Spray-Drying Microencapsulation of Artemisia herba-alba Phenolic Extract: Physicochemical Properties, Structural Characterization, and Bioactivity
Abstract
1. Introduction
2. Results and Discussion
2.1. Phenolic Content
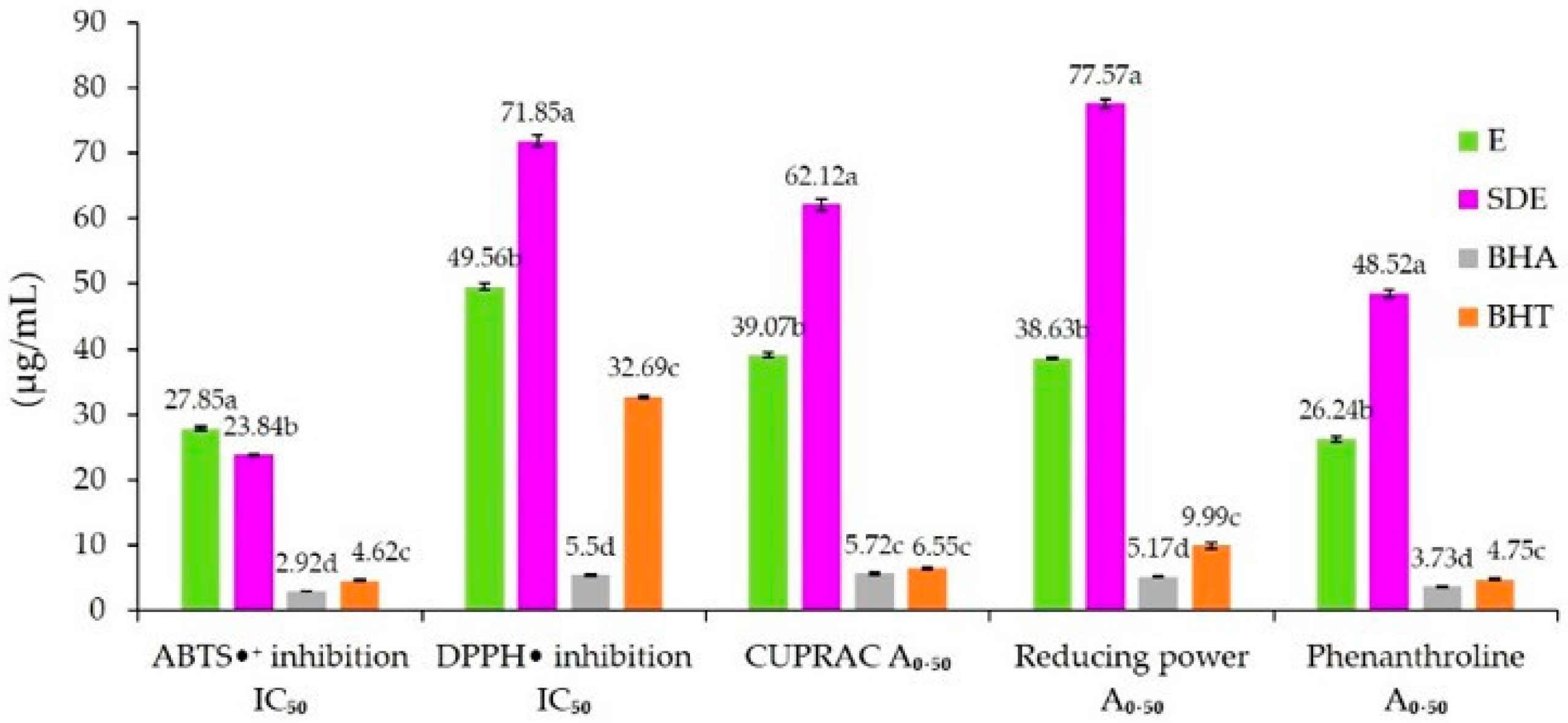
2.2. Antioxidant Activity
2.3. Antibacterial Activity
2.4. Physicochemical Characterizations of Powder
2.4.1. Encapsulation Yield
2.4.2. Encapsulation Efficiency
2.4.3. Moisture Content
2.4.4. Water Activity
2.4.5. Hygroscopicity
2.4.6. Particle Size
2.5. Structural Characterizations of Powder
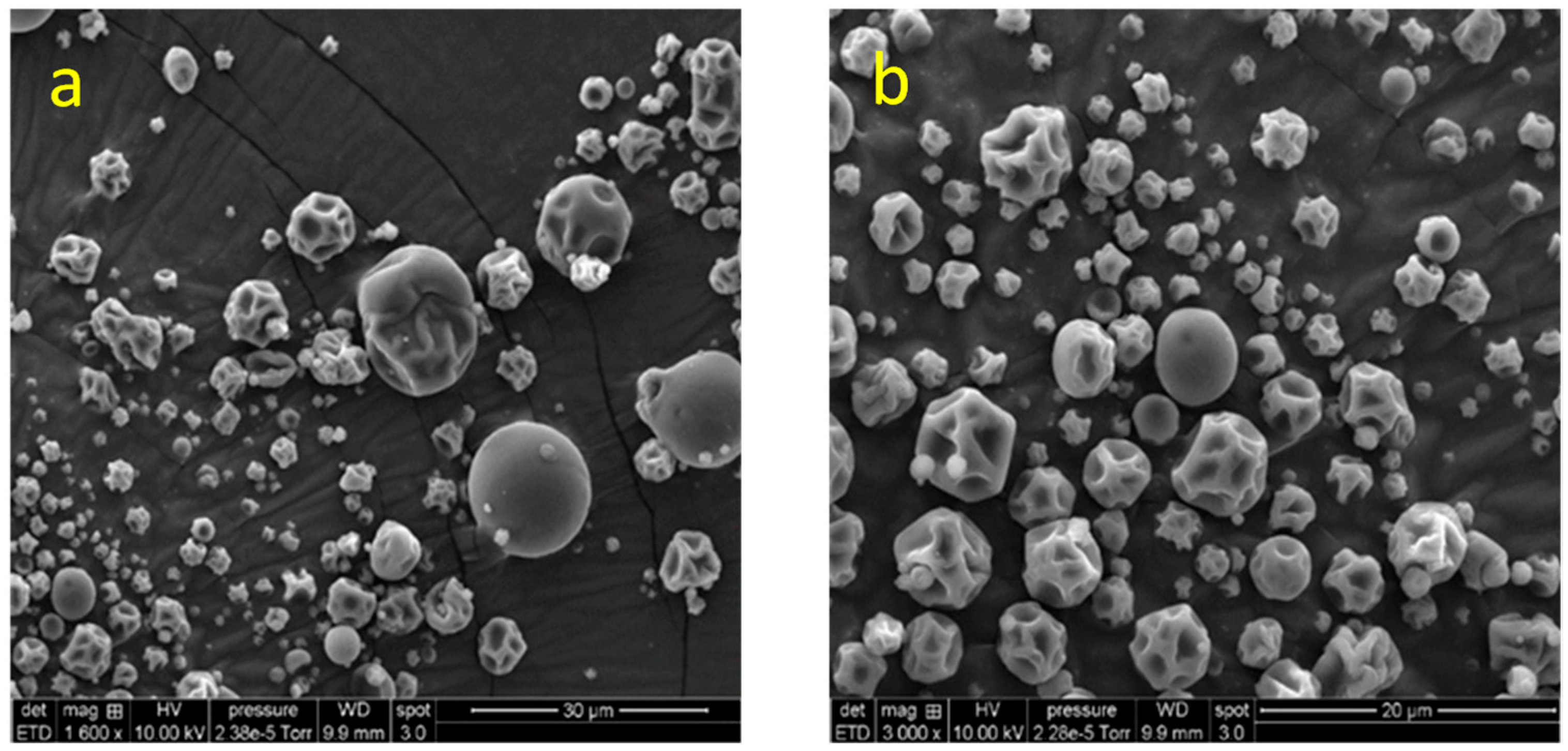
2.5.1. Morphology
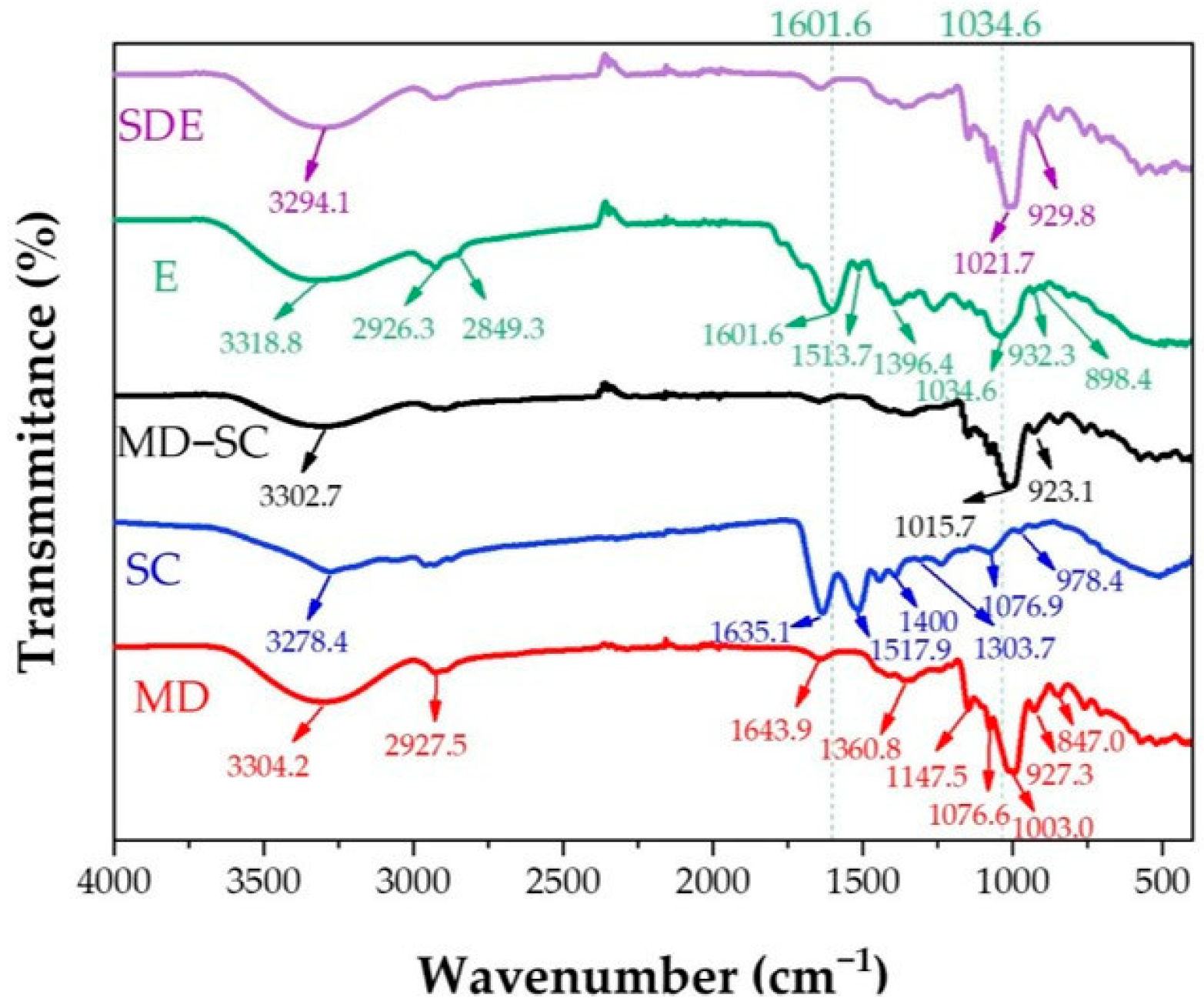
2.5.2. ATR-FTIR Analysis
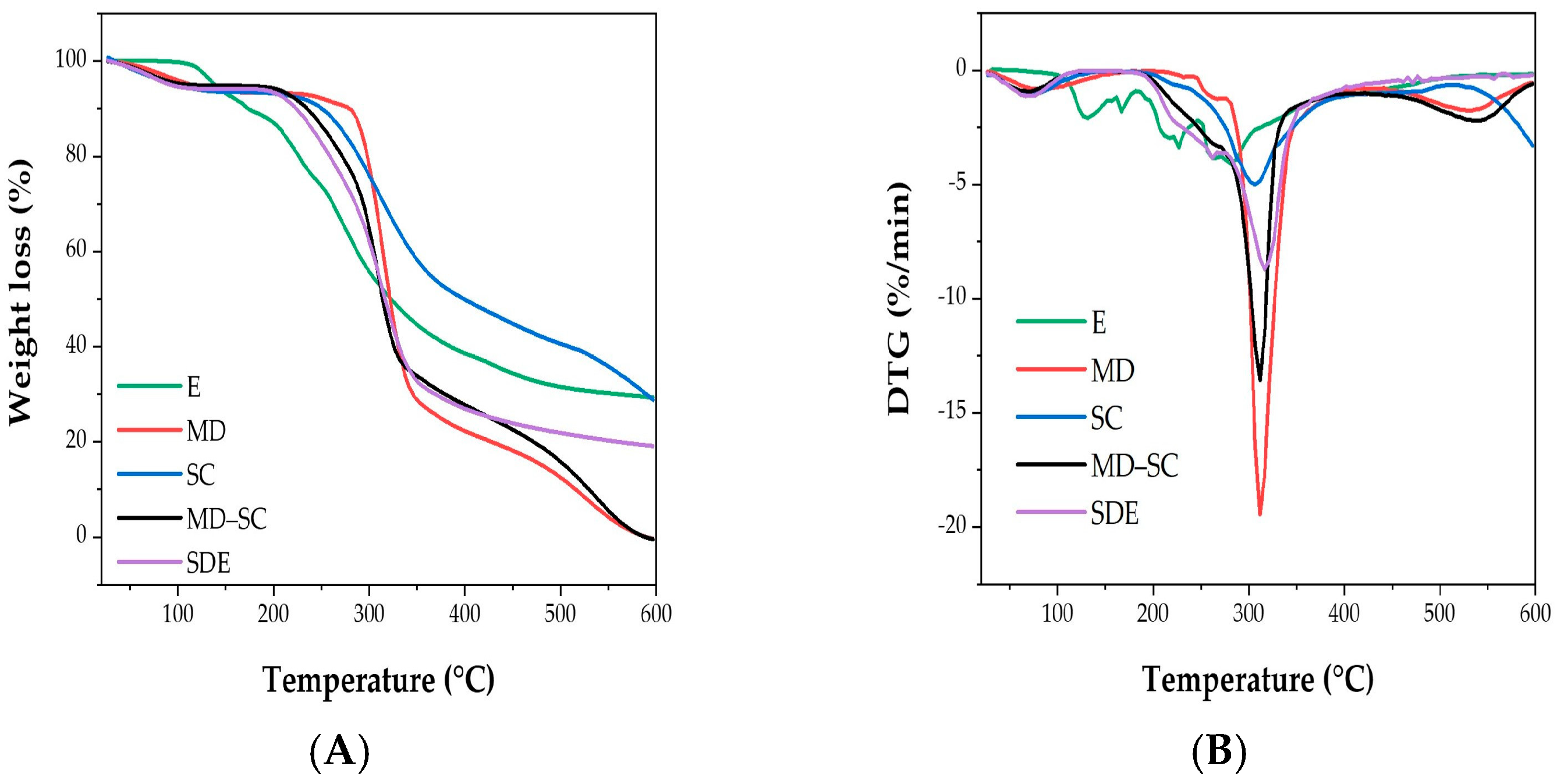
2.5.3. Thermal Stability
3. Materials and Methods
3.1. Chemicals
3.2. Plant Material
3.3. Extraction of Phenolic Compounds
3.4. Encapsulation of Phenolic Compounds
3.5. Determination of Phenolic Content
3.5.1. Total Phenolic Content (TPC)
3.5.2. Surface Phenolic Content (SPC)
3.6. Evaluation of Antioxidant Activity
3.6.1. ABTS•+ Radical Scavenging Assay
3.6.2. DPPH• Radical Scavenging Assay
3.6.3. CUPRAC Assay
3.6.4. Reducing Power Assay
3.6.5. Phenanthroline Assay
3.7. Evaluation of Antibacterial Activity
3.7.1. Source and Selection of Bacterial Strains
3.7.2. Inoculum Preparation
3.7.3. Agar Well Diffusion Assay
3.8. Determination of Physicochemical Characteristics of Powder
3.8.1. Determination of Encapsulation Yield
3.8.2. Determination of Encapsulation Efficiency
3.8.3. Moisture Content Measurement
3.8.4. Water Activity Measurement
3.8.5. Hygroscopicity Measurement
3.8.6. Particle Size Measurement
3.9. Determination of Structural Characterstics of Powder
3.9.1. Scanning Electron Microscopy (SEM)
3.9.2. Attenuated Total Reflectance–Fourier Transform Infrared (ATR-FTIR) Spectroscopy
3.9.3. Thermogravimetric and Derivative Thermogravimetric Analysis (TGA/DTG)
3.10. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dhifallah, A.; Selmi, H.; Ouerghui, A.; Sammeri, H.; Aouini, D.; Rouissi, H. Comparative Study of Phenolic Compounds and Antiradical Activities of Four Extracts of Tunisian Artemisia Herba Alba. Pharm. Chem. J. 2022, 56, 226–232. [Google Scholar] [CrossRef]
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and Anti-Oxidant, Anti-Cancer and Anti-Inflammatory Activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013, 55, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Mrabti, H.N.; Hachlafi, N.E.; Al-Mijalli, S.H.; Jeddi, M.; Elbouzidi, A.; Abdallah, E.M.; Flouchi, R.; Assaggaf, H.; Qasem, A.; Zengin, G.; et al. Phytochemical Profile, Assessment of Antimicrobial and Antioxidant Properties of Essential Oils of Artemisia herba-alba Asso., and Artemisia dracunculus L.: Experimental and Computational Approaches. J. Mol. Struct. 2023, 1294, 136479. [Google Scholar] [CrossRef]
- Bertella, A.; Benlahcen, K.; Abouamama, S.; Pinto, D.C.G.A.; Maamar, K.; Kihal, M.; Silva, A.M.S. Artemisia herba-alba Asso. Essential Oil Antibacterial Activity and Acute Toxicity. Ind. Crops Prod. 2018, 116, 137–143. [Google Scholar] [CrossRef]
- Mighri, H.; Hajlaoui, H.; Akrout, A.; Najjaa, H.; Neffati, M. Antimicrobial and Antioxidant Activities of Artemisia herba-alba Essential Oil Cultivated in Tunisian Arid Zone. Comptes Rendus Chim. 2009, 13, 380–386. [Google Scholar] [CrossRef]
- Mohammed, J.K.; Mahdi, A.A.; Ma, C.; Elkhedir, A.E.; Al-Maqtari, Q.A.; Al-Ansi, W.; Mahmud, A.; Wang, H. Application of Argun Fruit Polysaccharide in Microencapsulation of Citrus Aurantium L. Essential Oil: Preparation, Characterization, and Evaluating the Storage Stability and Antioxidant Activity. J. Food Meas. Charact. 2021, 15, 155–169. [Google Scholar] [CrossRef]
- Younsi, F.; Trimech, R.; Boulila, A.; Ezzine, O.; Dhahri, S.; Boussaid, M.; Messaoud, C. Essential Oil and Phenolic Compounds of Artemisia herba-alba (Asso.): Composition, Antioxidant, Antiacetylcholinesterase, and Antibacterial Activities. Int. J. Food Prop. 2016, 19, 1425–1438. [Google Scholar] [CrossRef]
- Adel, K.; Zied, Z.; Ahmed, B.K.; Neacute Ji, G.; Mohamed, D.; Radhouane, G. Chemical Constituents and Antioxidant Activity of the Essential Oil from Aerial Parts of Artemisia herba-alba Grown in Tunisian Semi-Arid Region. Afr. J. Biotechnol. 2011, 10, 2923–2929. [Google Scholar] [CrossRef]
- Souhila, T.; Fatma Zohra, B.; Tahar, H.S. Identification and Quantification of Phenolic Compounds of Artemisia herba-alba at Three Harvest Time by HPLC–ESI–Q-TOF–MS. Int. J. Food Prop. 2019, 22, 843–852. [Google Scholar] [CrossRef]
- Sendi, N.; Mkadmini-Hammi, K.; Ben Mansour, R.; Selmi, S.; Trabelsi, N.; Isoda, H.; Ksouri, R.; Megdiche-Ksouri, W. Simultaneous Optimization of Ultrasound-Assisted Extraction of Flavonoid Compounds and Antiradical Activity from Artemisia herba-alba Using Response Surface Methodology. Prep. Biochem. Biotechnol. 2020, 50, 943–953. [Google Scholar] [CrossRef]
- Bourgou, S.; Bettaieb Rebey, I.; Mkadmini, K.; Isoda, H.; Ksouri, R.; Ksouri, W.M. LC-ESI-TOF-MS and GC-MS Profiling of Artemisia herba-alba and Evaluation of Its Bioactive Properties. Food Res. Int. 2017, 99, 702–712. [Google Scholar] [CrossRef] [PubMed]
