scholar] [CrossRef]
- Msaada, K.; Salem, N.; Tammar, S.; Hammami, M.; Saharkhiz, M.J.; Debiche, N.; Limam, F.; Marzouk, B. Essential oil composition of Lavandula dentata, L. stoechas and L. multifida cultivated in Tunisia. J. Essent. Oil-Bear. Plants 2012, 15, 1030–1039. [Google Scholar] [CrossRef]
- Plants of the World Online (POWO). Lavandula multifida L. 2025. Available online: https://powo.science.kew.org/taxon/449064-1 (accessed on 14 September 2025).
- World Flora Online (WFO). Lavandula multifida L. 2025. Available online: http://www.worldfloraonline.org/taxon/wfo-0000224197 (accessed on 14 September 2025).
- Fazio, A.; Cerezuela, R.; Panuccio, M.R.; Cuesta, A.; Esteban, M.Á. In vitro effects of Italian Lavandula multifida L. leaf extracts on gilthead seabream (Sparus aurata) leucocytes and SAF-1 cells. Fish Shellfish Immunol. 2017, 66, 334–344. [Google Scholar] [CrossRef]
- Lis-Balchin, M. Lavender: The Genus Lavandula; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Upson, T.M.; Jury, S.L. A revision of native Moroccan species of Lavandula L. section Pterostoechas Ging. (Lamiaceae). Taxon 2002, 51, 309–327. [Google Scholar] [CrossRef]
- Panuccio, M.R.; Fazio, A.; Musarella, C.M.; Mendoza-Fernández, A.J.; Mota, J.F.; Spampinato, G. Seed germination and antioxidant pattern in Lavandula multifida (Lamiaceae): A comparison between core and peripheral populations. Plant Biosyst. 2018, 152, 398–406. [Google Scholar] [CrossRef]
- Chograni, H.; Zaouali, Y.; Rajeb, C.; Boussaid, M. Essential oil variation among natural populations of Lavandula multifida L. (Lamiaceae). Chem. Biodivers. 2010, 7, 933–942. [Google Scholar] [CrossRef]
- Ghadiri, M.K.; Gorji, A. Lavender for medicine: A brief review of clinical effects. Avicenna 2002, 1, 23–27. [Google Scholar]
- El Rhaffari, L.; Ismaïli-Alaoui, M.; Belkamel, J.; Jeannot, V. Chemical composition and antibacterial properties of the essential oil of Lavandula multifida L. Int. J. Essent. Oil Ther. 2007, 1, 122–125. [Google Scholar]
- Karous, O.; Jilani, I.B.H.; Ghrabi-Gammar, Z. Ethnobotanical study on plant used by semi-nomad descendants’ community in Ouled Dabbeb—Southern Tunisia. Plants 2021, 10, 642. [Google Scholar] [CrossRef]
- Martínez-Lirola, M.J.; González-Tejero, M.R.; Molero-Mesa, J. Ethnobotanical resources in the province of Almería, Spain: Campos de Nijar. Econ. Bot. 1996, 50, 40–56. [Google Scholar] [CrossRef]
- Bourlière, F.; Quezel, P.; Santa, S. Nouvelle Flore de l’Algérie et de Ses Régions Désertiques Méridionales. Tome II; CNRS Éditions: Paris, France, 1963. [Google Scholar]
- El-Hilaly, J.; Hmammouchi, M.; Lyoussi, B. Ethnobotanical Studies and Economic Evaluation of Medicinal Plants in Taounate Province (Northern Morocco). J. Ethnopharmacol. 2003, 86, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Fatma, G.; Sami, B.H.A.; Ahmed, L. Investigation of extracts from Tunisian ethnomedicinal plants as antioxidants, cytotoxins, and antimicrobials. Biomed. Environ. Sci. 2017, 30, 811–824. [Google Scholar]
- Koblovská, R.; Macková, Z.; Vítková, M.; Kokoška, L.; Klejdus, B.; Lapčík, O. Isoflavones in the rutaceae family: Twenty selected representatives of the genera Citrus, Fortunella, Poncirus, Ruta, and Severinia. Phytochem. Anal. 2008, 19, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Chen, L.G.; Chang, T.L.; Ke, W.M.; Lo, Y.F.; Wang, C.C. The correlation between skin-care effects and phytochemical contents in lamiaceae plants. Food Chem. 2011, 124, 833–841. [Google Scholar] [CrossRef]
