Abstract
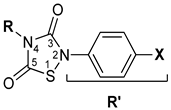
A focused library of 1,2,4-thiadiazolidin-3,5-diones (THIA-1–10), previously characterized as hydrogen sulfide (H2S) donors, was evaluated for inhibitory activity against cysteine proteases. We included two key cysteine proteases aiming at antiviral drug development—SARS-CoV-2 3CLpro (Mpro) and PLpro—alongside reference enzymes Papain and Cathepsin L. The compounds exhibited distinct selectivity profiles and inhibition mechanisms. The ability to act as covalent inhibitors of 3CLpro in the nanomolar range is of particular interest, with compounds THIA-6, -7, and -10 proving to be the most potent inhibitors of the series, and compounds THIA-1, -2, and -8 proving to be the most selective with respect to the other proteases. We explored the molecular bases of the observed activity profile of THIA-1–10 through computational studies, which supported and complemented the experimental findings, paving the way for future structure optimization. The results highlight that inhibitory potency depends not only on electrophilicity but also on the ability to access the catalytic cysteine within the active site. The dual functionality of THIA-1–10 as H2S donors and selective cysteine protease inhibitors underscores its potential as a promising lead for therapeutic development.
1. Introduction
Cysteine proteases have emerged as critical therapeutic targets, particularly in the context of viral infections such as SARS-CoV-2. These enzymes play essential roles in viral replication and immune evasion, making them attractive candidates for the development of selective covalent inhibitors. 1,2,4-Thiadiazolidin-3,5-diones (THIAs) are a versatile class of heterocycles with diverse pharmacological applications. They have been reported as inhibitors of glycogen synthase kinase 3β (GSK3β) [1], regulators of G protein signaling (RGS) proteins [2], and hydrogen sulfide (H2S) donors [3]. The underlying mechanisms of these activities are based on the reaction of the sulfur atom of THIAs with free sulfhydryl groups (SH) contained in compounds that release H2S [3] or with the catalytic cysteine residue in enzymatic reactions [2]. Given this reactivity, inhibition of cysteine proteases is an expected activity for THIA derivatives. However, 4-(4-fluorobenzyl)-2-p-tolyl-1,2,4-thiadiazolidine-3,5-dione (CCG-50014) failed to inhibit Papain [4]. This intriguing observation led us to consider that the covalent inhibition requires, besides the reaction of the THIA ring with the catalytic cysteine, the adequate fitting of the compound in the active site, as defined for Papain by Schechter and Berger [5] and mapped in other cysteine proteases with libraries and systematic series of synthetic peptide substrates [6,7,8,9,10].
Based on these considerations, we selected a focused library of ten THIA derivatives (THIA-1–10) (Table 1), previously characterized as H2S donors [3], to evaluate their inhibitory activity against cysteine proteases with distinct substrate specificities. We chose two reference enzymes—Papain and Cathepsin L—and two SARS-CoV-2 proteases: the Chymotrypsin-like protease (3CLpro or Mpro), essential for viral polyprotein processing, and the papain-like protease (PLpro), which exhibits deubiquitinating and deISGylating activities. The aim of this study is twofold: (i) to assess the potential of THIA-1–10 as selective covalent inhibitors of cysteine proteases and (ii) to elucidate the molecular basis of their activity through computational approaches, thereby providing a foundation for future structure-based optimization.

Table 1.
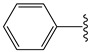
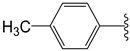
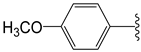
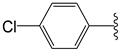
The 1,2,4-thiadiazolidine-3,5-diones: THIA 1–10.
Papain and Cathepsin L share 41% sequence identity and exhibit similar crystal structures [11,12], with well-characterized S1 to S1’ subsites that accommodate substrate residues and contribute to binding specificity [13,14,15]. 3CLpro is active only in its dimeric form [16]. Each homodimer comprises three domains: domains I and II consist of antiparallel β-barrels that form the chymotrypsin-like fold, while domain III facilitates dimerization. The catalytic center consists of a Cys145–His41 dyad. Crystal structures of 3CLpro–inhibitor complexes reveal that the hydrophobic residues from P3 to P1′ of the inhibitors fit into the corresponding protease subsites S3 to S1’ [17,18,19,20,21]. PLpro is a cysteine protease with a canonical catalytic triad composed of Cys111, His272, and Asp289. Its S2 and S1 subsites are located within a narrow channel that accommodates the Gly–Gly motif, enabling the enzyme to perform deubiquitinating and deISGylating activities [22,23,24,25,26,27].
We initially evaluated the stability of THIA-1–10 in organic solvents and buffer solutions. Subsequently, we determined the inhibition type for each protease using THIA-3 as a reference compound, followed by the calculation of IC50 values for all other THIA derivatives. Despite the limited number of compounds investigated in the present work, they exhibited notable in vitro potency and selectivity toward therapeutically relevant cysteine proteases. Accordingly, computational studies—including conformational analysis, density functional theory (DFT) calculations, molecular docking simulations, and structural and bioinformatics analyses—were performed to investigate the molecular bases of the observed activity, laying the groundwork for future optimization of the identified hits.
2. Results and Discussion
2.1. Stability of THIA Derivatives
We chose THIA-3 as a reference due to its simplicity in larger-scale synthesis to evaluate the stability of this class of compounds. In DMSO with 1% water, THIA-3 decomposed to 1,3-diphenylurea, a surprising result that occurred surprisingly quickly (Figure S1A). However, in acetonitrile (ACN), THIA-3 remained stable even when diluted in water or phosphate buffer. In the Tris buffer, decomposition to 1,3-diphenylurea was very slow, with some decomposition only apparent after 12 h of incubation (Figure S1B). We also evaluated the stability of 10 mM stock solutions of the ten THIAs in DMSO stored in a freezer (−20 °C) by Liquid Chromatography Mass Spectrometry (LC-MS). THIA-1, THIA-2, THIA-7, THIA-8, and THIA-10 were the most resistant, indicating that the stability of the THIA derivatives in this condition depends on R or R’ substituents (Figure S2). DMSO is also considered a reagent [28], but we have not explored the decomposition of THIA compounds in DMSO further, as it falls outside the scope of this work. These results justified using ACN as the organic solvent of the THIA derivatives and 100 mM phosphate as the buffer in all experiments with the cysteine proteases.
2.2. Inhibitory Activities of THIAs on Cysteine Proteases
2.2.1. Selection of Substrates
Z-Phe-Arg-MCA is a commercially available fluorescent substrate for Papain and Cathepsin L. Both proteases hydrolyze it at the Arg-MCA peptide bond with high kcat and Km values. 3CLpro and PLpro cleave the SARS-CoV-2 polyprotein into sixteen mature components, termed nonstructural proteins (NSPs). We synthesized the fluorescence resonance energy transfer (FRET) peptide Abz-SAVLQSGFRK(Dnp)NH2 [Abz = ortho-aminobenzoic acid; K(Dnp) = N-ε-(2,4-dinitrophenyl) Lys]) based on the SARS-CoV-2 3CLpro optimum substrate sequence, as we previously reported [16]. For PLpro, we initially synthesized and assayed a series of FRET peptides of general structure Abz-peptidyl-Q-EDDnp (EDDnp = ethylene diamine 2,4-dinitrophenyl) [29] with sequences of SARS-CoV-2 polyprotein containing the sites nps2/3 and nps3/4 cleaved by PLpro and four peptides containing the C- terminal sequence of two ubiquitins linked to εNH2-caproic acid carrying Q-EDDnp or the peptide sequence of two ubiquitinated protein (Table S1). Based on these results, we selected Abz-TLKGGAPIK-Q-EDDnp as the substrate for PLpro.
2.2.2. Kinetic Mechanism of Inhibition
Initially, we identified the type of inhibition in each protease using THIA-3 as a reference compound for all the tested THIA derivatives. The Papain activity decreased with progressive THIA-3 pre-incubation time (Figure S3A), indicating irreversible inhibition that also occurred with Cathepsin L. Using a previously reported protocol [30], we observed a covalent irreversible inhibition of THIA-3 on Papain and 3CLpro, reverted by reducing agents (Figure S4), and reversible inhibition of PLpro (Figure S3B,C). Due to the susceptibility of the THIA derivatives to reducing agents, such as Cys or DTT (Figure S4), the proteases Papain, Cathepsin L, 3CLpro, and PLpro were separately pre-activated by 5 mM DTT during 30 min, which was removed by gel filtration, forming stock solutions of the activated proteases, and then kept at −20 °C under an N2 atmosphere for future use.
Figure 1 shows the reactions of THIA-3 and THIA-10 with Papain, as well as THIA-3 with 3CLpro, which are compatible with a two-step covalent enzyme inactivation mechanism. This is confirmed by kinetic analyses of the reaction of THIA-3 with Papain, used as a diagnostic method as previously reported [31]. The kinetic constant values of the reaction of the ten THIA compounds with Papain, for the reaction paths shown below, are reported in Table S2.
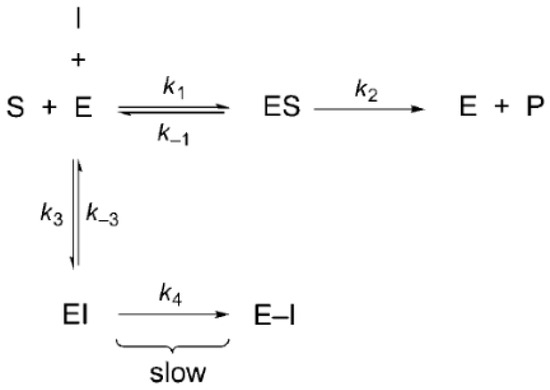
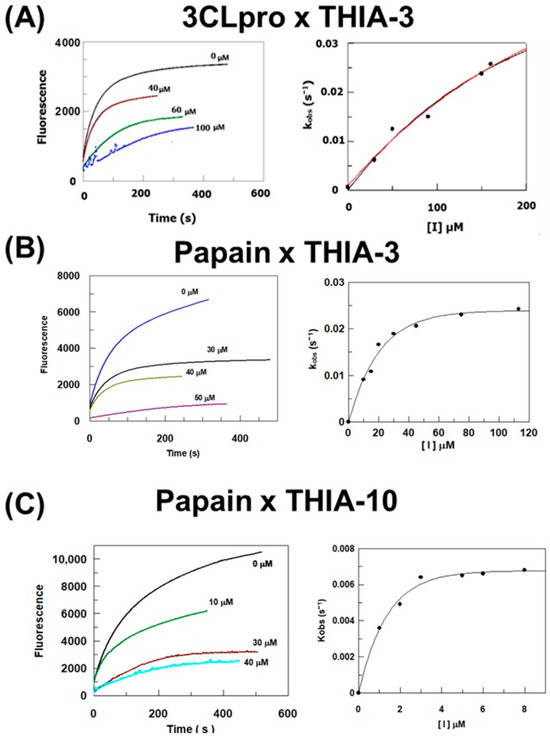
Figure 1.
(A) Left panel: Time course of the hydrolysis of Abz-SAVLQSGFRK(Dnp)-NH2 by 3CLpro in the absence and presence of THIA-3 (concentrations indicated near the curves). Right panel: Inactivation rate constants as a function of THIA-3 concentration. (B,C) Left panels: Time course of the hydrolysis of Z-FR-MCA by Papain in the absence and presence of THIA-3 or THIA-10 (concentrations indicated near the curves). Right panels: Inactivation rate constants as a function of THIA-3 or THIA-10 concentrations.
These results confirmed a two-step, irreversible bimolecular reaction, namely, inhibitor binding and the formation of a covalent inhibitory complex, as shown in Scheme 1A. The assistance in this reaction of the imidazole group of His that belongs to the cysteine protease cysteine catalytic triad is shown in Scheme 1B.
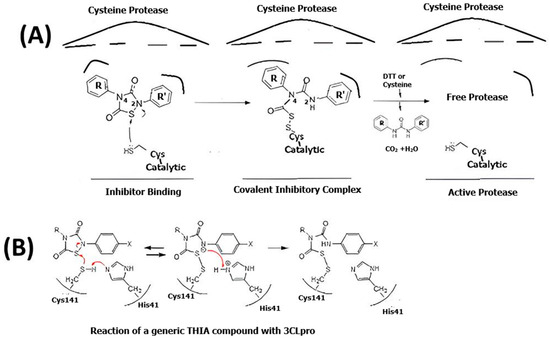
Scheme 1.
(A) Schematic representation of a putative two-step mechanism for irreversible cysteine protease modification by THIA derivatives. First step: inhibitor binding. Second Step: covalent complex formation. The reductive agents (DTT or Cysteine) reactivate the protease. (B) Chemical reactions of the covalent inhibition at the cysteine protease active site.
The nucleophilic attack of the catalytic cysteine to the S1 sulfur atom of the THIA ring and the subsequent adduct formation is superimposable to what was previously proposed by us for the reaction with free L-cysteine in solution [3] (Scheme 1). However, the reaction of the resulting adducts with a second cysteine observed in the solution of this free amino acid, resulting in the release of H2S, is not conceivable with the cysteine protease.
To compare the inhibition efficiency of the THIA derivatives on the four cysteine proteases, we fixed the pre-incubation time at 10 min to obtain the IC50 values. Table 2 shows the obtained results.

Table 2.
IC50 for Inhibition of Papain, 3CLpro, PLpro, and Cathepsin L by THIA derivatives.
2.3. IC50 Values with Papain and Cathepsin L
The inhibitory activity trend of THIA-1–10 is very parallel for papain and cathepsin L, even though most of the compounds were more active on papain, as reported in Table 2. In particular, THIA-1, THIA-2, and THIA-8 were the worst inhibitors on both proteases, with IC50 in the μM range, while all other THIAs showed IC50 values in the nM range. The common feature that distinguishes THIA-1, THIA-2, and THIA-8 from the other tested compounds is the presence of an alkyl group as an R substituent. In contrast, in the other compounds, R is a phenyl ring (with or without para-substitution). In the papain family, the S1 subsite is strictly conserved (Table S3), showing a preference for positively charged residues (i.e., arginine and lysine). On the contrary, the S2 subsite is quite different in shape and size and represents the predominant factor in defining substrate or inhibitor specificities [7,11,12,14,32] (for more details, see the paragraph “Investigation of the molecular bases of THIA selectivity”). Finally, papain and cathepsin L also present some differences in the S3 subsite (Table S3) due to their broader interaction specificity [8]. THIA-1 and THIA-2, with a benzyl group and THIA-8, with a cyclohexyl group, as R substituents, proved to be the least effective inhibitors on both enzymes, suggesting that the effect exerted by the presence of an alkyl carbon at N4 on the reactivity of the THIA ring may be involved in the drop in inhibitory activity. Interestingly, compound CCG-50014, which exhibits a complete absence of papain inhibition [4], has a benzyl group as an R substituent, similar to THIA-1 and THIA-2, but with fluorine in the para position. Possibly, fluorine in the para position of the benzyl group further alters its properties [33,34,35], impairing the CCG-50014 reaction with papain. In contrast, THIA-7 with the 4-chlorophenyl group as the R substituent inhibited papain and cathepsin L with IC50 values in the nanomolar range, similar to the unsubstituted analog THIA-4. The halogen at the para position of the phenyl ring did not impair the inhibition processes, indicating that the drop in the activity is related to the presence of the benzyl group.
THIA-6, bearing 4-methoxyphenyl substituents at both N2 and N4, is one of the most effective inhibitors of Papain. Notably, its IC50 (0.01 μM) is lower than that of THIA-4 (0.07 μM), containing just one 4-methoxyphenyl at N2 (R’ substituent), which is, in turn, lower than that of THIA-3 (0.14 μM), presenting two unsubstituted phenyl rings as substituents. These results suggest that both 4-methoxyphenyl groups of THIA-6, at R and R’ positions, may have favorable interactions with Papain.
Finally, THIA-10 is another of the best inhibitors of Papain. Its IC50 (0.01 μM) is lower than that of THIA-3 (0.14 μM), and they differ only by the presence of fluorine in the para position of the phenyl group at the R’ substituent. The electron-negative inductive effect of fluorine in the para position of the phenyl group in the R’ substituent is supposed to speed up the nucleophilic attack of the papain catalytic Cys on the sulfur atom (S1) of the THIA ring. This electronic effect of the R’ substituent was evident in the release of H2S by reaction of THIA-10 with Cys [3]. On the other hand, the inhibition of Cathepsin L by THIA-10 yielded an IC50 similar to that obtained with THIA-3, while the IC50 values of THIA-1, -2, and -8 are ten times higher than those obtained with Papain.
