Synthesis, Characterization and Optical Behavior of Nanocrystalline CoWO4
Abstract
1. Introduction
2. Results and Discussion
2.1. Phase Formation and Characterization
2.1.1. X-Ray Diffraction Analysis
2.1.2. TEM Analysis
2.1.3. Infrared Investigation
2.2. Optical Properties of CoWO4 Obtained Through Mechanochemical and Solid-State Approaches
2.2.1. UV–Vis Defuses Reflectance
2.2.2. Photoluminescence Emission Spectra
3. Materials and Methods
3.1. Direct Mechanochemical Synthesis
3.2. Solid-State Synthesis
3.3. Characterization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rostampour, M.; Eavani, S. Synthesis and characterization of the novel nano composite pigments using CoWO4 on different silica sources: A comparative study. Powder Techn. 2020, 363, 86–94. [Google Scholar] [CrossRef]
- Cid-Dresdner, H.; Escobar, C. The crystal structure of ferberite, FeWO4. Zeitschtift Fur. Kristallographie 1968, 127, 61–72. [Google Scholar] [CrossRef]
- Trots, D.M.; Senyshyn, A.; Vasylechko, L.; Niewa, R.; Vad, T.; Mikhailik, V.B.; Kraus, H. Crystal structure of ZnWO4 scintillator material in the range of 3–1423 K. J. Phys. Condens. Matter 2009, 21, 325402–325411. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.I.; Adam, A.; Khan, A.; Rehman, A.U.; Qamaruddin, M.; Siddiqui, M.N.; Qamar, M. Improved photoelectrochemical water oxidation un dervisible light with mesoporous CoWO4. Mater. Lett. 2016, 183, 281–284. [Google Scholar] [CrossRef]
