Saponin from Tea (Camellia sinensis) Seed Meal Attenuates Cortisol-Induced Lipogenesis and Inflammation in Human Cells
Abstract
1. Introduction
2. Results
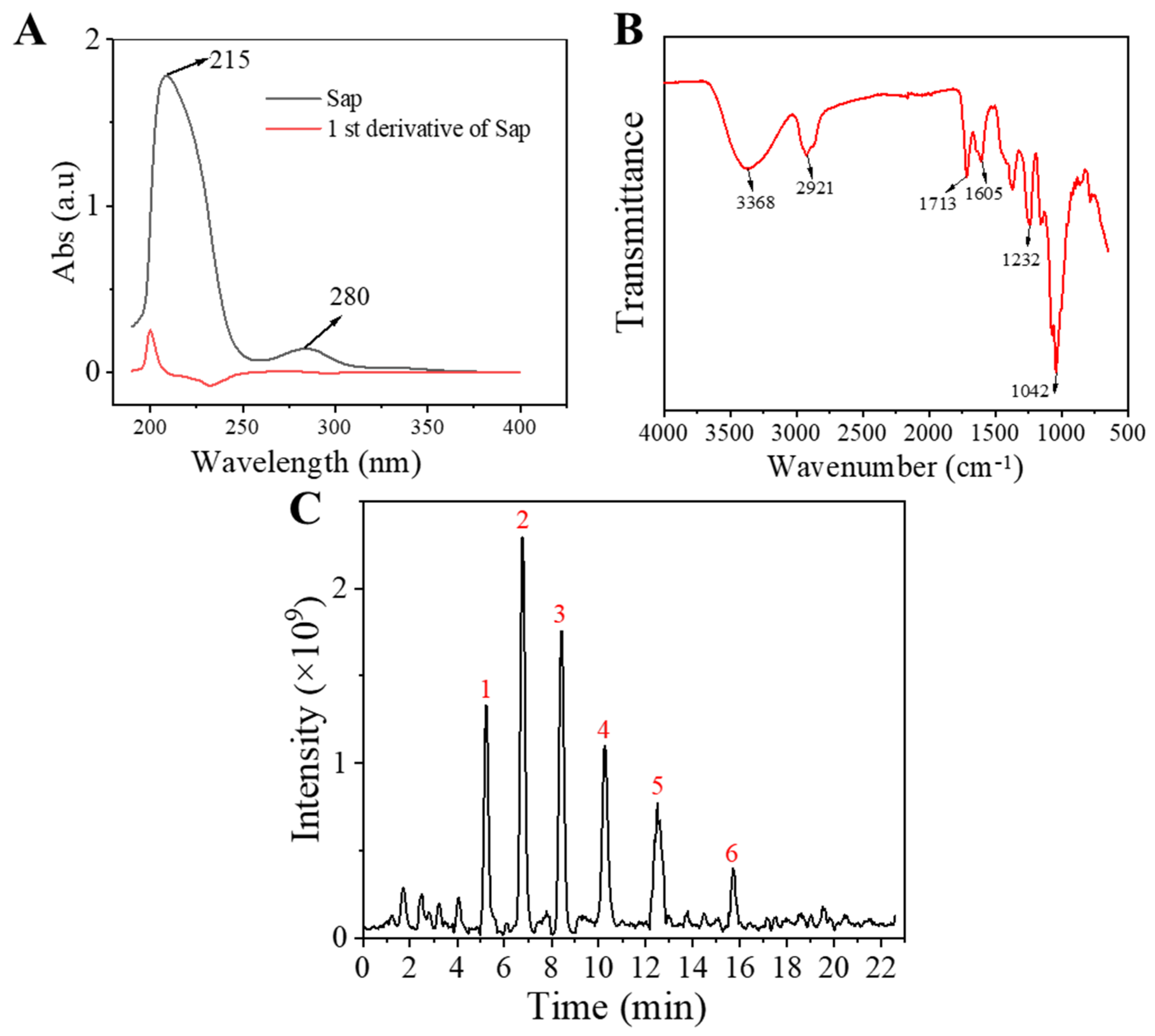
2.1. Characterization of Sap in Tea Seed Meal
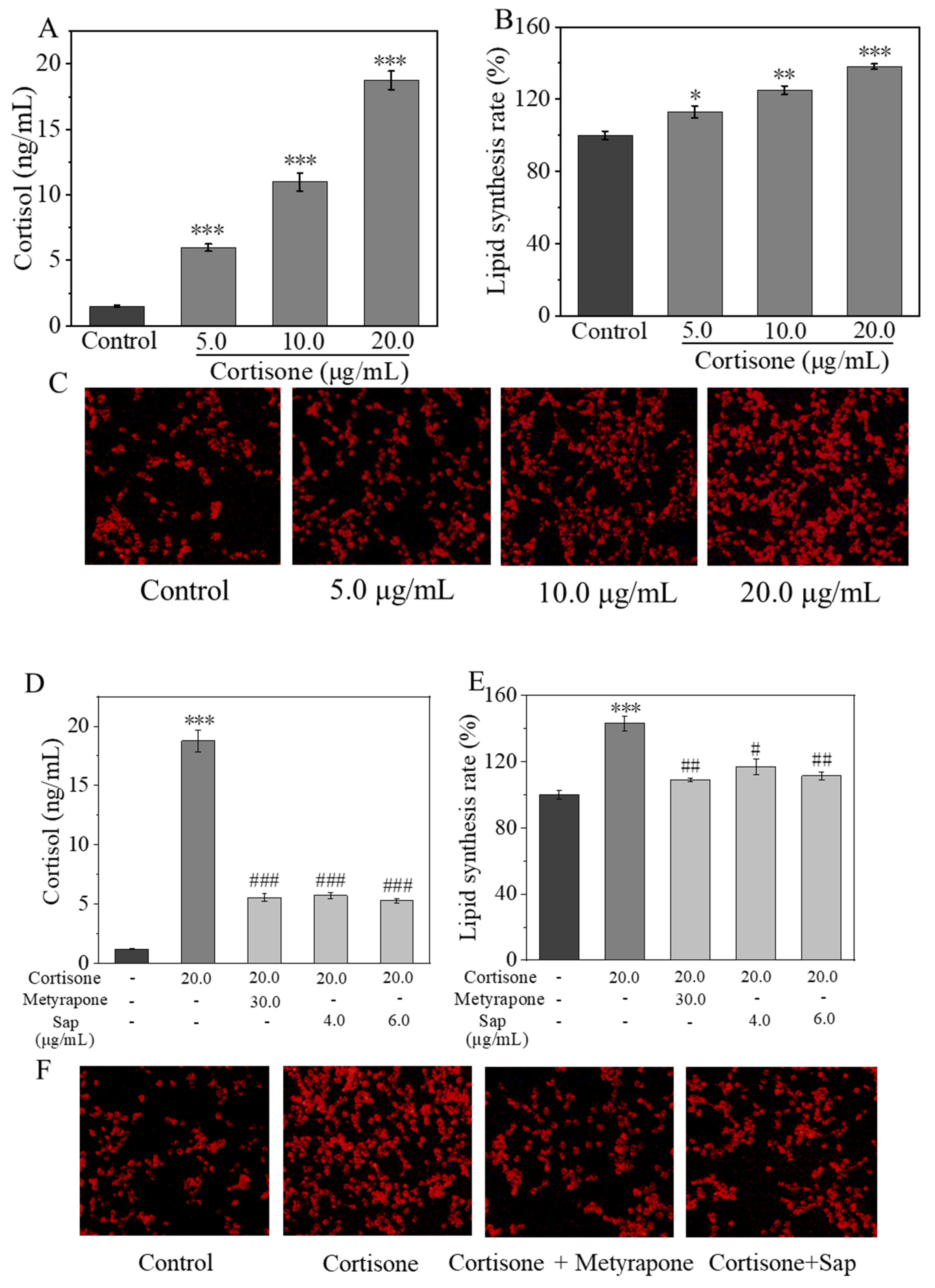
2.2. Sap Inhibits Cortisone-Induced Cortisol Secretion and Lipid Production in SZ95 Sebocytes
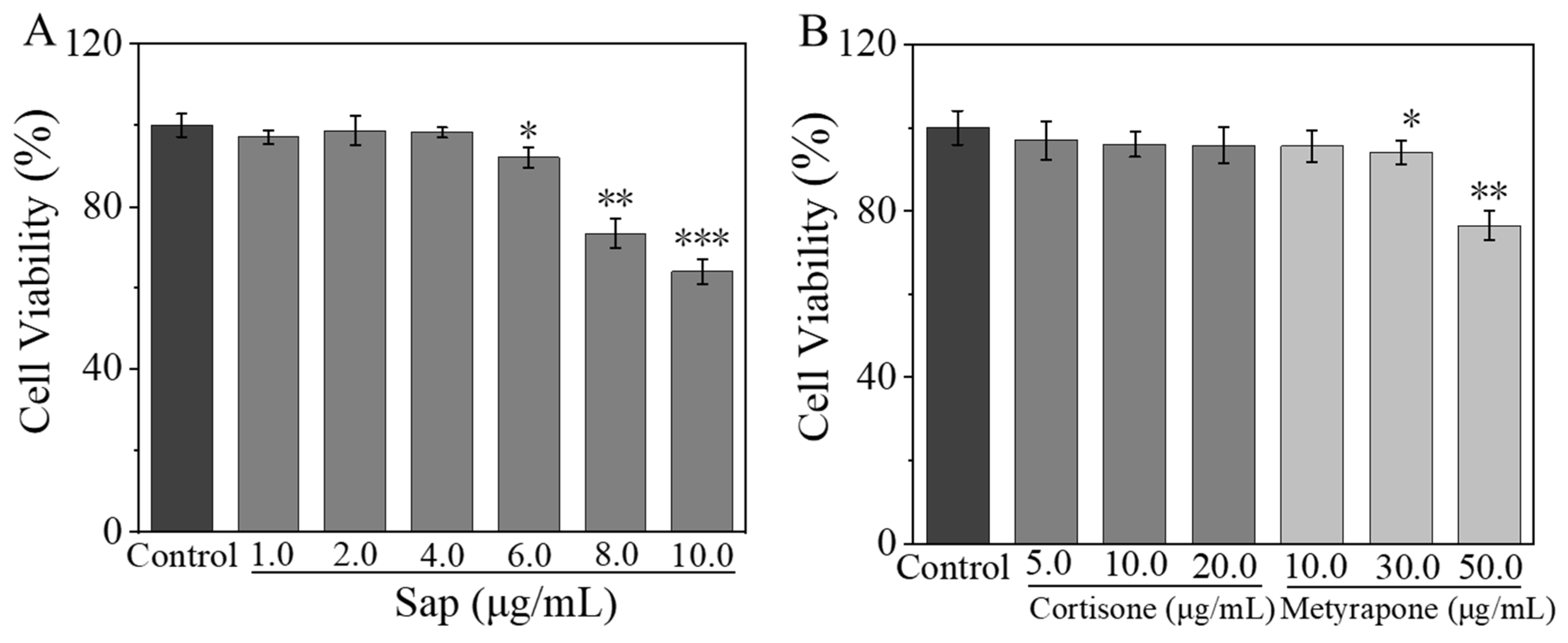
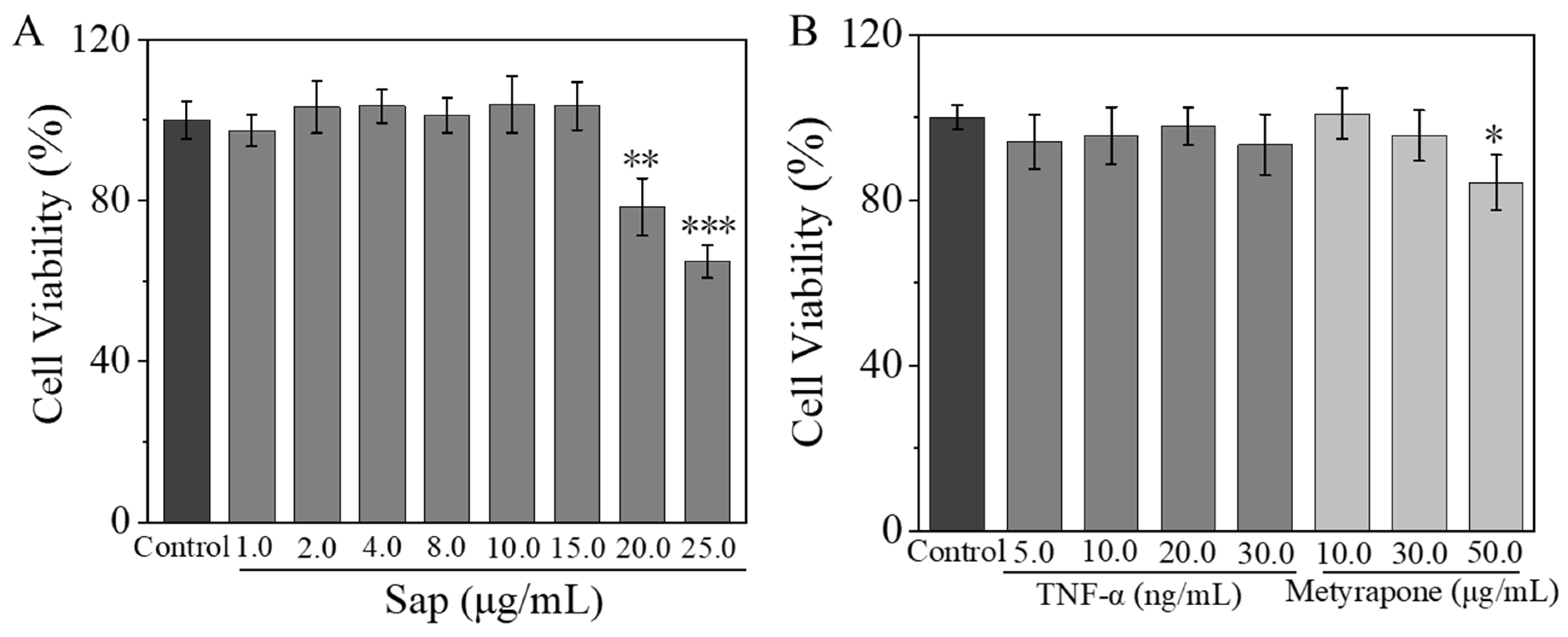
2.2.1. Effects of Sap, Cortisone, and Metyrapone on SZ95 Sebocyte Viability
2.2.2. Inhibitive Effect of Sap on Cortisone-Induced Cortisol and Lipid Secretion
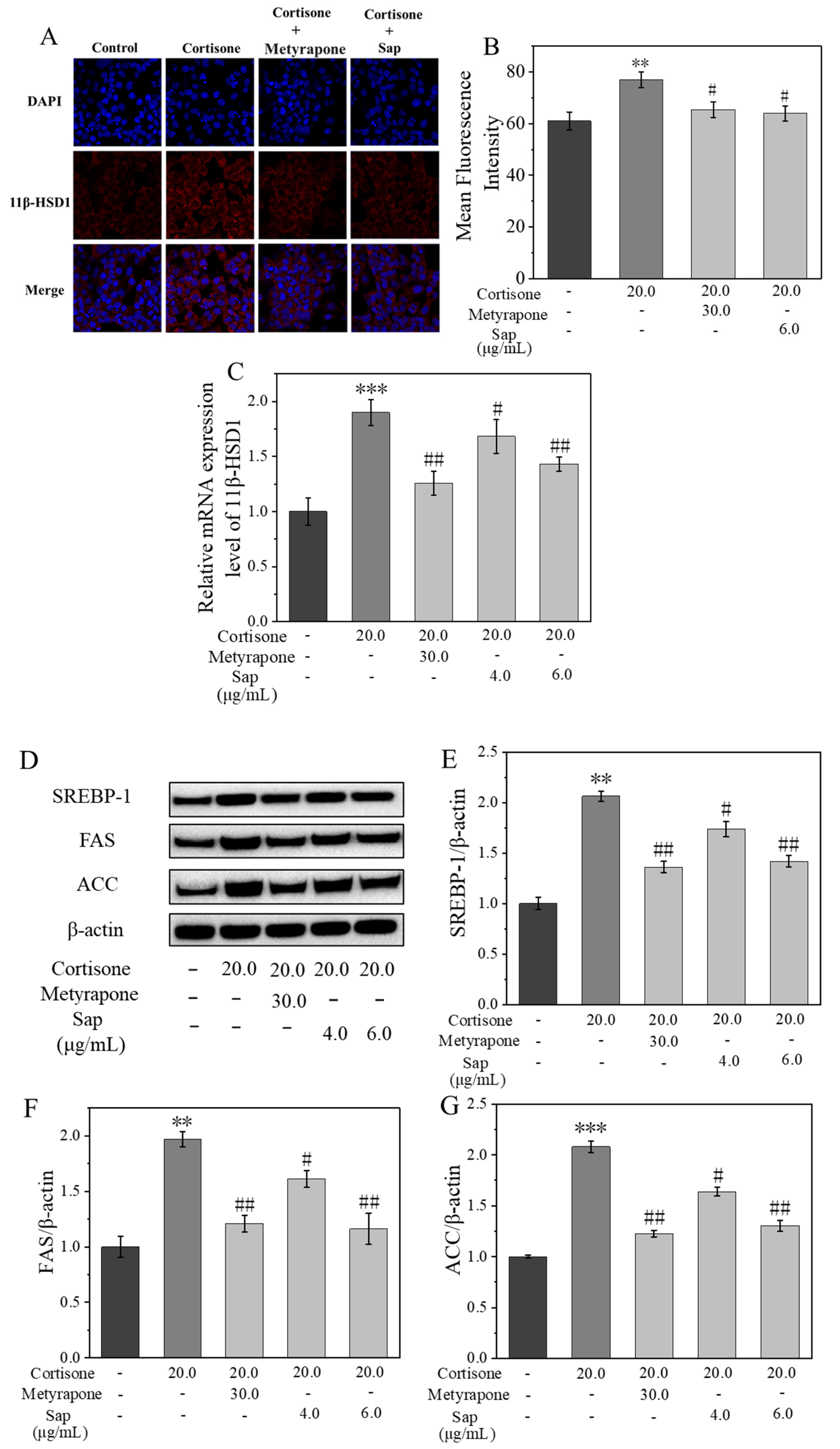
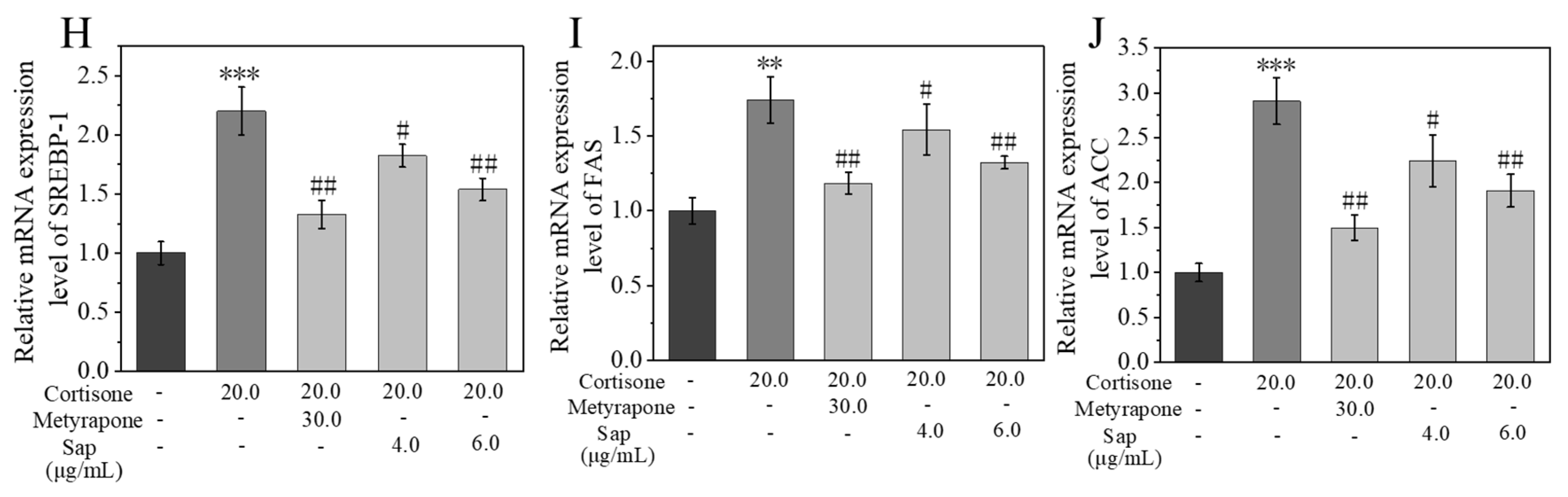
2.3. Sap Modulates Lipogenesis in SZ95 Sebocytes via the 11β-HSD1/SREBP-1 Pathways
2.4. Sap Inhibits TNF-α-Stimulated Inflammation in NHEKs
2.4.1. Effects of Sap, TNF-α, and Metyrapone on NHEK Viability
2.4.2. Sap Inhibits TNF-α-Stimulated Cortisol, IL-1β, IL-6, and IL-8 Production in NHEKs
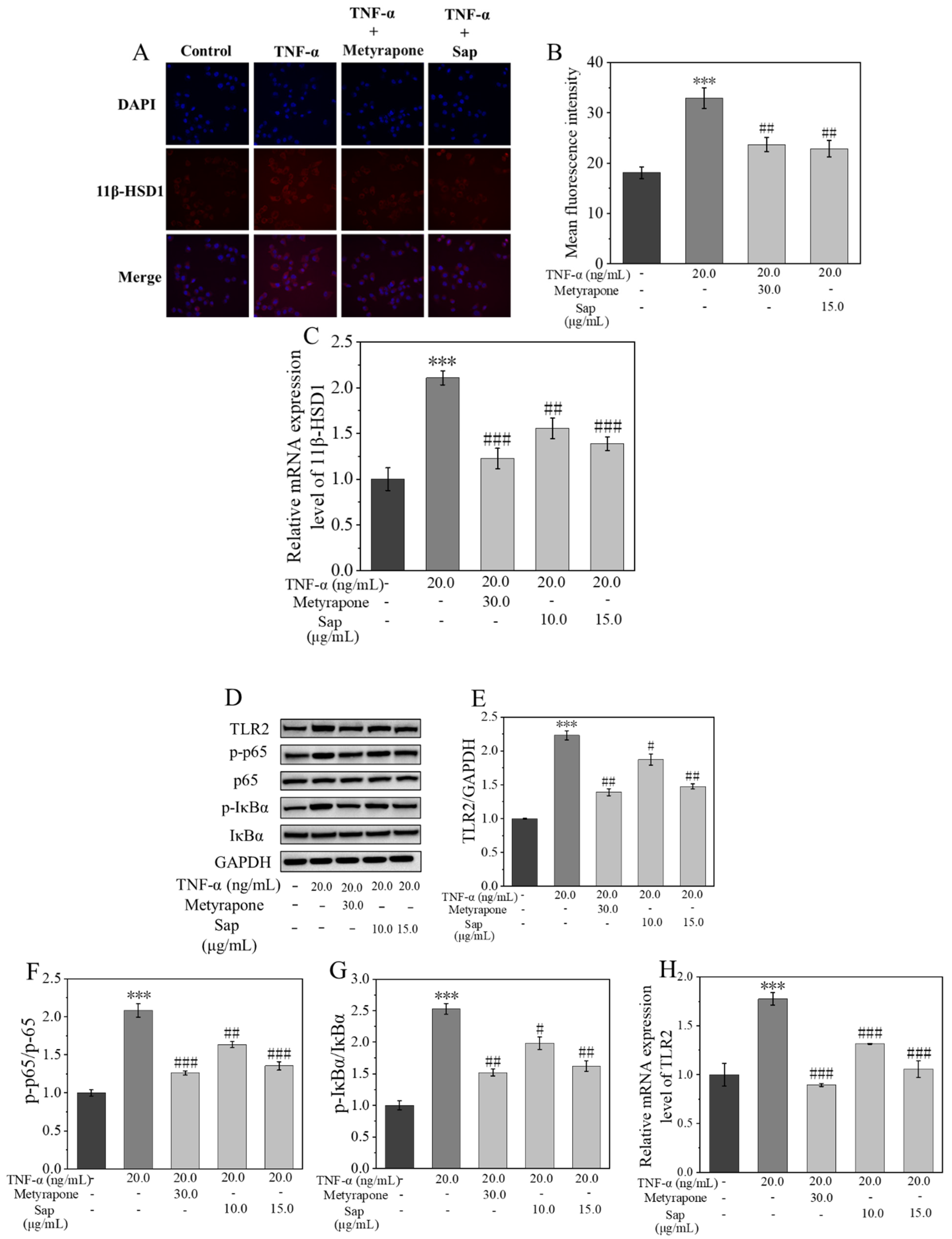
2.4.3. Sap Inhibits Stress-Induced Inflammation via the 11β-HSD1/TLR2/NF-κB Pathways
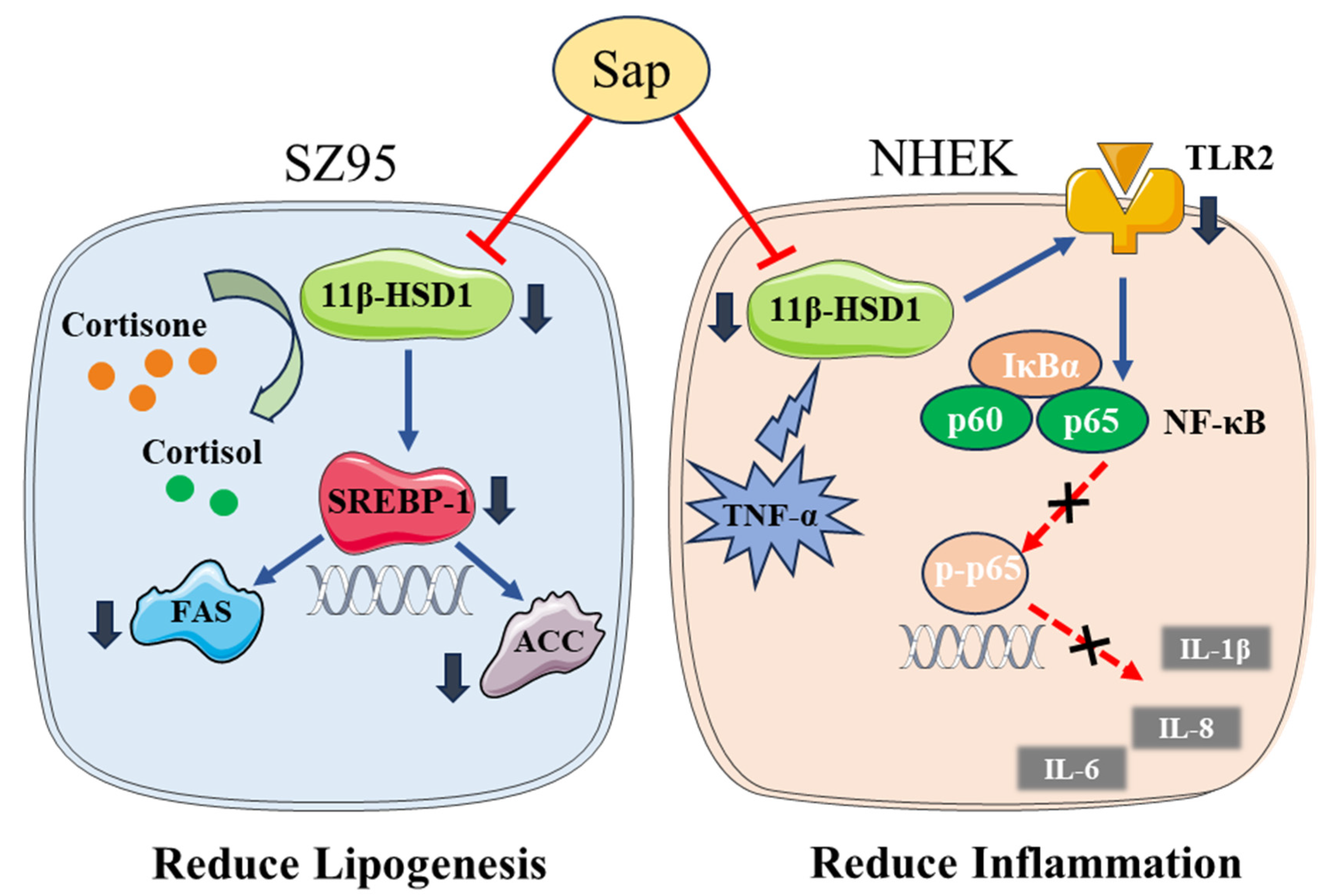
3. Discussion
4. Materials and Methods
4.1. Extraction and Purification of Sap from Tea Seed Meal
4.1.1. Extraction of Sap
4.1.2. Isolation and Purification of Sap
4.1.3. Total Saponin Content Determination
4.1.4. Determination of Total Phenol Content
4.1.5. Structure Identification of Sap from Tea Seed Meal
4.2. Cell Culture
4.3. MTT Assay for Cell Viability
4.4. Effects of Sap on Cortisone-Induced Cortisol Conversion and Lipid Secretion in SZ95
4.5. Effects of Sap on the Secretion of Cortisol and Inflammatory Factors in NHEKs Induced by TNF-α
4.6. Immunofluorescence
4.7. RT-PCR Analysis of mRNA Expression of Related Genes
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Sap | Saponin |
| SZ95 | Human sebaceous gland cell line SZ95 |
| NHEKs | Normal human epidermal keratinocytes |
References
- Papadimitriou, A.; Priftis, K.N. Regulation of the hypothalamic-pituitary-adrenal axis. Neuroimmunomodulation 2009, 16, 265–271. [Google Scholar] [PubMed]
