An Assessment of the Public Health Risk Associated with Consumption of Imported Fish Based on the Intake of Essential and Harmful Elements
Abstract
1. Introduction
2. Results and Discussion
| Nile Tilapia | Panga | Alaska Pollock | Hake | Yellowfin Sole | Pacific Cod | Flounder | Mackerel | Rainbow Trout | Salmon | Bream | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean, mean ± SD, or range of analyzed elements * (mg/kg w.w.) | |||||||||||
| Zn | 3.89 ± 0.74 2.59–5.38 6.28 ± 0.39 [26] 58.3 ± 0.6 [31] 14.0 ** [32] 1.31–3.9 [33] 3.6–11.0 [34] | 2.08 ± 1.13 0.94–5.92 3.57 ± 0.02 [35] | 2.31 ± 0.65 1.11–3.41 3.44 ** [36] | 2.18 ± 0.30 1.77–2.80 3.34 ± 0.38 [37] 3.21 ** [36] 3.30 ** [38] | 2.72 ± 0.23 2.34–3.03 | 3.19 ± 0.45 2.63–3.72 0.35 ± 0.01 [39] | 3.47 ± 0.32 3.14–3.88 2.41 ± 0.88 [40] 4.62 ± 0.71 [41] | 1.83 ± 0.54 1.38–2.49 0.27 ± 0.005 [39] 2.0 ± 0.3 [42] 8.74 ** [36] 8.95 ± 4.30 [40] 66.8 ± 1.6 [31] | 7.54 ± 1.00 6.10–8.40 5.6 ± 1.9 [26] 4.7–8.6 [43] 16.98 ** [44] | 4.86 ± 0.76 4.18–5.58 1.7–9.9 [43] 1.96 ± 0.04 [45] 3.20 ** [36] | 5.60 ± 0.54 5.06–6.25 2.3–3.5 [46] |
| Ni | 0.073 ± 0.06 0.03–0.37 <0.01–0.07 [34] 5.75 ± 1.04 [26] 0.03 ± <0.01 [35] | 0.08 ± 0.03 0.04–0.14 0.10–0.17 [47] | 0.08 ± 0.03 0.02–0.13 0.090 ** [36] | 0.08 ± 0.06 <LOD–0.17 <0.05 ** [38] 0.035 ** [36] | 0.09 ± 0.04 0.05–0.14 | 0.10 ± 0.03 0.10–0.11 0.10 ± 0.01 [39] | 0.33 ± 0.02 0.31–0.35 0.02 ± 0.02 [41] 0.04 ± 0.06 [40] | 0.33 ± 0.03 0.30–0.36 0.070 ** [36] 0.82 ± 0.15 [42] 0.16 ± 0.12 [40] 0.03 ± 0.01 [39] 0.04 ± <0.01 [31] | 0.27 ± 0.02 0.25–0.30 2.38 ± 0.28 [26] 5.25 ** [44] | 0.33 ± 0.02 0.31–0.36 0.038 ** [36] | 0.32 ± 0.02 0.29–0.34 <LOD–0.13 [46] |
| Fe | 1.84 ± 0.29 1.04–2.29 <LOQ–91.5 [48] 2.4–17.0 [34] 28.0 ** [32] | 1.14 ± 0.39 0.31–1.78 9.35 ± 0.03 [35] | 1.45 ± 0.45 0.96–2.58 1.35 ** [36] | 1.42 ± 0.20 1.06–1.70 1.35 ** [36] 1.7 ** [38] | 1.32 ± 0.42 0.85–1.77 | 1.53 ± 0.15 1.31–1.62 2.37 ± 0.10 [39] | 1.73 ± 0.28 1.35–1.96 3.52 ± 1.51 [40,41] | 1.61 ± 0.35 1.17–1.90 8.98 ** [36] 9.22 ± 0.11 [40,42] 3.16 ± 0.12 [39] | 1.57 ± 0.08 1.48–1.67 40.02 ** [44] 3.09–5.59 [43] | 1.48 ± 0.10 1.35–1.60 1.87 ** [36] 2.6 ± 0.21 [45] 2.46–11.90 [43] | 1.41 ± 0.10 1.32–1.56 1.3–1.5 [46] |
| Mn | 0.12 ± 0.03 0.08–0.21 <LOQ–17.7 [48] 0.05–1.41 [33] 0.40 ± 0.02 [26] 1.0 ** [32] | 0.07 ± 0.03 0.02–0.12 0.14–0.20 [47] 0.22 ± 0.01 [35] | 0.05 ± 0.01 0.02–0.07 0.059 ** [36] | 0.05 ± 0.01 0.02–0.07 0.09 ** [38] 0.054 ** [36] | 0.06 ± 0.03 0.02–0.08 | 0.06 ± 0.01 0.05–0.07 | 0.12 ± 0.01 0.11–0.14 0.19 ± 0.12 [41] 0.07 ± 0.09 [40] | 0.07 ± 0.01 0.06–0.07 0.16 ** [36] 0.46 ± 0.11 [42] 0.03 ± 0.03 [40] | 0.08 ± 0.01 0.07–0.09 1.37 ± 0.12 [26] 0.25–0.36 [43] | 0.09 ± 0.01 0.08–0.09 0.110 ** [36] 0.14–0.87 [43] | 0.09 ± <0.01 0.09–0.10 0.11–0.39 [46] |
| Cr | 0.08 ± 0.03 0.04–0.13 0.007–0.8 [34] 3.6 ± 0.45 [39] 0.03 ± 0.06 [35] 2.79 ± 0.95 [49] | 0.09 ± 0.04 0.04–0.17 | 0.08 ± 0.04 0.02–0.15 0.22 ** [36] | 0.09 ± 0.03 0.04–0.13 0.10 ** [38] 0.114 ** [36] | 0.08 ± 0.01 0.06–0.10 | 0.08 ± 0.01 0.07–0.09 0.08 ** [50] | 0.1 ± <0.01 0.09–0.10 0.32 ± 0.04 [40] | 0.01 ± 0.01 0.08–0.11 0.18 ** [36] 0.87 ± 0.11 [42] 0.44 ± 0.04 [40] 0.03 ± <0.01 [31] | 0.13 ± 0.04 0.10–0.17 3.14 ** [44] | 0.13 ± <0.01 0.012–0.013 0.314 ** [36] | 0.11 ± <0.01 0.012–0.014 0.02–0.11 [46] |
| Cu | 0.15 ± 0.07 0.06–0.33 <LOQ–42.9 [48] 0.23–0.47 [33] 0.2–9.0 [34] 1.6 ** [32] | 0.19 ± 0.04 0.09–0.25 0.16–0.23 [47] 0.64 ± 0.01 [35] | 0.20 ± 0.10 0.07–0.39 0.227 ** [36] | 0.13 ± 0.04 0.08–0.19 0.12 ± 0.04 [37] 0.16 ** [38] 0.136 ** [36] | 0.15 ± 0.02 0.12–0.18 | 0.20 ± 0.01 0.9–0.21 0.127 ± 0.01 [39] | 0.19 ± 0.02 0.02–0.22 0.33 ± 0.16 [41] | 0.19 ± 0.02 0.17–0.21 1.03 ** [36] 0.72 ± 0.02 [42] 0.28 ± 0.003 [39] | 0.21 ± 0.01 0.20–0.23 2.06 ** [44] 0.19–0.55 [43] | 0.21 ± 0.01 0.20–0.21 0.580 ** [36] 0.98 ± 0.02 [45] 0.12–0.90 [43] | 0.21 ± <0.01 0.20–0.21 0.11–0.24 [46] |
| Al | 0.24 ± 0.05 0.13–0.32 0.3–8.7 [34] | 0.14 ± 0.03 0.09–0.19 | 0.24 ± 0.09 0.10–0.42 2.53 ** [36] | 0.19 ± 0.06 0.01–0.28 0.834 ** [36] | 0.18 ± 0.03 0.1–0.22 | 0.14 ± 0.02 0.11–0.15 | 0.12 ± 0.01 0.11–0.13 1.72 ± 0.58 [41] | 0.13 ± 0.01 0.12–0.14 1.16 ** [36] | 0.11 ± 0.02 0.08–0.13 1.89–2.75 50] | 0.11 ± 0.03 0.07–0.15 0.529 ** [36] 0.23–3.72 [43] | 0.11 ± 0.01 0.10–0.13 |
| Li | 0.03 ± 0.02 0.01–0.08 0.004–0.013 [34] | 0.04 ± 0.02 0.01–0.08 | 0.03 ± 0.01 0.01–0.05 0.014 ** [36] | 0.02 ± 0.01 0.01–0.04 0.017 ** [36] | 0.04 ± 0.02 0.02–0.07 | 0.04 ± 0.02 0.02–0.06 | 0.05 ± 0.01 0.04–0.06 0.017 ± 0.007 [41] | 0.05 ± 0.02 0.04–0.07 0.018 ** [36] | 0.06 ± 0.01 0.04–0.07 | 0.06 ± 0.01 0.01–0.07 0.005 ** [36] | 0.05 ± 0.01 0.04–0.06 0.01–0.05 [46] |
| As | 0.21 ± 0.08 0.11–0.39 0.01–0.06 [34] <0.001 ** [35] | 0.24 ± 0.08 0.11–0.39 <0.017 ** [47] | 0.17 ± 0.05 0.08–0.26 | 0.18 ± 0.05 0.09–0.29 7.86 ± 2.83 [37] | 0.23 ± 0.02 0.20–0.27 | 0.16 ± 0.03 0.12–0.19 1.59 ** [50] | 0.21 ± 0.07 0.12–0.28 5.29 ± 1.87 [40] | 0.13 ± 0.03 0.10–0.16 2.51 ± 0.75 [40] <LOD [31] | 0.14 ± 0.01 0.13–0.15 | 0.16 ± 0.05 0.09–0.22 | 0.10 ± 0.03 0.05–0.12 0.0–0.3 [51] |
| Pb | 0.026 ± 0.009 0.013–0.047 <LOQ–0.3 [48] 0.34–2.7 [33] 0.001–0.041 [34] 0.094 ± 0.055 [35] 3.49 ± 1.86 [49] | 0.024 ± 0.010 0.010–0.014 0.12–0.17 [47] | 0.019 ± 0.005 0.011–0.031 0.010 ** [36] | 0.017 ± 0.004 0.012–0.024 0.016 ± 0.005 [37] 0.002 ± 0.001 [52] 0.010 ** [36] | 0.013 ± 0.04 0.007–0.016 | 0.011 ± 0.002 0.010–0.014 0.024 ** [50] 0.202 ± 0.01 [39] | 0.010 ± 0.003 0.125–0.276 0.053 ± 0.052 [41] 0.021 ± 0.059 [40] | 0.008 ± 0.003 0.103–0.163 0.009 ** [36] 0.122 ± 0.021 [42] 0.075 ± 0.024 [40] 0.457 ± 0.016 [39] 0.046 ± 0.004 [31] | 0.009 ± 0.002 0.129–0.152 <LOD [44] 0.017–0.035 [43] | 0.010 ± 0.003 0.093–0.222 0.005 ** [36] <0.01–0.02 [43] | 0.011 ± 0.004 0.053–0.123 0–0.115 [7,51] 0–0.02 ** [46] |
