Antioxidant Potential and Volatile Aroma Profiling of Red Wines from the Tarnave Vineyard
Abstract
1. Introduction
2. Results and Discussion
2.1. Climatic Parameter Characterization for the Mica Vineyard Region
2.2. Polyphenolic Composition and Antioxidant Implications
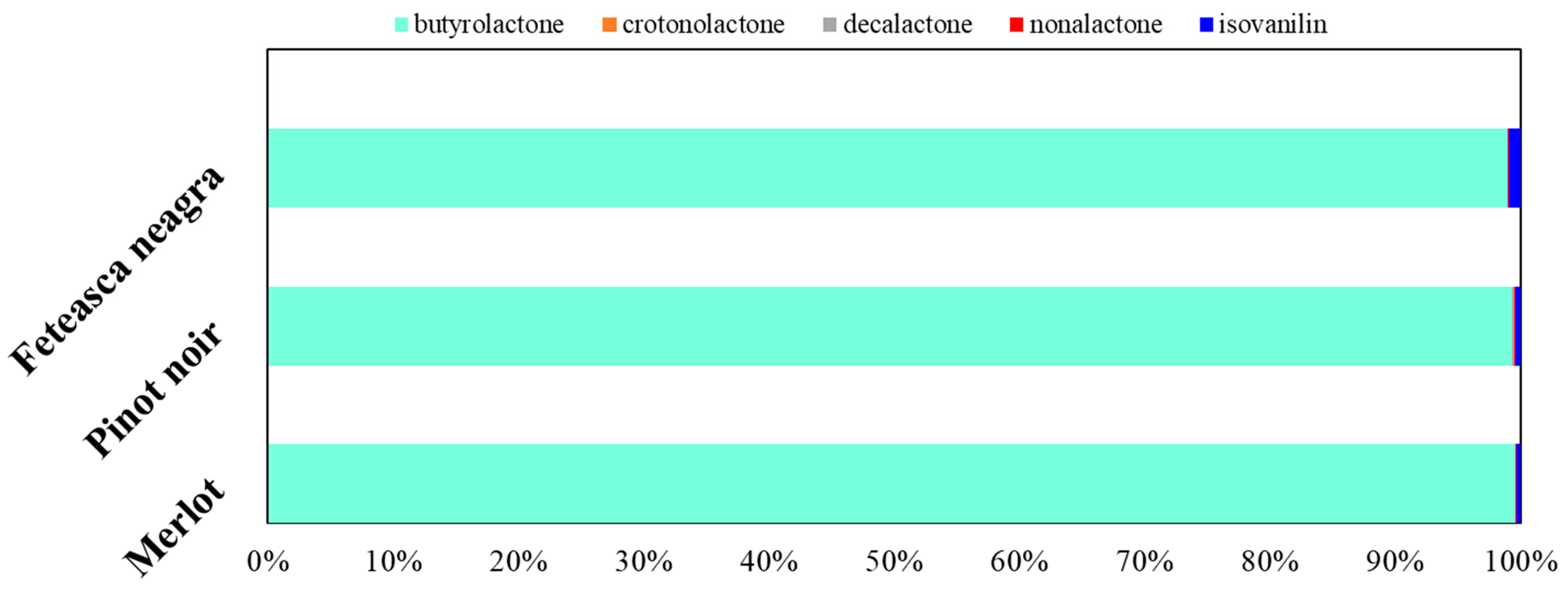
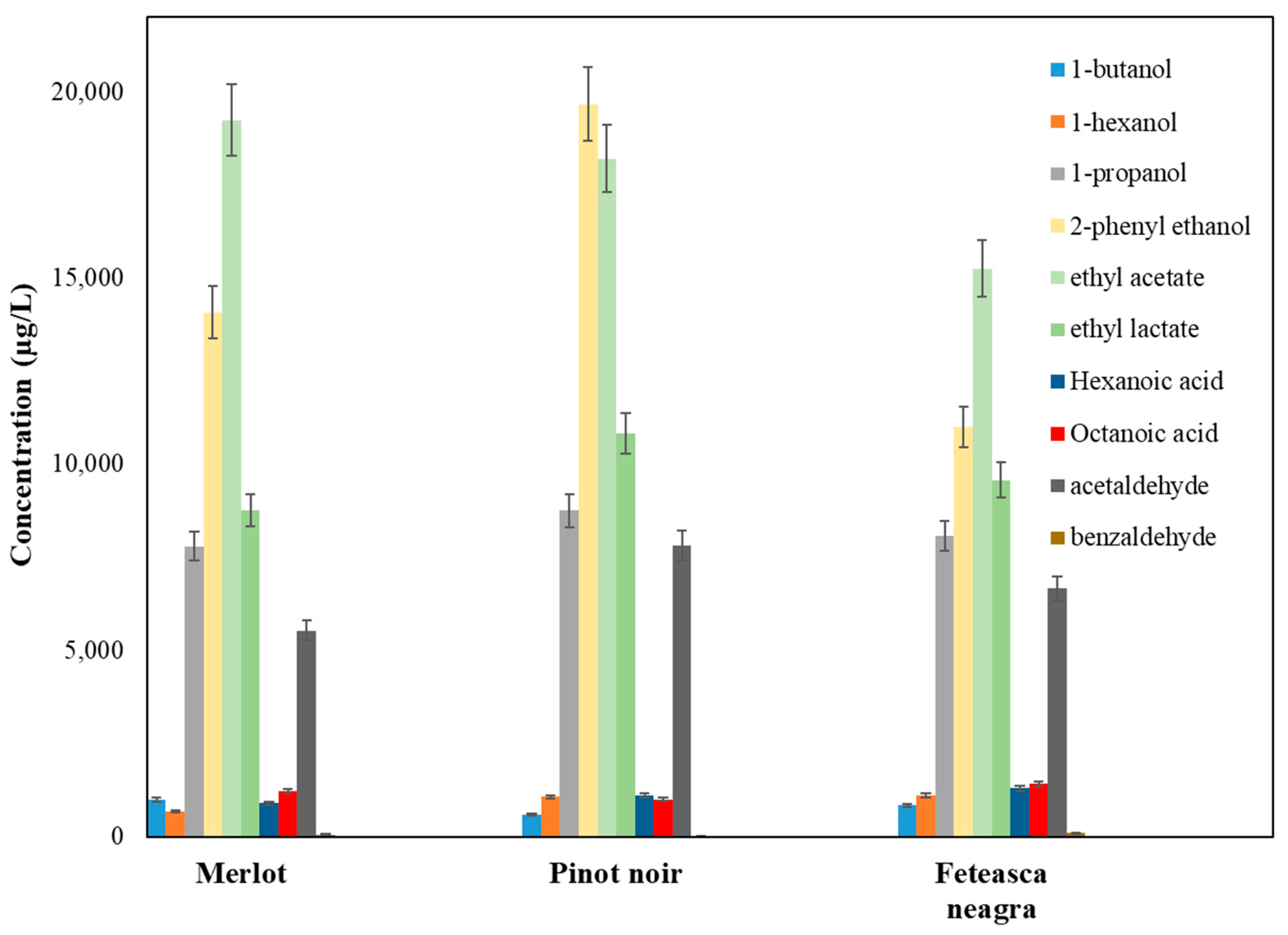
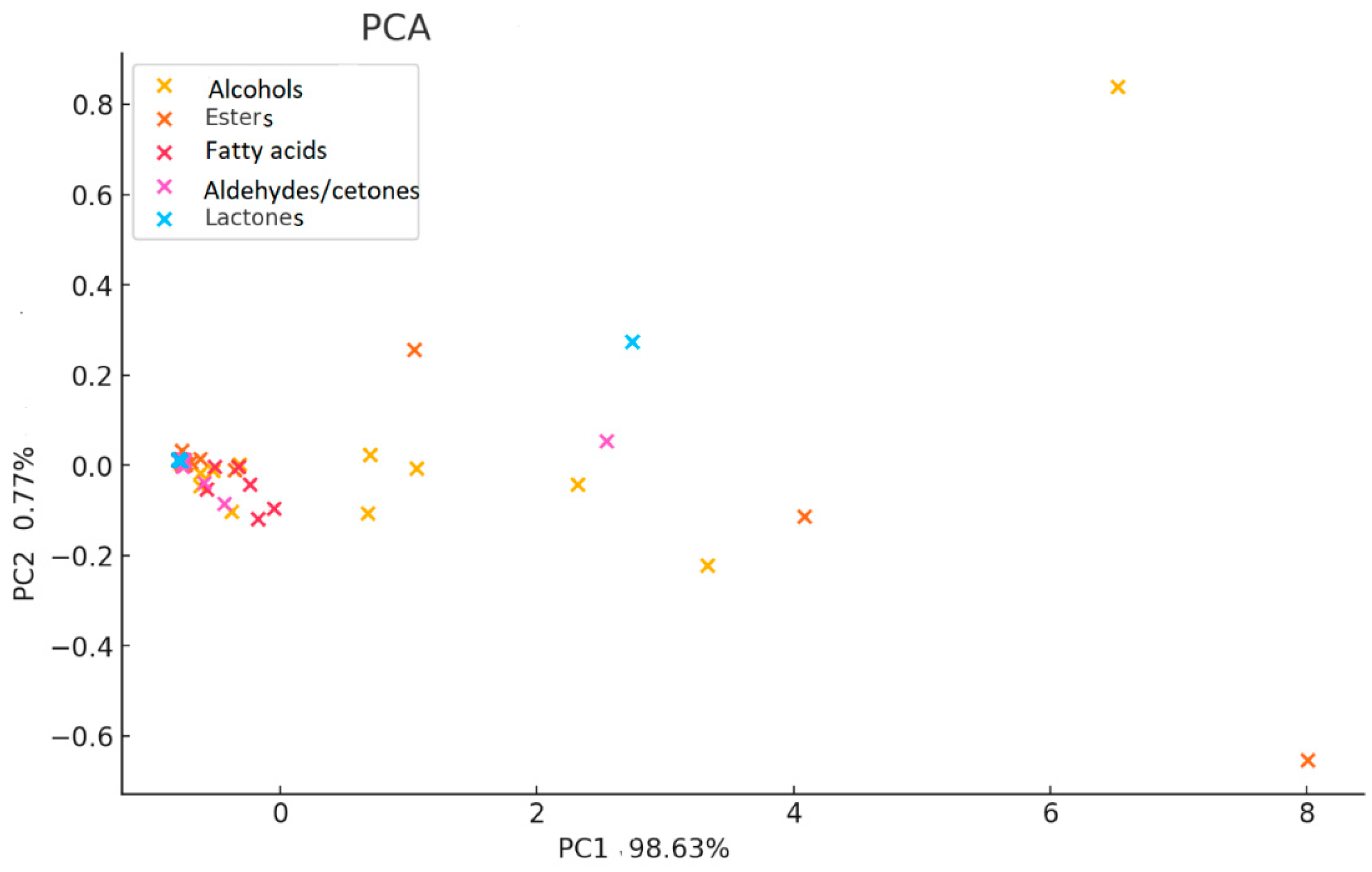
2.3. Volatile Compounds Quantification
2.4. Sensory Implications and Varietal Typicity
3. Materials and Methods
3.1. Reference Zone
3.2. Evaluation of Climatic Parameters for Vineyard Management and Regional Climate Zoning
- Global radiation (MJ/m2/day), measured via pyranometer during the growing season (1 April–30 September).