- Filaretova, L.; Morozova, O.; Komkova, O.; Yarushkina, N. Pharmacological preconditioning by corticotropin releasing factor (CRF) or ACTH protects the gastric mucosa against ulcerogenic action of indomethacin through involvement of glucocorticoids. FASEB J. 2022, 36, 3155. [Google Scholar] [CrossRef]
- Freel, E.M.; Ingram, M.; Wallace, A.M.; White, A.; Fraser, R.; Davies, E.; Connell, J.M.C. Effect of variation in CYP11B1 and CYP11B2 on corticosteroid phenotype and hypothalamic-pituitary-adrenal axis activity in hypertensive and normotensive subjects. Clin. Endocrinol. 2008, 68, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, T.; Xu, X.; Wan, S.; Wang, Y. Review of skin problems caused by stress. J. Clin. Med. Res. 2022, 3, 116–122. [Google Scholar] [CrossRef]
- Chen, Y.; Lyga, J. Brain-skin connection: Stress, inflammation and skin aging. Inflamm. Allergy Drug Targets 2014, 13, 177–190. [Google Scholar] [CrossRef]
- Zouboulis, C.C.; Seltmann, H.; Hiroi, N.; Chen, W.; Young, M.; Oeff, M.; Scherbaum, W.A.; Orfanos, C.E.; McCann, S.M.; Bornstein, S.R. Corticotropin-releasing hormone: An autocrine hormone that promotes lipogenesis in human sebocytes. Proc. Natl. Acad. Sci. USA 2002, 99, 7148–7153. [Google Scholar] [CrossRef]
- Krause, K.; Schnitger, A.; Fimmel, S.; Zouboulis, C.C. Corticotropin-releasing hormone skin signaling is receptor-mediated and is predominant in the sebaceous glands. Horm. Metab. Res. 2007, 39, 166–170. [Google Scholar] [CrossRef]
- Schagen, S.K.; Ganceviciene, R.; Krause, K.; Angres, S.; Fimmel, S.; Bornstein, S.R. Update on cutaneous stress: The sebocyte own corticotropin-releasing hormone system is an amplifier of inflammation. Cosmoderm XVI-Eur. Soc. Cosmet. Aesthet. Dermatol (ESCAD) Int. Proc. 2011, 63–66. [Google Scholar]
- Zouboulis, C.C.; Angres, S. Macrophage-activating lipopeptide-2 and corticotropin-releasing hormone stimulate the inflammatory signalling in human sebocytes through activation of stearoyl-CoA desaturase and fatty acid desaturase 2. J. Eur. Acad. Dermatol. Venereol. 2021, 35, 493–501. [Google Scholar]