To investigate the role of S2 subsite fitting in the selective inhibition of cysteine proteases by THIA derivatives, we evaluated their activity against bromelain. Indeed, this protease is unique among cysteine proteases, as it requires substrates containing a positively charged residue at the P2 position due to the presence of Glu68 in its S2 subsite [36]. Notably, we observed inhibition with the pre-incubation during 10 min of 10 μM only with THIA-7 (IC50 = 2.0 ± 0.1μM), which contains the para-Cl-phenyl group in the R position. The Cl at the para position induces dipole charge resonance structures in the phenyl group, establishing a favorable interaction with the Glu68 carboxylate group in the S2 subsite (Figure S16A; and see Section 2.5 “Molecular Modeling Studies”).
THIA compounds seem to have comparable reactivity as the classical known irreversible cysteine protease inhibitors, such as L-trans-Epoxysuccinyl-leucylamido (4-guanidino)butane (E-64) and its analogues, which are also more effective irreversible inhibitors of papain than cathepsin L [37], similar to what we observed with the THIA compounds. The poor inhibition of Bromelain by E-64 [36], due to the presence of the negative charge of Glu68 at the bottom of S2 subsite, was also observed with THIA compounds since bromelain was inhibited only by THIA-7, possibly due to the Cl substitution in the para position of the phenyl group at N4 of THIA-7, which allows better fitting to the S2 subsite. Another relevant comparison is with the peptidyl diazomethane Z-Phe-Phe-CHN2, which is a better inhibitor of cathepsin L than Z-Phe-Ala-CHN2 [37], because Z-Phe-Phe-CHN2 occupies both the S1 and S2 subsites of cathepsin L. A similar situation is observed with THIA-3 (IC50 = 0.12µM), which has two aromatic substituents, in contrast to THIA-8 (IC50 = 27 µM), which has an aromatic substituent only in N2 of the THIA ring.
2.4. IC50 Values with SARS-CoV-2 Cysteine Proteases
The IC50 inhibition values of 3CLpro with the ten THIA derivatives (Table 2) are in the nanomolar (nM) range, demonstrating remarkable performance as protease inhibitors. THIA-6, THIA-7, and THIA-10 are the best inhibitors, with IC50 values ten times lower than those of the other assayed THIAs, except for THIA-5. In contrast with Papain and Cathepsin L, the proteases 3CLpro and PLpro have, respectively, 12 and 11 Cys residues (Figure 2). The room-temperature crystal structure of 3CLpro demonstrated that only catalytic Cys145 is oxidized. At the same time, the other surface Cys remains reduced, and only Cys145 and Cys156 react with the alkylating agent N-ethylmaleimide [20]; then, the irreversible inhibition of 3CLpro by THIA derivatives must be due to their reaction with Cys145 in the catalytic site [21].
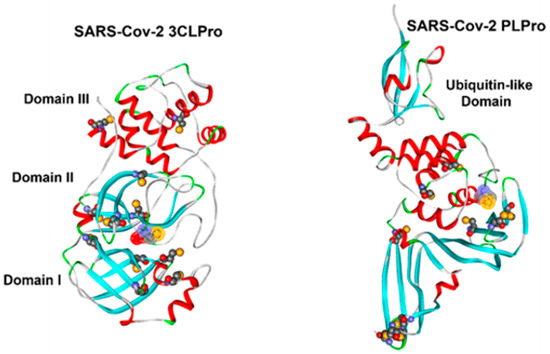
Figure 2.
Free cysteine residues in SARS-CoV-2 3CLpro (PDB ID: 7JKV) and PLpro (PDB ID: 6WX4). Cysteines are shown in CPK representation and colored by atom type. The solvent-accessible surface of the catalytic cysteine is highlighted. The protein backbone is displayed as ribbons and colored according to secondary structure (white = loop, green = turn, red = α-helix, cyan = β-strand).
To correlate the 3CLpro substrate specificity with its inhibition by the THIA derivatives, we synthesized and assayed a library of FRET peptides with 3CLpro, using Abz-SAVQSSGFRK(Dnp)-NH2 as the reference sequence that spans the S4 to S3’ subsites. The amino acid sequence of this peptide spans the cleavage between the nonstructural protein 4 (nsp4) and the N-terminal side of 3CLpro (SAVLQ3240↓S3241GFRK), which is highly susceptible to 3CLpro [16]. Figure 3 shows the hydrolytic activity of 3CLpro on each peptide.
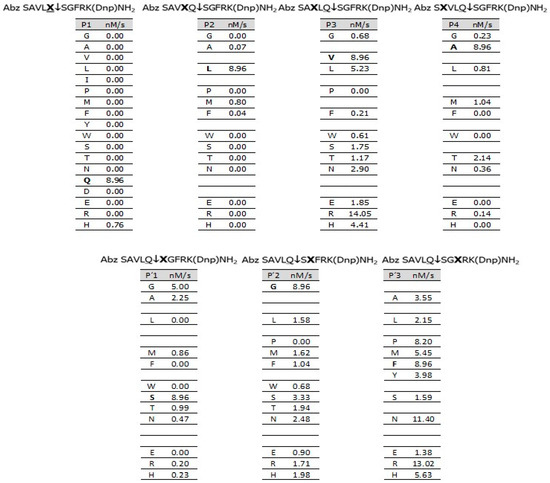
Figure 3.
Hydrolytic activity (nM/s) of 3CLpro on a FRET peptide library, using Abz-SAVQSSGFRK(Dnp)-NH2 as the reference sequence. Experimental conditions: 50 mM Tris, 1 mM EDTA, pH 7.5, 5 mM DTT, at 37 °C. [3CLpro] = 54 nM; [Peptides] = 12 μM. ↓ indicates cleavage site.
At position P1, 3CLpro hydrolyzed only peptides containing Gln and His, with the latter being a very poor substrate. The carboxamide nitrogen of Gln is isosterically equivalent to Nτ-imidazole; in contrast, the nitrogen of Asn is isosteric to Nπ-imidazole of His (Scheme 2), but the peptide with Asn is resistant to 3CLpro. Notably, there is a very restrictive specificity to the carboxamide group of Gln of the S1 subsite of 3CLpro.
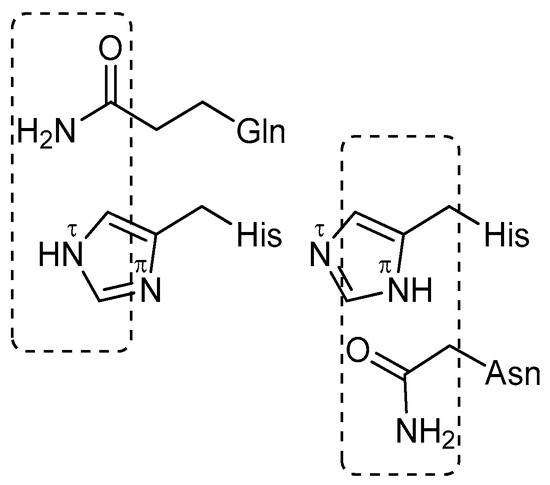
Scheme 2.
Structural comparison of the side chains of histidine, asparagine, and glutamine, highlighting their chemical relationships and functional group similarities.
The S2 subsite of 3CLpro is also highly selective, efficiently cleaving only the peptide with Leu. Little hydrolysis occurred with the hydrophobic amino acids Met and Phe, and complete resistance to hydrolysis was observed with all other amino acids. The S3 subsite is more tolerant and accepts almost all classes of amino acids, with the highest hydrolysis with Val, Leu, and Arg, while S4 significantly prefers Ala, suggesting it is a shallow subsite. The subsites S1’ and S2’ exhibit selectivity for amino acids with small side chains, with a preference for Gly, and finally, S3’ accepts any amino acid. We also synthesized and assayed a second library containing His at the P1 position with 3CLpro (Figure S5), and the profile of specificity was very similar to that of Gln in the P1 position, except that peptides with Met, Phe, and Thr at the P2 position were more efficiently hydrolyzed. The THIA derivatives are short compared to the FRET peptides; still, as covalent inhibitors, the THIA ring must occupy the S1 subsite for the nucleophilic attack by Cys145 and, due to the hydrophobicity of the S2 subsite, it very probably binds one of the THIA substituents (R or R’).
While we were developing this work, a detailed analysis of the specificity of the S6-S6’ subsites of 3CLpro using a comprehensive methodology called Proteomic Identification of Cleavage site Specificity (PICS) was published [38]. Overall, our results align with those obtained by PICS, and the substrate Abz-SAVLQSGFRK(Dnp)-NH2 used for 3CLpro assays proved adequate.
The IC50 values of THIA derivatives with PLpro are all in the μM range, with about half of them exceeding 10 μM. (Table 2). The observed reversible inhibition (Figure S3C) is due to the inaccessibility of subsites S1 and S2 to larger molecular structures than glycyl-glycine, resulting in weaker inhibition of THIAs compared to the covalent inhibitors that resulted from the nucleophilic reaction with Cys111 in the catalytic site [39]. This reversible inhibition also indicates that the other ten Cys residues of PLpro, as shown in Figure 2, are not reactive toward THIA derivatives under the conditions we tested. The noncovalent deubiquitinase inhibitor 5-amino-2-methyl-N-[(1R)-1-(1-naphthalenyl)ethyl]benzamide (GRL0617) inhibited PLpro (IC50 = 0.6 μM) through an unusual mechanism, binding to the S4–S3 subsites and inducing a conformational change in the loop that closes the access to the catalytic site [23]. The THIA-5 and THIA-8, with lower IC50 values (3.9 μM and 4.2 μM, respectively), contain some components, such as GRL0617. The good structural overlap between GRL0617 and THIA-5 in complex with PLpro supports the putative binding of both compounds to the same allosteric site (for more details, see the paragraph “Investigation of the molecular bases of THIA selectivity”; Figure S14).
2.5. Molecular Modeling Studies
To elucidate the molecular basis of the inhibitory activity of THIA derivatives and to gain critical insights for future structural optimization, a comprehensive set of computational studies was conducted. Initially, the conformational and electronic properties of the compounds listed in Table 1 were evaluated using a combination of Molecular Mechanics (MM) and Density Functional Theory (DFT) calculations. Subsequently, molecular docking simulations with 3CLpro were performed on a representative subset of derivatives, ranging from the most active (THIA-7) to the least active (THIA-8). These initial docking studies were designed to simulate the adaptation of the inhibitor within the enzyme’s active site, with the specific aim of positioning the reactive warhead in proximity to the catalytic cysteine, thereby facilitating the nucleophilic attack required for covalent bond formation. To rationalize the observed selectivity with respect to other cysteine proteases, a bioinformatic and structural analysis was conducted. Finally, covalent docking techniques were employed to explore the potential adaptation of the inhibitor within the enzyme’s active site following the formation of a covalent bond.
2.5.1. Ligand Analysis
A stochastic conformational search procedure sampled the conformational space of THIA derivatives [40], followed by DFT full optimization of the MM conformers using the conductor-like polarizable continuum model (C-PCM) to mimic an aqueous environment [41]. The comparison of the starting MM conformers with the resulting DFT ones highlights significant structural changes, and different MM structures converge towards the same DFT conformer (Tables S4–S13; Figure S6). These outcomes underline the need to use DFT methods to model highly conjugated structures, such as THIA derivatives.
In particular, the planes of the (substituted)-phenyl rings at N2 (τ1) and N4 (τ2) always assume a perpendicular orientation with respect to that of the 1,2,4-thiadiazolidin-3,5-dione ring (τ1 and τ2 ~90°). These observations indicate that the conjugation of N2 and N4 with the adjacent carbonyl groups prevails over the conjugation with the phenyl substituents, as confirmed by the nitrogen-carbonyl carbon bond length and order (partial double) compared to those of the nitrogen-phenyl carbon bond (single). Accordingly, the most negative values of the molecular electrostatic potentials are placed on the oxygen atoms of the carbonyl functions, particularly on O3 (as shown in Figure 4 and Figure 5).
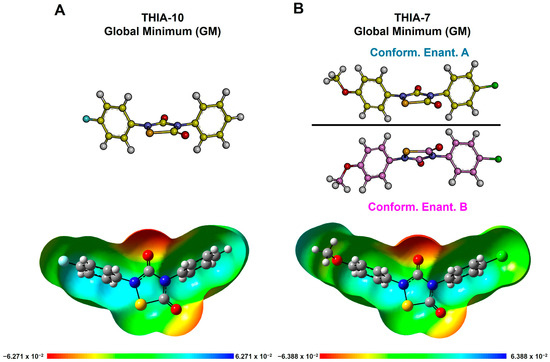
Figure 4.
DFT-optimized conformers of (A) THIA-10 and (B) THIA-7, including conformational enantiomers A and B. Structures are shown in ball-and-stick representation; heteroatoms are colored by atom type: oxygen = red, nitrogen = blue, sulfur = yellow, chlorine = green. Bottom: molecular electrostatic potential (MEP) surfaces (isovalue: 0.0004 e−/a.u.3). Bond order is inferred from bond length. The frontmost portions of the MEP surfaces have been clipped (z-clip option) to allow visualization of the molecular interior.
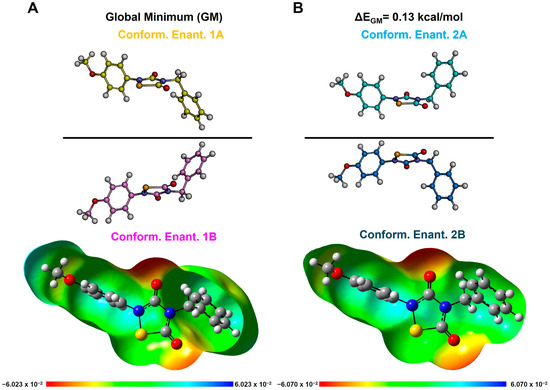
Figure 5.
DFT-optimized conformers of THIA-2. Conformational enantiomers 1A and 1B are shown in (A), while enantiomers 2A and 2B are shown in (B). Structures are displayed in ball-and-stick representation; heteroatoms are colored by atom type: oxygen = red, nitrogen = blue, sulfur = yellow. Bottom: molecular electrostatic potential (MEP) surfaces (isovalue: 0.0004 e−/a.u.3). Bond order is inferred from bond length. The frontmost portions of the MEP surfaces have been clipped (z-clip option) to allow visualization of the molecular interior.
According to the results of our conformational analysis, compounds with two rotatable bonds, such as THIA-3, THIA-9, and THIA-10, show just one conformer (Tables S6, S12 and S13; Figure 4A), while compounds with three rotatable bonds, such as THIA-1, THIA-4, THIA-5, THIA-7, present a couple of specular, not superimposable, and isoenergetic conformers (i.e., conformational enantiomers; namely A and B in Figure 4B; Tables S4, S7, S8 and S10). Finally, compounds with four rotatable bonds (THIA-2 and THIA-6) together with THIA-8, whose cyclohexyl ring can adopt two almost isoenergetic orientations, show two couples of conformational enantiomers (namely, 1A-B and 2A-B; τ2 = 180° or 0°, respectively) (Figure 5; Tables S5, S9, and S11).
The limited energetically accessible conformational space of our THIA derivatives, as well as the presence of conformational enantiomers, are likely to play a role in their ability to fit into the cysteine protease active site. According to the frontier orbital theory, the energy of the lowest unoccupied molecular orbital (LUMO) accounts for the propensity of a molecule to acquire electrons. Hence, in order to evaluate the tendency of THIA compounds to undergo nucleophilic attack by the sulfur atom of the catalytic cysteine [42], the LUMO energy of the DFT conformers was calculated (Table 3).

Table 3.
Lowest unoccupied molecular orbital (LUMO) energy of the DFT conformers of THIA1–10.
The results evidence that the propensity to acquire electrons decreases (LUMO energy increases ~ 3 kcal/mol) in compounds presenting a benzyl (THIA-1 and THIA-2) or a cyclohexyl (THIA-8) ring at N4 compared to those presenting a (substituted)-phenyl ring (THIA-3, THIA-4, THIA-5, THIA-6, THIA-7, THIA-9, and THIA-10). Moreover, among these latter, the value of the LUMO energy varies (~1 kcal/mol) depending on the nature of the para-substituents on the two phenyl rings. Electron-withdrawing substituents (i.e., p-Cl (THIA-7) and p-F (THIA-10)) decrease LUMO energy, while electron-donating substituents (such as p-OMe or p-Me; THIA-4, THIA-5, THIA-6, and THIA-9) determine an increase in LUMO energy (Table 3). By comparing the data in Table 3 with those in Table 2, it can be observed that the ability of THIA-1, THIA-2, and THIA-8 to act as covalent inhibitors is notably lower than that of the other THIA derivatives in the case of Papain and Cathepsin L. At the same time, the inhibitory activity toward our target 3CLpro is scarcely affected. These observations suggest that, besides compound reactivity, the role of the R and R’ substituents in the ability of the compound to interact with the 3CLpro active site and correctly orient the reaction partner with respect to the catalytic cysteine plays a crucial role in the inhibitory activity toward this enzyme (see next paragraph).