- Lopez, X.A.; Fuentes, A.F.; Zaragoza, M.M.; Guillen, J.A.D.; Gutierrez, J.S.; Ortiz, A.L.; Collins-Martınez, V. Synthesis, characterization and photocatalytic evaluation of MWO4 (M = Ni, Co, Cu and Mn) tungstates. Inter. J. Hydrogen Energy 2016, 41, 23312–23317. [Google Scholar] [CrossRef]
- Srirapu, V.K.V.P.; Kumar, A.; Srivastava, P.; Singha, R.N.; Sinha, A.S.K. Nanosized CoWO4 and NiWO4 as efficient oxygen-evolving electrocatalysts. Electrochim. Acta 2016, 209, 75–84. [Google Scholar] [CrossRef]
- Taneja, P.; Sharma, S.; Umar, A.; Mehta, S.K.; Ibhadon, A.O.; Kansal, S.K. Visible-light driven photocatalytic degradation of brilliant green dye based on cobalt tungstate (CoWO4) nanoparticles. Mater. Chem. Phys. 2018, 211, 335–342. [Google Scholar] [CrossRef]
- Alagumalai, K.; Balamurugan, M.; Chen, S.M.; Selvaganapathy, M. One-pot engineering of novel cashew like cobalt tungstate; dynamic electrocatalyst for the selective detection of promethazine hydrochloride. Microchem. J. 2020, 159, 105381–105389. [Google Scholar] [CrossRef]
- Diao, Q.; Yang, F.; Yin, C.; Li, J.; Yang, S.; Liang, X.; Lu, G. Ammonia sensors based on stabilized zirconia and CoWO4 sensing electrode. Solid State Ion. 2012, 225, 328–331. [Google Scholar] [CrossRef]
- Joy, J.J.; Jaya, N.V. Structural, magnetic and optical behavior of pristine and Yb-doped CoWO4 nanostructure. J. Mater. Sci. Mater. Electron. 2013, 24, 1788–1795. [Google Scholar]
- Han, S.; Xiao, K.; Liu, L.; Huang, H. Zn1-xCoxWO4 (0 ≤ x ≤ 1) full range solid solution: Structure, optical properties, and magnetism. Mater. Res. Bull. 2016, 74, 436–440. [Google Scholar] [CrossRef]
