The Use of Non-Conventional Yeast in Sake Production
Abstract
1. Introduction
2. Results and Discussion
2.1. Alcoholic Fermentation Kinetics and Basic Chemical Composition of Sake
2.2. Ethyl Carbamate and Its Precursors
2.3. Aroma of Sake
2.4. Color of Sake
2.5. Sensory Analysis
3. Materials and Methods
3.1. Raw Materials and Microorganisms
3.2. Sake Preparation
3.3. Basic Chemical Composition of Sake
3.4. Gas Chromatography GC/MS
3.5. Urea and L-Arginine
3.6. Antiradical Activity Against ABTS Radical
3.7. Color Determination Using the CIE Lab Model
3.8. Sensory Evaluation
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yoshizawa, K. Sake: Production and flavour. Food Rev. Int. 1999, 15, 83–107. [Google Scholar] [CrossRef]
- Fleet, G.H. Yeast interactions and wine flavour. Int. J. Food Microbiol. 2003, 86, 11–22. [Google Scholar] [CrossRef]
- Takemitsu, H.; Amako, M.; Sako, Y.; Shibakusa, K.; Kita, K.; Kitamura, S.; Inui, H. Analysis of Volatile Odor Components of Superheated Steam-Cooked Rice with a Less Stale Flavor. Food Sci. Technol. Res. 2016, 22, 771–778. [Google Scholar] [CrossRef]
- Yoshizaki, Y.; Furusawa, H.; Okutsu, K.; Hashizume, K.; Tanaka, T.; Hoshino, T.; Iwashita, K. Analysis of volatile compounds in shochu koji, sake koji, and steamed rice by GC–MS. J. Inst. Brew. 2010, 116, 49–55. [Google Scholar] [CrossRef]
- Verma, D.K.; Srivastav, P.P. Introduction to Rice Aroma, Flavor, and Fragrance. In Science and Technology of Aroma, Flavor, and Fragrance in Rice; Verma, D.K., Srivastav, P.P., Eds.; Apple Academic Press: Oakville, ON, Canada; Waretown, NJ, USA, 2018; pp. 3–34. [Google Scholar]
- Zhang, K.; Wu, W.; Yan, Q. Research advances on sake rice, koji, and sake yeast: A review. Food Sci. Nutr. 2020, 8, 2995–3003. [Google Scholar] [CrossRef]
- Katou, T.; Namise, M.; Kitagaki, H.; Akao, T.; Shimoi, H. QTL mapping of sake brewing characteristics of yeast. J. Biosci. Bioeng. 2009, 107, 383–393. [Google Scholar] [CrossRef][Green Version]
- Kitagaki, H.; Kitamoto, K. Breeding research on sake yeasts in Japan: History, recent technological advances, and future perspectives. Annu. Rev. Food Sci. Technol. 2013, 4, 215–235. [Google Scholar] [CrossRef]
- Takao, Y.; Takahashi, T.; Yamada, T.; Goshima, T.; Isogai, A.; Sueno, K.; Fujii, T.; Akao, T. Characteristic features of the unique house sake yeast strain Saccharomyces cerevisiae Km67 used for industrial sake brewing. J. Biosci. Bioeng. 2018, 126, 617–623. [Google Scholar] [CrossRef]
- Yamasaki, R.; Goshima, T.; Oba, K.; Kanai, M.; Ohdoi, R.; Hirata, D.; Akao, T. Development of sake yeast haploid set with diverse brewing properties using sake yeast strain Hiroshima no. 6 exhibiting sexual reproduction. J. Biosci. Bioeng. 2020, 129, 706–714. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H. Discrimination for sake brewing methods by compound-specific isotope analysis and formation mechanism of organic acids in sake. Food Chem. 2022, 381, 132295. [Google Scholar] [CrossRef]
- Baba, S.; Hamasaki, T.; Sawada, K.; Orita, R.; Nagano, Y.; Kimura, K.; Goto, M.; Kobayashi, G. Breeding sake yeast and identification of mutation patterns by synchrotron light irradiation. J. Biosci. Bioeng. 2021, 132, 265–270. [Google Scholar] [CrossRef]
- Takahashi, T.; Ohara, Y.; Sawatari, M.; Sueno, K. Isolation and characterization of sake yeast mutants with enhanced isoamyl acetate productivity. J. Biosci. Bioeng. 2017, 123, 71–77. [Google Scholar] [CrossRef]
- Takahashi, T.; Ohara, Y.; Sueno, K. Breeding of a sake yeast mutant with enhanced ethyl caproate productivity in sake brewing using rice milled at a high polishing ratio. J. Biosci. Bioeng. 2017, 123, 707–713. [Google Scholar] [CrossRef]
- Ikeda, Y.; Isogai, A.; Moriyoshi, Y.; Kanda, R.; Iwashita, K.; Fujii, T. Construction of sake yeast with low production of dimethyl trisulfide precursor by a self-cloning method. J. Biosci. Bioeng. 2018, 125, 419–424. [Google Scholar] [CrossRef]
- Fukuda, K.; Watanabe, M.; Asano, K.; Ouchi, K.; Takasawa, S. Isolation and genetic study of p-fluoro-DL-phenylalanine-resistant mutants overproducing β-phenethyl-alcohol in Saccharomyces cerevisiae. Curr. Genet. 1991, 20, 449–452. [Google Scholar] [CrossRef]
- Fukuda, K.; Watanabe, M.; Asano, K.; Ueda, H.; Ohta, S. Breeding of brewing yeast producing a large amount of β-phenylethyl alcohol and β-phenylethyl acetate. Agric. Biol. Chem. 1990, 54, 269–271. [Google Scholar] [CrossRef]
- Ouchi, K.; Akiyama, H. Non-foaming mutants of sake yeasts selection by cell agglutination method and by froth flotation method. Agric. Biol. Chem. 1971, 35, 1024–1032. [Google Scholar] [CrossRef][Green Version]
- Shimoi, H.; Sakamoto, K.; Okuda, M.; Atthi, R.; Iwashita, K.; Ito, K. The AWA1 gene is required for the foam-forming phenotype and cell surface hydrophobicity of sake yeast. Appl. Environ. Microbiol. 2002, 68, 2018–2025. [Google Scholar] [CrossRef]
- Kuribayashi, T.; Sato, K.; Joh, T.; Kaneoke, M. Breeding of a non-urea-producing sake yeast carrying a FAS2 mutation. Mycoscience 2017, 58, 302–306. [Google Scholar]
- Hara, M.; Sasaki, M.; Obata, T.; Nojiro, K. Isolation of an ethanol-resistant strain from sake yeast Kyokai no. 7. J. Brew. Soc. Jpn. 1976, 71, 301–304. [Google Scholar]
- Aikawa, M.; Suizu, T.; Ichikawa, E.; Kawato, A.; Abe, Y.; Imayasu, S. Breeding of higher malic acid–productive mutants from Saccharomyces cerevisiae Kyokai no. 7 (K-7). Hakkokogaku Kaishi 1992, 70, 473–477. [Google Scholar] [CrossRef]
- Asano, T.; Kurose, N.; Tarumi, S. Isolation of high-malate-producing sake yeasts from low-maltose-assimilating mutants. J. Biosci. Bioeng. 2001, 92, 429–433. [Google Scholar] [CrossRef]
- Kitagaki, H.; Kato, T.; Isogai, A.; Mikami, S.; Shimoi, H. Inhibition of mitochondrial fragmentation during sake brewing causes high malate production in sake yeast. J. Biosci. Bioeng. 2008, 105, 675–678. [Google Scholar] [CrossRef]
- Koseki, T.; Kudo, S.; Matsuda, Y.; Ishigaki, H.; Ajiki, Y.; Muraoka, Y.; Wada, Y. Tyrosol High-Productivity Yeast Mutant and Method for Producing Fermented Alcoholic Beverage Using the Mutant. Jap. Open Pat. Gaz. 2004, 2004, 215644. Available online: https://patents.google.com/patent/JP3898652B2/en (accessed on 10 July 2025).
