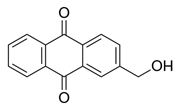
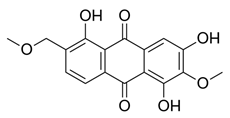
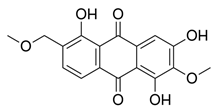
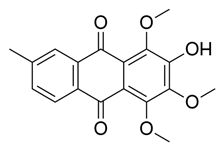
2.4.1. Antioxidant Activity
Antioxidant activity refers to the ability of a substance to inhibit or delay the oxidation of other molecules, preventing damage caused by reactive oxygen species (ROS) and reactive nitrogen species (RNS). These species can oxidize essential cellular components such as lipids, proteins, carbohydrates, and nucleic acids, resulting in oxidative stress, which is related to aging and various diseases [
48]. In this sense, antioxidant activity plays a fundamental role in maintaining cellular homeostasis and protecting against degenerative processes. In practical terms, it represents the ability of natural or synthetic compounds to neutralize free radicals before they cause irreversible damage to cells. This ability is particularly relevant in tissues with a high metabolic rate or vulnerable to the accumulation of reactive species, such as the brain, where oxidative stress is implicated in the etiology of neurodegenerative diseases such as Alzheimer’s [
49].
Antioxidant activity, when analyzed under a molecular and functional approach, should be understood as an integrative parameter, with the ability to predict the therapeutic potential of bioactive compounds, representing an essential mechanism of biological defense, whose stimulation or supplementation, through natural compounds, contributes significantly to health promotion and the prevention of diseases associated with oxidative stress [
50]. Antioxidant activity has great relevance in the biological and pharmacological context due to its ability to modulate the oxidation reaction in the body and in experimental systems [
51]. In this sense, the half maximal inhibitory concentration (IC50) is widely employed as a standard parameter to quantify antioxidant potential, since it represents the concentration of a compound required to inhibit 50% of oxidative activity, being a reliable indicator of potency in comparative studies [
52].
Phytochemical prospecting of the
M. citrifolia revealed the presence of several bioactive secondary metabolites. The analysis allowed the identification and isolation of lignans, including americanin, which is recognized for its structure that confers antioxidant and anti-inflammatory properties, and two additional lignans not yet named, in addition to scopoletin, which are secondary metabolites derived from the life of shikimate. In addition, glycosides were identified, classified as compounds A-D, which may influence their solubility, transport, and bioavailability in the body [
37,
53].
Biologically, antioxidants participate in maintaining the cellular redox balance, regulating processes such as intracellular signaling, gene expression, and the structural integrity of biomolecules such as lipids, proteins, and nucleic acids. From a pharmacological point of view, these compounds act as functional agents in therapeutic formulations, contributing to the chemical stability of active ingredients, protecting them against oxidative degradation, and enhancing their bioavailability and efficacy [
54]. In this context, the search for natural sources of antioxidants has intensified, especially due to the need for safe and bioactive alternatives to antioxidants (
Table 3 and
Table 4).
The study conducted by Sevalho et al. (2017) reinforces this trend by highlighting the high antioxidant potential of
M. citrifolia extracts, especially the ethyl acetate extract of the seeds, using the Thin-layer chromatography (TLC) with 1,1-diphenyl-2-picrylhydrazyl (DPPH), which is a bioautographic technique used to separate the compounds in an extract and reveal the compounds with significant free radical scavenging activity, even in the absence of classical phenolics or flavonoids [
55]. In parallel with its stabilizing function in pharmaceutical formulations, antioxidant activity also plays a profound physiological role by protecting cells against the harmful effects of free radicals, which can trigger highly damaging chain reactions.