- Soro, N.K.; Majdouli, K.; Moutaouakil, K.; Elhilali, F.; Khabbal, Y.; Bentayeb, A.; Zaïr, T. Chemical composition and antibacterial power of Lavandula multifida L. essential oil against multiresistant strains of Escherichia coli, Pseudomonas aeruginosa, and Klebsiella pneumoniae isolated in hospital. Int. J. Innov. Sci. Res. 2014, 9, 464–472. [Google Scholar]
- Molina-Tijeras, J.A.; Ruiz-Malagón, A.J.; Hidalgo-García, L.; Diez-Echave, P.; Rodríguez-Sojo, M.J.; Cádiz-Gurrea, M.L.; Segura-Carretero, A. The antioxidant properties of Lavandula multifida extract contribute to its beneficial effects in High-Fat Diet-Induced obesity in mice. Antioxidants 2023, 12, 832. [Google Scholar] [CrossRef]
- Ramchoun, M.; Harnafi, H.; Alem, C.; Benlyas, M.; Elrhaffari, L.; Amrani, S. Study on antioxidant and hypolipidemic effects of polyphenol-rich extracts from Thymus vulgaris and Lavandula multifida. Pharmacogn. Res. 2009, 1, 106–112. [Google Scholar]
- Mammeri, A.; Bendif, H.; Bensouici, C.; Benslama, A.; Rebas, K.; Bouasla, A.; Rebaia, I.; Souilah, N.; Miara, M.D. Total phenolic contents, in vitro antioxidant activity, enzyme inhibition and anti-inflammatory effect of the selective extracts from the algerian Lavandula multifida. Acta Pharm. Sci. 2022, 60, 1–23. [Google Scholar]
- Soudani, L.; Nabi, F.; Bendif, H.; Dilaycan, Ç.; Bouriah, N.; Öztürk, M. In-Depth Chemical profile by GC–MS, ICP–OES and HPLC–DAD, and In Vitro antioxidant properties of Lavandula multifida. Food Anal. Methods 2025, 18, 634–645. [Google Scholar] [CrossRef]
- Sosa, S.; Altinier, G.; Politi, M.; Braca, A.; Morelli, I.; Della Loggia, R. Extracts and constituents of Lavandula multifida with topical anti-inflammatory activity. Phytomedicine 2005, 12, 271–277. [Google Scholar] [CrossRef]
- Saadi, A.; Brada, M.; Kouidri, M.; Dekkiche, H.; Attar, F. Chemical composition and content of essential oil of Lavandula multifida from Algeria. Chem. Nat. Compd. 2016, 52, 162–164. [Google Scholar] [CrossRef]
- Khadir, A.; Bendahou, M.; Benbelaid, F.; Abdoune, M.A.; Bellahcene, C.; Zenati, F.; Muselli, A.; Paolini, J.; Costa, J. Chemical composition and Anti-MRSA activity of essential oil and ethanol extract of Lavandula multifida L. from Algeria. J. Essent. Oil-Bear. Plants 2016, 19, 712–718. [Google Scholar] [CrossRef]
- Alves-Silva, J.; Zuzarte, M.; Cavaleiro, C.; Salgueiro, L. Antibiofilm effect of Lavandula multifida essential oil: A new approach for chronic infections. Pharmaceutics 2023, 15, 2142. [Google Scholar] [CrossRef] [PubMed]
- Messaoud, C.; Chograni, H.; Boussaid, M. Chemical composition and antioxidant activities of essential oils and methanol extracts of three wild Lavandula L. Species. Nat. Prod. Res. 2013, 27, 37–41. [Google Scholar]
- Douhri, B.; Douhri, H.; Farah, A.; Idaomar, M.; Senhaji, N.S.; Abrini, J. Phytochemical analysis and antibacterial activity of essential oil of Lavandula multifida L. Int. J. Innov. Sci. Res. 2014, 1, 116–126. [Google Scholar]
- Znini, M.; Paolini, J.; Majidi, L.; Desjobert, J.-M.; Costa, J.; Lahhit, N.; Bouyanzer, A. Evaluation of the inhibitive effect of Lavandula multifida L. essential oil on the corrosion behavior of C38 steel in 0.5 M H2SO4 medium. Res. Chem. Intermed. 2012, 38, 669–683. [Google Scholar] [CrossRef]
- PubChem. PubChem Database. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 21 September 2025).
