Abstract
The reactivity and stereoselectivity in the inverse-electron-demand Diels–Alder reaction between 4-methoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene and methoxyethene was examined using density functional theory (DFT) calculations at the M06-2X level. The formation of the two bonds in this reaction was calculated to be asynchronous. The formation of the C−C bond occurs first and is driven by electron delocalization from the dienophile to the diene, a process which simultaneously governs the regioselectivity. Moreover, the endo selectivity of the reaction was found to arise from non-bonding-orbital interactions, electrostatic attractions, and dispersion interactions. The sulfonyl group attached to the diene influences the selectivity and the reactivity. In contrast, when a methoxycarbonyl group is attached to the diene, it affects the selectivity in a different way depending on the position where it is attached.
1. Introduction
The Diels–Alder reaction is one of the most useful reactions in organic synthesis for the synthesis of cyclic compounds [1]. The normal-electron-demand Diels–Alder (NEDDA) reaction, which is a cycloaddition reaction between an electron-rich diene and an electron-deficient dienophile, usually proceeds with endo selectivity [2]. Since the proposition of the rule of “maximum accumulation of double bonds” by Alder and Stein [3,4], the endo selectivity of the reaction has been discussed from a variety of viewpoints [5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29]. In particular, secondary orbital interactions (SOIs) between the non-bonded atoms in the diene and dienophile, as proposed by Hoffmann and Woodward, have been widely accepted as the origin of the selectivity [30,31]. The concept of SOI has, in many cases, been used to explain the selectivity of various cycloaddition reactions in terms of the frontier-orbital theory [32]. However, the effect of SOIs turns out to be much weaker than suggested by the frontier-orbital argument when the contribution of other orbitals is taken into consideration [33]. We have previously examined the NEDDA reaction between cyclopentadiene and maleic anhydride using density-functional-theory (DFT) calculations and revealed that the origin of the endo selectivity in the reaction arises not from the orbital interactions but from the electrostatic attractions caused by the highly polarized C=O bonds, which play important roles for the reactivity [34]. The effects of Lewis acids on a highly endo-selective Lewis-acid-catalyzed NEDDA reaction have also been investigated [35].
Meanwhile, the inverse-electron-demand Diels–Alder (IEDDA) reaction, which employs an electron-deficient diene and an electron-rich dienophile, has recently emerged as an important tool in both chemical biology and organic synthetic chemistry [36,37,38]. Boger and co-workers have developed IEDDA reactions between N-sulfonyl-1-aza-1,3-butadine and electron-rich dienophiles [39,40]. In the reaction of 4-ethoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene with ethoxyethane, for example, high regio- and endo-selectivity (endo:exo ratio is >20:1) was reported (Scheme 1) [40].
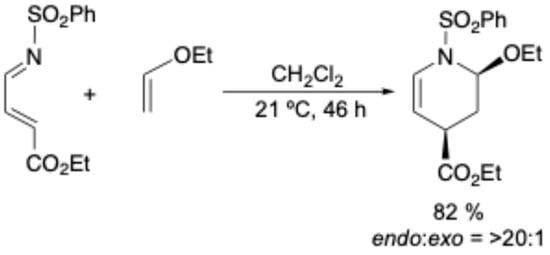
Scheme 1.
The reaction of 4-ethoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene with ethoxyethane reported by Boger and co-workers.
It has been argued that the endo selectivity is a result of not only the interaction between the alkoxy substituent of the dienophile and the carbon atom adjacent to the nitrogen atom in the azadiene, i.e., SOI, but also a result of the n-σ* interaction (or anomeric effect) that arises from the interaction between the lone-pair electron of the nitrogen atom and the σ* orbital of the C-O bond. Therefore, it is of great interest to compare the origin of the stereoselectivity in the IEDDA reaction with that of the NEDDA reaction, although some DFT studies on the stereoselectivity in IEDDA reactions were already reported [41,42,43]. In the present study, we have examined in detail a model reaction between 4-methoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene (1) and methoxyethene (2) to clarify the origin of the selectivity of the reaction (Scheme 2).
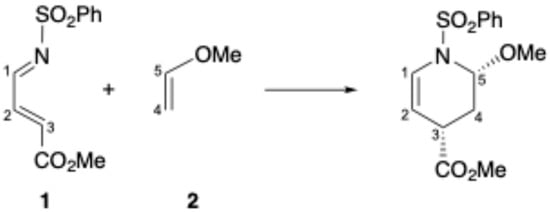
Scheme 2.
The model reaction examined in the present study. Carbon atoms are numbered as shown.
2. Results and Discussion
2.1. Transition-State (TS) Structures
A variety of transition-state (TS) structures for the cycloaddition (TSn, n = 1–32) were explored (Figure S1). TS structures TS1–TS16 lead to the major regioisomer where the nitrogen atom in 1 binds to the carbon atom attached to the methoxy group in 2 (Scheme 3). Conversely, TS17–TS32 give the minor regioisomer where the nitrogen atom in 1 binds to the carbon atom located further away from the methoxy group. Our calculations showed that the latter structures have ca. 10 kcal/mol higher free energies than the former structures, showing that the reaction is highly regioselective (Figure S1 and Table S1). Of the TS structures that lead to the major regioisomer, TS1–TS8 correspond to an endo addition, while TS9–TS16 give exo adducts. TS1, which has the lowest free energy of the endo TS structures, was calculated to have 2.8 kcal/mol lower free energy than TS9, which has the lowest free energy of the exo TSs (Figure 1). A probability distribution of structures of TS1–TS16 provides an endo:exo ratio of 136:1, showing that the endo addition is kinetically preferred over the exo addition. These results are consistent with the experimental results reported by Boger and co-workers [40].