| Cd | 0.040 ± 0.013 0.011–0.073 0.09–1.17 [33] <0.0025 [34] 0.051 ± 0.004 [26] 0.003 ± <0.001 [31] 0.40 ± 0.01 [49] | 0.043 ± 0.011 0.026–0.072 <0.006 [47] | 0.032 ± 0.007 0.020–0.048 | 0.036 ± 0.008 0.021–0.048 0.01 ± 0.01 [37] 0.003 ± 0.001 [52] | 0.032 ± 0.010 0.013–0.045 | 0.010 ± 0.006 0.005–0.018 <0.002 [53] 0.030 ± 0.001 [39] | 0.011 ± 0.002 0.008–0.012 0.009 ± 0.006 [41] 0.002 ± <0.001 [40] | 0.011 ± 0.004 0.006–0.016 0.007 ± 0.002 [42] 0.003 ± 0.003 [40] 0.488 ± 0.012 [39] <LOD [31] | 0.013 ± 0.004 0.008–0.017 0.007 ± 0.001 [26] <LOD [44] <LOD–0.012 [43] | 0.020 ± 0.006 0.012–0.027 <LOD–0.008 [43] | 0.019 ± 0.004 0.016–0.024 <LOD–0.060 [7,52] <LOD–0.003 [46] |
| Hg | 0.055 ± 0.082 0.025–0.058 <LOD–0.16 [48] 0.001–0.16 [34] | 0.054 ± 0.010 0.031–0.070 0.10–0.69 [54] 0.002–0.018 [47] | 0.043 ± 0.008 0.028–0.058 0.037 ** [55] | 0.040 ± 0.011 0.031–0.070 0.44 ± 0.06 [56] 0.023 ± 0.002 [52] 0.041 ** [55] | 0.045 ± 0.005 0.036–0.051 | 0.031 ± 0.009 0.022–0.043 0.049 ** [55] 0.128 ** [50] | 0.058 ± 0.002 0.055–0.060 0.036 ± 0.017 [41] 0.016 ± 0.017 [40] 0.056 ** [55] | 0.037 ± 0.004 0.033–0.040 0.152 ± 0.096 [40] 0.058 ** [55] | 0.035 ± 0.003 0.033–0.038 0.010 ** [55] | 0.041 ± 0.002 0.039–0.042 | 0.029 ± 0.007 0.023–0.039 <LOD–0.391 [7,51] <LOD–0.01 [46] |
| Zn Mean ± SD (Range) | Fe Mean ± SD (Range) | Mn Mean ± SD (Range) | Cu Mean ± SD (Range) | ||||
|---|---|---|---|---|---|---|---|
| Women | Men | Women | Men | Women | Men | Men/Women | |
| Polish
Recommendations | RDA
8.0 mg/day | RDA
11.0 mg/day | RDA
18.0 mg/day | RDA
10.0 mg/day | AI
1.8 mg/day | AI
2.3 mg/day | RDA
0.9 mg/day |
| Nile tilapia | 1.7 ± 0.3 (1.1–2.3) | 1.2 ± 0.2 (0.8–1.7) | 0.36 ± 0.06 (0.20–0.44) | 0.64 ± 0.10 (0.36–0.80) | 0.23 ± 0.06 (0.17–0.41) | 0.18 ± 0.05 (0.13–0.32) | 0.58 ± 0.28 (0.24–1.27) |
| Pacific cod | 1.4 ± 0.2 (1.1–1.6) | 1.0 ± 0.1 (0.8–1.2) | 0.30 ± 0.03 (0.25–0.31) | 0.53 ± 0.05 (0.46–0.56) | 0.12 ± 0.02 (0.10–0.14) | 0.09 ± 0.02 (0.08–0.11) | 0.78 ± 0.05 (0.72–0.82) |
| Panga | 0.9 ± 0.5 (0.4–2.6) | 0.7 ± 0.4 (0.3–1.9) | 0.22 ± 0.08 (0.06–0.34) | 0.40 ± 0.14 (0.11–0.62) | 0.14 ± 0.06 (0.03–0.23) | 0.11 ± 0.05 (0.03–0.18) | 0.72 ± 0.15 (0.36–0.96) |
| Alaska pollock | 1.0 ± 0.3 (0.5–1.5) | 0.7 ± 0.2 (0.4–1.1) | 0.28 ± 0.09 (0.19–0.50) | 0.50 ± 0.16 (0.33–0.90) | 0.10 ± 0.02 (0.04–0.14) | 0.08 ± 0.02 (0.03–0.11) | 0.76 ± 0.36 (0.26–1.49) |
| Yellowfin sole | 1.2 ± 0.1 (1.0–1.3) | 0.9 ± 0.1 (0.7–1.0) | 0.26 ± 0.08 (0.16–0.34) | 0.46 ± 0.15 (0.29–0.61) | 0.11 ± 0.06 (0.04–0.16) | 0.08 ± 0.04 (0.03–0.13) | 0.57 ± 0.08 (0.45–0.69) |
| North-Pacific hake | 0.9 ± 0.1 (0.8–1.2) | 0.7 ± 0.1 (0.6–0.9) | 0.27 ± 0.04 (0.21–0.33) | 0.49 ± 0.07 (0.37–0.59) | 0.10 ± 0.03 (0.04–0.13) | 0.08 ± 0.02 (0.03–0.10) | 0.49 ± 0.15 (0.32–0.72) |
| Flounder | 1.5 ± 0.1 (1.7–1.7) | 1.1 ± 0.1 (1.0–1.2) | 0.33 ± 0.05 (0.26–0.38) | 0.60 ± 0.10 (0.47–0.68) | 0.22 ± 0.03 (0.21–0.26) | 0.17 ± 0.02 (0.16–0.21) | 0.74 ± 0.07 (0.67–0.84) |
| Mackerel | 0.8 ± 0.2 (0.6–1.1) | 0.6 ± 0.2 (0.4–0.8) | 0.31 ± 0.07 (0.23–0.37) | 0.56 ± 0.12 (0.41–0.66) | 0.13 ± 0.01 (0.12–0.14) | 0.10 ± 0.01 (0.10–0.11) | 0.73 ± 0.06 (0.67–0.81) |
| Rainbow trout | 3.3 ± 0.4 (2.7–3.7) | 2.4 ± 0.3 (1.9–2.7) | 0.30 ± 0.02 (0.29–0.32) | 0.55 ± 0.03 (0.52–0.58) | 0.16 ± 0.01 (0.14–0.16) | 0.12 ± 0.01 (0.11–0.13) | 0.83 ± 0.05 (0.79–0.90) |
| Salmon | 2.1 ± 0.3 (1.8–2.4) | 1.5 ± 0.2 (1.3–1.8) | 0.29 ± 0.02 (0.26–0.31) | 0.52 ± 0.03 (0.47–0.56) | 0.17 ± 0.01 (0.17–0.18) | 0.13 ± 0.01 (0.13–0.14) | 0.79 ± 0.02 (0.77–0.81) |
| Bream | 2.4 ± 0.2 (2.2–2.7) | 1.8 ± 0.2 (1.6–2.0) | 0.27 ± 0.02 (0.25–0.30) | 0.49 ± 0.04 (0.46–0.54) | 0.18 ± <0.01 (0.17–0.18) | 0.14 ± 0.01 (0.14–0.14) | 0.79 ± 0.02 (0.78–0.81) |
3. Materials and Methods
3.1. Characteristics of the Tested Material
3.2. Determination of Elements
3.3. Evaluation of Element Concentrations in Relation to Nutrient Requirement
3.4. Assessment of Concentrations of Harmful Elements in Relation to Standards and Risk Factors
3.5. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AI | Adequate Intake |
| BMDL | Benchmark Dose Lower Confidence Limit |
| CV-AAS | Cold Vapor Atomic Absorption Spectrometry |
| EDI | Estimated Daily Intake |
| EUMOFA | European Observatory on the Market in Fisheries and Aquaculture |
| FAO/WHO | Food and Agriculture Organization (FAO) and the World Health Organization (WHO) |
| GF-AAS | Graphite Furnace–Atomic Absorption Spectrometry |
| ICP-AES | Inductively Coupled Plasma Atomic Emission Spectrophotometry |
| JECFA | Joint FAO/WHO Expert Committee on Food Additives |
| LOD | Limits of Detection |
| LOQ | Limits of Quantification |
| NaBH4 | Sodium borohydride |
| NIH | National Institutes of Health |
| PTMI | Provisional Tolerable Monthly Intake |
| RDA | Recommended Dietary Allowance |
| RfD | Reference Dose |
| SD | Standard Deviation |
| THQ | Target Hazard Quotient |
| TTHQ | Total Target Hazard Quotient |
| TWI | Tolerable Weekly Intake |
| w.w. | Wet weight |
Appendix A
| Zn Fish Species | Nile Tilapia | Pacific Cod | Panga | Alaska Pollock | Yellowfin Sole | North-Pacific Hake | Flounder | Mackerel | Rainbow Trout | Salmon |
|---|---|---|---|---|---|---|---|---|---|---|
| Pacific cod | 0.1420 | |||||||||
| Panga | 0.0002 | 0.0252 | ||||||||
| Alaska pollock | 0.0010 | 0.0642 | 0.6285 | |||||||
| Yellowfin sole | 0.0152 | 0.2933 | 0.1992 | 0.3674 | ||||||