- Elwardani, H.; Oubihi, A.; Haida, S.; Ez-Zriouli, R.; Kabous, K.E.; Ouhssine, M. Seasonal Variation in Essential Oil Composition of Artemisia herba-alba and Their Effects on Antioxidant, Antibacterial, and Antifungal Activities. Chem. Data Collect. 2024, 50, 101118. [Google Scholar] [CrossRef]
- Khojasteh, A.; Mirjalili, M.H.; Alcalde, M.A.; Cusido, R.M.; Eibl, R.; Palazon, J. Powerful Plant Antioxidants: A New Biosustainable Approach to the Production of Rosmarinic Acid. Antioxidants 2020, 9, 1273. [Google Scholar] [CrossRef]
- Tajner-Czopek, A.; Gertchen, M.; Rytel, E.; Kita, A.; Kucharska, A.Z.; Sokół-Łętowska, A. Study of Antioxidant Activity of Some Medicinal Plants Having High Content of Caffeic Acid Derivatives. Antioxidants 2020, 9, 412. [Google Scholar] [CrossRef]
- Chamali, S.; Bendaoud, H.; Bouajila, J.; Camy, S.; Saadaoui, E.; Condoret, J.-S.; Romdhane, M. Optimization of Accelerated Solvent Extraction of Bioactive Compounds from Eucalyptus Intertexta Using Response Surface Methodology and Evaluation of Its Phenolic Composition and Biological Activities. J. Appl. Res. Med. Aromat. Plants 2023, 35, 100464. [Google Scholar] [CrossRef]
- Alves, D.; Pinho, E. Encapsulation of Polyphenols, Plant Bioactive Compounds. In Functionality of Cyclodextrins in Encapsulation for Food Applications; Ho, T.M., Yoshii, H., Terao, K., Bhandari, B.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 91–113. ISBN 978-3-030-80055-0. [Google Scholar]
- Garavand, F.; Jalai-Jivan, M.; Assadpour, E.; Jafari, S.M. Encapsulation of Phenolic Compounds within Nano/Microemulsion Systems: A Review. Food Chem. 2021, 364, 130376. [Google Scholar] [CrossRef]
- Sansone, F.; Mencherini, T.; Picerno, P.; d’Amore, M.; Aquino, R.P.; Lauro, M.R. Maltodextrin/Pectin Microparticles by Spray Drying as Carrier for Nutraceutical Extracts. J. Food Eng. 2011, 105, 468–476. [Google Scholar] [CrossRef]
- Stanković, N.; Mihajilov-Krstev, T.; Zlatković, B.; Stankov-Jovanović, V.; Mitić, V.; Jović, J.; Čomić, L.; Kocić, B.; Bernstein, N. Antibacterial and Antioxidant Activity of Traditional Medicinal Plants from the Balkan Peninsula. NJAS—Wagening. J. Life Sci. 2016, 78, 21–28. [Google Scholar] [CrossRef]
- Mighri, H.; Akrout, A.; Bennour, N.; Eljeni, H.; Zammouri, T.; Neffati, M. LC/MS Method Development for the Determination of the Phenolic Compounds of Tunisian Ephedra Alata Hydro-Methanolic Extract and Its Fractions and Evaluation of Their Antioxidant Activities. S. Afr. J. Bot. 2019, 124, 102–110. [Google Scholar] [CrossRef]
- Hcini, K.; Lozano-Pérez, A.A.; Luis Cenis, J.; Quílez, M.; José Jordán, M. Extraction and Encapsulation of Phenolic Compounds of Tunisian Rosemary (Rosmarinus officinalis L.) Extracts in Silk Fibroin Nanoparticles. Plants 2021, 10, 2312. [Google Scholar] [CrossRef]
- Alvarez Gaona, I.J.; Fanzone, M.L.; Galmarini, M.V.; Chirife, J.; Ferreras-Charro, R.; García-Estévez, I.; Escribano-Bailón, M.T. Encapsulation of Phenolic Compounds by Spray Drying of Ancellotta and Aspirant Bouchet Wines to Produce Powders with Potential Use as Natural Food Colorants. Food Biosci. 2022, 50, 102093. [Google Scholar] [CrossRef]
- da Rosa, C.G.; Borges, C.D.; Zambiazi, R.C.; Rutz, J.K.; da Luz, S.R.; Krumreich, F.D.; Benvenutti, E.V.; Nunes, M.R. Encapsulation of the Phenolic Compounds of the Blackberry (Rubus Fruticosus). LWT—Food Sci. Technol. 2014, 58, 527–533. [Google Scholar] [CrossRef]
- Mehta, N.; Kumar, P.; Verma, A.K.; Umaraw, P.; Kumar, Y.; Malav, O.P.; Sazili, A.Q.; Domínguez, R.; Lorenzo, J.M. Microencapsulation as a Noble Technique for the Application of Bioactive Compounds in the Food Industry: A Comprehensive Review. Appl. Sci. 2022, 12, 1424. [Google Scholar] [CrossRef]
- Ferro, D.M.; Müller, C.M.O.; Ferreira, S.R.S. Photostability and Characterization of Spray-Dried Maltodextrin Powders Loaded with Sida Rhombifolia Extract. Biocatal. Agric. Biotechnol. 2020, 27, 101716. [Google Scholar] [CrossRef]
- Vergara, L.P.; Dos Santos Hackbart, H.C.; Jansen Alves, C.; Reissig, G.N.; Wachholz, B.S.; Borges, C.D.; Chim, J.F.; Zambiazi, R.C. Encapsulation of Phenolic Compounds through the Complex Coacervation Technique for the Enrichment of Diet Chewable Candies. Food Biosci. 2023, 51, 102256. [Google Scholar] [CrossRef]
- Klein, T.; Longhini, R.; Bruschi, M.L.; De Mello, J.C.P. Microparticles Containing Guaraná Extract Obtained by Spray-Drying Technique: Development and Characterization. Rev. Bras. Farmacogn. 2015, 25, 292–300. [Google Scholar] [CrossRef]
- Labuschagne, P. Impact of Wall Material Physicochemical Characteristics on the Stability of Encapsulated Phytochemicals: A Review. Food Res. Int. 2018, 107, 227–247. [Google Scholar] [CrossRef]
- Saenz, C.; Tapia, S.; Chavez, J.; Robert, P. Microencapsulation by Spray Drying of Bioactive Compounds from Cactus Pear (Opuntia ficus-indica). Food Chem. 2009, 114, 616–622. [Google Scholar] [CrossRef]
- Pudziuvelyte, L.; Marksa, M.; Jakstas, V.; Ivanauskas, L.; Kopustinskiene, D.M.; Bernatoniene, J. Microencapsulation of Elsholtzia Ciliata Herb Ethanolic Extract by Spray-Drying: Impact of Resistant-Maltodextrin Complemented with Sodium Caseinate, Skim Milk, and Beta-Cyclodextrin on the Quality of Spray-Dried Powders. Molecules 2019, 24, 1461. [Google Scholar] [CrossRef]
