Synthetic Routes to, Stabilities and Transformations of, and Characterization of (Carbamoyl)disulfanyl Chlorides and Related Compounds1,2
Abstract
1. Introduction
2. Results and Discussion
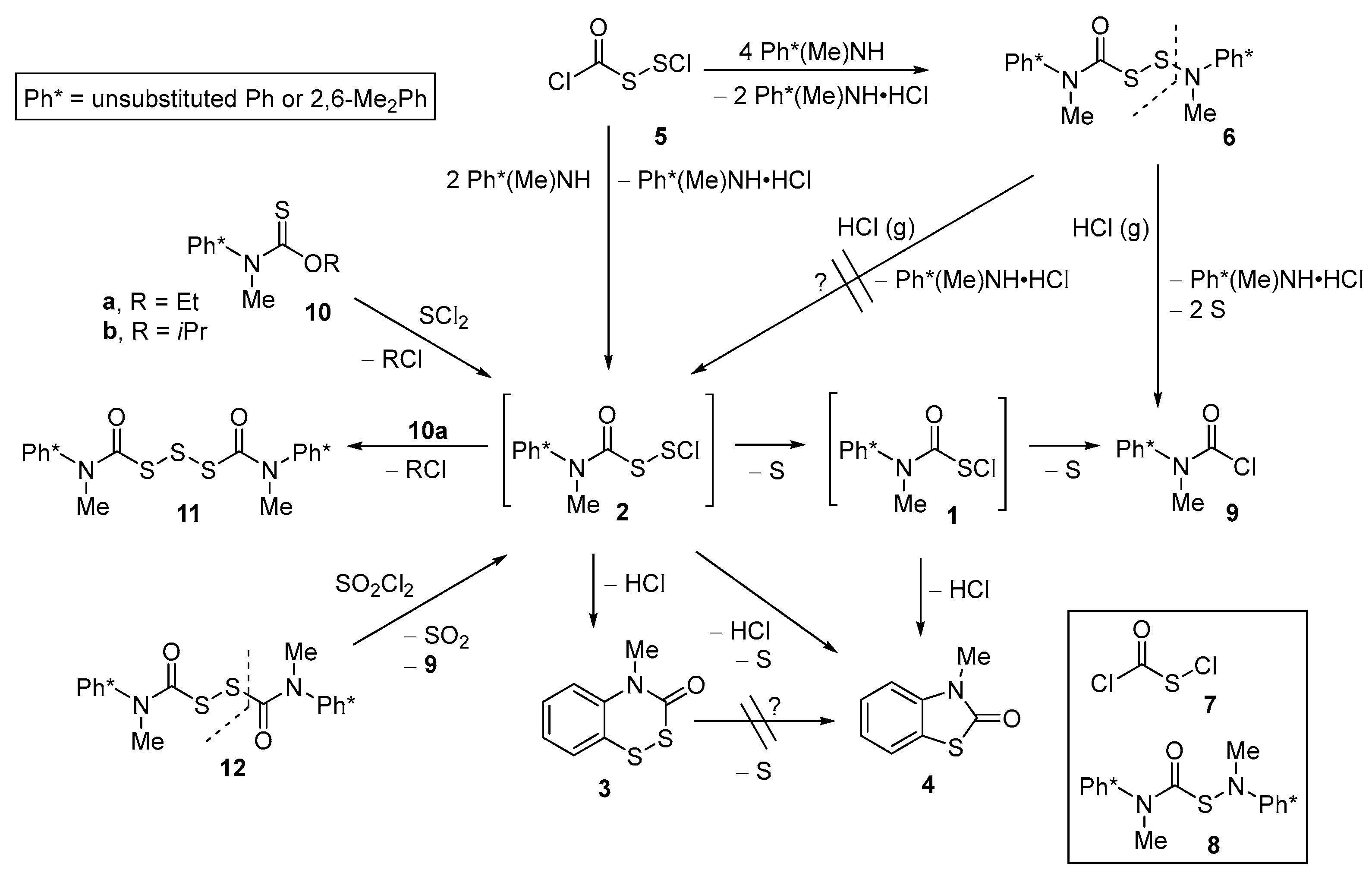
2.1. Generation, Stability, and Decomposition Pathways of (Carbamoyl)disulfanyl Chlorides (2)
2.2. Trapping of (Carbamoyl)disulfanyl Chlorides (2)
2.3. Reaction of 4-Methyl-2(3H)-benzo-1,2,4-dithiazinone (3) with Triphenylphosphine
2.4. Crystal Structure of 4-Methyl-2(3H)-benzo-1,2,4-dithiazinone (3) and Comparison to Linear and Cyclic Compounds with One or Two Sulfur Atoms
| Name or Number CSD Refcode [32] CCDC Number | 3 2470096 | PyDITCN SUJQAR 1013809 | 6 VUYBIC 1428652 | 4 COSFAR01 711838 | EDITH 2479242 | DtsNH NAHMUE 123954 |
|---|---|---|---|---|---|---|
| S1–C1 | 1.7976 (18) | 1.784 (3) | 1.8273 (13) | 1.776 (2) | 1.806 (7) | 1.764 (2) |
| S1–S2 | 2.0580 (6) | 2.0655 (9) | 2.0625 (5) | NR | 2.051 (2) | 2.0584 (8) |
| S2–C16 | 1.7627 (18) | 1.754 (3) | 1.6660 (11) a | 1.744 (2) d | 1.757 (7) | 1.761 (2) |
| N1–C1 | 1.363 (2) | 1.287 (3) | 1.3569 (16) | 1.367 (2) | 1.385 (10) | 1.367 (2) |
| N1–C11 | 1.427 (2) | 1.375 (3) | 1.4429 (15) | 1.392 (2) | 1.272 (9) | 1.369 (2) |
| C11–C16 | 1.403 (2) | 1.389 (4) | 1.3865 (18) | 1.392 (3) | NR | NR |
| C1–S1–S2 | 98.78 (6) | 98.13 (9) | 102.60 (4) | NR | 94.5 (3) | 95.42 (6) |
| C16–S2–S1 | 97.43 (6) | 95.67 (9) | 108.37 (4) b | NR | 91.6 (2) | 95.45 (6) |
| C1–N1–C11 | 126.11 (15) | 117.2 (2) | 123.17 (10) | 115.4 (2) | 115.1 (6) | 121.99 (14) |
| N1–C1–S1 | 117.55 (13) | 126.9 (2) | 112.55 (9) | 109.17 (15) | 115.3 (5) | 113.51 (12) |
| C16–C11–N1 | 122.13 (15) | 131.2 (2) | NR | 113.2 (2) | NR | NR |
| C11–C16–S2 | 118.89 (13) | 120.3 (2) | NR | 110.42 (14) d | NR | NR |
| C1–S1–S2–C16 | –58.36 (8) | 51.72 (12) | –92.62 (6) c | NR | 1.6 (4) | –1.94 (7) |
| C2–N1–C1–O1 | 0.5 (3) | NR | 4.27 (19) | 1.1 (2) | NR | NR |
| N1–C11–C16–S2 | 0.8 (2) | 0.2 (4) | NR | 0.21 (14) d | NR | NR |
| Ring deviation from planarity | 0.328 | 0.292 | NR | 0.008 | 0.011 | 0.013 |
3. Materials and Methods
3.1. General
3.2. X-Ray Data Collection, Solution, and Refinement
3.3. Experimental
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DtsNH | 1,2,4-dithiazolidine-3,5-dione |
| EDITH | 3-ethoxy-1,2,4-dithiazolin-5-one |
| HMB | hexamethylbenzene |
| NMA | N-methylaniline |
| PyDITCN | 1,3-dimethyl-5H-pyrazolo [3,4-e][1,2,4]dithiazine-3-carbonitrile |
| 1 | P.T.G. and T.R.T. contributed equally to this work. |