- Naik, S.J.; Salker, A.V. Solid state studies on cobalt and copper tungstates nano materials. Solid State Sci. 2010, 12, 2065–2072. [Google Scholar] [CrossRef]
- Azevedo, H.V.S.B.; Raimundo, R.A.; Ferreira, L.S.; Silva, M.M.S.; Morales, M.A.; Macedo, D.A.; Gomes, U.U.; Cavalcante, D. Green synthesis of CoWO4 powders using agar-agar from red seaweed (Rhodophyta): Structure, magnetic properties and battery-like behavior. Mater. Chem. Phys. 2020, 242, 122544–122554. [Google Scholar] [CrossRef]
- Oliveira, Y.L.; Gouveia, A.F.; Costa, M.J.S.; Lopes, F.H.P.; Sczancoski, J.C.; Longo, E.; Luz, G.E.; Santos, R.S.; Cavalcante, L.S. Investigation of electronic structure, morphological features, optical, colorimetric, and supercapacitor electrode properties of CoWO4 crystals. Mater. Sci. Energy Techn. 2022, 5, 125–144. [Google Scholar] [CrossRef]
- Xiao, E.C.; Liu, M.; Ren, Q.; Cao, Z.; Guo, M.; Dou, G.; Qi, Z.M.; Shi, F. Phonon characteristics and dielectric properties of a phase pure CoWO4 ceramic. Cer. Inter. 2020, 46, 15705–15708. [Google Scholar] [CrossRef]
- Song, X.C.; Yang, E.; Ma, R.; Chen, H.F.; Zhao, Y. Sodium dodecyl sulfate-assisted synthesis of CoWO4 nanorods. J. Nanopart. Res. 2008, 10, 709–713. [Google Scholar] [CrossRef]
- Thongtem, S.; Wannapop, S.; Thongtem, T. Characterization of CoWO4 nano-particles produced using the spray pyrolysis. Cer. Inter. 2009, 35, 2087–2091. [Google Scholar] [CrossRef]