- Soejima, H.; Tsuge, K.; Yoshimura, T.; Koganemaru, K.; Kitagaki, H. Breeding of a high tyrosol-producing sake yeast by isolation of an ethanol-resistant mutant from trp3 mutant. J. Inst. Brew. 2012, 118, 264–268. [Google Scholar] [CrossRef]
- Yamada, T.; Furukawa, K.; Hara, S.; Mizoguchi, H. Isolation of copper-tolerant mutants of sake yeast with defective peptide uptake. J. Biosci. Bioeng. 2005, 100, 460–465. [Google Scholar] [CrossRef]
- Akao, T.; Yashiro, I.; Hosoyama, A.; Kitagaki, H.; Horikawa, H.; Watanabe, D.; Akada, R.; Ando, Y.; Harashima, S.; Inoue, T.; et al. Whole-genome sequencing of sake yeast Saccharomyces cerevisiae Kyokai no. 7. DNA Res. 2011, 18, 423–434. [Google Scholar] [CrossRef]
- Kanai, M.; Shibata, T.; Zhou, Y.; Hayashi, R.; Fukuba, I.; Kochi, T.; Teramoto, S.; Shimoi, H.; Takahashi, H.; Akao, T. Efficient gene identification via quantitative trait loci analysis by crossbreeding of sake and laboratory yeast. Appl. Microbiol. Biotechnol. 2025, 109, 84. [Google Scholar] [CrossRef]
- Chadani, T.; Ohnuki, S.; Isogai, A.; Goshima, T.; Kashima, M.; Ghanegolmohammadi, F.; Nishi, T.; Hirata, D.; Watanabe, D.; Kitamoto, K.; et al. Genome editing to generate sake yeast strains with eight mutations that confer excellent brewing characteristics. Cells 2021, 10, 1299. [Google Scholar] [CrossRef]
- Hirosawa, I.; Aritomi, K.; Hoshida, H.; Kashiwagi, S.; Nishizawa, Y.; Akada, R. Construction of a self-cloning sake yeast that overexpresses alcohol acetyltransferase gene by a two-step gene replacement protocol. Appl. Microbiol. Biotechnol. 2004, 65, 68–73. [Google Scholar] [CrossRef]
- Nakagawa, T.; Yoshimura, A.; Sawai, Y.; Hisamatsu, K.; Akao, T.; Masaki, K. Japanese sake making using wild yeasts isolated from natural environments. Biosci. Biotechnol. Biochem. 2024, 88, 231–236. [Google Scholar] [CrossRef]
- Varela, C. The impact of non-Saccharomyces yeasts in the production of alcoholic beverages. Appl. Microbiol. Biotechnol. 2016, 100, 9861–9874. [Google Scholar] [CrossRef] [PubMed]
- Benito, S.; Ruiz, J.; Belda, I.; Kiene, F.; Beisert, B.; Navascués, E.; Marquina, D.; Calderón, F.; Santos, A.; Rauhut, D. Application of non-Saccharomyces yeasts in wine production. In Non-conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 75–90. [Google Scholar]
- Sannino, C.; Mezzasoma, A.; Buzzini, P.; Turchetti, B. Non-conventional yeasts for producing alternative beers. In Non-conventional Yeasts: From Basic Research to Application; Sibirny, A., Ed.; Springer Nature: Cham, Switzerland, 2019; pp. 361–388. [Google Scholar]
- Dashko, S.; Zhou, N.; Compagno, C.; Piškur, J. Why, When, and How Did Yeast Evolve Alcoholic Fermentation? Appl. Environ. Microbiol. 2014, 80, 3047–3055. [Google Scholar] [CrossRef]
- Walker, G.M. Yeast Physiology and Biotechnology. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Roca-Mesa, H.; Sendra, S.; Mas, A.; Portillo, M.C. Nitrogen Preferences during Alcoholic Fermentation of Non-Saccharomyces Wine Yeasts. Microorganisms 2020, 8, 1570. [Google Scholar] [CrossRef]
- Crépin, L.; Nidelet, T.; Sanchez, I.; Dequin, S.; Camarasa, C. Sequential Use of Nitrogen Compounds by Saccharomyces cerevisiae during Wine Fermentation: A Model Based on Kinetic and Regulation Characteristics of Nitrogen Permeases. Appl. Environ. Microbiol. 2012, 78, 8102–8111. [Google Scholar] [CrossRef]
- Sahana, T.G.; Ganesan, K.; Venugopal, V.; Rajendran, R.; Xu, J.; Balasubramanian, G. Mechanisms of Ethanol Tolerance in Yeast: A Review. Microb. Cell Fact. 2024, 23, 12. [Google Scholar]
- Riles, L.; Fay, J.C. Genetic Basis of Variation in Saccharomyces cerevisiae Sensitivity to Ethanol Stress. G3 Genes|Genomes|Genetics 2018, 8, 17–27. [Google Scholar]
- Bideaux, C.; Alfenore, S.; Cameleyre, X.; Molina-Jouve, C.; Guillouet, S.E. Short-Term Dynamics of Glycerol Production in Saccharomyces cerevisiae. FEMS Yeast Res. 2006, 6, 147–159. [Google Scholar]
- Navarrete, C.; Treu, L.; Campanaro, S.; da Silva, L.V.; Quirós, M.; Lopandić, K.; Ciani, M.; Pretorius, I.S.; Dequin, S.; Morales, P. Metabolic Engineering of Glycerol Production in Saccharomyces cerevisiae for Industrial Applications. AMB Express 2014, 4, 86. [Google Scholar] [CrossRef] [PubMed]
- de Smidt, O.; du Preez, J.C.; Albertyn, J. The Alcohol Dehydrogenases of Saccharomyces cerevisiae: A Comprehensive Review. FEMS Yeast Res. 2008, 8, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Nakatani, M.; Ohtani, R.; Umezawa, K.; Uchise, T.; Matsuo, Y.; Fukuta, Y.; Obata, E.; Katabuchi, A.; Kizaki, K.; Kitazume, H.; et al. Characterization and application of Lachancea thermotolerans isolates for sake brewing. J. Biosci. Bioeng. 2024, 139, 30–35. [Google Scholar] [CrossRef]