2.5.2. Docking Calculations
Docking studies were conducted to simulate the accommodation of THIA derivatives within the 3CLpro active site immediately prior to nucleophilic attack by the catalytic cysteine residue. Calculations were performed taking into account the activation of Cys145 and the interaction with His41, and the results were filtered based on the orientation of the reacting atoms (Scheme 1). To achieve this aim, we applied a shape-based grid docking procedure (LigandFit), combined with MM energy minimization (CFF forcefield; Dassault Systèmes BIOVIA, San Diego, 2023) [43].
In order to consider all possible ligand conformers and, at the same time, preserve the DFT-calculated ligand conformation during docking (force-field-based) simulations, all conformers of THIA-2 (four), THIA-4 (two), THIA-7 (two), THIA-8 (four), and THIA-10 (one) were used to generate separate ligand-protein starting complexes for a total of thirteen docking runs, during which the ligand conformation was tethered.
A structural and bioinformatic analysis guided the definition of the area of the protein to be explored during docking simulations (namely, the ligand binding domain area). The tertiary structure of 3Clpro consists of three domains: Domain I and II, connected by a long loop region (named LH-Loop) to Domain III (Figure S7A). The substrate-binding site is present in a cleft between Domains I and II and buries the catalytic dyad (i.e., Cys145 and His41). Upon reaction of the sulfur atom of Cys145, the hydrogen is transferred as a proton to the nitrogen atom of the scissile peptide bond via protonation of His41; this latter, along with the backbone amides of Gly143, Ser144, and Cys145, form the so-called oxyanion hole, which assists the stabilization of the thiohemiketal intermediate [44]. The catalytic site can be divided into subsites, namely, S1-S5 and S1’-S2’ [45,46] (Figure S7B); accordingly, to fully cover these subsites, the ligand binding domain area was defined by a binding site sphere of 12 Å around the mass center of the co-crystalized inhibitor of the 3CLpro-GRL-2420 complex (PDB ID: 7JKV) (Volume: 522.5 Å3; Figure S8). Moreover, in order to preserve the activation of the catalytic cysteine during docking simulations, we applied a tethering restraint to the hydrogen bond between Cys145 and His41.
Each of the thirteen docking runs generated up to 20 complexes (Figure S8), which were subjected to energy minimization and subsequently filtered based on the maximum distance between the ligand and enzyme reactive atoms required for nucleophilic attack (i.e., the distance between the sulfur atom S1 of the THIA derivative and the sulfur atom of Cys145 ≤ 4 Å) [47,48]. Selection criteria also included the activation of Cys145 by His41. The ligand–protein interaction energy of the selected complexes was calculated, and the resulting structures were ranked and analyzed.
Analysis of the docked THIA-2/3CLpro complexes shows that the ligands could approach the catalytic Cys145 by placing the R and R’ substituents in different subsites, resulting in four possible binding modes (named BM-I, BM-II, BM-III, and BM-IV; Figure 6, Tables S14–S18).
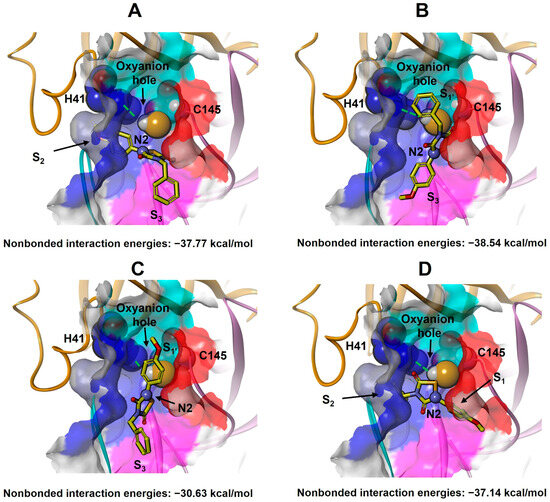
Figure 6.
Docking calculations obtained four binding modes of THIA-2 to 3CLpro: BM-I (A), BM-II (B), BM-III (C), and BM-IV (D). The protein backbone is displayed as ribbons and colored by protein domains: orange (Domain I: aa 1–101), pink (Domain II: aa 102–184), and cyan (LH-Loop: aa 185–200). The solvent-accessible surfaces of S1’ (cyan), S1 (red), S2 (blue), and S3 (magenta) subsites are displayed in solid (transparency 50%). The catalytic residues His41 and Cys145 are displayed in ball-and-stick models. The hydrogen bond between the catalytic dyad, His41, and Cys145, is shown as a green dashed line. THIA-2 (yellow) is displayed on a stick. Atoms are colored by type (N: blue; O: red; S: orange). THIA-2 N2 nitrogen atom is evidenced in a ball.
However, the reaction mechanism of THIA derivatives with thiols involves cleavage of the S1–N2 bond, accompanied by protonation of N2 [2] (Scheme 1), analogous to the protonation of the scissile peptide bond nitrogen [42]. As previously described, in the 3CLpro active site, this proton transfer occurs in the oxyanion hole, where a proton shifts from Cys145 to the substrate nitrogen via His41. Accordingly, for THIA N2 to be protonated, it must be positioned within the oxyanion hole—a spatial arrangement achievable only in binding mode BM-I, where the N2 and N4 substituents occupy the S2 and S3 subsites, respectively (Figure 6A). Based on this rationale, the BM-I complex with the most favorable ligand–protein binding energy was identified as the best-docked structure (Table 4 and Tables S14–S19; Figure 7 and Figure 8 and Figure S9).

Table 4.
THIA-3CLpro best docked complexes. IC50 for inhibition of 3CLpro, the ligand-protein non-bond interaction energy, the distance between the sulfur atoms of THIA and Cys145, the ligand conformation and LUMO energy are reported.
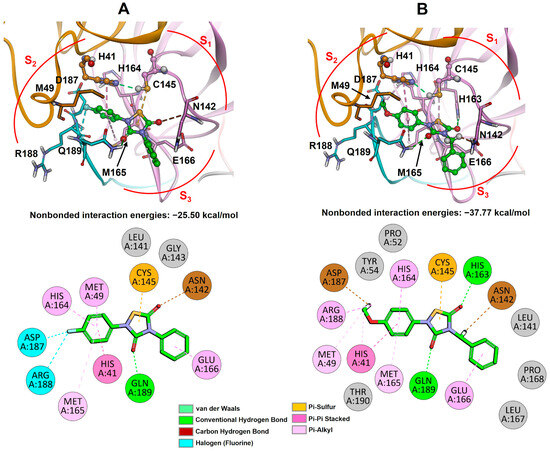
Figure 7.
Best-docked complexes of 3CLpro with THIA-10 (A) and THIA-2 (B). Top panels: ligand binding sites; bottom panels: 2D representations of ligand–protein interactions. The protein is shown as a ribbon, and the ligand is displayed as a ball-and-stick (top) or stick (bottom) model and colored green. Protein residues with interaction energies > 1 kcal/mol are shown and labeled in black. 3CLpro substructures are colored as follows: orange (Domain I, residues 1–101), pink (Domain II, residues 102–184), and cyan (LH-loop, residues 185–200). Atoms are colored by type: carbon = green, oxygen = red, nitrogen = blue, fluorine = cyan, sulfur = yellow, hydrogen = white.
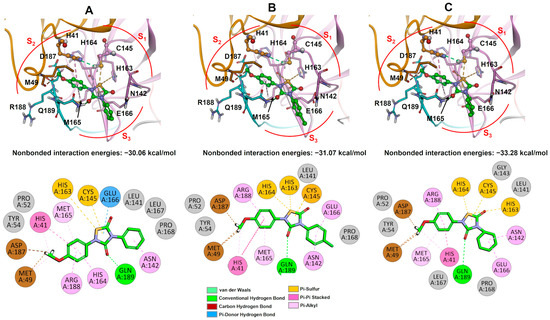
Figure 8.
Best-docked complexes of 3CLpro with THIA-4 (A), THIA-7 (B), and THIA-8 (C). Top panels: ligand binding sites; bottom panels: 2D representations of ligand–protein interactions. The protein is shown as ribbons, and the ligand is displayed as a ball-and-stick (top) or stick (bottom) model and colored green. Protein residues with interaction energies > 1 kcal/mol are shown and labeled in black. 3CLpro substructures are colored as follows: orange (Domain I, residues 1–101), pink (Domain II, residues 102–184), and cyan (LH-loop, residues 185–200). Atoms are colored by type (C = green, O = red, N = blue, Cl = light green, S = yellow, H = white).
The contribution of each protein residue to the binding energy was assessed (Tables S20–S24), and the number and types of ligand-protein interactions were also estimated using a geometry-based method [49]. It is worth noting that all THIA derivatives interact with several residues also involved in the interaction with the substrate, such as Gln189, His41, Met49, His163, His164, Met165, and Glu166 (Figure 7 and Figure 8 vs. Figure S7B), with the strongest contribution to the binding energy (~−4 kcal/mol) being represented by the hydrogen bond between O3 and the side chain of Gln189 (S2 subsite; Figure 7 and Figure 8; Tables S20–S24). Going into details, the OMe group at R’ of THIA-2, THIA-4, THIA-7, and THIA-8 always presents the same conformation (corresponding to conformational enantiomer B) and contributes to the ligand-binding energy by establishing favourable interaction with the S2 subsite (particularly with Asp187, Met49, and Arg188; Figure 7B and Figure 8; Tables S20–S23). The other carbonyl group (C5=O5) points toward S1, partially occupying and interacting (His163) with this subsite, more (THIA-2) or less (THIA-10), depending on the considered THIA compound (Figure 7 and Figure 8). In particular, THIA-2, thanks to the presence of the flexible methylene linker, shows the best accommodation with the catalytic site (best binding energy), establishing a hydrogen bond between O5 and the side chain of His163 (Figure 7B), thus, mimicking the interaction of the side chain of the P1 residue (Gln) of the substrate (Figure S7B).
THIA-2 and THIA-8 show, indeed, the most favourable ligand-protein binding energies (Table 4). Interestingly, THIA-1, THIA-2, and THIA-8, characterized by the presence of a sp3 carbon attached to N4, present the highest LUMO energy among the studied THIAs, that is, the lower propensity to acquire electrons (Table 3). Our docking results suggest that their ability to fit into the 3CLpro active site by establishing strong interactions with the protein could “compensate” for their relatively lower reactivity (Figure 7B and Figure 8C; Tables S20 and S23). Indeed, THIA-2 and THIA-8 (as well as THIA-1) remain 3CLpro covalent inhibitors at submicromolar concentrations, while retaining potency against the other proteases. Hence, the presence of a sp3 carbon at N4 of the THIA ring decreases electron affinity but increases the fitting of the R substituent into 3CLpro S3 subsite, resulting in compounds (THIA-1, THIA-2, and THIA-8) with comparable 3CLpro inhibitory potency with respect to their phenyl counterparts (THIA-3 and THIA-4) but showing improved selectivity.
On the other hand, the THIA-10/3CLpro complex exhibits the lowest ligand–protein binding energy among all THIA derivatives (still indicating a favorable interaction; Table 4). Interestingly, while all other THIA compounds also adopt “unproductive” binding modes (BM-II to BM-IV)—often with more favorable interaction energies than BM-I (Tables S14–S17)—THIA-10 is restricted to BM-I only (Table S18). This exclusive adoption of the productive binding mode increases the likelihood of a successful interaction with the protein and may contribute to entropic advantages. In addition, the THIA-10/3CLpro complex displays the shortest distance between the S1 sulfur atom of the ligand and the sulfur atom of the catalytic residue Cys145 (Table 4). This proximity is likely favored by an interaction between the fluorine atom and the backbone of Asp187 and Arg188, which induces a shift in the ligand toward the S2 subsite, bringing the reactive centers closer together compared to other docked derivatives. Finally, the electron-withdrawing effect of the para-fluorine substituent, which increases the electrophilicity of the THIA core and facilitates nucleophilic attack (Table 3 and Table 4), may further contribute to the high inhibitory potency of this compound toward 3CLpro (IC50 = 0.02 μM; Table 2).
Thus, the ability of the studied THIAs to act as 3CLpro covalent inhibitors seems to be the result of a balance between chemical reactivity and the ability to interact with the catalytic site properly, orienting the reacting partners. In line with this hypothesis, THIA-7, presenting the best compromise between the calculated LUMO energy and the ligand-protein interaction energy (Table 4), resulted in the most potent 3CLpro inhibitor among the tested THIAs (IC50 = 0.01 μM; Table 2). On the other hand, THIA-4, with relatively low LUMO energy (lower than THIA-2 and THIA-8, higher than THIA-7 and THIA-10) and a ligand-protein binding energy value lower than THIA-10 but higher than the other THIAs, showed an inhibitory potency against 3CLpro (IC50 = 0.13 μM), comparable to that of THIA-2 and THIA-8. The introduction of a para-halogen substituted phenyl ring at either N4 (R; S3 subsite; THIA-7) or N2 (R’; S2 subsite; THIA-10) significantly increases electron affinity (Table 3), speeding up the reaction with the catalytic cysteine. Accordingly, THIA-7 and THIA-10 exhibit increased potency as 3CLpro covalent inhibitors but decreased selectivity, being also very potent against Papain and Cathepsin L (see the following paragraph).
The positive effect of introducing a para-methoxy (p-OMe) substituent on the phenyl ring at N4 (R substituent) on 3CLpro inhibitory activity (THIA-6 vs. THIA-4; Table 2) was investigated by fitting THIA-6 into the 3CLpro active site, based on docking results obtained for THIA-4 (pharmacophore fitting). The calculation of the ligand-protein interactions for the resulting THIA-6/3CLpro complex revealed that the p-OMe group of the R substituent could establish favorable hydrophobic contacts with Pro168 in the S3 subsite (Figure S10). However, this substituent does not enhance compound selectivity, as it can also be accommodated within the active sites of Cathepsin L and Papain (see the following paragraph).
2.5.3. Steered Molecular Dynamics Simulations
To elucidate the dynamic nature of ligand–protein interactions and validate docking results, Steered Molecular Dynamics (SMD) simulations were performed using the final docked complexes as starting conformations. SMD simulations of ligand unbinding from catalytic sites offer valuable insights into the molecular recognition process and can indirectly inform the binding mechanism [50]. In our case, however, we are not mimicking the unbinding process itself, but rather the reverse of the binding event, by guiding the ligand away from the catalytic site along a plausible dissociation pathway. This approach is particularly useful for exploring the conformational states and energetic routes that the ligand may traverse to reach the catalytic site [51].
The best pulling pathway was directed through the S3 subsite, the widest channel leading out of the catalytic pocket. For all compounds, the interaction energy with the protein decreased going from the starting (docked) to the final (unbound) conformation (Figure S11). At the end of the simulations, all compounds exceeded the 20 Å distance between S1 and the sulfur atom of Cys145 (Figure S11), which represents the threshold beyond which a ligand can be considered unbound from the protein site [52].
Importantly, the complexes retaining the BM-I binding modes resulted in the ones with the best interaction energy, further supporting the results of our docking studies, as reported in Figure 9.

Figure 9.
Graphs showing the relationship between S1-SCys distance (Å) and the ligand-protein interaction energy (kcal/mol) calculated considering all the resulting SMD structures of THIA-10, THIA-2, THIA-4, THIA-7 and THIA-8. Yellow circles highlight the complexes adopting BM-I.
SMD simulations showed that THIA-2 is the most effective compound in maintaining the binding mode identified by docking studies (BM-I) with interaction energies ranging from –48.66 to –38.07 kcal/mol and the longest (65 ps) retention time of this binding mode among all THIA derivatives (Figure 9). This good fit with the catalytic site is in line with docking results and, as previously observed, could compensate for the low propensity to acquire electrons of THIA-2 (the highest LUMO energy among THIAs).
Finally, local motions and conformational changes induced by THIA binding were assessed through the calculation of Root Mean Square Fluctuation (RMSF), by aligning each trajectory frame to the initial structure using all Cα atoms. Residues with RMSF values greater than 2 Å were considered to exhibit notable flexibility.
Results (Figure 10 and Figures S12–S15) revealed that THIA-4 displayed the highest fluctuation in the upper loop region (residues 44–54). This region plays a key role in stabilizing the active conformation of the catalytic residues as well as the ligand binding [53]
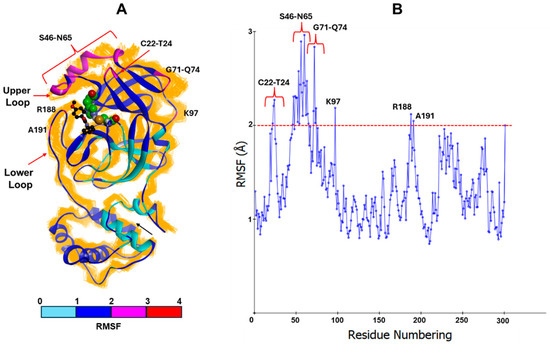
Figure 10.