- Song, Z.; Ma, J.; Sun, H.; Sun, Y.; Fang, J.; Liu, Z.; Gao, C.; Liu, Y.; Zhao, J. Low-temperature molten salt synthesis and characterization of CoWO4 nano-particles. Mater. Sci. Engin. B 2009, 163, 62–65. [Google Scholar] [CrossRef]
- Subramanian, U.; Naik, S.J.; Tangsali, R.B.; Salker, A.V. Upconversion luminescence of cerium doped CoWO4 nanomaterials. J. Lumin. 2013, 134, 464–468. [Google Scholar] [CrossRef]
- Zhang, D.; Guo, C.; Hu, Y.; Chen, H.; Liu, H.; Zhang, H.; Wang, X. Large-scale synthesis and photoluminescence of cobalt tungstate nanowires. Phys. Rev. B 2013, 87, 035416–035424. [Google Scholar] [CrossRef]
- Jeyakanthan, M.; Subramanian, U.; Tangsali, R.B. Enhanced photoluminescence of CoWO4 in CoWO4/PbWO4 nanocomposites. J. Mater. Sci. Mater. Electron. 2018, 29, 1914–1924. [Google Scholar] [CrossRef]
- Amouzegara, Z.; Naghizadeh, R.; Rezaie, H.R.; Ghahari, M.; Aminzare, M. Microwave engineering of ZnWO4 nanostructures: Towards morphologically favorable structures for photocatalytic activity. Cer. Inter. 2015, 41, 8352–8359. [Google Scholar] [CrossRef]
- Hojamberdieva, M.; Kanakala, R.; Ruzimuradov, O.; Yan, Y.; Zhu, G.; Xu, Y. Besom-like CdWO4 structures composed of single-crystalline nanorods grown under a simple hydrothermal process in ultra-wide pH range. Opt. Mater. 2012, 34, 1954–1957. [Google Scholar] [CrossRef]
- Dimitriev, Y.; Gancheva, M.; Iordanova, R. Mechanochemical synthesis of nanocrystalline ZnWO4 at room temperature. J. Alloys Compd. 2012, 519, 161–166. [Google Scholar] [CrossRef]