- Yamaguchi, S. Basic properties of umami and effects on humans. Physiol. Behav. 1991, 49, 833–841. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, C.V.; Olsen, K.; Mouritsen, O.G. Umami potential of fermented beverages: Sake, wine, champagne, and beer. Food Chem. 2021, 360, 128971. [Google Scholar] [CrossRef]
- Okada, K.; Gogami, Y.; Oikawa, T. Principal component analysis of the relationship between the D-amino acid concentrations and the taste of the sake. Amino Acids 2013, 44, 489–498. [Google Scholar] [CrossRef]
- Iwano, K.; Takahashi, K.; Ito, T.; Nakazawa, N. Search for amino acids affecting the taste of Japanese sake. J. Brew. Soc. Jpn. 2004, 99, 659–664. [Google Scholar] [CrossRef]
- El Hosry, L.; Elias, V.; Chamoun, V.; Halawi, M.; Cayot, P.; Nehme, A.; Bou-Maroun, E. Maillard reaction: Mechanism, influencing parameters, advantages, disadvantages, and food industrial applications—A review. Foods 2025, 14, 1881. [Google Scholar] [CrossRef]
- Boerzhijin, S.; Isogai, A.; Mukai, N. Impact of storage conditions on the volatile aroma compounds of aged sake. J. Food Compos. Anal. 2023, 121, 105351. [Google Scholar] [CrossRef]
- Isogai, A.; Utsunomiya, H.; Kanda, R.; Iwata, H. Changes in the aroma compounds of sake during aging. J. Agric. Food Chem. 2005, 53, 4118–4123. [Google Scholar] [CrossRef]
- Swiegers, J.; Pretorius, I. Modulation of volatile sulfur compounds by wine yeast. Appl. Microbiol. Biotechnol. 2007, 74, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Mendoza-Cózatl, D.; Loza-Tavera, H.; Hernández-Navarro, A.; Moreno-Sánchez, R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005, 29, 653–671. [Google Scholar] [CrossRef] [PubMed]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial contribution to wine aroma and its intended use for wine quality improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef] [PubMed]
- Ogata, K. Hydrogen sulphide production by bottom-fermenting yeast is related to nitrogen starvation signalling. J. Inst. Brew. 2013, 119, 62–68. [Google Scholar] [CrossRef]
- Zhang, H.; Cui, Y. Study of sulphides production affecting the fermentation process of lager beer. Int. J. Biol. Life Sci. 2023, 3, 4–8. [Google Scholar] [CrossRef]
- Andorrà, I.; Martín, L.; Nart, E.; Puxeu, M.; Hidalgo, C.; Ferrer-Gallego, R. Effect of grape juice composition and nutrient supplementation on the production of sulfur dioxide and carboxylic compounds by Saccharomyces cerevisiae. Aust. J. Grape Wine Res. 2018, 24, 260–266. [Google Scholar] [CrossRef]
- Wells, A.; Osborne, J.P. Production of SO2 binding compounds and SO2 by Saccharomyces during alcoholic fermentation and the impact on malolactic fermentation. S. Afr. J. Enol. Vitic. 2011, 32, 204–213. [Google Scholar] [CrossRef][Green Version]
- Regulation (EU) No 2021/2117 of the European Parliament and of the Council of 2 December 2021 amending Regulations (EU) No 1308/2013, (EU) No 1151/2012, (EU) No 251/2014 and (EU) No 228/2013. Off. J. Eur. Union 2021, L 430, 1–23. Available online: https://eur-lex.europa.eu/eli/reg/2021/2117/oj/eng (accessed on 10 July 2025).
- Regulation (EU) No 1308/2013 of the European Parliament and of the Council of 17 December 2013 establishing a common organisation of the markets in agricultural products. Off. J. Eur. Union 2013, L 347, 671–854. Available online: https://eur-lex.europa.eu/eli/reg/2013/1308/oj/eng (accessed on 11 July 2025).
- Abe, Y.; Saito, T.; Okamoto, T.; Sasaki, T.; Hoshi, Y.; Sugie, M.; Hagiwara, S.; Shiga, T. Investigation of changes in antioxidant activity during sake brewing by the ORAC method. J. Brew. Soc. Jpn. 2012, 107, 693–698, (In Japanese with English abstract, figures and tables). [Google Scholar] [CrossRef]
- Kitagaki, H.; Tsugawa, M. 1,1-Diphenyl-2-picrylhydrazyl radical (DPPH) scavenging ability of sake during storage. J. Biosci. Bioeng. 1999, 87, 328–332. [Google Scholar] [CrossRef]
- Tsuji, A.; Koyanagi, T. Significant contribution of amino acids to antioxidant capacity of Japanese rice wine (sake). J. Sci. Food Agric. 2025, 105, 227–234. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol Consumption and Ethyl Carbamate. In IARC Monographs on the Evaluation of Carcinogenic Risks to Humans; International Agency for Research on Cancer: Lyon, France, 2010; Volume 96, ISBN 978-92-832-1296-6. Available online: https://publications.iarc.who.int/Book-And-Report-Series/Iarc-Monographs-On-The-Identification-Of-Carcinogenic-Hazards-To-Humans/Alcohol-Consumption-And-Ethyl-Carbamate-2010 (accessed on 1 July 2025).