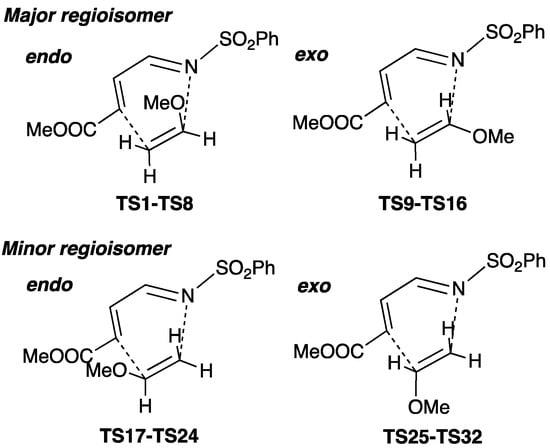
Scheme 3.
Transition-state structures TS1–TS32 for the cycloaddition reaction between 1 and 2.
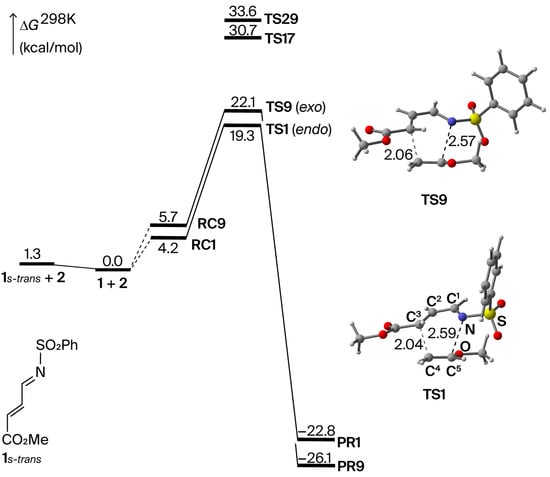
Figure 1.
Energy diagram (kcal/mol) and transition-state structures for TS1 and TS9 with bond distances in Å.
2.2. Asynchronous Bond Formations and Regioselectivity
Intrinsic reaction coordinate (IRC) [44,45] calculation was performed for TS1 (Figure S2). The C3−C4 bond, which is located furthest from the methoxy group, is 2.04 Å in the TS (s = 0 amu1/2∙bohr), whilst the N−C5 bond reaches the same bond length at a much later stage of the reaction (s ~ 4.0 amu1/2∙bohr). Thus, the C3−C4 bond is formed much faster than the N−C5 bond, demonstrating that the formation of the two bonds is asynchronous. The fragment charges based on a natural population analysis (NPA) [46] show that the C3−C4 bond formation is brought about primarily by electron delocalization from the dienophile to the diene, while the N−C5 bond formation is associated mainly with electron delocalization in the opposite direction (Figure S3). This fragment-charge profile thus indicates the opposite behavior of the NEDDA reactions examined in our previous studies [35,47].
Then, the change in the Mulliken overlap population [48,49,50,51] for the C3−C4 and N−C5 bonds along the IRC was further examined (Figure 2A and Figure 2B, respectively). The population of the C3−C4 bond is slightly negative when s < −1 amu1/2∙bohr and after this increases positively. The decomposition in terms of fragment Kohn–Sham orbitals [47,52] showed that the component between the occupied orbitals of the dienophile fragment and unoccupied orbitals of the diene fragment (2(Occ)−1(Unocc) in Figure 2A) contributes to the increase in the overlap population. Thus, the electron delocalization from the dienophile to the diene plays an important role in the formation of the C3−C4 bond. In contrast, the increase in the overlap population between the N and C5 atoms (s > 0 amu1/2∙bohr) depends largely on the contribution of the unoccupied orbitals of the dienophile fragment and the occupied orbitals of the diene fragment (2(Unocc)−1(Occ) in Figure 2B). This observation indicates that electron delocalization in the direction opposite to the C3−C4 bond formation process is essential for the formation of the N−C5 bond.
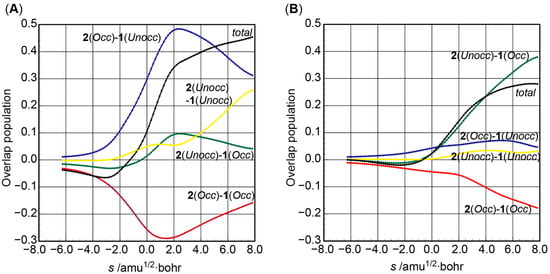
Figure 2.
Changes in the Mulliken overlap population for the (A) C3−C4 and (B) N−C5 bonds along the IRC obtained from TS1.
