Polyamine Induction of Secondary Metabolite Biosynthetic Genes in Fungi Is Mediated by Global Regulator LaeA and α-NAC Transcriptional Coactivator: Connection to Epigenetic Modification of Histones
Abstract
1. Introduction
Historical Overview of the Studies of the Role of Polyamines in Animal and Fungal Cells
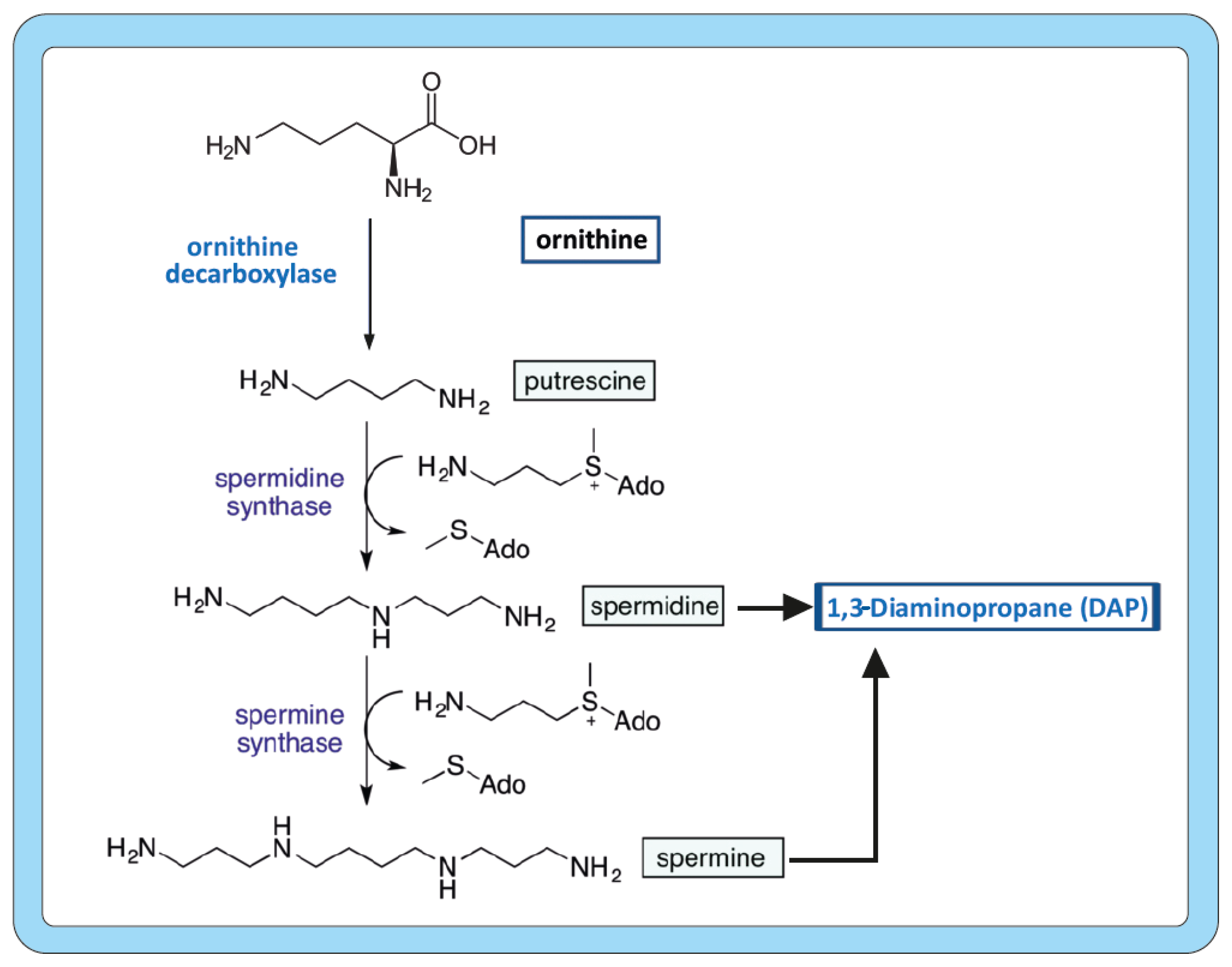
2. Biosynthesis of Polyamines
3. Role of Polyamines in the Biosynthesis of Fungal Secondary Metabolites
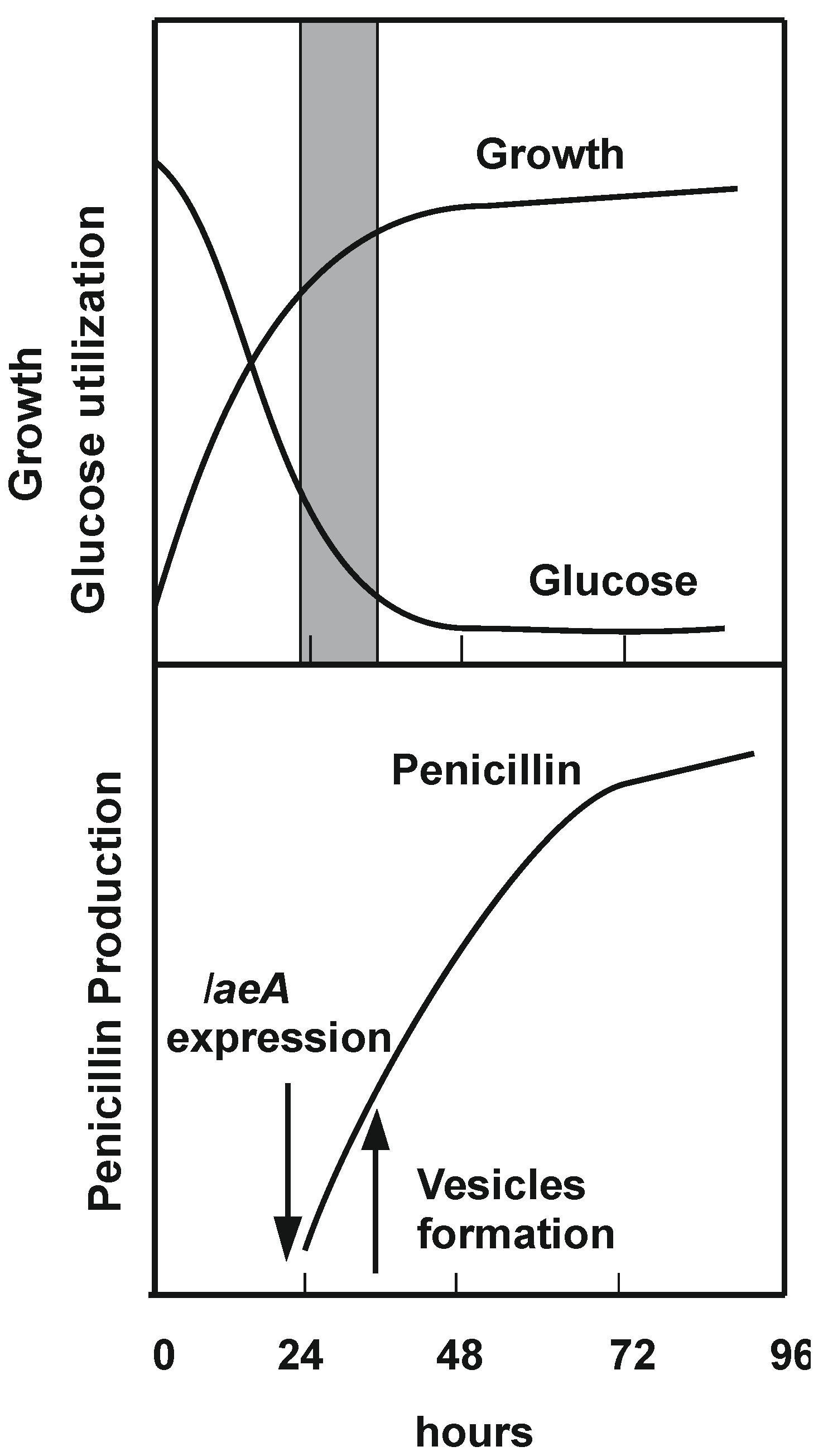
3.1. Induction of Penicillin Gene Expression by Polyamines
3.2. Polyamines Trigger the Biosynthesis of Trichothecenes, Aflatoxins, Lovastatin, Cephalosporin, and Other Secondary Metabolites in Filamentous Fungi
4. Spermidine Increases Yeast Lifespan and Prolongs the Lifetime of Penicillin Gene Transcripts in P. chrysogenum
5. DAP and Spermidine Regulate the LaeA-Mediated Control of Secondary Metabolites
5.1. DAP and Spermidine Positively Regulate Expression of the LaeA Gene in P. chrysogenum
5.2. DAP and Spermidine Simultaneously Regulate LaeA Formation and Secondary Metabolite Biosynthesis in Different Fungi
6. DAP and Spermidine Induce the α-NAC Co-Activator
6.1. The α-NAC Co-Activator in Mammals and Yeasts
6.2. The Transcriptional Co-Activator Function of the Nascent Polypeptide α-NAC Subunit in Different Filamentous Fungi
7. The Metabolic Switch from Growth to Secondary Metabolites’ Production Phase Is Connected to Histone Modifications
8. Heterochromatic Markers Are Associated with Repression of Secondary Metabolism Biosynthesis Genes
9. Conclusions and Future Outlook
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AflR | aflatoxins regulatory protein |
| cAMP | cyclic AMP |
| CoA | coenzyme A |
| CPC | cephalosporin C |
| CRE | cAMP receptor element |
| DAC | Deacetylcephalosporin C |
| DAP | 1,3-diaminopropane |
| dcSAM | decarboxyl-SAM |
| DON | deoxynivalenol |
| EDFG2 | α-NAC component in S. cerevisiae |
| EDFG1 | β-NAC component in S. cerevisiae |
| H3K9 | histone3 lysine9 |
| HAT | histone acetyltransferase |
| HDAC | histone deacetylase |
| HPA1 | Heterochromatin-associated protein 1 |
| HY | high-yield-producing strain |
| laeA | Mutant producing “loss of aflR expression” |
| IAT | isopenicillin N-acyltransferase |
| NAC | nascent polypeptide-associated complex |
| NLS | nuclear localization signal |
| NMR | nuclear magnetic resonance |
| NRPS | Non-ribosomal peptide synthetase |
| PAL | phenylalanine ammonia lyase |
| PKS | polyketide synthase |
| SAM | S-adenosylmethionine |
| SAR | system-acquired resistance |
| SMs | secondary metabolites |
| TBP | TATA-box binding protein |
| TMV | tobacco mosaic virus |
| UBA | ubiquitin-associated domain |
References
- Demain, A.L. Importance of microbial natural products and the need to revitalize their discovery. J. Ind. Microbiol. Biotechnol. 2014, 41, 185–201. [Google Scholar] [CrossRef]
- Martín, J.F.; García-Estrada, C.; Zeilinger, S. (Eds.) Biosynthesis and Molecular Genetics of Fungal Secondary Metabolites; Springer: New York, NY, USA, 2014. [Google Scholar]
- Wang, J.-T.; Shi, T.-T.; Ding, L.; Xie, J.; Zhao, P.-J. Multifunctional Enzymes in Microbial Secondary Metabolic Processes. Catalysts 2023, 13, 581. [Google Scholar] [CrossRef]
- Bergmann, S.; Schümann, J.; Scherlach, K.; Lange, C.; Brakhage, A.A.; Hertweck, C. Genomics-driven discovery of PKS-NRPS hybrid metabolites from Aspergillus nidulans. Nat. Chem. Biol. 2007, 3, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-M.; Szewczyk, E.; Nayak, T.; Davidson, A.D.; Sanchez, J.F.; Lo, H.-C.; Ho, W.-Y.; Simityan, H.; Kuo, E.; Praseuth, A.; et al. Molecular Genetic Mining of the Aspergillus Secondary Metabolome: Discovery of the Emericellamide Biosynthetic Pathway. Chem. Biol. 2008, 15, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A.; Schroeckh, V. Fungal secondary metabolites—Strategies to activate silent gene clusters. Fungal Genet. Biol. 2011, 48, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F.; Liras, P. Novel Antimicrobial and other Bioactive Metabolites obtained from Silent Gene Clusters. In Antibiotics: Current Innovations and Future Trends; Demain, A.L., Sanchez, S., Eds.; Horizon Scientific Press and Caister Academic Press: Norfolk, UK, 2015; pp. 275–292. [Google Scholar]
- Chattopadhyay, M.K.; Park, M.H.; Tabor, H. Hypusine modification for growth is the major function of spermidine in Saccharomyces cerevisiae polyamine auxotrophs grown in limiting spermidine. Proc. Natl. Acad. Sci. USA 2008, 105, 6554–6559. [Google Scholar] [CrossRef]
