New Aluminum Complexes with an Asymmetric Amidine–Imine Ligand: Synthesis, Characterization, and Application in Catalysis
Abstract
1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Amidine–Imine Ligand
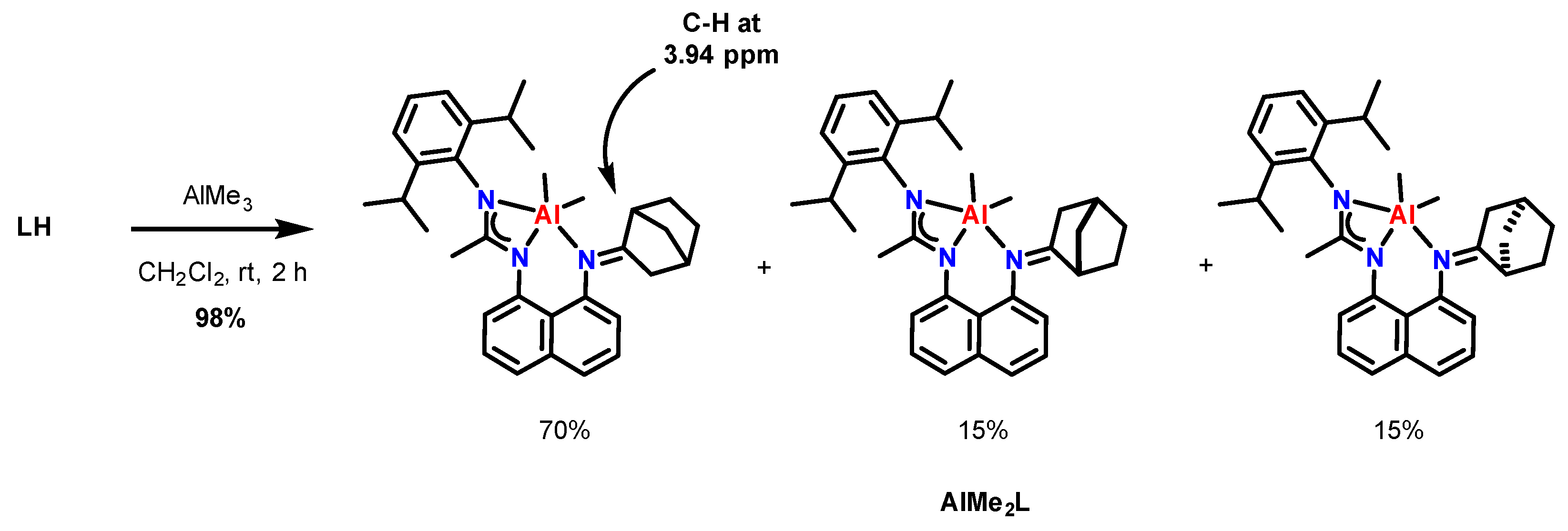
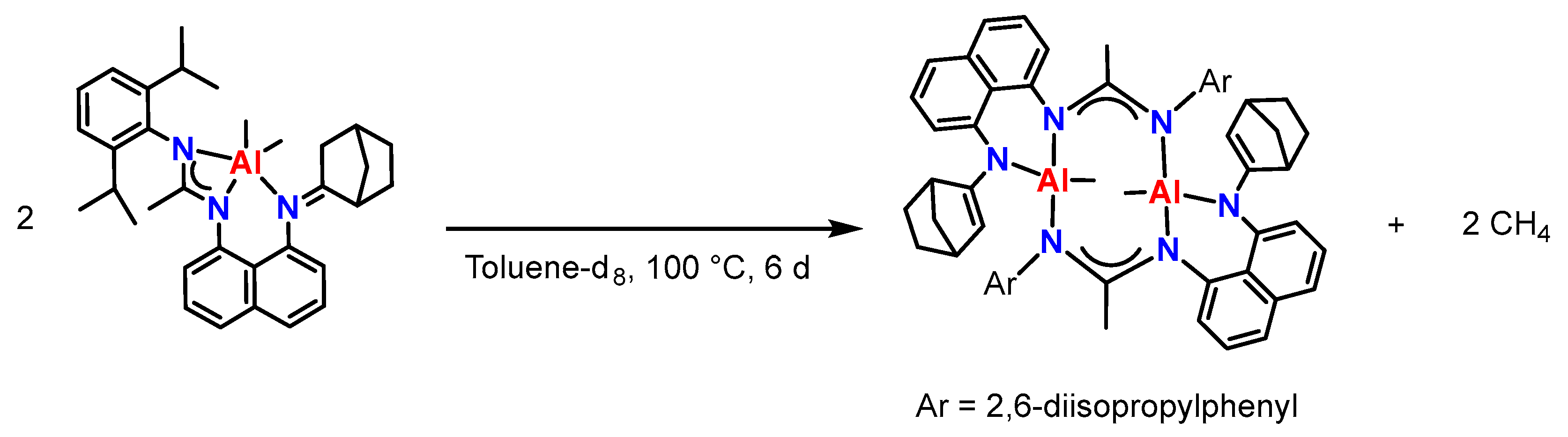
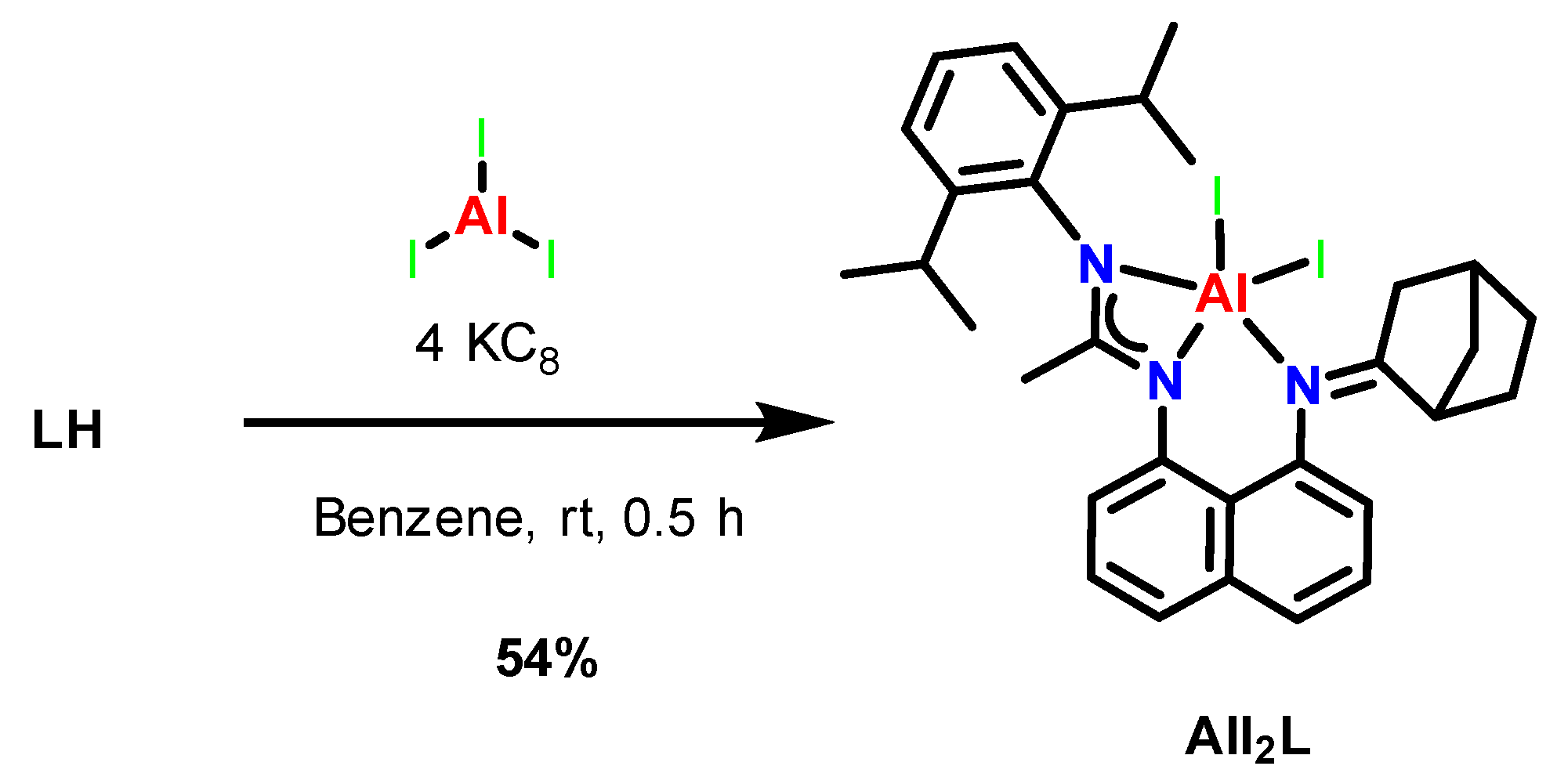
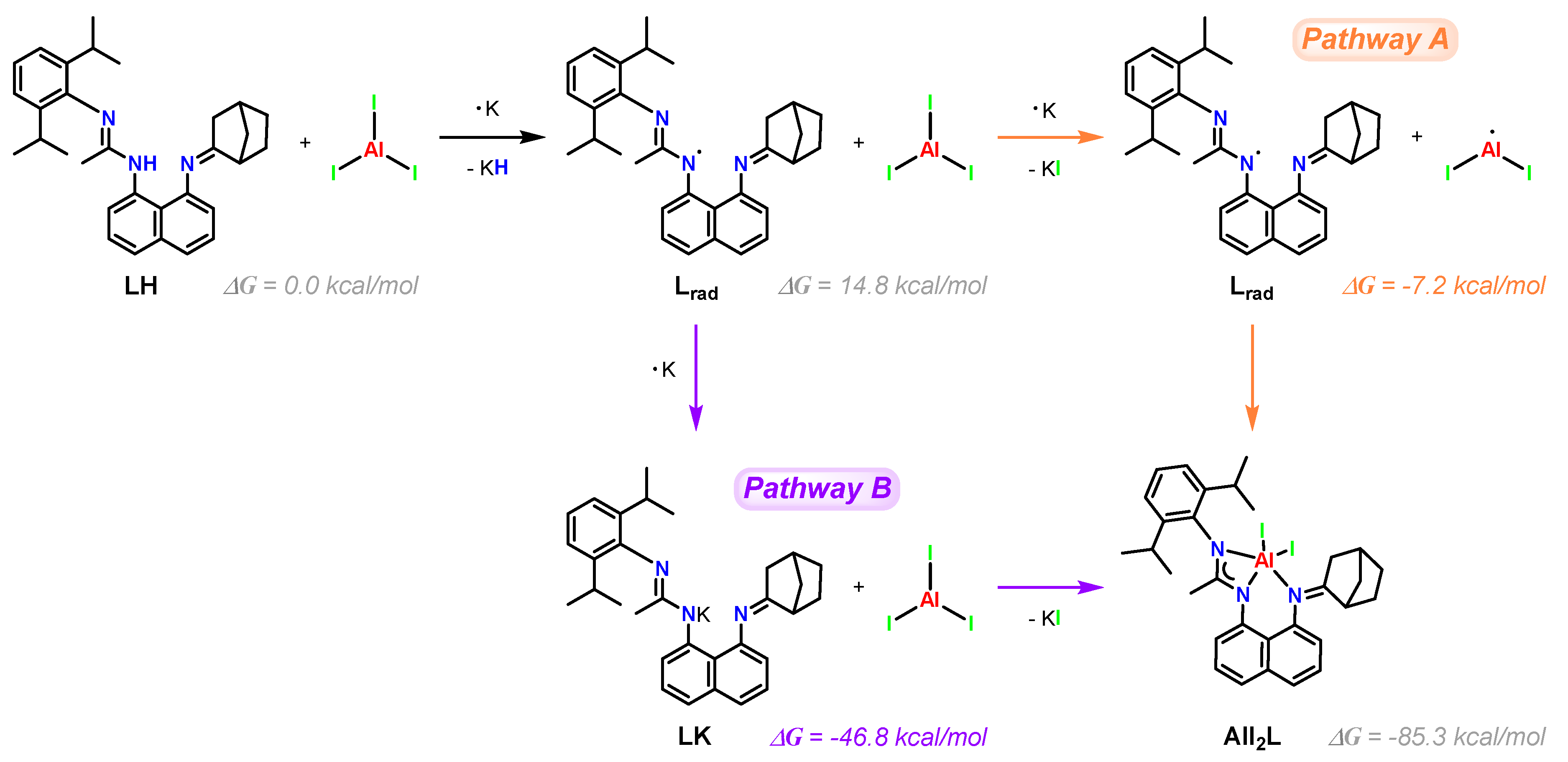
2.2. Synthesis and Characterization of New Aluminum(III) Complexes with an Asymmetric Amidine–Amine Ligand
2.3. Catalytic Evaluation of AlMe2L as a Two-Component System for the Synthesis of Cyclic Carbonates
2.4. Catalytic Evaluation of AlI2L as a Single-Component Catalyst for the Synthesis of Cyclic Carbonates
2.5. Conclusions
3. Materials and Methods
3.1. General Comments
3.2. Synthesis
General Catalysis
3.3. X-Ray Data
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pospech, J.; Fleischer, I.; Franke, R.; Buchholz, S.; Beller, M. Alternative Metals for Homogeneous Catalyzed Hydroformylation Reactions. In Angewandte Chemie—International Edition; Wiley-VCH Verlag: Weinheim, Germany, 2013; pp. 2852–2872. [Google Scholar] [CrossRef]
- Godoy, F.; Segarra, C.; Poyatos, M.; Peris, E. Palladium Catalysts with Sulfonate-Functionalized-NHC Ligands for Suzuki-Miyaura Cross-Coupling Reactions in Water. Organometallics 2011, 30, 684–688. [Google Scholar] [CrossRef]
- Que, L.; Tolman, W.B. Biologically Inspired Oxidation Catalysis. Nature 2008, 455, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, T. (Ed.) Superbases for Organic Synthesis; Wiley: Hoboken, NJ, USA, 2009. [Google Scholar] [CrossRef]
- Aly, A.A.; El-Din, A.M.N. Functionality of Amidines and Amidrazones. Arkivoc 2008, 2008, 153–194. [Google Scholar] [CrossRef]
- Rajak, S.; Chair, K.; Rana, L.K.; Kaur, P.; Maris, T.; Duong, A. Amidine/Amidinate Cobalt Complexes: One-Pot Synthesis, Mechanism, and Photocatalytic Application for Hydrogen Production. Inorg. Chem. 2020, 59, 14910–14919. [Google Scholar] [CrossRef]