Subsequently, orbital interactions were analyzed in terms of the interaction frontier orbitals (IFOs) [53,54]. One pair of orbitals, (ψ′1; φ′1), is mainly responsible for the electron delocalization from the dienophile fragment to the diene fragment in TS1 (Figure 3). The orbital ψ′1, obtained from the linear combination of the occupied Kohn–Sham orbitals of the dienophile fragment, consists of the π bonding orbital of the C=C bond of the dienophile and the amplitude of the C4 atom is larger than that of the C5 atom. The orbital φ′1, which consists of the unoccupied orbitals of the diene fragment, is similar to the LUMO of butadiene and the amplitude of the C3 atom is slightly larger than that of the N atom. Orbitals ψ′1 and φ′1 have energies of −7.62 eV and −2.05 eV, respectively. The interaction between these two orbitals is important for the C3−C4 bond formation process.
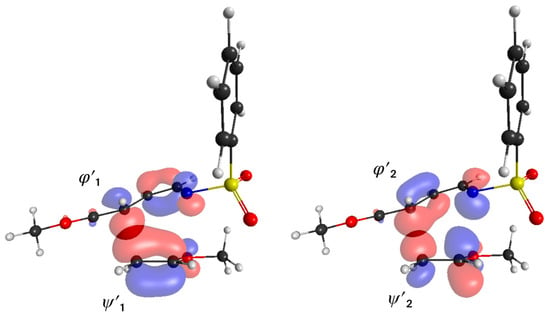
Figure 3.
Pairs of interaction frontier orbitals (IFOs) (ψ′1; φ′1) and (ψ′2; φ′2) in TS1.
Another pair of orbitals, (ψ′2; φ′2), governs the electron delocalization from the diene to the dienophile (Figure 3). The orbital ψ′2 is the unoccupied π orbital of the dienophile, while the orbital φ′2 is the occupied π orbital of the diene. Orbitals ψ′2 and φ′2 have energies of +3.85 eV and −9.90 eV, respectively. This pair is responsible for the N−C5 bond formation process, although the interaction is weaker than that of the (ψ′1; φ′1) pair of orbitals due to the larger energy difference between the ψ′2 and φ′2 orbitals. We also investigated other pairs of orbitals but did not find any that corresponded to an n-σ* interaction.
The regioselectivity in this reaction is closely related to the asynchronous bond-formation process. The electron-withdrawing SO2Ph group in the diene fragment both lowers the energy levels of the occupied and unoccupied π orbitals and prompts the polarization of the π orbitals (Figure S4). The SO2Ph group enhances the overlap repulsion at the N atom, while the repulsion at the C3 atom is diminished. At the same time, the electron-accepting ability of the pπ orbital at the C3 atom increases. On the other hand, the electron-donating ability of the pπ orbital at the C4 atom in the dienophile fragment is strengthened by the methoxy group [2]. Thus, the combination of the C3 atom and the C4 atom favors electron delocalization from the dienophile fragment to the diene fragment, controlling the regioselectivity of the reaction.
2.3. Stereoselectivity
In the reaction involving N-(phenylsulfonyl)-1-aza-1,3-butadiene (3) (Scheme 4), the relative free energies of the endo and exo TSs, TS1‴ and TS9‴, were calculated to be 17.9 and 20.3 kcal/mol, respectively. Thus, the free energy of the endo TS TS1‴ is by 2.4 kcal/mol lower than that of the exo TS TS9‴, indicating that the effect that the CO2Me group on the C3 atom has on the endo selectivity is relatively small (Figure S5a). In contrast, the relative free energies of the endo and exo TSs, TS1⁗ and TS9⁗, were found to be 30.3 and 29.8 kcal/mol, respectively, in the reaction involving 1-aza-1,3-butadiene (4) (Figure S5b). It shows that the energy of the endo structure, TS1⁗, is 0.5 kcal/mol higher than that of the exo structure, TS9⁗. These results demonstrate that, in addition to resulting in higher reactivity, the SO2Ph group also dictates the stereochemical outcome of the reaction. The results of an energy decomposition analysis (EDA) [55] suggested that, although the group directly interacts with the dienophile, the SO2Ph group facilitates the interaction between the diene group and the dienophile (Figure S6).
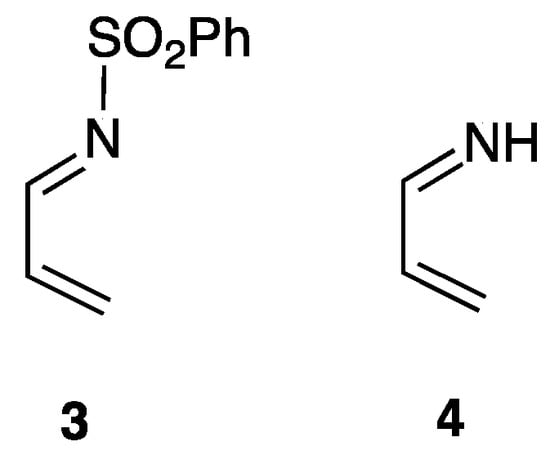
Scheme 4.
N-(phenylsulfonyl)-1-aza-1,3-butadiene (3) and 1-aza-1,3-butadiene (4).
