Fluorescent Dihomooxacalix[4]arenes for the Detection of Nitroaromatic Compounds in Solution and in the Vapour Phase: Structural and Supramolecular Insights
Abstract
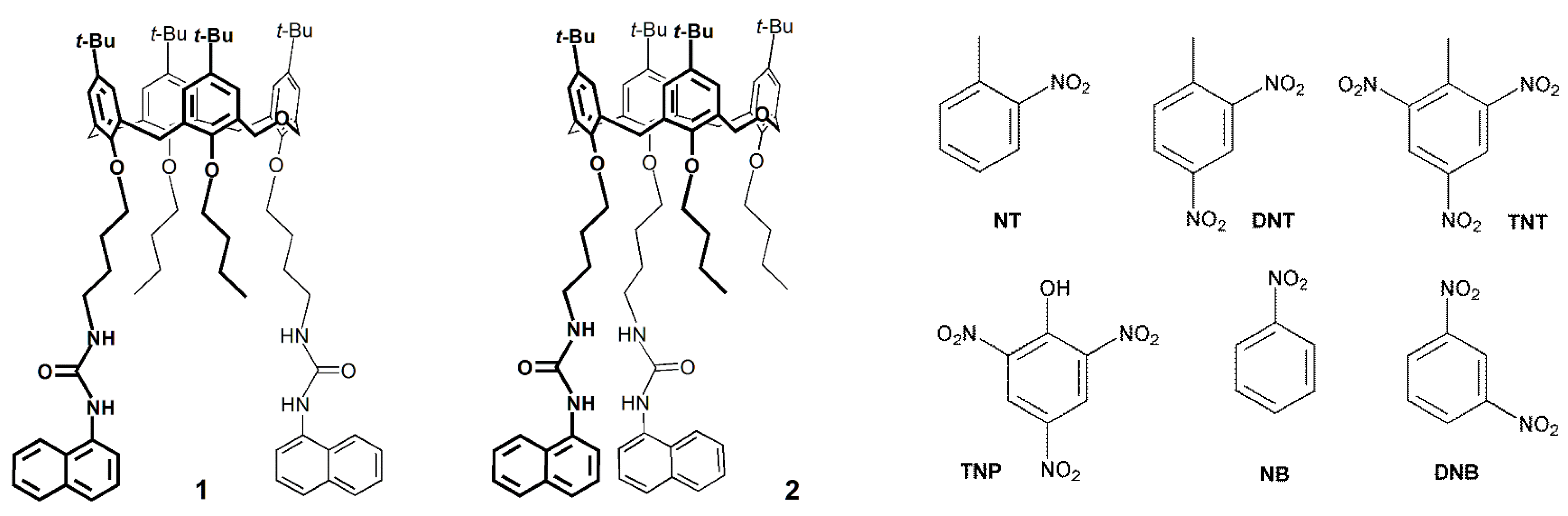
1. Introduction
2. Results and Discussion
2.1. Single-Crystal X-Ray Diffraction Studies
2.2. Detection of Nitroaromatic Compounds
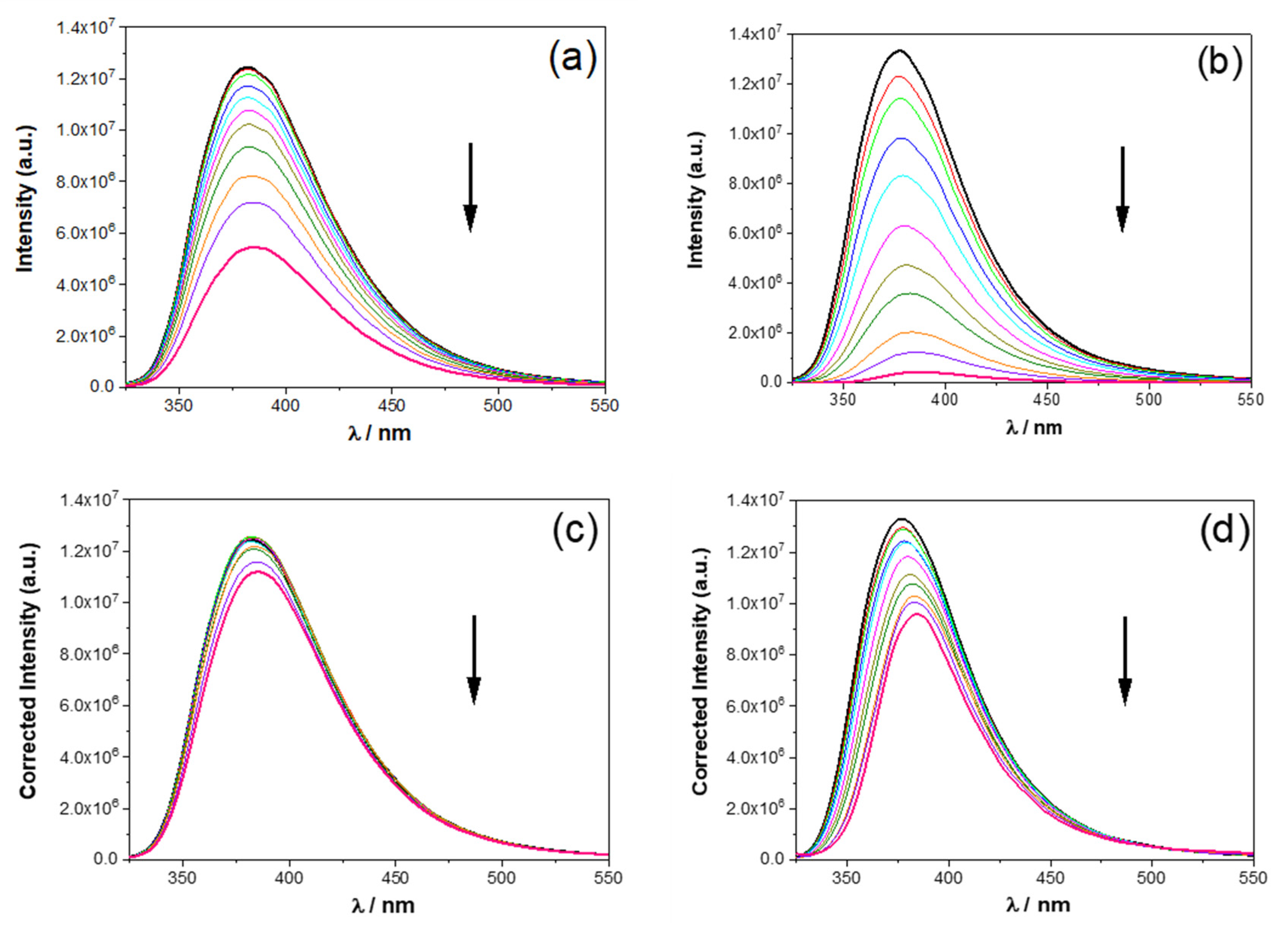
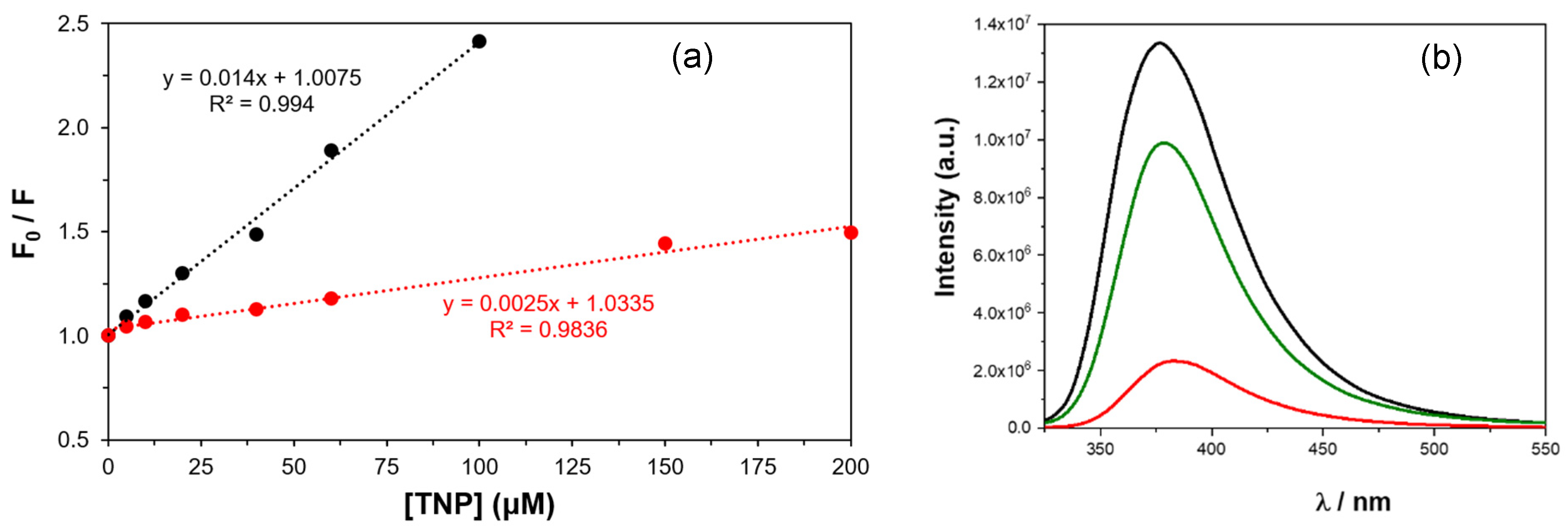
2.2.1. UV-Vis Absorption and Fluorescence Studies in Solution
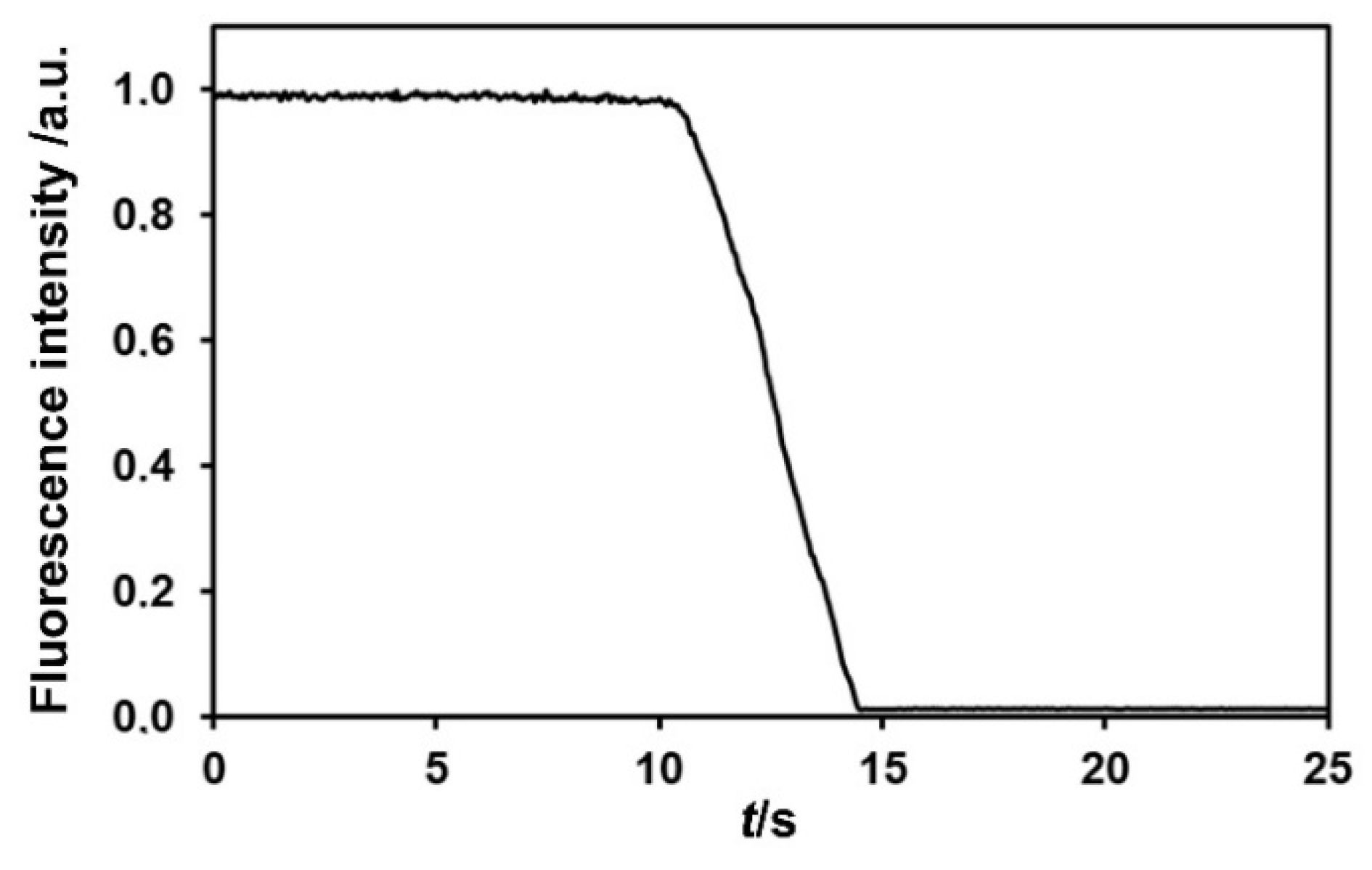
2.2.2. Quenching Studies with NAC Vapours
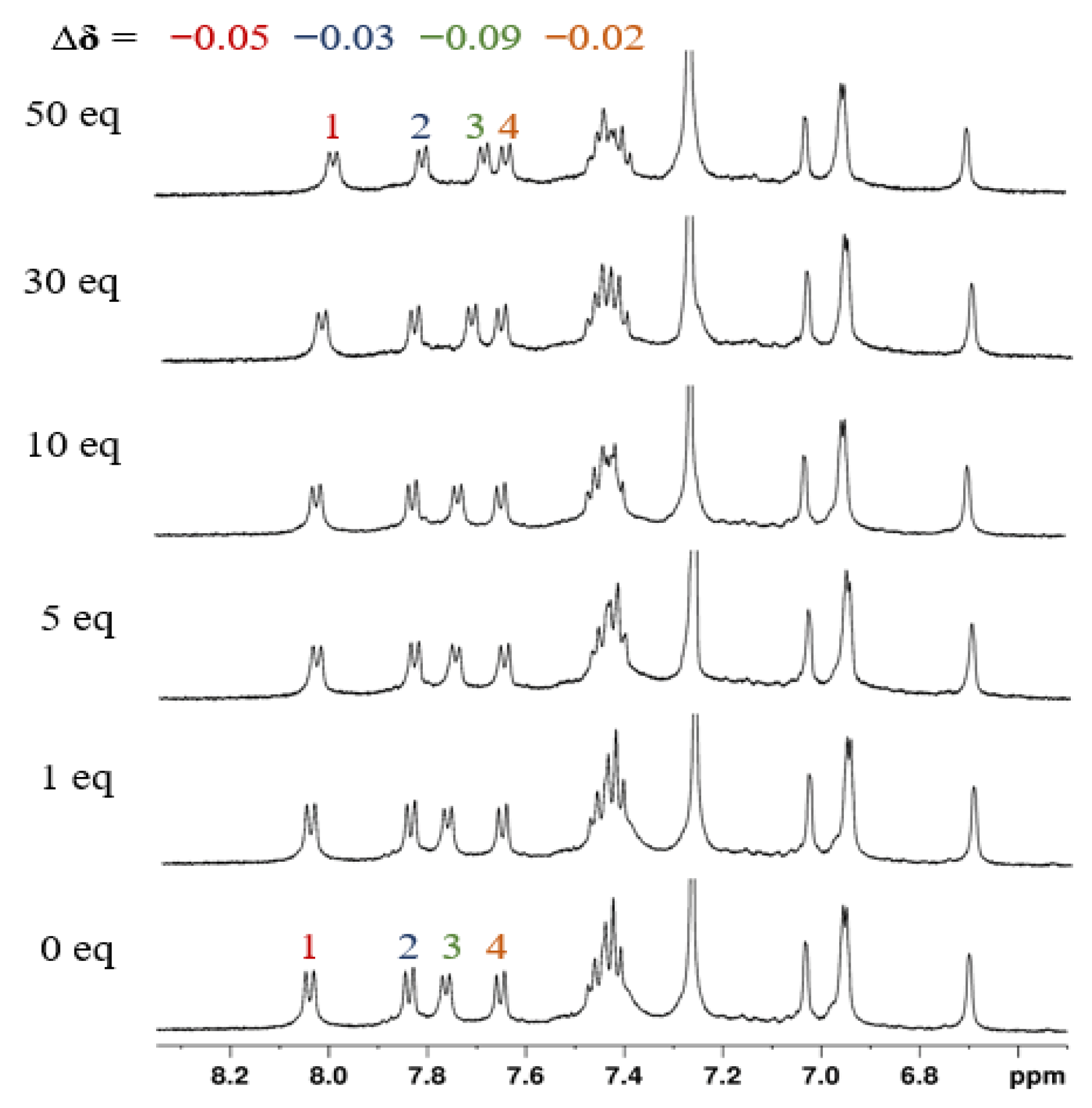
2.2.3. NMR Studies
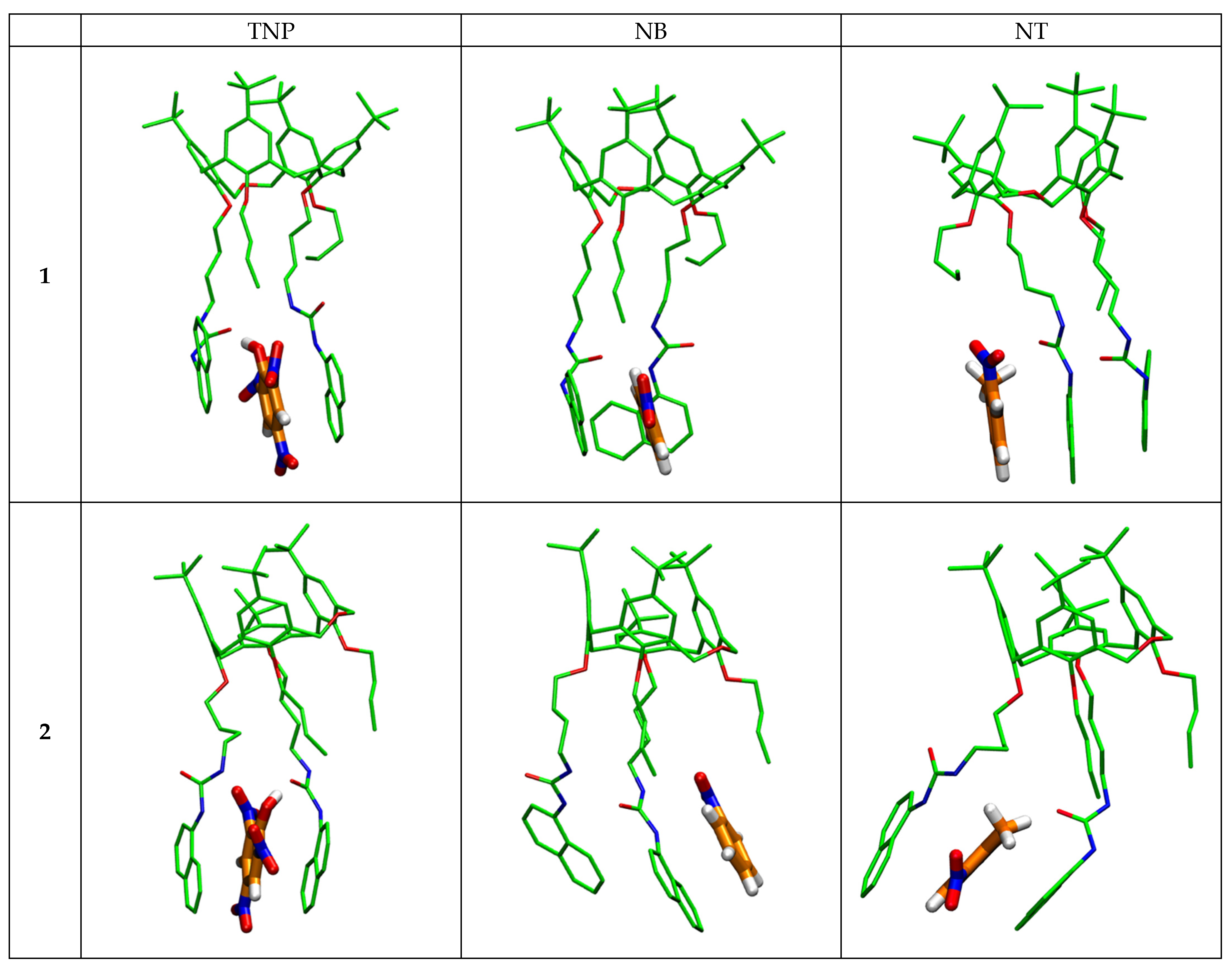
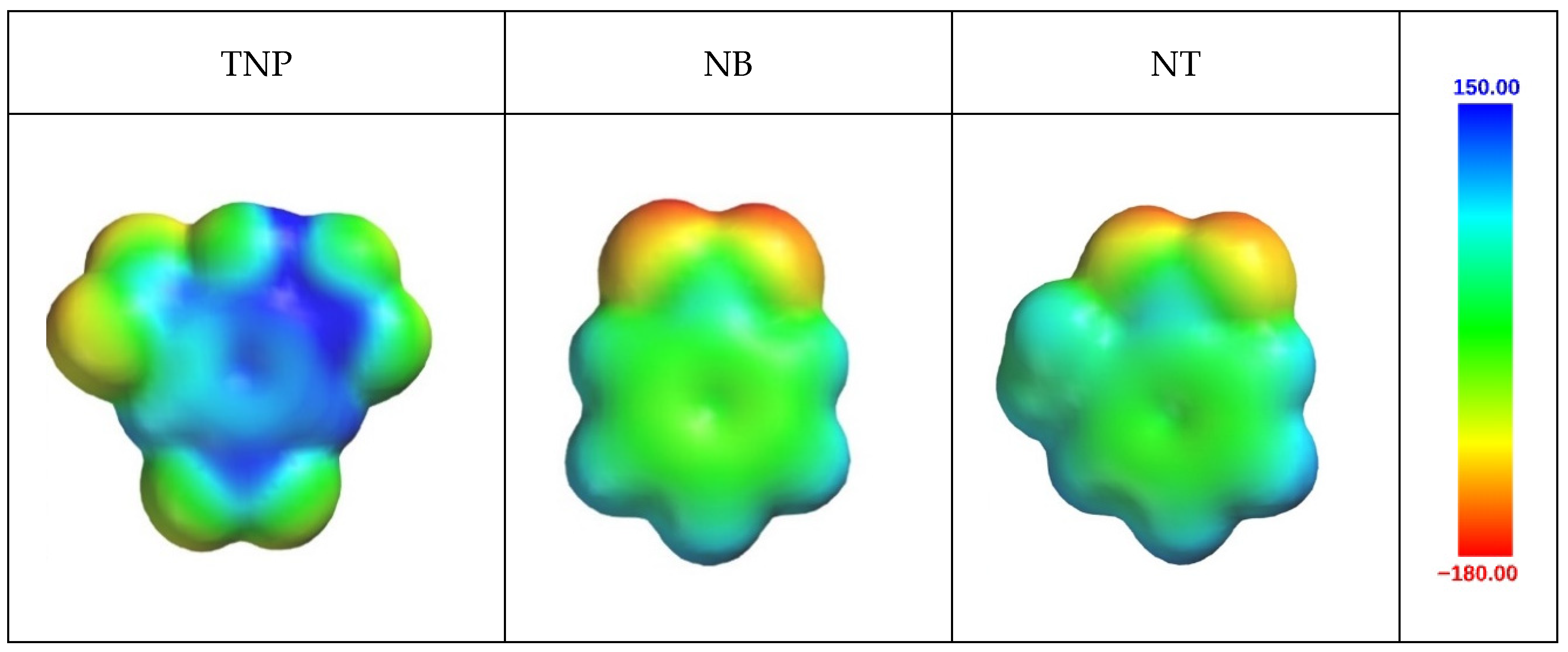
2.2.4. Theoretical Calculations
3. Materials and Methods
3.1. Determination of Crystallographic Structures of 2
3.2. UV-Vis Absorption and Fluorescence Studies
3.3. 1H NMR Studies
3.4. DFT Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sun, X.; Wang, Y.; Lei, Y. Fluorescence based explosive detection: From mechanisms to sensory materials. Chem. Soc. Rev. 2015, 44, 8019–8061. [Google Scholar] [CrossRef]
- Martelo, L.M.; Marques, L.F.; Burrows, H.D.; Berberan-Santos, M.N. Explosive detection: From sensing to response. In Fluorescence in Industry; Springer Series on Fluorescence; Pedras, B., Ed.; Springer: Cham, Switzerland, 2019; Volume 18. [Google Scholar]
- Bilal, M.; Bagheri, A.R.; Bhatt, P.; Bhatt, P.; Chen, S. Environmental occurrence, toxicity concerns, and remediation of recalcitrant nitroaromatic compounds. J. Environ. Manag. 2021, 291, 112685. [Google Scholar] [CrossRef]
- To, K.C.; Ben-Jaber, S.; Parkin, I.P. Recent developments in the field of explosive trace detection. ACS Nano 2020, 14, 10804–10833. [Google Scholar] [CrossRef]