- Effective temperature sum (ƩTe, °C), calculated as the cumulative daily temperature increments exceeding the 10 °C biological threshold for vine development during the growing season:
3.3. Reagents and Equipment
3.4. Determination of Total Polyphenols (Folin-Ciocâlteu Method)
3.5. Total Anthocyanins Assessment (pH Differential Method)
3.6. Antioxidant Activity Assessment (DPPH, TEAC, FRAP)
3.6.1. DPPH Radical Scavenging Assay
3.6.2. Trolox Equivalent Antioxidant Capacity (TEAC)
3.6.3. Ferric Reducing Antioxidant Power (FRAP)
3.7. GC–MS Determination of Aroma Compounds
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| TEAC | Trolox Equivalent Antioxidant Capacity |
| FRAP | Ferric Reducing Antioxidant Potential |
| GC–MS | Gas Chromatography/Mass Spectrometry |
| PCA | Principal Component Analysis |
| PC | Principal Component |
| VOCs | Volatile Organic Compounds |
| RHI | Real Heliothermal Index |
| Ibcv | Bioclimatic Index |
| HTC | Hydro-Thermal Coefficient of Selyaninov |
| DMI | The De Martonne Aridity Index |
| IAOe | Oenoclimatic Aptitude Index |
| TPTZ | 2,4,6-tripyridyl-s-triazine |
| ABTS | 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid |
| GAE | Gallic Acid Equivalents |
| A | Absorbance |
| MW | Molecular Weight |
| DF | Dilution Factor |
| TE | Trolox Equivalents |
| l | Path Length of the Cuvette |
| ε | Molar Extinction Coefficient |
| PTFE | Polytetrafluoroethylene |
| DCM | Dichloromethane |
References
- Rouxinol, M.I.; Martins, M.R.; Barroso, J.M.; Rato, A.E. Wine Grapes Ripening: A Review on Climate Effect and Analytical Approach to Increase Wine Quality. Appl. Biosci. 2023, 2, 347–372. [Google Scholar] [CrossRef]
- Niculescu, V.-C.; Sandru, D.; Botoran, O.R.; Sutan, N.A.; Popescu (Stegarus), D.I. Red Wines from Consecrated Wine-Growing Area: Aromas Evolution Under Indigenous and Commercial Yeasts. Appl. Sci. 2024, 14, 10239. [Google Scholar] [CrossRef]
- Trujillo, M.; Bely, M.; Albertin, W.; Masneuf-Pomarède, I.; Colonna-Ceccaldi, B.; Marullo, P.; Barbe, J.-C. Impact of Grape Maturity on Ester Composition and Sensory Properties of Merlot and Tempranillo Wines. J. Agric. Food Chem. 2022, 70, 11520–11530. [Google Scholar] [CrossRef] [PubMed]
- Mota, R.V.D.; Peregrino, I.; Silva, C.P.C.; Raimundo, R.H.P.; Fernandes, F.D.P.; Souza, C.R.D. Row Orientation Effects on Chemical Composition and Aromatic Profile of Syrah Winter Wines. Food Sci. Technol. 2021, 41, 412–417. [Google Scholar] [CrossRef]
- Jordão, A.; Vilela, A.; Cosme, F. From Sugar of Grape to Alcohol of Wine: Sensorial Impact of Alcohol in Wine. Beverages 2015, 1, 292–310. [Google Scholar] [CrossRef]
- Hewitt, S.; Hernández-Montes, E.; Dhingra, A.; Keller, M. Impact of Heat Stress, Water Stress, and Their Combined Effects on the Metabolism and Transcriptome of Grape Berries. Sci. Rep. 2023, 13, 9907. [Google Scholar] [CrossRef]
- De Rességuier, L.; Inchboard, L.; Parker, A.K.; Petitjean, T.; Van Leeuwen, C. Drivers of Grape Berry Sugar Accumulation in Field Conditions at Local Scale: This Article Is Published in Cooperation with the XVth International Terroir Congress, 18–22 November 2024, Mendoza, Argentina. Guest Editors: Federico Berli, Jorge Prieto and Martín Fanzone. OENO One 2024, 58. [Google Scholar] [CrossRef]
- Chacón-Vozmediano, J.L.; Martínez-Gascueña, J.; García-Romero, E.; Gómez-Alonso, S.; García-Navarro, F.J.; Jiménez-Ballesta, R. Effects of Water Stress on the Phenolic Compounds of ‘Merlot’ Grapes in a Semi-Arid Mediterranean Climate. Horticulturae 2021, 7, 161. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Intrigliolo, D.S.; Almajano, M.P.; Rubio-Bretón, P.; Garde-Cerdán, T. Effects of Water Deficit Irrigation on Phenolic Composition and Antioxidant Activity of Monastrell Grapes under Semiarid Conditions. Antioxidants 2021, 10, 1301. [Google Scholar] [CrossRef]
- He, Y.; Wang, X.; Li, P.; Lv, Y.; Nan, H.; Wen, L.; Wang, Z. Research Progress of Wine Aroma Components: A Critical Review. Food Chem. 2023, 402, 134491. [Google Scholar] [CrossRef]
- Jiang, B.; Xi, Z.; Luo, M.; Zhang, Z. Comparison on Aroma Compounds in Cabernet Sauvignon and Merlot Wines from Four Wine Grape-Growing Regions in China. Food Res. Int. 2013, 51, 482–489. [Google Scholar] [CrossRef]
- Jagatić Korenika, A.-M.; Preiner, D.; Tomaz, I.; Jeromel, A. Volatile Profile Characterization of Croatian Commercial Sparkling Wines. Molecules 2020, 25, 4349. [Google Scholar] [CrossRef] [PubMed]
- Longo, R.; Carew, A.; Sawyer, S.; Kemp, B.; Kerslake, F. A Review on the Aroma Composition of Vitis vinifera L. Pinot noir Wines: Origins and Influencing Factors. Crit. Rev. Food Sci. Nutr. 2021, 61, 1589–1604. [Google Scholar] [CrossRef] [PubMed]
- Nan, L.; Liu, L.; Li, Y.; Huang, J.; Wang, Y.; Wang, C.; Wang, Z.; Xu, C. Comparison of Aroma Compounds in Cabernet Sauvignon Red Wines from Five Growing Regions in Xinjiang in China. J. Food Qual. 2021, 2021, 1–16. [Google Scholar] [CrossRef]
- Petronilho, S.; Lopez, R.; Ferreira, V.; Coimbra, M.A.; Rocha, S.M. Revealing the Usefulness of Aroma Networks to Explain Wine Aroma Properties: A Case Study of Portuguese Wines. Molecules 2020, 25, 272. [Google Scholar] [CrossRef]
- Pichler, A.; Ivić, I.; Mesić, J.; Drenjančević, M.; Kujundžić, T.; Marković, T.; Kopjar, M. Aroma Profile of Merlot Red Wine Stored in Stainless-Steel Tanks and Wooden Barrels with Different Toasting Methods. Foods 2024, 13, 45. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Seguin, G. The Concept of Terroir in Viticulture. J. Wine Res. 2006, 17, 1–10. [Google Scholar] [CrossRef]
- Li, N.; Li, G.; Guan, X.; Li, A.; Tao, Y. Volatile Aroma Compound-Based Decoding and Prediction of Sweet Berry Aromas in Dry Red Wine. Food Chem. 2025, 463, 141248. [Google Scholar] [CrossRef]
- Yao, X.-C.; Zhang, H.-L.; Ma, X.-R.; Xia, N.-Y.; Duan, C.-Q.; Yang, W.-M.; Pan, Q.-H. Leaching and Evolution of Anthocyanins and Aroma Compounds during Cabernet Sauvignon Wine Fermentation with Whole-Process Skin-Seed Contact. Food Chem. 2024, 436, 137727. [Google Scholar] [CrossRef]
- Zhang, T.; Liao, Z.; Li, Z.; Liu, Y.; Bi, J.; Liu, Y.; Song, Y.; Qin, Y. Revealing the Flavor Differences of Sauvignon Blanc Wines Fermented in Different Oak Barrels and Stainless-Steel Tanks through GC-MS, GC-IMS, Electronic, and Artificial Sensory Analyses. Food Chem. X 2025, 25, 102188. [Google Scholar] [CrossRef]
- Ayestarán, B.; Martínez-Lapuente, L.; Guadalupe, Z.; Canals, C.; Adell, E.; Vilanova, M. Effect of the Winemaking Process on the Volatile Composition and Aromatic Profile of Tempranillo Blanco Wines. Food Chem. 2019, 276, 187–194. [Google Scholar] [CrossRef]
- Qu, J.; Chen, X.; Wang, X.; He, S.; Tao, Y.; Jin, G. Esters and Higher Alcohols Regulation to Enhance Wine Fruity Aroma Based on Oxidation-Reduction Potential. LWT 2024, 200, 116165. [Google Scholar] [CrossRef]
- Dennis, E.G.; Keyzers, R.A.; Kalua, C.M.; Maffei, S.M.; Nicholson, E.L.; Boss, P.K. Grape Contribution to Wine Aroma: Production of Hexyl Acetate, Octyl Acetate, and Benzyl Acetate during Yeast Fermentation Is Dependent upon Precursors in the Must. J. Agric. Food Chem. 2012, 60, 2638–2646. [Google Scholar] [CrossRef]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Ju, Y.; Cui, Y.; Wei, S.; Xu, H.; Zhang, Z. Evolution of Green Leaf Volatile Profile and Aroma Potential during the Berry Development in Five Vitis vinifera L. Cultivars. Food Chem. X 2023, 18, 100676. [Google Scholar] [CrossRef]