- Mainente, F.; Piovan, A.; Zanoni, F.; Chignola, R.; Cerantola, S.; Faggin, S.; Giron, M.C.; Filippini, R.; Seraglia, R.; Zoccatelli, G. Spray-Drying Microencapsulation of an Extract from Tilia Tomentosa Moench Flowers: Physicochemical Characterization and in Vitro Intestinal Activity. Plant Foods Hum. Nutr. 2022, 77, 467–473. [Google Scholar] [CrossRef]
- Savan, E.K. Encapsulation of Cardamom Extracts. In Cardamom (Elettaria cardamomum): Production, Processing and Properties; Ramadan, M.F., Ed.; Springer International Publishing: Cham, Switzerland, 2023; pp. 255–276. ISBN 978-3-031-35425-0. [Google Scholar]
- Ștefănescu, B.E.; Nemes, S.A.; Teleky, B.E.; Călinoiu, L.F.; Mitrea, L.; Martău, G.A.; Szabo, K.; Mihai, M.; Vodnar, D.C.; Crișan, G. Microencapsulation and Bioaccessibility of Phenolic Compounds of Vaccinium Leaf Extracts. Antioxidants 2022, 11, 674. [Google Scholar] [CrossRef]
- Eghbal, N.; Liao, W.; Dumas, E.; Azabou, S.; Dantigny, P.; Gharsallaoui, A. Microencapsulation of Natural Food Antimicrobials: Methods and Applications. Appl. Sci. 2022, 12, 3837. [Google Scholar] [CrossRef]
- Kuhn, F.; Santos Dorneles, M.; Pelayo Zapata Noreña, C. Accelerated Stability Testing and Simulated Gastrointestinal Release of Encapsulated Betacyanins and Phenolic Compounds from Bougainvillea Glabra Bracts Extract. Food Chem. 2022, 393, 133391. [Google Scholar] [CrossRef]
- Moro, K.I.B.; Bender, A.B.B.; Da Silva, L.P.; Penna, N.G. Green Extraction Methods and Microencapsulation Technologies of Phenolic Compounds From Grape Pomace: A Review. Food Bioprocess Technol. 2021, 14, 1407–1431. [Google Scholar] [CrossRef]
- Cilek, B.; Luca, A.; Hasirci, V.; Sahin, S.; Sumnu, G. Microencapsulation of Phenolic Compounds Extracted from Sour Cherry Pomace: Effect of Formulation, Ultrasonication Time and Core to Coating Ratio. Eur. Food Res. Technol. 2012, 235, 587–596. [Google Scholar] [CrossRef]
- Sylla, N.; Bouyahya, A.; Taha, D.; Dakka, N.; Elhajji, H. Study of the Antioxidant and Antidiabetic Activity in Vitro of Free and Encapsulated Phenolic Compounds of Olive Pomace. Biocatal. Agric. Biotechnol. 2021, 36, 102126. [Google Scholar] [CrossRef]
- Aguiar, J.; Estevinho, B.N.; Santos, L. Microencapsulation of Natural Antioxidants for Food Application—The Specific Case of Coffee Antioxidants—A Review. Trends Food Sci. Technol. 2016, 58, 21–39. [Google Scholar] [CrossRef]
- Gaber Ahmed, G.H.; Fernández-González, A.; Díaz García, M.E. Nano-Encapsulation of Grape and Apple Pomace Phenolic Extract in Chitosan and Soy Protein via Nanoemulsification. Food Hydrocoll. 2020, 108, 105806. [Google Scholar] [CrossRef]
- Herman-Lara, E.; Rivera-Abascal, I.; Gallegos-Marín, I.; Martínez-Sánchez, C.E. Encapsulation of Hydroalcoholic Extracts of Moringa Oleifera Seed through Ionic Gelation. LWT—Food Sci. Technol. 2024, 203, 116368. [Google Scholar] [CrossRef]
- Pashazadeh, H.; Zannou, O.; Ghellam, M.; Koca, I.; Galanakis, C.M.; Aldawoud, T.M.S. Optimization and Encapsulation of Phenolic Compounds Extracted from Maize Waste by Freeze-Drying, Spray-Drying, and Microwave-Drying Using Maltodextrin. Foods 2021, 10, 1396. [Google Scholar] [CrossRef]
- Luca, A.; Cilek, B.; Hasirci, V.; Sahin, S.; Sumnu, G. Storage and Baking Stability of Encapsulated Sour Cherry Phenolic Compounds Prepared from Micro- and Nano-Suspensions. Food Bioprocess Technol. 2014, 7, 204–211. [Google Scholar] [CrossRef]
- Avilés-Betanzos, K.A.; Cauich-Rodríguez, J.V.; Ramírez-Sucre, M.O.; Rodríguez-Buenfil, I.M. Optimization of Spray-Drying Conditions of Microencapsulated Habanero Pepper (Capsicum chinense Jacq.) Extracts and Physicochemical Characterization of the Microcapsules. Processes 2023, 11, 1238. [Google Scholar] [CrossRef]
- Silva, P.I.; Stringheta, P.C.; Teófilo, R.F.; de Oliveira, I.R.N. Parameter Optimization for Spray-Drying Microencapsulation of Jaboticaba (Myrciaria jaboticaba) Peel Extracts Using Simultaneous Analysis of Responses. J. Food Eng. 2013, 117, 538–544. [Google Scholar] [CrossRef]
- Simon-Brown, K.; Solval, K.M.; Chotiko, A.; Alfaro, L.; Reyes, V.; Liu, C.; Dzandu, B.; Kyereh, E.; Goldson Barnaby, A.; Thompson, I.; et al. Microencapsulation of Ginger (Zingiber officinale) Extract by Spray Drying Technology. LWT—Food Sci. Technol. 2016, 70, 119–125. [Google Scholar] [CrossRef]
- Tran, T.T.; Nguyen, V.H. Effects of Spray-Drying Temperatures and Carriers on Physical and Antioxidant Properties of Lemongrass Leaf Extract Powder. Beverages 2018, 4, 84. [Google Scholar] [CrossRef]
- Gomes, W.F.; França, F.R.M.; Denadai, M.; Andrade, J.K.S.; Da Silva Oliveira, E.M.; De Brito, E.S.; Rodrigues, S.; Narain, N. Effect of Freeze- and Spray-Drying on Physico-Chemical Characteristics, Phenolic Compounds and Antioxidant Activity of Papaya Pulp. J. Food Sci. Technol. 2018, 55, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Shugulí, C.; Vidal, C.P.; Cantero-López, P.; Lopez-Polo, J. Encapsulation of Plant Extract Compounds Using Cyclodextrin Inclusion Complexes, Liposomes, Electrospinning and Their Combinations for Food Purposes. Trends Food Sci. Technol. 2021, 108, 177–186. [Google Scholar] [CrossRef]