- Li, L.; Lyu, X.; Liang, S.; Liu, Z. Application of fluorescence sensing technology in trace detection of explosives. Dye. Pigment. 2023, 220, 111651. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Rizwan, K.; Bilal, M.; Hussain, T.; Shehzad, S.A. Conjugated supramolecular architectures as state-of-the-art materials in detection and remedial measures of nitro based compounds: A review. TrAC Trends Anal. Chem. 2020, 129, 115958. [Google Scholar] [CrossRef]
- Nguyen, Y.T.; Shin, S.; Kwon, K.; Kim, N.; Bae, S.W. BODIPY-based fluorescent sensors for detection of explosives. J. Chem. Res. 2023, 47. [Google Scholar] [CrossRef]
- Kumar, R.; Jung, Y.; Kim, J.S. Fluorescent calixarene hosts. In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 743–760. [Google Scholar]
- Kumar, R.; Sharma, A.; Singh, H.; Suating, P.; Kim, H.S.; Sunwoo, K.; Shim, I.; Gibb, B.C.; Kim, J.S. Revisiting fluorescent calixarenes: From molecular sensors to smart materials. Chem. Rev. 2019, 119, 9657–9721. [Google Scholar] [CrossRef] [PubMed]
- Desai, V.; Panchal, M.; Dey, S.; Panjwani, F.; Jain, V.K. Recent advancements for the recognization of nitroaromatic explosives using calixarene based fluorescent probes. J. Fluoresc. 2022, 32, 67–79. [Google Scholar] [CrossRef]
- Ren, H.; Wang, H.; Wen, W.; Li, S.; Li, N.; Huo, F.; Yin, C. A summary of calixarene-based fluorescent sensors developed during the past five years. Chem. Commun. 2023, 59, 13790–13799. [Google Scholar] [CrossRef] [PubMed]
- Gutsche, C.D. Calixarenes, an Introduction; Monographs in Supramolecular Chemistry; The Royal Society of Chemistry: Cambridge, UK, 2008. [Google Scholar]
- Neri, P.; Sessler, J.L.; Wang, M.-X. (Eds.) Calixarenes and Beyond; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Lee, Y.H.; Liu, H.; Lee, J.Y.; Kim, S.H.; Kim, S.K.; Sessler, J.L.; Kim, Y.; Kim, J.S. Dipyrenylcalix[4]arene—A fluorescence-based chemosensor for trinitroaromatic explosives. Chem. Eur. J. 2010, 16, 5895–5901. [Google Scholar] [CrossRef]