- Conde-Guadano, S.; Hanton, M.; Tooze, R.P.; Danopoulos, A.A.; Braunstein, P. Amidine- and Amidinate-Functionalised N-Heterocyclic Carbene Complexes of Silver and Chromium. Dalton Trans. 2012, 41, 12558. [Google Scholar] [CrossRef]
- Lane, A.C.; Vollmer, M.V.; Laber, C.H.; Melgarejo, D.Y.; Chiarella, G.M.; Fackler, J.P.; Yang, X.; Baker, G.A.; Walensky, J.R. Multinuclear Copper(I) and Silver(I) Amidinate Complexes: Synthesis, Luminescence, and CS2 Insertion Reactivity. Inorg. Chem. 2014, 53, 11357–11366. [Google Scholar] [CrossRef]
- Collins, R.A.; Russell, A.F.; Scott, R.T.W.; Bernardo, R.; van Doremaele, G.H.J.; Berthoud, A.; Mountford, P. Monometallic and Bimetallic Titanium κ1 -Amidinate Complexes as Olefin Polymerization Catalysts. Organometallics 2017, 36, 2167–2181. [Google Scholar] [CrossRef]
- Bag, P.; Weetman, C.; Inoue, S. Experimental Realisation of Elusive Multiple-Bonded Aluminium Compounds: A New Horizon in Aluminium Chemistry. Angew. Chem. Int. Ed. 2018, 57, 14394–14413. [Google Scholar] [CrossRef] [PubMed]
- Qian, F.; Liu, K.; Ma, H. Amidinate Aluminium Complexes: Synthesis, Characterization and Ring-Opening Polymerization of Rac-Lactide. Dalton Trans. 2010, 39, 8071. [Google Scholar] [CrossRef]
- Hobson, K.; Carmalt, C.J.; Bakewell, C. Aluminum Amidinates: Insights into Alkyne Hydroboration. Inorg. Chem. 2021, 60, 10958–10969. [Google Scholar] [CrossRef]
- Rios Yepes, Y.; Martínez, J.; Rangel Sánchez, H.; Quintero, C.; Ortega-Alfaro, M.C.; López-Cortés, J.G.; Daniliuc, C.G.; Antiñolo, A.; Ramos, A.; Rojas, R.S. Aluminum Complexes with New Non-Symmetric Ferrocenyl Amidine Ligands and Their Application in CO2 Transformation into Cyclic Carbonates. Dalton Trans. 2020, 49, 1124–1134. [Google Scholar] [CrossRef] [PubMed]