- Laghchimi, A.; Znini, M.; Majidi, L.; Renucci, F.; El Harrak, A.; Costa, J. Composition chimique et effet des phases liquide et vapeur de l’huile essentielle de Lavandula multifida sur la croissance mycélienne des moisissures responsables de la pourriture de la pomme. J. Mater. Environ. Sci. 2014, 5, 1770–1780. [Google Scholar]
- Awad, M.; Moustafa, M.A.M.; Alfuhaid, N.A.; Amer, A.; Ahmed, F.S. Toxicological, biological, and biochemical impacts of the egyptian lavender (Lavandula multifida) essential oil on two lepidopteran pests. J. Plant Prot. Res. 2024, 64, 127–138. [Google Scholar] [CrossRef]
- Wells, R.; Truong, F.; Adal, A.M.; Sarker, L.S.; Mahmoud, S.S. Lavandula essential oils: A current review of applications in medicinal, food, and cosmetic industries of lavender. Nat. Prod. Commun. 2018, 13, 1403–1417. [Google Scholar] [CrossRef]
- Zenão, S.; Aires, A.; Dias, C.; Saavedra, M.J.; Fernandes, C. Antibacterial potential of Urtica dioica and Lavandula angustifolia extracts against methicillin-resistant Staphylococcus aureus isolated from diabetic foot ulcers. J. Herb. Med. 2017, 10, 53–58. [Google Scholar] [CrossRef]
- Bouyahya, A.; Et-Touys, A.; Abrini, J.; Talbaoui, A.; Fellah, H.; Bakri, Y.; Dakka, N. Lavandula stoechas essential oil from Morocco as novel source of antileishmanial, antibacterial, and antioxidant activities. Biocatal. Agric. Biotechnol. 2017, 12, 179–184. [Google Scholar] [CrossRef]
- Pombal, S.; Rodrigues, C.F.; Araújo, J.P.; Rocha, P.M.; Rodilla, J.M.; Diez, D.; Granja, Á.P.; Gomes, A.C.; Silva, L.A. Antibacterial and antioxidant activity of Portuguese Lavandula luisieri (Rozeira) Rivas-Martinez and its relation with their chemical composition. Springerplus 2016, 5, 1711. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zuzarte, M.; Gonçalves, M.J.; Cruz, M.T.; Cavaleiro, C.; Canhoto, J.; Vaz, S.; Pinto, E.; Salgueiro, L. Lavandula luisieri essential oil as a source of antifungal drugs. Food Chem. 2012, 135, 1505–1510. [Google Scholar] [CrossRef]
- Carrasco Ruiz, A.; Tomas, V.; Tudela, J.; Miguel, M.G. Comparative study of GC-MS characterization, antioxidant activity, and hyaluronidase inhibition of different species of Lavandula and Thymus essential oils. Flavour Fragr. J. 2016, 31, 57–69. [Google Scholar] [CrossRef]
- Nikolova, G.; Karamalakova, Y.; Kovacheva, N.; Stanev, S.; Zheleva, A.; Gadjeva, V. Protective effect of two essential oils isolated from Rosa damascena Mill. and Lavandula angustifolia Mill., and two classic antioxidants against L-dopa oxidative toxicity induced in healthy mice. Regul. Toxicol. Pharmacol. 2016, 81, 1–7. [Google Scholar] [CrossRef]
- Georgiev, Y.N.; Paulsen, B.S.; Kiyohara, H.; Ciz, M.; Ognyanov, M.H.; Vasicek, O.; Rise, F. The common lavender (Lavandula angustifolia Mill.) pectic polysaccharides modulate phagocytic leukocytes and intestinal Peyer’s Patch Cells. Carbohydr. Polym. 2017, 174, 948–959. [Google Scholar] [CrossRef]
- Husseini, Y.; Sahraei, H.; Meftahi, G.H.; Dargahian, M.; Mohammadi, A.; Hatef, B.; Zardooz, H. Analgesic and anti-inflammatory activities of hydro-alcoholic extract of Lavandula officinalis in mice: Possible involvement of the cyclooxygenase Type 1 and 2 Enzymes. Rev. Bras. Farmacogn. 2016, 26, 102–108. [Google Scholar] [CrossRef]