- Zhan, J.; Zhu, X.; Fang, F.; Miao, F.; Tian, D.; Li, H. Sensitive fluorescence sensor for nitroaniline isomers based on calix[4]arene bearing naphthyl groups. Tetrahedron 2012, 68, 5579–5582. [Google Scholar] [CrossRef]
- Zhang, F.; Luo, L.; Sun, Y.; Miao, F.; Bi, J.; Tan, S.; Tian, D.; Li, H. Synthesis of a novel fluorescent anthryl calix[4]arene as picric acid sensor. Tetrahedron 2013, 69, 9886–9889. [Google Scholar] [CrossRef]
- Cao, X.; Luo, L.; Zhang, F.; Miao, F.; Tian, D.; Li, H. Synthesis of a deep cavity calix[4]arene by fourfold Sonogashira cross-coupling reaction and selective fluorescent recognition toward p-nitrophenol. Tetrahedron Lett. 2014, 55, 2029–2032. [Google Scholar] [CrossRef]
- Boonkitpatarakul, K.; Yodta, Y.; Niamnont, N.; Sukwattanasinitt, M. Fluorescent phenylethynylene calix[4]arenes for sensing TNT in aqueous media and vapor phase. RSC Adv. 2015, 5, 33306–33311. [Google Scholar] [CrossRef]
- Dinda, S.K.; Hussain, M.A.; Upadhyay, A.; Rao, C.P. Supramolecular sensing of 2,4,6-trinitrophenol by a tetrapyrenyl conjugate of calix[4]arene: Applicability in solution, in solid state, and on the strips of cellulose and silica gel and the image processing by a cellular phone. ACS Omega 2019, 4, 17060–17071. [Google Scholar] [CrossRef] [PubMed]
- Narula, A.; Hussain, M.A.; Upadhyay, A.; Rao, C.P. 1,3-Di-naphthalimide conjugate of calix[4]arene as a sensitive and selective sensor for trinitrophenol and this turns reversible when hybridized with carrageenan as beads. ACS Omega 2020, 5, 25747–25756. [Google Scholar] [CrossRef]