- Martinez-Rocha, A.L.; Woriedh, M.; Chemnitz, J.; Willingmann, P.; Kröger, C.; Hadeler, B.; Hauber, J.; Schäfer, W. Posttranslational hypusination of the eukaryotic translation initiation factor-5A regulates Fusarium graminearum virulence. Sci. Rep. 2016, 6, 24698. [Google Scholar] [CrossRef]
- Igarashi, K.; Kashiwagi, K. Modulation of protein synthesis by polyamines. IUBMB Life 2015, 67, 160–169. [Google Scholar] [CrossRef]
- Carruthers, L.M.; Marton, L.J.; Peterson, C.L. Polyamine analogues: Potent inducers of nucleosomal array oligomerization and inhibitors of yeast cell growth. Biochem. J. 2007, 405, 541–545. [Google Scholar] [CrossRef]
- Casero, R.A., Jr.; Murray Stewart, T.; Pegg, A.E. Polyamine metabolism and cancer: Treatments, challenges and opportunities. Nat. Rev. Cancer 2018, 18, 681–695. [Google Scholar] [CrossRef]
- Han, M.; Jia, L.; Lv, W.; Wang, L.; Cui, W. Epigenetic Enzyme Mutations: Role in Tumorigenesis and Molecular Inhibitors. Front. Oncol. 2019, 9, 194. [Google Scholar] [CrossRef]
- Morgan, J.E.; Blankenship, J.W.; Matthews, H.R. Polyamines and Acetylpolyamines Increase the stability and Alter the Con-formation of Nucleosome Core Particles. Biochemistry 1987, 26, 3643–3649. [Google Scholar] [CrossRef]
- Pollard, K.J.; Samuels, M.L.; Crowley, K.A.; Hansen, J.C.; Peterson, C.L. Functional interaction between GCN5 and polyamines: A new role for core histone acetylation. EMBO J. 1999, 18, 5622–5633. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.A.; Gilmour, S.K. High levels of intracellular polyamines promote histone acetyltransferase activity resulting in chromatin hyperacetylation. J. Cell. Biochem. 2000, 77, 345–360. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, Y.; Liu, Y.; Li, B.; Tian, S.; Zhang, Z. Research Progress on the Mechanism and Function of Histone Acetylation Regulating the Interaction between Pathogenic Fungi and Plant Hosts. J. Fungi 2024, 10, 522. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Chung, Y.-M.; Wu, Y.-C.; Hunyadi, A.; Wang, C.C.C.; Chang, F.-R. Natural products development under epigenetic modulation in fungi. Phytochem. Rev. 2020, 19, 1323–1340. [Google Scholar] [CrossRef]
- Alejandro-Osorio, A.L.; Huebert, D.J.; Porcaro, D.T.; Sonntag, M.E.; Nillasithanukroh, S.; Will, J.L.; Gasch, A.P. The histone deacetylase Rpd3p is required for transient changes in genomic expression in response to stress. Genome Biol. 2009, 10, R57. [Google Scholar] [CrossRef]
- Hou, X.; Liu, L.; Li, Y.; Wang, P.; Pan, X.; Xu, D.; Lai, D.; Zhou, L. Regulation of Histone Acetylation Modification on Biosynthesis of Secondary Metabolites in Fungi. Int. J. Mol. Sci. 2024, 26, 25. [Google Scholar] [CrossRef]
- Freeman-Cook, L.L.; Sherman, J.M.; Brachmann, C.B.; Allshire, R.C.; Boeke, J.D.; Pillus, L. The Schizosaccharomyces pombe hst4+ gene is a SIR2 homologue with silencing and centromeric functions. Mol. Cell. Biol. 1999, 10, 3171–3186. [Google Scholar] [CrossRef]
- Yi, C.; Ma, M.; Ran, L.; Zheng, J.; Tong, J.; Zhu, J.; Ma, C.; Sun, Y.; Zhang, S.; Feng, W.; et al. Function and Molecular Mechanism of Acetylation in Autophagy Regulation. Science 2012, 336, 474–477. [Google Scholar] [CrossRef] [PubMed]
- Robbins, N.; Leach, M.D.; Cowen, L.E. Lysine Deacetylases Hda1 and Rpd3 Regulate Hsp90 Function thereby Governing Fungal Drug Resistance. Cell Rep. 2012, 2, 878–888. [Google Scholar] [CrossRef]
- Smith, K.M.; Kothe, G.O.; Matsen, C.B.; Khlafallah, T.K.; Adhvaryu, K.K.; Hemphill, M.; Freitag, M.; Motamedi, M.R.; Selker, E.U. The fungus Neurospora crassa displays telomeric silencing mediated by multiple sirtuins and by methylation of histone H3 lysine 9. Epigenetics Chromatin 2008, 1, 5. [Google Scholar] [CrossRef] [PubMed]
- Strauss, J.; Reyes-Dominguez, Y. Regulation of secondary metabolism by chromatin structure and epigenetic codes. Fungal Genet. Biol. 2011, 48, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Pasini, A.; Caldarera, C.M.; Giordano, E. Chromatin remodeling by polyamines and polyamine analogs. Amino Acids 2014, 46, 595–603. [Google Scholar] [CrossRef]
- Kumar, N.; Basundra, R.; Maiti, S. Elevated polyamines induce c-MYC overexpression by perturbing quadruplex–WC duplex equilibrium. Nucleic Acids Res. 2009, 37, 3321–3331. [Google Scholar] [CrossRef] [PubMed]
- Miller-Fleming, L.; Olin-Sandoval, V.; Campbell, K.; Ralser, M. Remaining misteries of Molecular Biology: The role of polyamines in the cell. J. Mol. Biol. 2014, 427, 3389–3406. [Google Scholar] [CrossRef]