- Panahi, Y.; Akhavan, A.; Sahebkar, A.; Hosseini, S.M.; Taghizadeh, M.; Akbari, H.; Sharif, M.R.; Imani, S. Investigation of the effectiveness of Syzygium aromaticum, Lavandula angustifolia, and Geranium robertianum essential oils in the treatment of acute external otitis: A comparative trial with ciprofloxacin. J. Microbiol. Immunol. Infect. 2014, 47, 211–216. [Google Scholar] [CrossRef]
- Al Sufyani, N.M.; Hussien, N.A.; Hawsawi, Y.M. Characterization and anticancer potential of silver nanoparticles biosynthesized from Olea chrysophylla and Lavandula dentata leaf extracts on HCT116 colon cancer cells. J. Nanomater. 2019, 2019, 7361695. [Google Scholar] [CrossRef]
- Miastkowska, M.; Kantyka, T.; Bielecka, E.; Kałucka, U.; Kamińska, M.; Kucharska, M.; Kilanowicz, A.; Cudzik, D.; Cudzik, K. Enhanced biological activity of a novel preparation of Lavandula angustifolia essential oil. Molecules 2021, 26, 2458. [Google Scholar] [CrossRef]
- Rabiei, Z.; Rafieian-Kopaei, M.; Mokhtari, S.; Alibabaei, Z.; Shahrani, M. The Effect of pretreatment with different doses of Lavandula officinalis ethanolic extract on memory, learning, and nociception. Biomed. Aging Pathol. 2014, 4, 71–76. [Google Scholar] [CrossRef]
- Costa, P.; Sarmento, B.; Gonçalves, S.; Romano, A. Protective effects of Lavandula viridis L’Hér extracts and rosmarinic acid against H2O2-induced oxidative damage in A172 Human astrocyte cell line. Ind. Crops Prod. 2013, 50, 361–365. [Google Scholar] [CrossRef]
- Videira, R.; Castanheira, P.; Grãos, M.; Salgueiro, L.; Faro, C.; Cavaleiro, C. A Necrodane monoterpenoid from Lavandula luisieri essential oil as a cell-permeable inhibitor of BACE-1, the β-Secretase in Alzheimer’s disease. Flavour Fragr. J. 2013, 28, 380–388. [Google Scholar] [CrossRef]
- Seyyed-Rasooli, A.; Salehi, F.; Mohammadpoorasl, A.; Goljaryan, S.; Seyyedi, Z.; Thomson, B. Comparing the effects of aromatherapy massage and inhalation aromatherapy on anxiety and pain in burn patients: A single-blind randomized clinical trial. Burns 2016, 42, 1774–1780. [Google Scholar] [CrossRef]
- Bagheri-Nesami, M.; Espahbodi, F.; Nikkhah, A.; Shorofi, S.A.; Charati, J.Y. The effects of lavender aromatherapy on pain following needle insertion into a fistula in hemodialysis patients. Complement. Ther. Clin. Pract. 2014, 20, 1–4. [Google Scholar] [CrossRef]
- Soltani, R.; Soheilipour, S.; Hajhashemi, V.; Asghari, G.; Bagheri, M.; Molavi, M. Evaluation of the effect of aromatherapy with lavender essential oil on post-tonsillectomy pain in pediatric patients: A randomized controlled trial. Int. J. Pediatr. Otorhinolaryngol. 2013, 77, 1579–1581. [Google Scholar] [CrossRef]
- İşlek, Z.; Şahin, F. In Vitro Antileishmanial Activity of Lavandula angustifolia Essential Oil on Leishmania infantum Parasites. Experimed 2023, 13, 115–120. [Google Scholar] [CrossRef]