| 2 | Dedicated to Michael Bárány (29 October 1921–24 July 2011), pioneering scientist and brilliant scholar, who in the last months of his life encouraged G.B. to persevere with this research and his career. Dedicated further to Douglas L. Thompson (25 November 1939–5 November 2024), a curious and playful man who through his example encouraged T.R.T. to be both independent and a team player, disciplined and kind, as she worked with G.B. to revisit and close out this project. |
| 3 | Footnote 14 in [3] drew compound 19, a putative (carbamoyl)disulfanyl chloride, and spectral data deduced for “19” was included in the experimental section for compound 16 (compound numberings from [3]). Thus, it was claimed that freshly generated ROCCl2SSS(C=O)N(Me)Ph rapidly reached equilibrium with RO(C=S)Cl + ClSS(C=O)N(Me)Ph. With the benefit of the comprehensive results reported in the present work, we now realize that the species encountered and described briefly in our previous work was not (carbamoyl)disulfanyl chloride (2), but in fact heterocycle 4 (compound numberings in the current sentence correspond to the text and Schemes of this paper). |
| 4 | Sulfurization reagents that convert P(III) to P(V)=S, along with the applications of this chemistry for the creation of potential anti-sense drugs based on DNA or RNA, have been reviewed relatively recently [9,10]. Our contributions to this area [11,12] center around ethoxy-1,2,4-dithiazolin-5-one (EDITH) and 1,2,4-dithiazolidine-3,5-dione (DtsNH), both of which (especially EDITH) effect this transformation with extraordinarily high efficiency. For chemical structures of DtsNH and EDITH, please refer to Scheme 5, later in the paper. |
| 5 | To put this result in context, the usual N-methylaniline assay [6] of (chlorocarbonyl)disulfanyl chloride (5), using the secondary amine in excess, rapidly gives (carbamoyl)disulfenamide 6 [3,13] in excellent yield and purity (Scheme 1, top line, right side). For further context, methods described in the text to create 2 and 6 are based on our previous precedents [1,3,6] that use (chlorocarbonyl)sulfenyl chloride (7) plus limiting or excess N-methylaniline to create (carbamoyl)sulfenamides 1 or 8, respectively. |
| 6 | There can be little doubt that 1 and 2 are intermediates in the reactions of 7 and 5 with excess N-methylaniline or N,2,6-trimethylaniline to provide 8 and 6, respectively. The interception of 1 with a different secondary amine to create unsymmetrical “crossover” (carbamoyl)sulfenamide products 8, with one sulfur, has been precedented [1], but the obvious homologation of such reactions in two-sulfur homologues (i.e., 2, 6) was not explicitly pursued in the present work. |