- Allen, M.S.; Lacey, M.J. Methoxypyrazines of Grapes and Wines. In Chemistry of Wine Flavor; American Chemical Society: Washington, DC, USA, 1998; pp. 31–38. [Google Scholar] [CrossRef]
- Dumitriu (Gabur), G.-D.; Sánchez-Suárez, F.; Peinado, R.A.; Cotea, V.V.; De Lerma, N.L.; Gabur, I.; Simioniuc, V. Metabolomics of Red Wines Aged Traditionally, with Chips or Staves. Foods 2024, 13, 196. [Google Scholar] [CrossRef] [PubMed]
- Nevares, I.; del Alamo-Sanza, M. New Materials for the Aging of Wines and Beverages: Evaluation and Comparison. In Food Packaging and Preservation; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 375–407. [Google Scholar] [CrossRef]
- Zhao, L.-Z.; Chen, J.; Wei, X.-Y.; Lin, B.; Zheng, F.-J.; Verma, K.K.; Chen, G.-L. Response of Alcohol Fermentation Strains, Mixed Fermentation and Extremozymes Interactions on Wine Flavor. Front. Microbiol. 2025, 16, 1–13. [Google Scholar] [CrossRef]
- Rayne, S.; Eggers, N.J. 4-Ethylphenol and 4-Ethylguaiacol in Wines: Estimating Non-Microbial Sourced Contributions and Toxicological Considerations. J. Environ. Sci. Health Part B 2007, 42, 887–897. [Google Scholar] [CrossRef]
- Gonzalez, R.; Morales, P. Wine Secondary Aroma: Understanding Yeast Production of Higher Alcohols. Microb. Biotechnol. 2017, 10, 1449–1450. [Google Scholar] [CrossRef]
- Wagner, M.; Zaldarriaga Heredia, J.; Segura-Borrego, M.P.; Morales, M.L.; Camiña, J.M.; Azcarate, S.M.; Callejón, R.M.; Ríos-Reina, R. Identification of Potential Volatile Markers for Characterizing Argentine Wine Vinegars Based on Their Production Process. Talanta Open 2024, 10, 100370. [Google Scholar] [CrossRef]
- Copernicus Climate Change Service (C3S); ECMWF. Global Climate Highlights 2024—ERA5 Reanalysis; Copernicus Climate Change Service: Reading, UK, 2025; Available online: https://climate.copernicus.eu/global-climate-highlights-2024 (accessed on 14 September 2025).
- Buljeta, I.; Pichler, A.; Šimunović, J.; Kopjar, M. Beneficial Effects of Red Wine Polyphenols on Human Health: Comprehensive Review. Curr. Issues Mol. Biol. 2023, 45, 782–798. [Google Scholar] [CrossRef]
- Perestrelo, R.; Bordiga, M.; Locatelli, M.; Silva, C.; Câmara, J.S. Polyphenols, Biogenic Amines and Amino Acids Patterns in Verdelho Wines According to Vintage. Microchem. J. 2020, 153, 104383. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Rebenciuc, I.; Lengyel, E.; Stegăruş, D.I.; Alexe, P.; Călugăr, A. Identification and Quantification of Total Polyphenols in Musts and Aromatic and Semiaromatic Wines. Adv. Agric. Bot. 2020, 12, 106–111. [Google Scholar]
- Tedesco, I.; Spagnuolo, C.; Russo, G.L.; Russo, M.; Cervellera, C.; Moccia, S. The Pro-Oxidant Activity of Red Wine Polyphenols Induces an Adaptive Antioxidant Response in Human Erythrocytes. Antioxidants 2021, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Tzanova, M.; Atanassova, S.; Atanasov, V.; Grozeva, N. Content of Polyphenolic Compounds and Antioxidant Potential of Some Bulgarian Red Grape Varieties and Red Wines, Determined by HPLC, UV, and NIR Spectroscopy. Agriculture 2020, 10, 193. [Google Scholar] [CrossRef]
- Ubeda, C.; Kania-Zelada, I.; Del Barrio-Galán, R.; Medel-Marabolí, M.; Gil, M.; Peña-Neira, Á. Study of the Changes in Volatile Compounds, Aroma and Sensory Attributes during the Production Process of Sparkling Wine by Traditional Method. Food Res. Int. 2019, 119, 554–563. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef]
- Nel, A.P. Tannins and Anthocyanins: From Their Origin to Wine Analysis—A Review. South Afr. J. Enol. Vitic. 2018, 39, 1–20. [Google Scholar] [CrossRef]
- Mislata, A.M.; Puxeu, M.; Tomás, E.; Nart, E.; Ferrer-Gallego, R. Influence of the Oxidation in the Aromatic Composition and Sensory Profile of Rioja Red Aged Wines. Eur. Food Res. Technol. 2020, 246, 1167–1181. [Google Scholar] [CrossRef]
- Casassa, L.F.; Bolcato, E.A.; Sari, S.E.; Barda, N. Effects of Maceration Length after Prefermentative Cold Soak: Detailed Chromatic, Phenolic and Sensory Composition of Cabernet Sauvignon, Malbec and Merlot Wines. J. Food Compos. Anal. 2021, 104, 104168. [Google Scholar] [CrossRef]
- Gombau, J.; Pons-Mercadé, P.; Conde, M.; Asbiro, L.; Pascual, O.; Gómez-Alonso, S.; García-Romero, E.; Miquel Canals, J.; Hermosín-Gutiérrez, I.; Zamora, F. Influence of Grape Seeds on Wine Composition and Astringency of Tempranillo, Garnacha, Merlot and Cabernet Sauvignon Wines. Food Sci. Nutr. 2020, 8, 3442–3455. [Google Scholar] [CrossRef] [PubMed]
- Mannino, G.; Gentile, C.; Ertani, A.; Serio, G.; Bertea, C.M. Anthocyanins: Biosynthesis, Distribution, Ecological Role, and Use of Biostimulants to Increase Their Content in Plant Foods—A Review. Agriculture 2021, 11, 212. [Google Scholar] [CrossRef]
- Banc, R.; Loghin, F.; Miere, D.; Ranga, F.; Socaciu, C. Phenolic Composition and Antioxidant Activity of Red, Rosé and White Wines Originating from Romanian Grape Cultivars. Not. Bot. Horti Agrobo 2020, 48, 716–734. [Google Scholar] [CrossRef]
- Moreno-Arribas, M.V.; Bartolomé, B.; Peñalvo, J.L.; Pérez-Matute, P.; Motilva, M.J. Relationship between Wine Consumption, Diet and Microbiome Modulation in Alzheimer’s Disease. Nutrients 2020, 12, 3082. [Google Scholar] [CrossRef]
- Zhou, D.-D.; Li, J.; Xiong, R.-G.; Saimaiti, A.; Huang, S.-Y.; Wu, S.-X.; Yang, Z.-J.; Shang, A.; Zhao, C.-N.; Gan, R.-Y.; et al. Bioactive Compounds, Health Benefits and Food Applications of Grape. Foods 2022, 11, 2755. [Google Scholar] [CrossRef]
- Rodriguez-Lopez, P.; Rueda-Robles, A.; Borrás-Linares, I.; Quirantes-Piné, R.M.; Emanuelli, T.; Segura-Carretero, A.; Lozano-Sánchez, J. Grape and Grape-Based Product Polyphenols: A Systematic Review of Health Properties, Bioavailability, and Gut Microbiota Interactions. Horticulturae 2022, 8, 583. [Google Scholar] [CrossRef]
- Nicolescu, C.M.; Bumbac, M.; Radulescu, C.; Buruleanu, C.L.; Olteanu, R.L.; Stanescu, S.G.; Gorghiu, L.M.; Serban, B.C.; Buiu, O. Phytochemical Statistical Mapping of Red Grape Varieties Cultivated in Romanian Organic and Conventional Vineyards. Plants 2023, 12, 4179. [Google Scholar] [CrossRef]
- Navarro-Masip, È.; Manocchio, F.; Colom-Pellicer, M.; Escoté, X.; Iglesias-Carrés, L.; Calvo, E.; Bravo, F.I.; Muguerza, B.; Desjardins, Y.; Aragonès, G. Vitis vinifera L. Bioactive Components Modulate Adipose Tissue Metabolic Markers of Healthy Rats in a Photoperiod-Dependent Manner. Mol. Nutr. Food Res. 2023, 67, e2300074. [Google Scholar] [CrossRef]
- Ilak Peršurić, A.S.; Rossi, S.; Bestulić, E.; Radeka, S. Perceptions of Wine Health Benefits AND Effects of Wine Consumption on Well-Being. EA 2023, 70, 145–167. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate Change and Global Wine Quality. Clim. Change 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Selyaninov GL About the agricultural evaluation of the climate. TrudyGGO 1928, 20, 177–185.