| North-Pacific hake | 0.0004 | 0.0382 | 0.8241 | 0.7666 | 0.2612 | |||||
| Flounder | 0.3530 | 0.5291 | 0.0048 | 0.0164 | 0.1127 | 0.0083 | ||||
| Mackerel | 0.0000 | 0.0062 | 0.5862 | 0.3394 | 0.0799 | 0.4742 | 0.0008 | |||
| Rainbow trout | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||
| Salmon | 0.0301 | 0.0004 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0028 | 0.0000 | 0.0000 | |
| Bream | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0975 |
| Ni Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.3042 | |||||||||
| Panga | 0.9050 | 0.3355 | ||||||||
| Alaska pollock | 0.8991 | 0.3489 | 0.9988 | |||||||
| Yellowfin sole | 0.4953 | 0.6777 | 0.5403 | 0.5566 | ||||||
| North-Pacific hake | 0.8159 | 0.3799 | 0.8942 | 0.9004 | 0.6007 | |||||
| Flounder | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | ||||
| Mackerel | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.9770 | |||
| Rainbow trout | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0098 | 0.0091 | ||
| Salmon | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.9360 | 0.9542 | 0.0087 | |
| Bream | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.4880 | 0.4862 | 0.0409 | 0.4910 |
| Fe Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.1579 | |||||||||
| Panga | 0.0016 | 0.0904 | ||||||||
| Alaska pollock | 0.0844 | 0.7057 | 0.1676 | |||||||
| Yellowfin sole | 0.0230 | 0.3641 | 0.3641 | 0.5568 | ||||||
| North-Pacific hake | 0.0646 | 0.6142 | 0.2039 | 0.8736 | 0.6424 | |||||
| Flounder | 0.5586 | 0.3621 | 0.0097 | 0.2217 | 0.0803 | 0.1797 | ||||
| Mackerel | 0.2620 | 0.7067 | 0.0432 | 0.4817 | 0.2205 | 0.4090 | 0.5410 | |||
| Rainbow trout | 0.2049 | 0.8438 | 0.0642 | 0.5905 | 0.2884 | 0.5083 | 0.4447 | 0.8360 | ||
| Salmon | 0.1095 | 0.8107 | 0.1333 | 0.8682 | 0.4741 | 0.7621 | 0.2719 | 0.5653 | 0.6843 | |
| Bream | 0.0635 | 0.6058 | 0.1962 | 0.8599 | 0.6400 | 0.9756 | 0.1769 | 0.4021 | 0.5006 | 0.7511 |
| Mn Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.0004 | |||||||||
| Panga | 0.0039 | 0.5127 | ||||||||
| Alaska pollock | 0.0000 | 0.5333 | 0.2293 | |||||||
| Yellowfin sole | 0.0001 | 0.6506 | 0.2990 | 0.8204 | ||||||
| North-Pacific hake | 0.0000 | 0.5774 | 0.2548 | 0.9210 | 0.8849 | |||||
| Flounder | 0.7734 | 0.0011 | 0.0082 | 0.0001 | 0.0002 | 0.0001 | ||||
| Mackerel | 0.0022 | 0.6173 | 0.8399 | 0.2953 | 0.3731 | 0.3241 | 0.0051 | |||
| Rainbow trout | 0.0203 | 0.2365 | 0.5414 | 0.0838 | 0.1177 | 0.0959 | 0.0364 | 0.4478 | ||
| Salmon | 0.0629 | 0.1024 | 0.2865 | 0.0280 | 0.0430 | 0.0332 | 0.0998 | 0.2267 | 0.5983 | |
| Bream | 0.0935 | 0.0662 | 0.2083 | 0.0155 | 0.0253 | 0.0189 | 0.1371 | 0.1594 | 0.4653 | 0.7986 |
| Cr Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.8799 | |||||||||
| Panga | 0.5692 | 0.6407 | ||||||||
| Alaska pollock | 0.9722 | 0.8948 | 0.5716 | |||||||
| Yellowfin sole | 0.9772 | 0.8956 | 0.5776 | 0.9930 | ||||||
| North-Pacific hake | 0.5800 | 0.6396 | 0.9741 | 0.5755 | 0.5862 | |||||
| Flounder | 0.3902 | 0.4499 | 0.7320 | 0.3933 | 0.3972 | 0.7211 | ||||
| Mackerel | 0.4271 | 0.4862 | 0.7815 | 0.4281 | 0.4336 | 0.7729 | 0.9280 | |||
| Rainbow trout | 0.0099 | 0.0130 | 0.0395 | 0.0097 | 0.0101 | 0.0396 | 0.0728 | 0.0668 | ||
| Salmon | 0.0146 | 0.0186 | 0.0519 | 0.0141 | 0.0148 | 0.0528 | 0.0887 | 0.0843 | 0.8721 | |
| Bream | 0.0907 | 0.1083 | 0.2249 | 0.0891 | 0.0920 | 0.2267 | 0.3286 | 0.3177 | 0.3573 | 0.4105 |
| Cu Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.2130 | |||||||||
| Panga | 0.2964 | 0.7273 | ||||||||
| Alaska pollock | 0.2612 | 0.8693 | 0.8319 | |||||||
| Yellowfin sole | 0.9180 | 0.1885 | 0.2824 | 0.2338 | ||||||
| North-Pacific hake | 0.4943 | 0.0627 | 0.1056 | 0.0829 | 0.5291 | |||||
| Flounder | 0.2877 | 0.8047 | 0.8984 | 0.9196 | 0.2605 | 0.0952 | ||||
| Mackerel | 0.2912 | 0.7753 | 0.9342 | 0.8848 | 0.2681 | 0.0986 | 0.9564 | |||
| Rainbow trout | 0.1285 | 0.7378 | 0.5212 | 0.6397 | 0.1106 | 0.0314 | 0.5864 | 0.5613 | ||
| Salmon | 0.1868 | 0.9043 | 0.6575 | 0.7934 | 0.1632 | 0.0523 | 0.7325 | 0.7033 | 0.8055 | |
| Bream | 0.1898 | 0.9217 | 0.6710 | 0.8066 | 0.1666 | 0.0535 | 0.7456 | 0.7170 | 0.7964 | 0.9754 |
| Al Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.0008 | |||||||||
| Panga | 0.0008 | 0.9534 | ||||||||
| Alaska pollock | 0.9697 | 0.0007 | 0.0008 | |||||||
| Yellowfin sole | 0.0558 | 0.1437 | 0.1353 | 0.0520 | ||||||
| North-Pacific hake | 0.0650 | 0.1245 | 0.1239 | 0.0563 | 0.8903 | |||||
| Flounder | 0.0001 | 0.6244 | 0.6021 | 0.0001 | 0.0632 | 0.0511 | ||||
| Mackerel | 0.0003 | 0.8034 | 0.7747 | 0.0003 | 0.1007 | 0.0842 | 0.7823 | |||
| Rainbow trout | 0.0000 | 0.4592 | 0.4370 | 0.0000 | 0.0368 | 0.0281 | 0.7588 | 0.5904 | ||
| Salmon | 0.0001 | 0.5077 | 0.4849 | 0.0001 | 0.0440 | 0.0342 | 0.8252 | 0.6466 | 0.9148 | |
| Bream | 0.0001 | 0.5068 | 0.4865 | 0.0001 | 0.0428 | 0.0335 | 0.8284 | 0.6458 | 0.9056 | 0.9837 |
| Li Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.8431 | |||||||||
| Panga | 0.7130 | 0.8418 | ||||||||
| Alaska pollock | 0.5942 | 0.4953 | 0.4105 | |||||||
| Yellowfin sole | 0.8362 | 0.9807 | 0.8492 | 0.4995 | ||||||
| North-Pacific hake | 0.1651 | 0.1284 | 0.1001 | 0.3460 | 0.1332 | |||||
| Flounder | 0.0818 | 0.1118 | 0.1368 | 0.0269 | 0.1073 | 0.0018 | ||||
| Mackerel | 0.0854 | 0.1174 | 0.1499 | 0.0282 | 0.1150 | 0.0019 | 0.9928 | |||
| Rainbow trout | 0.0291 | 0.0433 | 0.0593 | 0.0074 | 0.0425 | 0.0003 | 0.6222 | 0.6033 | ||
| Salmon | 0.0034 | 0.0058 | 0.0091 | 0.0005 | 0.0057 | 0.0000 | 0.2366 | 0.2212 | 0.4334 | |