- Costa, A.I.; Pinto, H.D.; Ferreira, L.F.V.; Prata, J.V. Solid-state sensory properties of calix-poly(phenylene ethynylene)s towards nitroaromatic explosives. Sens. Actuators B Chem. 2012, 161, 702–713. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Cooperative effects in the detection of a nitroaliphatic liquid explosive and an explosive taggant in the vapor phase by calix[4]arene-based carbazole-containing conjugated polymers. ChemPlusChem 2014, 79, 83–89. [Google Scholar] [CrossRef]
- Prata, J.V.; Costa, A.I.; Teixeira, C.M. A solid-state fluorescence sensor for nitroaromatics and nitroanilines based on a conjugated calix[4]arene polymer. J. Fluoresc. 2020, 30, 41–50. [Google Scholar] [CrossRef]
- Barata, P.D.; Prata, J.V. Fluorescent calix[4]arene-carbazole-containing polymers as sensors for nitroaromatic explosives. Chemosensors 2020, 8, 128. [Google Scholar] [CrossRef]
- Marcos, P.M. Functionalization and properties of homooxacalixarenes. In Calixarenes and Beyond; Neri, P., Sessler, J.L., Wang, M.-X., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 445–466. [Google Scholar]
- Marcos, P.M.; Berberan-Santos, M.N. Fluorescent homooxacalixarenes: Recent applications in supramolecular systems. Front. Chem. 2023, 11, 1258026. [Google Scholar] [CrossRef]
- Wu, C.; Zhao, J.-L.; Jiang, X.-K.; Ni, X.-L.; Zeng, X.; Redshaw, C.; Yamato, T. Click-modified hexahomotrioxacalix[3]arenes as fluorometric and colorimetric dual-modal chemosensors for 2,4,6-trinitrophenol. Anal. Chim. Acta 2016, 936, 216–221. [Google Scholar] [CrossRef]
- Miranda, A.S.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Cragg, P.J.; Schurhammer, R.; Gourlaouen, C. Critical Analysis of Association Constants between Calixarenes and Nitroaromatic Compounds Obtained by Fluorescence. Implications for Explosives Sensing. Molecules 2023, 28, 3052. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Wu, L.