As shown in Figure 3, the small pπ amplitude at the oxygen atom of the methoxy group in ψ′1 can be in-phase with the amplitudes around the C1−C2 bond in φ′1 when the pπ amplitude of the C4 atom in ψ′1 overlaps with the same phase of the C3 atom in φ′1. This may suggest the presence of a non-bonding orbital interaction between the oxygen atom in ψ′1 and the C1 or C2 atom in the endo TS (in TS1, the distances between the oxygen atom and the C1 atom and between the oxygen atom and the C2 atom are 2.90 and 3.03 Å, respectively). Thus, we examined the overlap population between the O and C1 atoms and between the O and C2 atoms in TS1 (Figure S7a,b). The overlap population between the O and C1 atoms has a small positive value of +0.020. Here, the contribution between the occupied orbitals of the dienophile fragment and the unoccupied orbitals of the diene fragment has a positive value, +0.035, while the contribution between the unoccupied orbitals of the dienophile fragment and the occupied orbitals of the diene fragment has a negative value of −0.010. The contribution in between the occupied orbitals also has a negative value of −0.008. On the other hand, the overlap population between the O and C2 atoms also has a positive value of +0.042. This is because the contribution between the occupied orbitals of the dienophile and the unoccupied orbitals of the diene is +0.037, while the contribution between the unoccupied orbitals of the dienophile and the occupied orbitals of the diene is +0.009. Thus, the non-bonding orbital interaction between the methoxy oxygen atom and C1 or C2 atom exists, but the value is relatively small.
In the case where the SO2Ph group is removed from the diene, the overlap population between the O and C1 atoms decreases (Figure S6). The value of the contribution between the occupied orbitals of the diene and the unoccupied orbitals of the dienophile becomes negative, making the total overlap between these atoms smaller. In contrast, the overlap population between the O and C2 atoms increases because of the increase in the contribution value between the occupied orbitals of the diene and the unoccupied orbitals of the dienophile. The introduction of an SO2Ph group into the diene changes the non-bonding orbital interaction by diminishing the electron delocalization from the diene to the dienophile.
The polarization of the π orbitals induced by the SO2Ph group of the diene causes a decrease in the electron population at the C1 atom as well as an increase in the electron-accepting ability of the pπ orbital at the C3 atom (Figure S8). This change affects the weak attractive electrostatic interaction between the non-bonded atoms. The electrostatic potential (ESP) maps on the isodensity surfaces of 1 and 2 (Figure S9) show that the methoxy oxygen atom is responsible for the negatively charged region in 2 (the atomic charge calculated by NPA is −0.53), while the C1−C2 bond is the location of the positively charged region in 1. The attraction between these two regions increases in the endo TS. Thus, the electrostatic attraction between the methoxy oxygen atom and the C1−C2 bond contributes to the endo preference. Furthermore, the results of the EDA also suggested that the dispersion energy has an effect in this context. A non-covalent interaction (NCI) plot for TS1 shows interactions not only between the dienophile and the SO2Ph group in the diene but also between the non-bonded atoms (Figure S10).
2.4. Effect of the Position of the Methoxycarbonyl Group on the Reactivity and Selectivity
In 1, the electron-withdrawing CO2Me group is attached to the C3 atom. Boger and co-workers have reported that the introduction of the electron-withdrawing group at the C2 atom enhances the reactivity [40]. We therefore investigated the effect of the position of the methoxycarbonyl group on both the reactivity and the endo selectivity (Scheme 5). In the case of 1′, TS3′ has the lowest free energy of the endo attack TSs studied here (Figure 4 and Figure S11). The free energy of TS3′ relative to the initial state (1′ + 2) (11.1 kcal/mol) is much lower than that of TS1 in the reaction of 1 (19.3 kcal/mol). This result suggests that the reactivity of 1′ is much higher than that of 1. In addition, TS3′ has 3.3 kcal/mol lower free energy than the lowest energy exo transition state (TS11′). The endo preference in the reaction of 1′ is slightly higher than that in the reaction of 1 (the probability distributions of the structures TS1′–TS16′ afford an endo:exo ratio of 236:1).
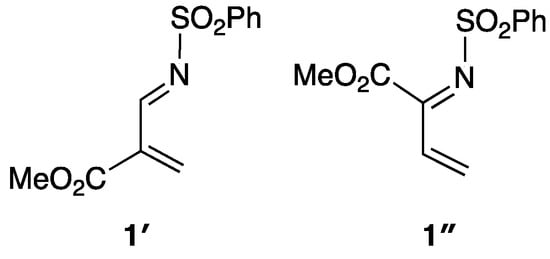
Scheme 5.
3-Methoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene (1′) and 2-methoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene (1″).
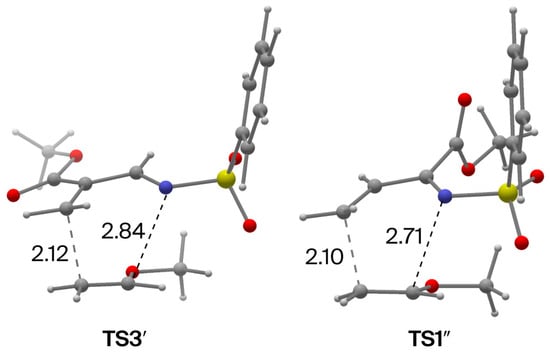
Figure 4.
TS structures TS3′ and TS1″ with bond distances in Å.