| Bream | 0.1915 | 0.2461 | 0.2837 | 0.0776 | 0.2362 | 0.0082 | 0.6126 | 0.6310 | 0.3528 | 0.1065 |
| As Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.2318 | |||||||||
| Panga | 0.4363 | 0.0561 | ||||||||
| Alaska pollock | 0.2822 | 0.8416 | 0.0777 | |||||||
| Yellowfin sole | 0.6039 | 0.0997 | 0.7547 | 0.1316 | ||||||
| North-Pacific hake | 0.3733 | 0.6736 | 0.1190 | 0.7970 | 0.1907 | |||||
| Flounder | 0.9928 | 0.2387 | 0.4217 | 0.2981 | 0.5841 | 0.4005 | ||||
| Mackerel | 0.0450 | 0.3782 | 0.0055 | 0.3118 | 0.0128 | 0.2238 | 0.0469 | |||
| Rainbow trout | 0.1047 | 0.6161 | 0.0181 | 0.5219 | 0.0369 | 0.3964 | 0.1087 | 0.6591 | ||
| Salmon | 0.2236 | 0.9887 | 0.0546 | 0.8410 | 0.0968 | 0.6696 | 0.2336 | 0.3909 | 0.6310 | |
| Bream | 0.0062 | 0.1174 | 0.0004 | 0.0911 | 0.0011 | 0.0574 | 0.0066 | 0.4360 | 0.2524 | 0.1243 |
| Pb Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.0009 | |||||||||
| Panga | 0.5072 | 0.0076 | ||||||||
| Alaska pollock | 0.0877 | 0.1090 | 0.2533 | |||||||
| Yellowfin sole | 0.0042 | 0.6304 | 0.0243 | 0.2258 | ||||||
| North-Pacific hake | 0.0526 | 0.1741 | 0.1732 | 0.7602 | 0.3246 | |||||
| Flounder | 0.0007 | 0.9072 | 0.0060 | 0.0935 | 0.5717 | 0.1532 | ||||
| Mackerel | 0.0001 | 0.5839 | 0.0014 | 0.0375 | 0.3346 | 0.0690 | 0.6459 | |||
| Rainbow trout | 0.0003 | 0.7290 | 0.0030 | 0.0606 | 0.4410 | 0.1053 | 0.8002 | 0.8080 | ||
| Salmon | 0.0006 | 0.8597 | 0.0052 | 0.0860 | 0.5398 | 0.1430 | 0.9416 | 0.6808 | 0.8427 | |
| Bream | 0.0012 | 0.9184 | 0.0091 | 0.1213 | 0.6790 | 0.1885 | 0.8384 | 0.5329 | 0.6706 | 0.7943 |
| Cd Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.0000 | |||||||||
| Panga | 0.5182 | 0.0000 | ||||||||
| Alaska pollock | 0.2294 | 0.0006 | 0.0782 | |||||||
| Yellowfin sole | 0.2264 | 0.0006 | 0.0764 | 0.9522 | ||||||
| North-Pacific hake | 0.5533 | 0.0000 | 0.2451 | 0.4915 | 0.4852 | |||||
| Flounder | 0.0000 | 0.9110 | 0.0000 | 0.0007 | 0.0007 | 0.0000 | ||||
| Mackerel | 0.0000 | 0.9156 | 0.0000 | 0.0007 | 0.0008 | 0.0001 | 0.9887 | |||
| Rainbow trout | 0.0000 | 0.6835 | 0.0000 | 0.0020 | 0.0020 | 0.0002 | 0.7363 | 0.7438 | ||
| Salmon | 0.0015 | 0.1531 | 0.0001 | 0.0430 | 0.0386 | 0.0084 | 0.1655 | 0.1733 | 0.2628 | |
| Bream | 0.0013 | 0.1643 | 0.0001 | 0.0426 | 0.0416 | 0.0080 | 0.1705 | 0.1833 | 0.2628 | 0.9407 |
| Hg Fish species | Nile tilapia | Pacific cod | Panga | Alaska pollock | Yellowfin sole | North-Pacific hake | Flounder | Mackerel | Rainbow trout | Salmon |
| Pacific cod | 0.0539 | |||||||||
| Panga | 0.0479 | 0.0001 | ||||||||
| Alaska pollock | 0.9665 | 0.0550 | 0.0510 | |||||||
| Yellowfin sole | 0.6851 | 0.0222 | 0.0937 | 0.6767 | ||||||
| North-Pacific hake | 0.6420 | 0.1216 | 0.0201 | 0.6555 | 0.4199 | |||||
| Flounder | 0.0067 | 0.0000 | 0.4179 | 0.0070 | 0.0174 | 0.0019 | ||||
| Mackerel | 0.3176 | 0.3120 | 0.0036 | 0.3221 | 0.1813 | 0.5334 | 0.0002 | |||
| Rainbow trout | 0.1842 | 0.4939 | 0.0010 | 0.1875 | 0.0950 | 0.3406 | 0.0000 | 0.6924 | ||
| Salmon | 0.6959 | 0.1095 | 0.0234 | 0.7058 | 0.4601 | 0.9193 | 0.0024 | 0.4992 | 0.3141 | |
| Bream | 0.0252 | 0.7220 | 0.0000 | 0.0260 | 0.0091 | 0.0658 | 0.0000 | 0.1943 | 0.3304 | 0.0576 |
References
- EFSA (European Food Safety Authority). Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 2017, e15121. [Google Scholar] [CrossRef]
- Kotlęga, D.; Peda, B.; Palma, J.; Zembroń-Łacny, A.; Gołąb-Janowska, M.; Masztalewicz, M.; Nowacki, P.; Szczuko, M. Free Fatty Acids Are Associated with the Cognitive Functions in Stroke Survivors. Int. J. Environ. Res. Public Health 2021, 18, 6500. [Google Scholar] [CrossRef] [PubMed]
- Noreen, S.; Hashmi, B.; Aja, P.M.; Atoki, A.V. Health benefits of fish and fish by-products—A nutritional and functional perspective. Front. Nutr. 2025, 12, 1564315. [Google Scholar] [CrossRef] [PubMed]
- Skałecki, P.; Florek, M.; Kędzierska-Matysek, M.; Poleszak, E.; Domaradzki, P.; Kaliniak-Dziura, A. Mineral and trace element composition of the roe and muscle tissue of farmed rainbow trout (Oncorhynchus mykiss) with respect to nutrient requirements: Elements in rainbow trout products. J. Trace Elem. Med. Biol. 2020, 62, 126619. [Google Scholar] [CrossRef]
- Abera, B.; Adimas, M. Health benefits and health risks of contaminated fish consumption: Current research outputs, research approaches, and perspectives. Heliyon 2024, 10, e33905. [Google Scholar] [CrossRef]
- Herrera-Herrera, C.; Fuentes-Gandara, F.; Zambrano-Arévalo, A.; Higuita, F.B.; Hernández, J.P.; Marrugo-Negrete, J. Health Risks Associated with Heavy Metals in Imported Fish in a Coastal City in Colombia. Biol. Trace Elem. Res. 2019, 190, 526–534. [Google Scholar] [CrossRef]
- Stanek, M.; Janicki, B. Distribution of heavy metals in the meat, gills and liver of common bream (Abramis brama L.) caught from Żnińskie Duże Lake (Poland). J. Elem. 2016, 21, 1141–1150. [Google Scholar] [CrossRef]
- Younis, A.M.; Hanafy, S.; Elkady, E.M.; Alluhayb, A.H.; Alminderej, F.M. Assessment of health risks associated with heavy metal contamination in selected fish and crustacean species from Temsah Lake, Suez Canal. Sci. Rep. 2024, 14, 18706. [Google Scholar] [CrossRef]
- Dourson, M.L. Let the IRIS Bloom:Regrowing the integrated risk information system (IRIS) of the U.S. Environmental Protection Agency. Regul. Toxicol. Pharmacol. RTP 2018, 97, A4–A5. [Google Scholar] [CrossRef]
- USEPA. Integrated Risk Information System (IRIS). United States Environmental Protection. Available online: https://www.epa.gov/iris (accessed on 13 April 2025).