-F.; Wu, C.; Rahman, S.; Alodhayb, A.; Redshaw, C.; Georghiou, P.E.; Yamato, T. A facile and sensitive hexahomotrioxacalix[3]arene-based fluorescent sensor for the detection of trace amounts of 2,4,6-trinitrophenol. Sci. Total Environ. 2024, 908, 168209. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.S.; Martelo, L.M.; Fedorov, A.A.; Berberan-Santos, M.N.; Marcos, P.M. Fluorescence properties of p-tert-butyldihomooxacalix[4]arene derivatives and the effect of anion complexation. New J. Chem. 2017, 41, 5967–5973. [Google Scholar] [CrossRef]
- Miranda, A.S.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Schurhammer, R.; Hickey, N.; Geremia, S. Dihomooxacalix[4]arene-based fluorescent receptors for anion and organic ion pair recognition. Molecules 2020, 25, 4708. [Google Scholar] [CrossRef] [PubMed]
- Miranda, A.S.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Menezes, F. Anion binding by fluorescent ureido-hexahomotrioxacalix[3]arene receptors: An NMR, absorption and emission spectroscopic study. Molecules 2022, 27, 3247. [Google Scholar] [CrossRef] [PubMed]
- Gattuso, G.; Notti, A.; Parisi, M.F.; Pisagatti, I.; Marcos, P.M.; Ascenso, J.R.; Brancatelli, G.; Geremia, S. Selective recognition of biogenic amine hydrochlorides by heteroditopic dihomooxacalix[4]arenes. New J. Chem. 2015, 39, 817–821. [Google Scholar] [CrossRef]
- Miranda, A.S.; Serbetci, D.; Marcos, P.M.; Ascenso, J.R.; Berberan-Santos, M.N.; Hickey, N.; Geremia, S. Ditopic receptors based on dihomooxacalix[4]arenes bearing phenylurea moieties with electron-withdrawing groups for anions and organic ion pairs. Front. Chem. 2019, 7, 758. [Google Scholar] [CrossRef]