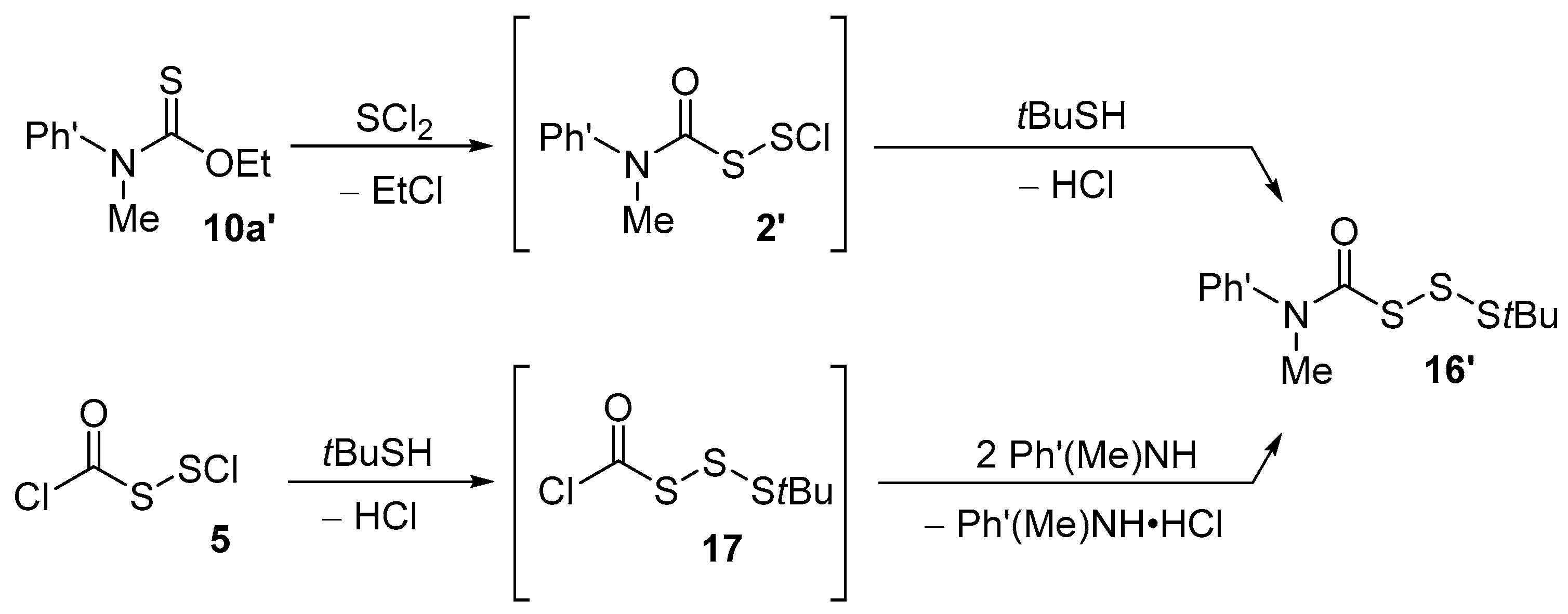
| 7 | Careful examination of the 1H NMR signal that we assign to 2 reveals two closely adjacent singlets, most likely due to the presence of both protonated and non-protonated species. It was not possible to characterize compound 2 by 13C NMR, since reaction conditions required for reliable generation of 2 were too dilute to allow acquisition of spectral data on a time frame that preceded further transformations and/or decomposition of 2. As reported later in this paper, when the N-methylaniline moiety of 2 is replaced by an N,2,6-trimethylaniline moiety, the resultant 2’ is considerably more stable, and could be characterized by both 1H and 13C NMR. |
| 8 | In contrast, heterocyclization of (carbamoyl)sulfenyl chloride 1 to provide the one-sulfur heterocycle 4, as reported in [1], was a function of both the method of generating 1 and the concentration of this species. In typical experiments, when 1 (1 M or 0.1 M) had been generated by reaction of limiting N-methylaniline with (chlorocarbonyl)sulfenyl chloride (7) in CDCl3 at 25 °C, further conversion of 1 to 4 occurred with t½~1 h or 24 h, respectively. |
| 9 | |
| 10 | The reaction of equimolar amounts of thiocarbamate 10b with SCl2, somewhat analogous to the reaction of 10b with SO2Cl2 that reliably gave (carbamoyl)sulfenyl chlorides 1 (plus iPrCl and SO2) as documented in [1], was reasonably expected to provide (carbamoyl)disulfanyl chloride 2 plus an equiv of iPrCl—especially in view of the fact that reaction of 10b (2 equiv) with SCl2 is a reliable, high-yield route to trisulfane 11 (see [3]). |
| 11 | Prior to numerous examples from ref. [2] and from our own laboratory (e.g., [1,3,6]), the reactions of sulfenyl chlorides with O-alkyl thiocarbamates to generate carbamoyl disulfide moieties (with concomitant loss of alkyl chloride) were described in 1960 by Harris [20]—hence our reference to “Harris reactions”. |
| 12 | |
| 13 | When this manuscript was peer reviewed, two separate anonymous referees suggested potentially productive avenues to further develop and extend the new chemistry reported herein. One proposed replacing the chlorine of 2 with fluorine, while another speculated a connection to Bunte salts. We believe that compelling cases can be made to explore these and other avenues, and hope that other laboratories will rise to these challenges. |
References