- Díaz, G.E.E.; Orellana, F.R.S.; Vega, R.E.Y.; Valdiviezo-Rivera, J.S.; Ríos-Touma, B.P. Trace Metal Contamination in Commercial Fish from the Ecuadorian Amazon: Preliminary Health Risk Assessment in a Local Market. Fishes 2025, 10, 392. [Google Scholar] [CrossRef]
- Jamil Emon, F.; Rohani, M.F.; Sumaiya, N.; Tuj Jannat, M.F.; Akter, Y.; Shahjahan, M.; Abdul Kari, Z.; Tahiluddin, A.B.; Goh, K.W. Bioaccumulation and Bioremediation of Heavy Metals in Fishes—A Review. Toxics 2023, 11, 510. [Google Scholar] [CrossRef]
- Radwan, M.; Abbas, M.M.M.; Afifi, M.A.M.; Mohammadein, A.; Al Malki, J.S. Fish Parasites and Heavy Metals Relationship in Wild and Cultivated Fish as Potential Health Risk Assessment in Egypt. Front. Environ. Sci. 2022, 10, 890039. [Google Scholar] [CrossRef]
- Canli, M.; Atli, G. The relationships between heavy metal (Cd, Cr, Cu, Fe, Pb, Zn) levels and the size of six Mediterranean fish species. Environ. Pollut. 2003, 121, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Varol, M.; Kaçar, E. Analysis of metal(loid)s and minerals in tissues of fish from a reservoir on the Euphrates River by ICP-MS/MS: Health risks, nutritional benefits and correlations with fish size. Food Chem. Toxicol. 2025, 204, 115652. [Google Scholar] [CrossRef]
- Dogruyol, H.; Can Tunçelli, İ.; Özden, Ö.; Erkan, N.; Karakulak, F.S. Bioaccumulation of Mercury, Cadmium, Lead, and Arsenic in Whiting and Tub Gurnard From the Sea of Marmara: Implications for Human Health. Food Sci. Nutr. 2025, 13, e70370. [Google Scholar] [CrossRef]
- Yildiz, H.; Bayrakli, B.; Altuntas, M.; Celik, I. Concentrations, selenium-mercury balance, and potential health risk assessment for consumer of whiting (Merlangius merlangus euxinus L., 1758) from different regions of the southern Black Sea. Environ. Sci. Pollut. Res. Int. 2023, 30, 65059–65073. [Google Scholar] [CrossRef]
- Jarosz-Krzemińska, E.; Mikołajczyk, N.; Adamiec, E. Content of toxic metals and As in marine and freshwater fish species available for sale in EU supermarkets and health risk associated with its consumption. J. Sci. Food Agric. 2021, 101, 2818–2827. [Google Scholar] [CrossRef] [PubMed]
- Nghia, N.D.; Lunestad, B.T.; Trung, T.S.; Son, N.T.; Maage, A. Heavy metals in the farming environment and in some selected aquaculture species in the Van Phong Bay and Nha Trang Bay of the Khanh Hoa Province in Vietnam. Bull. Environ. Contam. Tox. 2009, 82, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Sakultantimetha, A.; Bangkedphol, S.; Lauhachinda, N.; Homchan, U.; Songsasen, A. Environmental fate and transportation of cadmium, lead and manganese in a river environment using the EPISUITE Program. Agric. Nat. Resour. 2009, 43, 620–627. [Google Scholar]
- Commission Regulation (EC). No 1881/2006 of 19 December 2006 Setting Maximum Levels for Certain Contaminants in FOODSTUFFS. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32006R1881 (accessed on 28 April 2025).
- Zambelli, S. Question for Written Answer E-000134/23 to the Commission. 2023. Available online: https://www.europarl.europa.eu/RegData/questions/reponses_qe/2023/000134/P9_RE(2023)000134_EN.pdf (accessed on 10 May 2025).
- Ristea, E.; Pârvulescu, O.C.; Lavric, V.; Oros, A. Assessment of Heavy Metal Contamination of Seawater and Sediments Along the Romanian Black Sea Coast: Spatial Distribution and Environmental Implications. Sustainability 2025, 17, 2586. [Google Scholar] [CrossRef]
- Siddique, A.B.; Al Helal, A.S.; Patindol, T.A.; Lumanao, D.M.; Longatang, K.J.G.; Rahman, M.A.; Catalvas, L.P.A.; Tulin, A.B.; Shaibur, M.R. Assessment of Heavy Metal Contamination and Ecological Risk in Urban River Sediments: A Case Study from Leyte, Philippines. Pollutants 2025, 5, 7. [Google Scholar] [CrossRef]
- Özçelik, Ş.; Tekin-Özan, S. Evaluation of selected heavy metal and selenium pollution in water and sediments of Lake Eğirdir (Isparta/Türkiye) using statistical analysis and pollution indices. Oceanol. Hydrobiol. Stud. 2024, 53, 186–207. [Google Scholar] [CrossRef]
- Łuszczek-Trojnar, E.; Błoniarz, P.; Winiarski, B.; Drąg-Kozak, E.; Popek, W. Comparison of cadmium, zinc, manganese and nickel concentrations in fillets of selected species of food fish. Sci. Ann. Pol. Soc. Anim. Prod. 2015, 11, 75–84. (In Polish) [Google Scholar]
- Witczak, A.; Harada, D.; Aftyka, A.; Cybulski, J. Endocrine-disrupting organochlorine xenobiotics in fish products imported from Asia—An assessment of human health risk. Environ. Monit. Assess. 2021, 193, 132. [Google Scholar] [CrossRef]
- NIH (National Institutes of Health). Available online: https://ods.od.nih.gov/factsheets/Zinc-HealthProfessional/#en (accessed on 14 July 2025).