- Gancheva, M.; Iordanova, R.; Mihailov, L. Direct mechanochemical synthesis and characterization of SrWO4 nanoparticles. Bulg. Chem. Commun. 2022, 54, 337–342. [Google Scholar]
- Gancheva, M.; Koseva, I.; Iordanova, R.; Tzvetkov, P.; Ivanov, P. The Influence of High-Energy Milling on the Phase Formation, Structural, and Photoluminescent Properties of CaWO4 Nanoparticles. Materials 2024, 17, 3724–3738. [Google Scholar]
- Gancheva, M.; Iordanova, R.; Ivanov, P.; Yordanova, A. Effect of Ball Milling Speeds on the Phase Formation and Optical Properties of α-ZnMoO4 and ß-ZnMoO4 Nanoparticles. J. Manuf. Mater. Process. 2025, 9, 118. [Google Scholar] [CrossRef]
- Gancheva, M.; Iordanova, R.; Avdeev, G.; Piroeva, I.; Ivanov, P. Direct mechanochemical synthesis of SrMoO4: Structural and luminescence properties. RSC Mechanochem. 2025, 2, 459–467. [Google Scholar] [CrossRef]
- Gancheva, M.; Iordanova, R.; Koseva, I.; Piroeva, I.; Ivanov, P. Structural and optical properties of BaWO4 obtained by fast mechanochemical treatment. Inorganics 2025, 13, 172. [Google Scholar] [CrossRef]
- Guia, L.; Yanga, H.; Zhao, Q.; Li, E. Synthesis of low temperature firing scheelite-type BaWO4 microwave dielectric ceramics with high performances. Ceram. Int. 2018, 46, 1360–1365. [Google Scholar] [CrossRef]
- Jena, P.; Gupta, S.K.; Verma, N.K.; Singh, A.K.; Kadam, R.M. Energy transfer dynamics and time resolved photolumines-cence in BaWO4: Eu3+ nanophosphors synthesized by mechanical activation. New J. Chem. 2017, 41, 8947–8958. [Google Scholar] [CrossRef]
