The Effect of Two Preservation Techniques on the Yield, Percentage Solids, Electrophoretic Profile, Gelatinolytic Activity, and Brine Shrimp Lethality of Bitis arietans Venom
Abstract
1. Introduction
2. Results
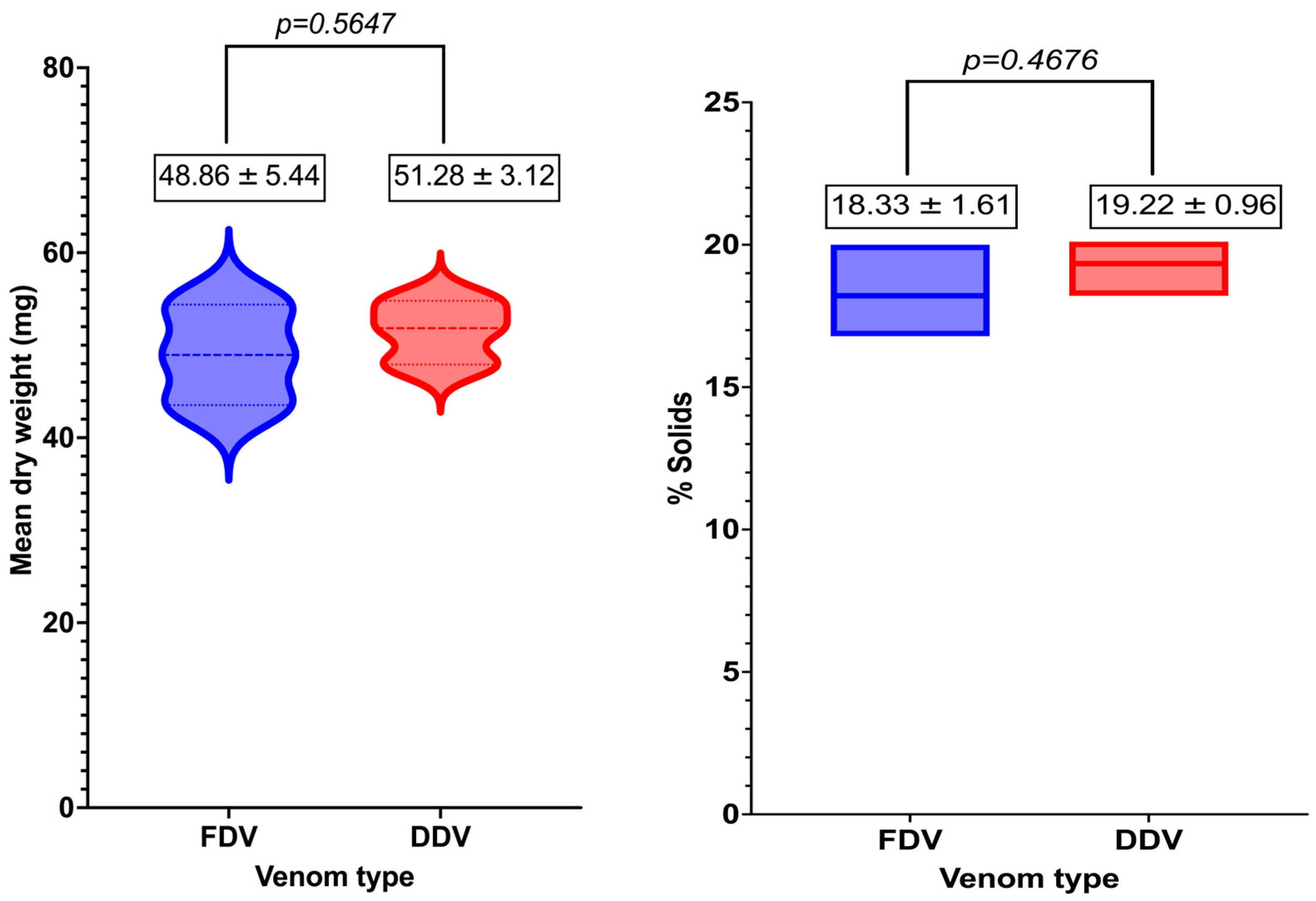
2.1. Yield and Percentage Solids in Venom
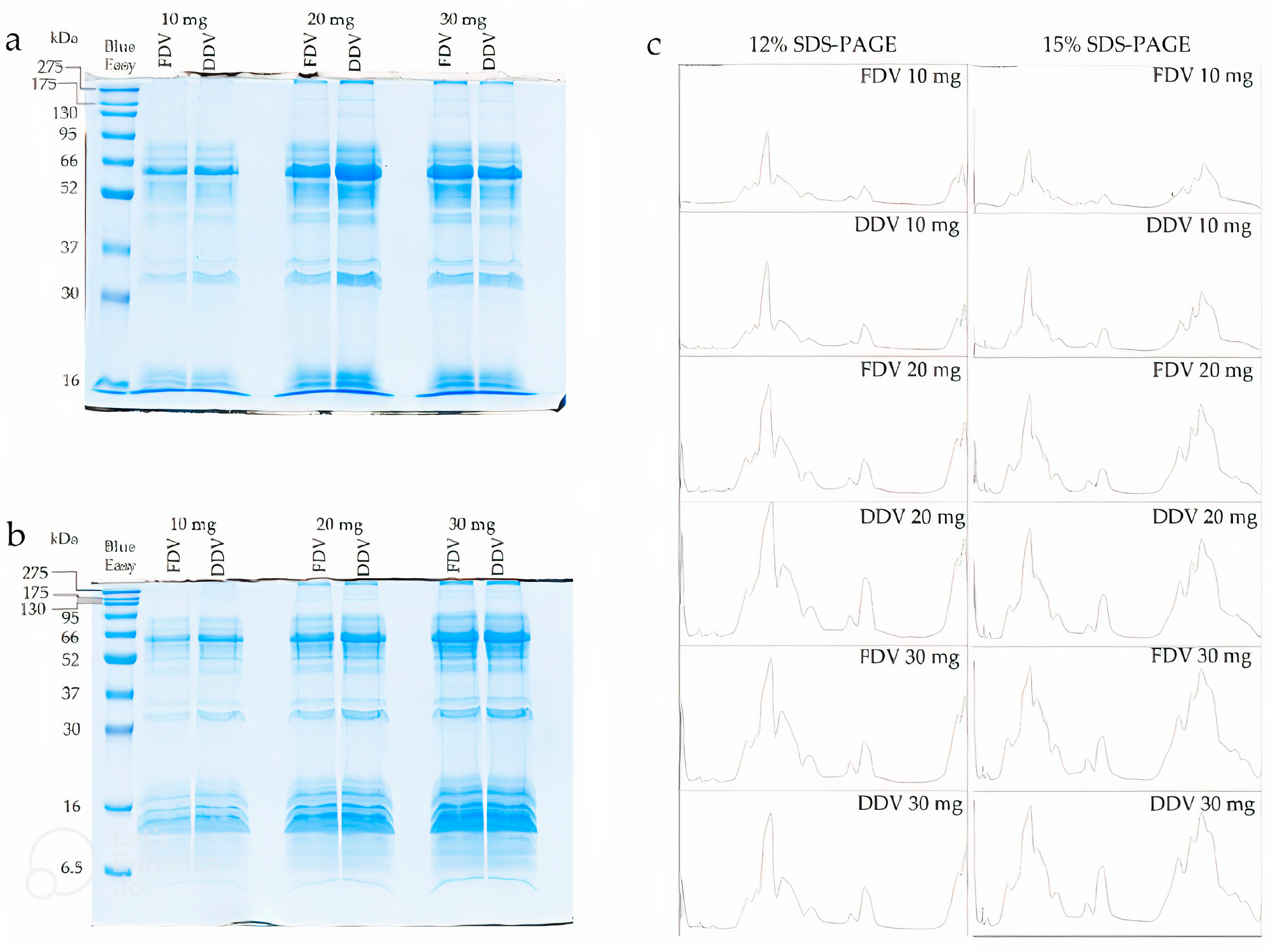
2.2. SDS-PAGE Electrophoresis
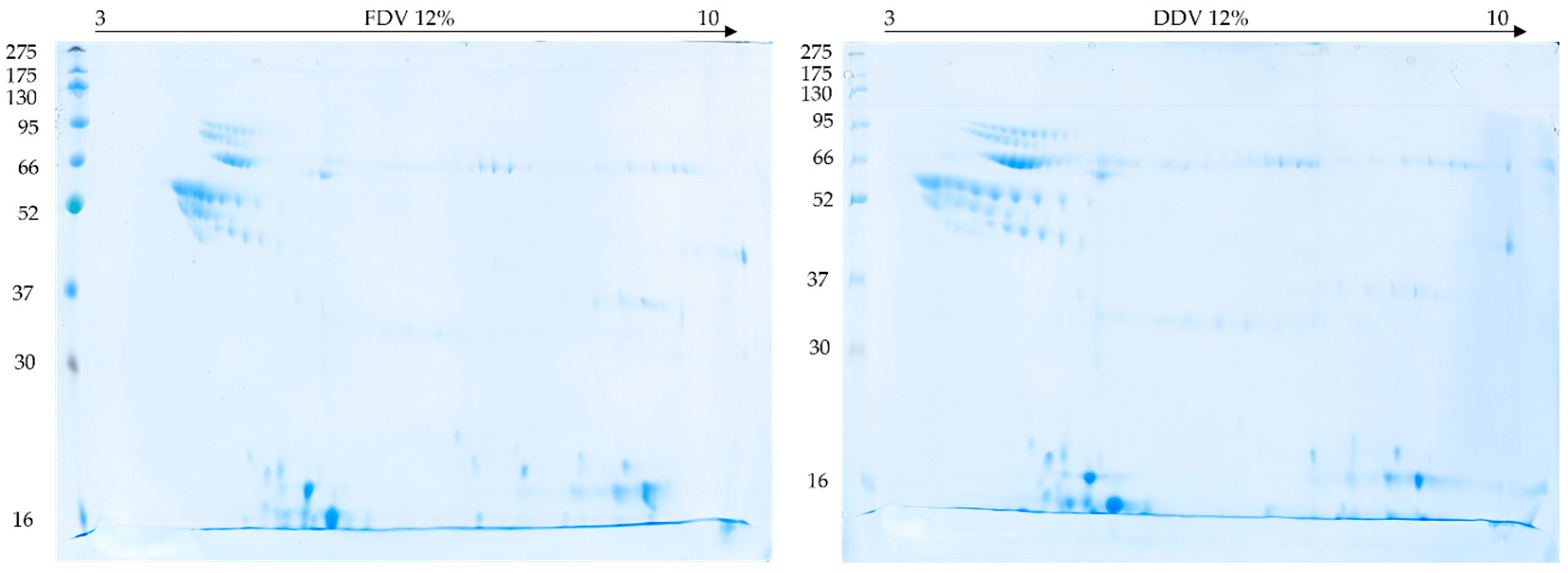
2.3. 2D Electrophoresis
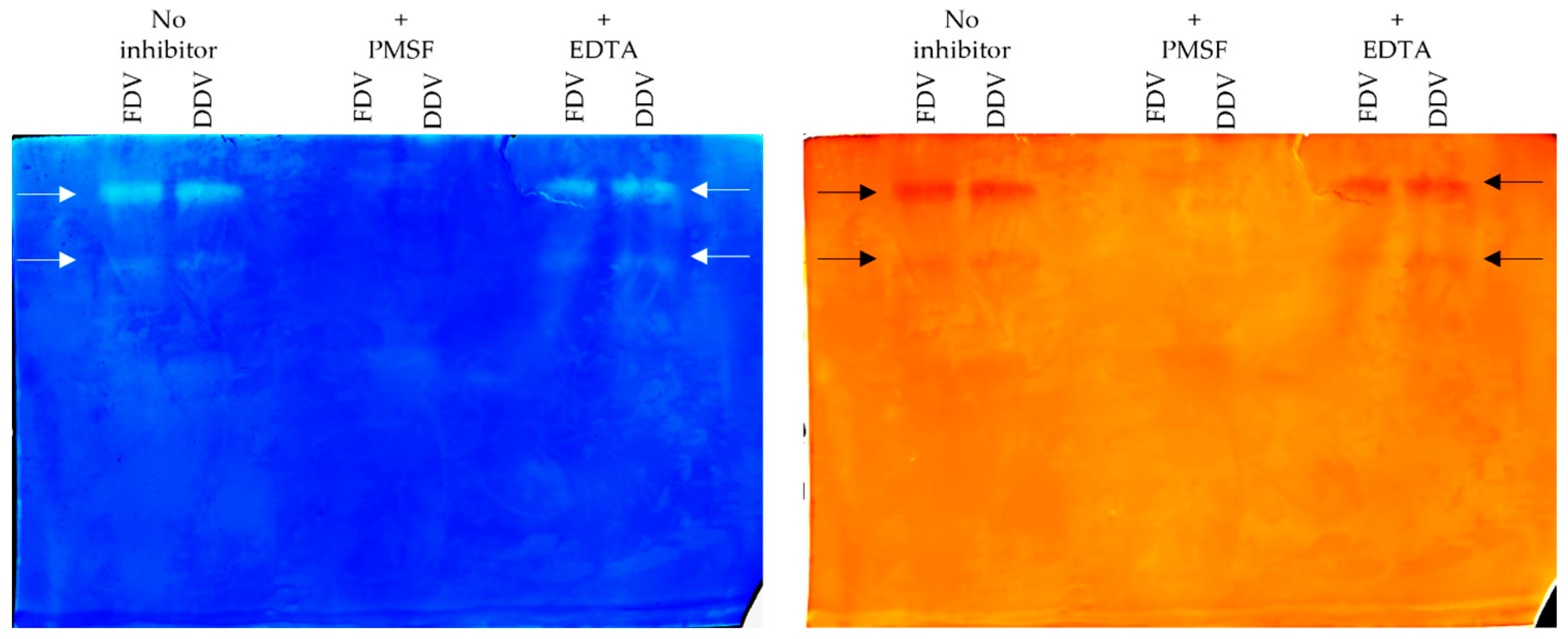
2.4. Gelatinolytic Activity Assay
2.5. Brine Shrimp Lethality Assay
3. Discussion
4. Materials and Methods
4.1. Collection and Preservation of Venom
4.2. SDS-PAGE Electrophoresis
4.3. Two-Dimensional Electrophoresis
4.4. Gelatinolytic Activity Assay
4.5. Brine Shrimp Lethality Assay
4.6. Ethical Considerations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DDV | Desiccator dried venom |
| FDV | Freeze-dried venom |
| 2D | Two-dimensional |
| SDS-PAGE | Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis |
| KDa | Kilodaltons |
| LC50 | Lethal concentration responsible for the death of 50% of brine shrimp |
| PLA2s | Phospholipase A2’s |
| 3FTXs | Three Finger Toxins |
| SVMPs | Snake Venom Metalloproteases |
| SVSPs | Snake Venom Serine Proteases |
| KUN | Kunitz Peptides |
| CRISP | Cysteine Rich Secretory Protein |
| LAAO | L-Amino Acid Oxidase |
| CTL | C-type Lectins |
| DIS | Disintegrins |
| NP | Natriuretic peptide |
| IEF | Isoelectric Focusing |
| EDTA | Ethylene Diamine Tetra Acetic Acid |
| PMSF | Phenylmethylsulphonyl fluoride |
| CBB | Coomassie Brilliant Blue |
| SPSS | Statistical Package for the Social Sciences |
| FVM | Faculty of Veterinary Medicine |
| BAUEC | Biosafety, Animal Use, and Ethics Committee |
References