- Martín, J.; García-Estrada, C.; Kosalková, K.; Ullán, R.V.; Albillos, S.M.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine produce a drastic increase in the expression of the penicillin biosynthetic genes for prolonged time, mediated by the LaeA regulator. Fungal Genet. Biol. 2012, 49, 1004–1013. [Google Scholar] [CrossRef]
- Rocha, R.O.; Wilson, R.A. Essential, deadly, enigmatic: Polyamine metabolism and roles in fungal cells. Fungal Biol. Rev. 2019, 33, 47–57. [Google Scholar] [CrossRef]
- Sun, W.; Zhang, X.; Chen, D.; Murchie, A.I.H. Interactions between the 5′ UTR mRNA of the spe2 gene and spermidine regulate translation in S. pombe. RNA 2020, 26, 137–149. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Diamine Fungal Inducers of Secondary Metabolism: 1,3-Diaminopropane and Spermidine Trigger Enzymes Involved in β-Alanine and Pantothenic Acid Biosynthesis, Precursors of Phosphopantetheine in the Activation of Multidomain Enzymes. Antibiotics 2024, 13, 826. [Google Scholar] [CrossRef]
- Fierro, F.; García-Estrada, C.; Castillo, N.I.; Rodríguez, R.; Velasco-Conde, T.; Martín, J.-F. Transcriptional and bioinformatic analysis of the 56.8kb DNA region amplified in tandem repeats containing the penicillin gene cluster in Penicillium chrysogenum. Fungal Genet. Biol. 2006, 43, 618–629. [Google Scholar] [CrossRef]
- van den Berg, M.A.; Westerlaken, I.; Leeflang, C.; Kerkman, R.; Bovenberg, R.A. Functional characterization of the penicillin biosynthetic gene cluster of Penicillium chrysogenum Wisconsin54-1255. Fungal Genet. Biol. 2007, 44, 830–844. [Google Scholar] [CrossRef] [PubMed]
- Martín, J.F. Molecular Control of Expression of Penicillin Biosynthesis Genes in Fungi: Regulatory Proteins Interact with a Bidirectional Promoter Region. J. Bacteriol. 2000, 182, 2355–2362. [Google Scholar] [CrossRef] [PubMed]
- Brakhage, A.A. Molecular Regulation of β-Lactam Biosynthesis in Filamentous Fungi. Microbiol. Mol. Biol. Rev. 1998, 62, 547–585. [Google Scholar] [CrossRef]
- Martín, J.; García-Estrada, C.; Rumbero, Á.; Recio, E.; Albillos, S.M.; Ullán, R.V.; Martín, J.-F. Characterization of an Autoinducer of Penicillin Biosynthesis in Penicillium chrysogenum. Appl. Environ. Microbiol. 2011, 77, 5688–5696. [Google Scholar] [CrossRef]
- García-Estrada, C.; Barreiro, C.; Jami, M.-S.; Martín-González, J.; Martín, J.-F. The inducers 1,3-diaminopropane and spermidine cause the reprogramming of metabolism in Penicillium chrysogenum, leading to multiple vesicles and penicillin overproduction. J. Proteom. 2013, 85, 129–159. [Google Scholar] [CrossRef]
- García-Estrada, C.; Ullán, R.V.; Velasco-Conde, T.; Godio, R.P.; Teijeira, F.; Vaca, I.; Feltrer, R.; Kosalková, K.; Mauriz, E.Y.; Martín, J.F. Post-translational enzyme modification by the phosphopantetheinyl transferase is required for lysine and penicillin biosynthesis but not for roquefortine or fatty acid formation in Penicillium chrysogenum. Biochem. J. 2008, 415, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Gardiner, D.M.; Kazan, K.; Praud, S.; Torney, F.J.; Rusu, A.; Manners, J.M. Early activation of wheat polyamine biosynthesis during Fusarium head blight implicates putrescine as an inducer of trichothecene mycotoxin production. BMC Plant Biol. 2010, 10, 289. [Google Scholar] [CrossRef]
- Crespo-Sempere, A.; Marín, S.; Sanchis, V.; Ramos, A.J. VeA and LaeA transcriptional factors regulate ochratoxin A biosynthesis in Aspergillus carbonarius. Int. J. Food Microbiol. 2013, 166, 479–486. [Google Scholar] [CrossRef]
- Majumdar, R.; Lebar, M.; Mack, B.; Minocha, R.; Minocha, S.; Carter-Wientjes, C.; Sickler, C.; Rajasekaran, K.; Cary, J.W. The Aspergillus flavus Spermidine Synthase (spds) Gene, Is Required for Normal Development, Aflatoxin Production, and Pathogenesis During Infection of Maize Kernels. Front. Plant Sci. 2018, 9, 317. [Google Scholar] [CrossRef]
- Kennedy, J.; Auclair, K.; Kendrew, S.G.; Park, C.; Vederas, J.C.; Hutchinson, C.R. Modulation of Polyketide Synthase Activity by Accessory Proteins During Lovastatin Biosynthesis. Science 1999, 284, 1368–1372. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Nuraeva, G.K.; Eldarov, M.A. The Role of LaeA and LovE Regulators in Lovastatin Biosynthesis with Exogenous Polyamines in Aspergillus terreus. Appl. Biochem. Microbiol. 2019, 55, 639–648. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Nuraeva, G.K.; Dumina, M.V.; Voinova, T.M.; Dzhavakhiya, V.V.; Eldarov, M.A. 1,3-Diaminopropane and Spermidine Upregulate Lovastatin Production and Expression of Lovastatin Biosynthetic Genes in Aspergillus terreus via LaeA Regulation. Appl. Biochem. Microbiol. 2019, 55, 243–254. [Google Scholar] [CrossRef]