- Saltarini, S.; Villegas-Escobar, N.; Martínez, J.; Daniliuc, C.G.; Matute, R.A.; Gade, L.H.; Rojas, R.S. Toward a Neutral Single-Component Amidinate Iodide Aluminum Catalyst for the CO2 Fixation into Cyclic Carbonates. Inorg. Chem. 2021, 60, 1172–1182. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y.; Laaksonen, A.; Krook-Riekkola, A.; Yang, Z.; Lu, X.; Ji, X. Carbon Recycling—An Immense Resource and Key to a Smart Climate Engineering: A Survey of Technologies, Cost and Impurity Impact. Renew. Sustain. Energy Rev. 2020, 131, 110010. [Google Scholar] [CrossRef]
- Pescarmona, P.P. Cyclic Carbonates Synthesised from CO2: Applications, Challenges and Recent Research Trends. Curr. Opin. Green Sustain. Chem. 2021, 29, 100457. [Google Scholar] [CrossRef]
- Yakovenko, M.V.; Cherkasov, A.V.; Fukin, G.K.; Cui, D.; Trifonov, A.A. Lanthanide Complexes Coordinated by a Dianionic Bis(Amidinate) Ligand with a Rigid Naphthalene Linker. Eur. J. Inorg. Chem. 2010, 2010, 3290–3298. [Google Scholar] [CrossRef]
- Ciou, J.-M.; Zhu, H.-F.; Chang, C.-W.; Chen, J.-Y.; Lin, Y.-F. Physical Organic Studies and Dynamic Covalent Chemistry of Picolyl Heterocyclic Amino Aminals. RSC Adv. 2020, 10, 40421–40427. [Google Scholar] [CrossRef]
- Mandal, D.; Demirer, T.I.; Sergeieva, T.; Morgenstern, B.; Wiedemann, H.T.A.; Kay, C.W.M.; Andrada, D.M. Evidence of Al II Radical Addition to Benzene. Angew. Chem. Int. Ed. 2023, 62, e202217184. [Google Scholar] [CrossRef]
- Bhattacharyya, U.; Thomas, R.; Puchta, R. The Proton Sponge 1,8-Bis(Dimethylamino)Naphthalene: The Quicker-Picker-Upper Also for s-Block Metal Cations? Chem. Phys. Lett. 2021, 777, 138735. [Google Scholar] [CrossRef]