- Shi, L.; Zhou, J.; Guo, J.; Gladden, I.; Kong, L. Starch Inclusion Complex for the Encapsulation and Controlled Release of Bioactive Guest Compounds. Carbohydr. Polym. 2021, 274, 118596. [Google Scholar] [CrossRef] [PubMed]
- Ayar-Sumer, E.N.; Nyambe, C.; Hashim, M.A.; Altin-Yavuzarslan, G.; El-Messery, T.M.; Ozçelik, B. Optimizing Encapsulation of Black Carrot Extract Using Complex Coacervation Technique: Maximizing the Bioaccessibility and Release Kinetics in Different Food Matrixes. LWT—Food Sci. Technol. 2024, 198, 115995. [Google Scholar] [CrossRef]
- Bhagya Raj, G.V.S.; Dash, K.K. Microencapsulation of Betacyanin from Dragon Fruit Peel by Complex Coacervation: Physicochemical Characteristics, Thermal Stability, and Release Profile of Microcapsules. Food Biosci. 2022, 49, 101882. [Google Scholar] [CrossRef]
- Figueroa-Robles, A.; Antunes-Ricardo, M.; Guajardo-Flores, D. Encapsulation of Phenolic Compounds with Liposomal Improvement in the Cosmetic Industry. Int. J. Pharm. 2021, 593, 120125. [Google Scholar] [CrossRef]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of Rosemary Extract by Liposomes Formulated by Mozafari Method: Physicochemical Characterization and Optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Parhizkary, M.; Jafari, S.M.; Assadpour, E.; Enayati, A.; Kashiri, M. Pea Protein-Coated Nanoliposomal Encapsulation of Jujube Phenolic Extract with Different Stabilizers; Characterization and In Vitro Release. Food Chem. X 2024, 23, 101771. [Google Scholar] [CrossRef]
- Chanioti, S.; Katsouli, M.; Tzia, C. Novel Processes for the Extraction of Phenolic Compounds from Olive Pomace and Their Protection by Encapsulation. Molecules 2021, 26, 1781. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.D.; Secco, M.C.; De Cezaro, A.M.; Fischer, B.; Cansian, R.L.; Junges, A.; Franceschi, E.; Backes, G.T.; Valduga, E. Encapsulation of Olive Leaf (Olea europaea) Extract Using Solution-Enhanced Dispersion by Supercritical Fluids (SEDS) Technique. J. Supercrit. Fluids 2023, 198, 105922. [Google Scholar] [CrossRef]
- Wen, P.; Zong, M.H.; Linhardt, R.J.; Feng, K.; Wu, H. Electrospinning: A Novel Nano-Encapsulation Approach for Bioactive Compounds. Trends Food Sci. Technol. 2017, 70, 56–68. [Google Scholar] [CrossRef]
- Bratovcic, A.; Suljagic, J. Micro- and Nano-Encapsulation in Food Industry. Croat. J. Food Sci. Technol. 2019, 11, 113–121. [Google Scholar] [CrossRef]
- Rajapaksha, D.S.W.; Shimizu, N. Valorization of Spent Black Tea by Recovery of Antioxidant Polyphenolic Compounds: Subcritical Solvent Extraction and Microencapsulation. Food Sci. Nutr. 2020, 8, 4297–4307. [Google Scholar] [CrossRef]
- Aliakbarian, B.; Sampaio, F.C.; de Faria, J.T.; Pitangui, C.G.; Lovaglio, F.; Casazza, A.A.; Converti, A.; Perego, P. Optimization of Spray Drying Microencapsulation of Olive Pomace Polyphenols Using Response Surface Methodology and Artificial Neural Network. LWT—Food Sci. Technol. 2018, 93, 220–228. [Google Scholar] [CrossRef]
- Geranpour, M.; Assadpour, E.; Jafari, S.M. Recent Advances in the Spray Drying Encapsulation of Essential Fatty Acids and Functional Oils. Trends Food Sci. Technol. 2020, 102, 71–90. [Google Scholar] [CrossRef]
- Di Battista, C.A.; Constenla, D.; Ramírez Rigo, M.V.; Piña, J. Process Analysis and Global Optimization for the Microencapsulation of Phytosterols by Spray Drying. Powder Technol. 2017, 321, 55–65. [Google Scholar] [CrossRef]
- Hoyos Merlano, N.T.; Borroni, V.; Giménez, R.B.; Herrera, M.L. Encapsulation of Hydrophobic Compounds in Sugar Matrixes by Freeze-Drying. In Basic Protocols in Encapsulation of Food Ingredients; Gomez-Zavaglia, A., Ed.; Springer: New York, NY, USA, 2021; pp. 1–10. ISBN 978-1-07-161648-2. [Google Scholar]
- Leyva-Jiménez, F.J.; Lozano-Sánchez, J.; Cádiz-Gurrea, M.D.L.L.; Fernández-Ochoa, Á.; Arráez-Román, D.; Segura-Carretero, A. Spray-Drying Microencapsulation of Bioactive Compounds from Lemon Verbena Green Extract. Foods 2020, 9, 1547. [Google Scholar] [CrossRef]
- Yu, F.; Li, Z.; Zhang, T.; Wei, Y.; Xue, Y.; Xue, C. Influence of Encapsulation Techniques on the Structure, Physical Properties, and Thermal Stability of Fish Oil Microcapsules by Spray Drying. J. Food Process Eng. 2017, 40, e12576. [Google Scholar] [CrossRef]
- Paini, M.; Aliakbarian, B.; Casazza, A.A.; Lagazzo, A.; Botter, R.; Perego, P. Microencapsulation of Phenolic Compounds from Olive Pomace Using Spray Drying: A Study of Operative Parameters. LWT—Food Sci. Technol. 2015, 62, 177–186. [Google Scholar] [CrossRef]
- Sadiq, U.; Gill, H.; Chandrapala, J.; Shahid, F. Influence of Spray Drying on Encapsulation Efficiencies and Structure of Casein Micelles Loaded with Anthraquinones Extracted from Aloe Vera Plant. Appl. Sci. 2022, 13, 110. [Google Scholar] [CrossRef]
- Zhu, J.; Li, X.; Liu, L.; Li, Y.; Qi, B.; Jiang, L. Preparation of Spray-Dried Soybean Oil Body Microcapsules Using Maltodextrin: Effects of Dextrose Equivalence. LWT—Food Sci. Technol. 2022, 154, 112874. [Google Scholar] [CrossRef]