- Rychlik, E.; Stoś, K.; Woźniak, A.; Mojska, H. Normy Żywienia dla Populacji Polski; Narodowy Instytut Zdrowia Publicznego PZH—Państwowy Instytut Badawczy: Warszawa, Poland, 2024; ISBN 978-83-65870-78-0. (In Polish) [Google Scholar]
- Begum, W.; Rai, S.; Banerjee, S.; Bhattacharjee, S.; Mondal, M.H.; Bhattarai, A.; Saha, B. A comprehensive review on the sources, essentiality and toxicological profile of nickel. RSC Adv. 2022, 12, 9139. [Google Scholar] [CrossRef]
- Gaidam, M.B.; Sadauki, M.A.; Dauda, A.B.; Idris, B.M.G.; Yagana, A.; Ali, J.; Bichi, A.H. Analysis if heavy metals in some fresh and marine water fish species sold in Damaturu Metropolies. LSIJ 2023, 1, 29–37. [Google Scholar]
- Bayissa, T.N.; Geerardyn, M.; Gobena, S.; Vanhauteghem, D.; Du Laing, G.; Wakijra Kabeta, M.; Janssens, G.P.J. Impact of species and their edible parts on the macronutrient and mineral composition of fish from the same aquatic environment, the Gilgel Gibe Reservoir, Ethiopia. J. Anim. Physiol. Anim. Nutr. 2022, 106, 220–228. [Google Scholar] [CrossRef]
- El-Sappah, A.H.; Seif, M.M.; Abdel-Kader, H.H.; Soaud, S.A.; Elhamid, M.A.A.; Abdelghaffar, A.M.; El-Sappah, H.H.; Sarwar, H.; Yadav, V.; Maitra, P.; et al. Genotoxicity and Trace Elements Contents Analysis in Nile Tilapia (Oreochromis niloticus) Indicated the Levels of Aquatic Contamination at Three Egyptian Areas. Front. Vet. Sci. 2022, 9, 818866. [Google Scholar] [CrossRef] [PubMed]
- Simukoko, C.K.; Mwakalapa, E.B.; Bwalya, P.; Muzandu, K.; Berg, V.; Mutoloki, S.; Polder, A.; Lyche, J.L. Assessment of heavy metals in wild and farmed tilapia (Oreochromis niloticus) on Lake Kariba, Zambia: Implications for human and fish health. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess. 2022, 39, 74–91. [Google Scholar] [CrossRef] [PubMed]
- Artar, E.; Olgunoglu, M.P.; Olgunoglu, I.A. Mineral Contents and Fatty Acids Compositions of Fillets of Female and Male Pangas (Pangasıus hypophthalmus, Sauvage 1878) Cultured in Turkey. Prog. Nutr. 2022, 24, e2022056. [Google Scholar] [CrossRef]
- Guérin, T.; Chekri, R.; Vastel, C.; Sirot, V.; Volatier, J.L.; Leblanc, J.C.; Noël, L. Determination of 20 trace elements in fish and other seafood from the French market. Food Chem. 2011, 127, 934–942. [Google Scholar] [CrossRef]
- Bergés-Tiznado, M.E.; Bojórquez-Sánchez, C.; Acosta-Lizárraga, L.G.; Zamora-García, O.G.; Márquez-Farías, J.F.; Páez-Osuna, F. Tissue dynamics of potential toxic elements in the Pacific hake (Merluccius productus): Distribution and the public health risk assessment. Environ. Sci. Pollut. Res. 2022, 29, 77945–77957. [Google Scholar] [CrossRef]
- Antovic, I.; Sukovic, D.; Andjelic, S.; Svrkota, N. Heavy metals and radionuclides in muscles of Fish species in the south Adriatic—Montenegro. RAP Conference Proceedings. Bull. Vet. Inst. Pulawy 2019, 4, 589–595. [Google Scholar] [CrossRef]
- Ogundiran, M.A.; Adewoye, S.O.; Ayandiran, T.A.; Dahunsi, S. Heavy metal, proximate and microbial profile of some selected commercial marine fish collected from two markets in south western Nigeria. Afr. J. Biotechnol. 2014, 13, 1147–1153. [Google Scholar] [CrossRef]
- Copat, C.; Grasso, A.; Fiore, M.; Cristaldi, A.; Zuccarello, P.; Signorelli, S.S.; Conti, G.O.; Ferrante, M. Trace elements in seafood from the Mediterranean sea: An exposure risk assessment. Food Chem. Toxicol. 2018, 115, 13–19. [Google Scholar] [CrossRef]
- Pokorska, K.; Protasowicki, M.; Bernat, K.; Kucharczyk, M. Content of metals in flounder, Platichthys flesus L., and Baltic herring, Clupea harengus membras L., from the southern Baltic Sea. Arch. Pol. Fish. 2012, 20, 51–53. [Google Scholar] [CrossRef]
- Demirezen, D.; Uruç, K. Comparative study of trace elements in certain fish, meat and meat products. Meat Sci. 2006, 74, 255–260. [Google Scholar] [CrossRef]
- Rajkowska-Myśliwiec, M.; Pokorska-Niewiada, K.; Witczak, A.; Balcerzak, M.; Ciecholewska-Juśko, D. Health benefits and risks associated with element uptake from grilled fish and fish products. J. Sci. Food Agric. 2022, 102, 957–964. [Google Scholar] [CrossRef] [PubMed]
- Al-Dahhan, W.H.; Ahmad, S.M.; Mansour, O.; Jebali, J.; Yousif, E.A. Determination of Heavy Metals in Some Types of Imported Fish from Local Markets. ANJS 2022, 25, 14–19. [Google Scholar] [CrossRef]
- Atanassoff, A.; Nikolov, G.; Staykov, Y.; Zhelyazkov, G.; Sirakov, I. Proximate and mineral analysis of Atlantic Salmon (Salmo salar) cultivated in Bulgaria. Biotechnol. Anim. Husb. 2013, 29, 571–579. [Google Scholar] [CrossRef]
- Lidwin-Kaźmierkiewicz, M.; Pokorska, K.; Protasowicki, M.; Rajkowska, M.; Wechterowicz, Z. Content of selected essential and toxic metals in meat of freshwater fish from West Pomerania, Poland. Pol. J. Food Nutr. Sci. 2009, 59, 219–224. [Google Scholar]
- Kilercioglu, S.; Kosker, A.R.; Evliyaoglu, E. Public health risk assessments associated with heavy metal levels in panga fish fillets imported from Vietnam. Int. J. Agric. Environ. Food Sci. 2022, 6, 568–578. [Google Scholar] [CrossRef]
- Al-Sisi, M.; Elhawat, N.; Alshaal, T.; Eissa, F. Assessment of trace element occurrence in Nile Tilapia from the Rosetta branch of the River Nile, Egypt: Implications for human health risk via lifetime consumption. Ecotoxicol. Environ. Saf. 2024, 285, 117079. [Google Scholar] [CrossRef] [PubMed]
- Ekweozor, I.K.E.; Ugbomeh, A.P.; Ogbuehi, K.A. Zn, Pb, Cr and Cd concentrations in fish, water and sediment from the Azuabie Creek, Port Harcourt. J. Appl. Sci. Environ. Manag. 2017, 21, 87–91. [Google Scholar] [CrossRef]
- Burger, J.; Gochfeld, M.; Shukla, T.; Jeitner, C.; Burke, S.; Donio, M.; Shukla, S.; Snigaroff, R.; Snigaroff, D.; Stamm, T.; et al. Heavy Metals in Pacific Cod (Gadus macrocephalus) from the Aleutians: Location, Age, Size, and Risk. J. Toxicol. Environ. Health Part A 2007, 70, 1897–1911. [Google Scholar] [CrossRef] [PubMed]
- Szkoda, J.; Żmudzki, J.; Nawrocka, A.; Kmiecik, M. Toxic elements in free-living freshwater fish, water and sediments in Poland. Bull. Vet. Inst. Pulawy 2014, 58, 589–595. [Google Scholar] [CrossRef][Green Version]
- Djedjibegovic, J.; Marjanowic, A.; Tahirovic, D.; Caklovica, K.; Turalic, A.; Lugusic, A.; Omeragic, E.; Sober, M.; Caklovica, F. Heavy metals in commercial fish and seafood products and risk assessment in adult population in Bosnia and Herzegovina. Sci. Rep. 2020, 10, 13238. [Google Scholar] [CrossRef]
- Li, L.; Yang, X. The Essential Element Manganese, Oxidative Stress, and Metabolic Diseases: Links and Interactions. Oxidative Med. Cell. Longev. 2018, 2018, 7580707. [Google Scholar] [CrossRef]
- Rodríguez, M.; Gutiérrez, Á.J.; Rodríguez, N.; Rubio, C.; Paz, S.; Martín, V.; Revert, C.; Hardisson, A. Assessment of mercury content in Panga (Pangasius hypophthalmus). Chemosphere 2018, 196, 53–57. [Google Scholar] [CrossRef]
- Brodziak-Dopierała, B.; Fischer, A. Analysis of the Mercury Content in Fish for Human Consumption in Poland. Toxics 2023, 11, 717. [Google Scholar] [CrossRef]
- Acosta-Lizárraga, L.G.; Bergés-Tiznado, M.E.; Bojórquez-Sánchez, C.; Osuna-Martínez, C.C.; Páez-Osuna, F. Bioaccumulation of mercury and selenium in tissues of the mesopelagic fish Pacific hake (Merluccius productus) from the northern Gulf of California and the risk assessment on human health. Chemosphere 2020, 255, 126941. [Google Scholar] [CrossRef]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic Mechanisms of Five Heavy Metals: Mercury, Lead, Chromium, Cadmium, and Arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Wysocka, D.; Snarska, A.; Sobiech, P. Copper—An essential micronutrient for calves and adult cattle. J. Elem. 2019, 24, 101–110. [Google Scholar] [CrossRef]
- Sirisangarunroj, P.; Monboonpitak, N.; Karnpanit, W.; Sridonpai, P.; Singhato, A.; Laitip, N.; Ornthai, N.; Yafa, C.; Judprasong, K. Toxic Heavy Metals and Their Risk Assessment of Exposure in Selected Freshwater and Marine Fish in Thailand. Foods 2023, 12, 3967. [Google Scholar] [CrossRef]
- Ferrantelli, V.; Giangrosso, G.; Cicero, A.; Naccari, C.; Macaluso, A.; Galvano, F.; D’Orazio, N.; Arcadipane, G.E.; Naccari, F. Evaluation of mercury levels in Pangasius and Cod fillets traded in Sicily (Italy). Food Addit. Contam. Part A 2012, 29, 1046–1051. [Google Scholar] [CrossRef]
- Staszowska A, Skałecki P, Florek M and Litwińczuk A, Impact of fish species and their living environment on concentration of lead and estimated intake thereof from muscle tissue. Food Sci. Technol. Qual. 2013, 20, 60–68. (In Polish) [CrossRef]
- Brázová, T.; Syrota, Y.; Oros, M.; Uhrovič, D. Heavy Metal Accumulation in Freshwater Fish: The Role of Species, Age, Gender, and Parasites. Bull. Environ. Contam. Toxicol. 2025, 114, 92. [Google Scholar] [CrossRef] [PubMed]
- Gholamhosseini, A.; Shiry, N.; Soltanian, S.; Banaee, M. Bioaccumulation of metals in marine fish species captured from the northern shores of the Gulf of Oman, Iran. Reg. Stud. Mar. Sci. 2021, 41, 101599. [Google Scholar] [CrossRef]
- Kawarazuka, N.; Béné, C. The potential role of small fish species in improving micronutrient deficiencies in developing countries: Building evidence. Public Health Nutr. 2011, 14, 1927–1938. [Google Scholar] [CrossRef]
- Phogat, S.; Dahiya, T.; Jangra, M.; Kumari, A.; Kumar, A. Nutritional Benefits of Fish Consumption for Humans: A Review. Int. J. Environ. Clim. Change 2022, 12, 1443–1457. [Google Scholar] [CrossRef]
- Lofstedt, A.; de Roos, B.; Fernandes, P.G. Less than half of the European dietary recommendations for fish consumption are satisfied by national seafood supplies. Eur. J. Nutr. 2021, 60, 4219–4228. [Google Scholar] [CrossRef] [PubMed]
- Utri-Khodadady, Z.; Skolmowska, D.; Głąbska, D. Determinants of Fish Intake and Complying with Fish Consumption Recommendations—A Nationwide Cross-Sectional Study among Secondary School Students in Poland. Nutrients 2024, 16, 853. [Google Scholar] [CrossRef]
- Jarosz, M.; Rychlik, E.; Stoś, K.; Charzewska, J. Nutrition Recommendations for Polish Population. In Normy Żywienia Dla Populacji Polski i ich Zastosowanie; NIZP-PZH; Narodowy Instytut Zdrowia Publicznego—Państwowy Zakład Higieny: Warsaw, Poland, 2020; Volume 83, pp. 1–465. (In Polish) [Google Scholar]
- EUMOFA—European Market Observatory for Fisheries and Aquaculture. The EU Fish Market, 2023rd ed.; Publications Office of the European Union: Luxemburg, 2023; Available online: https://eumofa.eu/documents/20124/35668/EFM2023_EN.pdf/95612366-79d2-a4d1-218b-8089c8e7508c?t=1699541180521 (accessed on 10 May 2025).