- Lyubov, D.M.; Cherkasov, A.V.; Fukin, G.K.; Lyssenko, K.A.; Rychagova, E.A.; Ketkov, S.Y.; Trifonov, A.A. Rare-Earth Metal-Mediated PhC≡N Insertion into N,N-Bis(Trimethylsilyl)Naphthalene-1,8-Diamido Dianion—A Synthetic Approach to Complexes Coordinated by Ansa-Bridged Amido-Amidinato Ligand. Dalton Trans. 2018, 47, 438–451. [Google Scholar] [CrossRef] [PubMed]
- Bag, P.; Porzelt, A.; Altmann, P.J.; Inoue, S. A Stable Neutral Compound with an Aluminum–Aluminum Double Bond. J. Am. Chem. Soc. 2017, 139, 14384–14387. [Google Scholar] [CrossRef]
- Hobson, K.; Carmalt, C.J.; Bakewell, C. Recent Advances in Low Oxidation State Aluminium Chemistry. Chem. Sci. 2020, 11, 6942–6956. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Kundu, S.; Stückl, A.C.; Zhu, H.; Keil, H.; Herbst-Irmer, R.; Stalke, D.; Schwederski, B.; Kaim, W.; Andrada, D.M.; et al. A Stable Neutral Radical in the Coordination Sphere of Aluminum. Angew. Chem. Int. Ed. 2017, 56, 397–400. [Google Scholar] [CrossRef]
- Kulkarni, A.; Arumugam, S.; Francis, M.; Reddy, P.G.; Nag, E.; Gorantla, S.M.N.V.T.; Mondal, K.C.; Roy, S. Solid-State Isolation of Cyclic Alkyl(Amino) Carbene (CAAC)-Supported Structurally Diverse Alkali Metal-Phosphinidenides. Chem. A Eur. J. 2021, 27, 200–206. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R.; Young, C. Synthesis of Cyclic Carbonates from Epoxides and CO2. Green Chem. 2010, 12, 1514. [Google Scholar] [CrossRef]
- Rios Yepes, Y.; Quintero, C.; Osorio Meléndez, D.; Daniliuc, C.G.; Martínez, J.; Rojas, R.S. Cyclic Carbonates from CO2 and Epoxides Catalyzed by Tetra- and Pentacoordinate Amidinate Aluminum Complexes. Organometallics 2019, 38, 469–478. [Google Scholar] [CrossRef]
- North, M.; Pasquale, R. Mechanism of Cyclic Carbonate Synthesis from Epoxides and CO2. Angew. Chem. Int. Ed. 2009, 48, 2946–2948. [Google Scholar] [CrossRef]
- Tian, D.; Liu, B.; Gan, Q.; Li, H.; Darensbourg, D.J. Formation of Cyclic Carbonates from Carbon Dioxide and Epoxides Coupling Reactions Efficiently Catalyzed by Robust, Recyclable One-Component Aluminum-Salen Complexes. ACS Catal. 2012, 2, 2029–2035. [Google Scholar] [CrossRef]