- Pflaum, T.; Hausler, T.; Baumung, C.; Ackermann, S.; Kuballa, T.; Rehm, J.; Lachenmeier, D.W. Carcinogenic compounds in alcoholic beverages: An update. Arch. Toxicol. 2016, 90, 2349–2367. [Google Scholar] [CrossRef]
- Ough, C.S.; Crowell, E.A.; Gutlove, B.R. Carbamyl compound reactions with ethanol. Am. J. Enol. Vitic. 1988, 39, 239–242. [Google Scholar] [CrossRef]
- Abt, E.; Incorvati, V.; Posnick, R.L.; Redan, B.W. Occurrence of ethyl carbamate in foods and beverages: Review of the formation mechanisms, advances in analytical methods, and mitigation strategies. J. Food Prot. 2021, 84, 2195–2212. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.G.; Lyu, J.; Kim, M.K.; Lee, K.-G. Effect of citrulline, urea, ethanol, and urease on the formation of ethyl carbamate in soybean paste model system. Food Chem. 2015, 189, 74–79. [Google Scholar] [CrossRef]
- Wang, P.; Sun, J.; Li, X.; Wu, D.; Li, T.; Lu, J.; Chen, J.; Xie, G. Contribution of citrulline to the formation of ethyl carbamate during Chinese rice wine production. Food Addit. Contam. A 2014, 31, 587–592. [Google Scholar]
- Stewart, G.G. Saccharomyces species in the production of beer. Beverages 2016, 2, 34. [Google Scholar] [CrossRef]
- Fu, M.-L.; Liu, J.; Chen, Q.-H.; Liu, X.-J.; He, G.-Q.; Chen, J.-C. Determination of Ethyl Carbamate in Chinese Yellow Rice Wine Using High-Performance Liquid Chromatography with Fluorescence Detection. Int. J. Food Sci. Technol. 2010, 45, 1297–1302. [Google Scholar]
- Wu, Q.; Cui, K.; Lin, J.; Zhu, Y.; Xu, Y. Urea production by yeasts other than Saccharomyces in food fermentation. FEMS Yeast Res. 2017, 17. [Google Scholar] [CrossRef] [PubMed]
- Benito, Á.; Jeffares, D.; Palomero, F.; Calderón, F.; Bai, F.-Y.; Bähler, J.; Benito, S. Selected Schizosaccharomyces pombe strains have characteristics that are beneficial for winemaking. PLoS ONE 2016, 11, e0151102. [Google Scholar] [CrossRef]
- Larcher, R.; Moser, S.; Menolli, A.U.; Tonidandel, L.; Nicolini, G. Ethyl carbamate formation in sub-optimal wine storage conditions and influence of the yeast starter. J. Int. Sci. Vigne Vin 2013, 47, 65–68. [Google Scholar] [CrossRef]
- Fang, F.; Qiu, Y.; Du, G.; Chen, J. Evaluation of ethyl carbamate formation in Luzhou-flavor spirit during distillation and storage processes. Food Biosci. 2018, 23, 137–141. [Google Scholar] [CrossRef]
- Leça, J.M.; Pereira, V.; Miranda, A.; Vilchez, J.L.; Marques, J.C. New insights into ethyl carbamate occurrence in fortified wines. LWT-Food Sci. Technol. 2021, 150, 111566. [Google Scholar] [CrossRef]
- Liu, X.; Qian, M.; Dong, H.; Bai, W.; Zhao, W.; Li, X.; Liu, G. Effect of ageing process on carcinogen ethyl carbamate (EC), its main precursors and aroma compound variation in Hakka Huangjiu produced in southern China. Int. J. Food Sci. Technol. 2020, 55, 1773–1780. [Google Scholar] [CrossRef]