- Balamurugan, S.; Thomas, S.M.; Ashika, S.A.; Sana Fathima, T.K. Profound impact on different properties of calcium tungstatescheelite, CaWO4 phase stabilized via wider synthesis conditions. Inorg. Chem. Commun. 2023, 155, 111090–111099. [Google Scholar] [CrossRef]
- Gancheva, M.; Naydenov, A.; Iordanova, R.; Nihtianova, D.; Stefanov, P. Mechanochemically assisted solid state synthesis, characterization, and catalytic properties of MgWO4. J. Mater. Sci. 2015, 50, 3447–3456. [Google Scholar] [CrossRef]
- Michalska, M.; Jasinski, J.B.; Pavlovsky, J.; Zurek-Siworska, P.; Sikora, A.; Gołębiewski, P.; Szysiak, A.; Matejka, V.; Seidlerova, J. Solid state-synthesized lanthanum orthovanadate (LaVO4) Co-doped with Eu as efficient photoluminescent material. J. of Lumin. 2021, 233, 117934–117946. [Google Scholar] [CrossRef]
- Shekarnoush, M.; Aguirre-Tostado, F.S.; Fernandez-Izquierdo, L.; Lopez, M.Q. Band gap tuning in inorganic chlorine-based halide perovskites via solid-state synthesis at room temperature. J. Solid State Chem. 2025, 348, 125341–125349. [Google Scholar] [CrossRef]
- Yang, H.K.; Moon, B.K.; Choi, B.C.; Jeong, J.H.; Kim, J.H.; Kim, K.H. Up-converted luminescence in Yb, Tm co-doped LaGaO3 phosphors by high-energy ball milling and solid state reaction. Solid State Sci. 2012, 14, 236–240. [Google Scholar] [CrossRef]