(A) Steered Molecular Dynamics (SMD)results obtained for THIA-4. The resulting complexes were superimposed on the starting structure by using Cα. The starting complex is displayed as solid ribbons and colored according to the calculated RMSF values. The Cα of the resulting complexes is displayed as line ribbons and colored in orange. The ligand (black; ball-and-stick model) and the catalytic residues (CPK and green) are colored by atom type (O: red; S: orange; N: blue). (B) Mean Square Fluctuation (RMSF) values were calculated considering all the resulting SMD structures. The red dashed line represents the 2 Å RMSF threshold, which is used to highlight the residues with greater flexibility. The residues with an RMSF value > 2 Å are labelled.
This result may account for the low inhibitory potency against 3CLpro of THIA-4, comparable to that of THIA-2 and THIA-8 (Table 2), despite the higher propensity to accept electrons (LUMO energy) compared to THIA-2 and THIA-8.
2.5.4. Investigation of the Molecular Bases of THIA Selectivity
To rationalize the selectivity profiles exhibited by the THIA derivatives against the tested cysteine proteases (Table 2), we conducted a comparative analysis between our computational findings and the experimentally resolved structures of the corresponding enzymes. Initially, we analyzed the size, shape, and relative orientation of the catalytic subsites of 3CLpro, PLpro, Papain, Cathepsin L, and Bromelain (Table S25). Subsequently, the THIA scaffold was fitted into the active sites of PLpro (PDB ID: 6WX4), Papain (PDB ID: 6TCX), Cathepsin L (PDB ID: 3OF8), and Bromelain (PDB ID: 6YCG) by superimposing it onto the co-crystallized peptide ligands (pharmacophore fitting).
Papain, Cathepsin L, and Bromelain share a common fold topology and exhibit ~48% sequence similarity, allowing structural superimposition based on sequence alignment (Figure S16). In contrast, PLpro and 3CLpro show less than 30% sequence similarity with these proteases and cannot be structurally superimposed by their sequence alignment. However, PLpro retains a similar active site architecture and catalytic residue orientation (Figure S17), enabling its inclusion in the superimposition by aligning its catalytic triad (Cys111, His272, Asp286) with that of Cathepsin L (Cys26, His164, Asn188). 3CLpro, which differs significantly in both fold topology and catalytic residue orientation (Cys145, His41), was superimposed by aligning the backbone atoms of its co-crystallized peptide ligand (PDB ID: 7JKV) with those of the peptide ligand co-crystallized with PLpro (PDB ID: 6WX4).
This ligand-based structural alignment enabled a comparative analysis of the positioning of the subsites relative to the catalytic cysteine and the scissile peptide bond. While the S1 and S2 subsites were found to be roughly superimposed across all proteases, the S3 subsite exhibited distinct orientations. A schematic representation is reported in Figure 11.
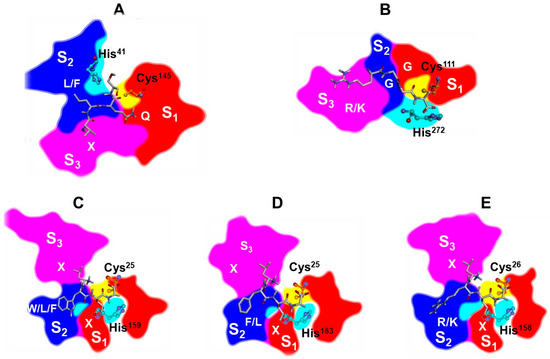
Figure 11.
Schematic representations of the catalytic subsites S1 (red), S2 (blue), and S3 (magenta) of five proteases, 3CLpro (A), PLpro (B), Papain (C), Cathepsin L (D), and Bromelain (E), generated from their Connolly surfaces. The spatial arrangement of the subsites was obtained using the superimposition method described in the Materials and Methods section. Representations were created by posterizing the corresponding 3D molecular structures using Inkscape (version 1.3). Catalytic residues Cys and His are shown in ball-and-stick models, labeled and colored in orange and cyan, respectively. The enzyme substrate is shown in stick (C: white; N: blue; O: red) and the consensus substrate sequence is labelled (X = any residue).
Notably, the oxyanion holes of Papain, Cathepsin L, Bromelain, and PLpro are differently oriented compared to those of 3CLpro. In 3CLpro, it is located between the S1 and S2 subsites, whereas in the other proteases it is positioned within the S1 subsite, on the opposite side relative to the catalytic cysteine (Figure 11, Figure 12A–C and Figure S18A,B). This implies a different spatial arrangement of the ligand-reacting atoms, as confirmed by the analysis of the X-ray structures. Accordingly, the fitting of the THIA skeleton into the active sites of Papain (PDB ID: 6TCX), Cathepsin L (PDB ID: 3OF8), Bromelain (PDB ID: 6YCG), and PLpro (PDB ID: 6WX4) places both the sulfur (S1) and nitrogen (N2) atoms in the S1 subsite, whereas in 3CLpro, N2 is located in the S2 subsite (Figure 12D–F and Figure S18C,D).
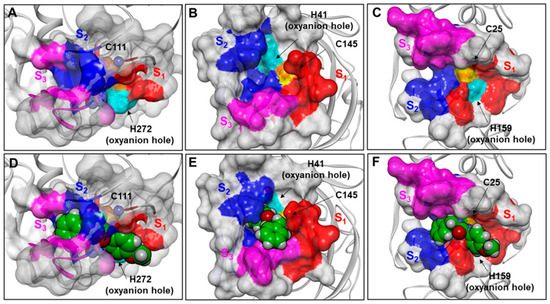
Figure 12.
(A): x-ray structure of SARS-CoV-2 PLpro (PDB ID: 6WX4). (B): x-ray structure of SARS-CoV-2 3CLpro (PDB ID: 7JKV). (C): x-ray structure of Papain (PDB ID: 6TCX). (D): fitting of THIA-2 in the active site of PLpro (PDB ID: 6WX4). (E): 3CLpro-THIA-2 best-docked complex. (F): fitting of THIA-2 in the active site of Papain (PDB ID: 6TCX). The proteins are displayed as grey ribbons. The catalytic residues Cys and His are displayed in CPK, labeled and colored in orange and cyan, respectively. The Connolly surfaces of the proteases are displayed in solid. The residues of S1, S2, and S3 subsites are colored red, blue, and magenta, respectively. The solvent-accessible surface (SASA) of the sulfur atom of the catalytic cysteine is displayed and colored in yellow. THIA-2 is displayed in CPK and colored by atom type (C = green, O = red, N = blue, S = yellow).
In PLpro, the catalytic residues are located at the interface between the thumb (aa 62–178) and palm (aa 241–315) domains, and the S1 and S2 subsites are very narrow (Figure 8 A), in line with the substrate specificity of this enzyme at positions P1 and P2 where only glycine residues can be accepted [25,38,54]. Accordingly, the THIA derivatives cannot fit into the PLpro active site (Figure 12D). However, they could occupy the PLpro S3 and S4 subsites, thus closing the access to the catalytic site without forming a covalent bond with the catalytic Cys111, similar to what has been demonstrated for GRL0617 (IC50: 2.3 μM) and its derivatives, a class of potent SARS-CoV-2 PLpro allosteric inhibitors characterized by a structure similar to THIAs (Figure 13) [23,55,56].
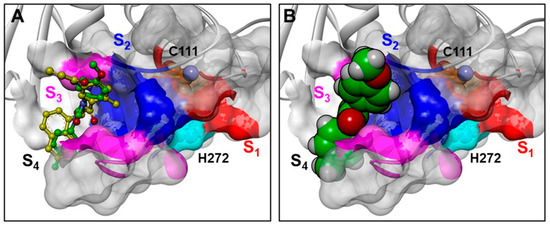
Figure 13.
(A): structural superimposition of THIA-5 (ball-and-stick model; green) on a GRL0617 derivative in complex with PLpro (yellow; PDB ID: 7JIW); (B): van der Waals volume of THIA-5 is displayed. The protein is displayed as grey ribbons. The catalytic residues Cys and His are displayed in CPK, labeled and colored in orange and cyan, respectively. The Connolly surface of the protease is displayed in solid. The residues of S1, S2, and S3 subsites are colored red, blue, and magenta, respectively. THIA-5 is colored by atom type (C = green, O = red, N = blue, S = yellow).
In Figure 10A, the structural superimposition of THIA-5 with a GRL0617 derivative in complex with PLpro (PDB ID: 7JIW) is reported; it is worth noting that THIA-5 may interact with most of the residues also involved in the binding of the GRL0617 derivative (Figure S19). This accounts for the observed reversible Inhibition of PLpro by THIA compounds (Figure S3C).
On the contrary, the active sites of Papain and Cathepsin L are less constrained (Figure 12C and Figure S13A), allowing them to accommodate bulkier substrates, in line with the broader specificity of these proteases [8,57]. In particular, the catalytic cysteine is more solvent-exposed and accessible than in the other proteases, as shown by the solvent-accessible surface area (SASA) analysis (Table S26). Accordingly, THIA derivatives—including those bearing a benzyl group such as THIA-2—can fit into the active sites of Papain and Cathepsin L (Figure 12F and Figure S18C). As previously discussed, their different ability to inhibit these proteases appears to depend primarily on their distinct reactivity (ELUMO; Table 3).
The overall inactivity of THIA derivatives against Bromelain is likely due to the peculiar amino acid composition of its S2 subsite. Indeed, while the S1 subsite is strictly conserved, the S2 subsite is very different among Papain, Cathepsin L, and Bromelain (Figure S20 and Table S3). The S2 subsites of Papain and Cathepsin L are hydrophobic and, accordingly, bind to substrate/inhibitors presenting hydrophobic residues at P2. On the contrary, the S2 subsite of Bromelain is polar and negatively charged due to the presence of Asp209 and Glu68, in line with the ability of this enzyme to recognize substrates containing a positively charged residue at the P2 position [8,36]. The unique capacity of THIA-7 to inhibit Bromelain (IC50 = 2.0 ± 0.1μM) could be due to the ability of the chlorine atom at the para position of the phenyl ring at N4 (R substituent) to establish a halogen bond with the Glu68 carboxylate group in the S2 subsite of this enzyme (Figure S21A), as resulted from the calculation of ligand-protein interactions using a nonbonded geometric interaction criteria [47].
Our binding hypothesis could also account for the overall higher inhibitory activity of all THIA derivatives on Papain compared to Cathepsin L (Table 2). Indeed, the S2 subsite of Papain is characterized by less bulky residues compared to those present in Cathepsin L (Papain: Pro68, Val157 vs. Cathepsin L: Met70, Met161; Table S3), leading to a better fit of the R substituent (Figure S20). In particular, the methoxyl group of THIA-6 could establish a hydrogen bond with Ser205, a residue exclusive to Papain (subsite S2; Figures S20 and S21B; Table S3) in line with the highest Papain inhibitory activity of THIA-6 among the tested compounds (Table 2).
2.5.5. Covalent Docking Simulation
In order to obtain the molecular models of the covalent adducts of 3CLpro, Papain, and Cathepsin L with THIA-3, -4, -6, and -8, we investigated the ability of the catalytic cysteine-THIA covalent adduct (Scheme 1) to adapt to the catalytic sites of the different proteases by applying the covalent docking procedure in AutoDock4 [58].
No specific starting ligand/protein complex was considered, and the adduct was randomly generated to obtain the best complex after product formation. Moreover, the conformation of the resulting adduct (resulting from the opening of the THIA ring) was allowed to change in order to find the best adaptation to the enzyme site.
Since THIA-6, THIA-7, and THIA-10 exhibit stronger inhibitory effects (IC50 < 0.05) compared to the other THIAs with similar IC50 values, we focused on comparing the covalent binding of 3CLpro with THIA-6 and THIA-4, as they differ only by the substitution of a p-methoxyphenyl group in THIA-6 versus a phenyl group in THIA-4 at the N4 substituent (R). Covalent docking revealed that, following reaction with the catalytic Cys145, both compounds occupy the S1–S2–S4 subsites and one hydrogen bond with the Nδ atom of His41. In this binding mode, the N4 substituent (R) is accommodated within the S1 subsite (Phe140, Leu141, Asn142, Met165, Glu166) while the N2 substituent (R′) bearing a p-methoxyphenyl group penetrates deeply into the hydrophobic S2 pocket (Met49, Asp187, Arg188), with the p-methoxy substituent extending additional interactions toward the S4 subsite (Met165, Glu166, Gln189 and Gln192) (Figure 14A).
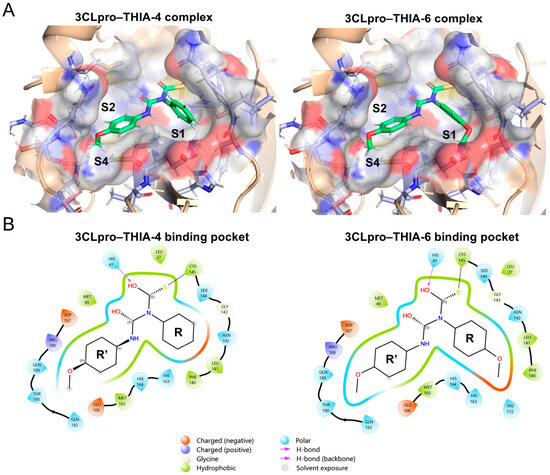
Figure 14.
(A) 3D molecular visualization of the 3CLpro–THIA-4 (Lowest binding energy of −6.48 kcal/mol) and 3CLpro–THIA-6 (Lowest binding energy of −6.43 kcal/mol) complexes. Ligands are shown in green within the active site of 3CLpro. Subsites S1, S2, and S4 are highlighted, illustrating the spatial arrangement of the binding pocket and indicating specific interactions with each ligand. (B) 2D interaction diagrams of the 3CLpro binding pockets for THIA-4 and THIA-6. Each diagram highlights the specific interactions of the ligands within the subsites. Hydrogen bonds, hydrophobic contacts, metal coordination, and solvent exposure are represented using standardized symbols and color codes.
Although both compounds adopt a similar positioning within the 3CLpro binding pocket, the p-methoxy substituent at the N4 position of THIA-6 enabled additional interactions within the S1 subsite, including contacts with His172. This deep interaction with the S1 subsite allowed an improved fit across the S2–S4 subsites and conferred slight solvent accessibility compared to THIA-4 (Figure 14B). It is noteworthy that the covalent docking differs from the docking complex shown in Figure 8 regarding the position of N4 substituents (R substituent) that occupy the S3 subsite in all complexes.
Next, we evaluated THIA-4, THIA-6, and THIA-8 using covalent docking simulations with Papain adducts. These compounds were selected because, although they differ only at the N4 substituent, THIA-6 exhibits a sevenfold higher inhibitory potency than THIA-4 and a 240-fold increase compared to THIA-8. The covalent docking simulations further revealed that the N4 substituent (R) generally occupies the S2 subsite and the N2 substituent (R’) occupies the S3 subsite, except in the complex with THIA-8, where this orientation is inverted (Figure 15A).
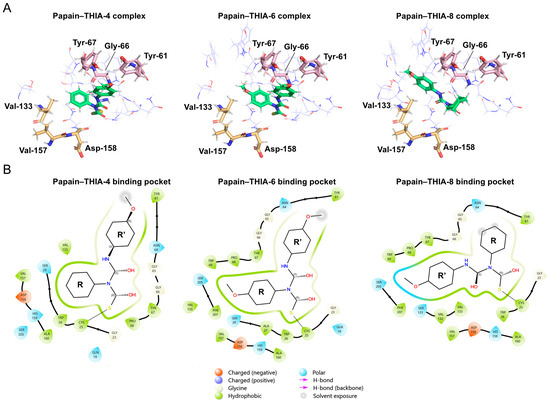
Figure 15.
(A) Representation of 3D molecular structure of Papain bound to THIA-4 (Lowest binding energy of −6.28 kcal/mol), THIA-6 (Lowest binding energy of −6.19 kcal/mol), and THIA-8 (Lowest binding energy of −5.94 kcal/mol). Key residues involved in ligand binding corresponding to S2-S3 subsites are labeled. (B) 2D interaction diagrams of Papain–THIA complexes. Each diagram illustrates the binding pocket and interaction profile for THIA-4, THIA-6, and THIA-8. Hydrogen bonds, hydrophobic contacts, and solvent exposure are indicated using standardized symbols. Amino acid residues and ligand structures are annotated to show the molecular basis of binding specificity.