- Clegg, W.; Harrington, R.W.; North, M.; Pasquale, R. Cyclic Carbonate Synthesis Catalysed by Bimetallic Aluminium–Salen Complexes. Chem. A Eur. J. 2010, 16, 6828–6843. [Google Scholar] [CrossRef] [PubMed]
- Rulev, Y.A.; Gugkaeva, Z.; Maleev, V.I.; North, M.; Belokon, Y.N. Robust Bifunctional Aluminium–Salen Catalysts for the Preparation of Cyclic Carbonates from Carbon Dioxide and Epoxides. Beilstein J. Org. Chem. 2015, 11, 1614–1623. [Google Scholar] [CrossRef] [PubMed]
- Castro-Osma, J.A.; Lara-Sánchez, A.; North, M.; Otero, A.; Villuendas, P. Synthesis of Cyclic Carbonates Using Monometallic, and Helical Bimetallic, Aluminium Complexes. Catal. Sci. Technol. 2012, 2, 1021. [Google Scholar] [CrossRef]
- Boeré, R.T.; Klassen, V.; Wolmershäuser, G. Synthesis of Some Very Bulky N,N′-Disubstituted Amidines and Initial Studies of Their Coordination Chemistry. J. Chem. Soc. Dalton Trans. 1998, 24, 4147–4154. [Google Scholar] [CrossRef]
- Bruker, S. SADABS, Bruker; AXS Inc.: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Hohenberg, P.; Kohn, W. Inhomogeneous Electron Gas. Phys. Rev. 1964, 136, B864–B871. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133–A1138. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16, Revision C.01; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian—Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The Influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theor. Chim. Acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Hay, P.J.; Wadt, W.R. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for the Transition Metal Atoms Sc to Hg. J. Chem. Phys. 1985, 82, 270–283. [Google Scholar] [CrossRef]