- Desai, N.M.; Haware, D.J.; Basavaraj, K.; Murthy, P.S. Microencapsulation of Antioxidant Phenolic Compounds from Green Coffee. Prep. Biochem. Biotechnol. 2019, 49, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Bednarska, M.A.; Janiszewska-Turak, E. The Influence of Spray Drying Parameters and Carrier Material on the Physico-Chemical Properties and Quality of Chokeberry Juice Powder. J. Food Sci. Technol. 2020, 57, 564–577. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Yang, X.; Shen, J.; Li, Z.; Tan, S.; Liu, W.; Cheng, Z. Choosing the Appropriate Wall Materials for Spray-Drying Microencapsulation of Natural Bioactive Ingredients: Taking Phenolic Compounds as Examples. Powder Technol. 2021, 394, 562–574. [Google Scholar] [CrossRef]
- Šturm, L.; Osojnik Črnivec, I.G.; Istenič, K.; Ota, A.; Megušar, P.; Slukan, A.; Humar, M.; Levic, S.; Nedović, V.; Kopinč, R.; et al. Encapsulation of Non-Dewaxed Propolis by Freeze-Drying and Spray-Drying Using Gum Arabic, Maltodextrin and Inulin as Coating Materials. Food Bioprod. Process. 2019, 116, 196–211. [Google Scholar] [CrossRef]
- Tao, Y.; Wang, P.; Wang, J.; Wu, Y.; Han, Y.; Zhou, J. Combining Various Wall Materials for Encapsulation of Blueberry Anthocyanin Extracts: Optimization by Artificial Neural Network and Genetic Algorithm and a Comprehensive Analysis of Anthocyanin Powder Properties. Powder Technol. 2017, 311, 77–87. [Google Scholar] [CrossRef]
- Hamed, I.; Moradi, M.; Ezati, P.; O’Higgins, L.; Meléndez-Martínez, A.J.; Frleta Matas, R.; Šimat, V.; McClements, D.J.; Jakobsen, A.N.; Lerfall, J. Encapsulation of Microalgal-Based Carotenoids: Recent Advances in Stability and Food Applications. Trends Food Sci. Technol. 2023, 138, 382–398. [Google Scholar] [CrossRef]
- Sekiou, O.; Boumendjel, M.; Taibi, F.; Tichati, L.; Boumendjel, A.; Messarah, M. Nephroprotective Effect of Artemisia Herba Alba Aqueous Extract in Alloxan-Induced Diabetic Rats. J. Tradit. Complement. Med. 2021, 11, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction Methods, Antioxidative Action, Bioavailability and Anticarcinogenic Effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Chepel, V.; Lisun, V.; Skrypnik, L. Changes in the Content of Some Groups of Phenolic Compounds and Biological Activity of Extracts of Various Parts of Heather (Calluna vulgaris (L.) Hull) at Different Growth Stages. Plants 2020, 9, 926. [Google Scholar] [CrossRef]
- Vagiri, M.; Conner, S.; Stewart, D.; Andersson, S.C.; Verrall, S.; Johansson, E.; Rumpunen, K. Phenolic Compounds in Blackcurrant (Ribes nigrum L.) Leaves Relative to Leaf Position and Harvest Date. Food Chem. 2015, 172, 135–142. [Google Scholar] [CrossRef]
- Staszowska-Karkut, M.; Materska, M. Phenolic Composition, Mineral Content, and Beneficial Bioactivities of Leaf Extracts from Black Currant (Ribes nigrum L.), Raspberry (Rubus idaeus), and Aronia (Aronia melanocarpa). Nutrients 2020, 12, 463. [Google Scholar] [CrossRef]
- Kuck, L.S.; Noreña, C.P.Z. Microencapsulation of Grape (Vitis Labrusca Var. Bordo) Skin Phenolic Extract Using Gum Arabic, Polydextrose, and Partially Hydrolyzed Guar Gum as Encapsulating Agents. Food Chem. 2016, 194, 569–576. [Google Scholar] [CrossRef]
- Tolun, A.; Altintas, Z.; Artik, N. Microencapsulation of Grape Polyphenols Using Maltodextrin and Gum Arabic as Two Alternative Coating Materials: Development and Characterization. J. Biotechnol. 2016, 239, 23–33. [Google Scholar] [CrossRef]
- Akdeniz, B.; Sumnu, G.; Sahin, S. Microencapsulation of Phenolic Compounds Extracted from Onion (Allium cepa) Skin. J. Food Process. Preserv. 2018, 42, e13648. [Google Scholar] [CrossRef]
- Carra, J.B.; Matos, R.L.N.D.; Novelli, A.P.; Couto, R.O.D.; Yamashita, F.; Ribeiro, M.A.D.S.; Meurer, E.C.; Verri, W.A.; Casagrande, R.; Georgetti, S.R.; et al. Spray-Drying of Casein/Pectin Bioconjugate Microcapsules Containing Grape (Vitis labrusca) by-Product Extract. Food Chem. 2022, 368, 130817. [Google Scholar] [CrossRef]
- Soleimanifar, M.; Jafari, S.M.; Assadpour, E.; Mirarab, A. Electrosprayed Whey Protein Nanocarriers Containing Natural Phenolics; Thermal and Antioxidant Properties, Release Behavior and Stability. J. Food Eng. 2021, 307, 110644. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Papp, N.; Kőszegi, T. Cytotoxic, Antimicrobial, Antioxidant Properties and Effects on Cell Migration of Phenolic Compounds of Selected Transylvanian Medicinal Plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef]
- Wang, X.; Liu, X.; Shi, N.; Zhang, Z.; Chen, Y.; Yan, M.; Li, Y. Response Surface Methodology Optimization and HPLC-ESI-QTOF-MS/MS Analysis on Ultrasonic-Assisted Extraction of Phenolic Compounds from Okra (Abelmoschus esculentus) and Their Antioxidant Activity. Food Chem. 2023, 405, 134966. [Google Scholar] [CrossRef]
- Ciric, A.; Krajnc, B.; Heath, D.; Ogrinc, N. Response Surface Methodology and Artificial Neural Network Approach for the Optimization of Ultrasound-Assisted Extraction of Polyphenols from Garlic. Food Chem. Toxicol. 2020, 135, 110976. [Google Scholar] [CrossRef] [PubMed]