This capacity was evidenced in the study by Ezenwaka et al. (2018) [
56], which compared the antioxidant potential of the methanolic extracts of the fruits of
M. citrifolia, demonstrating significant action in the neutralization of various radicals such as superoxide, hydroxyl, and nitric oxide in a dose-dependent pattern. Notably, the
Morinda extract showed better performance against the superoxide radical, surpassing vitamin C, which reinforces its effectiveness as a reducing agent in intense oxidative environments [
56]. In addition, many natural antioxidants demonstrate multiple bioactivities, including anti-inflammatory, metal chelating, and enzyme modulating properties, which make them promising targets for the development of new pharmaceutical and nutraceutical products [
57]. The enhanced antioxidant capacity of
M. citrifolia in comparison to vitamin C may be attributed to the nature of the solvent employed during the extraction process. As reported by Jelena Mitrovic et al. (2024) [
58], extracts obtained using polar solvents, particularly methanol, demonstrate superior antioxidant potential. This phenomenon is primarily associated with the increased efficiency of polar solvents in extracting phenolic compounds and flavonoids, which are bioactive constituents well recognized for their significant contribution to antioxidant activity [
58].
The ethanolic extract of
M. citrifolia Linn. leaves showed significant antioxidant activity in in vitro tests using the DPPH method, which is a stable free radical widely used as a marker for antioxidant activity tests, due to its intense color and reactivity with hydrogen or electron donors. In the present study, Rodrigues et al. (2017) identified an inhibitory concentration (IC 50) value of 4.27 ± 0.004 g/L, with a linear coefficient of determination of 0.9954, indicating a high capacity of the extract to neutralize free radicals, an action that was mainly attributed to the presence of flavonoids detected in the phytochemical prospection of the leaf, which are known for their antioxidant properties [
59].
The antioxidant activity of
M. citrifolia has been highlighted as a plant of great relevance due to its expressiveness, as it is widely associated with a variety of pharmacological and biological effects. According to Choi et al. (2020) [
60], in particular, the aqueous extract of
M. citrifolia fruit showed high antioxidant activity in a cellular model; through DPPH assays, the extract showed efficacy of up to 97%, even surpassing ascorbic acid, which is used as a positive control. Treatment with the extract resulted in a significant reduction in inflammatory mediators, such as interleukin-6 (IL-6), which acts as a pleiotropic molecule that promotes lymphocyte differentiation, and tumor necrosis factor alpha (TNF-α), which is a potent inducer of inflammation, promoting the expression of adhesion molecules, chemokines, and metalloproteinases, contributing to tissue damage and cell apoptosis [
61]. Beyond macrophages, other studies report immunomodulatory actions involving adaptive immune cells. For instance, polysaccharide-rich fractions of
M. citrifolia were shown to enhance CD8
+ T lymphocyte activity and stimulate NK cell proliferation and cytotoxicity, suggesting broader immunostimulatory effects than previously recognized. This highlights the potential of
M. citrifolia not only in innate immune modulation but also in adaptive responses, which could be therapeutically relevant in infection control and cancer immunotherapy [
60,
62].
From a pharmacological point of view,
M. citrifolia L. has great potential, which is largely justified by its rich phytochemical composition and potent antioxidant activity. According to Fontes et al. (2023) [
63], this matrix has been shown to have high levels of phenolic compounds (7486.38 µg GAE/g) and flavonoids (385.57 µg QE/g), as well as high levels of vitamin C (336.62 µg QE/g) and carotenoids. The high antioxidant capacity was confirmed by in vitro Ferric Reducing Antioxidant Power (FRAP) and 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) methods, which are methods used to assess the in vitro antioxidant activity of compounds, with values of up to 467970.40 mmol/100g of ascorbic acid equivalence standing out, showing the efficiency of the freeze-dried pulp in neutralizing free radicals [
63].
At the same time, according to Semwal et al. (2021), rutin, which is a major flavonoid found in the pulp and bark of noni, has a great ability to inhibit the production of pro-inflammatory cytokines such as TNF-α and IL-6 due to its potent antioxidant action, with the ability to stabilize reactive oxygen species and protect cell membranes against lipid peroxidation [
64]. In addition, Wang et al. (2022), demonstrated the action of vanillin in experimental models, which in turn acted as an antioxidant agent, reducing cell damage mediated by free radicals, which in turn stands out as a phenolic compound, acting in the neutralization of reactive oxygen species and in the reduction of cellular oxidative damage; its protective effects are attributed to the ability to modulate biochemical pathways involved in oxidative stress, conferring stability to cellular structures [
65].