- Hickey, N.; Iuliano, V.; Talotta, C.; De Rosa, M.; Soriente, A.; Gaeta, C.; Neri, P.; Geremia, S. Solvent and guest-driven supramolecular organic frameworks based on a calix[4]arene-tetrol: Channels vs molecular cavities. Cryst. Growth Des. 2021, 21, 6357–6363. [Google Scholar] [CrossRef]
- Widegren, J.A.; Bruno, T.J. Gas saturation vapor pressure measurements of mononitrotoluene isomers from (283.15 to 313.15) K. J. Chem. Eng. Data 2010, 55, 159–164. [Google Scholar] [CrossRef]
- Gogoi, B.; Paul, N.; Chowdhury, D.; Sarma, N.S. Instant detection of picric acid vapour by developing layer by layer polymer detectors and an electronic prototype. J. Mater. Chem. C 2015, 3, 11081–11089. [Google Scholar] [CrossRef]
- Pushkarsky, M.B.; Dunayevskiy, I.G.; Prasanna, M.; Tsekoun, A.G.; Go, R.; Patel, C.K.N. High-sensitivity detection of TNT. Proc. Natl. Acad. Sci. USA 2006, 103, 19630–19634. [Google Scholar] [CrossRef]
- Kabsch, W. XDS. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 125–132. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, Scaling, Space-Group Assignment and Post-Refinement. Acta Crystallogr. Sect. D Struct. Biol. 2010, 66, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Menezes, F.; Fedorov, A.; Baleizão, C.; Valeur, B.; Berberan-Santos, M.N. Methods for the analysis of complex fluorescence decays: Sum of Becquerel functions versus sum of exponentials. Methods Appl. Fluoresc. 2013, 1, 015002. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. B.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]


| Co-Crystallized Solvent | Unit Cell | S.G. | Z’ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| a (Å) | b (Å) | c (Å) | α (°) | β (°) | γ (°) | ||||
| 2α * | — | 11.473 | 55.43 | 12.189 | 90 | 102.08 | 90 | P21/n | 1 |
| 2β | DMSO/CHCl3 | 20.486 | 16.422 | 24.136 | 90 | 100.186 | 90 | P21/c | 1 |
| 2γ | EtOH | 18.218 | 27.893 | 30.697 | 93.026 | 92.572 | 91.321 | P-1 | 4 |