- Schrader, A.M.; Schroll, A.L.; Barany, G. Synthetic Routes to, Transformations of, and Rather Surprising Stabilities of (N-Methyl-N-phenylcarbamoyl)sulfenyl Chloride, ((N-Methyl-N-phenylcarbamoyl)dithio)carbonyl Chloride, and Related Compounds. J. Org. Chem. 2011, 76, 7882–7892. [Google Scholar] [CrossRef]
- Zumach, G.; Kühle, E. Chlorosulfenylated Carbonic Acid Derivatives. Angew. Chem. Int. Ed. Engl. 1970, 9, 54–63. [Google Scholar] [CrossRef]
- Schroll, A.L.; Barany, G. Novel Symmetrical and Mixed Carbamoyl and Amino Polysulfanes by Reactions of (Alkoxydichloromethyl)polysulfanyl Substrates with N-Methylaniline. J. Org. Chem. 1986, 51, 1866–1881. [Google Scholar] [CrossRef]
- Besthorn, E. Über Derivate des Benzthiazols. Berichte Dtsch. Chem. Ges. 1910, 43, 1519–1526. [Google Scholar] [CrossRef]
- Fife, T.H.; Hutchins, J.E.; Wang, M.S. Highly efficient intramolecular nucleophilic reactions. The cyclization of p-nitrophenyl N-(2-mercaptophenyl)-N-methylcarbamate and phenyl N-(2-aminophenyl)-N-methylcarbamate. J. Am. Chem. Soc. 1975, 97, 5878–5882. [Google Scholar] [CrossRef]
- Barany, G.; Schroll, A.L.; Mott, A.W.; Halsrud, D.A. A General Strategy for Elaboration of the Dithiocarbonyl Functionality, –(C=O)SS–: Application to the Synthesis of Bis(chlorocarbonyl)disulfane and Related Derivatives of Thiocarbonic Acids. J. Org. Chem. 1983, 48, 4750–4761. [Google Scholar] [CrossRef]
- Rudd, S.; Barany, G. 3-Methyl-2(3H)-benzothiazolone, C8H7NOS. Acta Cryst. 1984, 40, 2118–2120. [Google Scholar] [CrossRef]
- Yosef, H.A.A. Preparation, Thermolysis, and Photolysis of Some New Thiophosphoramidates Derived from 3-Methyl-2-Benzothiazolinone Hydrazone. Phosphorus Sulfur Silicon Relat. Elem. 2010, 185, 890–897. [Google Scholar] [CrossRef]
- Volk, D.; Lokesh, G. Development of Phosphorothioate DNA and DNA Thioaptamers. Biomedicines 2017, 5, 41. [Google Scholar] [CrossRef]
- Ren, Q.; Osawa, T.; Tatsuno, M.; Obika, S. THF Peroxide as a Factor in Generating Desulphurised Products from the Solid-Phase Synthesis of Phosphorothioate-Modified Oligonucleotides. RSC Adv. 2024, 14, 21590–21596. [Google Scholar] [CrossRef]
- Xu, Q.; Musier-Forsyth, K.; Hammer, R.P.; Barany, G. Use of 1,2,4-Dithiazolidine-3,5-Dione (DtsNH) and 3-Ethoxy-1,2,4-Dithiazoline-5-One (EDITH) for Synthesis of Phosphorothioate-Containing Oligodeoxyribonucleotides. Nucleic Acids Res. 1996, 24, 1602–1607. [Google Scholar] [CrossRef]
- Xu, Q.; Barany, G.; Hammer, R.P.; Musier-Forsyth, K. Efficient Introduction of Phosphorothioates into RNA Oligonucleotides by 3-Ethoxy-1,2,4-Dithiazoline-5-One (EDITH). Nucleic Acids Res. 1996, 24, 3643–3644. [Google Scholar] [CrossRef]