- De Martonne, E. Aerisme, et Índices d’aridite. Comptesrendus De L’Academie Des Sci. 1926, 182, 1395–1398. [Google Scholar]
- Jones, G.V.; Davis, R.E. Climate Influences on Grapevine Phenology, Grape Composition, and Wine Production and Quality for Bordeaux, France. Am. J. Enol. Vitic. 2000, 51, 249–261. [Google Scholar] [CrossRef]
- Downey, M.O.; Dokoozlian, N.K.; Krstic, M.P. Cultural Practice and Environmental Impacts on the Flavonoid Composition of Grapes and Wine: A Review of Recent Research. Am. J. Enol. Vitic. 2006, 57, 257–268. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A.; Dubernet, M.; Duchêne, E.; Gowdy, M.; Marguerit, E.; Pieri, P.; Parker, A.; De Rességuier, L.; Ollat, N. An Update on the Impact of Climate Change in Viticulture and Potential Adaptations. Agronomy 2019, 9, 514. [Google Scholar] [CrossRef]
- Van Leeuwen, C.; Destrac-Irvine, A. Modified Grape Composition under Climate Change Conditions Requires Adaptations in the Vineyard. OENO One 2017, 51, 147–154. [Google Scholar] [CrossRef]
- Hofmann, M.; Lux, R.; Schultz, H.R. Constructing a Framework for Risk Analyses of Climate Change Effects on the Water Budget of Differently Sloped Vineyards with a Numeric Simulation Using the Monte Carlo Method Coupled to a Water Balance Model. Front. Plant Sci. 2014, 5. [Google Scholar] [CrossRef]
- Jones, G.V.; Schultz, H.R. Climate Change: Climate Change and Emerging Cool Climate Wine Regions. Wine Vitic. J. 2016, 31. [Google Scholar]
- Vršič, S.; Pulko, B.; Vodovnik-Plevnik, T.; Perko, A. The Impact of Climatic Warming on Earlier Wine-Grape Ripening in Northeastern Slovenia. Horticulturae 2024, 10, 611. [Google Scholar] [CrossRef]
- Gowdy, M.; Destrac-Irvine, A.; Marguerit, E.; Pieri, P.; Gambetta, G.; van Leeuwen, C. Carbon Isotope Discrimination in Berry Juice Sugars: Changes in Response to Soil Water Deficits across a Range of Vitis vinifera Cultivars. In Proceedings of the A Multidisciplinary Vision Towards Sustainable Viticulture, Thessaloniki, Greece, 23–28 June 2019. [Google Scholar]
- Mendieta Herrera, J.; Iñiguez Armijos, C.; Rosado Alcarria, D.; Aguilar Ramírez, S. Toxicity of Difenoconazole and Atrazine and Their Photodegradation Products on Aquatic Biota: Environmental Implications in Countries Lacking Good Agricultural Practices. Toxics 2023, 11, 213. [Google Scholar] [CrossRef] [PubMed]
- Irimia, L.M. Changes in oenoclimate aptitude index characterizing climate suitability of Romanian wine growing regions. Appl. Ecol. Environ. Res. 2017, 15, 755–767. [Google Scholar] [CrossRef]
- Filimon, R.M.; Bunea, C.I.; Filimon, R.V.; Bora, F.D.; Damian, D. Long-Term Evolution of the Climatic Factors and Its Influence on Grape Quality in Northeastern Romania. Horticulturae 2024, 10, 705. [Google Scholar] [CrossRef]
- Waterhouse, A.L. Wine Phenolics. Ann. N. Y. Acad. Sci. 2002, 957, 21–36. [Google Scholar] [CrossRef]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A Review of the Effect of Winemaking Techniques on Phenolic Extraction in Red Wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar] [CrossRef]
- Paixao, N.; Perestrelo, R.; Marques, J.; Camara, J. Relationship between Antioxidant Capacity and Total Phenolic Content of Red, Rosé and White Wines. Food Chem. 2007, 105, 204–214. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Miao, Y. Can Chemical Analysis Predict Wine Aging Capacity? Foods 2021, 10, 654. [Google Scholar] [CrossRef]
- Deshaies, S.; Cazals, G.; Enjalbal, C.; Constantin, T.; Garcia, F.; Mouls, L.; Saucier, C. Red Wine Oxidation: Accelerated Ageing Tests, Possible Reaction Mechanisms and Application to Syrah Red Wines. Antioxidants 2020, 9, 663. [Google Scholar] [CrossRef]
- Bai, S.; Tao, X.; Hu, J.; Chen, H.; Wu, J.; Zhang, F.; Cai, J.; Wu, G.; Meng, J. Flavonoids Profile and Antioxidant Capacity of Four Wine Grape Cultivars and Their Wines Grown in the Turpan Basin of China, the Hottest Wine Region in the World. Food Chem. X 2025, 26, 102301. [Google Scholar] [CrossRef]
- Tena, N.; Martín, J.; Asuero, A.G. State of the Art of Anthocyanins: Antioxidant Activity, Sources, Bioavailability, and Therapeutic Effect in Human Health. Antioxidants 2020, 9, 451. [Google Scholar] [CrossRef]
- Strati, I.F.; Tataridis, P.; Shehadeh, A.; Chatzilazarou, A.; Bartzis, V.; Batrinou, A.; Sinanoglou, V.J. Impact of Tannin Addition on the Antioxidant Activity and Sensory Character of Malagousia White Wine. Curr. Res. Food Sci. 2021, 4, 937–945. [Google Scholar] [CrossRef]
- Büyüktuncel, E.; Porgalı, E.; Çolak, C. Comparison of Total Phenolic Content and Total Antioxidant Activity in Local Red Wines Determined by Spectrophotometric Methods. Food Nutr. Sci. 2014, 05, 1660–1667. [Google Scholar] [CrossRef]
- Romano, C.A.; Neto, J.R. de O.; da Cunha, L.C.; dos Santos, A.H.; de Paula, J.R. Essential Oil-Based Nanoemulsion of Murraya Koenigii Is an Efficient Larvicidal against Aedes Aegypti under Field Conditions. Ind. Crops Prod. 2024, 208. [Google Scholar] [CrossRef]
- Radeka, S.; Rossi, S.; Bestulić, E.; Budić-Leto, I.; Kovačević Ganić, K.; Horvat, I.; Lukić, I.; Orbanić, F.; Zaninović Jurjević, T.; Dvornik, Š. Bioactive Compounds and Antioxidant Activity of Red and White Wines Produced from Autochthonous Croatian Varieties: Effect of Moderate Consumption on Human Health. Foods 2022, 11, 1804. [Google Scholar] [CrossRef]
- Onache, P.A.; Florea, A.; Geana, E.-I.; Ciucure, C.T.; Ionete, R.E.; Sumedrea, D.I.; Tița, O. Assessment of Bioactive Phenolic Compounds in Musts and the Corresponding Wines of White and Red Grape Varieties. Appl. Sci. 2023, 13, 5722. [Google Scholar] [CrossRef]