The binding site of papain should not be divided simply into S2 (Val133, Val157, and Asp158) and S3 (Gly66 and Tyr67) subsites, but rather as a single large hydrophobic pocket encompassing both regions [59]. We observed that THIA-4 and THIA-6 displayed similar interactions of the p-methoxyphenyl group at the N2 substituent within the S3 subsite and showed greater occupancy of the S2 subsite core due to the phenyl substituent at N4 near the S2 residues. THIA-6, in particular, exhibited a higher number of interactions within the S2 subsite than THIA-4 owing to the O-methyl group, including additional contacts with Phe297 and Trp69.
In contrast, the p-methoxyphenyl group at the N2 position of THIA-8 was deeply inserted into the S2 subsite. In contrast, the cyclohexyl at the N4 substituent extended toward the S3 region, remaining distant from Gly66 and Tyr67, and instead being solvent-exposed (Figure 15B). Based on the structural features of both the receptor and the ligands, we hypothesize that the papain binding pocket is not deep enough to accommodate the bulky cyclohexyl group of THIA-8. This steric limitation likely results in an incorrect geometric alignment of the warhead within the active site, redirecting the binding orientation and forcing the N2 substituent into the S2 subsite while leaving the warhead poorly positioned in the S3 pocket.
Cathepsin L (CatL) contains a deep and narrow hydrophobic S2 subsite defined by the side chains of Leu69, Met70, Ala135, and Met161, together with the backbone atoms of Met161, Asp162, His163, and Gly164, highlighting their essential roles in substrate specificity [60], as exemplified in the Cat L–E64d complex (PDB ID: 7ZXA). Moreover, the restricted architecture of the S2 pocket is reinforced by Asp160, Met161, Asp162, and Ala214, which collectively form a continuous steric hindrance barrier that prevents further extension of the S2 subsite [61]. In this context, the bulky cyclohexyl moiety may fit the S2 subsite, similar to that of Triazine, which addresses the hydrophobic S2 pocket [62] (PDB ID: 4AXM) (Figure 16A).
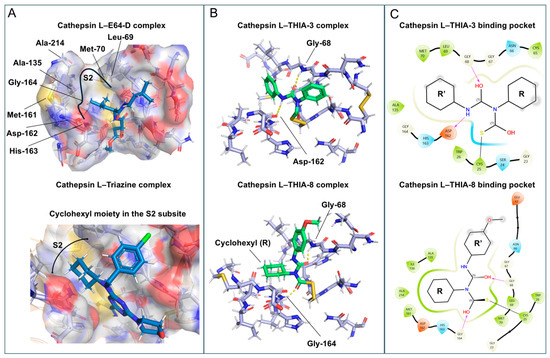
Figure 16.
(A) Structural representation of the Cathepsin L–E64-D complex (PDB ID: 7zxa), highlighting the residues of the S2 subsite and The Cyclohexyl moiety of Triazine occupying this pocket (PDB ID: 4axm). (B) Structural representation of Cathepsin L-THIA-3 complex (Lowest binding energy of −5.09 kcal/mol) and Cathepsin L-THIA-8 complex (Lowest binding energy of −6.18 kcal/mol), showing R and R’ positioning and respective H-bonds (lines yellow), in which the Cyclohexyl group from THIA-8 was positioned within the S2 subsite, similar to Triazine. (C) 2D interaction diagrams of Cathepsin L with THIA-3 and THIA-8. Hydrogen bonds, hydrophobic contacts, and other molecular interactions are illustrated. Ligand structures and binding residues are annotated to highlight key interactions within the binding pocket.
The distinct hydrophobicity, especially in the S2 and S3 subsites (Gly61, Glu63, Gly67, Gly68), favors different related hydrophobic side chains of the compounds, and hydrogen bond hotspots across the subsites of Cat L include Asp162 and Gly68 [63]. The calculated adducts of Cathepsin L with THIA-3 and -8 show that in the complex THIA-3—Cathepsin L, the phenyl substituent at N4 is located in the S1 subsite, and the phenyl group of N2 points to the entrance of the S2 subsite, having an H-bond with Gly68 and Asp162. In the complex THIA-8—Cathepsin L, the N4 substituent (cyclohexyl; R) points to S2 containing an H-bond with Gly68 and Gly164, and the N2 substituent is solvent-exposed (Figure 16B).
Although the cyclohexyl moiety fits well into the S2 pocket of Cat L, the p-methoxyphenyl group at the N2 substituent of THIA-8 exhibits suboptimal accommodation in the S3 subsite. Possibly the relatively bulky volume of this moiety, combined with the narrow geometry of the Cat L S3 pocket, which leads to a steric clash with the pocket wall, as indicated by Glu63, results in partial solvent exposure. In contrast, the smaller substituent composition of THIA-3 favors more extensive interactions within the S1 subsite and achieves a better fit inside the S3 subsite (Figure 16C).
It is noteworthy that the presence of the bulky side chain of Met70 and Met161 in the S2 subsite (Table S3) restricts its occupancy that may account for the overall weaker inhibitory activity of THIA derivatives on Papain compared to the other two proteases (3CLPro and Cathepsin L; Table 2), particularly THIA-1, THIA-2, and THIA-8, characterized by bulkier substituents at N4, compared to the other THIAs.
3. Materials and Methods
3.1. Chemistry
Anhydrous dimethyl sulfoxide (DMSO) and acetonitrile (ACN) were from Sigma-Aldrich (St. Louis, MO, USA). All buffer salts were reagent-grade and purchased from Fisher Scientific (Pittsburgh, PA, USA) or Sigma-Aldrich. The resins and all peptide synthesis-grade reagents (N-methylpyrrolidone (NMP), N-methylmorpholine (NMM), dichloromethane, piperidine, trifluoroacetic acid (TFA), anisole, thioanisole, and triisopropylsilane) and 5-carboxyfluorescein were purchased from Sigma (Saint-Quentin Fallavier, France).
The FRET peptides were obtained by solid-phase synthesis, using the Fmoc (N-(9-fluorenyl)-methoxycarbonyl) from Novabiochem (Darmstadt, Germany), on an automated multiple peptide synthesizer (PSSM-8 system; Shimadzu, Tokyo, Japan), as previously described [6,28]. Syntheses were carried out on a NovaSyn® TGR resin (Millipore, Burlington, MA, USA), using HBTU (N, N’-tetramethyl-O-benzotriazo-1-yluronium tetrafluoroborate)/HOBt (1-hydroxybenzotriazole) as coupling reagent. The removal of peptide from the resin was accomplished with TFA: thioanisole: 1,2-ethanedithiol: water (85:5:3:7). Semipreparative HPLC purified peptides on a C18 column (Econosil™; Fisher Scientific, Pittsburgh, PA, USA) and their molecular weight and purity (>95%) checked by reverse-phase chromatography and by mass spectrometry (Shimadzu, Tokyo, Japan). Stock solutions of peptides were prepared in ACN, and the concentration was measured spectrophotometrically using the Dnp molar extinction coefficient of 17,300 M−1 cm−1 at 365 nm.
All the 1,2,4-thiadiazolidin-3,5-diones were synthesized through oxidative condensation of the appropriate isothiocyanates and isocyanates in the presence of SO2Cl2, and then purified and characterized as previously reported [3]. THIA-3, selected as a reference compound because of its simplicity, was produced on a larger scale. As an alternative to crystallization, employed in the previously described procedure, and in order to achieve a higher degree of purity associated with high recovery efficiency of the desired product, THIA-3 was purified at >99% purity by direct phase flash chromatography (5–40% ethyl acetate/n-hexane) on a Biotage Selekt instrument (Biotage, Uppsala, Sweden) equipped with a Sfär Duo 10 g column.
3.2. Enzymes
Recombinant human Cathepsin L was expressed in Pichia pastoris following the previously described procedures [64]. The enzyme and substrate were used under the following conditions: 100 mM sodium acetate buffer containing 1 mM EDTA, pH 5.0, with substrate Z Phe-Arg-MCA.
Papain and Bromelain were purchased from Sigma. Papain was previously activated at 25 °C in a degassed 100 mM sodium phosphate, 1 mM EDTA, pH 6.0 buffer containing 5 mM DTT (dithiothreitol) for 10 min, as previously described [65]. To remove the DTT excess, a 1 mL solution of activated Papain was loaded on a 3 mL syringe with 1 mL Sephadex G25 size exclusion resin (Amersham Pharmacia, Amersham, UK). The enzyme was eluted with the same assay buffer, and its activity was monitored using Z-Phe-Arg-MCA as the substrate.
Recombinant SARS-CoV-2 proteases 3CLpro were expressed from E. coli as previously described [17,66] with some modifications. Briefly, the culture was pre-cultured in LB medium with ampicillin (100 μg/mL) and then transferred to ZYM 5052 self-inducing medium, shaking at 37 °C until OD 600 0.7–0.8, when the protein expression was conducted at 18 °C for 20 h at 180 rpm. Afterward, the cells were centrifuged, the bacterial pellet was resuspended, and the cells were lysed using French Pressure (3 times at 7000–10,000 psi) and 20 μL of Benzonase Nuclease (Millipore). The supernatant containing the soluble protein was then loaded onto a nickel affinity column. To cleave the His-6 Tag from the purified protein, the Pierce kit™ HRV3C Protease Solution, also known as PreScission Protease (Thermo Scientific, Waltham, MA, USA), was used according to the manufacturer’s instructions. The reaction was transferred into a dialysis cassette (Slide-A-Lyzer dialysis cassette, 10 K, Thermo Scientific) and dialyzed against buffer C (20 mM Tris-HCl, pH 7.8, containing 150 mM NaCl and 1 mM DTT) at 4 °C for 20 h. In order to remove the HRV 3C protease, His6-tag, and eventual non-cleft 3CLpro His-6-tag, the dialysis solution was applied to a GST Trap FF (GE Healthcare, Chicago, IL, USA) column connected to a nickel column HisTrap FF (GE Healthcare) [17]. The purified 3CLpro was stored in buffer D at 4 °C. Recombinant SARS-CoV-2 proteases PLpro were also expressed from E. coli, as previously described [67].
The activities of Cathepsin L and Papain were assessed using Z-Phe-Arg-MCA. Cathepsin L was assayed in the standard reaction buffer, 100 mM sodium acetate, 1 mM EDTA, pH 5.0, degassed, and Papain was assayed in 100 mM sodium phosphate, 1 mM EDTA, pH 6.0, degassed.
Bromelain substrate was Z Arg-Arg-MCA in 50 mM sodium acetate, 1 mM EDTA, pH 5.0. The SARS-CoV-2 3CLpro and PLpro assays were conducted using the FRET-based substrates, Abz SAVLQSGFRK(Dnp)NH2 and Abz TLKGGAPIKQ-EDDnp, respectively, as reference [16,68].
3.2.1. Determination of IC50
The assays of proteases were performed as described above, and the enzyme inhibition was expressed as the THIAs concentrations, causing a 50% decrease in enzyme activity (IC50). We calculated them by nonlinear regression, dose response curves using THIAs at different concentrations, and the data were analyzed in Grafit 5.0 software (Erithacus, London, UK) using Equation (1): [y = 100%/1 + (x/IC50)s], where 100% is the fitted uninhibited value and s is a slope factor. The equation assumes that y falls with increasing x. All the assays were done using a SpectraMaxM2e (Molecular Devices, San Jose, CA, USA) with 320 nm excitation and 420 nm emission for FRET peptides and 360 nm excitation and 480 nm emission for MCA substrates.
3.2.2. Statistical Analysis
IC50 data are reported as mean ± S.D. Statistical differences between groups were evaluated using Student’s t-test. p < 0.05 was considered significant.
3.3. Molecular Modeling Studies
Molecular modeling calculations were performed on a CPU/GPU hybrid High-Performance Computing Cluster (10 Twin servers (DoIT Systems Srl, Torino, Italy), for a total of 560 Intel® Xeon® Gold processors (128 GB RAM), 64 AMD® EPYC® processors, and 2 GPU NVIDIA® Tesla® V100). The molecular modeling graphics were carried out on a personal computer equipped with an Intel(R) Core (TM) i7-8700 processor.
3.3.1. Conformational Analysis
The THIA derivatives were built using the Small Molecule tool of Discovery Studio 2023 (Dassault Systèmes BIOVIA, San Diego, CA, USA). Atomic potentials and charges were assigned using the cff force field [69]. The conformational space of the compounds was sampled using the random search algorithm, Boltzmann Jump, for the random generation of a maximum of 300 conformations. Applying this method, each random perturbation is either accepted or rejected according to the Metropolis selection criterion with a ratio according to the Boltzmann distribution (T = 300 K). Finally, an energy threshold value of 106 kcal/mol was used as the selection criterion. The generated structures were then subjected to Molecular Mechanics (MM) energy minimization (ε = 8 × r) until the average RMS gradient during a cycle of minimization was less than 0.001 kcal/Å, using Conjugate Gradient [70] as minimization algorithm. The resulting conformers were ranked by their potential energy values (i.e., energy difference from the global minimum (ΔEGM)) and classified according to the values of the torsion angles.
All the MM energy conformers were then subjected to DFT calculations (Gaussian 16 package) [71]. All structures were fully optimized at the B3LYP/6–311++G(d,p) using the conductor-like polarizable continuum model (C-PCM) [41]. The C-PCM method allows the calculation of the energy in the presence of a solvent. In this case, all structures were optimized as a solute in an aqueous solution. In order to characterize every structure as a minimum, a vibrational analysis was carried out (keyword = freq). The RMS force criterion was set to 3 × 10−4 a.u. Molecular orbitals have been calculated using the natural bond orbital (NBO) method [72]. The resulting DFT conformers were ranked by their potential energy values (i.e., ΔE from the global energy minimum) and classified according to the values of the torsion angles.
3.3.2. Docking Studies on 3CLpro
The experimentally determined structure of 3CLpro with the best resolution (1.25 Å) in complex with the covalent peptidomimetic inhibitor GRL-2420 (PDB ID: 7JKV) [73] was selected as the starting structure for the docking studies.
The coordinates of 7JKV were downloaded from the Protein Data Bank (PDB; https://www.rcsb.org/, accessed on 1 April 2025). The structure was refined by using the “Prepare protein” tool of Discovery Studio 2023 (Dassault Systèmes BIOVIA, San Diego, CA, USA). In particular, the alternate conformations of side chains of the residues A: Asp245, A: Thr196, A: Val186, A: Met165, A: Met162, A: Ser139, A: Val125, A: Ser121, B: Ser254, B: Thr226, B: Thr196, B: Met162, B: Val125, B: Leu87, B: Val86, B: Arg76, B: Ser62 were deleted. The hydrogens were added to the structure, assuming a pH of 7.2 by using the “Calculate Protein Ionization and Residue pK” command, while the N-terminal and C-terminal residues were considered charged. The inhibitor of 7JKV was removed, and atomic potentials and partial charges were assigned using the cff force field.
Docking studies were conducted on THIA-2, THIA-4, THIA-7, THIA-8, and THIA-10 using the LigandFit docking procedure, a shape-based grid docking combined with MM energy minimization (cff forcefield; Dassault Systèmes BIOVIA, San Diego, 2023) [43]. To increase the variance of the starting complexes (i.e., starting ligand poses), the resulting DFT conformers of THIA-2 (four conformers), THIA-4 (two conformers), THIA-7 (two conformers), THIA-8 (four conformers) and THIA-10 (one conformer) were placed in the catalytic site of 3CLpro. According to the molecular mechanism of proteolysis of 3CLpro and considering the positioning of substrate [42], each starting conformer was placed in the catalytic site of 3CLpro, positioning the R substituent into the S1 subsite, the R’ substituent into the S2 subsite and the N2 in the oxyanion hole [74].
Starting complexes were then subjected to docking studies. Docking parameters (i.e., RMS threshold for ligand-site matching, dimensions of the grid, dielectric constant) were appropriately tailored to the system on the basis of a series of “test docking” calculations performed on 7JKV. In particular, the position of the peptidomimetic inhibitor was manually modified by placing its substituents in different subsites compared to the starting X-ray structure. Then, the parameters able to reproduce the X-ray binding mode of GRL-2420 (PDB ID: 7JKV) were chosen.
During the docking calculations, the protein was held rigid, while the ligands were allowed to move by a random combination of translation and rotation changes to sample their orientation within the defined binding domain area without changing their conformation. According to the results of the structural and bioinformatics analysis, to properly investigate the binding mode of THIA, for each starting conformation of the ligands, the binding domain area was defined using a binding site sphere of 12 Å around the mass center of the inhibitor GRL-2420, covering the S1-S5 and S1’-S2’ subsites (Volume: 522.5 Å3). Moreover, in order to investigate the first approach of our compounds to the catalytic site before the nucleophilic attack, a tethering restraint was applied on the hydrogen bond between the catalytic residues Cys145 and His41 (constrained within 3.0 Å using a force constant of 100 (kcal/mol)/Å). Firstly, random orientations of the starting conformation in the binding domain area were generated using an RMS threshold for ligand-site matching of 10 Å. To increase the sampling of ligands in the binding site, a softened Lennard-Jones 9–6 potential was used for calculating the van der Waals energy in the energy calculation.