- Daniel, M.F.; Desbat, B.; Lassegues, J.C.; Gerand, B.; Figlarz, M. Infrared and Raman study of WO3 tungsten trioxides and WO3, xH2O tungsten trioxide tydrates. J. Solid State Chem. 1987, 67, 235–247. [Google Scholar] [CrossRef]
- Rahimi-Nasrabadi, M.; Naderi, H.R.; Karimi, M.S.; Ahmadi, F.; Pourmortazavi, S.M. Cobalt carbonate and cobalt oxide nanoparticles synthesis, characterization and supercapacitive evaluation. Mater. Sci. Mater. Electron. 2017, 28, 1877–1888. [Google Scholar] [CrossRef]
- Yang, J.; Cheng, H.; Frost, R.L. Synthesis and characterisation of cobalt hydroxy carbonate Co2CO3(OH)2 nanomaterials. Spectrochim. Acta. A 2011, 78, 420–428. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Barwar, B.; Barbar, S.K. Impact of Ni doping into Co2SiO4 compound: Synthesis, structural, morphological, dielectric, optical, photoluminescence, and magnetic properties. Cer. Inter. 2025, 51, 20839–20853. [Google Scholar]
- Tauc, J. Absorption edge and internal electric fields in amorphous semiconductors. Mater. Res. Bull. 1970, 5, 721–729. [Google Scholar] [CrossRef]
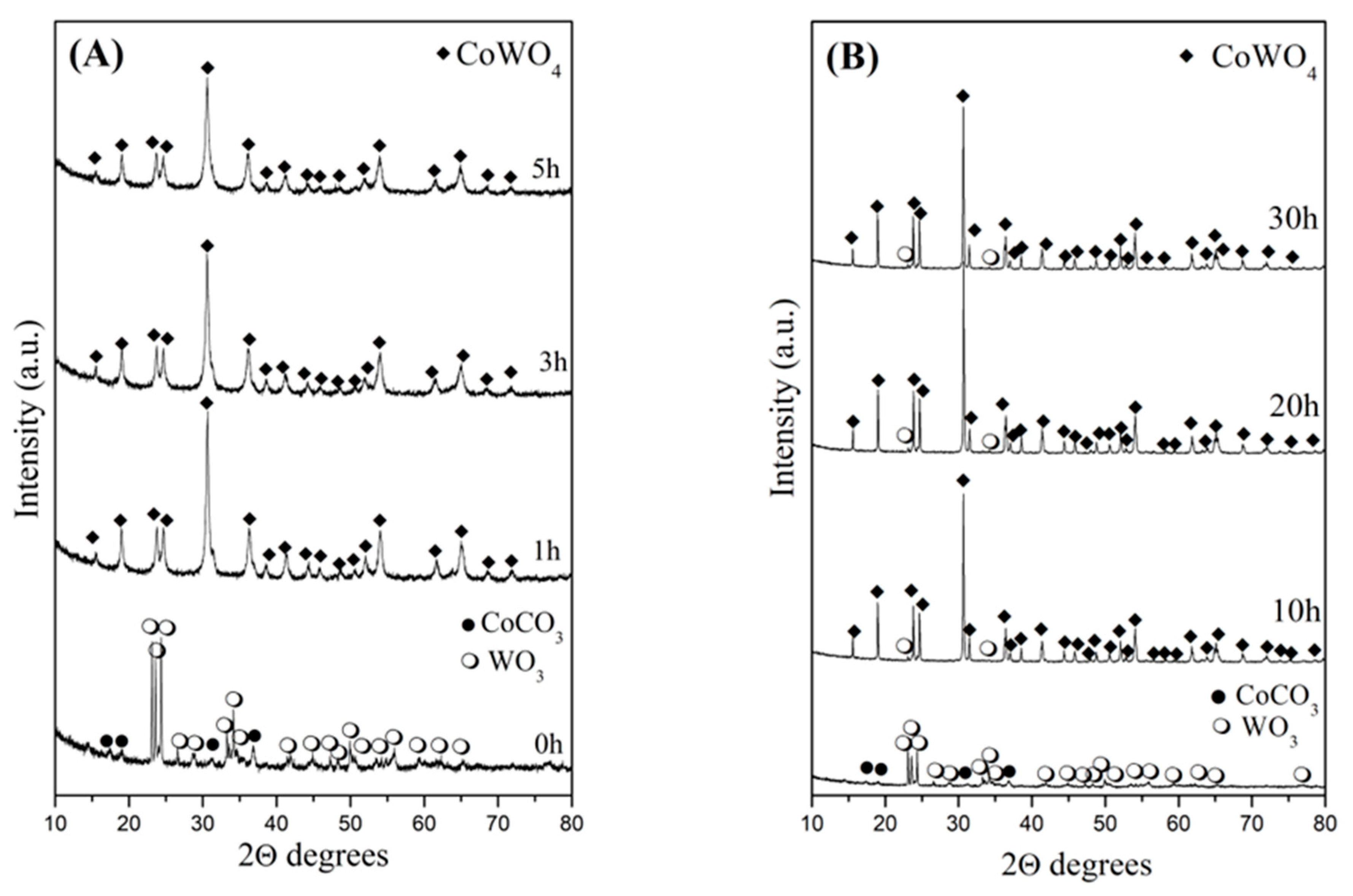

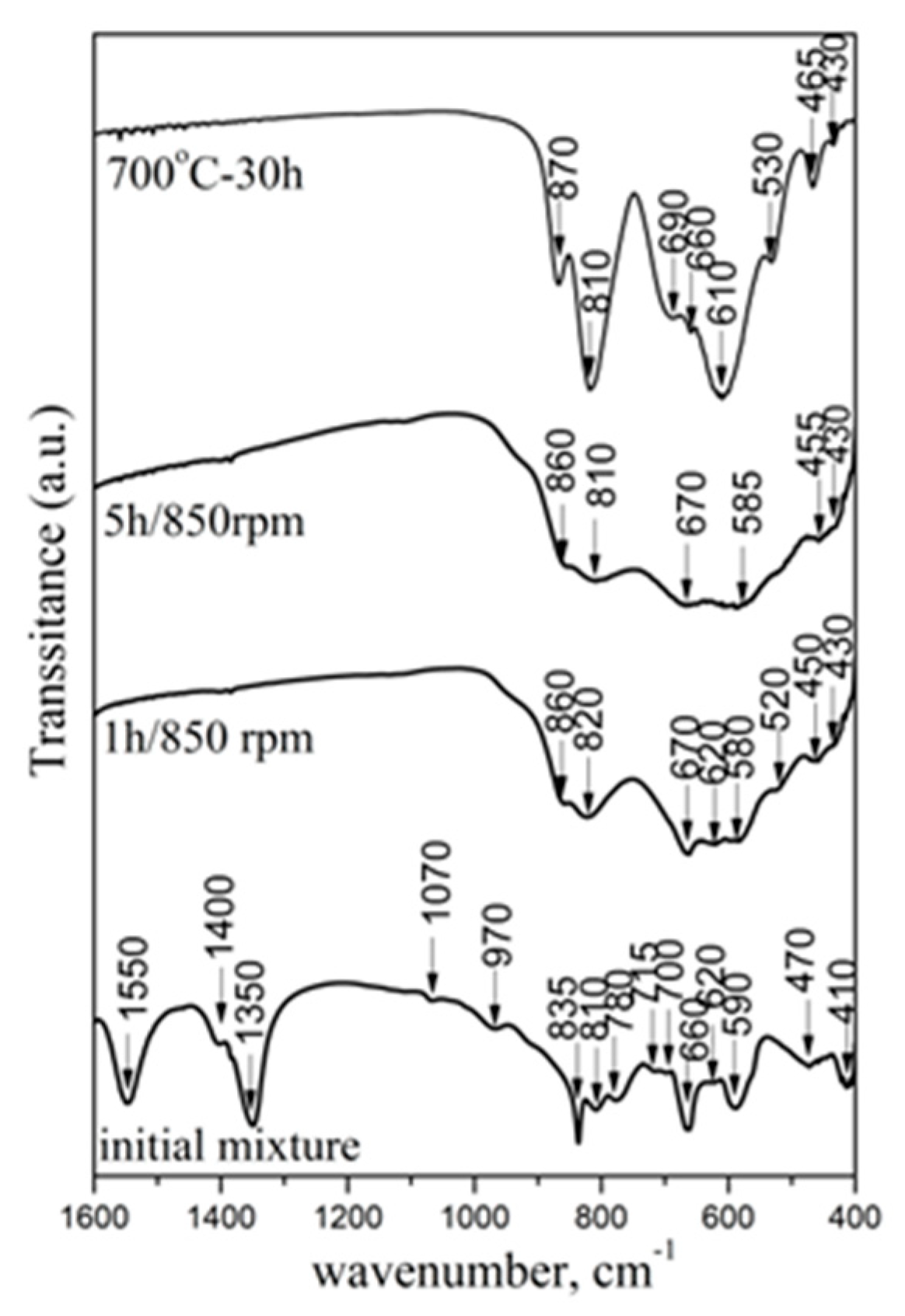
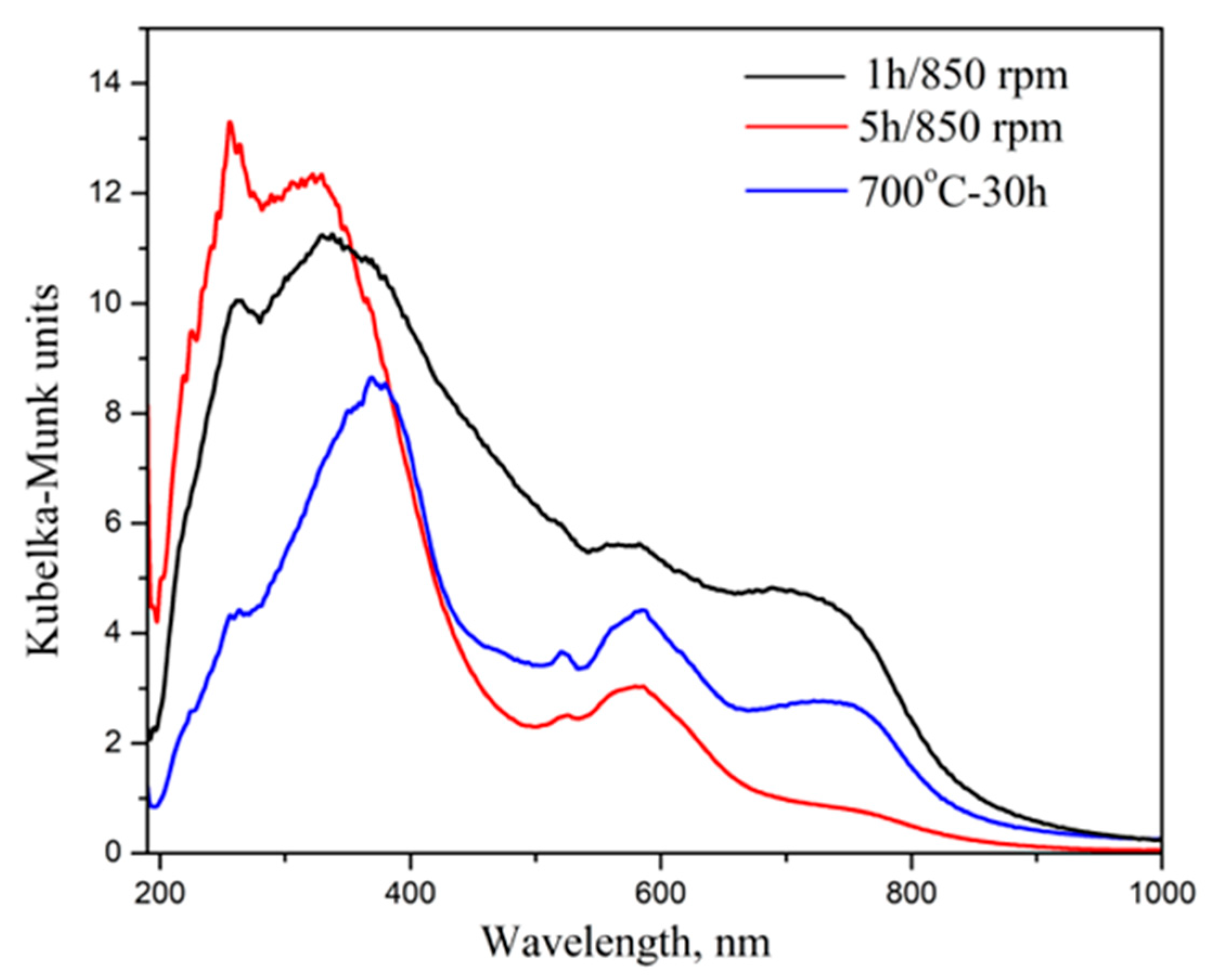
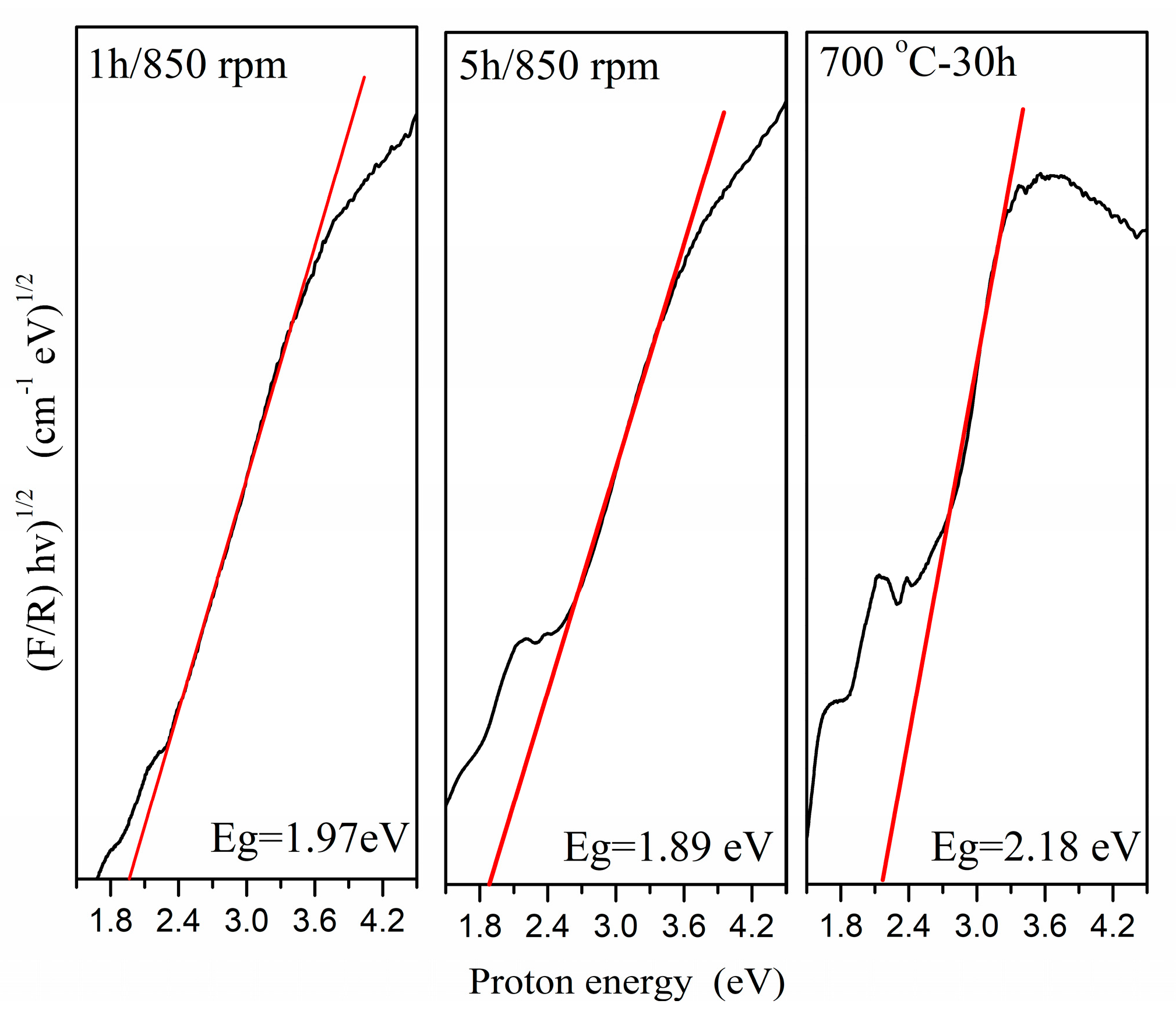

| Samples | V/106 pm3 | a/Å | b/Å | c/Å | Strain % | Crystallite Size, nm |
|---|---|---|---|---|---|---|
| CoWO4-1 h/850 rpm | 131.59 | 4.6630 (±6) | 5.6899 (±7) | 4.9599 (±6) | 0.107 | 17 |
| CoWO4-5 h/850 rpm | 132.64 | 4.6593 (±2) | 5.7141 (±1) | 4.9810 (±1) | 0.069 | 22 |