- Kratchanova, M.; Denev, P.; Ciz, M.; Lojek, A.; Mihailov, A. Evaluation of Antioxidant Activity of Medicinal Plants Containing Polyphenol Compounds. Comparison of Two Extraction Systems. Acta Biochim. Pol. 2010, 57, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Benamar-Aissa, B.; Gourine, N.; Ouinten, M.; Harrat, M.; Benarfa, A.; Yousfi, M. Synergistic Effects of Essential Oils and Phenolic Extracts on Antioxidant Activities Responses Using Two Artemisia Species (A. campestris and A. herba alba) Combined with Citrus Aurantium. Biocatal. Agric. Biotechnol. 2023, 47, 102570. [Google Scholar] [CrossRef]
- Dif, M.M.; Benali Toumi, F.; Boukaaza, H.; Mokaddem, F.; Benyahia, M.; Bouazza, S. Phenolic content and antioxidant activity of Artemisa herba-alba, a medicinal plant from Algerian arid zone. Phytothérapie 2018, 16, 1–5. [Google Scholar] [CrossRef]
- Bouchara, N.; Senejoux, F.; Fraisse, D.; Felgines, C.; Caldéfie-Chezet, F.; Vasson, M.-P.; Madani, K.; Rossary, A. Anti-Inflammatory and Prolonged Protective Effects of Artemisia herba-alba Extracts via Glutathione Metabolism Reinforcement. S. Afr. J. Bot. 2021, 142, 206–215. [Google Scholar] [CrossRef]
- Ayad, N.; Benaraba, R.; Hemida, H.; Abdellah, F. Biological Activities of Phenolic Extracts from Artemisia herba-alba Asso Grown in Western Algeria. Eur. J. Biol. Res. 2022, 12, 46–61. [Google Scholar] [CrossRef]
- Ghandehari Yazdi, A.P.; Barzegar, M.; Sahari, M.A.; Ahmadi Gavlighi, H. Encapsulation of Pistachio Green Hull Phenolic Compounds by Spray Drying. J. Agric. Sci. Technol. 2021, 23, 51–64. [Google Scholar]
- Ferreira, L.M.D.M.C.; Pereira, R.R.; Carvalho-Guimarães, F.B.D.; Remígio, M.S.D.N.; Barbosa, W.L.R.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Microencapsulation by Spray Drying and Antioxidant Activity of Phenolic Compounds from Tucuma Coproduct (Astrocaryum vulgare Mart.) Almonds. Polymers 2022, 14, 2905. [Google Scholar] [CrossRef] [PubMed]
- Akbarmehr, A.; Peighambardoust, S.H.; Soltanzadeh, M.; Jafari, S.M.; Sarabandi, K. Microencapsulation of Yerba Mate Extract: The Efficacy of Polysaccharide/Protein Hydrocolloids on Physical, Microstructural, Functional, and Antioxidant Properties. Int. J. Biol. Macromol. 2023, 234, 123678. [Google Scholar] [CrossRef] [PubMed]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic Profile (HPLC-UV) of Olive Leaves According to Extraction Procedure and Assessment of Antibacterial Activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef]
- Tsali, A.; Goula, A.M. Valorization of Grape Pomace: Encapsulation and Storage Stability of Its Phenolic Extract. Powder Technol. 2018, 340, 194–207. [Google Scholar] [CrossRef]
- Navarro-Flores, M.J.; Ventura-Canseco, L.M.C.; Meza-Gordillo, R.; Ayora-Talavera, T.D.R.; Abud-Archila, M. Spray Drying Encapsulation of a Native Plant Extract Rich in Phenolic Compounds with Combinations of Maltodextrin and Non-Conventional Wall Materials. J. Food Sci. Technol. 2020, 57, 4111–4122. [Google Scholar] [CrossRef]
- Medfai, W.; Oueslati, I.; Dumas, E.; Harzalli, Z.; Viton, C.; Mhamdi, R.; Gharsallaoui, A. Physicochemical and Biological Characterization of Encapsulated Olive Leaf Extracts for Food Preservation. Antibiotics 2023, 12, 987. [Google Scholar] [CrossRef]
- Tu, Q.B.; Wang, P.Y.; Sheng, S.; Xu, Y.; Wang, J.Z.; You, S.; Zhu, A.H.; Wang, J.; Wu, F.A. Microencapsulation and Antimicrobial Activity of Plant Essential Oil Against Ralstonia Solanacearum. Waste Biomass Valorization 2020, 11, 5273–5282. [Google Scholar] [CrossRef]
- Maqsoudlou, A.; Sadeghi Mahoonak, A.; Mohebodini, H.; Koushki, V. Stability and Structural Properties of Bee Pollen Protein Hydrolysate Microencapsulated Using Maltodextrin and Whey Protein Concentrate. Heliyon 2020, 6, e03731. [Google Scholar] [CrossRef]
- Cassol, L.; Noreña, C.P.Z. Microencapsulation and Accelerated Stability Testing of Bioactive Compounds of Hibiscus Sabdariffa. J. Food Meas. Charact. 2021, 15, 1599–1610. [Google Scholar] [CrossRef]
- Ćujić-Nikolić, N.; Stanisavljević, N.; Šavikin, K.; Kalušević, A.; Nedović, V.; Bigović, D.; Janković, T. Application of Gum Arabic in the Production of Spray-Dried Chokeberry Polyphenols, Microparticles Characterisation and in Vitro Digestion Method. Lek. Sirovine 2018, 38, 9–16. [Google Scholar] [CrossRef]
- Fazaeli, M.; Emam-Djomeh, Z.; Kalbasi Ashtari, A.; Omid, M. Effect of Spray Drying Conditions and Feed Composition on the Physical Properties of Black Mulberry Juice Powder. Food Bioprod. Process. 2012, 90, 667–675. [Google Scholar] [CrossRef]
- Başyiğit, B.; Sağlam, H.; Kandemir, Ş.; Karaaslan, A.; Karaaslan, M. Microencapsulation of Sour Cherry Oil by Spray Drying: Evaluation of Physical Morphology, Thermal Properties, Storage Stability, and Antimicrobial Activity. Powder Technol. 2020, 364, 654–663. [Google Scholar] [CrossRef]
- Baghi, F.; Ghnimi, S.; Dumas, E.; Gharsallaoui, A. Microencapsulation of Antimicrobial Trans-Cinnamaldehyde: Effect of Emulsifier Type, pH, and Drying Technique. Appl. Sci. 2023, 13, 6184. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Influence of Process Conditions on the Physicochemical Properties of Açai (Euterpe oleraceae Mart.) Powder Produced by Spray Drying. J. Food Eng. 2008, 88, 411–418. [Google Scholar] [CrossRef]