Moreover, in order to ensure that the energy for ligand atoms outside of the defined binding domain area was calculated, the dimensions of the grid were extended to 3.0 Å from the defined binding domain area. Then, in order to refine the resulting poses, these latter were minimized using steepest descent (1000 steps) and Broyden–Fletcher–Goldfarb–Shanno (BFGS; 2000 steps) as minimization algorithms (ε = 80 × r). The poses were selected using an RMS threshold of 1.50 Å. For each ligand conformation, 20 poses were generated.
The generated complexes were then subjected to MM energy minimization until the average RMS gradient during a minimization cycle was less than 0.1 kcal/Å, using the Conjugate Gradient algorithm (cff; ε = 80 × r). During the minimization, all the residues of 3CLpro were left free to move. In order to investigate the first approach of our compounds to the catalytic site before the nucleophilic attack, a tethering restraint was applied on the hydrogen bond between the catalytic residues Cys145 and His41 (constrained within 3.0 Å using a force constant of 100 (kcal/mol)/Å). In order to preserve the THIA conformation obtained from DFT calculations, dihedral restraints were applied on the torsion angles using a force constant of 100 kcal/mol.
For each docking calculation, the complexes, characterized by the distance between the S1 sulfur atom of THIA and the sulfur atom of Cys145 < 4 Å, were selected. The structures were ranked by their ligand-protein non-bond interaction energy values (vdW and electrostatic energy contribution; atom-based method; Nonbond List Radius = 15.0; Higher Cutoff Distance = 14.0; Lower Cutoff Distance = 11.0; ε = 2 × r; Simulation tool, Discovery Studio 2023) and classified by the positioning of the R and R’ substituents in the 3CLpro subsites, defining the different ligand binding modes: BM-I, BM-II, BM-III, and BM-IV. The orientation and the distance (<4.5 Å) of the N2 nitrogen with respect to the hydrogen catalytic cysteine were checked, and the binding mode BM-I was accordingly selected.
3.3.3. Analysis of the Selected Docked Complexes
The protein structural quality of the selected complexes was assessed using Procheck structure evaluator software, version 3.5.4 [75] and compared to that of the reference structure (PDB ID: 7JKV). Structural analyses of the selected docked complexes were performed using Macromolecules and Receptor-Ligand Interaction tools of Discovery Studio 2023 (Dassault Systèmes BIOVIA, San Diego, 2023) and taking into account the results of the structural and bioinformatics analysis. Finally, the ligand-protein non-bond interaction energy (i.e., the van der Waals term and the electrostatic term; kcal/mol) was calculated, and the contribution of each protein residue within 5 Å from any ligand atom was assessed (vdW and electrostatic energy contribution; atom-based method; Nonbond List Radius = 15.0; Higher Cutoff Distance = 14.0; Lower Cutoff Distance = 11.0; ε = 2 × r; Simulation tool, Discovery Studio 2023). The residues with a favorable non-bond interaction ≥ 1 kcal/mol were considered key interaction residues. The types of ligand-protein interactions were evaluated using the nonbonded geometric interaction criteria (Receptor-Ligand Interaction tool; Discovery Studio 2023) [49].
3.3.4. Steered Molecular Dynamics
To validate docking results, the selected docked complexes were subjected to Steered Molecular Dynamics (SMD). As in the docking studies, SMD was employed to explore the initial approach of the compounds to the catalytic site prior to nucleophilic attack. Accordingly, during all the following calculations, a tethering restraint was applied on the hydrogen bond between the catalytic residues Cys145 and His41 (constrained within 3.0 Å using a force constant of 100 (kcal/mol)/Å). To preserve the THIA conformation obtained from DFT calculations, dihedral restraints were applied on the torsion angles using a force constant of 100 (kcal/mol)/Å.
The starting structures were solvated into a cubic periodic box using the TIP3P water model [76]. The box edges were set at least 12 Å around the protein, and periodic boundary conditions (PBC) were applied. To keep the electroneutrality of the system, eight Cl− ions were added. All structures were parametrized using the CHARMM force field [77]. To eliminate steric clashes, the solvated systems were subjected to energy minimization using the Conjugate Gradient algorithm [70] until the average RMS gradient was less than 0.1 kcal/mol·Å. Subsequently, solvent molecules were equilibrated for 50 ps under an isothermal–isobaric (NPT) ensemble, with protein and ligand atoms held fixed. Temperature was maintained at 298 K using the Nosé–Hoover thermostat [78] and pressure was controlled at 1 atm using the Langevin piston method [79]. During the simulation, the SHAKE algorithm [80] was utilized to constrain the hydrogen-involved bonds, and the particle mesh Ewald method [81] was used for the calculation of electrostatic contributions to the nonbonded interactions, with the nonbonded cutoff distance set to 12 Å. The final equilibrated structures were used as starting points for SMD simulations. Each compound underwent a 500 ps SMD run with a 1 fs time step. Based on the active site geometry and the positioning of THIA derivatives after docking studies, the pulling pathway was directed (1) through the S3 subsite, the widest channel leading out of the catalytic pocket or (2) through the upper and lower loops as defined in Figure 10. Steering forces were applied between the sulfur atom of Cys145 and either the para-position carbon of the R substituent (THIA-4, THIA-7, THIA-8, THIA-10) or the methylene carbon (THIA-2). A spring constant of 350 pN/Å (equivalent to 5 kcal/mol·Å) [50,51] and a pulling speed of 0.04 Å/ps were applied. Trajectories were saved every 5 ps for subsequent analysis.
Structural analyses of the resulting conformations were performed using Receptor-Ligand Interaction and Simulation tools of Discovery Studio 2023 (Dassault Systèmes BIOVIA, San Diego, 2023). The ligand-protein non-bond interaction energy (i.e., the van der Waals term and the electrostatic term; kcal/mol) was calculated (vdW and electro-static energy contribution; atom-based method; Nonbond List Radius = 15.0; Higher Cut-off Distance = 14.0; Lower Cutoff Distance = 11.0; ε = 2 × r; Simulation tool, Discovery Studio 2023) including the water molecules involved in the interaction between THIA and the protein binding site.
The types of ligand-protein interactions were evaluated using the nonbonded geometric interaction criteria (Receptor-Ligand Interaction tool; Discovery Studio 2023) [49]. Since the CFF force field used in docking is not compatible with PBC [82], nonbonded interaction energies for the docked complexes were recalculated using the CHARMM force field to ensure consistency with the SMD simulations. THIA compounds were considered to preserve the BM-I binding mode when the following criteria were met: (i) the distance between the S1 sulfur atom of THIA and the sulfur atom of Cys145 was less than 4 Å; (ii) the N2 nitrogen was within 4.5 Å of the catalytic cysteine hydrogen; (iii) N2 and N4 substituents occupied the S2 and S3 subsites; (iv) hydrogen bond with Gln189 was maintained. Finally, Root Mean Square Fluctuation (RMSF) values were calculated for each residue. RMSF was computed by aligning each trajectory frame to the initial structure using all Cα atoms. Residues with RMSF values greater than 2 Å were considered to exhibit notable flexibility.
3.3.5. Bioinformatics and Structural Analysis
The experimentally determined structures of (i) 3CLpro (PDB IDs: 7JKV and 7N89), (ii) PLpro (PDB IDs: 6WX4 and 7JIW), (iii) Papain (PDB IDs: 6TCX), (iv) Cathepsin L (PDB ID: 3OF8) and, (v) Bromelain (PDB ID: 6YCG) were downloaded from the Protein Data Bank (PDB; http://www.rcsb.org/pdb/).
All structures were parametrized using the CHARMM force field [77] and analyzed by using Macromolecules, Simulation, and Receptor-Ligand Interaction tools of Discovery Studio 2023 (Dassault Systèmes BIOVIA, San Diego, 2023).
To perform the analysis, the structures were superimposed using the following procedure. A secondary structure sequence alignment among Cathepsin L (PDB ID: 3OF8), Papain (PDB ID: 6TCX), and Bromelain (PDB ID: 6YCG) was performed using the Align123 algorithm [83] (Secondary structure: DSC Pairwise Alignment method: Slow; Scoring Matrix: BLOSUM; Gap Open Penalty: 10; Gap Extension Penalty: 0.05; Macromolecules tool; Discovery Studio 2023). The structures were superimposed by fitting all Cα atoms of the aligned residues, and the RMSD value of each Cα pair was calculated; then, a final superimposition was performed using only the Cα pairs with an RMSD < 1Å. The PLpro structure (PDB ID: 6WX4) was added to the superimposition by fitting the Cα atoms of its catalytic residues (Cys111, His272, and Asp286) on those of Cathepsin L (Cys26, His164, and Asn188). Finally, the 3CLpro structure (PDB ID: 7JKV) was added to the superimposition by fitting the backbone atoms of the P1 and P2 residues of the co-crystallized peptide ligand (GRL-2420) with those of the peptide ligand (VIR251) of PLpro (PDB ID: 6WX4). The other 3CLpro (PDB ID: 7N89) and PLpro (PDB ID: 7JIW) structures were also included and superimposed on 7JKV and 6WX4, respectively, according to their secondary structure sequence alignment using the same procedure reported above for Cathepsin L, Papain, and Bromelain.
The DFT conformers of THIA derivatives were fitted into the active site of PLpro, Cathepsin L, Papain, and Bromelain in order to properly orient S1 and N2 with respect to the catalytic dyad (Cys and His; oxyanion hole). The global minimum DFT conformer of THIA-2 and THIA-3 was superimposed on the peptide inhibitor VIR251 in complex with SARS-CoV-2 PLpro (PDB ID: 6WX4) by fitting: (i) the centroid of phenyl ring at N2 on the centroid of methyl ester group (subsite S1); (ii) the centroid of phenyl ring at N4 on the Cα of glycine (subsite S2), and (iii) the sulfur atom on the carbon atom covalently linked to the catalytic cysteine. The lowest energy DFT conformers (i.e., conformers 1A/B) of THIA-2, THIA-6, and THIA-7 were superimposed on Phe-Tyr(OBut)-COCHO inhibitor in complex with Cathepsin L (PDB ID: 3OF8) by fitting: (i) the centroid of phenyl ring at N2 on the centroid of the side chain of tyrosine (subsite S1); (ii) the centroid of phenyl ring at N4 on the centroid of the side chain of phenylalanine (subsite S2), and (iii) the sulfur atom on the carbon atom covalently linked to the catalytic cysteine. According to this latter superimposition, THIA-6 and THIA-7 were also fitted into the active site of Papain and Bromelain.
The global minimum DFT conformer of THIA-5 was fitted into the allosteric site of PLpro by superimposition with the noncovalent inhibitor (PDB ID: 7JIW) (Molecular Overlay; 50% steric, 50% electrostatic components; Discovery Studio 2023).
In all the obtained complexes, the number and types of ligand-protein interactions were evaluated using the nonbonded geometric interaction criteria (Receptor-Ligand Interaction tool; Discovery Studio 2023).
The surfaces of subsites S1, S2, and S3 were created using the residues within 5 Å from any atom of the P1, P2, or P3 substrate/inhibitor residues (Receptor-Ligand Interaction tool; Discovery Studio 2023). Solvent accessible surface area (SASA) calculations were performed using Discovery Studio 2023. The SASA of the catalytic cysteine sulfur atom was calculated for the following experimentally determined structures of 3CLpro (PDB IDs: 7JKV), PLpro (PDB IDs: 6WX4), Papain (PDB IDs: 6TCX), Cathepsin L (PDB ID: 3OF8), and Bromelain (PDB ID: 6YCG).
3.3.6. Covalent Docking
Previously reported structures of 3CLpro (PDB: 7SF1), Papain (PDB: 1PE6), and Cathepsin L (PDB: 7W34) were adapted for covalent docking using PyMOL (version 3.0.3). Covalent inhibitors were present in these structures. Ligands, ions, and water molecules were removed, and hydrogens were added to the receptors using PyMOL. THIA-3, -4, -6, and -8 were designed, and their topology parameters were generated via the SwissParam [84] and LigParGen [85] web servers. The inhibitors were then converted into mol2 format using Openbabel (version 3.1.1) for covalent docking. Covalent docking of the hypothetical Cysteine protease-THIA complexes was performed with the flexible side-chain method in AutoDock4 to predict the binding modes of the covalent complexes. Docking simulations utilized the default settings of the Lamarckian Genetic Algorithm, and ligand docking conformations were ranked based on a semi-empirical force field-derived scoring function [86]. Manual iterative adjustments of the covalent binding were subsequently performed in Coot [87] using the docked protease-inhibitor models. Visualization and preparation of structural figures were carried out using Schrödinger Maestro v. 14.5] and PyMOL (https://pymol.org/2/, accessed on 1 September 2025). Below, we present the structures of the simulation for covalent adduct formation between 3CLpro, Papain, and Cathepsin L with THIA-3, -4, -6, and -8.
4. Conclusions
The diversity among the ten THIA compounds assayed is still limited, which hinders a comprehensive analysis of the factors involved in the reactions studied, as well as other structures we plan to synthesize based on the data reported in this paper. However, we demonstrate that 1,2,4-thiadiazolidin-3,5-diones (THIA-1–10) can act as selective covalent inhibitors of specific cysteine proteases, including SARS-CoV-2 3CLpro, with varying degrees of activity also observed against reference enzymes such as Papain and Cathepsin L. Computational studies provided support for the experimental findings and offered a rationale for their selectivity profiles. The inhibition mechanism followed a two-step irreversible bimolecular process, but was reversible in the presence of reducing agents. The inhibitory activity seems to depend not only on the electrophilic reactivity of the THIA core, which enables covalent binding to the catalytic cysteine, but also on the conformational features of the scaffold and the ability of the R and R′ substituents to direct the compound toward specific protease subsites, promoting productive hydrophobic and electrostatic interactions. The most potent inhibitors (THIA-6, -7, and -10) exhibited nanomolar IC50 values against 3CLpro, while THIA-1, -2, and -8 displayed enhanced selectivity. The benzyl or cyclohexyl group at the N4 nitrogen favored selectivity toward 3CLpro, whereas para-halogenated phenyl substituents increased overall potency. In the case of Cathepsin L, the reduced activity may be attributed to steric hindrance within the S2 subsite. The observed reversible inhibition of PLpro is likely due to the inaccessibility of subsites S1 and S2; the good structural overlap between the non-covalent inhibitor GRL0617 and THIA-5 in complex with PLpro supports the putative binding of both compounds to the same allosteric site.
The dual functionality of THIA derivatives—as both H2S-releasing agents and covalent inhibitors—justifies the development of multifunctional therapeutic agents targeting cysteine proteases involved in viral replication and inflammatory processes.