- Ganceviciene, R.; Graziene, V.; Fimmel, S.; Zouboulis, C.C. Involvement of the corticotropin-releasing hormone system in the pathogenesis of acne vulgaris. Br. J. Dermatol. 2009, 160, 345–352. [Google Scholar] [CrossRef]
- Elewa, R.M.; Abdallah, M.; Youssef, N.; Zouboulis, C.C. Aging-related changes in cutaneous corticotropin-releasing hormone system reflect a defective neuroendocrine-stress response in aging. Rejuvenation Res. 2012, 15, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Terao, M.; Katayama, I. Local cortisol/corticosterone activation in skin physiology and pathology. J. Dermatol. Sci. 2016, 84, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Pereira, M.T.; Schuler, G.; Aslan, S.; Payan-Carreira, R.; Reichler, I.M.; Reynaud, K. Utero-placental expression and functional implications of HSD11B1 and HSD11B2 in canine pregnancy. Biol. Reprod. 2023, 108, 645–658. [Google Scholar] [CrossRef] [PubMed]
- Pujos, M.; Chamayou-Robert, C.; Parat, M.; Bonnet, M.; Couret, S.; Robiolo, A.; Doucet, O. Impact of chronic moderate psychological stress on skin aging: Exploratory clinical study and cellular functioning. J. Cosmet. Dermatol. 2025, 24, e16634. [Google Scholar] [CrossRef]
- Zhang, X.; Meng, L.; Xiao, Y.; Chen, Z. Case report: Radiofrequency ablation combined with biopsy for Cushing’s syndrome due to ectopic ACTH lesions in the lung. Front. Oncol. 2022, 12, 1059308. [Google Scholar] [CrossRef]
- Matsuda, N.; Yamamoto, L.; Masuda, R.; Tagawa, M. Cortisol promotes staining-type hypermelanosis in juvenile Japanese flounder. Aquaculture 2018, 497, 147–154. [Google Scholar] [CrossRef]
- Chae, M.; Bae, I.H.; Lim, S.; Jung, K.; Roh, J.; Kim, W. AP collagen peptides prevent cortisol-induced decrease of collagen type I in human dermal fibroblasts. Int. J. Mol. Sci. 2021, 22, 4788. [Google Scholar] [CrossRef]
- Choo, J.H.; Lee, H.G.; Lee, S.Y.; Kang, N.G. Iris pallida extract alleviates cortisol-induced decrease in type 1 collagen and hyaluronic acid syntheses in human skin cells. Curr. Issues Mol. Biol. 2023, 45, 353–363. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Meunier, M.; Scandolera, A.; Sandre, J.; Lambert, C.; Chapuis, E.; Auriol, D.; Reynaud, R. Well-aging: A new strategy for skin homeostasis under multi-stressed conditions. J. Cosmet. Dermatol. 2020, 19, 444–455. [Google Scholar] [CrossRef]