- Huang, X.; Li, H.-M. Cloning and bioinformatic analysis of lovastatin biosynthesis regulatory gene lovE. Chin. Med. J. 2009, 122, 1800–1805. [Google Scholar]
- García-Estrada, C.; Domínguez-Santos, R.; Kosalková, K.; Martín, J.-F. Transcription Factors Controlling Primary and Secondary Metabolism in Filamentous Fungi. Fermentation 2018, 4, 47. [Google Scholar] [CrossRef]
- Zhgun, A.A.; Eldarov, M.A. Spermidine and 1,3-Diaminopropane Have Opposite Effects on the Final Stage of Cephalosporin C Biosynthesis in High-Yielding Acremonium chrysogenum Strain. Int. J. Mol. Sci. 2022, 23, 14625. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez, S.; Velasco, J.; Marcos, A.T.; Fernández, F.J.; Fierro, F.; Barredo, J.L.; Díez, B.; Martín, J.F. Expression of the cefG gene is limiting for cephalosporin biosynthesis in Acremonium chrysogenum. Appl. Microbiol. Biotechnol. 1997, 48, 606–614. [Google Scholar] [CrossRef] [PubMed]
- Boubakri, H.; Seghezzi, N.; Duchateau, M.; Gominet, M.; Kofroňová, O.; Benada, O.; Mazodier, P.; Pernodet, J.-L. The Absence of Pupylation (Prokaryotic Ubiquitin-Like Protein Modification) Affects Morphological and Physiological Differentiation in Streptomyces coelicolor. J. Bacteriol. 2015, 197, 3388–3399. [Google Scholar] [CrossRef]
- Compton, C.L.; Fernandopulle, M.S.; Nagari, R.T.; Sello, J.K. Genetic and proteomic analyses of pupylation in Streptomyces coelicolor. J. Bacteriol. 2015, 197, 2747–2753. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P.; Sánchez, S. Modulation of Gene Expression in Actinobacteria by Translational Modification of Transcriptional Factors and Secondary Metabolite Biosynthetic Enzymes. Front. Microbiol. 2021, 12, 630694. [Google Scholar] [CrossRef]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Büttner, S.; Ruckenstuhl, C.; Kroemer, G. Spermidine: A novel autophagy inducer and longevity elixir. Autophagy 2010, 6, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Madeo, F.; Bauer, M.A.; Carmona-Gutierrez, D.; Kroemer, G. Spermidine: A physiological autophagy inducer acting as an anti-aging vitamin in humans? Autophagy 2019, 15, 165–168. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Targeting of Specialized Metabolites Biosynthetic Enzymes to Membranes and Vesicles by Posttranslational Palmitoylation: A Mechanism of Non-Conventional Traffic and Secretion of Fungal Metabolites. Int. J. Mol. Sci. 2024, 25, 1224. [Google Scholar] [CrossRef]
- Campos, C.; Lázaro-Rodríguez, T.G.; Fragoso-Soriano, R.; Fernández, F.J. Vesicular transport and secretion of penicillin G in Penicillium rubens P2-32-T. Arch. Microbiol. 2020, 202, 1257–1262. [Google Scholar] [CrossRef]
- Chanda, A.; Roze, L.V.; Kang, S.; Artymovich, K.A.; Hicks, G.R.; Raikhel, N.V.; Calvo, A.M.; Linz, J.E. A key role for vesicles in fungal secondary metabolism. Proc. Natl. Acad. Sci. USA 2009, 106, 19533–19538. [Google Scholar] [CrossRef] [PubMed]
- Linz, J.E.; Chanda, A.; Hong, S.-Y.; Whitten, D.A.; Wilkerson, C.; Roze, L.V. Proteomic and Biochemical Evidence Support a Role for Transport Vesicles and Endosomes in Stress Response and Secondary Metabolism in Aspergillus parasiticus. J. Proteome Res. 2012, 11, 767–775. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.Y.; Linz, J.E. Functional expression and sub-cellular localization of the aflatoxin pathway enzyme Ver-1 fused to en-hanced green fluorescent protein. Appl. Env. Microbiol. 2008, 74, 6385–6396. [Google Scholar] [CrossRef]
- Bok, J.W.; Keller, N.P. LaeA, a regulator of secondary metabolism in Aspergillus spp. Eukaryot. Cell 2004, 3, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Bayram, O.; Krappmann, S.; Ni, M.; Bok, J.; Helmstaedt, K.; Valerius, O.; Braus-Stromeyer, S.; Kwon, N.J.; Keller, N.P.; Yu, J.H.; et al. VelB/VeA/LaeA complex coordinates light signal with fungal development and secondary metabolism. Science 2008, 320, 1504–1506. [Google Scholar] [CrossRef]
- Kosalková, K.; García-Estrada, C.; Ullán, R.V.; Godio, R.P.; Feltrer, R.; Teijeira, F.; Mauriz, E.; Martín, J.F. The global regulator LaeA controls penicillin biosynthesis, pigmentation and sporulation, but not roquefortine C synthesis in Penicillium chrysogenum. Biochimie 2009, 91, 214–225. [Google Scholar] [CrossRef]
- Fierro, F.; Barredo, J.L.; Díez, B.; Gutierrez, S.; Fernández, F.J.; Martín, J.F. The penicillin gene cluster is amplified in tandem repeats linked by conserved hexanucleotide sequences. Proc. Natl. Acad. Sci. USA 1995, 92, 6200–6204. [Google Scholar] [CrossRef] [PubMed]