- Rao, S.; Reghu, N.; Nair, B.G.; Vanuopadath, M. The Role of Snake Venom Proteins in Inducing Inflammation Post-Envenomation: An Overview on Mechanistic Insights and Treatment Strategies. Toxins 2024, 16, 519. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Isbister, G.K. A Current Perspective on Snake Venom Composition and Constituent Protein Families. Arch. Toxicol. 2023, 97, 133–153. [Google Scholar] [CrossRef]
- Tasoulis, T.; Isbister, G.K. A Review and Database of Snake Venom Proteomes. Toxins 2017, 9, 290. [Google Scholar] [CrossRef] [PubMed]
- Tasoulis, T.; Pukala, T.L.; Isbister, G.K. Investigating Toxin Diversity and Abundance in Snake Venom Proteomes. Front Pharmacol. 2022, 12, 768015. [Google Scholar] [CrossRef] [PubMed]
- Willemse, G.T.; Hattingh, J. Snake Venom Instability. Afr. Zool 1978, 13, 293–303. [Google Scholar] [CrossRef]
- Brunton, T.L.; Fayrer, J. On the Nature and Physiological Action of the Poison of Naja Tripudians and Other Indian Venomous Snakes. Ind. Med. Gaz. 1875, 10, 136. [Google Scholar]
- Rabe, M.; Verdes, D.; Seeger, S. Understanding Protein Adsorption Phenomena at Solid Surfaces. Adv. Colloid Interface Sci. 2011, 162, 87–106. [Google Scholar] [CrossRef]
- Vogler, E.A. Protein Adsorption in Three Dimensions. Biomaterials 2012, 33, 1201–1237. [Google Scholar] [CrossRef]
- Grzeskowiak, R.; Hamels, S.; Gancarek, E. Comparative Analysis of Protein Recovery Rates in Eppendorf LoBind® and Other “Low Binding” Tubes; Eppendorf: Hamburg, Germany, 2016. [Google Scholar]
- Munekiyo, S.M.; Mackessy, S.P. Effects of Temperature and Storage Conditions on the Electrophoretic, Toxic and Enzymatic Stability of Venom Components. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 119, 119–127. [Google Scholar] [CrossRef]
- Jesupret, C.; Baumann, K.; Jackson, T.N.W.; Ali, S.A.; Yang, D.C.; Greisman, L.; Kern, L.; Steuten, J.; Jouiaei, M.; Casewell, N.R. Vintage Venoms: Proteomic and Pharmacological Stability of Snake Venoms Stored for up to Eight Decades. J. Proteom. 2014, 105, 285–294. [Google Scholar] [CrossRef]