- Daza, L.D.; Fujita, A.; Fávaro-Trindade, C.S.; Rodrigues-Ract, J.N.; Granato, D.; Genovese, M.I. Effect of Spray Drying Conditions on the Physical Properties of Cagaita (Eugenia dysenterica DC.) Fruit Extracts. Food Bioprod. Process. 2016, 97, 20–29. [Google Scholar] [CrossRef]
- Quek, S.Y.; Chok, N.K.; Swedlund, P. The Physicochemical Properties of Spray-Dried Watermelon Powders. Chem. Eng. Process. Process Intensif. 2007, 46, 386–392. [Google Scholar] [CrossRef]
- Hoyos-Leyva, J.D.; Bello-Perez, L.A.; Agama-Acevedo, J.E.; Alvarez-Ramirez, J.; Jaramillo-Echeverry, L.M. Characterization of Spray Drying Microencapsulation of Almond Oil into Taro Starch Spherical Aggregates. LWT—Food Sci. Technol. 2019, 101, 526–533. [Google Scholar] [CrossRef]
- Ocak, B. Gum Arabic and Collagen Hydrolysate Extracted from Hide Fleshing Wastes as Novel Wall Materials for Microencapsulation of Origanum onites L. Essential Oil through Complex Coacervation. Environ. Sci. Pollut. Res. 2020, 27, 42727–42737. [Google Scholar] [CrossRef] [PubMed]
- Goula, A.M.; Adamopoulos, K.G. A New Technique for Spray Drying Orange Juice Concentrate. Innov. Food Sci. Emerg. Technol. 2010, 11, 342–351. [Google Scholar] [CrossRef]
- Botrel, D.A.; de Barros Fernandes, R.V.; Borges, S.V.; Yoshida, M.I. Influence of Wall Matrix Systems on the Properties of Spray-Dried Microparticles Containing Fish Oil. Food Res. Int. 2014, 62, 344–352. [Google Scholar] [CrossRef]
- Feihrmann, A.C.; Da Silva, N.M.; De Marins, A.R.; Antônio Matiucci, M.; Nunes, K.C.; Nakamura, C.V.; De Souza, M.L.R.; De Oliveira, O.; Gomes, R.G. Ultrasound-Assisted Extraction and Encapsulation by Spray Drying of Bioactive Compounds from Tradescantia Zebrina Leaves. Food Chem. Adv. 2024, 4, 100621. [Google Scholar] [CrossRef]
- Joye, I.J.; McClements, D.J. Biopolymer-Based Nanoparticles and Microparticles: Fabrication, Characterization, and Application. Curr. Opin. Colloid Interface Sci. 2014, 19, 417–427. [Google Scholar] [CrossRef]
- Sarabandi, K.; Peighambardoust, S.H.; Mahoonak, A.S.; Samaei, S.P. Effect of Carrier Types and Compositions on the Production Yield, Microstructure and Physical Characteristics of Spray Dried Sour Cherry Juice Concentrate. J. Food Meas. Charact. 2017, 11, 1602–1612. [Google Scholar] [CrossRef]
- Medina-Torres, L.; Santiago-Adame, R.; Calderas, F.; Gallegos-Infante, J.A.; González-Laredo, R.F.; Rocha-Guzmán, N.E.; Núñez-Ramírez, D.M.; Bernad-Bernad, M.J.; Manero, O. Microencapsulation by Spray Drying of Laurel Infusions (Litsea glaucescens) with Maltodextrin. Ind. Crops Prod. 2016, 90, 1–8. [Google Scholar] [CrossRef]
- Ballesteros, L.F.; Ramirez, M.J.; Orrego, C.E.; Teixeira, J.A.; Mussatto, S.I. Encapsulation of Antioxidant Phenolic Compounds Extracted from Spent Coffee Grounds by Freeze-Drying and Spray-Drying Using Different Coating Materials. Food Chem. 2017, 237, 623–631. [Google Scholar] [CrossRef]
- Do Nascimento, T.G.; Borges, A.L.T.F.; De Almeida, L.M.; Ribeiro, Ê.A.N.; Silva, F.G.C.; Da Costa Silva, V.; Do Nascimento Prata, A.P.; Basílio-Júnior, I.D.; Goulart, M.O.F.; Morilla, D.P.; et al. Preparation and Characterization of Microcapsules Loaded with Polyphenols-Enriched Uncaria Tomentosa Extract Using Spray-Dryer Technique. J. Therm. Anal. Calorim. 2022, 147, 11949–11963. [Google Scholar] [CrossRef]
- De Abreu Figueiredo, J.; Andrade Teixeira, M.; Henrique Campelo, P.; Maria Teixeira Lago, A.; Pereira De Souza, T.; Irene Yoshida, M.; Rodrigues De Oliveira, C.; Paula Aparecida Pereira, A.; Maria Pastore, G.; Aparecido Sanches, E.; et al. Encapsulation of Camu-Camu Extracts Using Prebiotic Biopolymers: Controlled Release of Bioactive Compounds and Effect on Their Physicochemical and Thermal Properties. Food Res. Int. 2020, 137, 109563. [Google Scholar] [CrossRef] [PubMed]
- Estupiñan-Amaya, M.; Fuenmayor, C.A.; López-Córdoba, A. New Freeze-Dried Andean Blueberry Juice Powders for Potential Application as Functional Food Ingredients: Effect of Maltodextrin on Bioactive and Morphological Features. Molecules 2020, 25, 5635. [Google Scholar] [CrossRef] [PubMed]
- Tirgarian, B.; Farmani, J.; Farahmandfar, R.; Milani, J.M.; Van Bockstaele, F. Switchable pH-Responsive Biopolymeric Stabilizers Made by Sonothermal Glycation of Sodium Caseinate with Κappa-Carrageenan. Food Biophys. 2023, 18, 362–378. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Peltzer, M.A.; Garrigós, M.D.C.; Jiménez, A. Structure and Mechanical Properties of Sodium and Calcium Caseinate Edible Active Films with Carvacrol. J. Food Eng. 2013, 114, 486–494. [Google Scholar] [CrossRef]
- Otálora, M.C.; Wilches-Torres, A.; Gómez Castaño, J.A. Microencapsulation of Betaxanthin Pigments from Pitahaya (Hylocereus megalanthus) By-Products: Characterization, Food Application, Stability, and In Vitro Gastrointestinal Digestion. Foods 2023, 12, 2700. [Google Scholar] [CrossRef]
- Xue, R.; Yuan, X.; Jiang, H.; Huang, H.; Luo, X.; Li, P. Preparation and Physicochemical Analysis of Camellia Sinensis Cv. ‘Ziyan’ Anthocyanin Microcapsules. Foods 2024, 13, 618. [Google Scholar] [CrossRef]