- Gao, G.; Fu, J.; Cui, J.; Zhang, T.; Zouboulis, C.C.; Wang, J.; Yan, S. Effects and stress-relieving mechanisms of dark tea polysaccharide in human HaCaT keratinocytes and SZ95 sebocytes. Molecules 2023, 28, 6128. [Google Scholar] [CrossRef]
- Hu, J.-L.; Nie, S.-P.; Huang, D.-F.; Li, C.; Xie, M.Y. Extraction of saponin from camellia oleifera cake and evaluation of its antioxidant activity. Int. J. Food Sci. Technol. 2012, 47, 1676–1687. [Google Scholar] [CrossRef]
- Yang, W.S.; Ko, J.; Kim, E.; Kim, J.H.; Park, J.G.; Sung, N.Y.; Kim, H.G.; Yang, S.; Rho, H.S.; Hong, Y.D. 21-O-angeloyltheasapogenol E3, a novel triterpenoid saponin from the seeds of tea plants, inhibits macrophage-mediated inflammatory responses in a NF-κB-dependent manner. Mediat. Inflamm. 2014, 2014, 658351. [Google Scholar] [CrossRef]
- Guo, N.; Tong, T.; Ren, N.; Tu, Y.; Li, B. Saponins from seeds of genus camellia: Phytochemistry and bioactivity. Phytochemistry 2018, 149, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huang, Y.C.; Deng, J.M.; Yu, M.; Zouboulis, C.C.; Wang, G.L.; Wang, J. Tea (Camellia sinensis) seed saponins act as sebosuppression agents via the AMPK/mTOR pathway. J. Cosmet. Dermatol. 2025, 24, e16793. [Google Scholar] [CrossRef] [PubMed]
- Yuan, C.; Li, Y.; Li, Q.; Jin, R.S.; Ren, L.L. Purification of tea saponins and evaluation of its effect on alcohol dehydrogenase activity. Open Life Sci. 2018, 13, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.L.; Zhang, W.N.; Hu, Z.X.; Qi, Y.; Gong, R. Quantitative determination of tea saponin in tea seed meal by high performance liquid chromatography. J. Food Sci. 2013, 34, 153–156. [Google Scholar]
- Yu, X.; Zhao, Z.; Yan, X.; Xie, J.H.; Yu, Q.; Chen, Y. Extraction optimization of tea saponins from camellia oleifera seed meal with deep eutectic solvents: Composition identification and properties evaluation. Food Chem. 2023, 427, 136681. [Google Scholar] [CrossRef]