- Ge, Y.-L.; Xia, N.-Y.; Wang, Y.-C.; Zhang, H.-L.; Yang, W.-M.; Duan, C.-Q.; Pan, Q.-H. Evolution of Aroma Profiles in Vitis vinifera L. Marselan and Merlot from Grapes to Wines and Difference between Varieties. Molecules 2024, 29, 3250. [Google Scholar] [CrossRef] [PubMed]
- Alonso González, P.; Parga Dans, E.; Ballester, J. Volatile Composition of Light Red Wines Aged in Canary Pine Barrels from La Palma (Canary Islands, Spain). OENO One 2022, 56, 29–40. [Google Scholar] [CrossRef]
- Mihail, M.; Călugăr, A.; Pop, N.; Gal, E.; Pop, T.I. Odor activity value in red wines aroma from three wine regions in Romania. Agrucultura 2019, 109, 29–38. [Google Scholar]
- Feng, Y.; Liu, M.; Ouyang, Y.; Zhao, X.; Ju, Y.; Fang, Y. Comparative Study of Aromatic Compounds in Fruit Wines from Raspberry, Strawberry, and Mulberry in Central Shaanxi Area. Food Nutr. Res. 2015, 59, 29290. [Google Scholar] [CrossRef]
- Martínez-Avila, O.; Sánchez, A.; Font, X.; Barrena, R. 2-Phenylethanol (Rose Aroma) Production Potential of an Isolated Pichia kudriavzevii through Solid-State Fermentation. Process Biochem. 2020, 93, 94–103. [Google Scholar] [CrossRef]
- Welke, J.E.; Zanus, M.; Lazzarotto, M.; Alcaraz Zini, C. Quantitative Analysis of Headspace Volatile Compounds Using Comprehensive Two-Dimensional Gas Chromatography and Their Contribution to the Aroma of Chardonnay Wine. Food Res. Int. 2014, 59, 85–99. [Google Scholar] [CrossRef]
- Antal, E.; Kállay, M.; Varga, Z.; Nyitrai-Sárdy, D. Effect of Botrytis cinerea Activity on Glycol Composition and Concentration in Wines. Fermentation 2023, 9, 493. [Google Scholar] [CrossRef]
- Delgado, J.A.; Sánchez-Palomo, E.; Osorio Alises, M.; González Viñas, M.A. Chemical and Sensory Aroma Typicity of La Mancha Petit Verdot Wines. LWT 2022, 162, 113418. [Google Scholar] [CrossRef]
- Martelanc, M.; Slaghenaufi, D.; Radovanović Vukajlović, T.; Martin Rojas, D.A.; Suklje, K.; Sternad Lemut, M.; Mozetič Vodopivec, B.; Butinar, L.; Antalick, G. A Unique Mixture of Monoterpenes and Volatile Phenols Characterises Zelen Wine’s Aromatic Profile. OENO One 2025, 59. [Google Scholar] [CrossRef]
- Arcari, S.G.; Caliari, V.; Sganzerla, M.; Godoy, H.T. Volatile Composition of Merlot Red Wine and Its Contribution to the Aroma: Optimization and Validation of Analytical Method. Talanta 2017, 174, 752–766. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary Aroma: Influence of Wine Microorganisms in Their Aroma Profile. Foods 2020, 10, 51. [Google Scholar] [CrossRef]
- Marais, J. Terpenes in the Aroma of Grapes and Wines: A Review. South Afr. J. Enol. Vitic. 2017, 4, 49–58. [Google Scholar] [CrossRef]
- Rous, C.V.; Snow, R.; Kunkee, R.E. Reduction of higher alcohols by fermentation with a leucine-auxotrophic mutant of wine yeast. J. Inst. Brew. 1983, 89, 274–278. [Google Scholar] [CrossRef]
- Pereira, V.; Cacho, J.; Marques, J.C. Volatile Profile of Madeira Wines Submitted to Traditional Accelerated Ageing. Food Chem. 2014, 162, 122–134. [Google Scholar] [CrossRef]
- Del Fresno, J.M.; Morata, A.; Loira, I.; Escott, C.; Suarez Lepe, J.A. Evolution of the Phenolic Fraction and Aromatic Profile of Red Wines Aged in Oak Barrels. ACS Omega 2020, 5, 2788–2796. [Google Scholar] [CrossRef]
- Garbay, J.; Cameleyre, M.; Le Menn, N.; Riquier, L.; Barbe, J.-C.; Lytra, G. Study of the Fruity Aroma of Red Wines Made from Grape Varieties Potentially Adapted to Climate Change Using a Semi-Preparative HPLC Method. LWT 2024, 204, 116481. [Google Scholar] [CrossRef]
- Sánchez-Palomo, E.; Trujillo, M.; García Ruiz, A.; González Viñas, M.A. Aroma Profile of Malbec Red Wines from La Mancha Region: Chemical and Sensory Characterization. Food Res. Int. 2017, 100, 201–208. [Google Scholar] [CrossRef]
- Gambetta, J.M.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. Factors Influencing the Aroma Composition of Chardonnay Wines. J. Agric. Food Chem. 2014, 62, 6512–6534. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.-T.; Wen, Y.; Tao, Y.-S.; Lan, Y.-Y. Modulating the Formation of Meili Wine Aroma by Prefermentative Freezing Process. J. Agric. Food Chem. 2013, 61, 1542–1553. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.L.; Pan, Q.H.; Tang, X.J.; Yang, Q.; Jiang, R.; Shi, Y.; Duan, C.Q. Characteristic Aroma Compounds in Two New Vitis vinifera Cultivars (Table Grapes) and Impact of Vintage and Greenhouse Cultivation. South Afr. J. Enol. Vitic. 2016, 35, 264–277. [Google Scholar] [CrossRef]
- Tufariello, M.; Fragasso, M.; Pico, J.; Panighel, A.; Castellarin, S.D.; Flamini, R.; Grieco, F. Influence of Non-Saccharomyces on Wine Chemistry: A Focus on Aroma-Related Compounds. Molecules 2021, 26, 644. [Google Scholar] [CrossRef]
- Ferreira, A.C.S.; de Pinho, P.G.U. Analytical Method for Determination of Some Aroma Compounds on White Wines by Solid Phase Microextraction and Gas Chromatography. J. Food Sci. 2003, 68, 2817–2820. [Google Scholar] [CrossRef]
- Cutzach, I.; Chatonnet, P.; Dubourdieu, D. Study on Sweet Natural Non Muscat Wine Aroma 1st Part: Qualitative Analysis of Sweet Natural Wines Aroma Found during Ageing. OENO One 1998, 32, 99. [Google Scholar] [CrossRef]
- Câmara, J.S.; Alves, M.A.; Marques, J.C. Changes in Volatile Composition of Madeira Wines during Their Oxidative Ageing. Anal. Chim. Acta 2006, 563, 188–197. [Google Scholar] [CrossRef]
- McCormick, A. Characterisation of Vanilla Extracts Based on Sensory Properties and Chemical Composition. Ph.D. Thesis, Massey University, Palmerston North, New Zealand, 2015. Available online: https://mro.massey.ac.nz/bitstream/10179/15120/2/McCormickPhDThesis.pdf (accessed on 24 July 2025).