- Ministry of Health, Labour and Welfare (Japan). Food Sanitation Act; Ministry of Health, Labour and Welfare (Japan): Tokyo, Japan, 2020; Available online: https://www.japaneselawtranslation.go.jp/en/laws/view/3687/en (accessed on 10 July 2025).
- Ministry of Agriculture, Forestry and Fisheries (Japan). Japanese Agricultural Standards (JAS); Ministry of Agriculture, Forestry and Fisheries (Japan): Tokyo, Japan, 2022; Available online: https://www.maff.go.jp/e/policies/standard/jas/index.html (accessed on 10 July 2025).
- National Tax Agency (Japan). Standards for Sake Production and Labeling (Notification No. 8 of 1989); National Tax Agency (Japan): Tokyo, Japan, 1989. [Google Scholar]
- Health Canada. Health Canada’s Maximum Levels for Chemical Contaminants in Foods; Health Canada: Ottawa, ON, Canada, 2020; Available online: https://www.canada.ca/en/health-canada/services/food-nutrition/food-safety/chemical-contaminants/maximum-levels-chemical-contaminants-foods.html (accessed on 10 July 2025).
- Heller, L. Canada Approves GM Yeast That Combats Cancer Compound. Available online: https://www.beveragedaily.com/Article/2006/10/06/Canada-approves-GM-yeast-that-combats-cancer-compound/ (accessed on 10 July 2025).
- Coulon, J.; Husnik, J.I.; Inglis, D.L.; van der Merwe, G.K.; Lonvaud, A.; Erasmus, D.J.; van Vuuren, H.J.J. Metabolic engineering of Saccharomyces cerevisiae to minimize the production of ethyl carbamate in wine. Am. J. Enol. Vitic. 2006, 57, 113–124. [Google Scholar] [CrossRef]
- OIV. International Code of Oenological Practices; OIV Code Sheet–Issue 2022/01; OIV: Paris, France, 2022; ISBN 978-2-85038-059-4. Available online: www.oiv.int/public/medias/8630/code-2022-en.pdf (accessed on 10 July 2025).
- Huang, D.; Zhong, Y.; Liu, Y.L.; Song, Y.Y.; Zhao, X.X.; Qin, Y. Reducing higher alcohols by integrating indigenous Saccharomyces cerevisiae, nitrogen compensation, and chaptalization methods during fermentation of kiwifruit wine. LWT-Food Sci. Technol. 2023, 184, 115059. [Google Scholar] [CrossRef]
- Fikselová, K.; Mátéová, K.; Špánik, I. Effect of indigenous S. cerevisiae strains on higher alcohols, volatile acids and esters in wine. Czech J. Food Sci. 2017, 35, 131–142. [Google Scholar] [CrossRef]
- Belda, I.; Navascués, E.; Marquina, D.; Santos, A.; Calderón, F.; Benito, S. Dynamic analysis of physiological properties of Torulaspora delbrueckii in wine fermentations and its incidence on wine quality. Appl. Microbiol. Biotechnol. 2015, 99, 1911–1922. [Google Scholar] [CrossRef]
- Azzolini, M.; Tosi, E.; Lorenzini, M.; Finato, F.; Zapparoli, G. Contribution to the aroma of white wines by controlled Torulaspora delbrueckii cultures in association with Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2015, 31, 277–293. [Google Scholar] [CrossRef]
- Chen, L.; Ren, X.; Wang, Y.; Hao, D.; Liang, Y.; Qin, Y. Transcriptomic identification of core regulatory genes for higher alcohol production in Saccharomyces cerevisiae at different sugar concentrations in wine fermentation. Foods 2025, 14, 1479. [Google Scholar] [CrossRef]