- Almeida, J.R.; Mendes, B.; Patiño, R.S.P.; Pico, J.; Laines, J.; Terán, M.; Mogollón, N.G.S.; Zaruma-Torres, F.; Caldeira, C.A.D.S.; da Silva, S.L. Assessing the Stability of Historical and Desiccated Snake Venoms from a Medically Important Ecuadorian Collection. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2020, 230, 108702. [Google Scholar] [CrossRef]
- Shukla, S. Freeze Drying Process: A Review. Int. J. Pharm. Sci. Res. 2011, 2, 3061. [Google Scholar]
- Hower, R.O. The Freeze-Dry Preservation of Biological Specimens; The Office of Exhibits Central: Washington, DC, USA, 1967. [Google Scholar]
- Santos, L.; Oliveira, C.; Vasconcelos, B.M.; Vilela, D.; Melo, L.; Ambrósio, L.; da Silva, A.; Murback, L.; Kurissio, J.; Cavalcante, J. Good Management Practices of Venomous Snakes in Captivity to Produce Biological Venom-Based Medicines: Achieving Replicability and Contributing to Pharmaceutical Industry. J. Toxicol. Environ. Health Part B 2021, 24, 30–50. [Google Scholar] [CrossRef]
- Herrera, M.; Solano, D.; Gómez, A.; Villalta, M.; Vargas, M.; Sánchez, A.; Gutiérrez, J.M.; León, G. Physicochemical Characterization of Commercial Freeze-Dried Snake Antivenoms. Toxicon 2017, 126, 32–37. [Google Scholar] [CrossRef]
- Brülls, M.; Rasmuson, A. Heat Transfer in Vial Lyophilization. Int. J. Pharm. 2002, 246, 1–16. [Google Scholar] [CrossRef]
- Wei, W.; Mo, C.; Guohua, C. Issues in Freeze Drying of Aqueous Solutions. Chin. J. Chem. Eng. 2012, 20, 551–559. [Google Scholar] [CrossRef]
- Patra, A.; Herrera, M.; Gutiérrez, J.M.; Mukherjee, A.K. The Application of Laboratory-Based Analytical Tools and Techniques for the Quality Assessment and Improvement of Commercial Antivenoms Used in the Treatment of Snakebite Envenomation. Drug Test Anal. 2021, 13, 1471–1489. [Google Scholar] [CrossRef] [PubMed]