- Hoff, B.; Kamerewerd, J.; Sigl, C.; Mitterbauer, R.; Zadra, I.; Kürnsteiner, H.; Kück, U. Two Components of a velvet -Like Complex Control Hyphal Morphogenesis, Conidiophore Development, and Penicillin Biosynthesis in Penicillium chrysogenum. Eukaryot. Cell 2010, 9, 1236–1250. [Google Scholar] [CrossRef]
- Martín, J.F. Key role of LaeA and velvet complex proteins on expression of β-lactam and PR-toxin genes in Penicillium chrysogenum: Cross-talk regulation of secondary metabolite pathways. J. Ind. Microbiol. Biotechnol. 2016, 44, 525–535. [Google Scholar] [CrossRef]
- Patananan, A.N.; Palmer, J.M.; Garvey, G.S.; Nancy, P.; Keller, N.P.; Clarke, S.G. A Novel Automethylation Reaction in the Aspergillus nidulans LaeA Protein Generates S-Methylmethionine. J. Biol. Chem. 2013, 288, 14032–14045. [Google Scholar] [CrossRef]
- Kadooka, C.; Nakamura, E.; Mori, K.; Okutsu, K.; Yoshizaki, Y.; Takamine, K.; Goto, M.; Tamaki, H.; Futagami, T. LaeA Controls Citric Acid Production through Regulation of the Citrate Exporter-Encoding cexA Gene in Aspergillus luchuensis mut. kawachii. Appl. Environ. Microbiol. 2020, 86, e01950-19. [Google Scholar] [CrossRef]
- Wiedmann, B.; Sakai, H.; Davis, T.A.; Wiedmann, M. A protein complex required for signal-sequence-specific sorting and translocation. Nature 1994, 370, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Yotov, W.V.; Moreau, A.; St-Arnaud, R. The alpha chain of the nascent polypeptide-associated complex functions as a tran-scriptional coactivator. Mol. Cell Biol. 1998, 18, 1303–1311. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, X.; Liu, Q.; Qiu, D.; Zhang, Y.; Zeng, H.; Yuan, J.; Mao, J. Purification of novel protein elicitor from Botrytis cinerea that induces disease resistance and drought tolerance in plants. Microbiol. Res. 2010, 165, 142–151. [Google Scholar] [CrossRef]
- Li, X.; Guo, M.; Xu, D.; Chen, F.; Zhang, H.; Pan, Y.; Li, M.; Gao, Z. The nascent-polypeptide-associated complex alpha subunit regulates the polygalacturonases expression negatively and influences the pathogenicity of Sclerotinia sclerotiorum. Mycologia 2015, 107, 1130–1137. [Google Scholar] [CrossRef]
- Van Dyke, N.; Baby, J.; Van Dyke, M.W. Stm1p, a ribosome-associated protein, is important for protein synthesis in Saccharomyces cerevisiae under nutritional stress conditions. J. Mol. Biol. 2006, 358, 1023–1031. [Google Scholar] [CrossRef]
- Spreter, T.; Pech, M.; Beatrix, B. The Crystal Structure of Archaeal Nascent Polypeptide-associated Complex (NAC) Reveals a Unique Fold and the Presence of a Ubiquitin-associated Domain. J. Biol. Chem. 2005, 280, 15849–15854. [Google Scholar] [CrossRef]
- Gamerdinger, M.; Kobayashi, K.; Wallisch, A.; Kreft, S.G.; Sailer, C.; Schlömer, R.; Sachs, N.; Jomaa, A.; Stengel, F.; Ban, N.; et al. Early Scanning of Nascent Polypeptides inside the Ribosomal Tunnel by NAC. Mol. Cell 2019, 75, 996–1006.e8. [Google Scholar] [CrossRef] [PubMed]
- Jomaa, A.; Gamerdinger, M.; Hsieh, H.H.; Wallisch, A.; Chandrasekaran, V.; Ulusoy, Z.; Scaiola, A.; Hegde, R.S.; Shan, S.O.; Ban, N.; et al. Mechanism of signal sequence handover from NAC to SRP on ribosomes during ER-protein targeting. Science 2022, 375, 839–844. [Google Scholar] [CrossRef]
- Andersen, K.M.; Hofmann, K.; Hartmann-Petersen, R. Ubiquitin binding proteins: Similar, but different. Essays Biochem. 2005, 41, 49–67. [Google Scholar] [CrossRef]
- Quélo, I.; Akhouayri, O.; Prud’HOmme, J.; St-Arnaud, R. GSK3β-Dependent Phosphorylation of the αNAC Coactivator Regulates Its Nuclear Translocation and Proteasome-Mediated Degradation. Biochemistry 2004, 43, 2906–2914. [Google Scholar] [CrossRef]
- Franke, J.; Reimann, B.; Hartmann, E.; Köhlerl, M.; Wiedmann, B. Evidence for a nuclear passage of nascent polypep-tide-associated complex subunits in yeast. J. Cell Sci. 2001, 114, 2641–2648. [Google Scholar] [CrossRef] [PubMed]
- Al-Shanti, N.; Aldahoodi, Z. Inhibition of Alpha Nascent Polypeptide Associated Complex Protein May Induce Proliferation, Differentiation and Enhance the Cytotoxic Activity of Human CD8+ T Cells. J. Clin. Immunol. 2006, 26, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Alamo, M.; del Hogan, D.J.; Pechmann, S.; Albanese, V.; Brown, P.O.; Frydman, J. Defining the specificity of co-translationally acting chaperones by systematic analysis of mRNAs associated with ribosome-nascent chain complexes. PLoS Biol. 2011, 9, e1001100. [Google Scholar] [CrossRef]
- Ott, A.-K.; Locher, L.; Koch, M.; Deuerling, E. Functional Dissection of the Nascent Polypeptide-Associated Complex in Sac-charomyces cerevisiae. PLoS ONE 2015, 10, e0143457. [Google Scholar] [CrossRef]