- Wadt, W.R.; Hay, P.J. Ab Initio Effective Core Potentials for Molecular Calculations. Potentials for Main Group Elements Na to Bi. J. Chem. Phys. 1985, 82, 284–298. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian Basis Sets for Molecular Calculations. I. Second Row Atoms, Z = 11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient Diffuse Function-augmented Basis Sets for Anion Calculations. III. The 3-21 + G Basis Set for First-row Elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- Peterson, K.A.; Figgen, D.; Goll, E.; Stoll, H.; Dolg, M. Systematically Convergent Basis Sets with Relativistic Pseudopotentials. II. Small-Core Pseudopotentials and Correlation Consistent Basis Sets for the Post-d Group 16–18 Elements. J. Chem. Phys. 2003, 119, 11113–11123. [Google Scholar] [CrossRef]
- Miertuš, S.; Scrocco, E.; Tomasi, J. Electrostatic Interaction of a Solute with a Continuum. A Direct Utilization of AB Initio Molecular Potentials for the Prevision of Solvent Effects. Chem. Phys. 1981, 55, 117–129. [Google Scholar] [CrossRef]
- Barone, V.; Cossi, M. Quantum Calculation of Molecular Energies and Energy Gradients in Solution by a Conductor Solvent Model. J. Phys. Chem. A 1998, 102, 1995–2001. [Google Scholar] [CrossRef]
- Hiroaki, S.; Shohei, S.; Sumihiro, A.; Akio, H. Near Infrared Absorption Dye and Its Use. JP 2017165857, 21 September 2017. [Google Scholar]











| Entry | Epoxides | Temperature [°C] | Yield [%] b | TOF c [h–1] |
|---|---|---|---|---|
| 1 | 1a (R = Ph) | 80 | 90 | 2.50 |
| 2 | 90 | 98 | 2.73 | |
| 3 | 1b (R = CH2Cl) | 80 | 86 | 2.39 |
| 4 | 90 | 99 | 2.75 | |
| 5 | 1c (R = n-bu) | 80 | 83 | 2.31 |
| 6 | 90 | 87 | 2.42 | |
| 7 | 1d (R = CH2OCH2(CF2)3CHF2) | 80 | 32 | 0.89 |
| 8 | 90 | 80 | 2.23 | |
| 9 | 1e (R = 4-ClPh) | 80 | 22 | 0.61 |
| 10 | 90 | 37 | 1.00 | |
| 11 | 1f (R = 4-BrPh) | 80 | 36 | 1.00 |
| 12 | 90 | 58 | 1.60 |