- Tang, Y.; He, X.; Sun, J.; Liu, G.; Li, C.; Li, L.; Sheng, J.; Zhou, Z.; Xin, M.; Ling, D.; et al. Comprehensive evaluation on tailor-made deep eutectic solvents (DESs) in extracting tea saponins from seed pomace of camellia oleifera Abel. Food Chem. 2021, 342, 128243. [Google Scholar] [CrossRef]
- Myose, M.; Warashina, T.; Miyase, T. Triterpene saponins with hyaluronidase inhibitory activity from the seeds of camellia sinensis. Chem. Pharm. Bull. 2012, 60, 612–623. [Google Scholar] [CrossRef]
- Yoshikawa, M.; Morikawa, T.; Nakamura, S.; Li, N.; Li, X.; Matsuda, H. Bioactive saponins and glycosides. XXV. Acylated oleanane-type triterpene saponins from the seeds of tea plant (Camellia sinensis). Chem. Pharm. Bull. 2007, 55, 57–63. [Google Scholar] [CrossRef]
- Morikawa, T.; Li, N.; Nagatomo, A.; Matsuda, H.; Li, X.; Yoshikawa, M. Triterpene saponins with gastroprotective effects from tea seed (the seeds of Camellia sinensis). J. Nat. Prod. 2006, 69, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Shi, V.Y.; Leo, M.; Hassoun, L.; Chahal, D.S.; Maibach, H.I.; Sivamani, R.K. Role of sebaceous glands in inflammatory dermatoses. J. Am. Acad. Dermatol. 2015, 73, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Drost, L.; Finke, J.B.; Behrje, A.; Rebeck, D.; Domes, G.; Schaechinger, H. Optimal timing of oral metyrapone intake for the suppression of cold-pressor stress-induced cortisol release. Psychoneuroendocrinology 2023, 156, 106328. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Kim, J.M.; Jeong, M.K.; Zouboulis, C.C.; Lee, S.H. 11β-hydroxysteroid dehydrogenase type 1 is expressed in human sebaceous glands and regulates glucocorticoid-induced lipid synthesis and toll-like receptor 2 expression in SZ95 sebocytes. Br. J. Dermatol. 2013, 168, 47–55. [Google Scholar] [CrossRef]
- Lu, Y.; Xu, X.; Jiang, T.; Jin, L.; Zhao, X.D.; Cheng, J.H.; Xin, X.J.; Ma, J.; Piao, H.N.; Piao, X.X. Sertraline ameliorates inflammation in CUMS mice and inhibits TNF-α-induced inflammation in microglia cells. Int. Immunopharmacol. 2019, 67, 119–128. [Google Scholar] [CrossRef]
- Itoi, S.; Terao, M.; Murota, H.; Katayama, I. 11β-Hydroxysteroid dehydrogenase 1 contributes to the proinflammatory response of keratinocytes. Biochem. Biophys. Res. Commun. 2013, 440, 265–270. [Google Scholar] [CrossRef]
