The Effect of Organic Materials on the Response of the Soil Microbiome to Bisphenol A
Abstract
1. Introduction
2. Results
2.1. Plant
2.2. Culturable Bacteria
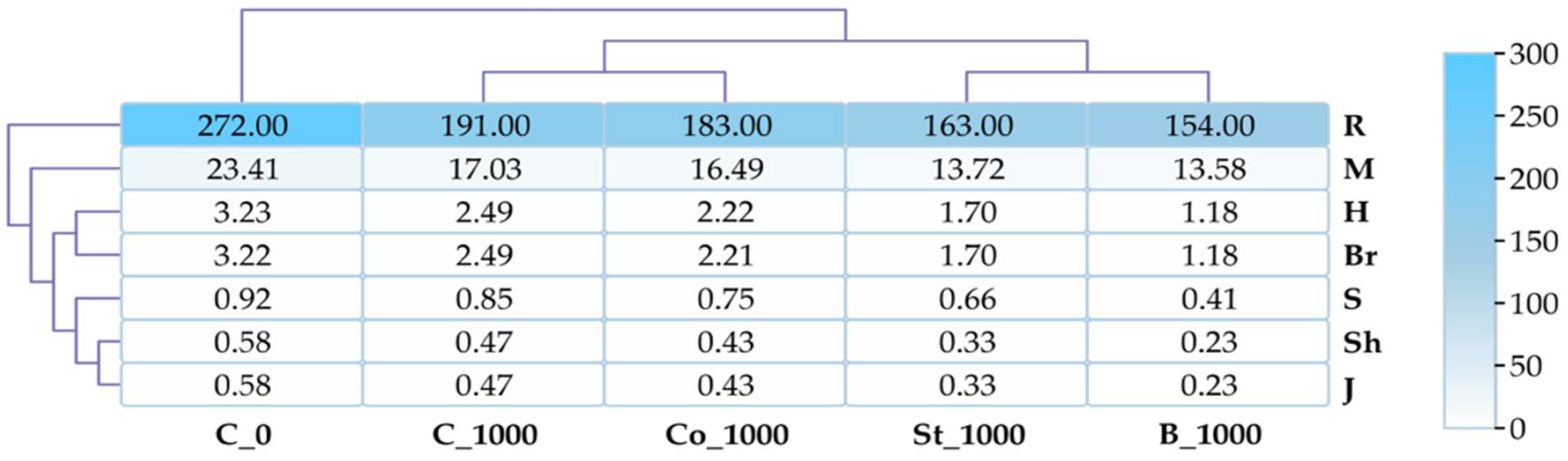
2.3. Non-Culturable Bacteria
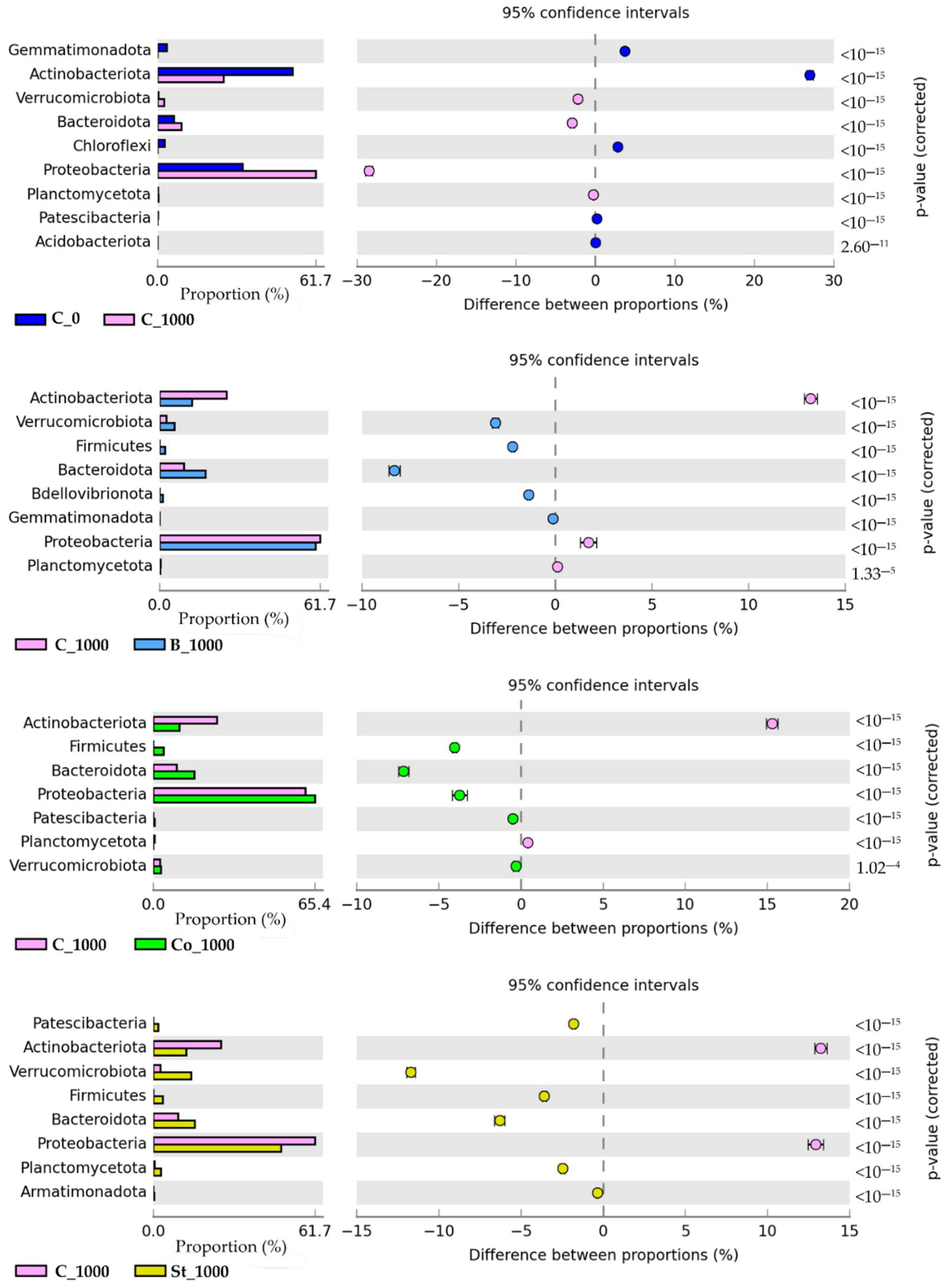
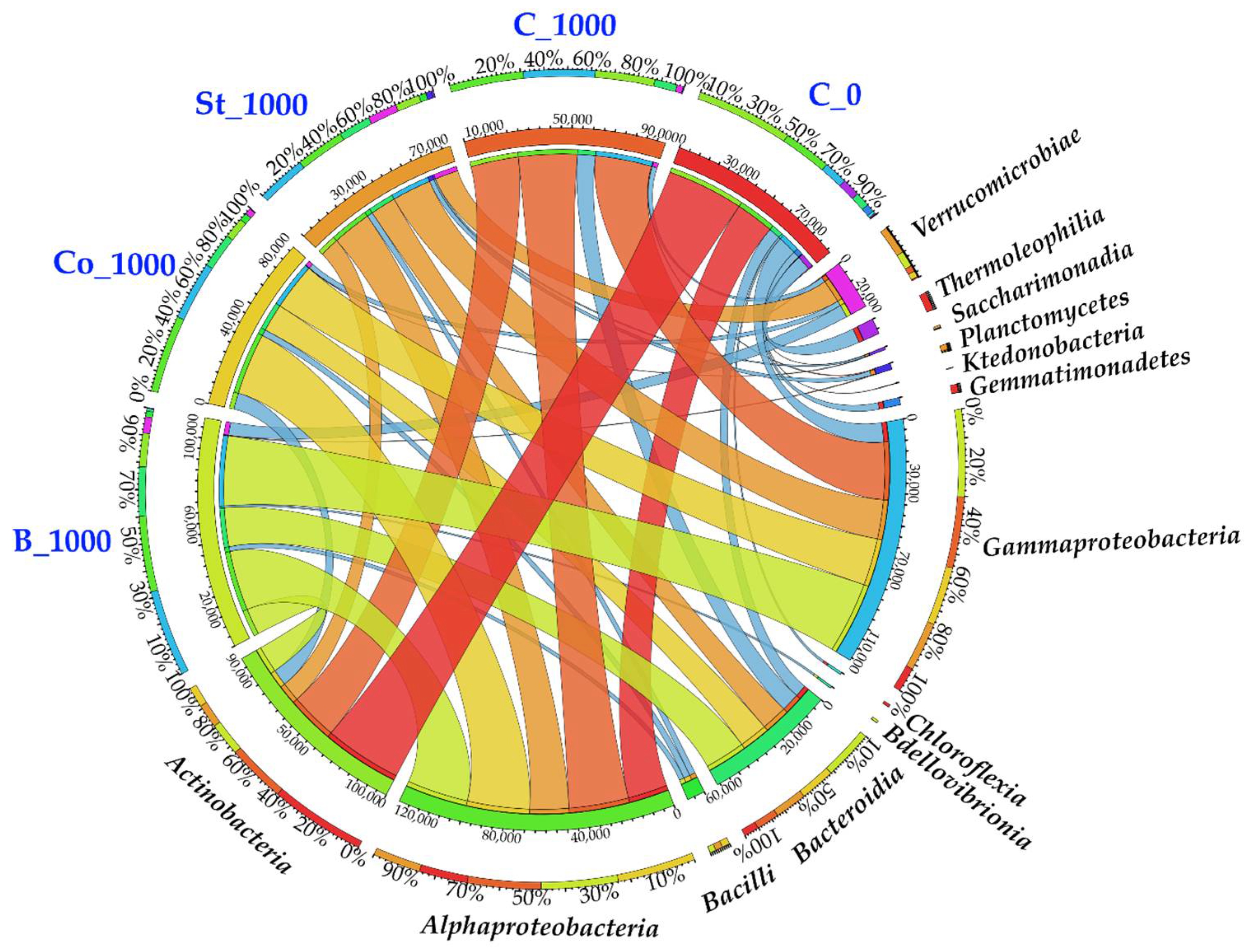
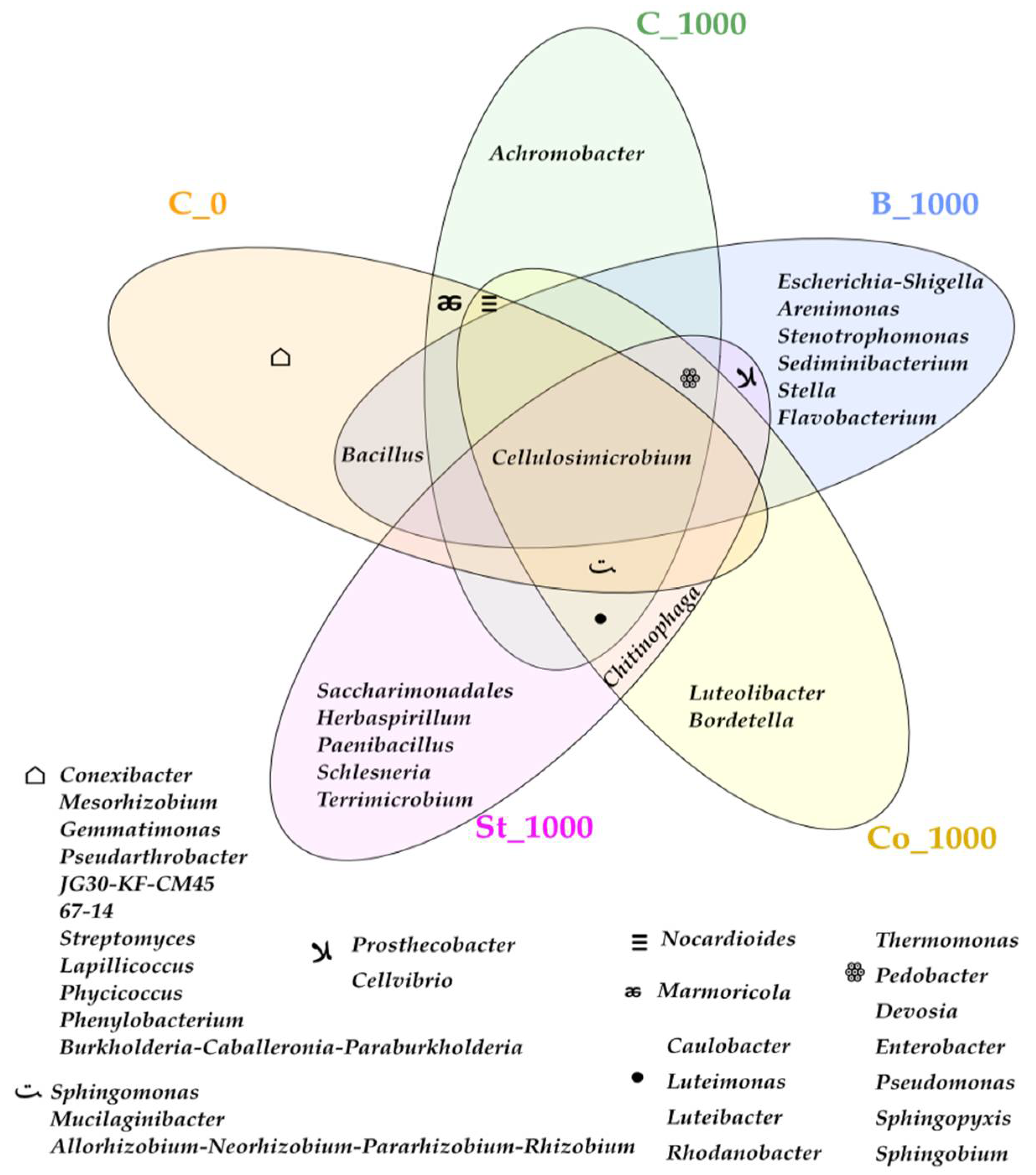
2.3.1. Bacteria
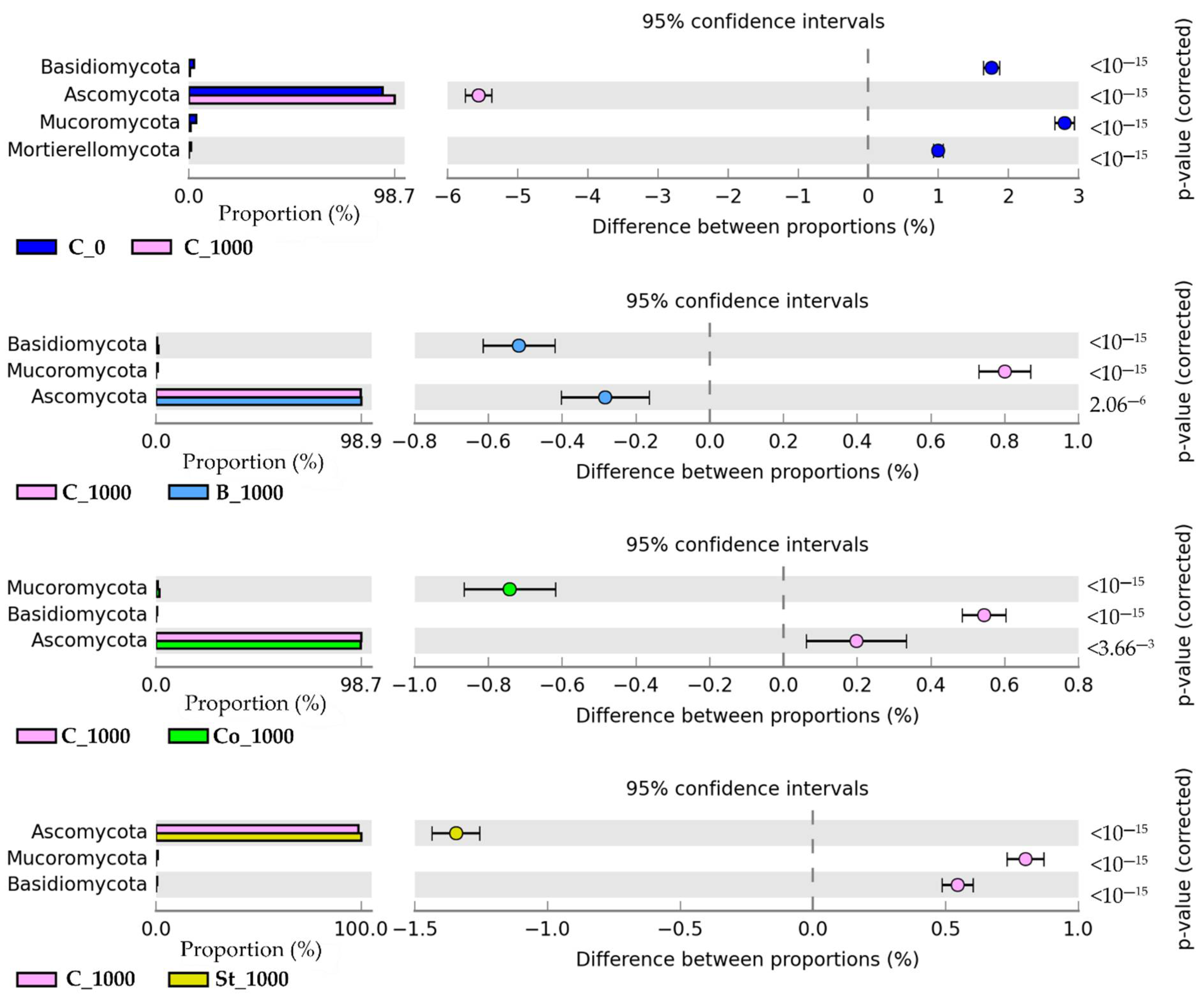
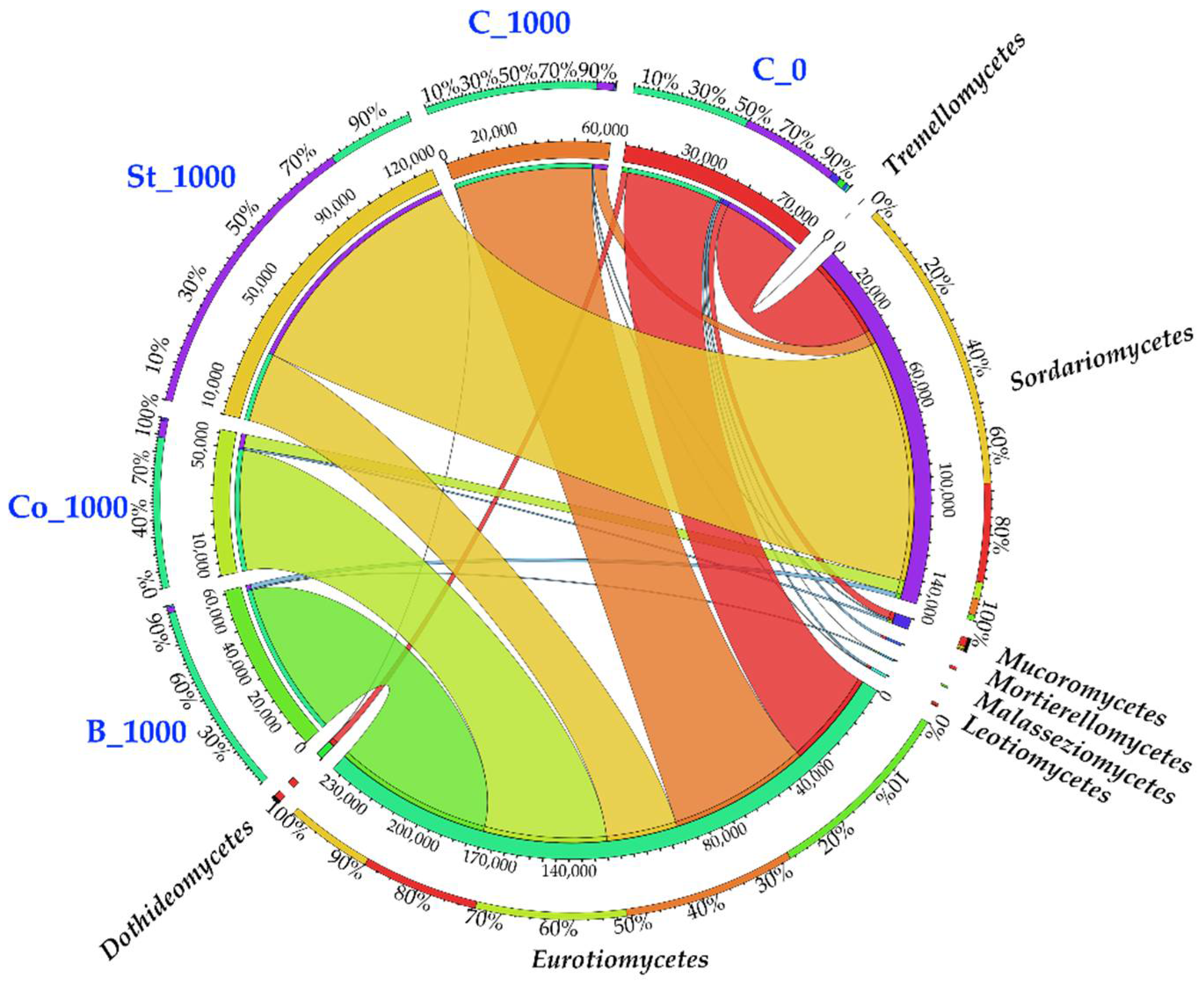
2.3.2. Fungi
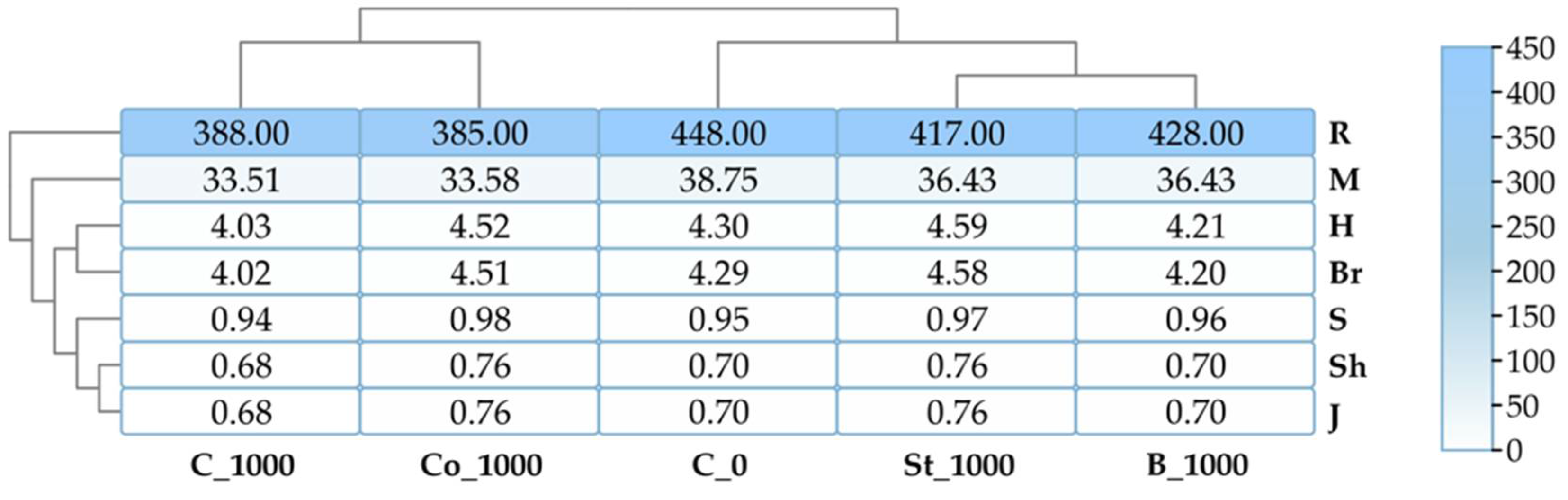
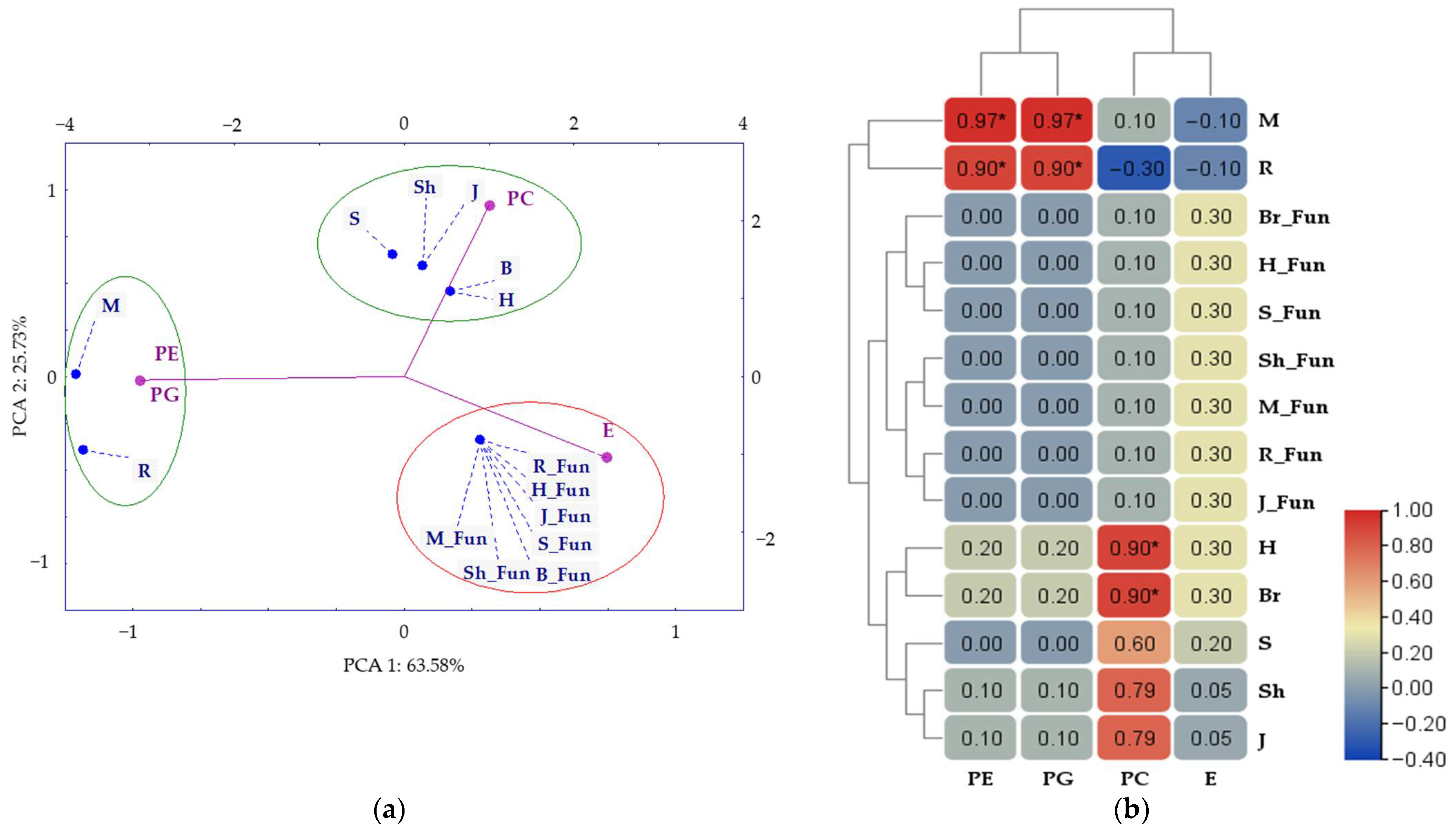