5. Future Directions
We will focus on the design and synthesis of new THIA derivatives and on their encapsulation (to protect the reaction from reducing agents, such as glutathione) for biological assays. Based on our experience in the field [88], we will investigate THIA compounds also as inhibitors of human cysteine proteases (cathepsins B, K, L, and S) and cysteine proteases associated with parasitic infectious diseases, including cruzipain from Trypanosoma cruzi (Chagas disease), falcipain-2 from Plasmodium falciparum (malaria), and CPB2.8DCTE cysteine proteinase from Leishmania mexicana (leishmaniosis).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules30193896/s1, Table S1: Initial rates of SARS-CoV-2 PLpro using different substrates; Table S2: Values of the kinetic constants for Papain inactivation kinetics analyses by all THIA derivatives; Table S3: List of amino acids located in the subsites S1, S2, and S3 of 3CLpro, PLpro, Cathepsin L, Papain, and Bromelain; Table S4: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-1; Table S5: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-2; Table S6: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-3; Table S7: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-4; Table S8: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-5; Table S9: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-6; Table S10: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-7; Table S11: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-8; Table S12: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-9; Table S13: ΔEGM values (kcal/mol) and torsional angle values (degrees) of MM and DFT conformers of THIA-10; Table S14: Selected 3CLpro and THIA-2 docked complexes; Table S15: DFT conformer, binding mode, ligand-protein non-bond interaction energies (kcal/mol), distance (Å) between the THIA sulfur atom S1 and Cys145 sulfur atom of the selected 3CLpro and THIA-4 docked complexes; Table S16: DFT conformer, binding mode, ligand-protein non-bond interaction energies (kcal/mol), distance (Å) between the THIA sulfur atom S1 and Cys145 sulfur atom of the selected 3CLpro and THIA-7 docked complexes; Table S17: DFT conformer, binding mode, ligand-protein non-bond interaction energies (kcal/mol), distance (Å) between the THIA sulfur atom S1 and Cys145 sulfur atom of the selected 3CLpro and THIA-8 docked complexes; Table S18: DFT conformer, binding mode, ligand-protein non-bond interaction energies (kcal/mol), distance (Å) between the THIA sulfur atom S1 and Cys145 sulfur atom of the selected 3CLpro and THIA-10 docked complexes; Table S19: Summary of Procheck results obtained for the selected docked complexes and the reference structure PDB ID: 7JKV; Table S20: Ligand-residue nonbonded interaction energies (kcal/mol) of the THIA-2/3CLPro docked complex; Table S21: Ligand-residue nonbonded interaction energies (kcal/mol) of the THIA-4/3CLPro docked complex; Table S22: Ligand-residue nonbonded interaction energies (kcal/mol) of the THIA-7/3CLPro docked complex; Table S23: Ligand-residue nonbonded interaction energies (kcal/mol) of the THIA-8/3CLPro docked complex; Table S24: Ligand-residue nonbonded interaction energies (kcal/mol) of the THIA-10/3CLPro docked complex; Table S25: Experimentally determined structures of 3CLpro, PLpro, Papain, Cathepsin L, and Bromelain used in the structural and bioinformatics analysis; Table S26: Solvent accessible surface (SASA) of the sulfur atom of the catalytic cysteine; Figure S1: (A)—Stability of THIA-3 in DMSO with 1% H2O. (B)—Stability of THIA-3 in water,100 mM Na Phosphate, Tris buffer pH 7.4, with 1 to 40% CAN; Figure S2: Stability of THIAs in DMSO and stored at −20 °C; Figure S3: Irreversible inhibition of papain, 3CLpro, and reversible inhibition of PLpro by THIA-3; Figure S4: Inhibition of Papain by THIA-3 and reversibility of the activity using cysteine (Cys) as a reducing agent; Figure S5: Assay with 3CLpro of FRET peptides library having Abz-SAVLHSGFRK(Dnp)-NH2 as the reference sequence; Figure S6: Structural comparison between the MM and DFT conformers of THIA-7; Figure S7: X-ray structure of SARS-CoV-2 3CLpro C145A mutant in complex with substrate Ac-SAVLQSGF-CONH2 (PDB ID: 7N89); Figure S8: X-ray structure of SARS-CoV-2 3CLpro used as protein starting structure in docking studies (PDB ID: 7JKV); Figure S9: 3CLPro-THIA best docked complexes superimposed on the starting structure (PDB ID: 7JKV; Cα pairs with a RMSD < 1Å); Figure S10: 3CLpro/THIA-6 molecular interaction model obtained by the superimposition of THIA-6 on THIA-4 in the best docked complex with 3CLpro; Figure S11: Graphs showing the relationship between time (ps) and interaction energy (kcal/mol) calculated considering all the resulting SMD structures; Figure S12: (A) Steered Molecular Dynamics (SMD)results obtained for THIA-10. (B) Mean Square Fluctuation (RMSF) values calculated considering all the resulting SMD structures; Figure S13: (A) Steered Molecular Dynamics (SMD)results obtained for THIA-2. (B) Mean Square Fluctuation (RMSF) values calculated considering all the resulting SMD structures; Figure S14: (A) Steered Molecular Dynamics (SMD)results obtained for THIA-7. (B) Mean Square Fluctuation (RMSF) values calculated considering all the resulting SMD structures; Figure S15: (A) Steered Molecular Dynamics (SMD)results obtained for THIA-8. (B) Mean Square Fluctuation (RMSF) values calculated considering all the resulting SMD structures; Figure S16: superimposition among x-ray structure of Papain, x-ray structure of Cathepsin L, and x-ray structure of Bromelain; Figure S17: X-ray structure of SARS-CoV-2 PLpro (PDB ID: 6WX4); Figure S18: X-ray structure of Cathepsin L (PDB ID: 3OF8), Bromelain (PDB ID: 6YCG). Fitting of THIA-2 in the active site of Cathepsin L (PDB ID: 3OF8). Fitting of THIA-2 in the active site of Bromelain (PDB ID: 6YCG); Figure S19: X-ray structure of SARS-CoV-2 PLpro in complex with a GRL0617 derivative (PDB ID: 7JIW). Fitting of THIA-5 in the allosteric site of PLpro (PDB ID:7JIW); Figure S20: Superimposition among x-ray structure of Papain (PDB ID: 6TCX), x-ray structure of Cathepsin L (PDB ID: 3OF8), and x-ray structure of Bromelain (PDB ID: 6YCG); Figure S21: fitting of THIA-7 in the active site of Bromelain (PDB ID:6YCG).
Author Contributions
Conceptualization, C.F., G.C., M.A.J. and L.J.; Data curation, M.P., G.T. (Giuseppe Tumbarello), B.S. and F.F. (Francesco Frecentese); Formal analysis, M.P., C.F., O.T., A.C., A.S., S.V., M.A.J. and L.J.; Investigation M.P., G.T. (Giuseppe Tumbarello), C.F., A.C., A.S., S.V., M.A.J., K.R.F. and G.T. (Gabriel Trigo); Methodology C.F., M.P., B.S., F.F. (Ferdinando Fiorino), M.A.J., L.J., D.O. and A.S.T.; Funding acquisition L.J.; Project administration C.F. and L.J.; Software M.P., G.T. (Giuseppe Tumbarello) and O.T.; Supervision C.F., L.J., G.C. and V.S.; Validation, G.C., V.S., F.F. (Ferdinando Fiorino), F.F. (Francesco Frecentese), C.F. and M.P.; Visualization M.P., G.T. (Giuseppe Tumbarello), O.T. and J.T.L.; Writing—original draft C.F., B.S., M.A.J. and L.J.; Writing—review & editing, M.A.J., M.P., B.S., G.T. (Giuseppe Tumbarello), D.O., K.R.F., G.T. (Gabriel Trigo), A.S., J.T.L., O.T., A.C., F.F. (Ferdinando Fiorino), A.S., F.F. (Francesco Frecentese), V.S., S.V., G.C., L.J. and C.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by FAPESP 2018-13588-0.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article and Supplementary Material. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| THIA | 1,2,4-thiadiazolidin-3,5-diones |
| RGS | Regulators of G protein signaling |
| SAR | Structure-activity relationships |
| 3CLpro | chymotrypsin-like cysteine protease |
| Mpro | Main protease |
| PLpro | Papain-like protease |
| DFT | Density functional theory |
| LC-MS | Liquid Chromatography Mass Spectrometry |
| FRET | Fluorescence resonance energy transfer |
| PICS | Proteomic Identification of Cleavage site Specificity |
| MM | Molecular Mechanics |
| C-PCM | Conductor-like polarizable continuum model |
| LUMO | Lowest unoccupied molecular orbital |
| NBO | Natural bond orbital |
| PDB | Protein Data Bank |
| ΔEGM | Energy difference from the global minimum |
| SASA | Solvent accessible surface area |
| SMD | Steered Molecular Dynamics |
References
- Martinez, A.; Alonso, M.; Castro, A.; Dorronsoro, I.; Gelpí, J.L.; Luque, F.J.; Pérez, C.; Moreno, F.J. SAR and 3D-QSAR studies on thiadiazolidinone derivatives: Exploration of structural requirements for glycogen synthase kinase 3 inhibitors. J. Med. Chem. 2005, 48, 7103–7112. [Google Scholar] [CrossRef] [PubMed]
- Turner, E.M.; Blazer, L.L.; Neubig, R.R.; Husbands, S.M. Small Molecule Inhibitors of Regulator of G Protein Signalling (RGS) Proteins. ACS Med. Chem. Lett. 2012, 9, 146–150. [Google Scholar] [CrossRef] [PubMed]
- Severino, B.; Corvino, A.; Fiorino, F.; Luciano, P.; Frecentese, F.; Magli, E.; Saccone, P.; Di Vaio, I.; Citi, V.; Calderone, V.; et al. 1,2,4-Thiadiazolidin-3,5-diones as novel hydrogen sulfide donors. Eur. J. Med. Chem. 2018, 143, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Blazer, L.L.; Zhang, H.; Casey, E.M.; Husbands, S.M.; Neubig, R.R. A nanomolar-potency small molecule inhibitor of regulator of G-protein signaling proteins. Biochemistry 2011, 50, 3181–3192. [Google Scholar] [CrossRef]
- Schechter, I.; Berger, I.A. On the size of the active site in proteases. I. Papain. Biochem. Biophys. Res. Commun. 2012, 425, 497–502. [Google Scholar] [CrossRef]
- St Hilaire, P.M.; Willert, M.; Juliano, M.A.; Juliano, L.; Meldal, M. Fluorescence-Quenched Solid Phase Combinatorial Libraries in the Characterization of Cysteine Protease Substrate Specificity. J. Comb. Chem. 1999, 1, 509–523. [Google Scholar] [CrossRef]
- Turk, D.; Guncar, G.; Podobnik, M.; Turk, B. Revised definition of substrate binding sites of papain-like cysteine proteases. Biol. Chem. 1998, 379, 137–147. [Google Scholar] [CrossRef]
- Choe, Y.; Leonetti, F.; Greenbaum, D.C.; Lecaille, F.; Bogyo, M.; Brömme, D.; Ellman, J.A.; Craik, C.S. Substrate Profiling of Cysteine Proteases Using a Combinatorial Peptide Library Identifies Functionally Unique Specificities. J. Biol. Chem. 2006, 281, 12824–12832. [Google Scholar] [CrossRef]
- Gosalia, D.N.; Salisbury, C.M.; Ellman, J.A.; Diamond, S.L. High throughput substrate specificity profiling of serine and cysteine proteases using solution-phase fluorogenic peptide microarrays. Mol. Cell. Proteom. 2005, 4, 626–636. [Google Scholar] [CrossRef]
- Alves, L.C.; Melo, R.L.; Sanderson, S.J.; Mottram, J.C.; Coombs, G.H.; Caliendo, G.; Santagada, V.; Juliano, L.; Juliano, M.A. S1 subsite specificity of a recombinant cysteine proteinase, CPB, of Leishmania mexicana compared with cruzain, human cathepsin L and Papain using substrates containing non-natural basic amino acids. Eur. J. Biochem. 2001, 268, 1206–1212. [Google Scholar] [CrossRef]
- Fujishima, A.; Imai, Y.; Nomura, T.; Fujisawa, Y.; Yamamoto, Y.; Sugawara, T. The crystal structure of human cathepsin L complexed with E-64. FEBS Lett. 1997, 407, 47–50. [Google Scholar] [CrossRef] [PubMed]
- Varughese, K.I.; Ahmed, F.R.; Carey, P.R.; Hasnain, S.; Huber, C.P.; Stored, A.C. Crystal Structure of a Papain-E-64 Complex. Biochemistry 1989, 28, 1330–1332. [Google Scholar] [CrossRef] [PubMed]
- Tsuge, H.; Nishimura, T.; Tada, Y.; Asao, T.; Turk, D.; Turk, V.; Katunuma, N. Inhibition Mechanism of Cathepsin L-Specific Inhibitors Based on the Crystal Structure of Papain–CLIK148 Complex. Biochem. Biophys. Res. Commun. 1999, 266, 411–416. [Google Scholar] [CrossRef] [PubMed]
- Gour-Salin, B.J.; Lachance, P.; Magny, M.C.; Plouffe, C.; Ménard, R.; Storer, A.C. E64 [trans-epoxysuccinyl-L-leucylamido-(4-guanidino)butane] analogues as inhibitors of cysteine proteinases: Investigation of S2 subsite interactions. Biochem. J. 1994, 299 Pt 2, 389–392. [Google Scholar] [CrossRef][Green Version]
- Coulombe, R.; Grochulski, P.; Sivaraman, J.; Ménard, R.; Mort, J.S.; Cygler, M. Structure of human procathepsin L reveals the molecular basis of Inhibition by the prosegment. EMBO J. 1996, 15, 5492–5503. [Google Scholar] [CrossRef]
- Okamoto, D.N.; Oliveira, L.C.; Kondo, M.Y.; Cezari, M.H.; Szeltner, Z.; Juhász, T.; Juliano, M.A.; Polgár, L.; Juliano, L.; Gouvea, I.E. Increase of SARS-CoV 3CL peptidase activity due to macromolecular crowding effects in the milieu composition. Biol. Chem. 2010, 391, 1461–1468. [Google Scholar] [CrossRef]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved alpha-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef]
- Konno, S.; Kobayashi, K.; Senda, M.; Funai, Y.; Seki, Y.; Tamai, I.; Schäkel, L.; Sakata, K.; Pillaiyar, T.; Taguch, A.; et al. 3CL Protease inhibitors with an electrophilic arylketone moiety as anti-SARS-CoV-2 agents. J. Med. Chem. 2022, 65, 2926–2939. [Google Scholar] [CrossRef]
- Kitamura, N.; Sacco, M.D.; Ma, C.; Hu, Y.; Townsend, J.A.; Meng, X.; Zhang, F.; Zhang, X.; Ba, M.; Szeto, T.; et al. Expedited Approach toward the Rational Design of Noncovalent SARS-CoV-2 Main Protease Inhibitors. J. Med. Chem. 2022, 65, 2848–2865. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Tan, K.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Room-temperature X-ray crystallography reveals the oxidation and reactivity of cysteine residues in SARS-CoV-2 3CL Mpro: Insights into enzyme mechanism and drug design. IUCrJ. 2020, 7 Pt 6, 1028–1035. [Google Scholar] [CrossRef]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Jedrzejczak, R.; Stols, L.; Langan, P.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Structural plasticity of SARS-CoV-2 3CL Mpro active site cavity revealed by room temperature X-ray crystallography. Nat. Commun. 2020, 11, 3202. [Google Scholar] [CrossRef]
- Barretto, N.; Jukneliene, D.; Ratia, K.; Chen, Z.; Mesecar, A.D.; Baker, S.C. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005, 79, 15189–15198. [Google Scholar] [CrossRef] [PubMed]
- Ratia, K.; Pegan, S.; Takayama, J.; Sleeman, K.; Coughlin, M.; Baliji, S.; Chaudhuri, R.; Fu, W.; Prabhakar, B.S.; Johnson, M.E.; et al. A noncovalent class of papain-like protease/deubiquitinase inhibitors blocks SARS virus replication. Proc. Natl. Acad. Sci. USA 2008, 105, 16119–16124. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.-Y.; Lai, H.-Y.; Chen, H.-Y.; Cheng, S.-C.; Cheng, K.-W.; Chou, Y.-W. Structural basis for catalysis and ubiquitin recognition by the severe acute respiratory syndrome coronavirus papain-like protease. Acta Crystallogr. D Biol. Crystallogr. 2014, 70 Pt 2, 572–581. [Google Scholar] [CrossRef]