- Tang, Z.L.; Zhang, K.; Lv, S.C.; Xu, G.W.; Zhang, J.F.; Jia, H.Y. LncRNA MEG3 suppresses PI3K/AKT/mTOR signalling pathway to enhance autophagy and inhibit inflammation in TNF-α-treated keratinocytes and psoriatic mice. Cytokine 2021, 148, 155657. [Google Scholar] [CrossRef]
- Ishii, T.; Masuzaki, H.; Tanaka, T.; Arai, N.; Yasue, S.; Kobayashi, N.; Tomita, T.; Noguchi, M.; Fujikura, J.; Ebihara, K.; et al. Augmentation of 11β-hydroxysteroid dehydrogenase type 1 in LPS-activated J774.1 macrophages—Role of 11β-HSD1 in pro-inflammatory properties in macrophages. FEBS Lett. 2007, 581, 349–354. [Google Scholar] [CrossRef]
- Ishii-Yonemoto, T.; Masuzaki, H.; Yasue, S.; Okada, S.; Kozuka, C.; Tanaka, T.; Noguchi, M.; Tomita, T.; Fujikura, J.; Yamamoto, Y.; et al. Glucocorticoid reamplification within cells intensifies NF-κB and MAPK signaling and reinforces inflammation in activated preadipocytes. Am. J. Physiol.-Endocrinol. Endocrinol. Metab. 2010, 298, 930–940. [Google Scholar] [CrossRef][Green Version]
- Mempel, M.; Voelcker, V.; Köllisch, G.; Plank, C.; Rad, D.; Gerhard, M.; Schnopp, C.; Fraunberger, P.; Walli, A.; Ring, J. Toll-like receptor expression in human keratinocytes: Nuclear factor κB controlled gene activation by Staphylococcus aureus is Toll-like receptor 2 but not toll-like receptor 4 or platelet activating factor receptor dependent. J. Investig. Dermatol. 2003, 121, 1389–1396. [Google Scholar] [CrossRef]
- Khan, S.; Shafiei, M.S.; Longoria, C.; Schoggins, J.; Savani, R.C.; Zaki, H. SARS-CoV-2 spike protein induces inflammation via TLR2-dependent activation of the NF-κB pathway. Elife 2021, 10, e68563. [Google Scholar] [CrossRef] [PubMed]
- Zong, K.; Xu, K.; Zhang, X.; Wang, P.; Wang, Z.; Yang, S.; Li, H.; He, H.; He, S.; Hu, Y. Exploring the mechanism of huangqin tang against skin lipid accumulation through network pharmacology and experimental validation. J. Ethnopharmacol. 2023, 313, 116581. [Google Scholar] [CrossRef] [PubMed]