- Yao, N.; He, J.K.; Pan, M.; Hou, Z.F.; Xu, J.J.; Yang, Y.; Tao, J.P.; Huang, S.Y. In Vitro Evaluation of Lavandula angustifolia Essential Oil on Anti-Toxoplasma Activity. Front. Cell. Infect. Microbiol. 2021, 11, 755715. [Google Scholar] [CrossRef]
- Badreddine, B.S.; Olfa, E.; Samir, D.; Hnia, C.; Lahbib, B.J.M. Chemical composition of Rosmarinus and Lavandula essential oils and their insecticidal effects on Orgyia trigotephras (Lepidoptera, Lymantriidae). Asian Pac. J. Trop. Med. 2015, 8, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Al-Ansari, M.M.; Andeejani, A.M.I.; Alnahmi, E.; AlMalki, R.H.; Masood, A.; Vijayaraghavan, P.; Abdel Rahman, A.; Choi, K.C. Insecticidal, antimicrobial and antioxidant activities of essential oil from Lavandula latifolia L. and its deterrent effects on Euphoria leucographa. Ind. Crops Prod. 2021, 170, 113740. [Google Scholar] [CrossRef]
- Radi, F.-Z.; El Hamzaoui, N.; Margier, A.; Auckle, T.; Oulhaj, H.; Zair, T. The antibacterial effect of essential oils of Satureja calamintha subsp. Nepeta (L.) Briq, Lavandula multifida L., and Mentha pulegium L., tested against some multiresistant strains that are involved in nosocomial infections. Phytothérapie 2020, 18, 375. [Google Scholar] [CrossRef]
- Alabdullatif, M.; Boujezza, I.; Mekni, M.; Taha, M.; Kumaran, D.; Yi, Q.-L.; Landoulsi, A.; Ramirez-Arcos, S. Enhancing blood donor skin disinfection using natural oils. Transfusion 2017, 57, 2920–2927. [Google Scholar] [CrossRef]
- Moustafa, M.A.M.; El-said, N.A.; Ahmed, F.S.; Amer, A.; Awad, M. In Vitro and Silico exploration of the insecticidal properties of Lavandula multifida L. essential oil and its binary combinations with cyantraniliprole and emamectin benzoate on Spodoptera frugiperda (Lepidoptera: Noctuidae). Crop Prot. 2025, 187, 106969. [Google Scholar] [CrossRef]
- El-Bokl, M.M. Toxicity and bioefficacy of selected plant extracts against the mosquito vector Culex pipiens L. (Diptera: Culicidae). J. Entomol. Zool. Stud. 2016, 4, 483–488. [Google Scholar]
- Hamad Al-Mijalli, S.; Elsharkawy, E.R.; Abdallah, E.M.; Hamed, M.; El Omari, N.; Mahmud, S.; Bouyahya, A. Determination of volatile compounds of Mentha piperita and Lavandula multifida and investigation of their antibacterial, antioxidant, and antidiabetic properties. Evid. Based Complement. Alternat. Med. 2022, 2022, 9306251. [Google Scholar] [CrossRef]
- Mączka, W.; Duda-Madej, A.; Grabarczyk, M.; Wińska, K. Natural Compounds in the Battle against Microorganisms—Linalool. Molecules 2022, 27, 6928. [Google Scholar] [CrossRef]
- Ramdani, C.; El Fakhouri, K.; Sbaghi, M.; Bouharroud, R.; Boulamtat, R.; Aasfar, A.; Mesfioui, A.; El Bouhssini, M. Chemical Composition and Insecticidal Potential of Six Essential Oils from Morocco against Dactylopius opuntiae (Cockerell) under Field and Laboratory Conditions. Insects 2021, 12, 1007. [Google Scholar] [CrossRef]
- Henni, A.; Chahbar, M.; Tefiel, H.; Touahri, A.; Chouhim, K.M.A. Chemical Composition and Efficacy of Essential Oil from Thymus lancéolatus Desf. Against Paenibacillus larvae and Ascosphaera apis in Honeybees (Apis mellifera). Braz. J. Anim. Environ. Res. 2024, 7, e73605. [Google Scholar] [CrossRef]