- Yang, H.; Wang, X.; Liu, P.; Niu, H.; Wang, L.; Wang, Z. Research progress of wine aroma components: A critical review. Food Chem. 2023, 402, 134491. [Google Scholar]
- Lambrechts, M.G.; Pretorius, I.S. Yeast and its importance to wine aroma—A review. S. Afr. J. Enol. Vitic. 2000, 21, 97–129. [Google Scholar] [CrossRef]
- Swiegers, J.H.; Pretorius, I.S. Yeast modulation of wine flavor. Adv. Appl. Microbiol. 2005, 57, 131–175. [Google Scholar]
- Saerens, S.M.G.; Delvaux, F.R.; Verstrepen, K.J.; Thevelein, J.M. Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- Mimura, N.; Isogai, A.; Iwashita, K.; Bamba, T.; Fukusaki, E. Gas chromatography/mass spectrometry based component profiling and quality prediction for Japanese sake. J. Biosci. Bioeng. 2014, 118, 406–414. [Google Scholar] [CrossRef]
- Lin, C.; de la Cerda García-Caro, R.; Zhang, P.; Carlin, S.; Gottlieb, A.; Petersen, M.; Vrhovsek, U.; Bond, U. Packing a punch: Understanding how flavours are produced in lager fermentations. FEMS Yeast Res. 2021, 21, foab040. [Google Scholar] [CrossRef]
- Puligundla, P.; Smogrovicova, D.; Mok, C.; Obulam, V.S.R. Recent developments in high gravity beer-brewing. Innov. Food Sci. Emerg. Technol. 2020, 64, 102399. [Google Scholar] [CrossRef]
- Satō, S.; Nakamura, K.; Tadenuma, M.; Yoda, M.; Koseki, T.; Hamachi, M. Studies on Changes in Sake Caused by Light and Storage (Part XI). Formation of Kynurenic Acid by Yeast. J. Soc. Brew. Jpn. 1971, 66, 404–410. [Google Scholar] [CrossRef]
- Nishidono, Y.; Misaka, S.; Maejima, Y.; Shimomura, K.; Tanaka, K. Comparative Analysis of Functional Components in Sakekasu (Sake Lees). Funct. Foods Health Dis. 2023, 14, 74–86. [Google Scholar] [CrossRef]
- Nose, A.; Hamasaki, T.; Hojo, M. Effect of Humic Acid in Water on the Coloring of Japanese Sakes. J. Brew. Soc. Jpn. 2015, 110, 315–322. [Google Scholar] [CrossRef]
- Ichikawa, E.; Hirata, S.; Hata, Y.; Yazawa, H.; Tamura, H.; Kaneoke, M.; Iwashita, K.; Hirata, D. Analysis of metabolites in Japanese alcoholic beverage sake made from the rice Koshitanrei. J. Biosci. Bioeng. 2019, 83, 1570–1582. [Google Scholar]
- Kotani, A.; Watanabe, J.; Machida, K.; Yamamoto, K.; Hakamata, H. Determination of amino acidity in Japanese sake based on the voltammetric measurement of surplus acid by quinone reduction. Electrochemistry 2022, 90, 117002. [Google Scholar] [CrossRef]
- Utsunomiya, H.; Isogai, A.; Iwata, H.; Nakano, S. Flavor terminology and reference standards for sensory analysis of sake. Rep. Res. Inst. Brew. 2006, 178, 45–52. (in Japanese). [Google Scholar]
- Available online: https://www.theiwsr.com/insight/growth-of-4bn-expected-from-no-alcohol-category-by-2028/ (accessed on 25 August 2025).
- Agra CEAS Consulting, S.A.; Areté, s.r.l.; Directorate-General for Agriculture and Rural Development (European Commission). Study on low/no alcohol beverages—Final report, Publications Office of the European Union. 2023. Available online: https://data.europa.eu/doi/10.2762/315469 (accessed on 25 August 2025).