In the case of 1″, the free energies of the lowest energy endo and exo TSs relative to the initial state (1″ +2), i.e., TS1″ and TS9″, are 15.1 and 18.9 kcal/mol, respectively (the energy difference is 3.8 kcal/mol; Figure 4 and Figure S12). Thus, the endo preference in this case (endo:exo = 707:1) was found to be higher than that of 1 and 1′. This result is in agreement with the experimentally observed endo:exo ratio (>20:1) in the reaction between 4-phenyl-2-ethoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene and ethoxyethane [40].
As has already been shown, the electron-accepting ability at the C3 atom in the diene plays an important role in the reaction. To investigate further, we used the 2 pπ orbital at the C3 atom as the reference orbital, δr, and then projected this reference orbital onto the unoccupied orbital spaces of 3, 4, 1, 1′, and 1″. The orbital has energy expectation values, λunoc(δr), of +0.67 and +1.53 eV for 3 and 4, respectively, showing that the SO2Ph group greatly strengthens the electrophilicity of the C3 atom. Certainly, the reactivity of 3 is much higher than that of 4 in terms of activation energy. Moreover, the λunoc(δr) values of 1, 1′, and 1″ were estimated to be +1.14, −0.05, +0.55 eV, respectively. The attachment of a CO2Me group to the C3 atom weakens the electrophilicity of the C3 atom, while when this group is attached to the C2 atom it strengthens the electrophilicity of the C3 atom. These results show that the electron-accepting ability of the dienes increases in the order 1′ > 1″ > 1, which is in agreement with their order of reactivity in the IEDDA reaction.
With regard to the endo selectivity in the reaction of 1′, the positively charged carbon atom in the CO2Me group attached to the C2 atom (+0.80 for NPA atomic charge) increases the electrostatic attractive interaction with the methoxy oxygen atom. However, the slightly larger deformation of the dienophile in the endo structure compensates for the larger interaction energy. Consequently, the endo preference slightly increases in comparison to that in the reaction of 1. On the other hand, the larger deformation of the diene in the exo structure increases the endo preference in the case of 1″, because the CO2Me group, which is roughly orthogonal to the diene backbone, causes the geometrical position of the SO2Ph group to change slightly (Table S2 and Figure S13). Thus, the influence that a CO2Me group attached to the C1 atom has on the selectivity is different from that when it is attached to the C2 atom.
3. Computational Details
DFT calculations were carried out using the Gaussian16 [56] and GAMESS [57] packages. Geometry optimizations and analytical vibrational-frequency analyses were performed using restricted Kohn–Sham DFT calculations [58,59] based on the M06-2X functional [60,61,62,63]. For the numerical integration, a larger grid (superfinegrid) was used in the Gaussian16 program [56]. Pople’s 6-311G(d,p) basis set was used for the Gaussian basis functions (6d polarization functions) [64].
4. Conclusions
The inverse-electron-demand Diels–Alder reaction between 4-methoxycarbonyl-N-(phenylsulfonyl)-1-aza-1,3-butadiene and methoxyethene was examined using DFT calculations at the M06-2X level. The formation of the two bonds in this reaction is asynchronous, i.e., the formation of the C−C bond proceeds that of the N−C bond. The formation of the C−C bond is driven by electron delocalization from the dienophile to the diene, indicating that the reaction is an inverse-electron-demand reaction. The endo structure is preferred over the exo structure due to a weak non-bonding orbital interaction, electrostatic attractions, and dispersion interactions. The presence of an electron-withdrawing SO2Ph group in the diene results in higher reactivity and endo selectivity. The presence of a CO2Me group in the diene affects the selectivity in a different way depending on the position where it is attached.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/molecules30193861/s1. Table S1: Total electronic energy (E) and Gibbs free energy (G); Table S2: EDA for the reactions of 3, 1, 1′, and 1″ with 2; Figure S1: Transition state structures of TS1–TS32; Figure S2: Change in bond distances along the IRC of TS1; Figure S3: The fragment charge based on natural population analysis along the IRC of TS1; Figure S4: Natural population atomic charges in 1-aza-1,3-butadiene and N-(phenylsulfonyl)-1-aza-1,3-butadiene; Figure S5: Transition state structures for (a) the reaction between 3 and 2 and (b) the reaction between 4 and 2; Figure S6: Energy decomposition analysis and overlap populations for TS1‴ and TS9‴; Figure S7: Change in the Mulliken overlap populations (a) between O and C1 atoms and (b) between O and C2 atoms along the IRC of TS1; Figure S8: Natural population atomic charges in 1 and 2; Figure S9: The electrostatic potential maps on the isodensity (0.0004 e/au3) surfaces of 1 and 2; Figure S10: NCI plot for TS1; Figure S11: Transition state structures of TS1′–TS32′; Figure S12: Transition state structures of TS1″–TS32″; Figure S13: Dihedral angle of C(Ph gorup)−S−N−C1, ϕ (degree); Cartesian coordinates of stationary points.
Author Contributions
Conceptualization, K.S.; methodology, K.S.; software, K.S. and T.Y.; validation, K.S. and T.Y.; formal analysis, K.S., Y.G. and T.Y.; investigation, K.S., Y.G. and T.Y.; resources, K.S.; data curation, Y.G.; writing—original draft preparation, K.S.; writing—review and editing, K.S.; visualization, K.S.; supervision, K.S. and T.Y.; project administration, K.S.; funding acquisition, K.S. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the JSPS via KAKENHI grants 24H00049 and 24K08418.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All related data and methods are presented in this paper and the Supplementary Materials.