- Dalhat, M.M.; Potet, J.; Mohammed, A.; Chotun, N.; Tesfahunei, H.A.; Habib, A.G. Availability, Accessibility and Use of Antivenom for Snakebite Envenomation in Africa with Proposed Strategies to Overcome the Limitations. Toxicon X 2023, 18, 100152. [Google Scholar] [CrossRef]
- Dorce, V.A.C.; da Rocha, M.M.T.; Candido, D.M.; Nencioni, A.L.A.; Auada, A.V.V.; Barbaro, K.C.; Lebrun, I. Influence of Different Processing Techniques on the Toxicity and Biochemical Characteristics of Tityus Serrulatus Scorpion Venom. Toxicon 2018, 156, 41–47. [Google Scholar] [CrossRef]
- Egen, N.B.; Russell, F.E. Effects of Preparatory Procedures on the Venom from a Rattlesnake (Crotalus Molossus Molossus), as Determined by Isoelectric Focusing. Toxicon 1984, 22, 653–657. [Google Scholar] [CrossRef]
- Mirtschin, P.J.; Shine, R.; Nias, T.J.; Dunstan, N.L.; Hough, B.J.; Mirtschin, M. Influences on Venom Yield in Australian Tigersnakes (Notechis scutatus) and Brownsnakes (Pseudonaja textilis: Elapidae, Serpentes). Toxicon 2002, 40, 1581–1592. [Google Scholar] [CrossRef]
- Sahyoun, C.; Rima, M.; Mattei, C.; Sabatier, J.-M.; Fajloun, Z.; Legros, C. Separation and Analytical Techniques Used in Snake Venomics: A Review Article. Processes 2022, 10, 1380. [Google Scholar] [CrossRef]
- Hus, K.K.; Buczkowicz, J.; Pietrowska, M.; Petrilla, V.; Petrillová, M.; Legáth, J.; Litschka-Koen, T.; Bocian, A. Venom Diversity in Naja Mossambica: Insights from Proteomic and Immunochemical Analyses Reveal Intraspecific Differences. PLoS Negl. Trop. Dis. 2024, 18, e0012057. [Google Scholar] [CrossRef]
- Every, D. Quantitative Measurement of Protease Activities in Slab Polyacrylamide Gel Electrophoretograms. Anal. Biochem. 1981, 116, 519–523. [Google Scholar] [CrossRef]