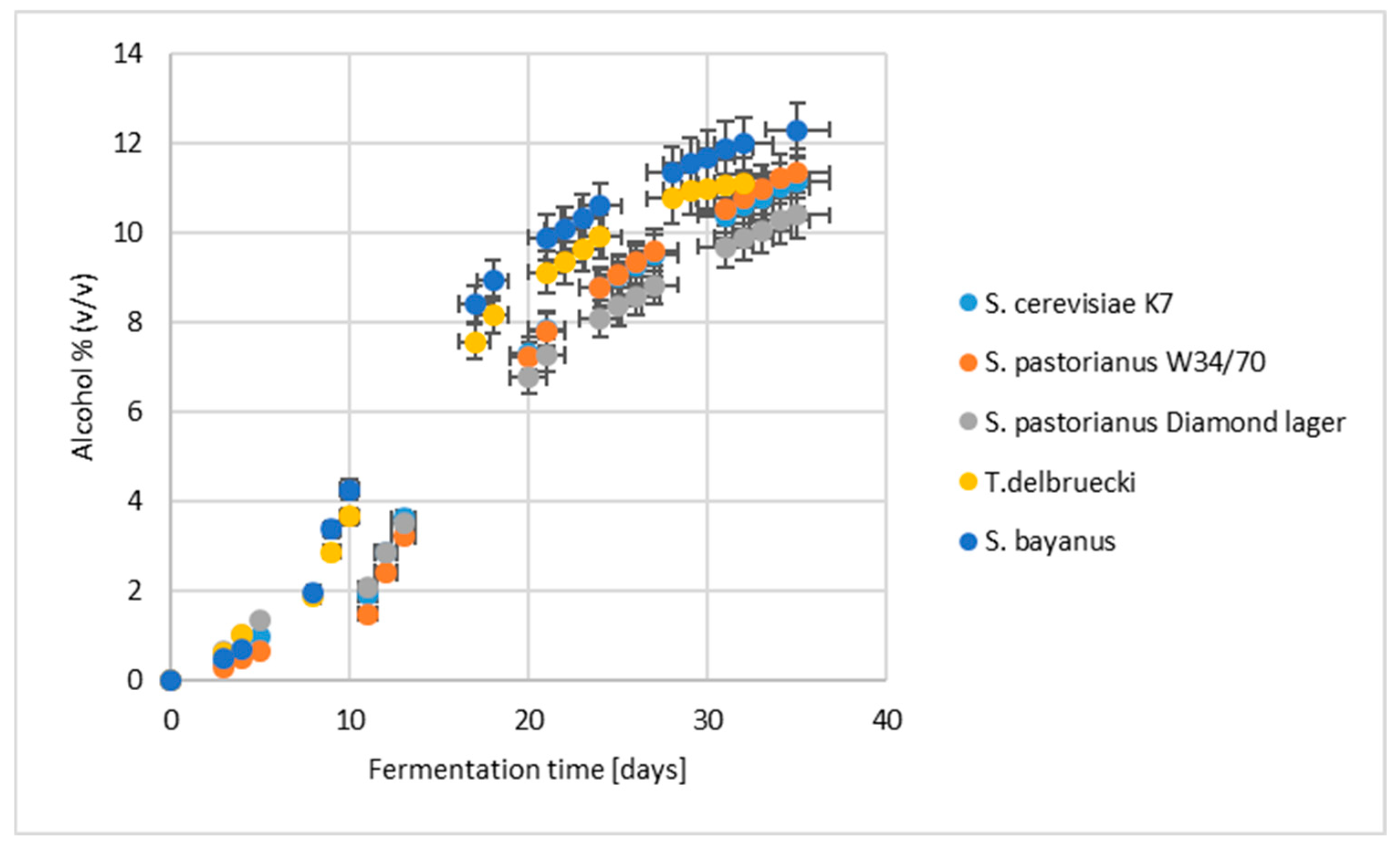

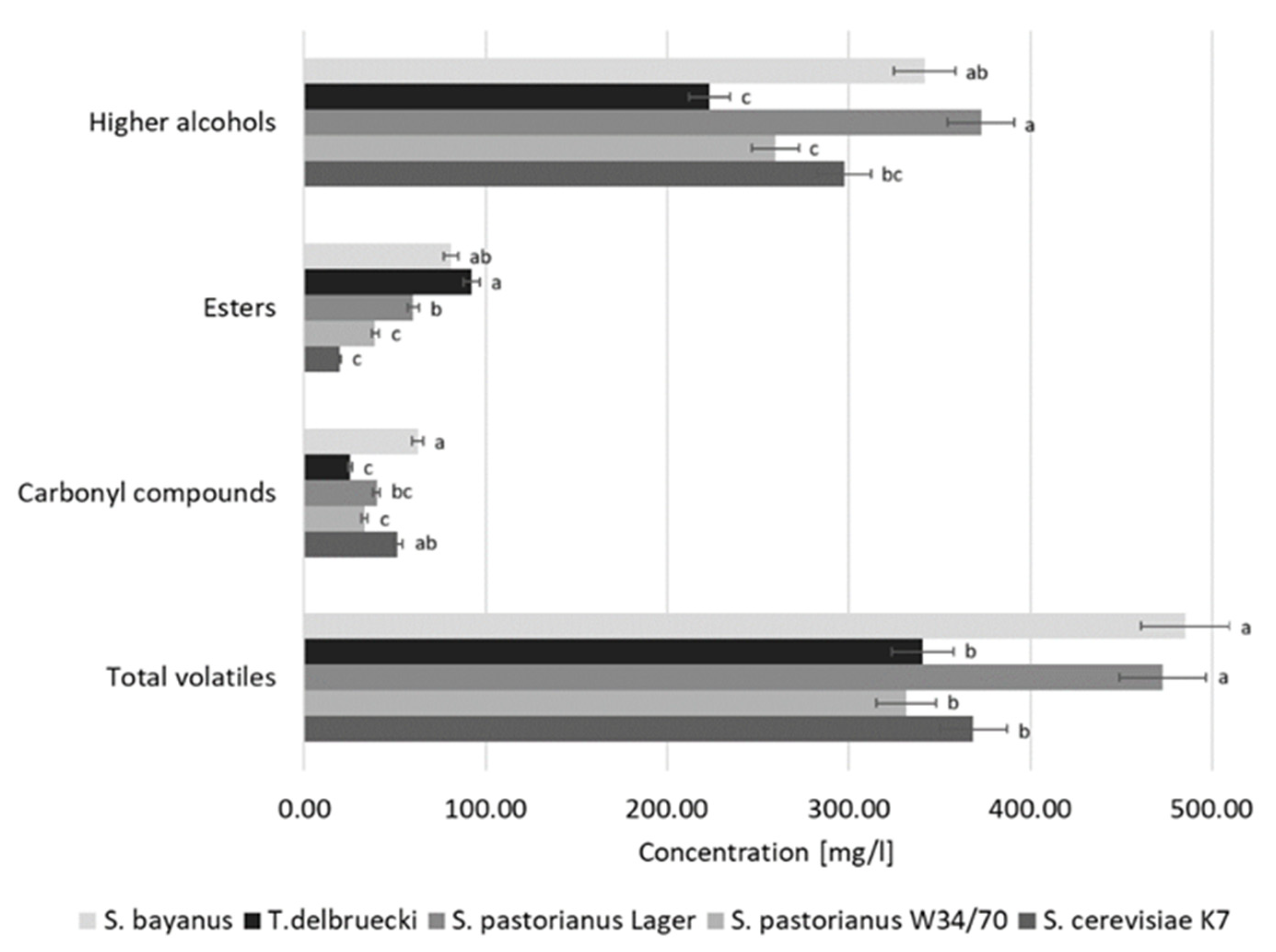
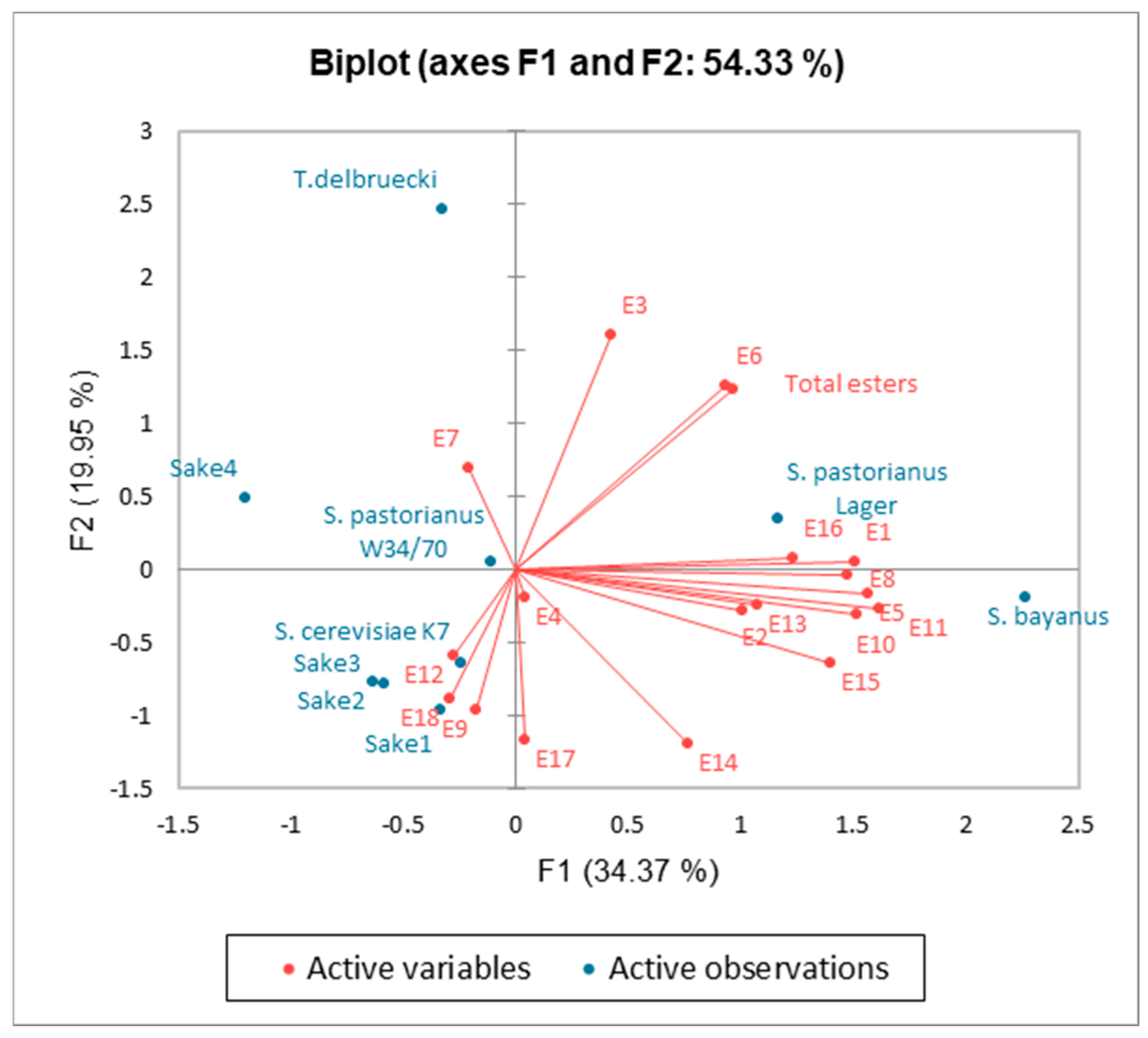
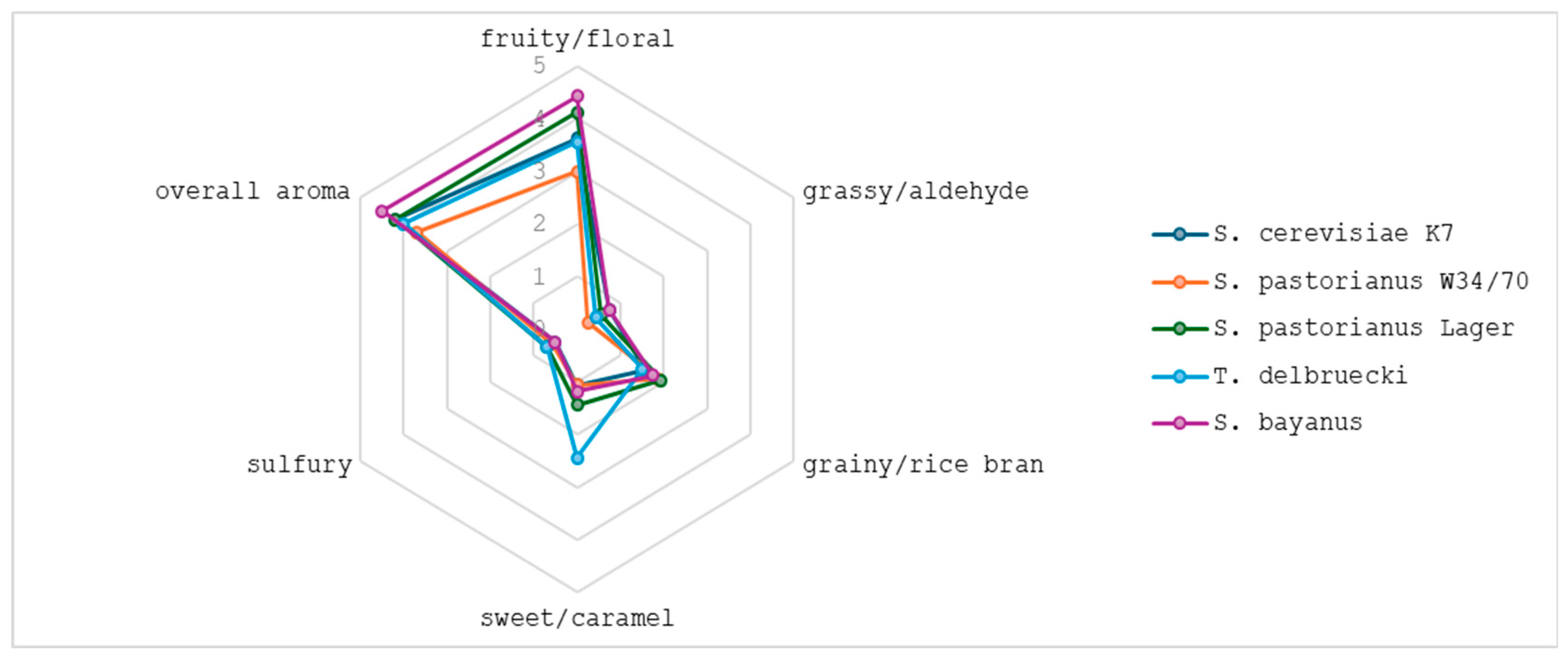
| S. cerevisiae K7 | S. pastorianus W34/70 | S. pastorianus Lager | T. delbrueckii | S. bayanus | |
|---|---|---|---|---|---|
| Alcohol [% (v/v)] | 11.6 ± 0.3 b | 11.8 ± 0.0 b | 10.7 ± 0.1 c | 11.1 ± 0.1 bc | 12.3 ± 0.4 a |
| Total extract [g/L] | 22.14 ± 0.00 a | 21.13 ± 0.10 b | 20.12 ± 0.00 c | 23.23 ± 0.42 d | 18.09 ± 0.00 e |
| Total acidity [g/L] | 1.87 ± 0.05 bc | 1.83 ± 0.09 c | 1.94 ± 0.12 b | 2.42 ± 0.07 a | 1.95 ± 0.04 b |
| Volatile acidity [g/L] | 0.39 ± 0.04 a | 0.33 ± 0.03 a | 0.36 ± 0.00 a | 0.54 ± 0.04 b | 0.50 ± 0.00 b |
| Amino acidity [mL] | 2.55 ± 0.07 a | 2.45 ± 0.07 ab | 2.05 ± 0.06 c | 2.20 ± 0.00 bc | 2.05 ± 0.07 c |
| Free SO2 [mg/L] | 2.74 ± 0.09 ab | 2.83 ± 0.24 a | 2.34 ± 0.21 b | 2.33 ± 0.25 b | 2.30 ± 0.15 b |
| Total SO2 [mg/L] | 13.49 ± 0.55 b | 13.73 ± 0.41 ab | 13.67 ± 0.55 ab | 13.94 ± 0.38 ab | 14.07 ± 0.70 a |
| Antiradical activity [mM/L] | 0.32 ± 0.04 a | 0.33 ± 0.01 a | 0.25 ± 0.01 a | 0.23 ± 0.01 a | 0.29 ± 0.02 a |
| S. cerevisiae K7 | S. pastorianus W34/70 | S. pastorianus Lager | T. delbrueckii | S. bayanus | |
|---|---|---|---|---|---|
| Arginine [mg/L] | nd | nd | nd | nd | nd |
| Urea [mg/L] | 6.62 ± 0.28 a | 4.23 ± 0.20 b | 5.80 ± 0.27 ab | 6.25 ± 0.32 a | 4.42 ± 0.17 b |
| Ethyl carbamate LOD 4.89 µg/L LOQ 14.82 µg/L | nd | nd | nd | nd | nd |
| Compound | S. cerevisiae K7 | S. pastorianus W34/70 | S. pastorianus Lager | T. delbrueckii | S. bayanus | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | Mean | ±SD | |
| Acetaldehyde | 51.125 ab | 3.935 | 33.149 c | 0.577 | 39.680 bc | 2.478 | 25.122 c | 6.382 | 61.992 a | 0.914 |
| Methyl acetate | 0.009 c | 0.001 | 0.033 b | 0.005 | 0.072 a | 0.004 | 0.011 | 0.002 | 0.058 ac | 0.006 |
| 1-Propanol | 46.187 bc | 2.448 | 35.613 dc | 1.600 | 51.006 b | 4.614 | 27.837 d | 3.542 | 68.962 a | 1.215 |
| n-Propyl acetate | 0.013 b | 0.000 | 0.015 b | 0.001 | 0.035 b | 0.007 | 0.023 b | 0.001 | 0.092 ac | 0.009 |
| Ethyl isobutyrate | 0.003 d | 0.000 | 0.008 cd | 0.000 | 0.017 b | 0.002 | 0.032 a | 0.001 | 0.009 | 0.000 |
| Butyl acetate | 0.000 c | 0.000 | 0.002 a | 0.000 | 0.001 b | 0.000 | 0.000 c | 0.000 | 0.000 c | 0.000 |
| Ethyl formate | 0.149 b | 0.017 | 0.159 b | 0.014 | 0.139 b | 0.032 | 0.032 c | 0.014 | 0.523 a | 0.009 |
| Isobutyraldehyde | 0.182 bc | 0.009 | 0.133 cd | 0.003 | 0.200 b | 0.019 | 0.106 d | 0.011 | 0.270 a | 0.004 |
| 2,3-Butanedione | 0.011 a | 0.002 | 0.008 a | 0.001 | 0.013 a | 0.002 | 0.074 a | 0.040 | 0.011 a | 0.001 |
| Ethyl acetate | 17.372 d | 1.231 | 36.980 c | 1.072 | 57.139 b | 6.219 | 90.869 a | 3.653 | 76.712 a | 3.916 |