2.3.3. Phospholipid and Ergosterol Composition of Soil
2.3.4. Interdependence Between Membrane Lipid Composition and Indicators of Fungal and Bacterial Diversity
3. Discussion
3.1. Zea mays
3.2. Culturable Bacteria
3.3. Non-Culturable Microorganisms
3.3.1. Bacteria
3.3.2. Fungi
3.3.3. Phospholipid and Ergosterol Composition of Soil
4. Materials and Methods
4.1. Design and Procedure for Conducting Research on Soil Sown with Zea mays
4.2. Characteristics of the Soil Sampling Area for Research
4.3. Selected Chemical, Physicochemical, and Microbiological Properties of Soil
4.4. BPA
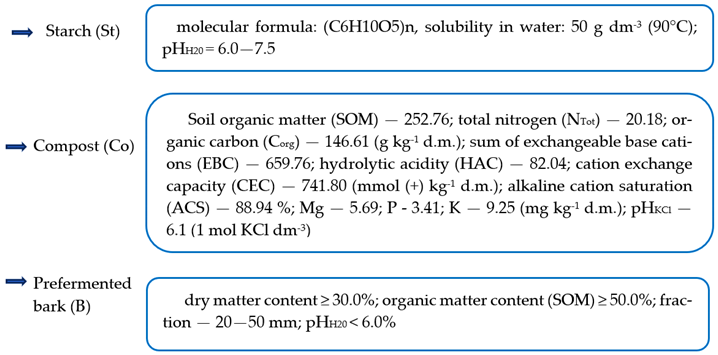
4.5. Characteristics of Organic Remediation Substances
4.6. Methodology for the Determination of Cultivable Bacteria and Fungi
4.7. Isolation of Bacterial and Fungal DNA
4.8. Methodology for the Identification of Non-Culturable Bacteria and Fungi
4.9. Analysis of Fatty Acid Profiles
4.9.1. Soil Preparation
4.9.2. Lipids Analysis
4.10. Calculation Methodology and Statistical Data Analysis
- H′—Shannon Wiener index,
- S—number of genera (genus richness).