2.4.2. Antimicrobial Activity
Antimicrobial activity refers to the ability of a natural or synthetic compound to inhibit the growth of or eliminate pathogenic microorganisms such as bacteria, fungi, viruses, and parasites [
66]. According to Zhang et al. (2021), the importance of this property lies in its essential role in the prevention and treatment of infections, especially in the face of the growing problem of antimicrobial resistance, which renders many conventional antibiotics ineffective [
67]. Corroborating this relevance, Dian et al. (2020) [
68] showed that the combination of organic acids such as benzoic, formic, and fumaric acid alone had a significant inhibitory effect against strains of
Escherichia coli and
Salmonella typhi in an in vitro test, with the compounds tested having a minimum inhibitory concentration (MIC) of 50% for
E. coli and 100% for
Salmonella typhi. coli and 100% for Salmonella, with a total reduction in microbial viability after 13 to 14 h of exposure, depending on the formulation. Results such as these reinforce that natural substances such as phenolic extracts and organic acids can represent effective alternatives to conventional antimicrobials [
68]. Regarding antiviral potential, evidence remains less developed, but several phytochemicals from
M. citrifolia, including iridoids, flavonoids, terpenoids, and lignans, exhibit structural features that may interact with viral enzymes and inhibit key steps of viral replication. Notably, bisabolane-type sesquiterpenes isolated from
M. citrifolia demonstrated significant inhibitory activity against HIV-1 reverse transcriptase, with median effective concentration (EC
50) values ranging from 0.16 to 6.29 μM. These findings suggest that beyond theoretical interactions,
M. citrifolia-derived compounds hold antiviral potential, opening promising avenues for deeper investigation into their use as scaffolds for novel antiviral drug development [
44].
The antimicrobial effect is attributed to the presence of bioactive compounds such as flavonoids, alkaloids, lignins, coumarins, and antimicrobial peptides (
Table 5 and
Table 6). Research has shown that the methanolic extracts of
M. citrifolia seeds have significant activity against clinical strains of methicillin-resistant
Staphylococcus aureus (MRSA), with MICs ranging from 16 to 200 μg/mL. Isolated compounds such as americanin A and scopoletin have been identified as responsible for this activity, suggesting that they have great potential as natural antibacterial agents [
69].
Medrano-Colmenares et al. (2024) [
70] showed that the methanolic extract of the bark, pulp, and seeds of
M. citrifolia has fungistatic and fungicidal effects, inhibiting fungal growth and killing the fungus
Candida albicans. The seed extract showed high sensitivity against this type of fungus with a minimum inhibitory concentration (MIC) of 1366.25 mg/mL [
70]. In addition, Kaul et al. (2023) incorporated
M. citrifolia extracts in the form of silver nanoparticles (AgNPs) into dental prosthesis base resin, observing a significant reduction in
Candida albicans adhesion on surfaces treated with 0.1% AgNPs from the plant, indicating its antifungal and antimicrobial potential applied to clinical biomaterials [
71]. In studies carried out by De La Cruz-Sánchez et al. (2019) [
43], the methanolic extract of
M. citrifolia seeds showed significant activity against multidrug-resistant clinical strains of
Staphylococcus aureus,
S. epidermids, and S. haemolyticus. The MIC of the crude extract was 16 mg/mL, with the methanolic extract being the most potent among the solvents tested, with purified fractions containing scopoletin and americanin showing MICs between 25 and 100 µg/mL, indicating high efficacy even at low concentration levels [
43].