| 2δ | CH3CN | 18.199 | 30.879 | 30.919 | 63.535 | 87.731 | 89.789 | P-1 | 4 |
| 2ε | CH3CN | 11.411 | 12.425 | 27.438 | 84.719 | 83.052 | 83.765 | P1 | 2 |
| Molecule | A (°) | B (°) | C (°) | D (°) | Nap-Nap (°) |
|---|---|---|---|---|---|
| 2α | 125 | 83 | 140 | 92 | 88 |
| 2β | 116 | 71 | 130 | 100 | 57 |
| 2γ-1 | 120 | 73 | 151 | 98 | 87 |
| 2γ-2 | 117 | 76 | 144 | 101 | 75 |
| 2γ-3 | 120 | 74 | 147 | 96 | 81 |
| 2γ-4 | 118 | 76 | 153 | 100 | 86 |
| 2δ-1 | 119 | 74 | 153 | 98 | 84 |
| 2δ-2@CH3CN | 117 | 93 | 141 | 101 | 64 |
| 2δ-3@CH3CN | 108 | 95 | 138 | 103 | 88 |
| 2δ-4 | 119 | 73 | 145 | 95 | 75 |
| 2ε-1@CH3CN * | 118 | 97 | 137 | 98 | 80 |
| 2ε-2@CH3CN | 118 | 97 | 135 | 99 | 78 |
| τ1/ns (%) | τ2/ns (%) | τ3/ns (%) | τ4/ns (%) | /ns | |
|---|---|---|---|---|---|
| 1 * | 0.27 (9) | 2.21 (13) | 11.49 (78) | — | 9.4 |
| 1 + NT | 0.27 (9) | 2.14 (14) | 9.43 (77) | — | 7.6 |
| 1 + DNT | 0.28 (9) | 2.19 (14) | 10.11 (77) | — | 8.1 |
| 1 + TNT | 0.27 (10) | 2.02 (12) | 9.97 (78) | — | 8.0 |
| 1 + NB | 0.25 (10) | 2.04 (12) | 9.54 (78) | — | 7.7 |
| 1 + DNB | 0.26 (10) | 2.04 (11) | 10.69 (79) | — | 8.7 |
| 1 + TNP | 0.27 (10) | 2.01 (14) | 10.84 (77) | — | 8.6 |
| 2 * | 0.20 (6) | 0.99 (7) | 3.77 (31) | 11.96 (56) | 7.9 |
| 2 + NT | 0.20 (7) | 0.77 (6) | 3.35 (28) | 9.86 (59) | 6.7 |
| 2 + DNT | 0.24 (10) | — | 2.00 (12) | 9.52 (78) | 7.7 |
| 2 + TNT | 0.23 (7) | 1.23 (8) | 3.83 (38) | 11.17 (47) | 6.8 |
| 2 + NB | 0.20 (6) | 0.75 (6) | 3.09 (35) | 9.46 (53) | 6.1 |
| 2 + DNB | 0.21 (7) | 0.81 (6) | 3.36 (34) | 10.45 (53) | 6.8 |
| 2 + TNP | 0.21 (7) | 0.94 (7) | 3.51 (34) | 10.43 (52) | 6.7 |
| NT | DNT | TNT | NB | DNB | TNP | ||
|---|---|---|---|---|---|---|---|
| 1 | KSV | 1550 | 490 | 440 | 1200 | 270 | 3000 |
| DL | 31 | 105 | 153 | 52 | 212 | 18 | |