| Isobutyl alcohol | 82.345 ab | 4.253 | 55.023 b | 2.724 | 97.395 a | 9.859 | 57.951 b | 7.633 | 93.051 a | 3.181 |
| 3-Methylbutanal | 0.041 a | 0.002 | 0.026 b | 0.001 | 0.041 a | 0.004 | 0.017 b | 0.003 | 0.047 a | 0.002 |
| 2-Methylbutanal | 0.006 b | 0.000 | 0.004 c | 0.000 | 0.008 a | 0.000 | 0.002 d | 0.000 | 0.009 a | 0.000 |
| Ethyl propionate | 0.021 d | 0.000 | 0.159 b | 0.015 | 0.178 b | 0.010 | 0.223 a | 0.004 | 0.099 c | 0.001 |
| Acetal | 1.022 b | 0.083 | 0.604 c | 0.027 | 0.613 c | 0.041 | 0.417 c | 0.109 | 1.450 a | 0.009 |
| 3-Methylbutanol | 118.207 b | 1.860 | 122.088 b | 2.743 | 150.481 a | 3.266 | 95.574 c | 4.434 | 113.396 b | 3.027 |
| 2-Methylbutanol | 30.401 bc | 0.311 | 28.138 c | 0.804 | 50.189 a | 3.191 | 23.578 c | 2.384 | 39.228 b | 0.657 |
| Isobutyl acetate | 0.032 b | 0.000 | 0.026 b | 0.001 | 0.064 b | 0.008 | 0.048 b | 0.015 | 0.187 a | 0.033 |
| Ethyl butyrate | 0.085 b | 0.005 | 0.074 b | 0.001 | 0.107 b | 0.012 | 0.077 b | 0.005 | 0.195 a | 0.021 |
| 3-Methylbutanol acetate | 0.441 c | 0.019 | 0.674 bc | 0.008 | 1.340 a | 0.117 | 0.165 c | 0.057 | 1.100 ab | 0.185 |
| 2-Methylbutanol acetate | 0.023 b | 0.001 | 0.027 b | 0.001 | 0.075 b | 0.007 | 0.022 b | 0.009 | 0.137 a | 0.023 |
| Ethyl hexanoate | 0.066 b | 0.001 | 0.058 b | 0.004 | 0.085 b | 0.012 | 0.011 c | 0.002 | 0.131 a | 0.011 |
| 2-Phenylethanol | 20.488 a | 5.105 | 18.562 a | 2.282 | 23.698 a | 2.287 | 18.358 a | 0.535 | 27.261 a | 5.639 |
| Ethyl octanoate | 0.070 ab | 0.002 | 0.055 b | 0.010 | 0.070 ab | 0.004 | 0.003 c | 0.001 | 0.098 a | 0.011 |
| 2-Phenethyl acetate | 0.098 ab | 0.001 | 0.056 b | 0.002 | 0.100 ab | 0.015 | 0.051 b | 0.004 | 0.120 a | 0.016 |
| Ethyl decanoate | 0.004 a | 0.000 | 0.006 a | 0.001 | 0.005 a | 0.001 | 0.000 a | 0.000 | 0.005 a | 0.000 |
| L | a* | b* | ΔE | |
|---|---|---|---|---|
| S. cerevisiae K7 | 96.26 ± 0.12 a | −0.44 ± 0.04 ab | 2.70 ± 0.06 a | - |
| S. pastorianus W34/70 | 96.22 ± 0.12 a | −0.44 ± 0.04 ab | 2.61 ± 0.12 a | 0.15 ± 0.04 |
| S. pastorianus Diamond Lager | 96.24 ± 0.04 a | −0.36 ± 0.02 a | 2.63 ± 0.09 a | 0.12 ± 0.06 |
| T. delbrueckii | 96.12 ± 0.20 a | −0.36 ± 0.01 a | 2.66 ± 0.15 a | 0.23 ± 0.09 |
| S. bayanus | 96.27 ± 0.13 a | −0.48 ± 0.02 b | 2.79 ± 0.04 a | 0.14 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wilkowska, A.; Dzwonnik, Z. The Use of Non-Conventional Yeast in Sake Production. Molecules 2025, 30, 3786. https://doi.org/10.3390/molecules30183786
Wilkowska A, Dzwonnik Z. The Use of Non-Conventional Yeast in Sake Production. Molecules. 2025; 30(18):3786. https://doi.org/10.3390/molecules30183786
Chicago/Turabian StyleWilkowska, Agnieszka, and Zuzanna Dzwonnik. 2025. "The Use of Non-Conventional Yeast in Sake Production" Molecules 30, no. 18: 3786. https://doi.org/10.3390/molecules30183786
APA StyleWilkowska, A., & Dzwonnik, Z. (2025). The Use of Non-Conventional Yeast in Sake Production. Molecules, 30(18), 3786. https://doi.org/10.3390/molecules30183786