Obeng-Boateng et al. (2024) [
72] observed the antibacterial activity of
M. citrifolia extracts against eight pathogenic bacterial species. For this experiment, different parts of the plant were used, such as the root, leaf, and fruit in different forms (fresh, dried, and fermented). The extracts were prepared with distilled water and ethanol in concentrations of 60%, 80%, and 100%, and their efficacy was assessed using the agar diffusion method, with ciprofloxacin as a positive control. The results showed that, in general, the extracts made with pure ethanol were more effective in inhibiting the bacteria, showing larger zones of inhibition than the extracts made with water alone. The root extract prepared with 80% ethanol had the best results, forming an average inhibition zone of 21.33 ± 1.80 mm against the
Campylobacter spp. bacteria. In addition, the
Enterococcus faecium and
Bacillus cereus bacteria were very sensitive to all the extracts tested, while
Higella spp. and
Klebsiella spp. were more resistant [
72].
Complementing these findings, Oliveira et al. (2022) [
73] investigated the antimicrobial potential of endophytic fungi isolated from
M. citrifolia against
Xanthomonas axonopodis, which is the causative agent of bacterial blotch in passion fruit trees. The fungus
Guignardia mangiferae produced two compounds, Sydowinol and Sydowimim A, which showed bactericidal activity up to the lowest concentration tested (3.125 µg/mL), revealing the potential of
M. citrifolia as a source of agricultural antimicrobial agents of natural origin [
73].
2.4.3. Healing and Anti-Inflammatory Activity
In the study conducted by Ly et al. (2020) [
28], it was observed that a 70% ethanolic extract of
M. citrifolia leaves had a significant healing effect on an excisional wound model in mice. The animals treated topically with 1% and 5% concentrations of the extract demonstrated wound contractions of 92.45% and 87.18%, respectively, after 11 days, while the control group exhibited only 54.46% contraction. Furthermore, histological analysis revealed that the groups treated with the
M. citrifolia leaf extract exhibited normal regeneration of the epidermis and reorganization of the skin tissue, accompanied by fibroblast proliferation and a reduction in the inflammatory infiltrate [
28].
Although most of the evidence on the healing potential of
M. citrifolia comes from animal models, some preliminary clinical studies also show benefits in humans. A relevant example is described by West (2018) [
74] in an open-label prospective study conducted with 30 children with burn injuries, aged 2 to 9 years, admitted to a hospital in Bolivia. In this trial, patients received 30 mL of noni juice twice a day for up to 30 days in combination with conventional treatment. The results indicated faster granulation, improved quality of the granulation tissue formed, and reduced hospitalization time when compared to the usual recovery pattern. Although there are methodological limitations, such as the low number of participants, the results reinforce the hypothesis that noni may have beneficial effects on healing in humans, in line with the mechanisms already observed in experimental studies [
74].
The study conducted by researchers Kim, Seong, and Choung (2020) [
53] investigated the effects of treatment with fermented
M. citrifolia on skin lesions similar to atopic dermatitis in mice. Oral administration of fermented
M.a citrifolia at doses of 250, 500, and 1000 mg/kg significantly reduced lesions and symptoms associated with atopic dermatitis, including ear thickness, dermatitis score, the number of infiltrated mast cells and eosinophils, as well as scratching behavior, when compared to the control group. Serum IgE levels in the treatment group decreased approximately twofold, from 2400 µg/mL to 1270 µg/mL, relative to the control group. Furthermore, the treatment reduced histamine levels and inhibited the expression of inflammatory cytokines such as IL-4, IL-17, and IL-22, while restoring the expression of proteins important for the skin barrier, such as filaggrin, loricrin, and involucrin. Thus, according to the authors, the beneficial effects of fermented noni on lesions similar to atopic dermatitis result from synergistic actions of multiple compounds in the extract, which simultaneously modulate Th2-, Th17-, and Th22-mediated inflammation and promote skin barrier restoration [
53].
Alkausar et al. (2024) [
75] observed that a gel formulation containing extracts of noni and
Aloe vera significantly enhanced the healing of burns in white mice (
Mus musculus). The gel, which contained 5% noni and 0.5%
Aloe vera, demonstrated results comparable to a commercial gel. After 15 days, the average wound diameter in the treatment group was 4.04 cm, significantly smaller than the untreated group, which measured 6.53 cm. This finding suggests that the combination of these plants acts synergistically, attributed to active compounds such as mannose-6-phosphate from
Aloe vera, which stimulates fibroblast and collagen synthesis, and antioxidants from noni, including flavonoids, tannins, triterpenes, and polyphenols, which exhibit anti-inflammatory and antimicrobial properties [
75].