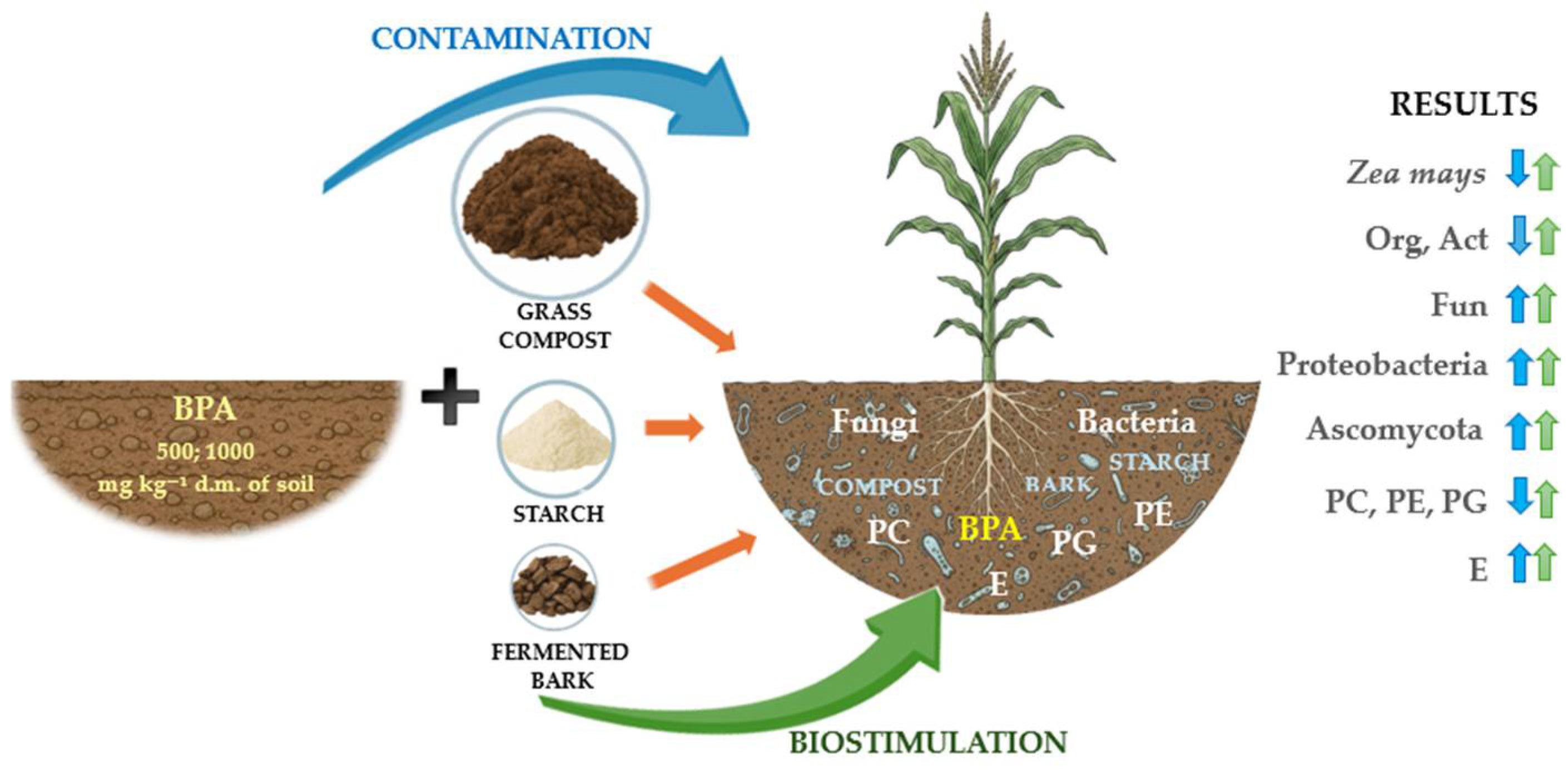
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Das, S.; Kim, P.J.; Nie, M.; Chabbi, A. Soil organic matter in the Anthropocene: Role in climate change mitigation, carbon sequestration, and food security. Agric. Ecosyst. Environ. 2024, 375, 109180. [Google Scholar] [CrossRef]
- Wu, G.C.; Baker, J.S.; Wade, C.M.; McCord, G.C.; Fargione, J.E.; Havlik, P. Contributions of healthier diets and agricultural productivity toward sustainability and climate goals in the United States. Sustain. Sci. 2023, 18, 539–556. [Google Scholar] [CrossRef]
- Purohit, H.J.; Pandit, P.; Pal, R.; Warke, R.; Warke, G.M. Soil microbiome: An intrinsic driver for climate smart agriculture. J. Agric. Food Res. 2024, 18, 101433. [Google Scholar] [CrossRef]
- Roß, C.-L.; Baumecker, M.; Ellmer, F.; Kautz, T. Organic manure increases carbon sequestration far beyond the “4 per 1000 initiative” goal on a sandy soil in the Thyrow Long-Term Field Experiment DIV.2. Agriculture 2022, 12, 170. [Google Scholar] [CrossRef]
- United Nations. The “4 per 1000” Initiative and Its Implementation. Department of Economic and Social Affairs, Sustainable Development. 2025. Available online: https://sdgs.un.org/partnerships/4-1000-initiative-and-its-implementation (accessed on 10 March 2025).
- Hermans, S.M.; Lear, G.; Case, B.S.; Buckley, H.L. The soil microbiome: An essential, but neglected, component of regenerative agroecosystems. iScience 2023, 26, 106028. [Google Scholar] [CrossRef]
- Willer, H.; Travníček, J.; Meier, C.; Schlatter, B. The World of Organic Agriculture. Statistics and Emerging Trends 2021; FiBL & IFOAM: Bonn, Germany, 2021; Available online: https://orgprints.org/id/eprint/45973/ (accessed on 7 March 2025).
- Long, F.; Ren, Y.; Bi, F.; Wu, Z.; Zhang, H.; Li, J.; Gao, R.; Liu, Z.; Li, H. Contamination characterization, toxicological properties, and health risk assessment of bisphenols in multiple media: Current research status and future perspectives. Toxics 2025, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Abu Hasan, H.; Muhamad, M.H.; Budi Kurniawan, S.; Buhari, J.; Husain Abuzeyad, O. Managing bisphenol A contamination: Advances in removal technologies and future prospects. Water 2023, 15, 3573. [Google Scholar] [CrossRef]
- Wang, J.; Chan, F.K.S.; Johnson, M.F.; Chan, H.K.; Cui, Y.; Chen, J.; Chen, W.Q. Material cycles, environmental emissions, and ecological risks of bisphenol A (BPA) in China and implications for sustainable plastic management. Environ. Sci. Technol. 2025, 59, 1631–1646. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, M.; Scheringer, M.; McKone, T.E.; Hungerbuhler, K. The state of multimedia mass-balance modeling in environmental science and decision-making. Environ. Sci. Technol. 2010, 44, 8360–8364. [Google Scholar] [CrossRef]
- Mordor Intelligence. Bisphenol-A Market Size & Share Analysis—Growth Trends & Forecasts (2025–2030). Available online: https://www.mordorintelligence.com/industry-reports/bisphenol-a-bpa-market (accessed on 15 March 2025).