- Khalid, S.; Keller, N.P. Chemical Signals Driving Bacterial–Fungal Interactions. Environ. Microbiol. 2021, 23, 1334–1347. [Google Scholar] [CrossRef] [PubMed]
- Domingues, J.; Delgado, F.; Gonçalves, J.C.; Zuzarte, M.; Duarte, A.P. Mediterranean Lavenders from Section Stoechas: An Undervalued Source of Secondary Metabolites with Pharmacological Potential. Metabolites 2023, 13, 337. [Google Scholar] [CrossRef] [PubMed]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Dinis, A.M.; Canhoto, J.M.; Salgueiro, L.R. Chemical Composition and Antifungal Activity of the Essential Oils of Lavandula pedunculata (Miller) Cav. Chem. Biodivers. 2009, 6, 1283–1292. [Google Scholar] [CrossRef] [PubMed]
- Karaca, N.; Demirci, B.; Demirci, F. Evaluation of Lavandula stoechas L. subsp. Stoechas L., Mentha spicata L. subsp. Spicata L. Essential Oils and Their Main Components against Sinusitis Pathogens. Z. Naturforsch. C 2018, 73, 353–360. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.J.; Cavaleiro, C.; Canhoto, J.; Vale-Silva, L.; Silva, M.J.; Pinto, E.; Salgueiro, L. Chemical Composition and Antifungal Activity of the Essential Oils of Lavandula viridis L’Hér. J. Med. Microbiol. 2011, 60, 612–618. [Google Scholar] [CrossRef]
- Shakeri, A.; Sahebkar, A.; Javadi, B. Melissa officinalis L.—A review of its traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2016, 188, 204–228. [Google Scholar] [CrossRef]
- Deng, B.; Kong, W.; Suo, H.; Shen, X.; Newton, M.A.; Burkett, W.C.; Zhao, Z.; John, C.; Sun, W.; Zhang, X.; et al. Oleic Acid Exhibits Anti-Proliferative and Anti-Invasive Activities via the PTEN/AKT/mTOR Pathway in Endometrial Cancer. Cancers 2023, 15, 5407. [Google Scholar] [CrossRef]
- Kodati, B.R.; Sampathkumar, Y.; Godishala, V. Gas Chromatography-Mass Spectrometry Analysis of Anabaena Extract: Identification of Bioactive Compounds and Their Therapeutic Potential. J. Food Chem. Nanotechnol. 2025, 11, 67–74. [Google Scholar] [CrossRef]
- Fahmy, M.A.; Farghaly, A.A.; Hassan, E.E.; Hassan, E.M.; Hassan, Z.M.; Mahmoud, K.; Omara, E.A. Evaluation of the Anti-Cancer/Anti-Mutagenic Efficiency of Lavandula officinalis Essential Oil. Asian Pac. J. Cancer Prev. 2022, 23, 1215. [Google Scholar] [CrossRef]
- Dalilan, S.; Rezaei-Tavirani, M.; Nabiuni, M.; Heidari-Keshel, S.; Zamanian Azodi, M.; Zali, H. Aqueous Extract of Lavender Angustifolia Inhibits Lymphocytes Proliferation of Hodgkin’s Lymphoma Patients. Iran. J. Cancer Prev. 2013, 6, 201. [Google Scholar]
- Zhao, Y.; Chen, R.; Wang, Y.; Qing, C.; Wang, W.; Yang, Y. In Vitro and In Vivo efficacy studies of Lavender angustifolia essential oil and its active constituents on the proliferation of human prostate cancer. Integr. Cancer Ther. 2017, 16, 215. [Google Scholar] [CrossRef]
- Ahmed, S.B.H.; Sghaier, R.M.; Guesmi, F.; Kaabi, B.; Mejri, M.; Attia, H.; Laouini, D.; Smaali, I. Evaluation of Antileishmanial, Cytotoxic and Antioxidant Activities of Essential Oils Extracted from Plants Issued from the Leishmaniasis-Endemic Region of Sned (Tunisia). Nat. Prod. Res. 2011, 25, 1195–1201. [Google Scholar] [CrossRef]