In addition, Sousa et al. (2018) [
76] used an excision wound model in streptozotocin-induced diabetic rats to investigate the healing effects of noni fruit juice. The animals were divided, and a group of diabetics was treated with noni juice at a dosage of 100 mL/kg, administered via drinking water for 10 days. The results indicated that the group treated with noni juice experienced a 73% reduction in wound area, which was significantly higher than the 63% reduction observed in the diabetic control group. Furthermore, there was a notable increase in granulation tissue weight and hydroxyproline content, suggesting enhanced collagen deposition. Although the study primarily focused on wound healing, the authors proposed that these benefits may be linked to the anti-inflammatory properties of noni, which contribute to reducing the inflammation and oxidative stress that typically impede healing in diabetic wounds [
76]. Compared to standard therapies such as metformin, which inhibits hepatic glucose production and improves insulin sensitivity in peripheral tissues, noni juice stands out for its ability to modulate inflammation and accelerate tissue healing [
77,
78]. Thus, while metformin has a direct metabolic effect on controlling hyperglycemia, noni has a complementary effect, contributing to the reduction of secondary complications of diabetes, such as delayed wound healing [
77,
78].
West et al. (2009) [
17] found in their studies that the ethanolic extracts and juice of
M. citrifolia leaves exhibit significant topical anti-inflammatory activity. In a model of erythema induced by UVB radiation in volunteers, the application of the extracts increased the minimum dose required to induce erythema by nearly 3.5 times compared to untreated skin. This effect was comparable to that of formulations containing 8% homosalate, highlighting the photoprotective and soothing potential of noni extracts. Additionally, in vitro tests demonstrated that the ethanolic extract of the leaves inhibited binding to the histamine H-1 receptor by 57%, suggesting a possible mechanism of action related to the modulation of histamine-mediated inflammatory pathways [
17].
2.4.4. Gastroprotective Activity
The gastrointestinal effects of the polyphenols found in the fruit of
M. citrifolia were investigated in a study conducted by Chen et al. (2024) [
79]. Dried noni fruit powder was utilized for the experiments, allowing for enhanced control and standardization in simulating human digestive conditions while preserving bioactive compounds that are sensitive to humidity and temperature during storage. The compounds passed through the oral, gastric, and intestinal phases of digestion before undergoing colonic fermentation for up to 24 h. Throughout this process, the release of polyphenols, their antioxidant activity, and changes in the composition of the microbiota were analyzed. The results indicated that the phenolic compounds associated with the fibers were primarily released during colonic fermentation, which also promoted beneficial changes in the intestinal microbiota and an increase in bacterial genera, suggesting a significant prebiotic effect on gastrointestinal health [
79].
Gadicherla et al. (2019) [
80] investigated the therapeutic effect of
M. citrifolia fruit extract for the treatment of L-arginine-induced acute pancreatitis in male rats. The positive control group received melatonin, while the treatment groups received
M. citrifolia extract at doses of 200 and 400 mg/kg six days before disease induction. Twelve hours after induction, blood samples were collected and demonstrated that administration of
M. citrifolia fruit extract promoted a significant reduction (
p < 0.001) in amylase levels (from 330.5 ± 3.23 IU/L in the disease group to 118.8 ± 4.99 IU/L with 400 mg/kg in the extract) and lipase (from 83.17 ± 1.13 U/L to 44.33 ± 1.63 U/L). Histological analysis showed less damage to pancreatic tissue, and DNA assays showed that the extract prevented DNA fragmentation, indicating protection against apoptosis [
80].
In the study by Lina et al. (2017) [
78], the hepatoprotective effects of naturally fermented noni juice (NJ) against liver fibrosis in rodents were observed. The animals were divided, and fibrosis was induced with thioacetamide. They were subsequently treated with noni juice at different concentrations: low dose (2.51 mL NJ/kg), medium dose (5.02 mL NJ/kg), and high dose (7.52 mL NJ/kg). Finally, it was observed that fermented noni juice showed a protective potential against liver fibrosis through increased antioxidant capacities.