- Chen, X.; Chen, C.-E.; Cheng, S.; Sweetman, A.J. Bisphenol A sorption on commercial polyvinyl chloride microplastics: Effects of UV-aging, biofilm colonization and additives on plastic behaviour in the environment. Environ. Pollut. 2024, 356, 124218. [Google Scholar] [CrossRef]
- Tian, Y.; Zhou, X.; Miao, M.; Li, D.; Wang, Z.; Li, R.; Liang, H.; Yuan, W. Association of bisphenol A exposure with LINE-1 hydroxymethylation in human semen. Int. J. Environ. Res. Public Health 2018, 15, 1770. [Google Scholar] [CrossRef] [PubMed]
- Costa, H.E.; Cairrao, E. Effect of bisphenol A on the neurological system: A review update. Arch. Toxicol. 2024, 98, 1–73. [Google Scholar] [CrossRef]
- Perera, F.; Nolte, E.L.R.; Wang, Y.; Margolis, A.E.; Calafat, A.M.; Wang, S.; Garcia, W.; Hoepner, L.A.; Peterson, B.S.; Rauh, V.; et al. Bisphenol A exposure and symptoms of anxiety and depression among inner city children at 10–12 years of age. Environ. Res. 2016, 151, 195–202. [Google Scholar] [CrossRef]
- Zhang, N.; Zhao, Y.; Zhai, L.; Bai, Y.; Jia, L. Urinary bisphenol A and S are associated with diminished ovarian reserve in women from an infertility clinic in Northern China. Ecotoxicol. Environ. Saf. 2023, 256, 114867. [Google Scholar] [CrossRef]
- Bono, R.; Bellisario, V.; Tassinari, R.; Squillacioti, G.; Manetta, T.; Bugiani, M.; Migliore, E.; Piccioni, P. Bisphenol A, tobacco smoke, and age as predictors of oxidative stress in children and adolescents. Int. J. Environ. Res. Public Health 2019, 16, 2025. [Google Scholar] [CrossRef]
- Vasiljevic, T.; Harner, T. Bisphenol A and its analogues in outdoor and indoor air: Properties, sources and global levels. Sci. Total Environ. 2021, 789, 148013. [Google Scholar] [CrossRef]
- Wang, H.; Tang, Z.; Liu, Z.H.; Zeng, F.; Zhang, J.; Dang, Z. Occurrence, spatial distribution, and main source identification of ten bisphenol analogues in the dry season of the Pearl River, South China. Environ. Sci. Pollut. Res. 2022, 29, 27352–27365. [Google Scholar] [CrossRef]
- Xie, J.; Zhao, N.; Zhang, Y.; Hu, H.; Zhao, M.; Jin, H. Occurrence and partitioning of bisphenol analogues, triclocarban, and triclosan in seawater and sediment from East China Sea. Chemosphere 2022, 287, 132218. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, L.; Jia, Y.; Zhang, Y.; Dong, Q.; Huang, C. A study on environmental bisphenol A pollution in plastics industry areas. Water Air Soil Pollut. 2017, 228, 98. [Google Scholar] [CrossRef]
- Huang, D.Y.; Zhao, H.Q.; Liu, C.P.; Sun, C.X. Characteristics, sources, and transport of tetrabromobisphenol A and bisphenol A in soils from a typical e-waste recycling area in South China. Environ. Sci. Pollut. Res. 2014, 21, 5818–5826. [Google Scholar] [CrossRef]
- Gewurtz, S.B.; Tardif, G.; Power, M.; Backus, S.M.; Dove, A.; Dubé-Roberge, K.; Garron, C.; King, M.; Lalonde, B.; Letcher, R.J.; et al. Bisphenol A in the Canadian environment: A multimedia analysis. Sci. Total Environ. 2021, 755, 142472. [Google Scholar] [CrossRef]
- Clifton, M.S.; Wargo, J.P.; Weathers, W.S.; Colón, M.; Bennett, D.H.; Tulve, N.S. Quantitative analysis of organophosphate and pyrethroid insecticides, pyrethroid transformation products, polybrominated diphenyl ethers and bisphenol A in residential surface wipe samples. J. Chromatogr. A 2013, 1273, 1–11. [Google Scholar] [CrossRef]
- Cao, S.; Wang, S.; Zhao, Y.; Wang, L.; Ma, Y.; Schäffer, A.; Ji, R. Fate of bisphenol S (BPS) and characterization of non-extractable residues in soil: Insights into persistence of BPS. Environ. Int. 2020, 143, 105908. [Google Scholar] [CrossRef]
- Wang, D.; Peng, Q.; Yang, W.X.; Dinh, Q.T.; Tran, T.A.T.; Zhao, X.D.; Wu, J.T.; Liu, Y.X.; Liang, D.L. DOM derivations determine the distribution and bioavailability of DOM-Se in selenate applied soil and mechanisms. Environ. Pollut. 2020, 259, 113899. [Google Scholar] [CrossRef] [PubMed]
- Ou, Y.H.; Chang, Y.J.; Lin, F.; Chang, M.; Yang, C.Y.; Shih, Y. Competitive sorption of bisphenol A and phenol in soils and the contribution of black carbon. Ecol. Eng. 2016, 92, 270–276. [Google Scholar] [CrossRef]
- Dai, Y.; Sun, Q.; Wang, W.; Lu, L.; Liu, M.; Li, J.; Yang, S.; Sun, Y.; Zhang, K.; Xu, J.; et al. Utilizations of agricultural waste as adsorbent for the removal of contaminants: A review. Chemosphere 2018, 211, 235–253. [Google Scholar] [CrossRef] [PubMed]
- Mpatani, F.M.; Han, R.; Aryee, A.A.; Kani, A.N.; Li, Z.; Qu, L. Adsorption performance of modified agricultural waste materials for removal of emerging micro-contaminant bisphenol A: A comprehensive review. Sci. Total Environ. 2021, 780, 146629. [Google Scholar] [CrossRef]
- Parab, C.; Yadav, K.D.; Prajapati, V. Genomics and microbial dynamics in green waste composting: A mini review. Ecol. Genet. Genom. 2023, 29, 100206. [Google Scholar] [CrossRef]
- Keel, S.G.; Anken, T.; Büchi, L.; Chervet, A.; Fliessbach, A.; Flisch, R.; Huguenin-Elie, O.; Mäder, P.; Mayer, J.; Sinaj, S.; et al. Loss of soil organic carbon in Swiss long-term agricultural experiments over a wide range of management practices. Agric. Ecosyst. Environ. 2019, 286, 106654. [Google Scholar] [CrossRef]
- Supriyadi, D.; Damayanti, D.; Veigel, S.; Hansmann, C.; Gindl-Altmutter, W. Unlocking the potential of tree bark: Review of approaches from extractives to materials for higher-added value products. Mater. Today Sustain. 2025, 29, 101074. [Google Scholar] [CrossRef]
- Bartley, P.C.; Amoozegar, A.; Fonteno, W.C.; Jackson, B.E. Particle densities of horticultural substrates. HortScience 2022, 57, 379–383. [Google Scholar] [CrossRef]
- Dou, J.; Wang, J.; Hietala, S.; Evtuguin, D.V.; Vuorinen, T.; Zhao, J. Structural features of lignin–hemicellulose–pectin (LHP) orchestrate a tailored enzyme cocktail for potential applications in bark biorefineries. Green Chem. 2023, 25, 5661–5678. [Google Scholar] [CrossRef]
- Kumar, Y.; Singh, S.; Saxena, D.C. A comprehensive review on methods, mechanisms, properties, and emerging applications of crosslinked starches. Int. J. Biol. Macromol. 2025, 306, 141526. [Google Scholar] [CrossRef]
- Meticulous Research. Modified Starch Market Size, Share, Forecast, & Trends Analysis by Product Type (Acetylated Distarch Adipate, Dextrin, Maltodextrin), Raw Material (Corn, Cassava), Production Method (Chemical), Function, Form, End-use Industry—Global Forecast to 2031. 2023. Available online: https://www.meticulousresearch.com/product/modified-starch-market-2718 (accessed on 19 March 2025).