- Rathod, N.B.; Kulawik, P.; Ozogul, F.; Regenstein, J.M.; Ozogul, Y. Biological activity of plant-based carvacrol and thymol and their impact on human health and food quality. Trends Food Sci. Technol. 2021, 116, 733–748. [Google Scholar] [CrossRef]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From Theory to Practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Dias, M.C.; Pinto, D.C.; Silva, A.M. Plant Flavonoids: Chemical Characteristics and Biological Activity. Molecules 2021, 26, 5377. [Google Scholar] [CrossRef]
- Sales-Campos, H.; de Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An Overview of the Modulatory Effects of Oleic Acid in Health and Disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [PubMed]
- Aryal, P.; Syed, I.; Lee, J.; Patel, R.; Nelson, A.T.; Siegel, D.; Saghatelian, A.; Kahn, B.B. Distinct Biological Activities of Isomers from Several Families of Branched Fatty Acid Esters of Hydroxy Fatty Acids (FAHFAs). J. Lipid Res. 2021, 62, 100108. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, Y.; Yang, K.; Jiao, J.; Zhan, H.; Yang, Y.; Lv, D.; Li, W.; Ding, W. Maslinic Acid: A New Compound for the Treatment of Multiple Organ Diseases. Molecules 2022, 27, 8732. [Google Scholar] [CrossRef] [PubMed]
- Wimmer, Z. Selected Pentacyclic Triterpenoids and Their Derivatives as Biologically Active Compounds. Molecules 2025, 30, 3106. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]


| Countries | Local Names | Plant Parts Used | Traditional Uses | Preparation Form | Ref. |
|---|---|---|---|---|---|
| Algeria | Kammoun el djmel, Tay djebal, Zeriga, Khzama | Aerial parts, leaf | Influenza, hypotensive, Sedative, stomachic, antispasmodic, astringent and cancer | Infusion, Cataplasm for head, Ocular drip | [38,39,67] |
| Spain | Cantueso, Cantagueso, Cantigueso | Aerial parts | Digestive problem and fatigue | Infusion | [66] |
| Morocco | Kohyla, Kohayla, Hlihla | Aerial parts | Broncho-pulmonary affections, Rheumatism, chill, digestive system, cough, cold, hepatitis, icterus | Decoction, Infusion, Pow | [41,42,68] |
| Portugal | alfazema-de-folhas-recortadas, Alfazema de folha recortada | Flowering aerial parts, flowers and stems. | bronchitis, asthma, and cough; digestion and bile stimulation; nervousness and dizziness; soporific; carminative; tonic; and hair stimulation | * NI | [34,39] |
| Tunisia | Soltan oud, Kammoun | Leaves, Flowers | Hypotensive, emmenagogue, antidiabetic, and hair perfume | Decoction, Maceration | [65,69] |
| Country of Origin | Plant Part | Used Extract | Analytical Method | Chemical Classes | Identified Compounds | Ref. |
|---|---|---|---|---|---|---|
| Algeria | Aerial parts | Methanol extract | HPLC–DAD | Phenolic acids | Protocatechuic acid, caffeic acid | [76] |
| Flavonoids | Apigenin, rutin, catechin | |||||
| Coumarin | Coumarin | |||||
| Petroleum Ether extract | GC–FID | Fatty acids | Palmitic acid, stearic acid, myristic acid, caprylic acid, dodecanoic acid, pentadecanoic acid, 14-methyl-hexadecanoic acid, margaric acid, nonadecanoic acid, eicosanoic acid, oleic acid, linoleic acid, palmitoleic acid. | |||