| 2 | KSV | 1200 | 465 | 320 | 1000 | 150 | 2500 |
| DL | 39 | 135 | 189 | 48 | 214 | 21 | |
| ∆E (kJ mol−1) | ||
|---|---|---|
| 1 | 2 | |
| TNP | −92.09 | −109.33 |
| NB | −42.59 | −73.72 |
| NT | −56.23 | −36.99 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gil, B.V.; Miranda, A.S.; Marcos, P.M.; Ascenso, J.R.; Palmeira, T.; Berberan-Santos, M.N.; Schurhammer, R.; Hickey, N.; Joshi, S.; Geremia, S. Fluorescent Dihomooxacalix[4]arenes for the Detection of Nitroaromatic Compounds in Solution and in the Vapour Phase: Structural and Supramolecular Insights. Molecules 2025, 30, 3901. https://doi.org/10.3390/molecules30193901
Gil BV, Miranda AS, Marcos PM, Ascenso JR, Palmeira T, Berberan-Santos MN, Schurhammer R, Hickey N, Joshi S, Geremia S. Fluorescent Dihomooxacalix[4]arenes for the Detection of Nitroaromatic Compounds in Solution and in the Vapour Phase: Structural and Supramolecular Insights. Molecules. 2025; 30(19):3901. https://doi.org/10.3390/molecules30193901
Chicago/Turabian StyleGil, Beatriz V., Alexandre S. Miranda, Paula M. Marcos, José R. Ascenso, Tiago Palmeira, Mário N. Berberan-Santos, Rachel Schurhammer, Neal Hickey, Siddharth Joshi, and Silvano Geremia. 2025. "Fluorescent Dihomooxacalix[4]arenes for the Detection of Nitroaromatic Compounds in Solution and in the Vapour Phase: Structural and Supramolecular Insights" Molecules 30, no. 19: 3901. https://doi.org/10.3390/molecules30193901
APA StyleGil, B. V., Miranda, A. S., Marcos, P. M., Ascenso, J. R., Palmeira, T., Berberan-Santos, M. N., Schurhammer, R., Hickey, N., Joshi, S., & Geremia, S. (2025). Fluorescent Dihomooxacalix[4]arenes for the Detection of Nitroaromatic Compounds in Solution and in the Vapour Phase: Structural and Supramolecular Insights. Molecules, 30(19), 3901. https://doi.org/10.3390/molecules30193901