| CoWO4-700 °C-30 h | 131.19 | 4.6679 (±1) | 5.6808 (±1) | 4.9473 (±1) | 0.008 | 178 |
| (2 wt% WO3) | - | - | - | - | - | - |
| CoWO4 (PDF-98-0015851) | 131.52 | 4.6700 | 5.6870 | 4.9520 | - | - |
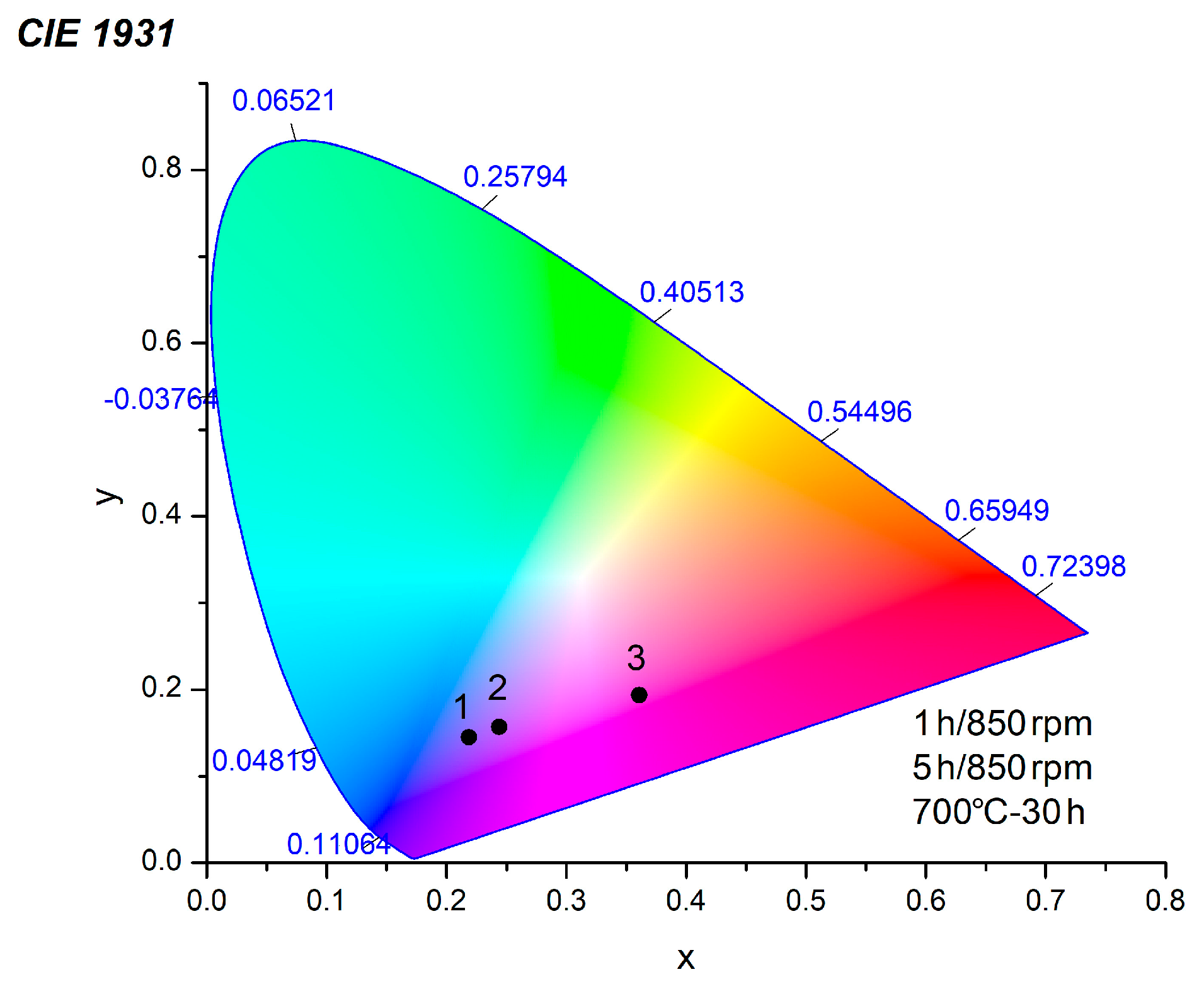
| Samples | x | y | QY % | τ [ns] | [ns] |
|---|---|---|---|---|---|
| CoWO4, 1 h, 850 rpm | 0.2186 | 0.1447 | 0.34 | = 0.43, = 1.60, = 0.72 | 0.40 |
| CoWO4, 5 h, 850 rpm | 0.2439 | 0.1569 | 0.67 | = 0.83, = 3.00, = 0.94 | 0.65 |
| CoWO4, 700 °C, 30 h | 0.3609 | 0.1938 | 0.60 | = 0.75, = 2.56, = 0.86 | 0.60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iordanova, R.; Gancheva, M.; Koseva, I.; Avdeev, G.; Ivanov, P. Synthesis, Characterization and Optical Behavior of Nanocrystalline CoWO4. Molecules 2025, 30, 3843. https://doi.org/10.3390/molecules30193843
Iordanova R, Gancheva M, Koseva I, Avdeev G, Ivanov P. Synthesis, Characterization and Optical Behavior of Nanocrystalline CoWO4. Molecules. 2025; 30(19):3843. https://doi.org/10.3390/molecules30193843
Chicago/Turabian StyleIordanova, Reni, Maria Gancheva, Iovka Koseva, Georgi Avdeev, and Petar Ivanov. 2025. "Synthesis, Characterization and Optical Behavior of Nanocrystalline CoWO4" Molecules 30, no. 19: 3843. https://doi.org/10.3390/molecules30193843
APA StyleIordanova, R., Gancheva, M., Koseva, I., Avdeev, G., & Ivanov, P. (2025). Synthesis, Characterization and Optical Behavior of Nanocrystalline CoWO4. Molecules, 30(19), 3843. https://doi.org/10.3390/molecules30193843