- Vandooren, J.; Geurts, N.; Martens, E.; Van den Steen, P.E.; Opdenakker, G. Zymography Methods for Visualizing Hydrolytic Enzymes. Nat. Methods 2013, 10, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Meléndez-Martínez, D.; Plenge-Tellechea, L.F.; Gatica-Colima, A.; Cruz-Pérez, M.S.; Aguilar-Yáñez, J.M.; Licona-Cassani, C. Functional Mining of the Crotalus Spp. Venom Protease Repertoire Reveals Potential for Chronic Wound Therapeutics. Molecules 2020, 25, 3401. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Stetlerstevenson, W.G. Quantitative Zymography: Detection of Picogram Quantities of Gelatinases. Anal. Biochem. 1994, 218, 325–329. [Google Scholar] [CrossRef]
- Okumu, M.O.; Mbaria, J.M.; Gikunju, J.K.; Mbuthia, P.G.; Madadi, V.O.; Ochola, F.O.; Jepkorir, M.S. Artemia Salina as an Animal Model for the Preliminary Evaluation of Snake Venom-Induced Toxicity. Toxicon X 2021, 12, 100082. [Google Scholar] [CrossRef] [PubMed]
- Araya, X.; Okumu, M.; Durán, G.; Gómez, A.; Gutiérrez, J.M.; León, G. Assessment of the Artemia Salina Toxicity Assay as a Substitute of the Mouse Lethality Assay in the Determination of Venom-Induced Toxicity and Preclinical Efficacy of Antivenom. Toxicon X 2024, 22, 100195. [Google Scholar] [CrossRef]
- Mallow, D.; Ludwig, D.; Nilson, G. True Vipers: Natural History and Toxinology of Old-World Vipers; CAB International: Wallingford, UK, 2003; ISBN 0894648772. [Google Scholar]
- Brown, J.H. Toxicology and Pharmacology of Venoms from Poisonous Snakes; Thomas: New York, NY, USA, 1973. [Google Scholar]
- Fox, J.W.; Serrano, S.M.T. Exploring Snake Venom Proteomes: Multifaceted Analyses for Complex Toxin Mixtures. Proteomics 2008, 8, 909–920. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Clemetson, K.J. Snake Venom L-Amino Acid Oxidases. Toxicon 2002, 40, 659–665. [Google Scholar] [CrossRef]