- Panasenko, O.; Landrieux, E.; Feuermann, M.; Finka, A.; Paquet, N.; Collart, M.A. The yeast Ccr4-Not complex controls ubiq-uitination of the nascent-associated polypeptide (NAC-EGD) complex. J. Biol. Chem. 2006, 281, 31389–31398. [Google Scholar] [CrossRef]
- Chen, J.J.; Stermer, D.; Tanny, J.C. Decoding histone ubiquitylation. Front. Cell Dev. Biol. 2022, 10, 968398. [Google Scholar] [CrossRef] [PubMed]
- Lenssen, E.; James, N.; Pedruzzi, I.; Dubouloz, F.; Cameroni, E.; Bisig, R.; Maillet, L.; Werner, M.; Roosen, J.; Petrovic, K.; et al. The Ccr4-Not Complex Independently Controls both Msn2-Dependent Transcriptional Activation—via a Newly Identified Glc7/Bud14 Type I Protein Phosphatase Module—and TFIID Promoter Distribution. Mol. Cell. Biol. 2005, 25, 488–498. [Google Scholar] [CrossRef]
- Laribee, R.N. Ccr4-Not as a mediator of environmental signaling: A jack of all trades and master of all. Curr. Genet. 2021, 67, 707–713. [Google Scholar] [CrossRef] [PubMed]
- Collart, M.A.; Audebert, L.; Bushell, M. Roles of the CCR4-Not complex in translation and dynamics of co-translation events. Wiley Interdiscip. Rev. RNA 2023, 15, e1827. [Google Scholar] [CrossRef]
- Schilke, B.A.; Ziegelhoffer, T.; Domanski, P.; Marszalek, J.; Tomiczek, B.; Craig, E.A. Functional similarities and differences among subunits of the nascent polypeptide-associated complex (NAC) of Saccharomyces cerevisiae. Cell Stress Chaperon- 2024, 29, 721–734. [Google Scholar] [CrossRef]
- Andersen, M.; Semple, C.A.; Hartmann-Petersen, R.K. Characterization of the nascent polypeptide-associated complex in fission yeast. Mol. Biol. Rep. 2007, 34, 275–281. [Google Scholar] [CrossRef]
- Wang, P.-H.; Wu, P.-C.; Huang, R.; Chung, K.-R. The Role of a Nascent Polypeptide-Associated Complex Subunit Alpha in Siderophore Biosynthesis, Oxidative Stress Response, and Virulence in Alternaria alternata. Mol. Plant-Microbe Interact. 2020, 33, 668–679. [Google Scholar] [CrossRef]
- Lin, T.-C.; Hshie, T.Y.; Lin, C.-L.; Chung, W.-C.; Chung, K.-R.; Huang, J.-W. Pathogenic fungal protein-induced resistance and its effects on vegetable diseases. J. Agric. Sci. 2017, 155, 1069–1081. [Google Scholar] [CrossRef]
- Zhang, W.; Yang, X.; Qiu, D.; Guo, L.; Zeng, H.; Mao, J.; Gao, Q. PeaT1-induced systemic acquired resistance in tobacco follows salicylic acid-dependent pathway. Mol. Biol. Rep. 2011, 8, 2549–2556. [Google Scholar] [CrossRef]
- Cánovas, D.; Marcos, A.T.; Gacek, A.; Ramos, M.S.; Gutiérrez, G.; Reyes-Domínguez, Y.; Strauss, J. The Histone Acetyltransferase GcnE (GCN5) Plays a Central Role in the Regulation of Aspergillus Asexual Development. Genetics 2014, 197, 1175–1189. [Google Scholar] [CrossRef]
- Garcia-Brugger, A.; Lamotte, O.; Vandelle, E.; Bourque, S.; Lecourieux, D.; Poinssot, B.; Wendehenne, D.; Pugin, A. Early Signaling Events Induced by Elicitors of Plant Defenses. Mol. Plant-Microbe Interact. 2006, 19, 711–724. [Google Scholar] [CrossRef]
- Martín, J.F.; Liras, P. Comparative Molecular Mechanisms of Biosynthesis of Naringenin and Related Chalcones in Actinobacteria and Plants: Relevance for the Obtention of Potent Bioactive Metabolites. Antibiotics 2022, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Yue, L.; Muhammad, H.; Stephen, E.; Nat, N. Developmentally induced changes in the sclerotial proteome of Sclerotinia sclerotiorum. Fungi Biol. 2010, 114, 619–627. [Google Scholar]
- Yu, Y.; Jiang, D.; Xie, J.; Cheng, J.; Li, G.; Yi, X.; Fu, Y. Ss-Sl2, a Novel Cell Wall Protein with PAN Modules, Is Essential for Sclerotial Development and Cellular Integrity of Sclerotinia sclerotiorum. PLOS ONE 2012, 7, e34962. [Google Scholar] [CrossRef]
- Chen, C.; Arye, H.; Rena, G.; Oded, Y.; Martin, B.D. MAPK regulation of sclerotial development in Sclerotinia sclerotiorum is linked with pH and CAMP sensing. Mol. Plant-Microb. Interact. 2004, 17, 404–413. [Google Scholar] [CrossRef]
- Calvo, A.M.; Cary, J.W. Association of fungal secondary metabolism and sclerotial biology. Front. Microbiol. 2015, 6, 62. [Google Scholar] [CrossRef]
- Takaoka, S.; Kurata, M.; Harimoto, Y.; Hatta, R.; Yamamoto, M.; Akimitsu, K.; Tsuge, T. Complex regulation of secondary metabolism controlling pathogenicity in the phytopathogenic fungus Alternaria alternata. New Phytol. 2014, 202, 1297–1309. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, Y.; Masunaka, A.; Tsuge, T.; Yamamoto, M.; Ohtani, K.; Fukumoto, T.; Gomi, K.; Peever, T.L.; Tada, Y.; Ichimura, K.; et al. ACTTS3 Encoding a Polyketide Synthase Is Essential for the Biosynthesis of ACT-Toxin and Pathogenicity in the Tangerine Pathotype of Alternaria alternata. Mol. Plant-Microbe Interact. 2010, 23, 406–414. [Google Scholar] [CrossRef]