Acknowledgments
Some of the calculations were performed at the Research Center for Computational Science, Okazaki, Japan. T.Y. and K.S. are grateful for the generous permission to use these computing facilities under project number 25-IMS-C048.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nicolaou, K.C.; Snyder, S.A.; Montagnon, T.; Vassilikogiannakis, G. The Diels-Alder Reaction in Total Synthesis. Angew. Chem. Int. Ed. 2002, 41, 1668–1698. [Google Scholar] [CrossRef]
- Fleming, I. Molecular Orbitals and Organic Chemical Reactions, Reference Edition; Wiley: Hoboken, NJ, USA, 2010; pp. 253–368. [Google Scholar]
- Alder, K.; Stein, G. Untersuchungen über den Verlauf der Diensynthese. Angew. Chem. 1937, 50, 510–519. [Google Scholar] [CrossRef]
- Alder, K. Über den sterischen Verlauf von Dien-Synthesen mit acyclischen Dienen. Die allgemeine sterische Formel. Eur. J. Org. Chem. 1951, 571, 157–166. [Google Scholar] [CrossRef]
- Salem, L. Intermolecular orbital theory of the interaction between conjugated systems. II. Thermal and photochemical cycloadditions. J. Am. Chem. Soc. 1968, 90, 553–566. [Google Scholar] [CrossRef]
- Houk, K. The role of secondary orbital interactions in cycloaddition reactions. Tetrahedron Lett. 1970, 11, 2621–2624. [Google Scholar] [CrossRef]
- Houk, K.N.; Strozier, R.W. Lewis acid catalysis of Diels-Alder reactions. J. Am. Chem. Soc. 1973, 95, 4094–4096. [Google Scholar] [CrossRef]
- McCarrick, M.A.; Wu, Y.D.; Houk, K.N. exo-Lone-pair effect on hetero-Diels-Alder cycloaddition stereochemistry. J. Am. Chem. Soc. 1992, 114, 1499–1500. [Google Scholar] [CrossRef]
- McCarrick, M.A.; Wu, Y.D.; Houk, K.N. Hetero-Diels-Alder reaction transition structures: Reactivity, stereoselectivity, catalysis, solvent effects, and the exo-lone-pair effect. J. Org. Chem. 1993, 58, 3330–3343. [Google Scholar] [CrossRef]
- Suarez, D.; Sordo, T.L.; Sordo, J.A. Ab initio study of the Lewis acid-catalyzed Diels-Alder reaction of sulfur dioxide with isoprene: Regioselectivity and stereoselectivity. J. Am. Chem. Soc. 1994, 116, 763–764. [Google Scholar] [CrossRef]
- Suarez, D.; Gonzalez, J.; Sordo, T.L.; Sordo, J.A. Ab Initio Study of the Thermal and Lewis Acid-Catalyzed Hetero Diels-Alder Reactions of 1,3-Butadiene and Isoprene with Sulfur Dioxide. J. Org. Chem. 1994, 59, 8058–8064. [Google Scholar] [CrossRef]
- Apeloig, Y.; Matzner, E. Evidence for the Dominant Role of Secondary Orbital Interactions in Determining the Stereochemistry of the Diels-Alder Reaction: The Case of Cyclopropene. J. Am. Chem. Soc. 1995, 117, 5375–5376. [Google Scholar] [CrossRef]
- Okamoto, I.; Ohwada, T.; Shudo, K. Orbital Unsymmetrization Affects Facial Selectivities of Diels–Alder Dienophiles. J. Org. Chem. 1996, 61, 3155–3166. [Google Scholar] [CrossRef]
- Domingo, L.R.; Picher, M.T.; Andrés, J.; Safont, V.S. Ab Initio Study of Stereo- and Regioselectivity in the Diels–Alder Reaction between 2-Phenylcyclopentadiene and α-(Methylthio)acrylonitrile. J. Org. Chem. 1997, 62, 1775–1778. [Google Scholar] [CrossRef]
- Imade, M.; Hirao, H.; Omoto, K.; Fujimoto, H. Theoretical study of endo selectivity in the Diels–Alder reactions between bu-tadienes and cyclopropane. J. Org. Chem. 1999, 64, 6697–6701. [Google Scholar] [CrossRef]
- García, J.I.; Mayoral, J.A.; Salvatella, L. Do Secondary Orbital Interactions Really Exist? Accounts Chem. Res. 2000, 33, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Calbo-Losada, S.; Suárez, D. Stereochemistry of the furan−maleic anhydride cycloaddition: a theoretical study. J. Am. Chem. Soc. 2000, 122, 390–391. [Google Scholar] [CrossRef]
- Arrieta, A.; Cossío, F.P.; Lecea, B. Direct Evaluation of Secondary Orbital Interactions in the Diels–Alder Reaction between Cyclopentadiene and Maleic Anhydride. J. Org. Chem. 2001, 66, 6178–6180. [Google Scholar] [CrossRef] [PubMed]
- Birney, D.; Lim, T.-K.; Koh, J.H.P.; Pool, B.R.; White, J.M. Structural investigations into the retro-Diels-Alder reaction. Experimental and theoretical studies. J. Am. Chem. Soc. 2002, 124, 5091–5099. [Google Scholar] [CrossRef]
- Kiri, S.; Odo, Y.; Omar, H.I.; Shimo, T.; Somekawa, K. Origin of the Endo/Exo Stereoselectivity and Syn/Anti Face-Selectivity in Diels–Alder Reactions, Determined by Transition State Energy Partitioning. Bull. Chem. Soc. Jpn. 2004, 77, 1499–1504. [Google Scholar] [CrossRef]