- Kohli, D.; Garg, S.; Jana, A.K. Synthesis of cross-linked starch based polymers for sorption of organic pollutants from aqueous solutions. Indian Chem. Eng. 2012, 54, 210–222. [Google Scholar] [CrossRef]
- Wyszkowska, J.; Borowik, A.; Zaborowska, M.; Kucharski, J. Sensitivity of Zea mays and soil microorganisms to the toxic effect of chromium (VI). Int. J. Mol. Sci. 2023, 24, 178. [Google Scholar] [CrossRef] [PubMed]
- de Morais Farias, J.; Krepsky, N. Bacterial degradation of bisphenol analogues: An overview. Environ. Sci. Pollut. Res. 2022, 29, 76543–76564. [Google Scholar] [CrossRef]
- Cafaro, V.; Notomista, E.; Capasso, P.; Donato, D. Phenol hydroxylase and toluene/o-xylene monooxygenase from Pseudomonas stutzeri OX1: Interplay between two enzymes. Appl. Environ. Microbiol. 2004, 70, 2211–2219. [Google Scholar] [CrossRef]
- Das, R.; Li, G.; Mai, B.; An, T. Spore cells from BPA-degrading Bacillus sp. GZB displaying high laccase activity and stability for BPA degradation. Sci. Total Environ. 2018, 640–641, 798–806. [Google Scholar] [CrossRef]
- Hugentobler, K.G.; Müller, M. Towards semisynthetic natural compounds with a biaryl axis: Oxidative phenol coupling in Aspergillus niger. Bioorg. Med. Chem. 2018, 26, 1374–1377. [Google Scholar] [CrossRef]
- Ding, S.; Lange, M.; Lipp, J.; Schwab, V.F.; Chowdhury, S.; Pollierer, M.M.; Krause, K.; Li, D.; Kothe, E.; Scheu, S.; et al. Characteristics and origin of intact polar lipids in soil organic matter. Soil Biol. Biochem. 2020, 151, 108045. [Google Scholar] [CrossRef]
- Ge, L.; Xie, Q.; Wei, X.; Li, Y.; Shen, W.; Hu, Y.; Yao, J.; Wang, S.; Du, X.; Zeng, X. Five undescribed plant-derived bisphenols from Artemisia capillaris aerial parts: Structure elucidation, anti-hepatoma activities and plausible biogenetic pathway. Arab. J. Chem. 2023, 16, 104580. [Google Scholar] [CrossRef]
- Kondo, Y.; Shimoda, K.; Miyahara, K.; Hamada, H.; Hamada, H. Regioselective hydroxylation, reduction, and glycosylation of diphenyl compounds by cultured plant cells of Eucalyptus perriniana. Plant Biotechnol. 2006, 23, 291–296. [Google Scholar] [CrossRef][Green Version]
- Zhao, C.; Shi, Y.; Xu, Y.; Lin, N.; Dong, H.; Bei, L. Effects of Bisphenol A on Antioxidation and Nitrogen Assimilation of Maize Seedling Roots. Ecotoxicol. Environ. Saf. 2022, 247, 114255. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, L.; Shen, F.; Zhou, Q.; Huang, X. Impacts of exogenous pollutant bisphenol A on characteristics of soybeans. Ecotoxicol. Environ. Saf. 2018, 157, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Bahmani, R.; Kim, D.; Modareszadeh, M.; Thompson, A.J.; Park, J.H.; Yoo, H.H.; Hwang, S. The Mechanism of Root Growth Inhibition by the Endocrine Disruptor Bisphenol A (BPA). Environ. Pollut. 2020, 257, 113516. [Google Scholar] [CrossRef]
- Xiao, C.; Wang, L.; Hu, D.; Zhou, Q.; Huang, X. Effects of exogenous bisphenol A on the function of mitochondria in root cells of soybean (Glycine max L.) seedlings. Chemosphere 2019, 222, 619–627. [Google Scholar] [CrossRef]
- Jadhav, V.V.; Jadhav, A.S.; Chandagade, C.A.; Raut, P.D. Genotoxicity of Bisphenol A on Root Meristem Cells of Allium cepa: A Cytogenetic Approach. Asian J. Water Environ. Pollut. 2012, 9, 39–43. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Bisphenol A—A Dangerous Pollutant Distorting the Biological Properties of Soil. Int. J. Mol. Sci. 2021, 22, 12753. [Google Scholar] [CrossRef]
- Saleem, M.; Fariduddin, Q.; Castroverde, C.D.M. Salicylic Acid: A Key Regulator of Redox Signalling and Plant Immunity. Plant Physiol. Biochem. 2021, 168, 381–397. [Google Scholar] [CrossRef]
- Ali, I.; Jan, M.; Wakeel, A.; Azizullah, A.; Liu, B.; Islam, F.; Ali, A.; Daud, M.K.; Liu, Y.; Gan, Y. Biochemical responses and ultrastructural changes in ethylene insensitive mutants of Arabidopsis thialiana subjected to bisphenol A exposure. Ecotoxicol. Environ. Saf. 2017, 144, 62–71. [Google Scholar] [CrossRef]
- Adamakis, I.D.S.; Panteris, E.; Cherianidou, A.; Eleftheriou, E.P. Effects of bisphenol A on the microtubule arrays in root meristematic cells of Pisum sativum L. Mut. Res. 2013, 750, 111–120. [Google Scholar] [CrossRef]
- Goeppert, N.; Dror, I.; Berkowitz, B. Fate and transport of free and conjugated estrogens during soil passage. Environ. Pollut. 2015, 206, 80–87. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A.; Kucharski, J. Evaluation of the Effectiveness of Innovative Sorbents in Restoring Enzymatic Activity of Soil Contaminated with Bisphenol A (BPA). Molecules 2024, 29, 3113. [Google Scholar] [CrossRef]
- Alshaal, T.; Alharbi, K.; Naif, E.; Rashwan, E.; Omara, A.E.-D.; Hafez, E.M. Strengthen Sunflowers Resilience to Cadmium in Saline-Alkali Soil by PGPR-Augmented Biochar. Ecotoxicol. Environ. Saf. 2024, 280, 116555. [Google Scholar] [CrossRef] [PubMed]
- El-Akhdar, I.; Shabana, M.M.A.; El-Khateeb, N.M.M.; Elhawat, N.; Alshaal, T. Sustainable Wheat Cultivation in Sandy Soils: Impact of Organic and Biofertilizer Use on Soil Health and Crop Yield. Plants 2024, 13, 3156. [Google Scholar] [CrossRef]
- Aguilar-Paredes, A.; Valdés, G.; Araneda, N.; Valdebenito, E.; Hansen, F.; Nuti, M. Microbial Community in the Composting Process and Its Positive Impact on the Soil Biota in Sustainable Agriculture. Agronomy 2023, 13, 542. [Google Scholar] [CrossRef]
- Zühlke, M.K.; Schlüter, R.; Mikolasch, A.; Henning, A.K.; Giersberg, M.; Lalk, M.; Kunze, G.; Schweder, T.; Urich, T.; Schauer, F. Biotransformation of bisphenol A analogues by the biphenyl-degrading bacterium Cupriavidus basilensis—A structure-biotransformation relationship. Appl. Microbiol. Biotechnol. 2020, 104, 3569–3583. [Google Scholar] [CrossRef]
- Tian, K.; Gu, J.; Wang, Y.; Zhang, F.; Zhou, D.; Qiu, Q.; Yu, Y.; Sun, X.; Chang, M.; Zhang, X.; et al. Removal of BPA by Pseudomonas asiatica P1: Synergistic response mechanism of toxicity resistance and biodegradation. Ecotoxicol. Environ. Saf. 2024, 288, 117410. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Wu, Q.; Chen, H.; Song, Y.; Zhang, X.; Guo, Y.; Hu, F.; Li, H. Examining the Shift in the Decomposition Channel Structure of the Soil Decomposer Food Web: A Methods Comparison. Microorganisms 2023, 11, 2589. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Alimadadi, N.; AbuQamar, S.; Alhoraibi, H.; Saeed, E.E.; El-Tarabily, K.A. Fungal–Bacterial Combinations in Plant Health under Stress. Microorganisms 2024, 12, 147. [Google Scholar] [CrossRef]
- Zaborowska, M.; Wyszkowska, J.; Borowik, A. Soil microbiome response to contamination with Bisphenol A, Bisphenol F and Bisphenol S. Int. J. Mol. Sci. 2020, 21, 3529. [Google Scholar] [CrossRef]
- Heider, J.; Fuchs, G. Microbial anaerobic aromatic metabolism. Anaerobe 1997, 3, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Dercová, K.; Certík, M.; Malová, A.; Sejáková, Z. Effect of chlorophenols on the membrane lipids of bacterial cells. Int. Biodeterior. Biodegrad. 2004, 54, 251–254. [Google Scholar] [CrossRef]
- Razia, S.; Hadibarata, T.; Lau, S.Y. A review on biodegradation of Bisphenol A (BPA) with bacteria and fungi under laboratory conditions. Int. Biodeterior. Biodegrad. 2024, 195, 105893. [Google Scholar] [CrossRef]
- Xie, N.; Chapeland-Leclerc, F.; Silar, P.; Ruprich-Robert, G. Systematic gene deletions evidences that laccases are involved in several stages of wood degradation in the filamentous fungus Podospora anserina. Environ. Microbiol. 2014, 16, 141–161. [Google Scholar] [CrossRef]
- Zhang, C.; Li, M.; Chen, X.; Li, M. Edible fungus degrade bisphenol A with no harmful effect on its fatty acid composition. Ecotoxicol. Environ. Saf. 2015, 118, 126–132. [Google Scholar] [CrossRef]
- Mitbaá, R.; Hernández, D.R.O.; Pozo, C.; Nasri, M.; Mechichi, T.; González, J.; Aranda, E. Degradation of bisphenol A and acute toxicity reduction by different thermo-tolerant ascomycete strains isolated from arid soils. Ecotoxicol. Environ. Saf. 2018, 156, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Siedt, M.; Schäffer, A.; Smith, K.E.C.; Nabel, M.; Roß-Nickoll, M.; van Dongen, J.T. Comparing straw, compost, and biochar regarding their suitability as agricultural soil amendments to affect soil structure, nutrient leaching, microbial communities, and the fate of pesticides. Sci. Total Environ. 2021, 751, 141607. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Rene, E.R.; Wang, J.; Ma, W. Biodegradation of polyaromatic hydrocarbons and the influence of environmental factors during the co-composting of sewage sludge and green forest waste. Bioresour. Technol. 2020, 297, 122434. [Google Scholar] [CrossRef]