| Entry | Epoxides | CO2 Pressure [bar] | Yield [%] b | TOF c [h–1] |
|---|---|---|---|---|
| 1 | 1a (R = Ph) | 1 | 21 | - |
| 2 | 5 | 80 | 2.22 | |
| 3 | 1b (R = CH2Cl) | 1 | <5 | - |
| 4 | 5 | 33 | 0.92 | |
| 5 | 1c (R = n-bu) | 1 | 17 | - |
| 6 | 5 | 78 | 2.16 | |
| 7 | 1d (R = CH2OCH2(CF2)3CHF2) | 1 | <5 | - |
| 8 | 5 | 42 | 1.17 | |
| 9 | 1e (R = 4-ClPh) | 1 | <5 | - |
| 10 | 5 | 28 | 0.78 | |
| 11 | 1f (R = 4-BrPh) | 1 | 11 | - |
| 12 | 5 | 71 | 1.94 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez Zamorano, F.; Rojas, M.J.; Mallet-Ladeira, S.; Cabrera, A.R.; Garo, J.; Sotiropoulos, J.-M.; Maerten, E.; Madec, D.; Rojas, R.S. New Aluminum Complexes with an Asymmetric Amidine–Imine Ligand: Synthesis, Characterization, and Application in Catalysis. Molecules 2025, 30, 3842. https://doi.org/10.3390/molecules30193842
Gómez Zamorano F, Rojas MJ, Mallet-Ladeira S, Cabrera AR, Garo J, Sotiropoulos J-M, Maerten E, Madec D, Rojas RS. New Aluminum Complexes with an Asymmetric Amidine–Imine Ligand: Synthesis, Characterization, and Application in Catalysis. Molecules. 2025; 30(19):3842. https://doi.org/10.3390/molecules30193842
Chicago/Turabian StyleGómez Zamorano, Fernando, María José Rojas, Sonia Mallet-Ladeira, Alan R. Cabrera, Jordan Garo, Jean-Marc Sotiropoulos, Eddy Maerten, David Madec, and René S. Rojas. 2025. "New Aluminum Complexes with an Asymmetric Amidine–Imine Ligand: Synthesis, Characterization, and Application in Catalysis" Molecules 30, no. 19: 3842. https://doi.org/10.3390/molecules30193842
APA StyleGómez Zamorano, F., Rojas, M. J., Mallet-Ladeira, S., Cabrera, A. R., Garo, J., Sotiropoulos, J.-M., Maerten, E., Madec, D., & Rojas, R. S. (2025). New Aluminum Complexes with an Asymmetric Amidine–Imine Ligand: Synthesis, Characterization, and Application in Catalysis. Molecules, 30(19), 3842. https://doi.org/10.3390/molecules30193842