- Ferreira, W.Q.; Alves, B.S.; Dantas, K. Health risk assessment attributed the consumption of fish and seafood in Belém, Pará, Brazil. J. Trace Elem. Miner. 2023, 6, 100103. [Google Scholar] [CrossRef]
- Hashempour-Baltork, F.; Jannat, B.; Tajdar-Oranj, B.; Aminzare, M.; Sahebi, H.; Mirza Alizadeh, A.; Hosseini, H. A comprehensive systematic review and health risk assessment of potentially toxic element intakes via fish consumption in Iran. Ecotoxicol. Environ. Saf. 2023, 1, 114349. [Google Scholar] [CrossRef]
- Yardım, Ö.; Bat, L. Human Health Risk Assessment of Heavy Metals Via Dietary Intake of Rainbow Trout from Samsun Fish Markets. J. Anatol. Environ. Anim. Sci. 2020, 5, 260–263. [Google Scholar] [CrossRef]
- Adebiyi, F.M.; Ore, O.T.; Owolafe, O.S. Human health risk assessment of potentially toxic metals in fish (Cynoglossus sp.) commonly consumed in Nigeria. Discov. Toxicol. 2024, 1, 7. [Google Scholar] [CrossRef]
- Rajkowska-Myśliwiec, M.; Ciemniak, A.; Karp, G. Arsenic in Rice and Rice-Based Products with Regard to Consumer Health. Foods 2024, 13, 3153. [Google Scholar] [CrossRef]
- Statistical Yearbook of the Republic of Poland; Statistics Poland: Warsaw, Poland, 2024. (In Polish)
- US EPA. Risk-Based Concentration Table; United States Environmental Protection Agency: Washington, DC, USA, 2000. [Google Scholar]
- US EPA. Quantitative Risk Assessment Calculations; Environmental Protection Agency: Washington, DC, USA, 2015. [Google Scholar]
- Ravanbakhsh, M.; Javid, A.Z.; Hadi, M.; Fard, N.J.H. Heavy metals risk assessment in fish species (Johnius belangerii (C) and Cynoglossus arel) in Musa Estuary. Persian Gulf. Environ. Res. 2020, 188, 109560. [Google Scholar] [CrossRef] [PubMed]
- JECFA. Evaluations of the Joint FAO/WHO Expert Committee on Food Additives (JECFA). Includes All Updates Up to the 89th JECFA (June 2020). 2021. Available online: https://apps.who.int/food-additives-contaminants-jecfa-database/search.aspx?fcc=2 (accessed on 17 July 2025).
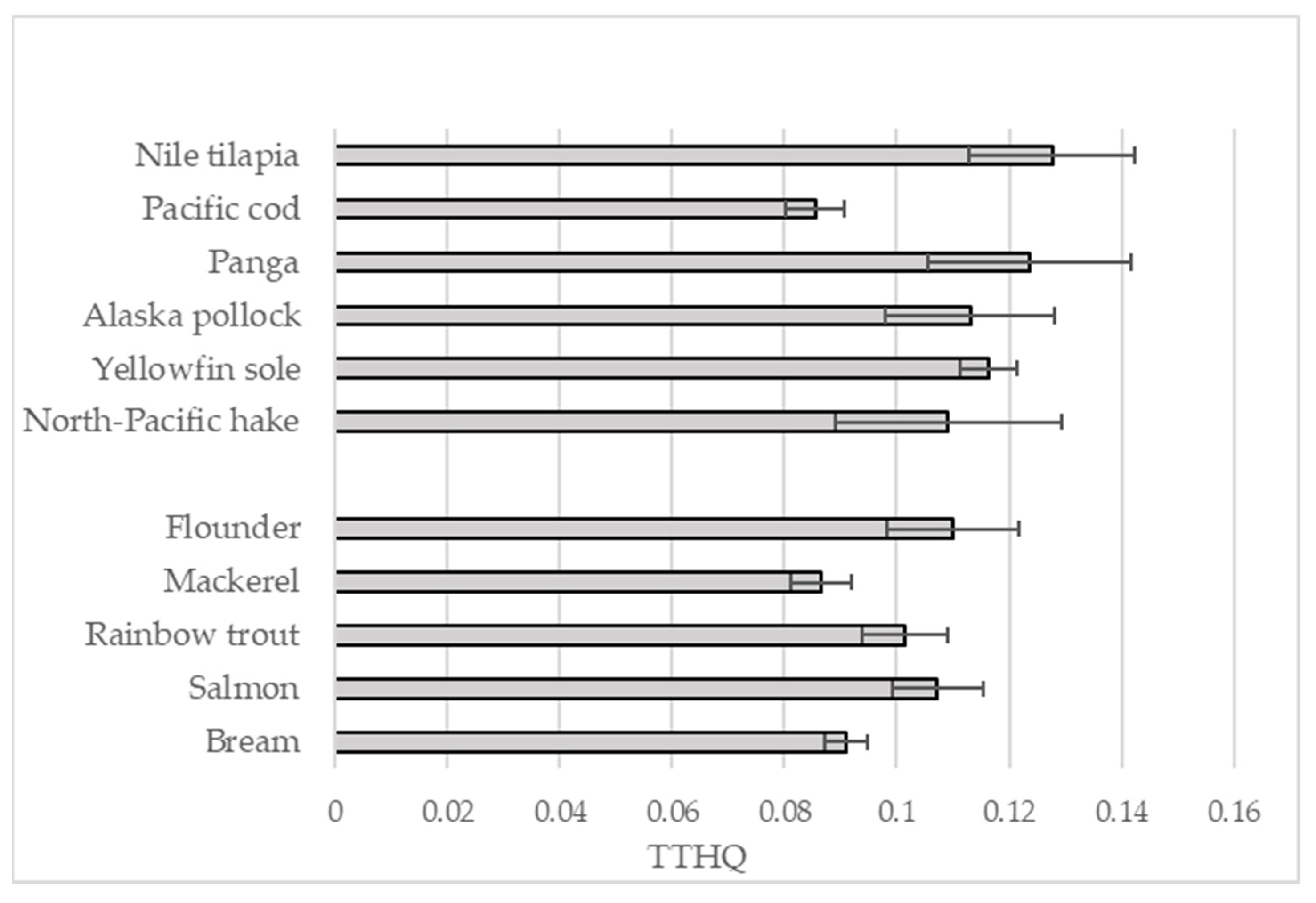
| EDI | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Fish Species | Zn | Ni | Fe | Mn | Cr | Cu | Al | Li | As | Pb | Cd | Hg | |
| Nile tilapia | x | 1.93 | 0.04 | 0.92 | 0.059 | 0.039 | 0.075 | 0.121 | 0.017 | 0.104 | 0.013 | 0.020 | 0.021 |
| Pacific cod | x | 1.58 | 0.05 | 0.76 | 0.031 | 0.041 | 0.100 | 0.067 | 0.018 | 0.079 | 0.005 | 0.005 | 0.015 |
| Panga | x | 1.03 | 0.04 | 0.57 | 0.036 | 0.045 | 0.093 | 0.068 | 0.019 | 0.120 | 0.012 | 0.022 | 0.027 |
| Alaska pollock | x | 1.15 | 0.04 | 0.72 | 0.026 | 0.040 | 0.097 | 0.120 | 0.015 | 0.083 | 0.009 | 0.016 | 0.021 |
| Yellowfin sole | x | 1.35 | 0.05 | 0.66 | 0.027 | 0.040 | 0.073 | 0.090 | 0.018 | 0.115 | 0.006 | 0.016 | 0.022 |
| North-Pacific hake | x | 1.08 | 0.04 | 0.71 | 0.026 | 0.045 | 0.062 | 0.092 | 0.010 | 0.088 | 0.009 | 0.018 | 0.020 |
| Flounder | x | 1.72 | 0.17 | 0.86 | 0.057 | 0.048 | 0.095 | 0.059 | 0.026 | 0.105 | 0.005 | 0.005 | 0.029 |
| Mackerel | x | 0.91 | 0.17 | 0.80 | 0.034 | 0.047 | 0.094 | 0.063 | 0.026 | 0.062 | 0.004 | 0.005 | 0.018 |
| Rainbow trout | x | 3.75 | 0.13 | 0.78 | 0.040 | 0.065 | 0.106 | 0.054 | 0.028 | 0.070 | 0.005 | 0.006 | 0.017 |
| Salmon | x | 2.41 | 0.16 | 0.74 | 0.044 | 0.063 | 0.102 | 0.056 | 0.032 | 0.080 | 0.005 | 0.010 | 0.020 |
| Bream | x | 2.78 | 0.16 | 0.70 | 0.046 | 0.056 | 0.102 | 0.056 | 0.024 | 0.048 | 0.006 | 0.010 | 0.014 |
| THQ | |||||||||||||
| Fish species | Zn | Ni | Fe | Mn | Cr | Cu | Al | Li | As | Pb | Cd | Hg | |
| Nile tilapia | x | 0.006 | 0.002 | 0.001 | <0.001 | 0.013 | 0.002 | 0.030 | 0.001 | 0.035 | 0.020 | 0.013 | |
| Pacific cod | x | 0.005 | 0.003 | 0.001 | <0.001 | 0.014 | 0.002 | 0.017 | 0.001 | 0.026 | 0.005 | 0.010 | |
| Panga | x | 0.003 | 0.002 | 0.001 | <0.001 | 0.015 | 0.002 | 0.017 | 0.001 | 0.040 | 0.022 | 0.017 | |
| Alaska pollock | x | 0.004 | 0.002 | 0.001 | <0.001 | 0.013 | 0.002 | 0.030 | 0.001 | 0.028 | 0.016 | 0.013 | |
| Yellowfin sole | x | 0.004 | 0.002 | 0.001 | <0.001 | 0.013 | 0.002 | 0.023 | 0.001 | 0.038 | 0.016 | 0.014 | |