- Henley, M.J.; Schroll, A.L.; Young, V.G., Jr.; Barany, G. Crystal structures of (N-methyl-N-phenylamino)(N-methyl-N-phenylcarbamoyl)sulfide and the corresponding disulfane. Acta Crystallogr. 2015, 71, 1371–1374. [Google Scholar] [CrossRef] [PubMed]
- Böhme, H.; Brinkmann, M.; Steudel, H.-P. Gewinnung und Umsetzungen von Chlordisulfanylderivaten der Kohlensäure. Liebigs Ann. Chem. 1981, 1244–1251. [Google Scholar] [CrossRef]
- Mott, A.W.; Barany, G. A New Method for the Synthesis of Unsymmetrical Trisulfanes. Synthesis 1984, 8, 657–660. [Google Scholar] [CrossRef]
- Chen, L.; Zoulíková, I.; Slaninová, J.; Barany, G. Synthesis and Pharmacology of Novel Analogues of Oxytocin and Deaminooxytocin: Directed Methods for the Construction of Disulfide and Trisulfide Bridges in Peptides. J. Med. Chem. 1997, 40, 864–876. [Google Scholar] [CrossRef]
- Tobón, Y.A.; Cozzarín, M.V.; Wang, W.G.; Ge, M.F.; Della Védova, C.O.; Romano, R.M. Vibrational and Valence Photoelectron Spectroscopies, Matrix Photochemistry, and Conformational Studies of ClC(O)SSCl. J. Phys. Chem. A 2011, 115, 10203–10210. [Google Scholar] [CrossRef]
- Hammer, R.P.; Butrie, M.A.; Davidson, K.; Goldblatt, P.T.; Schrader, A.M.; Dalluge, J.J.; Becker, A.; Barany, G. Scaled-up Synthesis and Characterization of Oxytocin Trisulfide. Int. J. Pept. Ther. Res. 2024, 30, 5. [Google Scholar] [CrossRef]
- Schroll, A.; Eastep, S.J.; Barany, G. Synthesis and Characterization of (Methoxy(thiocarbonyl))sulfenyl Chloride. J. Org. Chem. 1990, 55, 1475–1479. [Google Scholar] [CrossRef]
- Harris, J.F. The Reactions of Sulfenyl Chlorides with Thionocarbamates. J. Am. Chem. Soc. 1960, 82, 155–158. [Google Scholar] [CrossRef]
- Sievertsson, H.; Nilsson, J.L.G.; Hjeds, H.; Ragnarsson, U.; Rasmussen, S.E.; Sunde, E.; Sørensen, N.A. Phenylcarbamic Acid Anhydrides; a New Class of Compounds. Acta Chem. Scand. 1970, 24, 939–945. [Google Scholar] [CrossRef]
- Review: Kühle, E. One Hundred Years of Sulfenic Acid Chemistry II a. Oxidation, Reduction, and Addition Reactions of Sulfenyl Halides 1. Synthesis 1971, 1971, 563–586. [Google Scholar] [CrossRef]
- Hanusek, J.; Russell, M.A.; Laws, A.P.; Jansa, P.; Atherton, J.H.; Fettes, K.; Page, M.I. Mechanism of the Sulfurisation of Phosphines and Phosphites Using 3-Amino-1,2,4-Dithiazole-5-Thione (Xanthane Hydride). Org. Biomol. Chem. 2007, 5, 478–484. [Google Scholar] [CrossRef] [PubMed]
- Ponomarov, O.; Laws, A.P.; Hanusek, J. 1,2,4-Dithiazole-5-Ones and 5-Thiones as Efficient Sulfurizing Agents of Phosphorus(III) Compounds—A Kinetic Comparative Study. Org. Biomol. Chem. 2012, 10, 8868–8876. [Google Scholar] [CrossRef]