- García, J.I.; Mayoral, J.A.; Salvatella, L. The Source of the endo Rule in the Diels–Alder Reaction: Are Secondary Orbital Interactions Really Necessary? Eur. J. Org. Chem. 2004, 2005, 85–90. [Google Scholar] [CrossRef]
- Lubomír, R.; Šebek, P.; Havlas, Z.; Hrabal, R.; Čapek, P.; Svatoš, A.; Svatoš, A. An Experimental and Theoretical Study of Stereoselectivity of Furan−Maleic Anhydride and Furan−Maleimide Diels–Alder Reactions. J. Org. Chem. 2005, 70, 6295–6302. [Google Scholar]
- Meir, R.; Chen, H.; Lai, W.; Shaik, S. Oriented electric fields accelerate diels–alder reactions and control the endo/exo selectivity. Chem. Phys. Chem. 2010, 11, 301–310. [Google Scholar] [CrossRef]
- Fernández, I.; Bickelhaupt, F.M. Origin of the “endo rule” in Diels–Alder reactions. J. Comput. Chem. 2014, 35, 371–376. [Google Scholar] [CrossRef]
- Gayatri, G.; Sastry, G.N. Estimating Regio and Stereoselectivity in [4 + 2] Cycloadditions of Vinyl-Substituted Cyclic Dienes with Maleic Anhydride. J. Phys. Chem. A 2009, 113, 12013–12021. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.W.; Pool, B.R.; White, J.M. Structural Studies on Cycloadducts of Furan, 2-Methoxyfuran, and 5-Trimethylsilylcyclopentadiene with Maleic Anhydride and N-Methylmaleimide. J. Org. Chem. 2007, 73, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Boutelle, R.C.; Northrop, B.H. Substituent Effects on the Reversibility of Furan–Maleimide Cycloadditions. J. Org. Chem. 2011, 76, 7994–8002. [Google Scholar] [CrossRef]
- Qiu, Y. Substituent effects in the Diels–Alder reactions of butadienes, cyclopentadienes, furans and pyroles with maleic anhydride. J. Phys. Org. Chem. 2015, 28, 370–376. [Google Scholar] [CrossRef]
- Molina-Espíritu, M.; Esquivel, R.O.; Kohout, M.; Angulo, J.C.; Dobado, J.A.; Dehesa, J.S.; Lópezrosa, S.; Soriano-Correa, C. Insight into the informational-structure behavior of the Diels-Alder reaction of cyclopentadiene and maleic anhydride. J. Mol. Model. 2014, 20, 2361. [Google Scholar] [CrossRef]
- Hoffmann, R.; Woodward, R.B. Orbital Symmetries and endo-exo Relationships in Concerted Cycloaddition Reactions. J. Am. Chem. Soc. 1965, 87, 4388–4389. [Google Scholar] [CrossRef]
- Woodward, R.B.; Hoffmann, R. The Conservation of Orbital Symmetry; Verlag Chemie: Weinheim, Germany, 1970. [Google Scholar]
- Wannere, C.S.; Paul, A.; Herges, R.; Houk, K.N.; Schaefer, H.F., III; Schleyer, P.V.R. The existence of secondary orbital interactions. J. Comput. Chem. 2007, 28, 344–361. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, A.; Fujimoto, H. Reexamination of orbital interactions in Diels–Alder reactions. Tetrahedron Lett. 2002, 43, 2055–2057. [Google Scholar] [CrossRef]
- Sakata, K.; Fujimoto, H. Origin of the endo selectivity in the Diels–Alder reaction between cyclopentadiene and maleic anhydride. Eur. J. Org. Chem. 2016, 2016, 4275–4278. [Google Scholar] [CrossRef]
- Sakata, K.; Fujimoto, H. Roles of Lewis Acid Catalysts in Diels-Alder Reactions between Cyclopentadiene and Methyl Acrylate. ChemistryOpen 2020, 9, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.L.; Guo, Z.; Bernardes, G.J.L. Inverse electron demand Diels–Alder reactions in chemical biology. Chem. Soc. Rev. 2017, 46, 4895–4950. [Google Scholar] [CrossRef] [PubMed]
- Png, Z.M.; Zeng, H.; Ye, Q.; Xu, J. Inverse-Electron-Demand Diels–Alder Reactions: Principles and Applications. Chem. Asian J. 2017, 12, 2142–2159. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, R. Recent Developments in Catalytic Asymmetric Inverse-Electron-Demand Diels–Alder Reaction. Chem. Rev. 2013, 113, 5515–5546. [Google Scholar] [CrossRef]
- Zhang, J.; Shukla, V.; Boger, D.L. Inverse Electron Demand Diels–Alder Reactions of Heterocyclic Azadienes, 1-Aza-1,3-Butadienes, Cyclopropenone Ketals, and Related Systems. A Retrospective. J. Org. Chem. 2019, 84, 9397–9445. [Google Scholar] [CrossRef]
- Boger, D.L.; Corbett, W.L.; Curran, T.T.; Kasper, A.M. Inverse electron-demand Diels-Alder reactions of N-sulfonyl .alpha.,.beta.-unsaturated imines: A general approach to implementation of the 4.pi. participation of 1-aza-1,3-butadienes in Diels-Alder reactions. J. Am. Chem. Soc. 1991, 113, 1713–1729. [Google Scholar] [CrossRef]