| Morocco | Aerial parts | Ethanol extract | HPLC, TLC, RP-HPLC, NMR | Monoterpenes | Carvacrol, Carvacrol-3-glucoside | [77] |
| Diterpenes | 15S,16-dihydroxy-7-oxopimar-8(9)-ene, 15,16,17-trihydroxy-7-oxopimar-8(9)-ene, 15,16-dihydroxy-7,11-dioxopimar-8(9)-ene, 15,16,17-trihydroxypimar-8(9)-ene, 15S,16-dihydroxyisopimar-8(9)-ene | |||||
| Triterpenes | Maslinic acid, Oleanolic acid, Ursolic acid, 3β,19α,23-trihydroxy-urs-12-en-28-oic acid | |||||
| Spain | Aerial parts | Hydrometh- anolic extract | UHPLC-MS | Phenolic acids | Mucic acid lactone gallate, dihydroferulic acid glucuronide, chicoric acid, amurensin | [73] |
| Flavonoids | Hesperetin, quercetin glucoside, quercetin glucuronide, luteolin-7-O-glucoside, kaempferol acetyl-glucopyranoside, isorhamnetin-3-O-glucuronide, isoscutellarin-8-O-glucuronide, rutin, (epi)catechin digallate | |||||
| Triterpenes | Maslinic acid (and isomers), madecassic acid (and isomers), asiatic acid (and isomers), quillaic acid (and isomers), glycyrrhetinic acid, camellenodiol | |||||
| Saponins | Licoricesaponin J2 (or isomer), bryoamaride (or isomer), yunganoside G2 (or isomer), hovenidulcigenin B (or isomer) | |||||
| Fatty acids | Fatty acid (C18H32O3), fatty acid (C18H30O3), fatty acid (C18H30O2), fatty acid (unspecified formulas) | |||||
| Sterols/Steroidal derivatives | Stigmastene dione, hydroxyecdysone monoacetonide | |||||
| Other metabolites | Citreaglycon A, malic acid, | |||||
| Italy | Fresh leaves | Methanolic extract | RP-DAD-HPLC | Monoterpene | Carvacrol | [42] |
| Flavonoids (flavones and derivatives) | Vitexin (apigenin-8-C-glucoside), hypolaetin-7-O-glucoside, scutellarein-7-O-glucoside, luteolin-7-O-glucoside, isoscutellarein-7-O-glucoside, apigenin-7-O-glucoside, chrysoeriol-7-O-glucoside, isoscutellarein-8-O-glucoside, apigenin |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allouani, M.; Hendel, N.; Moutassem, D.; Sarri, M.; Sarri, D.; D’Anneo, A.; Gallo, G.; Palumbo Piccionello, A. Traditional Applications, Phytochemical Constituents, and Pharmacological Properties of Lavandula multifida L.: A Review. Molecules 2025, 30, 3906. https://doi.org/10.3390/molecules30193906
Allouani M, Hendel N, Moutassem D, Sarri M, Sarri D, D’Anneo A, Gallo G, Palumbo Piccionello A. Traditional Applications, Phytochemical Constituents, and Pharmacological Properties of Lavandula multifida L.: A Review. Molecules. 2025; 30(19):3906. https://doi.org/10.3390/molecules30193906
Chicago/Turabian StyleAllouani, Mohammed, Noui Hendel, Dahou Moutassem, Madani Sarri, Djamel Sarri, Antonella D’Anneo, Giuseppe Gallo, and Antonio Palumbo Piccionello. 2025. "Traditional Applications, Phytochemical Constituents, and Pharmacological Properties of Lavandula multifida L.: A Review" Molecules 30, no. 19: 3906. https://doi.org/10.3390/molecules30193906
APA StyleAllouani, M., Hendel, N., Moutassem, D., Sarri, M., Sarri, D., D’Anneo, A., Gallo, G., & Palumbo Piccionello, A. (2025). Traditional Applications, Phytochemical Constituents, and Pharmacological Properties of Lavandula multifida L.: A Review. Molecules, 30(19), 3906. https://doi.org/10.3390/molecules30193906