- Crisafulli, C.; Galuppo, M.; Cuzzocrea, S. Effects of genetic and pharmacological inhibition of TNF-α in the regulation of inflammation in macrophages. Pharmacol. Res. 2009, 60, 332–340. [Google Scholar] [CrossRef] [PubMed]
- Jang, K.J.; Choi, S.H.; Yu, G.J. Anti-inflammatory potential of total saponins derived from the roots of Panax ginseng in lipopolysaccharide-activated RAW 264.7 macrophages. Exp. Ther. Med. 2015, 11, 1109–1115. [Google Scholar] [CrossRef]
- Wan, K.; Li, J.; Ma, L.; Chen, T.M.; Chen, Y.; Li, Z.Z.; Zouboulis, C.C.; Wang, G.L.; Wang, J. Camellia saponin modulates oleic acid/linoleic acid-induced lipogenesis in human sebocytes through lipophagy activation. Int. J. Cosmet. Sci. 2025, 47, 497–509. [Google Scholar] [CrossRef]
- Ben Lagha, A.; Azelmat, J.; Vaillancourt, K.; Grenier, D. A polyphenolic cinnamon fraction exhibits anti-inflammatory properties in a monocyte/macrophage model. Public Libr.Sci. ONE 2021, 16, e0244805. [Google Scholar] [CrossRef]
- Lee, K.E.; Youm, J.K.; Lee, W.J.; Kang, S.; Kim, Y.J. Polyphenol-rich apple extract inhibits dexamethasone-induced sebaceous lipids production by regulating SREBP1 expression. Exp. Dermatol. 2017, 26, 958–960. [Google Scholar] [CrossRef]
- Ding, Y.L.; Wang, Y.Z.; Zhang, J.; Zhang, Q.Z.; Zhang, J.Y.; Jin, H. Application of the vanillin sulfuric acid colorimetry-ultraviolet spectrometry on quality evaluation of panax notoginseng. Spectrosc. Spectr. Anal. 2013, 33, 471–475. [Google Scholar]
- Chang, Q.; Su, M.; Chen, Q.; Zeng, B.; Wang, W.; Li, H. Effects of two extraction methods on the polyphenols and antioxidant activity from cauarium album. Chin. J. Trop. Crops 2016, 37, 1575–1581. [Google Scholar]
- Zouboulis, C.C.; Seltmann, H.; Neitzel, H.; Orfanos, C.E. Establishment and characterization of an immortalized human sebaceous gland cell line (SZ95). J. Investig. Dermatol. 1999, 113, 1011–1020. [Google Scholar] [CrossRef]

| Compound | Rt/(min) | Molecular Formula | Structural Formula |
|---|---|---|---|
| 1 | 5.57 | C47H74O17 | 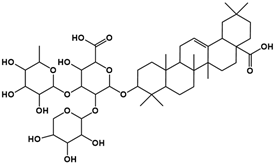 |
| 2 | 6.72 | C43H68O14 | 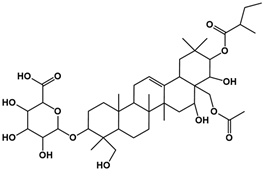 |
| 3 | 8.56 | C42H68O14 | 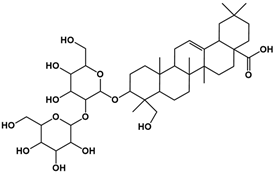 |
| 4 | 10.29 | C48H78O19 | 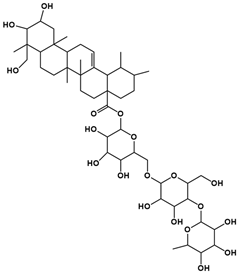 |