- Ponomarov, O.; Padělková, Z.; Hanusek, J. Mechanism of Sulfur Transfer from 1,2,4-Dithiazolidine-3,5-Diones to Triphenylphosphines. J. Phys. Org. Chem. 2013, 26, 560–564. [Google Scholar] [CrossRef]
- Chen, L.; Thompson, T.R.; Hammer, R.P.; Barany, G. Synthetic, Mechanistic, and Structural Studies Related to 1,2,4-Dithiazolidine-3,5-Dione. J. Org. Chem. 1996, 61, 6639–6645. [Google Scholar] [CrossRef]
- Young, V.G., Jr.; Goebel, E.S.; Barany, G. CCDC 2479242: Experimental Crystal Structure Determination of 3-Ethoxy-1,2,4-Dithiazoline-5-one; Cambridge Crystallographic Data Centre: Cambridge, UK, 2025. [Google Scholar] [CrossRef]
- Steudel, R. Properties of Sulfur-Sulfur Bonds. Angew. Chem. Int. Engl. 1975, 14, 655–664. [Google Scholar] [CrossRef]
- Cayón, V.M.; Erben, M.F.; Romano, R.M.; Stammler, H.; Mitzel, N.W.; Ge, M.F.; Védova, C.O.D. Simple Disulfides: Studies of Some Exponents of a Family Involved in Prominent Processes. ChemistrySelect 2023, 8, e202302560. [Google Scholar] [CrossRef]
- Oughton, B.M.; Harrison, P.M. The crystal structure of hexagonal L-cystine. Acta Crystallogr. 1959, 12, 396. [Google Scholar] [CrossRef]
- Thornton, J.M. Disulphide Bridges in Globular Proteins. J. Mol. Biol. 1981, 151, 261–287. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Crystallogr. 2016, 72, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Koyioni, M.; Manoli, M.; Koutentis, P.A. Synthesis of Fused 1,2,4-Dithiazines and 1,2,3,5-Trithiazepines. J. Org. Chem. 2014, 79, 9717–9727. [Google Scholar] [CrossRef]
- Ruff, B.M.; Zhong, S.; Nieger, M.; Bräse, S. Thiolation of Symmetrical and Unsymmetrical Diketopiperazines. Org. Biomol. Chem. 2012, 10, 935–940. [Google Scholar] [CrossRef]
- Baumann, M.; Dieskau, A.P.; Loertscher, B.M.; Walton, M.C.; Nam, S.; Xie, J.; Horne, D.; Overman, L.E. Tricyclic analogues of epidithiodioxopiperazine alkaloids with promising in vitro and in vivo antitumor activity. Chem. Sci. 2015, 6, 4451–4457. [Google Scholar] [CrossRef]
- Asquith, C.R.M.; Sil, B.C.; Laitinen, T.; Tizzard, G.J.; Coles, S.J.; Poso, A.; Hofmann-Lehmann, R.; Hilton, S.T. Novel Epidithiodiketopiperazines as Anti-Viral Zinc Ejectors of the Feline Immunodeficiency Virus (FIV) Nucleocapsid Protein as a Model for HIV Infection. Bioorg. Med. Chem. 2019, 27, 4174–4184. [Google Scholar] [CrossRef]
- Kilgore, H.R.; Olsson, C.R.; D’Angelo, K.A.; Movassaghi, M.; Raines, R.T. n →π* Interactions Modulate the Disulfide Reduction Potential of Epidithiodiketopiperazines. J. Am. Chem. Soc. 2020, 142, 15107–15115. [Google Scholar] [CrossRef]