Table 7 shows the levels of antioxidant capacity and reduction in liver enzymes in rats [
78].
2.4.5. Other Activities
Ratnoglik et al. (2014) [
81] evaluated the antidiabetic activity of
M. citrifolia fruit extract and its bioactive compound, scopoletin, through in vitro enzyme inhibition and glucose uptake assays in HepG2 cells. First, to determine the cytotoxicity of the compounds, cell viability tests were conducted using the 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT), with a concentration of 1 mg/mL defined for the noni extract and 0.2 μM for scopoletin. Subsequently, the potential of the tested compounds to stimulate glucose uptake by HepG2 cells was investigated by measuring the glucose remaining in the medium after treatment. A decrease in glucose levels indicated greater cellular uptake. These experiments demonstrated the potential of noni extract as an aid in controlling blood glucose levels, both by slowing the digestion of carbohydrates and by promoting glucose utilization by the cells [
81].
Sasnan, Hanani, and Kristianto (2014) [
82] evaluated the effects of
M. citrifolia extract on individuals with hypercholesterolemia through a double-blind clinical trial. This randomized, placebo-controlled study involved 60 volunteers who were equally divided into a control group and an experimental group. Participants in the experimental group received capsules of noni extract (500 mg, three times a day) for 14 days, while the control group received a placebo, following the same regimen as the experimental group. Blood samples were collected before and after treatment, and the results indicated that, at the end of the trial, the group that consumed the extract experienced a significant reduction in total cholesterol and LDL levels, while the placebo group showed no significant changes. None of the participants reported any adverse effects during the study, suggesting that the use of the extract was safe throughout the trial. The authors concluded that
M. citrifolia extract may be a promising natural alternative for managing hypercholesterolemia but emphasized the need for further research, particularly long-term studies, to confirm its clinical efficacy and safety with prolonged use [
82].
Wan Osman et al. (2019) [
83] investigated the therapeutic effects of
Morinda leaf extract, which is rich in epicatechin and scopoletin, using a monoiodoacetate-induced osteoarthritis model in rats. The animals were divided into control groups and were administered noni extract orally for a duration of 28 days. The results showed that the extract significantly reduced the levels of pro-inflammatory cytokines, such as TNF-α and IL-1β, as well as matrix-degrading enzymes, including MMP-3, in the serum of the treated animals. Furthermore, there was an increase in the levels of endogenous antioxidants, such as glutathione, and a decrease in oxidative stress markers. Histopathological analysis revealed significant preservation of cartilage integrity in the knees of rats treated with noni extract [
83].
Moh et al. (2024) [
84] investigated the effects of the methanolic extract of
M. citrifolia fruit on the resistance of
Penaeus vannamei shrimp to infection by
Vibrio parahaemolyticus. The post-larval shrimp were fed for 30 days with rations supplemented with varying concentrations of the extract. Following this period, they were challenged with the pathogenic bacteria. The results indicated that the shrimp receiving the highest dosage of the extract exhibited a survival rate increase of 26.7% compared to the control group. Additionally, there was a significant enhancement in the activity of digestive enzymes and antioxidants, along with a reduction in the degeneration of hepatopancreatic cells [
84].
Finally, Prompipak et al. (2021) [
85] demonstrated that ethanolic extracts of noni fruit combined with 5-fluorouracil (5-FU) exerted a synergistic anticancer effect against cholangiocarcinoma cells both in vitro and in vivo. The combined treatment enhanced apoptosis, increased reactive oxygen species (ROS) production, and significantly reduced tumor growth in xenograft models compared to either treatment alone. While the study did not provide a fractional inhibitory concentration (FIC) index, the quantitative data showed that tumor volume in the combination group decreased by nearly 60% relative to control, whereas 5-FU alone reduced it by 35%. These findings provide strong evidence of the pharmaceutical potential of
M. citrifolia extracts as adjuvants in combination therapies, supporting the need for further investigations into standardized synergy metrics [
85].