- Jackson, R.S. Wine Science: Principles, Practice, Perception, 2nd ed.; Food science and technology international series; Academic Press: San Diego, CA, USA, 2000; ISBN 978-0-08-048986-5. [Google Scholar]
- Baker, A.K.; Ross, C.F. Wine Finish in Red Wine: The Effect of Ethanol and Tannin Concentration. Food Qual. Prefer. 2014, 38, 65–74. [Google Scholar] [CrossRef]
- Romano, G.; Taurino, M.; Gerardi, C.; Tufariello, M.; Lenucci, M.; Grieco, F. Yeast Starter Culture Identification to Produce of Red Wines with Enhanced Antioxidant Content. Foods 2024, 13, 312. [Google Scholar] [CrossRef]
- Monagas, M.; Bartolomé, B. Anthocyanins and Anthocyanin-Derived Compounds. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009. [Google Scholar] [CrossRef]
- Moio, L.; Etievant, P.X. Ethyl Anthranilate, Ethyl Cinnamate, 2,3-Dihydrocinnamate, and Methyl Anthranilate: Four Important Odorants Identified in Pinot noir Wines of Burgundy. Am. J. Enol. Vitic. 1995, 46, 392–398. [Google Scholar] [CrossRef]
- Vilanova, M.; Genisheva, Z.; Graña, M.; Oliveira, J.M. Determination of Odorants in Varietal Wines from International Grape Cultivars (Vitis vinífera) Grown in NW Spain. South Afr. J. Enol. Vitic. 2016, 34. [Google Scholar] [CrossRef]
- Yu, H.; Xie, T.; Xie, J.; Chen, C.; Ai, L.; Tian, H. Aroma Perceptual Interactions of Benzaldehyde, Furfural, and Vanillin and Their Effects on the Descriptor Intensities of Huangjiu. Food Res. Int. 2020, 129, 108808. [Google Scholar] [CrossRef] [PubMed]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and Its Importance to Wine Aroma—A Review. SAJEV 2019, 21, 97–129. [Google Scholar] [CrossRef]
- Bayraktar, V.N. Organic acids concentration in wine stocks after Saccharomyces cerevisiae fermentation. Biotechnol. Acta 2013, 6, 97–106. [Google Scholar] [CrossRef]
- Noguerol-Pato, R.; Sieiro-Sampredro, T.; González-Barreiro, C.; Cancho-Grande, B.; Simal-Gándara, J. Effect on the Aroma Profile of Graciano and Tempranillo Red Wines of the Application of Two Antifungal Treatments onto Vines. Molecules 2014, 19, 12173–12193. [Google Scholar] [CrossRef]
- Jiang, B.; Zhang, Z. Volatile Compounds of Young Wines from Cabernet Sauvignon, Cabernet Gernischet and Chardonnay Varieties Grown in the Loess Plateau Region of China. Molecules 2010, 15, 9184–9196. [Google Scholar] [CrossRef]
- Zhu, J.; Shi, J.; Lu, Y.; Liu, L.; Liu, Y. Application of Strains of Geotrichum Spp. to Decrease Higher Alcohols and to Increase Esters: Application of Geotrichum Spp. Strains to Decrease Higher Alcohols and to Increase Esters. J. Inst. Brew. 2016, 122, 147–155. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Tonizzo, A.; Battistutta, F. Yeast Derivatives (Extracts and Autolysates) in Winemaking: Release of Volatile Compounds and Effects on Wine Aroma Volatility. Food Chem. 2006, 99, 217–230. [Google Scholar] [CrossRef]
- Ferreira, V.; de la Fuente, A.; Sáenz-Navajas, M.P. Wine aroma vectors and sensory attributes. In Managing Wine Quality, 2nd ed.; Reynolds, A.G., Ed.; Woodhead Publishing: Cambridge, UK, 2022; pp. 3–39. [Google Scholar] [CrossRef]
- Administratia Nationala de Meteorologie. Available online: https://www.meteoromania.ro/ (accessed on 15 May 2025).