- Rigolon, T.C.B.; Silva, R.R.A.; De Oliveira, T.V.; Nascimento, A.L.A.A.; De Barros, F.A.R.; Martins, E.; Campelo, P.H.; Stringheta, P.C. Exploring Anthocyanins-Polysaccharide Synergies in Microcapsule Wall Materials via Spray Drying: Interaction Characterization and Evaluation of Particle Stability. Meas. Food 2024, 13, 100126. [Google Scholar] [CrossRef]
- Bisinella, R.Z.B.; De Oliveira, C.S.; Zappani, P.S.C.; Schnitzler, E.; Masson, M.L. Thermal Analysis as Screening Technique to Assess Spray-Drying Process of Encapsulated “Yacon” Juice. J. Therm. Anal. Calorim. 2016, 126, 1841–1849. [Google Scholar] [CrossRef]
- Nunes, G.L.; Boaventura, B.C.B.; Pinto, S.S.; Verruck, S.; Murakami, F.S.; Prudêncio, E.S.; De Mello Castanho Amboni, R.D. Microencapsulation of Freeze Concentrated Ilex Paraguariensis Extract by Spray Drying. J. Food Eng. 2015, 151, 60–68. [Google Scholar] [CrossRef]
- Castro-López, C.; Espinoza-González, C.; Ramos-González, R.; Boone-Villa, V.D.; Aguilar-González, M.A.; Martínez-Ávila, G.C.G.; Aguilar, C.N.; Ventura-Sobrevilla, J.M. Spray-Drying Encapsulation of Microwave-Assisted Extracted Polyphenols from Moringa oleifera: Influence of Tragacanth, Locust Bean, and Carboxymethyl-Cellulose Formulations. Food Res. Int. 2021, 144, 110291. [Google Scholar] [CrossRef]
- Zahnit, W.; Smara, O.; Bechki, L.; Bensouici, C.; Messaoudi, M.; Benchikha, N.; Larkem, I.; Awuchi, C.G.; Sawicka, B.; Simal-Gandara, J. Phytochemical Profiling, Mineral Elements, and Biological Activities of Artemisia campestris L. Grown in Algeria. Horticulturae 2022, 8, 914. [Google Scholar] [CrossRef]
- Khenifi, M.L.; Serseg, T.; Migas, P.; Krauze-Baranowska, M.; Özdemir, S.; Bensouici, C.; Alghonaim, M.I.; Al-Khafaji, K.; Alsalamah, S.A.; Boudjeniba, M.; et al. HPLC-DAD-MS Characterization, Antioxidant Activity, α-Amylase Inhibition, Molecular Docking, and ADMET of Flavonoids from Fenugreek Seeds. Molecules 2023, 28, 7798. [Google Scholar] [CrossRef]
- Benouchenne, D.; Bellil, I.; Akkal, S.; Bensouici, C.; Khelifi, D. LC–MS/MS Analysis, Antioxidant and Antibacterial Activities of Algerian Fir (Abies numidica de LANNOY Ex CARRIÈRE) Ethylacetate Fraction Extracted from Needles. J. King Saud Univ.—Sci. 2020, 32, 3321–3327. [Google Scholar] [CrossRef]
- Djermane, N.; Gali, L.; Arhab, R.; Gherraf, N.; Bensouici, C.; Erenler, R.; Gok, M.; Abdessamed, A. Chemical Composition and in Vitro Evaluation of Antioxidant, Antimicrobial, and Enzyme Inhibitory Activities of Erucaria Uncata and Thymeleae Hirsuta. Biocatal. Agric. Biotechnol. 2020, 29, 101834. [Google Scholar] [CrossRef]
- Bendjedid, S.; Lekmine, S.; Tadjine, A.; Djelloul, R.; Bensouici, C. Analysis of Phytochemical Constituents, Antibacterial, Antioxidant, Photoprotective Activities and Cytotoxic Effect of Leaves Extracts and Fractions of Aloe Vera. Biocatal. Agric. Biotechnol. 2021, 33, 101991. [Google Scholar] [CrossRef]
- Guebebia, S.; Gharsallaoui, A.; Dumas, E.; Baghi, F.; Zourgui, L.; Romdhane, M.; Agusti, G.; Ghnimi, S. Microencapsulation of Phenolic Compounds Extracted from Okra (Abelmoschus esculentus L.) Leaves, Fruits and Seeds. Appl. Sci. 2023, 13, 12273. [Google Scholar] [CrossRef]

| Bacteria | Inhibition Zone Diameter for (E) Sample (mm) | Inhibition Zone Diameter for (SDE) Sample (mm) | Inhibition Zone Diameter for Chloramphenicol (mm) |
|---|---|---|---|
| Listeria innocua | - | - | 29.21 ± 0.16 |
| Brochothrix thermosphacta | 11.56 ± 0.49 | 8.92 ± 0.05 | 32.93 ± 0.59 |
| Pseudomonas aeruginosa | 11.00 ± 0.72 | 10.59 ± 0.12 | 12.00 ± 0.59 |
| Salmonella enterica | - | - | 30.12 ± 0.03 |
| Escherichia coli | - | - | 27.78 ± 0.47 |
| Physicochemical Properties | SDE |
|---|---|
| Encapsulation yield (%) | 69.40 ± 0.25 |
| Encapsulation efficiency (%) | 96.39 ± 0.11 |
| Moisture (%) | 4.34 ± 0.10 |
| Water activity | 0.415 ± 0.01 |
| Hygroscopicity (%) | 12.67 ± 0.75 |
| Particle size D [4,3] (µm) | 10.05 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lemmadi, S.; Dumas, E.; Adoui, F.; Agusti, G.; Vessot-Crastes, S.; Medfai, W.; Gharsallaoui, A. Spray-Drying Microencapsulation of Artemisia herba-alba Phenolic Extract: Physicochemical Properties, Structural Characterization, and Bioactivity. Molecules 2025, 30, 3904. https://doi.org/10.3390/molecules30193904
Lemmadi S, Dumas E, Adoui F, Agusti G, Vessot-Crastes S, Medfai W, Gharsallaoui A. Spray-Drying Microencapsulation of Artemisia herba-alba Phenolic Extract: Physicochemical Properties, Structural Characterization, and Bioactivity. Molecules. 2025; 30(19):3904. https://doi.org/10.3390/molecules30193904
Chicago/Turabian StyleLemmadi, Sara, Emilie Dumas, Faïza Adoui, Géraldine Agusti, Séverine Vessot-Crastes, Wafa Medfai, and Adem Gharsallaoui. 2025. "Spray-Drying Microencapsulation of Artemisia herba-alba Phenolic Extract: Physicochemical Properties, Structural Characterization, and Bioactivity" Molecules 30, no. 19: 3904. https://doi.org/10.3390/molecules30193904
APA StyleLemmadi, S., Dumas, E., Adoui, F., Agusti, G., Vessot-Crastes, S., Medfai, W., & Gharsallaoui, A. (2025). Spray-Drying Microencapsulation of Artemisia herba-alba Phenolic Extract: Physicochemical Properties, Structural Characterization, and Bioactivity. Molecules, 30(19), 3904. https://doi.org/10.3390/molecules30193904