- Koning, N.R.; Sundin, A.P.; Strand, D. Total Synthesis of (−)-Glionitrin A and B Enabled by an Asymmetric Oxidative Sulfenylation of Triketopiperazines. J. Am. Chem. Soc. 2021, 143, 21218–21222. [Google Scholar] [CrossRef]
- Harris, R.K.; Becker, E.D.; De Menezes, S.M.C.; Granger, P.; Hoffman, R.E.; Zilm, K.W. Further Conventions for NMR Shielding and Chemical Shifts (IUPAC Recommendations 2008). Magn. Reson. Chem. 2008, 46, 582–598. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.K.; Becker, E.D.; Menezes, S.M.C.; Goodfellow, R.; Granger, P. NMR Nomenclature. Nuclear Spin Properties and Conventions for Chemical Shifts (IUPAC Recommendations 2001). Pure Appl. Chem. 2001, 73, 1795–1818. [Google Scholar] [CrossRef]
- Bruker AXS. APEX5; Bruker AXS: Madison, WI, USA, 2023. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Bruker. SAINT; Bruker AXS Inc.: Madison, WI, USA, 2023. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goldblatt, P.T.; Thompson, T.R.; Brennessel, W.W.; Smith, T.G.; Schrader, A.M.; Goebel, E.S.; Henley, M.J.; Lovstedt, A.; Young, V.G., Jr.; Barany, G. Synthetic Routes to, Stabilities and Transformations of, and Characterization of (Carbamoyl)disulfanyl Chlorides and Related Compounds1,2. Molecules 2025, 30, 3892. https://doi.org/10.3390/molecules30193892
Goldblatt PT, Thompson TR, Brennessel WW, Smith TG, Schrader AM, Goebel ES, Henley MJ, Lovstedt A, Young VG Jr., Barany G. Synthetic Routes to, Stabilities and Transformations of, and Characterization of (Carbamoyl)disulfanyl Chlorides and Related Compounds1,2. Molecules. 2025; 30(19):3892. https://doi.org/10.3390/molecules30193892
Chicago/Turabian StyleGoldblatt, Phillip T., Tracy R. Thompson, William W. Brennessel, Thomas G. Smith, Alex M. Schrader, Erik S. Goebel, Madeleine J. Henley, Alex Lovstedt, Victor G. Young, Jr., and George Barany. 2025. "Synthetic Routes to, Stabilities and Transformations of, and Characterization of (Carbamoyl)disulfanyl Chlorides and Related Compounds1,2" Molecules 30, no. 19: 3892. https://doi.org/10.3390/molecules30193892
APA StyleGoldblatt, P. T., Thompson, T. R., Brennessel, W. W., Smith, T. G., Schrader, A. M., Goebel, E. S., Henley, M. J., Lovstedt, A., Young, V. G., Jr., & Barany, G. (2025). Synthetic Routes to, Stabilities and Transformations of, and Characterization of (Carbamoyl)disulfanyl Chlorides and Related Compounds1,2. Molecules, 30(19), 3892. https://doi.org/10.3390/molecules30193892