- De Corato, U.; Patruno, L.; Avella, N.; Lacolla, G.; Cucci, G. Composts from green sources show an increased suppressiveness to soilborne plant pathogenic fungi: Relationships between physicochemical properties, disease suppression, and the microbiome. Crop Prot. 2019, 124, 104870. [Google Scholar] [CrossRef]
- Liu, J.; Jiang, S.; Chen, D.; Dai, G.; Wei, D.; Shu, Y. Activation of persulfate with biochar for degradation of bisphenol A in soil. Chem. Eng. J. 2020, 381, 122637. [Google Scholar] [CrossRef]
- Hussain, A.; Wu, S.C.; Le, T.-H.; Huang, W.-Y.; Lin, C.; Bui, X.-T.; Ngo, H.H. Enhanced Biodegradation of Endocrine Disruptor Bisphenol A by Food Waste Composting without Bioaugmentation: Analysis of Bacterial Communities and Their Relative Abundances. J. Hazard. Mater. 2023, 460, 132345. [Google Scholar] [CrossRef]
- Loffredo, E.; Campanale, E.; Cocozza, C.; Denora, N. Kompost pofermentacyjny moduluje proces retencji/uwalniania ksenobiotyków organicznych w zmodyfikowanej glebie. Agriculture 2025, 15, 1925. [Google Scholar] [CrossRef]
- Čekstere, G.; Laiviņš, M.; Osvalde, A. Chemical composition of Scots pine bark as a bioindicator of environmental quality in Riga, Latvia. Proc. Latv. Acad. Sci. Sect. B Nat. Exact Appl. Sci. 2015, 69, 87–97. [Google Scholar] [CrossRef][Green Version]
- Barbini, S.; Sriranganadane, D.; España Orozco, S.; Kabrelian, A.; Karlström, K.; Rosenau, T.; Potthast, A. Tools for bark biorefineries: Studies toward improved characterization of lipophilic lignocellulosic extractives by combining supercritical fluid and gas chromatography. ACS Sustain. Chem. Eng. 2021, 9, 1323–1332. [Google Scholar] [CrossRef]
- Siczek, A.; Frąc, M.; Gryta, A.; Kalembasa, S.; Kalembasa, D. Variation in soil microbial population and enzyme activities under faba bean as affected by pentachlorophenol. Appl. Soil Ecol. 2020, 150, 103466. [Google Scholar] [CrossRef]
- Patil, J.S.; Anil, A.C. Efficiency of copper and cupronickel substratum to resist development of diatom biofilms. Int. Biodeter. Biodegrad. 2015, 15, 203–214. Available online: http://drs.nio.org/drs/handle/2264/4852 (accessed on 24 August 2025). [CrossRef]
- Behera, S.; Das, S. Potential and prospects of Actinobacteria in the bioremediation of environmental pollutants: Cellular mechanisms and genetic regulations. Microbiol. Res. 2023, 273, 127399. [Google Scholar] [CrossRef]
- Vandana; Das, S. Genetic regulation, biosynthesis and applications of extracellular polysaccharides of the biofilm matrix of bacteria. Carbohydr. Polym. 2022, 291, 119536. [Google Scholar] [CrossRef] [PubMed]
- Thathola, P.; Agnihotri, V.; Pandey, A.; Upadhyay, S.K. Biodegradation of bisphenol A using psychrotolerant bacterial strain Pseudomonas palleroniana GBPI_508. Arch. Microbiol. 2022, 204, 272. [Google Scholar] [CrossRef]
- Shobnam, N.; Sun, Y.; Mahmood, M.; Löffler, F.E.; Im, J. Biologically mediated abiotic degradation (BMAD) of bisphenol A by manganese-oxidizing bacteria. J. Hazard. Mater. 2021, 417, 125987. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Dub, D.; Zhou, W.; Zeng, X.; Cheng, G. Phenol degradation and genotypic analysis of dioxygenase genes in bacteria isolated from sediments. Braz. J. Microbiol. 2017, 48, 305–313. [Google Scholar] [CrossRef]
- Zhang, C.; Zeng, G.; Yuan, L.; Yu, J.; Li, J.; Huang, G.; Xi, B.; Liu, H. Aerobic degradation of bisphenol A by Achromobacter xylosoxidans strain B-16 isolated from compost leachate of municipal solid waste. Chemosphere 2007, 68, 181–190. [Google Scholar] [CrossRef]
- Pugazhendi, A.; Rajesh Banu, J.; Dhavamani, J.; Yeom, I.T. Biodegradation of 1,4-dioxane by Rhodanobacter AYS5 and the role of additional substrates. Ann. Microbiol. 2015, 65, 2201–2208. [Google Scholar] [CrossRef]
- Cetiner, I.; Shea, A.D. Wood waste as an alternative thermal insulation for buildings. Energy Build. 2018, 168, 374–384. [Google Scholar] [CrossRef]
- Li, X.; Yang, Y.; Fan, X.; Hu, X. Microbial community dynamics and metabolite changes during wheat starch slurry fermentation. Foods 2024, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Harms, H.; Schlosser, D.; Wick, L. Untapped potential: Exploiting fungi in bioremediation of hazardous chemicals. Nat. Rev. Microbiol. 2011, 9, 177–192. [Google Scholar] [CrossRef]
- Eltoukhy, A.; Mohamed, H.; Abo-Kadoum, M.A.; Khalid, H.; Ramadan, A.S.; Hassane, A.M.A.; Zhang, H.; Song, Y. Biodegradation and mineralization of bisphenol A by a novel soil-derived fungus Paraconiothyrium brasiliense mediated by extracellular laccase. J. Hazard. Mater. 2025, 488, 137460. [Google Scholar] [CrossRef]
- Leitão, A.L. Potential of Penicillium Species in the Bioremediation Field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef]
- Koleva, Z.; Abrashev, R.; Angelova, M.; Stoyancheva, G.; Spassova, B.; Yovchevska, L.; Dishliyska, V.; Miteva-Staleva, J.; Krumova, E. A Novel Extracellular Catalase Produced by the Antarctic Filamentous Fungus Penicillium Rubens III11-2. Fermentation 2024, 10, 58. [Google Scholar] [CrossRef]
- Shedbalkar, U.; Dhanve, R.; Jadhav, J. Biodegradation of triphenylmethane dye cotton blue by Penicillium ochrochloron MTCC 517. J. Hazard. Mater. 2008, 157, 472–479. [Google Scholar] [CrossRef]
- Chhaya, U.; Gupte, A. Possible Role of Laccase from Fusarium incarnatum UC-14 in Bioremediation of Bisphenol A Using Reverse Micelles System. J. Hazard. Mater. 2013, 254–255, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Geiger, O.; López-Lara, I.M.; Sohlenkamp, C. Phosphatidylcholine biosynthesis and function in bacteria. Biochim. Biophys. Acta 2013, 1831, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Epand, R.M.; Walker, C.; Epand, R.F.; Magarvey, N.A. Molecular mechanisms of membrane targeting antibiotics. Biochim. Biophys. Acta 2016, 1858, 980–987. [Google Scholar] [CrossRef]
- Hąc-Wydro, K.; Połeć, K.; Broniatowski, M. The comparative analysis of the effect of environmental toxicants: Bisphenol A, S and F on model plant, fungi and bacteria membranes. The studies on multicomponent systems. J. Mol. Liq. 2019, 289, 111136. [Google Scholar] [CrossRef]
- Chen, L.; Chen, J.; Zhou, G.; Wang, Y.; Xu, C.; Wang, X. Molecular dynamics simulations of the permeation of bisphenol A and pore formation in a lipid membrane. Sci. Rep. 2016, 6, 33399. [Google Scholar] [CrossRef]
- Dunder, L.; Lejonklou, M.; Lind, L.; Risérus, U.; Lind, P.M. Low-dose developmental bisphenol A exposure alters fatty acid metabolism in Fischer 344 rat offspring. Environ. Res. 2018, 166, 117–129. [Google Scholar] [CrossRef]
- Zeng, J.; Li, J.; Liu, S.; Yang, Z.; Zhong, Y.; Chen, X.; Li, G.; Li, J. Lipidome disturbances in preadipocyte differentiation associated with bisphenol A and replacement bisphenol S exposure. Sci. Total Environ. 2021, 753, 141949. [Google Scholar] [CrossRef]
- Chen, P.; An, B.; Hu, Y.; Tao, Y. 2,4-Bisphenol S triggers physiological changes, oxidative stress and lipidome alterations in Gram-positive Enterococcus faecalis at environmental concentrations. Environ. Pollut. 2025, 366, 125475. [Google Scholar] [CrossRef]
- Jasińska, A.; Soboń, A.; Różalska, S.; Średnicka, P. Bisphenol A removal by the fungus Myrothecium roridum IM 6482—Analysis of the cellular and subcellular level. Int. J. Mol. Sci. 2021, 22, 10676. [Google Scholar] [CrossRef]
- Camenzind, T.; Haslwimmer, H.; Rillig, M.C.; Ruess, L.; Finn, D.R.; Tebbe, C.C.; Hempel, S.; Marhan, S. Revisiting soil fungal biomarkers and conversion factors: Interspecific variability in phospholipid fatty acids, ergosterol and rDNA copy numbers. Soil Ecol. Lett. 2024, 6, 24024. [Google Scholar] [CrossRef]
- Riesbeck, S.; Petruschke, H.; Rolle-Kampczyk, U.; Schori, C.; Ahrens, C.H.; Eberlein, C.; Heipieper, H.J.; von Bergen, M.; Jehmlich, N. Adaptation and resistance: How Bacteroides thetaiotaomicron copes with the bisphenol A substitute bisphenol F. Microorganisms 2022, 10, 1610. [Google Scholar] [CrossRef]
- Bonkowski, M.; Tarkka, M.; Razavi, B.S.; Schmidt, H.; Blagodatskaya, E.; Koller, R.; Yu, P.; Knief, C.; Hochholdinger, F.; Vetterlein, D. Spatiotemporal dynamics of maize (Zea mays L.) root growth and its potential consequences for the assembly of the rhizosphere microbiota. Front. Microbiol. 2021, 12, 619499. [Google Scholar] [CrossRef]
- FAO. FAOSTAT: Production: Crops and Livestock Products; Food and Agriculture Organization: Rome, Italy, 2023; Available online: https://www.fao.org/3/cc9205en/cc9205en.pdf (accessed on 20 March 2025).