- Rut, W.; Lv, Z.; Zmudzinski, M.; Patchett, S.; Nayak, D.; Snipas, S.J.; El Oualid, F.; Huang, T.T.; Bekes, M.; Drag, M.; et al. Activity profiling and crystal structures of inhibitorbound SARS-CoV-2 papain-like protease: A framework for anti–COVID-19 drug design. Sci. Adv. 2020, 6, eabd4596. [Google Scholar] [CrossRef]
- Baez-Santos, Y.M.; St. John, S.E.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and Inhibition by designed antiviral compounds. Antivir. Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- Lv, Z.; Cano, K.E.; Jia, L.; Drag, M.; Huang, T.T.; Olsen, S.K. Targeting SARS-CoV-2 Proteases for COVID-19 Antiviral Development. Front. Chem. 2022, 9, 819165. [Google Scholar] [CrossRef]
- Tashrifi, Z.; Khanaposhtani, M.M.; Larijani, B.; Mahdav, M. Dimethyl Sulfoxide: Yesterday’s Solvent, Today’s Reagent. Adv. Synth. Catal. 2019, 361, 65–86. [Google Scholar] [CrossRef]
- Korkmaz, B.; Attucci, S.; Juliano, M.A.; Kalupov, T.; Jourdan, M.L.; Juliano, L.; Gauthier, F. Measuring elastase, proteinase 3 and cathepsin G activities at the surface of human neutrophils with fluorescence resonance energy transfer substrate. Nat. Protoc. 2008, 3, 991–1000. [Google Scholar] [CrossRef]
- Niesor, E.J.; Boivin, G.; Rhéaume, E.; Shi, R.; Lavoie, V.; Goyette, N.; Picard, M.E.; Perez, A.; Thode, F.L.; Tardif, J.C. Inhibition of the 3CL Protease and SARS-CoV-2 Replication by Dalcetrapib. ACS Omega 2021, 6, 16584–16591. [Google Scholar] [CrossRef]
- Baici, A.; Schenker, P.; Wächter, M.; Rüedi, P. 3-Fluoro-2,4-dioxa-3-phosphadecalins as inhibitors of acetylcholinesterase. A reappraisal of kinetic mechanisms and diagnostic methods. Chem. Biodivers. 2009, 6, 261–282. [Google Scholar] [CrossRef]
- Portaro, F.C.; Santos, A.B.; Cezar, M.H.; Juliano, M.A.; Juliano, L.; Carmona, E. Probing the specificity of cysteine proteinases at subsites remote from the active site analysis of P4, P3, P2′ and P3′ variations in extended substrates. Biochem. J. 2000, 347 Pt 1, 123–129. [Google Scholar] [CrossRef]
- Abeles, R.H.; Alston, T.A. Enzyme inhibition by fluoro compounds. J. Biol. Chem. 1990, 265, 16705–16708. [Google Scholar] [CrossRef] [PubMed]
- Böhm, H.J.; Banner, D.; Bendels, S.; Kansy, M.; Kuhn, B.; Müller, K.; Obst-Sander, U.; Stahl, M. Fluorine in medicinal chemistry. Chembiochem 2004, 5, 637–643. [Google Scholar] [CrossRef] [PubMed]
- Olsen, J.A.; Banner, D.W.; Seiler, P.; Obst Sander, U.; D’Arcy, A.; Stihle, M.; Müller, K.; Diederich, F. A fluorine scan of thrombin inhibitors to map the fluorophilicity/fluorophobicity of an enzyme active site: Evidence for C-F…C=O interactions. Angew. Chem. Int. Ed. Eng. 2003, 42, 2507–2511. [Google Scholar] [CrossRef] [PubMed]
- Azarkan, M.; Maquoi, E.; Delbrassine, F.; Herman, R.; M’Rabet, N.; Esposito, R.C.; Charlier, P.; Kerff, F. Structures of the free and inhibitors-bound forms of Bromelain and ananain from Ananas comosus stem and in vitro study of their cytotoxicity. Sci. Rep. 2020, 10, 19570. [Google Scholar] [CrossRef]
- Barrett, A.J.; Kembhavi, A.A.; Brown, M.A.; Kirschke, H.; Knight, C.G.; Tamai, M.; Hanada, K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem. J. 1982, 201, 189–198. [Google Scholar] [CrossRef]
- Jesus, H.C.R.; Solis, N.; Machado, Y.; Pablos, I.; Bell, P.A.; Kappelhoff, R.; Grin, P.M.; Sorgi, C.A.; Butler, G.S.; Overall, C.M. Optimization of quenched fluorescent peptide substrates of SARS-CoV-2 3CLpro main protease (Mpro) from proteomic identification of P6-P6′ active site specificity. J. Virol. 2024, 98, e0004924. [Google Scholar] [CrossRef]
- Osipiuk, J.; Azizi, S.A.; Dvorkin, S.; Endres, M.; Jedrzejczak, R.; Jones, K.A.; Kang, S.; Kathayat, R.S.; Kim, Y.; Lisnyak, V.G.; et al. Structure of papain-like protease from SARS-CoV-2 and its complexes with noncovalent inhibitors. Nat. Commun. 2021, 12, 743. [Google Scholar] [CrossRef]
- Nicholls, A.; Honig, B.A. Rapid Finite Difference Algorithm, Utilizing Successive Over-Relaxation to Solve Poisson-Boltzmann Equations. J. Comp. Chem. 1991, 12, 435–445. [Google Scholar] [CrossRef]
- Cossi, M.; Rega, N.; Scalmani, G.; Barone, V. Energies, structures, and electronic properties of molecules in solution with the C-PCM solvation model. J. Comp. Chem. 2003, 24, 669–681. [Google Scholar] [CrossRef]
- Ren, P.; Li, H.; Nie, T.; Jian, X.; Yu, C.; Li, J.; Su, H.; Zhang, X.; Li, S.; Yang, X.; et al. Discovery and Mechanism Study of SARS-CoV-2 3C-like Protease Inhibitors with a New Reactive Group. J. Med. Chem. 2023, 66, 12266–12283. [Google Scholar] [CrossRef]
- Venkatachalam, C.M.; Jiang, X.; Oldfield, T.; Waldman, M. LigandFit: A novel method for the shape-directed rapid docking of ligands to protein active sites. J. Mol. Graph. Model. 2003, 21, 289–307. [Google Scholar] [CrossRef] [PubMed]
- Świderek, K.; Moliner, V. Revealing the molecular mechanisms of proteolysis of SARS-CoV-2 Mpro by QM/MM computational methods. Chem Sci. 2020, 11, 10626–10630. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Xiong, Y.; Zhu, G.H.; Zhang, Y.N.; Zhang, Y.W.; Huang, P.; Ge, G.B. The SARS-CoV-2 main protease (Mpro): Structure, function, and emerging therapies for COVID-19. MedComm 2020, 3, e151. [Google Scholar] [CrossRef]
- Cho, E.; Rosa, M.; Anjum, R.; Mehmood, S.; Soban, M.; Mujtaba, M.; Bux, K.; Moin, S.T.; Tanweer, M.; Dantu, S.; et al. Dynamic Profiling of β-Coronavirus 3CL Mpro Protease Ligand-Binding Sites. J. Chem. Inf. Model. 2021, 61, 3058–3073. [Google Scholar] [CrossRef]
- Lodola, A.; Branduardi, D.; De Vivo, M.; Capoferri, L.; Mor, M.; Piomelli, D.; Cavalli, A. A catalytic mechanism for cysteine N-terminal nucleophile hydrolases, as revealed by free energy simulations. PLoS ONE 2012, 7, e32397. [Google Scholar] [CrossRef]
- Arafet, K.; Ferrer, S.; González, F.V.; Moliner, V. Quantum mechanics/molecular mechanics studies of the mechanism of cysteine protease inhibition by peptidyl-2,3-epoxyketones. Phys. Chem. Chem. Phys. 2017, 19, 12740–12748. [Google Scholar] [CrossRef]
- Bissantz, C.; Kuhn, B.; Stahl, M. A medicinal chemist’s guide to molecular interactions. J. Med. Chem. 2010, 53, 5061–5084. [Google Scholar] [CrossRef]
- Okimoto, N.; Suenaga, A.; Taiji, M. Evaluation of Protein-Ligand Affinity Prediction Using Steered Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2017, 35, 3221–3231. [Google Scholar] [CrossRef]
- Hu, X.; Hu, S.; Wang, J.; Dong, Y.; Zhang, L.; Dong, Y. Steered Molecular Dynamics for Studying Ligand Unbinding of Ecdysone Receptor. J. Biomol. Struct. Dyn. 2018, 36, 3819–3828. [Google Scholar] [CrossRef]
- Patel, J.S.; Berteotti, A.; Ronsisvalle, S.; Rocchia, W.; Cavalli, A. Steered Molecular Dynamics Simulations for Studying Protein-Ligand Interaction in Cyclin-Dependent Kinase 5. J. Chem. Inf. Model. 2014, 54, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.; Rauscher, S. The Conformational Space of the SARS-CoV-2 Main Protease Active Site Loops Is Determined by Ligand Binding and Interprotomer Allostery. Biochemistry 2025, 64, 32–46. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, S.; Nitsche, C. SARS-CoV-2 Papain-Like Protease: Structure, Function and Inhibition. Chembiochem 2022, 23, e202200327. [Google Scholar] [CrossRef] [PubMed]
- Fu, Z.; Huang, B.; Tang, J.; Liu, S.; Liu, M.; Ye, Y.; Liu, Z.; Xiong, Y.; Zhu, W.; Cao, D.; et al. The complex structure of GRL0617 and SARS-CoV-2 PLpro reveals a hot spot for antiviral drug discovery. Nat. Commun. 2021, 12, 488. [Google Scholar] [CrossRef]
- Shao, Q.; Xiong, M.; Li, J.; Hu, H.; Su, H.; Xu, Y. Unraveling the catalytic mechanism of SARS-CoV-2 papain-like protease with allosteric modulation of C270 mutation using multiscale computational approaches. Chem. Sci. 2023, 14, 4681–4696. [Google Scholar] [CrossRef]
- Biniossek, M.L.; Nägler, D.K.; Becker-Pauly, C.; Schilling, O. Proteomic identification of protease cleavage sites characterizes prime and non-prime specificity of cysteine cathepsins B, L, and S. J. Proteome Res. 2011, 10, 5363–5373. [Google Scholar] [CrossRef]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef]
- Kim, M.J.; Yamamoto, D.; Matsumoto, K.; Inoue, M.; Ishida, T.; Mizuno, H.; Sumiya, S.; Kitamura, K. Crystal structure of papain-E64-c complex. Binding diversity of E64-c to papain S2 and S3 subsites. Biochem. J. 1992, 287 Pt 3, 797–803. [Google Scholar] [CrossRef]
- Xie, X.; Lan, Q.; Zhao, J.; Zhang, S.; Liu, L.; Zhang, Y.; Xu, W.; Shao, M.; Peng, J.; Xia, S.; et al. Structure-based design of pan-coronavirus inhibitors targeting host cathepsin L and calpain-1. Sig. Transduct. Target Ther. 2024, 9, 54. [Google Scholar] [CrossRef]
- Li, Q.; Wang, H.; Yang, W.L.; Yang, J.K. An approach combining deep learning and molecule docking for drug discovery of cathepsin L. Expert Opin. Drug Discov. 2023, 18, 347–356. [Google Scholar] [CrossRef]
- Ehmke, V.; Winkler, E.; Banner, D.W.; Haap, W.; Schweizer, W.B.; Rottmann, M.; Kaiser, M.; Freymond, C.; Schirmeister, T.; Diederich, F. Optimization of triazine nitriles as rhodesain inhibitors: Structure-activity relationships, bioisosteric imidazopyridine nitriles, and X-ray crystal structure analysis with human cathepsin L. ChemMedChem 2013, 8, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Falke, S.; Lieske, J.; Herrmann, A.; Loboda, J.; Karničar, K.; Günther, S.; Reinke, P.Y.A.; Ewert, W.; Usenik, A.; Lindič, N.; et al. Structural Elucidation and Antiviral Activity of Covalent Cathepsin L Inhibitors. J. Med. Chem. 2024, 67, 7048–7067. [Google Scholar] [CrossRef]
- Linnevers, C.J.; McGrath, M.E.; Armstrong, R.; Mistry, F.R.; Barnes, M.G.; Klaus, J.L.; Palmer, J.T.; Klatz, B.A.; Bromme, D. Expression of human cathepsin K in Pichia pastoris and preliminary crystallographic studies of an inhibitor complex. Protein Sci. 1997, 6, 919–921. [Google Scholar] [CrossRef]
- Cunha, R.L.O.R.; Urano, M.E.; Chagas, J.R.; Almeida, P.C.; Bincoletto, C.; Tersariol, I.L.S.; Comasseto, J.V. Tellurium-based cysteine protease inhibitors: Evaluation of novel organotellurium(IV) compounds as inhibitors of human cathepsin B. Bioorg. Med. Chem. Lett. 2005, 15, 755–760. [Google Scholar] [CrossRef]
- van de Plassche, M.A.T.; Barniol-Xicota, M.; Verhelst, S.H.L. Peptidyl Acyloxymethyl Ketones as Activity-Based Probes for the Main Protease of SARS- CoV-2. ChemBioChem 2020, 21, 3383–3388. [Google Scholar] [CrossRef]
- Puhl, A.C.; Gomes, G.F.; Damasceno, S.; Godoy, A.S.; Noske, G.D.; Nakamura, A.M.; Gawriljuk, V.O.; Fernandes, R.S.; Monakhova, N.; Riabova, O.; et al. Pyronaridine Protects against SARS-CoV-2 Infection in Mouse. ACS Inf. Dis. 2022, 8, 1147–1160. [Google Scholar] [CrossRef]
- Fuzo, C.A.; Martins, R.B.; Fraga-Silva, T.F.C.; Amstalden, M.K.; DeLeo, T.C.; Souza, J.P.; Lima, T.M.; Faccioli, L.H.; Okamoto, D.N.; Juliano, M.A.; et al. Celastrol: A lead compound that inhibits SARS-CoV-2 replication, the activity of viral and human cysteine proteases, and virus-induced IL-6 secretion. Drug Dev. Res. 2022, 83, 1623–1640. [Google Scholar] [CrossRef]
- Ewig, C.S.; Berry, R.; Dinur, U.; Hill, J.R.; Hwang, M.J.; Li, H.; Liang, C.; Maple, J.; Peng, Z.; Stockfisch, T.P.; et al. Derivation of class II force fields. VIII. Derivation of a general quantum mechanical force field for organic compounds. J. Comput. Chem. 2001, 22, 1782–1800. [Google Scholar] [CrossRef]
- Fletcher, R. Unconstrained optimization. In Practical Methods of Optimization, 1st ed.; John Wiley & Sons Ltd.: New York, NY, USA, 1980; Volume 1, pp. 1–128. ISBN 978-0471277118. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Reed, E.; Weinstock, R.B.; Weinhold, F. Natural Population Analysis. J. Chem. Phys. 1985, 83, 735–746. [Google Scholar] [CrossRef]
- Hattori, S.I.; Higashi-Kuwata, N.; Hayashi, H.; Allu, S.R.; Raghavaiah, J.; Bulut, H.; Das, D.; Anson, B.J.; Lendy, E.K.; Takamatsu, Y.; et al. A small molecule compound with an indole moiety inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2021, 12, 668. [Google Scholar] [CrossRef] [PubMed]
- Jaskolski, M.; Dauter, Z.; Shabalin, I.G.; Gilski, M.; Brzezinski, D.; Kowiel, M.; Rupp, B.; Wlodawer, A. Crystallographic models of SARS-CoV-2 3CLpro: In-depth assessment of structure quality and validation. IUCrJ 2021, 8 Pt 2, 238–256. [Google Scholar] [CrossRef] [PubMed]
- Laskowski, R.A.; MacArthur, M.W.; Moss, D.S.; Thornton, J.M. PROCHECK: A program to check the stereochemical quality of protein structures. J. Appl. Crystallogr. 1993, 26, 283–291. [Google Scholar] [CrossRef]
- Jorgensen, W.L.; Chandrasekhar, J.; Madura, J.D.; Impey, R.W.; Klein, M.L. Comparison of Simple Potential Functions for Simulating Liquid Water. J. Chem. Phys. 1983, 79, 926–935. [Google Scholar] [CrossRef]
- Vanommeslaeghe, K.; Hatcher, E.; Acharya, C.; Kundu, S.; Zhong, S.; Shim, J.E.; Darian, E.; Guvench, O.; Lopes, P.; Vorobyov, I.; et al. CHARMM General Force Field (CGenFF): A force field for drug-like molecules compatible with the CHARMM all-atom additive biological force fields. J. Comput. Chem. 2010, 31, 671–690. [Google Scholar] [CrossRef]
- Hoover, W.G. Canonical Dynamics: Equilibrium Phase-Space Distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef]
- Feller, S.E.; Zhang, Y.; Pastor, R.W.; Brooks, B.R. Constant Pressure Molecular Dynamics Simulation: The Langevin Piston Method. J. Chem. Phys. 1995, 103, 4613–4621. [Google Scholar] [CrossRef]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N·log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef]
- Maple, J.R.; Hwang, M.J.; Stockfisch, T.P.; Dinur, U.; Waldman, M.; Ewig, C.S.; Hagler, A.T. Derivation of Class II Force Fields. I. Methodology and Quantum Force Field for the Alkyl Functional Group and Alkane Molecules. J. Comput. Chem. 1994, 15, 162–182. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucl. Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Dodda, L.S.; de Vaca, I.C.; Tirado-Rives, J.; Jorgensen, W.L. LigParGen web server: An automatic OPLS-AA parameter generator for organic ligands. Nucleic Acids Res. 2017, 45, W331–W336. [Google Scholar] [CrossRef] [PubMed]
- Bianco, G.; Forli, S.; Goodsell, D.S.; Olson, A.J. Covalent docking using autodock: Two-point attractor and flexible side chain methods. Protein Sci. 2016, 25, 295–301. [Google Scholar] [CrossRef]
- Emsley, P.; Lohkamp, B.; Scott, W.G.; Cowtan, K. Features and development of Coot. Acta Crystallogr. D Biol. Crystallogr. 2010, 66 Pt 4, 486–501. [Google Scholar] [CrossRef]
- Cianni, L.; Feldmann, C.W.; Gilberg, E.; Gütschow, M.; Juliano, L.; Leitão, A.; Bajorath, J.; Montanari, C.A. Can Cysteine Protease Cross-Class Inhibitors Achieve Selectivity? J. Med. Chem. 2019, 62, 10497–10525. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).