| 5 | 12.36 | C41H66O13 | 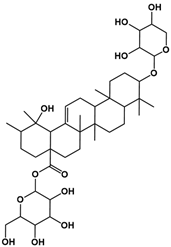 |
| 6 | 15.87 | C42H70O12 |  |
| Primer Name | Primer Sequence |
|---|---|
| 11β-HSD1 | F: 5′-TGGAGCAGCCTCAGCACACTAC-3′ |
| R: 5′-GTGTTGGTGATGTGGTTGAGAATG-3′ | |
| SREBP-1 | F: 5′-CCCACTTCATCAAGGCAGACTCG-3′ |
| R: 5′-CACTCACCAGGGTCGGCAAAG-3′ | |
| FAS | F: 5′-TGGCACACATCCTGGGCATC-3′ |
| R: 5′-CAGACCATCCTCCTTGGGCGT-3′ | |
| ACC | F: 5′-ACAACGCAGGCATCAGAAGATTATT-3′ |
| R: 5′-CCACCATTTTGGCAAGTTTCACC-3′ | |
| GAPDH | F: 5’-GAAGGTGAAGGTCGGAGTC-3’ |
| R: 5’-GAAGATGGTGATGGGATTTC-3’ |
| Primer Name | Primer Sequence |
|---|---|
| 11β-HSD1 | F: 5′-TGGAGCAGCCTCAGCACACTAC-3′ |
| R: 5′-GTGTTGGTGATGTGGTTGAGAATG-3′ | |
| TLR2 | F: 5′-CTTCTCCCATTTCCGTCTTTTTG-3′ |
| R: 5′-TCTTGGTGTTCATTATCTTCCGC-3′ | |
| β-actin | F: 5′-TGGCACCCAGCACAATGAA-3′ |
| R: 5′-GAAGCATTTGCGGTGGACG-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhang, L.-Y.; Huang, Y.-C.; Deng, J.-M.; Yu, M.; Zouboulis, C.C.; Li, J.-H.; Wang, G.-L.; Wang, J. Saponin from Tea (Camellia sinensis) Seed Meal Attenuates Cortisol-Induced Lipogenesis and Inflammation in Human Cells. Molecules 2025, 30, 3844. https://doi.org/10.3390/molecules30193844
Li J, Zhang L-Y, Huang Y-C, Deng J-M, Yu M, Zouboulis CC, Li J-H, Wang G-L, Wang J. Saponin from Tea (Camellia sinensis) Seed Meal Attenuates Cortisol-Induced Lipogenesis and Inflammation in Human Cells. Molecules. 2025; 30(19):3844. https://doi.org/10.3390/molecules30193844
Chicago/Turabian StyleLi, Jian, Lu-Yao Zhang, Yuan-Cheng Huang, Jian-Ming Deng, Min Yu, Christos C Zouboulis, Jin-Hua Li, Guang-Li Wang, and Jing Wang. 2025. "Saponin from Tea (Camellia sinensis) Seed Meal Attenuates Cortisol-Induced Lipogenesis and Inflammation in Human Cells" Molecules 30, no. 19: 3844. https://doi.org/10.3390/molecules30193844
APA StyleLi, J., Zhang, L.-Y., Huang, Y.-C., Deng, J.-M., Yu, M., Zouboulis, C. C., Li, J.-H., Wang, G.-L., & Wang, J. (2025). Saponin from Tea (Camellia sinensis) Seed Meal Attenuates Cortisol-Induced Lipogenesis and Inflammation in Human Cells. Molecules, 30(19), 3844. https://doi.org/10.3390/molecules30193844