- Cheng, Y.; Cao, M.; Shi, X.; Chen, X.; Li, Z.; Ma, Y. Mitigating salt stress in Zea mays: Harnessing Serratia nematodiphila-biochar-based seed coating for plant growth promotion and rhizosphere microecology regulation. Ind. Crops Prod. 2025, 223, 120164. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. Lecture Notes on the Major Soils of the World—Cambisols; International Soil Reference and Information Centre (ISRIC): Wageningen, The Netherlands, 2022; Available online: https://www.isric.org/sites/default/files/major_soils_of_the_world/set5/cm/cambisol.pdf (accessed on 1 June 2024).
- ISO 10390; Soil Quality—Determination of pH. International Organization for Standardization: Geneva, Switzerland, 2005.
- ISO 11261; Soil Quality—Determination of Total Nitrogen—Modified Kjeldahl Method. International Organization for Standardization: Geneva, Switzerland, 1995.
- Nelson, D.W.; Sommers, L.E. Total carbon, organic carbon, and organic matter. In Methods of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 1201–1229. [Google Scholar]
- Klute, A. Methods of Soil Analysis; American Society of Agronomy: Madison, WI, USA, 1996; Agronomy Monograph 9. [Google Scholar]
- Bunt, J.S.; Rovira, A.D. Microbiological studies of some subantarctic soils. J. Soil Sci. 1955, 6, 119–128. [Google Scholar] [CrossRef]
- Küster, E.; Williams, S.T. Selection of media for isolation of Streptomycetes. Nature 1964, 202, 928–929. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.P. Use of acid rose bengal and streptomycin in the plate method for estimating soil fungi. Soil Sci. 1950, 69, 215–232. [Google Scholar] [CrossRef]
- Hu, Y.; Zhu, Q.; Yan, X.; Liao, C.; Jiang, G. Occurrence, fate and risk assessment of BPA and its substituents in wastewater treatment plant: A review. Environ. Res. 2019, 178, 108732. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Zhang, J.; Huang, R.; Yin, H.; Dang, Z.; Wu, P.; Liu, Y. Insights into removal mechanisms of bisphenol A and its analogues in municipal wastewater treatment plants. Sci. Total Environ. 2019, 692, 107–116. [Google Scholar] [CrossRef]
- Schniete, J.K.; Fernández-Martínez, L.T. Natural product discovery in soil actinomycetes: Unlocking their potential within an ecological context. Curr. Opin. Microbiol. 2024, 79, 102487. [Google Scholar] [CrossRef]
- Rashid, M.I.; Mujawar, L.H.; Shahzad, T.; Almeelbi, T.; Ismail, I.M.I.; Oves, M. Bacteria and fungi can contribute to nutrients bioavailability and aggregate formation in degraded soils. Microbiol. Res. 2016, 183, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Sarathchandra, S.U.; Burch, G.; Cox, N.R. Growth patterns of bacterial communities in the rhizoplane and rhizosphere of white clover (Trifolium repens L.) and perennial ryegrass (Lolium perenne L.) in long-term pasture. Appl. Soil Ecol. 1997, 6, 293–299. [Google Scholar] [CrossRef]
- De Leij, F.A.M.; Whipps, J.M.; Lynch, J.M. The use of colony development for the characterization of bacterial communities in soil and on roots. Microb. Ecol. 1993, 27, 81–97. [Google Scholar] [CrossRef]
- Bernat, P.; Jasińska, A.; Niedziałkowska, K.; Słaba, M.; Różalska, S.; Paraszkiewicz, K.; Sas-Paszt, L.; Heipieper, H.J. Adaptation of the metolachlor-degrading fungus Trichoderma harzianum to the simultaneous presence of low-density polyethylene (LDPE) microplastics. Ecotoxicol. Environ. Saf. 2023, 267, 115656. [Google Scholar] [CrossRef] [PubMed]
- Rusetskaya, V.; Różalska, S.; Słaba, M.; Bernat, P. Degradation ability of Trichoderma spp. in the presence of poly(butylene adipate-co-terephthalate) microparticles. Int. Biodeterior. Biodegrad. 2024, 193, 105829. [Google Scholar] [CrossRef]
- Tibco Software Inc. Statistica, Version 13; Data Analysis Software System; Tibco Software Inc.: Palo Alto, CA, USA, 2021. Available online: https://www.tibco.com/products/ebx (accessed on 11 March 2025).
- Parks, D.H.; Tyson, G.W.; Hugenholtz, P.; Beiko, R.G. STAMP: Statistical analysis of taxonomic and functional profiles. Bio-informatics 2014, 30, 3123–3124. [Google Scholar] [CrossRef]
- RStudio Team. RStudio: Integrated Development for R; RStudio, Inc.: Boston, MA, USA, 2019; Available online: http://www.rstudio.com/ (accessed on 15 March 2025).
- Krzywinski, M.; Schein, J.; Birol, İ.; Connors, J.; Gascoyne, R.; Horsman, D.; Jones, S.J.; Marra, M.A. Circos: An Information Aesthetic for Comparative Genomics. Genome Res. 2009, 19, 1639–1645. [Google Scholar] [CrossRef] [PubMed]
- Heberle, H.; Meirelles, G.V.; da Silva, F.R.; Telles, G.P.; Minghim, R. InteractiVenn: A web-based tool for the analysis of sets through Venn diagrams. BMC Bioinform. 2015, 16, 169. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef]
- Magurran, A.E. Measuring Biological Diversity; Wiley-Blackwell: Oxford, UK, 2004; ISBN 0-632-05633-9. [Google Scholar]

| Lipids Content | C_0 | BPA_1000 | St_1000 | Co_1000 | B_1000 |
|---|---|---|---|---|---|
| PC (%) | 30.34 c ± 3.37 | 43.64 ab ± 1.85 | 49.41 a ± 3.87 | 40.54 b ± 2.63 | 19.34 d ± 1.61 |
| PC (µg g−1 of soil) | 0.34 b ± 0.03 | 0.33 b ± 0.02 | 0.64 a ± 0.01 | 0.36 b ± 0.01 | 0.15 c ± 0.01 |
| PE (%) | 28.04 b ± 0.68 | 25.25 bc ± 2.84 | 24.77 bc ± 0.75 | 22.93 c ± 0.72 | 35.86 a ± 3.47 |
| PE (µg g−1 of soil) | 0.26 a ± 0.05 | 0.17 c ± 0.04 | 0.22 b ± 0.02 | 0.20 b ± 0.02 | 0.25 a ± 0.00 |
| PG (%) | 41.34 ab ± 2.25 | 31.10 c ± 1.32 | 25.80 d ± 2.55 | 36.47 b ± 1.14 | 44.74 a ± 2.54 |
| PG (µg g−1 of soil) | 0.46 a ± 0.01 | 0.24 d ± 0.05 | 0.32 b ± 0.03 | 0.29 c ± 0.01 | 0.34 b ± 0.01 |
| E (µg g−1 of soil) | 1.33 b ± 0.12 | 1.69 a ± 0.18 | 1.88 a ± 0.07 | 1.30 b ± 0.17 | 0.80 c ± 0.09 |
| UI | 1.02 bc ± 0.05 | 1.10 b ± 0.03 | 1.25 a ± 0.08 | 1.06 bc ± 0.04 | 0.97 c ± 0.01 |
| PC/PE | 1.10 b ± 0.14 | 1.73 a ± 0.13 | 2.00 a ± 0.15 | 1.77 a ± 0.07 | 0.55 c ± 0.10 |
| Parameters | Terms/Values |  |
| BPA synonyms | 4,4′-isopropylidenediphenol 2,2-bis(4-hydroxyphenyl)-propane) | |
| logKOC | 4.88 | |
| VP (Pa) | 5.6 × 10−6 | |
| SW mg dm−3 | 120 | |
| BCF | 71.85 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaborowska, M.; Wyszkowska, J.; Słaba, M.; Borowik, A.; Kucharski, J.; Bernat, P. The Effect of Organic Materials on the Response of the Soil Microbiome to Bisphenol A. Molecules 2025, 30, 3868. https://doi.org/10.3390/molecules30193868
Zaborowska M, Wyszkowska J, Słaba M, Borowik A, Kucharski J, Bernat P. The Effect of Organic Materials on the Response of the Soil Microbiome to Bisphenol A. Molecules. 2025; 30(19):3868. https://doi.org/10.3390/molecules30193868
Chicago/Turabian StyleZaborowska, Magdalena, Jadwiga Wyszkowska, Mirosława Słaba, Agata Borowik, Jan Kucharski, and Przemysław Bernat. 2025. "The Effect of Organic Materials on the Response of the Soil Microbiome to Bisphenol A" Molecules 30, no. 19: 3868. https://doi.org/10.3390/molecules30193868
APA StyleZaborowska, M., Wyszkowska, J., Słaba, M., Borowik, A., Kucharski, J., & Bernat, P. (2025). The Effect of Organic Materials on the Response of the Soil Microbiome to Bisphenol A. Molecules, 30(19), 3868. https://doi.org/10.3390/molecules30193868