| North-Pacific hake | x | 0.004 | 0.002 | 0.001 | <0.001 | 0.015 | 0.002 | 0.023 | 0.001 | 0.029 | 0.018 | 0.012 | |
| Flounder | x | 0.006 | 0.008 | 0.001 | <0.001 | 0.016 | 0.002 | 0.015 | 0.001 | 0.035 | 0.005 | 0.018 | |
| Mackerel | x | 0.003 | 0.008 | 0.001 | <0.001 | 0.016 | 0.002 | 0.016 | 0.001 | 0.021 | 0.005 | 0.011 | |
| Rainbow trout | x | 0.012 | 0.007 | 0.001 | <0.001 | 0.022 | 0.003 | 0.014 | 0.001 | 0.023 | 0.006 | 0.011 | |
| Salmon | x | 0.008 | 0.008 | 0.001 | <0.001 | 0.021 | 0.003 | 0.014 | 0.002 | 0.027 | 0.010 | 0.013 | |
| Bream | x | 0.009 | 0.008 | 0.001 | <0.001 | 0.019 | 0.003 | 0.014 | 0.001 | 0.016 | 0.010 | 0.009 | |
| Cd | Cd | Pb | |
|---|---|---|---|
| Fish Species | % TWI x ± SD | % PTMI x ± SD | % BMDL0.1 x ± SD |
| Nile tilapia | 5.53 ± 1.82 | 2.37 ± 0.78 | 0.87 ± 0.30 |
| Pacific cod | 1.39 ± 0.87 | 0.59 ± 0.37 | 0.36 ± 0.06 |
| Panga | 6.05 ± 1.52 | 2.59 ± 0.65 | 0.78 ± 0.33 |
| Alaska pollock | 4.48 ± 1.00 | 1.92 ± 0.43 | 0.62 ± 0.16 |
| Yellowfin sole | 4.43 ± 1.39 | 1.90 ± 0.60 | 0.43 ± 0.12 |
| North-Pacific hake | 5.04 ± 1.09 | 2.16 ± 0.47 | 0.57 ± 0.12 |
| Flounder | 1.48 ± 0.26 | 0.64 ± 0.11 | 0.34 ± 0.09 |
| Mackerel | 1.47 ± 0.55 | 0.63 ± 0.24 | 0.27 ± 0.09 |
| Rainbow trout | 1.76 ± 0.54 | 0.75 ± 0.23 | 0.30 ± 0.07 |
| Salmon | 2.74 ± 0.84 | 1.17 ± 0.36 | 0.33 ± 0.08 |
| Bream | 2.68 ± 0.50 | 1.15 ± 0.21 | 0.37 ± 0.12 |
| Species | Amount of Material Tested | Amount of Analysed Samples | Country of Origin | Fat Content % a | Dry Weight % a |
|---|---|---|---|---|---|
| Nile tilapia | 18 | 180 | China | 1.39 ± 0.47 | 18.39 ± 1.71 |
| Panga | 27 | 270 | Vietnam | 1.09 ± 0.73 | 10.36 ± 2.19 |
| Alaska Pollock | 12 | 120 | China | 0.67 ± 0.09 | 8.60 ± 1.42 |
| North-Pacific hake | 3 | 12 | China | 2.05 ± 0.12 | 6.81 ± 1.02 |
| Yellowfin sole | 9 | 90 | China | 1.07 ± 0.17 | 11.25 ± 1.13 |
| Pacific cod | 13 | 130 | China | 0.50 ± 0.06 | 16.79 ± 0.29 |
| Flounder | 10 | 100 | Poland | 2.08 ± 0.05 | 20.08 ± 1.22 |
| Mackerel | 30 | 300 | Poland | 13.01 ± 0.11 | 27.14 ± 2.06 |
| Rainbow trout | 12 | 120 | Poland | 4.7 ± 0.12 | 24.03 ± 1.33 |
| Salmon | 8 | 80 | Poland | 13.5 ± 0.32 | 18.5 ± 0.1.12 |
| Bream | 22 | 220 | Poland | 3.5 ± 0.32 | 17.51 ± 0.98 |
| Trace Element | LOD µg/kg | LOQ µg/kg | Recovery % | Trace Element | LOD µg/kg | LOQ µg/kg | Recovery % |
|---|---|---|---|---|---|---|---|
| Zn | 20.4 | 62.8 | 98.8 | Al | 5.5 | 18.8 | 96.9 |
| Ni | 1.4 | 4.1 | 96.9 | Li | 1.3 | 4.0 | 97.6 |
| Fe | 19.8 | 60.4 | 98.4 | As | 0.3 | 1.0 | 95.9 |
| Mn | 1.2 | 3.9 | 97.7 | Pb | 0.8 | 3.1 | 99.2 |
| Cr | 1.4 | 4.5 | 98.2 | Cd | 0.31 | 1.1 | 98.9 |
| Cu | 8.6 | 26.1 | 99.1 | Hg | 1.6 | 5.2 | 96.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Witczak, A.; Ciemniak, A.; Więcaszek, B.; Keszka, S.; Protasowicki, M.; Pokorska-Niewiada, K. An Assessment of the Public Health Risk Associated with Consumption of Imported Fish Based on the Intake of Essential and Harmful Elements. Molecules 2025, 30, 3836. https://doi.org/10.3390/molecules30183836
Witczak A, Ciemniak A, Więcaszek B, Keszka S, Protasowicki M, Pokorska-Niewiada K. An Assessment of the Public Health Risk Associated with Consumption of Imported Fish Based on the Intake of Essential and Harmful Elements. Molecules. 2025; 30(18):3836. https://doi.org/10.3390/molecules30183836
Chicago/Turabian StyleWitczak, Agata, Artur Ciemniak, Beata Więcaszek, Sławomir Keszka, Mikołaj Protasowicki, and Kamila Pokorska-Niewiada. 2025. "An Assessment of the Public Health Risk Associated with Consumption of Imported Fish Based on the Intake of Essential and Harmful Elements" Molecules 30, no. 18: 3836. https://doi.org/10.3390/molecules30183836
APA StyleWitczak, A., Ciemniak, A., Więcaszek, B., Keszka, S., Protasowicki, M., & Pokorska-Niewiada, K. (2025). An Assessment of the Public Health Risk Associated with Consumption of Imported Fish Based on the Intake of Essential and Harmful Elements. Molecules, 30(18), 3836. https://doi.org/10.3390/molecules30183836