- Eichberg, S.; Sanz, L.; Calvete, J.J.; Pla, D. Constructing Comprehensive Venom Proteome Reference Maps for Integrative Venomics. Expert Rev. Proteom. 2015, 12, 557–573. [Google Scholar] [CrossRef]
- Ghezellou, P.; Albuquerque, W.; Garikapati, V.; Casewell, N.R.; Kazemi, S.M.; Ghassempour, A.; Spengler, B. Integrating Top-down and Bottom-up Mass Spectrometric Strategies for Proteomic Profiling of Iranian Saw-Scaled Viper, Echis Carinatus Sochureki, Venom. J. Proteome Res. 2020, 20, 895–908. [Google Scholar] [CrossRef]
- Offor, B.C.; Muller, B.; Piater, L.A. A Review of the Proteomic Profiling of African Viperidae and Elapidae Snake Venoms and Their Antivenom Neutralisation. Toxins 2022, 14, 723. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, C.A.H.; Borges, R.J.; Lomonte, B.; Fontes, M.R.M. A Structure-Based Proposal for a Comprehensive Myotoxic Mechanism of Phospholipase A2-like Proteins from Viperid Snake Venoms. Biochim. Biophys. Acta (BBA)—Proteins Proteom. 2014, 1844, 2265–2276. [Google Scholar] [CrossRef]
- Gasanov, S.E.; Dagda, R.K.; Rael, E.D. Snake Venom Cytotoxins, Phospholipase A2s, and Zn2+-Dependent Metalloproteinases: Mechanisms of Action and Pharmacological Relevance. J. Clin. Toxicol. 2014, 4, 1000181. [Google Scholar] [CrossRef]
- de Oliveira, A.L.N.; Lacerda, M.T.; Ramos, M.J.; Fernandes, P.A. Viper Venom Phospholipase A2 Database: The Structural and Functional Anatomy of a Primary Toxin in Envenomation. Toxins 2024, 16, 71. [Google Scholar] [CrossRef]
- Gutiérrez, J.M.; Rucavado, A.; Escalante, T. Snake Venom Metalloproteinases. In Handbook of Venoms and Toxins of Reptiles; CRC Press: Boca Raton, FL, USA, 2016; pp. 115–138. [Google Scholar]
- Bermúdez-Méndez, E.; Fuglsang-Madsen, A.; Føns, S.; Lomonte, B.; Gutiérrez, J.M.; Laustsen, A.H. Innovative Immunization Strategies for Antivenom Development. Toxins 2018, 10, 452. [Google Scholar] [CrossRef] [PubMed]