- Li, L.; Ma, H.; Zheng, F.; Chen, Y.; Wang, M.; Jiao, C.; Li, H.; Gai, Y. The transcription regulator ACTR controls ACT-toxin biosynthesis and pathogenicity in the tangerine pathotype of Alternaria alternata. Microbiol. Res. 2021, 248, 126747. [Google Scholar] [CrossRef] [PubMed]
- Haas, H.; Eisendle, M.; Turgeon, B.G. Siderophores in Fungal Physiology and Virulence. Annu. Rev. Phytopathol. 2008, 46, 149–187. [Google Scholar] [CrossRef]
- Liras, P.; Martín, J.F. Interconnected Set of Enzymes Provide Lysine Biosynthetic Intermediates and Ornithine Derivatives as Key Precursors for the Biosynthesis of Bioactive Secondary Metabolites. Antibiotics 2023, 12, 159. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.H.; Lin, C.H.; Chung, K.R. A nonribosomal peptide synthetase mediates siderophore production and virulence in the citrus fungal pathogen Alternaria alternata. Mol. Plant Pathol. 2013, 14, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Yang, S.L.; Chung, K.-R. Resistance to oxidative stress via regulating siderophore-mediated iron acquisition by the citrus fungal pathogen Alternaria alternata. Microbiology 2014, 160, 970–979. [Google Scholar] [CrossRef]
- Chung, K.-R. Reactive oxygen species in the citrus fungal pathogen Alternaria alternata: The roles of NADPH-dependent oxidase. Physiol. Mol. Plant Pathol. 2014, 88, 10–17. [Google Scholar] [CrossRef]
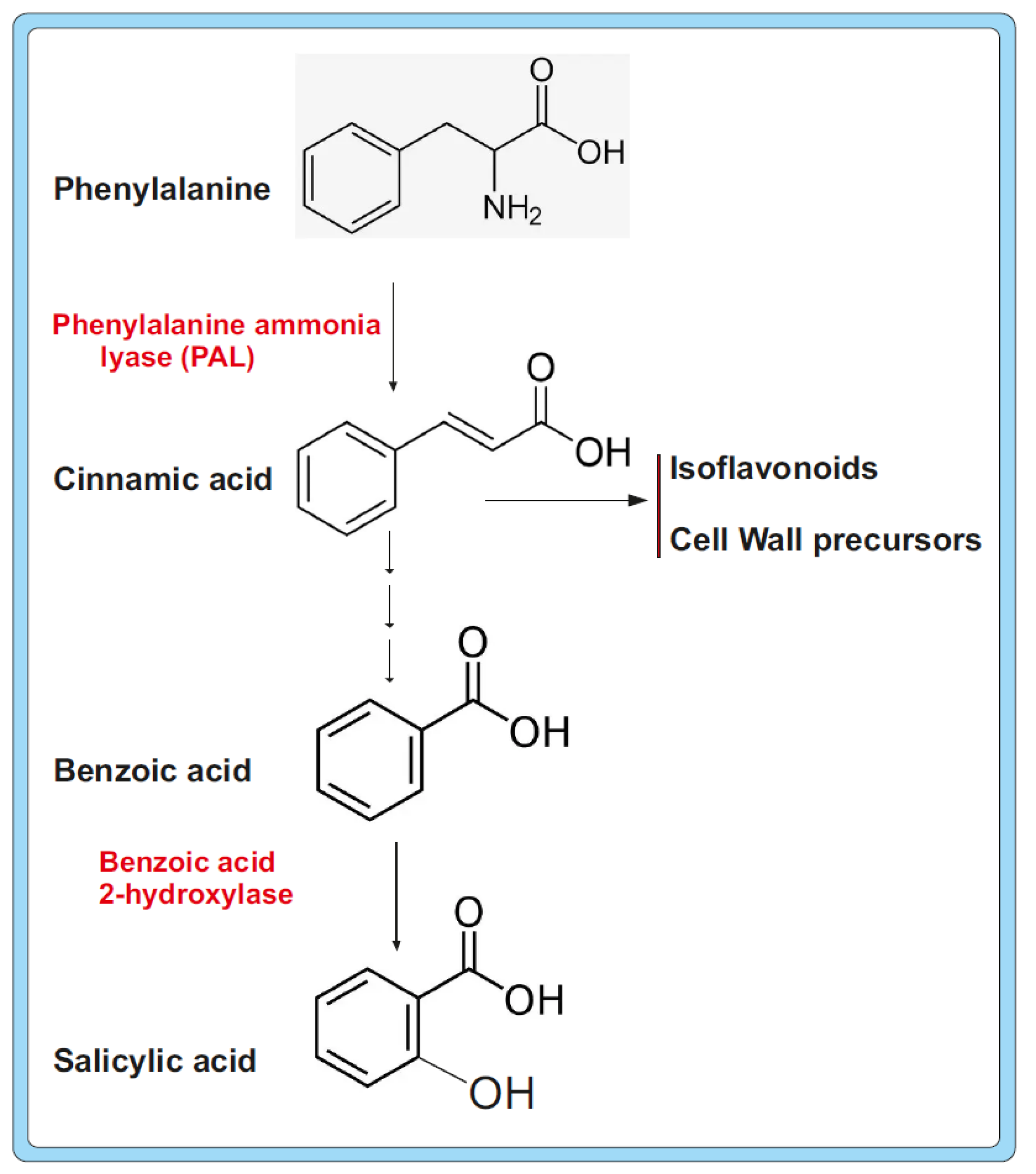
- Dempsey, D.A.; Shah, J.; Klessig, D.F. Salicylic Acid and Disease Resistance in Plants. Crit. Rev. Plant Sci. 1999, 18, 547–575. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Slusarenko, A.J. Production of salicylic acid precursors is a major function of phenylalanine ammonialyase in the resistance of Arabidopsis to Peronospora parasitica. Plant Cell 1996, 8, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Roze, L.V.; Chanda, A.; Linz, J.E. Compartmentalization and molecular traffic in secondary metabolism: A new understanding of established cellular processes. Fungal Genet. Biol. 2011, 48, 35–48. [Google Scholar] [CrossRef]
- Roze, L.V.; Arthur, A.E.; Hong, S.Y.; Chanda, A.; Linz, J.E. The initiation and pattern of spread of histone H4 acetylation parallel the order of transcriptional activation of genes in the aflatoxin cluster. Mol. Microbiol. 2007, 66, 713–726. [Google Scholar] [CrossRef]
- Roze, L.V.; Miller, M.J.; Rarick, M.; Mahanti, N.; Linz, J.E. A Novel cAMP-response Element, CRE1, Modulates Expression of nor-1 in Aspergillus parasiticus. J. Biol. Chem. 2004, 279, 27428–27439. [Google Scholar] [CrossRef]
- Miller, M.J.; Roze, L.V.; Trail, F.; Linz, J.E. Role of cis-acting sites NorL, a TATA box, and AflR1 in nor-1transcriptional activation in Aspergillus parasiticus. Appl. Environ. Microbiol. 2005, 71, 1539–1545. [Google Scholar] [CrossRef] [PubMed]
- Shahbazian, M.D.; Grunstein, M. Functions of Site-Specific Histone Acetylation and Deacetylation. Annu. Rev. Biochem. 2007, 76, 75–100. [Google Scholar] [CrossRef]
- Reyes-Dominguez, Y.; Bok, J.W.; Berger, H.; Shwab, E.K.; Basheer, A.; Gallmetzer, A.; Scazzocchio, C.; Keller, N.; Strauss, J. Heterochromatic marks are associated with the repression of secondary metabolism clusters in Aspergillus nidulans. J. Mol. Microbiol. 2010, 76, 1376–1386. [Google Scholar] [CrossRef]
- Toghueo, R.M.K.; Sahal, D.; Boyom, F.F. Recent advances in inducing endophytic fungal specialized metabolites using small molecule elicitors including epigenetic modifiers. Phytochemistry 2020, 174, 112338. [Google Scholar] [CrossRef]
- Xue, M.; Hou, X.; Fu, J.; Zhang, J.; Wang, J.; Zhao, Z.; Xu, D.; Lai, D.; Zhou, L. Recent Advances in Search of Bioactive Secondary Metabolites from Fungi Triggered by Chemical Epigenetic Modifiers. J. Fungi 2023, 9, 172. [Google Scholar] [CrossRef]
- Okada, B.K.; Seyedsayamdost, M.R. Antibiotic dialogues: Induction of silent biosynthetic gene clusters by exogenous small molecules. FEMS Microbiol. Rev. 2017, 41, 19–33. [Google Scholar] [CrossRef]
- Bind, S.; Bind, S.; Sharma, A.K.; Chaturvedi, P. Epigenetic Modification: A Key Tool for Secondary Metabolite Production in Microorganisms. Front. Microbiol. 2022, 13, 784109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, J.; Wei, C.; Deng, X.; Xu, J. Chemical epigenetic modifiers enhance the production of immunosuppressants from the endophytic fungus Aspergillus fumigatus isolated from Cynodon dactylon. J. Nat. Prod. Res. 2022, 36, 4481–4485. [Google Scholar] [CrossRef]