- Rooshenas, P.; Hof, K.; Schreiner, P.R.; Williams, C.M. 1,2,4-Triazine vs. 1,3- and 1,4-oxazinones in normal- and inverse-electron-demand hetero-Diels–Alder reactions: Establishing a status quo by computational analysis. Eur. J. Org. Chem. 2011, 2011, 983–992. [Google Scholar] [CrossRef]
- Domingo, L.R.; Pérez, P. A molecular electron density theory study of the higher-order cycloaddition reactions of tropone with electron-rich ethylenes. The role of the Lewis acid catalyst in the mechanism and pseudocyclic selectivity. New J. Chem. 2021, 46, 294–308. [Google Scholar] [CrossRef]
- Domingo, L.R.; Aurell, M.; Pérez, P.; Contreras, R. Quantitative characterization of the global electrophilicity power of common diene/dienophile pairs in Diels–Alder reactions. Tetrahedron 2002, 58, 4417–4423. [Google Scholar] [CrossRef]
- Fukui, K. The path of chemical reactions-the IRC approach. Accounts Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Hratchian, H.P.; Schlegel, H.B. Accurate reaction paths using a Hessian based predictor–corrector integrator. J. Chem. Phys. 2004, 120, 9918–9924. [Google Scholar] [CrossRef]
- Reed, A.E.; Curtiss, L.A.; Weinhold, F. Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev. 1988, 88, 899–926. [Google Scholar] [CrossRef]
- Sakata, K.; Fujimoto, H. Quantum Chemical Study of Diels–Alder Reactions Catalyzed by Lewis Acid Activated Oxazaborolidines. J. Org. Chem. 2013, 78, 3095–3103. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. I. J. Chem. Phys. 1955, 23, 1833–1840. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO–MO Molecular Wave Functions. II. Overlap Populations, Bond Orders, and Covalent Bond Energies. J. Chem. Phys. 1955, 23, 1841–1846. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. III. Effects of Hybridization on Overlap and Gross AO Populations. J. Chem. Phys. 1955, 23, 2338–2342. [Google Scholar] [CrossRef]
- Mulliken, R.S. Electronic Population Analysis on LCAO-MO Molecular Wave Functions. IV. Bonding and Antibonding in LCAO and Valence-Bond Theories. J. Chem. Phys. 1955, 23, 2343–2346. [Google Scholar] [CrossRef]
- Sakata, K.; Eda, M.; Kitaoka, Y.; Yoshino, T.; Matsunaga, S. Cp*CoIII-catalyzed C–H alkenylation/annulation reactions of indoles with alkynes: A DFT study. J. Org. Chem. 2017, 82, 7379–7387. [Google Scholar] [CrossRef]
- Fukui, K.; Koga, N.; Fujimoto, H. Interaction frontier orbitals. J. Am. Chem. Soc. 1981, 103, 196–197. [Google Scholar] [CrossRef]
- Fujimoto, H. Paired interacting orbitals: A way of looking at chemical interactions. Accounts Chem. Res. 1987, 20, 448–453. [Google Scholar] [CrossRef]
- Su, P.; Li, H. Energy decomposition analysis of covalent bonds and intermolecular interactions. J. Chem. Phys. 2009, 131, 014102. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16; revision A.03; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Schmidt, M.W.; Baldridge, K.K.; Boatz, J.A.; Elbert, S.T.; Gordon, M.S.; Jensen, J.H.; Koseki, S.; Matsunaga, N.; Nguyen, K.A.; Su, S.; et al. General atomic and molecular electronic structure system. J. Comput. Chem. 1993, 14, 1347–1363. [Google Scholar] [CrossRef]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-consistent equations including exchange and correlation effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. Density Functionals with Broad Applicability in Chemistry. Acc. Chem. Res. 2008, 41, 157–167. [Google Scholar] [CrossRef]
- Riley, K.E.; Op’t Holt, B.T.; Merz, K.M., Jr. Critical Assessment of the Performance of Density Functional Methods for Several Atomic and Molecular Properties. J. Chem. Theory Comput. 2007, 3, 407–433. [Google Scholar] [CrossRef] [PubMed]
- Pieniazek, S.N.; Clemente, F.R.; Houk, K.N. Sources of Error in DFT Computations of C-C Bond Formation Thermochemistries: π→σ Transformations and Error Cancellation by DFT Methods. Angew. Chem. 2008, 120, 7860–7863. [Google Scholar] [CrossRef]
- Plumley, J.A.; Evanseck, J.D. Hybrid Meta-Generalized Gradient Functional Modeling of Boron−Nitrogen Coordinate Covalent Bonds. J. Chem. Theory Comput. 2008, 4, 1249–1253. [Google Scholar] [CrossRef]
- Hehre, W.J.; Radom, L.; Schleyer, P.v.R.; Pople, J.A. Ab Initio Molecular Orbital Theory; Wiley: New York, NY, USA, 1986. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).