- Alomran, N.; Alsolaiss, J.; Albulescu, L.-O.; Crittenden, E.; Harrison, R.A.; Ainsworth, S.; Casewell, N.R. Pathology-Specific Experimental Antivenoms for Haemotoxic Snakebite: The Impact of Immunogen Diversity on the in Vitro Cross-Reactivity and in Vivo Neutralisation of Geographically Diverse Snake Venoms. PLoS Negl. Trop. Dis. 2021, 15, e0009659. [Google Scholar] [CrossRef] [PubMed]
- WHO. Guidelines for the Production, Control and Regulation of Snake Antivenom Immunoglobulins; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Fix, J.D. Short Communication Cryosorptive Pumping as a Method of Lyophilization and Vacuum Desiccation of Snake Venom; Pergamon Press: Oxford, UK, 1976; Volume 14. [Google Scholar]
- Schwick, G.; Dickgiesser, F. Probleme Der Antigen-Und Fermentanalyse Im Zusammenhang Mit Der Herstellung Polyvalenter Schlangengiftseren. Gift. Erde 1963, 227, 35–66. [Google Scholar]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Meyer, B.N.; Ferrigni, N.R.; Putnam, J.E.; Jacobsen, L.B.; Nichols, D.E.J.; McLaughlin, J.L. Brine Shrimp: A Convenient General Bioassay for Active Plant Constituents. Planta Med. 1982, 45, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Okumu, M.; Mbaria, J.; Gikunju, J.; Mbuthia, P.; Madadi, V.; Ochola, F.; Oketch-Rabah, H.; Dhanani, T.; Kalita, B.; Vishwa Vidyapeetham, A.; et al. Exploring Nature’s Antidote: Unveiling the Inhibitory Potential of Selected Medicinal Plants from Kisumu, Kenya against Venom from Some Snakes of Medical Significance in Sub-Saharan Africa. Front. Pharmacol. 2024, 15, 1369768. [Google Scholar] [CrossRef] [PubMed]



| Venom Type | Concentration (µg/mL) | Number of Deaths per 5 mL Sample Tube (Out of 10 Larvae) | Mean % Number of Deaths ± SEM | LC50 (µg/mL) | Toxicity | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | Meyer’s Toxicity Index | Clarkson’s Toxicity Index | ||||