- Branas, J.; Bernon, G.; Levadoox, L. Élements de Viticulture Genéralé. 1946. Available online: https://www.mikeparkbooks.com/product/37111/Elements-de-Viticulture-Generale (accessed on 15 May 2025).
- Constantinescu, G.; Donaud, A.; Dragomir, E. Determination of the Value of the Vine Bioclimatic Index for the Main Vineyards of the R.P. Roumanie. Rev. Roum. Biol. Série Bot. 1964, 9, 35–40. [Google Scholar]
- Teodorescu, S.; Popa, A.; Sandu, G. Oenoclimatul României; Editura Ştiinţifică şi Enciclopedică: Bucharest, Romania, 1987. [Google Scholar]
- Popescu (Stegarus), D.I.; Botoran, O.R.; Ionete, R.E.; Sandru, D.; Sutan, N.A.; Niculescu, V.-C. Highlighting the Terroir Influence on the Aromatic Profile of Two Romanian White Wines. Appl. Sci. 2023, 14, 19. [Google Scholar] [CrossRef]
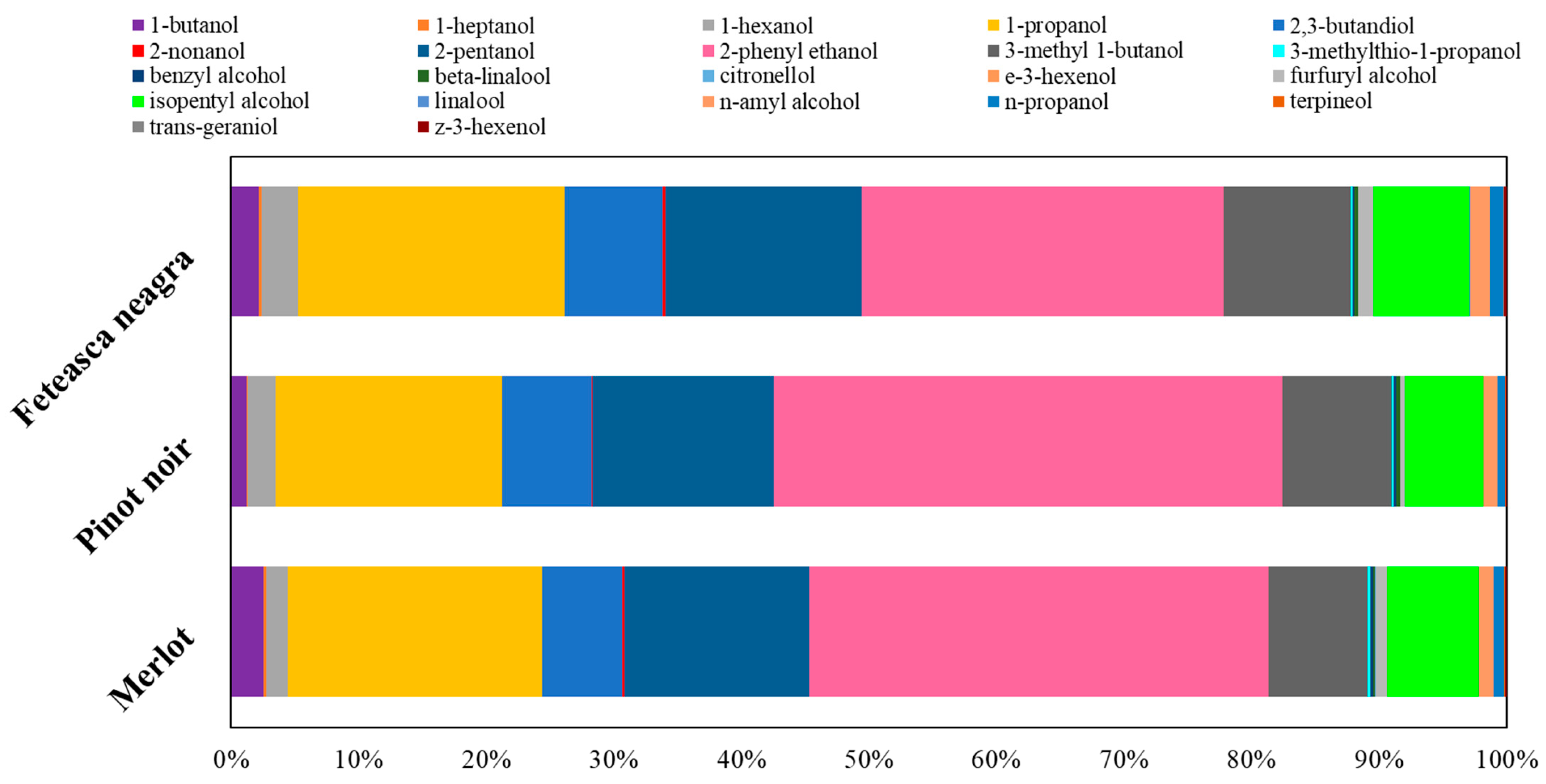
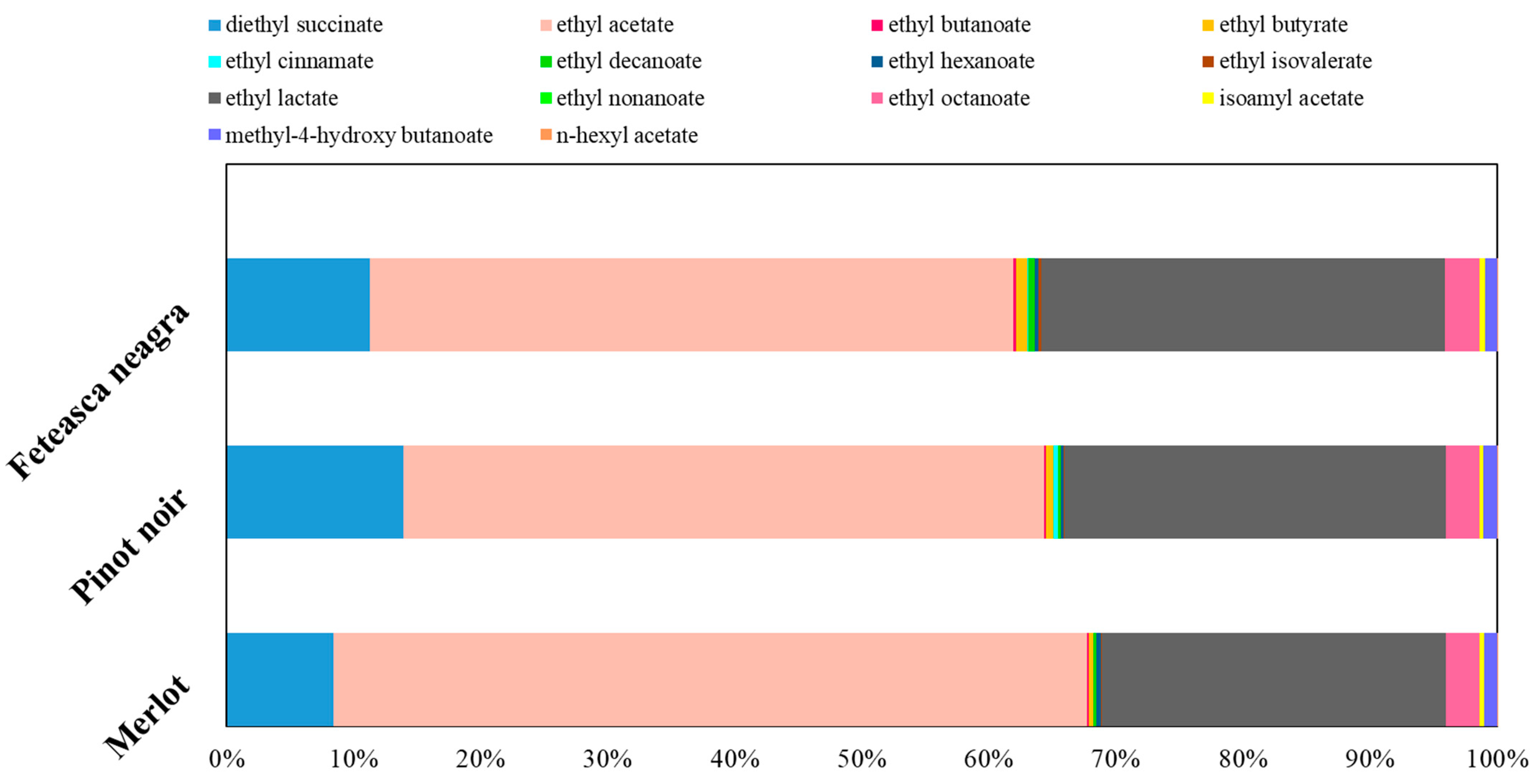
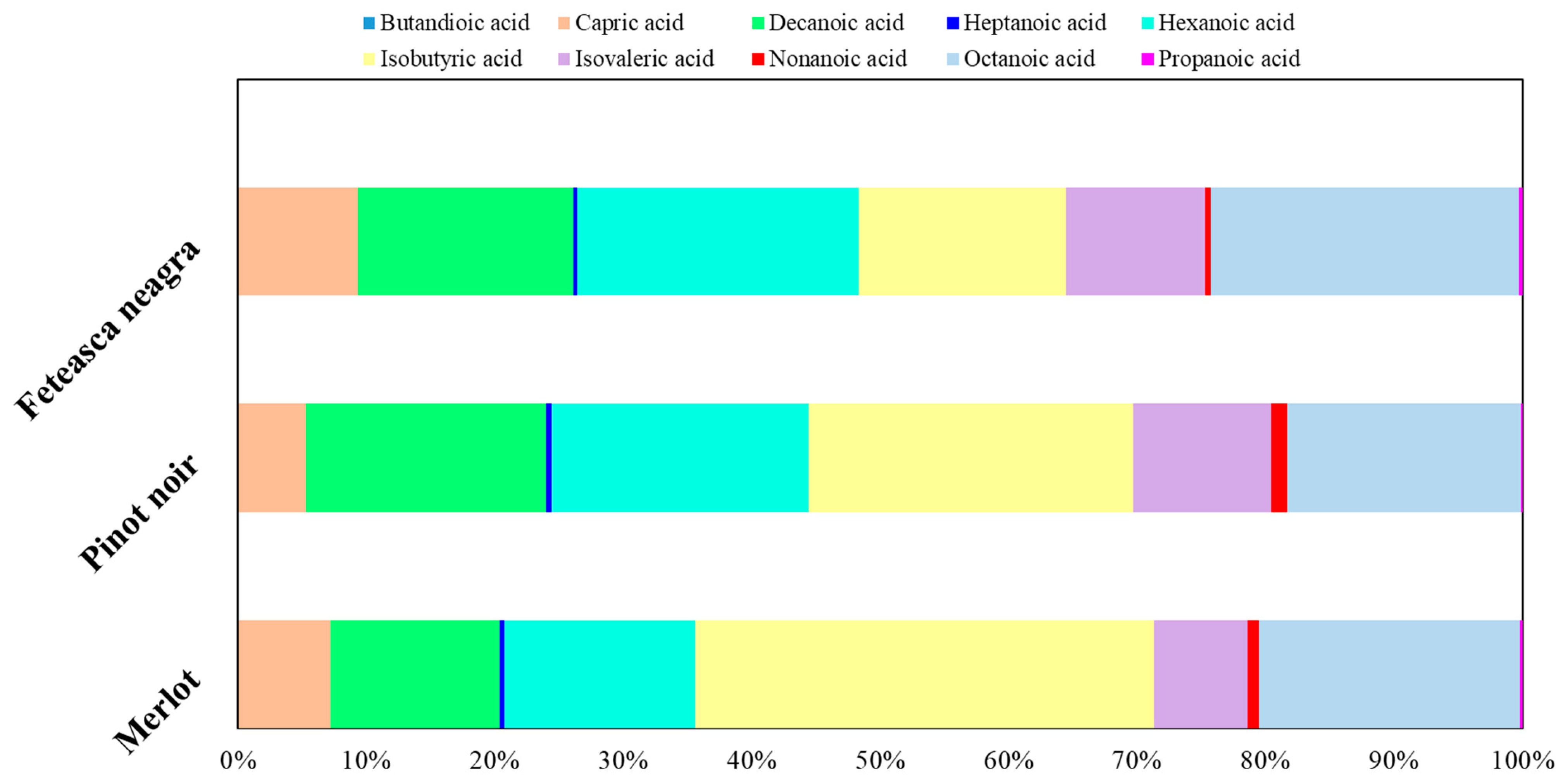
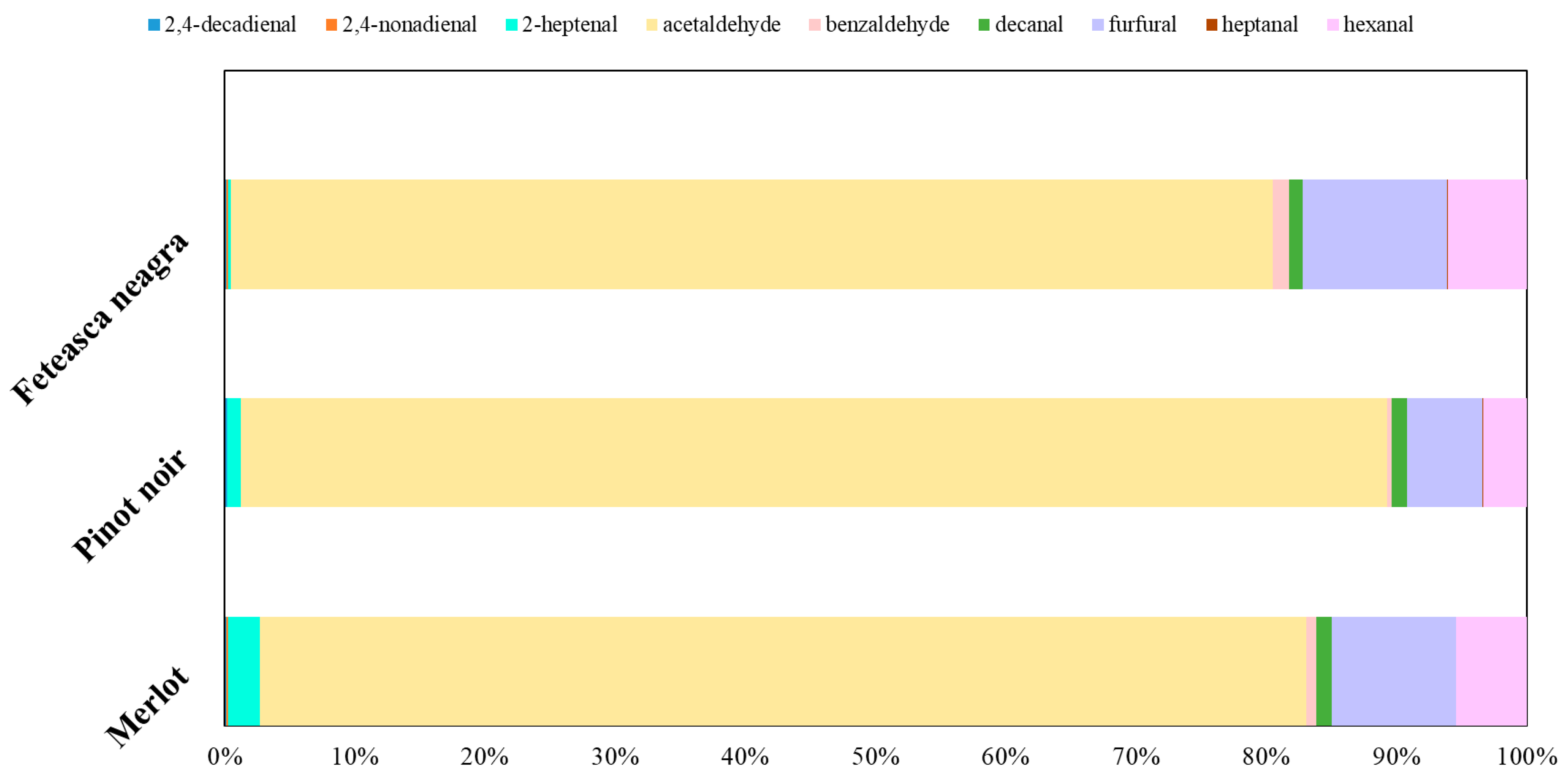

| Indicators | Mean Value of Indicators | ||||
|---|---|---|---|---|---|
| 1950–1963 | 1964–1978 | 1979–1993 | 1994–2008 | 2009–2023 | |
| Global radiation (MJ/m2/day) | 20.56 | 21.01 | 21.09 | 22.19 | 22.24 |
| Effective temperature sum | 1533.32 | 1547.88 | 1539.76 | 1602.33 | 1612.47 |
| Real heliothermal index | 1.79 | 1.83 | 1.88 | 1.89 | 2.01 |
| bioclimatic index | 7.42 | 7.48 | 8.02 | 8.93 | 9.12 |
| Hydro-thermal coefficient of Selyaninov | 1.27 | 1.78 | 1.77 | 1.43 | 1.32 |
| De Martonne aridity index | 17.43 | 15.38 | 17.17 | 18.47 | 19.96 |
| Oenoclimatic aptitude index | 29.22 | 28.36 | 28.91 | 30.03 | 32.67 |
| Parameters | Wine | ||
|---|---|---|---|
| Merlot | Pinot Noir | Feteasca Neagra | |
| Total polyphenols (mg GAE/L) | 2328.34 ± 1.18 | 2539.19 ± 1.07 | 2814.22 ± 1.21 |
| Total anthocyanins (mg M3GE/L) | 423.32 ± 0.73 | 477.77 ± 0.84 | 526.33 ± 0.72 |
| DPPH (%) | 68 | 75 | 84 |
| IC50 (µg/mL) | 228.12 | 196.45 | 115.32 |
| Antioxidant activity (mmol TE/L) | 7.47 | 9.32 | 12.92 |
| TEAC (mmol TE/L) | 10.18 ± 0.51 | 12.02 ± 0.29 | 13.62 ± 1.16 |
| FRAP (mmol TE/L) | 10.97 ± 0.83 | 12.39 ± 0.47 | 13.45 ± 1.22 |
| Class | Compound | Aroma Descriptor | Odor Series * | Reference |
|---|---|---|---|---|
| Higher alcohols | 1-Butanol | Intoxicating aroma, alcoholic odor | 1 | [83] |
| 1-Heptanol | Bouquet plant odor, grape odor | 6 | [83] | |
| 1-Hexanol | Leafy and fruity notes | 4 | [83] | |
| 1-Propanol | Bouquet, ripe fruity notes | 6 | [83] | |
| 2,3-Butanediol | – | - | - | |
| 2-Nonanol | Strong fruity notes, rose odor | 2 | [83] | |
| 2-Pentanol | – | - | - | |
| 2-Phenylethanol | Rose aroma | 2 | [84] | |
| 3-Methyl-1-butanol | Cheese odor | 5 | [83] | |
| 3-(Methylthio)-1-propanol | Meaty or onion-like aromas | 8 | [82] | |
| Benzyl alcohol | Candy, fruity | 3 | [85] | |
| β-Linalool | Floral and citrus-like aromas | 2 | [91] | |
| Citronellol | Floral | 2 | [87] | |
| (E)-3-Hexen-1-ol | Freshly cut grass | 8 | [88] | |
| Furfuryl alcohol | Burned | 9 | [89] | |
| Isoamyl alcohol | Fruit aroma | 6 | [90] | |
| Linalool | Muscat | 2 | [91] | |
| 1-Pentanol (n-Amyl alcohol) | Floral | 2 | [2] | |
| n-Propanol | Ripe fruit | 6 | [93] | |
| α-Terpineol | Floral, candy | 2 | [85] | |
| (E)-Geraniol (Trans-Geraniol) | Muscat | 2 | [91] | |