- Guzman-Chavez, F.; Salo, O.; Samol, M.; Ries, M.; Kuipers, J.; Bovenberg, R.A.L.; Vreeken, R.J.; Driessen, A.J.M. Deregulation of secondary metabolism in a histone deacetylase mutant of Penicillium chrysogenum. Microbiologyopen 2018, 7, e00598. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.W.; Zhou, H.C.; Zhang, P.; Wang, X.; Li, W.; Zhang, W.; Liu, X.; Liu, H.-W.; Keller, N.P.; An, Z.; et al. Polyketide Production of Pestaloficiols and Macrodiolide Ficiolides Revealed by Manipulations of Epigenetic Regulators in an Endophytic Fungus. Org. Lett. 2016, 18, 1832–1835. [Google Scholar] [CrossRef]
- Mao, X.M.; Xu, W.; Li, D.; Yin, W.B.; Chooi, Y.H.; Li, Y.Q.; Tang, Y.; Hu, Y. Epigenetic Genome Mining of an Endophytic Fungus Leads to the Pleiotropic Biosynthesis of Natural Products. Angew. Chem. Int. Ed. Engl. 2015, 54, 7592–7596. [Google Scholar] [CrossRef] [PubMed]
- Lind, A.L.; Lim, F.Y.; Soukup, A.A.; Keller, N.P.; Rokas, A. An LaeA- and BrlA-Dependent Cellular Network Governs Tissue-Specific Secondary Metabolism in the Human Pathogen Aspergillus fumigatus. mSphere 2018, 3, e00050-18. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-H.; Lin, C.-H.; Chung, K.-R. Roles for SKN7 response regulator in stress resistance, conidiation and virulence in the citrus pathogen Alternaria alternata. Fungal Genet. Biol. 2012, 49, 802–813. [Google Scholar] [CrossRef] [PubMed]
| Origin | Sequence | α-NAC a.a. | GeneBank Access |
|---|---|---|---|
| Mus musculum | 70SRSEKKARK78 | 215 | AAB80961 |
| Penicillium chrysogenum | 45SRGEKKARK52 | 203 | CAP86656 |
| Aspergillus nidulans | 46SRNEKKARK54 | 203 | Q5AYK0 |
| Sclerotinia sclerotiorum | 52SRNEKKARK60 | 214 | A7EIZ1 |
| Alternaria tenuissima | 48SRNEKKARK56 | 207 | ABM54184 |
| Botrytis cinerea | 51SRNEKKARK59 | 212 | A6SB28 |
| Saccharomyces cerevisiae | 14NKNEKKARE22 | 174 | P38879 |
| Fungi/Yeasts | Amino Acids Number | Characteristics | References |
|---|---|---|---|
| Saccharomyces cerevisiae | 174 | Ubiquitin binding domain (UBA) in the C-terminal region | [81,82,88] |
| Schizosaccharomyces pombe | 174 | UBA in the C-terminal region. α-NAC mutants are resistant to the arginine analogue canavanine | [89] |
| Penicillium chrysogenum | 203 | Nac motif that allows formation of the α-β heterodimer. UBA at the C-terminal end | [38], This article |
| Botrytis cinerea | 212 | Triggers germination of wheat seeds and growth of the germline Protects wheat against draught Increases drastically the phenylalanine ammonia lyase (PAL) activity involved in flavin and isoflavonoids biosynthesis and in lignin precursor formation | [71] |
| Sclerotinia sclerotiorum | 214 | α-NAC silencing results in slow maturation of sclerotia and increases plant-cell-wall-degrading enzymes | [72] |
| Alternaria alternata | 208 | Contains the UBA and α-Nac domains α-NAC is required for biosynthesis of the dimethylcoprogen siderophore, synthesized by the NRPS np6 Siderophore-mediated iron acquisition plays an important role in ROS formation. α-NAC mutants are unable to detoxify ROS α-NAC is required for conidia development, plant pathogenicity, and resistance to osmotic stress factors | [90,91] |
| Alternaria tenuissima | 207 | Confers systemic acquired resistance (SAR) in infected plants 1 Protect tobacco plants against tobacco mosaic virus (TMV) | [92,93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, J.F. Polyamine Induction of Secondary Metabolite Biosynthetic Genes in Fungi Is Mediated by Global Regulator LaeA and α-NAC Transcriptional Coactivator: Connection to Epigenetic Modification of Histones. Molecules 2025, 30, 3903. https://doi.org/10.3390/molecules30193903
Martín JF. Polyamine Induction of Secondary Metabolite Biosynthetic Genes in Fungi Is Mediated by Global Regulator LaeA and α-NAC Transcriptional Coactivator: Connection to Epigenetic Modification of Histones. Molecules. 2025; 30(19):3903. https://doi.org/10.3390/molecules30193903
Chicago/Turabian StyleMartín, Juan F. 2025. "Polyamine Induction of Secondary Metabolite Biosynthetic Genes in Fungi Is Mediated by Global Regulator LaeA and α-NAC Transcriptional Coactivator: Connection to Epigenetic Modification of Histones" Molecules 30, no. 19: 3903. https://doi.org/10.3390/molecules30193903
APA StyleMartín, J. F. (2025). Polyamine Induction of Secondary Metabolite Biosynthetic Genes in Fungi Is Mediated by Global Regulator LaeA and α-NAC Transcriptional Coactivator: Connection to Epigenetic Modification of Histones. Molecules, 30(19), 3903. https://doi.org/10.3390/molecules30193903