| DDV | 10 100 1000 | 1 5 10 | 3 4 10 | 7 0 10 | 0 4 10 | 0 2 10 | 22.00 ± 13.19 30.00 ± 8.94 100 ± 0.00 | 86.57 | Toxic | Highly cytotoxic |
| FDV | 10 100 1000 | 0 0 6 | 1 1 5 | 0 1 7 | 1 0 7 | 0 0 7 | 4.00 ± 2.45 4.00 ± 2.45 64.00 ± 4.00 | 460.37 | Toxic | Slightly (low) cytotoxic |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okumu, M.; Nieczaj, A.; Hassan, F.; Ooko, S.; Sande, E.; Chinheya, R.; Manjia, J.; Bocian, A. The Effect of Two Preservation Techniques on the Yield, Percentage Solids, Electrophoretic Profile, Gelatinolytic Activity, and Brine Shrimp Lethality of Bitis arietans Venom. Molecules 2025, 30, 3827. https://doi.org/10.3390/molecules30183827
Okumu M, Nieczaj A, Hassan F, Ooko S, Sande E, Chinheya R, Manjia J, Bocian A. The Effect of Two Preservation Techniques on the Yield, Percentage Solids, Electrophoretic Profile, Gelatinolytic Activity, and Brine Shrimp Lethality of Bitis arietans Venom. Molecules. 2025; 30(18):3827. https://doi.org/10.3390/molecules30183827
Chicago/Turabian StyleOkumu, Mitchel, Anna Nieczaj, Farhan Hassan, Selline Ooko, Ebrahim Sande, Rosa Chinheya, Jacqueline Manjia, and Aleksandra Bocian. 2025. "The Effect of Two Preservation Techniques on the Yield, Percentage Solids, Electrophoretic Profile, Gelatinolytic Activity, and Brine Shrimp Lethality of Bitis arietans Venom" Molecules 30, no. 18: 3827. https://doi.org/10.3390/molecules30183827
APA StyleOkumu, M., Nieczaj, A., Hassan, F., Ooko, S., Sande, E., Chinheya, R., Manjia, J., & Bocian, A. (2025). The Effect of Two Preservation Techniques on the Yield, Percentage Solids, Electrophoretic Profile, Gelatinolytic Activity, and Brine Shrimp Lethality of Bitis arietans Venom. Molecules, 30(18), 3827. https://doi.org/10.3390/molecules30183827