| (Z)-3-Hexen-1-ol | Fresh, fruity | 6 | [95] | |
| Esters | 2-Phenylethyl acetate | Floral | 2 | [96] |
| Diethyl succinate | Caramel, fruity | 3 | [97,98] | |
| Ethyl acetate | Fruity odor, ester odor | 6 | [83] | |
| Ethyl butanoate | Fruity, strawberry | 6 | [95] | |
| Ethyl butyrate | Fruity | 6 | [87] | |
| Ethyl cinnamate | Honey, cinnamon, floral | 3 | [97] | |
| Ethyl decanoate | Caramel, fruity | 3 | [87] | |
| Ethyl hexanoate | Green apple aroma, fruity odor | 4 | [83] | |
| Ethyl isovalerate | Fruity, apple | 6 | [93] | |
| Ethyl lactate | Buttery or subtle fruity aroma | 6 | [93] | |
| Ethyl nonanoate | Fruity, floral | 6 | [85] | |
| Ethyl octanoate | Fruity odor, caramel | 6 | [83] | |
| Isoamyl acetate | Banana and pear-like notes | 6 | [93,97] | |
| Methyl 4-hydroxybutanoate | Strawberries, bananas | 6 | [82] | |
| n-Hexyl acetate | Pleasant fruity, green apple odor | 6 | [83] | |
| Volatile fatty acids | Butanedioic acid | – | [18] | |
| Capric acid | Rancid fat | 5 | [114] | |
| Decanoic acid | Rancid fat | 5 | [87] | |
| Heptanoic acid | Fatty, dry | 5 | [115] | |
| Hexanoic acid | Copra oil, sweat | 5 | [83] | |
| Isobutyric acid | Rancid, butter, cheese | 5 | [87] | |
| Isovaleric acid | Acidic, rancid | 5 | [87] | |
| Nonanoic acid | Fruity, fatty, joyful | 6 | [116] | |
| Octanoic acid | Light fruity acid odor | 6 | [83] | |
| Propanoic acid | Cheese | 5 | [117] | |
| Aldehydes and ketones | 2,4-Decadienal | Fatty and citrus-like aromas | 4 | [101] |
| 2,4-Nonadienal | Fatty and citrus-like aromas | 4 | [99] | |
| 2-Heptenal | Fatty and green odor | - | [100] | |
| Acetaldehyde | Pungent, ripe apple | 6 | [87] | |
| Benzaldehyde | Almonds | 3 | [85] | |
| Decanal | Grassy, orange skin-like | 8 | [115] | |
| Furfural | Burned | 9 | [89] | |
| Heptanal | – | - | - | |
| Hexanal | Herbaceous | 4 | [99] | |
| Lactones | γ-Butyrolactone | Plum aroma | 6 | [102] |
| Crotonolactone | – | - | - | |
| δ-Decalactone | Fruity, sweet, floral | 2 | [103] | |
| γ-Nonalactone | Coconut, peach | 6 | [93] | |
| Isovanillin | Sweet, vanilla, and slightly smoky | 2 | [104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Popescu, D.I.; Wilhelmine, C.N.; Tița, O.; Niculescu, V.-C.; Ionescu, N.A. Antioxidant Potential and Volatile Aroma Profiling of Red Wines from the Tarnave Vineyard. Molecules 2025, 30, 3853. https://doi.org/10.3390/molecules30193853
Popescu DI, Wilhelmine CN, Tița O, Niculescu V-C, Ionescu NA. Antioxidant Potential and Volatile Aroma Profiling of Red Wines from the Tarnave Vineyard. Molecules. 2025; 30(19):3853. https://doi.org/10.3390/molecules30193853
Chicago/Turabian StylePopescu (Stegarus), Diana Ionela, Claudia Nicoleta Wilhelmine, Ovidiu Tița, Violeta-Carolina Niculescu, and Nicoleta Anca Ionescu (Șuțan). 2025. "Antioxidant Potential and Volatile Aroma Profiling of Red Wines from the Tarnave Vineyard" Molecules 30, no. 19: 3853. https://doi.org/10.3390/molecules30193853
APA StylePopescu, D. I., Wilhelmine, C. N., Tița, O., Niculescu, V.-C., & Ionescu, N. A. (2025). Antioxidant Potential and Volatile Aroma Profiling of Red Wines from the Tarnave Vineyard. Molecules, 30(19), 3853. https://doi.org/10.3390/molecules30193853








